This work was produced by Brunt et al. under the terms of a commissioning contract issued by the Secretary of State for Health and Social Care. This is an Open Access publication distributed under the terms of the Creative Commons Attribution CC BY 4.0 licence, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. See: https://creativecommons.org/licenses/by/4.0/. For attribution the title, original author(s), the publication source – NIHR Journals Library, and the DOI of the publication must be cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Brunt AM, Haviland JS, Wheatley DA, et al. One versus three weeks hypofractionated whole breast radiotherapy for early breast cancer treatment: the FAST-Forward phase III RCT. Southampton (UK): National Institute for Health and Care Research; 2023 Nov. (Health Technology Assessment, No. 27.25.)

One versus three weeks hypofractionated whole breast radiotherapy for early breast cancer treatment: the FAST-Forward phase III RCT.
Show detailsRecruitment
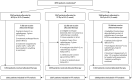
Between November 2011 and June 2014, 4110 patients were enrolled in the FAST-Forward Trial from 97 UK centres (47 RT centres and 50 referring hospitals; Appendix 2, Table 31). The trial completed recruitment 17 months ahead of schedule (see Figure 5). One-ninety patients were recruited into acute toxicity study 1, 161 patients into acute toxicity study 2, 1798 patients into the PRO sub-study, 1737 patients into the photographic assessment sub-study, 3878 patients consented to donate a blood sample and 4077 patients consented to donate their primary tissue sample.

FIGURE 5
Target and actual recruitment for FAST-Forward Main Trial.
The original target for recruitment was based on five centres opening to recruitment once the trial opened with a further one centre expected to open per month until a total of 25 centres were open to recruitment. Each centre was predicted to recruit four patients per month. Using these figures it was predicted that recruitment would take 54 months to attain. Appendix 3, Figure 20 shows that over 100 centres opened to recruitment which allowed the target to be reached 17 months early. Seventy-two centres recruited at a rate of at least one patient a month.
Deviations and withdrawals
Fourteen patients withdrew consent for use of data and were removed from the intention-to-treat population, therefore results are reported for 4096 consenting participants. A total of 40 patients (7 in 40 Gy, 12 in 27 Gy, 21 in 26 Gy) did not receive the allocated therapy (see Figure 6); compliance with allocated treatment was therefore 99% (4056/4096). These 40 patients included six found to be ineligible after randomisation: two in the 27 Gy group (one with relapse detected after recruitment so RT cancelled, and one with tissue expander in situ) and four in the 26 Gy group (three with metastases detected before start of RT, and one with previous DCIS in contralateral breast).
A total of 22 patients withdrew from clinical follow-up before 5 years, and one was lost to follow-up. Of the 1798 patients who consented to participate in the PRO sub-study, 181 had withdrawn from the PRO questionnaires by 5 years.
Data returns
Baseline forms were received for 100% out of those expected. Data return rates of annual follow-up forms were over 90%, with 5-year visit forms available for 3733 (98%) patients out of 3798 still in follow-up (not died, withdrawn or lost). Data returns for individual forms are shown in Appendix 2, Table 39 and are correct up to 1 March 2022.
Baseline data
Demographic and clinical characteristics at baseline were well-balanced between groups (see Table 4). Overall, 2551/4096 (62%) were classified as low-risk (age > 50 and grade 1 or 2); the majority were ER positive/HER-2 negative (3335/4077 with data, 82%), and 407/4077 with data (10%) were HER-2 positive. 3832 (93%) had BCS, 1011 (25%) received a RT boost to tumour bed, 1174 (29%) had neo-adjuvant or adjuvant chemotherapy, 3512/3649 (96%) ER positive patients had endocrine therapy and 311/407 (76%) HER-2 positive patients received trastuzumab. Medical history details at randomisation are presented in Appendix 2, Table 40 and RT details are in Table 5.
