23.2. HIV Synthesis Model
To conduct this evaluation the HIV Synthesis transmission model is used. It is an individual-based stochastic model of heterosexual transmission, progression and treatment of HIV infection (9, 10). All variables in the model are updated in 3 month periods. The model includes an age-structure and sexual risk behaviour is modelled as the number of condom-less sex short-term partners and presence of a condom-less sex long-term partner in each period. In any given period, the probability of an uninfected person having a condom-less sex partner who is infected with HIV depends on their number of partners and on the prevalence of HIV amongst partnerships formed by those of the opposite gender, accounting for patterns of age mixing. Given exposure to an infected partner, the probability of transmission depends on the viral load level of the partner (obtained by sampling from the distribution of viral load levels in partnerships formed by HIV infected people, accounting for gender and age), on the estimated risk of transmission at that viral load, presence of a concurrent sexually transmitted infection and on gender. The presence or not of resistance mutations does not influence the risk of transmission (i.e. virus with resistance mutations present is assumed equally transmissible as virus without such mutations, for a given viral load). For people who have become infected with HIV the variables modelled include viral load, CD4 count, presence of resistance mutations, risk of AIDS and death, whether they have tested HIV positive and whether they are in care and on treatment. The model of progression of HIV and the effect of ART has been shown to provide a generally close fit to observed data relating to the natural progression of HIV infection and the effect of ART (11). People who have not tested HIV-positive and who have access to HIV testing have an increasing chance over calendar years to be tested for HIV up to the end of 2017. In addition pregnant women can access HIV testing through antenatal clinics. Only people who are diagnosed with HIV can be linked to care (60% chance within 3 months of diagnosis) and if eligible initiate ART or be monitored with 6 monthly CD4 measurements.
This version of the model HIV Synthesis Transmission model has been amended compared to the version previously published (9, 10). The main amendments include the definition of women who are considered female sex workers, so that interventions can be targeted at this group, the roll out of voluntary medical male circumcision in men over the age of 15 and testing pre-circumcision. In addition, all the HIVST scenarios described below have been newly programmed, including some extra details such as the probability of failing at performing a test. Since this analysis was conducted, the process of circumcision at birth, testing of people who are not living with HIV presenting with HIV like symptoms and repeat testing of women attending ANC have been included.
Each simulation generates a population of 100,000 – each person is aged 15 to 65 years in some period between 1989 (the start of the simulation) and 2035, but the population size in this age band at any one point is lower than 100,000 because, for example, people who will be 15 years old in 2030 are not included in the adult population until this date.
The model is in the process of being re-calibrated using an Approximate Bayesian Computation Approach and using the following data:
- -
size of the female sex worker population (NAC) estimated in 2002 and 2012
- -
gender-specific HIV prevalence in adults (age 15–49)(DHS in 2005/06, 2010/11and 2015 and ZIMPHIA in 2015/16 (12, 13))
- -
gender-specific HIV prevalence in people aged 15–24 (DHS in 2005/06, 2010/11 and 2015 and ZIMPHIA in 2015/16 (12–14))
- -
HIV prevalence among female sex workers (RDS conducted at the end of 2013 within the SAPPH-Ire trial)
- -
gender-specific HIV incidence in adults (age 15–49) (ZIMPHIA in 2015/16 (14))
- -
gender-specific proportion tested for HIV in the last year in adults (age 15–49)(DHS in 2005/06, 2010/11 and 2015 (12, 13))
- -
number of HIV test performed per year in adults (age 15–49) (Global AIDS Response Country Progress Report in in 2007, 2009, 2012–2015 (15)
- -
proportion of people (age 15–64) living with HIV reporting knowing their HIV status (ZIMPHIA in 2015/16 (14))
- -
gender-specific number of adults on ART (age 15–49; 2004–2015 (16))
- -
Number of adults on second line (end of 2014, Ministry of Health (17))
- -
Gender-specific prevalence of viral load suppression among HIV-positive adults aged 15 to 64 years (ZIMPHIA in 2015/16 (14))
- -
Proportion with reverse transcriptase resistant mutations at ART initiation in 2008–2010 (Report on the National HIV Drug Resistance. Monitoring at Sentinel sites 2009–2011 (18))
The calibration for this exercise did not include the data from the DHS 2015 and ZIMPHIA because they were not available at the time. In addition, the proportion tested for HIV in the last year and the number of HIV test performed per year were not included but instead the gender-specific proportion ever tested for HIV from the DHS in 2005/06 and 2010/11 and the proportion ever tested among FSW estimated at baseline in the SAPPH-Ire trial were included. Finally, the number of people on ART was included but only for the years 2010–2014 and it was not gender specific.
The model is programmed in SAS 9.4.
23.3. Setting
For this project, we want to reflect the HIV epidemic in a specific country. This decision was driven by the necessity to model realistic gender and age specific levels of HIV testing and prevalence to accurately predict the impact of introducing self-testing in groups defined on the basis of age and gender. We are finalizing the calibration of the Synthesis model to Zimbabwe using an Approximate Bayesian Computational Approach. The Synthesis model was previous calibrated manually to the HIV epidemic in Zimbabwe.
The choice of Zimbabwe as the setting was guided by several factors: first, by the fact that the Zimbabwean government is interested in exploring self-testing; second, because we are involved in other projects in Zimbabwe and we have links with the Zimbabwe Ministry of Health and health economists working there and third, because it is one of the countries where the STAR project is taking place.
