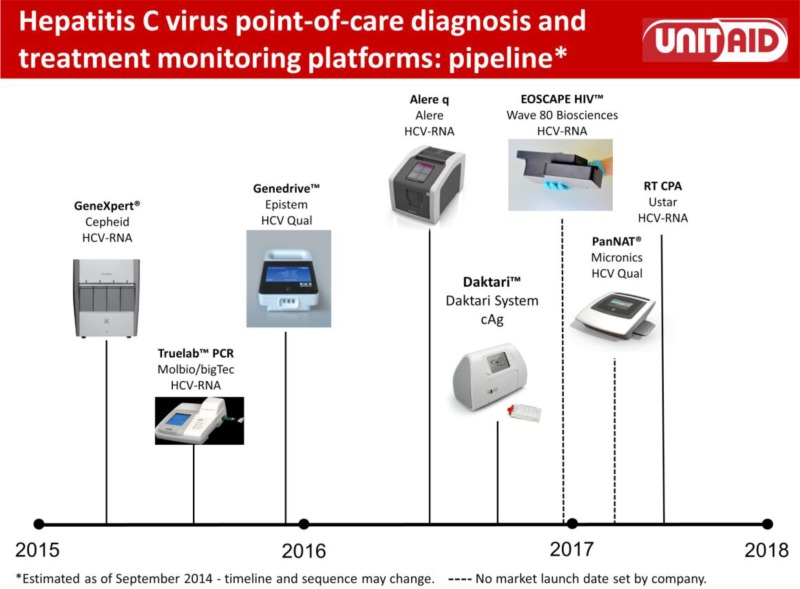
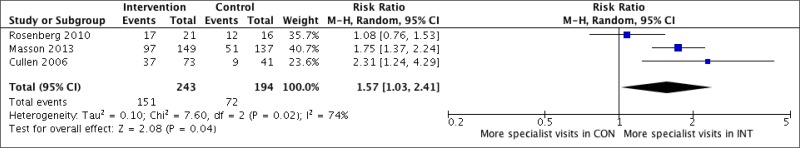
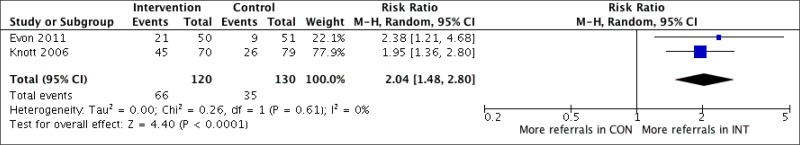
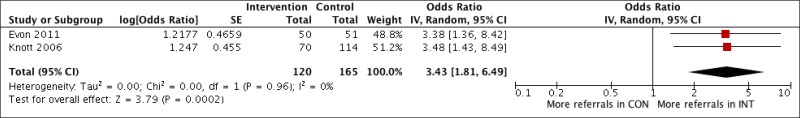
What is the impact, cost and cost–effectiveness of different HCV testing approaches and scenarios?
Topic for analysis: who to screen?
Population
Risk-based screening in different high-risk populations
Injecting drug users (IDUs), men who have sex with men (MSM), immigrants, recipients of blood transfusion and blood products, sex workers, and health-care workers (HCW), HIV-infected persons
General population (excluding blood donors) or selected subpopulations of general population (women during pregnancy, those with raised alanine aminotransferase (ALT), infants, schoolchildren and adolescents)
Other approaches: Birth cohort screening (based on different age cut-offs; born between 1945–1960 or 1965 or 1970)
One-off screening vs repeat screening every five years.
Intervention
Testing strategies for HBV in different populations (risk-based and general population and birth cohort); and at different prevalence thresholds
Comparator
No testing or current practice or comparison of different testing strategies
Outcomes
Benefits, harms and costs, and cost-effectiveness with different screening strategies for different target populations
Individual patient outcomes: Number of cases detected, overall mortality, liver-related mortality, cirrhosis, end-stage liver disease, rate of hospitalizations, serious adverse events, quality of life
Prevention: New infections (mother to child, horizontal (IDUs needle sharing and sexual; and sexual, especially MSM)
Cost-effectiveness: Cost and incremental cost per case diagnosed; cost and incremental cost per case screened and treated; cost and incremental cost per life saved; cost and incremental cost per infections averted; quality-adjusted life-years (QALYs) gained
Background
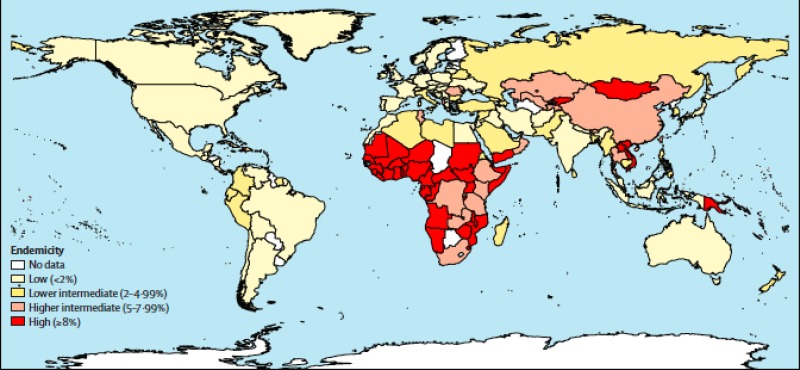
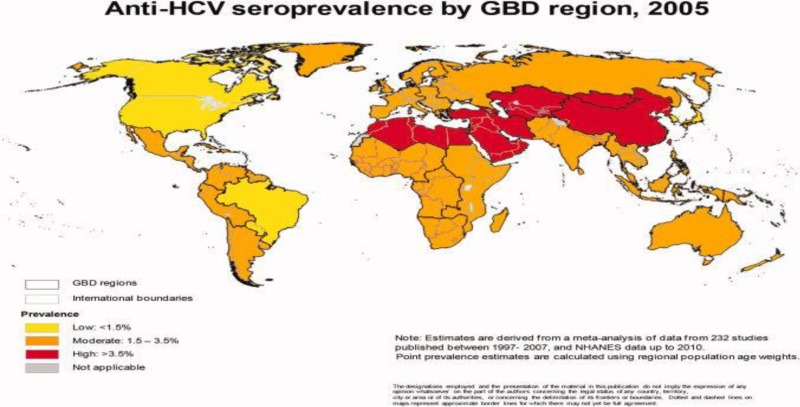
Hepatitis C virus (HCV) is a global public health burden and major cause of morbidity and mortality including liver failure and hepatocellular carcinoma. Current global HCV seroprevalence is estimated to be 2.8%, or >185 million infected individuals worldwide.
Routes of transmission
In many countries, HBV, HCV and HIV transmission occurs predominantly in high-risk key populations, often via common routes of transmission. Key populations include people who inject drugs (PWID), people in prisons and other closed settings, some mobile populations, some indigenous populations, MSM and sex workers. PWID are a key population who are at particularly high risk of HCV infection. In many high-income countries and some developing countries, ongoing HCV transmission is driven mainly by PWID populations.
