6.1. Introduction
Less than 30% of people diagnosed with HIV in resource-limited settings navigate the full continuum of care (1,2). Worldwide, less than 50% of adults are retained in care four years after initiation of antiretroviral therapy (ART) (3). Simple and standardized first-line ART now directly supports adherence, decentralization, task shifting to nurse-led teams and community delivery and more efficient procurement and supply management. In 2013, for the first time, WHO published operational recommendations on how to implement clinical recommendations on the use of antiretroviral (ARV) drugs. These guidelines further expand on the implementation aspects of the clinical recommendations and present 10 new recommendations, three good practices, consensus-derived definitions and a new framework for ART service delivery intended to help countries and programme managers improve the quality and efficiency of services.
This chapter provides guidance in three service delivery areas:
Differentiated care:
Recommendations to strengthen the continuum of treatment and care:
linkage from HIV testing to enrolment in care
retention
adherence
frequency of clinic visits and medication pickup
task shifting
decentralization
integration
adolescent-friendly health services.
Considerations for continuity and high quality of service delivery:
New service delivery recommendations made in 2015 are included with recommendations from the 2013 WHO Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. The inclusion criteria of all systematic reviews conducted to support the decision-making process included adults, key populations, pregnant and breastfeeding women, children and adolescents. In general, there were limited data to inform specific recommendations for these important populations. However, learning from programme implementation has helped to highlight specific challenges and some potential solutions to improve service delivery.
Applicability of service delivery recommendations
In contrast to most clinical interventions, service delivery interventions are generally highly context specific in terms of both relative effectiveness and relative importance in a given context. Consistent with the burden of disease, much of the evidence supporting the recommendations in this chapter comes from studies undertaken in sub-Saharan Africa. Nevertheless, for the majority of interventions, evidence was also available from other regions. Where this is not the case, the need for evidence from a broader range of settings has been indicated in the corresponding section as a research gap.
Many of the recommendations in the service delivery section are conditional and represent interventions that have been shown to have benefit in some settings and thus represent approaches that programme managers can consider in designing their care packages.
6.2. Differentiated care
It is estimated that 95% of HIV service delivery is currently facility based (4). In nearly all countries, the delivery of HIV care in the initial phase of rapid scale-up has been based on a “one-size-fits-all”, clinic-based model, largely undifferentiated for individual needs (5). As national guidelines evolve towards initiating ART for all people living with HIV regardless of clinical and immunological status (6), HIV programmes will be challenged to manage an increasingly diverse set of patient needs. There is now a growing cohort of patients who have been on treatment for several years. At the same time, there is a need to expand timely access to ART for those who have yet to start. While implementation of the recommendations in these guidelines will mean that more people will start earlier, programmes must also retain the capacity to respond to the needs of patients who present with advanced disease, and are at heightened risk of morbidity and mortality (7).
During a scoping consultation on care packages for people living with HIV, WHO reviewed the growing diversity of patient needs and assessed how programmes can treat and care for people differentially within a public health approach (8). Broadly, four groups of patients with specific needs can be identified. First, people who present when well, potentially with higher CD4 cell counts, may require additional and targeted adherence and retention support in order to commit to lifelong ART. Second, people presenting to care with advanced disease require a fast-tracked clinical and care package to initiate ART and prevent death and reduce ill health. A third group of individuals are those who are already on ART but need careful monitoring to ensure timely action as required; this may include clinical care, additional adherence support and timely switch to second-line ART regimens in the case of treatment failure. A final group of stable individuals are likely to represent the majority of people on ART and they can safely reduce the frequency of clinic visits, potentially receiving ART in community settings. Such an approach can relieve overburdened health-care settings and enable more attention to be paid to patients with more complex conditions who require prompt diagnosis and treatment of opportunistic infections, enhanced adherence support, viral load testing and potential changes of regimen, HIV drug resistance testing or other specialized care (8). Receiving care closer to their home can also reduce direct and indirect costs related to transport and long facility waiting time for patients and their families. While these four groups have distinct needs, patients may move between the groups over the course of their lifetime in care.
The care package elements for people living with HIV () are a minimum and may be expanded according to the epidemic, clinical setting and health-care system. These packages are further supported by the differentiated care framework, which proposes the delivery of care in facilities for those who need clinic-based services, with less frequent clinical contact for those who are stable. Differentiating between the service needs of those who are unwell – either because they present late for care or due to treatment failure – and those who are stable on ART, and determining where and how those services are to be delivered, are key to maximizing treatment outcomes and efficiencies. A number of national HIV programmes have already adopted differentiated approaches to care as they scale up ART.
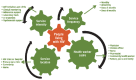
Diversity of care needs for people living with HIV.
The differentiated care framework () is characterized by four delivery components: (i) the types of services delivered; (ii) the location of service delivery; (iii) the provider of services; and (iv) the frequency of services. How these components are combined into a service delivery framework will vary across countries and populations, but the common intention should be to improve acceptability and care outcomes (5).
Key factors in differentiated approaches to HIV care (5).
Two key groups of people require specific approaches that will involve different resource requirements – people who present late to care and people who are stable on ART. To support differentiated care approaches for these two groups, the following WHO consensus definitions and related package of care were reached in a Delphi survey of experts.1
People with advanced disease are defined as those presenting to care with a CD4 count below 200 cells/mm3 or WHO disease stages 3 and 4. The package of care for these people should include the following:
Stable individuals are defined as those who have received ART for at least one year and have no adverse drug reactions that require regular monitoring, no current illnesses or pregnancy, are not currently breastfeeding and have good understanding of lifelong adherence and evidence of treatment success (i.e. two consecutive viral load measurements below 1000 copies/mL). In the absence of viral load monitoring, rising CD4 cell counts or CD4 counts above 200 cells/mm3, an objective adherence measure, can be used to indicate treatment success. The package of care for stable individuals can include the following:
less frequent (3–6-monthly) clinic visits;
less frequent (3–6-monthly) medication pickup;
community-based care; and
cessation of CD4 count monitoring if viral load testing is available.
While less frequent clinic visits are recommended for stable individuals, rapidly growing children (0–5 years old) and adolescents will need to be monitored more frequently for treatment dosing/weight changes and adherence support.
6.5. Retention in care
Recommendations
NEW
Programmes should provide community support for people living with HIV to improve retention in HIV care (strong recommendation, low-quality evidence).
The following community-level interventions have demonstrated benefit in improving retention in care:
- a
Patient advocates, treatment and peer support interventions providing adherence and psychosocial support in the community.
- b
Peer support, distribution of ARV drugs and assessment by non-clinical or lay providers.
Background
Poor patient retention undermines programme and patient outcomes, including achieving sustained viral suppression. The global effort to increase the number of people on ART needs to ensure that people taking ART are retained in chronic care for life. Systematic reviews show that retention rates are estimated to be as low as 64% to as high as 94% at 12 months after ART initiation (33,34). Several countries report a lower than 60% retention rate after 3 years on ART, with high loss to follow-up representing unknown outcomes. Retention in ART programmes is a major challenge in all settings and across populations, specifically paediatric and adolescent populations, postpartum women and men. Multiple factors may play a role in loss to follow-up, including distance to health facilities, lack of transport or inability to cover travel expenses, stigma and disclosure-related issues, being too sick and lack of understanding of the need for lifelong care.
Rationale and supporting evidence
A systematic review (35) identified six studies (one randomized controlled trial and five cohort studies) that evaluated community-level interventions to strengthen retention in HIV care: a package of community-based interventions, adherence support clubs and provision of extra care for high-risk patients. A strong recommendation was made despite the overall low quality of evidence due to the balance of benefit greatly outweighing potential harm, the degree of acceptability to people living with HIV and the programmatic benefits of implementing interventions that result in positive patient and programmatic outcomes.
Package of community-based interventions
Community-based interventions identified with beneficial impact on retention in HIV care included: support centred on the needs of the individual, counselling and psychosocial support by lay adherence counsellors or patient advocates and family and peer support. The lay counsellors or patient advocates assisted patients by linking health facilities with communities, providing counselling and patient-centred support and visiting patients in their home environment. One cohort study (36) included children and adolescents (aged <16 years) and demonstrated significantly increased retention at 36 months with overall low quality of evidence. Bringing services closer to communities through decentralization has also improved retention in HIV care and has been recommended by WHO since 2013 (see
section 6.9 “Decentralization”) (37).
Adherence clubs
The systematic review identified one retrospective cohort study (38) evaluating the impact of facility-based adherence clubs on loss to follow-up or death at 40 months, with a significant reduction compared to standard care. No studies were identified in the review evaluating the impact of adherence clubs on outcomes for adolescents or children.
Extra care for high-risk people
The systematic review identified one cohort study from Kenya that evaluated the impact of nurses and non-clinical health workers providing weekly or biweekly contact with patients with advanced HIV infection (i.e. CD4 count <100 cells/mm3) on mortality (39). Stable individuals on ART met for brief assessments by a non-clinical health worker, referral to a nurse where necessary, in addition to peer support and distribution of ART every 2 months. The study also included a yearly follow-up review with a clinician. Long-term follow-up and death were significantly reduced at 40 months, with an overall very low quality of evidence.
Cost and cost–effectiveness
The cost of implementing community-level interventions varies by setting and depends on whether community-based health programmes are already established. Generally, costs related to training and remunerating lay providers are much less than the cost of care provided at facility level and care provided by health workers. Cost related to community support, peer support and support by patient advocates is often related to training and orienting these cadres and support groups.
Equity and acceptability
A qualitative evidence synthesis (40) highlighted key interventions that were acceptable, related to improving retention in HIV care. These included lay health workers providing support (moderate confidence), particularly if they were also living with HIV; family and friend support (moderate confidence); mobile health (mHealth) interventions (moderate confidence) and developing positive and non-judgemental relationships with those implementing the intervention (moderate confidence).
Population considerations
Pregnant and breastfeeding women
For pregnant women living with HIV, the transition from antenatal care and maternal, newborn and child health (MNCH) services to ART care is a potential point for loss to follow-up. A systematic review (41) identified 10 studies (one cluster randomized controlled trial, three individual randomized controlled trials and six cohort studies) that evaluated interventions to improve postpartum retention of women living with HIV. The majority of the studies reported outcomes in the immediate postpartum period. There was moderate-quality evidence to support the use of phone calls and SMS/GSM/GPRS to improve retention in the early postpartum period (6–10 weeks). Given the paucity of data on the long-term impact of these interventions, limited conclusions can be derived from this result. There is also very limited evidence on the cost–effectiveness of interventions to improve retention in care of postpartum women. However, a systematic review attests to the positive impact of various mHealth approaches (42). Given the low costs of technologies such as SMS or phone, it can be inferred that this would likely be a cost-effective intervention.
