Copyright © Queen’s Printer and Controller of HMSO 2020. This work was produced by Appleton et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Appleton RE, Rainford NEA, Gamble C, et al. Levetiracetam as an alternative to phenytoin for second-line emergency treatment of children with convulsive status epilepticus: the EcLiPSE RCT. Southampton (UK): NIHR Journals Library; 2020 Nov. (Health Technology Assessment, No. 24.58.)

Levetiracetam as an alternative to phenytoin for second-line emergency treatment of children with convulsive status epilepticus: the EcLiPSE RCT.
Show detailsParts of this chapter have been reused from Lyttle et al.1 This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) license, which permits others to copy and redistribute the material in any medium or format, provided the original work is properly cited. See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
Recruitment
Participants were recruited from a total of 30 sites, all EDs. Although 30 sites were formally opened, two subsequently closed with neither site having recruited any participants. The first participant was recruited from Alder Hey Children’s Hospital, Liverpool, on 22 July 2015 under version 3.0 of the protocol and the last participant was recruited from Southampton General Hospital, Southampton, on 7 April 2018 under version 5.0 of the protocol.
The five top-recruiting sites recruited 124 out of the 286 (43.4%) participants.
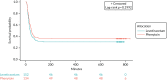
The observed site opening and participant recruitment rates closely followed those predicted (Figure 2).
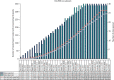
FIGURE 2
Recruitment graph.
Study commencement was delayed by approximately 6 weeks because of contractual issues between the two co-sponsors of the study and, therefore, recruitment closed 6 weeks later than planned, on 10 April 2018.
Participant screening and throughput are summarised in Figure 3.
FIGURE 3
Consolidated Standards of Reporting Trials (CONSORT) flow diagram. ITT, intention to treat.
Of the 1432 children screened for eligibility, 404 were randomised (i.e. the randomisation pack was opened). A total of 1028 children were excluded before randomisation, including 972 children who did not meet the eligibility criteria (see Tables 1 and 2). Reasons for not randomising 53 children who were considered eligible included no trial-trained doctor available, loss of, or failure to, achieve i.v. access, clinical judgement (e.g. child too sick) and treatment given before random allocation.
Inclusion criterion 2 (see Table 1) combined seizure type with the need for second-line treatment. Table 2 looks at this inclusion criteria in greater detail to distinguish eligibility owing to seizure type and ongoing need for second-line treatment.
Of the 404 children randomised, 93 did not require a second-line treatment. Of those children randomised who did require a second-line treatment, 25 were excluded from the analysis as consent was either incompletely documented (n = 6) or declined (n = 19). Table 3 provides the reasons why consent was declined.
TABLE 3
Reasons for consent declined
Compliance with the intervention
Data on compliance (adherence) with treatment are shown in Tables 4 and 5.
TABLE 4
Treatment received by allocation
TABLE 5
Trial compliance data
Baseline characteristics
The baseline characteristics of the study participants were comparable (Table 6). However, the levetiracetam-treated group comprised more participants in whom the presenting episode of CSE [n = 69 (45%) vs. n = 49 (37%)] represented their first convulsive seizure ever, with a lower proportion of participants with a chronic epilepsy, as evidenced by participants taking oral maintenance antiepileptic drugs [n = 51 (34%) vs. n = 55 (41%)].
TABLE 6
Baseline demographic and seizure characteristics of the trial population
Protocol deviations
Protocol deviations were comparable across both treatment groups. Major and minor deviations are shown in Table 7.
TABLE 7
Major and minor protocol deviations
Major protocol deviations comprised:
- premature discontinuation of randomised treatment (none in the levetiracetam-treated group and two in the phenytoin-treated group)
- duration of infusion shorter than expected (none in the levetiracetam-treated group and one in the phenytoin-treated group)
- lower dose of the intervention administered (eight in the levetiracetam-treated group and four in the phenytoin-treated group)
- missing data for primary outcome (two in both the levetiracetam- and phenytoin-treated groups).
Primary outcome
Seizure cessation was achieved in 106 out of the 152 (70%) levetiracetam-treated participants and in 86 out of the 134 (64%) phenytoin-treated participants.
Table 8 provides the median time to seizure cessation from randomisation and Figure 4 shows the Kaplan–Meier curve and log-rank test. As the event of interest (i.e. seizure cessation) is positive, the lower curve indicates a shorter time to seizure cessation; however, there is no statistically significant difference between the treatment arms (log-rank p-value > 0.05).
TABLE 8
Time to cessation of all visible signs of convulsive seizure activity
The unadjusted HR was 1.2 (95% CI 0.91 to 1.6; p = 0.2) in favour of levetiracetam. The Schoenfeld residuals for the unadjusted model (p = 0.72) indicated the independency of time and the validity of the proportionality assumption. The Schoenfeld residuals for the adjusted model indicated that the assumption of proportionality for weight was not met (p = 0.05, p-value ranged from 0.27 to 0.71 for other variables). The data were subgrouped according to weight category as per the baseline table (i.e. < 12 kg, 12–36 kg and > 36 kg) and estimates from the adjusted model calculated (see Appendix 6, Tables 15–29). The proportionality assumption within each subgroup of data was supported by the Schoenfeld residuals. Direction of treatment effect was consistent across subgroups, CIs were wide and results were not statistically significant. The treatment effect was increased for children in the > 36 kg subgroup, but remained non-significant and numbers within this group are small.
A range of sensitivity analyses were considered to assess seizure cessation from the start of the infusion, rather than from randomisation (see Appendix 7, Table 32) and the impact censoring regarding RSI and death. The results demonstrated robustness of conclusions and are presented in Appendices 6 and 7.