The advent of high-efficacy, low duration therapy, however, generates prioritization for testing for HCV infection, linking infected patients to care, and curing HCV before patients begin to experience the consequences of cirrhosis and end-stage liver disease.
Low rates of diagnosis and linkage to care
The majority of people are unaware of their HCV infection, and therefore often present with advanced disease. Based on still limited studies, overall <15% of the estimated 180 million who are chronically infected with HCV are aware of their diagnosis, based on data from higher-income settings - United States, Europe and China.
In addition to the very low access to and uptake of testing, there is also further attrition on the care cascade with very poor linkage to care and therefore treatment, among those who test positive.
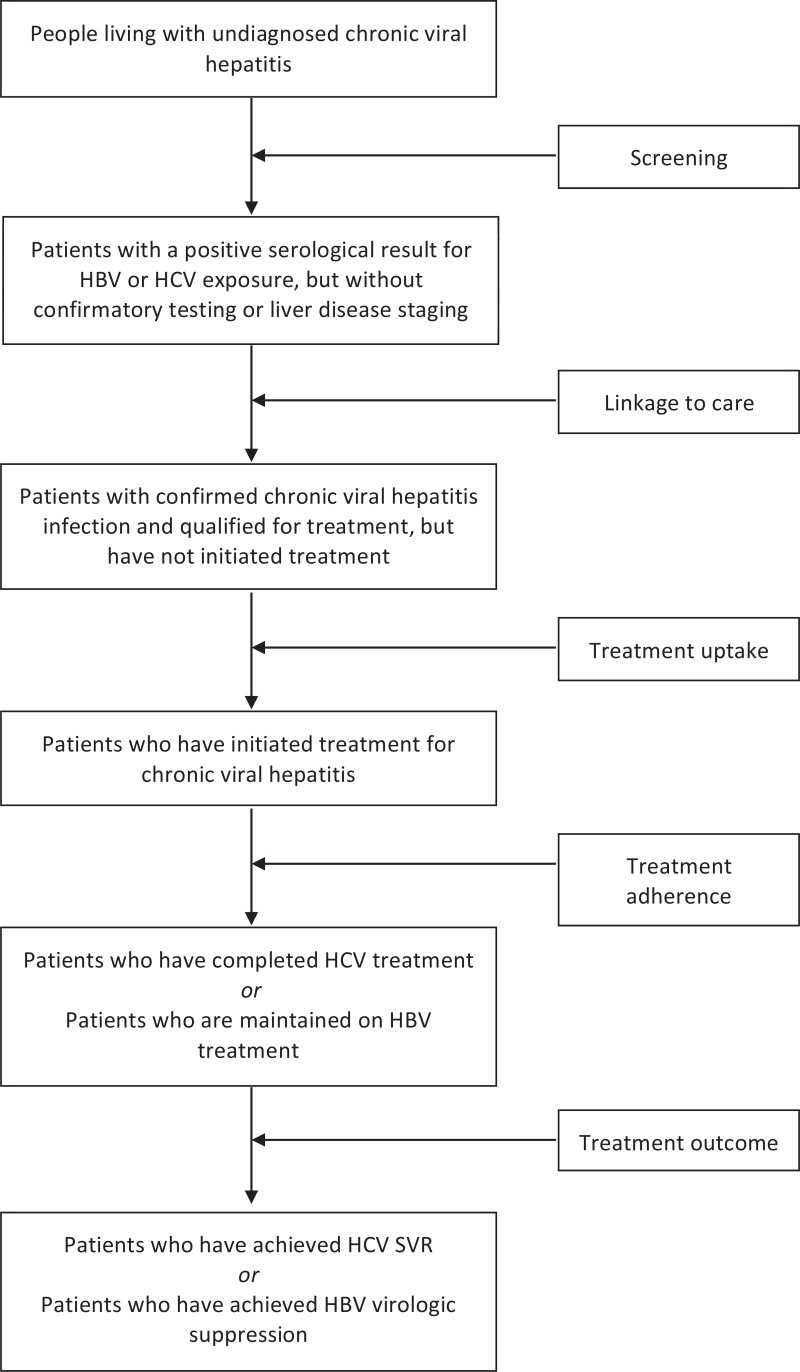
Hepatitis B and C testing and diagnosis are at the core of entry to both the prevention and treatment cascade. Testing is required to identify those with are positive, linking them with care, counselling them on measures to reduce transmission to others then assessing who needs treatment, initiating treatment, achieving treatment response (sustained virological response [SVR] for hepatitis C). Hepatitis testing is also needed to identify those who are negative, to provide hepatitis B vaccination, and the opportunity to implement individual or facility level prevention measures counsel to reduce risk behaviours.
There are three key approaches to HCV screening
Targeted risk factor-based screening. This refers to screening of specific groups including key populations, who are generally at higher risk of being infected than the general population. This includes PWID, people in prisons and other closed settings, migrant populations, some indigenous populations, MSM and sex workers, but may also include health-care workers. People attending services providing care and treatment for viral hepatitis or HIV can be encouraged to bring their partners to be tested.
This involves screening those with high-risk behaviours, exposures and other conditions. Most countries have based their list of high-risk groups as defined by the Centers for Disease Control and Prevention (CDC), and are largely based on known modes of transmission.
Family members and household contacts of hepatitis B patients
MSM
PWID
HIV-positive patients
Patients on immunosuppression or chemotherapy
Persons with liver disease of unclear etiology
Health-care workers.
Birth cohort screening for HBV and HCV
Population or community-based screening (including antenatal). Routine general population screening: i.e. testing among the general population without attempt to identify high-risk behaviours or characteristics (“routine testing”). This means that all members of the population have access to the screening programme under consideration. It may also include home-based testing (house to house); campaigns (e.g. HTC plus - malaria, safe water, non-communicable diseases (diabetes and hypertension); outreach (mobile) in general and key populations; workplaces and schools; and health-care facility-based screening.
Health-care facilities. Testing could also be offered in special dedicated clinics, e.g. HIV, STI clinics. Screening at health-care facilities may include primary care settings, inpatient and outpatient settings, and may involve screening on the basis of clinical presentation or focus on only those with abnormal liver function tests, abnormal ultrasound scan, family history of liver disease or other clinical suspicion of liver function test.
Persons in whom there is clinical suspicion of viral hepatitis: even when risk factors for HBV and/or HCV are not present, screening is indicated wherever there is clinical suspicion of viral hepatitis infection. This may occur, for example, where there is existing liver disease, including liver cirrhosis or hepatocellular carcinoma, or where there is unexplained liver disease including abnormal liver function tests.
Existing guidelines: what are countries doing?
The main approach to HCV testing is a targeted risk factor-based testing for those with high-risk behaviours, exposures and other conditions, e.g. (PWID, liver disease, renal dialysis) and use of a birth cohort approach in the US and Japan. At present, no country guidelines recommend routine testing for all individuals regardless of demographics or specific behavioural risk.
Screen those with high-risk behaviours, exposures and other conditions. Most countries have based their list of high-risk groups as defined by CDC, and are largely based on known modes of transmission.