A review of country programme experience of delivery of ART in antenatal care settings shows a range of practices to address women who transition from MNCH-based services to HIV care clinics. This transition period is often a critical point in which a substantial number of women and their infants discontinue care. Several countries are implementing interventions with evidence of benefit, including assigning district-level focal points, active patient tracing and financial support for transportation. Many programmes are implementing community-based interventions: peer support such as mothers-to-mothers programmes and peer adolescent support groups for adolescent pregnant women living with HIV. Structured counselling sessions and telephone reminders may also have the potential to support the process of transition.
Children
Caregivers are responsible for understanding the importance of retaining children in care, especially younger children. Disclosure to children typically occurs late, making it challenging to discuss the importance of follow-up. WHO recommends age-appropriate disclosure to children (43). Solutions include:
supporting caregivers to attend for regular follow-up; and
reinforcing to caregivers the importance of the process of disclosing to the child, which can begin early with age-appropriate messaging and tools.
Adolescents
Frequent clinic visits, time spent waiting for services and having to miss school discourages adolescents' engagement in care. Negative attitudes of health workers, concerns regarding privacy and confidentiality and limited opportunity to discuss their concerns also act as barriers to retention for younger people. Distance to facilities and out-of-pocket expenses may restrict their engagement. Service delivery models beyond the facility, which support adolescents to engage in care, such as peer-based interventions and community-based services, should be considered. Peer interventions are highly valued by young people. Adolescent-friendly health services should be implemented to improve quality (see
section 6.11 “Delivering HIV services to adolescents”).
Consider:
providing adolescent services at specific times or in separate areas with flexible appointment systems that accommodate school hours;
comprehensive services that address multiple needs, including psychosocial support and sexual and reproductive health (SRH); and
close monitoring of adolescents' engagement in care, rapid proactive follow-up and implementation of strategies for re-engagement.
Men
A systematic review (44) identified 69 studies demonstrating that men had a 37% increased risk of death while on ART compared to women, after adjusting for baseline characteristics. This is partly explained by the fact that men tend to be diagnosed later and are more likely to start ART late. In several settings, initiatives to improve men's engagement in care have focused on engaging them in services for PMTCT. Innovative service delivery models are essential to improve men's access to HIV care services and ART initiation. Programmes need to routinely disaggregate data by sex in order to better monitor access to and outcomes of treatment for both men and women.
Implementation considerations
There is no single model of community or peer support that works in all settings, and programmes need to adapt such interventions to the local context. Some patients may choose not to receive services in the community due to concern over stigma and discrimination. Community interventions require linkage with health facilities for smooth transfer and referral of patients, when necessary, and strategic planning and resourcing for sustainability. Community-level programmes still need to be integrated into national health sector plans in many settings.
Research gaps
Implementation research and evaluation of the different models of community-level support in different contexts are necessary to further guide programmes. Innovative approaches and effective strategies are needed to support transitioning across different care-delivery points for men, postpartum women, adolescents and children. Further data on the cost of implementing community-level interventions in different settings will guide national policy.
6.6. Adherence
Recommendations
NEW
Adherence support interventions should be provided to people on ART (strong recommendation, moderate-quality evidence).
The following interventions have demonstrated benefit in improving adherence and viral suppression:
peer counsellors (moderate-quality evidence)
mobile phone text messages (moderate-quality evidence)
reminder devices (moderate-quality evidence)
cognitive-behavioural therapy (moderate-quality evidence)
behavioural skills training and medication adherence training (moderate-quality evidence)
fixed-dose combinations and once-daily regimens (moderate-quality evidence).
a
- a
Refer to section 4.4.2 “Fixed-dose combinations and once-daily regimens” for detail.
Background
Adherence to ART is the primary determinant of viral suppression and the risk of transmission, disease progression and death (45–47). Suboptimal adherence is a major challenge worldwide and is associated with a diversity of patient- and programme-related causes. Individual factors may include forgetting doses, being away from home, changes in daily routine, depression or other illness and substance or alcohol use. Adherence to ART may also be challenging in the absence of supportive environments for people living with HIV and in the presence of HIV-related stigma and discrimination. Medication-related factors may include adverse events and the complexity of dosing regimens, such as those for children. Health system factors include distance to health services, long waiting times to receive care and obtain prescription refills, receiving only one month's supply of drugs, pharmacy stock-outs and the burden of direct and indirect costs of care (48,49).
Specific population groups face additional challenges to adherence, and these should be considered when implementing the recommended interventions.
Pregnant and postpartum women
The pregnancy and postpartum period presents significant biological, social and economic challenges that may affect treatment adherence. It is estimated that around a quarter of pregnant women have inadequate ART adherence, and this is higher during the postpartum period (50). Pregnancy-related conditions such as nausea and vomiting may negatively affect treatment adherence. Other individual factors include suboptimal understanding of HIV, ART and PMTCT, lack of partner disclosure and support, and fear of stigma and discrimination. Service delivery barriers include poor-quality clinical practices, gaps in provider knowledge and training, poor access to services and health worker attitudes (51,52).
Adolescents
It is estimated that over one third (38%) of adolescents globally are suboptimally adherent to ART, with substantial regional variation (53). In addition to common challenges to adherence, adolescents face specific challenges, including psychosocial issues such as peer pressure, the perceived need to conform and inconsistent daily routine (54,55). Adolescents are often left out of decisions and have limited opportunities to discuss their concerns, and there is limited availability of adolescent-specific treatment literacy and adherence counselling tools. For adolescents who are transitioning from paediatric to adolescent care, additional challenges may include assuming increased responsibility for their own care, issues relating to disclosure to peers or partners, difficulties in navigating the health-care system, lack of links between adult and paediatric services and inadequately skilled health workers (56).
Infants and young children
Successfully treating a child requires the commitment and involvement of a responsible caregiver. Parents and other family members of children living with HIV may themselves be living with HIV, and suboptimal HIV care and treatment for family members could result in suboptimal care for the child. Other challenges include lack of nutrition support, limited choice of paediatric formulations, poor palatability of liquid formulations, high pill or liquid volume burden, large pill size, frequent dosing requirements and difficulties in swallowing tablets (57–59).
People with mental health conditions and substance use
People with HIV with uncontrolled depressive symptoms are more likely to have poor adherence to ART (60,61). Adherence is complicated by mental health comorbidity that results in forgetfulness, poor organization and poor comprehension of treatment plans. Counselling for HIV and depression and appropriate medical therapies for people with mental disorders can help to improve adherence. WHO recommends that assessment and management of depression should be included in care services for all people living with HIV (see
section 5.3.2 “Assessment and management of depression in people living with HIV”).
Use of alcohol and other substances may also contribute to poor adherence to ART. Alcohol and substance use can lead to forgetfulness, poor organization and diversion of monetary resources (62,63). Treatment of depression and management of substance use disorders can improve HIV treatment outcomes (64,65). WHO recommends treatment of depression and substance use disorders regardless of HIV status. Other key services for people with HIV who use drugs, such as needle and syringe programmes and drug substitution therapy, provide further opportunities to support adherence.
Key populations
In many settings, key populations face multiple challenges related to stigma and discrimination that can affect access to health services, all of which may impact negatively on adherence (66,67). WHO Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations include specific considerations for adherence support for these populations.
Rationale and supporting evidence
A systematic review and network meta-analysis identified 84 randomized controlled trials of interventions to improve adherence (68). Forty-seven trials reported on overall viral suppression and 71 on adherence. The review found overall moderate-quality evidence to support a range of interventions that demonstrate the benefits of improving adherence and viral suppression. Interventions were grouped by components of similarity, given below:
Peer counsellors: there is direct evidence of higher levels of adherence compared to standard care.
Mobile phone text messages: text messages sent to the client's phone one way or two ways. There is direct evidence that text messages result in higher adherence compared to standard care.
Reminder devices: interventions using calendars, alarms, system devices for disease management assistance and pagers. There is direct evidence that supporters and device reminders result in increased viral suppression.
Cognitive-behavioural therapy: there is direct evidence of a higher rate of viral suppression compared to standard care.
Behavioural skills training: provision of training or medication adherence training sessions. These included module-based interventions and those designed to improve life skills, attitudes, behaviour and knowledge.
The benefits significantly outweigh any potential harm for all interventions identified. Peer counsellors are generally considered to be a low-cost intervention and, in some settings, have been highly cost-effective. Interventions such as cognitive-behavioural therapy and behavioural skills training require initial increased investment for training and resources. Reminder devices, telephone interventions and mobile phone text messages are low cost in the majority of low- and middle-income countries. Cost–effectiveness will vary depending on the setting and epidemic context. A strong recommendation was made by the Operational Guideline Development Group, given the potential benefits for patient outcomes, feasibility in a variety of settings and low cost.
Supportive interventions
Several interventions may also be of value in addressing specific challenges that impact on adherence and/or viral suppression. Nutritional assessment, care and support are essential components of HIV care. HIV programmes should ensure that existing national policies on nutritional support are observed to maximize adherence to ART and achieve optimal health outcomes, particularly in food-insecure settings. Nutritional support could include nutritional counselling, cash transfers, subsidizing food costs and/or food vouchers. Studies suggest that providing nutritional support to people receiving ART reduces the risk of non-adherence among food-insecure individuals (69).
Financial support can reduce the risk of non-adherence (70). Programmes and care providers should consider a broader programmatic approach to reducing the costs of care for people living with HIV. This includes avoiding out-of-pocket payments at the point of care (such as drugs, diagnostics and clinical services), supporting transport costs, decentralizing care and reducing the frequency of health facility visits. Programmes need to consider the ethical and equity implications of providing financial support and non-monetary incentives for people living with HIV. Standardized criteria for supporting people receiving ART may need to be developed based on national income levels.
Equity and acceptability
A qualitative evidence review (71) of the acceptability of interventions to improve adherence identified intervention-specific themes. These included educational programmes for individuals and families (high confidence), mobile phone text messages (high confidence), health worker outreach (moderate confidence) and complex interventions (low confidence). Confidentiality and privacy and strengthening social networks were identified as cross-cutting themes from a total of 31 studies. Interventions may be more acceptable if they consider local cultural customs and religious beliefs. Peer-based interventions are generally well accepted, in particular among adolescents, who find that hearing experiences and learning from others facing the same challenges is critical for supporting adherence and engagement in care (72).
Implementation considerations – monitoring adherence
Effective monitoring of adherence requires a combination of approaches based on human and financial resource capacity, acceptability to people with HIV and health workers and an understanding of the local context.
