NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Brazzelli M, Aucott L, Aceves-Martins M, et al. Biomarkers for assessing acute kidney injury for people who are being considered for admission to critical care: a systematic review and cost-effectiveness analysis. Southampton (UK): NIHR Journals Library; 2022 Jan. (Health Technology Assessment, No. 26.7.)

Biomarkers for assessing acute kidney injury for people who are being considered for admission to critical care: a systematic review and cost-effectiveness analysis.
Show detailsThis chapter assesses the cost-effectiveness of alternative biomarkers (NephroCheck, ARCHITECT urine NGAL and BioPorto urine and plasma NGAL assays) used in combination with standard clinical assessment (i.e. serum creatinine and urine output), compared with standard clinical assessment alone, for evaluating critically ill people who are at risk of developing AKI and are being considered for possible critical care admission in an NHS hospital setting. The specific objectives were to review the existing cost-effectiveness evidence base for these tests and to develop a de novo economic model to assess cost-effectiveness from an NHS and Personal Social Services perspective.
Systematic review of existing cost-effectiveness evidence
Objective
The aim of the review of economic evaluations was to identify, report and critically appraise existing economic evaluations of NephroCheck, ARCHITECT urine NGAL, and BioPorto urine and plasma NGAL assays for evaluating critically ill people (adults and children) at risk of developing AKI.
Search strategies
Comprehensive electronic searches were conducted to identify economic evaluations of the candidate tests. Highly sensitive search strategies were developed, including index terms, free-text words, abbreviations and synonyms. The following electronic databases were searched: Ovid MEDLINE, Ovid EMBASE, NHS Economic Evaluation Database, HTA Database, Research Papers in Economics, and International Society for Pharmacoeconomics and Outcomes Research (ISPOR) Presentations Database, with no restriction on date, language or publication type. The searches were undertaken on 27 May 2019, with additional searches on 11 September 2019.
Inclusion and exclusion criteria
Studies were deemed appropriate for inclusion in the review of economic evaluations if they (1) were full economic evaluations, defined as a comparative assessment of costs and outcomes in the framework of cost–utility, cost-effectiveness, cost–benefit or cost-minimisation analyses; (2) assessed the cost-effectiveness of the candidate tests within the population defined in the NICE scope (i.e. critically ill people, both adults and children, at risk of AKI who are being considered for admission to ICUs); and (3) provided sufficient information to judge the quality of the study and obtain any relevant data (i.e. conference abstracts alone were unlikely to meet this criterion). Economic evaluations conducted alongside single effectiveness studies (e.g. RCTs) and decision-analytic models were all deemed relevant for inclusion. Studies were excluded if they were methodological studies, systematic reviews of cost-effectiveness studies (although these were retained for reference) or cost-of-illness studies. Studies were also excluded if they assessed tests/biomarkers outside the NICE scope (e.g. cystatin C) only or used the candidate tests for a purpose other than determining risk of AKI.
Quality assessment of included studies
Included studies were appraised against the NICE reference case for the assessment of cost-effectiveness of diagnostic tests.96
Evidence synthesis of cost-effectiveness studies
The main findings are summarised in a narrative review, with key study characteristics and findings tabulated for ease of comparison.
Results
Figure 19 illustrates the PRISMA flow diagram for the review of economic evaluations. The searches identified 125 potentially relevant abstracts. After abstract screening, 99 (79.2%) studies were excluded because they did not meet the inclusion criteria. Full-text articles were sought for the remaining 26 (20.8%) studies for further assessment against the inclusion/exclusion criteria. Of those 26 studies, four studies were ultimately included in the review.95,97–99 A tabulated summary of the study characteristics and results is provided in Table 13, and a quality assessment against the NICE reference case is provided in Table 14.
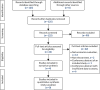
FIGURE 19
The PRISMA flow diagram for the review of cost-effectiveness studies. CA, conference abstract; EE, economic evaluation.
TABLE 13
Summary of study characteristics and results from the review of economic evaluations
TABLE 14
Appraisal of included studies against the NICE reference case and scope
Relevance of the included studies for the current decision problem
Of the four studies identified in the review, three conducted cost-effectiveness analyses based on decision-analysis modelling.97–99 Two of these studies included both a decision tree to capture the diagnostic phase of the model and a Markov cohort model to capture the long-term sequelae of diagnosis and possible prevention of AKI.97,98 Both modelling strategies were similar and appropriate for the current decision problem in that they modelled the progression of AKI to CKD, end-stage renal disease (ESRD), transplantation and death. Although two studies97,99 were conducted in the UK, only one study97 was deemed directly relevant for informing the decision model developed as part of this assessment. Hall et al.97 was also the only study to assess all of the candidate tests specified in the NICE scope. Although Hall et al.97 provide a comprehensive and high-quality assessment of the cost-effectiveness of the relevant tests, the setting of the study relates to AKI occurring in people already admitted to ICUs and is therefore outside the scope of this assessment. Therefore, substantial revision of the Hall et al.97 model is required, particularly for the early acute phase, to generate results that are appropriate for decision-making in critically ill patients who are at risk of AKI and are being considered for possible admission to ICU, but are not yet in the ICU setting.
Additional literature searches
Further searches were conducted to help develop the economic model. Broader searches were carried out to identify existing economic models in the area of AKI in addition to those identified for the candidate biomarker tests. A separate search was also developed for health-state utility data relevant to the health states included in the economic model. As searches for models and parameters were conducted by Hall et al.97 up to 2016, our searches aimed to identify any relevant studies published after this date. Supplementary searches were carried out in MEDLINE, EMBASE, NHS Economic Evaluation Database, HTA Database, Research Papers in Economics, and ISPOR Scientific Presentations. The searches were undertaken on 11 September 2019, with no language restrictions. The relevant data are discussed in the subsections to follow.
Independent assessment of cost-effectiveness
A two-phase model was developed using TreeAge Pro 2018 (TreeAge Software, Inc., Williamstown, MA, USA) to assess the cost-effectiveness of using biomarker tests to help detect the risk of AKI development and to help initiate early preventative care.
As described in Chapter 3, there was no direct evidence regarding the clinical effectiveness of biomarker-guided preventative care, compared with standard monitoring-guided preventative care, on final health outcomes (e.g. AKI status, mortality, need for RRT). Therefore, a linked-evidence approach was required to determine the potential value of the tests. The model structure was therefore built to reflect hypothesised associative benefits of averting AKI or reducing its severity through biomarker-guided early intervention. The structure was informed by the review of cost-effectiveness studies and was based largely on Hall et al.,97 who kindly provided access to their model [built in R (The R Foundation for Statistical Computing, Vienna, Austria)] under a Creative Commons licence. The appropriateness of the model structure was validated with the External Assessment Group’s (EAG’s) clinical experts. Data sources to populate the model are described in the sections that follow. The model was built and analysed following the guidelines stipulated in the NICE reference case for diagnostic test evaluation.101
Methods
Relevant population(s)
The baseline population and prevalence of CKD in hospital for the model were obtained from a Grampian population cohort (described in Model structure: initial decision tree phase). The model base-case analysis is therefore based on a mixed cohort of CKD and non-CKD patients, with an average age of 63 years, and a 54.3% female population.
Diagnostic biomarkers evaluated
The model aims to assess the cost-effectiveness of the NephroCheck test, the ARCHITECT urine NGAL assay and the BioPorto urine and plasma NGAL assays in combination with standard clinical assessment, compared with standard clinical assessment alone (including serum creatinine and urine output), for evaluating critically ill people at risk of developing AKI who are being assessed for possible critical care admission.
Model structure: initial decision tree phase
The systematic review did not identify any randomised trials providing causal evidence for the effect of biomarker-guided care versus standard monitoring (serum creatinine)-guided care on patient-relevant outcomes such as peak AKI severity, admission to ICU, need for RRT, CKD or mortality.
In the absence of such data, the initial decision tree phase of the model used a linked-evidence approach to capture the potential impact of diagnostic test accuracy (sensitivity and specificity) on the probability of averting AKI or reducing its severity through earlier adoption of a KDIGO care bundle triggered by a positive biomarker test result. The model then captured possible effects on changes in health outcomes through associative links between AKI severity and the relevant outcomes [need for ICU care, length of stay (LOS), 90-day mortality, and development of CKD].
These associative links have been built up in the decision tree by reanalysis of observational data from Grampian.102 The data set includes 17,630 adult patients admitted to hospital in Grampian in 2003 and is the complete population of all patients who had an abnormal kidney function blood test on hospital admission, including all patients who developed AKI. The study methodology is described in detail by Sawhney et al.,103 but the data derived from the data set used to populate the model are unpublished. These observational, population-level data were used to define the starting age, sex and underlying proportion of prevalent CKD cases in the modelled cohort. The data were also used to populate the model with respect to the distribution of peak AKI severity, as well as LOS in hospital, probability of admission to ICU and 90-day mortality parameters (by KDIGO AKI stage) for the decision tree phase of the model.
