6.1. Introduction
ART has reduced mortality and morbidity associated with HIV and transformed HIV into a chronic disease requiring lifetime care. Coinfections and comorbidities, including physical and mental health conditions and substance use disorders, are common among people living with HIV. Comprehensive HIV care includes combination HIV prevention, the promotion of general health and well-being, maintaining quality of life and screening, ART, and the prevention and management of coinfections and comorbidities.
This chapter provides a brief overview of common and important concomitant conditions among people living with HIV. This includes information on co-trimoxazole prophylaxis, the diagnosis, prevention and treatment of TB, viral hepatitis, malaria, sexually transmitted infections, cervical cancer prevention, nutrition, vaccinations, mental health and substance use. It summarizes selected key recommendations, good practice statements and related materials from existing WHO guidelines. Sources are provided for relevant previously published recommendations where more detailed information on their management can be found.
6.2. General care for people living with HIV
Recommendations (2020)
Children and adolescents
Children and adolescents should do at least an average of 60 minutes per day of moderate- to vigorous-intensity, mostly aerobic, physical activity, across the week
(strong recommendation, moderate-certainty evidence).
Vigorous-intensity aerobic activities, as well as those that strengthen muscle and bone, should be incorporated at least three days a week
(strong recommendation, moderate-certainty evidence).
Children and adolescents should limit the amount of time spent being sedentary, particularly the amount of recreational screen time
(strong recommendation, low-certainty evidence).
Adults (18–64 years old) and older adults (65 years and older), including those with chronic conditions
All adults should undertake regular physical activity
(strong recommendation, moderate-certainty evidence).
Adults should do at least 150–300 minutes of moderate-intensity aerobic physical activity; or at least 75–150 minutes of vigorous intensity aerobic physical activity; or an equivalent combination of moderate- and vigorous-intensity activity throughout the week, for substantial health benefits
(strong recommendation, moderate-certainty evidence).
Adults should also do muscle strengthening activities at moderate or greater intensity that involve all major muscle groups on two or more days a week, since these provide additional health benefits
(strong recommendation, moderate-certainty evidence).
Adults may increase moderate-intensity aerobic physical activity to more than 300 minutes; or do more than 150 minutes of vigorous-intensity aerobic physical activity; or an equivalent combination of moderate- and vigorous-intensity activity throughout the week for additional health benefits
(conditional recommendation, moderate-certainty evidence).
Adults should limit the amount of time spent being sedentary. Replacing sedentary time with physical activity of any intensity (including light intensity) provides health benefits
(strong recommendation, moderate-certainty evidence).
To help reduce the detrimental effects of high levels of sedentary behaviour on health, adults should aim to do more than the recommended levels of moderate- to vigorous-intensity physical activity
(strong recommendation, moderate-certainty evidence).
Additional recommendation for older adults (65 years and older)
As part of their weekly physical activity, older adults should do varied multicomponent physical activity that emphasizes functional balance and strength training at moderate or greater intensity, on three or more days a week, to enhance functional capacity and to prevent falls
(strong recommendation, moderate-certainty evidence).
Source: Guidelines on physical activity and sedentary behaviour (1).
One in four adults and four of five adolescents do not get enough physical activity and 4–5 million deaths per year could be averted if global populations were more physically active (2). WHO has produced evidence-informed guidelines and recommendations on the health effects of physical activity and sedentary behaviour that governments can adopt as part of their national policy frameworks (1). The guidelines provide a cost-effective option that regions, countries or subnational authorities can adapt and use. For adults, physical activity confers benefits for the following health outcomes: reduced all-cause mortality, cardiovascular disease mortality, incident hypertension, incident site-specific cancer,1 incident type 2 diabetes, improved mental health (reduced symptoms of anxiety and depression), cognitive health and sleep; measures of adiposity may also improve (1).
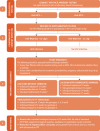
Countries should establish a package of general HIV care interventions, in addition to ART, for people living with HIV to reduce HIV transmission, prevent illness and improve their quality of life. General care includes combination HIV prevention, promoting the health of people living with HIV and screening for, prophylaxis for and management of coinfections and comorbidities. WHO has produced summary guidance on general care and prevention interventions (3–5) and recommends a package of 13 prevention interventions for adults and adolescents living with HIV in resource-limited settings:
psychosocial counselling and support;
disclosure and partner notification;
co-trimoxazole prophylaxis;
TB counselling, screening and preventive therapy;
preventing common fungal infections;
preventing sexually transmitted infections and supporting reproductive health needs, including preventing and screening for cervical cancer;
malaria: co-trimoxazole, bed nets and preventing malaria among pregnant women;
selected vaccine-preventable diseases;
nutrition;
family planning;
prevention of mother-to-child HIV transmission;
needle and syringe programmes for people who inject drugs; and
water sanitation and hygiene.
A general care package will vary according to the type of epidemic, populations affected and prevalence of coinfections, other comorbidities and health conditions. provides an overview of elements of a general care package for people living with HIV. In the era of universal treatment for all people living with HIV, the time between HIV diagnosis, enrolment into care and initiation of ART may be limited to a single visit to reduce loss to follow-up and to provide life-saving ART as soon as possible. WHO no longer recommends the need for preparatory visits before initiating ART; many of the care aspects outlined in can be accomplished once ART has started (4,5).
Overview of key elements of general care over the continuum of HIV care for people living with HIV.
Research gaps
Despite the large quantity of supporting data relating to physical activity and, increasingly, sedentary behaviour to health outcomes across the lifespan, important evidence gaps remain. There is less evidence from low- and middle-income countries and economically disadvantaged or underserved communities and a dearth of evidence from subpopulations, including people with disabilities. In addition, greater investment is needed in research to build evidence on the precise shape of the dose–response curve between physical activity and/or sedentary behaviour and health outcomes; the health benefits of light-intensity physical activity; and the joint association between physical activity and sedentary behaviour and health outcomes across the life-course.
6.3. Co-trimoxazole prophylaxis
Recommendation (2014)
Co-trimoxazole prophylaxis is recommended for adults (including pregnant women) with severe or advanced HIV clinical disease (WHO stage 3 or 4) and/or with CD4 cell count ≤350 cells/mm3
(strong recommendation, moderate-certainty evidence).
In settings where malaria and/or severe bacterial infections are highly prevalent, co-trimoxazole prophylaxis should be initiated regardless of CD4 cell count or WHO stage
(conditional recommendation, moderate-certainty evidence).
Co-trimoxazole prophylaxis may be discontinued for adults (including pregnant women) with HIV who are clinically stable on ART, with evidence of immune recovery and viral suppression
(conditional recommendation, low-certainty evidence).
In settings where malaria and/or severe bacterial infections are highly prevalent, co-trimoxazole prophylaxis should be continued regardless of CD4 cell count or WHO clinical stage
(conditional recommendation, moderate-certainty evidence).
Co-trimoxazole prophylaxis is recommended for infants, children and adolescents with HIV, regardless of clinical and immune conditions. Priority should be given to all children younger than five years old regardless of CD4 cell count or clinical stage and children with severe or advanced HIV clinical disease (WHO clinical stage 3 or 4) and/or those with CD4 cell count ≤350 cells/mm3
(strong recommendation, high-certainty evidence).
In settings where malaria and/or severe bacterial infections are highly prevalent, co-trimoxazole prophylaxis should be continued until adulthood whether or not ART is being taken
(conditional recommendation, moderate-certainty evidence).
In settings of low prevalence for both malaria and bacterial infections, co-trimoxazole prophylaxis may be discontinued for children five years of age and older who are clinically stable and/or virally suppressed on ART for at least six months and CD4 cell count >350 cells/mm3
(strong recommendation, very-low-certainty evidence).
Co-trimoxazole prophylaxis is recommended for HIV-exposed infants from four to six weeks of age and should be continued until HIV infection has been excluded by an age-appropriate HIV test to establish final diagnosis after complete cessation of breastfeeding
(strong recommendation, very-low-certainty evidence).
Routine co-trimoxazole prophylaxis should be given to all people living with HIV with active TB disease regardless of CD4 cell count
(strong recommendation, high-certainty evidence).
Source: Guidelines on post-exposure prophylaxis for HIV and the use of co-trimoxazole prophylaxis for HIV-related infections among adults, adolescents and children: recommendations for a public health approach – December 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection (6).
Background and rationale
Co-trimoxazole is a fixed-dose combination of two antimicrobial agents (sulfamethoxazole and trimethoprim) used to treat a variety of bacterial, fungal and protozoan infections. Co-trimoxazole prophylaxis is a feasible, well-tolerated and inexpensive intervention to reduce HIV-related morbidity and mortality among people living with HIV. Co-trimoxazole is an off-patent drug and is widely available in resource-limited settings.
In 2006, the first WHO guidelines on co-trimoxazole prophylaxis in resource-limited settings recommended co-trimoxazole prophylaxis as an integral component of HIV care (7). These guidelines were reviewed in 2014 and updated in the context of expanded access to and earlier initiation of ART (6). In recent years, new evidence has emerged showing that, with expanded access to ART, co-trimoxazole prophylaxis has broader benefits beyond preventing some AIDS-associated opportunistic diseases (Pneumocystis jirovecii pneumonia and toxoplasmosis) and reducing HIV-associated mortality among people with low CD4 cell counts. These benefits relate to preventing malaria and severe bacterial infections among adults and children living with HIV.
Nine observational studies (8–16) provide moderate-certainty evidence to support the effectiveness of co-trimoxazole prophylaxis in reducing death among people starting ART with CD4 cell count at or below 350 cells/mm3 and/or WHO clinical stage 3 or 4 disease. In addition, a new expanded recommendation for using co-trimoxazole prophylaxis is based on a systematic review showing the effectiveness of co-trimoxazole prophylaxis in reducing mortality, severe bacterial infections, malaria and hospitalization among adults and adolescents with HIV regardless of clinical and immunological parameters (17). One randomized clinical trial involving children living with HIV showed survival benefits regardless of age and CD4 cell count and also supports the expansion of co-trimoxazole prophylaxis to children, especially in settings with high prevalence of malaria and/or severe bacterial infections (18,19).
Continuing co-trimoxazole prophylaxis regardless of ART status, age, CD4 cell count or WHO clinical stage in settings with high prevalence of malaria and/or severe bacterial infections is also recommended based on data from randomized controlled trials that show a significant reduction in the risk of hospitalization, malaria and diarrhoea among adults and children with HIV in settings with high prevalence of malaria and/or severe bacterial infections (20,21). In addition, the recommendation to continue co-trimoxazole prophylaxis in settings with a high prevalence of malaria and/or severe bacterial infections may simplify HIV management, forecasting and supply management issues and improve access to co-trimoxazole prophylaxis access for people living with HIV.
The risks and benefits of continuing versus stopping co-trimoxazole prophylaxis after viral suppression induced by ART were also evaluated in settings with a low burden of malaria and severe bacterial infections. Two studies found that the rates of Pneumocystis jirovecii pneumonia and death were similar for people receiving ART who achieved suppressed viral loads and had CD4 cell counts above 100 cells/mm3 in study arms (22,23). In these settings, discontinuing co-trimoxazole prophylaxis for adults based on clinical, immunological and virological parameters indicating ART immune recovery can be considered, although the certainty of the evidence is low to very low (6). However, in settings with a low prevalence of malaria and/or severe bacterial infections and limited or no access to CD4 cell testing, co-trimoxazole prophylaxis should not be discontinued.
The recommendation on women and adolescents living with HIV using co-trimoxazole prophylaxis during pregnancy to prevent malaria complications and avoid simultaneous intermittent preventive treatment is based on a systematic review showing that co-trimoxazole prophylaxis is not inferior to intermittent preventive treatment of malaria in pregnancy with respect to mortality, low birth weight, placental malaria, maternal deaths and severe adverse events (24). The recommendation to discontinue co-trimoxazole prophylaxis at the end of the risk period for transmission in infants who are HIV-exposed and uninfected is also maintained, but new evidence has emerged on the lack of clinical benefits of co-trimoxazole prophylaxis for HIV-exposed infants who are uninfected and on the potential harm associated with disrupting the microbiome and selecting antibiotic resistance. Where HIV vertical transmission is very uncommon and HIV infection can be reliably excluded, consideration has been given to shorten administration of co-trimoxazole in infants who are HIV exposed but uninfected (see Box 6.1). WHO anticipates reviewing this recommendation as further evidence is gathered and increasing progress is made to rapidly identify infants living with HIV and to retain those exposed to HIV in the testing cascade until final diagnosis is ascertained.
Box 6.1Using co-trimoxazole among infants who are HIV exposed but uninfected
Since WHO revised the co-trimoxazole guidelines in 2013, new evidence has emerged on the lack of clinical benefits of co-trimoxazole prophylaxis for HIV-exposed infants. A systematic review (25) was undertaken to assess the effect of co-trimoxazole prophylaxis on morbidity and mortality among HIV-exposed and uninfected infants. Only two trials from Botswana and South Africa were identified (26,27).
The randomized trial in Botswana (co-trimoxazole n = 1423; placebo n = 1425) gave co-trimoxazole from 14–34 days until 15 months and showed no evidence of benefit of co-trimoxazole for HIV-exposed and uninfected children for cumulative mortality to 18 months (30 deaths [2.4%] for co-trimoxazole versus 34 deaths [2.6%] for placebo; difference −0.2%, 95% CI −1.5% to 1.0%; primary outcome) or hospitalization, diarrhoea or pneumonia (secondary outcomes; P > 0.05). The randomized trial in South Africa (co-trimoxazole n = 611; no co-trimoxazole n = 609), designed as a non-inferiority trial, gave co-trimoxazole from six weeks until infants were confirmed HIV-uninfected at the end of the at-risk HIV period and showed non-inferiority of not giving co-trimoxazole to HIV-exposed and uninfected children on combined grade 3 and 4 pneumonia, diarrhoea and all-cause mortality by 12 months (primary outcome 49 [8%] events [co-trimoxazole] versus 39 [6%] events [no co-trimoxazole]; risk difference no co-trimoxazole minus co-trimoxazole −0.032, 95% CI −0.075 to 0.011) or pneumonia, diarrhoea and mortality separately (P > 0.05). The groups did not differ in anaemia, but one study found that neutropaenia was more frequent in the co-trimoxazole group (26).
Substudies within these trials investigated bacterial resistance and found that the proportion of co-trimoxazole-resistant gastrointestinal bacteria was higher in the co-trimoxazole group (28), and co-trimoxazole prophylaxis decreased gut microbiome β-diversity and increased antibiotic resistance gene α-diversity and prevalence (29). Three further studies examined using co-trimoxazole prophylaxis to prevent malaria (30–32), all in Uganda. These studies found that co-trimoxazole prophylaxis protected against malaria among HIV-exposed and uninfected children when continued after breastfeeding ended, but mortality, hospitalization, diarrhoea and pneumonia were unaffected.
To critically review the evidence and explore potential implications for country programmes, WHO convened a technical expert group in March 2021. The group examined current estimates and trends for preventing HIV vertical transmission and coverage of infant testing, noting persisting gaps in timely identification and retention of infants in the testing-to-treatment cascade, with vertical transmission increasingly occurring postnatally. Current co-trimoxazole prophylaxis guidelines provide protection for children at high risk of acquiring HIV who may be missed by infant testing services, but as the systematic review findings suggest, if HIV infection can be reliably excluded, co-trimoxazole does not provide additional benefit to HIV-exposed but uninfected infants and children and may disrupt their microbiomes and increase antibiotic resistance to co-trimoxazole and other widely used antibiotics. This led the South African Thoracic Society to change their guidelines to no longer recommend co-trimoxazole prophylaxis for HIV-exposed and uninfected children (33). However, the studies included in the systematic review were undertaken in Botswana and South Africa, and these findings have limited generalizability to epidemic settings with higher vertical HIV transmission rates, poorer infant testing coverage, higher burden of malaria and other severe bacterial infections and higher infant mortality.
To further explore the potential impact of different approaches across epidemic settings, the group examined the output of a modelling study designed to help quantify the predicted impact of alternative co-trimoxazole strategies on death among HIV-exposed and uninfected children at age two years (34). Assuming full co-trimoxazole uptake, changing current guidelines was predicted to increase mortality in all settings. However, the benefits of the current policy are expected to be greatest in settings with substantial vertical transmission and poor infant testing coverage, in contrast to settings with low vertical transmission and very good infant testing, in which a strategy of shorter co-trimoxazole administration may be a reasonable alternative. This model did not include potential harm associated with disrupting the microbiome and selecting for antibiotic resistance because of the lack of clear clinical correlates.
Current WHO guidelines recommend starting co-trimoxazole for all HIV-exposed infants at age 4–6 weeks and stopping after the period of risk and final confirmation of a negative HIV status, defined as a negative 18-month test or testing after breastfeeding ends if breastfed longer than 18 months. Overall, the expert group thought that the evidence reviewed is compelling, but programme implementation remains challenging in many settings. The group considered fully revising the current recommendations to be premature but the group acknowledged that co-trimoxazole prophylaxis may be discontinued at the end of the at-risk period for HIV transmission (after breastfeeding ends and once HIV infection is ruled out by age-appropriate HIV testing), which may occur before 18 months.
Further, in settings with low vertical transmission rates, high HIV infant diagnosis coverage and strong retention in the testing-to-treatment cascade, country programmes may consider stopping providing routine co-trimoxazole as soon as HIV infection is ruled out by age-appropriate HIV testing (see Chapter 2).
Several gaps remain on how to optimize the use of co-trimoxazole prophylaxis among HIV-exposed infants to provide the highest impact. These gaps include optimal timing to start co-trimoxazole; clinical and programmatic impact of shorter duration of co-trimoxazole prophylaxis in different epidemic contexts and programmatic realities; the added value of potential differentiated approaches to co-trimoxazole prophylaxis delivery; the potential impact of shorter co-trimoxazole strategies on retention in the testing-to-treatment cascade; alternative antibiotic prophylactic regimens (other antibiotics); the clinical relevance of the selection of antibiotic resistance associated with co-trimoxazole prophylaxis; and the short- and long-term clinical relevance of the microbiome disruption resulting from co-trimoxazole prophylaxis. Although a randomized blinded clinical trial may not be required to address some of these questions, well-conducted operational research will be critical to innovate and better inform children’s use of co-trimoxazole prophylaxis in the future.
summarizes the criteria for initiating and discontinuing co-trimoxazole prophylaxis for adults, adolescents, pregnant women and children living with HIV.
Criteria for initiating and discontinuing co-trimoxazole prophylaxis.
Implementation considerations
Some of the major barriers to implementing co-trimoxazole include supply chain and management issues leading to stock-outs; imposing user charges for medication and/or monitoring; inadequate training, supervision and/or mentoring of health-care workers; low coverage of HIV testing and counselling; and lack of coordination across programmes. National programmes can implement co-trimoxazole prophylaxis policy and guidelines more effectively by using the approaches shown in Box 6.2.
Box 6.2Steps to improve the implementation of co-trimoxazole prophylaxis policy and guidelines at the national level
Adapt WHO guidelines to the national context.
Strengthen national and local drug supply management systems to ensure sustained availability of co-trimoxazole at health-care facilities.
Secure funding for providing co-trimoxazole prophylaxis to ensure that no user charges are imposed.
Coordinate with malaria programmes at the country level with regard to recommendations related to intermittent preventive treatment of malaria in pregnancy and seasonal malaria chemoprophylaxis for children younger than five years.
Provide co-trimoxazole prophylaxis to eligible people at TB, maternal, newborn and child health and opioid substitution therapy services.
Scale up the training and sensitization of health-care workers.
Increase co-trimoxazole prophylaxis knowledge at the community level.
Ensure that a human rights framework is used: for example, people living with HIV should always consent before co-trimoxazole prophylaxis is administered.
Ensure that high-quality co-trimoxazole formulations are provided.
Monitor the toxicity of adverse reactions, especially for chronic co-trimoxazole prophylaxis.
Assess adherence to policies and the impact on population health.
6.4. Tuberculosis
Background
An estimated one fourth of the world’s population is infected with TB, and about 5–10% of those infected develop active TB disease in their lifetime. The risk for active TB disease after infection depends on several factors, the most important being the person’s immune status (38). People living with HIV are 15–22 times more likely to develop active TB than people without HIV, and TB is the leading cause of death among people living with HIV worldwide (39,40).
WHO has developed and published consolidated guidelines on TB in four modules, designed as living documents that will be updated as new information becomes available:
Module 1: Prevention (
38);
Module 2: Screening: systematic screening for tuberculosis disease (
41);
Module 3: Diagnosis: rapid diagnostics for tuberculosis detection (
42); and
Module 4: Treatment: drug-resistant tuberculosis treatment (
43).
Key information from each module is summarized below.
WHO is developing new consolidated guidelines on managing TB among children and adolescents. These guidelines, along with an operational handbook, are expected to be released at the end of 2021; they will consolidate all TB-related recommendations relevant for children (0–9 years old) and adolescents (10–19 years old) in TB, HIV and nutrition guidelines. New evidence will be reviewed on diagnostic approaches (using treatment decision algorithms and using Xpert® Ultra in gastric aspirate and stool specimens), treatment shortening for drug-susceptible TB, treatment of drug-resistant TB, treatment of TB meningitis and models of care (decentralization and family-centred, integrated approaches) among children and adolescents, including those living with HIV.
6.4.1. Screening and diagnosis
Systematic screening for TB among people living with HIV
Recommendation (2021)
People living with HIV should be systematically screened for TB disease at each visit to a health facility
(strong recommendation, very-low-certainty evidence).
Source: WHO consolidated guidelines on tuberculosis. Module 2: Screening: systematic screening for tuberculosis disease (41).
Tools for screening for TB among people living with HIV
Recommendations (2021)
Among adults and adolescents living with HIV, systematic screening for TB disease should be conducted using the WHO-recommended four-symptom screen, and those who report any one of the symptoms of current cough, fever, weight loss or night sweats may have TB and should be evaluated for TB and other diseases
(strong recommendation, moderate-certainty evidence).
Among children younger than 10 years who are living with HIV, systematic screening for TB disease should be conducted using a symptom screen including any one of the symptoms of current cough, fever, poor weight gain or close contact with a person with TB disease
(strong recommendations, low-certainty evidence for test accuracy).
Among adults and adolescents living with HIV, C-reactive protein with a cut-off of >5 mg/L may be used to screen for TB disease
(conditional recommendation, low-certainty evidence for test accuracy).
Among adults and adolescents living with HIV, chest X-ray may be used to screen for TB disease
(conditional recommendation, moderate-certainty evidence for test accuracy).
Among individuals aged 15 years and older in populations in which TB screening is recommended, computer-aided detection software programmes may be used in place of human readers for interpreting digital chest X-rays for screening and triage for TB disease
(conditional recommendation, low-certainty evidence).
Among adults and adolescents living with HIV, molecular WHO-recommended rapid diagnostic tests may be used to screen for TB disease
(conditional recommendation, moderate-certainty evidence for test accuracy).
Adult and adolescent inpatients with HIV in medical wards where the TB prevalence is >10% should be tested systematically for TB disease with a molecular WHO-recommended rapid diagnostic test
(strong recommendation, moderate-certainty evidence for test accuracy).
Source: WHO consolidated guidelines on tuberculosis. Module 2: Screening: systematic screening for tuberculosis disease (41).
Summary of evidence and rationale
In 2019, an estimated 44% of people living with HIV who also had TB disease did not reach care, and TB caused 30% of all HIV-related deaths (2). Thus, ensuring early detection and treatment for TB among all people living with HIV is crucial for reducing morbidity and mortality.
The recommendation to systematically screen for TB disease at each visit to a health facility, which applies to people of all ages, along with the recommendations on related symptom screening algorithms for adults and adolescents and for children, was first published in 2011 in WHO’s Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings (19,44). For adults and adolescents, the WHO-recommended four-symptom screen is recommended. If an individual screens positive on any of the following four symptoms: current cough, fever, night sweats and weight loss, they should receive further diagnostic work-up. For children, any of the following symptoms would indicate diagnostic work-up for TB: current cough, fever, poor weight gain or close contact with a person with TB disease.
An individual participant data meta-analysis was conducted in 2020 to review the accuracy of the WHO-recommended four-symptom screen and other tools for TB screening among adults and adolescents, including C-reactive protein, chest X-ray and molecular WHO-recommended rapid diagnostic tests.
WHO four-symptom screen
The meta-analysis of individual patient data found no alternative screening tools or strategies that were significantly higher in both sensitivity and specificity than the WHO-recommended four-symptom screen. In all cases, when sensitivity was higher and met the minimal requirements of the target product profile for a screening test, the specificity was compromised and vice versa. Although the WHO-recommended four-symptom screen may have real-life limitations in terms of consistency and quality of delivery that might not be reflected in studies, it remains the simplest non-invasive tool to implement in any setting, requiring no infrastructure. However, the high proportion of positivity (94%) and very low specificity among medical inpatients living with HIV in settings where the TB prevalence among study participants was >10% gives it limited utility as a screen to rule in TB before diagnostic confirmation by molecular WHO-recommended rapid diagnostic tests in this very ill population. The review also found that the WHO-recommended four-symptom screen had reduced specificity for people not receiving ART (37%, 95% CI 25–59%) and reduced sensitivity for outpatients receiving ART (53%, 95% CI 36–69%). Programmes might therefore want to supplement the WHO-recommended four-symptom screen with other screening tools.
