This chapter summarizes guidance on managing people presenting for health care with advanced HIV disease. For the full set of guidelines, please see WHO’s previous guidelines for managing advanced HIV disease (1).
5.1. Introduction
In 2015, WHO recommended that all people living with HIV start ART irrespective of clinical or immune status. Most national guidelines have adopted this recommendation (2). However, despite this progress, up to half the people living with HIV continue to present to care with advanced HIV disease.
WHO defines advanced HIV disease for adults and adolescents (and children five years and older) as having a CD4 cell count of less than 200 cells/mm3 or WHO clinical stage 3 or 4 disease (3). All children younger than five years living with HIV are considered to have advanced HIV disease.
Children older than two years who have been receiving ART for more than one year and are clinically stable should not be considered to have advanced disease and should be eligible for multimonth ART dispensing (subsection 5.6).
Advanced HIV disease includes people presenting to care for the first time following an HIV diagnosis and people who have treatment failure and consequent decline in CD4 cell count. Individuals who had previously initiated ART and are re-engaging with care after a period of ART interruption should be assessed for advanced HIV disease and should be offered the advanced HIV disease package as appropriate.
People presenting with advanced HIV disease are at high risk of death, even after starting ART, with the risk increasing with decreasing CD4 cell count, especially with CD4 cell count <100 cells/mm3 (3–6). Advanced HIV disease is also associated with increased health-care costs (7), increased risk of opportunistic infections, immune reconstitution inflammatory syndrome, incomplete immune reconstitution, higher viral reservoirs, higher inflammation, increased risk of AIDS-related and non-AIDS-related comorbidities, use of more health-care services and more frequent monitoring needs.
5.2. Causes of morbidity and mortality among adults with advanced HIV disease
Leading causes of mortality among adults with advanced HIV disease globally include TB, severe bacterial infections, cryptococcal disease, histoplasmosis, toxoplasmosis and Pneumocystis jirovecii pneumonia. Other invasive fungal infections have been recently estimated as contributing significantly to the number of people dying from AIDS-related causes (8).
TB
TB is the leading cause of morbidity and mortality among people living with HIV (9). In 2019, an estimated 1.2 million (range, 1.1 million–1.3 million) HIV-negative people died from TB (a reduction from 1.7 million in 2000), and an additional 208 000 HIV-positive people died from TB (range, 177 000–242 000) (a reduction from over 678 000 in 2000) (10). TB also remains a leading cause of HIV-associated hospitalization among adults and children living with HIV worldwide (11). See section 6.5 for more information on managing people coinfected with TB and HIV.
Severe bacterial infections
People with advanced HIV disease frequently have severe bacterial infections, including bloodstream, respiratory, central nervous system and gastrointestinal infections (12). The burden of mortality and morbidity attributable to severe bacterial infections is poorly characterized, largely because appropriate diagnostic testing facilities are lacking. Severe bacterial infections are estimated to cause more than one third of the hospitalizations among adults and children living with HIV worldwide (13).
Invasive fungal infections
Cryptococcal disease
By far the most common presentation of cryptococcal disease is cryptococcal meningitis, which accounts for an estimated 15% of all people dying from AIDS-related causes globally, three quarters of which are in sub-Saharan Africa (14). Less common presentations of cryptococcal disease include pulmonary disease, skin, lymph node and bone involvement. Cryptococcal disease is less common among young children than among adults. Subsection 5.4 provides more details about managing cryptococcal disease among people with advanced HIV disease.
Histoplasmosis
Histoplasmosis is a fungal disease mostly reported in the WHO Region of the Americas, but it has also been reported in countries in Asia and Africa (15). Histoplasmosis is highly endemic in some regions of Central and South America and is a major opportunistic infection among people living with HIV (15). Thousands of people living with HIV with advanced disease are estimated to die from histoplasmosis each year (8). A major concern about histoplasmosis is misdiagnosing it as TB and the high frequency of co-occurrence (about 20%) because of lack of rapid and accurate diagnosis (16). Subsection 5.5 provides more details about managing histoplasmosis among people with advanced HIV disease.
Pneumocystis jirovecii pneumonia
Pneumocystis jirovecii pneumonia is a leading cause of mortality among hospitalized adults (13%) and children (29%) living with HIV (13). However, the global burden of morbidity and mortality attributable to P. jirovecii pneumonia is poorly characterized because appropriate diagnostic testing facilities are lacking in most settings.
Toxoplasmosis
Cerebral toxoplasmosis is the most frequent cause of expansive brain lesions among adults living with HIV not receiving co-trimoxazole. Toxoplasmosis is a common protozoan infection among people with HIV, with the prevalence of coinfection especially high in sub-Saharan Africa (45%), Latin America and the Caribbean (49%) and North Africa and the Middle East (61%) (17). People with latent toxoplasmosis infection are at risk of developing cerebral toxoplasmosis when their CD4 count falls below 200 cells/mm3.
Other important fungal infections
Fungal infections other than those caused by Cryptococcus species and P. jirovecii, notably histoplasmosis and talaromycosis, are associated with advanced HIV disease in specific geographical areas.
Talaromycosis (formerly known as penicilliosis) is a systemic mycosis that is endemic to many countries in South-East Asia, including parts of China and India, and is a leading cause of HIV-associated mortality, especially among individuals with a CD4 cell count <100 cells/mm3. Untreated disseminated infection is usually fatal, and even when appropriate therapy is provided mortality rates among hospitalized people are up to 30% (18,19).
Emergomycosis and other dimorphic fungal pathogens are emerging around the world. The emergence of novel species, such as Emergomyces africanus, is adding challenges to the clinical care of immunocompromised people, including those with advanced HIV disease (20). Lack of knowledge about diagnosis, treatment and care are key aspects for further work.
Cytomegalovirus disease
Cytomegalovirus infection is a systemic viral infection that usually manifests as cytomegalovirus retinitis among severely immunocompromised people; the reported prevalence of cytomegalovirus retinitis is highest in Asia and appears to be low in Africa (21).
Wasting syndrome and malnutrition
Malnutrition and wasting are an important cause of hospitalization, responsible for 3% of hospitalizations overall, rising to 12% in the WHO African Region (13). Nutritional assessment (anthropometry and clinical and dietary assessment), counselling and support should be an integral component of HIV care and conducted at enrolment in care and monitored across the care continuum. Children with advanced HIV disease commonly present with malnutrition.
Assessing advanced HIV disease
CD4 cell count is the best indicator of disease stage and immediate risk of death and thus should be used to identify people with advanced HIV disease. If access to CD4 count is limited or unavailable, WHO staging should be used. For children from five years of age, adolescents and adults, advanced HIV disease is defined as the presence of a CD4 cell count <200 cells/mm3 or WHO clinical stage 3 or 4. All children younger than five years (who are not already receiving ART and clinically stable) are considered to have advanced HIV disease.
Everyone entering or re-entering care should receive a CD4 test at treatment baseline and as clinically indicated for people who are severely ill, clinically unstable or have advanced HIV disease. A person receiving ART is considered clinically stable based on the following criteria: receiving ART for at least six months, no current illnesses, good understanding of lifelong adherence and evidence of treatment success within the past six months (such as all viral load measurements <1000 copies/mL).
