8.2. Background
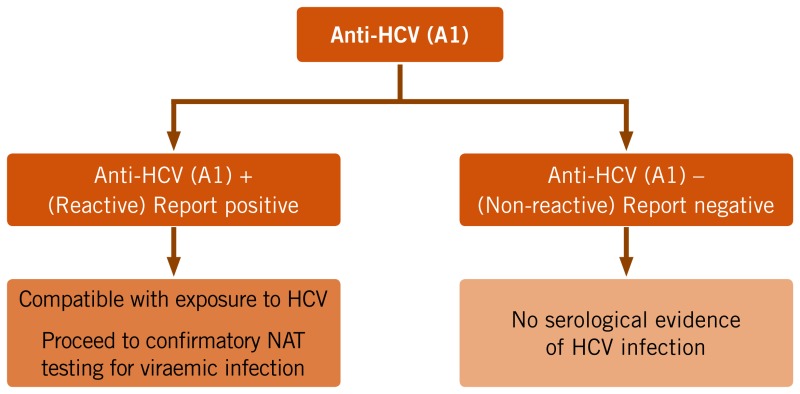
The principal assays used to determine exposure to HCV infection and evidence of past or current HCV infection rely on detection of antibodies to HCV using relatively inexpensive serological assays. Such antibody-based assays are unable to detect infection soon after acquisition of HCV infection, as antibodies may not be detected for 2–3 months in an individual who has been recently infected (135). This diagnostic window period can be shortened by using assays that also directly detect HCV antigen. Assessing HCV exposure typically involves either a one- or two-serological assay testing strategy. The main rationale for the use of a second HCV antibody test is to minimize false-positive results and reduce the number of people referred for more costly NAT technologies to confirm viraemic HCV infection. If HCV antibody positivity is established consistent with past or current infection, testing for current viraemic HCV infection is performed to ascertain viral replication through the detection of HCV RNA or HCV (p22) core antigen (HCVcAg). Aside from blood and organ donation screening, HCV RNA is not currently used to determine exposure to HCV, in spite of the shorter window period (1–2 weeks after the onset of acute infection) primarily for reasons of access and cost (135).
8.3. Summary of the evidence
Which serological assay to use
A systematic review (see Web annex 5.4) compared the diagnostic performance (sensitivity, specificity, positive and negative predictive values) of commercially available serological assays (RDTs and EIAs) for the detection of HCV antibody, when compared to a laboratory-based immunoassay reference standard. Five studies evaluated RDTs compared to an EIA reference (201, 232–235), 13 studies compared RDT results to NAT or immunoblot (236–248) and 14 studies compared RDTs with a combination of EIA, immunoblot or NAT (197, 201, 232–235, 247, 249–255). Twelve studies compared RDTs using oral fluid to RDTs using whole blood (as well as serum or plasma) (235, 238, 240, 241, 246–248, 250, 251, 255–257). The studies were carried out on different source populations, including the general population, key populations and hospital patients. The sample sizes of the included studies ranged from 37 to 17 894. All studies used a cross-sectional or case–control design.
RDTs. Based on the five studies of RDTs compared to EIA-only reference standard, the pooled RDT sensitivity and specificity were, respectively, 99% (95% CI: 98–100) and 100% (95% CI: 100–100), but sensitivities in individual studies ranged from 83% to 100%, and specificities from 99% to 100%.
Brands. There was significant heterogeneity between studies and variable performance across RDT brands and even within the same brand. Although use of NAT or immunoblot is not an appropriate reference to assess RDT diagnostic performance, the pooled sensitivity and specificity of RDTs were 93% (95% CI: 91–95%) and 98% (95% CI: 97–99%), respectively (Table 8.1).
TABLE 8.1
Summary diagnostic accuracy of HCV antibody tests (different assay format and comparators, populations, specimen type and oral kit brand).
Populations. A high sensitivity (>95%) and specificity (>99%) of RDTs for HCV antibody were observed across populations screened (general population, key populations, hospital patients) using different reference standards (EIA, immunoblot), but a patient selection bias was evident in around a third of studies.
Specimen type. RDTs using oral fluid showed a lower sensitivity but higher specificity compared to the reference standard, respectively, at 94% (95% CI: 93–96%) and 100% (95% CI: 100–100%). However, eight studies that examined OraQuick ADVANCE® HCV Rapid Antibody Test (OraSure Technologies, Inc.) had a higher sensitivity of 98% (95% CI: 97–98) compared to the other brands examined in six studies (pooled sensitivity of 88% [95% CI: 84–92]). There were insufficient data for other key brands, including the SD-Bioline which is now WHO prequalified.
