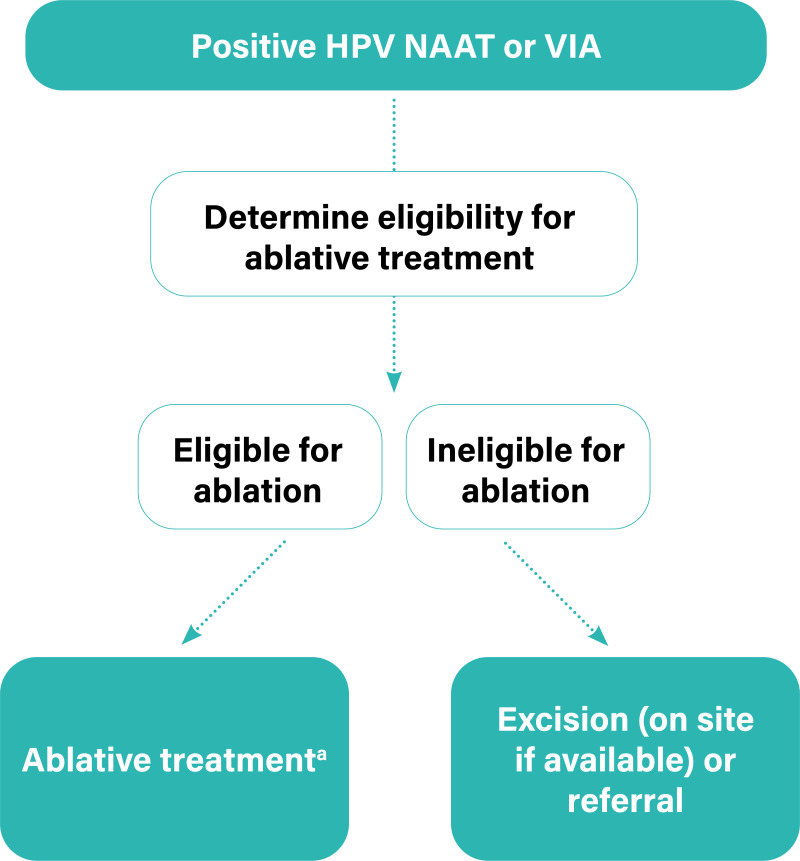
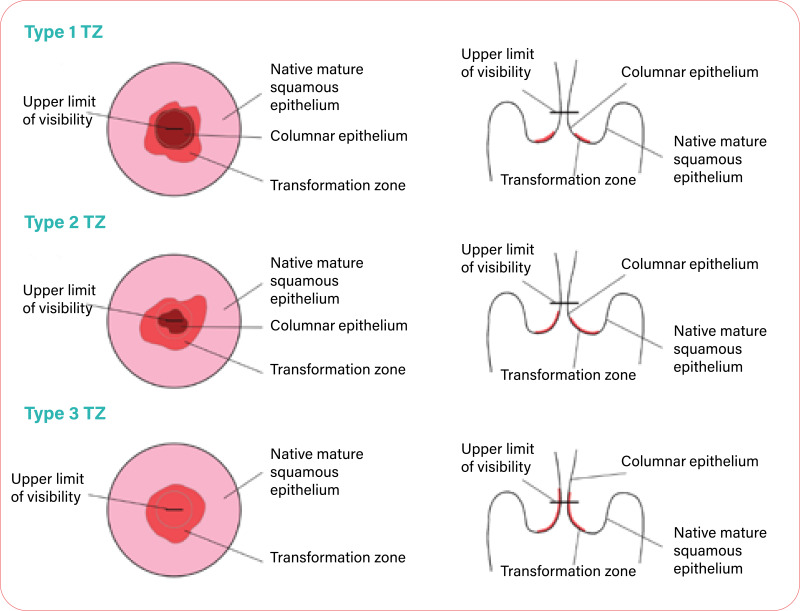
5.2.1. Persistent, residual or recurrent disease (histopathologically verified)
Definition: Persistent or residual disease is the detection of a histopathological high-grade lesion – including histological high-grade squamous intraepithelial lesions (HSIL) as per LAST terminology2 (HSIL-cervical intraepithelial neoplasia grade 2 [CIN2] and HSIL-CIN3); high-grade CIN as per CIN terminology (CIN2 and CIN3); HSIL not specified; and AIS – diagnosed from a punch biopsy or a subsequent surgical specimen (including hysterectomy, cold knife conization [CKC] and LLETZ) at any time interval after the initial treatment was performed with no intervening documented absence of disease. Persistent or residual disease excludes cancers that have been confirmed after diagnosis with surgical specimen.
