This document represents WHO's normative support for using evidence-informed policies and practices in all countries. This document was developed using the standard operating procedures described in the WHO handbook for guideline development (20). In summary, the process included: (i) identifying priority questions and outcomes; (ii) retrieval of the evidence; (iii) assessment and synthesis of the evidence; (iv) formulation of the recommendations; and (v) planning for the dissemination, implementation, impact evaluation and updating of the guideline.
2.1. WHO Steering Group
The WHO Steering Group, comprising staff members from the WHO Department of Reproductive Health and Research (RHR) and the WHO Department of Maternal, Newborn, Child and Adolescent Health (MCA), of the Family, Women's and Children's Health (FWC) Cluster, supervised the guideline development process. The group drafted the initial scope of the guideline, identified priority questions and outcomes, prepared the guideline planning proposal, and identified systematic review teams, guideline methodologists and members of the Guideline Development Group (GDG). Additionally, the Steering Group supervised the evidence retrieval, assessment and synthesis, organized the GDG meetings (technical consultations), prepared draft recommendations for the GDG to review, prepared the final guideline document, and managed its publication and dissemination. The members of the Steering Group are listed in Annex 2.
2.2. Guideline Development Group
The WHO Steering Group identified 18 external experts and stakeholders from the six WHO regions to form the GDG. This was a diverse group of individuals with expertise in research, clinical practice, policy and programmes, and guideline development methods relating to intrapartum care practices and service delivery, in addition to two patient/consumer representatives. The members were identified in a way that ensured geographic representation and gender balance, and they had no important conflicts of interest (see section 2.13). A short biography of the GDG members was published on the WHO RHR departmental website for public review and comment prior to the first GDG meeting.
Selected members of this group participated in a scoping meeting held in April 2016, and provided input into the final version of the priority questions and outcomes that guided the evidence review. The GDG examined and interpreted the evidence and formulated the final recommendations at two face-to-face meetings in May and September 2017. The group also reviewed and approved the final guideline document. The list of GDG members can be found in Annex 2.
2.3. External Review Group
This group included six technical experts and stakeholders with an interest in the provision of evidence-based intrapartum care. The group was geographically representative and gender balanced, and the members had no important conflicts of interest (see section 2.13). The External Review Group (ERG) peer-reviewed the final guideline document to identify any factual errors and comment on clarity of the language, contextual issues and implications for implementation. The ERG ensured that the guideline decision-making processes considered and incorporated the contextual values and preferences of persons affected by the recommendations, including pregnant women and adolescent girls, health care professionals and policy-makers. It was not within the remit of this group to change recommendations that were formulated by the GDG. The members of the ERG are listed in Annex 2.
2.4. Technical Working Group
The Technical Working Group (TWG) comprised guideline methodologists and systematic review teams. An independent consultant from the Evidence-Based Medicine Consultancy in Bath, United Kingdom, and technical experts from Centro Rosarino de Estudios Perinatales (CREP) in Rosario, Argentina, served as guideline methodologists. In relation to quantitative evidence on the effects of different prioritized interventions, the Cochrane Pregnancy and Childbirth Group (PCG) provided input on the scoping of the guideline priority questions and supervised the updating of relevant systematic reviews following the standard processes of the Cochrane Collaboration. The methodologists from CREP appraised the evidence from these systematic reviews using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (21).
Where there were no suitable systematic reviews (Cochrane or non-Cochrane) for priority questions and other considerations relevant to the domains of the GRADE evidence-to-decision (EtD) frameworks, new systematic reviews of quantitative or qualitative studies were conducted by experts from CREP, Argentina, and from the University of Central Lancashire and King's College London, United Kingdom, in collaboration with the WHO Steering Group.
The Steering Group worked closely with members of the TWG to review the evidence and prepare the GRADE EtD frameworks. Members of the TWG are listed in Annex 2.