Viral load monitoring
Viral load monitoring is considered the gold standard for monitoring adherence and confirming treatment response. Although treatment failure is often caused by lapses in adherence to ART, it may also result from other factors, including drug resistance, malabsorption, drug–drug interactions and other patient-, disease- and drug-associated effects. Other approaches to monitoring adherence should therefore be considered as a way to provide additional information about possible causes of virological failure or to support adherence monitoring in settings where viral load testing is not available. WHO recommends that, following an initial high viral load (>1000 copies/mL), an adherence intervention be carried out prior to conducting a second viral load test. This has been shown to lead to re-suppression in over 70% of patients (73). Viral load monitoring also has a high potential to motivate adherence.
Pharmacy refill records
Pharmacy refill records provide information on when people pick up their ARV drugs (74,75). Pharmacy records are more reliable than self-reporting (76) and are already a part of national monitoring and evaluation frameworks in many settings.
Self-reporting
Self-reported data are easy to collect and can be a useful adjunct to estimating non-adherence but are subject to recall bias (77). Counselling on the importance of remembering ART doses and an environment that promotes and enables honest reporting of non-adherence are critical components of monitoring adherence to ART in routine care settings.
Pill counts
Counting the remaining pills in bottles may help to assess adherence. Pill counts usually take place at routine health-care visits. However, some people discard tablets prior to health-care visits, leading to overestimated adherence. Counting pills also requires health-care personnel to invest significant time and may not be feasible in routine care settings. Pill count has been found to perform better when combined with self-reported adherence (78).
Research gaps
Further research is needed to determine:
optimal ways to proactively monitor adherence and identify through simple triage those patients in greatest need of adherence support;
interventions to support adherence in populations at heightened risk of suboptimal adherence (children and adolescents, pregnant women, men who have sex with men, people who inject drugs);
potential synergistic effects of combining two or more interventions that could impact individual, social support and health system factors; and
the effectiveness of long-acting ART in improving adherence and viral suppression.
6.7. Frequency of clinical visits and medication pick-up
Recommendations
NEW
Less frequent clinical visits (3–6 months) are recommended for people stable on ART (strong recommendation, moderate-quality evidence).
aLess frequent medication pickup (3–6 months) is recommended for people stable on ART (strong recommendation, low-quality evidence).
b
- a
When routine clinical consultations are due, they should be coordinated with planned medication pickup to reduce visit frequency.
- b
ARV supply management should be strengthened to ensure availability of ARV medicines and prevent stock-outs in the context of less frequent medication pickup.
Background
In the early period of ART scale-up in low- and middle-income settings, the majority of people seeking HIV care presented with advanced disease, often requiring intensive clinical management (79). This led to the typical practice of monthly visits to health facilities for clinical review (80). As guidelines now recommend treatment at earlier stages of the disease, there is a growing appreciation that, with effective ART and good adherence, people can have a near-normal life expectancy (81–83). HIV programmes are providing ART to increasing numbers of people who are stable on ART, and there is a need to rationalize the delivery of care to reduce the burden on health systems, particularly in countries with a high HIV prevalence. Frequent clinical visits also place a burden on people taking ART, and the opportunity and financial costs of travelling to the clinic have been associated with an increased risk of poor adherence and poor retention in care (84). Correspondingly, approaches to reducing the frequency of clinical visits, including community ART dispensing, have been associated with improved retention (85,86).
Rationale and supporting evidence
Because clinical visits and medication pickup are often linked, both were assessed in a combined systematic review that found eight studies (one cluster randomized controlled trial, two randomized controlled trials and five cohort studies) from Kenya, Malawi, South Africa, Uganda and the United States, providing comparative data on the impact of reduced frequency of clinical visits. Overall, the review found that reduced frequency of clinical visits among stable individuals was associated with significantly better retention, with no difference in mortality outcomes. The same review identified three studies from Malawi, South Africa and Spain, which reported that reduced frequency of ART refills was also associated with improved retention in care. The studies also reported a positive trend towards improved adherence (87). There was no evidence that reduced frequency of ART refills led to additional complications or disengagement from care.
The strong recommendation to reduce the frequency of clinical visits and drug refills is supported by the expanding availability of ART and the fact that people are presenting to care earlier and require less intensive clinical care. This trend is expected to continue as countries move towards initiating ART for all people living with HIV regardless of CD4 cell count. In addition, there is substantial cost to people and health services of monthly clinical visits for those who are stable on ART. Reducing the frequency of clinical and ART pickup visits will be cost saving for both patients and health services. All cost analyses conducted within the systematic review point towards reduced cost per patient with reduced visits.
Equity and acceptability
Implementation of these recommendations is likely to improve equity as it relieves the burden placed on people to attend for frequent clinical assessments and to pick up drugs. This burden is greatest on people in rural areas (distance to travel) and those with very low income (costs associated with time off work). This recommendation is supported by the reported acceptability by young people and adults, citing increased convenience and fewer interruptions of daily schedules.
Implementation considerations
Reduced frequency of clinical and ART pickup visits has already been implemented for HIV and other chronic diseases in a variety of low- and middle-income settings. In implementing these recommendations, programmes need to consider coordinating routine clinical consultations with planned medicine pickup to reduce visit frequency. ARV drug procurement and supply management should be strengthened to ensure availability of ARV drugs and prevent stock-outs in the context of less frequent medication pickup (see
section 6.10 “Integrating and linking services”).
In some settings, consideration is being given to implementing even less frequent (i.e. annual) clinical visits for people who are stable on ART. While there is currently no published evidence to support this approach as a formal recommendation in these guidelines, it is recognized that this may be a reasonable approach in certain contexts and for certain populations.
Population considerations
Pregnant and breastfeeding women
Pregnant or breastfeeding women on ART may require closer follow-up and more frequent visits than other populations. Psychosocial support and counselling requirements may need to be considered, especially with regard to infant feeding and postpartum care. Differential care models should be used from the beginning of pregnancy until the end of the breastfeeding period.
Children
The needs of the children evolve as they grow, especially during rapid phases of growth and development, early adolescence and around the time of disclosure. Differential care models should be modified to the child's needs. Growth monitoring is an important component of paediatric HIV care and is necessary for dose adjustment of ART. This should be emphasized within the differential care model.
Adolescents
Rapid development may impact on adherence, retention and support requirements for adolescents. The evolving capacities and emerging independence of adolescents need to be recognized as well as the involvement of caregivers. Busy and changing daily routines and competing priorities can make frequent visits challenging for adolescents. Close monitoring of adolescents' engagement in care, rapid and proactive follow up and implementation of strategies for re-engagement are critical. Facilitating independence and self-management can support differential care for adolescents. Peers and other community-based services can also facilitate early identification of adolescents requiring additional support and follow-up. (See
section 6.11 “Delivering HIV services to adolescents”.)
Research gaps
Further evidence of the feasibility and benefits of increasingly spaced clinical visits and medication pickup (greater than 6 months) is needed, and this should be studied across various patient populations.
6.8. Task shifting and task sharing
Recommendations
NEW
Trained and supervised lay providers can distribute ART to adults, adolescents and children living with HIV (strong recommendation, low-quality evidence).
Trained non-physician clinicians, midwives and nurses can initiate first-line ART (strong recommendation, moderate-quality evidence).
Trained non-physician clinicians, midwives and nurses can maintain ART (strong recommendation, moderate-quality evidence).
Trained and supervised community health workers can dispense ART between regular clinical visits (strong recommendation, moderate-quality evidence).
These recommendations apply to all adults, adolescents and children living with HIV.
Source:
Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013. (http://www.who.int/hiv/pub/guidelines/arv2013/download/en) [PubMed: 24716260].
Background
The number of available health workers remains inadequate in many settings with a high burden of HIV. Task shifting and task sharing involve the redistribution of tasks within health workforce teams. Specific tasks are reassigned to health workers with shorter training and fewer qualifications to optimize the available human resources. Although increasing the number of health-care personnel is also crucial, clinical tasks need to be shared and shifted to ensure that enough health workers are available.
In 2013, WHO made recommendations on task shifting relating to initiation, maintenance and dispensing of ART. These recommendations are supported by a systematic review of four randomized controlled trials and six observational studies (88). Overall, the data showed no difference in mortality and some evidence of reduced rates of loss to follow-up when nurses or non-physician clinicians initiated or maintained people on ART or when community health workers maintained people on ART relative to physicians performing these tasks. The quality of care in these studies was ensured by providing training, mentoring and supervision for nurses, non-physician clinicians and community health workers, clear referral pathways and effective monitoring and evaluation systems (88).
These guidelines provide a new recommendation for trained and supervised lay providers to distribute ART to adults, adolescents and children living with HIV. Several high HIV-burden settings are experiencing a critical shortage of pharmacy personnel to undertake this task. Apart from the absolute shortage of such staff, pharmacy personnel tend to be concentrated in urban settings, large hospitals and private pharmacies, accentuating shortages in rural and primary-care settings (89–91). Community distribution of ART for stable individuals can ease the burden of care on people with HIV and health services by reducing the frequency of clinical consultation and drug pick-up visits. Additionally, retrospective cohort studies from Uganda, the United Republic of Tanzania and Zambia demonstrate that community ARV distribution results in significantly less patient attrition than health facility-based services (92).
Rationale and supporting evidence
Indirect evidence from a systematic review comparing dispensing of ARV drugs by non-pharmacists compared to pharmacists identified two cluster randomized trials that compared the outcomes of community-based HIV care with clinic-based care (93). The first trial evaluated patient outcomes (viral load, CD4 count, opportunistic infections and change in ART regimen) when ART was provided in community settings by community care counsellors living with HIV compared to clinic-based ART delivery where care was provided by health professionals. In the community-based settings, ART was distributed by community care counsellors who were trained and mentored for patient triaging, referral when necessary and distribution of ARV drugs prefilled by pharmacy personnel. People receiving community-based care had been on ART for more than six months and had no clincial conditions and high levels of self-reported adherence to treatment (94). People in the standard-of-care arm visited health facilities on a monthly basis, picked up their ARV drugs from a health facility pharmacy and were provided care by health workers. The second trial evaluated virological failure among those accessing ART from lay providers in the community compared to the standard ART care delivery by health professionals at health facilities (95).
The meta-analysis identified no difference in mortality and virological failure between people who received care from a community-based team and those who received care from health professionals at a health facility. Fewer people were lost to follow-up in the community-based care group. Both groups demonstrated high levels of self-reported medication adherence, with no statistical differences between the groups. The overall quality of evidence is low. The lay providers were also part of a wider community-based intervention with additional confounders that supported the success of the intervention, not solely the distribution of ART.
A strong recommendation was made that trained and supervised lay providers can distribute ART to adults, adolescents and children living with HIV despite the low quality of evidence, considering existing programme experience of trained and supervised community health workers and lay workers providing and performing other tasks within the HIV testing, treatment and care continuum. Additionally, there are important examples within the broader health sector where community health workers, with minimal training, are entrusted to provide curative and preventive care in maternal and child health services, malaria diagnosis and treatment and TB care.