Recurrent disease is defined as detection of a histopathological high-grade lesion (as described above) that was diagnosed from a biopsy or a subsequent surgical specimen (including hysterectomy, CKC and LLETZ) following a documented absence of high-grade lesions at any time interval after the initial treatment was performed.
Caveats: Studies have used the terms “persistent/residual disease” and “recurrence” to indicate the detection of disease post-treatment without making any distinction between them. Sub-analysis may be considered to distinguish between them. However, both conditions are treatment failures. Some studies use the terms “cure” or “treatment success” to indicate the absence of persistent/residual/recurrent disease following initial treatment and have estimated the rates. The specific location of the lesion on the cervix detected at follow-up is not important since the aim of the treatment of cervical pre-cancer is to treat the entire transformation zone and not any particular lesion. Any lesion detected at follow-up should be considered as representing treatment failure. The final diagnosis is the worst outcome found on histopathology.
Symptoms: Non-specific.
Consequences: Patient needs retreatment for high-grade lesions; adverse psychological impact; resource implications (including cost, human resources, hospital services).
Assessed by: Different studies may use different assessment protocols at follow-up. Some studies may take random punch biopsies from all the treated women undergoing follow-up. Other studies may have considered negative HPV test, cytology, VIA or normal colposcopy as evidence of absence or persistent/residual/recurrent disease. Such studies do biopsies only on selected patients who are positive on HPV test, cytology, VIA and/or colposcopy. These different methods of assessment should not exclude any studies, but a sensitivity analysis should then be conducted. Diagnosis of cancer at follow-up should ideally be excluded from verification of treatment outcome, as cancer should have been ruled out at baseline diagnosis.
Threshold: HSIL-CIN2 and HSIL-CIN3; high-grade CIN as per CIN terminology (CIN2 and CIN3); HSIL not specified; AIS. Some studies may consider low-grade squamous intraepithelial lesions (LSIL) or CIN1 as the threshold. Data that use LSIL+ or CIN1+ as the threshold should not be included but, if presented separately, data for HSIL-CIN2+ should be used.
Treatment: Treatment of recurrent or persistent HSIL/CIN2/CIN3 includes LLETZ or ablation (depending on the eligibility for ablation and the availability of treatment techniques). AIS is treated by LLETZ or CKC. Such treatment may be administered by a colposcopist, a general practitioner or a nurse.
Time horizon: In assessing research, the presence of disease should be measured beyond six months after treatment – and 1–3 years is preferred. A sensitivity analysis should exclude six months. Recurrence may be detected several years after treatment.
5.2.2. Persistent, residual or recurrent disease (not histopathologically verified)
Definition: Persistence or residual disease may be defined as a positive follow-up screening test (e.g. VIA, HPV test or cytology) at any time after the initial treatment. The women with positive tests were not further verified by histopathology. This is usually applicable in settings that use screen-and-treat approaches.
Recurrent disease may be defined as a positive follow-up screening test (e.g. VIA, HPV test or cytology) following a documented negative test at any time after the initial treatment in a screen-and-treat approach setting. There was no histopathological verification after a positive follow-up screening test.
Caveats: Studies have used the terms “persistent/residual disease” and “recurrence” to indicate the detection of disease post-treatment without making any distinction between them. Sub-analysis may be considered to distinguish between them. However, both conditions are treatment failures.
Symptoms: Non-specific.
Consequences: Patient needs retreatment, or verification with colposcopy and/or histopathology; adverse psychological impact; resource implications (including cost, human resources, hospital services).
Assessed by: Usually the same screening test at baseline is used to detect persistent/residual/recurrent disease at follow-up. HPV type-specific persistence/recurrence (detection of same HPV genotype at different time points) may be studied with an appropriate HPV detection test.