Secondary outcomes
The study comprised four secondary outcomes. These assessed the efficacy and safety of the two trial interventions (Table 9).
TABLE 9
Secondary outcomes and 14-day follow-up
One participant who was allocated and received phenytoin experienced a SAR. This was profound hypotension, which was considered to be immediately life-threatening and responded to emergency treatment. This participant also experienced a severe unexpected serious adverse reaction (SUSAR), which manifested as a large increase in seizure frequency and marked sedation within 24 hours of receiving phenytoin. The SUSAR was considered medically significant and the participant required admission to the intensive care unit. The SUSAR resolved without complication.
Safety, tolerability and compliance
Two patients died in the study, but neither death was considered related to the randomised treatment. One participant presented to the ED in a generalised tonic–clonic seizure and unconscious. Resuscitation was immediate. The participant received levetiracetam (the randomised treatment) and then phenytoin, followed by RSI with thiopentone (Archimedes, Reading, UK) because of abnormal posturing. The participant died 36 hours following admission and a post-mortem examination revealed severe brain oedema secondary to encephalitis. Consent for recruitment into the study was subsequently obtained from the participant’s carers. The death was considered to be unrelated to the randomised treatment by the principal investigator and chief investigator.
The second participant received phenytoin. The participant died and the results of the post-mortem examination were not available prior to closure to recruitment to the study. The principal investigator and chief investigator considered the death to be unrelated to the randomised treatment. Consent was sought from the participant’s carers but had not been obtained by the time recruitment to the study closed on 10 April 2018 and, therefore, this participant’s data are not included in the analysis.
Adverse events and serious adverse events
A total of 51 AEs were reported in 39 out of the 286 participants. Some participants experienced more than one AE. Forty-one of the AEs were classified as mild, nine as moderate and one as severe. Sixteen out of 130 levetiracetam-treated participants, 18 out of 132 phenytoin-treated participants and four out of 24 participants who received both drugs experienced at least one AE. Each individual AE had a prevalence of < 10%. In the levetiracetam-treated group (20 AEs in 16 participants), a psychiatric AE was reported in 12 participants (agitation in 11 and hallucinations in one). In the phenytoin-treated group (23 AEs in 18 participants), a cardiovascular AE was reported in eight participants, an extravasation/administration site reaction AE in seven (severe in one) and an agitation AE in four. In the group that received levetiracetam and phenytoin (eight AEs in four participants), an extravasation/administration site reaction was reported in three. The full list of reported AEs is shown in Table 10.
TABLE 10
Adverse events
Five SAEs were reported in four participants [including one participant who experienced two SAEs (participant 00133027)]. In three participants, the SAE was considered unrelated to the intervention, and one each was considered to be possible and probable (Table 11).
TABLE 11
Serious adverse events
An additional follow-up questionnaire was completed by sites and the families of participants who had been randomised, treated and consented to take part in the study. The questionnaire was completed 2 weeks following randomisation. Results are shown in Table 12.
TABLE 12
Follow-up questionnaires
Only 74 (25.9%) families completed their 14-day follow-up questionnaire. As documented in the internal meeting minutes, 8 May 2018, details for these questionnaires are not presented within this report because of the low response rate.
Laboratory parameters (haematological, biochemical analysis and urinalysis)
Laboratory analyses in the trial protocol were limited to measurements of blood levels of levetiracetam and phenytoin in the randomised, treated and consented participants. Blood samples were obtained between 1 and 2 hours after completion of infusion of the two treatments. Biochemistry laboratories in each site processed the samples in accordance with their standard operating procedures. The reference ranges for blood levels of levetiracetam were provided by a single central laboratory that analysed all of the samples of levetiracetam-treated participants. The reference ranges for blood levels of phenytoin were provided by the biochemistry department of each participating site that analysed samples of its phenytoin-treated participants.
A total of 192 participants underwent measurement of a blood level [96 participants in the levetiracetam-treated group (63.2%) and 96 participants in the phenytoin-treated group (77.7%)] and the results are shown in Figures 5 and 6.

FIGURE 5
Levetiracetam blood levels.
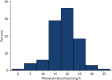
FIGURE 6
Phenytoin blood levels.
Copyright © Queen’s Printer and Controller of HMSO 2020. This work was produced by Appleton et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
- Clinical effectiveness results - Levetiracetam as an alternative to phenytoin fo...Clinical effectiveness results - Levetiracetam as an alternative to phenytoin for second-line emergency treatment of children with convulsive status epilepticus: the EcLiPSE RCT
- Discussion - Levetiracetam as an alternative to phenytoin for second-line emerge...Discussion - Levetiracetam as an alternative to phenytoin for second-line emergency treatment of children with convulsive status epilepticus: the EcLiPSE RCT
- Mus musculus predicted gene 9222 (Gm9222), transcript variant 13, non-coding RNAMus musculus predicted gene 9222 (Gm9222), transcript variant 13, non-coding RNAgi|1845792405|ref|NR_168605.1|Nucleotide
- Mus musculus predicted gene 9222 (Gm9222), transcript variant 9, non-coding RNAMus musculus predicted gene 9222 (Gm9222), transcript variant 9, non-coding RNAgi|1845792094|ref|NR_168601.1|Nucleotide
- Cladonia diversa voucher DFC9 small subunit ribosomal RNA gene, partial sequence...Cladonia diversa voucher DFC9 small subunit ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence; and large subunit ribosomal RNA gene, partial sequencegi|2305955872|gb|OP479900.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...