NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Khunti K, Griffin S, Brennan A, et al. Behavioural interventions to promote physical activity in a multiethnic population at high risk of diabetes: PROPELS three-arm RCT. Southampton (UK): NIHR Journals Library; 2021 Dec. (Health Technology Assessment, No. 25.77.)

Behavioural interventions to promote physical activity in a multiethnic population at high risk of diabetes: PROPELS three-arm RCT.
Show detailsParticipant recruitment commenced in December 2013 and was completed in February 2015, with data collection completed in July 2019. Overall, 12,417 individuals from 47 general practices were identified as potentially eligible to take part and were sent invitation letters, with a further 746 being identified and invited from previous research databases. Of these, 1563 individuals consented to take part and were screened for inclusion. Eighty were subsequently withdrawn because they were diagnosed with T2D at baseline and a further 117 were excluded because of ineligibility following the baseline visit, or otherwise withdrew from the trial before randomisation, leaving 1366 randomised and, therefore, included in this analysis. The overall flow of participants is highlighted in Figure 2. For the flow stratified by each site (Leicester and Cambridge), see Appendix 2, Figures 20 and 21.
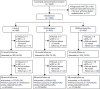
FIGURE 2
Participant flow.
The sociodemographic and clinical characteristics of those included in the trial, stratified by randomised study arm, are presented in Table 5. Study arms were well matched. The median age ranged from 60 to 61 years across the arms.
TABLE 5
Sociodemographic and clinical characteristics of trial participants, stratified by randomised study arm
In total, 993 (72.7%) individuals had valid primary outcome data at the 48-month follow-up and were, therefore, included in the primary analysis. Generally, those with complete data had similar characteristics to those with missing data, including baseline prediabetes status, although those with missing data in the intervention study arms were more likely to be smokers, less likely to be university educated and less likely to have access to the internet. For the specific values for the characteristics of those with and those without complete primary data, stratified by intervention study arm, see Appendix 4, Tables 29–31.
Intervention engagement for each intervention study arm is shown in Table 6. Approximately 80% attended the initial education programme in both arms and over two-thirds attended at least one annual group-based follow-on session. The majority also engaged with the key elements of the telephone and text messaging intervention in the Walking Away Plus study arm. On average, participants were sent a mean of 266 (SD 75) text messages (approximately five per month) (see Appendix 17 for the array of text messages used).
TABLE 6
Engagement rates with the key components of the intervention (per protocol criteria)
Primary outcome
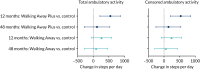
Over the 48 months of the trial, participants in all study arms experienced small reductions in ambulatory activity, with no difference between either of the intervention study arms and the control arm (see Figure 4). However, at 12 months, participants in the Walking Away Plus study arm had increased their total ambulatory activity by 547 (97.5% CI 211 to 882) steps per day compared with those in the control study arm (see Figure 4).
The results for total ambulatory activity were consistent with those for censored ambulatory activity, in which an increase in the Walking Away Plus study arm compared with the control arm of 531 (97.5% CI 201 to 86) steps per day was observed at 12 months. This indicates that the increase in ambulatory activity at 12 months was primarily because of purposeful movement.
When the 278 participants (62%) in the Walking Away and the 235 (52%) participants in the Walking Away Plus study arms who met the pre-protocol definition were analysed, or when missing data were replaced with multiple imputation (Table 7), the results for total ambulatory activity at 48 months – the primary end point – were not affected.
TABLE 7
Change in ambulatory activity at 48 months using a per-protocol definition or when replacing missing data with multiple imputation
The average levels of total ambulatory activity (primary outcome) and censored ambulatory activity at each assessment time point in each study arm are shown in Table 8. The change in ambulatory activity in intervention study arms compared with the control arm at follow-up is shown in Figure 3.
TABLE 8
Ambulatory activity at baseline and follow-up in each study arm
The results for the primary outcome were not modified by sex, age, ethnicity, family history of diabetes, prediabetes at baseline or obesity status, suggesting that the results for the primary outcome were consistent across these categories, including ethnicity (see Appendix 7, Table 36). However, there was evidence that the primary outcome was modified by social deprivation (p = 0.035 for interaction). In the Walking Away Plus study arm, compared with the control study arm, those above the median level of social deprivation increased their ambulatory activity (480 steps/day, 97.5% CI –73 to 1033 steps/day), whereas those below the median level had a decrease in activity level at 48 months (–370 steps/day, 97.5% CI –945 to 205 steps/day).