2.5. External partners and observers
Representatives of the International Federation of Gynecology and Obstetrics (FIGO), the International Confederation of Midwives (ICM), the Royal College of Obstetricians and Gynaecology (RCOG), the United Nations Population Fund (UNFPA) and the United States Agency for International Development (USAID) were invited to the final face-to-face GDG meeting in September 2017 to serve as observers (see Annex 2). These organizations are potential implementers of the guideline with a history of collaboration with the WHO RHR and MCA Departments in guideline dissemination and implementation.
2.6. Identifying priority questions and outcomes
The WHO Steering Group, in consultation with the systematic review teams, guideline methodologists and selected members of the GDG, drafted the priority questions for this guideline. To develop these questions, a rigorous scoping exercise to identify and map clinical practices, interventions and health outcomes related to intrapartum care commenced in January 2016. First, a scoping literature review was performed to define the population of interest for the guideline and to explore what constitutes “normal” labour and childbirth in clinical practice across settings, based on a search of the PubMed and Latin American and Caribbean Health Sciences Literature (LILACS) databases. Next, a preliminary literature search of existing clinical guidelines and key systematic reviews on intrapartum interventions was performed, using the following sources: Cochrane Database of Systematic Reviews, LILACS, National Guidelines Clearinghouse, PubMed, and web pages of professional societies (including FIGO, the European Board & College of Obstetrics and Gynaecology [EBCOG], the American College of Obstetricians and Gynecologists [ACOG], RCOG, the Royal Australian and New Zealand College of Obstetricians and Gynaecologists [RANZCOG] and the ICM) and health agencies (including the United Kingdom's National Institute for Health and Care Excellence [NICE], the Agency for Healthcare Research & Quality [AHRQ] of the United States Department of Health & Human Services, and the Institute for Clinical Systems Improvement [ICSI], based in the United States of America].
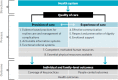
This exercise generated about 140 potential interventions that could be applied during the intrapartum period, starting before labour admission through to the immediate postpartum period. The interventions were then classified according to the WHO quality of care framework for maternal and newborn health () (12) to ensure that the ensuing recommendations would respond to the domains of intrapartum care quality in terms of both provision and experience of care.
WHO quality of care framework for maternal and newborn health.
The scoping exercise also informed the choice of potential outcomes for the guideline, particularly through the review of outcomes used in Cochrane systematic reviews related to intrapartum care interventions. To prioritize outcomes, a total of 44 international experts and stakeholders in the field of maternal and child health, including those who later participated in a guideline scoping meeting, were invited to rank the potential outcomes identified through the above exercise, using an electronic survey. Survey participants ranked the relative importance of outcomes on a 9-point scale ranging from 1 (not important) to 9 (critical). Using all the responses, the median score was calculated for each outcome, to identify a set of outcomes that are “critical” (median scores ≥ 7) and “important but not critical” (median scores 4–6) as a basis for making decisions about the recommendations.
Based on these initial steps, the WHO Steering Group developed a framework for discussion at a guideline scoping meeting, held in Geneva in April 2016, the aim of which was to prioritize guideline questions and to define the scope of the guideline in terms of focus, population of interest, interventions and outcomes. At this meeting, it was decided that the scope of this guideline should prioritize essential interventions that can be applied in low-, middle- and high-income settings, and that would be applicable to all pregnant women, regardless of their risk status (“low” or “high”) at the beginning of labour. Highly specialized labour interventions for the management of complications such as labour dystocia, fetal distress and meconium staining were considered beyond the scope of this guideline.
The key thematic areas for essential intrapartum care were discussed in the light of interventions that are already covered in existing WHO guidelines. Considering the resources available, the group agreed to limit the scope of prioritized questions to those that have not already been addressed by existing WHO guidelines, with the caveat that existing recommendations (that were developed according to WHO standard procedures) would be integrated into the final guideline document. However, the exception to this was the prioritization of the question related to companionship during labour and childbirth, for which several new trials were identified following the publication of the supporting Cochrane review (22).