Potential benefits and harm
The public health benefits of lay providers distributing ART are an overall increase in the number of providers to overcome the shortage of facility personnel, reduced clinic congestion and services provided closer to communities, which support retention in care. Where ARV drug supply and stock management are not reliable, distributing ARV drugs outside health facilties may increase distribution sites and potentially aggravate stock-outs. Neither of the two studies in the systematic review included people with comorbidities or children. While the recommendation is relevant to these populations, necessary dose adjustments, as the child grows, can be safely done during clinic visits, with maintenance treatment provided at the community level.
Cost and cost–effectiveness
There may be initial increased costs for training and other costs associated with introduction of community ART distribution. However, studies report substantial costs to patients when they are required to travel to centralized health services to collect medication. A study from Uganda reported that the cost to people receiving hospital-based care was three times higher than for people who received home-based care (95).
Equity and acceptability
A qualitative evidence synthesis identified that task shifting can enhance linkage to care and treatment adherence and assists HIV programmes to cope with shortages of professional health workers (18). Strengthened relationships between people with HIV and local communities can empower individuals. In Malawi, people from the community were hired as pharmacy attendants to pre-package and distribute ARV drugs to enhance the capacity of pharmaceutical services, an approach that has facilitated visit spacing and reduced waiting times and health providers' workload at health facilities (96).
Implementation considerations
Both initial and ongoing training and mentoring, supportive supervision and administrative planning have been critical to the success of programmes that have implemented task shifting. Programmes adopting these recommendations need to train and establish a system for routine supportive supervision of health workers, including lay providers. Adopting task shifting and sharing strategies often requires the revision of regulatory frameworks and national policies so that new cadres of health workers can perform new tasks. WHO recommends that all health workers, including lay providers, should be adequately remunerated (97), and programme experience indicates that it is difficult to sustain health services based on volunteerism alone. National regulatory bodies, professional associations and other stakeholders need to be involved when addressing the scope of practice, roles and responsibilities of health workers.
It is important that the local supply chain system take into account task shifting and sharing responsibilities, and community models of ARV drug delivery to ensure adequate stock. The supply management logistic information system needs to incorporate all ARV drug distribution outlets, including community lay providers' distribution sites (see
section 6.13 “Procurement and supply management systems for HIV health products”).
Good practice statement
NEW
Trained and supervised non-laboratory staff, including laypeople, can undertake blood finger-prick for sample collection.
WHO recommends that trained and supervised lay providers be used to provide safe and effective HIV testing services using rapid diagnostic tests (see
section 2.3.1 “Pre- and post-test services: overview). A systematic review (98) was conducted to evaluate the outcomes of shifting tasks of finger-prick specimen collection for HIV-related diagnostic tests to non-laboratory staff (i.e. clinicians, nurses and midwives) and lay providers compared to laboratory professionals doing the same task. The review identified 27 studies (observational and technical evaluations), the majority based in sub-Saharan Africa (88%). The study results were inconsistent due to various cadres of health workers, test types utilized and the outcomes assessed. All of the studies reported a high degree of acceptability by clients and health workers, which was consistent with findings from the qualitative evidence synthesis on the acceptability of task shifting (see recommendations for task shifting). The inconsistent study results, high risk of bias and limitations of the reported data reduce the ability to make definitive conclusions from the systematic review alone.
A good practice statement was developed on task shifting for conducting blood samples by finger-prick testing, due to the overall benefits outweighing any potential harm. Nurses, midwives and clinicians readily perform HIV testing and are involved in EID sample collection and CD4 point-of-care testing in many settings, which form the basis of this good practice statement. Expanding finger-prick testing to lay providers will support increased access to essential diagnostics and decentralization of HIV care. In several settings, trained and supervised lay providers are already conducting HIV testing and performing sample collection using the finger-prick method. No harm was identified from this approach; however, a system of quality assurance is necessary for effective and safe implementation. The WHO Consolidated guidelines on HIV testing services provide additional considerations and key steps for successful implementation related to performing testing and sample collection by lay providers (http://www.who.int/hiv/pub/guidelines/hiv-testing-services/en).
Building human resource capacity
As HIV treatment and care have been scaled up in the past decade, in-service training has assumed a key role in rapidly upgrading the competencies of health-care providers. All health workers, including community health workers and lay providers, need to be regularly trained, mentored and supervised to ensure high-quality care and timely implementation of updated national policies. Given the rapidly evolving knowledge on HIV care and treatment, countries need to consider a system for supporting health workers' continuing education, including through clinical mentoring and regular supportive supervision (99). The use of new technologies, such as computer-based self-learning, distance education, online courses and phone-based consultation, may supplement classroom in-service training and enable efficient use of health workers' time and other resources. It is equally important to strengthen the HIV treatment and care components of existing pre-service courses for health workers graduating and receiving certification in a range of disciplines. Health-care workers also need to be equipped to manage HIV as a chronic condition, work in a team and implement new national guidelines and protocols.
Programme managers should also support the development and implementation of policies to create a suitable environment for recruiting, retaining, remunerating and motivating health workers in rural or remote areas, where turnover and attrition may be considerably higher than in urban settings.
In many countries, people living with HIV, community workers and volunteers are involved in delivering HIV testing, counselling, care, treatment and social support services. People with HIV are also involved in training health workers as expert trainers. Involving people living with HIV in both training health workers and delivering HIV services may have the additional benefit of overcoming HIV-related stigma and discrimination.
Research gaps
Research is needed to determine the feasibility and safety of further task shifting to improve access to treatment, including initiation and maintenance of ART at the community level, with spaced clinical visits to a health facility.
High-quality evidence is lacking for the initiation of second-line ART by trained non-physician clinicians, midwives and nurses. Further research in this area would identify the benefits and harm of implementing this approach in settings where there are clinician shortages.
6.9. Decentralization
Recommendations
Decentralization of HIV treatment and care should be considered as a way to increase access to and improve retention in care:
initiation of ART in hospitals with maintenance of ART in peripheral health facilities (strong recommendation, low-quality evidence);
initiation and maintenance of ART in peripheral health facilities (strong recommendation, low-quality evidence); and
initiation of ART at peripheral health facilities with maintenance at the community level (strong recommendation, moderate-quality evidence).
a
Source:
Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization; 2013. (http://www.who.int/hiv/pub/guidelines/arv2013/download/en/ [PubMed: 24716260].
- a
Community level includes external outreach sites, health posts, home-based services or community-based organizations. Frequency of clinical visits will depend on health status.
Background
The rapid scale-up of ART programmes has posed significant challenges to health systems in high-burden, resource-limited settings. In many settings with a high burden of HIV infection, clinics have long waiting times due to the volume of patients needing care. Decentralizing HIV treatment and care reduces waiting times for people receiving care in facilities and brings HIV services closer to people's homes. Decentralizing HIV treatment and care also strengthens community engagement by linking community-based interventions with health facilities and can optimize access to services, care-seeking behaviour and retention in care. People living with HIV, affected communities and community-based interventions play a key role in providing HIV testing, treatment, care and support.
Rationale and supporting evidence
This recommendation, made in 2013, is supported by a systematic review identifying 16 studies (two cluster randomized trials and 14 cohort studies) assessing models of decentralized HIV treatment and care. The review provides evidence that decentralization of HIV care, either from hospitals to health centres or from health centres to community-based care, improves patient access and retention in care without compromising clinical outcomes. All but one of the studies was carried out in sub-Saharan Africa, and the benefits of decentralization may differ in different contexts (100).
Implementation considerations
The optimal model for ART decentralization (partial or full) depends on the local context, including the burden of HIV infection and the health-care delivery system. Programmes should determine which clinical and laboratory services will be available at what level of the health-care delivery system.
Programme managers should consider the values and preferences of those receiving care, the number of people likely to attend decentralized settings and whether decentralization brings services closer to people who would otherwise travel long distances to receive ART.
Decentralization should be accompanied by efforts to strengthen linkage and referral systems. Community-based treatment programmes should be linked with regular care at health facilities and with adequate laboratory facilities, diagnostics, monitoring and evaluation and drug and supply management systems.
Standards of care should be defined for each level of the health system. The role of each level should match its capacity, and the lines of authority and accountability should be clear and well understood. In many settings, decentralizing ART requires task shifting to ensure an appropriate mix of health workers at peripheral facilities. An appropriate regulatory framework (laws, regulations, policies and guidelines) is needed to allow tasks to be performed by different cadres of health workers.
Adaptations may be needed for specific populations. HIV care and treatment services for pregnant and postpartum women and HIV-exposed and -infected children can be provided in decentralized settings and is a preferred option where the HIV burden is high and a large number of women and children access health services in primary care settings. In settings with a low HIV burden, a centralized service delivery model with community linkage may be more appropriate. Some groups, such as adolescents and key populations, may choose to receive HIV services in a facility that is not close to their homes due to stigma and disclosure-related concerns. In such settings, programme managers should incorporate the values and preferences of their clients in designing appropriate service delivery models.
6.11. Delivering HIV services to adolescents
Recommendations
NEW
Adolescent-friendly health services should be implemented in HIV services to ensure engagement and improved outcomes (strong recommendation, low-quality evidence).
Community-based approaches can improve treatment adherence and retention in care of adolescents living with HIV (conditional recommendation, very low-quality evidence).
Training of health-care workers can contribute to treatment adherence and improvement in retention in care of adolescents living with HIV (conditional recommendation, very low-quality evidence).
Adolescents should be counselled about the potential benefits and risks of disclosure of their HIV status to others and empowered and supported to determine if, when, how and to whom to disclose (conditional recommendation, very low-quality evidence).
Source:
HIV and adolescents: guidance for HIV testing and counselling and care for adolescents living with HIV. Geneva: World Health Organization; 2013. (http://www.who.int/hiv/pub/guidelines/adolescents/en) [PubMed: 25032477].
Background
These guidelines recommend ART initiation in all adolescents (10–19 years old) living with HIV, regardless of CD4 count or clinical stage of disease (see
section 4.3.3 “When to start ART in adolescents (10–19 years of age)”). There is a growing cohort of adolescents living with HIV, which includes those infected from birth and those who have acquired HIV later in childhood and adolescence. While there is still a lack of health outcome data for this age group, emerging evidence indicates that adolescents living with HIV are underserved by current HIV services and have significantly worse access to and coverage of ART (139). Adolescents are at high risk of loss to follow-up both before and after ART initiation; people aged 15–24 years and those attending services for the PMTCT of HIV are particularly at risk (140–146).
Rationale and supporting evidence
All adolescents, including those living with HIV, face significant barriers to accessing health services, due to inadequate health literacy, limited ability to navigate health systems, legal requirements for parental or caregiver consent, and insufficient resources to pay direct and associated service costs (147,148). Adolescents face significant levels of stigma and discrimination, particularly those from key populations, among whom criminalization of behaviour such as sex work, drug use and same-sex activity further perpetuates social exclusion and hinders access to health and support services (149,150).