Family members and household contacts of hepatitis B patients
MSM
PWID
HIV-positive patients
Patients on immunosuppression or chemotherapy
Persons with liver disease of unclear etiology
Health-care workers.
Survey of guidelines (Surjo De)
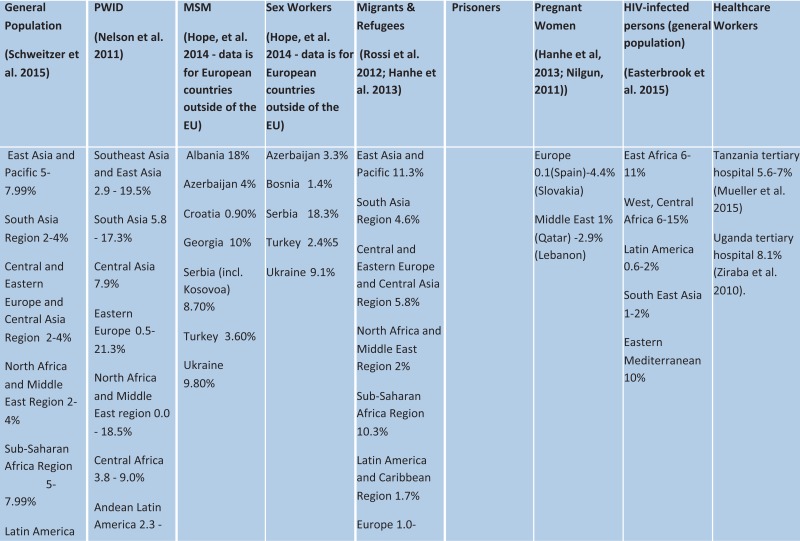
Global prevalence of hepatitis C virus
View in own window
| General population (Hanafiah et al. 2013) | PWID (Nelson et al. 2011) | MSM (Hope, et al. 2014 – data is for European countries outside of the EU) | Sex workers (Hope, et al. 2014 – data is for European countries outside of the EU) | Migrants and refugees (Hanhe et al. 2013) | Prisoners (Larney et al. 2013) | Pregnant women (Hanhe et al., 2013) | HIV-infected persons | Health-care workers |
|---|
Southeast Asia 2%
East Asia 3.7%
Oceana 2.6%
South Asia 3.4%
Central Asia 3.8%
Central Europe 2.4%
Eastern Europe 2.9%
North Africa and Middle East region 3.6%
Central Africa 2.3%
East Africa 2%
Southern Africa 2.1%
West Africa 2.8%
Andean Latin America 2%
Central Latin America 1.6%
Southern Latin America 1.6%
Tropical Latin America 1.2%
Caribbean 2.1%
Asia Pacific High income 1.4%
Australasia 2.7%
Western Europe 2.4%
North America High Income 1.3% | Southeast Asia and East Asia 41–89.8%
South Asia 36.0–87.3%
Central Asia 51.7–61.3%
Eastern Europe 22.6–90.5%
North Africa and Middle East region 28.–67.6%
West Africa 22.2 - 97.3%
Andean Latin America 9.8 – 97.4%
Australasia 51.9–54.6%
Western Europe 20.7 – 86.2% | Azerbaijan 14%
Bosnia 12%
Croatia 3%
Georgia 16%
Kazakhstan 4.20%
Kyrgyzstan 1.20%
Republic of Moldova 11%
Russian Federation 2.30%
Ukraine 20% | Azerbaijan 9.30%
Bosnia and Herzegovina 4.30%
Croatia 4%
Kazakhstan 11%
Kyrgyzstan 3.9–28%
Republic of Moldova 13%
Russian Federation 14–40%
Serbia 3.30%
Tajikistan 6.30%
Turkey 2.40%
Ukraine 32%
Uzbekistan 11–12.8% | Europe 0–23.4%
(Hungary) | SSA 7–26%
Western Europe 26–34%
Eastern Europe 14–31%
Latin America 8–19%
Australasia 28–43%
North America 24–34%
South Asia 4–11%
Middle East and North Africa 1–5%
East and SE Asia 13–38%
Central Asia 32–43%
Extrapolated global 23–29% | Europe 0
(Slovakia) −1.7%
(Italy) | | United States Hospital workers 1%
(Alter, 1997)
Egypt National Liver Institute HCWs 16.8%
(Adelwahab et al. 2013) |
Global epidemiology of hepatitis C virus infection: new estimates of age - specific antibody to HCV seroprevalence (Hanafiah, 2013)
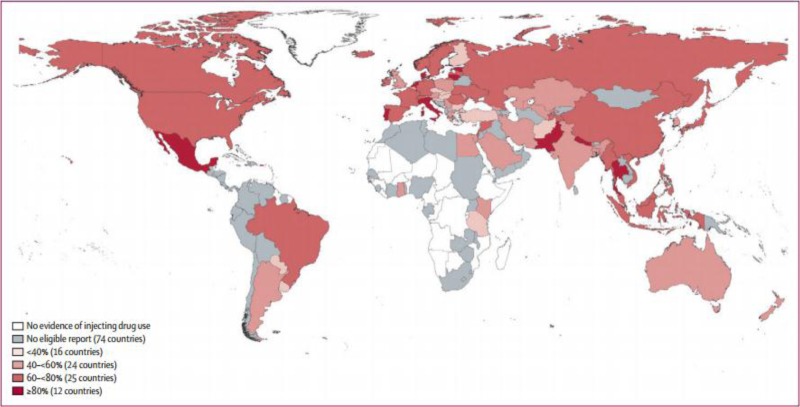
Prevalence of hepatitis C antibodies in injecting drug users
A review of global prevalence data from 77 countries estimated that exposure to HCV (anti-HCV positive) among PWID is estimated to be between 60% and 80% in 25 countries and over 80% in 12 countries.
MSM
MSM can acquire HBV and HCV sexually. MSM who are HIV positive are at significantly higher risk of acquiring HCV infection than HIV negative MSM. Incidence rates of sexually acquired HCV among MSM have been rising in several industrialized countries since 2000, and outbreaks have been described in some less industrialized nations.