Threshold: The cytology threshold may vary between atypical squamous cells of uncertain significance (ASCUS), LSIL and HSIL. Ideally, HSIL should be used (LSIL could be used if identified). Other thresholds: VIA-positive and HPV-positive tests (any high-risk type detected by a validated HPV test).
Treatment: Treatment may include ablation or excision. Such treatment may be administered by a colposcopist, a general practitioner or a nurse.
Time horizon: In assessing research, the presence of disease should be measured beyond six months of treatment – and 1–3 years is preferred. There should be a sensitivity analysis to exclude six months. Recurrence may be detected several years after treatment.
5.2.3. Incidence of histopathological high-grade lesions and cancers
Definition: New (incident) cases of histopathological high-grade lesions and cancers (HSIL+ or CIN2+) detected following treatment. This includes histological HSIL as per LAST terminology (HSIL-CIN2 and HSIL-CIN3); high-grade CIN as per CIN terminology (CIN2 and CIN3); HSIL not specified; AIS, invasive squamous cell cancer, adenocarcinoma, carcinoma unspecified. All categories are combined together as HSIL+ or CIN2+.
Symptoms: Non-specific.
Consequences: Patient needs treatment for high-grade lesions or invasive cancer; adverse psychological consequences; resource implications (including cost, human resources, hospital services).
Assessed by: New cases are detected either through linkage with the screening registry or by active follow-up of the screening cohort. The treated women without any disease at first follow-up are advised to attend regular screening. Further evaluation and histopathology are performed if the screening test is positive (any screening tests at any follow-up visit).
Rates of HSIL+ or CIN2+ may be computed by each year since the index treatment on the basis of the number of women under active surveillance, with the number of women in active surveillance diagnosed with CIN2/3 in that year divided by the number in active surveillance screened in that year.
The cumulative rates of HSIL+ or CIN2+ or of invasive cancer alone may be estimated as number per 100 000 person-years of observation.
Threshold: The studies may have used HSIL+/CIN2+ or CIN3+ (HSIL-CIN3/CIN3, AIS, invasive cancer) or only invasive cancer as the end point. The studies should be analysed separately.
Treatment: The treatment of high-grade lesions requires ablation or excision depending on eligibility. AIS/microinvasive cancer requires excision (CKC or LLETZ). Such treatment may be administered by a colposcopist, a general practitioner or a nurse. Invasive cancer needs radical surgery or radiation therapy in a specialized setting. Additional testing for metastasis may be needed in patients with invasive cancers.
Time horizon: Incident CIN/cancer may be detected between six months and several years after treatment.
5.2.4. Incidence of cervical cancer (includes squamous cell carcinoma, adenocarcinoma or invasive cancer unspecified)
Definition: Newly diagnosed cases of invasive cervical cancer in the population under consideration within a defined time period, typically at least six months after screening and treatment.
Symptoms: Symptoms such as vaginal discharge, irregular bleeding, pain.
Consequences: Needs further investigations and treatment; adverse psychological consequences; disability; may be fatal, depending on stage at diagnosis; resource implications (including cost, human resources, hospital services).
Assessed by: The new cases of cancers are histopathologically diagnosed through active follow-up and/or linkage to the screening registry and/or population-based cancer registry. The incidence is usually documented as number per 100 000 person-years of observation.
Thresholds: Not applicable.
Treatment: Surgery or radiation therapy with concomitant chemotherapy, depending on stage.
Time horizon: Any time after initial treatment of premalignant lesions.
5.2.9. Treatment side-effects and complications
Definition: A complication is a condition or an event unfavourable to the patient’s health, causing irreversible damage or needing a change in therapeutic approach, including prolonged hospital stay. Side-effects are unwanted symptoms due to treatment that are mostly temporary and resolve spontaneously. These two terms are often used interchangeably.
Complications are graded as grade I (no pharmacological treatment or surgical intervention other than mild analgesics, antipyretics or antiemetics needed), grade II (needing pharmacological treatment, including intravenous fluid or blood transfusion), grade III (needing surgical intervention, e.g. cervical stiches), grade IV (life-threatening) and grade V (death). Most studies evaluating cervical pre-cancer treatment do not use such systematic classification or distinguish clearly between side-effects and complications. The most commonly evaluated significant treatment complications are described below. The proportion of treated women having one or several of these complications is assessed.
a. Major infections (grade II+)
Definition: Post-operative infection of the genital tract needing antibiotics and/or hospital admission.