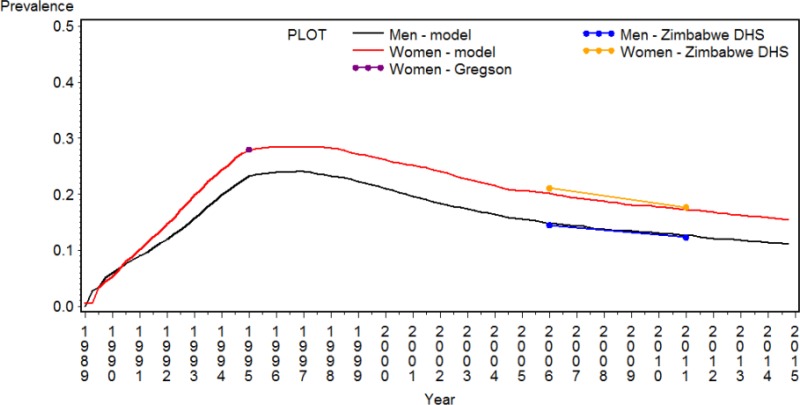
summarizes demographic information for Zimbabwe and its response to the HIV epidemic. The population the HIV Synthesis model focuses on is the adult population aged 15 to 65 years old, which was estimated to be 8.5 million in mid-2016 (19). The HIV prevalence in people aged 15 to 49 years old reached levels of 15% in 2011 (12) and a similar estimates was obtained at the last DHS in 2015/16 (13.8%) (13). But as shown in , it peaked in the mid-90s and then declined since then. In 2015/16, 49% of women and 36% of men tested for HIV in the last year and therefore were aware of their HIV status (13) and in 2011 60% of women attending antenatal clinic (12). Availability of ART has increased dramatically in the last few years, by the 1st quarter of 2013 over 500,000 people were receiving antiretroviral treatment (20) and by mid of 2015 over 800,000 (16). Retention on ART is relatively high: 86% at 12 months from start of ART (15).
Table 23.1AInformation on the HIV epidemic in Zimbabwe
View in own window
| Value | Year | Source |
|---|
| Population 0+ (in million) | 14.5 | 2016.5 | CIA (19) |
|---|
| Population 15–65, n (in million) | 8.5 | 2016.5 | CIA (19) |
|---|
| HIV prevalence, 15–49 | 13.8% | 2015/16 | DHS (13) |
|---|
| % ever tested | 64% M, 81% W | 2015/6 | DHS (13) |
|---|
| % of pregnant women attending ANC tested for HIV | 59% | 2011 | DHS (12) |
|---|
| % tested in the last year | 36% M, 49% W | 2015/6 | DHS (13) |
|---|
| Number of adults on ART | 818,207 | Sept 2015 | Apollo (16) |
|---|
| ART coverage | 85% | 2012 | 2016 (15) |
|---|
| 73% | End 2015 |
| Of those who started ART 12 months ago, % known to be still on | 86% | 2015 |
|---|
ANC: Antenatal Clinic; ART: antiretroviral therapy; CIA: Central Intelligence Agency; DHS: Demographic and Health Survey;
shows the HIV prevalence, gender-specific, in red for women and in black for men, reconstructed by the Synthesis model together with prevalence data estimated from nationally representative surveys (12) and other sources (21). This is based on the calibration previously published (9) as the new calibration is still ongoing.
23.4. Summary of assumptions
23.4.1. Assumptions on scale up of HIV testing
HIV testing was assumed introduced in 1997. At that time we assumed 20% of the population were resistant to be tested for HIV unless symptomatic and this decreased linearly to 5% by the end of 2010. In the Synthesis model this group has no possibility of getting tested for HIV unless symptomatic. Limited data are available to inform this parameter (proxy variables are the proportion who reported never being tested for HIV and, more precisely, the proportion who refuse HIV testing), nevertheless we considered it important to take this into account, given the evidence that not everyone accepts HIV testing for various reasons. The level of acceptability of provider initiated HIV testing and counselling (PITC) in LIC is extremely variable from levels of 99%, observed in inpatients in Uganda (22) to 31% among outpatients in South Africa (23). Among pregnant women the level of acceptability of PITC seems to be higher, varying from 76 to 99.9% (24), while the estimated acceptability of home-based counselling and testing has been estimated in a meta-analysis to be 83% (25). This huge variability seems to be related mainly to the quality of the intervention delivered and on calendar time. Acceptability seems to have increased over time due to the reduction in stigma and higher availability of ART, therefore we thought it was reasonable to assume a decline in the proportion resistant to be tested for HIV down to 5% in 2010.
For the remainder of the population (non-resistant to HIV testing), increasing gender and age-specific rates of HIV testing (for the 1st time and for repeat testing) since 1997 were assumed, to reflect the level of testing observed in the DHS (12). Pregnant women experience an additional probability of being tested in the ANC, which increases over calendar year (12) and it is assumed that since the start of the roll-out of voluntary medical male circumcision men are tested for HIV before getting circumcised.
For people who have never had condom-less sex (the model does not distinguish between not having sexual intercourse and having a sexual intercourse using a condom), a 3-fold reduction in the rate of testing was assumed. This has been observed in the Zimbabwe DHS where people who reported never having had sex (whether with or without condom) are less likely to test for HIV. In the DHS in 2011 the proportion ever tested for HIV in this subgroup was 15% in men and 17% in women and the proportion tested in the last year 8% and 9% compared to 20% and 34% in the population 15–49 years old overall (12).
People with acute symptoms (WHO stage 4, 3 or active TB) are assumed to have a higher chance of testing for HIV in that 3 month period and a higher chance of being linked to care once diagnosed. In 2015 the probability of testing as a result of symptoms was 0.8, 0.48 and 0.12 for people with a WHO stage 4 event, tuberculosis and WHO stage 3 event.
and DHS: Demographic and Health Survey;
show, respectively, the proportion ever tested for HIV and the proportion tested for HIV in the last year in 2006 and 2011, as assumed by the model and as observed in the DHS (12) in the different age groups (15–19, 20–24, 25–29, 30–39, 40–49 and overall) among men and women. These illustrations are based on the previous calibration, but we aim to have a similar outcome using the new calibration.
23.4.2. HIVST device and scenarios compared
The device considered is OraQuick In-Home Oral HIV Test, because it is the device for which there are more data available.