Poor quality of services also limits adolescent engagement in health care. Adolescents often perceive health services as unacceptable due to concerns about confidentiality and negative health provider attitudes (150,151,153). Services are often not organized to accommodate adolescent needs and routines and have inconvenient service schedules, inflexible appointments and unwelcoming environments (150,151). Without sufficient consideration and support, adolescents can be lost between paediatric and adult services. The rapid developmental and social changes that occur during adolescence exacerbate the impact of such barriers and can have a profound impact on the way adolescents engage with health services (151).
Due to their unique needs, adolescents living with HIV require quality comprehensive services and care to support access, retention and adherence. This includes psychosocial support, SRH and mental health care (151,152). The WHO quality-of-care framework provides a useful working definition of adolescent-friendly health services (Box 6.1) (153,154). Additionally, global standards for quality health-care services for adolescents have been developed to support implementation (Box 6.2) (155).

WHO-defined characteristics of adolescent-friendly health services. Equitable: all adolescents, not just certain groups, are able to obtain the health services they need. Accessible: adolescents are able to obtain the services that are provided.

Global standards for quality of health-care services for adolescents.
A systematic review (156) was conducted to assess the effectiveness of adolescent-friendly health services for adolescents living with HIV compared to standard care. Due to the limited evidence available, the review was expanded to include the general adolescent population and young people up to and including 24 years of age. Adolescent-friendly health services were defined according to the WHO characteristics and the global standards for quality health-care services for adolescents. Eleven randomized control trials (157–167) and eight observational studies (168–175) from four of the six WHO regions were identified. Four studies focused on adolescents living with HIV; the remainder focused on SRH, HIV prevention, mental health, diabetes, general health and smoking cessation. All studies included two or more of the WHO characteristics, and global standards for quality health-care services for adolescents. Only one study included all WHO characteristics and no study addressed all global standards.
Young people engaged in adolescent-friendly health services compared to standard care showed small but significant improvements in various outcomes, including health outcomes (lower pregnancy rates); health-care utilization (presentation at a clinic for mental health, HIV counselling and testing and outpatient visits); uptake (HIV testing); knowledge (HIV and STI acquisition, pregnancy prevention and sexual health); attitudes (towards sex and HIV testing); sexual risk-reduction behaviour (condom use); self-efficacy (condom use or diabetes management); and service acceptability. No differences were seen with respect to healthy lifestyle or quality of life outcomes. Among HIV-positive young people exposed to adolescent-friendly health services compared to the standard care, there were small but significant improvements with respect to short-term viral load reduction and long-term ART adherence. The overall quality of evidence is low. A strong recommendation was made despite low-quality data, citing the promising improvement in outcomes, the existing programme experience, and evidence of feasibility and acceptability by the end users.
Cost and cost–effectiveness
No studies have assessed the resource requirements or cost–effectiveness of adolescent-friendly health services within HIV service settings. However, a cost modelling study and a retrospective cost analysis for adolescent-friendly health services in low- and middle-income countries reported that, although additional resources are required to ensure delivery of quality adolescent-friendly health services, investments to implement and scale up these services – in particular, services providing multiple interventions – appear to have value and impact for adolescents (176,177).
Equity and acceptability
Adolescent-friendly approaches aim to ensure that all adolescents obtain the health services that they need, that policies and procedures are in place to facilitate provision of health services to adolescents and that all health service providers treat adolescents with equal care and respect, regardless of HIV status, behaviour or other characteristics.
A global consultation of 470 young people living with HIV and a situational analysis of over 200 facilities in the WHO African Region was undertaken to prepare for the development of these guidelines (150,178). Additional inputs were also considered from two unpublished, multicountry longitudinal qualitative studies with 147 adolescents living with HIV (179).
Key acceptability themes and suggested strategies for improving service delivery focused on empowered and solution-oriented information and support; opportunities for open and honest discussion; skills development on SRH and HIV disclosure from an early age; comprehensive care that addresses issues beyond HIV, including support for adolescents from key populations; flexible scheduling of clinic visits to accommodate school hours; free services closer to home and community-based services; dedicated hours and spaces for adolescents; peer-led interventions and services; and an adolescent-competent workforce.
Feasibility
The programmatic and facility-level feasibility of adolescent-friendly health services was explored. The situational analysis of facilities assessed the availability of appropriate HIV treatment services for adolescents (181). Thirty-five per cent of facilities reported that adolescent patients were attended to separately from adult and/or paediatric patients through the use of dedicated schedules, staff or spaces. A case study of a government ART clinic in KwaZulu-Natal province in South Africa found that it is feasible to provide adolescent-friendly health service approaches in an HIV service setting. Although financial costs were low, consideration needs to be given to ensure engagement of stakeholders, including adolescents, adolescent-specific training, minimal provider rotation and adequate time for planning of restructured services. A survey of HIV programme managers highlighted the lack of appropriately trained health service providers and the need for greater preparation of health-care services as key challenges in delivering HIV services to adolescents (180).
Experience in Zimbabwe has identified several requirements for scaling up adolescent-friendly HIV services. These include a national, multisectoral, coordinated response; adolescent-sensitive policies and guidelines; meaningful involvement of adolescents; training and sustained mentorship of health service providers; community systems strengthening; linking community interventions with health facilities; and a national monitoring and evaluation framework with disaggregation and clear indicators for adolescents.
Implementation considerations
The global standards for quality health-care services for adolescents provide an approach to implementing health-care services for adolescents (Box 6.2) (152). The standards outline the desired level of care and what needs to be done for the standards to be achieved.
Further actions to implement the standards at the national, district and facility levels are outlined within the implementation guide (158). These standards and actions are relevant to HIV services for adolescents.
HIV-specific implementation considerations include the following:
aligning approaches for HIV service delivery with WHO and national adolescent-friendly health service standards, protocols and activities;
including implementation of adolescent-friendly approaches in HIV health service supervisory and monitoring systems;
ensuring training, research and personal development opportunities for health service providers on adolescent HIV treatment and care;
engaging service providers, adolescents and other key stakeholders to identify acceptable and feasible activities;
implementing adolescent-friendly health service approaches in all HIV services used by adolescents, including antenatal care for pregnant adolescents living with HIV;
establishing linkages and referral pathways to ensure a comprehensive continuum of care, especially for the transition from paediatric to adult HIV services; and
addressing the needs and vulnerabilities of adolescents from key populations (
181).
Research gaps
Research should contribute to a better understanding of how to implement adolescent-friendly health services at a programmatic level and the cost–effectiveness of these approaches in HIV services in low- and middle-income countries. Research on the service delivery needs of adolescents living with HIV should examine the minimum package of care; models of delivery at different service levels, including for key populations and pregnant adolescents living with HIV; the integration of SRH in ART services for adolescents; interventions to support safe disclosure; treatment literacy; interventions to address mental health; and the impact of provider training and peer interventions.
6.12. Improving the quality of HIV care services
Background
This section provides brief guidance for programme managers and health-care providers on improving the quality of HIV care services. It focuses on key principles, approaches and interventions, highlighting QA and quality improvement (QI) practices based on implementation and programme experience. HIV programmes should also be innovative in addressing local challenges and aim to strengthen programme monitoring and the routine use of programme data to improve the quality of services. Quality of care emphasizes that services should be effective in achieving desired health outcomes and that health-care practices should be people-centred and safe (182). The WHO global strategy on people-centred and integrated health services outlines the strategy and provides an overview of evidence and good practices (183,184). Strategies to improve the quality of HIV care services are needed both at the programme management level and at health facility and community levels where HIV care services are provided (185). If an intervention is to achieve the desired health outcomes, it needs to be evidence based, of high quality and achieve a level of coverage that brings desired outcomes at the population level.
Rationale for strengthening the quality of HIV care services
Quality care means that people living with HIV receive the care they require to maintain their health and quality of life. For HIV programmes and health-care providers, quality HIV care optimizes programme effectiveness and efficiency. For policy-makers and funding agencies, quality care is an important requirement for maintaining health at the population level and ensuring the optimal use of available resources.
HIV programmes should plan to provide quality HIV care services from the outset by incorporating quality in the national policy, strategic plan, strategic information framework and operational and service delivery plans. Quality of care should not be seen as an additional activity to routine HIV services or a short-term project to redress implementation issues and gaps; it should be incorporated into daily activities at all levels, from service delivery to national programme management.
The WHO global strategy on people-centred and integrated health services
The WHO global strategy on people-centreda
and integrated health servicesb represents a fundamental shift in the way health services should be funded, managed and delivered. Without a people-centred and integrated health services approach, health care will become increasingly fragmented, inefficient and unsustainable. The strategy proposes that all people have access to health services provided in a way that responds to their needs and that are equitable, safe, effective, efficient, timely and of an acceptable quality.
Within the context of HIV care service delivery, people-centred care includes:
building health-care providers' skills for effective communication with people;
providing information and supporting people to make informed decisions and for their active engagement in their own care and self-management;
offering a patient appointment system and acceptable frequency of facility visits;
avoiding long health facility waiting times during clinical consultations, medication pick-up or laboratory services;
coordinating care when people require multiple services (e.g. TB and HIV treatment, family-centred care); and
providing comprehensive integrated services, as appropriate and relevant.
Source:
WHO global strategy on people-centred and integrated health services. (http://www.who.int/servicedeliverysafety/areas/people-centred-care/en).
- a
People-centred health services involve an approach to care that consciously adopts the perspectives of individuals, families and communities and sees them as participants as well as beneficiaries of trusted health systems that respond to their needs and preferences in humane and holistic ways. People-centred care requires that people have the education and support they need to make decisions and participate in their own care. It is organized around the health needs and expectations of people rather than diseases.
- b
Integrated health services are health services that are managed and delivered in a way that ensures that people receive a continuum of health promotion, disease prevention, diagnosis, treatment, disease management, rehabilitation and palliative care services at the different levels and sites of care within the health system and according to their needs, throughout their whole life.
WHO, the participants of a scoping consultation on strengthening quality of HIV clinical services and members of the Operational Guideline Development Group developed the following good practice statements for HIV care services that are reflective of the broader WHO global strategy on people-centred and integrated health services (187).
Good practice statements
NEW
HIV programmes should:
provide people-centred care that is focused and organized around the health needs, preferences and expectations of people and communities, upholding individual dignity and respect, especially for vulnerable populations, and engage and support people and families to play an active role in their own care by informed decision-making;
offer safe, acceptable and appropriate clinical and non-clinical services in a timely fashion, aiming to reduce morbidity and mortality associated with HIV infection, and to improve health outcomes and quality of life in general; and
promote efficient and effective use of resources.