In determining the guideline focus, the scoping process highlighted the need to identify womencentred interventions and outcomes for intrapartum care. To this end, a qualitative systematic review was conducted to understand what women want, need and value during childbirth (23). The findings of this review suggested that the primary outcome for all pregnant women undergoing childbirth is a “positive childbirth experience” (as defined in Box 2.1).
BOX 2.1Positive childbirth experience
Women want a positive childbirth experience that fulfils or exceeds their prior personal and sociocultural beliefs and expectations. This includes giving birth to a healthy baby in a clinically and psychologically safe environment with continuity of practical and emotional support from birth companion(s) and kind, technically competent clinical staff. Most women want a physiological labour and birth, and to have a sense of personal achievement and control through involvement in decisionmaking, even when medical interventions are needed or wanted.
Based on the outcome prioritization exercise described above and discussions at the scoping meeting, a set of outcomes that were considered critical and important to women (and their families) was prioritized for the intrapartum period. However, due to important differences between the types of prioritized interventions and the range of potential outcomes, and with due consideration for what matters to pregnant women undergoing labour, these outcomes were further prioritized separately for individual guideline questions. Informed by the qualitative review of women's views, the list of outcomes was complemented with the outcome “maternal birth experience” (including maternal satisfaction with care, women's mental and psychological health assessment, rating of childbirth experience, and sense of control) to reflect women's perception of the quality of care for all interventions prioritized. For questions related to definitions and duration of phases and stages of labour and diagnostic performance of 1-cm/hour cervical dilatation threshold, the outcomes include characteristic features and duration of phases of labour, and sensitivity and specificity of test thresholds, respectively.
In summary, this scoping and consultation process led to the identification of priority questions and outcomes related to the effectiveness of clinical and non-clinical practices aimed at achieving a positive childbirth experience that includes a healthy mother and a healthy baby. These questions and outcomes are listed in Annex 1.
2.7. Integration of recommendations from published WHO guidelines
In order to harmonize and consolidate all recommendations that are relevant to the care of healthy pregnant women and their newborn babies into a single document, existing WHO recommendations that were within the scope of essential intrapartum care were identified and integrated into this guideline. Only recommendations published from 2012 onwards in other WHO guidelines approved by the Guidelines Review Committee (GRC) were included. These integrated recommendations cover other critical components of intrapartum care for which questions were not prioritized. These include third stage of labour, care of the newborn immediately after birth, and care of the woman after birth. Recommendations and their corresponding remarks have been integrated from their parent guidelines without modification, as these recommendations were considered to be current.
2.8. Focus and approach
The focus of this guideline is on the essential intrapartum care practices that all pregnant women and adolescent girls should receive to facilitate a positive childbirth experience. To help decision-makers consider a range of factors relating to each intervention or option evaluated, the GRADE EtD framework tool was used, which includes the following domains: effects (benefits and harms), values, resources, equity, acceptability and feasibility (24). The preparatory work for the guideline was organized into five work streams to synthesize and examine evidence across the EtD framework domains ().
WHO intrapartum care guideline work streams.
2.9. Evidence identification and retrieval
Evidence to support this guideline was derived from a number of sources by the systematic review teams and methodologists working in collaboration with the WHO Steering Group. Evidence on effects was mainly derived from Cochrane systematic reviews of randomized controlled trials (RCTs). The Steering Group, in collaboration with the Cochrane PCG and methodologists from CREP, first identified all relevant Cochrane systematic reviews that addressed the prioritized questions. The Cochrane systematic reviews were based on studies identified from searches of the Cochrane PCG Trials Register.1 In instances where the Cochrane reviews identified were found to be out of date, review authors were invited to update their Cochrane reviews in accordance with the standard process of the Cochrane PCG and with the support of Cochrane PCG staff.
