Recruitment
This chapter uses material from an Open Access article previously published by the research team [see Kyle SD, Siriwardena AN, Espie CA, Yang Y, Petrou S, Ogburn E, et al. Clinical and cost-effectiveness of nurse-delivered sleep restriction therapy for insomnia in primary care (HABIT): a pragmatic, superiority, open-label, randomised controlled trial. Lancet 2023;402(10406):975–87. https://doi.org/10.1016/S0140-6736(23)00683-9. Epub
10 Aug 2023. PMID: 37573859]. This article is published under licence to The Lancet. This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY 4.0) licence, which permits others to distribute, remix, adapt and build upon this work, for commercial use, provided the original work is properly cited. See: https://creativecommons.org/licenses/by/4.0/.
We recruited participants from 35 practices (average patient list size = 11,802) across three sites (Thames Valley, Greater Manchester, Lincolnshire) between 29 August 2018 and 23 March 2020. A total of 31,464 invitation letters were sent out from practices; 3171 people entered the screening phase and 642 participants were randomised (321 to intervention and 321 to control; ). Main reasons for exclusion following eligibility assessment were not meeting insomnia criteria, shift work and suspected sleep disorder other than insomnia (see Appendix 1, Table 33).
Baseline data
Baseline characteristics by randomised group are presented in Tables 4–6. Mean age (range) was approximately 55 (19–88) years old, 76% were female, 97% were from a white ethnic background, and nearly 50% had a university degree. Mean (SD) ISI scores were in the clinical range (17.5–4.1), median duration of insomnia was 10 years, 76% had previously consulted their doctor for insomnia, and 25% reported current use of prescribed sleep medication. The sample had a range of comorbid conditions. For example, 41% had a mental health problem, 30% had a musculoskeletal disorder and 20% had a respiratory illness. Seventy-one per cent had two or more medical conditions. Consistent with these data, mean SF-36 scores for mental health and physical health were lower than normative values58 and 49% met ‘caseness’ for depression on the PHQ-9 (score ≥ 10). Baseline characteristics were similar between the two groups, with a slightly higher percentage of participants in the SRT group having consulted for insomnia (78% vs. 74% for SH).

Participant baseline characteristics

Sleep diary and actigraphy outcomes at baseline

Primary and secondary questionnaire outcomes at baseline
Treatment receipt and fidelity
Sleep hygiene
All participants in the SH group were sent their SH booklet by e-mail or postal mail. No participant in the SH group met criteria for contamination (i.e. receiving nurse-delivered SRT) at 3 months (0/265) or 6 months (0/285).
Sleep restriction therapy
Sleep restriction therapy sessions were provided by 40 nurses (31 PNs and 9 research nurses). The median number of participants treated per nurse was 10 (min = 1, max = 24). Median time between randomisation and first treatment session was 23 days (min = 2, max = 306).
Table 7 summarises the number of treatment sessions attended by participants in the SRT arm: 92% attended one or more nurse sessions, while 65% attended all four treatment sessions; 8% did not attend any SRT sessions.

Number of participants who attended SRT intervention sessions
Table 8 provides a breakdown of reasons for withdrawal from SRT. The most common reasons were (1) finding implementation of SRT challenging, (2) not finding SRT useful and (3) personal circumstances.

Reasons for withdrawal from SRT intervention
Fidelity of sleep restriction therapy sessions
Seventy-nine audio recordings of therapy sessions (53 session 1, 26 session 3) were sampled and reviewed by a clinical psychologist experienced in sleep medicine. Fidelity ratings were high for session 1 [median % = 100, interquartile range (IQR) 96.2–100] and session 3 (median % = 87.5, IQR 75–100).
Numbers analysed
Table 9 summarises data on completion of follow-up assessments, withdrawals (and reasons) and analysis population. Five hundred and eighty participants (90.3%) provided data at a minimum of one follow-up time point.

Outcome completion and withdrawal from trial by time point
Table 10 summarises the availability of data for the primary and secondary outcomes at each time point by randomised group and overall. Eighty-five per cent of participants provided data on the primary outcome (ISI) at 6 months post randomisation. Of note, data completion for sleep diaries and actigraphy at 6 and 12 months was low (≤ 41%), chiefly due to the pandemic, which precluded sending out watches and diaries. Data on absenteeism, presenteeism and work productivity loss (from the WPAI) were only available for those in employment.