CD4 cell count testing can be performed using a variety of technologies, including laboratory-based CD4 analysers, point-of-care technologies, and device-free semi-quantitative rapid tests (22). Many countries have one or more of these options already available from previous investments made when CD4 cell count was used to set priorities among people living with HIV initiating ART. It is suggested that countries map their CD4 network and identify the best technologies and potential mix useful for their context, considering testing volume needs, health-care facility distribution and key characteristics of each assay, such as time to obtain results, throughput and costs. Although same-day point-of-care CD4 cell count testing supported more rapid ART initiation before the “treat all” policy was adopted (23), the clinical benefits of using same-day point-of-care CD4 cell count testing to more rapidly and effectively identify people living with advanced HIV disease has not yet been studied. However, given the high rates of morbidity and mortality observed among people living with advanced HIV disease, more rapidly identifying people with advanced HIV disease and providing the advanced HIV disease package of care are likely to improve outcomes. To support rapid and, ideally, same-day identification, several technologies are available, both with and without devices (24). As with any diagnostic assay, careful consideration should be given to human resource requirements, quality assurance and service and maintenance (if device-based). Lack of same-day availability of CD4 count results should not be a barrier to initiating ART on the same day. In settings with limited or no access to laboratory-based CD4 cell count and available point-of-care CD4, it may be considered acceptable for use in the context of the advanced HIV disease package, noting the limitation that a point-of-care test is unable to differentiate between an individual who has a CD4 cell count of less than 100 cells/mm3 and a cell count between 100 and 200 cells/mm3.
5.3. Providing a package of care
To address these leading causes of morbidity and mortality among people with advanced HIV disease, WHO recommends that a package of interventions, including screening, treatment and/or prophylaxis for major opportunistic infections, rapid ART initiation and intensified adherence support interventions, be offered to everyone (all populations and age groups) living with HIV presenting with advanced HIV disease (1).
Recommendation (2017)
A package of interventions including screening, treatment and/or prophylaxis for major opportunistic infections, rapid ART initiation and intensified adherence support interventions should be offered to everyone presenting with advanced HIV disease(strong recommendation, moderate-certainty evidence).
Source: Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy (26).
Rationale for this recommendation
The rationale for this recommendation is based on two randomized controlled trials: REMSTART (25) and REALITY (26).
REMSTART (25) was conducted in the United Republic of Tanzania and Zambia and randomized 1999 ART-naive adults living with HIV with CD4 count <200 cells/mm3 to either standard care or standard care plus enhanced clinic-based care with serum cryptococcal antigen screening and pre-emptive antifungal treatment for those who tested cryptococcal antigen–positive and additional community support (comprising a weekly home or community visit by trained and paid lay workers who delivered ART, provided adherence support and monitored participants for signs and symptoms of drug toxicity or new symptoms). The intervention group had 28% fewer people dying: mortality was 13% in the intervention group versus 18% in the group receiving standard care.
REALITY (26) enrolled 1805 mainly adults living with HIV (72 were 5–17 years old) with CD4 counts <100 cells/mm3 in Kenya, Malawi, Uganda and Zimbabwe. All underwent screening for active TB at enrolment and then were randomized to the standard of care (co-trimoxazole) according to national guidelines or an enhanced prophylaxis package: 12 weeks of fluconazole (100 mg once daily), 12 weeks of a fixed-dose combination of co-trimoxazole (800 + 160 mg) + isoniazid (300 mg) + pyridoxine (25 mg) as a scored once-daily tablet, five days of 500 mg of azithromycin once daily and a single dose of 400 mg of albendazole. All drugs were started simultaneously, and ART was offered on the same day as the prophylaxis package.
The enhanced prophylaxis package at the time of ART initiation reduced mortality by 27% (from 12.2% to 8.9%) over 24 weeks. Mortality from Cryptococcus species declined considerably, from 1.5% to 0.4%, and mortality from unascertained causes (most people died at home) declined from 6.0% to 3.8%. TB incidence was reduced by 28%, cryptococcal disease by 62% and hospitalization by 17% in the enhanced prophylaxis group versus the standard-of-care group. Most of the deaths in this study occurred within the first three weeks, highlighting the value of early prophylaxis for people with advanced disease.
Implementation considerations
Providing a package of essential interventions focuses attention on preventing, diagnosing and treating the most common causes of morbidity and mortality among people with advanced HIV disease. Identifying people with advanced HIV disease who are eligible for elements of a package of care requires CD4 testing. In addition, determining the immune status of people whose treatment is failing according to virological criteria can help in guiding clinical management decisions. See Chapter 4 on the treatment monitoring algorithm.
Attention should also be paid to other important causes of severe illness not covered by the package, especially in regions in which specific comorbidities and coinfections are prevalent. Of note, increased pill burden and side-effects may affect treatment adherence. To support treatment adherence, shorter regimens for TB preventive treatment are recommended (27). Identifying suitable screening tools for use is also an important research gap.
summarizes the specific components of the package of interventions that should be offered to people presenting with advanced HIV disease. For detailed guidance on using systematic TB screening for people, including screening tools recommended for people living with HIV and diagnostic tools such as lateral flow urine lipoarabinomannan assay (LF-LAM), WHO-approved molecular rapid diagnostics and TB preventive treatment, see the consolidated guidelines and operational handbooks for TB modules 1, 2 and 3 (27–29).
Clinical considerations
The role of presumptive treatment in managing cryptococcal disease and histoplasmosis as well as preventive therapy for TB, P. jirovecii pneumonia and bacterial infections should be considered in settings in which access to diagnostic tests is limited and people present with typical or possible signs and symptoms (especially when accompanied by clinical signs indicating severe illness). A seriously ill adult is defined as having any of the following danger signs: respiratory rate ≥30 breaths per minute; heart rate ≥120 beats per minute; or unable to walk unaided. Other clinical conditions, such as body temperature ≥39°C, can also be considered based on local epidemiology and clinical judgement.
People with advanced HIV disease may start both ART and prophylaxis at the same time (26). However, ART initiation should be deferred when clinical symptoms suggest TB meningitis or cryptococcal meningitis to avoid paradoxical worsening of the existing infection which can be life-threatening (30).
Components of the package of care for people with advanced HIV disease.
5.4. Overview of clinical management of cryptococcal disease
Cryptococcal disease is one of the most important opportunistic infections among people living with advanced HIV disease and is a major contributor to mortality (14,31–33). Cryptococcus neoformans, the causative agent of cryptococcal disease, is present in the environment worldwide. Exposure occurs through inhalation.
In 2018, WHO published Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children (34).
Early diagnosis and treatment of cryptococcal meningitis is key to reducing mortality from cryptococcal disease. Health-care professionals should have a low threshold for suspecting cryptococcal meningitis among people with advanced HIV disease. Countries should give priority to reliable access to rapid diagnostic cryptococcal antigen assays, preferably lateral flow assays, for use in CSF, serum, plasma or whole blood.
Of importance, immediate ART initiation is not recommended for adults, adolescents and children living with HIV who have cryptococcal meningitis because of the risk of increased mortality and ART initiation should be deferred 4–6 weeks from the initiation of antifungal treatment.
Box 5.1 summarizes the recommendations, which are all based on evidence reviewed by the Guideline Development Group (34).