Where no systematic review was identified for a priority question, a new systematic review was commissioned from external experts. In this instance, the external experts were asked to prepare a standard protocol before embarking on the review, including: a clear PICO (population, intervention, comparator, outcome) question; criteria for identification of studies, including search strategies for different bibliographic databases; methods for assessing risk of bias; and a data analysis plan. The protocol was reviewed and endorsed by the Steering Group and selected content experts among the GDG members. The entire systematic review development process was iterative, with the methodologists in constant communication with the Steering Group to discuss challenges and agree on solutions.
Qualitative reviews focused on: what matters to women and health care providers in terms of intrapartum care; health care professionals’ views of barriers and facilitators to uptake and delivery of intrapartum care interventions; acceptability of practices to women and health care professionals; feasibility of implementing the interventions; how the outcomes impacted by an intervention are valued by women and other stakeholders; and general or specific perceptions on equity relating to the interventions prioritized (26). In addition, qualitative evidence related to labour companionship and respectful maternity care (RMC) were derived from two qualitative systematic reviews specifically addressing these questions (27, 28). To inform the question on effective communication by health care providers, a further mixed-methods review was conducted. The search strategies for evidence identification and retrieval for these reviews can be found in the respective publications.
Evidence on cost-effectiveness was identified by a systematic review of the literature, from 1 January 1996 to 20 February 2017, using the MEDLINE electronic database. Evidence was retrieved on costs and cost-effectiveness of intrapartum care in general, and cost-effectiveness of specific intrapartum interventions, including fetal monitoring, clinical pelvimetry, communication, companionship, birth positions, episiotomy and pain relief methods. The “related articles” feature of PubMed was used to identify additional relevant studies.
2.10. Quality assessment and grading of the evidence
Quality assessment of primary studies included in the reviews
The assessment of the quality of individual studies included in Cochrane reviews follows a specific and explicit method of risk-of-bias assessment using six standard criteria outlined in the Cochrane handbook for systematic reviews of interventions (29). Each included study is assessed and rated by reviewers to be at low, high or unclear risk of bias for sequence generation, allocation concealment, blinding of study personnel and participants, attrition, selective reporting and other sources of bias, such as publication bias. The assessment along these domains provides an overall risk of bias for each included study that indicates the likely magnitude and direction of the bias and how it is likely to impact the review findings. For the new systematic reviews on effectiveness of interventions, which were commissioned by the WHO Steering Group, each included study was assessed for risk of bias according to the Cochrane review methodology.
Studies identified for qualitative reviews were subjected to a simple quality appraisal system using a validated instrument that rated studies against 11 pre-defined criteria and then allocated a score ranging from A to D, with D indicating the presence of significant flaws that are very likely to affect the credibility, transferability, dependability and/or confirmability of the study. Studies scoring D were excluded on grounds of poor quality (30).
Quality assessment of the review evidence
The GRADE approach to appraising the quality of quantitative evidence (21) was used for all the critical outcomes identified in the PICO questions, and a GRADE evidence profile was prepared for each quantitative outcome for each priority question. Accordingly, the certainty of evidence for each outcome was rated as “high”, “moderate”, “low” or “very low”, based on a set of criteria. By default, RCTs were considered to provide highcertainty evidence, while non-randomized trials and observational studies provide low-certainty evidence. This baseline quality rating was then downgraded based on consideration of study design limitations (risk of bias), inconsistency, imprecision, indirectness and publication bias. For observational studies, other considerations, such as magnitude of effect, could lead to upgrading of the rating if there were no limitations that indicated a need for downgrading. The systematic review teams and methodologists from CREP graded the quantitative review evidence in accordance with standard operating procedures approved by the WHO Steering Group.