TABLE 4
Demographic, clinical and treatment characteristics at randomisation of the 4096 patients consenting to the FAST-Forward Main Trial
TABLE 5
Radiotherapy details for the 4090 treated patients in the FAST-Forward Main Trial
Follow-up
Calculating length of follow-up as the time between randomisation and date last seen (censoring at date of death or withdrawal of consent from follow-up where applicable), median follow-up in Main Trial patients at the time of the primary analysis data snapshot (22 November 2019) was 71.5 months (IQR 71.3–71.7). Five-year visit forms were available for 3733 (98%) patients out of 3798 still in follow-up (not died, withdrawn or lost).
Five-year cancer outcomes in the Main Trial
After a median follow-up of 71.5 months (IQR 71.3–71.7), IBTR was recorded in 79 patients (40 Gy: 31, 27 Gy: 27, 26 Gy: 21) Table 6. Estimated cumulative incidence of IBTR up to 5 years was 2.1% (95% CI 1.4 to 3.1) for 40 Gy (expected incidence 2%), 1.7% (1.2 to 2.6) for 27 Gy and 1.4% (0.9 to 2.2) for 26 Gy (see Table 7, Figures 7 and 8). Estimated absolute differences in IBTR versus 40 Gy were −0.3% (−1.0 to 0.9) for 27 Gy and −0.7% (−1.3 to 0.3) for 26 Gy. Since the upper confidence limits excluded an increase in IBTR of >1.6%, non-inferiority can be claimed for both 5-fraction schedules compared with 40 Gy in 15 fractions. This is confirmed by a test against the critical HR > 1.81, with p = 0.0022 for 27 Gy and p = 0.00019 for 26 Gy compared with 40 Gy. Analyses in the per-protocol population were consistent [estimated absolute difference vs. 40 Gy −0.4%; (−1.0 to 0.8); p = 0.0017 for 27 Gy and −0.6% (−1.2 to 0.4); p = 0.00037 for 26 Gy; full data for per-protocol analyses not shown as 99% treatment compliance]. Comparing the 5-fraction schedules, the estimated absolute difference in IBTR cumulative incidence up to 5 years was −0.4% (−1.0 to 0.6) for 26 Gy versus 27 Gy. The unadjusted α/β estimate for IBTR was 3.7 Gy (0.3, 7.1 Gy), with EQD2 estimates of 44.7 Gy for 40 Gy, 43.1 Gy for 27 Gy and 40.6 Gy for 26 Gy with no correction for treatment time. Adjusting for risk group and ER/HER-2 status made minimal difference (adjusted α/β estimate 3.7 Gy; 0.4, 6.9). HRs obtained from a competing risks analysis of IBTR with death from any cause as a competing event were almost identical to those from the primary analysis reported in Table 19 [HRs from competing risks model: 0.85 (95% CI 0.51 to 1.43) for 27 Gy vs. 40 Gy; 0.67 (0.38 to 1.16) for 26 Gy vs. 40 Gy].
TABLE 6
Relapses, second primary cancers and deaths by fractionation schedule, in 4096 patients consenting to the FAST-Forward Main Trial
TABLE 7
Relapse and mortality by fractionation schedule: results of time to event analyses for 4096 patients consenting to the FAST-Forward Trial
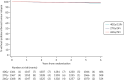
FIGURE 7
Kaplan–Meier plot of IBTR in Main Trial.

FIGURE 8
Cumulative risk of IBTR in Main Trial.
Regional relapses occurred in 34/4096 (1%) patients (40 Gy: 13, 27 Gy: 11, 26 Gy: 10; see Table 6), 6 of which were concurrent with IBTR. Incidence of locoregional relapse, distant relapse, disease-free and overall survival were similar between groups, with no statistically significant differences (see Table 7, Figures 9 and 10, Appendix 3, Figures 21–26). Invasive contralateral breast cancer was reported for 55/4096 (1%) patients (40 Gy: 18, 27 Gy: 17, 26 Gy: 20; see Table 18), and non-breast second primary cancers for 123/4096 (3%) patients (40 Gy: 42, 27 Gy: 37, 26 Gy: 44; see Table 18), the most common being colorectal cancer with 25 cases in total.