In the decision tree, patients who are critically ill in hospital, are at risk of developing AKI and are having their kidney function monitored are divided into two cohorts, those with AKI and those without AKI, depending on the underlying prevalence.102,103 The underlying prevalence of AKI was calculated directly from a more recent version of the Grampian data set,102 describing all hospital admissions with at least one overnight stay in 2012 (for patients having their kidney function monitored). The base-case prevalence of AKI generated from these data was 9.2%, sampled probabilistically from a beta distribution in the model based on count data. A sensitivity analysis uses prevalence data directly from the systematic review studies used to generate the diagnostic test accuracy parameters.
Acute kidney injury is defined in the model as having, or being destined to develop, AKI while in hospital and is classified based on the peak severity of AKI. There is an assumption in the model that it is possible to avert AKI with early biomarker-guided treatment in people who would otherwise develop it under standard care. However, it should be noted that, in some circumstances, it may not be possible to avoid AKI by earlier detection, as AKI may not always be modifiable.3 The probability of averting AKI is zero in the standard-care arm. AKI is split into four KDIGO-defined stages (stages 1–3), with stage 3 split by the proportion of patients receiving RRT or not. The initial phase of the model describes the associations between peak AKI classification and probability of admission to ICU, LOS in ICU, LOS in hospital and 90-day mortality. These associative effects are all derived from the Grampian population cohort described previously. At the end of the 90 days, costs and QALY payoffs are assigned based on the decision tree pathway followed, before surviving patients enter the Markov cohort model.
The standard-care cohort is assumed to be perfectly identified as having or not having AKI, based on a combination of serum creatinine levels, other diagnostic workup and clinical expert opinion, which represents clinical practice. The hypothesised advantage of the biomarkers is that they may help to detect AKI earlier, but will not detect additional cases of AKI not detected by current practice. Figure 20 provides an illustration of the initial decision tree pathways for the standard-care arm of the model.
Participants in the intervention (test) arms of the model are similarly split into those with and without AKI, according to the same prevalence data, but all participants receive additional testing. It is assumed that the background diagnostic workup is similar for all arms of the model (i.e. all patients will continue to have their serum creatinine and urine output monitored). As the diagnostic accuracy test data are primarily based on single use of the test, it is assumed, in the base-case model, that each test will be administered only once. It is assumed that the test is administered as soon as possible after a patient has been determined to be at risk of AKI to enable early detection and preventative measures to be implemented. A sensitivity analysis explores the impact of more frequent multiple-use tests on the results.
The diagnostic accuracy of the candidate tests in addition to serum creatinine, compared with serum creatinine alone, was obtained from the results of the systematic review and meta-analysis described in Chapter 3. Table 15 describes the diagnostic accuracy parameters, namely sensitivity and specificity, used in the modelling. All diagnostic data are incorporated probabilistically in the model, accounting for the joint uncertainty in sensitivity and specificity for each biomarker test. The logit of the sensitivity/specificity for each of the biomarker tests was derived from the meta-analysis of diagnostic accuracy studies. The model specified the correlation between sensitivity and specificity parameters (on the logit scale). These parameters were converted to Cholesky decomposition matrices, with the decomposed data referenced by multinormal distributions, sampling from the mean and standard error (on the logit scale). The probabilistic draws were back-transformed from the logit scale for application in the model. It should be noted that diagnostic accuracy data obtained from the meta-analyses are based on heterogeneous studies with different thresholds; this is particularly true for the NGAL assays. Therefore, the results of the economic model, particularly for comparisons between different NGAL assays, should be interpreted cautiously. Further details have been provided in Chapter 3.
TABLE 15
Sensitivity and specificity data used in the model
For each biomarker test group, the proportions of true AKI cases that are true positive and false negative are determined by test sensitivity, whereas the proportion of non-AKI cases that are true negatives or false positives are determined by test specificity.
Based on the EAG’s own clinical expert opinion (Simon Sawhney and Callum Kaye, University of Aberdeen, 2019, personal communication), it is assumed in the base case that patients testing negative would not have any adaptions made to their care pathway. This is because it would be unlikely that care would be de-escalated based solely on a negative NephroCheck or NGAL result, as the conservative practitioner would wait to ensure that there was no rise in serum creatinine before concluding that no AKI was present and stepping down the patient’s care.
The model assumes that all patients will receive the KDIGO care bundle once they are defined as AKI positive using current standard practice methods (i.e. monitoring serum creatinine and urine output), regardless of their NephroCheck or NGAL test result. The potential to benefit from use of the biomarkers, therefore, lies in the early adoption of a preventative care bundle. For patients testing positive, the model includes the functionality to reflect uncertainty in clinical decision-making, that is the probability that a positive test would be acted on. This parameter is assumed to take a value of 100%, in accordance with best practice guidance whereby positive biomarker tests should have a preventative KDIGO care bundle implemented, with the associated costs. Although all positive test results will trigger the KDIGO bundle, only those patients whose tests are true positive will accrue any potential benefits of having their AKI averted or having reduced severity (i.e. peak KDIGO stage) AKI. For exploratory scenarios in which a test might not be acted on in practice, the cohort would follow the standard-care pathways according to whether or not they had AKI, as measured using current clinical practice.
There is limited direct evidence to describe the impact of the use of the AKI biomarkers on important health outcomes (such as need for ICU care, length of hospital stay, risk of 90-day mortality or development of new/progression of existing CKD). Therefore, a linked-evidence approach was required, whereby we relied on observational associations to infer how prevention or mitigation of AKI may affect changes in health outcomes. The associative effects are the benefits of averting or mitigating AKI that lead to better health outcomes (i.e. need for ICU care, CKD and mortality).
These associations necessitate causal assumptions, but, although a causal link between AKI and poor outcomes is plausible, the extent of this causal relationship is uncertain and controversial. It cannot necessarily be assumed that, by averting or changing the severity of AKI, a patient would have the exact same risks (associative effects from the Grampian observational data described previously) of ICU and mortality as a patient who was never going to develop AKI in the first place.
As the true causal relationship between AKI and health outcomes is unknown, the model includes the functionality to apply none, all or a proportion of the relative risk (RR) of health outcomes such as need for ICU care, mortality and CKD (AKI vs. none) to the AKI-averted proportion of the cohort. This is achieved while maintaining the observational associations in the standard-care arm of the model.
The base-case analysis assumes that there are no adverse health consequences of a false-positive test on either NephroCheck or NGAL. Clinical expert opinion (Simon Sawhney and Callum Kaye, personal communication) indicates that there may be a risk to a patient’s health of inappropriate fluid resuscitation; delay of access to appropriate imaging because of concerns regarding contrast exposure; or removal of the most effective, but potentially nephrotoxic, treatments for a critically ill patient. However, the magnitude of this negative effect is difficult to quantify. Therefore, a sensitivity analysis explores scenarios in which an additional mortality risk is added for false-positive tests.
In summary, the early-stage (up to 90 days) costs and outcomes depend on (1) the diagnostic accuracy of the test, (2) clinical decision-making in the presence of positive or negative test results, (3) the initiation of a KDIGO care bundle to avert AKI and amend the distribution of peak AKI severity and (4) the degree to which the hypothesised associative effects between AKI and final health outcomes, such as length of hospital stay, admission to ICU, need for RRT, 90-day mortality and risk of CKD, can be modified simply by amending the AKI distribution.
Model structure: follow-up Markov model
One potential route to patient benefit is that avoiding AKI or reducing its severity may reduce the risk of later developing CKD. As CKD is defined as a minimum of 3 months of persistent reduced renal function,105,106 progression from AKI to CKD is incorporated into the Markov phase of the model.
Figure 21 illustrates the long-term follow-up model structure.
After 90 days, the surviving cohort from each of the decision tree pathways enters a lifetime Markov model. The model follows a similar structure to those of Hall et al.97 and Parikh et al.,95 with six mutually exclusive health states: outpatient follow-up, CKD (stages 1–4), ESRD not requiring dialysis, ESRD requiring dialysis, post transplant and death. Members of the cohort enter the model either in the outpatient follow-up state, where they experience an annual baseline risk of developing CKD, or directly in the CKD state, with the proportion starting in the CKD state determined by the underlying CKD prevalence and the severity of AKI from the acute (decision tree) phase of the model. The base-case model assumes that the outpatient cohort have an increased risk of CKD in the first cycle that is dependent on their AKI experience, but, thereafter, the transition between the outpatient follow-up and CKD states is independent of whether or not a patient had AKI in the hospitalisation period. A sensitivity analysis explores the impact of an increased CKD risk applied for the full lifetime time horizon, as per Hall et al.97
The cohort is then modelled to transition through the disease pathway, starting with CKD stages 1–4 (defined as a single Markov state), to ESRD, with or without the requirement for dialysis, the need for transplant, the success or failure of that transplant, and, ultimately, progression to death, with state-specific mortality probabilities. Those members of the cohort who experience transplant failure are assumed to return to the dialysis health state, in which the probability of a second transplant is the same as the probability of a first transplant. The cohort is exposed to a probability of all-cause mortality from each model state and assigned mortality probabilities based on the higher value of age- and sex-adjusted all-cause mortality or the disease state-specific mortality obtained from the literature.