Box 5.1Summary of recommendations (2018)
Diagnosis of cryptococcal meningitis
For adults, adolescents and children living with HIV suspected of having a first episode of cryptococcal meningitis, prompt lumbar puncture with measurement of CSF opening pressure and rapid cryptococcal antigen assay is recommended as the preferred diagnostic approach (34)
(strong recommendation, moderate-certainty evidence for adults and adolescents).
The following diagnostic approaches are recommended, according to the context.
Settings with ready access to and no contraindication for lumbar puncture
- 1.
If both access to a cryptococcal antigen assay (either lateral flow assay or latex agglutination assay) and rapid results (less than 24 hours) are available: lumbar puncture with rapid CSF cryptococcal antigen assay is the preferred diagnostic approach (34)
(strong recommendation, moderate-certainty evidence for adults and adolescents).
- 2.
If access to a cryptococcal antigen assay is not available and/or rapid results are not available: lumbar puncture with CSF India ink test examination is the preferred diagnostic approach (34)
(strong recommendation, moderate-certainty evidence for adults and adolescents).
Settings without immediate access to lumbar puncture or when lumbar puncture is clinically contraindicated such as significant coagulopathy or suspected space-occupying lesion based on focal nervous system signs or recurrent seizures
- 1.
If both access to a cryptococcal antigen assay and rapid results (less than 24 hours) are available: rapid serum, plasma or whole-blood cryptococcal antigen assays are the preferred diagnostic approaches (34)
(strong recommendation, moderate-certainty evidence for adults and adolescents).
- 2.
If a cryptococcal antigen assay is not available and/or rapid access to results is not ensured: prompt referral for further investigation and treatment as appropriate (34)
(strong recommendation, moderate-certainty evidence for adults and adolescents).
Prevention and screening
Overarching principle
Screening for cryptococcal antigen is the optimal approach for guiding resources in a public health approach and is the preferred approach for identifying infection when managing people aged 10 years or older presenting with advanced HIV disease (25).
Recommendations
Screening for cryptococcal antigen followed by pre-emptive antifungal therapy (35)a among cryptococcal antigen–positive people to prevent the development of invasive cryptococcal disease are recommended before initiating or reinitiating ART for adults and adolescents living with HIV who have a CD4 count <100 cells/mm3 (36)
(strong recommendation, moderate-certainty evidence).
This may be considered at a higher CD4 cell count threshold of <200 cells/mm3 (36)
(conditional recommendation, moderate-certainty evidence).
All people living with HIV with a positive cryptococcal antigen result on screening should be carefully evaluated for signs and symptoms of meningitis and undergo a lumbar puncture, if feasible, with CSF examination and India ink or CSF cryptococcal antigen assay to exclude active cryptococcal disease. India ink has low sensitivity, and a negative result on India ink should be confirmed by CSF cryptococcal antigen testing.
When cryptococcal antigen screening is not available, fluconazole primary prophylaxis should be given to adults and adolescents living with HIV who have a CD4 count <100 cells/mm3 (37)
(strong recommendation, moderate-certainty evidence).
This may be considered at a higher CD4 cell count threshold of <200 cells/mm3 (36)
(conditional recommendation, moderate-certainty evidence).
Treatment
Induction
The following is recommended as the preferred induction regimen.
The following induction regimens are recommended as alternative options.
Two weeks of fluconazole (1200 mg daily, 12 mg/kg per day for children and adolescents) + flucytosine (100 mg/kg per day, divided into four doses per day) (
39)
(strong recommendation, moderate-certainty evidence).
Two weeks of amphotericin B deoxycholate (1.0 mg/kg per day) + fluconazole (1200 mg daily, 12 mg/kg per day for children and adolescents up to a maximum of 800 mg daily) (
39)
(strong recommendation, moderate-certainty evidence).
Consolidation
Fluconazole (400–800 mg daily for adults or 6–12 mg/kg per day for children and adolescents up to a maximum of 800 mg daily) is recommended for the consolidation phase (for eight weeks following the induction phase) (40,41)
(strong recommendation, low-certainty evidence).
Maintenance (or secondary prophylaxis)
Fluconazole (200 mg daily for adults or 6 mg/kg per day for adolescents and children) is recommended for the maintenance phase (42–44)
(strong recommendation, high-certainty evidence).
Using adjunctive systemic corticosteroids in treating cryptococcal meningitis
Routine use of adjunctive corticosteroid therapy during the induction phase is not recommended in treating adults, adolescents and children who have HIV-associated cryptococcal meningitis (45)
(strong recommendation, high-certainty evidence for adults and adolescents).
Timing of ART
Immediate ART initiation is not recommended among adults, adolescents and children living with HIV who have cryptococcal meningitis because of the risk of increased mortality and should be deferred 4–6 weeks from the initiation of antifungal treatment (46–49)
(strong recommendation, low-certainty evidence for adults).
- a
The Southern African HIV Clinicians’ Society recommends starting ART two weeks after starting fluconazole, and consideration is being given to starting ART immediately if lumbar puncture excludes cryptococcal meningitis among people who test positive for whole-blood cryptococcal antigen.
The Guidelines on the diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children (34) also set out good practice principles ().
Preventing, monitoring and managing amphotericin B toxicity. For people living with HIV receiving amphotericin B–containing regimens for treating cryptococcal disease, a minimum package of preventing, monitoring and managing toxicity is recommended to minimize the serious types of amphotericin B–related toxicity, especially hypokalaemia, nephrotoxicity and anaemia (50–53).
Monitoring for and managing raised intracranial pressure. Adults, adolescents and children living with HIV with suspected cryptococcal meningitis should have an initial lumbar puncture and an early repeat lumbar puncture (within 3–5 days) with measurement of CSF opening pressure to assess for raised intracranial pressure regardless of the presence of symptoms or signs of raised intracranial pressure (54,55).
Managing raised intracranial pressure. Therapeutic lumbar puncture: relieve pressure by draining a volume sufficient to reduce the CSF pressure to <20 cm or halving the baseline pressure if extremely high; the persistence or recurrence of symptoms or signs of raised intracranial pressure should determine the frequency of repeat therapeutic lumbar puncture. For people with persistent symptoms of intracranial pressure, repeat daily therapeutic lumbar puncture (with measurement of CSF opening pressure where available) and CSF drainage, if required, are recommended until the symptoms resolve or the opening pressure is normal for at least two days (34).
Monitoring treatment response. Clinical response (including resolution or recurrence of fever, headache and symptoms or signs of raised intracranial pressure) should be assessed daily during the initial two weeks of induction therapy. Among people for whom evidence indicates a sustained clinical response, routine follow-up lumbar puncture after completing induction treatment to assess antifungal treatment response (CSF fungal culture and CSF cryptococcal antigen) or serum or plasma cryptococcal antigen is not recommended in low- and middle-income countries (34).
Managing treatment failure. For people who present with cryptococcal meningitis relapse, the following steps are recommended: start or restart induction treatment according to the recommendations for induction treatment; manage raised intracranial pressure with therapeutic lumbar puncture; and provide adapted adherence support. If ART has not already started, initiating ART after 4–6 weeks of optimal antifungal therapy is recommended (34).
Paradoxical cryptococcal immune reconstitution inflammatory syndrome occurs among 10–50% of people with cryptococcal disease initiating ART (56) and is associated with high mortality (57). The median time to onset in reported cohort studies is 1–10 months but typically is 3–12 weeks after initiating ART (56).