Components of quality assurance and quality improvement
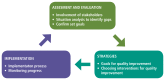
QI is the process of improving services and care through the routine use of patient and programme data. Several contextual factors, such as leadership and teamwork, also have a bearing on the quality of HIV care. Programme managers and health-care providers need to continuously monitor the quality of HIV care services (). This can be done by comparing implementation against set standards, analysis and use of routinely collected data at facility level and consultation with service users or community networks on the needs, values and preferences related to HIV care services that they receive. Some quality interventions require broader system-level interventions to ensure sustained improvement. For example, the delivery of community ART, triaging patients to reduce clients' waiting time for clinical consultation and use of lay providers in patient education and counselling all require a supportive programme and resources for sustained implementation. Lessons from the QI process in HIV care service delivery and management should feed back into subsequent policy and programme formulation and planning processes.
Key enablers of quality HIV care services.
Enablers of quality improvement
HIV programmes should provide a national framework for strengthening the quality of HIV care services. The framework should also facilitate accountability and resource mobilization at different levels of the health-care delivery system. Integration of quality HIV care in national policies and strategic plans can also facilitate partnership with other health programmes and stakeholders, including professional associations, funding agencies, research institutions, communities and networks of people living with HIV. A national framework should also highlight the needs of marginalized people or groups, address resource needs to reach underserved populations and address health worker capacity to bridge gaps in quality. Comprehensive quality-of-care activities should include both QA and QI activities. summarizes enablers for delivering high-quality HIV care services.
Several key considerations for integrating QI of HIV care into national policies, programmes and strategic plans are given below.
Ensure involvement of stakeholders, including networks of people with HIV, communities and health-care workers, for building a common understanding on strengthening quality HIV care services and efficient use of resources.
Identify elements of national policies and strategies to address the quality of HIV care services and HIV-related stigma and its impact on accessing HIV care services.
Prioritize strategies for QA and QI of HIV care ().
Identify nationally harmonized key indicators and reporting requirements for monitoring the quality of HIV care.
Define roles and responsibilities of stakeholders at different levels of the health-care delivery system in strengthening the quality of HIV care services.
Allocate resources for the development of mechanisms to support QI; for management and information systems that report, monitor and improve the quality of HIV care services; and for alignment of performance with implementation of evidence-based good practices.
Include high-quality, people-centred HIV care service delivery in the principles, values and strategies of national polices and the HIV strategic plan.
Set standards for evidence-based HIV care through national guidelines by defining the package of HIV care services to be offered, including at what level of the health-care delivery system and for which population or geographical location.
Provide a national monitoring framework for continuous QI of HIV care services and assure that the practice of care adheres to national, evidence-based guidelines and standards.
Process of building a strategy for continuous quality improvement.
6.13. Procurement and supply management systems for HIV health products
6.13.1. Overview
This section provides operational guidance on procurement and supply management (PSM), with a focus on how PSM systems can respond to new recommendations in these guidelines. Comprehensive advice on the general management of PSM systems is readily available in existing publications and training materials. References to relevant publications and materials are provided at the end of this section.
The overarching objective of PSM systems is to support national policy with the adequate and continuous availability of the most effective, heat-stable, fixed-dose, quality-assured ARV drug formulations, diagnostics and other consumables at service delivery sites, in the right quantities, at the lowest possible cost and in a timely manner.
The new recommendation that ART should be initiated in all people living with HIV regardless of CD4 cell count will require an integrated national strategic response that considers the resources available and enables strong PSM systems at all levels of the health system. In addition, the fact that all people living with HIV need to be on ART will accelerate the scaling up of ART programmes. Procurement managers and ART programme managers will need to work together to ensure that the national supply system is forecasting, procuring and distributing the quantities of ARV drug and other health commodities required to meet the increasing national demand and the 90–90–90 target.
While there are no major changes in the ART regimens recommended in these guidelines, new drugs are recommended as components of alternative regimen choices, with potentially important implications for PSM.
6.13.2. Implementation considerations
Most national HIV programmes will need to plan a phased approach to the adoption of the new recommendations, notably the expansion of ART to all people living with HIV and the introduction of pre-exposure prophylaxis (PrEP) for groups at substantial risk of HIV infection. Where programme resources are already stretched, unplanned adoption of these new recommendations with the associated increase in demand for drugs and diagnostics may lead to stock-outs and imbalances.
Challenges and opportunities associated with implementing new WHO recommendations include the following:
product selection;
quantification and demand forecast;
procurement planning and execution, including delivery and timeliness of orders;
the ability of global supply to cope with the increasing global demand;
storage and distribution, including logistics constraints;
monitoring of consumption and demand changes;
frequency of ARV drug pickup;
information flow between stakeholders at different levels;
costs and opportunities for cost saving;
shelf-life of ARV drugs;
risk of stock-outs if procurement does not meet demand; and
risk of expired health products if the quantities procured have been overestimated.
National HIV programmes should consider establishing a stakeholder working group to develop a plan to address these issues, with participation by health policy-makers, implementers, PSM specialists and representatives from central medical stores management and the finance ministry.
Selecting pharmaceutical and diagnostic products
Medicines should be selected in accordance with national ART guidelines and programme needs.
National ART guidelines should provide guidance on alternative regimens in case of drug toxicities and treatment failure or stock-outs (e.g. lopinavir/ritonavir [LPV/r] versus atazanavir (ATV)/r; lamivudine (3TC)-containing versus emtricitabine (FTC)-containing formulations).
It is recommended that the overall number of ARV drug regimens be minimized in order to optimize treatment and sourcing. According to WHO country surveys conducted in 2015, 30% of reporting countries use over 10 different first-line ARV drug regimens in adults and adolescents.
Before new products are included in national essential medicines lists, registration and intellectual property status should be verified to ensure that the product can be imported.
If a selected ARV drug is not on the national list and/or registered in the country, HIV programme managers should coordinate with the national drug regulatory authority and request that these drugs be put on the list and registered. Pharmaceutical companies also have a responsibility to register products in countries where they market their products.
Drugs no longer recommended by WHO for any ARV drug treatment regimen should be removed from the national ART guidelines and essential medicines lists, and plans should be made to transition patients to more effective regimens, using existing stocks as appropriate.
The synchronized introduction of new guidelines with planning for forecasting, procurement and distribution will minimize the wastage of products that are being phased out and shortages of newly recommended products.
Quantification and forecasting of demand
To assess the volume of products required to meet programme demand, PSM managers need to know:
the number of patients on treatment, disaggregated by age group;
the ARV drug regimens in use by those patients;
proposed changes in ARV drug regimens, if any;
the expected rate of scale-up of treatment, i.e. the increase in numbers of patients on each regimen in a given period of time;
the numbers of rapid test kits required to identify people with HIV in line with scale-up targets;
the forecasted uptake of PrEP; and
the frequency of ARV drug pickup by patients.
The process of quantification of needs can be highly complex. Good practice suggests that quantification should be undertaken annually, projecting for at least two years with a quarterly or semi-annual review to determine if any significant upward or downward adjustments are needed. Assuming that financial resources are available, PSM managers can then plan ahead and place long-term orders while allowing sufficient flexibility to adjust for potential changes in the pace of scale-up, regimen switching and/or other unforeseen events affecting consumption.
When demand for a specified period has been quantified, procurement managers should develop a supply plan that takes into account:
the months of stock currently available;
the existing orders yet to be fulfilled by vendors;
the budget available for new orders;
the volume of new orders required to satisfy the forecasted demand, including provision of a safety buffer stock; and
the required delivery dates for new shipments.
It is recommended that this process of supply planning be conducted on a quarterly or half-yearly basis to accommodate changes in demand and any delivery delays from suppliers.
Procurement
A uniform and harmonized procurement system is required to efficiently procure quality-assured, affordable ARV drugs and diagnostics. Procurement should be based on selection of appropriate products and forecasted needs, considering consumption, expanding services, phasing in and phasing out of formulations and implementing new recommendations. Transparent procedures should be adopted to achieve best-value procurement and a QA system implemented to procure, store and distribute quality-assured pharmaceuticals, diagnostics and other health products.
National procurement programmes should:
request that partners supporting the national HIV programme consolidate PSM systems and pool procurement for ARV drugs and diagnostics;
consider joining other pooled procurement mechanisms to increase economies of scale and minimize the risk of long delivery times observed with small orders (second-line regimens and paediatric ART);
establish agreed specifications for selected products to ensure a common basis for procurement competition, QA standards of the products, and any special needs such as packaging or identification, with special requirements likely leading to price increases;
use a competitive process to ensure value for money;
establish long-term framework contracts against which call-down orders can be placed for commodities in regular and repeated demand, such as medicines and diagnostics. This will reduce procurement transaction costs as well as the time taken from identification of need to fulfilment of order and help to build collaborative relationships between buyers (national procurement managers) and sellers (suppliers);
wherever possible, implement a multisupplier procurement strategy to support a healthy market and avoid dependence on a single supplier, which will also provide flexibility during periods of supply constraints or where individual suppliers face production or logistical difficulties that lead to delays;
use a publicly accessible database to facilitate access to information about prices and to support competition; and
follow the principles described in the United Nations interagency guidelines for donated drugs and WHO model quality assurance system for procurement (MQAS) (
186).
National HIV programmes should be aware that other countries and programmes will be ordering the same or similar formulations at the same time, and manufacturers may already have orders that will account for current production, possibly for several months ahead. Working with their suppliers, procurement managers will be able to place their orders according to agreed volumes and delivery schedules. Advice on current production and any existing supply constraints and opportunities may also be available from the organizations and contacts provided at the end of this chapter (see
section 6.13.6 “Useful PSM resources”).
Storage and distribution
Appropriate storage and distribution of HIV medicines, diagnostics and other commodities is essential. The recommendation to offer ART to people with HIV regardless of CD4 cell count will significantly increase commodity volumes and the demands on storage and distribution. Countries may need to plan for new public facilities or examine alternative approaches, including leveraging additional resources by outsourcing to private sector facilities, provided that they are appropriate for storing pharmaceuticals. Neither of these options is a quick fix; contracting with private sector providers may take many months to complete the appropriate tendering, contracting and QA of providers before available facilities can be activated for use. Countries may also wish to explore the potential of existing parallel systems, such as cold-chain facilities in immunization programmes for products that require temperature control.
PSM systems should consider planned programmatic changes in service delivery related to the frequency of clinical visits and location where patients receive their ARV drugs. For example, community distribution of ARV drugs to stable patients and community-based HIV testing may involve another step in the local supply chain and potentially increase the quantity of ARV drugs and diagnostics to be procured and distributed. Changes that impact positively on the amount of stock retained at each level, including by the patient, need to take into account the shelf-life of the ARV drugs. For example, the most common first-line ARV drugs currently have a 24-month shelf-life only, and many rapid test kits have a 12- to 18-month shelf-life only, which may impact storage and distribution plans.