Model parameters: probabilities and duration of length of stay
Table 16 summarises the probability, LOS and relative effect size parameters used to populate the economic model. Further details and description are provided in the sections that follow.
TABLE 16
Probability, LOS and RR parameters used in the economic model
Early phase probabilities and length of stay
The potential associative links between AKI and ICU admission, ICU LOS, hospital LOS and 90-day mortality are all sourced from the Grampian data set.102 For chance nodes in the decision tree with only two possible branches, probabilities are sampled from beta distributions. Where there are three or more branches, probabilities are incorporated using Dirichlet distributions.
The model assumes, based on expert opinion, and consistent with Hall et al.,97 that RRT is provided in AKI stage 3 only; this is deemed reflective of most current clinical practice. Assuming no RRT for patients who have a peak AKI of stage 1 or 2 might be considered a favourable scenario for biomarker tests that can reduce AKI severity, thereby generating reductions in cost. In the absence of published UK data, the proportion of AKI stage 3 patients requiring RRT is taken from a retrospective analysis of 5242 ICU survivors with AKI across 23 French ICUs.108 A total of 1603 of these survivors had KDIGO AKI stage 3, of whom 55.2% received RRT. It is assumed that the French ICU setting is broadly transferable to a UK pre-ICU setting for critically ill patients and is therefore appropriate for populating the model. Data reported from Hall et al.97 are not used because they relate to only a single UK ICU setting with a small sample of patients with AKI 3 patients (n = 18). The EAG’s clinical experts validated these data as relevant to the UK setting and noted that the probability was lower than that applied in Hall et al.,97 which was consistent with clinical experience outside the ICU setting. Moreover, more detailed data on the need for RRT in England are currently being collected by the UK Renal Registry, but are not yet publicly available.
There are potentially strong associations between AKI status or severity of AKI and the probability of needing ICU care and of dying within 90 days of hospital admission. However, these data should not be interpreted as definitive causative effects and a sensitivity analysis explores the application of different assumptions around these highly uncertain associations.
Data for LOS in hospital are obtained from the Grampian data set,102 but ICU LOS was unavailable by peak AKI status. ICU LOS data were therefore obtained from an alternative source, Bastin et al.,109 a large cohort study of 1881 adults who had cardiac surgery (and who were, therefore, deemed critically ill and sufficiently matched the scope for this assessment). Bastin et al.109 reported median LOS in ICU by AKI stage (according to AKIN and KDIGO criteria). Given the likely skewed distribution of LOS data, a log-normal distribution fitted to mean and median days’ duration is used to generate the simulated draws for the probabilistic analysis. As mean LOS in ICU was not available to parameterise the log-normal distribution, it was assumed that the mean was twice the median, reflecting the ratio of mean to median days’ stay as reported in Hall et al.,97 who obtained the data from the Leeds Teaching Hospitals NHS Trust, AKI registry data, for ICU patients.
As the variable ‘hospital LOS’ also includes the time spent in ICU, the time on a hospital ward is obtained by subtracting the ICU LOS from the total hospital LOS for the application of costs and utilities in the model. As the probabilistic analysis samples independently from these distributions, an additional correction is added to the model to ensure that LOS in an ICU cannot exceed total hospital LOS in any of the sampled draws. The average LOS in hospital/ICU for those with a peak AKI of 3 is applied to both those requiring and those not requiring RRT. The assumption that requirement for RRT would not usually extend the hospital admission for this patient cohort has been validated by the EAG’s clinical experts.
The relative effects of diagnostic biomarkers on acute kidney injury and clinical outcomes: the impact of early adoption of a Kidney Disease: Improving Global Outcomes care bundle
The impact of an early Kidney Disease: Improving Global Outcomes care bundle on acute kidney injury
National-level guidelines16 indicate that, in a patient defined as being at risk of developing AKI through a positive biomarker result, all appropriate efforts should be made to ensure that AKI does not develop, and, if it does, it should be minimised in terms of severity (i.e. providing the maximum support possible for the kidneys). The model therefore assumes that all AKI patients will receive a KDIGO care bundle; the only difference between the testing strategies is the duration for which that bundle is implemented, with earlier implementation assumed to incur additional resource use in terms of fluid management, nephrologist review and pharmacist review of medications, as well as the removal of any potentially nephrotoxic agents when necessary.5
There are two potential mechanisms by which early adoption of a KDIGO care bundle might lead to patient benefit. These are to (1) avert AKI in people in whom it would otherwise develop and (2) shift the distribution of AKI severity (between KDIGO AKI stages 1–3), if AKI occurs.
Hall et al.97 conducted a review of the literature to identify studies testing the impact of early preventative intervention for AKI. Their searches identified eight studies relevant to early intervention in the UK setting (excluding early RRT, which was deemed contentious). Four studies explored the impact of early nephrologist involvement, which was deemed to be the most reflective proxy for the non-specific care bundles that a patient may access as part of the KDIGO care bundle recommendations.5 The largest of these four studies, with a sample of 1096 participants, was used in the Hall et al.97 economic model, and found that early nephrologist consultation reduced AKI incidence: adjusted odds ratio (early involvement vs. not) 0.71 (95% CI 0.53 to 0.95).
The EAG has conducted a supplementary targeted search of trials for the post-Hall et al.97 period to identify any further potentially relevant studies exploring the impact of early preventative intervention or application of AKI care bundles on the probability of developing AKI and/or the severity of peak AKI. In brief, 39 additional titles and abstracts were identified from the targeted searches, of which 17 (44%) were full-text assessed. Based on the NICE scope,100 KDIGO care guidelines5 and clinical expert opinion (Simon Sawhney, University of Aberdeen, 2019, personal communication), it was decided that studies testing the impact of a KDIGO care bundle provided the most appropriate source of data to populate the economic model. Three trials110,116,117 (18%) assessed the effect of NephroCheck-guided application of a KDIGO bundle compared with standard care where information about the NephroCheck test result was not available to a patient’s hospital care team. No studies assessed the impact of NGAL-guided treatment.
All three studies reported results in terms of the probability of developing AKI.110,116,117 However, only one study110 described the impact on both the incidence and severity of AKI. Meersch et al.110 reported the results of a single-centre trial, with a sample of 276 participants, in a German setting. All of the population had positive NephroCheck test results, using a 0.3 mg/dl threshold, consistent with the sources of diagnostic accuracy data obtained from the systematic review of diagnostic accuracy studies (see Chapter 3). Patients were then randomised to receive a strict implementation of the KDIGO guidelines or standard care. The intervention group included avoidance of nephrotoxic agents, discontinuation of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), close monitoring of urine output, close monitoring of serum creatinine levels, avoidance of hyperglycaemia (for 72 hours), consideration of alternatives to radiocontrast agents, and fluid optimisation. In the control (standard-care) group, Meersch et al.110 state that the recommendations of the American College of Cardiology Foundation 2011 were followed, including keeping mean arterial pressure at > 65 mmHg and central venous pressure at between 8 and 10 mmHg. ACEIs and ARBs were used only when the haemodynamic situation stabilised and hypertension occurred. It is unclear whether or not knowledge of the NephroCheck test result was revealed to the treating hospital team for patients in the standard-care arm of the study. The primary outcome in Meersch et al.110 was 72-hour AKI and the trial found an absolute risk reduction of 16.6% (95% CI 5.5% to 27.99%). The Meersch et al.110 study was supported by the German Research Foundation (Bonn, Germany), the European Society of Intensive Care Medicine (Brussels, Belgium), the Innovative Medizinische Forschung (Münster, Germany) and an unrestricted research grant from Astute Medical, Inc.
A second, smaller study (121 participants),116 also in a German setting, showed that NephroCheck-guided care demonstrated a trend towards a lower probability of AKI, although the results were not statistically significant, with an OR for standard care versus NephroCheck of 1.96 (95% CI 0.93 to 4.10). The study did, however, find significantly greater odds of AKI (defined as stage 2 and 3 combined) in the standard care group than in the NephroCheck group: OR for standard care versus NephroCheck 3.43 (95% CI 1.04 to 11.32). A third study,117 with only 100 participants, compared the effect of a NephroCheck-triggered consultation implementing KDIGO recommendations for AKI with the effect of standard care in an ED in Germany. AKI outcomes were similar in both groups. The probability of AKI stage 2 or 3 at day 1 post admission was 32.1% in the intervention group and 33.3% in the control group; at day 3, it was 38.9% in the intervention group and 39.1% in the control group. Neither the Göcze et al.116 study nor the Schanz et al.117 study reports any funding involvement from the test manufacturers.