C-reactive protein
The analysis found that C-reactive protein was most accurate among outpatients living with HIV not receiving ART. When performed after a positive WHO-recommended four-symptom screen, C-reactive protein with a cut-off of >5 mg/L was found to be as sensitive as the WHO-recommended four-symptom screen, with a sensitivity of 78% (95% CI 70–85%) but with significantly higher specificity (73%; 95% CI 66–79%) than the WHO-recommended foursymptom screen, which had a sensitivity of 84% (95% CI 75–90%) and specificity of 37% (95% CI 25–50%).
Chest X-ray
The parallel combination of the WHO-recommended four-symptom screen and chest X-ray, in which a positive result from either tool should be followed up by diagnostic confirmation, had the highest sensitivity (85%, 95% CI 69–94%) compared with other tools including the WHO-recommended four-symptom screen (53%, 95% CI 36–69%) when used to screen for TB among outpatients receiving ART.
Computer-aided detection of chest X-ray
Studies comparing the accuracy of computer-aided detection software showed considerable variability among readers, but the substantial overlap of confidence intervals between computer-aided detection software and human readers suggested little difference in accuracy. Limited data were available for comparing computer-aided detection to human interpretation of chest X-ray among people living with HIV; further evidence is needed about the performance of computer-aided detection software among people living with HIV, to enable better setting-specific and patient-specific calibration of computer-aided detection software.
Diagnosing TB
The clinical picture of TB disease is often non-specific and in isolation does not enable its accurate diagnosis, requiring bacteriological testing for all people with signs and symptoms of TB disease. People living with HIV may have an atypical clinical picture, especially those with advanced disease, further complicating the clinical diagnosis of pulmonary and extrapulmonary forms of TB disease.
The diagnostic options recommended by WHO (42) are of two broad groups, either initial test for diagnosing TB, often with at least rifampicin resistance detection or those used for follow-on testing after TB confirmation. The latter aimed at detecting additional drug resistance once a TB diagnosis is made and is not covered in this section but covered in the relevant TB guidelines.
Rapid and accurate diagnosis is essential to ensure that people with TB are effectively treated and cured. shows the initial tests WHO currently recommends. All have recommendations for use among people living with HIV and are considered as WHO rapid diagnostic tests. Furthermore, all are molecular WHO-recommended rapid diagnostic tests except for the lateral flow lipoarabinomannan (LF-LAM) test. Molecular WHO-recommended rapid diagnostic tests are recommended as an initial test rather than smear microscopy or culture, and the diagnostic algorithm 1 provided in the appropriate operational handbook should be followed (42).
LF-LAM is an add-on test specifically for people living with HIV, and the recommendations vary by the presence or absence of symptoms, CD4 cell count and severity of disease requiring hospitalization or not. It is a point-of-care test performed on a urine sample and suited for use as part of the standard package of care for advanced HIV disease. The respective TB guidelines and the accompanying handbook (42) provide details. In the latter, algorithms 2a and 2b provide the patient pathways for using LF-LAM for inpatients and outpatients. A positive LF-LAM test predicts mortality, and using the test in advanced HIV accompanied by appropriate and effective treatment saves lives. Next-generation tests with improved sensitivity, including patients with CD4 counts greater than 200 cells/mm3, have not yet reached commercialization. However, once available and reviewed, these tests offer the potential for broader use within people living with HIV.
Molecular WHO-recommended rapid diagnostic tests are the essential starting-point for diagnosing TB. They include nine different products, with most including simultaneous detection of at least rifampicin resistance. The Xpert® MTB/RIF and Xpert® MTB/RIF Ultra (Xpert® Ultra) have specific recommendations for people living with HIV and extrapulmonary TB. The Xpert® Ultra is more sensitive than the Xpert® MTB/RIF, including for people living with HIV, accompanied by slightly lower specificity. The lower specificity is associated with a previous history of TB treatment in the past five years, mainly when a very low bacterial load is detected, and this semiquantitative result is called “trace”. TB DNA may trigger this test result from non-viable organisms among people previously treated, thus being false positive for TB disease.
The Truenat™ and Truenat™ plus tests are suited to similar care levels to the Xpert® and Ultra. Testing for rifampicin is performed as a follow-on reflex test on the same instruments. The TB-LAMP does not test for rifampicin resistance and is thus best suited for areas with a low prevalence of multidrug- and rifampicin-resistant TB. In addition, it requires more hands-on time than other molecular WHO-recommended rapid diagnostic tests. However, it is less expensive both in test costs and equipment than other molecular WHO-recommended rapid diagnostic tests.
The latest addition to the recommended group of molecular WHO-recommended rapid diagnostic tests as initial diagnostic test is the class of moderate complexity automated nucleic acid amplification tests (NAATs). These tests have comparable sensitivity and specificity with other molecular WHO-recommended rapid diagnostic tests in detecting TB and detect resistance to rifampicin and isoniazid. However, this class of tests requires laboratory infrastructure with a rapid and reliable specimen transport system. The class include systems that can perform between 24 and 96 samples in a single run, making it suitable for use in higher-throughput and urban settings. Importantly, this class now includes four new products, all of which have SARS-CoV-2 testing available. Two are widely used for HIV testing and thus facilitate the use of common platforms where capacity exists. The list of products is Abbott RealTime RealTime MTB and MTB RIF/INH assays (Abbott Laboratories, Abbott Park, IL, USA), the BD MAX™ multidrug-resistant TB assay (Becton, Dickinson and Company, Franklin Lakes, NJ, USA), the Hain FluoroType® MTBDR assay (Bruker/Hain Lifescience, Nehren, Germany) and the Roche COBAS® MTB and MTB-RIF/INH assays (F. Hoffmann-La Roche, Basel, Switzerland).
All the molecular WHO-recommended rapid diagnostic tests are recommended for diagnosing pulmonary TB. However, for extrapulmonary TB and children, specific recommendations are only provided for Xpert® MTB/RIF, and Xpert® Ultra data for all other tests were limited when the review was conducted. Nevertheless, Xpert® Ultra has higher sensitivity in these groups, and trace positives are considered positive for these populations. Further details on the WHO-recommended diagnostic tools should be consulted in the latest TB consolidated guidelines and operational handbook on diagnostics (42,45).
Molecular WHO-recommended rapid diagnostic tests
Data from the individual participant data analysis found that 94% of medical inpatients had a positive WHO-recommended four-symptom screen, with a specificity of 11%. Thus, the difference in accuracy was minimal between the full screening and diagnostic strategy of using the WHO-recommended four-symptom screen followed by molecular WHO-recommended rapid diagnostic tests and using molecular WHO-recommended rapid diagnostic tests alone. WHO therefore recommends that medical inpatients be screened and tested with a molecular WHO-recommended rapid diagnostic test, regardless of symptoms, to inform a decision about whether to treat for TB. A 10% threshold TB prevalence among hospital inpatients living with HIV is recommended, considering the TB prevalence among the participants studied and striking a balance between ensuring rapid diagnosis in this critically ill population and the need to avoid overtreatment.
Because of the increased sensitivity of molecular WHO-recommended rapid diagnostic tests, but considering the likely challenges relating to access, high costs and feasibility in many countries, molecular WHO-recommended rapid diagnostic tests are also recommended as an option for screening for TB disease among all adults and adolescents living with HIV who are not medical inpatients in settings where the TB prevalence exceeds 10%. In this case, and as with all screening tools, a positive molecular WHO-recommended rapid diagnostic test screen should be followed by a diagnostic assessment to prevent the potential harm of overtreatment. In addition, due consideration should be made to giving priority to molecular WHO-recommended rapid diagnostic tests as a diagnostic test for all people with presumptive TB before scaling up molecular WHO-recommended rapid diagnostic tests as a screening test.
WHO-recommended rapid diagnostic tests as initial tests for the diagnosis of TB.
Implementation considerations
Countries should position the WHO-recommended four-symptom screen, C-reactive protein, chest X-ray and molecular WHO-recommended rapid diagnostic tests in combination with diagnostic evaluation using molecular WHO-recommended rapid diagnostic tests and LF-LAM within national TB screening and diagnostic algorithms according to their feasibility, the level of the health facility, resources and equity. Although all the screening tools presented are recommended for all people living with HIV, evidence showed notable accuracy of C-reactive protein for TB screening for people not yet receiving ART and that chest X-ray enhanced the sensitivity of the WHO-recommended four-symptom screen among people receiving ART, both of which might be considered when choosing algorithms. Among inpatients in medical wards in settings with a high TB burden, evidence showed that the WHO-recommended four-symptom screen, C-reactive protein and chest X-ray had limited accuracy because of either extremely low specificity or suboptimal sensitivity and that using molecular WHO-recommended rapid diagnostic tests as an upfront screening and diagnostic test is warranted, particularly given the urgency of timely diagnosis in this population.
Data from the WHIP3TB trial highlight the need to conduct more intensified screening in addition to the WHO-recommended four-symptom screen. Programmes might consider using additional screening tools at the time of initial diagnosis of HIV or during the first antenatal care visit for pregnant women and then annually thereafter. To reduce the burden on the person living with HIV, such screening should be aligned with other routine HIV care visits, such as those for viral load monitoring or for ruling out TB disease before initiating TB preventive treatment, depending on the setting and the national guidelines on HIV. Where applicable, the WHO-recommended four-symptom screen should also be conducted as part of a comprehensive clinical evaluation and to inform the need for increased infection control and for other diagnostic tests, such as LF-LAM. Otherwise screening with the WHO-recommended four-symptom screen alone should be carried out during all other interactions between patients and health-care workers.
Consideration should also be given to the added benefit of including C-reactive protein for ruling out TB disease before initiating TB preventive treatment among people living with HIV. In a setting of 1% TB prevalence, among 1000 outpatients screened with the WHO-recommended four-symptom screen followed by C-reactive protein, 742 would be true negatives and eligible for TB preventive treatment versus only 416 found eligible by the WHO-recommended four-symptom screen. Similar to the case for using chest X-ray for ruling out TB disease before initiating TB preventive treatment, restricted access to C-reactive protein or chest X-ray should not be a barrier to initiating TB preventive treatment. When using a molecular WHO-recommended rapid diagnostic tests as a TB screening tool among people living with HIV is considered, it should be ensured that universal access to molecular WHO-recommended rapid diagnostic tests for everyone with presumptive TB is achieved first. The use of a molecular WHO-recommended rapid diagnostic test as a screening tool requires significant resources for implementation, including increasing the capacity of diagnostic networks and expanding sample transport networks. Depending on the feasibility and resources available, countries may choose to give priority to TB screening using molecular WHO-recommended rapid diagnostic tests among certain subpopulations, such as all medical inpatients, people with advanced disease or pregnant women living with HIV.
To inform programming and resource planning, countries are encouraged to monitor and evaluate the yield of TB screening among people living with HIV, disaggregated by screening tool. Additionally, more data are needed on the effectiveness, cost–effectiveness, feasibility and acceptability, frequency and optimal periodicity of routine, regular screening with the WHO-recommended four-symptom screen, C-reactive protein, chest X-ray and molecular WHO-recommended rapid diagnostic tests among people living with HIV. More studies are needed that explore the optimal placement of molecular WHO-recommended rapid diagnostic tests for screening in antenatal care settings versus within ART clinics. Lastly, more research is needed on the potential for screening people living with HIV with molecular WHO-recommended rapid diagnostic tests using specimens other than sputum.
Extrapulmonary TB among people living with HIV
The risk of extrapulmonary TB is higher among people living with HIV, especially those with lower CD4 cell counts. People living with HIV with extrapulmonary TB often have disseminated disease and are at high risk of rapid clinical deterioration and death. The commonest forms include lymph node (especially in the neck or under the arms), pleural (usually one-sided pleural effusion) and disseminated TB (disease that is not limited to one site in the body). Pericardial and meningeal TB are less frequent forms of extrapulmonary TB but are more likely to result in fatal outcomes (46). The diagnosis of extrapulmonary TB is challenging. Lack of pulmonary findings is not uncommon among people living with HIV with advanced immunosuppression, and disseminated TB can manifest as non-specific febrile illness. Extrapulmonary TB can be suspected among all people living with HIV presenting with TB symptoms. Further, symptoms suggesting specific organ involvement, such as breathlessness (pleural effusion or pericarditis), enlarged glands in the neck or armpit (lymphadenitis) and chronic headache or altered mental status (meningitis) should prompt further investigation for extrapulmonary TB (42). Bacterial confirmation is often difficult because of low sensitivity of smear microscopy and difficulty in obtaining samples from extrapulmonary sites. If possible, extrapulmonary specimens should be obtained. For people with suspected TB meningitis, a molecular WHO-recommended rapid diagnostic test is the preferred initial diagnostic test for cerebrospinal fluid (42). If lymphadenitis is suspected, molecular WHO-recommended rapid diagnostic tests may be used to test for samples obtained from lymph node biopsies or fine-needle aspiration. LF-LAM may also assist in the diagnosis because these people living with HIV are likely to have low CD4 cell counts (25). The accurate diagnosis of extrapulmonary TB is complex and difficult, especially in peripheral health facilities with limited support and diagnostic infrastructure.
6.4.2. Timing of ART for adults and children with TB
Early initiation of ART among people with both TB and HIV is critical for reducing mortality. Section 4.4.3 provides more detailed information and recommendations on the co-treatment of TB and HIV.
6.4.3. Treatment
Presumptive treatment of TB for people living with HIV
The rationale for presumptive TB treatment, also referred to as empirical treatment, is to prevent the death of people living with HIV in situations when expedited diagnosis of TB is not possible or feasible because of the person’s clinical condition or limited access to TB diagnostic services. Although presumptive TB has no case definition, WHO algorithms include initiating TB treatment for people living with HIV in peripheral facilities based exclusively on clinical suspicion (without TB investigations) for seriously ill people2 based on the judgement of the clinician (47). This approach is based on expert opinion and emphasizes that every effort should be made to confirm the diagnosis of TB after initiating presumptive treatment and that treatment should be stopped only if bacteriological, histological or strong clinical evidence indicates an alternative diagnosis.
In 2015, a systematic review was performed to assess the role of presumptive TB treatment for people living with HIV, with a particular focus on its efficacy in reducing mortality and the risk of severe treatment adverse events. Three randomized controlled trials (48–50) were identified.
In the REMEMBER trial, empirical TB therapy did not reduce mortality at 24 weeks among outpatient adults initiating ART with advanced HIV disease. The low mortality rate of the trial supports the implementation of systematic TB screening and intermittent preventive treatment among outpatients with advanced HIV disease (48). In the PrOMPT trial, despite limited enrolment, the study did not suggest that empirical TB treatment among severely immunosuppressed people with low BMI decreased mortality (49). In the STATIS trial, systematic treatment for TB among severely immunosuppressed adults with HIV infection who had not previously received ART was not superior to test-guided treatment in reducing the rate of death or invasive bacterial disease over 24 or 48 weeks and was associated with more grade 3 or 4 adverse events (50).
Based on the available evidence, WHO made no new recommendation on presumptive TB treatment for people living with HIV and noted the importance of further research on this issue, including research on the clinical predictors for selecting people living with HIV for presumptive treatment and whether nurses or clinical officers can initiate it. Nevertheless, expert opinion continues to support presumptive TB treatment in peripheral health facilities in HIV-prevalent settings for people living with HIV who are seriously ill because of suspected TB.
Implementation considerations
People living with HIV should be closely followed up to assess the occurrence of side-effects related to co-treatment and of TB-associated immune reconstitution inflammatory syndrome, which is common among people with TB starting ART but is usually self-limited (46). Stakeholders and service providers should establish mechanisms to ensure that people living with HIV receive TB treatment along with ART, emphasizing integrated and patient-centred care, preferably at the same location.
Treatment of drug-sensitive TB
Early initiation of ART among people with TB and HIV is critical for reducing mortality. Chapter 4 provides more detailed information and recommendations on the co-treatment of TB and HIV.
At the time of publication, the only current recommended regimen for drug-sensitive TB is a six-month TB regimen containing two months of isoniazid, rifampicin, pyrazinamide and ethambutol followed by four months of rifampicin and isoniazid (46).
However, a recent randomized, multinational, open-label controlled Phase 3 trial, Study 31/A5349, compared the efficacy of a shorter four-month rifapentine-containing regimen comprising rifapentine, isoniazid, pyrazinamide and moxifloxacin with the standard six-month control regimen. As part of the 2020 update of Module 4 of the WHO consolidated guidelines on tuberculosis, the data from the trial were reviewed and the efficacy of the four-month rifapentine-based regimen was found to be noninferior to the standard six-month regimen for the treatment of drug-susceptible pulmonary TB and the regimen was equally well tolerated. The available evidence supports using this regimen as a possible alternative to the current standard six-month regimen, including among people living with HIV. The shorter regimen has shown similar performance to the current standard regimen, both in terms of both efficacy and safety. The four-month regimen, which is shorter, effective and all-oral, would be a preference for many people and also national TB and HIV programmes, enabling more rapid cure and easing the burden on both these people and the health-care system. However, implementation and uptake of the new regimen will be more feasible if the cost of rifapentine is reduced and availability improved. It will also require rigorous antibacterial stewardship to ensure the appropriate use of the first-line regimen since it contains moxifloxacin, an antibiotic usually used for drug-resistant TB. Further details, including on eligibility for the shorter treatment regimen for drug-susceptible TB, are available in the 2020 update of Module 4 of the WHO consolidated guidelines on tuberculosis.
Significant progress in the availability of improved diagnostics and more effective medicines in recent years have led to earlier detection and higher success rates among people with multidrug- and rifampicin-resistant TB in a number of countries. However, these achievements have not been reproduced globally, and the overall treatment success rate reported in 2018 reached only 56% for people with multidrug- and rifampicin-resistant TB and 39% for people with extensively drug-resistant TB (51). Further information is available in Module 4 of the WHO consolidated guidelines on tuberculosis, which replaces all previous and current WHO guidelines on drug-resistant TB treatment (43). Further information, including on drug–drug interactions, is also available in the WHO operational handbook on tuberculosis. Module 4: treatment – drug-resistant tuberculosis treatment (52).
Treatment of people with drug-resistant TB
Multidrug-resistant TB is TB that is resistant to at least isoniazid and rifampicin. People with both HIV and multidrug-resistant TB face complicated clinical management, fewer treatment options and poorer treatment outcomes (53). Systematic reviews have shown an association between HIV and multidrug-resistant TB (54,55). Outbreaks of multidrug-resistant TB among people living with HIV have been documented in hospital and other settings, especially in eastern Europe and central Asia and in southern African countries with a high HIV prevalence (56).
Recommendation (2020)
WHO recommends ART for all people with HIV and drug-resistant TB, requiring second-line anti-TB drugs irrespective of CD4 cell count, as early as possible (within the first eight weeks) following initiation of anti-TB treatment
(strong recommendation, very-low-certainty evidence).
Source: WHO consolidated guidelines on tuberculosis. Module 4: treatment: drug-resistant tuberculosis treatment (43).
WHO has recently updated guidance on drug-resistant TB, including recommendations on regimens on rifampicin-susceptible isoniazid-resistant TB, a shorter all-oral bedaquiline-containing regimen for multidrug- or rifampicin-resistant TB and longer regimens for multidrug- or rifampicin-resistant TB. There may be a potential for overlapping, additive types of toxicity or for drug–drug interactions between some ARV drugs and the injectable agents moxifloxacin and clofazimine; however, there are usually no grounds to warrant modifying the regimens for multidrug-resistant TB or ART. WHO does not recommend using bedaquiline and efavirenz in combination. ART regimens need to be optimized, and should be initiated early, in accordance with WHO recommendations. Close monitoring for response and toxicity is advised for people receiving both TB and HIV treatment. Other comorbidities (such as diabetes and mental health disorders) should be managed accordingly.
Supporting evidence and rationale
Evidence was reviewed from 10 studies to assess treatment outcomes when ART and second-line anti-TB drugs were used together. None of the data were from randomized controlled trials. Individual data were available for 217 people with drug-resistant TB, of whom 127 received ART. The evidence in individual observational studies varied from low to very low certainty (43).
6.4.4. Prevention
TB preventive treatment
Latent TB is defined as a state of persistent immune response to stimulation by Mycobacterium tuberculosis antigens with no evidence of clinically manifest active TB disease. An estimated quarter of the world’s population is infected with TB.
Among people living with HIV, the combined use of TB preventive treatment and ART has been shown to benefit both TB prevention and mortality, including for people with a higher CD4 cell count (57–59).
Recommendations (2020)
Identifying populations for latent TB infection testing and TB preventive treatment
People living with HIV
Adults and adolescents living with HIV who are unlikely to have active TB should receive TB preventive treatment as part of a comprehensive package of HIV care. Treatment should also be given to those receiving ART, to pregnant women and to those who have previously been treated for TB, irrespective of the degree of immunosuppression and even if latent TB infection testing is unavailable
(strong recommendation, high-certainty evidence).
Infants aged <12 months living with HIV who are in contact with a person with TB and who are unlikely to have active TB on an appropriate clinical evaluation or according to national guidelines should receive TB preventive treatment
(strong recommendation, moderate-certainty evidence)
Children aged ≥12 months living with HIV who are considered unlikely to have active TB on an appropriate clinical evaluation or according to national guidelines should be offered TB preventive treatment as part of a comprehensive package of HIV prevention and care if they live in a setting with high TB transmission, regardless of contact with a person with TB
(strong recommendation, low-certainty evidence).
All children living with HIV who have successfully completed treatment for TB disease may receive TB preventive treatment
(conditional recommendation, low-certainty evidence).
For more information on identifying household contacts (regardless of HIV status) for latent TB infection testing and TB preventive treatment: see WHO consolidated guidelines on tuberculosis: Module 1: prevention: tuberculosis preventive treatment (38).
Algorithms to rule out active TB disease
Adults and adolescents living with HIV should be screened for TB according to a clinical algorithm. Those who do not report any of the symptoms of current cough, fever, weight loss or night sweats are unlikely to have active TB and should be offered preventive treatment, regardless of their ART status
(strong recommendation, moderate-certainty evidence).
Adults and adolescents living with HIV who are screened for TB according to a clinical algorithm and who report any of the symptoms of current cough, fever, weight loss or night sweats may have active TB and should be evaluated for TB and other diseases and offered preventive treatment if active TB is excluded
(strong recommendation, moderate-certainty evidence).
Chest radiography may be offered to people living with HIV receiving ART and TB preventive treatment given to those with no abnormal radiographic findings
(conditional recommendation, low-certainty evidence).
Infants and children living with HIV who have poor weight gain, fever or current cough or who have a history of contact with a person with TB should be evaluated for TB and other diseases that cause such symptoms. If TB disease is excluded after an appropriate clinical evaluation or according to national guidelines, these children should be offered TB preventive treatment, regardless of their age
(strong recommendation, low-certainty evidence).
The absence of any symptoms of TB and the absence of abnormal chest radiographic findings may be used to rule out active TB disease among HIV-negative household contacts aged ≥5 years and other risk groups before TB preventive treatment
(conditional recommendation, very-low-certainty evidence).
Testing for latent TB infection
TB preventive treatment options
The following options are recommended for the treatment of latent TB infection regardless of HIV status: six or nine months of daily isoniazid, or a three-month regimen of weekly rifapentine plus isoniazid, or a three-month regimen of daily isoniazid plus rifampicin
(strong recommendation, moderate- to high-certainty evidence in the estimates of effect).
A one-month regimen of daily rifapentine plus isoniazid or four months of daily rifampicin alone may also be offered as alternatives
(conditional recommendation, low- to moderate-certainty evidence).
In settings with high TB transmission, adults and adolescents living with HIV who have an unknown or a positive latent TB infection test and are unlikely to have active TB disease should receive at least 36 months of daily isoniazid preventive therapy. Daily isoniazid preventive therapy for 36 months should be given whether or not the person is receiving ART and irrespective of the degree of immunosuppression, history of previous TB treatment and pregnancy in settings considered to have high TB transmission as defined by national authorities
(conditional recommendation, low-certainty evidence).
Source: WHO consolidated guidelines on tuberculosis: Module 1: prevention: tuberculosis preventive treatment (38).
Rationale and evidence
WHO published the recommendation to give TB preventive treatment for all people living with HIV in 2011 (60). A systematic review of 12 randomized controlled trials found that TB preventive treatment reduced the overall risk of TB by 33% (RR 0.67, 95% CI 0.51–0.87) (61).
Pregnancy should not disqualify women living with HIV from receiving preventive treatment with medicines commonly used to treat active TB that are generally considered safe for use in pregnancy, such as isoniazid and rifampicin.