Physical activity and sedentary behaviour
The other objective measures of physical activity and posture are presented in Tables 9 and 10, respectively. Time in moderate to vigorous physical activity increased by 3.5 (97.5% CI 0.6 to 6.5) minutes per day and time spent walking increased by 8.5 (97.5% CI 3.3 to 13.7) minutes per day in the Walking Away Plus study arm compared with the control study arm at 12 months, but the differences were not sustained at 48 months. The self-reported measures of physical activity are presented in Appendix 8, Table 37. There was an increase in total physical activity energy expenditure in the Walking Away Plus study arm compared with the control arm of 4.4 kJ/kg/day (97.5% CI 0.0 to 8.8 kJ/kg/day) at 48 months; no other differences were detected.
TABLE 9
Baseline and follow-up physical activity and sedentary behaviour data, with corresponding intervention effects
TABLE 10
Baseline and follow-up posture and walking time data, with corresponding intervention effects
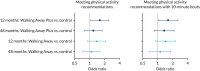
At 12 months, the proportions of participants meeting the physical activity guidelines of 150 minutes per week of objectively measured physical activity of at least moderate intensity in the control, Walking Away and Walking Away Plus study arms were 50.1% (n = 190), 59.5% (n = 194) and 60.2% (n = 205), respectively. The odds ratio of meeting the physical activity guidelines was 1.61 (97.5% CI 1.05 to 2.45) higher in the Walking Away Plus study arm than in the control arm at 12 months (Figure 4), with the results maintained when considering 150 minutes accumulated in at least 10-minute bouts (see Figure 4). However, no differences were observed at 48 months.
Other secondary outcomes
The anthropometric outcomes at baseline and follow-up, along with the associated intervention effects, are presented in Appendix 9, Tables 38–47. There was a reduction in body mass of 0.6 kg (97.5% CI 0.03 to 1.18 kg), a reduction in waist circumference of 1.23 cm (97.5% CI 0.38 to 2.18 cm) and a reduction in body fat percentage of 0.50% (97.5% CI 0.03% to 0.98%) in the Walking Away study arm compared with the control study arm at 12 months. These effects were sustained at 48 months, with a reduction in body mass of 1.00 kg (97.5% CI 0.07 to 1.92 kg), a reduction in waist circumference of 1.57 cm (97.5% CI 0.45 to 2.70 cm) and a reduction in body fat percentage of 1.06% (97.5% CI 0.33% to 1.79%) observed in the Walking Away study arm compared with the control arm. The results for weight and waist circumference are displayed in Figure 5. There were no other changes to assessed outcomes at 12 or 48 months in the Walking Away study arm or the Walking Away Plus arm.

FIGURE 5
Change in ambulatory activity in intervention arms compared with control at follow-up. Data are mean (97.5% CI). Adjusted for randomisation stratification variables (centre, ethnicity, sex) and baseline value.
The biochemical outcomes at baseline and follow-up, along with the associated intervention effects, are presented in Appendix 9, Tables 40 and 42. Triglycerides were reduced by –0.15 mmol/l (97.5% CI –0.29 to –0.01 mmol/l) in the Walking Away Plus study arm compared with the control arm at 12 months, with effects sustained at 48 months (–0.11 mmol/l, 97.5% CI –0.21 to –0.00 mmol/l). Liver enzymes alanine aminotransferase (ALT) and alkaline phosphatase (ALP) followed the pattern of weight loss in the Walking Away study arm, with reductions of 1.79 (97.5% CI 0.07 to 3.51) IU/l and 3.70 (97.5% CI 0.96 to 6.45) IU/l, respectively, compared with the control study arm observed at 48 months. There were no other changes to the assessed biochemical outcomes at 12 or 48 months.
During the trial, 39 (9.3%) individuals in the control study arm, 30 (7.8%) individuals in the Walking Away arm and 41 (10.4%) individuals in the Walking Away plus arm developed T2D. There was no difference in the odds of developing diabetes in either intervention arm compared with the control arm.