Raised intracranial pressure is a common feature of cryptococcal immune reconstitution inflammatory syndrome and an important contributor to high mortality (58). Multiple repeat lumbar puncture may be necessary. Optimizing antifungal therapy and reinduction with an amphotericin-based regimen are important if suboptimal antifungal treatment is considered to contribute to developing immune reconstitution inflammatory syndrome (34).
Scenarios for cryptococcal diagnostic testing.
5.5. Overview of clinical management of histoplasmosis
Histoplasmosis is a disease caused by the fungus Histoplasma capsulatum; the most frequent clinical presentation among people living with HIV is disseminated histoplasmosis. Symptoms of disseminated histoplasmosis are nonspecific and may be indistinguishable from those of other infectious diseases, especially TB, thus complicating diagnosis and treatment (59). Histoplasmosis is highly endemic in some regions of North America, Central America and South America and is also reported in certain countries of Asia and Africa.
The lack of access to appropriate antifungal therapies and in vitro diagnostics for rapid detection of histoplasmosis and the co-occurrence of other infectious diseases, especially TB, may affect clinical outcomes and underlie the high mortality of disseminated histoplasmosis among people living with HIV (16,60).
Severe or moderately severe histoplasmosis is defined as the presence of at least one sign or symptom involving vital organs: respiratory or circulatory failure, nervous system signs, renal failure, coagulation anomalies and a general alteration of the WHO performance status greater than 2, in which the person is confined to a bed or chair more than half of the waking hours and only capable of limited self-care (61).
In 2020, WHO published Guidelines on diagnosing and managing disseminated histoplasmosis among people living with HIV (61). Box 5.2 summarizes the recommendations, which are all based on evidence reviewed by the Guideline Review Committee (61).
Box 5.2Summary of recommendations (2020)
Diagnosis of disseminated histoplasmosis among people living with HIV
Among people living with HIV, disseminated histoplasmosis should be diagnosed by detecting circulating Histoplasma antigens (62)
(conditional recommendation, low-certainty evidence).
Induction therapy
Treating people living with HIV for severe or moderately severe histoplasmosis: liposomal amphotericin B, 3.0 mg/kg, for two weeks is recommended. In settings in which liposomal amphotericin B is unavailable, deoxycholate amphotericin B, 0.7–1.0 mg/kg, is recommended for two weeks (63–66)
(conditional recommendation, very-low-certainty evidence).
As a good practice for people with renal failure, or at risk of renal injury, measures to prevent or treat toxicity are recommended.
Induction therapy should be given for two weeks. Since deoxycholate amphotericin B may be associated with renal toxicity, therapy may need to be shorter than two weeks based on the clinical assessment of how the person responds to treatment. Involvement of the central nervous system may require extending induction therapy or increasing dosage.
Treating people living with HIV for mild to moderate histoplasmosis: itraconazole 200 mg three times daily for three days and then 200 mg twice daily is recommended (67,68)
(conditional recommendation, very-low-certainty evidence).
Maintenance therapy
Itraconazole 200 mg twice daily for 12 months is recommended (69–71)
(conditional recommendation, very-low-certainty evidence).
Less than 12 months of therapy can be considered when the person is clinically stable, receiving ART, has suppressed viral load and the immune status has improved (72)
(conditional recommendation, very-low-certainty evidence).
Timing of ART initiation
ART should be initiated as soon as possible among people with disseminated histoplasmosis for whom central nervous system involvement is not suspected or proven (48)
(conditional recommendation, very-low-certainty evidence).
TB therapy for people coinfected with TB, HIV and histoplasmosis
People living with HIV who also have TB and histoplasmosis coinfection should receive TB therapy according to WHO treatment guidelines (61)
(conditional recommendation, very-low-certainty evidence).
5.6. Advanced HIV disease among children and adolescents
All children younger than five years (who are not already receiving ART and clinically stable) are considered to have advanced disease because evidence shows that 80% of all children initiating ART have severe immunosuppression.
Advanced HIV disease is defined as WHO stage 3 or 4 or a CD4 count <200 cells/mm3 for children five years or older (the same definition used for adults). All children younger than five years living with HIV are considered as having advanced HIV disease, although those who have been receiving ART for more than one year and are established on ART and older than two years should not be considered to have advanced disease and should be eligible for multimonth dispensing.
Children and adolescents who had previously initiated ART and are re-engaging with care after a period of ART interruption should be assessed for advanced HIV disease and should be offered the advanced HIV disease package as appropriate.
Major causes of morbidity and mortality
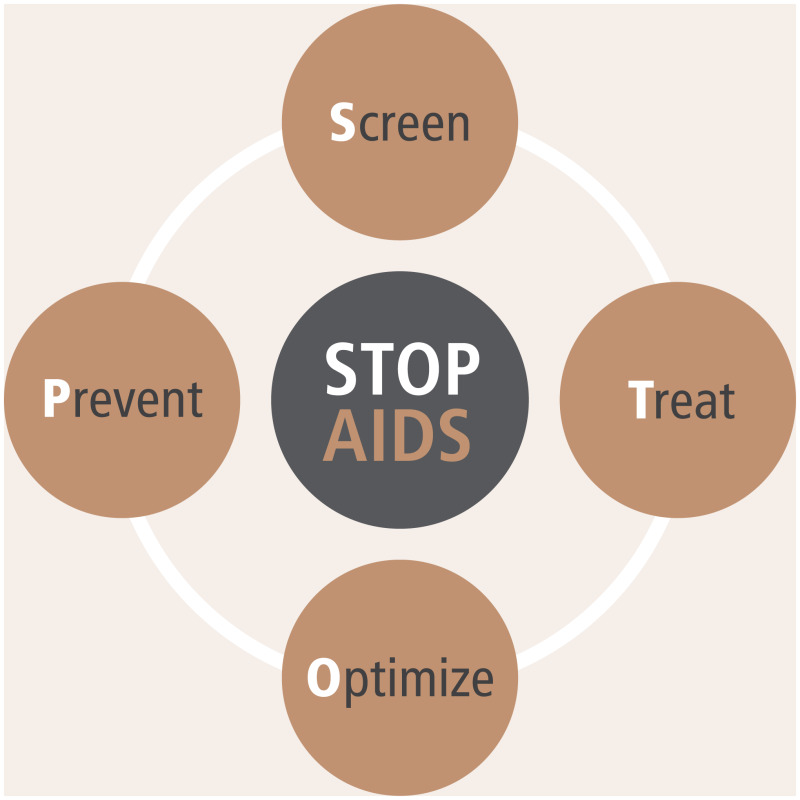
The major causes of morbidity and mortality among children living with HIV in low- and middle-income countries are pneumonia (including P. jirovecii pneumonia), TB, bloodstream infections, diarrhoeal disease and severe acute malnutrition. No randomized controlled trials have included children to determine the optimal package of care for advanced HIV disease for children. However, the main interventions known to reduce morbidity and mortality among children living with HIV can be summarized as screen, treat, optimize and prevent AIDS ( and Box 5.3).
These recommendations include screening for TB (), severe malnutrition and (for adolescents) cryptococcal meningitis; treatment of TB, severe pneumonia, severe bacterial infections and malnutrition (as well as cryptococcal meningitis); rapid ART unless there are signs of meningitis (as for adults) with appropriate measures to prevent TB disease, pneumococcal disease and other vaccine-preventable diseases. In addition, routine interventions recommended by WHO for children in general such as deworming, malaria prophylaxis, iron and vitamin A supplementation and growth monitoring should all be provided.