For infants younger than 12 months living with HIV, TB preventive treatment should be given only to those who have a history of household contact with a person with TB and do not have TB disease according to investigations conducted in accordance with national guidelines because of limited data on the benefits (38). TB preventive treatment is strongly recommended for children 12 months or older living with HIV without clinical manifestations suggesting active TB, despite the low certainty of the evidence, because of the clear benefits for adults living with HIV and the high risk of active TB among people living with HIV (38). Children 12 months and older living with HIV who have clinical manifestations or who have contact with a person with TB should be evaluated further and treated for active TB or latent TB infection as indicated. Although the evidence for the efficacy of preventive treatment for children receiving ART is limited, it is biologically plausible given the evidence of additive effects for adults living with HIV receiving ART. Thus, TB preventive treatment is recommended for children living with HIV (38).
Implementation considerations
TB preventive treatment for people living with HIV should be a core component of the HIV package of care and should be primarily the responsibility of national HIV and AIDS programmes and HIV service providers (51). In situations where these tests are not available, TB preventive treatment should not be withheld from eligible people if active disease has been excluded on clinical grounds alone, and chest radiography should not be a requirement for initiating preventive treatment.
Ruling out active TB disease
Excluding active TB disease before initiating preventive treatment is one of the critical steps in the latent TB infection care pathway. For adults and adolescents living with HIV, the four-symptom screen – current cough, fever, weight loss and night sweats – is useful for ruling out active TB, regardless of ART use. WHO consolidated guidelines on tuberculosis: Module 1: prevention: tuberculosis preventive treatment (38) includes an algorithm for latent TB infection testing and TB preventive treatment for individuals at risk.
TB preventive treatment options
TB preventive treatment for an infection with strains presumed to be drug-susceptible can be broadly categorized into two types: monotherapy with isoniazid for at least six months (isoniazid preventive therapy) and treatment with regimens containing a rifamycin (rifampicin or rifapentine). Isoniazid preventive therapy has been the most widely used type of TB preventive treatment, but the shorter duration of rifamycin regimens presents a clear advantage (38). Preventive treatment for multidrug-resistant TB requires a different regimen using a fluoroquinolone or other second-line agents (38).
WHO has included both recommendations for regimens containing isoniazid or rifamycins in guidance since 2015 (62). Previous WHO guidance included a strong recommendation for TB preventive treatment alternatives to six months of isoniazid monotherapy based on evidence of low to high certainty. In 2019, WHO made two new conditional recommendations for daily rifapentine plus isoniazid for one month and daily rifampicin monotherapy for four months in all settings. These new recommendations are based on low- to moderate-certainty evidence. In addition, instead of a previous range of 3–4 months, WHO now recommends a duration of three months for daily isoniazid plus rifampicin and of four months of daily rifampicin alone to reflect the usual length of time for which these regimens are currently used.
Moreover, three previous recommendations on using six months of isoniazid monotherapy, three months of daily isoniazid plus rifampicin for people younger than 15 years and daily rifapentine plus isoniazid for three months in high-TB-prevalence settings that featured separately in previous guidance are now proposed as alternative options. The revised recommendation makes all latent TB infection options applicable to all settings (38).
Implementation considerations
The recommendation to give at least 36 months of daily isoniazid monotherapy to people living with HIV in high-TB-transmission settings is conditional and based on evidence that longer-term isoniazid preventive therapy significantly adds benefit to ART. The efficacy, safety and convenience of repeated treatment with shorter rifapentine regimens is being studied among people living with HIV in such settings. WHO defines high-TB-transmission settings as those with a high frequency of individuals with undetected or undiagnosed active TB or in which people with infectious TB are present and there is a high risk of TB transmission, but the national authorities should establish the definition. Testing for latent TB infection is not a prerequisite for TB preventive treatment for people living with HIV, but using it is encouraged because people who are positive on a tuberculin skin test have a greater protective benefit from TB preventive treatment. People living with HIV with a negative tuberculin skin test should not receive 36 months of daily isoniazid preventive therapy.
The benefits of three months of daily isoniazid plus rifampicin for infants and children younger than 15 years outweigh the harm, given its safety profile, the higher rate of completion compared with isoniazid monotherapy and the availability of child-friendly, fixed-dose combinations of rifampicin and isoniazid.
All the treatment options can be self-administered. WHO consolidated guidelines on tuberculosis: Module 1: prevention: tuberculosis preventive treatment (38) outlines the recommended dosages of medicines for TB preventive treatment.
Drug–drug interactions
Regimens containing rifamycins should be prescribed with caution to people living with HIV who are receiving ART because of potential drug–drug interactions. These regimens should not be administered to people receiving PIs or NVP, including HIV-exposed infants receiving TB preventive treatment. Rifampicin can decrease the concentrations of ATV, DRV, LPV and other PIs. No dose adjustment is required when rifampicin is co-administered with EFV. The dose of DTG needs to be increased to 50 mg twice daily when given together with rifampicin and twice daily dosing should be continued for an additional two weeks following stop of rifampicin use (63). Results from a recent Phase 1/2 trial of daily rifapentine plus isoniazid for three months and DTG for adults living with HIV reported good tolerability and viral load suppression. However, the Guideline Development Group stressed the continued need for studying the pharmacokinetics of daily rifapentine plus isoniazid for three months concomitantly with other medicines, especially ART.
6.4.5. Infection control
The WHO End TB Strategy calls for a 90% reduction in TB deaths and an 80% decrease in the TB incidence rate by 2030. The strategy emphasizes the need for prevention across all approaches, including infection prevention and control in health-care services and other settings with a high risk of M. tuberculosis transmission. Infection prevention and control practices are vital to reduce the risk of M. tuberculosis transmission, by reducing the concentration of infectious droplet nuclei in the air and the exposure of susceptible individuals to such aerosols.
Details on WHO infection control recommendations are available in the WHO guidelines on tuberculosis infection prevention and control: 2019 update (64).
6.5. Hepatitis B and C
Introduction
Chronic HBV infection (defined as persistence of hepatitis B surface antigen (HBsAg) for more than six months) and chronic HCV infection (defined as HCV antibody–positive with viraemic HCV infection) are major global public health problems (65,66). WHO estimates that, in 2019, 71 million people had chronic HCV infection and 257 million people chronic HBV worldwide, and 820 000 people died from HBV and 290 000 from HCV, mainly from cirrhosis or hepatocellular carcinoma (67). In 2019, there were 1.5 million new chronic HCV infections (65). Transmission of HCV infection has been most commonly associated with unsafe injection or inadequate infection control practices in health-care facilities as well as sharing of needles and syringes among people who inject drugs and transmission among people who inject drugs. HCV viraemic prevalence among people who inject drugs is 39% (67), which accounts for about one third of new HCV infections globally (68,69). There are important differences across countries and regions in the relative contribution of these routes of transmission (65,66,68). The regions with the highest prevalence of chronic HCV infection in the general population (>3.5%) are central and east Asia and North Africa and the Middle East. For HBV infection, perinatal or horizontal transmission is the main route of transmission globally, but transmission also occurs via injecting drug use and high-risk sexual behaviour (65,66). The highest prevalence of HBsAg (>5%) is in sub-Saharan Africa and east Asia, and worldwide, most people with chronic HCB infection were infected at birth or in early childhood, leading to high rates of chronic infection. Between 20% and 30% of those with chronic HBV infection develop complications, mainly cirrhosis and hepatocellular carcinoma. For HCV infection, the risk of cirrhosis ranges from 15% to 30% after 20 years of HCV infection (70,71).
6.5.1. HIV and HBV or HCV coinfection
Globally, the estimated prevalence and burden of HCV coinfection among people living with HIV are 6.2% (interquartile range 3.4–11.9%) and 2.3 million (interquartile range 1.3 million–4.4 million), of which 1.3 million are people who inject drugs. The numbers for HBV coinfection are 7.6% (interquartile range 5.6–12.1%) and 2.7 million (interquartile range 2.0 million–4.2 million) (71,72). Although sub-Saharan Africa has the greatest burden of HIV and HBV coinfection (69% of cases; 1.9 million), for HIV and HCV coinfection, it is in the concentrated epidemic settings of central Asia and eastern Europe among people who inject drugs, which account for 27% of the HIV and HCV burden. HIV and HCV have common routes of transmission, and people living with HIV, especially people who inject drugs (67) and gay men and other men who have sex with men (73), have an increased risk of HCV infection. In sub-Saharan Africa, HBV infection is predominantly acquired perinatally or in early childhood. As a result, most people have already been HBV-infected for many years by the time they are exposed to HIV in adulthood (72).
Liver disease caused by coinfection with HBV or HCV is an increasing cause of morbidity and mortality among people living with HIV in some regions, including among people receiving ART. Concurrent infection with HIV usually results in more severe and progressive liver disease and a higher incidence of cirrhosis, hepatocellular carcinoma and mortality (74,75). People living with HIV are therefore a priority group for early diagnosis of viral hepatitis coinfection and provision of both ART and specific antiviral therapy. In particular, HCV-related liver disease progresses more rapidly among people coinfected with HIV and HCV than among people solely infected with HCV. Even among people for whom ART successfully controls HIV infection (based on undetectable HIV viral load), the risk of hepatic decompensation among coinfected people is higher than among people solely infected with HCV. For these reasons, HCV treatment is a priority for people with HIV and HCV coinfection (75). A comprehensive approach includes prevention, HBV and HCV testing, HBV vaccination and treatment and care for people living with HIV who are coinfected with HBV and/or HCV.
6.5.2. Testing for HBV and HCV infection
Testing and diagnosis of HBV and HCV infection is the gateway for access to both prevention and treatment services. Early identification of people with chronic HBV or HCV infection enables them to receive the necessary care and treatment to prevent or delay the progression of liver disease. Testing also provides an opportunity to link people to interventions to reduce transmission, through counselling on risk behaviour and provision of prevention commodities (such as sterile needles and syringes) and HBV vaccination.
The 2017 testing guidelines recommend offering focused testing to individuals from populations most affected by HBV or HCV infection (either part of a population with higher seroprevalence or have a history of exposure to or high-risk behaviour for HBV or HCV infection) (70). This includes all adults and adolescents living with HIV. For HBV and HCV, other priority groups are mobile and migrant populations from high- and intermediate-endemic countries and certain indigenous populations or those with a history of exposure or high-risk behaviour for HBV infection (such as people who inject drugs; people in prisons and other closed settings; gay men and other men who have sex with men; sex workers; people living with HIV; and the partners, family members and children of people with HBV infection) and health-care workers in all settings. This is in addition to adults, adolescents and children for whom chronic viral hepatitis is clinically suspected (through symptoms, signs or laboratory markers) (70,76).
In settings with a ≥2% or ≥5% seroprevalence of HBsAg or HCV antibody (anti-HCV) (based on existing published thresholds for intermediate or high seroprevalence), it is recommended that all adults have routine access to and be offered testing (a general population testing approach) or use birth cohort testing for specific age groups with higher anti-HCV seroprevalence. In settings with a ≥2% or ≥5%% HBsAg seroprevalence (depending on the epidemic profile and country infrastructure) in the general population, it is recommended that HBsAg serological testing be routinely offered to all pregnant women in antenatal clinics, with linkage to prevention, care and treatment services. Couples and partners in antenatal care settings should also be offered HBV testing services. Overall, these different testing approaches should make use of existing facility-based services (such as outpatient clinics, antenatal clinics, HIV or TB services).
There is also a recent caution on the need to test for HBV infection and consider antiviral therapy before starting direct-acting antiviral therapy among people coinfected with HBV and HCV, because of a potential risk of HBV reactivation and worsening of liver disease. For people living with HIV, ART with a TDF + 3TC or FTC–based regimen should be initiated before starting direct-acting antiviral therapy.
The guidelines recommend using a single quality-assured serological in vitro diagnostic test (either a laboratory-based immunoassay such as enzyme immunoassay or chemiluminescence immunoassay or rapid diagnostic test) to detect HBsAg and HCV antibody. The rapid diagnostic tests used should meet minimum performance standards and be delivered at the point of care to improve access and linkage to care and treatment. Following a reactive HCV antibody serological test result, a quantitative or qualitative RNA NAT is recommended to diagnose viraemic infection. Detecting core HCV antigen, in which the assay has comparable clinical sensitivity to NAT technologies, may be considered as an alternative. The use of HBV DNA NAT following a reactive HBsAg serological test result is recommended to help further guide whom to treat or not treat if no evidence indicates cirrhosis and to monitor for treatment response, based on existing recommendations from the 2015 WHO guidelines on managing HBV (77). There are several WHO-prequalified rapid diagnostic tests for both HCV antibody and HBsAg and one point-of-care HCV RNA viral load NAT assay but not yet for HBV DNA viral load (78,79).
6.5.3. Managing HIV and HCV coinfection
The global response and opportunities for eliminating HCV infection have been transformed by the introduction of curative, short-course direct-acting antiviral therapy, the widespread availability of rapid diagnostic testing for HCV antibody, the availability of NAT for HCV viraemia and the 2018 updated WHO recommendation of a “treat-all” approach regardless of stage of disease using three pangenotypic regimens (see Box 6.3) (74). This is further supported by a simplified public health approach for HCV testing and treatment and good practice principles of decentralization, integration and task sharing to promote the scale-up of testing and treatment.
In general, clinical stabilization of HIV disease with ART is advisable before starting treatment for HCV, especially for people with advanced immunosuppression (CD4 count below 200 cells/mm3). HCV treatment outcomes with direct-acting antiviral therapy are comparable for people with HIV and HCV coinfection to those solely infected with HCV (75). Because direct-acting antiviral therapy is safe and effective for people with HIV and HCV, they no longer need to be considered as a special or difficult-to-treat population (80). However, pangenotypic HCV regimens and ART have important drug–drug interactions. Checking for drug–drug interactions between HIV and HCV medications is therefore important.
Box 6.3Pangenotypic regimens currently available for adults 18 years and older
For adults without cirrhosis, the following pangenotypic regimens can be used:
For adults with compensated cirrhosis, the following pangenotypic regimens can be used:
- a
People with HCV genotype 3 infection who have received interferon and/or ribavirin in the past should be treated for 16 weeks.
- b
May be considered in countries where the genotype distribution is known and the genotype 3 prevalence is <5%.
Pretreatment evaluation
Women of childbearing age may be offered pregnancy testing and be informed about the lack of available data on the safety and efficacy of direct-acting antiviral therapy during pregnancy. In addition, WHO recommends an alcohol intake assessment before initiating treatment and a fibrosis assessment using non-invasive tests such as the AST to platelet ratio index score or FIB-4 test to determine whether there is cirrhosis (66,74). This information will allow clinicians to decide on the appropriate treatment duration of the pangenotypic regimen of their choice based on the absence or presence of cirrhosis. The treatment duration of the recommended pangenotypic regimens sofosbuvir + daclatasvir and glecaprevir + pibrentasvir depends on the absence or presence of cirrhosis (Box 6.3 and ).
The association between recommended pangenotypic regimens and EFV is either contraindicated (for sofosbuvir + velpatasvir and glecaprevir + pibrentasvir) or requires dose adjustment (for sofosbuvir + daclatasvir). Chapter 4 includes a summary of the drug–drug interactions between WHO-recommended HIV ARV drugs and HCV drugs as do the annexes. Where drug–drug interactions are likely, ARV drug substitutions may be considered before initiating HCV therapy. Prescribers may consult the University of Liverpool webpage on hepatitis drug interactions (81) before prescribing, since the details of interactions are frequently updated. This website includes details of interactions with prescribed and non-prescribed medicines.
Algorithm for the diagnosis, treatment and monitoring of chronic HCV infection among adults and adolescents. a People with HCV genotype 3 infection who have received interferon and/or ribavirin in the past should be treated for 16 weeks. b May be considered (more...)
6.5.4. Managing HIV and HBV coinfection
HBV vaccination. Universal infant and perinatal HBV vaccination remains the key strategy for preventing mother-to-child transmission and controlling the HBV epidemic. Although high uptake of infant vaccination has been achieved, leading to substantial decreases in incidence in recent years, HBV birth-dose vaccination is being implemented by less than half of countries. The risk of HBV infection may be higher for adults living with HIV, and therefore everyone newly diagnosed with HIV should be screened for HBsAg and anti-HBs to identify those with chronic HCB infection and propose vaccination if non-immune, especially among high-risk groups such as people who inject drugs and gay men and other men who have sex with men. People living with HIV may respond more poorly to HBV vaccine, especially those with a low CD4 cell count. A schedule using four double (40 µg) doses of the vaccine may provide a higher protective anti-HBs titre than the regular three 20 µg dose schedule.
Treatment. In the absence of treatment, HIV coinfection profoundly influences the course of HBV infection, including more rapid progression to cirrhosis and hepatocellular carcinoma, higher liver-related mortality and decreased treatment response compared with people who do not have HIV. All people newly diagnosed with HIV should therefore be screened for HBsAg and vaccinated if HBsAg negative and non-immune (HbsAB <10 IU/L). The recommended NRTI drugs for ART – TDF with 3TC or FTC – are also active against HBV. Fortunately, TDF, a drug widely included in ART regimens, is also the most effective drug for long-term treatment of HBV, leading to sustained HBV viral suppression, reversal of cirrhosis and fibrosis and reduction in HBV-related mortality. WHO guidelines recommend using TDF or entecavir for the long-term treatment of people with chronic HBV infection (77). All people coinfected with HIV and HBV should therefore receive a TDF-based ART regimen in combination with 3TC (or FTC), as the NRTI backbone of an ART regimen, regardless of stage of disease or HBV DNA level. HIV treatment among people coinfected with HBV without using TDF in the regimen may lead rarely to flares of HBV because of ART-associated immune reconstitution. If ARV drugs need to be changed because of HIV drug resistance or toxicity, then TDF with 3TC or FTC should be continued together with the new ARV drugs. Similarly, abrupt treatment discontinuation of TDF or 3TC, may be associated with HBV reactivation, hepatic flares and, in rare cases, hepatic decompensation.
6.5.5. Preventing mother-to-child transmission of HBV infection
Among people living with HIV, HBV coinfection is associated with a higher rate of HBV e-antigen positivity, higher HBV viraemia and increased perinatal transmission of perinatal HBV infection (13). Eliminating HBV infection as a public health threat requires reducing HBsAg prevalence to less than 0.1% among children five years old. This can be achieved by universally immunizing newborns against HBV and other interventions to prevent the mother-to-child transmission of HBV. The 2020 WHO guidelines on antiviral prophylaxis for hepatitis B in pregnancy (76) included the following recommendations.
Routinely testing pregnant women for HIV, HBV and syphilis. All pregnant women should be tested for HIV, syphilis and HBsAg at least once and as early as possible in the pregnancy (HIV standing recommendation since 2007; syphilis: strong recommendation, moderate-certainty evidence; HBsAg: strong recommendation, low-certainty evidence).
Existing recommendations on immunization from the WHO position paper (
80). All infants should receive their first dose of HBV vaccine as soon as possible after birth, preferably within 24 hours. Delivery of HBV vaccine within 24 hours of birth should be a performance indicator for all immunization programmes, and reporting and monitoring systems should be strengthened to improve the quality of data on the birth dose. The birth dose should be followed by two or three doses to complete the primary series.
Tenofovir prophylaxis to prevent mother-to-child transmission of HBV. Women coinfected with HIV and HBV should be receiving TDF-based ART, which will provide prophylaxis to prevent the mother-to-child transmission of HBV. This is in addition to three-dose HBV vaccination for all infants, including timely birth dose (conditional recommendation, moderate-certainty evidence).
Summary of recommendations on testing for chronic HBV and HCV infection.
6.6. Malaria
Introduction
Malaria continues to cause high levels of morbidity and mortality. Malaria is preventable and treatable, but according to the latest World Malaria Report (83), there were an estimated 22 000 cases and 409 000 deaths globally in 2019. In 2021, WHO published guidelines for malaria (84) as a comprehensive resource for advice on malaria.
There is significant geographical overlap between HIV and malaria. People living with HIV have increased risk of more frequent and higher-density infection, severe malaria and malaria-related death, depending on the malaria transmission intensity of the area.
Key interventions to control malaria include early diagnosis, prompt and effective treatment with artemisinin-based combination therapies and use of insecticide-treated nets and indoor residual insecticide spraying to control the vector mosquitoes. In areas of stable malaria transmission, people living with HIV (as for the general population) should routinely use insecticide-treated bed nets or have access to indoor residual spraying to reduce their exposure to malaria. Intermittent preventive treatment during pregnancy and seasonal malaria chemoprophylaxis are also recommended in areas of high transmission. Treatment or intermittent preventive treatment with sulfadoxine-pyrimethamine should not be given to people living with HIV or HIV-exposed infants who are taking co-trimoxazole prophylaxis. Intermittent preventive treatment of malaria in pregnancy should not be provided in addition to co-trimoxazole prophylaxis.
People living with HIV who develop malaria should receive prompt, effective antimalarial treatment regimens. Parasitological confirmation should be undertaken for all suspected malaria cases using either microscopy or a rapid diagnostic test. However, the absence or delay of parasitological diagnosis should not delay the immediate start of antimalarial treatment.
Limited information is available on how HIV infection modifies therapeutic responses to artemisinin-based combination therapies. Early studies suggested that increasing HIV-related immunosuppression was associated with decreased treatment response to antimalarial drugs. There is presently insufficient information to modify the general malaria treatment recommendations for people living with HIV.
Good practice statement (2021)
For people who have HIV and uncomplicated Plasmodium falciparum malaria, avoid artesunate + sulfadoxine-pyrimethamine if they are being treated with co-trimoxazole and avoid artesunate + amodiaquine if they are being treated with efavirenz or zidovudine.
Source: WHO guidelines for malaria (84).
Supporting evidence and rationale (84)
WHO recommends DTG-based regimens as first-line therapy for HIV. In two Phase 2 healthy volunteer studies, participants received 50 mg of DTG once daily alone or in combination with standard treatment doses of artemether + lumefantrine (80/480 mg) or artesunate + amodiaquine (200/540 mg) (85). Co-administration increased DTG clearance by 10.6% (95% CI 4.1–34.5%) and 26.4% (95% CI 14.3–51.4%), respectively. Simulations showed that simulated trough concentrations of DTG alone or in combination with artemether/lumefantrine or artesunate/amodiaquine are maintained above the DTG protein-adjusted concentration required for 90% inhibition of 0.064 mg/L for more than 99% of the individuals. DTG dose adjustments are not necessary for people taking standard three-day treatment doses of artemether + lumefantrine or artesunate + amodiaquine.
A study of children with uncomplicated malaria in a high-transmission area of Africa showed a decreased risk for recurrent malaria after treatment with artemether + lumefantrine for children receiving LPV/r-based ART versus NNRTI-based ART. Evaluation of pharmacokinetics for these children and for healthy volunteers showed significantly higher exposure to lumefantrine and lower exposure to dihydroartemisinin with LPV/r-based ART but no adverse effects. Conversely, EFV-based ART was associated with a two- to fourfold decrease in exposure to lumefantrine in healthy volunteers and malaria-infected adults and children, with increased rates of recurrent malaria after treatment. Close monitoring is required. Increasing artemether + lumefantrine dosing with EFV-based ART has not yet been studied. Exposure to lumefantrine and other NNRTI-based ART, namely NVP and ETR, did not show consistent changes that would require dose adjustment.
Studies of administration of quinine with LPV/r or RTV alone among healthy volunteers gave conflicting results. The combined data are insufficient to justify dose adjustment. Single-dose atovaquone–proguanil with EFV, LPV/r or ATV/r were all associated with a significantly decreased area under the concentration–time curve for atovaquone (two- to fourfold) and proguanil (twofold), which could well compromise treatment or prophylactic efficacy. There is insufficient evidence to change the current mg/kg body weight dosing recommendations; however, these people should also be monitored closely.
6.7. Buruli ulcer
Introduction
Buruli ulcer, caused by Mycobacterium ulcerans, is largely a health problem among poor people in remote rural areas of sub-Saharan Africa and is the third most common mycobacterial disease after TB and leprosy (86,87). Nearly 50% of the people affected are younger than 15 years, live in remote rural areas and have little or no access to health services (88).
Buruli ulcer and HIV
Areas of sub-Saharan Africa in which Buruli ulcer is endemic also have a high prevalence of HIV, with adult prevalence rates between 1% and 5% (89). Preliminary evidence suggests that HIV infection may increase the risk of Buruli ulcer disease (90–92). Prevalence studies in Benin, Cameroon and Ghana showed that people with Buruli ulcer were 3–8 times more likely to be living with HIV than those without Buruli ulcer (90,92). HIV may affect the clinical presentation of the severity of Buruli ulcer disease, with a reported increased incidence of multiple larger and ulcerated Buruli ulcer lesions among people living with HIV. Buruli ulcer is more common among people living with HIV with low CD4 cell counts, and the size of the Buruli ulcer lesions may increase with decreasing CD4 cell counts (91–96).