The dietary and sleep outcomes at baseline and follow-up, along with the associated intervention effects, are presented in Appendix 9, Tables 44 and 46. At 12 months, both intervention arms reported an increase in fresh fruit consumption of 0.21 (97.5% CI 0.07 to 0.35) and 0.17 (97.5% CI 0.04 to 0.31) portions per week in the Walking Away and Walking Away Plus arm, respectively, compared with control. In addition, the Walking Away Plus arm reported an increase in green leafy vegetable consumption of 0.14 (97.5% CI 0.00 to 0.29) portions per week and a reduction in cheese intake of 0.16 (97.5% CI 0.1 to 0.31) portions per week. At 48 months, participants in both intervention arms also reported an increase in green leafy vegetables of 0.24 (97.5% CI 0.07 to 0.41) and 0.24 (97.5% CI 0.08 to 0.41) along with an increase in other vegetables of 0.20 (97.5% CI 0.05 to 0.35) and 0.20 (97.5% CI 0.06 to 0.35) in the Walking Away and Walking Away Plus arms, respectively, compared with the control arm. In addition, the Walking Away arm maintained their increased fruit consumption at 48 months, reporting 0.22 (97.5% CI 0.05 to 0.40) more portions per week. At 48 months, changes to fruit and vegetable consumption were matched by dietary restraint, where those in the Walking Away and Walking Away plus arms reported actively trying to limit the amount of total fat in their diet on 0.32 (97.5% CI 0.09 to 0.55) and 0.32 (97.5% CI 0.09 to 0.55) more days per week and saturated fat by 0.41 (97.5% CI 0.18 to 0.65) and 0.37 (97.5% CI 0.12 to 0.61) more days per week, respectively, than those in the control arm. No other differences in other dietary variables or sleep time were observed.
The quality-of-life, depression and anxiety outcomes at baseline and follow-up, along with the associated intervention effects, are presented in Appendix 9, Table 46. There was a small increase in the EQ-5D quality-of-life score of 0.02 (97.5% CI 0.00 to 0.04) units in the Walking Away arm at 12 months compared with the control arm; no other differences were observed at either time point.
See Appendix 5 for the self-efficacy and illness perception scores at baseline and follow-up in each study arm (see Tables 32–34). See Appendix 6, Table 35, for self-reported use of behaviour change strategies. Self-efficacy for walking was high in all study arms at all time points. At the 48-month follow-up, participants in the control study arm were 90% confident that they could walk for 60 minutes per day, compared with an average confidence rating of 100% in the Walking Away arm and 95% in the Walking Away Plus study arm. Illness perception scores indicated that those in Walking Away and Walking Away Plus arms increased their perceived understanding of their risk of diabetes at 12 months and 48 months following the intervention, whereas understanding remained stable in the control study arm. However, there was no consistent evidence that other key illness perceptions were systematically different between the control and the intervention study arms, including perception of the degree of agency (control) over diabetes risk or the degree to which treatment can be used to alter risk, which largely remained stable across time in all study arms.
However, the intervention study arms did differentially affect the use of behaviour change strategies, especially using pedometers over the course of the intervention. At 48 months, 64.2% of particiapnts in the Walking Away Plus arm and 49.7% in the Walking Away study arm reported using a pedometer at least some of the time, compared with 19.7% of participants in the control study arm, who had not received a pedometer as part of the study. Similarly, 40.9% and 30.6% in the Walking Away Plus and Walking Away study arms, respectively, reported keeping an exercise log at least some of the time, compared with 11.1% in the control study arm. Furthermore, 78.8% and 73.0% in the Walking Away Plus and Walking Away study arm, respectively, reported setting themselves exercise goals, compared with 64.0%% in the control study arm. Similar differences between study arms were also observed at 12 months. Full data are displayed in Appendix 6.
In the control study arm, there were seven (1.5%) serious and 47 (3.4%) non-serious adverse events. The equivalent values for Walking Away were 15 (3.3%) and 14 (3.11%), respectively, and for Walking Away Plus they were 28 (6.4%) and 16 (3.5%), respectively. Additional details and a breakdown of adverse reporting in each study arm are displayed in Appendix 15, Tables 71–73.
- Results - Behavioural interventions to promote physical activity in a multiethni...Results - Behavioural interventions to promote physical activity in a multiethnic population at high risk of diabetes: PROPELS three-arm RCT
- Economic evaluation - Patient-reported outcome measures for monitoring primary c...Economic evaluation - Patient-reported outcome measures for monitoring primary care patients with depression: the PROMDEP cluster RCT and economic evaluation
- The FEVER observational study - Different temperature thresholds for antipyretic...The FEVER observational study - Different temperature thresholds for antipyretic intervention in critically ill children with fever due to infection: the FEVER feasibility RCT
Your browsing activity is empty.
Activity recording is turned off.
See more...