Scenarios to be compared (from 2017):
Standard of care: current rate of testing continues in the future (current HIV testing services are available including testing in pregnant women attending ANC, testing pre-circumcision, testing of symptomatic people and testing of the general population) without introduction of HIVST, indicated as a reference scenario (RS)
Secondary distribution: HIVST is given to women attending antenatal care (ANC) to bring the kit at home and test their male sex partner(s) (indicated in orange)
Pharmacy distribution: HIVST is introduced in pharmacies, adult men (aged 25 to 49 years old), young people (aged 15 to 25 years old) and FSW are eligible to access them (PB, indicated in grey)
Community-based - young: HIVST is introduced in young people (aged 15–25 years old) using a community based approach (CBY, indicated in green). HIVST is limited to this group.
Community-based - FSW: HIVST is introduced among FSW (male sex workers are not included, because not modelled in the Synthesis model and not much information available on them) using a community based approach (CBSW, indicated in pink). HIVST is limited to this group.
Community-based – adult men: HIVST is introduced among adult men (aged 25–49 years old) using a community based approach (CBM, indicated in blue). HIVST is limited to this group.
Community-based: HIVST is introduced among young people (aged 15–25 years old), FSW, adult men (aged 25–49 years old) using a community based approach (CBM, indicated in purple). HIVST is limited to these groups.
The main assumptions are indicated with the colour scheme indicated above and black if the assumption is the same regardless of the target group. The only assumptions that will vary by target group are those on the uptake of HIVST and the proportion of tests conducted using HIVST (indicated in ).
The shows the size of the populations benefitting from the introduction of HIVST, and the estimated HIV prevalence and incidence in 2017 in absence of HIVST (using the Synthesis Model).
Table 23.2ASize of the population benefitting from HIVST and HIV prevalence and incidence in 2017 (using the Synthesis model)
View in own window
| Population considered | Size in 2017 | % of the population 15–64 | HIV prevalence in 2017 | HIV incidence (per 100 py) in 2017 |
|---|
| Adults 15–64 | 8.02 m | 100% | 13% | 0.9 |
| Young 15–24 | 3.12 m | 39% | 3% | 0.6 |
| Adult men 25–49 | 2.00 m | 25% | 17% | 1.4 |
| Partners (15–49) of pregnant women | 179 k | 2.2% | 14% | 0.4 |
| FSW | 94 k | 1.1% | 31% | 12 |
| FSW not using condom consistently | 51 k | 0.6% | 48% | 22 |
FSW: female sex worker; py: person-year;
23.4.3. Assumptions relating to HIV self-testing (HIVST) uptake and substitution of HTS test with HIVST
shows the uptake assumed in the different target group, the level of substitution of HTS with HIVST and the assumptions on the mean number of tests performed over the 20 year time frame.
In the last few years the number of test performed in Zimbabwe has increased dramatically: 1.8 million tested in 2014, 2.2 million in 2015 (15) and the target for 2016 is 2.8 million (26). These have been based on the evidence from the literature published by November 2016 on the uptake of HIVST (see ) but taking into account the fact that if HIVST was introduced nationally it would be unlikely for the programme to reach the same uptake observed in research studies. We believe that if anything these assumptions are overestimates of what could be achieved on the ground.
Table 23.3AAssumptions on uptake of HIVST and level of substitution of HTS with HIVST
View in own window
| Delivery model | Population benefitting from HIVST introduction | Uptake of HIVST (% who HIVST per year) | Proportion of tests done using HIVST out of all test that would have been done in absence of HIVST | Number of tests in the population 15–64 (by HCW or HIVST) per year (mean over 2017–2036; in million)* |
|---|
| Secondary distribution | Partners (15–49) of pregnant women | 40% | No substitution, although men going for circumcision do not receive testing if tested or self-tested in the previous 3m | 3.72 |
| Pharmacy-based | Young people (15–24) | 5% | 20% | 4.03 |
| FSW | 25% |
| Adult men (25–49) | 20% |
| Community based | Young people (15–24) | 65% | 30% | 9.57 |
| Community based | FSW | 45% | 50% | 3.79 |
| Community based | Adult men (25–49) | 55% | 30% | 6.21 |
| Community based | Young people (15–24), FSW and adult men (25–49) | 48% | Assumptions above for community based | 12.24 |
FSW: female sex worker; HIVST: HIV self-testing;
- *
In the reference scenario is 3.69 million
23.4.4. Assumptions on the performance of HIVST, HTS and on the behaviour following the test result
summarizes the assumptions on the sensitivity, specificity, failure at performing the test and behaviour following an HIV test, whether positive or negative for the HIVST and with the standard HTS. For further details on the literature to inform these assumptions see .
Table 23.4AAssumptions on the performance of HIVST and HTS and the behaviour following the test result
View in own window
| Parameter | Value assumed for base case | Source |
|---|
| Sensitivity of HIVST | 93.9% | (27) |
| Specificity of HIVST | 99.2% | (27) |
| Sensitivity of HTS* | 98% | (28) |
| Specificity of HTS* | 99.2% | (29) |
| Failure at performing supervised HIVST | 0.3% | (30) |
| Failure at performing unsupervised HIVST | 0.3% | In (31) it was less than supervised HIVST, |
| Failure at performing HTS | 0.15% | Assumption** |
| Confirmatory HTS following positive HIVST | 80% by 1 year from positive HIVST | Evidence on disclosure from (32) |
| Change in sexual behaviour (SB) in those who are tested HIV+ by HTS | with long-term partner: −13%, with short-term partner: −17% in the first 6 months, −9% after | (33, 34) |
| Change in SB in those tested HIV- by HTS | No change (in sensitivity 5% increase in CLS with short-term partner and 10% with long-term) | (35) |
| Change in SB after HIVST | No change | It does occur only once they have the confirmatory positive HTS |
| CD4 threshold for ART | All diagnosed | Zimbabwe MoH |
HIVST: HIV self-testing; HTS: HIV testing and counselling; SB: sexual behaviour;
- *
assumed as facility based RDT;
- **
50% compared to HIVST. This was based on opinion of Cheryl Johnson and Carmen Figueroa from WHO
23.4.5. Assumptions relating to quality of life and costs of HIVST and HTS
In order to estimate the cost-effectiveness of a certain intervention it is necessary to estimate a measure of health benefit, in this case the number of disability adjusted life-years averted and the cost of that intervention. The disability adjusted life-years take into account the number of deaths averted as a consequence of a certain intervention but as well the quality of life of the years lived. People who do not have those condition are assumed to have a perfect health and so a disability weight of 0. summarizes the assumptions on the disability weights to be applied to certain conditions and the cost components of HIVST.