Systematic review of HCV prevalence in HIV-infected persons (Platt et al. 2015)
Coinfection estimates were identified for 78 of the 194 countries (40%). There were 760 HIV/HCV coinfection prevalence estimates. Findings suggest that globally HCV/HIV coinfection is 1.9% (IQR = 0·4–6·6%) among general population samples, 7% (IQR = 2·6–11·1%) among people living with HIV (PLHIV) who are pregnant or where heterosexual transmission is reported, 6.2% (IQR = 3·3–13·5%) among men who have sex with men (MSM) and 83% (IQR = 55·2–94·1%) among people who inject drugs (PWID). Odds of HCV infection are 11 times higher in the presence of HIV infection, but varied by risk group. The global estimate of HCV coinfections among PLHIV is 3.2 million (IQR = 1.4–4.3 million) of whom 1.2 million (IQR = 0.9–1.4 million) are PWID
View in own window
| Mid-point co-infection prevalence (Interquartile range) Number of studies |
|---|
| Gen pop | PWID | MSM | Hetero | Pregnant |
|---|
| East Africa | 1.3% (0–4.9) 5 | 71 % (42–99) 2 | 20%(1–38) 2 | 4% (3–9) 10 | 0.6% (0.1–5) 3 |
| Cental and West Africa | 5 % (2–12) 9 | | 8% 1 | 8% (4–12.4) 19 | 10.1 (5–16) 4 |
| South Africa | | | 2% 1 | 0.5% (0–1) 3 | |
| Latin America | 7% (0.8–16.1) 3 | 82% (52–88) 4 | 4% (0–16) 6 | 11% (8–15) 2 | 10% (5–18) 4 |
| North America | | 84 (41–89) 25 | 13 (8–15) 16 | 12 (9–25) 9 | 4% 1 |
| South East Asia | 5% (3–29) 7 | 90 (86–97) 18 | 6% (5–8) 5 | 5% (1.5–7) 5 | |
| Eastern Europe and Central Asia | | 82% (68–95) 8 | | | |
| Europe | 6% (0.3–30) 3 | 82% (53–91) 41 | 8% (4–17) 40 | 11% (4–23) 11 | 3% 1 |
| East Med | 1% 1 | 81% (74–89) 7 | | | |
| East Asia | | 96% (80–98) 15 | 4% (2–9) 3 | 51% (6–89) 7 | |
| Western Pacific | | | 9% (7–10) 4 | | |
We compared the prevalence of HCV among 105 samples of HIV-positive and -negative population groups (general population, PWID, MSM, sex workers, prison inmates and high-risk populations). This is summarized in Figure 4 and in the online table. Overall, there was a 12-fold (95% CI 11·2–11·8) increased odds for HCV positivity across all population groups among HIV-positive compared to HIV-negative persons. Odds of HCV were highest among HIV-positive prison inmates (OR=16·5 95% CI 15·9–17·1) and other high-risk populations (OR = 11·7 95% CI 11·0–12·4), PWID (OR = 5·1 95% CI 4·7–5·5), followed by MSM (OR = 3·8 95% CI 3·1–4·5) and general population samples (OR = 3·7 95% CI 3·3–4·3) and sex workers (OR = 2·5 95% CI 2·0–3·2).
Epidemic scenarios hepatitis C virus
Generally, HCV epidemics around the world are heterogeneous and represent mixtures of three core epidemic components:
Infection related to high-risk behaviours: In essentially every geographical region, the highest prevalence of HCV infection is among persons who use injection drugs (PWID). The prevalence of injection drug use differs between countries and regions, but within those who do inject drugs, HCV prevalence is nearly universally high. Commercial sex workers and prisoners also have increased prevalence (presumably related to both drug use and perhaps sexual transmission) as do men who have sex with men, especially those who are HIV infected. In many cohorts of PWID in North America, Europe and Asia, HCV prevalence ranges from 30% to 75%.
Infection related to past generalized exposures that have since been identified and removed: this epidemic pattern, in which there is a high prevalence of HCV within a given age group, is commonly referred to as a “birth cohort epidemic.” While typically identified as being the infection pattern in North America and Europe, many nations have some element of birth cohort epidemics with their unique HCV epidemiology (). Birth cohort epidemics reflect an HCV exposure source that was once present and to which a large portion of the population was exposed, but that has since been identified and removed. For example, before it was identified and sequenced, HCV infected the blood supply of many countries in all regions of the world. When the blood supply began to be screened for the presence of HCV, the exposure was removed. As a result, the incidence of HCV fell dramatically among the general population, but there remains a burden of prevalent, chronic HCV among patients who were alive and likely to get a blood transfusion during the time that HCV existed in the blood supply.
Generalized population epidemic: This pattern is related to a widespread exposure, often iatrogenic, that results in high prevalence (8–10%) across essentially all age groups. Note that the primary difference between a “birth cohort” pattern and a generalized pattern of infection is the duration of time that the generalized exposure existed and whether it has been removed or mitigated. An example of a generalized exposure is the common use of reusable hypodermic syringes and needles in medical settings without adequate sterilization between uses.
Few epidemics fall into one of the above three categories. Rather, most are mixed, and represent some combination of all components (Table).
View in own window
| Epidemic scenarios | | | 1.1.2 |
|---|
| Definition | Disaggregation | Country example |
|---|
| Generalized | High (>5%) | With birth cohort | Egypt, Pakistan |
|---|
| Without birth cohort | 1.1.3 |
| High intermediate (3–5%) | With birth cohort | Congo, Ukraine |
| Without birth cohort | 1.1.4 |
| Low intermediate (2–3%) | With birth cohort | Cote d’Ivoire, Thailand |
| Without birth cohort | 1.1.5 |
| Mixed | Generalized population prevalence, low, moderate or high with a sizeable risk population (PWID) | High generalized | Uzbekistan |
|---|
| High intermediate generalized | Taiwan |
| Low intermediate generalized | Syria, Hong Kong |
| Low (1–2%) with PWID | Mexico, Switzerland |
| Low (1–2%) without PWID | The Gambia, Singapore |
| To check (UNDOC % of population) | 1.1.6 |
| Concentrated | Generalized population prevalence <1% with high-risk groups | | United Kingdom, Turkey |
|---|
View in own window
| Extra risk classification | Country example |
|---|
| Unsafe blood transfusions prior to 1990 | Brazil, Portugal |
Draft recommendation(s)
Existing recommendations (Source: WHO, 20149)
Risk-based: It is recommended that HCV serology testing be offered to individuals who are part of a population with high HCV seroprevalence or who have a history of HCV risk exposure/behaviour. These include:
Persons who inject drugs (PWID)
Persons with HIV infection (HIV-positive men who have sex with men)
Prisoners and persons previously incarcerated
Persons who have had tattoos, body piercing or scarification procedures where infection control is not guaranteed
Children born to mothers infected with HCV
Close contacts of persons infected with HCV
Persons who have used intranasal drugs
Persons from a country with intermediate or high prevalence (2% or greater) of hepatitis C
Persons who have received medical or dental interventions in health-care settings where infection control is not guaranteed
Persons who have received blood transfusions prior to the time when HCV serologic testing of blood donors was initiated or in countries where HCV serologic testing of blood donations is not routinely performed.
General population and birth cohort: It is recommended that in settings with a high HCV seroprevalence (>8%) in the general population, testing be offered, especially at least once, to persons born between 1945 and 1955.
Existing WHO recommendations
HIV testing services should be routinely offered to all key populations in the community, closed settings such as prisons, and clinical settings.
PWID
WHO recommends delivery of a comprehensive package of nine evidence-based interventions for HIV prevention, treatment and care for PWID, all of which are also directly relevant to prevention, treatment and care for HBV and HCV, and one of which is specific to viral hepatitis testing (vaccination, diagnosis and treatment of viral hepatitis, see Box).
The nine interventions in the comprehensive package for HIV prevention, treatment and care for people who inject drugs
Needle and syringe programmes
Opioid substitution therapy and other drug dependence treatment
HIV testing and counselling
Antiretroviral therapy
Prevention and treatment of sexually transmitted infections
Condom programmes for people who inject drugs and their sexual partners
Targeted information, education and communication for people who inject drugs and their sexual partners
Vaccination, diagnosis and treatment of viral hepatitis
Prevention, diagnosis and treatment of tuberculosis.