RDTs and EIAs in HIV-positive persons. The number of studies was insufficient to undertake subanalyses based on HIV coinfection (256, 260–263). However, one recent study has reported that HCV EIAs may be associated with high rates of false positivity among HIV-infected persons in Africa (258).
The overall quality of the evidence for the recommendation to use RDTs was rated from low to moderate with downgrading mainly due to a serious risk of bias based on cross-sectional study design, and heterogeneity of results.
Which testing strategy to use
There was a single cost-effectiveness analysis that compared three testing strategies in a Brazilian population (259) (see Web annex 5.6). They found that a one-serological assay testing strategy for detection of anti-HCV followed by HCV RNA NAT to establish viraemic HCV infection was more cost–effective than a two-serological assay testing strategy. A predictive modelling study was also undertaken, which examined the diagnostic accuracy of a one- or two-assay HCV antibody testing strategy based on a hypothetical population of 1000 individuals across both a range of HCV antibody seroprevalence levels that reflect typical high prevalence rates among PWID [45%], intermediate prevalence among HIV-infected MSM [10%] and moderate [2%] to low [0.4%] endemicity in a general population), and across a range of assay performance characteristics (sensitivity of 98% and 90%, and specificity of 99% and 98% derived from the systematic review pooled sensitivity and specificity for HCV antibody RDTs, Table 8.1).
The outcomes show the strong influence of prevalence and assay specificity on PPV (see Web annex 6.2). The use of a highly sensitive and specific (98% and 99%) single assay yields a high PPV in excess of 90% at prevalences of 40% and 10%, and 67% at a 2% prevalence, and only a small number of false-positive diagnoses. Only at the lowest prevalence (e.g. 0.4%) does the PPV fall below 50%. Since overall the PPV is high at all prevalence levels with a single assay, the use of a second test would have a significant impact only in the lowest-prevalence populations (0.4%), especially if the initial assay was of lower performance.
8.4. Rationale for the recommendations on which assay to use
Overall, the Guidelines Development Group made a strong recommendation for the use of serological assays, particularly RDTs, based on moderate/low-quality evidence for diagnostic performance. As for HBsAg, the selection of assay format (either EIA4 or RDT) to test for HCV antibody in a particular setting will depend first on the performance characteristics of the assay, cost and also on key operational considerations, such as accessibility and ease of use in the intended-use setting, such as a community-based drug treatment programme versus a hospital-based clinic.
Balance of benefits and harms
Use of RDTs. In settings where access to laboratory services is limited, or where existing testing services do not have the capacity for conducting EIA, and for hard-to-reach and rural populations, the Guidelines Development Group recommended (as for HIV and HBsAg) the use of quality-assured RDTs rather than conventional laboratory-based EIAs. This was due mainly to their simplicity, relatively low cost and rapid turnaround time, and therefore their potential to substantially improve access to HCV testing, enhance linkage to care and reduce loss to follow up.
Other reasons for the preferred use of RDTs include the following:
- RDTs for the detection of antibodies to HCV have acceptable sensitivity and specificity compared to laboratory-based EIAs across a wide range of settings and different populations and for different brands. RDTs that use oral fluid are also available, which have adequate sensitivity and specificity, and may therefore be particularly useful where collection of venous or capillary whole blood is challenging.
- RDTs performed at the point of care, using less invasively collected specimens than venous whole blood, may allow for results to be available on the same day as testing, and so avoid the need for multiple follow-up appointments and reduce loss to follow up.
- For national programmes in resource-limited settings, expanded use of RDTs may mitigate the challenges of specimen collection, processing and transportation to laboratory services, and allow for the simplification and decentralization of testing.
- RDTs can also be used in outreach programmes (e.g. prison services, substance use/treatment services) in HICs to increase the uptake of hepatitis screening. Well-trained community health workers can perform testing accurately and reliably.
Use of EIAs. In settings with existing laboratory infrastructure or where many tests are carried out per day, testing by laboratory-based methods, such as EIAs, may be cost–effective and appropriate.
Although RDTs and EIAs had similar clinical sensitivity and specificity, testing using laboratory-based EIAs was recommended as the more appropriate and cost–effective assay in settings where suitable laboratory infrastructure is available, and where there is likely to be high-volume throughput, with many tests performed per day (>40 per day per operator), and in individuals who have good access to laboratory-based testing.