It is assumed there is no cost of targeting specific groups, but the service is available only for those groups. For community-based distribution it is assumed that 15% of first time HIVST users opt for supervised HIVST while the remaining and the subsequent HIVST are assumed to be unsupervised. The cost of HIVST per person tested will differ by delivery model (secondary distribution, community-based or pharmacy-based) and by whether it is the first HIVST (and therefore supervised in 15% of HIVST users if the distribution is community-based) or not, but not across different target groups.
The cost of an HIVST, regardless of the delivery model amounts to US$4.84 (US$3.5 for the kit, ~US0.84 for the international shipping, insurance, clearing of HIVST kit and ~US0.5 HIVST kit domestic distribution). In addition, there is a component of the overall cost of delivering an HIVST (indicated as “other cost components”) which is specific to each delivery model. The evidence to inform this assumption was very limited. We started from the fact that Maheswran and colleagues (4) estimated the fully loaded cost of supervised community-based HIVST to be US$8.78. We removed the cost of the kit, international shipping and domestic distribution (indicated as “Consumable and equipment” in of (4)) amounting to US$5.41 and added our estimate based on the current cost of the kit and the indication given by CHAI given a fully loaded cost for the supervised community-based HIVST. We have assumed in discussion with experts (Cheryl Johnson from WHO) that the cost of the supervision per HIVST was around US $1, this is envisaged to be the cost of pre-test counselling and post-test counselling. This gives an estimate for the “Other cost components of HIVST” for the community based approach of $2.37, for a fully loaded cost of the unsupervised community-based HIVST of $7.21. The secondary-based approached was considered to be the potentially cheapest way of delivering HIVST, so “other cost component” were assumed to amount to US$0.16 amounting to an overall cost of US$ 5 and the pharmacy based to be an intermediate cost between the two (US$6).
The cost estimate of HIV testing and counselling, for comparability, was taken from the same study that had evaluated the cost of community-based HIVST: US $8.89. As you can notice, this cost is only slightly higher than the cost per HIVST, However, there is evidence from Zimbabwe that cost of community based HIV testing performed by HCW is significantly higher (US $24.5) and this was taken into account. Other sources have reported significantly lower cost for HTS.
Both costs and disability adjusted life years are discounted at 3.5%.
Table 23.5AKey parameters relating to quality of life and costs of HIVST
View in own window
| Parameter | Value assumed | Source |
|---|
| Disability weights | WHO 4 event: 0.55;
Acute TB: 0.40; WHO 3 event: 0.22 | (36) |
| In 3% with HIVST (for 3m): 0.2 | Assumption |
| HIV self-testing | OraQuick In-Home HIV test kit~ | US$ 3.5 | (37) |
| international shipping, insurance, clearing of HIVST kit~ | US$ 0.84 (24% of kit cost) | (38) |
| HIVST kit domestic distribution~ | US$ 0.5 (15% of kit cost) | (38) |
| Other cost components of HIVST* | Secondary dist. | US$ 0.16 (Tot: US$ 5) | Assumption |
| Pharmacy-based | US$ 1.16 (Tot: US$ 6) | Assumption** |
| Community-based | US$2.37 (Tot: $7.21) | Assumption |
| Additional cost of supervised vs unsupervised*** | US$ 1 (Tot: $ 8.21) | (4) |
| HIV testes tana counselling | OraQuick In-Home HIV test kit~ | US$ 8.89 | Facility-based HTS in (4) |
| international shipping, insurance, clearing of HIVST kit~ | US$ 24.5 | PSI Zimbabwe 2015 (39) |
| Cost of HIV testing pre-circumcision | Same as facility-based HTS | Assumption |
| Cost of HIV testing performed in ANC | Same as facility-based HTS | Assumption |
| Additional cost if the results is positive (including false positive) | $5.1 (Total cost of HIV positive test $14 if facility based; ~29.6 if community based) | Assumption |
- ~
apply to all delivery models;
- *
Staff salaries; Overheads (Infrastructure); Quality Control for HIVST; Training; Monitoring and evaluation; Indirect cost (time off work for person attending or accompanying);
- **
Mugo suggested an estimate of US$4.41 for the other cost;
- ***
it applies only to 15% of first CB HIVST;
Table 23.6AKey parameters relating to costs other than HIVST and HCT
View in own window
| Parameter | Value assumed | Source / explanation |
|---|
Drug costs per year (including supply chain):
First-line: tenofovir/3TC/nevirapine
Second-line: zidovudine/3TC/atazanavir |
$136
$317 | Untangling the web of antiretroviral price reductions. 17th Edition – July 2013. www.msfaccess.org. |
Cost of treatment of a WHO stage 4 condition over 3 months (cost is incurred for 3 months)
Cost of treatment of a WHO stage 3 condition over 3 months (cost is incurred for 3 months)
Cost of treatment of TB per 3 months (cost is incurred for 6 months)
Cotrimoxazole annual cost | $200
$20
$50
$5 | Specific data not available on average unit costs of treating WHO stage 3 and 4 conditions and per clinic visit costs - costs used are informed by evidence synthesis from studies that cost according to current CD4 count of those in pre-ART care, cost of ART initiation, which also include costs of CD4 tests (40) |
| CD4 count measurement | $10 | (41, 42) |
| Viral load measurement: | $22 | Human resource costs $3, sample collection consumables $2, relaying of results $2 (this costing information was provided by Medecin Sans Frontiers (MSF) (43), with the most recent information update October 2014), running the test (including equipment and other costs such as consumables, maintenance and shipping) $15 ((44, 45) |