The findings of the qualitative reviews were appraised for quality using the GRADE-CERQual (Confidence in the Evidence from Reviews of Qualitative research) tool (25). The GRADE-CERQual tool, which uses a similar approach conceptually to other GRADE tools, provides a transparent method for assessing and assigning the level of confidence that can be placed in evidence from reviews of qualitative research. The systematic review team used the GRADE-CERQual tool to assess the confidence in qualitative review findings – a level of confidence was assigned to the evidence domains on values, acceptability and feasibility according to four components: methodological limitations of the individual studies; adequacy of data; coherence; and relevance to the review question of the individual studies contributing to a review finding.
2.11. Formulation of the recommendations
The WHO Steering Group supervised and finalized the preparation of evidence profiles and evidence summaries in collaboration with the TWG using the GRADE EtD framework. The EtD tool includes explicit and systematic consideration of evidence on prioritized interventions in terms of specified domains: effects, values, resources, equity, acceptability and feasibility. For each priority question, judgements were made on the impact of the intervention on each domain, in order to inform and guide the decision-making process. Using the EtD framework template, the Steering Group and TWG created summary documents for each priority question covering evidence on each domain, as described below.
Effects: The evidence on the critical outcomes was summarized in this domain to answer the questions: “What are the desirable and undesirable effects of the intervention/option?” and “What is the certainty of the evidence on effects?” Where benefits clearly outweighed harms for outcomes that are highly valued by pregnant women, or vice versa, there was a greater likelihood of a clear judgement in favour of or against the intervention, respectively. Uncertainty about the net benefits or harms, and small net benefits usually led to a judgement that did not favour the intervention or the comparator. The higher the certainty of evidence of benefits across outcomes, the higher the likelihood of a judgement in favour of the intervention. In the absence of evidence of benefits, evidence of potential harm led to a recommendation against the option. Where evidence of potential harm was found for interventions that were also found to have evidence of important benefits, depending on the level of certainty and likely impact of the harm, such evidence of potential harm was more likely to result to a context-specific recommendation for the intervention (and the context is explicitly stated within the recommendation).
Values: This relates to the relative importance assigned to the outcomes of the intervention by those affected by them, how such importance varies within and across settings, and whether this importance is surrounded by any uncertainty. The question asked was: “Is there important uncertainty or variability in how much women value the main outcomes associated with the intervention/option?” Interventions that resulted in outcomes that most women consistently value regardless of settings were more likely to lead to a judgement in favour of the intervention. This domain, together with the “effects” domain (see above), informed the “balance of effects” judgement.
Resources: This domain addressed the questions: “What are the resources associated with the intervention/option?” and “Is the intervention/option cost-effective?” The resources required to implement the reviewed intrapartum care interventions mainly include the costs of providing supplies, training, equipment and skilled human resources. A judgement in favour of or against the intervention was likely where the resource implications were clearly advantageous or disadvantageous, respectively. Cost evaluation relied on reported estimates obtained during the evidence retrieval process; the OneHealth Model: intervention treatment assumptions report (31); the WHO compendium of innovative health technologies for low-resource settings (32); as well as the experiences and opinions of the GDG members. Where available, direct evidence from systematic reviews of costeffectiveness informed this domain.
Acceptability: This domain addressed the question: “Is the intervention/option acceptable to women and health care providers?” Qualitative evidence from the systematic reviews on women's and providers’ views and experiences across different labour practices informed the judgements for this domain. The lower the acceptability, the lower the likelihood of a judgement in favour of the intervention. If it was deemed necessary to recommend an intervention that was associated with low acceptability, the recommendation is accompanied by a strategy to address concerns about acceptability during implementation.
Feasibility: The feasibility of implementing an intervention depends on factors such as the resources, infrastructure and training requirements. This domain addressed the question: “Is it feasible for the relevant stakeholders to implement the intervention/option?” Qualitative evidence from the systematic reviews on women's and providers’ views and experiences across different labour practices was used to inform judgements for this domain. Where barriers were identified, it was less likely that a judgement would be made in favour of the intervention.