Symptoms: Systemic: fever, nausea, vomiting, dehydration; local: foul-smelling discharge, pain.
Consequences: Hospitalization, antibiotic therapy, loss of workdays, psychological harm, long-term sequelae such as infertility, chronic pelvic inflammatory disease.
Assessed by: The diagnosis of major infection is mostly based on history and clinical assessment during follow-up. Acute pelvic inflammatory disease (presence of fever and/or lower abdominal pain and/or foul-smelling purulent vaginal discharge and/or adnexal tenderness and/or pain during cervical movement) is included in major infection. The diagnosis of major infection can also require blood tests, ultrasound and/or culture/sensitivity testing to adjust treatment.
Thresholds: Minor infections causing vaginal discharge and mild pain are often indistinguishable from side-effects and are not usually considered. Major infections can be distinguished from minor infections as they may include foul-smelling discharge plus other signs such as fever, tenderness on examination or severe pain.
Treatment: Hospitalization, antibiotics and rehydration, depending on the severity of the condition.
Time horizon: Usually 3–4 days after treatment and within one month; may take a few days to a few weeks to recover.
b. Major bleeding (grade II+)
Definition: Post-operative bleeding needing intervention (vaginal packing, cervical sutures), with or without hospitalization, with or without intravenous fluid or blood transfusion.
Symptoms: Vaginal bleeding, usually with the passage of clots, with or without pain. May have symptoms due to hypotension and shock (weakness, giddiness, vomiting, loss of consciousness).
Consequences: Shock, death in severe cases; may lead to anaemia; psychological harms; loss of workdays.
Assessed by: History and clinical examination, routine blood tests.
Thresholds: Major bleeding may be distinguished from minor bleeding as major bleeding may be accompanied by the passage of clots, a fall in blood pressure and/or significant drop in haemoglobin levels, or it may require intervention, such as vaginal packing or cervical sutures.
Treatment: Hospitalization followed by vaginal packing and antibiotics, and treatment of any shock; stitches may sometimes be needed under anaesthesia, and very rarely hysterectomy will be needed.
Time horizon: During the treatment and within one month of treatment. Recovery depends on severity – bleeding usually stops within a few days.
c. Procedure-associated pain
Symptoms: Pain, lower abdominal cramps during and/or immediately after the procedure.
Consequences: Medications; psychological harm; loss of workdays.
Assessed by: Feedback from the patient using a visual assessment scale; assessment by the treatment provider.
Thresholds: Exceeds pain that is mild and tolerable or the pain experienced during normal menstrual cycles.
Treatment: Analgesics advised depending on the severity of the pain.
Time horizon: During the procedure and up to one week after.
d. Cervical stenosis
Definition: Narrowing of the endocervical canal of the cervix to the extent that a cotton bud/swab or a uterine sound (diameter 3 mm) cannot be introduced.
Symptoms: May lead to dysmenorrhoea (rare) and/or haematocolpos.
Consequences: Dysmenorrhoea, decreased fertility (no consistent evidence), difficulty in obtaining endocervical samples during screening, difficulty in VIA or colposcopic assessment.
Assessed by: Clinical examination
Thresholds: Not applicable.
Treatment: May need dilatation of cervix.
Time horizon: Up to three years.
e. Spontaneous abortions
Definition: Clinically recognized spontaneous pregnancy loss before 20 weeks of gestation, or spontaneous expulsion of an embryo or fetus weighing 500 g or less. Also referred to as miscarriage.
Symptoms: In pregnant women, spotting, bleeding, pain, passage of fleshy mass through the vagina.
Consequences: Loss of pregnancy, hospitalization, psychological harm, loss of workdays.
Assessed by: History, clinical examination and ultrasound, or as reported by the patient, which may be less reliable.
Thresholds: Pregnancy may or may not be confirmed.
Treatment: Bed rest, hospitalization, tocolytic medications, evacuation in the case of incomplete abortion.
Time horizon: Not applicable.
f. Premature rupture of membranes
Definition: Rupture of membrane before the onset of labour at any gestational age (also known as pre-labour rupture of membranes).