| Non-ART programme costs per year, $40 per year if on tiered care due to viral load < 1000 | $80 | Bill and Melinda Gates Foundation tiered care meeting report (the per client cost of running the Khayelitsha adherence clubs was $58 per client per year compared to standard clinic care of $108 per client per year. At the Infectious Disease Institute in Kampala, the annual costs per client for physician, nurse, and pharmacy only visits were $60, $45, and $19, respectively). |
| Cost of voluntary medical male circumcision | US $100 | Estimates vary from $61 (46) to $125 (47) |
Table 23.7ASummary of the literature to inform the main assumptions on implementation of HIVST
View in own window
| Item reported in the literature | Literature | Source |
|---|
| Uptake of HIVST using secondary distribution approach | Kenya HIV negative women 18–39 at ANC, post-partum clinic (PPC) distributing HIVST to male partner:
Women at ANC (18–29) – 91.6%
Women at PPC (18–29) – 88.8%
FSW at Drop in Centers-87.1%
Women at ANC 18–39 – 62% self-tested
Women at PPC 18–39 – 76.4% self-tested
FSW at drop-in centre 18–39 – 71% self-tested | (48) |
| Uptake of HIVST using a PB approach | Kenya
Uptake-Prelim Data Pharmacy adults
32% uptake of HIVST- 76% of those eligible for and who received HIV testing at pharmacy with counsel also accessed HIVST
Male 18+: 31% Female 18+: 35%
Age 18–24: 42% Age 25–35: 36% | (49) |
2 provinces in Kenya (1 rural and 1 urban)*:
FSW 95 % would procure and perform the test on their own; 75 % preferred to obtain the kits from private chemists/pharmacies | (50) |
| Uptake of HIVST using a community based approach | Malawi (Gen Pop)
76.5% in year 1, 70.9% following 6m | (3) |
Malawi (Gen Pop)
91.7% - opted for supervised HIVST | (51) |
Zimbabwe:
70% chose HIVST when option of HIVST or HTS by HCW | (52) |
Kenya (15–49 year old men and women)
91% of respondents were willing to use an oral fluid-based HIVST kit compared to other HIV testing alternatives. | (53) |
| Lesotho (Basotho women and men 15–49, n=45): 75% self-tested | (54) |
2 provinces in Kenya (1 rural and 1 urban)*: likely users of HIVST kits include those who have ever tested, but 87% of general population, and over 90% among MSM and FSWs had ever tested.
FSW 95 % would procure and perform the test on their own; 75 % preferred to obtain the kits from private chemists/pharmacies; 53 % in government facilities and 13 % in supermarkets/shops | (50) |
India (General population, sex workers and high risk populations)
54% HIVST uptake | (50) |
| China: HIVST acceptability rate among FSW is 72.1% | (56) |
| Zimbabwe: 62.9% of HIV-negative FSW reported being very interested in HIVST, and 67.6% of HIV-positive women would have preferred to test themselves rather than be tested by a health care worker if a simple-to-use home HIV test had been available to them. | (57) |
Malawi
1–12 Month Uptake among Men (16–49) 70.8% (5603/7910) | (3) |
Malawi
1–12 Month Uptake Young people
< 20 (22.2%)
20–29 (45.6%)
16–29 (67.8%)
13–24 Month Uptake Young People
< 20 (24.7%)
20–29 (46.3%)
16–29 (71%) | (3) |
| USA: Uptake in young adults (n=21, 18–24) recruited in bars, dance clubs, community events and community-based organizations in New York | (58) |
USA (African American young people/adolescents)
65% favoured HIV self-testing and 76% favoured it for repeat testing | (59) |
| Peru: Nearly 82% of participants were willing to self-test (95% transgender women and 78% MSM) | (8) |
| Singapore (200 known HIV-positive patients and 794 unknown HIV status at-risk participants): 87% would purchase the kit over-the-counter; 89% preferred to take HIV tests in private. | (30) |
| Kenya (healthcare workers): 89 % took the HIVST kits and of those, 85 % of those reportedly self-tested | (60) |
South Africa (50 lay users were purposively selected in rural KwaZulu-Natal)
Majority of participants said they would self-test again if it was free (98%) with most being willing to buy a test (86%). | (61) |
| Proportion of tests done using HIVST using a FB approach | Kenya:
Women at ANC – 98% had HIV test in last 12 months & 82% of primary male partner had an HIV test in last 12 months
Women at PPC (18–29)- 97% had HIV test in last 12 months & 64% of primary male partner had an HIV test in last 12 months
FSW at Drop in Centers- 100% had HIV test in last 12 months & 70% of primary male partner had an HIV test in last 12 months | (48) |
| Proportion of tests done using HIVST using a PB approach | No data available | |
| Proportion of tests done using HIVST using a community based approach | Kenya (formative research 3ie): 80% of respondents had tested for HIV before- 91% said they would purchase an HIVST kit | (62) |
Kenya (15–49 year old men and women)
91% of respondents were willing to use an oral fluid-based HIVST kit compared to other HIV testing alternatives | (53) |
| Kenya (240 adults from general population):90% of participants had tested for HIV before, 94% found HIVST acceptable | (63) |
| Malawi (Gen Pop) 216 participants who self-tested, 137 (63.4%) had previously tested for HIV, with 47 (21.8%) having tested in the past twelve months. | (51) |
Malawi (Gen Pop)
1–12 Mo 72.7% of HIVSTers had not tested for HIV in past 12 months
1–12 Mo 35.1% of HIVSTers had never tested for HIV before
13–24 Mo 38.8% of HIVSTers had not tested for HIV in the past 12 months
13–24 Mo 17.8% of HIVSTers had not tested for HIV before | (3) |
USA (n=161 MSM)
56.5% preferred HIVST, 23.6% preferred facility testing | (64) |