Equity: This domain encompasses evidence or considerations as to whether or not an intervention would reduce health inequities. Therefore, this domain addressed the question: “What is the anticipated impact of the intervention/option on equity?” The findings of qualitative systematic reviews on women's and providers’ views and experiences, the 2015 WHO report on inequalities in reproductive, maternal, newborn and child health (33), and a review on facilitators and barriers to facility-based birth (8), as well as the experiences and opinions of the GDG members, were used to inform this domain. An intervention was likely to be recommended if its proven (or anticipated) effects reduce (or could reduce) health inequalities among different groups of women and their families.
For each of the above domains, additional evidence of potential harms or unintended consequences are described in the “additional considerations” subsections. Such considerations were derived from studies that might not have directly addressed the priority question but provided pertinent information in the absence of direct evidence. These were extracted from single studies, systematic reviews or other relevant sources.
The WHO Steering Group provided the EtD frameworks, including evidence summaries, GRADE evidence profiles, and other documents related to each recommendation, to GDG members as soon as the documents were drafted, and several weeks in advance of the face-to-face meetings. The GDG members were asked to review and electronically provide comments on the documents before the GDG meetings. During the face-to-face meetings at the WHO headquarters in Geneva, Switzerland, in May and September 2017, under the leadership of the GDG chairperson for each meeting, GDG members collectively reviewed the frameworks, the draft recommendations and any comments received through preliminary feedback. The purpose of the meetings was to reach consensus on each recommendation, including its direction and in some instances the specific context, based on explicit consideration of the range of evidence presented in each EtD framework and the judgement of the GDG members. In line with other recently published WHO guidelines using EtD frameworks (34-36), the GDG classified each recommendation into one of the following categories defined below.
- ▪
Recommended: This category indicates that the intervention or option should be implemented.
- ▪
Not recommended: This category indicates that the intervention or option should not be implemented.
- ▪
Recommended only in specific contexts: This category indicates that the intervention or option is applicable only to the condition, setting or population specified in the recommendation, and should only be implemented in these contexts.
- ▪
Recommended only in the context of rigorous research: This category indicates that there are important uncertainties about the intervention or option. In such instances, implementation can still be undertaken on a large scale, provided that it takes the form of research that is able to address unanswered questions and uncertainties related both to effectiveness of the intervention or option, and its acceptability and feasibility.
For recommendations integrated from existing guidelines, information on the strength and quality of the evidence from the source guideline document has been presented in the accompanying remarks. For consistency, integrated recommendations have also been categorized according to the typology described above.
2.12. Decision-making during the GDG meetings
The GDG meetings were guided by the following protocol: the meetings were designed to allow participants to discuss the supporting evidence and each of the recommendations drafted by the WHO Steering Group, and to reach a consensus on the final wording of each recommendation after revision. Consensus was defined as the agreement by three quarters or more of the GDG, provided that those who disagreed did not feel strongly about their position. Strong disagreements would have been recorded as such in the guideline (there was no record of such disagreement in any of the GDG meetings). Where required, the GDG determined the context of recommendations by the same process of consensus, based on discussions about the balance of evidence on effects (benefits and harms) of the interventions across different contexts.
If the participants were unable to reach a consensus, the disputed recommendation, or any other decision, would be put to a vote. Voting would have been by a show of hands among members of the GDG. A recommendation or decision would stand if more than two thirds of the GDG voted in support of it, unless the disagreement was related to a safety concern, in which case the WHO Secretariat could choose not to issue a recommendation on the subject. WHO staff at the meetings, external technical experts involved in the collection and grading of the evidence, and observers were not eligible to vote. If the issue to be voted upon involved primary research or systematic reviews conducted by any of the participants who had declared an academic conflict of interest, those individuals were allowed to participate in the discussion, but were not allowed to vote on the issue in question.