As the Meersch et al.110 study has a larger sample, and reports data for both the probability of AKI and the distribution of AKI severity, given that it occurs, these data were used for the model base-case analysis. Although the clinical context of the immediate postoperative period after cardiac surgery from Meersch et al.110 is likely to be generalisable between the UK and other countries, the nature of the AKI insult (ischaemia/reperfusion, postoperative haemodynamic, oxidative stress, haemolysis, in people with cardiac comorbidity) is specific to this context, as is acknowledged by the authors. Accordingly, this study110 may not be generalisable to AKI in the context of other acute or critical illness circumstances in which biomarker performance and the potential for AKI prevention/mitigation may be different.
The model describes the potential impacts of a biomarker-guided care bundle on (1) the chance that patients may develop AKI and (2) the severity of AKI given that it occurs. The assumption is that early biomarker-guided implementation of the KDIGO care bundle may reduce the proportion of people who develop AKI and help ensure that, if they do develop AKI, it will be of reduced severity. These effects are applied probabilistically as RRs in the model for those with true-positive test results only, using log-normal distributions.
In the absence of any data on the impact of NGAL-guided KDIGO care bundles on the probability of developing AKI or the severity of AKI, the base-case model assumes that the potential to avert AKI is similar for both biomarkers. However, based on clinical expert opinion (Simon Sawhney, personal communication) and the manufacturer-described role of the tests, NGAL measures injury and can be used to define AKI, whereas NephroCheck can identify stress, thereby enabling intervention before AKI develops. Therefore, a sensitivity analysis explores a scenario in which the RR of AKI for NGAL-guided care is equal to 1, while retaining the same effect on the AKI distribution, given that AKI occurs as for NephroCheck. It is acknowledged that these assumptions are uncertain, and the sensitivity analysis may present a bias against NGAL if data were to become available to suggest an effect on AKI prevention.
It was assumed that there are no negative health effects of early intervention for the proportion of each test group with false-positive results, but the additional costs of the bundle were still incurred. The model also includes the functionality to explore the impact of an additional mortality risk, for example because of excessive resuscitation as a result of fluid administration or removal of effective, but nephrotoxic, treatments in patients with a false-positive test result.
Although the model describes the impact of biomarker-guided early intervention on the distribution of AKI, it is unclear whether or not these effects translate into final clinical and patient-relevant health outcomes, such as need for ICU care, need for RRT, mortality or the development of CKD. The limited evidence that exists from Meersch et al.110 suggests that, although there is a significant reduction in the primary study outcome of AKI within 72 hours for NephroCheck-guided implementation of a care bundle, compared with standard care (OR 0.483, 95% CI 0.293 to 0.796), this ability to avert AKI was not demonstrated to translate into improvements in a range of clinical and patient-relevant outcomes, including need for RRT therapy in hospital (OR 1.618, 95% CI 0.676 to 3.874), 90-day all-cause mortality (OR 1.213, 95% CI 0.486 to 3.028), ICU LOS (median difference 0 days, 95% CI –1 to 0 days) or hospital LOS (median difference 0 days, 95% CI –1 to 1 days). Although the study was not powered to detect differences in these outcomes, there are no trends in the data that are suggestive of an effect size. Furthermore, the uncertainty regarding the link between increased resource use and clinical outcomes is emphasised by Wilson et al.,118 who demonstrated in their RCT of an electronic alert system for AKI that an early warning system increases resource use (e.g. renal consultation), but with no evidence that this translates into measurable clinical or patient benefit in terms of mortality or LOS. Indeed, for a subgroup on a surgical ward, the mortality rate was significantly higher in the electronic alert group. As these causal links between AKI and changes in health outcomes are highly uncertain and hypothesised based on observational data in the model, extensive sensitivity analyses are conducted to test the impact of a range of plausible assumptions on cost-effectiveness.
Follow-up phase probabilities
Starting proportions applied in Markov cohorts
One plausible route to patient benefit from averting or reducing the severity of AKI is through the prevention of new CKD and the indirectly associated longer-term progression to ESRD and transplant. It should be noted that the model does not assume a direct effect of peak AKI on ESRD at 90 days; therefore, patients can enter the Markov model in either the outpatient follow-up or CKD (stages 1–4) health states only. A reanalysis of 2012 data from the Grampian cohort102 indicated that only a very small proportion [13/4314 (≈ 0.03%)] of patients with AKI, almost all of whom had underlying CKD, progressed directly to ESRD at 90 days. Therefore, we assumed no direct transition from the decision tree to the ESRD state in the Markov model. The starting proportions (after 90 days) for each health state are dependent on the decision tree pathway through which the cohort has come, and the peak AKI severity the cohort experienced. The baseline prevalence of CKD in the UK general population has been estimated from Kerr119 at 6.1%. However, in a group of critically ill, hospitalised patients, this prevalence may be substantially higher. For the base-case analysis, we use the underlying prevalence of CKD in the Grampian data set,102 calculated as the prevalence of CKD in all hospitalised patients having their kidney function monitored. Multiplying through by the sampling fraction for no CKD (20%) and taking the proportion of CKD/full sample gives the baseline prevalence in this group, calculated as (5935/53,691) × 100% = 11.05%.
Health-state transition probabilities
The baseline incidence of new-onset CKD for the Markov model uses the same source as Hall et al.,97 with an annual probability of progressing from the outpatient state to the CKD state of 0.0044 (95% CI 0.0039 to 0.0049) for patients in the no-AKI cohort.111 The data are obtained from a large cohort study of 97,782 ICU patients enrolled on the Swedish intensive care register. The parameter value 0.0044 reflects the CKD incidence at 1 year post ICU admission for the proportion of patients with no AKI. The same baseline proportion of CKD was applied for those without AKI and for those modelled to have had AKI averted as a result of early preventative treatment. The proportion of the no-AKI cohort starting in the CKD state at day 90 was calculated as the underlying prevalence plus the new annual incidence, adjusted to the 90-day time horizon of the decision tree component of the model.
Hazard ratios for AKI 1, AKI 2 and AKI 3 on the development of CKD (defined as CKD stage ≥ 3) were obtained from a systematic review by See et al.112 The review included a total of 82 studies quantifying the association between AKI and longer-term renal outcomes (including CKD) and mortality. However, only three studies reported the impact of each stage of AKI on CKD development.120–122 One study (104,764 participants) in a US setting generated slightly counterintuitive results, with point estimates of the HR reducing as AKI stage increased.120 However, two other studies in Asian settings (with 77122 and 1363121 participants) illustrated an increasing HR for more severe AKI stages. The systematic review has meta-analysed these three studies; the summary effects by AKI stage on CKD, defined as CKD stage 3, are used in the base-case analysis. The advantage of these studies is that they allow a demonstration of the impact of adapting the distribution of AKI severity on longer-term development of CKD. However, they are not conducted in a UK setting and may lack relevance. An alternative source, reporting the HR for the association between AKI and CKD that is constant across all AKI stages, is reported by Sawhney et al.102 for 9004 hospitalised patients with AKI in Grampian. The HR for development of stage 4 CKD (AKI vs. no AKI) was 2.55 (95% CI 1.41 to 4.64). This study has the advantage of relevance to the setting, but does not include risks by AKI severity. However, it should be noted that the definition of CKD is stage 4 in Sawhney et al.,102 compared with stage 3 in the meta-analysed studies, which may limit the comparability of the reported HRs.
The HRs of CKD by AKI stage are applied to the new incidence over the first 90 days and to the first annual transition in the model. Thereafter, the transition probabilities from outpatient follow-up to CKD follow the baseline 0.0044 per year. This approach is based on expert opinion (Simon Sawhney, personal communication) that any longer-term effect of AKI on CKD development will become attenuated over time, particularly if it has not occurred in the first year following hospital discharge. A sensitivity analysis explores a scenario in which the HR of CKD is applied for the full duration of the model, reflecting the assumption applied in Hall et al.97
Prevalence of CKD and incidence of new-onset CKD are parameterised in the model using beta distributions, and the HRs for the effect of peak AKI severity on CKD incidence (i.e. transition probabilities to CKD state) are parameterised using log-normal distributions.
Progression from chronic kidney disease
The transition probability from outpatient follow-up to CKD is 0.0044, as described previously. The model cohort can then progress from CKD to ESRD, with or without dialysis, and from ESRD to transplant according to the modelled transition probabilities. It is assumed that AKI can influence only the number of people who get CKD and then has no further direct effect on how fast they progress through the CKD stages to ESRD, dialysis or transplant. The cohort is also exposed to an increasing mortality risk as it progresses through more severe disease states from CKD (stages 1–4) to ESRD without dialysis and ESRD with dialysis. Transitions from CKD (stages 1–4) to ESRD, from ESRD (no dialysis) to ESRD (with dialysis), and from CKD (stages 1–4)/ESRD to death are obtained from Kent et al.,113 who reported data on progression of kidney disease from the large (7246 participants), international (Europe, North America and Australasia) Study of Heart and Renal Protection (SHARP) RCT. The median study follow-up was 4.9 years, participants had a mean age of 63 years and 64% of participants were male.