| Singapore (n=350 systematically sampled participants across 2 HIV testing centers): 89% preferred HIVST in private to other testing | (65) |
USA Gen Pop
Participants who requested a HIVST kit had longer duration since last HIV test than those who did not (greater than 6 months, 48.4% vs. 26.7%). More participants who requested HIVST kit (27.8%) than those who did not (9.7%) were unsure about their current HIV status. | (66) |
| MSM China- Among those who had used HIVST, 58.7% (200/341) reported their first-time HIV test using HIVST. | (67) |
Zimbabwe - FSW
67.6% of HIV-positive FSW would have preferred to test themselves rather than be tested by a health care worker if a simple-to-use home HIV test had been available to them. | (57) |
| Sensitivity of HIVST | 93.9% (pooled estimate)
fingerstick/whole blood-based RDTs: 96.4%-98.8%
oral fluid-based RDTs: 66.7%-97.9% | (27).** |
| Specificity of HIVST | Range across studies: 94.7%-100% | (27) |
| HIVST kit failure | Supervised HIVST ranges from 0.3% (30) up to 56.3% (65). When excluding (65), as suggested by Figueroa, the upper limit is 3.5% (56). When restricting to Ora-Quick the upper limit is 3.2% (52) | (68) |
| Unsupervised HIVST ranges from 0.2% in (31) and Phase III FDA up to 15.1%. When excluding (63), as suggested by Figueroa, the upper limit is 7.9% (69), 4.1% (69) when restricting to Oraquick and excluding (63) |
| Supervised and unsupervised HIVST ranges from 0.8% (70) up to 8.3% when using Determine Combo (71) |
| Sensitivity of HTS (assumed the same as facility based RDT) | 98% | (28) |
| 97.6% to 100% sensitivity | (29) |
Cameroon
Algorithm 1 sensitivity 94.7%, Algorithm 2 sensitivity 100% | (72) |
Uganda
Sensitivity: 97.3% (95% CI: 96.0–98.3) (weak reactive excluded)
Sensitivity: 97.4% (95% CI: 96.1–98.4) (weak reactive included) | (73) |
Uganda
Sensitivity: 98.6% (95% CI: 91.4 to 99.9); 1 false-negative results | (74) |
Uganda
Sensitivity 97.7% (95% CI: 94.1%-99.4) | (75) |
Botswana
Sensitivity 100.0% (95% CI: 88.8 to 100.0)
Sensitivity 98.1% (95% CI: 96.5 to 99.1) | (76) |
| Specificity of HTS | 100% | (28) |
| 95.3% to 100% | (29) |
Cameroon
Algorithm specificity 98.8%, Algorithm 2 specificity 91.5% | (72) |
Uganda
99.9% (95% CI 99.6–99.99%)
99.7% (95% CI 99.3–99.9%) | (73) |
Uganda
99.9% (95% CI, 99.76 to 99.96%); 5 false-positive results | (74) |
Uganda
90.4% (95% CI: 88.7%-91.9%). | (75) |
Botswana
Specificity 98.5% (95% CI: 96.2 to 99.6)
Specificity 98.2% (95% CI: 96.4 to 99.3) | (76) |
| Probability of having a confirmatory test as a consequence of a positive HIVST | Around 80% disclosure HIV positive result using HIVST | (32) |
Kenya
100% male partners went for confirmatory testing following reactive HIVST | (48) |
| Linkage to care following diagnosis | % of people who received CD4 count results or clinical staging by one year since diagnosis was 59% (median; range: 35%-88%) | (77) |
| Linkage to care following diagnosis (consequence of a positive HIVST) | Linkage to care among people found HIV-positive through HIV self-testing and who were not already on ART was 56.4% | (3) |
| In the previous modelling exercise we have assumed that the linkage to care, once diagnosed, is the same regardless of whether the first positive test was an HIVST or not. | (9) |
Kenya
89.2% of male partners enrolled in care after confirming HIV positive diagnosis after initial reactive HIVST result | (48) |
| Change in sexual behaviour following positive HIV test | OR=0.61 (95%CI: 0.37–0.997; p=0.048) of reporting increased number of partners,
OR=3.24 (95%CI: 2.29–4.58; p<0.001) of reporting condom use vs those who did not receive VCT | (33)*** |
| Change in sexual behaviour following negative HIV test | No evidence of a higher odds of reporting increased number of sexual partners or decreased condom use when comparing participants who received VCT to those who did not | (34) |
| Change in sexual behaviour following positive HIVST | Sexual intercourse was significantly less likely after a
sexual partner self-tested HIV-positive versus HIV-negative (18% vs. 62%, p<0.0001), while condom use was significantly more likely (100% vs. 44%, p=0.0018). | (48) |
| Change in sexual behaviour following negative HIVST | Sexual intercourse was significantly less likely after a
sexual partner self-tested HIV-positive versus HIV-negative (18% vs. 62%, p<0.0001), while condom use was significantly more likely (100% vs. 44%, p=0.0018). | (48) |
| Psychological negative consequences of HIVST | 2.9% (95% CI 2.6%-3.2%) reported being “forced to test” but HIVST satisfaction high (94.4%) and no HIVST-related partner violence or suicides reported | (3) |
IPV occurred 4 times as a result of distributing HIVST
2 in women from PPC and 2 from FSW | (48) |
- *
It included general population (782 sexually active men and women aged 18 to 49), MSM (n=100), FSW (n=100). FSWs reported a preference for private clinics or pharmacies (74%) compared to public sector clinics (54%) and approximately 20% of the general population and 15% of key populations preferred procuring a kit at a supermarket/shop.
- **
In the abstract referenced some values are slightly different because the methods used to calculate them slightly changed.
- ***
(containing (35) (Manicaland, Zimbabwe) & (78) (Thailand.)) Same findings in Fedor et al. 2015 (Malawi)
Note: Regarding the frequency of HIVST, we found only one study that reported on frequency of HIVST. This is among MSM in Seattle and found that those with access to HIVST tested for HIV more frequently, with 76% testing at least every 3 months, compared to 54% of those in control.