2.13. Declaration of interests by external contributors
In accordance with the WHO handbook for guideline development (20), all GDG, TWG and ERG members, and external collaborators were asked to declare in writing any competing interests (whether academic, financial or other) at the time of the invitation to participate in the guideline development process. The standard WHO form for declaration of interests (DOI) was completed and signed by each expert and sent electronically to the responsible technical officer. The WHO Steering Group reviewed all the DOI forms before finalizing experts’ invitations to participate. All experts were instructed to notify the responsible technical officer of any change in relevant interests during the course of the process, in order to review and update conflicts of interest accordingly. In addition, experts were requested to submit an electronic copy of their curriculum vitae along with the completed DOI form. The Steering Group collated and reviewed signed DOI forms and curriculum vitae, and determined whether a conflict of interest existed. Where any conflict of interest was declared, the Steering Group determined whether it was serious enough to affect the individual's ability to make objective judgements about the evidence or recommendations. To ensure consistency, the Steering Group applied the criteria for assessing the severity of a conflict of interest as provided in the WHO handbook for guideline development (20).
All findings from the received DOI statements were managed in accordance with the WHO DOI guidelines on a case-by-case basis. Where a conflict of interest was not considered significant enough to pose any risk to the guideline development process or reduce its credibility, the expert was only required to declare the conflict of interest at the GDG meeting and no further action was taken. Conflicts of interest that warranted action by WHO staff arose where experts had performed primary research or a systematic review related to any guideline recommendations; in such cases, the experts were restricted from participating in discussions and/or formulating any recommendation related to the area of their conflict of interest. At the GDG face-to-face meetings, members were required again to state any conflicts of interest openly to the entire group, and were required to submit a signed and updated version of their earlier DOI statements. A summary of the DOI statements and information on how conflicts of interest were managed are included in Annex 3.
2.14. Document preparation and peer review
Following the final GDG meeting, an independent consultant and the responsible technical officer from the WHO Steering Group prepared a draft of the full guideline document to accurately reflect the deliberations and decisions of the GDG. Other members of the Steering Group provided comments on the draft guideline document before it was sent electronically to the GDG members for further comments. The document was revised based on the feedback received from the GDG and then sent to the ERG for peer review. The ERG members were asked to review the revised draft of the guideline to identify any errors of fact, comment on the clarity of the language, and to raise any issues related to implementation, adaptation and contextual considerations. The Steering Group carefully evaluated the input of the peer reviewers for inclusion in the final guideline document and made further revisions to the draft as needed. After the GDG meetings and external peer review, further modifications to the guideline by the Steering Group were limited to corrections of factual errors and improvements in language to address any lack of clarity. The revised final version was returned electronically to the GDG for their approval.
2.15. Presentation of guideline content
A summary list of the recommendations is presented in the executive summary of this guideline. For each recommendation, a summary of the evidence on effects, values, resources, equity, acceptability, feasibility, and other considerations reviewed at the two GDG meetings can be found in the “Evidence and recommendations” section (Section 3). The language used to interpret the evidence on effects is consistent with the Cochrane Effective Practice and Organization of Care (EPOC) approach (37).
The WHO Steering Group has integrated into this guideline a number of existing WHO recommendations that are relevant to routine intrapartum care from other recent WHO guidelines. In all instances, these recommendations are identical to those published in the respective source guidelines. To ensure that the integrated information is complete, the strength of the recommendation and certainty of the evidence as originally published for the existing recommendation has been included in the remarks section. Such recommendations include an additional remark providing a direct web address for the source guideline. Guideline users are referred to the respective WHO source guidelines for more details on these integrated recommendations.
- 1
The Cochrane Pregnancy and Childbirth Group (PCG) Trials Register is maintained by the Cochrane PCG's Trial Search Coordinator and contains trials identified from: monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL); weekly searches of MEDLINE; weekly searches of Embase; hand-searches of 30 journals and the proceedings of major conferences; weekly “current awareness” alerts for a further 44 journals; and monthly BioMed Central email alerts. For further information, see: http://pregnancy.cochrane.org/pregnancy-and-childbirth-groups-trials-register