Screening, diagnosis and prevention components of the package of care for children and adolescents with advanced HIV disease.
The main differences in the package of care for children compared with adolescents and adults is that routine cryptococcal antigen screening and pre-emptive therapy are not recommended for children younger than 10 years because of the low prevalence of cryptococcal meningitis in this age group. However, if a child younger than 10 years presents with signs and symptoms of meningitis, cryptococcal meningitis should still be considered and the appropriate investigations and treatment for this should be implemented ().
The burden of TB is still high among children living with HIV. and Box 5.3 highlight the main recommendations for TB screening. Data on LF-LAM among children is limited and recommendations are largely extrapolated from adults. Treatment for drug-sensitive TB among children comprises a four-drug regimen that includes rifampicin (R), isoniazid (H), pyrazinamide (Z) and ethambutol (E) to be provided with available child-friendly, fixed-dose combinations in dispersible formulations to decrease the pill burden and facilitate administration for young children. Drug–drug interactions between rifampicin and LPV/r or DTG need to be considered and ART dosing adjusted accordingly.
Although rapid ART initiation within seven days of diagnosis is a priority, especially for children older than five years, children who require hospitalization for severe acute malnutrition, TB meningitis or other illnesses need to be clinically stabilized first. However, initiating ART is encouraged as part of the child’s hospital admission, since referral after discharge may lead to loss to follow-up and failure to initiate ART. Among children with signs of or confirmed TB meningitis, the start of ART should be delayed in accordance with existing guidelines. Similarly, ensuring linkage to the facility in which the child will receive ongoing HIV care on discharge is critical.
Prevention of opportunistic infections in advanced HIV disease among children consists mostly of rapid and optimal ART initiation, preventing severe TB disease with BCG and TB preventive treatment (mainly with isoniazid while drug–drug interactions with rifapentine are ruled out), preventing P. jirovecii pneumonia with co-trimoxazole prophylaxis and administering age-appropriate vaccinations and catch-up vaccine administration when indicated ( and Box 5.3).
Box 5.3Screen, Treat, Optimize and Prevent AIDS among children
View in own window
| Screena |
|---|
|
TB
|
Screen for TB using available screening tools as indicated bFor those who screen positive, use the following diagnostic tests to confirm TB as applicable c:
- –
Rapid molecular diagnostic on (induced) sputum, stool, gastric aspirate or nasopharyngeal aspirate or other extrapulmonary samples if relevant - –
LF-LAM assayd
|
|
Cryptococcal infection among adolescents
| Serum or plasma or blood cryptococcal antigen screening followed by lumbar puncture if positive or symptomatic |
|
Malnutrition
|
|
| Treat |
|---|
|
TB, severe pneumonia, severe bacterial infections, cryptococcal meningitis and severe acute malnutrition
| In accordance with WHO guidelines |
| Optimize |
|---|
|
Rapid ART start
| Preferably same-day but no later than seven days after diagnosis with optimal regimense |
|
ART counselling
| In accordance with WHO guidelines |
| Prevent |
|---|
|
Bacterial infections and P. jirovecii pneumonia
| Co-trimoxazole prophylaxis |
|
TB
| TB preventive treatment |
|
Cryptococcal meningitis among adolescents
| Fluconazole pre-emptive therapy if cryptococcal antigen positive or cryptococcal antigen unavailable |
|
Vaccinations
|
Pneumococcal vaccine Human papillomavirus Measles BCG
|
- a
Screening refers to screening and diagnostics throughout this publication.
- b
For screening algorithms and screening tools, see WHO consolidated guidelines on tuberculosis: module 1: prevention: tuberculosis preventive treatment (28) and WHO operational handbook on tuberculosis: module 1: prevention: tuberculosis preventive treatment (75). Screening and diagnosis of TB for adolescents is the same as for adults.
- c
A negative test result does not exclude TB for children living with HIV for whom there is a strong clinical suspicion of TB.
- d
Package of care for children and adolescents with advanced HIV disease: stop AIDS: technical brief (76).
- e
Unless TB or cryptococcal meningitis is diagnosed (77).
Implementation considerations
Aligning recommendations across multiple guidelines is essential. Guidelines relating not only to HIV (for example, TB and HIV guidelines relating to TB preventive treatment) but also to routine child health and development interventions (vitamin A, deworming and the Expanded Programme on Immunization) should align as much as possible to prevent multiple visits to health services.
At the facility level, centres introducing the advanced HIV disease package for children should provide a child-friendly environment and ensure access to child-specific resources such as drug formulations for children, a mid-upper arm circumference tape, stadiometer, appropriate scales and expertise in phlebotomy for children. Health-care providers should be sensitized on child-specific issues such as growth monitoring and other routine child health interventions. Efforts should additionally be put in place to support and equip parents and caregivers to recognize warning signs and be able to reliably administer the prescribed medications Country-specific programmes with advanced HIV disease services specifically for children have been successfully implemented (76).
Research gaps
Multiple research gaps exist in addressing prevention and care for children living with advanced HIV disease. Better tools are needed to screen and diagnose TB among children living with HIV. Better diagnostics, including the need to develop simplified point-of-care diagnostics for pneumonia (including P. jirovecii pneumonia) and for cytomegalovirus disease, whether to empirically treat for TB and/or cytomegalovirus disease among children living with HIV who present with severe pneumonia and what the optimal package of prophylactic interventions for children living with HIV younger than five years should be are all example of critical knowledge gaps.
5.7. Supporting decision-making for providing a package of care
The algorithm for providing a package of care for people with advanced HIV disease () helps to support decision-making for providing care for people with advanced HIV disease (1).
Considerations for specific adult groups and populations
Pregnant and breastfeeding women
The package of care for pregnant and breastfeeding women with advanced HIV is the same as for non-pregnant adults. However, more evidence is needed to support the use of shorter TB preventive treatment regimens in this population. In addition, WHO guidelines on antenatal care provide recommendations on nutrition support, disease prevention and managing common physical symptoms and infant feeding support for women who cannot breastfeed (78).
Region-specific comorbidities and coinfections
Consideration should be given to regional differences in comorbidities and coinfections that may require additional prophylactic, diagnostic and therapeutic options not covered by the package.
5.7.1. People re-engaging with care after treatment interruption or treatment failure
People re-engaging with care after treatment interruption with advanced HIV disease should be offered comprehensive clinical assessment. The package should be given to people who are re-engaging with care after a period of ART interruption or when ART fails and they have developed advanced HIV disease, since such people are likely to benefit from the same set of interventions as ART-naive people with advanced HIV disease.
People interrupting treatment on a NNRTI– containing regimen are at risk of drug resistance and may require more intensive virological monitoring, and consideration should be given to restarting ART using a different regimen – whenever possible a DTG-containing regimen – with a goal of re-establishing viral suppression (79).
For people presenting with diagnoses consistent with treatment failure (defined as a new or recurrent clinical event indicating severe immunodeficiency), WHO recommends viral load testing; CD4 cell count testing is no longer recommended for ART monitoring for people receiving ART who are clinically stable where viral load monitoring is available (77); however, CD4 cell count testing should be specifically prompted for people with a viral load exceeding 1000 copies/mL and for everyone whose clinical presentation suggests advanced HIV disease regardless of ART exposure. For people with suspected treatment failure and advanced HIV disease, CD4 cell count and viral load should be carried out in parallel.