Buruli ulcer may occur in the context of immune reconstitution inflammatory syndrome after initiating ART (97).
Diagnosis
In an area of known endemicity, an experienced health-care worker can usually diagnose Buruli ulcer on clinical grounds (86). Molecular detection of M. ulcerans by PCR is used to confirm the diagnosis (89,98). If PCR is not available, any of the following or a combination may be used: direct smear examination, PCR, histopathology and culture (not for diagnosis and treatment). For ulcerative lesions, at the start of antibiotic treatment, swabs should be taken from the undermined edges of the ulcer for direct smear examination, culture and PCR. Swabs should also be taken at the end of antibiotic treatment (if the lesion has not healed or surgery is indicated) to enable analysis of the response to treatment. For non-ulcerative lesions, before the start of antibiotic treatment, a fine-needle aspirate should be taken from the estimated centre of the lesion for microbiological analysis (direct smear examination, PCR and culture). Other procedures that can be used to obtain specimens include punch and surgical biopsy if histopathological analysis is strongly required. WHO guidance for obtaining specimens for laboratory confirmation is available (99).
Common differential diagnoses include tropical phagedenic ulcer, necrotizing fasciitis, venous ulcer (especially among older people), diabetic ulcer, sickle-cell disease–related ulcers, yaws, cutaneous TB, leprosy, cutaneous leishmaniasis and malignant skin ulcer (86).
Treatment considerations
The current recommended antibiotic treatment for Buruli ulcer is a combination of rifampicin with clarithromycin or moxifloxacin (4). DTG-based ART is recommended as a preferred first-line regimen for adults, adolescents and children living with HIV who are initiating ART, as recommended for those without Buruli ulcer disease. Because of the pharmacokinetic interaction with rifampicin, it is recommended that the dose of DTG be increased to 50 mg twice daily when both drugs are used concomitantly. The use and management of alternative first-line and second-line regimens should also follow the same principles adopted for people living with HIV without Buruli ulcer disease (see Chapter 4). Clarithromycin and EFV interact to significantly reduce the dose of clarithromycin, potentially reducing its effectiveness and increasing the risk of toxicity (skin rash). Rifampicin and clarithromycin should therefore be used with caution when combined with EFV. The concentrations of most PIs are significantly reduced when combined with rifampicin and should therefore be avoided during antibiotic treatment of Buruli ulcer. If the person is already receiving a PI-based regimen, then the PI should be changed to DTG (with dose adjustment) as the preferred approach (4).
Collaboration with TB programmes at all levels is recommended, especially in areas such as coordinating drug procurement, using laboratory facilities and networks and monitoring for potential antibiotic resistance. Collaboration with HIV and AIDS programmes at all levels is important in managing people with Buruli ulcer, who may be living with HIV. A network of laboratories conducting high-performance PCR-based diagnosis of Buruli ulcer is essential in endemic African countries (98).
Box 6.4 lists the main treatment considerations for Buruli ulcer for people living with HIV.
Box 6.4HIV and Buruli ulcer coinfection: guiding principles
Everyone with Buruli ulcer should be offered high-quality facility-based HIV testing and counselling.
Combination antibiotic treatment for Buruli ulcer should begin before starting ART and given for eight weeks.
The recommended combination is rifampicin plus clarithromycin. The DTG dose needs to be adjusted because of drug–drug interaction with rifampicin, and this antibiotic regimen should be used with caution when used with ARV drug regimens containing EFV. An alternative antibiotic regimen is rifampicin plus moxifloxacin.
Rapid ART initiation is recommended for everyone coinfected with Buruli ulcer and HIV, regardless of clinical stage and/or CD4 cell count.
Everyone coinfected with Buruli ulcer and HIV should be actively screened for TB before beginning Buruli ulcer treatment and before starting ART.
Everyone coinfected with Buruli ulcer and HIV who has advanced HIV disease should be offered a package of care interventions including screening, treatment and/or prophylaxis for major opportunistic infections, rapid ART initiation and intensified adherence support interventions.
Programmes should implement a monitoring and reporting system to monitor and evaluate the outcomes of Buruli ulcer and HIV interventions.
Source: Management of Buruli ulcer–HIV coinfection: technical update (100).
6.8. Leishmaniasis
Introduction
Leishmaniasis is a group of diseases caused by Leishmania species. The three main forms of leishmaniasis are visceral (also known as kala-azar, the most serious form of the disease), cutaneous (the most common) and mucocutaneous (101). Leishmaniasis is caused by the protozoan Leishmania parasites, which are transmitted by the bite of infected female phlebotomine sandflies. It is a neglected tropical disease that disproportionately affects poor and marginalized populations with limited access to health care. In 2018, more than 95% of new cases reported to WHO occurred in 10 countries: Brazil, China, Ethiopia, India, Iraq, Kenya, Nepal, Somalia, South Sudan and Sudan (101). Although only a small fraction of those infected by Leishmania parasites eventually develops the disease, an estimated 700 000 to 1 million new cases occur annually. The burden of visceral leishmaniasis occurring among people living with HIV has increased in recent decades (102).
Leishmaniasis and HIV
Visceral leishmaniasis is an important opportunistic infection associated with HIV infection in some regions (103). The two diseases are mutually reinforcing, with people living with HIV being especially vulnerable to visceral leishmaniasis, and visceral leishmaniasis accelerating HIV replication and disease progression (103). Concomitant HIV infection increases the risk of developing active visceral leishmaniasis by between 100 and 2320 times (103). In areas endemic for visceral leishmaniasis, many people have asymptomatic infection (104). To date, as many as 42 countries throughout the world have reported HIV and leishmaniasis coinfection cases since the first case was reported in 1985 (105). In southern Europe, up to 70% of cases of visceral leishmaniasis among adults are associated with HIV infection (103), although the number of new cases has significantly declined since the end of the 1990s, mainly because of greater access to ART. In parts of the world in which access to ART is limited or leishmaniasis frequently occurs in advanced HIV disease, the prevalence of visceral leishmaniasis coinfection is steadily rising. Northern Ethiopia has a documented high rate of HIV infection among people with visceral leishmaniasis, ranging between 15% and 35% (106). In India, the prevalence of visceral leishmaniasis and HIV coinfection in total reported cases increased from 0.9% in 2000 to 3.8% in 2018. A study from Bihar reported that 5.6% of 2077 consecutive people with confirmed visceral leishmaniasis 14 years and older were living with HIV; half of these were unaware of their HIV status (107).
Clinical presentation of HIV and visceral leishmaniasis coinfection
Visceral leishmaniasis clinical presentations among people living with HIV are frequently atypical and involve various organs (such as the gastrointestinal tract, peritoneal space, lung, pleural space and skin), especially among people with advanced HIV disease and /or low CD4 cell counts. The presence of other concomitant opportunistic infections is common and may make the early clinical diagnosis of visceral leishmaniasis difficult. Cases of visceral leishmaniasis have been described in association with immune reconstitution inflammatory syndrome among people living with HIV with latent Leishmania infection or among people already treated for visceral leishmaniasis receiving ART (103).
Diagnosis
Laboratory diagnosis of visceral leishmaniasis is based on positive parasitology (stained smears from bone marrow, spleen, liver, lymph node, blood or culture of the organism from a biopsy or aspirated material) and/or positive serology (indirect fluorescent antibody test, ELISA, rK39 or direct agglutination test) or PCR (103). For visceral leishmaniasis, diagnosis is made by combining clinical signs with parasitological or serological tests (such as rapid diagnostic tests). In cutaneous and mucocutaneous leishmaniasis, serological tests have limited value and clinical manifestation with parasitological tests confirms the diagnosis (101). For people coinfected with HIV and visceral leishmaniasis, diagnostic tests perform less well, with lower sensitivity and specificity of serological tests. For people coinfected with HIV and visceral leishmaniasis, the parasite load is higher, and parasites may be found at unusual sites (103).
Treatment
WHO has guidance on the treatment of people with visceral leishmaniasis and HIV in eastern Africa and South-East Asia (103), recommending liposomal amphotericin B + miltefosine as the preferred treatment regimen to improve treatment efficacy and reduce toxicity. Secondary prophylaxis after the first episode of visceral leishmaniasis is recommended for all people coinfected with HIV and visceral leishmaniasis to reduce the risk of relapse and is summarized in this section. Optimal management of people coinfected with HIV and visceral leishmaniasis aims to cure visceral leishmaniasis and also make HIV viral load undetectable by rapidly initiating ART (104,108).
Pregnancy (103)
Little information is available on the treatment of visceral leishmaniasis in pregnancy. The threat of a fatal outcome of leishmaniasis for the mother, the fetus and the newborn is much greater than the risk for adverse drug effects. When untreated, spontaneous abortion, small for birth date and congenital leishmaniasis have been described. In general, amphotericin B deoxycholate and lipid formulations are the best therapeutic options for visceral leishmaniasis. No abortions or vertical transmission have been reported among mothers treated with liposomal amphotericin B. However, the combination regimen to treat visceral leishmaniasis among people coinfected with HIV includes miltefosine, which is potentially embryotoxic and teratogenic and thus should not be used during pregnancy. Experts have recommended to keeping a pregnancy register to evaluate the fetotoxicity of drugs in use.
Implementation considerations
Visceral leishmaniasis diagnostics and medicines need to be continuously available, affordable and accessible to the health systems and to all patients (109). Liposomal amphotericin B and miltefosine are included in the WHO Essential Model List for Medicines (110). Coordination between leishmaniasis and HIV programmes is essential (111).
Recommendations for treating people living with HIV for visceral leishmaniasis (2021)
People coinfected with visceral leishmaniasis and HIV in eastern Africa
Liposomal amphotericin B + miltefosine regimen
Liposomal amphotericin B (up to a total of 30 mg/kg at 5 mg/kg on days 1, 3, 5, 7, 9 and 11) + miltefosine (100 mg/day for 28 days)
(conditional recommendation, very-low-certainty evidence).
People coinfected with visceral leishmaniasis and HIV in South-East Asia
Liposomal amphotericin B + miltefosine regimen
Liposomal amphotericin B (up to a total of 30 mg/kg at 5 mg/kg on days 1, 3, 5, 7, 9 and 11) + miltefosine (100 mg/day for 14 days)
(conditional recommendation, very-low-certainty evidence).
Provide secondary prophylaxis after the first episode of visceral leishmaniasis for all people coinfected with visceral leishmaniasis and HIV.
(conditional recommendation, very-low-certainty evidence).
Key considerations
Routinely screen for TB at visceral leishmaniasis diagnosis and further follow-up.
Consider providing extended therapy for people who do not show good clinical response after ruling out other diagnoses.
When miltefosine is not available, consider using monotherapy with liposomal amphotericin B (up to a total of 40 mg/kg) in accordance with the liposomal amphotericin B regimen.
Provide comprehensive clinical management, including adequate HIV treatment and nutritional support.
Ensure access to contraception and pregnancy testing for women of childbearing age before administering miltefosine.
Source: WHO guidelines for the treatment of visceral leishmaniasis in HIV-coinfected persons in east Africa and South-East Asia (103).
6.9. Cervical cancer
Background
Cervical cancer, a preventable and treatable malignancy, is the fourth most commonly detected cancer among women worldwide, with an estimated half million new cases in 2018 (112). More than 311 000 women die from cervical cancer each year, with 87% of these deaths occurring among women living in low- and middle-income countries (104). Although an estimated 5% of all cervical cancer cases are attributable to HIV globally, the proportion of women living with HIV among people with cervical cancer varies widely by region because of the varying prevalence of HIV. In areas with high HIV prevalence, the fraction of cervical cancer attributable to HIV is high and is 40% or more in nine countries versus less than 5% in 122 countries with lower HIV prevalence (113).
Women living with HIV have a six-fold higher risk of cervical cancer than women without HIV (113), and cervical cancer is classified as an AIDS-defining condition (114). This higher risk starts with an increased risk of acquiring HPV infection, lower chances of regression of pre-cancer lesions, more rapid progression to cancer and higher rates of recurrence following treatment (115–117). ART has led to steep declines in AIDS-related mortality and has increased life expectancy, with more than 19 million women estimated to be living with HIV worldwide in 2019 (118).
Following a WHO call to action in 2018, 194 countries collectively resolved to eliminate cervical cancer as a public health problem (119,120). The 2030 targets of the WHO global strategy are to achieve: 90% of girls fully vaccinated with HPV vaccine by age 15 years, 70% of women are screened with a high-performance test by 35 years of age and again by 45 years of age, and 90% of women identified with cervical disease receive treatment.
New recommendations on screening and treatment to prevent cervical cancer
In 2021, WHO published updated guidance on screening and treatment recommendations to prevent cervical cancer (121) that included 14 new recommendations and good practice statements for women living with HIV. summarizes the recommendations for women living with HIV. Screening aims to detect precancerous lesions that can be treated before they progress to cancer. Women living with HIV with access to care have clinical appointments at least every six months, which provides an opportunity for delivering cervical cancer screening and treatment interventions, alongside appropriate follow-up.
WHO guidelines developed in 2021 (121) provide recommendations for screening and treatment programmes for cervical cancer prevention.
Supporting evidence
Systematic literature reviews were conducted for the effects of interventions (including tests) on outcomes, and for the accuracy of screening tests in the general population and among women living with HIV. An individual-patient data meta-analysis was performed to analyse age-specific data for cervical cancer and cervical intraepithelial neoplasia among women living with HIV.
A mathematical model, Policy1-Cervix, was also used to estimate the risk of important outcomes of the priority screening and treatment strategies across 78 low- and middle-income countries (122–124). A separate model for cervical cancer among women living with HIV in the United Republic of Tanzania was used to evaluate outcomes for women living with HIV (125). Outcomes were assessed over the lifetime of birth cohorts eligible for screening in 2030 onwards and included cervical cancer incidence and mortality, precancer treatment and additional preterm deliveries as a result of precancer treatment.
Women and girls 15 years and older, regardless of their previous cervical cancer screening or treatment status, were invited to participate in an anonymous, voluntary survey. The survey was promoted through the Union for International Cancer Control and the WHO advisory and advocacy groups for women living with HIV and shared through WHO regional focal points for cervical cancer elimination initiative.
Summary of WHO screening and treatment recommendations to prevent cervical cancer for women living with HIV.
Good practice statement for the general population and women living with HIV
Once a decision to treat a woman is made, treating as soon as possible within six months is good practice to reduce losses to treatment. However, for women who are pregnant, good practice includes deferral until after pregnancy.
In circumstances when treatment is not provided within this time frame, evaluating the woman before treatment is good practice.
Recommendation
WHO suggests large loop excision of the transformation zone or cold-knife conization for women who have histologically confirmed adenocarcinoma in situ.
Remarks: Loop excision may be preferred for women of reproductive age, in settings with greater availability of large loop excision of the transformation zone and by providers with greater expertise performing large loop excision of the transformation zone. Cold-knife conization may be preferred when interpretation of the margins of the histological specimen is imperative. (Conditional recommendation, low-certainty evidence).
Source: Guidelines for screening and treatment of precancerous lesions for cervical cancer prevention: WHO guidelines (126).
Summary of decision-making, strength and certainty of recommendations
For women living with HIV, a strong recommendation was made for using HPV DNA testing as a primary screening test because a higher value was placed on the reductions in cervical cancer and deaths that are likely with this approach than on the potential harm that may occur, such as preterm deliveries. Compared with VIA or cytology as a primary screening test, greater benefits are also more likely with HPV DNA testing. HPV DNA testing is acceptable to women and providers, is feasible and is not likely to lead to inequities. In some settings, HPV DNA testing is not yet available, however, and existing quality-assured programmes will need to remain until HPV DNA testing becomes operational.
A conditional recommendation was made to use HPV DNA testing with a triage test rather than HPV DNA testing followed by treatment because providing a triage test may lead to reduced potential harm, with minimal change in benefits. The feasibility and resources needed to provide different triage tests vary across settings, thus influencing which test is chosen.
Overall, with all screening and treatment strategies, there are greater reductions in cervical cancer, deaths and CIN2/3 lesions for women living with HIV compared with the general population of women. For women living with HIV receiving ART, there were few data on how ART affects HPV-associated lesions, although the evidence is growing; therefore, recommendations based on using ARV drugs were not made.
For the age at which to start screening, a meta-analysis of individual patient data, mathematical modelling and studies about cervical cancer incidence and cervical intraepithelial neoplasia by age provided low-certainty evidence supporting the initiation of screening at 25 years of age rather than at 20 or 30 years. Starting at this age is likely to be acceptable to stakeholders, is feasible and requires fewer resources than starting screening earlier. The studies mentioned above provided very-low-certainty evidence (given the small numbers of women followed and reporting cervical cancer or cervical intraepithelial neoplasia lesions) that the risk of cervical cancer and lesions may continue. Screening after 50 years of age was therefore suggested to continue at regular screening intervals until two consecutive negative screening results after 50 years. Conditional recommendations were made for screening intervals based on modelled evidence showing that three- or five-year screening intervals with HPV DNA testing (or cytology or VIA) may provide greater benefits, but there may be more treatments and therefore harm compared with a longer interval.
Conditional recommendations were made for HPV DNA testing 12 months after treatment and after a negative triage test, regardless of initial screening test, since there may be greater benefits and less harm.
HPV vaccines
HPV vaccines should be introduced as part of a coordinated strategy to prevent cervical cancer. Recommended target population for preventing cervical cancer: girls aged 9–14 years, before becoming sexually active (127). A three-dose schedule (0, 1–2 and 6 months) should be used for all vaccinations initiated at 15 years and older, including those younger than 15 years known to be immunocompromised and/or living with HIV (regardless of whether they are receiving ART) (127). Screening for HPV infection or HIV infection before HPV vaccination is not necessary (127).
Additional recommendations (126)
Low-certainty evidence from a systematic review found that there may be little to no difference in the recurrence rate of adenocarcinoma in situ with cold-knife conization or electrosurgical excision or in the incidence of complications such as major infection and bleeding and found that more women may have premature deliveries in subsequent pregnancies following cold-knife conization compared with electrosurgical excision. The studies included in the systematic review did not confirm HIV status, but the Guideline Development Group agreed that the data could be extrapolated to women living with HIV and applied directly. Cold-knife conization is performed in the operating theatre, so access to cold-knife conization may be limited in some settings, more costly and less preferred by women compared with large loop excision of the transformation zone. In addition, greater expertise may be needed for successful electrosurgical excision.
WHO guidelines for implementing these recommendations are expected in late 2021.
6.10. Noncommunicable diseases
Recommendation (2016)
Assessment and management of cardiovascular risk should be provided for all individuals living with HIV according to standard protocols recommended for general population
(conditional recommendation, very-low-certainty evidence).a
Good practice statement (2016)
Strategies for the prevention and risk reduction of cardiovascular diseases by addressing modifiable factors such as blood pressure, smoking, obesity status, unhealthy diet and lack of physical activity should be applied to all people living with HIV.
Source: Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach – second edition (51).
- a
The WHO Package of Essential Noncommunicable (PEN) disease interventions for primary health care (128) in low-resource settings targets the following populations for cardiovascular disease screening: age older than 40 years, smokers, people with known hypertension or diabetes, waist circumference (>90 cm for women and >110 cm for men) and family history of diabetes or premature cardiovascular disease.
Background and rationale
Noncommunicable diseases, including hypertension, cardiovascular disease, renal disease, cancer, chronic respiratory disease, diabetes and mental health disorders, account for 63% of global deaths (129). Low- and middle-income countries bear 86% of the burden of noncommunicable diseases (129). Compared with the general population, people living with HIV have increased risk of developing a range of chronic noncommunicable diseases, including cardiovascular disease, hypertension, diabetes, chronic obstructive pulmonary disease, kidney disease and cancer (130–135).
The intersection of HIV and noncommunicable diseases is strongly influenced by increasing survival because of effective ART, lifestyle factors, ART adverse events, chronic immune activation caused by HIV and other disease conditions associated with ageing (136,137). Cardiovascular disease is now one of the leading causes of non-AIDS-related morbidity and mortality among people living with HIV. Both HIV and noncommunicable diseases require health systems that can deliver effective acute and chronic care and support adherence to treatment. Chronic HIV care provides the opportunity for assessing, monitoring and managing noncommunicable diseases, especially through primary care. Integrating interventions such as nutrition assessment, dietary counselling and support, smoking cessation, exercise promotion, blood pressure monitoring and – when available – cholesterol management as part of HIV care can help to reduce the risks of noncommunicable diseases among people living with HIV and improve HIV treatment outcomes (138,139).
WHO has defined a package of essential noncommunicable disease interventions (128) and provides recommendations on assessing and managing the major noncommunicable diseases from the primary care level to the district hospital level. The interventions are mainly focused on assessing and managing cardiovascular disease risk, including high blood pressure, type 2 diabetes, chronic respiratory diseases (asthma and chronic obstructive pulmonary disease) and early identification of breast and cervical cancer.
Several studies have demonstrated that people living with HIV have increased risk of cardiovascular diseases compared with HIV-negative people in the same age ranges and that cardiovascular disease accounts for an increasing proportion of mortality observed in this population (140,141). Large cohort studies have confirmed that the risk of both myocardial infarction and cerebrovascular disease is 40–70% greater among people living with HIV than among age- and sex-matched HIV-uninfected controls (142–147). This association has been reported both among people receiving ART and among those who are treatment-naive. Similar findings have also been reported for children and adolescents with HIV (148). The mechanisms underlying the association between HIV and cardiovascular disease are multifactorial and include HIV-related chronic immune activation and inflammation, immunodeficiency and elevated burdens of traditional cardiovascular disease risk factors among people living with HIV (149–152).
Findings from observational studies have shown that the role of ART in cardiovascular disease risk and exposure to some classes of ARV drugs (PIs) causes lipid abnormalities and may increase the risk of premature cardiovascular disease (153–156). Associations between NRTIs and the risk of cardiovascular disease remain the subject of debate. Although recent and cumulative exposures to some NRTIs such as ABC have been associated with increased relative risk for cardiovascular disease (157–160), other reviews have not found such an association (161,162). Several studies have demonstrated an increased risk of cardiovascular disease events among people discontinuing ART and people with detectable viral load (163). It has been hypothesized that the increased attributable risk among people living with HIV results from increased immune activation and chronic inflammation, which remain abnormally high among people living with HIV even after suppression of viral loads (149,151). Both are associated with preclinical and clinical atherosclerosis. The overall beneficial role of ART on HIV morbidity and mortality has therefore been demonstrated to outweigh the potential risk of cardiovascular disease for people living with HIV.
Cardiovascular disease screening for people living with HIV has been recommended in several HIV clinical guidelines, and several risk tools for calculating cardiovascular disease probability have been used (164–168). Several studies have demonstrated that incorporating routine cardiovascular disease screening for people living with HIV could improve health outcomes and be cost effective (169–171).
A systematic review on using validated tools to identify the people at highest cardiovascular disease risk for primary prevention shows that there is potential to lower cardiovascular disease mortality and the incidence of cardiovascular events; this was especially evident in studies with high-intensity interventions (172). However, despite the overall consensus that the current cardiovascular disease screening tools designed for the general population have a moderate discriminatory power to determine which people living with HIV have a high risk for cardiovascular disease events or eligibility for therapeutic interventions, these tools frequently underestimate the cardiovascular disease risk of people living with HIV and need to be adjusted or validated in the HIV populations (173–180). The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study group has described a cardiovascular disease risk algorithm that incorporates some HIV-specific factors such as CD4 cell count and ARV drug use that has reported better accuracy in predicting serious cardiovascular disease events (181–183). Although this is an important step towards improving cardiovascular disease risk prediction, it still has limitations because the study populations – all in high-income settings – have different genetic and behavioural cardiovascular disease risk profiles than most people living with HIV in the world. In addition, as in the case of studies reporting less direct risk predictions, the D:A:D: instrument can also significantly overestimate and underestimate cardiovascular disease risk (176–178).
Implementation considerations
No specific WHO recommendation for the management of cardiovascular disease in people with HIV has been made in previous guidelines. However, since 2010, WHO has defined a package of essential noncommunicable disease interventions (WHO PEN), along with recommendations on screening for and treating noncommunicable diseases in the general population. WHO PEN (128) has several programmatic advantages in resource-limited settings, since it integrates other major noncommunicable diseases in addition to cardiovascular disease, can be implemented in primary health care, can be managed by non-physicians, consists of a minimal package and has good discrimination to identify those with high cardiovascular disease risk. The systematic review did not identify any studies assessing the impact or use of WHO PEN interventions for people living with HIV with any outcomes relevant to low- and middle-income countries. Studies on WHO PEN–based interventions in the general population from low- and middle-income countries were found, showing that the PEN protocol and universal risk assessment are cost-effective (184–186). Further, an evaluation of the short-term outcomes of PEN in pilot districts indicated a significant reduction of cardiovascular disease risk and increased healthy lifestyle of the target population (184).