It is important to note that the latest generation of assays designed to detect HCV antibody are also designed to detect HCVcAg in order to increase the sensitivity of the assay and reduce the diagnostic window period. However, these fourth-generation assays are not typically able to differentiate HCV exposure from chronic HCV infection.
In HIV-positive persons. Insufficient studies were retrieved for the systematic review of persons with HIV/HCV coinfection for a formal evaluation of the diagnostic accuracy of RDTs to detect antibodies to HCV in persons who are HIV coinfected. Theoretically, the sensitivity of serological assays that detect antibodies only may be reduced if the patient is immunocompromised, e.g. persons with HIV infection, those undergoing immunosuppressive therapy or renal dialysis, and therefore exposure to HCV may not be detected in these individuals. It is estimated that this may occur in up to 6% of HIV-infected persons who undergo testing using an EIA for the detection of antibodies to HCV (260, 261) but may occur more often among persons with advanced immunosuppression due to HIV and during early HCV infection (262, 263). Conversely, there are also reports of a large proportion of false-positive HCV serological tests among HIV-infected persons, especially in SSA (258).
Minimum performance criteria for EIAs and RDTs. The Guidelines Development Group decided against defining minimum performance characteristics for assays, but recommended that any assay used should meet the performance criteria for stringent regulatory authorities (see Chapter 15). The Group also recognized that performance of RDTs in the field (i.e. setting of intended use) may vary and that certain RDTs are not validated by the manufacturer for use on capillary whole blood. The issues of analytical sensitivity and LoD for HCV antibody assays is less relevant than for HBsAg for several reasons. First, there are no WHO reference standard materials for anti-HCV antibody, and so IU/mL cannot be applied. Second, in contrast to the 40–100-fold difference in LoD for HBsAg detection, there is a minimal difference in end-point titres for anti-HCV antibody between RDTs and EIAs.
8.5. Rationale for the recommendation for a one-assay serological testing strategy
Balance of benefits and harms
The Guidelines Development Group made a conditional recommendation for a single test using an RDT or EIA, followed by HCV RNA NAT or core antigen on reactive specimens as the simplest and most feasible testing strategy in all settings based on low-quality evidence for several reasons.
- Predictive modelling indicates that a one-assay testing strategy efficiently identifies all but a very few individuals likely to be infected and in need of NAT testing to confirm viraemic HCV infection, and similarly excludes nearly all HCV-uninfected individuals, even at low prevalence. The overall impact of the second assay on improving PPV was smaller compared to the situation with HBsAg because of the generally higher sensitivity and specificity of RDTs for HCV antibody.
- There are concerns about the cost implications and feasibility of implementing a second serological assay, particularly at the point of care and in resource-limited settings.
- In low-prevalence populations, a higher proportion of results would be false positive, and therefore individuals would undergo unnecessary and more expensive NAT to identify viraemic infection as a result of a single falsely reactive serological assay. In this situation, the two-assay serological testing strategy may be marginally cost saving. However, the Guidelines Development Group did not consider that the numbers of false- positive diagnoses were significant enough to justify a two-assay serological testing strategy. It was also recognized that many countries in SSA will fall into the low-seroprevalence category of less than 0.4%, and higher rates of false positivity have also been reported in these settings. As access to NAT remains very limited and costly at present, the use of a second serological test may be more cost–effective than performing NATs in multiple persons with false-positive results.
- The risks associated with a false positive HCV antibody result is minimal, as all individuals with a diagnosis of HCV exposure (HCV seropositive) will require supplemental testing to confirm viraemic HCV infection (by NAT to detect HCV RNA or serology to detect HCVcAg) before initiation of antiviral treatment.
Acceptability, values and preferences
The values and preferences survey showed strong support by the majority for the use of RDTs delivered at the point of care to promote access, and a simplified one-serological assay testing strategy for HCV exposure, followed by supplementary testing to detect viraemic HCV infection Providers and patients found RDTs that utilize oral fluid specimens to be more acceptable than capillary or venous whole blood specimens, especially in children.5
Resource use
The cost of RDTs for HCV antibodies ranges from US$ 0.50 to US$ 2.00 for blood-based assays, and US$ 10 for oral fluid RDTs. The cost of EIAs ranges from US$ 0.50 to US$ 1.70, but EIAs require additional laboratory infrastructure and equipment, with precision and expertise required in their operation. RDTs do not require capital investment in laboratory infrastructure, and so there is a concurrent reduction in maintenance costs and reagents. Using a second different RDT assay would at least double the costs.