People presenting with advanced HIV disease as a result of treatment failure should also benefit from the advanced HIV disease package, and if they are severely ill, an expedited switch to a new regimen should be considered by reducing the time between the first and second viral load tests (1–3 months) and by paying increased attention to ensuring rapid turnaround and action on the results. Where rapid viral load testing is not available, the decision to switch should be assessed according to the individual clinical presentation. Further research is required to demonstrate the impact of providing such a package of interventions to people presenting with treatment failure: for example, before switching to second-line ART.
5.7.2. Vaccination for people with advanced HIV disease
Providing vaccinations to people living with HIV does not accelerate HIV disease progression and is recommended as an important part of the HIV care package. However, people with severe immunosuppression may be at higher risk of complications from some live attenuated vaccines, and the response to other inactivated vaccines may be less effective because of their degree of immunosuppression. Additional doses or revaccination after immune reconstitution on ART may therefore be required. Nineteen of the 26 WHO vaccination position papers (80) provide guidance for people living with HIV.
Additional assessments
In addition to CD4 cell count testing or WHO clinical staging and TB and cryptococcal testing, the following additional assessments can be considered.
- □
Does the person have signs of being seriously ill? Should this person be admitted to an inpatient facility?
- □
Is the person receiving an ART regimen that may be failing (or has the person interrupted ART)? If so, additional diagnostic tests, particularly a rapid viral load test, and immediate adherence counselling may be considered, and ART regimen switch when appropriate.
Taking history and further investigations or presumptive treatment, as appropriate, for other illnesses should be considered, according to the local epidemiology or patient context if both TB and cryptococcal assessments are negative. This may include investigations for severe bacterial infections, cerebral toxoplasmosis, P. jirovecii pneumonia, other fungal infections (histoplasmosis and talaromycosis) and cytomegalovirus disease as well as lumbar puncture for those with symptoms or signs of meningitis. Additional assessments to consider include haematology to identify anaemia, liver function to identify high alkaline phosphatase that can prompt the diagnosis of hepatic granulomas, ultrasonography, full lymph node examination to assess for suspected lymphoma, skin examination to look for Kaposi’s sarcoma, anal and genital examination to identify severe HPV or anal cancer, examination of parasitic diarrhoea (stool) and neurological or vision examination.
Based on these additional assessments, appropriate and likely rapid treatment of any and all confirmed diagnoses should be considered. If rapid testing of additional potential comorbidities is not possible, consider presumptive treatment, especially if the person is seriously ill.
5.8. Programme considerations
Particular attention should be paid to people with advanced HIV disease who miss a clinic visit after initiating treatment for an opportunistic infection or during the initial months after starting or restarting ART, since they are at risk of high mortality.
Programmes should ensure capacity for actively tracing such people. Ideally, such people should consent to and be linked with a community-based health worker who may visit them at home.
People with advanced HIV disease require closer follow-up during the initial period of receiving ART to monitor the response to ART and to identify signs and symptoms of possible immune reconstitution inflammatory syndrome. The feasibility of the frequency of visits is context specific and may also depend on the person’s ability to travel to the clinical site. People missing appointments should also be rapidly traced by phone or through home visits. Where face-to-face contact is not feasible, distance contact through telephone consultation, mHealth, text messaging or other mobile interventions, or visits through a community health worker or home-based caregiver should be considered, with the consent of the client.
The package of care for people with advanced HIV disease should be offered at both hospitals and decentralized primary care clinics according to the clinical status of the person living with HIV (ambulatory or requiring hospital admission), the clinical skills of the health-care workers and access to diagnostics at the facilities. However, to increase access to the package, improving access at peripheral sites through mobile outreach or decentralization should be encouraged and may be enabled by providing point-of-care diagnostic tests at all levels where feasible (CD4 cell count, cryptococcal antigen testing, LF-LAM testing and molecular TB testing) or through expedited sample transport systems, where necessary.
Where care has been decentralized, clear referral criteria should be established to ensure that people requiring further investigation or specialist management receive services in a timely manner. Likewise, referral mechanisms and optimal communication following discharge back to the peripheral clinic must be implemented to ensure appropriate follow-up.
For hospitalized patients, programmes should provide measures to improve linkage and follow-up after discharge such as outpatient primary care clinic visits and home visits by community health workers to reduce the risks of loss to follow-up and of mortality after discharge.
Finally, programmes should provide guidance on reducing the provision of expanded care such as intensified adherence support and home visits for people receiving ART who are clinically stable with CD4 recovery.
Algorithm for providing a package of care for people with advanced HIV disease.
Any person who has signs of being seriously ill should be referred to the appropriate higher-lever facility for management. A seriously ill adult is defined as having any (more...)
Recommendations for the package of prophylaxis interventions for people with advanced HIV disease.
References
- 1.
- 2.
- 3.
Waldrop
G, Doherty
M, Vitoria
M, Ford
N. Stable patients and patients with advanced disease: consensus definitions to support sustained scale up of antiretroviral therapy. Trop Med Int Health. 2016;21:1124–30. [
PubMed: 27371814]
- 4.
Egger
M, May
M, Chêne
G, Phillips
AN, Ledergerber
B, Dabis
F
et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. [
PubMed: 12126821]
- 5.
Hogg
RS, Yip
B, Chan
KJ, Wood
E, Craib
KJ, O’Shaughnessy
MV
et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. [
PubMed: 11722271]
- 6.
Walker
AS, Prendergast
AJ, Mugyenyi
P, Munderi
P, Hakim
J, Kekitiinwa
A
et al. Mortality in the year following antiretroviral therapy initiation in HIV-infected adults and children in Uganda and Zimbabwe. Clin Infect Dis. 2012;55:1707–18. [
PMC free article: PMC3501336] [
PubMed: 22972859]
- 7.
Krentz
H, Auld
M, Gill
M. The high cost of medical care for patients who present late (CD4 <200 cells/μL) with HIV infection. HIV Med. 2004;5:93–8. [
PubMed: 15012648]
- 8.
Adenis
AA, Valdes
A, Cropet
C, McCotter
OZ, Derado
G, Couppie
P
et al. Burden of HIV-associated histoplasmosis compared with tuberculosis in Latin America: a modelling study. Lancet Infect Dis. 2018;18:1150–9. [
PMC free article: PMC6746313] [
PubMed: 30146320]
- 9.
Gupta
RK, Lucas
SB, Fielding
KL, Lawn
SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29:1987. [
PMC free article: PMC4568896] [
PubMed: 26266773]
- 10.
- 11.
Ford
N, Matteelli
A, Shubber
Z, Hermans
S, Meintjes
G, Grinsztejn
B
et al. TB as a cause of hospitalization and in-hospital mortality among people living with HIV worldwide: a systematic review and meta-analysis. J Int AIDS Soc. 2016;19:20714. [
PMC free article: PMC4712323] [
PubMed: 26765347]
- 12.
Gaskell
KM, Feasey
NA, Heyderman
RS. Management of severe non-TB bacterial infection in HIV-infected adults. Expert review of anti-infective therapy. 2015;13:183–95. [
PubMed: 25578883]
- 13.