Disparities in cardiovascular disease care among people living with HIV have been reported. In two studies, people living with HIV were significantly less likely to receive aspirin for cardiovascular disease prevention than those without HIV (187,188). Other data on medical management and outcomes following acute myocardial infarction showed that people living with HIV received significantly fewer cardiovascular procedures and/or therapeutics than people without HIV (189). Regularly assessing and managing cardiovascular disease risk among people living with HIV are expected to result in better and more equitable care.
One major remaining barrier to equitable access to cardiovascular disease prevention and care for people living with HIV is the lack of high-quality data assessing proven cardiovascular disease therapies in this population. For example, prospective trials on using statins for people living with HIV have generally been underpowered and have not assessed hard clinical endpoints (190).
Integrated multi-disease campaigns conducted in Lesotho and Uganda that included cardiovascular disease screening and HIV testing demonstrated the feasibility of integrated screening for communicable and noncommunicable diseases in community-based HIV programmes (191,192). Improved diagnosis and linkage to care for cardiovascular disease conditions have been shown to also improve linkage to HIV care and ART (193). Cardiovascular disease and HIV integration pilot services have been implemented in Kenya, Nigeria and Zambia since 2012 and shown to be feasible and acceptable, with cardiovascular disease integration implemented within the context of an HIV chronic care model (194).
Research gaps
Further research is needed to unravel the complex pathophysiology of atherogenesis among people living with HIV, to elucidate the relationship between traditional and HIV-associated cardiovascular disease risk factors and to investigate how ART alters these interactions. Studies on how early ART affects cardiovascular disease development, especially among adolescents and children living with HIV, are also needed. Studies are needed on cardiovascular disease treatments, such as ACE inhibitors and statins, which may be beneficial in reducing inflammation related to HIV. Clinical studies to assess methods of risk prediction and riskreduction strategies for cardiovascular disease applicable to people living with HIV would be of great use. There is a need to validate simplified cardiovascular disease screening protocols and risk assessment algorithms, appropriate to geographical regions, that include HIV-specific risk factors to improve accuracy. Using cardiovascular disease co-therapies such as statins, aspirin, antihypertensive drugs and metformin and measuring their impact on HIV mortality are also important. Assessments of the unique pathophysiology, related risk factors and optimal management of downstream cardiovascular disease complications associated with HIV, such as heart failure and malignant arrhythmia, are also needed. Such studies should be conducted in both high-income and resource-limited countries. In limited cohort data, integrase inhibitors have been reported to be associated with a decreased risk of cardiovascular disease. These findings require validation in other cohorts and with longer follow-up (195). Although integrase inhibitor–based regimens are highly efficacious for suppressing viral loads, they appear to cause more weight gain and treatment-emergent obesity than non-integrase inhibitor–based regimens and may increase the risk of weight-related comorbidities. More studies are needed to understand the pathogenesis of weight gain with INSTIs among people living with HIV to prevent this serious complication (196).
6.11. Mental health among people living with HIV
Recommendation (2016)
Assessment and management of depression should be included in the package of HIV care services for all individuals living with HIV
(conditional recommendation, very-low-certainty evidence).
Source: Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach – second edition (51).
Introduction
People living with HIV are at high risk of mental, nervous system and substance use disorders (197). A systematic review from both from low- and high-income countries show that depression is one of the most prevalent mental health comorbidities in people living with HIV (198,199). In a 2019 systematic review of the burden of depression among people living with HIV in Africa, the overall prevalence estimates were 36% (95% CI 32–41%) for depressive disorders and 15% (95% CI 12–18%) for probable major depressive disorders (200). Another 2019 systematic review reported the prevalence of depression among people living with HIV globally as 31% (95% CI 28–34%).
A systematic review conducted in 2015 reported depression prevalence rates as high as 80% among people living with HIV but with wide variation across studies that is attributed to the screening and diagnostic criteria used (201). Depressive symptoms have been reported as common in many studies in sub-Saharan Africa, where the HIV burden is also high (202,203).
People living with HIV who have depression are less likely to achieve optimal treatment adherence. Although chronic HIV care settings provide an opportunity to detect and manage depression among people living with HIV, it is often overlooked and unrecognized by health-care providers. Treatment or lack of treatment for mental health disorders can affect general health, adherence to ARV drugs and retention in care and may lead to potential side-effects and drug–drug interactions being overlooked (201,204–207).
The mhGAP intervention guide for mental, neurological and substance use disorders in non-specialized health settings: Mental Health Gap Action Programme (mhGAP), version 2 (208) provides evidence-informed comprehensive guidelines on the diagnosis and management of a range of mental, nervous system and substance use disorders, including developmental and behavioural disorders among children and adolescents, with a focus on nine priority mental health conditions, including diagnosing and managing people with depression. Key updates include a simplified clinical assessment algorithm for follow-up and the inclusion of new modules; an implementation module of necessary infrastructure and resources and revised modules on psychoses, behavioural disorders, disorders caused by substance use and child and adolescent mental health (208). Implementing mhGAP through primary care would improve the detection and management of depression among adults compared with standard-of-care approaches (209).
A systematic review conducted to support the guideline update in 2015 aimed to determine whether routine screening and management of depression (specifically with mhGAP criteria) improve ART adherence and treatment outcomes among people living with HIV (51,208). No studies were identified explicitly reporting on mhGAP for this specific population.
Indirect evidence from a systematic review on the accuracy of using screening tools to identify depression among people living with HIV in all settings identified 18 studies, using 25 different screening instruments, compared with criterion standards for diagnosing depression (51,208). Multiple index test and criterion standards were assessed, and the review evaluated each test’s area under the curve as a summary measure of accuracy (area under the curve above 0.9 is considered to be highly accurate; 0.7 to 0.9 to be moderately accurate; and 0.51 to 0.69 to be of low accuracy) (207). Although several instruments showed very good or even excellent performance diagnosing depression among people living with HIV, the overall certainty of evidence is very low. The Guideline Development Group, considering acceptability and feasibility from end-users and lack of harm, made a conditional recommendation.
Although depression is more common among people living with HIV than in the general population, there is less consistent and limited evidence to show that managing depression improves HIV treatment outcomes. However, management of depression improves the mental health and general well-being of people living with HIV.
The limited data on HIV and mental health service delivery models indicate that integration supports efficiency and does not increase the costs of care. A narrative review from sub-Saharan Africa suggests that integrating mental health care into existing health systems is an effective and cost-efficient approach to expand access to mental health services for people living with HIV in resource-limited settings (210). There is also an ongoing study on the cost–effectiveness of screening and treating people living with HIV for depression in sub-Saharan Africa (211). However, more evidence is needed on effective models of integrating HIV and mental health services in various settings (212).
A survey of national HIV programme managers found that 38% of respondents reported that mental health screening is performed in some HIV care settings with referral for treatment when indicated (51). Forty-three per cent did not have mental health screening and treatment available for people living with HIV. None of the countries reported countrywide implementation of mental health screening and treatment services in all HIV care settings. The top three challenges identified by programme managers for integrating mental health services in HIV care settings are shortage of human resources, skills and capacity of health-care providers and lack of funding. WHO estimates that up to 85% of people with severe mental disorders and 56% of people with depression in low- and middle-income countries do not have access to treatment (213).
Implementation considerations
Screening for depression may support adherence to ART, retention in care, suppression of viral loads and improve quality of life and, if implemented, depression should be managed according to national standards or mhGAP. Integration or linkage to the mental health services should be implemented in the settings in which health-care infrastructure and trained human resources are available. Implementing treatment for depression among people living with HIV may require task sharing, building health-care worker capacity, national adaptation of screening tools and simplifying tools for use by non-specialized primary care providers.
Research gaps
There are several research gaps related to screening and treating people living with HIV for mental health disorders and depression:
the accuracy of current estimates of HIV and depression because of the wide variability in reports, with packages of care for common mental disorders likely to be most effective among people living with HIV in low- and middle-income countries;
the long-term impact of depression interventions in relation to HIV outcomes; and
the optimal time-points for mental health interventions.
6.12. Drug use and drug use disorders
People who use drugs may experience a range of disorders related to drug use, including drug dependence, intoxication, withdrawal and overdose. Injecting drug use is associated with a range of diseases and infections, including HIV, viral hepatitis, TB, septicaemia and bacterial endocarditis.
WHO, UNODC and UNAIDS recommend a comprehensive package of interventions for HIV prevention, treatment and care for people who inject drugs, including needle and syringe programmes, opioid substitution therapy, HIV testing and counselling, ART, preventing and treating sexually transmitted infections, condom programmes, targeted behaviour change communication, preventing and treating viral hepatitis and preventing, diagnosing and treating TB. More recently, WHO updated this package to include community distribution of naloxone to manage opioid overdose as well as a set of enabling interventions to overcome the structural barriers for people who use drugs and other key populations to access these health interventions. These enabling interventions include revising laws and legislation that criminalize the consumption and possession of drugs and address violence, stigma and discrimination in health-care settings.
Although the focus with HIV has been injecting opioids, a link is becoming more evident between using other drugs such as amphetamine-like stimulants and sexual risk and transmission of HIV (214).
A form of sexualized drug use is also seen in subgroups of men who have sex with men, often referred to as chemsex, with increased risk of HIV and sexually transmitted infections, including HCV transmission.
6.13. Sexually transmitted infections
Recommendations
Sexually transmitted infection and family planning services can be integrated within HIV care settings
(conditional recommendation, very-low-certainty evidence).
For men who have sex with men and transgender people (2011)
Offering periodic testing for asymptomatic urethral and rectal N. gonorrhoeae and C. trachomatis infections using NAAT is suggested over not offering such testing for men who have sex with men and transgender people
(conditional recommendation, low-certainty evidence).
Not offering periodic testing for asymptomatic urethral and rectal N. gonorrhoeae infections using culture is suggested over offering such testing for men who have sex with men and transgender people
(conditional recommendation, low-certainty evidence).
Offering periodic serological testing for asymptomatic syphilis infection to men who have sex with men and transgender people is strongly recommended over not offering such screening
(strong recommendation, moderate-certainty evidence).
For sex workers and their clients in low- and middle-income countries (2012)
We suggest offering periodic screening for asymptomatic sexually transmitted infections to female sex workers
(conditional recommendation, low-certainty evidence).
We suggest offering female sex workers, in settings with high prevalence and limited clinical services, periodic presumptive treatment for asymptomatic sexually transmitted infections
(conditional recommendation, moderate- to high-certainty evidence).
For pregnant women (2017)
The WHO sexually transmitted infection guideline recommends screening all pregnant women for syphilis during the first antenatal care visit
(strong recommendation, moderate-certainty evidence).
This recommendation applies to all settings, including settings with high or low prevalence of syphilis.
Sources: Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach – second edition (51); Guidelines: prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender populations: recommendations for a public health approach (215); Prevention and treatment of HIV and other sexually transmitted infections for sex workers in low- and middle-income countries: recommendations for a public health approach (216); and WHO guidelines on syphilis screening and treatment for pregnant women (217).
Recommendations for the management of symptomatic sexually transmitted infections (2021)
Management of urethral discharge
For people who present with urethral discharge from the penis, management is recommended to be based on the results of quality-assured molecular assays. However, in settings with limited or no molecular tests or laboratory capacity, WHO recommends syndromic treatment to ensure treatment on the same day of the visit
(strong recommendation, moderate-certainty evidence).
Management of vaginal discharge
For people who present with vaginal discharge, WHO recommends treatment for
N. gonorrhoeae
and/or
C. trachomatis
and/or
T. vaginalis
on the same visit. WHO suggests treatment based on the results of quality-assured molecular assays for
N. gonorrhoeae
and/or
C. trachomatis
and/or
T. vaginalis. In settings in which treatment based on the results of molecular assay in the same visit is not feasible or that have limited or no molecular testing, WHO suggests treatment based on testing with quality-assured rapid point-of-care tests or on syndromic treatment
(strong recommendation, moderate-certainty evidence).
WHO suggests treating for bacterial vaginosis if vaginal discharge is present (for example, tenacious or thin) or based on the results of microscopy, if available
(conditional recommendation low-certainty evidence).
WHO suggests treating for candidiasis, where indicated by type of discharge (such as curd-like with vaginal itching) or by the results of microscopy, if available
(conditional recommendation low-certainty evidence).
For management of women with lower abdominal pain
For sexually active women who present with lower abdominal pain, WHO suggests assessing for pelvic inflammatory disease and treating syndromically.
For sexually active women with lower abdominal pain with either of the following features on clinical examination (bimanual palpation):
WHO suggests the following.
Treat for pelvic inflammatory disease on the same visit.
Test for infection with
N. gonorrhoeae
and
C. trachomatis
and, if available,
Mycoplasma genitalium, to support partner management when tests are available
(conditional recommendation, low-certainty evidence).
Management of genital ulcer disease, including anorectal ulcers
For people who present with genital ulcers (including anorectal ulcers), WHO recommends treatment based on quality-assured molecular assays of the ulcer. However, in settings with limited or no molecular tests or laboratory capacity, WHO recommends syndromic treatment to ensure treatment on the same day of the visit
(strong recommendation, moderate-certainty evidence).
Management of anorectal discharge
For people who present with anorectal discharge and report receptive anal sex, WHO recommends management based on the results of quality-assured molecular assays. However, in settings with limited or no molecular tests or laboratory capacity, WHO recommends syndromic treatment to ensure treatment on the same day of the visit
(strong recommendation, moderate-certainty evidence).
Good practice for men includes:
taking a medical and sexual history and assessing risk for sexually transmitted infections;
performing a physical examination of the genital and anal areas;
offering HIV and syphilis testing and other preventive services as recommended in other guidelines;
if symptoms persist at review, good practice includes checking partner notification and treatment history; and
for people with recurrent or persistent urethral discharge, referring people to a centre with laboratory capacity to diagnose infection with N. gonorrhoeae, C. trachomatis, M. genitalium and T. vaginalis and to test for antimicrobial-resistant N. gonorrhoeae and M. genitalium.
Good practice for women includes:
taking a medical and sexual history and assessing risk for sexually transmitted infections;
performing a physical examination, including abdominal and pelvic examination to assess for pelvic inflammatory disease, surgical conditions or pregnancy and external vulvo-vaginal examination to visualize any lesions, overt genital discharge or vulval erythema and excoriations;
bimanual digital examination of the vagina to (1) assess for cervical motion tenderness or pain with palpation of the pelvic area to exclude pelvic inflammatory disease; and (2) assess for the presence of vaginal discharge and the colour and consistency of the discharge on the glove;
offering HIV and syphilis testing and other preventive services as recommended in other guidelines; and
for people with recurrent or persistent vaginal discharge, referring to a centre with laboratory capacity to diagnose infection with N. gonorrhoeae, C. trachomatis, M. genitalium, T. vaginalis and bacterial vaginosis and to test for antimicrobial-resistant N. gonorrhoeae and M. genitalium (if there is a test) or for a specialist’s assessment (sexually transmitted infection expert and physician or a gynaecologist), when no such testing is available in primary health care centres.
Sources: Guidelines for the management of symptomatic sexually transmitted infections (218).
Introduction
The syndemics model of health describes the interaction of two or more concurrent or sequential epidemics and the additive effect, with each one intensifying the others (219). There is a high co-prevalence of HIV and the other sexually transmitted infections, particularly in vulnerable populations. Many of these infections are asymptomatic, especially among women (220).
Substantial evidence indicates that sexually transmitted infections increase HIV transmissibility and the risk of acquiring HIV by as much as 2–3 times in some populations (221,222). It has also been shown that infection with N. gonorrhoeae substantially increases shedding of HIV-1 from the male genital tract in seminal fluid (223). Genital herpes (HSV-2) almost triples the risk of acquiring HIV for both men and women (224,225). HIV increases the infectiousness and severity of sexually transmitted infections (226). HIV-seropositive women are at high risk of infection with human papillomavirus (HPV), including oncogenic HPV types (227). To reduce HIV transmission and optimize sexual and reproductive health, sexually transmitted infections need to be diagnosed and treated as a priority (228). Efforts should therefore be taken to strengthen sexually transmitted infection prevention, screening, diagnosis and treatment.
In a systematic review, the median prevalence of sexually transmitted infections among people living with HIV was 12.4%. The most common sexually transmitted infections were syphilis, gonorrhoea, chlamydia and trichomoniasis (229). Modelling has estimated that 10% of the cases in which gay men and other men who have sex with men acquire HIV are attributable to increased susceptibility because of N. gonorrhoeae or C. trachomatis infection (230). Reports in many settings indicate high sexually transmitted infection prevalence among users of PrEP at baseline screening and high incidence while taking PrEP. The reports also include high rates of gonorrhoea, especially among men who have sex with men (231).
For people living with HIV, a holistic and comprehensive approach to sexual and reproductive health and rights includes appropriate sexually transmitted infection services. People living with HIV should be screened and treated for sexually transmitted infections. Men who have sex with men and transgender people with symptomatic sexually transmitted infections should seek and be offered syndromic management and treatment, in line with existing WHO guidance.
Management
Symptomatic cases should either be managed syndromically and where feasible, etiological diagnosis obtained (218). To prevent future sexually transmitted infections, the promotion and provision of safer sex practices, including condom use, should be reenforced. Appropriate sexually transmitted infection treatment, including partner management, should be ensured. For people who have urethral discharge, vaginal discharge, lower abdominal pain, anorectal discharge or genital ulcer, WHO recommends management based on the results of quality-assured molecular assays. However, in settings with limited or no molecular tests or laboratory capacity, WHO recommend syndromic treatment to ensure treatment on the same day of the visit. Specific recommendations are available for each syndrome (232).
Sexually transmitted infection treatment for sex workers, men who have sex with men and transgender people
Anorectal symptoms and anorectal sexually transmitted infections are prevalent among men who have sex with men, female sex workers, transgender people and heterosexual women who engage in anal sexual intercourse. Asymptomatic anorectal infections are not uncommon, although precise data are scarce. The people at highest risk of asymptomatic anorectal infections are men who have sex with men, male and female sex workers, transgender people and women who have had receptive anal intercourse with men with sexually transmitted infections. For syndromic diagnosis and management, these infections have been grouped under anorectal infections. Anorectal infections may be associated with anorectal pain, itching, discharge, bleeding, sensation of rectal fullness, tenesmus, constipation and mucus streaking of stools.
Recommendations for the treatment of syphilis among pregnant women and of congenital syphilis have been previously published, updated in 2016 (233) and reprinted in 2017 (217).
Implementation considerations
A key approach to increasing the coverage of sexually transmitted infection services for people living with HIV is integration or linkage of these services within HIV care and treatment settings (51). It is necessary to promptly adopt service delivery models that integrate a people-centred approach to address sexually transmitted infections among people living with HIV.
Integration and linkage between HIV and sexual and reproductive health services require approaches that engage those at greatest risk for HIV and other sexually transmitted infections in any interventions and programmes. This will increase access to appropriate testing, linkage to treatment and further strengthening of preventive services. Frequently, stigma and discrimination result in reduced health-seeking behaviour, delaying diagnosis and effective partner notification and, consequently, impeding public health efforts to control the sexually transmitted infection and HIV epidemics. Health-care providers providing HIV prevention and treatment services need to provide culturally competent and sensitive sexually transmitted infection care, so that vulnerable populations seek clinical services more readily.
Research gaps
The challenge for researchers, clinicians and public health officials is to understand how best to promote sexual health (234). The improvements in HIV treatment, diagnostic capabilities for HIV and other sexually transmitted infections and educational digital media create new challenges and opportunities for key stakeholders, including civil society, to limit the spread of sexually transmitted infections while respecting individual decisions about sexual expression.
6.14. Vaccines for people living with HIV
Immunizations are an important component of the HIV care package in many international guidelines, and people living with HIV should be assessed for eligibility for vaccination at all stages of care (235–237). Vaccines usually have better safety and efficacy among people living with HIV who are receiving ART and those without significant immunosuppression, notably when the CD4 cell count is above 200 cells/mm3. People with more severe immunosuppression may be at higher risk of complications from some live attenuated vaccines. Inactivated vaccines, although safe, can be less effective among these people and may require supplemental doses or revaccination after ART-induced immune reconstitution. Transient increases in plasma HIV-RNA load have also been reported after the administration of several vaccines. Available evidence indicates that these transient increases are not clinically significant (238,239).
In general, HIV-exposed infants, children and adolescents with HIV should receive all vaccines under routine vaccination according to recommended national immunization schedules (240,241).
COVID-19 vaccination
Many of the COVID-19 vaccine studies have included a few people living with HIV in their trials. Despite limited data, the available information suggests that current WHO-recommended COVID-19 vaccines are safe for people living with HIV (242). No interactions have been reported between COVID-19 vaccines and ARV drugs, which people living with HIV should continue to take after vaccination to maintain health.
The source of most recommendations for vaccinating people living with HIV is WHO position papers and summary tables published in 2017 and 2018 (80,127,243–254). The position papers include guidance on BCG, HPV, HBV, measles, dengue, rubella, typhoid, pneumococcal and cholera vaccination for people living with HIV ().
HIV-specific guidance for selected vaccines from WHO vaccine position papers.
Further information on other vaccinations comes from a scoping review (255) that aims to identify and evaluate new evidence on using selected vaccines for children living with HIV concerning vaccine safety, efficacy, dosing and immunization schedule together with the effect of immune status and ART at the time of vaccination.
The scoping review for children living with HIV sought to identify safety and efficacy studies published since the last published systematic review (2012) of immunization of children living with HIV (254). The final scoping review included 19 articles.
The scoping review focused on:
vaccines recommended for all children: diphtheria, tetanus, oral polio, pertussis, Haemophilus influenzae type B, rotavirus, pneumococcal conjugate and rubella;
vaccines recommended for children in some high-risk populations: dengue, rabies, typhoid, meningococcal and hepatitis A;
vaccines for children receiving vaccinations from immunization programmes with certain characteristics: varicella, influenza and mumps; and
vaccines for children residing in certain regions: Japanese encephalitis, tick-borne encephalitis and yellow fever.
None of the studies assessing safety showed any significant difference in side-effects among children living with HIV compared with uninfected controls. Although in most of the studies, children living with HIV had lower antibody levels in response to vaccination, the proportion of children reaching the seroprotective levels was similar compared with uninfected children for most of the vaccines, except for pertussis vaccine and oral polio vaccine, for which children living with HIV reached lower levels. Eight of the 17 included studies had a sample size of 50 or fewer children living with HIV, most of whom were receiving ART.
Immunization against some diseases such as influenza, HBV, pneumococcal disease and tetanus is frequently indicated for adults living with HIV. Other immunizations may be recommended based on age, risk factors or travel plans.
For currently recommended vaccination schedules and detailed guidance on immunization for all age groups, see WHO recommendations for routine immunization – summary tables (254).
WHO has position papers on each vaccine and statements about them for people living with HIV (243–253).
6.15. HIV-related skin and oral conditions
HIV infection increases the prevalence and severity of skin and oral diseases, especially when the person’s CD4 cell count declines below 200 cells/mm3. As a result, skin and oral conditions affect up to 90% of adults and children with HIV in resource-limited settings (51). Adverse drug reactions of the skin are also 100 times more common among people living with HIV than among the general population, and their prevalence increases as immunodeficiency worsens. Immune reconstitution inflammatory syndrome occurs in 10–25% of people living with HIV starting ART. Cutaneous manifestations of immune reconstitution inflammatory syndrome, such as seborrhoeic dermatitis, anogenital herpes, acne, tinea, folliculitis, Kaposi’s sarcoma, herpes zoster, genital warts, molluscum contagiosum and pityriasis versicolor, are common, occurring among up to 50% of people living with HIV commencing ART with a low CD4 cell count (256).
Skin and oral manifestations of HIV infection can aggravate stigma in some societies as physical signs in the form of skin diseases, such as papular pruritic eruption, that suggest the possibility of HIV infection could make the affected person more vulnerable to discrimination (51).
WHO guidelines (257) describe common HIV-related skin disorders based on the burden of disease among adults and children living with HIV and the availability of evidence and effective interventions. These are Kaposi’s sarcoma, seborrhoeic dermatitis, papular pruritic eruption, eosinophilic folliculitis, tinea infections, herpes zoster, scabies, molluscum contagiosum, oropharyngeal candidiasis, Stevens-Johnson syndrome and toxic epidermal necrolysis.
Certain systemic diseases, such as Kaposi’s sarcoma, may initially be noted on the skin and may require urgent ART to reduce mortality. Others, while not always a major cause of mortality, can be a source of severe morbidity through, for example, itching that provokes scratching, secondary infections, disfigurement, sleep disturbance and mental stress. Candidiasis can cause pain on swallowing, limiting a person’s ability to take ARV drugs.
Because of a lack of services to promptly diagnose and manage skin and oral conditions, many people attempt to conceal the skin disease or avoid social contact. These could affect their health-seeking behaviour, negatively affecting their self-esteem and quality of life. Skin and oral conditions are one of the most common management problems faced by health-care workers caring for people living with HIV.