Storage and distribution plans should include:
QA of products on receipt at the warehouse;
secure storage facilities appropriate for pharmaceuticals;
cold storage for products that require temperature control;
rationalization of the number of storage levels to reduce the length of the supply pipeline;
inventory control systems with appropriate minimum and maximum levels that trigger reordering;
regular distribution patterns to service facilities, with increased frequency of potentially smaller deliveries supporting more effective use of existing limited space and distribution capacity; and
routine data reporting from facilities to monitor usage and identify changes in predicted consumption patterns that may risk excess stocks (which can be reallocated to avoid expiry) or stock-outs.
Ensuring a secure supply for programme flexibility and to avoid stock-outs and expired health products
It is essential to avoid stock-outs in order to prevent treatment discontinuation and achieve ART targets. Recommended actions to avoid the risk of stock-outs include the following.
There should be close coordination with HIV programme managers and policy-makers to understand the planned progression towards “treat all” targets. Programme managers and clinicians should agree on the speed at which new patients will be started on treatment to ensure that the required commodities are available. A faster-than-planned introduction of new patients will exhaust stocks and cause an increased risk of stock-out; a slower-than-planned introduction risks overstocking and wastage due to expiry of medications.
A clear understanding is needed of the supply chain implications of any proposed changes in the service delivery model (e.g. community distribution of ARV drugs).
It is important to ensure that the ARV drug and diagnostics supply chain – especially its distribution system and allocation of commodities by facility – reflects the geography of the HIV epidemic.
New patients should be initiated on the preferred first-line regimen, unless clinically contraindicated.
Quantification and ordering should include a rotating safety buffer to compensate for errors in forecasting and potential delivery delays. It is recommended that the buffer be part of the normal stock rotation, not a separate stockpile, to avoid the risk of retaining aged or expiring products. The level of buffer may vary but should cover at least one round of planned deliveries so that any delivery delay will not lead to a stock-out. Forecasts should be revisited at least semi-annually to adjust for variances between forecasts and actual demand and to review demand for the next 12–18 months, adjusting orders as necessary.
Orders should be placed in advance for timely delivery. Procurement managers should work closely with their suppliers to understand the suppliers' normal delivery periods and plan accordingly. It is recommended that orders be placed at least six months ahead of the required date of delivery, as this will allow adequate time for production and – where volumes allow delivery by sea freight – reduce the cost of shipping. It is also recommended that, where practical, deliveries be staged rather than arriving as a large shipment containing six months or more of stock. Staged deliveries allow for more flexible delivery schedules and enable PSM managers to make the best use of existing storage and distribution capacity.
PSM managers should be aware of potential and actual constraints in the global market. In 2015, there were some constraints in the availability of important active pharmaceutical ingredients. This impacts the ability of formulators to manufacture and deliver finished products and must be factored into requested delivery times for new orders.
Special consideration should be given to products that have a low demand, as in the case of many drugs used by adults or adolescents in second- or third-line ART and many paediatric ART regimens. Production of these medicines will be less frequent and many countries will require only low volumes, usually less than a full production batch. Pooled procurement at the national level and cooperation between countries and with suppliers may be appropriate in the case of these products. Buffer stocks may need to be higher to compensate for less regular deliveries and challenges in accurate forecasting of usage and uptake. Examples of such mechanisms include the Pan American Health Organization regional drug facility and the Paediatric ARV Procurement Working Group.
Where procurement regulations allow, it is recommended that framework contracts be placed to allow for call-down orders. This maximizes the flexibility in delivery schedules, which can be adapted to actual consumption, and reduces the need for frequent repeat procurement and bidding exercises without compromising value for money.
Several of the above actions will limit the risks of stock-outs as well as expired products (e.g. moving ARV drugs from low-volume treatment sites to high-volume treatment sites). However, when products expire, they should be destroyed according to the approaches and various disposal methods outlined in the WHO
Guidelines for safe disposal of unwanted pharmaceuticals in and after emergencies (
http://apps.who.int/iris/bitstream/10665/42238/1/WHO_EDM_PAR_99.2.pdf).
Use and monitoring
Robust information systems ensure the availability of accurate and timely consumption data on ARV drugs and diagnostics and other information required to effectively monitor the performance of the entire supply system and forecast the ARV drugs and diagnostics needed. Monitoring PSM through the effective use of early warning indicators prevents stock-outs and overstocks leading to expiry. Reliable capture and analysis of usage and consumption data from facilities will support a robust bottom-up approach to quantification and forecasting that will reflect changes in demand and support a flexible approach to the introduction of new recommendations in these guidelines.
6.13.3. Special considerations for adult and adolescent ART regimens
The preferred first-line ART regimen remains tenofovir (TDF) in combination with lamivudine and efavirenz, or TDF in combination with FTC and efavirenz (EFV), preferably as fixed-dose combinations (see
section 4.4 “What to start: first-line ART”). Regimens containing nevirapine (NVP) are retained in the guidelines as an alternative option where clinically required for patients who cannot tolerate or have contraindications to TDF or EFV.
Key changes compared to 2013 are:
Four key challenges for the supply chain arise as a result of the recommended ARV drug regimens.
The currently approved suppliers of preferred first-line FDC formulations are expected to have sufficient production capacity to satisfy the increased demand for these formulations as the number of patients on treatment increases, provided that increases are carefully phased to avoid sudden spikes in demand. However, short-term supply constraints may arise, highlighting the need for proper planning and maintenance of buffer stocks at the national level.
Delivery lead times for the recommended first-line drugs may become extended during peak periods of demand. PSM managers should be aware of current lead times and plan their orders and deliveries accordingly.
Purchasers and implementing partners who are distributing zidovudine (AZT)-based and NVP-based regimens to patients and have stocks and orders in process should consider how to manage their stocks to avoid stock-outs or wastage due to expiry of usable products from overstocking.
There may initially be limited demand for alternative first-line, second-line or third-line regimens, particularly those containing the newer medicines DTG, DRV/r and RAL. Pooling orders from several buyers is recommended to increase the volumes to be ordered and ensure that suppliers can deliver and adequately respond to the demand.
Transitioning to the recommended preferred regimens and preferred formulations
Programmes should plan carefully and discuss with their suppliers the pace at which increased quantities of TDF- and EFV-based products can be made available. This will require a graduated process of transition to the “test and offer” paradigm and for alternative scale-up targets. To ensure that supply is available to meet the anticipated demand, a phased programme is highly recommended. Suggested approaches are the following.
Initiate new patients eligible for ART on TDF-based regimens, with preference for FDCs of TDF + 3TC + EFV or TDF + FTC + EFV.
Include buffer stocks in supply plans and liaise closely with suppliers and the major pooled procurement mechanisms to understand global demand patterns and act accordingly.
Programmes should stop procuring the following:
Stavudine (d4T): in light of the cumulative mitochondrial toxicity of d4T, it should no longer be procured, and people currently receiving d4T-based regimens should transition to a TDF-based regimen.
Didanosine (ddI): ddI should no longer be procured as it is no longer recommended as an alternative nucleoside reverse-transcriptase inhibitor (NRTI) in adult and adolescent second-line regimens due to toxicity, lower efficacy and inconvenient dosing requirements.
People currently receiving first-line AZT- and/or NVP-based regimens should be transitioned to the preferred first-line FDCs in a phased manner to enable the use of current stocks and orders and taking into account the speed at which increased deliveries of TDF products can be ordered and delivered.
In areas with a high prevalence of HIV-2 infection, the procurement and use of formulations with two-drug FDCs (TDF with 3TC, TDF with FTC and AZT with 3TC) might be a preferred option, as this provides the flexibility to combine the NRTI backbone with protease inhibitors (PIs) or integrase strand transfer inhibitors (INSTIs) in first- and second-line therapy for people living with HIV-2 infection.
In the case of newer products such as DTG, DRV/r and RAL or existing products of low demand (e.g. for second- or third-line regimens), where feasible and practicable, procurement managers should consider pooling their demand with other domestic programmes, neighbouring countries or other regional programmes and/or collaborating with major purchasers to form a part of total orders that meet manufacturers' production batch sizes. Shelf-life, storage facilities and consumption patterns permitting, PSM managers should also plan to hold larger buffer stocks for essential products in low demand. In the case of newly introduced products, it should also be assumed that initial orders may require longer lead times.
Supply chain considerations for implementation of less frequent ARV drug refills, community ART delivery and lay providers distributing ARV drugs
There are several supply chain issues that programme managers and policy-makers need to consider when adopting and implementing new service delivery recommendations regarding less frequent ARV drug pickup, the use of community ART delivery models and lay providers distributing ARV drugs. PSM managers and policy-makers should examine the current ARV drug supply chain model and its performance to see what adaptations are needed to enable the supply chain to support these new recommendations. As no “one-size-fits-all” supply approach will meet the needs of differentiated care models, the local supply chain must be agile enough to serve a variety of service delivery models, including at the community level. In addition, programme managers should consider taking a phased approach that takes into account the following:
the additional ARV drugs needed at pickup sites, including the provision of a safety buffer stock;
the number of patients to be served by multi-month (3- to 6-month) prescribing and the regimens they currently use;
the capacity of local distribution sites to safely and securely store and handle the additional ARV drugs;
the additional reporting needed by the logistics information system to track the ARV drugs through these sites, including at community level;
any ARV drug shelf-life constraints;
overall supply chain performance where the recommendations will be implemented; and
incorporation of additional ARV drug requirements in the country's annual quantification, financing, procurement and supply plans.
Besides the quantity of additional products required to implement new recommendations, the manufacturing lead time may influence the pace at which programmes can take new recommendations to national scale.
6.13.4. Special considerations for paediatric ART
These guidelines make no changes to the first-line paediatric ART regimen recommended in 2013 (see
section 4.4.4 “First-line ART for children three to ten years of age” and section 4.4.5 “First-line ART for children younger than three years of age”). RAL is newly recommended in second-line paediatric ART for children younger or older than 3 years after failure of a first-line regimen containing LPV/r (see
section 4.8.3 “Third-line ART”).
Transitioning to recommended preferred regimens and preferred formulations
In general, these guidelines recommend once-daily FDCs to facilitate procurement and supply chain management, logistics and adherence. Additional logistic and programme factors should be addressed for national programmes to select optimal formulations. To ensure smooth implementation of recommended first-line regimens for children, it is critical for policy-makers and implementers to consider the availability of paediatric ARV drug formulations.
National programmes are urged to limit the procurement of ARV drug products for children to formulations on the Optimal Paediatric Formulary of the IATT on Prevention and Treatment of HIV Infection in Pregnant Women, Mothers and their Children. Complying with the IATT Optimal Formulary based on WHO recommendations will help to simplify the supply chain and aggregate global demand to stabilize the global supply of ARV drugs for children.