FIGURE 9
Kaplan–Meier plot of disease-free survival in Main Trial.
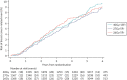
FIGURE 10
Cumulative risk of any breast cancer-related eventa in Main Trial. aAny breast cancer-related event includes local, regional or distant relapse, breast cancer death and contralateral breast cancer.
TABLE 18
Ipsilateral breast tumour relapse by higher risk subgroup in the Main Trial
A total of 287/4096 (7%) patients died, 151 (4%) from breast cancer, 125 (3%) from other causes (including 38 (1%) from second cancers and 27 (1%) from cardiac related) and 11 (0.3%) with unknown cause of death and no evidence of disease relapse (see Table 6). Of 27 patients with a cardiac-related death (40 Gy: 10, 27 Gy: 9, 26 Gy: 8), 15 (40 Gy: 7, 27 Gy; 4, 26 Gy: 4) had a history of cardiac disease reported at randomisation or was a current/ex-smoker in past year.
Late normal tissue effects up to 5 years’ follow-up in the Main Trial
Clinician assessments
At least one annual clinical assessment of NTE was available for 3975/4096 (97%) patients. Frequencies of clinician-assessed NTE according to year of follow-up (from 1 to 4 years) and fractionation schedule are shown in Appendix 2, Table 41. Bar charts of clinician-assessed NTE from 1 to 5 years are presented in Appendix 3, Figures 27–33. Cross-sectional analyses of clinician-assessed NTE at 5 years are in Table 8. At 5 years, any moderate/marked clinician-assessed NTE in the breast/chest wall was reported for 98/986 (10%) patients in the 40 Gy group, 155/1005 (15%) for 27 Gy and 121/1020 (12%) for 26 Gy (see Table 8, Figure 11), with a statistically significant difference between 40 Gy and 27 Gy (p = 0.0003) but not between 40 Gy and 26 Gy (p = 0.17). Breast shrinkage was the most prevalent moderate/marked effect at 5 years, reported in 50/916 (6%) for 40 Gy, 78/948 (8%) for 27 Gy and 65/954 (7%) for 26 Gy (see Table 8). Longitudinal analysis of all annual clinical assessments of NTE over follow-up showed a statistically significant increased risk of any moderate/marked effect in the breast/chest wall for the 27 Gy group compared with 40 Gy (OR 1.55, 1.32 to 1.83; p < 0.0001), with no statistically significant difference between 26 Gy and 40 Gy (OR 1.12, 0.94 to 1.34; p = 0.20; see Table 9). This pattern was similar for the individual effects of breast distortion, shrinkage, induration and breast/chest wall oedema, with statistically significant higher risk for 27 Gy compared with 40 Gy but not for 26 Gy (see Table 9, Appendix 3, Figures 34–40). Comparing the two 5-fraction schedules, 26 Gy had statistically significantly lower risk of any moderate/marked breast/chest wall NTE (p = 0.0001) and breast shrinkage (p = 0.0018) compared with 27 Gy. Estimates of 5-year cumulative incidence of any moderate/marked clinician-assessed NTE in the breast/chest wall were 26.8% (95% CI 24.4 to 29.4) for 40 Gy, 35.1% (32.4 to 37.9) for 27 Gy and 28.5% (26.0 to 31.1) for 26 Gy (see Table 10). Results for comparison of schedules from the analyses of time to first moderate/marked effect were similar to those from the longitudinal modelling of all annual clinical assessments.
TABLE 8
Cross-sectional analysis of clinician-assessed late NTE at 5 years according to fractionation schedule for 3626 patients with 5-year assessments
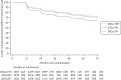
FIGURE 11
Kaplan–Meier plot of any moderate/marked clinician-assessed NTE in breast/chest wall (Main Trial).