For those with ESRD on dialysis, the proportions transitioning to kidney transplant and mortality were obtained from 5-year data published in the 2018 UK Renal Registry report (table 1.17),115 which provided information on transition from incident RRT in 2012 to transplant and mortality 1, 3 and 5 years later. The 3- and 5-year probabilities were annualised; the 3-year probability was applied to years 2 and 3, and the 5-year probability was applied to year 4 onwards. These probabilities were converted to the relevant annual cycle-specific probabilities and applied in the model using tunnel states to track time from entering a given health state. The UK Renal Registry also provided data on the probability of transition back to dialysis for failed transplants and the probability of death over 5 years following transplant. After 5 years post transplant, mortality is assumed to revert to the general population all-cause mortality probability and the annual probability of transplant failure remains at that reported from years 3–5 in the UK Renal Registry. It is further assumed that the proportion of the cohort with a transplant failure return to dialysis, and that their probability of progressing from ESRD on dialysis to a second transplant is the same as the probability of progression to the first transplant.
In the first 5 years of the follow-up phase of the model, mortality in all Markov states is modelled as the average mortality risk for patients discharged from hospital and ICU, unless health state-specific (ESRD, dialysis or transplant) mortality is higher, in which case the latter is applied. If, at any point, mortality falls below all-cause mortality, all-cause mortality is applied in the model. The 5-year post-discharge mortality data were based on Lone et al.,114 a matched UK cohort study (mean age of 60 years) using national registries: the Scottish Intensive Care Society Audit Group, the Scottish Morbidity Record (SMR) of acute hospital admissions (SMR01) and the Scottish death records. The model base case used an average of the ICU and non-ICU cohorts. Beyond 5 years, patients in the outpatient follow-up health state had the age- and sex-adjusted all-cause mortality probability applied,123 and those with CKD, ESRD, chronic dialysis or a transplant would be assigned the health state-specific mortality probability, unless the age- and sex-adjusted all-cause mortality was higher than the health state-specific mortality. A sensitivity analysis explores the impact of assigning long-term mortality risks that are dependent on whether or not the cohort had been admitted to ICU during the index hospitalisation.
Transition probabilities are incorporated into the model probabilistically using beta distributions. As the cycle lengths for the model in Hall et al.97 are the same as the current assessment (annual), it was not necessary to provide any further adjustment of the published transition probabilities.
Model parameters: costs
The health-care costs included are as follows: (1) the costs of conducting the tests, including equipment and staff resource use; (2) the costs of acute care in the first 90 days post hospital admission, including the additional cost of early application of a KDIGO care bundle, the cost of hospital/ICU LOS, and the cost of acute RRT; and (3) the annual, cycle-specific costs associated with Markov health states (CKD, ESRD, dialysis and transplant) over the longer-term follow-up phase. All costs are included from an NHS perspective and are reported in 2017/18 Great British pound values. When possible, resource use has been costed directly using 2017/18 UK national unit cost sources [the Personal Social Services Research Unit (PSSRU) for staff time,124 NHS reference costs for secondary care procedures125 and the British National Formulary for drugs126]. When this has not been possible, for example if total costs are reported in the literature without enough data regarding the underlying resource use to enable re-costing, these costs are inflated from their base year to 2017–18 values using the Campbell and Cochrane Economics Methods Group online inflation calculation tool.127 Table 17 details the cost parameters used in the economic model. Full details of the costing approach and associated assumptions are provided in the following sections.
TABLE 17
Cost parameters used in the economic model
Diagnostic test costs
NephroCheck testing is usually conducted on an Astute140 Meter, costing £3000, and an additional meter would need to be purchased. This cost was converted to an annuity, assuming that the platform’s lifetime is 5 years and an annual depreciation rate of 3.5%. The test could also be conducted on a VITROS Immunodiagnostic System, although, currently, there is a limited installed base of these in UK hospitals,97 which was confirmed at a NICE scoping workshop. The NGAL tests would not require a new platform for NGAL only, because it would be performed on platforms already available at the hospital laboratories. The capital costs of the laboratory analyser apportioned to each NGAL test are assumed to be negligible. The sensitivity analysis excludes capital and training costs to explore the impact on cost-effectiveness of scenarios in which a hospital might already have the required analyser in place and all staff are fully trained in its use.
The process of taking the sample for analysis, sending samples to the laboratory, processing at the laboratory and interpretation of test results would require the involvement of several members of the hospital team. A urine sample is first collected by a nurse, and then picked up by a porter, who takes it to the laboratory. It is assumed that, because the tests are classified as urgent samples, the porter would generally prioritise single test collection for the laboratory. A biomedical scientist conducts the diagnostic test in the laboratory. After completion of the test, the results from the laboratory would be authorised by a biochemist and released for review on the hospital information management system, where they can be interpreted by a nephrologist, an intensive care specialist or a junior doctor. The base-case analysis assumes an average of the three health-care professional costs for interpretation. Under some criteria (such as very abnormal results), a laboratory team might directly contact the care provider, but we assume that this approach would not be used routinely. For the purposes of test cost calculation, it is assumed that, on average, the role of interpreting the tests is equally split across the three specialist team members. The unit costs per hour for each of the staff resources involved in the testing process were obtained from PSSRU 2018:124 to conduct the test – band 5 nurse (£37.00), porter costed as health-care assistant (£27.26) and band 6 biomedical scientist (£44.00); to interpret the test – medical consultant/nephrologist (£108) and junior doctor foundation year 2 (£32.00). It is assumed that a hospital consultant, nephrologist and junior doctor are all equally likely to be the health-care professional interpreting the test.
The duration of resource use for each member of the team is based on a combination of information provided by the manufacturer and clinical expert opinion (Simon Sawhney and Callum Kaye, personal communication) regarding the flow from obtaining the test sample to result interpretation. The staff time to process the test in the laboratory was based on the NICE request for information documents to the different test manufacturers and the final scope (NICE technical team, 2019, personal communication). Estimates of the time taken to prepare the urine sample and interpret the test were based on the EAG’s clinical expert opinion (Simon Sawhney and Callum Kaye, personal communication).
Four test strategies were compared in the economic model: NephroCheck, BioPorto urine NGAL, ARCHITECT urine NGAL and BioPorto plasma NGAL. The NGAL test manufacturer BioPorto has not identified costs separately by sample type (plasma or urine). It is therefore assumed that these tests incur equal costs. The cost of the Alinity i urine NGAL test was not considered in the base-case economic evaluation because the review identified no diagnostic accuracy data for the test. Full costing of each test is provided in Appendix 13, Table 31. Further details of maintenance costs and consumables for each test can be found in Appendix 13, Table 34.
Cost of early treatment
The additional cost of early treatment with the KDIGO care bundle was calculated as £106.36 per patient treated, assuming an additional 3 days’ application of the care bundle in test-positive patients. An additional 3 days of treatment was assumed in line with the primary outcome from Meersch et al.110 (i.e. AKI at 72 hours) and based on clinical expert opinion (Simon Sawhney and Callum Kaye, personal communication) that a care bundle could be implemented for up to an extra 3 days. The care bundle cost is based on the NICE guidelines for preventing AKI,16 which state that measures to prevent AKI are avoidance of nephrotoxic agents, discontinuation of medication (ACEIs and ARBs), close monitoring of serum creatinine and urine output, avoidance of hyperglycaemia, alternatives to radiocontrast and close haemodynamic monitoring. The NICE recommendations for preventing AKI16 include seeking advice from a nephrology team with regard to giving ‘iodinated contrast agent to adults with contraindications to intravenous fluids’ (© NICE 2013 Acute Kidney Injury: Prevention, Detection and Management. Clinical Guideline [CG169].16 Available from www.nice.org.uk/Guidance/CG169. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication) and from a pharmacist with regards to medications (ACEIs, ARBs). Therefore, both nephrologist and pharmacist time are included in the cost of the care bundle. Further details of the cost calculation approach can be found in Appendix 13, Table 32. The additional cost of early adoption of the care bundle was applied to the proportion of the cohort with a positive biomarker test result, reflecting an assumption that care would be delivered for an additional 3 days over and above the cohort monitored using serum creatinine alone. The cost was applied using a gamma distribution with a standard deviation of 10% of the mean.
