1.6.1. Chapter contents
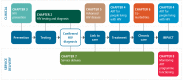
The structure of the guidelines is based on the continuum of HIV testing, prevention, treatment and care ().
Continuum of care and relevant sections of the guideline.
The chapters of the guidelines include the following information.
Chapter 2 summarizes existing WHO guidance on HIV testing services, including information to be provided during pre- and post-test counselling, approaches to service delivery and considerations for priority populations. New recommendations on the timing of and approaches to virological testing among infants and the use of new testing technologies are also provided.
Chapter 3 addresses the use of ARV drugs for HIV prevention. A new recommendation on the introduction of the dapivirine vaginal ring as an HIV combination prevention option for women with substantial risk of HIV infection is included (8,9). Recommendations on oral pre- and post-exposure prophylaxis, including infant ARV drug prophylaxis, are summarized and the importance of combination HIV prevention approaches is discussed.
Chapter 4 addresses ART for people living with HIV, including when to start treatment (first-line regimens for adults, adolescents and children) and what regimens to switch to (second- and third-line treatment). The chapter contains key recommendations on rapid ART initiation, the introduction of dolutegravir (DTG) as the preferred option in first-line regimens and in second-line regimens (if not previously used in first-line ART). Updated recommendations on the timing of ART for people with TB and infant feeding by women with HIV are summarized. The chapter includes new recommendations on the use of new point-of-care technologies for viral load testing and treatment monitoring as well as a detailed summary of guidance on managing toxicity related to ARV drugs and key ARV drug–drug interactions.
Chapter 5 summarizes the package of care for people with advanced HIV disease and the clinical management of cryptococcal disease and histoplasmosis. This package of advanced HIV care includes screening for TB, providing TB preventative therapy, testing and pre-emptive treatment for cryptococcal disease, providing co-trimoxazole and enhanced adherence counselling. It also links to the new WHO systematic screening guidance for TB and the most recent WHO guidance for TB preventive therapy.
Chapter 6 summarizes existing WHO guidance on the management of common coinfections and comorbidities associated with HIV, including the use of co-trimoxazole preventive therapy, TB case finding and treatment of latent and active TB and managing viral hepatitis. The chapter includes a new section on cervical cancer that introduces new recommendations and good practice statements on screening and treatment of cervical precancerous lesions among women living with HIV. New recommendations are presented for the diagnosis and management of HIV and Buruli ulcer coinfection and treatment of HIV and visceral leishmaniasis coinfection. The importance of assessing and managing the risk of noncommunicable diseases among people living with HIV is highlighted by recommendations on assessing and managing cardiovascular disease and mental health disorders.
Chapter 7 discusses key service delivery issues related to providing ART with a greater focus on person-centred care. Reduced frequency of clinic visits and medication refills for people established on ART and more convenient and accessible ARV drug distribution approaches are recommended to further reduce the burden on clients and health facilities. New recommendations are also provided to help strengthen linkage to care following HIV diagnosis and long-term retention in care, including community-based approaches to support adherence. Integration of sexual and reproductive health and rights services with HIV services has been revalidated, and a new recommendation on integrating noncommunicable disease services with HIV services has been made. Guidance on task sharing, integration and decentralization of services is summarized with new guidance on task sharing and integration of diagnostic services. New guidance in this chapter emphasizes the importance of providing psychosocial support interventions to meet the particular needs of adolescents. The chapter reiterates the importance that the health interventions for key populations, which do not differ from those for other people at risk of or living with HIV, reach these groups, whose access is often compromised, and the approaches for delivering services may therefore need to be adapted.
Chapter 8 summarizes a range of recommended approaches to monitoring and evaluating programmes along the continuum of testing, prevention and care, including using recommended programme indicators and strategies to monitor ARV toxicity and ARV drug resistance.
Chapter 9 outlines the processes for disseminating these new guidelines.
The annexes include reference tables to support key recommendations.
Supplementary materials will be forthcoming.