Data availability for primary and secondary outcomes
Table 11 shows that randomised group was associated with missingness of the primary outcome, with the SRT more likely to have missing data at 3, 6 and 12 months post randomisation.

Availability of primary outcome by randomised group
Outcomes and estimation
Primary outcome
The primary objective of the HABIT trial was to compare the effect of SRT versus SH on insomnia severity (assessed by the ISI) at baseline and 3, 6 and 12 months post randomisation. The primary end point was the 6-month time point.
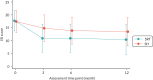
Table 12 summarises the adjusted treatment effect at each time point from the linear mixed-effect model (). At 6 months post randomisation, the estimated adjusted mean difference on the ISI was −3.05 (95% CI −3.83 to −2.28; p < 0.001, Cohen’s d = 0.74), indicating that participants in the SRT arm reported lower insomnia severity compared to the SH group. Treatment effects were also evident at 3 and 12 months. Mean differences between arms were reflected in the number of participants showing a treatment response (ISI change score reduction ≥ 8 points) and scoring in the non-clinical range (ISI absolute score < 11). At 6 months, 42% (108/257) of the SRT group met criteria for a clinically significant treatment response, while only 17% (49/291) of the SH arm did. Fifty per cent (128/257) of the SRT arm were in the non-clinical range at 6 months compared with 28% (80/291) in the SH arm.

Adjusted treatment effect for the primary outcome (insomnia severity)
Changes in the primary outcome, insomnia severity, across groups and time points. Raw means (±SD) are presented for both groups at each time point.
We performed sensitivity analyses to assess missingness of the primary outcome (ISI). Table 13 shows the results for the primary outcome when (1) adjusting for characteristics associated with non-completion of the ISI at 6 months and (2) performing multiple imputation. Both models yielded similar estimates as the primary analysis, demonstrating superiority of SRT over SH. A pattern mixture model was also conducted where missing ISI outcome values were imputed by up to five points either side of the observed average, both overall and in the SRT and SH arms separately. Even under these conservative assumptions the treatment effect and 95% CI would still not include 0 (see Appendix 2, ). Analyses assuming informative missingness of insomnia severity scores at 6 months [i.e. data missing not at random (MNAR)] indicated that even with asymmetrical differences between responders and non-responders conclusions are similar to the primary analysis (Appendix 2, Table 34).

Sensitivity analyses of the primary outcome at 6 months
Secondary outcomes
Adjusted treatment effects are presented for secondary outcomes in Tables 14 and 15.

Adjusted treatment effects for secondary questionnaire outcomes

Adjusted treatment effects for sleep diary and actigraphy outcomes
At 6 months, the SRT group relative to SH reported better mental HRQoL (SF-36 MCS) and sleep-related quality of life (GSII, patient-generated ranks 1–3), as well as lower depressive symptoms (PHQ-9) and activity impairment (WPAI). For employed participants, those in the SRT arm reported less absenteeism, presenteeism and work productivity loss (WPAI). Group effects on these measures were observed at all follow-up time points. Physical HRQoL (SF-36 PCS) was higher for the SRT group at 3 months but there was no evidence of group differences at 6 or 12 months. The SRT group also reported lower levels of cognitive and somatic arousal, and sleep effort, at 3, 6 and 12 months post randomisation.
All sleep diary metrics (SOL, WASO, SE, TST, SQ) were improve compared to control at 6 months (Table 15) and these effects were largely maintained at 12 months (except for SOL). Actigraphy-defined SE and WASO were improved, while TST was reduced, in the SRT group compared to control at 6 months. The only group difference at 12 months for actigraphy was lower TST for the SRT group relative to control. There was no evidence of group differences for use of prescribed sleep-promoting medication at 6 or 12 months.
Complier-average causal effects analyses
Baseline characteristics are presented for compliers and non-compliers in the treatment arm (Table 16). Compliance was defined as attending at least one SRT session.

Baseline characteristics by compliance
Table 17 summarises complier average causal effects for those attending a minimum of one, two, three, and four treatment sessions, respectively. Results show that attending more treatment sessions was associated with a larger treatment effect, relative to the primary analysis. For example, there is a > 1-point difference in the treatment effect on the ISI for those attending all four sessions (−4.10, 95% CI −5.06 to −3.14) versus the primary analysis (−3.05, 95% CI −3.83 to −2.28).