In 2014, WHO released guidelines for treating common HIV-associated skin and oral conditions in low- and middle-income countries (257). They are applicable for all adults, pregnant women, adolescents and children living with HIV and recommend HIV testing for everyone with unknown HIV status presenting with the discussed skin conditions, and if the HIV status is known, they should be evaluated to initiate ART.
ART is the initial treatment of choice for several of these conditions, such as Kaposi’s sarcoma, papular pruritic eruption, eosinophilic folliculitis and molluscum contagiosum.
6.16. Nutritional care and support
Low energy intake combined with increased energy demands from HIV infection (258–260) and related opportunistic and other infections may lead to HIV-related weight loss and wasting. In addition, an altered metabolism, reduced appetite and higher incidence of diarrhoea may lower nutrient intake and absorption and lead to nutrient losses. These effects may all be compounded in low-income, food-insecure contexts. Low body mass (BMI less than 18.5 kg/m2 for adults), weight loss and wasting in children are all independent risk factors for HIV disease progression and mortality (261,262). Nutritional assessment (anthropometry, clinical and dietary assessment), counselling and support should be an integral component of HIV care and conducted at enrolment in care and monitored across the care continuum. Malnourished people with HIV, especially in food-insecure contexts, may require food supplements in addition to ART to support nutritional recovery. Weight loss or failure to regain or maintain a healthy weight at any stage of HIV infection, including while receiving ART, should trigger further assessment and appropriate interventions.
Nutrition for children living with HIV
For infants and young children, ensuring optimal feeding, including exclusive breastfeeding for the first six months of life, followed by complementary feeding and breastfeeding through at least 24 months, within the context of HIV is important in all settings in which the prevalence of HIV is high and diarrhoea, pneumonia and undernutrition are common causes of infant and child mortality. Guidance on nutritional care for people living with HIV is summarized in section 6A of WHO’s Essential nutrition actions: mainstreaming nutrition through the life-course (263).
Nutritional assessment is essential to identify malnutrition and growth faltering early. Infants and children should undergo initial nutritional assessment (evaluation of nutritional status, diet and symptoms) and then be weighed and have height measured at each visit and monitored with reference to WHO or national growth curves. Growth monitoring should also be integrated into the assessment of response to ART (264). If poor growth is identified, then further assessment should be performed to determine the cause and appropriate response planned. WHO guidelines (265) provide details on nutritional interventions.
6.16.1. Infant feeding in the context of HIV
Recommendations (2016)
Duration of breastfeeding by mothers living with HIVa
Mothers living with HIV should breastfeed for at least 12 months and may continue breastfeeding for up to 24 months or longer (similar to the general population) while being fully supported for ART adherence (see
Chapter 7
for interventions to optimize adherence)
(strong recommendation, low-certainty evidence for 12 months; very-low-certainty evidence for 24 months).b
Remarks
In settings in which health services provide and support lifelong ART, including adherence counselling, and promote and support breastfeeding among women living with HIV, the duration of breastfeeding should not be restricted. Further, mothers living with HIV (and whose infants are HIV uninfected or of unknown HIV status) should exclusively breastfeed their infants for the first six months of life, introducing appropriate complementary foods thereafter and continue breastfeeding.
Breastfeeding should then only stop once a nutritionally adequate and safe diet without breast-milk can be provided.
Source: Guideline: updates on HIV and infant feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV (266).
Good practice statements (2010)
When mothers living with HIV do not exclusively breastfeed
Mothers living with HIV and health-care workers can be reassured that ART reduces the risk of postnatal HIV transmission in the context of mixed feeding. Although exclusive breastfeeding is recommended, practising mixed feeding is not a reason to stop breastfeeding in the presence of ARV drugs.
When mothers living with HIV do not plan to breastfeed for 12 months
Mothers living with HIV and health-care workers can be reassured that shorter durations of breastfeeding of less than 12 months are better than never initiating breastfeeding at all.
Source: Guidelines on HIV and infant feeding: principles and recommendations for infant feeding in the context of HIV and a summary of evidence (266).
- a
This recommendation updates the component of the 2010 recommendation on which breastfeeding practices and for how long related to the duration of breastfeeding. The components of the 2010 recommendation on breastfeeding practices and stopping breastfeeding remain unchanged and valid.
- b
WHO-recommended breastfeeding is defined as: (1) initiating breastfeeding within the first hour of life; (2) exclusive breastfeeding for the first six months of life (that is, the infant only receives breast-milk without any additional food or drink, not even water); followed by (3) continued breastfeeding for up to two years of age or beyond (with the introduction of appropriate complementary foods at six months); and (4) breastfeeding on demand – that is, as often as the child wants, day and night.
Introduction
Breastfeeding is the cornerstone of child survival, nutrition and development and maternal health and is one of the foundations of child health, development and survival, especially where diarrhoea, pneumonia and undernutrition are common causes of mortality among children younger than five years. WHO recommends exclusive breastfeeding for the first six months of life, followed by continued breastfeeding with appropriate complementary foods for up to two years or beyond. The evidence for the long-term benefits of longer durations of breastfeeding for both maternal and child health outcomes, including child development and preventing noncommunicable diseases, highlights the relevance of supporting breastfeeding in high-, middle- and low-income settings.
HIV infection both directly affects the nutritional status of women and children living with HIV and indirectly affects them by altering household food security and through inappropriate choices of infant-feeding practices.
In 2016, based on revisions to the ARV drug guidelines, a greater evidence base and programmatic experience, WHO updated its guidance on HIV and infant feeding. The recommendations and good practice statements are detailed below. For guidance on what to feed infants when mothers stop breastfeeding and conditions needed to safely formula feed should this be the mother’s choice, see the 2016 guidelines on HIV and infant feeding (266).
Implementing the following recommendations requires appropriately training and developing the capacity of health-care workers to supply them with the necessary skills and job aids and provide adequate supervision and oversight. Programmes need to be systematically monitored to ensure success and to identify and document challenges to implementation.
Clinical considerations for supporting mothers with HIV to breastfeed
Key clinical and implementation considerations for breastfeeding by mothers living with HIV while receiving ART include:
communicating clearly and effectively the effectiveness of ART and suppressing viral loads in reducing the postnatal transmission risks through breastfeeding to health-care workers, mothers and the community;
communicating the importance of viral load monitoring during breastfeeding to ensure that suppression is maintained;
highlighting the value of breastfeeding for the health, development and survival of mothers living with HIV and their children when the mother is receiving and adhering to ART and has suppressed viral loads;
implementing and sustaining specific interventions such as an integrated approach to delivering follow-up care for the mother and child as part of basic maternal, newborn and child health services (including immunization and other well-child services) to improve postpartum follow-up of mother–infant pairs and support breastfeeding practices and ART adherence;
emphasizing postnatal prophylaxis for infants: infants of mothers who are receiving ART and are breastfeeding should receive six weeks of infant prophylaxis with daily NVP, or if they are considered at high risk, enhanced infant prophylaxis using AZT and NVP for six weeks followed by either AZT and NVP or NVP alone for an additional six weeks (see section 4.4.6); and
linking the receipt of infant HIV testing and results with appropriate infant feeding counselling: infants living with HIV should continue breastfeeding until 24 months or longer.
For infants who acquire HIV despite interventions to prevent mother-to-child transmission, exposure to drugs through breastfeeding affects drug resistance and toxicity and may affect the success of ART regimens for the child (see section 4.8 on toxicity monitoring and drug resistance).
Implementation considerations
Investment and action to protect, promote and support breastfeeding in the general population should remain priorities of health ministries, nongovernmental organizations and other partners in all settings. HIV and maternal, newborn and child health programmes need to give priority to integrating ART services, including adherence counselling and support for infant and young child feeding, in all settings. These programmes and partner agencies need to ensure training and developing the capacity of health-care workers to implement the recommendations. Programmes and partner agencies should collect data to monitor the duration of breastfeeding by mothers living with HIV in addition to adherence to ART and the rates of retention in care of mothers and infants. Such data should be used to improve the quality of service delivery at district and local clinics.
Community and health facility approaches to support improved infant feeding practices include:
combining group education with individual counselling sessions;
building the skills and competencies of health-care workers to deliver infant feeding counselling;
involving fathers and other family members;
involving community health workers and trained health-care workers; and
integrating programmes for preventing mother-to-child transmission of HIV with access to ART.
National authorities need to create and sustain an enabling environment that encourages appropriate feeding practices for all infants and young children while scaling up interventions to reduce HIV transmission (266). As stated in the 2010 WHO guidelines on HIV and infant feeding (267), health services need to support mothers living with HIV in their chosen feeding practices even when these are inconsistent with nationally recommended practices. This principle is still endorsed by WHO and remains relevant.
In all settings, implementing recommendations for mothers living with HIV should be contextualized first by the optimal infant feeding practice recommended for all mothers and infants: exclusively breastfeeding for six months and then introducing appropriate complementary foods and continuing breastfeeding for 24 months or beyond. When implementing recommendations for mothers living with HIV, national health authorities need to clearly communicate the hierarchy of what is ideal and how the recommendations for mothers living with HIV are specific to their circumstances.
Simple, consistent messaging is essential to support breastfeeding in the general population, including mothers living with HIV. Programmes should develop clear messaging that addresses views and concerns related to previous recommendations to avoid misunderstandings among health-care workers, mothers living with HIV and the general population.
In settings in which national authorities recommend replacement feeding for mothers living with HIV, similar coordinated support can probably improve the safety of replacement feeding practices. WHO and Food and Agriculture Organization of the United Nations guidance on safe preparation of powdered infant formula (268) provides technical information that may be helpful in the context of HIV.
Research gaps
How does long-term postpartum exposure to low-dose ARV drugs in breast-milk affect the early and late health outcomes, especially growth, renal and bone metabolism and nervous system development, of HIV-exposed breastfeeding infants and children whose mothers are taking ART?
In the context of HIV, what support increases exclusive and continued breastfeeding in the general population?
How can mothers living with HIV who are receiving ARV drugs be supported to breastfeed for longer in circumstances such as returning to work or school?
6.17. Palliative care
Introduction
An essential component of comprehensive clinical management for people living with HIV is the palliative care to prevent and relieve suffering and optimize quality of life (269). Common problems among people living with HIV that are managed by palliative care include pain and other physical symptoms related to HIV infection or its treatment; mood disorders and psychosocial distress related to stigmatization and poverty; and spiritual distress. Successful ART has resulted in a growing population of ageing people living with HIV, many of whom have multimorbidity, meaning that the people who need palliative care are changing. Person-centred palliative care must value people’s social networks, promote quality of life and reform structurally to improve how people experience interacting with the health-care system (270).
Need for palliative care among people living with HIV
Epidemiology
An estimated 12.8 million of the 38 million people living with HIV need palliative care (271). This includes nearly all the 690 000 people living with HIV who died in 2019 (272). The great majority of people living with HIV who would benefit from palliative care are not at the end of life but are living with HIV and its comorbidities and biopsychosocial consequences. More than 90% of the total need for palliative care among people living with HIV is in low- and middle-income countries; among children, HIV represents about 30% of worldwide palliative care need (271).
Unrelieved suffering
Recent estimates of the burden of serious health-related suffering found that people living with HIV experienced almost 194 million days of serious health-related suffering (273). Symptoms of health-related suffering are highly prevalent from diagnosis and persist alongside treatment, with the prevalence of anxiety (28%) and depression (34%) among people living with HIV receiving ART being higher than among people with other chronic conditions such as cancer, diabetes and multiple sclerosis (274). These burdens are reflected in data showing that people living with HIV have poorer quality of life than the general population (275). Among people with AIDS, 43–95% report fatigue, 82% anorexia, 30–98% pain, 41–57% nausea and vomiting, 43–62% breathlessness, 40–74% insomnia and 29–53% diarrhoea (276).
Pain profoundly affects quality of life, and people living with HIV identify pain control as essential to enable a “life worth living” (277). Uncontrolled symptoms have severe clinical and public health implications, including poor adherence to ART (278), treatment switching (279), viral rebound (280), poorer quality of life (281), suicidal ideation and days out of work or education (282). Spiritual suffering is also burdensome among people living with HIV, especially in advanced HIV disease, when spiritual well-being can become people’s primary concern (283,284). Progressive illness in low- and middle-income countries places a huge mental, social, economic, physical and spiritual burden on people living with HIV and the largely female caregiving members of families (276,285).
Children and young people have some symptoms and concerns in common with adults but also face specific concerns (286). The palliative care needs of children and young people in sub-Saharan Africa include physical concerns (such as pain and symptom distress); psychosocial concerns (such as family and social relationships), ability to engage with age-appropriate activities (such as play and attending school); existential concerns (such as worry about death and loss of ambitions); and health-care quality (such as child- and adolescent-friendly services). Priority psychosocial concerns and health service factors vary by age (287,288). It is increasingly recognized that children living with HIV in low- and middle-income countries have multi-system chronic comorbidities despite ART (289). Thus, prompt diagnosis and treatment are a priority alongside managing pain and symptoms.
Ageing and comorbidities
The successes of ART have led to improved survival and quality of life, but an increasing proportion of deaths among people living with HIV is attributable to chronic noncommunicable diseases in many settings (290). By 2030, people living with HIV are estimated to be likely to have an average of three noncommunicable diseases (291). A longitudinal study of mortality among people living with HIV in the United Kingdom found that only 58% of the deaths were from AIDS-defining illness, 19% cancer, 19% cardiovascular diseases, 18% infections, 12% liver disease, 6% substance misuse, 5% suicide, 5% accidents and 17% other causes (more than one option and the total therefore exceeds 100%) (292). In a multisite study from Cambodia, India, Indonesia, Malaysia, Thailand and Viet Nam, 47% of deaths among adults and children with HIV were non-AIDS infections (293).
Palliative care in HIV treatment
Definition of palliative care
WHO defines palliative care as the prevention and relief of suffering of adults and children and their families facing the problems associated with life-threatening illness. These problems include physical, mental, social and spiritual suffering among the people with life-threatening illness and the mental, social and spiritual suffering of family members (294).
Palliative care:
entails early identification and accurate assessment and treatment of these problems;
enhances quality of life, promotes dignity and comfort and may also positively influence the course of illness;
provides accompaniment for the person with life-threatening illness and family throughout the course of illness and for bereaved family members;
should be integrated with and complement prevention, early diagnosis and treatment of HIV infection and associated conditions;
is applicable early in the course of illness in conjunction with ART and other therapies intended to prolong life;
is especially important near the end of life when the disease treatments may no longer be beneficial or desired by the person with life-threatening illness;
seeks to mitigate the pathogenic effects of poverty on people with life-threatening illness and their families and to protect them from financial hardship caused by illness or disability;
should be applied by health-care workers at all levels of health-care systems, including primary care providers, generalists and specialists in HIV or infectious disease;
encourages active involvement by communities and community members; and
should be accessible at all levels of health-care systems and in individuals’ homes.
Palliative care is applicable from the point of diagnosis, enabling people with life-threatening illness and their families to benefit from a holistic, people-centred approach focusing on individual preferences and priorities and controlling pain and symptoms. For people living with HIV at the end of life, symptoms may worsen, priorities change and decisions about place of death and treatment withdrawal become important (276).
Policy and human rights
Palliative care is recognized as a human right (295) and an essential component of integrated, people-centred health services. The World Health Assembly resolution on palliative care (resolution 67.19, 2014) calls for palliative care to be “integrated throughout the life-course” and recognizes it to be “fundamental to improving the quality of life, well-being, comfort and human dignity of individuals, being an effective person-centred health service” (296). The most recent version of the WHO goals for universal health coverage calls for the “full spectrum of essential, quality health services, from health promotion to prevention, treatment, rehabilitation and palliative care across the life-course” (297). WHO also calls for palliative care as an essential component of a comprehensive package of care for people living with HIV because of the variety of symptoms they can experience.
Although evidence consistently identifies that people with life-threatening illness prefer home death (298–300), a study across 11 countries found that people living with HIV are more likely to die in a hospital than people with cancer (301). Health policy should enable the preferences for care at the end of life to be honoured.
Evidence also indicates that individual members of key populations have a greater need for palliative care and worse bereavement outcomes than others (302–304) but may be excluded from palliative care services (305,306). Access to palliative care for children lags behind that of adults, and equitable access to palliative care that reflects children’s specific social, education and developmental needs is essential (286,307).
Evidence for the effectiveness and cost–effectiveness of palliative care
A systematic review found that home palliative care and inpatient hospice care significantly improve outcomes in the domains of pain and symptom control, anxiety, insight and spiritual well-being (308). Evidence from a randomized controlled trial of integrated palliative care for people living with HIV accessing ART in Kenya suggests that a simple intervention of brief training and using a holistic person-centred assessment tool with a proforma care plan and clinical mentorship improved quality of life, mental health, psychosocial concerns and stigma (309–313).
The available evidence suggests that palliative care in low- and middle-income countries saves money for health-care systems (314). Implementing a hospital-based palliative care team in a hospital in South Africa improved the proportion dying at home and reduced admissions and length of stay and costs per patient (315).
Programme considerations
Essential package of care
WHO has recommended an essential package of palliative care comprising four interventions: a list of safe, effective, inexpensive, off-patent and widely available medicines; simple and inexpensive equipment; basic social supports; and the human resources needed to provide the medicines, equipment and social supports effectively and safely () (273,316,317).
Essential medicines
The list of medicines in the essential package of palliative care is based on the 2019 WHO Model List of Essential Medicines for palliative care for adults and children (110,318). Medicines were selected based on their indispensability to prevent or relieve the most common types of suffering of people living with HIV and people with other serious illnesses, the ability of clinicians with basic palliative care training to prescribe them safely and their balance of clinical effectiveness, safety, ease of use, low cost and global market accessibility (316,319). Morphine, in oral fast-release and injectable formulations, is indispensable for treating pain and refractory terminal dyspnoea. Efforts to prevent the diversion of morphine and other controlled medicines should not result in inappropriate regulatory barriers to access to such medicines (319,320). The 2020 WHO guidelines on managing chronic pain among children (321) also state that access to pain management is a fundamental human right, and governments must “guarantee essential medicines, which include, among others, opioid analgesics as part of their minimum core obligations under the right to health”.
Non-pharmaceutical management
The availability of medicines for controlling pain and symptoms for people living with HIV is essential. In addition to pharmaceutical management, self-management may empower people living with HIV because of undertreatment stigma and challenges in accessing clinical support when pain is wrongly attributed (277). A systematic review found that self-management interventions delivered online, face-to-face or group-based comprising booklets, leaflets or manuals are effective in improving the relief of pain and physical symptoms among people living with HIV (322).
Clinical education
WHO recommends that all health–care providers be trained in at least basic palliative care because of the range of health-care providers who have a role in delivery, including community and primary care providers, paediatricians and HIV specialists (313). Basic HIV palliative care training should aim to create competencies in assessing and relieving pain and other physical symptoms, common types of mental distress such as anxiety and depression and common types of social suffering such as stigmatization, abandonment and extreme poverty. The training also should generate competencies in empathetic and culturally sensitive communication with people with life-threatening illness, medical ethics, advance care planning, interdisciplinary teamwork, end-of-life care, bereavement support and self-care. Outlines for such curricula are available (313,316).
The level of training that different types of health-care providers should require is as follows (316):
basic palliative care training (duration: 30–40 hours) for all health-care providers working at the primary care or community level who care for people living with HIV; and
intermediate-level palliative care training (duration: 60–80 hours) for health-care professionals specializing in such fields as HIV management, infectious disease, tropical medicine, TB, oncology, haematology, family medicine, internal medicine and critical care.
Place of care
The guidance for planning palliative care services states that people living with HIV should have access to palliative care at all levels of health-care systems and in the home (313). Central-level hospitals that care for people living with HIV should have a palliative care team comprising one or more doctors (ideally a palliative care specialist), nurses and a social worker or psychologist that provide inpatient and outpatient care and advice and support for clinicians providing palliative care at lower levels. District hospitals should have one or more doctors and nurses with at least basic palliative care training and a social worker who provides inpatient and outpatient palliative care as one official responsibility. In hospitals, health-care providers should initiate palliative care for people in need, relieve any type of suffering as completely as possible and create discharge plans for people returning home at the end of life to protect them from suffering. This may entail communicating with health-care providers at the community level (313).
Primary health-care centres would benefit from having at least one health-care worker with at least basic palliative care training. The tasks could include initiating palliative care and planning treatment for people living with HIV with uncomplicated needs, continuing palliative care begun in a hospital, surveillance for uncontrolled symptoms with help from community health workers, home visits when necessary and feasible, prescription refills and referral to higher levels as needed. If possible, primary health-care centres can provide inpatient hospice care for people living with HIV whose symptoms are well controlled but are unable to be cared for adequately at home. Community health workers can be taught in a few hours to recognize uncontrolled suffering among people at home, and simple valid person-centred outcome measures can enable lay community health workers to identify palliative care symptoms and concerns and communicate this information rapidly using mobile health technologies to palliative care teams (323). Health-care professionals should supervise community health workers who visit people who need palliative care. Person-centred HIV care delivered in the community by trained health-care professionals can improve outcomes in all four dimensions of well-being (physical, mental, social and spiritual) and retention in care (324) and is a core principle of WHO’s public health approach.
Social support
Social support is a basic component of the early palliative care consultation and should be accessible for anyone who needs palliative care and for their main caregiver in instances of extreme poverty (325–327). Given that extreme poverty is both a cause and an effect of HIV infection, it is crucial that meaningful social support be accessible (325). Such social support includes transport vouchers, cash payments, food packages and other types of in-kind support (326,327). In most cases, funding for social support should come not from health-care budgets but from antipoverty or social welfare programmes. Thus, implementing all aspects of the full essential package of palliative care requires intersectoral coordination (317).
6.18. Noncommunicable diseases among children and adolescents
As access to treatment improves, children and adolescents living with HIV receiving ART should have the chance to have an improved quality of life and reach their full potential. Service delivery platforms need to plan how to implement this, since screening for chronic comorbidities and disabilities, neural development and growth delays, promoting nurturing care and supporting the mental development of children and adolescents as they age are of paramount importance.
Worldwide, 530 000 children five years and younger are living with HIV, and 5.4 million children five years and younger are HIV-exposed and uninfected (328).
The early years lay a foundation for health, well-being and productivity that lasts throughout childhood, adolescence and adulthood (329). Failure to meet children’s needs in this critical window limits their ability to achieve their full developmental potential. Children living with HIV may be especially vulnerable during this period because of the following risk factors:
being born small for gestational age or prematurely (
330);
having more severe pneumonia, diarrhoeal disease and exposure to TB (
331);
receiving suboptimal breastfeeding and nutrition, resulting in poor growth;
being cared for by a mother or other caregiver who is experiencing health challenges, both physically and mentally;
being excluded from opportunities to interact with other children, adults and their surroundings;
being raised in extreme poverty or exposed to violence; and
being exposed to maternal HIV and to ARV medicine, resulting in worse developmental outcomes compared with their non-HIV-affected peers.
Enabling young children to achieve their full developmental potential is a human right and an essential requisite for sustainable development. Given the critical importance of enabling children to make the best start in life, the health sector, among other sectors, has an important role and responsibility to support nurturing care for early childhood development. WHO has produced Improving early childhood development: WHO guideline (332), which provides direction for strengthening policies and programmes to better address early childhood development.
The family primarily provides the nurturing care that children need to develop in the earliest years. Many parents and other caregivers need support to put this into practice. The guideline contains four recommendations for caregivers, health professionals and other workers who can assist them as well as policy-makers and other stakeholders. The recommendations relate to (1) providing responsive care and activities for early learning during the first three years of life; (2) including responsive care and early learning as part of interventions for optimizing the nutrition of infants and young children; and (3) integrating psychosocial interventions to support maternal mental health into early childhood health and development services (332).
To reach their full potential, children need the five interrelated components of nurturing care: good health, adequate nutrition, safety and security, responsive caregiving and opportunities for early learning. This begins in pregnancy and continues throughout the life-course. Adding a nurturing care lens to routine maternal, newborn and child health and HIV prevention and care services can improve the quality of the engagement between health-care workers and caregivers (334) ().
Adding a nurturing care lens to maternal, newborn and child health and HIV prevention services (334).
Service implications
Governments and facilities need to agree on, add and monitor indicators relevant to nurturing care through routine electronic health data systems and, if possible, disaggregate data by HIV infection and exposure status (333,334).
Chronic comorbidities and noncommunicable diseases
As children and adolescents age, comprehensive care remains critical. Recognition is now increasing that children living with HIV, including those taking ART, are at risk of developing chronic multisystem comorbidities and concomitant disability (335). To date, HIV care has mainly focused on delivery and adherence to ART, and the burden of comorbidities associated with HIV among children is insufficiently appreciated. For adolescents, some regions have shown a shift in trend from infectious causes of hospitalization towards noncommunicable diseases (336).
Common comorbidities include developmental delay and neurocognitive impairment, mental health disorders as well as certain organ system morbidities (chronic lung disease, heart disease and kidney disease) that are common among adolescents living with perinatally acquired HIV.