Acute phase costs
The base-case total cost in the acute phase (90 days) of the model depends on the number of days spent in hospital and the ICU. It also depends on the duration of acute RRT delivered to the proportion of AKI stage 3 patients receiving RRT. For the base-case analysis, data from the Adding Insult to Injury report show that 52% of RRT patients receive continuous RRT (daily) and 48% receive intermittent dialysis (an average of three sessions per week).3 The duration of RRT delivery is obtained from a randomised trial conducted in a US critical care setting129 comparing intensive (6 days per week; n = 563) with less intensive (3 days per week; n = 561) RRT strategies. The mean duration of RRT per patient was similar in both groups: intensive, 13.4 days (SD 9.6 days); n = 563; and less intensive, 12.8 days (SD 9.3 days); n = 561. The base-case model conservatively assumes the less intensive duration for the application of costs in the economic model. To incorporate the uncertainty and to reflect the likely skewed nature of the distribution, the duration of RRT is incorporated probabilistically into the model using a log-normal distribution. Data available from an alternative source,130 as used in NICE guidance for the comparison of early and late RRT, were not considered because median, rather than mean, durations were reported and the data were assessed as being of low quality in the NICE guidance.16 An additional daily excess cost of AKI was applied in a sensitivity analysis to capture the potential excess cost per day in hospital or an ICU of an AKI patient. This excess cost was not applied in the base-case scenarios because it was assumed that the cost of having AKI is captured in the cost of being in hospital or an ICU. All other acute costs and follow-up costs have a gamma distribution applied.
Long-term follow-up costs
There are four ways in which long-term follow-up costs may be driven by the proportion of the cohort that progress through different pathways from the initial decision tree. These are (1) whether or not long-term follow-up costs depend on whether or not a patient received ICU care in the initial decision tree, (2) whether or not there are additional follow-up costs beyond 5 years’ post index hospitalisation discharge, (3) whether or not an excess long-term cost is applied for the proportion of the cohort coming through AKI arms of the decision tree and (4) health state-specific costs incurred as the cohort progress through CKD stages to dialysis or transplant.
The outpatient follow-up costs in the Markov model post index hospitalisation discharge were obtained from Lone et al.,114 who reported 5 years of follow-up costs post index ICU and hospital discharge, using a matched cohort obtained from registries in Scotland [Scottish Intensive Care Society Audit Group, the SMR of acute hospital admissions (SMR01) and Scottish mortality data]. The base-case analysis assumes that the average of post-ICU and post-non-ICU admissions is applied in the Markov model. This is because patients in the cohort for this assessment are already deemed to be critically ill and at risk of needing ICU care, so might all be expected to have significant resource use post discharge. A sensitivity analysis allows the application of differential long-term costs that depend on whether or not a patient had received ICU care in the first 90 days.
The annual costs beyond 5 years are unknown. Therefore, the base-case analysis assumes no additional costs beyond year 5. A sensitivity analysis explores the impact of these assumptions by applying further costs between years 6 and 11 that reduce annually following a logarithmic function, with year 11 costs applied for the remaining duration of the model.
The base-case analysis assumes that there are no long-term excess follow-up costs as a result of having had AKI in the initial 90 days post hospitalisation. However, a sensitivity analysis explores a scenario in which patients entering the Markov model having had AKI in hospital incur an additional 15% of the non-AKI cohort costs for the first 5 years. The additional AKI cost factor was based on a proxy using the RR reported in Lone et al.114 on the number of admissions that patients on RRT had over 5 years, compared with those who were not on RRT. These additional costs are applied in the model as a sensitivity analysis, with a mean ratio of 1.15, log standard error 0.074, sampling from a log-normal distribution.
Annual cycle-specific health-state costs were applied to the proportion of the cohort transitioning through the CKD, ESRD, ESRD on dialysis and transplant health states. Costs were obtained from Kent et al.,113 using data from the SHARP trial reporting outpatient, day case and inpatient admissions. The CKD (stages 1–4) health-state cost applied in the model was calculated as the weighted average of CKD stages 1–3 and CKD stage 4, as reported in Kent et al.113 Therefore, the average weighted cost applied was £445.98 per cycle. The cost of medications (immunosuppressants for transplant patients, erythropoiesis-stimulating agents for dialysis patients and blood pressure medications for dialysis patients) were not captured in the study; therefore, these costs were added to the costs observed in Kent et al.113 The added transplant costs (immunosuppressants) were based on the approach applied in Scotland et al.131 for calculating the annual cost of immunosuppressants, using 2018 prices. The added costs to dialysis patients arising from blood pressure medications and erythropoiesis-stimulating agents are also based on the approach applied in Scotland et al.,131 with 2018 prices.
Health measurement and valuation
Table 18 summarises the utilities used throughout the economic model. These are described in more detail in the sections that follow. A full list of studies and utility values considered for population of the economic model can be found in Appendix 13, Tables 33, 37 and 38.
TABLE 18
Health-state utility values used in the economic model
Acute (decision tree) phase of the model
We have updated the searches from Hall et al.97 to identify studies that report utilities for the initial decision tree phase of the model. Our post-Hall et al.97 review identified four further potentially relevant studies. However, the only utilities that meet the NICE reference case are those proposed by Hall et al.97 All other studies identified from the literature review use non-UK value sets, so are not appropriate for UK decision-making. Given that there are no appropriate utility studies for AKI stage, the analysis uses the utilities identified in Hall et al.97 applied to the model based on LOS in hospital, LOS in ICU and duration discharged prior to 90 days following hospital admission. Owing to a lack of appropriate data and to avoid double-counting the utility impact of time in hospital/ICU, we have not attempted to apply any additional utility decrements by AKI stage (other than those on acute RRT). The application of utilities is consistent with that used by Hall et al.,97 with utilities age- and sex-adjusted when possible, with normal and beta distributions used to incorporate the data probabilistically in the model. It is difficult to find utility values for patients in an ICU. Two systematic reviews were consulted: one by Dritsaki et al.139 and one by Gerth et al.140 Both reviews focused on a population admitted to an ICU; however, no studies identified in the reviews were deemed suitable. Therefore, the utility value of an unconscious patient has been applied for the duration of ICU stay, using data sourced from Kind et al.132 and following the same approach as Hall et al.97 As a sensitivity analysis, we consider an alternative approach to calculate ICU utility to explore the substantial uncertainty in this parameter. The alternative value takes the average of the unconscious state (–0.402 from Kind et al.132) and the average post-ICU discharge from Hernández et al.133 from the Pragmatic Randomised, Controlled Trial of Intensive Care follow up programmes in improving Longer-term outcomes from critical illness (PRaCTICaL) (0.44), which followed up a cohort of ICU survivors reporting their quality of life using the EuroQol-5 Dimensions (EQ-5D) instrument. The calculated utility value applied in the sensitivity analysis was [(–0.402 + 0.44)/2] = 0.019.
Utility values for the chronic phase of the model
First, the Hall et al.97 HTA programme assessment and economic model for long-term follow-up post AKI and the Scotland et al.131 assessment for NICE of multiple-frequency bioimpedance devices to guide fluid management in people with CKD undergoing dialysis were consulted to obtain appropriate health-state utility values for application in the model. Hall et al.97 conducted a thorough review of the literature prior to 2016 for utility parameters. The authors identified two systematic reviews of utility data that provided data that could be used in the economic model. The first, a systematic review and meta-regression published by Wyld et al.,134 predicted utility according to treatment (transplant, dialysis, pre treatment, conservative management). This model predicted an EQ-5D utility value of 0.64 for patients on dialysis and an EQ-5D utility value of 0.75 for transplant patients. The utilities from Wyld et al.134 were used in the Hall et al.97 model.
However, a limitation of Wyld et al.134 is that some of the EQ-5D scores were calculated from mapping algorithms and the age to which the mean utility estimates applied was not reported. The earlier systematic review by Liem et al.137 restricted a meta-analysis to those studies using the EQ-5D index directly for each modality of chronic RRT, and reported the pooled mean age and sex distribution for the corresponding pooled EQ-5D values.
In addition to the two reviews identified by Hall et al.,97 a further structured literature search was conducted to obtain any more recent utility studies that match the NICE DAP reference case (i.e. studies that included EuroQol-5 Dimensions, three-level version, data for UK patients, valued using UK general population tariffs). A range of databases were searched for English language, full-text publications, published between 2016 (end data of Hall et al.97 searches) and 2019. Seven publications were identified that were deemed to meet the NICE reference case for the DAP; specifically, they reported EQ-5D-based utilities valued in accordance with the UK general population preference-based value sets. Studies in which the EQ-5D was administered to a non-UK population but the results were valued according to the UK tariff were also included.
The age- and sex-matched EQ-5D UK population norms were calculated using an equation published by Ara and Brazier141 and used to derive age-/sex-adjusted utility multipliers from the raw pooled estimates, based on the age and sex distribution of the source studies.138 The utility of the proportion of the cohort having a successful transplant is assumed to revert to that of the outpatient follow-up state. All utility data were incorporated into the model probabilistically using beta distributions.
Time horizon and discounting
The model was run over a lifetime time horizon, up to age 100 years (for a cohort with a starting age of 63 years in the model). The lifetime time horizon was chosen to ensure that all of the long-term costs and consequences of AKI-induced CKD were captured, including the long-term health effects of ultimate progression to ESRD, transplant and death. The cycle length for the model was annual, and half-cycle corrections have been applied to costs and utilities. All costs and outcomes accruing beyond the first yearly cycle of the model were discounted at a rate of 3.5% per annum, in line with the NICE reference case. The discount rate was varied between 0% and 6% in deterministic sensitivity analyses.