TABLE 9
Longitudinal analysis of moderate/marked clinician-assessed late NTE including all annual follow-up assessments for 3975 patients with at least one annual clinical assessment
TABLE 10
Survival analyses of moderate/marked clinician-assessed late NTE by fractionation schedule for 3975 patients in Main Trial with at least one annual clinical assessment
Patient self-assessments
One thousand seven hundred and ninety-six patients consented to the PRO sub-study, of whom 10 withdrew consent immediately after randomisation and eight were not given the baseline booklet. Questionnaires returned from those expected (patients alive and well, not withdrawn) totalled 1771/1778 (99%) at baseline, 1668/1733 (96%) at 3 months, 1622/1722 (94%) at 6 months, 1599/1707 (94%) at 1 year, 1531/1669 (92%) at 2 years and 1334/1589 (84%) at 5 years. Of the 1774 patients with at least one completed questionnaire, 1634 had BCS and 140 mastectomy.
Frequencies of patient-assessed breast/chest wall and arm/shoulder/hand symptoms are shown for each time point from baseline to 2 years and according to fractionation schedule in Appendix 2, Table 41. Cross-sectional analyses of patient-assessed symptoms at 5 years are in Table 11. Bar charts of patient-assessed late AE up to 5 years are presented in Appendix 3, Figures 41–51.
TABLE 11
Cross-sectional analysis of patient-assessed late NTE at 5 years according to fractionation schedule for 1338 patients in the Main Trial with 5-year questionnaire data available
Change in breast appearance had the highest 5-year prevalence, with moderate/marked change reported in 140/432 (32%) for 40 Gy, 158/440 (36%) for 27 Gy and 136/429 (32%) for 26 Gy. There were no statistically significant differences in 5-year prevalence of patient-reported AE between the schedules (see Table 11). There was some evidence of an increase in patient-reported moderate/marked breast hardness/firmness at 5 years for 27 Gy compared with 40 Gy and more breast swelling in both 5-fraction schedules, but these were not statistically significant at the pre-specified cut-off of p = 0.005. Longitudinal analyses of all patient assessments from baseline to 5 years showed a statistically significantly higher risk of moderate/marked breast hardness/firmness for 27 Gy compared with 40 Gy (OR 1.42, 1.17 to 1.72; p = 0.0003), and less change in breast appearance for 26 Gy compared with 27 Gy (p = 0.0018), but no statistically significant differences between schedules for the other NTE (see Table 12).
TABLE 12
Longitudinal analysis of moderate/marked patient-assessed late NTE from baseline to 5 years for 1774 patients in the Main Trial with at least one completed questionnaire
Photographic assessments of late adverse effects in the Main Trial
Of the 1737 patients (BCS and post-mastectomy) who consented to the photographic sub-study, baseline photographs were received for 1634 (94%), and 2- and/or 5-year photographs were available for 1385 (80%). The vast majority (1309) were patients who had BCS; for these patients, 2- and 5-year photographs were assessed in 1267 and 875, respectively (see Table 13). A total of 226 patients died or withdrew from the photographic sub-study by year 5, for the remainder the most common reasons for photographs not being taken were appointments not made due to clerical errors at the centres, patients not attending clinic visits, and patients withdrawing consent from the sub-study.
TABLE 13
Change in photographic breast appearance at 2 and 5 years (BCS patients) by fractionation schedule: results of longitudinal analysis for 1309 patients in the Main Trial with photographic assessments at 2 and/or 5 years
At 2 years, mild/marked change in photographic breast appearance was reported in 35/411 (8%) for 40 Gy, 67/429 (16%) for 27 Gy and 46/427 (11%) for 26 Gy; corresponding figures at 5 years were 34/283 (12%) for 40 Gy, 83/308 (27%) for 27 Gy and 37/284 (13%) for 26 Gy (see Table 13). Modelling 2- and 5-year photographic assessments together, 27 Gy had a statistically significantly increased risk of mild/marked change in breast appearance compared with 40 Gy (OR 2.29, 1.60 to 3.27; p < 0.0001), with no statistically significant difference between 26 Gy and 40 Gy (OR 1.26, 0.85 to 1.86; p = 0.24; see Table 13). Twenty-six Gy had a statistically significantly lower risk of change in photographic breast appearance compared with 27 Gy (p = 0.0006).