Growth
Although growth resumes after starting ART, children who have more profound stunting and begin ART in late childhood have a delayed growth spurt and are typically unable to reach their height potential (337–339). Age at initiating ART is an important predictor of bone density. In Zimbabwe, children living with HIV starting ART after the age of eight years had, on average, at least 1 standard deviation lower size-adjusted lumbar spine bone density (340). This level of bone density reduction doubles the fracture risk for adults (341).
Chronic comorbidities
Cardiac, renal and metabolic comorbidity has been described in adolescents with late access to suboptimal ART regimens. This should decline as less-toxic ART regimens become more widely available. However, these new regimens may require specific monitoring of other potential comorbidities, such as DTG and potential weight gain. Surveillance is crucial, including conventional growth monitoring.
Chronic lung disease
Reports of chronic lung disease associated with HIV among children mainly come from low-income settings. This difference in reporting might result from a high prevalence of risk factors in these settings, including recurrent lung infections in early life, delayed ART initiation, household air pollution, malnutrition and stunting (342). Malnutrition during the first year of life might be associated with reduced lung function at one year of age, and stunting is a marker of delayed somatic growth. Stunted children could therefore have smaller lungs and reduced lung volume (343).
Lung impairments and reduced lung function in childhood track through adult life, and lung injuries in childhood therefore not only prevent an individual from reaching full lung potential but also increase the risk of chronic lung disease in adult life. A study in South Africa showed a decline in lung function tracked over two years among adolescents living with HIV (9–16 years old) who were well established on ART (344).
Methods for diagnosing chronic lung disease, such as spirometry and high-resolution computed tomography, are scarce in low-income settings. Chronic respiratory symptoms are therefore often empirically treated with repeated antibiotics and anti-TB therapy in settings with a high prevalence of HIV and of TB. There are no specific guidelines for chronic lung disease associated with HIV. However, preventing lung infections can mitigate the burden of chronic lung disease among children living with HIV and optimize lung health. Lung infections can be prevented by ensuring routine vaccinations (including pneumococcal conjugate vaccine and annual influenza vaccine), early ART initiation, continued co-trimoxazole prophylaxis and use of isoniazid prophylactic therapy, avoiding exposure to tobacco smoke and indoor air pollution and optimizing nutrition.
Neurodevelopmental delay, neurocognitive disease and mental health
Children living with HIV who start ART after infancy can have subtle to severe neurocognitive deficits. A prospective study of children 5–11 years old from four countries in sub-Saharan Africa compared nervous system and mental outcomes of children living with HIV, children who had been exposed to HIV but were not infected and children who had not been exposed to HIV. The children living with HIV did worse in all cognitive domains than did the other two groups. More than 95% (239 of 246) of the children living with HIV had suppressed HIV viral load and good immune status (CD4 percentage greater than or equal to 25%), but only 1% (3 of 246) started ART in the first six months of life (335).
The causes of neurocognitive impairment despite effective ART are likely to be multifactorial, including ongoing viral replication in the CNS and resulting neuroinflammation, irreversible CNS injury before ART and neurotoxic effects of ART, and could be compounded by socioeconomic and psychosocial factors (345). Children with neurocognitive impairment can appear asymptomatic, with deficits missed by routine testing. Screening tools and standardized definitions that are context-specific and have been culturally validated are scarce. However, a study in South Africa in 2019 validated a an international HIV dementia screening tool for young people (346).
Several studies report a high prevalence of mental health disorders among children and adolescents living with HIV. A large study in Uganda (347) recruited more than 1300 children and adolescents living with HIV reported a 17.% (233 of 1339) prevalence of any mental health disorder and a 10% (128 of 1339) prevalence of any behavioural disorder, most commonly attention-deficit/hyperactivity disorder. These disorders were more common among adolescents than among children and commonly occurred concurrently with each other. Similarly, a study in South Africa (348) reported that adolescents living with HIV had poorer functional competence, self-concept and motivation and higher levels of depression, disruptive behaviour, attention-deficit/hyperactivity disorder symptoms and clinically significant anger compared with their HIV-negative peers. Children living with HIV face recurrent and cumulative psychosocial stressors that differ from other chronic childhood illnesses, such as stigma and discrimination, responsibility for the welfare of siblings or other family members who are ill, illness and the death of their parents and unstable guardianship. These stressors can hamper development of protective mechanisms and leave children mentally vulnerable and ill equipped for coping with challenges, most likely increasing the risk of mental health disorders. Mental health disorders affect an individual’s adherence to ART and are associated with impaired quality of life but typically receive little attention compared with physical health concerns.
Up to half of adult mental health problems begin during childhood and adolescence (349). This highlights the necessity to implement screening during adolescence. Promoting mental health and preventing mental health disorders and problems are being increasingly emphasized, with opportunities to integrate psychosocial support and mental health at key points, such as at the time HIV status is disclosed.
Cancer
Children living with HIV with advanced immunosuppression before initiating ART or who started ART at an older age have an increased risk of cancer compared with those with modest immunosuppression or who began ART in infancy (350). Linked data from five ART programmes for children and four paediatric oncology units in South Africa showed an overall incidence of cancer of 82 per 100 000 person-years. The most common types were Kaposi’s sarcoma, with an incidence of 34 per 100 000 person-years, and non-Hodgkin lymphoma, with an incidence of 31 per 100 000 person-years (351).
A study from the United States of America, which followed up children for 10 years, showed that, although the incidence of Kaposi’s sarcoma and non-Hodgkin lymphoma declined in the ART era, the risk of developing non-AIDS-defining cancer did not (352). This increased risk of non-AIDS-defining cancer highlights the need for continued monitoring of children growing up with HIV.
Multimorbidity
In a study in South Africa (353), more adults living with HIV receiving ART had multimorbidity in the younger age groups (18–35 and 36–45 years old) than in an older age group (46–55 years old), with 26% of 18- to 35-year-olds and 30% of 36- to 45-year olds having multimorbidity. Since the risk factors for multimorbidity include time on ART, adolescents living with perinatally acquired HIV may be at increased risk as they progress through their life-course. Adolescents in resource-limited settings may be specifically vulnerable because of a lack of primary prevention of common noncommunicable diseases and a high burden of inflammatory coinfections.
Service delivery
Various service delivery platforms already established could be used to screen for and manage noncommunicable diseases among children at different stages of their lives. For children younger than five years, examples include early childhood development, under-five clinics and HIV services. For children 5–9 years old, examples include integrated school and HIV services. For adolescents 10–19 years old, examples include adolescent-friendly services. Although implementing screening and prevention of noncommunicable diseases or comorbidities presents many challenges, opportunities to integrate this kind of care into existing adolescent activities are numerous. Some clinics have already established adolescent groups to address various health issues (354). HIV is one of the only conditions that results in an adolescent population interacting regularly with the health system. This should be leveraged to integrate a more comprehensive approach to health. summarizes the platforms, screening approaches, tools needed and interventions that are applicable across young children, children of school age and adolescents.
Comprehensive HIV care for children and adolescents includes:
earlier initiation of ART to prevent complications:
monitoring growth and musculoskeletal and neurocognitive development;
screening for heart, lung, kidney and neurocognitive disease;
assessing psychosocial status (schooling and guardianship) and mental health;
managing common mental health disorders and providing psychosocial support;
optimizing nutrition;
catching up or revaccinating according to WHO guidelines, such as pneumococcal and influenza vaccination;
HPV vaccination for adolescents;
cervical cancer screening after sexual debut;
referring to clinical specialties for management if feasible;
liaising with disability and rehabilitation services;
school-based programmes to provide educational support;
leveraging existing early child development platforms for supporting children living with HIV; and
linking to community-based psychosocial support services.
Service Delivery: Potential ways to integrate screening and possible interventions.
Research priorities
The epidemiology and clinical spectrum of noncommunicable diseases among children and adolescents living with HIV and the pathogenesis of these issues need further study. Standard definitions of comorbidities based on population-specific normative ranges are needed. Optimum screening, when to start screening and management strategies are not well defined and need further study. Validated age-appropriate and culturally appropriate screening tools specifically for mental health and neurocognitive disease are needed. Further research on interventions for prevention and treatment are needed, including antibiotics, antiviral agents, anti-inflammatory drugs and vitamin D as well as feasible and effective educational and mental health interventions. How the ARV drugs used for prevention and treatment interact among children needs to be studied.
Additional resources relating to Chapter 6
Sexual and reproductive health guidelines for women living with HIV
WHO, UNFPA. Sexual and reproductive health of women living with HIV/AIDS. Guidelines on care, treatment and support for women living with HIV/AIDS and their children in resource-constrained settings. Geneva: World Health Organization; 2006 (
https://apps.who.int/iris/handle/10665/43473).
References
- 1.
- 2.
- 3.
- 4.
- 5.
IMAI district clinician manual: hospital care for adolescents and adults – guidelines for the management of illnesses with limited resources. Geneva: World Health Organization; 2011 (
https://apps.who.int/iris/handle/10665/77751, accessed 1 June 2021).
- 6.
Guidelines on post-exposure prophylaxis for HIV and the use of co-trimoxazole prophylaxis for HIV-related infections among adults, adolescents and children: recommendations for a public health approach – December 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Geneva: World Health Organization; 2014 (
https://apps.who.int/iris/handle/10665/145719, accessed 1 June 2021). [
PubMed: 26042326]
- 7.
Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults: recommendations for a public health approach. Geneva: World Health Organization; 2006 (
https://apps.who.int/iris/handle/10665/43557, accessed 1 June 2021).
- 8.
- 9.
Amuron
B, Levin
J, Birunghi
J, Namara
G, Coutinho
A, Grosskurth
H
et al. Mortality in an antiretroviral therapy programme in Jinja, south-east Uganda: a prospective cohort study. AIDS Res Ther. 2011;8:39. [
PMC free article: PMC3210087] [
PubMed: 22018282]
- 10.
Auld
AF, Mbofana
F, Shiraishi
RW, Sanchez
M, Alfredo
C, Nelson
LJ
et al. Four-year treatment outcomes of adult patients enrolled in Mozambique’s rapidly expanding antiretroviral therapy program. PLoS One. 2011;6:e18453. [
PMC free article: PMC3070740] [
PubMed: 21483703]
- 11.
Hoffmann
CJ, Fielding
KL, Charalambous
S, Innes
C, Chaisson
RE, Grant
AD
et al. Reducing mortality with co-trimoxazole preventive therapy at initiation of antiretroviral therapy in South Africa. AIDS. 2010;24:1709–16. [
PMC free article: PMC2956913] [
PubMed: 20495439]
- 12.
Lim
PL, Zhou
J, Ditangco
RA, Law
MG, Sirisanthana
T, Kumarasamy
N
et al. Failure to prescribe pneumocystis prophylaxis is associated with increased mortality, even in the cART era: results from the Treat Asia HIV observational database. J Int AIDS Soc. 2012;15:1. [
PMC free article: PMC3354658] [
PubMed: 22281054]
- 13.
Lowrance
D, Makombe
S, Harries
A, Yu
J, Aberle-Grasse
J, Eiger
O
et al. Lower early mortality rates among patients receiving antiretroviral treatment at clinics offering co-trimoxazole prophylaxis in Malawi. J Acquir Immune Defic Syndr. 2007;46:56–61. [
PubMed: 17972365]
- 14.
Madec
Y, Laureillard
D, Pinoges
L, Fernandez
M, Prak
N, Ngeth
C
et al. Response to highly active antiretroviral therapy among severely immuno-compromised HIV-infected patients in Cambodia. AIDS. 2007;21:351–9. [
PubMed: 17255742]
- 15.
van Oosterhout
JJ, Ndekha
M, Moore
E, Kumwenda
JJ, Zijlstra
EE, Manary
M. The benefit of supplementary feeding for wasted Malawian adults initiating ART. AIDS Care. 2010;22:737–42. [
PubMed: 20467944]
- 16.
Walker
AS, Ford
D, Gilks
CF, Munderi
P, Ssali
F, Reid
A
et al. Daily co-trimoxazole prophylaxis in severely immunosuppressed HIV-infected adults in Africa started on combination antiretroviral therapy: an observational analysis of the DART cohort. Lancet. 2010;375:1278–86. [
PMC free article: PMC2858802] [
PubMed: 20347483]
- 17.
Suthar
AB, Vitoria
MA, Nagata
JM, Anglaret
X, Mbori-Ngacha
D, Sued
O
et al. Co-trimoxazole prophylaxis in adults, including pregnant women, with HIV: a systematic review and meta-analysis. Lancet HIV. 2015;2:e137–50. [
PubMed: 26424674]
- 18.
Walker
AS, Mulenga
V, Ford
D, Kabamba
D, Sinyinza
F, Kankasa
C
et al. The impact of daily co-trimoxazole prophylaxis and antiretroviral therapy on mortality and hospital admissions in HIV-infected Zambian children. Clin Infect Dis. 2007;44:1361–7. [
PubMed: 17443476]
- 19.
Mulenga
V, Ford
D, Walker
AS, Mwenya
D, Mwansa
J, Sinyinza
F
et al. Effect of co-trimoxazole on causes of death, hospital admissions and antibiotic use in HIV-infected children. AIDS. 2007;21:77–84. [
PubMed: 17148971]
- 20.
Campbell
JD, Moore
D, Degerman
R, Kaharuza
F, Were
W, Muramuzi
E
et al. HIV-infected ugandan adults taking antiretroviral therapy with CD4 counts >200 cells/muL who discontinue co-trimoxazole prophylaxis have increased risk of malaria and diarrhea. Clin Infect Dis. 2012;54:1204–11. [
PubMed: 22423133]
- 21.
Polyak
CS, Yuhas
K, Singa
B, Khaemba
M, Walson
J, Richardson
BA
et al. Co-trimoxazole prophylaxis discontinuation among antiretroviral-treated HIV-1-infected adults in Kenya: a randomized non-inferiority trial. PLoS Med. 2016;13:e1001934. [
PMC free article: PMC4701407] [
PubMed: 26731191]
- 22.
Bwakura-Dangarembizi
M, Kendall
L, Bakeera-Kitaka
S, Nahirya-Ntege
P, Keishanyu
R, Nathoo
K
et al. A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa. N Engl J Med. 2014;370:41–53. [
PMC free article: PMC4264559] [
PubMed: 24382064]
- 23.
Opportunistic Infections Project Team of the Collaboration of Observational HIVERiE, Mocroft
A, Reiss
P, Kirk
O, Mussini
C, Girardi
E
et al. Is it safe to discontinue primary Pneumocystis jirovecii pneumonia prophylaxis in patients with virologically suppressed HIV infection and a CD4 cell count <200 cells/µL?
Clin Infect Dis. 2010;51:611–9. [
PubMed: 20645862]
- 24.
Ford
N, Shubber
Z, Jao
J, Abrams
EJ, Frigati
L, Mofenson
L. Safety of co-trimoxazole in pregnancy: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2014;66:512–21. [
PMC free article: PMC4331013] [
PubMed: 24853309]
- 25.
- 26.
Lockman
S, Hughes
M, Powis
K, Ajibola
G, Bennett
K, Moyo
S
et al. Effect of co-trimoxazole on mortality in HIV-exposed but uninfected children in Botswana (the Mpepu Study): a double-blind, randomised, placebo-controlled trial. Lancet Global Health. 2017;5:e491–500. [
PMC free article: PMC5502726] [
PubMed: 28395844]
- 27.
Daniels
B, Coutsoudis
A, Moodley-Govender
E, Mulol
H, Spooner
E, Kiepiela
P
et al. Effect of co-trimoxazole prophylaxis on morbidity and mortality of HIV-exposed, HIV-uninfected infants in South Africa: a randomised controlled, non-inferiority trial. Lancet Global Health. 2019;7:e1717–27. [
PubMed: 31708152]
- 28.
Powis
KM, Souda
S, Lockman
S, Ajibola
G, Bennett
K, Leidner
J
et al. Co-trimoxazole prophylaxis was associated with enteric commensal bacterial resistance among HIV-exposed infants in a randomized controlled trial, Botswana. J Int AIDS Soc. 2017;20:11. [
PMC free article: PMC5810322] [
PubMed: 29119726]
- 29.
D’Souza
AW, Moodley-Govender
E, Berla
B, Kelkar
T, Wang
B, Sun
X
et al. Co-trimoxazole prophylaxis increases resistance gene prevalence and alpha-diversity but decreases beta-diversity in the gut microbiome of human immunodeficiency virus-exposed, uninfected infants. Clin Infect Dis. 2020;71:2858–68. [
PMC free article: PMC7778358] [
PubMed: 31832638]
- 30.
Sandison
TG, Homsy
J, Arinaitwe
E, Wanzira
H, Kakuru
A, Bigira
V
et al. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ. 2011;342:d1617. [
PMC free article: PMC3068910] [
PubMed: 21454456]
- 31.
Homsy
J, Dorsey
G, Arinaitwe
E, Wanzira
H, Kakuru
A, Bigira
V
et al. Protective efficacy of prolonged co-trimoxazole prophylaxis in HIV-exposed children up to age 4 years for the prevention of malaria in Uganda: a randomised controlled open-label trial. Lancet Global Health. 2014;2:e727–36. [
PubMed: 25433628]
- 32.
Kamya
MR, Kapisi
J, Bigira
V, Clark
TD, Kinara
S, Mwangwa
F
et al. Efficacy and safety of three regimens for the prevention of malaria in young HIV-exposed Ugandan children: a randomized controlled trial. AIDS. 2014;28:2701–9. [
PMC free article: PMC4487368] [
PubMed: 25493596]
- 33.
Zar
H, Moore
DP, Andronikou
S, Argent
AC, Avenant
T, Cohen
C
et al. Diagnosis and management of community-acquired pneumonia in children: South African Thoracic Society guidelines. Afr J Thoracic Crit Care Med. 2020;26:AJTCCM.2020.v26i3.104.
- 34.
Smith
C, Penazzato
M, Gibb
D, Slogrove
A, Evans
C, Prendergast
A. Co-trimoxazole prophylaxis for children born to mothers with HIV: predicted impact of different strategies on mortality up to the age of two years. International Workshop on HIV Pediatrics 2021, virtual, 16–17 July 2021.
- 35.
- 36.
- 37.
- 38.
- 39.
Bares
S, Swindells
S. Latent tuberculosis and HIV infection. Curr Infect Dis Rep. 2020;22:17.
- 40.
- 41.
- 42.
- 43.
- 44.
Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva: World Health Organization; 2011 (
https://apps.who.int/iris/handle/10665/44472, accessed 1 June 2021).
- 45.
- 46.
- 47.
Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents: recommendations for HIV-prevalent and resource-constrained settings. Geneva: World Health Organization; 2007 (
https://apps.who.int/iris/handle/10665/69463, accessed 1 June 2021).
- 48.
Hosseinipour
MC, Bisson
GP, Miyahara
S, Sun
X, Moses
A, Riviere
C
et al. Empirical tuberculosis therapy versus isoniazid in adult outpatients with advanced HIV initiating antiretroviral therapy (REMEMBER): a multicountry open-label randomised controlled trial. Lancet. 2016;387:1198–209. [
PMC free article: PMC4931281] [
PubMed: 27025337]
- 49.
Manabe
YC, Worodria
W, van Leth
F, Mayanja-Kizza
H, Traore
AN, Ferro
J
et al. Prevention of early mortality by presumptive tuberculosis therapy study: an open label, randomized controlled trial. Am J Trop Med Hyg. 2016;95:1265–71. [
PMC free article: PMC5154437] [
PubMed: 27928077]
- 50.
Blanc
FX, Badje
AD, Bonnet
M, Gabillard
D, Messou
E, Muzoora
C
et al. Systematic or test-guided treatment for tuberculosis in HIV-infected adults. N Engl J Med. 2020;382:2397–410. [
PubMed: 32558469]
- 51.
- 52.
- 53.
Post
FA, Grint
D, Werlinrud
AM, Panteleev
A, Riekstina
V, Malashenkov
EA
et al. Multi-drug-resistant tuberculosis in HIV positive patients in eastern Europe. J Infect. 2014;68:259–63. [
PubMed: 24247067]
- 54.
- 55.
Mesfin
YM, Hailemariam
D, Biadgilign
S, Kibret
KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PLoS One. 2014;9:e82235. [
PMC free article: PMC3885391] [
PubMed: 24416139]
- 56.
Dean
AS, Zignol
M, Falzon
D, Getahun
H, Floyd
K. HIV and multidrug-resistant tuberculosis: overlapping epidemics. Eur Respir J. 2014;44:251–4. [
PubMed: 24525438]
- 57.
Rangaka
MX, Wilkinson
RJ, Boulle
A, Glynn
JR, Fielding
K, van Cutsem
G
et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384:682–90. [
PMC free article: PMC4233253] [
PubMed: 24835842]
- 58.
Schechter
M. Prioritization of antiretroviral therapy in patients with high CD4 counts, and retention in care: lessons from the START and Temprano trials. J Int AIDS Soc. 2018;21:e25077. [
PMC free article: PMC5810323] [
PubMed: 29436779]
- 59.
Badje
A, Moh
R, Gabillard
D, Guehi
C, Kabran
M, Ntakpe
JB
et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health. 2017;5:e1080–9. [
PubMed: 29025631]
- 60.
Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva: World Health Organization; 2011 (
https://apps.who.int/iris/handle/10665/44472, accessed 1 June 2021).
- 61.
- 62.
Kwizera
A, Nabukenya
M, Agaba
P, Semogerere
L, Ayebale
E, Katabira
C
et al. Clinical characteristics and short-term outcomes of HIV patients admitted to an African intensive care unit. Crit Care Res Pract. 2016;2016:2610873. [
PMC free article: PMC5075298] [
PubMed: 27800179]
- 63.
- 64.
- 65.
- 66.
- 67.
Progress report on HIV, viral hepatitis and sexually transmitted infections 2019: accountability for the global health sector strategies, 2016–2021. Geneva: World Health Organization; 2019 (
https://apps.who.int/iris/handle/10665/324797, accessed 1 June 2021).
- 68.
Grebely
J, Larney
S, Peacock
A, Colledge
S, Leung
J, Hickman
M
et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction. 2019;114:150–66. [
PMC free article: PMC6657799] [
PubMed: 30035835]
- 69.
Trickey
A, Fraser
H, Lim
AG, Peacock
A, Colledge
S, Walker
JG
et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol. 2019;4:435–44. [
PMC free article: PMC6698583] [
PubMed: 30981685]
- 70.
- 71.
Easterbrook
PJ, WHO Guideline Development Group. Who to test and how to test for chronic hepatitis C infection – 2016 WHO testing guidance for low- and middle-income countries. J Hepatol. 2016;65(1 Suppl.):S46–66. [
PubMed: 27641988]
- 72.
Platt
L, Easterbrook
P, Gower
E, McDonald
B, Sabin
K, McGowan
C
et al. Prevalence and burden of HCV coinfection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16:797–808. [
PubMed: 26922272]
- 73.
Jin
F, Dore
GJ, Matthews
G, Luhmann
N, Macdonald
V, Bajis
S
et al. Prevalence and incidence of hepatitis C virus infection in men who have sex with men: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:39–56. [
PubMed: 33217341]
- 74.
- 75.
Sikavi
C, Chen
PH, Lee
AD, Saab
EG, Choi
G, Saab
S. Hepatitis C and human immunodeficiency virus coinfection in the era of direct-acting antiviral agents: no longer a difficult-to-treat population. Hepatology. 2018;67:847–57. [
PubMed: 29108121]
- 76.
- 77.
- 78.
- 79.
- 80.
- 81.
- 82.
- 83.
- 84.
- 85.
Kawuma
AN, Walimbwa
SI, Pillai
GC, Khoo
S, Lamorde
M, Wasmann
RE
et al. Dolutegravir pharmacokinetics during co-administration with either artemether/lumefantrine or artesunate/amodiaquine. J Antimicrob Chemother. 2021;76:1269–72. [
PubMed: 33550391]
- 86.
- 87.
O’Brien
DP, Ford
N, Vitoria
M, Christinet
V, Comte
E, Calmy
A
et al. Management of Buruli ulcer–HIV coinfection. Trop Med Int Health. 2014;19:1040–7. [
PubMed: 24946829]
- 88.
- 89.
Haas
AD, Ruffieux
Y, van den Heuvel
LL, Lund
C, Boulle
A, Euvrard
J
et al. Excess mortality associated with mental illness in people living with HIV in Cape Town, South Africa: a cohort study using linked electronic health records. Lancet Global Health. 2020;8:e1326–34. [
PMC free article: PMC7582785] [
PubMed: 32971055]
- 90.
Johnson
RC, Nackers
F, Glynn
JR, de Biurrun Bakedano
E, Zinsou
C, Aguiar
J
et al. Association of HIV infection and Mycobacterium ulcerans disease in Benin. AIDS. 2008;22:901–3. [
PubMed: 18427211]
- 91.