Feasibility
A survey of hepatitis testing programmatic experience across 19 LMICs found that a one-serological assay testing strategy using mainly RDTs was being implemented in a range of hospital-based services, including blood donor screening, harm-reduction services, and HIV treatment and care clinics.
8.6. Implementation considerations for HBsAg and HCV antibody serological testing
The most sensitive assay available, either RDT or EIA, in terms of both analytical sensitivity and clinical sensitivity, should be used. RDTs generally have a lower analytical sensitivity (IU/mL LoD) compared to EIAs. However, careful consideration should be given to ensure that the assay chosen has minimal rates of false positivity (both analytical and clinical). See also Chapter 15 for details on how to set up laboratory services for hepatitis testing and selection of an assay, and how to assure the quality of hepatitis B and hepatitis C testing.
- Quality-assured and fit-for-purpose assays. Access to a range of well-performing quality-assured assays is critical to the success of any hepatitis testing programme. While a wide variety of EIAs (and CLIAs, ECLs) are commercially available, there is a lack of HBsAg RDTs that meet minimum performance criteria, as well as safety and quality standards. National regulatory authorities are responsible for approving RDTs for sale and use after assessment of their quality, safety and performance.
- Accurate testing. All hepatitis B and C testing should be performed in accordance with the assay manufacturer's instructions, including an HBsAg neutralization step if it is being utilized. In addition, SOPs and job aids can help testing providers minimize testing and reporting errors, and thus improve the quality of the results.
- Staff training and supervision. The testing environment should operate according to quality management systems (see Chapter 15), and have access to qualified, proficient and motivated laboratory staff, trained specifically in performance of the various assays, with adequate and supervisory support. Health-care workers should understand the strengths and limitations of any given testing strategy, counsel patients who are screened, and be able to act appropriately on the results, both positive and negative. Delivery of RDTs requires appropriate training of test providers in performing the test, reading the test result, storage of test kits and other supplies, and interpreting and reporting the results.
- Linkage to care. As some preliminary results will be false positive, appropriate linkage will be needed to additional testing and clinical evaluation, especially where testing is conducted at the point of care in outreach programmes. Retesting strategies need to be implemented for those with a high risk of acquisition of HCV.
- Provision of NAT or HCVcAg testing at the same site as serological testing would be optimal, enabling rapid turnaround, reduced loss to follow up, and reduced personal and health-care costs of referral to a distant centre. The Guidelines Development Group did not consider in these guidelines the potential future testing scenario of a single NAT or HCVcAg for both diagnosis and confirmation of active infection.
Research gaps for HBsAg and HCV antibody serological testing
- The impact of HIV positivity (and of CD4 count, viral load and ART exposure, by regimen) on the diagnostic performance of RDTs for HBsAg and HCV antibody should be further evaluated.
- Evaluation should be done of the diagnostic performance, impact, cost and cost–effectiveness of a one- versus two-assay serological HBsAg or HCV testing strategy in diverse settings of both high and low HBsAg and HCV antibody prevalence.
- EIAs and RDTs assays should be validated using less invasive and simpler methods of sample collection, such as oral fluid and capillary whole blood and dried blood spots (DBS).
Footnotes
- 4
It is assumed that CLIA and ECL would have similar performance principles to EIAs.
- 5
Most RDTs using oral fluid are yet to be validated and then evaluated in children.
Publication Details
Copyright
Sales, rights and licensing. To purchase WHO publications, see http://apps.who.int/bookorders. To submit requests for commercial use and queries on rights and licensing, see http://www.who.int/about/licensing.
Third-party materials. If you wish to reuse material from this work that is attributed to a third party, such as tables, figures or images, it is your responsibility to determine whether permission is needed for that reuse and to obtain permission from the copyright holder. The risk of claims resulting from infringement of any third-party-owned component in the work rests solely with the user.
Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo).
Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “The translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”.
Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization.
Publisher
World Health Organization, Geneva
NLM Citation
WHO Guidelines on Hepatitis B and C Testing. Geneva: World Health Organization; 2017 Feb. 8, HOW TO TEST FOR CURRENT OR PAST HCV INFECTION (HCV EXPOSURE) – choice of serological assay and testing strategy.