Source: WHO, UNODC, UNAIDS,
Sex workers
WHO outlines a comprehensive set of interventions and approaches, both to promote enabling environments and to provide prevention, testing, care and treatment, in relation to HIV and STI programming for sex workers (see Box). These recommendations are directly relevant to the response to viral hepatitis among sex worker populations. Essential interventions include enabling sex workers to access and consistently use condoms, access prevention and care, treatment and support services, diagnosis and treatment of important comorbid conditions such as for TB (particularly in HIV endemic settings, incarcerated persons, PWID and sex workers living in exposed to poor cramped working and living conditions), other STIs, and access to harm reduction services for sex workers who inject drugs.
Importantly, as sex workers are a key population highly affected by the HBV epidemic, particularly in settings where vaccine coverage is suboptimal, and at high risk for HCV if they inject drugs, they must have access to hepatitis testing services (HepTS), repeat viral hepatitis testing, and partner and family testing wherever appropriate. The offer of HIV testing services (HTS) should be offered alongside HepTS, and HepTS can be integrated into HTS wherever possible. Delivery of HepTS should be informed by recommendations for the delivery of HTS. A variety of settings may be appropriate in which to implement testing services, including health-care settings as well as community settings, and via multiple different approaches. Outreach peer-led testing is likely to be particularly effective and acceptable in many sex worker populations.
Good practice recommendations
All countries should work toward decriminalization of sex work and elimination of the unjust application of non-criminal laws and regulations against sex workers.
Governments should establish antidiscrimination and other rights-respecting laws to protect against discrimination and violence and other violations of rights faced by sex workers in order to realize their human rights and reduce their vulnerability to HIV infection and the impact of AIDS. Antidiscrimination laws and regulations should guarantee sex workers’ right to health and financial services.
Health services should be made available, accessible and acceptable to sex workers based on the principles of avoidance of stigma, non-discrimination and the right to health.
Violence against sex workers is a risk factor for HIV and must be prevented and addressed in partnership with sex workers and sex worker-led organizations.
Evidence-based recommendations
Offer a package of interventions to enhance community empowerment among sex workers.
Promote correct and consistent condom use among sex workers and their clients.
Offer periodic screening for asymptomatic STIs to female sex workers.
Offer female sex workers, in settings with high prevalence and limited clinical services, periodic presumptive treatment for asymptomatic STIs.
Offer voluntary HIV testing and counselling to sex workers.
Use the current WHO recommendations on the use of antiretroviral therapy for HIV positive general populations for sex workers.
Use the current WHO recommendations on harm reduction for sex workers who inject drugs (in particular needle and syringe programme and opioid substitution therapy).
Include sex workers as targets of catch-up hepatitis B immunization strategies in settings where infant immunization has not reached full coverage.
Source: WHO, 2012
Prisons
Key WHO recommendations around testing services for people in prisons and closed settings have, to date, mostly focused on HIV prevention, testing and treatment. In 2013, UNODC and partners developed a comprehensive package of 15 key interventions for HIV prevention and treatment in prisons and other closed settings. Due to common transmission routes, these recommendations are equally applicable to viral hepatitis, and HBV vaccination and diagnosis and treatment of viral hepatitis is one of the specific recommendations (see box). This recommendation stipulates that “prisons should have a comprehensive hepatitis programme, including the provision of free hepatitis B vaccination for all prisoners, free hepatitis A vaccination to those at risk, and other interventions to prevent, diagnose and treat hepatitis B and C equivalent to those available in the community (including condom, needle and syringe programmes and drug dependence treatment as needed).”
Existing recommendations
Information, education and communication
Condom programmes
Prevention of sexual violence
Drug dependence treatment, including opioid substitution therapy
Needle and syringe programmes
Prevention of transmission through medical or dental services
Prevention of transmission through tattooing, piercing and other forms of skin penetration
Post-exposure prophylaxis
HIV testing and counselling
HIV treatment, care and support
Prevention, diagnosis and treatment of tuberculosis
Prevention of mother-to-child transmission of HIV
Prevention and treatment of sexually transmitted infections
Vaccination, diagnosis and treatment of viral hepatitis
Protecting staff from occupational hazards
Source: WHO, 2013
Recommendations and principles surrounding HIV testing in prisons apply equally to HBV and HCV testing in prisons (see box).
Existing recommendations
It is important to guard against negative consequences of testing in prisons - for example, segregation of prisoners - and to respect confidentiality.
It is also important that people who test positive have access and are linked to HIV care and treatment services.
HIV testing and counselling should be voluntary.
The use of HIV rapid testing can increase the likelihood of prisoners receiving their results.
Testing in conjunction with other risk-reduction services such as the provision of condoms with lubricants and STI screening can increase the benefits of testing and counselling.
Source: WHO, 2014.
View in own window
Summary and quality of evidence
Summary of evidence base for different screening approaches
Cost–effectiveness evidence summary: overview of report
Cost–effectiveness analyses of screening for HCV
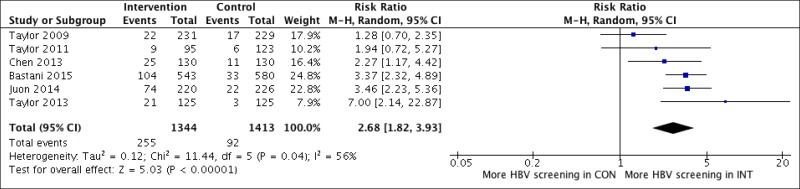
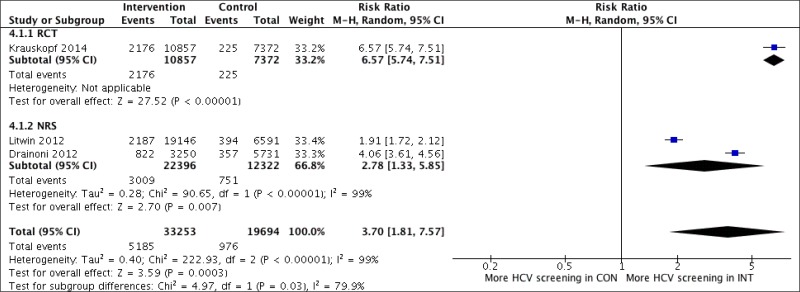
1. Gleue systematic search identified 19 studies. Majority of studies evaluated the cost–effectiveness of screening for HCV in Europe or the US; one study was carried out in Japan and another one in Italy. Ten studies evaluated screening in the general population; eleven studies screening IDUs; three studies looked at recipients of blood transfusions; one study evaluated screening in women during pregnancy and a further study looked at HCWs. Studies evaluated a one-off screening intervention, with the exception of one study that analysed screening every five years. Comparators were either: no screening or the status quo or different strategies were compared with each other. Recent studies concentrated on birth cohort screening in the US, evaluating the cost–effectiveness of oneoff screening for a cohort born between 1946 and 1970 and a cohort born between 1945 and 1965. HCV prevalence in this population was comparatively high. The initiation of a one-off screening intervention was assessed and compared with current risk-based screening interventions in these US studies.