Symptoms: Watery vaginal discharge in later gestational ages not associated with other signs of labour.
Consequences: Preterm labour, low birthweight, perinatal death, hospitalization, caesarean section, psychological harms.
Assessed by: Clinical examination, ultrasound, tests to detect amniotic fluid.
Thresholds: Not applicable.
Treatment: Bed rest, fetal monitoring, induction of labour, caesarean section.
Time horizon: Not applicable.
g. Preterm birth (early/late)
Definition: Delivery of fetus before 37 weeks of pregnancy. Early preterm birth is delivery before completion of 32 weeks of gestation.
Symptoms: Not applicable.
Consequences: Hospitalization, tocolytics, caesarean section, low birthweight, perinatal loss, psychological harms.
Assessed by: Gestational age is not always accurately determined; the best accuracy is obtained by ultrasound, but gestation could be clinically assessed or self-reported.
Thresholds: Not applicable.
Treatment: Vaginal delivery or caesarean section.
Time horizon: Not applicable.
h. Perinatal deaths
Definition: Death during the perinatal period between 22 completed weeks (154 days) of gestation and 7 days after birth.
Symptoms: Not applicable.
Consequences: Not applicable.
Assessed by: Standard definition as available from medical provider, but may be assessed by individual report, which is not confirmed by a medical provider.
Thresholds: Not applicable.
Treatment: Not applicable.
Time horizon: Not applicable.
i. Infertility
Definition: Women of reproductive age (15–49 years) at risk of becoming pregnant (not pregnant, sexually active, not using contraception and not lactating) who report trying unsuccessfully for a pregnancy for 12 months or more of regular unprotected sexual intercourse.
Symptoms: Not applicable.
Consequences: No pregnancy.
Assessed by: Self-reported.
Thresholds: Unsuccessful in becoming pregnant at 12 months.
Treatment: Various treatments can be provided to improve the chance of conception.
Time horizon: After treatment but before the first pregnancy following treatment.
j. Stigmatization
Definition: Stigma is a mark of disgrace associated with a particular circumstance or quality. Stereotypes, prejudice and discrimination are the main components of any type of stigma. Also, as defined by study authors.
Symptoms: Experience of fear, avoidance, depression, and so on.
Consequences: Stigma generally causes social and psychological harms, and may cause physical harm to the individual. The consequences include social isolation, intimate partner abuse, negative personal image, altered health-seeking behaviour.
Assessed by: There is no standardized tool. Assessment may be qualitative, by questionnaire surveys or the use of scales to measure stigma, which could be continuous or dichotomous.
Thresholds: To be determined.
Treatment: Community education and support for the individual experiencing stigma.
Time horizon: Any time after the receipt of screening results or after treatment.
k. Increased viral shedding in women living with HIV
Definition: Detection of HIV RNA in the genital tract of women living with HIV with or without detectable plasma viral loads.
Symptoms: Not applicable.
Consequences: Infection of non-infected partner.
Assessed by: mRNA-based viral load detection from vaginal fluid.
Thresholds: Vary from 40–200 copies per sample.
Treatment: None.
Time horizon: Usually estimated at baseline and then at 2 to 14 weeks at variable intervals.
5.2.10. Acceptability by women, providers or programme managers
Definition: The extent to which women receiving a screening test and/or treatment intervention consider it to be appropriate, based on anticipated or experienced cognitive and emotional responses to the intervention. This is a multicomponent construct based on an individual’s experience with side-effects, convenience, and perceived satisfaction with treatment and with services. It is also the extent to which providers and programme managers providing a screening test and/or treatment intervention consider it to be appropriate. Authors’ definitions of acceptability may also be used.
Symptoms: Not applicable.
Consequences: Better compliance with future screening and treatment, better community acceptance, better provision of screening and treatment interventions.
Assessed by: Questionnaires assessing the intensity of side-effects and complications, the level of satisfaction with the procedure and the services, and whether the treated person would recommend the services to others; visual scales are often used.
Thresholds: Scales may be dichotomized into acceptable or not acceptable.
Treatment: Not applicable.
Time horizon: May be immediately after treatment and/or during follow-up visits.