Programmes should not procure the following paediatric formulations:
Stavudine (d4T): d4T is no longer recommended due to its cumulative mitochondrial toxicity. Children currently receiving d4T-based regimens should transition to preferred paediatric ART regimens.
Didanosine (ddI): ddI should no longer be procured as it is no longer recommended as an alternative NRTI in paediatric second-line regimens due to toxicity, lower efficacy and inconvenient dosing requirements.
When available, age-appropriate FDCs for any recommended regimen are preferable.
Dispersible tablets (also known as tablets for oral solution) are the preferred solid oral formulations.
Oral liquid formulations should be avoided in favour of solid oral dosage forms when available:
In light of the continuing challenges of ensuring the availability of ARV drug formulations for children, the IATT provides guidance on optimal ARV drug products for children to promote a secure and sustainable supply. A new revision took place in December 2015 after these guidelines were finalized, and an updated version will be available at http://www.emtct-iatt.org/resources.
6.13.5. Checklist for introducing new products and phasing out old ones
These guidelines recommend new ARV drug formulations for adults and adolescents as well as for paediatric ART. Introduction of new medicinal or diagnostic products is one of the most complex and unpredictable activities in any HIV programme and, as such, presents a heightened challenge for policy-makers, PSM managers and manufacturers. When planning the introduction of new products, the following PSM-related factors should be taken into account.
Is the product subject to patent or other intellectual property protection that would restrict access to generic formulations of the product in your country? In many middle-income countries, access to generic versions of ARV drugs is restricted. Should this be the case, advice is available from the Public Health, Innovation and Intellectual Property Team of the WHO Department of Essential Medicines and Health Products.
Is the product registered for use in your country? If not, consider obtaining a temporary waiver and, in the meantime, accelerate the official registration processes for future procurement. This information should be available from the national drug regulatory authority. While it is the responsibility of the manufacturer to arrange registration, registering a drug can be a lengthy and expensive process.
What is the forecasted demand for the product, including the anticipated pace of adoption? The pace of adoption is very difficult to forecast accurately, and ordering and delivery schedules must take into account this unpredictability. Faster adoption may lead to stock-outs if the procurement plan has not taken this into account, whereas a slower pace could lead to expiry of stocks if the procurement plan assumed a faster adoption. PSM managers will need to monitor consumption closely and have risk mitigation strategies in place. In case of faster adoption, suppliers should be asked to be prepared to respond to urgent orders. In case of slower adoption, suppliers should be asked to deliver quantities gradually according to country requests until all the quantities ordered are consumed. Ordering in large volumes has the advantage of economies of scale, but suppliers should be flexible enough to send deliveries according to national demand to prevent wastage by expiry. PSM managers are encouraged to work with HIV programme managers in formulating risk mitigation plans to account for the difficulty of accurate forecasting of demand and the pace of adoption.
How will introduction of the new product impact the use of existing medicines or diagnostics? Unless it is recommended that a product be stopped due to severe toxicity or other reasons, PSM managers should always plan to optimize the use of existing stocks and orders before a full switchover to new products, in order to avoid wastage.
How will purchase of the new product affect procurement budgets? PSM managers may wish to consult with major global purchasers to gauge the expected price so that quantities of existing and new products can be accommodated within the available budget. In some cases, lack of sufficient funds has delayed a full transition to new products that were more expensive or led to stock-outs of existing products.
What is the shelf-life of the product and how might this affect the procurement strategy and the in-country distribution of the product? The “first expiry, first out” principle should be applied in stock management and distribution. Products with a shorter shelf-life should be distributed to treatment sites and dispensed as quickly as possible to allow their consumption before their expiry.
Does the new product require any special handling or storage, such as temperature control? Consider adjusting the capacity and storage conditions of current facilities.
What is the production status of the new product? What minimum order will the supplier accept and what is the anticipated lead time from order placement to delivery? In the case of products in lower demand, manufacturers may be willing to only commit to production once they are assured of commercially viable orders.
If small volumes of new products are required, PSM managers should consider collaborating with neighbouring countries or global pooled procurement mechanisms to reach total order volumes that are viable for the manufacturers. This strategy may be particularly appropriate for second- and third-line formulations and for paediatric ARVs.
Policy-makers and PSM managers may wish to consult with global purchasers and other knowledgeable entities to gain market intelligence on new formulations while they develop strategies to introduce these new formulations through national procurement.
6.13.6. Useful PSM resources
This document does not cover all technical issues related to PSM. The PSM Toolbox contains PSM tools that can be searched by technical area and by publishing organization: http://psmtoolbox.org/en.
6.14. Laboratory and diagnostic services
6.14.1. Overview
Implementing recommendations in these guidelines will require increased access to laboratory and diagnostic services.
To ensure that diagnostic services are accurate and reliable, relevant QA systems need to be developed and strengthened. Within a country, a multiplicity of diagnostic settings may exist, such as laboratories, maternal and child health clinics, HIV testing and counselling sites and community-based testing. A multipronged and networked approach to selecting diagnostics and laboratory systems should therefore be planned and adopted. Because many new diagnostic tests and point-of-care systems are entering the market, the use of only high-quality diagnostics and equipment needs to be ensured. Strategic planning for proper placement and harmonization of testing platforms should be carried out to ensure appropriate use and cost–effectiveness.
Effective laboratory and diagnostic services require sound leadership and governance to enable the following activities (188):
strengthening and expanding laboratory and diagnostic services;
supporting a dedicated specimen referral system;
appropriate availability of CD4 count testing;
increased access to HIV viral load testing for all people on ART, for monitoring purposes;
supporting the expansion of diagnostic services to include testing at the point of care;
training and certifying health-care workers who perform testing;
ensuring high-quality diagnostics and plans for implementing these, including QA; and
ensuring appropriate deployment of diagnostic technologies to increase their efficient and optimal use.
WHO and the United States Centers for Disease Control and Prevention (CDC) have developed a handbook on QA approaches to point-of-care testing and for laboratories in low- and middle-income countries (188). shows the three-phase approach to QA of laboratory programmes.
Three phases of laboratory quality assurance. Source:
WHO
US CDCImproving the quality of HIV-related point-of-care testing: ensuring the reliability and accuracy of test results
Geneva
World Health Organization2015(http://www.who.int/hiv/pub/toolkits/handbook-point-of-care-testing/en). (more...)
6.14.2. Strengthening and expanding laboratory and diagnostic services
The following areas are important for strengthening the network of laboratory and diagnostic services in order to implement the recommendations in these guidelines:
national laboratory strategic plans and policies;
evaluating diagnostics for their performance and operational characteristics to validate testing algorithms (with backup options) before introduction;
carrying out strategic planning in order to properly place and harmonize testing platforms to ensure appropriate use and cost–effectiveness;
expanding current laboratory networks to support and monitor the decentralization and integration of testing services and to provide access to testing when diagnostic services are unavailable at service delivery sites;
allocating appropriate resources to ensure the availability of testing services, including human and financial resources; and
guidance on operations and service delivery.
6.14.3. Supporting a dedicated specimen referral system
Laboratory referral systems and procedures for collecting and processing specimens need to be strengthened to increase access to viral load and other testing (e.g. CD4 testing at the point of care and EID). Providing for and strengthening a dedicated, efficient, safe and cost-effective specimen referral system requires reliable specimen transport with adequate conditions for whole blood, plasma and dried blood spot specimens as well as for rapidly and dependably reporting test results back to the referring site with linkage to care. Rapid reporting of results is essential for timely care.
6.14.4. Increasing access to HIV viral load testing
These guidelines recommend the use of viral load testing to monitor treatment response and diagnose treatment failure and the use of dried blood spot samples for viral load testing. This will require ongoing strengthening of existing laboratory services and phased expansion of monitoring services in peripheral facilities. It may involve:
strengthening and leveraging existing EID networks;
ensuring that laboratories have adequate infrastructure, technical expertise and QA and QI programmes;
ensuring an appropriate mix of high-volume centralized laboratory testing and testing at the point of care for facilities in remote locations; and
use of dried blood spot specimens to increase access to viral load testing.
6.14.5. Planning for appropriate use of CD4 count testing as access to viral load testing increases
As countries move to eliminate CD4 count-dependent treatment initiation thresholds and viral load monitoring replaces monitoring with CD4 cell count, it is anticipated that the demand for CD4 count testing will decrease. As this transition takes place, programme, laboratory and PSM staff should take into account the following programmatic considerations.
As demand decreases, reductions in CD4 count testing capacity can be staged through several strategies based on site-level demand, age of the instrument and failure rates.
Although sample referral networks for CD4 cell count and viral load testing may overlap, the sample types require different transport capabilities. Programmes need to ensure adequate network capability for sample referral for viral load testing prior to scaling down CD4 count testing.
Programme planning should include a realistic transition of financial support from CD4 count testing to viral load monitoring. Cost savings may not be evident immediately, as the cost per test for CD4 count testing will increase as volumes decrease.
Quantification and forecasting (active supply planning) will be essential to account for commodity shifts. This is particularly important in the early phases of the transition when historical data will not reflect current commodity needs. Supply chain needs, including cold-chain transport and storage, must also be considered during the transition.
Even in settings with full access to viral load testing, CD4 cell count testing capability will continue to be needed as part of HIV programmes for baseline risk and other clinical assessments. Depending on the context, as the transition to viral load monitoring from CD4 count testing for initiation and monitoring progresses, programmes may wish to consider centralizing the continued use of CD4 count testing.
6.14.6. Expanding diagnostic services to point-of-care settings
Decentralizing laboratory and diagnostic services requires that all aspects of testing be in place before services are implemented. This includes:
using only high-quality, evaluated and reliable diagnostic tests;
supervising and monitoring point-of-care testing for quality and reliability;
implementing a strategy for managing the supply chain and equipment service;
establishing data management systems for timely identification of quality issues; and
regional and national data reporting.
6.14.7. Ensuring appropriate deployment of diagnostic technologies
The WHO diagnostic use survey demonstrated that several technologies are available in the countries that reported their diagnostic usage. Their utilization is lower than 50% of their capacity for several reasons:
Deployment of high-throughput instruments in low-volume settings with no appropriate plan for sample transportation: CDC and WHO have produced a strategic planning manual since the Maputo Declaration on Strengthening of Laboratory Systems to ensure that equipment is deployed in a tiered laboratory network based on the volume of tests at the testing sites ().
Lack of maintenance and repair needs: maintenance should be embedded in the contractual agreement with the manufacturers supplying diagnostics.
Lack of installation of purchased equipment due to lack of trained personnel or adequate space for the size of the equipment: prior strategic planning is essential to ensure that appropriate space and a trained laboratory technician are ready when the procured equipment is delivered for use in the country.
Tiered laboratory network at various levels of the health-care delivery system.