Analyses
The model calculated the expected costs and expected QALYs over the lifetime of each cohort. This includes the costs and QALYs incurred in the first 90-day acute phase of the model, based on diagnostic test accuracy, preventative action to avert AKI, resultant peak AKI status and requirement for admission to an ICU. It also includes the longer-term extrapolations from the Markov cohort model, simulating the long-term transitions between progressive stages of CKD for those who develop it.
The model is fully probabilistic to simultaneously describe the impact of all parameter uncertainty on the model results. All model parameter estimates are sampled from their assigned distributions, as described in the preceding sections, using 1000 simulations. When it was not possible to derive a distribution, for example when insufficient information existed to determine the SD of the distribution, it was assumed that the SD of a parameter was equal to 10% of its mean, unless otherwise stated.
Results are reported as cost–utility analyses, in terms of incremental cost per QALY, expressed as the incremental cost-effectiveness ratio (ICER). Test strategies are plotted on the cost-effectiveness frontier. Tests are ranked in ascending order of benefit (QALYs), with results reported for all tests incrementally against each other to enable the exclusion of strictly dominated (less beneficial and more costly) alternatives from the ICER calculations. ICERs versus standard care are also reported. Results from the probabilistic analysis simulations are plotted using cost-effectiveness acceptability curves based on the net benefit calculation to identify the optimal diagnostic testing strategy at different threshold values of willingness to pay for a QALY.
Model validation
The economic model was checked for errors using the approach suggested by Tappenden and Chilcott,142 which specified verification tests. Components of the model tested were the estimation of the costs and QALYs, distributions of model parameters and other general tests for accuracy of the implementation of input parameters. No specific issues were identified through the verification tests.
Results
The model was developed and configured to assess the cost-effectiveness of the NephroCheck test, the ARCHITECT urine NGAL assay, the BioPorto urine NGAL test and the BioPorto plasma NGAL test in combination with standard clinical assessment, compared with standard clinical assessment alone.
There is no direct evidence to describe the impact of the use of the AKI biomarkers on important health outcomes (such as need for ICU care, length of hospital stay, risk of 90-day mortality or development of new/progression of existing CKD). Accordingly, the cost-effectiveness results are based on a linked-evidence approach whereby we have relied on observational associations to infer how prevention or mitigation of AKI may affect changes in health outcomes. These associations necessitate causal assumptions, but, although a causal link between AKI and poor outcomes is plausible, the extent of this causal relationship is uncertain and controversial. The cost-effectiveness results are therefore presented for a range of alternative, but potentially plausible, scenario analyses, ranging from a set of optimistic assumptions whereby biomarker-guided care bundles may lead to substantial improvements in health outcomes (need for ICU, CKD, mortality) to a set of more conservative assumptions where change in AKI status has no effect on health outcomes. It is likely that the true estimate of cost-effectiveness lies somewhere between these two extremes.
Furthermore, the model includes the following key assumptions:
- The model base-case analysis is run for a mixed cohort of CKD and non-CKD patients, average age 63 years, 54.3% female, based on the characteristics of hospitalised patients in Grampian, Scotland, who have at least a one-night hospital stay and are having their kidney function monitored, and so are deemed to be at risk of AKI.
- It is assumed that NephroCheck and NGAL can rise at similar time points; in the absence of any evidence to suggest otherwise, it is assumed that the time gain, relative to serum creatinine, in terms of early implementation of a KDIGO care bundle is equal for both.
- The base-case analyses assume that there are no adverse consequences, in terms of health effects, of false-positive or false-negative test results compared with standard care. False-positive results would incur the additional futile application of the care bundle costs, and clinical expert opinion (Simon Sawhney and Callum Kaye, personal communication) indicates that false negatives will be monitored until the negative test result is confirmed and would represent current practice without biomarkers. However, there is some concern that a false-positive test may lead to unnecessary fluid resuscitation, especially if encountered by inexperienced clinicians, which could lead to an increased mortality risk, although the magnitude of that risk is unknown. A sensitivity analysis explores this.
- For the Markov models, it is assumed that a patient can develop CKD linked to the index AKI event for the first cycle of the model only, reflecting a total exposure time to increased CKD risk of 1 year + 90 days. Thereafter, the background risk of developing CKD in the population is applied.
- It is assumed that the proportion of the cohort that experience graft failure post transplant return to the ‘ESRD on dialysis’ health state, where they are exposed to the same risks of transition to transplant/death as when they first entered the dialysis state.
- For the proportion of the cohort that do not develop long-term CKD, the base-case models assume that the longer-term follow-up costs and mortality risks are not dependent on events in the acute phase of the model (i.e. AKI severity and associated ICU admission). A sensitivity analysis explores the impact of applying additional costs and mortality risks for those admitted to ICU in the acute phase of the model.
- The model is run for a lifetime time horizon or 100 years, whichever comes first, with costs and QALYs discounted at an annual rate of 3.5% per annum.
Evidence from Meersch et al.110 shows that NephroCheck-guided early implementation of a KDIGO care bundle can avert AKI. However, the impact of NGAL-guided implementation of a care bundle is unknown. Therefore, two alternative base-case assumptions are considered. The first assumes that NGAL and NephroCheck have the same potential to avert AKI (based on Meersch et al.110). The second assumes that NGAL can reduce the severity of AKI (also from Meersch et al.110), but cannot prevent it from occurring. The rationale for the latter analysis is that NGAL detects injury to the kidneys, whereas NephroCheck can potentially detect stresses on the kidneys and may offer an earlier warning of impending AKI. The two base-case models and a range of scenario analyses conducted around important model assumptions are described in Table 19. A total of 15 scenario analyses are reported on each of these two plausible base-case configurations to illustrate the significant uncertainty in the cost-effectiveness findings. Table 20 reports the results for scenarios in which NGAL can avert AKI, and Table 21 reports results of scenarios in which NGAL cannot avert AKI. The results of additional scenario analyses requested by NICE are provided in Appendix 14 for completeness.
TABLE 19
Base-case model configuration and scenario analyses
TABLE 20
Scenario analyses assuming that the NGAL tests can avert AKI
TABLE 21
Scenario analyses assuming that the NGAL tests cannot avert AKI
Scenarios 1A and 2A describe two potential base-case analyses on which all the sensitivity analyses are conducted. These scenarios assume that there is a potential benefit of averting or having less severe AKI, in terms of improved outcomes (need for ICU care, risk of CKD and LOS), but the magnitude of that benefit may be less than that observed in observational data. Given the lack of direct evidence demonstrating the impact of biomarker tests on mortality, the base case assumes that there is no impact on 90-day mortality of averting AKI.
Scenarios B–E illustrate the impact of assumptions around the magnitude of the associative benefits of averting/experiencing less severe AKI on health outcomes. Scenarios F–P explore the impact of applying alternative follow-up costs and mortality, CKD projection, discount rate, alternative source data for AKI prevalence, test costs, excess mortality risk because of a false-positive result, and alternative utility sources.
The results are highly uncertain, with no clear optimal biomarker strategy. The findings are highly sensitive to each of the associative links applied between AKI and health outcomes, namely probability of ICU admission, LOS in hospital, probability of dying at 90 days and the risk of developing CKD.
In scenarios in which NGAL tests are assumed to be equally as effective as NephroCheck at averting AKI, the BioPorto urine NGAL test generally has the greatest probability of cost-effectiveness. This is because the main drivers of the relative cost-effectiveness of each of the biomarker tests against each other are the cost of the test and the diagnostic accuracy. The BioPorto urine NGAL test is slightly cheaper and the meta-analysis shows it as having slightly better diagnostic accuracy in the all-comers cohort. However, these findings should be interpreted cautiously because of the heterogeneity in the diagnostic test accuracy studies, which leads to further uncertainty in the cost-effectiveness results.
Conversely, the NephroCheck and ARCHITECT urine NGAL test are never the most cost-effective strategy when assuming that all tests are equally efficacious in averting AKI, because they are more costly tests, with comparatively poorer diagnostic accuracy. NephroCheck is estimated to have poorer specificity than the NGAL urine tests, thereby generating additional costs of treating false-positive test cases, who unnecessarily receive a KDIGO care bundle. However, under the alternative base-case assumptions, in which the NGAL tests are assumed to have no effect on averting AKI, the probability of NephroCheck being the most cost-effective test rises considerably. In the most optimistic scenario, NephroCheck is 100% cost-effective. In the most pessimistic scenario, standard care is the most cost-effective strategy.
Applying a daily excess cost of AKI in hospital or ICU (i.e. if the cost incurred by patients with AKI is not fully captured in the hospital/ICU daily cost) results in the tests being even more favourable than in the base case because more costs are offset by averting AKI or having less severe AKI in the test arms. This results in the NGAL tests being dominant and NephroCheck being cost-effective (ICER of < £20,000) compared with standard care.