Ford
N, Shubber
Z, Meintjes
G, Grinsztejn
B, Eholie
S, Mills
EJ
et al. Causes of hospital admission among people living with HIV worldwide: a systematic review and meta-analysis. Lancet HIV. 2015;2:e438–44. [
PubMed: 26423651]
- 14.
Rajasingham
R, Smith
RM, Park
BJ, Jarvis
JN, Govender
NP, Chiller
TM
et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017;17:873–81. [
PMC free article: PMC5818156] [
PubMed: 28483415]
- 15.
- 16.
- 17.
Wang
Z-D, Wang
S-C, Liu
H-H, Ma
H-Y, Li
Z-Y, Wei
F
et al. Prevalence and burden of
Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV. 2017;4:e177–88. [
PubMed: 28159548]
- 18.
Le
T, Wolbers
M, Chi
NH, Quang
VM, Chinh
NT, Huong Lan
NP
et al. Epidemiology, seasonality, and predictors of outcome of AIDS-associated
Penicillium marneffei infection in Ho Chi Minh City, Viet Nam. Clin Infect Dis. 2011;52:945–52. [
PMC free article: PMC3106230] [
PubMed: 21427403]
- 19.
Hu
Y, Zhang
J, Li
X, Yang
Y, Zhang
Y, Ma
J
et al. Penicillium marneffei infection: an emerging disease in mainland China. Mycopathologia. 2013;175:57–67. [
PubMed: 22983901]
- 20.
- 21.
Ford
N, Shubber
Z, Saranchuk
P, Pathai
S, Durier
N, O’Brien
DP
et al. Burden of HIV-related cytomegalovirus retinitis in resource-limited settings: a systematic review. Clin Infect Dis. 2013;57:1351–61. [
PubMed: 23899681]
- 22.
- 23.
Vojnov
L, Markby
J, Boeke
C, Harris
L, Ford
N, Peter
T. POC CD4 testing improves linkage to HIV care and timeliness of ART initiation in a public health approach: a systematic review and meta-analysis. PLoS One. 2016;11:e0155256. [
PMC free article: PMC4866695] [
PubMed: 27175484]
- 24.
- 25.
Mfinanga
S, Chanda
D, Kivuyo
SL, Guinness
L, Bottomley
C, Simms
V
et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet. 2015;385:2173–82. [
PubMed: 25765698]
- 26.
Hakim
J, Musiime
V, Szubert
AJ, Mallewa
J, Siika
A, Agutu
C
et al. Enhanced prophylaxis plus antiretroviral therapy for advanced HIV infection in Africa. N Engl J Med. 2017;377:233–45. [
PMC free article: PMC5603269] [
PubMed: 28723333]
- 27.
- 28.
- 29.
- 30.
Bahr
N, Boulware
DR, Marais
S, Scriven
J, Wilkinson
RJ, Meintjes
G. Central nervous system immune reconstitution inflammatory syndrome. Curr Infect Dis Rep. 2013;15:583–93. [
PMC free article: PMC3883050] [
PubMed: 24173584]
- 31.
Lawn
SD, Bekker
L-G, Myer
L, Orrell
C, Wood
R. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19:2050–2. [
PubMed: 16260920]
- 32.
Lawn
SD, Harries
AD, Anglaret
X, Myer
L, Wood
R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–908. [
PMC free article: PMC3816249] [
PubMed: 18784453]
- 33.
Park
BJ, Wannemuehler
KA, Marston
BJ, Govender
N, Pappas
PG, Chiller
TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. [
PubMed: 19182676]
- 34.
Guidelines for the diagnosis, prevention, and management of cryptococcal disease in HIV-infected adults, adolescents and children. Supplement to the 2016 consolidated guidelines of the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization; 2018 (
https://apps.who.int/iris/handle/10665/260399, accessed 1 June 2021). [
PubMed: 30285342]
- 35.
Southern African HIV Clinicians Society. Guideline for the prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons: 2013 update. S Afr J HIV Med. 2013;14:2
- 36.
Ford
N, Shubber
Z, Jarvis
JN, Chiller
T, Greene
G, Migone
C
et al. CD4 cell count threshold for cryptococcal antigen screening of HIV-infected individuals: a systematic review and meta-analysis. Clin Infect Dis. 2018;66:S152–9. [
PMC free article: PMC5850628] [
PubMed: 29514236]
- 37.
Awotiwon
AA JS, Rutherford
GW, Meintjes
G, Eshun-Wilson
I. Primary antifungal prophylaxis for cryptococcal disease in HIV-positive people. Cochrane Database Syst Rev. 2018;8:CD004773. [
PMC free article: PMC6513489] [
PubMed: 30156270]
- 38.
Molloy
SF, Kanyama
C, Heyderman
RS, Loyse
A, Kouanfack
C, Chanda
D
et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. N Engl J Med. 2018;378:1004–17. [
PubMed: 29539274]
- 39.
Tenforde
MW, Shapiro
AE, Rouse
B, Jarvis
JN, Li
T, Eshun-Wilson
I
et al. Treatment for HIV-associated cryptococcal meningitis. Cochrane Database Syst Rev. 2018;7;CD005647. [
PMC free article: PMC6513250] [
PubMed: 30045416]
- 40.
Mootsikapun
P, Chetchotisakd
P, Anunnatsiri
S, Choksawadphinyo
K. The efficacy of fluconazole 600 mg/day versus itraconazole 600 mg/day as consolidation therapy of cryptococcal meningitis in AIDS patients. J Med Assoc Thailand. 2003;86:293–8. [
PubMed: 12757072]
- 41.
Van der Horst
CM, Saag
MS, Cloud
GA, Hamill
RJ, Graybill
JR, Sobel
JD
et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med. 1997;337:15–21. [
PubMed: 9203426]
- 42.
Bozzette
SA, Larsen
RA, Chiu
J, Leal
MAE, Jacobsen
J, Rothman
P
et al. A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:580–4. [
PubMed: 1992319]
- 43.
Saag
MS, Cloud
GA, Graybill
JR, Sobel
JD, Tuazon
CU, Johnson
PC
et al. A comparison of itraconazole versus fluconazole as maintenance therapy for AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1999;28:291–6. [
PubMed: 10064246]
- 44.
Powderly
WG, Saag
MS, Cloud
GA, Robinson
P, Meyer
RD, Jacobson
JM
et al. A controlled trial of fluconazole or amphotericin B to prevent relapse of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:793–8. [
PubMed: 1538722]
- 45.
Beardsley
J, Wolbers
M, Kibengo
FM, Ggayi
A-BM, Kamali
A, Cuc
NTK
et al. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med. 2016;374:542–54. [
PMC free article: PMC4778268] [
PubMed: 26863355]
- 46.
Bisson
GP, Molefi
M, Bellamy
S, Thakur
R, Steenhoff
A, Tamuhla
N
et al. Early versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitis. Clin Infect Dis. 2013;56:1165–73. [
PubMed: 23362285]
- 47.
Boulware
DR, Meya
DB, Muzoora
C, Rolfes
MA, Huppler Hullsiek
K, Musubire
A
et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370:2487–98. [
PMC free article: PMC4127879] [
PubMed: 24963568]
- 48.
