5.1. Types of viral hepatitis assays
Serological assays are typically used as the first line of the testing strategy to screen for exposure to a virus because of their relatively low cost (compared to NAT), and are therefore used to rule in all individuals who might potentially be infected with HCV or HBV. Serological assays detect the host immune response (antibodies to HCV) or a viral antigen (HBsAg, HCVcAg). They are based on the immunoassay principle, and are available in the form of rapid diagnostic tests (RDTs) or laboratory-based enzyme immunoassays (EIAs), chemoluminescence immunoassays (CLIAs) and electrochemoluminescence immunoassays (ECLs).
In contrast, NAT technologies are typically used to detect the presence of the virus, determine if the infection is active and if the individual would benefit from antiviral treatment. NAT technologies are also used to determine when antiviral treatment should be discontinued (due to non-response or resistance) or to confirm virological cure (HCV) or effective suppression (HBV).
5.2. Serological assays
5.2.1. Rapid diagnostic tests
Rapid diagnostic tests (RDTs) are single-use disposable assays that are provided in simple-to-use formats that generally require no additional reagents except those supplied in the test kit. They are read visually and can give a simple qualitative result in under 30 minutes. Due to their simplicity, cost and rapid turnaround time, they can be performed by trained lay providers or health-care workers, without the need for venepuncture for specimen collection. Quality-assured RDTs are therefore particularly useful in settings where conventional laboratory-based testing services are not available or accessible. They can also be used in outreach programmes (e.g. prison services, prevention and treatment services for people who use drugs).
Most RDTs can be performed with capillary whole blood collected by a finger-stick procedure using a lancet, but many have also been developed for use with venous whole blood, serum or plasma. Certain ones have been validated for use with oral fluid specimens. It is critical to always refer to the manufacturer's instructions for use for specific recommendations on specimen collection. Rapid tests are generally not suitable for testing large numbers of blood samples. The reading of results is dependent on subjective evaluation and no permanent record of the original test results can be kept.
5.2.2. Laboratory-based immunoassays
Most laboratory-based serological immunoassays (EIAs, CLIAs and ECLs) detect antibodies, antigens or a combination of both and differ only in the mode of detection of immune complexes formed. A cut-off value, usually determined by the manufacturer of the assay, specifies the point at which the results are considered to be reactive, and therefore, EIA results are generally reported as optical density divided by the assay cut-off (OD/CO) values. These types of assays are best suited for and most cost–effective to perform in settings with a high throughput of specimens (in excess of 40 per day). They are meant for laboratory- or facility-based testing rather than for use in the community, where infrastructure (electricity, cold storage, climate-controlled rooms) and skilled staff are consistently available, as cold-chain storage of test kits and the use of precision pipettes are usually required. These assays are typically used only with serum or plasma specimens, and therefore require phlebotomy to collect an appropriate specimen.
These assays may be performed either manually or on non-dedicated automated assay or specific dedicated automated systems. Simple immunoanalysers automate a number of the processes and as such require less hands-on time than a manually run EIA. They can therefore be used in range of different situations from high-throughput laboratories for the screening of large numbers of samples with full automation, to medium-sized laboratories with semi-automation, to small laboratories, such as those in remote areas, which conduct a small number of tests manually.
5.2.3. Confirmatory assays
For HBsAg – neutralization assays are used to confirm if observed antigen reactivity is neutralizable upon repeat testing with the same specimen using a neutralization step in the laboratory-based immunoassays, with a specific anti-HBs-containing reagent in the same assay. The result is confirmed when this neutralization reagent can abolish reactivity in the assay in comparison with a control reaction.
For anti-HCV – line immunoassays or immunoblots are serological techniques to confirm the presence of antibodies to HCV that have already been detected by other serological assays. The use of confirmatory assays should be able to provide a definitive result, although these assays are more expensive than other assays and are prone to high rates of indeterminate results. These assays only confirm serostatus and cannot be used to diagnose viraemic active HCV infection.
5.3. Nucleic acid testing technologies
These assays detect the presence of viral nucleic acid – DNA or RNA – through targeting a specific segment of the virus, which is then amplified. The amplification step enables the detection of low levels of the virus in the original specimen, which might not otherwise have been detectable. Laboratory-based technologies for NAT require sophisticated equipment, rigorous laboratory conditions and specimen collection, and highly trained staff who can perform precision steps and avoid contamination. Not all NAT technologies detect all genotypes or subtypes equally well, unless they are optimized to do so. Newly developed NAT technologies that are simpler and more robust are intended for use at or near the point of care, and may avoid some of the logistical and technical disadvantages of laboratory-based NAT technologies.
In addition to NAT assays that target a single virus, multiplex NAT screening assays have been developed, which can detect DNA or RNA from multiple viruses simultaneously.
5.4. Choice of serological assays
describes the advantages and disadvantages of RDTs and laboratory-based immunoassays. The choice of assay format will depend on a variety of factors, most importantly, performance characteristics (sensitivity and specificity), cost, ease of use and the characteristics of the testing site, such as storage facilities, infrastructure, and level of staff skills. Chapter 15 provides further details on how to set up laboratory services for viral hepatitis testing and selection of an assay, and how to assure the quality of testing.
Advantages and disadvantages of different assay formats.
5.5. Selection of one- or two-assay serological testing strategy
A testing strategy defines the sequence of tests to be followed for a specific testing objective (i.e. to identify infected and non-infected individuals), taking into consideration the anticipated prevalence of HBsAg or HCV antibody in the population(s) to be tested. WHO recommends the use of standardized testing strategies to both maximize the accuracy of HBsAg or HCV antibody testing while simplifying the process through streamlining procurement and training (11). The choice between a one-assay versus two-assay serological testing strategy will depend on the seroprevalence in the population to be tested and diagnostic accuracy (sensitivity and specificity) of the assays used. In these guidelines, we refer to the use of testing strategy only in the context of serological testing and the use of a one- or two-serological assay testing strategy, though it is recognized that other sources refer to the use of a single HCV RNA NAT or core antigen as a one test strategy to replace the need for a two-step process of serological testing followed by NAT.
One-assay serological testing strategy
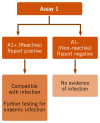
A one-assay serological testing strategy () is when a single serological test is performed. If the test result is reactive, a “compatible with positive infection” status is reported. If the initial test result is non-reactive, a “negative infection” status is reported. This testing strategy efficiently rules out most uninfected individuals correctly, and rules in those who are likely to be infected and therefore in need of further HBV DNA and HCV RNA NAT testing and staging of liver disease using NITs and clinical evaluation. This testing strategy is particularly suitable for high-prevalence settings due to the relatively higher positive predictive values (PPVs), but needs a highly sensitive and specific assay to maintain acceptable predictive values.
One-assay serological testing strategy.
Two-assay serological testing strategy
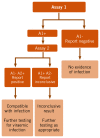
Two-assay serological testing strategy () differs in that two different assays are used sequentially, to improve the PPV of the testing strategy, and so reduce the number of individuals inappropriately referred on to more specialist services. This can be achieved by either (i) repeating the serological test using a different assay of similar sensitivity, or (ii) in the case of HBsAg, performing a neutralization test using a specific anti-HBs-containing reagent in the same first-line assay after appropriate dilution of the specimen under test.
Two-assay serological testing strategy.
If the first test result is non-reactive, a “negative infection” status is reported. If both test results are reactive, the status is reported as: “presumptive positive status infection for further diagnostic testing”. If the second test result is non-reactive, the status is reported as “infection inconclusive; requires additional testing”. If the second assay is less sensitive than the first, then it is likely that some true positives would be discarded if negative on the second test.