The ARCHITECT urine NGAL test is generally less likely to be cost-effective in all scenarios because of the test accuracy and cost. The ARCHITECT urine NGAL test is estimated to have lower sensitivity and specificity than the other tests, and costs more than the other NGAL tests.
In general, the results are also sensitive to the assumptions on having hospital-/ICU-specific follow-up costs and mortality (instead of an average of the two); increased long-term cost of AKI, including the linked effect between AKI and probability of CKD for the whole duration of the model (instead of for one cycle, as in the base-case); and using an alternative source of AKI prevalence data (with higher prevalence), with all scenarios favouring the test strategies, making them increasingly more cost-effective than standard care. In most of these cases, the BioPorto urine NGAL test is the most cost-effective test strategy; however, in the most optimistic scenario, the BioPorto plasma NGAL test is the most cost-effective choice of test. On the other hand, assuming that a false-positive test result can lead to an increased risk of mortality at 90 days (i.e. RR 1.5) favours standard care, which becomes the strategy with the highest probability of cost-effectiveness.
We have included an exploratory analysis in which the limited available diagnostic accuracy data for children are applied in the adult model. Diagnostic accuracy data were available for only two biomarkers (ARCHITECT urine NGAL and BioPorto urine NGAL). The following diagnostic accuracy estimates were included in this run of the model: BioPorto urine NGAL – sensitivity 0.77 (95% CI 0.70 to 0.84) and specificity 0.47 (95% CI 0.40 to 0.54); ARCHITECT urine NGAL – sensitivity 0.68 (95% CI 0.53 to 0.80) and specificity 0.79 (95% CI 0.63 to 0.89).
This analysis should be considered as speculative only, as to ensure a robust assessment of cost-effectiveness among children would require the reconfiguration of the model for a paediatric cohort, with appropriate care pathways and age-specific risks of transition between health states.
In summary, the results are highly uncertain and it is impossible to ascertain the most likely ICER given the available evidence. The range of ICERs across different plausible sets of assumptions is substantial and the probabilistic analyses indicate substantial uncertainties regarding the optimal test strategy. Any of the scenarios explored might be feasible, so it is important to consider these findings in the light of the substantial uncertainty underlying the impact of the tests on AKI and the causative links between AKI and changes in health outcomes. The substantial heterogeneity in the study populations for the diagnostic accuracy data for the candidate tests raises further concerns about the relative cost-effectiveness of the comparators in the absence of head-to-head trial comparisons across multiple candidate tests.
Cohort traces from the base-case Markov models
Figure 22 shows the Markov traces for the standard-care arm of the model under base case 1 assumptions. In the standard-care arm, at 10 years, the mortality for the cohort aged 63 years was 45% for the no-AKI cohort and 59% for the average of the AKI 1, 2 and 3 cohorts. The mortality for the no-AKI group is consistent with the observed 10-year mortality in the Grampian data.102 However, the mortality observed for the AKI cohorts at 10 years is lower than in the observational data from Grampian. This is because we did not apply an additional AKI-specific excess mortality risk beyond the first year of follow-up in the model, as to assume that such an additional risk is directly caused by AKI is questionable, based on existing evidence (e.g. Meersch et al.110).

FIGURE 22
Markov cohort traces for base-case model configuration. (a) No AKI; (b) AKI 1; (c) AKI 2; and (d) AKI 3.
Cost-effectiveness acceptability curves
Figures 23 and 24 report cost-effectiveness acceptability curves for the two potential base-case scenarios.
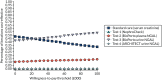
FIGURE 23
Cost-effectiveness acceptability curve: base case 1.

FIGURE 24
Cost-effectiveness acceptability curve: base case 2 – subgroup analyses.
Three subgroup analyses have been carried out on the two EAG-suggested base-case strategies (based on whether or not NGAL is assumed to be capable of averting AKI). The subgroups considered are adult critical care and adult post cardiac surgery. As there was an insufficient amount of data to populate a robust model for a children subgroup, this was considered as an exploratory analysis only (as per Tables 20 and 21).
Critical care subgroup
For the critical care subgroup, the same parameter values as the all-comers are used for the downstream model probabilities, costs and utilities. This subgroup may be useful for decision-making as it could be considered as an alternative, potentially more seriously ill, definition of the population in the NICE scope. Although the group is defined as ‘critical care’, the populations described in the source diagnostic accuracy studies are often more reflective of a seriously ill patient group that would not yet be in ICU in the UK setting. The diagnostic accuracy data used for this subgroup are described in Table 22.
TABLE 22
Diagnostic accuracy data used for the critical care subgroup analysis
The results of the critical care subgroup analysis are provided in Table 23.
TABLE 23
Results of the critical care subgroup analysis
Cardiac surgery subgroup
Diagnostic accuracy data were not available from the systematic review for all biomarker strategies for the cardiac surgery group, and were available from only single studies for some tests. When data were not available from the review, we used pooled estimates from Hall et al.,97 but note that this analysis should be considered with caution as it includes test manufacturers outside the scope of the NICE assessment. The diagnostic accuracy data for the cardiac surgery subgroup are provided in Table 24 and are included probabilistically in the model when possible.
TABLE 24
Diagnostic accuracy data used for cardiac surgery subgroup
Again, these results should be interpreted cautiously because of the lack of/limitations with the diagnostic accuracy data, and the questionable relevance of the downstream parameters/model structure for a cohort of post-cardiac patients only.
The results of the post-cardiac surgery subgroup analysis are provided in Table 25.
TABLE 25
Results of the post cardiac surgery subgroup analysis
Interpretation of the results
Published data show that NephroCheck-guided implementation of a KDIGO care bundle has the potential to avert AKI. However, no such data exist for the NGAL tests. Therefore, two base-case analyses are considered. Base case 1 can be considered an optimistic scenario for the NGAL assays and assumes that all NGAL tests are equally as effective as NephroCheck in terms of the potential to avert AKI. Base case 2 can be considered a more conservative approach, in the absence of evidence, and assumes that only NephroCheck can avert AKI, but that all tests have the potential to reduce AKI severity if AKI occurs.
Fifteen scenario analyses are provided for each potential base case, ranging from a set of optimistic assumptions whereby biomarker-guided care bundles may lead to substantial improvements in health outcomes (need for ICU, hospital LOS, CKD, mortality) to a set of more conservative assumptions whereby changing of AKI status has no effects on health outcomes.
Incremental cost-effectiveness ratios are highly uncertain and subject to wide variation depending on the set of scenarios chosen. The probability of cost-effectiveness at an ICER of < £20,000 per QALY gained for scenarios in which NGAL is assumed to be equally as effective as NephroCheck in preventing AKI ranged from 0% to 15% (NephroCheck), 0% to 55% (BioPorto urine NGAL), 0% to 2% (ARCHITECT urine NGAL) and 0% to 48% (BioPorto plasma NGAL). BioPorto urine NGAL was generally the test associated with the greatest probability of cost-effectiveness, albeit this was highly uncertain, when compared with standard care. This is because BioPorto urine NGAL had slightly better diagnostic test accuracy data and slightly lower test costs than the comparator tests. However, there is substantial uncertainty in the diagnostic test accuracy, driven by study heterogeneity; therefore, results should be interpreted cautiously.
When it is assumed that NGAL tests cannot avert AKI, but can only reduce its severity, the cost-effectiveness case for NephroCheck improves substantially. However, cost-effectiveness remains highly uncertain, with a probability of cost-effectiveness ranging from 0% to 99% across the explored scenarios.
Given the significant uncertainties across the range of scenario analyses undertaken, it is not possible to draw robust conclusions on the cost-effectiveness of the respective biomarkers.
- Assessment of cost-effectiveness - Biomarkers for assessing acute kidney injury ...Assessment of cost-effectiveness - Biomarkers for assessing acute kidney injury for people who are being considered for admission to critical care: a systematic review and cost-effectiveness analysis
- Development and validation of pre-eclampsia prediction models - Validation and d...Development and validation of pre-eclampsia prediction models - Validation and development of models using clinical, biochemical and ultrasound markers for predicting pre-eclampsia: an individual participant data meta-analysis
- Danio rerio hexosaminidase B (beta polypeptide) (hexb), transcript variant 2, mR...Danio rerio hexosaminidase B (beta polypeptide) (hexb), transcript variant 2, mRNAgi|836467586|ref|NM_001309863.1|Nucleotide
- Biomarkers for assessing acute kidney injury for people who are being considered...Biomarkers for assessing acute kidney injury for people who are being considered for admission to critical care: a systematic review and cost-effectiveness analysis
- Sec14l3 SEC14-like lipid binding 3 [Mus musculus]Sec14l3 SEC14-like lipid binding 3 [Mus musculus]Gene ID:380683Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...