Complier-average causal effects by treatment session and primary analysis effect for reference
Adherence to sleep restriction therapy
Implementation of SRT was indexed using self-reported bed and rise times from sleep diaries completed during the 4-week intervention. A percentage score was calculated for each participant, reflecting the number of bed and rise times adhered to within 15 minutes of the nurse prescription. One hundred and fifty-seven participants (49%) returned intervention diaries; 164 participants did not return diaries or returned incomplete diaries (i.e. < 50% of days with relevant questions completed). Mean adherence for returned diaries was 76.4% (SD = 21.6), with the majority of participants categorised as 60–100% adherent (Table 18).

Treatment effect as a function of adherence to SRT
Treatment effects on the change scores for different levels of adherence were estimated by fitting a group by adherence interaction in the model, with the reference category being the control group. The models are adjusted for baseline ISI score and a random effect was fitted for GP practice. Estimated treatment effects therefore reflect the difference in the change in ISI scores from baseline for each adherence category as compared to control.
At 6 months, those with higher diary-defined SRT adherence tended to display greater change from baseline and stronger estimated treatment effects. At 3 months the pattern appeared non-linear, with adherence categories > 40–≤ 60/> 60–≤ 80/> 80–≤ 100 separating and exhibiting stronger treatments relative to the 0–40% category.
Mediation and moderation analyses
Our proposed mediators, pre-sleep arousal (PSAS) and sleep effort (GSES), were significantly reduced in the SRT group relative to control at 3 months post randomisation (see Table 14). The extent to which these variables causally mediated 6-month ISI was investigated using the approach of Baron and Kenny adapted for linear mixed-effect models. Tables 19–21 summarise the direct and indirect effects for each mediator separately. There were statistically significant indirect effects for sleep effort, pre-sleep cognitive arousal and somatic arousal, which mediated between 15% and 36% of the total treatment effect at 6 months.

Mediating effect of sleep effort (3 months) on insomnia severity (6 months)

Mediating effect of somatic arousal (3 months) on insomnia severity (6 months)

Mediating effect of cognitive arousal (3 months) on insomnia severity (6 months)
We performed exploratory moderation analyses on the following subgroups at baseline for the ISI at 6 months:
actigraphy-defined
TST at baseline, categorised as either < 6 or ≥ 6 hours
chronotype (morning, intermediate or evening) defined by the
MEQr at baseline
age (18–65 years vs. > 65 years)
patient-reported prescribed sleep medication use at baseline (Yes vs. No)
depression ‘caseness’ (
PHQ-9 score < 10 vs. ≥ 10)
socialeconomic deprivation [Index of Multiple Deprivation (IMD) score: National quartiles 1 and 2 vs. 3 and 4].
summarises the adjusted mean differences between the randomised groups at 6 months for each level of the subgroup and the test of interaction. There were no significant subgroup differences for TST, chronotype, depression severity, age, sleep medication use, or level of deprivation.
Forest plot of the results from the subgroup analyses.
Adverse events
Pre-defined AEs (work-related accidents, falls, motor-vehicle accidents, near-miss driving incidents, falling asleep while driving) were assessed at baseline, 3, 6 and 12 months. Logistic mixed-effect models revealed no differences between groups for any outcome at any time point (Table 22).

Pre-defined AEs by randomised group at 3, 6 and 12 months
Serious adverse events
The number of SAEs is presented in Table 23. In total, 16 participants (8 in each arm) experienced at least one SAE. There was one death per group [one due to major haemorrhage (SH) and one due to pneumonia (SRT group)]. None of the SAEs were deemed to be related to the intervention or study.

Serious adverse events by randomised group
Impact of COVID-19
The final participant was randomised on 23 March 2020, which was the start date for the national UK lockdown due to COVID-19. The trial was able to continue with remote data collection for most outcomes during the pandemic. Sleep diaries and actigraphy watches were not sent out during lockdown because the research team could not access university buildings; this led to low completion rates for sleep diary, actigraphy and medication use outcomes. A small number of participants in the SRT arm were directly affected by the pandemic (n = 13) such that treatment sessions were adjusted so that they could be completed remotely. Because the lockdown and pandemic may have adversely affected sleep, a sensitivity analysis was conducted to explore whether there was a difference in treatment effect on the ISI between participants who completed the 6-month follow-up before the pandemic (< 23 March 2020) compared with participants whose follow-up was completed during the pandemic (≥ 23 March 2020). There was no evidence that treatment effects differed pre versus during the pandemic (see Appendix 3, Table 35).