Background
WHO estimates that in 2015, 257 million people were living with chronic hepatitis B virus (HBV) infection worldwide, and that 900 000 had died from HBV infection, mostly as a result of cirrhosis or hepatocellular carcinoma. Most HBV-associated deaths among adults are secondary to infections acquired at birth or in the first five years of life. In May 2016, the World Health Assembly endorsed the Global Health Sector Strategy on viral hepatitis, which calls for the elimination of viral hepatitis as a public health threat by 2030 (defined as a 90% reduction in incidence of new infections and a 65% reduction in mortality). Elimination of HBV infection as a public health threat requires a reduction in the prevalence of hepatitis B surface antigen (HBsAg) to below 0.1% in children 5 years of age. This can be achieved through universal immunization of newborns against hepatitis B and other interventions to prevent mother-to-child transmission of HBV.
Rationale for updating the recommendations on prevention of mother-to-child transmission of HBV to address peripartum prophylaxis with antivirals
The WHO position papers on immunization recommend that all infants receive their first dose of hepatitis B vaccine as soon as possible after birth, preferably within 24 hours, and that the birth dose be followed by two or three doses of hepatitis B vaccine at least four weeks apart to complete the primary series. Immunization against hepatitis B starting at birth is the foundation of the prevention of perinatal and horizontal transmission of HBV. In 2015, in the WHO Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection, no recommendation was made for the additional use of antiviral therapy to prevent mother-to-child HBV transmission. This was because of the still limited and low quality evidence base with several ongoing trials, and the lack of consensus as to the programmatic implications of a policy for more widespread use of antivirals in pregnancy. Three key developments prompted the consideration to now include the use of antiviral prophylaxis for pregnant women with HBV infection as an additional measure to prevent mother-to-child transmission of HBV. First, further evidence has become available on the efficacy and safety of antiviral prophylaxis in pregnant women and their children. Second, WHO has received requests from countries and regions with already high birth dose and infant vaccination coverage for updated guidance on the use of peripartum prophylaxis. Third, data from epidemiological studies and modelling suggest that infant vaccination alone would be insufficient to reach the 0.1% HBsAg prevalence goal in children by 2030, and that peripartum prophylaxis may also be needed.
Methods
In accordance with the procedures established by its Guidelines Review Committee (GRC), WHO commissioned systematic reviews, impact modelling and a cost–effectiveness analysis. A regionally representative and multidisciplinary Guidelines Development Group (GDG) met in September 2019 to formulate the recommendations using the GRADE (Grading of Recommendations Assessment, Development and Evaluation) approach. Evidence to inform the recommendations included two commissioned systematic reviews and meta-analyses, impact and cost–effectiveness modelling, an assessment of the overall balance of benefits and harms (at individual and population levels), patient/health worker values and preferences, resource use, cost–effectiveness, considerations on equity and human rights, and feasibility across the different WHO regions.
Summary of recommendations
Existing recommendations on immunization from the WHO position paper 2017 (6)
- a)
All infants should receive their first dose of hepatitis B vaccine as soon as possible after birth, preferably within 24 hours;
- b)
Delivery of hepatitis B vaccine within 24 hours of birth should be a performance indicator for all immunization programmes, and reporting and monitoring systems should be strengthened to improve the quality of data on the birth dose;
- c)
The birth dose should be followed by two or three doses to complete the primary series.
Existing recommendation on testing of pregnant women for HIV and syphilis from the 2019 Consolidated guidelines on HIV testing services (22), and for hepatitis B from the 2017 WHO Guidelines on hepatitis B and C testing (23)
All pregnant women should be tested for HIV, syphilis and hepatitis B surface antigen (HBsAg)* at least once and as early as possible in the pregnancy (HIV standing recommendation since 2007; syphilis: strong recommendation, moderate-quality evidence; HBsAg*: strong recommendation, low-quality evidence).
- *
Particularly in settings with a ≥2% seroprevalence in the general population.