2. Targeted review of the literature to determine the state of evidence about the cost–effectiveness of testing for HCV in different types of epidemics and among different risk groups. We provide a qualitative assessment of conclusions.
Testing in high-risk groups such as PWID, MSM, prisoners, HIV-infected persons, and commercial sex workers is likely cost–effective. Testing in settings with a high prevalence of high-risk patients is almost certainly cost–effective in all locations. It is important, however, to ensure adequate follow up after diagnosis. The best approach to testing outside of high-risk risk groups depends a great deal on a country’s unique HCV epidemiology. Most countries have at least some component of “birth cohort” epidemic, and “birth cohort” testing is likely cost–effective in most settings. In most epidemics, routine screening in the entire population is likely not to be cost–effective. The specific threshold at which a country should alter its approach to routine testing, however, is a function of multiple factors and cannot be identified more generally.
This report does not represent the results of a full systematic review. It is meant to serve as a summary of existing studies on cost–effectiveness of screening and treatment for HCV, with an analytic summary of key considerations. Due to the lack of relevant literature from low- and middle-income countries (LMICs), existing studies from high-income countries (HICs) are described and their potential uses and limitations, when drawing conclusions are discussed.
Summary of findings and conclusions
Testing in high-risk groups such as PWID, MSM, prisoners, HIV-infected persons, and commercial sex workers is likely cost–effective. Testing in settings with a high prevalence of high-risk patients is almost certainly cost–effective in all locations. It is important, however, to ensure adequate follow up after diagnosis. Persons who inject drugs: Multiple analyses in many geographical regions concur that routine testing for HCV in venues with a high prevalence of PWID is cost–effective, even when the studies assume very poor follow-up rates and limited access to therapy. Further, dynamic HCV transmission models suggest that aggressive diagnosis and treatment among current drug users could reduce the incidence of HCV – “cure as prevention”. With typical prevalence estimates of 40%, but ranging as high as 75% in some cohorts, routine screening for HCV is almost certainly cost–effective.
Men who have sex with men: Men who have sex with men (MSM) are also at an increased risk of HCV incidence, particularly if they are also HIV-positive. Cost–effectiveness modelling has found testing using liver function tests in combination with HCV Ab testing to be cost–effective in the HIV-positive MSM population. The results of these studies are dependent on appropriate linkage to effective therapy and retention in care. Prisoners: Prisons are likely to have a high HCV prevalence as the result of a high prevalence of PWID in prisons. A UK-based study, however, found that HCV case detection, using dried blood spot testing, was cost–effective, even when the model assumed low rates of HCV treatment initiation. A second study concurs that screening in prisons can be cost–effective, but this study concluded that targeting to screening to those prisoners with a history of injection drug use improves cost–effectiveness. HIV-infected persons: Although nearly every guideline for HCV care recommends HCV screening at enrolment in care, there are no cost–effectiveness analyses that address the specific question of the cost–effectiveness of HCV testing at enrollment in HCV care. Because the prevalence of HCV is known to be high in HIV-infected persons, the pace of fibrosis progression in HIV/HCV-coinfected patients is high, and new therapies to treat HCV are effective in HIV/HCV coinfection, testing for HCV at enrolment in HCV is almost certainly cost–effective. Sex workers: Because many sex workers are also PWID or noninjection drug users, the prevalence of HCV in this group is likely high. No studies were identified that address cost–effectiveness of HCV testing in sex workers, and therefore uncertain whether cost–effective to routinely screen all sex workers, compared to an approach that targets testing to sex workers who report a history of injection drug use.
Birth-cohort: i.e. testing among easily identified age or demographic groups known to have high HCV prevalence (“birth-cohort testing”). Most countries have at least some component of “birth cohort” epidemic, and “birth cohort” testing is likely cost–effective in most settings. Whenever there is an easily identified demographic group that has high HCV prevalence (for example, all individuals born in a certain time period) it is likely cost–effective to routinely test for HCV within that cohort. Several cost–effectiveness studies in the US and in Portugal show that birth cohort testing was cost–effective. Routine testing of the entire population: Routine general population screening: testing among the general population without attempt to identify high-risk behaviours or characteristics (“routine testing”). The data about population screening typically come from HICs such as the US and UK, and such studies find that routine testing in the general population is not cost–effective. When compared to “birth cohort testing”, however, universal testing resulted in worse outcomes and higher costs than the birth cohort approach. This analysis raises the spectre that in countries whose HCV epidemic is largely concentrated to a specific birth cohort or demographics group, attempting to identify cases by routine testing of the entire population can dilute the testing effort and result in fewer cases of HCV being identified. An older study, conducted in the UK, also found that although screening high-risk groups in primary care settings was cost–effective, extending screening beyond high-risk individuals was not.
Importantly, all of the above studies reflect the epidemiology of HCV in HICs. One recent paper explicitly studied the cost–effectiveness in Egypt of one-time, routine screening for HCV followed by treatment with either pegylated interferon and ribavirin (PEG-RBV) or PEG-RBV plus an HCV protease inhibitor. Given the very high prevalence of disease, screening was always cost–effective, and often cost-saving.
Drivers of cost–effectiveness
HCV prevalence: Screening provides increasing value as prevalence rises. In one U.S.-based study, screening was cost–effective (compared to no screening) at a US willingness-to-pay threshold down to a prevalence of 0.53%. In addition to the underlying prevalence of HCV infection, studies identified the rate of progression from chronic HCV to cirrhosis as an important factor together with levels of linkage to care and treatment that substantially influenced cost–effectiveness results.
|
□ High
□ Moderate
□ Low
□ Very low |
Risks/benefits
Targeted risk-based testing
Benefits
Key populations are disproportionately affected by hepatitis in all regions. Key populations are less likely to have received HBV vaccination, and offer of HBV testing will facilitate higher rates of completion of vaccination. In HIV-infected persons: Benefits of testing and treatment greatest in HIV-coinfected persons as they have more rapid progression of liver disease (HCV-associated liver disease in coinfected patients is emerging as a major cause of morbidity and mortality in HIV-infected persons) and risk of onward transmission than those without HIV infection. Leads to earlier diagnosis and access to treatment before development of cirrhosis. Some studies of PWID have shown that HCV testing and knowledge of serostatus results in reduction in injecting risk behaviours, including frequency of injecting and number of people injected with, in addition to other important risk behaviours, such as heavy alcohol consumption, and increases treatment uptake. Modelling studies suggest that scaling up treatment with DAAs, across different prevalence settings, would have a major impact on the prevalence of HCV. HCV treatment outcomes among people who actively inject drugs have been found to be acceptable. Community-based testing is a critical approach for reaching people from key populations and vulnerable populations who are unlikely to go to a facility, particularly those who are asymptomatic.