At 2 years, mild/marked retraction/distortion was reported in 14/411 (3.4%) for 40 Gy, 27/429 (6.3%) for 27 Gy and 14/427 (3.3%) for 26 Gy; corresponding figures at 5 years were 3/283 (1.1%) for 40 Gy, 25/308 (8.1%) for 27 Gy and 14/284 (4.9%) for 26 Gy (see Table 14). Modelling 2- and 5-year photographic assessments together, 27 Gy had a statistically significantly increased risk of mild/marked change in breast appearance compared with 40 Gy (OR 2.83, 1.50 to 5.34; p = 0.001), with no statistically significant difference between 26 Gy and 40 Gy (OR 1.59, 0.79 to 3.18; p = 0.190; see Table 14). There was no statistically significantly difference in risk of retraction/distortion for 26 Gy compared with 27 Gy (p = 0.056).
TABLE 14
Retraction/distortion at 2 and 5 years (BCS patients) by fractionation schedule: results of longitudinal analysis for 1309 patients in the Main Trial with photographic assessments at 2 and/or 5 years
Severe late adverse effects and specialist referrals for radiotherapy-related adverse effects in the Main Trial
The most common specialist referral for radiotherapy-related AE during follow-up was to lymphoedema clinics (see Table 15). Incidence of ischaemic heart disease, symptomatic rib fracture and symptomatic lung fibrosis was very low at this stage of follow-up (see Table 16).
TABLE 15
Specialist referrals for radiotherapy-related late AE during follow-up in the Main Trial
TABLE 16
Incidence of other late AE in the Main Trial
Estimation of radiobiology parameters for late adverse effects
The unadjusted α/β estimate for any moderate/marked clinician-assessed NTE in the breast/chest wall was 1.7 Gy (1.2–2.3), giving EQD2 estimates of 47.1 Gy for 40 Gy/15 fractions, 51.6 Gy for 27 Gy/5 fractions and 48.3 Gy for 26 Gy/5 fractions; adjusting for prognostic factors (age, boost, whole breast planning treatment volume as a proxy for breast size) made very little difference. α/β estimated from the photographic endpoint (adjusting for breast size and surgical deficit evaluated from the baseline photographs) was very similar (1.8 Gy; 1.1–2.4). The unadjusted α/β estimate for patient-reported change in breast appearance was 2.3 Gy (1.8–2.9), resulting in EQD2 estimates of 46.1 Gy, 48.2 Gy and 45.2 Gy for the 40 Gy, 27 Gy and 26 Gy schedules, respectively; as above, adjusting for covariates made minimal difference.
Assuming no clinically significant time effect for late AE between 1 and 3 weeks, complete sublethal damage repair between fractions and an α/β of 2.8 Gy for late NTE, the last assumption based on the combined estimates of α/β in START-A and FAST. On this basis, the relative EQD of the FAST-Forward schedules to 50 Gy in 25 fractions are shown in Table 17, where negative values indicate estimated AE rates lower than 50 Gy in 25 fractions.
TABLE 17
Relative EQD in 2 Gy fractions of FAST-Forward schedules and the absolute % difference in adverse events (ΔAE) expected compared to 50 Gy in 25 fractions assuming
Five-year subgroup analyses in the Main Trial
As stated in the Statistical Methods section in Chapter 2, the Main Trial protocol did not include pre-specified subgroup analyses, but exploratory post hoc subgroup analyses of the primary endpoint and of NTE were carried out.