23.5.1. Epidemiological impact
Without the introduction of HIVST (see “Reference” scenario in light blue in ) the (mean) proportion of people who tested for HIV in the last year over the next twenty years is around 0.38. The introduction of HIVST through secondary distribution to partners of pregnant women (in red in ), through pharmacies (in grey in ), or through community-based approached but only to FSW (in purple in ), does not affect significantly the proportion of the whole population living in Zimbabwe aged 15–64 who tested in the last year, simply because through these strategies only a limited number of people have access to HIVST (see ). By targeting larger groups such as adult men aged 25–49 (in dark blue in ), young people (in green in ) or these two groups and FSW (in violet in ), and using the community-based approach which allows to achieve high uptake, the proportion assumed to be tested in the last year is significantly higher, respectively 0.47, 0.57 and 0.68.
These correspond to mean proportion ever tested for HIV varying from around 0.7 (“Reference” scenario) up to around 0.9 in the widest scenario, where young people, 25–49 adult men and FSW are receiving HIVST through a community based approach.
Within the Synthesis model people who are diagnosed with HIV, by which we mean that have a positive test result using HTS, performed by a health care worker, cannot be tested again. They can be linked to care within 3 months or later and once in care they can be lost from care and come back to it. Therefore, with the term HIVST positivity rate we mean the ratio between the number of positive HIVST out of all the HIVST performed and in order to be eligible to use an HIVST people cannot have been diagnosed before.
The HIVST positivity rates for the difference scenarios are illustrated in . It is evident, and not surprising that the HIVST positivity rate is highest (0.13) when targeting FSW, given the high HIV incidence in this group (See ). This is followed by targeting partner of pregnant women and again this is not surprising as they are sexually active men not consistently using condom and it is know that adult men are generally low rate of testing compare the rest of the population.
By testing more people and testing them more frequently a greater proportion of people are diagnosed. This means that they experience a slight reduction in condomless sex (see assumption in ) and a greater proportion of people receives treatment. These have the following benefits:
- -
PLHIV diagnosed with HIV have reduced chance of transmitting because the CLS is reduced
- -
People on ART with viral load suppressed have very low chances of transmitting HIV
- -
Mortality in PLHIV is reduced if they are timely diagnosed and start receiving ART.
Through the first two mechanisms by introducing HIVST HIV infections are averted (see ). The greater number of HIV infections averted per year is around 670 per year and is in the scenario where more people are receiving HIVST. However the number of HIV infections averted does not fully mirror the number of HIVST (see ), as for example the scenario characterized by the lowest number of HIVST is the scenario where HIVST is available using a secondary distribution approach but this allows saving a greater number of HIV infections as it is in a group with a higher HIV incidence than for example the group that is assumed to access HIVST through pharmacies, which has been assumed here to be a random sample of young people, 25–49 adult mean and FSW. We are planning to modify this assumption as more evidence is accumulated.
23.5.2. Cost-effectiveness evaluation
23.5.3. Base case
As mentioned, the aim of this work was to evaluate the cost-effectiveness of introducing HIV self-testing (HIVST) in the context of Zimbabwe in specific groups of the population considering different HIVST delivery model(s). In order to address this we have used the HIV Synthesis model to estimate the health benefit over a 20 year time horizon, in terms of disability adjusted life-years (DALYs). These take into account the HIV infections averted, the deaths averted and the quality of life of the life years lived (See disability weights applied indicated in ) In terms of costs, we have calculated the cost accumulated over the next 20 years, by applying the unit costs to the items indicated in and . As mentioned, a health care payer prospective was taken, so only the cost incurred by the health care provider were included and both the costs and DALYs were discounted at 3.5% per year.
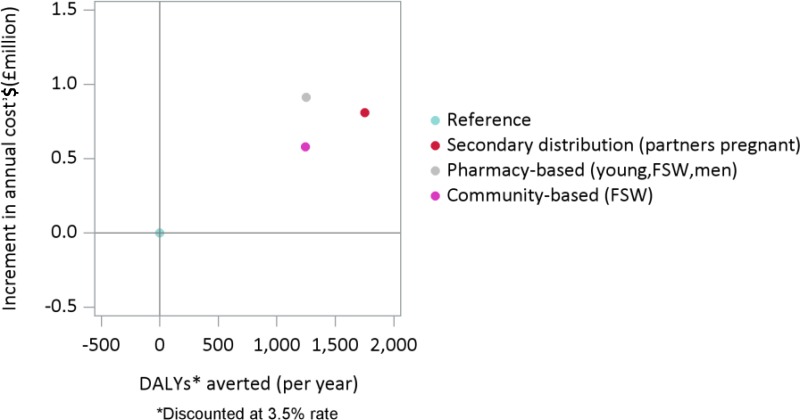
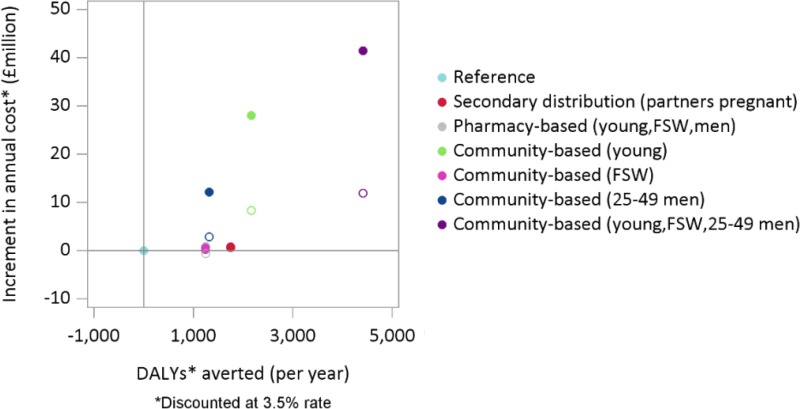
shows on the horizontal axis the discounted DALYs averted per year compared to the reference scenario (indicated with a light blue dot) and on the vertical axis the increment in annual cost, again compared to the reference scenario. By introducing HIVST using a community based approach for the next 20 years to young people adult men and FSW (indicated in violet) a substantial health benefit can be produced (4,400 DALYs averted per year), but this costs an additional US$41 million. This corresponds to an incremental cost-effectiveness ratio (ICER) of US$ 9,300 per DALY averted (US$ 41m/4,400). The scenarios where HIVST is introduced through a community based approach only to 25–49 adult men (in blue) or to young people (in green) have similar ICERs.