Tenofovir prophylaxis to prevent mother-to-child transmission of HBV
New recommendation
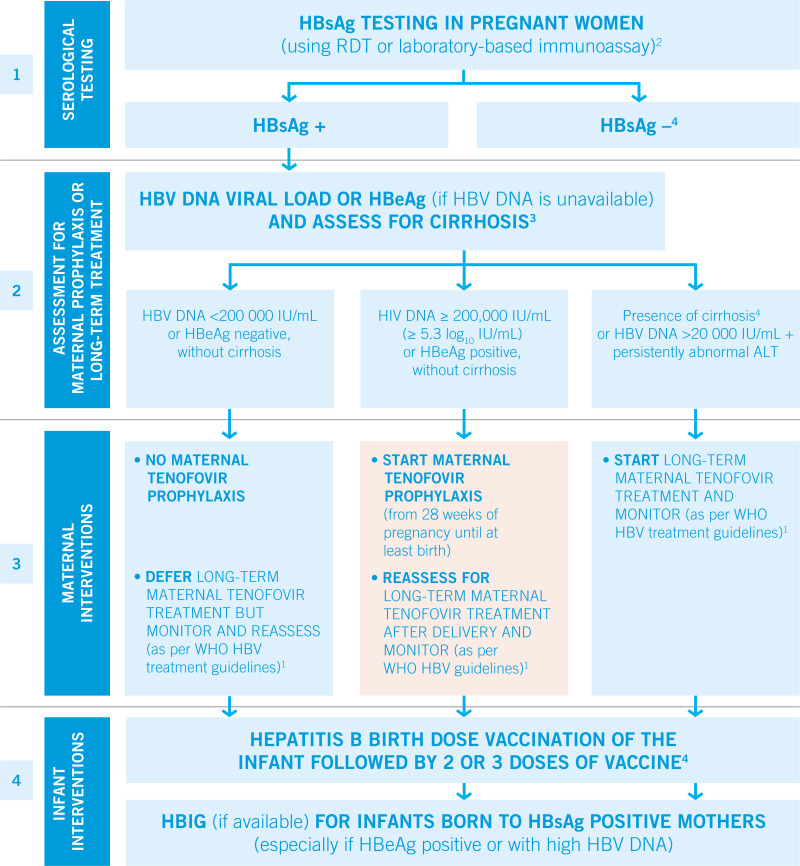
WHO recommends that pregnant women testing positive for HBV infection (HBsAg positive) with an HBV DNA ≥ 5.3 log10 IU/mL (≥ 200,000 IU/mL)1 receive tenofovir prophylaxis from the 28th week of pregnancy until at least birth, to prevent mother-to-child transmission of HBV. This is in addition to three-dose hepatitis B vaccination in all infants, including timely birth dose (conditional recommendation, moderate quality of evidence).
- 1
HBV DNA ≥5.3 log10 IU/mL is equivalent to ≥200 000 IU/mL.
Use of HBeAg testing, where HBV DNA testing is not available, to determine treatment eligibility for tenofovir prophylaxis to prevent mother-to-child transmission of HBV
New recommendation
WHO recommends that in settings in which antenatal HBV DNA testing is not available, HBeAg testing can be used as an alternative to HBV DNA testing to determine eligibility for tenofovir prophylaxis to prevent mother-to-child transmission of HBV2
(conditional recommendation, moderate quality of evidence).
- 2
The performance of HBeAg testing suggests that it is an acceptable alternative to diagnosing HBV DNA ≥5.3 log10 IU/mL.
Implementation considerations
Universal immunization of infants with hepatitis B vaccine, including a timely birth dose, is the foundation of programmes to prevent HBV infection at birth and in the first years of life. Countries that have not yet reached the 2020 goal of 1% HBsAg prevalence among children aged 5 years through vaccination need to focus their efforts on increasing their vaccination coverage, including timely birth dose.
The clinical trials that evaluated the efficacy and safety of tenofovir prophylaxis also included hepatitis B immune globulin (HBIG) as an additional preventive strategy in both trial arms. In a number of settings (mostly in high income countries) where it is available, HBIG is used in addition to hepatitis B vaccination, including birth dose, to reduce the risk of mother-to-child transmission of HBV. However, HBIG is a blood product that has to be screened for infectious diseases. The costs are high, a cold chain is required and HBIG can be in short supply. In low and middle-income setting, it may only be available when purchased by individuals.
As many countries are working towards dual elimination of perinatal HIV and syphilis infection, there are opportunities for efficiency gains and integration to also include elimination of mother-to-child transmission of HBV. Two WHO regions (Region of the Americas and the Western Pacific Region) already have plans and a framework for triple elimination.
Programmes to test and treat eligible pregnant women for HBV infection need to be implemented in the context of universal health coverage, aiming for covering the highest proportion of women while reducing financial hardship.
Testing of pregnant women needs to take place under circumstances that prevents stigma and discrimination and provides post-test counselling and education on measures to reduce the risk of transmitting HBV to the infant, encourage partner testing and ensure linkage to care of HBsAg positive women.
Clinical assessment should include an evaluation of whether HBsAg-positive pregnant women would be eligible for antiviral treatment for their own health. However, in accordance with criteria in the 2015 WHO
Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection (
20), only a small proportion of women of childbearing age would be eligible for long-term treatment.
HBV DNA quantification is the reference method to identify those HBsAg-positive pregnant women with a high viral load most at risk of transmitting HBV to their infants. Access to HBV DNA quantification (in terms of costs and availability of testing platforms) remains limited in low-income settings. Continuing efforts are needed to increase access to HBV DNA testing and reduce costs.
Diagnostic tests used need to meet quality, safety and performance standards (with regard to analytical, diagnostic and clinical sensitivity and specificity).
1
- 1
Assays should meet minimum acceptance criteria of either WHO prequalification of in vitro diagnostics (IVDs) or a stringent regulatory review for IVDs. All IVDs should be used in accordance with manufacturers’ instructions for use and, where possible, at testing sites enrolled in a national or international external quality assessment scheme.