Risks
Challenging to identify and engage high-risk groups People may be reluctant to admit risk behaviours, or may be unaware they are at risk, and so a screening approach that relies on history may miss a substantial proportion of cases. Much high-risk behaviour is stigmatized and underreported. Health-care workers are not always skilled at identifying high-risk behaviours. Because HCV risk behaviours are stigmatized and underreported, trying to identify high-risk individuals is difficult and prone to under testing high-risk patients. For key populations, especially those whose behaviour is criminalized, testing services are sometimes misused in punitive or coercive ways. As a result, people from key populations avoid the health services that they need. In addition, stigma, discrimination, lack of confidentiality, coercion and fear of repercussions, as well as lack of appropriate health services, resources and supplies, prevent people from testing and, if positive, linking to care. Provider-initiated testing and counselling (PITC) among key populations and vulnerable populations is recommended, so long as it is not compulsory or coercive and it is linked to treatment and care. In many settings, and in spite of guidelines, service providers are often reluctant to offer antiviral treatment to PWID.
PITC in health-care facilities
Benefits
89/117 LMICs recommend HIV PITC in all patient encounters. High HIV PITC acceptance in antenatal care (ANC) and TB settings Introduction of PITC increased paediatric HIV testing Many clinical settings in generalized epidemic settings not offering hepatitis testing – e.g. STI clinics, primary care, and so many missed opportunities for HBV diagnosis in health care facilities.
Partner testing
Benefits
Participating in couples and partner HBV testing has a number of benefits. These include adoption of prevention strategies by the couple (for example, condom use, safe injecting practices) and promotion of linkage to and retention in appropriate health-care services. Also applies to opportunistically offering HBV testing and vaccination to family members and other close household contacts of people diagnosed with CHB re access to vaccination and care. Couples and partner testing helps more people know their HBV and/or HCV status, particularly men, who in generalized epidemic settings may be less likely to test than women. Partners: <5% of people currently HIV test with their partners and similar low rates for HBV. Note: HIV serodiscordancy is common (half to two thirds of HIV-positive adults with cohabiting relationship have HIV-negative partner. Offering partner testing for persons with HBV and HCV – highest possible yield. Although risk of infection may be low, a negative test in the partner provides reassurance and the opportunity to provide counselling on reducing future risk, including vaccination.
Birth cohort testing
Benefits
Being a member of a birth cohort is easily determined. Screening by age group is less stigmatizing. Targeting testing to birth cohorts is feasible and often cost–effective. In countries with a strong birth cohort dynamic, birth cohort screening is likely preferred.
Risks
Routine generalized testing
Benefits
Leads to earlier diagnosis and access to treatment before development of cirrhosis Way of accessing missing populations, such as men, key populations and young women who are not pregnant Community-based testing is a critical approach for reaching people from key populations and vulnerable populations who are unlikely to go to a facility, particularly those who are asymptomatic.
Risks
When the HCV epidemic is concentrated to a specific age or risk group, routine generalized testing and screening may dilute the screening effort in the cohort with the highest prevalence of HCV and result in fewer cases of HCV identified and higher cost than “birth cohort testing.” If an epidemic is highly concentrated with a specific risk or demographic group, screening outside of that group can be inefficient and increase costs. In most epidemics, routine screening in the entire population may not be cost–effective. May lead to lower-than-expected positivity rates with home-based testing, testing within campaigns, key population outreach and testing of index partners. Countries with high HCV prevalence across the entire population should implement routine screening, but The specific threshold at which a country should alter its approach to routine testing, however, is a function of multiple factors and cannot be identified more generally. Suboptimal linkage to care is highly variable and may be problematic
| □ Benefits clearly outweigh harms
□ Benefits and harms are balanced
□ Potential harms clearly outweigh potential benefits
Are the desirable anticipated effects large?
□ No
□ Probably
□ Uncertain
□ Yes
□ Varies
|
Acceptability, values and preferences
A values and preferences survey of implementers and users of hepatitis B and C testing services was carried out by FIND in September 2015. A total of 104 respondents from 43 (20 high-income, 23 low- and middle-income) countries. Relating to this PICO,
Respondents from LMICs identified following target populations as priority for hepatitis B and C testing: blood donors (>85% for B and C), children born to HCV-infected mothers (55% vs 75% for HBV), persons living with HIV (50% vs 65%), and pregnant women (40% vs 78%), MSM (25% vs 45% for HBV), sex workers (<10% for HCV and 45% for HBV), and prisoners (25% for HCV and HBV) and those chronically ill (around 25% for HCV and HBV%). Population – wide screening was suggested by less than 10% for HCV and around 15% for HBV (30% of respondents), and in blood donors (22%) was less supported.
PITC
Partner testing
Targeted testing in drug treatment programmes, STI clinics, HIV clinics
| □ No major variability
□ Major variability
Is the option acceptable to key stakeholders?
□ No
□ Probably
□ Uncertain
□ Yes
□ Varies |
Equity, ethics and human right implications
Will recommendation raise questions around equity?
As for all testing services, programmes for key populations need to emphasize WHO’s “5 Cs” – particularly consent, confidentiality and connection to comprehensive prevention, care and treatment. The use of community-based and hepatitis B and C rapid testing can increase the likelihood of some key populations, such as prisoners receiving their results. Testing in certain populations, such as in prisons may increase chance for stigmatization.
Are there ethical implications to this recommendation?
| □ Less equitable
□ More equitable |
Resource use and financial implications
Resource use (see parameter matrix for sample HIV testing programme costs)
Estimating the costs associated with a given hepatitis testing approach can be challenging. Costs for similar hepatitis testing may differ significantly between countries and by programme type within a country. Differences in programme costs may be due to general cost differences between countries, in what specific services are provided (referral to clinic for those testing hepatitis-positive vs enhanced linkage support), cadre of staff employed (nurses vs community health workers), the ease of reaching different populations, the capacity of the health system, and the level of HIV testing coverage.
Standardized approach to costing of hepatitis testing: A common approach to estimating costs involves identifying and estimating costs incurred by the health-care provider within the following broad categories:
personnel (for example, health-care providers at facilities, counsellors, other paid programme staff, volunteers); recurrent costs (for example, HIV test kits and commodities, printed materials, office supplies); capital expenses, often amortized over their useful life and discounted annually at 3% (for example, office space, transportation, equipment);
Materials:
Cost of testing kits, buffer/reagents Cost of sterile lancets, pipettes, gloves, sharps-bins or other method of disposal of used kits Cost of automated reading machine, if applicable Quality-control reagents, if applicable (some kits are supplied with positive and negative controls)
Training and supervision:
Cost of training testing providers and appropriate assessment, validation and revalidation of their skills From included studies, excellent robust specificity of all tests is reassuring in terms of ensuring cost effective initiation of algorithms for further investigation and treatment. If being utilized at the point of care, it will be the responsibility of the testing provider to record and report the result appropriately.
| 1.1.9
Are the resources required small?
□ No
□ Probably
□ Uncertain
□ Yes
□ Varies |
• Feasibility and constraints to implementation
Are any major barriers expected for the implementation of this recommendation?