Risk group was a stratification factor for the Main Trial at randomisation, with low-risk defined as age ≥ 50 and grade 1 or 2, and high-risk defined as age < 50 and/or grade 3; 1545 (37.8%) patients were in the high-risk category. Retrospective subgroup analyses comparing IBTR in 26 Gy versus 40 Gy provide no evidence of a differential effect according to age, grade, pathological tumour size, nodal status, tumour bed boost, adjuvant chemotherapy, HER-2 status and in triple-negative patients (see Figure 12). CIs for the HRs overlap for the subgroups, although the number of events in these analyses was small (52), hence results should be interpreted with caution as the statistical power is low. Subgroup analysis according to type of primary surgery was not possible as there was only one IBTR event post-mastectomy in a control group patient (out of 91) and none in the 173 patients treated with 5 fractions. Subgroup analyses of IBTR for 27 Gy/5 fractions versus 40 Gy/15 fractions are shown in Figure 13. Table 18 and Appendix 2, Table 43 show the frequencies and number of patients according to subgroups defined by age, grade, primary surgery type and receptor status. No evidence to signal concern was seen for the 5-fraction schedules. The use of boost and dose/fractionation, both declared prior to randomisation, were balanced between the three treatment groups minimising risk of bias in dose intensity between trial groups.
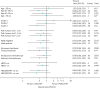
FIGURE 12
Subgroup analyses of time to IBTR for 26 Gy/5 fractions vs. 40 Gy/15 fractions in the Main Trial. Reproduced from Brunt et al. (CC BY 4.0).

FIGURE 13
Subgroup analyses of time to IBTR for 27 Gy/5 fractions vs. 40 Gy/15 fractions in the Main Trial. Reproduced from Brunt et al. (CC BY 4.0).
Retrospective subgroup analyses comparing time to first clinician-assessed moderate or marked AE in the breast or chest wall for 26 Gy versus 40 Gy provided no evidence of a differential effect of the 5-fraction schedule according to age, breast size, surgical deficit, tumour bed boost, or adjuvant chemotherapy, as CIs for subgroups overlap, although power for these retrospective subgroup analyses is low (see Figure 14). The corresponding figure for 27 Gy/5 fractions versus 40 Gy/15 fractions is shown in Figure 15.

FIGURE 14
Subgroup analyses of time to first moderate or marked clinician-assessed adverse event in breast/chest wall for 26 Gy/5 fractions vs. 40 Gy/15 fractions in the Main Trial. Reproduced from Brunt et al. (CC BY 4.0).

FIGURE 15
Subgroup analyses of time to first moderate or marked clinician-assessed adverse event in breast/chest wall for 27 Gy/5 fractions vs. 40 Gy/15 fractions in the Main Trial. Reproduced from Brunt et al. (CC BY 4.0).
Discussion of Main Trial results is in the main Discussion section (see Chapter 8).
- Recruitment
- Deviations and withdrawals
- Data returns
- Baseline data
- Follow-up
- Five-year cancer outcomes in the Main Trial
- Late normal tissue effects up to 5 years’ follow-up in the Main Trial
- Severe late adverse effects and specialist referrals for radiotherapy-related adverse effects in the Main Trial
- Estimation of radiobiology parameters for late adverse effects
- Five-year subgroup analyses in the Main Trial
- Main Trial - One versus three weeks hypofractionated whole breast radiotherapy f...Main Trial - One versus three weeks hypofractionated whole breast radiotherapy for early breast cancer treatment: the FAST-Forward phase III RCT
- Methods - A systematic review and individual patient data meta-analysis of progn...Methods - A systematic review and individual patient data meta-analysis of prognostic factors for foot ulceration in people with diabetes: the international research collaboration for the prediction of diabetic foot ulcerations (PODUS)
- List of abbreviations - Interferon gamma release assays for Diagnostic Evaluatio...List of abbreviations - Interferon gamma release assays for Diagnostic Evaluation of Active tuberculosis (IDEA): test accuracy study and economic evaluation
- Bronchiolitis of Infancy Discharge Study (BIDS): a multicentre, parallel-group, ...Bronchiolitis of Infancy Discharge Study (BIDS): a multicentre, parallel-group, double-blind, randomised controlled, equivalence trial with economic evaluation
- The effectiveness and cost-effectiveness of erythropoiesis-stimulating agents (e...The effectiveness and cost-effectiveness of erythropoiesis-stimulating agents (epoetin and darbepoetin) for treating cancer treatment-induced anaemia (including review of technology appraisal no. 142): a systematic review and economic model
Your browsing activity is empty.
Activity recording is turned off.
See more...