shows a detail of the cost-effectiveness plane illustrated in . The introduction of HIVST through secondary distribution (in red), pharmacy based approach (in grey) or a community based approach restricted to FSW produces a health benefit but lower than the benefit that could be achieved with a wide introduction. However, given the number of HIVST is significantly lower the additional costs is more limited. The estimated ICERs associated with these scenarios are respectively: $US 620 per DALY averted for the pharmacy based approach, $US 467 per DALY averted for the community based approach targeted to FSW and pharmacy based approach and $US 462 per DALY averted for the secondary distribution approach.
23.5.4. Univariate sensitivity analyses
In addition we considered a few univariate sensitivity analysis for the scenario where HIVST is introduced through community-based approach in young, FSW and 25–49 adult men (indicated in violet). The scenario indicated as “base case” in corresponds to the scenario indicated above in . The reason for conducting univariate sensitivity analysis on this scenario was due to the fact that this scenario is characterized by the highest number of HIVST and therefore is characterized by less stochastic variability.
First of all we considered increasing (doubling) the level of substitution of HTS with HIVST up to 60%. As can be seen see a higher level of substitution results in a lower number of discounted DALYs averted per year compared to the base case (3,800 vs 4,400) due to the less optimal performance of an HIVST compared to an HTS: the slightly lower sensitivity and the fact that in order to be diagnosed people who have a positive HIVST need to have a confirmatory HTS in order to be diagnosed.
Secondly, we considered a higher sensitivity for the HIVST (98% instead of 93.9%). This resulted in a slightly improvement in the health benefit (4,500 vs 4,400), but as well a higher additional cost and this is due to the fact that more people are correctly diagnosed and therefore put on treatment.
Thirdly, we considered a hypothetical scenario where it is assumed people with a positive HIVST have a 100% chance of having a confirmatory HTS and therefore to be diagnosed if living with HIV and 100% chance of being linked to care. This has a substantial beneficial effect on the number of DALYs averted per year (6,200, 40% more than in the base case) and only a slight higher additional cost (US$ 44 million, 7% higher than the base case). However with an ICER of US$7,100 per DALYs averted, this intervention is still far from being cost-effective. This sensitivity analyses shows that it is not HIVST per se (and the fact that it requires confirmatory test to provide a diagnosis) that is not cost-effective, but it is challenging for any forms of wide increase in HIV testing in a setting such as Zimbabwe with an already high proportion diagnoses to be cost-effective.
Finally we considered the potential benefit of HIVST to increase the demand for male circumcision. In particular, we investigated the impact of introducing HIVST to young, 25–49 adult men and FSW, assuming that 20% of men aged 15–30 who receive a negative HIVST get circumcised if HIV negative. This improves the cost-effectiveness of such an interventions even further reducing the ICER to US $5,600 underlying the importance to link not only HIV positive people but as well HIV- negative people in order to make this intervention as cost-effective as possible.
23.5.5. Sensitivity analysis on the cost of HIVST
Finally, we considered the impact of reducing the overall cost of HIVST kit from US$4.84 (US$3.5 for the kit, ~US0.84 for the international shipping, insurance, clearing of HIVST kit and ~US0.5 HIVST kit domestic distribution) to US$1.5. (See ). shows the cost-effectiveness of all the scenario as in the base case (see ), indicated with full circles and the cost-effectiveness of the same scenarios but assuming a substantial lower overall cost of the HIVST kit, indicated with empty circle. In this circumstance the ICERs corresponding to the largest introduction of HIVST (in violet) is reduced to US$2,700.
shows a detail of , for the scenario characterized by a relatively small introduction of HIVST and similarly to the graph above the empty circles indicate the cost-effectiveness assuming a reduced overall cost of the HIVST kit. The estimated ICERs associated with these scenarios are now respectively: $US 202 (instead of US$ 467) per DALY averted for the community based approach targeted to FSW and $US 364 (instead of US$ 462) per DALY averted for the secondary distribution approach. While the introduction of HIVST through a pharmacy based approach becomes cost-saving leading to a health benefit and reduction in the overall discounted cost over the next 20 years.
23.6. Summary and Conclusions
In conclusion, we found that given the low prevalence of undiagnosed HIV in Zimbabwe any substantial increase in relatively untargeted HIV testing it is unlikely to be cost-effective. This is an issue not specific to HIVST, because as shown in univariate sensitivity analysis the large introduction of HIVST even if all those with a positive HIVST results had a confirmatory HIVST and linked to care this would still not be cost-effective.
Secondly, given the current cost assumed per HIVST performed (US$ 4.8 for the kit, the international shipping, insurance, clearing of HIVST kit and HIVST kit domestic distribution), the introduction of HIVST is cost-effective (considering a cost-effective threshold of US$500) when considering secondary distribution, community distribution for FSW and borderline for pharmacy distribution (US$620 per DALY averted).
The cost of HIVST kit play an important role and if it can be reduced to US$1.5 (from current US$4.8), its introduction is not only cost-effective but cost-saving when considering pharmacy-based distribution approach, even if not targeted.
Improvements in the chance of having a confirmatory test following a positive HIVST & linkage to care and potential linkage to male circumcision for men with a negative HIVST aged 15 to 30 would improve the cost-effectiveness of HIVST.