Zolopa
A, Andersen
J, Powderly
W, Sanchez
A, Sanne
I, Suckow
C
et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4:e5575. [
PMC free article: PMC2680972] [
PubMed: 19440326]
- 49.
Makadzange
AT, Ndhlovu
CE, Takarinda
K, Reid
M, Kurangwa
M, Gona
P
et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub-Saharan Africa. Clin Infect Dis. 2010;50:1532–8. [
PubMed: 20415574]
- 50.
Bicanic
T, Bottomley
C, Loyse
A, Brouwer
AE, Muzoora
C, Taseera
K
et al. Toxicity of amphotericin B deoxycholate-based induction therapy in patients with HIV-associated cryptococcal meningitis. Antimicrob Agents Chemother. 2015;59:7224–31. [
PMC free article: PMC4649151] [
PubMed: 26349818]
- 51.
Bahr
NC, Rolfes
MA, Musubire
A, Nabeta
H, Williams
DA, Rhein
J
et al. Standardized electrolyte supplementation and fluid management improves survival during amphotericin therapy for cryptococcal meningitis in resource-limited settings. Open Forum Infect Dis. 2014;1:ofu170. [
PMC free article: PMC4281785] [
PubMed: 25734140]
- 52.
Girmenia
C, Cimino
G, Di Cristofano
F, Micozzi
A, Gentile
G, Martino
P. Effects of hydration with salt repletion on renal toxicity of conventional amphotericin B empirical therapy: a prospective study in patients with hematological malignancies. Support Care Cancer. 2005;13:987–92. [
PubMed: 15756584]
- 53.
Thakur
CP, Kumar
A, Mitra
DK, Roy
A, Sinha
AK, Ranjan
A. Improving outcome of treatment of kala-azar by supplementation of amphotericin B with physiologic saline and potassium chloride. Am J Trop Med Hyg. 2010;83:1040–3. [
PMC free article: PMC2963966] [
PubMed: 21036834]
- 54.
Graybill
JR, Sobel
J, Saag
M, Van Der Horst
C, Powderly
W, Cloud
G
et al. Diagnosis and management of increased intracranial pressure in patients with AIDS and cryptococcal meningitis. Clin Infect Dis. 2000;30:47–54. [
PubMed: 10619732]
- 55.
Rolfes
MA, Hullsiek
KH, Rhein
J, Nabeta
HW, Taseera
K, Schutz
C
et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clin Infect Dis. 2014;59:1607–14. [
PMC free article: PMC4441057] [
PubMed: 25057102]
- 56.
Haddow
LJ, Colebunders
R, Meintjes
G, Lawn
SD, Elliott
JH, Manabe
YC
et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10:791–802. [
PMC free article: PMC3026057] [
PubMed: 21029993]
- 57.
Kambugu
A, Meya
DB, Rhein
J, O’Brien
M, Janoff
EN, Ronald
AR
et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46:1694–701. [
PMC free article: PMC2593910] [
PubMed: 18433339]
- 58.
Shelburne
III
SA, Darcourt
J, White
Jr
AC, Greenberg
SB, Hamill
RJ, Atmar
RL
et al. The role of immune reconstitution inflammatory syndrome in AIDS-related
Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:1049–52. [
PubMed: 15825000]
- 59.
Adenis
A, Nacher
M, Hanf
M, Basurko
C, Dufour
J, Huber
F
et al. Tuberculosis and histoplasmosis among human immunodeficiency virus–infected patients: a comparative study. Am J Trop Med Hyg. 2014;90:216–23. [
PMC free article: PMC3919221] [
PubMed: 24394475]
- 60.
Pasqualotto
AC, Quieroz-Telles
F. Histoplasmosis dethrones tuberculosis in Latin America. Lancet Infect Dis. 2018;18:1058–60. [
PubMed: 30146319]
- 61.
PAHO, WHO. Guidelines for diagnosing and managing disseminated histoplasmosis among people living with HIV. Washington (DC): Pan American Health Organization; 2020 (
https://iris.paho.org/handle/10665.2/52304, accessed 1 June 2021).
- 62.
Caceres
DH, Knuth
M, Derado
G, Lindsley
MD. Diagnosis of progressive disseminated histoplasmosis in advanced HIV: a meta-analysis of assay analytical performance. J Fungi (Basel). 2019;5:76. [
PMC free article: PMC6787751] [
PubMed: 31426618]
- 63.
Wheat
LJ, Connolly-Stringfield
PA, Baker
RL, Curfman
MF, Eads
ME, Israel
KS
et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine. 1990;69:361–74. [
PubMed: 2233233]
- 64.
Johnson
PC, Wheat
LJ, Cloud
GA, Goldman
M, Lancaster
D, Bamberger
DM
et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med. 2002;137:105–9. [
PubMed: 12118965]
- 65.
Wheat
LJ, Freifeld
AG, Kleiman
MB, Baddley
JW, McKinsey
DS, Loyd
JE
et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807–25. [
PubMed: 17806045]
- 66.
- 67.
Wheat
J, MaWhinney
S, Hafner
R, McKinsey
D, Chen
D, Korzun
A
et al. Treatment of histoplasmosis with fluconazole in patients with acquired immunodeficiency syndrome. Am J Med. 1997;103:223–32. [
PubMed: 9316555]
- 68.
Wheat
J, Hafner
R, Korzun
AH, Limj
MT, Spencer
P, Larsen
RA
et al. Itraconazole treatment of disseminated histoplasmosis in patients with the acquired immunodeficiency syndrome. Am J Med. 1995;98:336–42. [
PubMed: 7709945]
- 69.
Norris
S, Wheat
J, McKinsey
D, Lancaster
D, Katz
B, Black
J
et al. Prevention of relapse of histoplasmosis with fluconazole in patients with the acquired immunodeficiency syndrome. Am J Med. 1994;96:504–8. [
PubMed: 8017447]
- 70.
Sharkey-Mathis
PK, Velez
J, Fetchick
R, Graybill
JR. Histoplasmosis in the acquired immunodeficiency syndrome (AIDS): treatment with itraconazole and fluconazole. J Acquir Immune Defic Syndr. 1993;6:809–19. [
PubMed: 8389850]
- 71.
Hecht
FM, Wheat
J, Korzun
AH, Hafner
R, Skahan
KJ, Larsen
R
et al. Itraconazole maintenance treatment for histoplasmosis in AIDS: a prospective, multicenter trial. J Acquir Immune Defic Syndr. 1997;16:100–7. [
PubMed: 9358104]
- 72.
Myint
T, Anderson
AM, Sanchez
A, Farabi
A, Hage
C, Baddley
JW
et al. Histoplasmosis in patients with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS): multicenter study of outcomes and factors associated with relapse. Medicine. 2014;93:11–8. [
PMC free article: PMC4616326] [
PubMed: 24378739]
- 73.
Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis of active tuberculosis in people living with HIV: policy update. Geneva: World Health Organization; 2019 (
https://apps.who.int/iris/handle/10665/329479, accessed 1 June 2021).
- 74.
User perspectives on LF-LAM testing: results from qualitative research. In: Lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis of active tuberculosis in people living with HIV. Geneva: World Health Organization; 2019 (
https://apps.who.int/iris/handle/10665/329513, accessed 1 June 2021).
- 75.
- 76.
- 77.
- 78.
- 79.
- 80.