As for all testing services, programmes for key populations need to emphasize WHO’s “5 Cs” – particularly consent, confidentiality and connection to comprehensive prevention, care and treatment. Need to overcome reluctance to provide partner testing/index partner testing Make use of lay providers/peer testing for outreach especially among key populations Viral hepatitis testing for key populations needs to be delivered alongside other key primary prevention interventions. Accessibility and coverage of testing would need to be high to have an impact on the prevalence of HBV among PWID and other key populations. Offering dried blood spot (DBS) testing for HCV to PWID attending drug treatment programmes increased uptake of testing services.
|
Is the option feasible to implement?
□ No
□ Probably
□ Uncertain
□ Yes
□ Varies |
Relevance to different settings/populations
Will this recommendation be most relevant for particular settings (e.g. endemicity)?
Key populations
In all settings, a number of social and structural barriers exist to PWID being able to access testing, health-care and harm reduction services, including inadequate coverage and delivery of interventions, stigma and discrimination, high incarceration rates, unstable living conditions, comorbid health problems, poor health literacy and social difficulties. This results in inadequate uptake of prevention, testing, treatment and HBV vaccination,
Viral hepatitis testing and treatment for PWID must always be delivered alongside other evidence-based essential primary prevention interventions also. Studies have shown that the uptake of opioid substitution therapy (OST) and adequate needle-syringe programme (NSP) coverage, in isolation, have been shown to reduce the odds of acquiring HCV, but the combination of both interventions together had a much larger impact. Additionally, the high prevalence of comorbidities in populations of PWID, including viral hepatitis/HIV coinfection, TB, mental health problems and poly-drug use alongside social and economic predictors of poor health means that it is particularly important that comprehensive prevention, treatment, care and social services are integrated and accessible to this population.
In many settings, responses to viral hepatitis, with particular regard for the need to reach key populations, can be integrated in order to be most effective. When this is not possible, strong links among health services working with priority populations should be established and maintained. Additionally, integration of viral hepatitis testing and treatment with existing services for HIV diagnosis and care is likely to be effective and less resource intensive.
Community-based testing is a critical approach for reaching people from key populations and vulnerable populations who are unlikely to go to a facility, particularly those who are asymptomatic. To improve access to and uptake of HBV, HCV and HIV testing, community-based testing services should be made available in settings acceptable and convenient to people from key populations and vulnerable populations
Accessibility and coverage of viral hepatitis testing will need to be high to have an impact on the prevalence of HBV and HCV among PWID. In order to achieve this, approaches must be acceptable to PWID populations. For example, studies from the UK suggest that offering DBS testing for HCV to PWID attending substance misuse services may increase uptake of testing services.
Prisoners
Many prisons around the world have implemented viral hepatitis and HIV prevention programmes, including HepTS and HTS as part of a comprehensive package of interventions; however, they are often small in scale and lack the necessary combination of essential interventions, greatly reducing their effectiveness.
Access to testing and counselling for viral hepatitis and HIV which is voluntary in nature and easily accessible alongside access to prevention, care and treatment interventions must be urgently scaled up in prisons. Particular attention must go to providing accurate information, obtaining informed consent and maintaining confidentiality. Additionally, there are often major challenges to continuity of care within closed settings and between prisons and the community that need to be addressed.
Pregnant women
Although the risk of mother-to-child transmission (MTCT) of HCV is much lower than that of HBV, perinatal transmission of HCV does occur in 4–6% of births, and the risk is two to three times higher if the mother is coinfected with HIV. MTCT is the most common cause of HCV infection in young children. In some settings, such as in west sub-Saharan Africa, there is a relatively high prevalence of HCV among young children, which may be a result of the high prevalence of HIV/HCV coinfection in that region.
HCV risk factor information should be elicited from pregnant women, and if present, testing of pregnant women for HCV should also be considered alongside testing initiatives for HIV and HBV. There is currently no effective public health intervention to decrease the risk of MTCT of HCV. However, as DAAs become more widely available, they may have a potential role to play in preventing MTCT of HCV if found to be safe and effective for use in pregnancy. Diagnosis of women with HCV before pregnancy should be prioritized so that appropriate treatment and potential HCV clearance can be achieved.
Children
Consideration should be given to testing children born to HCV-infected mothers, particularly if the mother is coinfected with HIV or has other risk factors for HCV infection, such as injecting drug use. Similar to HBV, the progression of HCV liver disease is usually slow in infected children, but early diagnosis is important to enable monitoring for liver disease and for linkage to appropriate care and treatment when necessary. As DAAs become more widely available, HCV testing in infants and children will become important to be able to offer curative treatment at an early stage before progression of liver disease.
Most infants whose mothers have been diagnosed with HBV or HCV should be followed up and routinely offered EID, and those diagnosed with either with should be regularly monitored for signs of liver disease so that treatment can be offered when necessary. However, some infants are lost to follow up, so additional paediatric case-finding is important. This can be achieved through the routine offer of PITC in health facilities, particularly in high-prevalence settings, and also through testing the family members of index cases where appropriate.
Integration of HBV and HCV testing into child health programmes
In high-prevalence settings: HBV and HCV testing of mothers and infants should be routinely available through a variety of services - child health services, immunization clinics, under-5 clinics, malnutrition services, well-child services and services for hospitalized and all sick children, TB clinics, and services for orphans and vulnerable children. For example, in Malawi it was reported that integrated testing for HIV-exposed infants at six weeks of age into routine postnatal, under-5 and immunization clinics was acceptable and feasible.
Potential viral hepatitis testing approaches to improve case-finding among infants and children
In all settings
Offer early infant diagnosis for HBV- and HCV-exposed infants.
Offer testing to all children and adolescents presenting with indicator conditions/signs and symptoms that suggest acute HBV or HCV, including anorexia, nausea, jaundice, right upper quadrant discomfort and abnormal liver function tests.
Consider offering viral hepatitis testing to all children and adolescents attending HIV services, STI clinics and TB clinics.
In high-prevalence settings and for high-risk individuals
Offer viral hepatitis testing or retesting to mothers or infants in immunization clinics or under-5 clinics. If mothers are not available for testing or refuse testing, infant testing is an acceptable alternative.
Offer viral hepatitis testing to all children with parents or siblings receiving any HIV service (for example, PMTCT, ART) through home-based or facility-based HTS.
Consider viral hepatitis testing in all children and adolescents attending paediatric inpatient health services.
Rationale for recommendation
Strength of recommendation
Implementation considerations
16. Research gaps
Large-scale implementation studies in range of different LMICs should be performed to evaluate different testing approaches and the extent to which providers can accurately identify and test high risk patients when employing a targeted approach or birth cohort approach, as well as estimate of linkage to HCV care and the HCV cascade. Terms of cost, impact and cost-effectiveness and evaluation of key drivers in a range of different high-endemic, low-income settings.
A formal cost-effectiveness analysis that compares “targeted” vs “birth cohort” vs “routine” testing requires estimates of the prevalence of high-risk behaviours, stratified by age, the prevalence of HCV among those with high- and low-risk behaviours, stratified by age, and the age structure of the population. This will need to be informed by cost of both HCV therapy in a country, as well as the costs associated with untreated HCV and end-stage liver disease.
What proportion of HCV-positive cases will be missed by a testing policy based on screening for at-risk behaviours and exposures?