Christinet
V, Comte
E, Ciaffi
L, Odermatt
P, Serafini
M, Antierens
A
et al. Impact of human immunodeficiency virus on the severity of Buruli ulcer disease: results of a retrospective study in Cameroon. Open Forum Infect Dis. 2014;1:ofu021. [
PMC free article: PMC4324202] [
PubMed: 25734094]
- 92.
Tuffour
J, Owusu-Mireku
E, Ruf
MT, Aboagye
S, Kpeli
G, Akuoku
V
et al. Challenges associated with management of Buruli ulcer/human immunodeficiency virus coinfection in a treatment center in Ghana: a case series study. Am J Trop Med Hyg. 2015;93:216–23. [
PMC free article: PMC4530737] [
PubMed: 26055745]
- 93.
- 94.
Johnson
RC, Ifebe
D, Hans-Moevi
A, Kestens
L, Houessou
R, Guedenon
A
et al. Disseminated Mycobacterium ulcerans disease in an HIV-positive patient: a case study. AIDS. 2002;16:1704–5. [
PubMed: 12172103]
- 95.
Toll
A, Gallardo
F, Ferran
M, Gilaberte
M, Iglesias
M, Gimeno
JL
et al. Aggressive multifocal Buruli ulcer with associated osteomyelitis in an HIV-positive patient. Clin Exp Dermatol. 2005;30:649–51. [
PubMed: 16197379]
- 96.
Bayonne Manou
LS, Portaels
F, Eddyani
M, Book
AU, Vandelannoote
K, De Jong
BC. [Mycobactium ulcerans disease (Buruli ulcer) in Gabon: 2005–2011.] Med Sante Trop. 2013;23:450–7. [
PubMed: 24413612]
- 97.
Amerson
EH, Maurer
TA. Immune reconstitution inflammatory syndrome and tropical dermatoses. Dermatol Clin. 2011;29:39–43. [
PubMed: 21095526]
- 98.
Buruli ulcer laboratory network and new external quality assessment programme for PCR-based diagnosis in the WHO African Region: terms of reference. Geneva: World Health Organization; 2020 (
https://apps.who.int/iris/handle/10665/333593, accessed 1 June 2021).
- 99.
- 100.
- 101.
Davy-Mendez
T, Napravnik
S, Wohl
DA, Durr
AL, Zakharova
O, Farel
CE
et al. Hospitalization rates and outcomes among persons living with human immunodeficiency virus in the southeastern United States, 1996–2016. Clin Infect Dis. 2020;71:1616–23. [
PMC free article: PMC7755093] [
PubMed: 31637434]
- 102.
Burza
S, Croft
SL, Boelaert
M. Leishmaniasis. Lancet. 2018;392:951–70. [
PubMed: 30126638]
- 103.
WHO guidelines for the treatment of visceral leishmaniasis in HIV-coinfected persons in east Africa and South-East Asia. Geneva: World Health Organization; in press.
- 104.
Guedes
DL, Justo
AM, Barbosa
Junior
WL, Silva
EDD, Aquino
SR, Lima Junior
M
et al. Asymptomatic Leishmania infection in HIV-positive outpatients on antiretroviral therapy in Pernambuco, Brazil. PLoS Negl Trop Dis. 2021;15:e0009067. [
PMC free article: PMC7853496] [
PubMed: 33476331]
- 105.
- 106.
- 107.
Burza
S, Mahajan
R, Sanz
MG, Sunyoto
T, Kumar
R, Mitra
G
et al. HIV and visceral leishmaniasis coinfection in Bihar, India: an underrecognized and underdiagnosed threat against elimination. Clin Infect Dis. 2014;59:552–5. [
PubMed: 24814660]
- 108.
Monge-Maillo
B, Lopez-Velez
R. Treatment options for visceral leishmaniasis and HIV coinfection. AIDS Rev. 2016;18:32–43. [
PubMed: 26936761]
- 109.
Sunyoto
T, Potet
J, den Boer
M, Ritmeijer
K, Postigo
JAR, Ravinetto
R
et al. Exploring global and country-level barriers to an effective supply of leishmaniasis medicines and diagnostics in eastern Africa: a qualitative study. BMJ Open. 2019;9:e029141. [
PMC free article: PMC6549606] [
PubMed: 31152044]
- 110.
- 111.
- 112.
Arbyn
M, Weiderpass
E, Bruni
L, de Sanjosé
S, Saraiya
M, Ferlay
J
et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Global Health. 2020;8:e191–203. [
PMC free article: PMC7025157] [
PubMed: 31812369]
- 113.
Stelzle
D, Tanaka
LF, Lee
KK, Khalil
AI, Baussano
I, Shah
AS
et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Global Health. 2021;9:e161–9. [
PMC free article: PMC7815633] [
PubMed: 33212031]
- 114.
WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: World Health Organization; 2007 (
https://apps.who.int/iris/handle/10665/43699, accessed 1 June 2021).
- 115.
Liu
G, Sharma
M, Tan
N, Barnabas
RV. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS. 2018;32:795–808. [
PMC free article: PMC5854529] [
PubMed: 29369827]
- 116.
- 117.
Debeaudrap
P, Sobngwi
J, Tebeu
P-M, Clifford
GM. Residual or recurrent precancerous lesions after treatment of cervical lesions in human immunodeficiency virus–infected women: a systematic review and meta-analysis of treatment failure. Clin Infect Dis. 2019;69:1555–65. [
PMC free article: PMC6792085] [
PubMed: 30602038]
- 118.
- 119.
- 120.
- 121.
Update of WHO screening and treatment recommendations to prevent cervical cancer. Geneva: World Health Organization; in press.
- 122.
Canfell
K, Kim
JJ, Brisson
M, Keane
A, Simms
KT, Caruana
M
et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:591–603. [
PMC free article: PMC7043006] [
PubMed: 32007142]
- 123.
Brisson
M, Kim
JJ, Canfell
K, Drolet
M, Gingras
G, Burger
EA
et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395:575–90. [
PMC free article: PMC7043009] [
PubMed: 32007141]
- 124.
Simms
KT, Steinberg
J, Caruana
M, Smith
MA, Lew
J-B, Soerjomataram
I
et al. Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: a modelling study. Lancet Oncol. 2019;20:394–407. [
PubMed: 30795950]
- 125.
Hall
MT, Smith
MA, Simms
KT, Barnabas
R, Murray
JM, Canfell
K. Elimination of cervical cancer in Tanzania: modelled analysis of elimination in the context of endemic HIV infection and active HIV control. Int J Cancer. 2021;149·297–306. [
PubMed: 33634857]
- 126.
- 127.
- 128.
- 129.
- 130.
Smith
CJ, Ryom
L, Weber
R, Morlat
P, Pradier
C, Reiss
P
et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384:241–8. [
PubMed: 25042234]
- 131.
Haregu
TN, Oldenburg
BF, Sestwe
G, Elliott
J, Nanayakkara
V. Epidemiology of comorbidity of HIV/AIDS and non-communicable diseases in developing countries: a systematic review. J Glob Health Care Syst. 2012;2:1–12.
- 132.
Crothers
K, Butt
AA, Gibert
CL, Rodriguez-Barradas
MC, Crystal
S, Justice
AC
et al. Increased chronic obstructive pulmonary disease among HIV-positive compared to HIV-negative veterans. Chest. 2006;130:1326–33. [
PubMed: 17099007]
- 133.
Peck
RN, Shedafa
R, Kalluvya
S, Downs
JA, Todd
J, Suthanthiran
M
et al. Hypertension, kidney disease, HIV and antiretroviral therapy among Tanzanian adults: a cross-sectional study. BMC Med. 2014;12:125. [
PMC free article: PMC4243281] [
PubMed: 25070128]
- 134.
Johnson
LF, Mossong
J, Dorrington
RE, Schomaker
M, Hoffmann
CJ, Keiser
O
et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10:e1001418. [
PMC free article: PMC3621664] [
PubMed: 23585736]
- 135.
Crum-Cianflone
N, Hullsiek
KH, Marconi
V, Weintrob
A, Ganesan
A, Barthel
RV
et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23:41–50. [
PMC free article: PMC2727153] [
PubMed: 19050385]
- 136.
- 137.
- 138.
- 139.
Rabkin
M, Nishtar
S. Scaling up chronic care systems: leveraging HIV programs to support noncommunicable disease services. J Acquir Immune Defic Syndr. 2011;57(Suppl. 2):S87–90. [
PubMed: 21857304]
- 140.
Currier
JS, Taylor
A, Boyd
F, Dezii
CM, Kawabata
H, Burtcel
B
et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–12. [
PubMed: 12869840]
- 141.
Islam
FM, Wu
J, Jansson
J, Wilson
DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13:453–68. [
PubMed: 22413967]
- 142.
Paisible
AL, Chang
CC, So-Armah
KA, Butt
AA, Leaf
DA, Budoff
M
et al. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68:209–16. [
PMC free article: PMC4441201] [
PubMed: 25588033]
- 143.
Chow
FC, Regan
S, Feske
S, Meigs
JB, Grinspoon
SK, Triant
VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60:351–8. [
PMC free article: PMC3670086] [
PubMed: 22580566]
- 144.
Triant
VA, Lee
H, Hadigan
C, Grinspoon
SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–12. [
PMC free article: PMC2763385] [
PubMed: 17456578]
- 145.
Marcus
JL, Leyden
WA, Chao
CR, Chow
FC, Horberg
MA, Hurley
LB
et al. HIV infection and incidence of ischemic stroke. AIDS. 2014;28:1911–9. [
PubMed: 24937309]
- 146.
- 147.
Patel
K, Wang
J, Jacobson
DL, Lipshultz
SE, Landy
DC, Geffner
ME
et al. Aggregate risk of cardiovascular disease among adolescents perinatally infected with the human immunodeficiency virus. Circulation. 2014;129:1204–12. [
PMC free article: PMC3991841] [
PubMed: 24366631]
- 148.
Cerrato
E, Calcagno
A, D’Ascenzo
F, Biondi-Zoccai
G, Mancone
M, Grosso Marra
W
et al. Cardiovascular disease in HIV patients: from bench to bedside and backwards. Open Heart. 2015;2:e000174. [
PMC free article: PMC4368980] [
PubMed: 25815207]
- 149.
Benchley
JM PD, Schacker
TW, Asher
TE, Silvestri
G, Rao
S
et al, . Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. [
PubMed: 17115046]
- 150.
Zanni
MV, Schouten
J, Grinspoon
SK, Reiss
P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11:728–41. [
PubMed: 25331088]
- 151.
Hunt
PW, Brenchley
J, Sinclair
E, McCune
JM, Roland
M, Page-Shafer
K
et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–33. [
PMC free article: PMC3466592] [
PubMed: 18171295]
- 152.
Duprez
DA, Neuhaus
J, Kuller
LH, Tracy
R, Belloso
W, De Wit
S
et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. [
PMC free article: PMC3438173] [
PubMed: 22970224]
- 153.
Iloeje
UH, Yuan
Y, L’Italien
G, Mauskopf
J, Holmberg
SD, Moorman
AC
et al. Protease inhibitor exposure and increased risk of cardiovascular disease in HIV-infected patients. HIV Med. 2005;6:37–44. [
PubMed: 15670251]
- 154.
Rhew
DC, Bernal
M, Aguilar
D, Iloeje
U, Goetz
MB. Association between protease inhibitor use and increased cardiovascular risk in patients infected with human immunodeficiency virus: a systematic review. Clin Infect Dis. 2003;37:959–72. [
PubMed: 13130409]
- 155.
D’Ascenzo
F, Cerrato
E, Biondi-Zoccai
G, Moretti
C, Omede
P, Sciuto
F
et al. Acute coronary syndromes in human immunodeficiency virus patients: a meta-analysis investigating adverse event rates and the role of antiretroviral therapy. Eur Heart J. 2012;33:875–80. [
PMC free article: PMC3345544] [
PubMed: 22187508]
- 156.
Bavinger
C, Bendavid
E, Niehaus
K, Olshen
RA, Olkin
I, Sundaram
V
et al. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS One. 2013;8:e59551. [
PMC free article: PMC3608726] [
PubMed: 23555704]
- 157.
Group
DADS, Friis-Moller
N, Reiss
P, Sabin
CA, Weber
R, Monforte
A
et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–35. [
PubMed: 17460226]
- 158.
Young
J, Xiao
Y, Moodie
EE, Abrahamowicz
M, Klein
MB, Bernasconi
E
et al. Effect of cumulating exposure to abacavir on the risk of cardiovascular disease events in patients from the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2015;69:413–21. [
PubMed: 25932884]
- 159.
Group
DADS, Sabin
CA, Worm
SW, Weber
R, Reiss
P, El-Sadr
W
et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371:1417–26. [
PMC free article: PMC2688660] [
PubMed: 18387667]
- 160.
Sabin
CA, Reiss
P, Ryom
L, Phillips
AN, Weber
R, Law
M
et al. Is there continued evidence for an association between abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med. 2016;14:61. [
PMC free article: PMC4815070] [
PubMed: 27036962]
- 161.
Cruciani
M, Zanichelli
V, Serpelloni
G, Bosco
O, Malena
M, Mazzi
R
et al. Abacavir use and cardiovascular disease events: a meta-analysis of published and unpublished data. AIDS. 2011;25:1993–2004. [
PubMed: 21716077]
- 162.
Ding
X, Andraca-Carrera
E, Cooper
C, Miele
P, Kornegay
C, Soukup
M
et al. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr. 2012;61:441–7. [
PubMed: 22932321]
- 163.
Strategies for Management of Antiretroviral Therapy Study G, El-Sadr
WM, Lundgren
J, Neaton
JD, Gordin
F, Abrams
D
et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. [
PubMed: 17135583]
- 164.
Krikke
M, Hoogeveen
RC, Hoepelman
AI, Visseren
FL, Arends
JE. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic Coronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) risk prediction models. HIV Med. 2016;17:289–97. [
PubMed: 26268806]
- 165.
Goff
DC
Jr, Lloyd-Jones
DM, Bennett
G, Coady
S, D’Agostino
RB, Sr., Gibbons
R
et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–59. [
PMC free article: PMC4700825] [
PubMed: 24239921]
- 166.
Edward
AO, Oladayo
AA, Omolola
AS, Adetiloye
AA, Adedayo
PA. Prevalence of traditional cardiovascular risk factors and evaluation of cardiovascular risk using three risk equations in Nigerians living with human immunodeficiency virus. N Am J Med Sci. 2013;5:680–8. [
PMC free article: PMC3877529] [
PubMed: 24404550]
- 167.
Edwards-Jackson
N, Kerr
S, Tieu
H, Ananworanich
J, Hammer
S, Ruxrungtham
K
et al. Cardiovascular risk assessment in persons with HIV infection in the developing world: comparing three risk equations in a cohort of HIV-infected Thais. HIV Med. 2011;12:510–5. [
PubMed: 21375686]
- 168.
Nery
MW, Martelli
CM, Silveira
EA, de Sousa
CA, Falco Mde
O, de Castro Ade
C
et al. Cardiovascular risk assessment: a comparison of the Framingham, PROCAM, and DAD equations in HIV-infected persons. ScientificWorldJournal. 2013;2013:969281. [
PMC free article: PMC3819022] [
PubMed: 24228022]
- 169.
Hsue
PY, Squires
K, Bolger
AF, Capili
B, Mensah
GA, Temesgen
Z
et al. Screening and assessment of coronary heart disease in HIV-infected patients. Circulation. 2008;118:e41–7. [
PubMed: 18566317]
- 170.
Nolte
JE, Neumann
T, Manne
JM, Lo
J, Neumann
A, Mostardt
S
et al. Cost-effectiveness analysis of coronary artery disease screening in HIV-infected men. Eur J Prev Cardiol. 2014;21:972–9. [
PMC free article: PMC3841232] [
PubMed: 23539717]
- 171.
Adeyemi
O. Cardiovascular risk and risk management in HIV-infected patients. Top HIV Med. 2007;15:159–62. [
PubMed: 18073451]
- 172.
- 173.
Friis-Moller
N, Worm
SW. Can the risk of cardiovascular disease in HIV-infected patients be estimated from conventional risk prediction tools?
Clin Infect Dis. 2007;45:1082–4. [
PubMed: 17879929]
- 174.
Knobel
H, Jerico
C, Montero
M, Sorli
ML, Velat
M, Guelar
A
et al. Global cardiovascular risk in patients with HIV infection: concordance and differences in estimates according to three risk equations (Framingham, SCORE, and PROCAM). AIDS Patient Care STDs. 2007;21:452–7. [
PubMed: 17651026]
- 175.
Parra
S, Coll
B, Aragones
G, Marsillach
J, Beltran
R, Rull
A
et al. Nonconcordance between subclinical atherosclerosis and the calculated Framingham risk score in HIV-infected patients: relationships with serum markers of oxidation and inflammation. HIV Med. 2010;11:225–31. [
PubMed: 19845792]
- 176.
Pirs
M, Jug
B, Erzen
B, Sabovic
M, Karner
P, Poljak
M
et al. Cardiovascular risk assessment in HIV-infected male patients: a comparison of Framingham, SCORE, PROCAM and DAD risk equations. Acta Dermatovenerol Alp Pannonica Adriat. 2014;23:43–7. [
PubMed: 25242159]
- 177.
Regan
S, Meigs
J, Grinspoon
SK, Triant
VA, Massaro
J, D’Agostino
R
et al. Evaluation of the ACC/AHA CVD risk prediction algorithm among HIV-infected patients. 19th Conference on Retroviruses and Opportunistic Infections, 23–26 February 2015, Seattle, WA, USA (
https://www.croiconference.org/abstract/evaluation-accaha-cvd-risk-prediction-algorithm-among-hiv-infected-patients-0, accessed 1 June 2021).
- 178.
Thompson-Paul
AM, Lichtenstein
KA, Armon
C, Palella
FJ, Jr., Skarbinski
J, Chmiel
JS
et al. Cardiovascular disease risk prediction in the HIV Outpatient Study. Clin Infect Dis. 2016;63:1508–16. [
PMC free article: PMC5624518] [
PubMed: 27613562]
- 179.
Markowicz
S, Delforge
M, Necsoi
C, De Wit
S. Cardiovascular risk evaluation of HIV-positive patients in a case-control study: comparison of the D:A:D and Framingham equations. J Int AIDS Soc. 2014;17(4 Suppl. 3):19515. [
PMC free article: PMC4224912] [
PubMed: 25394024]
- 180.
Begovac
J, Dragovic
G, Viskovic
K, Kusic
J, Perovic Mihanovic
M, Lukas
D
et al. Comparison of four international cardiovascular disease prediction models and the prevalence of eligibility for lipid lowering therapy in HIV infected patients on antiretroviral therapy. Croat Med J. 2015;56:14–23. [
PMC free article: PMC4364353] [
PubMed: 25727038]
- 181.
Friis-Moller
N, Thiebaut
R, Reiss
P, Weber
R, Monforte
AD, De Wit
S
et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. [
PubMed: 20543702]
- 182.
Friis-Moller
N, Ryom
L, Smith
C, Weber
R, Reiss
P, Dabis
F
et al. An updated prediction model of the global risk of cardiovascular disease in HIV-positive persons: the Data-collection on Adverse Effects of Anti-HIV Drugs (D:A:D) study. Eur J Prev Cardiol. 2016;23:214–23. [
PubMed: 25882821]
- 183.
Serrano-Villar
S, Estrada
V, Gomez-Garre
D, Avila
M, Fuentes-Ferrer
M, San
RJ
et al. Diagnosis of subclinical atherosclerosis in HIV-infected patients: higher accuracy of the D:A:D risk equation over Framingham and SCORE algorithms. Eur J Prev Cardiol. 2014;21:739–48. [
PubMed: 22718798]
- 184.
Wangchuk
D, Virdi
NK, Garg
R, Mendis
S, Nair
N, Wangchuk
D
et al. Package of essential noncommunicable disease (PEN) interventions in primary health-care settings of Bhutan: a performance assessment study. WHO South East Asia J Public Health. 2014;3:154–60. [
PubMed: 28607301]
- 185.
Wei
X, Zou
G, Gong
W, Yin
J, Yu
Y, Walley
J
et al. Cardiovascular disease risk reduction in rural China: a clustered randomized controlled trial in Zhejiang. Trials. 2013;14:354. [
PMC free article: PMC4015636] [
PubMed: 24160442]
- 186.
Zou
G, Zhang
Z, Walley
J, Gong
W, Yu
Y, Hu
R
et al. Use of medications and lifestyles of hypertensive patients with high risk of cardiovascular disease in rural China. PLoS One. 2015;10:e0124484. [
PMC free article: PMC4416726] [
PubMed: 25932640]
- 187.
Burkholder
GA, Tamhane
AR, Salinas
JL, Mugavero
MJ, Raper
JL, Westfall
AO
et al. Underutilization of aspirin for primary prevention of cardiovascular disease among HIV-infected patients. Clin Infect Dis. 2012;55:1550–7. [
PMC free article: PMC3491860] [
PubMed: 22942209]
- 188.
Suchindran
S, Regan
S, Meigs
JB, Grinspoon
SK, Triant
VA. Aspirin use for primary and secondary prevention in human immunodeficiency virus (HIV)-infected and HIV-uninfected patients. Open Forum Infect Dis. 2014;1:ofu076. [
PMC free article: PMC4324233] [
PubMed: 25734156]
- 189.
Pearce
D, Ani
C, Espinosa-Silva
Y, Clark
R, Fatima
K, Rahman
M
et al. Comparison of in-hospital mortality from acute myocardial infarction in HIV sero-positive versus sero-negative individuals. Am J Cardiol. 2012;110:1078–84. [
PubMed: 22762716]
- 190.
Feinstein
MJ, Achenbach
CJ, Stone
NJ, Lloyd-Jones
DM. A systematic review of the usefulness of statin therapy in HIV-infected patients. Am J Cardiol. 2015;115:1760–6. [
PubMed: 25907504]
- 191.
Tiam
A, Khonyana
J, Oyebanji
O, Ahimbisibwe
A, Pakela
R, Isavwa
A
et al. Family health days: an innovative approach to providing integrated health services for HIV and non-communicable diseases among adults and children in hard-to-reach areas of Lesotho. J Int AIDS Soc. 2012;15(Suppl. 3):18443–01.
- 192.
Chamie
G, Kwarisiima
D, Clark
TD, Kabami
J, Jain
V, Geng
E
et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS One. 2012;7:e43400. [
PMC free article: PMC3423366] [
PubMed: 22916256]
- 193.
Kotwani
P, Balzer
L, Kwarisiima
D, Clark
TD, Kabami
J, Byonanebye
D
et al. Evaluating linkage to care for hypertension after community-based screening in rural Uganda. Trop Med Int Health. 2014;19:459–68. [
PMC free article: PMC4118739] [
PubMed: 24495307]
- 194.
FHI360 fact sheet. Integration of HIV and noncommunicable disease care. Arlington (VA): FHI360; 2014
- 195.
O’Halloran
JA, Sahrmann
J, Butler
AM, Olsen
MA, Powderly
WG. Integrase strand transfer inhibitors are associated with lower risk of incident cardiovascular disease in people living with HIV. J Acquir Immune Defic Syndr. 2020;84:396–9. [
PMC free article: PMC7401319] [
PubMed: 32243280]
- 196.
- 197.
Bing
EG, Burnam
MA, Longshore
D, Fleishman
JA, Sherbourne
CD, London
AS
et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–8. [
PubMed: 11483137]
- 198.
Ciesla
JA, Roberts
JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158:725–30. [
PubMed: 11329393]
- 199.
Rabkin
JG. HIV and depression: 2008 review and update. Curr HIV/AIDS Rep. 2008;5:163–71. [
PubMed: 18838056]
- 200.
Bigna
JJ, Tounouga
DN, Kenne
AM, Djikeussi
TK, Foka
AJ, Um
LN
et al. Epidemiology of depressive disorders in people living with HIV in Africa: a systematic review and meta-analysis: burden of depression in HIV in Africa. Gen Hosp Psychiatry. 2019;57:13–22. [
PubMed: 30654293]
- 201.
- 202.
Brandt
R. The mental health of people living with HIV/AIDS in Africa: a systematic review. Afr J AIDS Res. 2009;8:123–33. [
PubMed: 25875564]
- 203.
Nakimuli-Mpungu
E, Bass
JK, Alexandre
P, Mills
EJ, Musisi
S, Ram
M
et al. Depression, alcohol use and adherence to antiretroviral therapy in sub-Saharan Africa: a systematic review. AIDS Behav. 2012;16:2101–18. [
PubMed: 22116638]
- 204.
Berg
CJ, Michelson
SE, Safren
SA. Behavioral aspects of HIV care: adherence, depression, substance use, and HIV-transmission behaviors. Infect Dis Clin North Am. 2007;21:181–200. [
PubMed: 17502235]
- 205.
Springer
SA, Dushaj
A, Azar
MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behav. 2012;16:2119–43. [
PMC free article: PMC3481055] [
PubMed: 22644066]
- 206.
Cook
JA, Grey
D, Burke
J, Cohen
MH, Gurtman
AC, Richardson
JL
et al. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94:1133–40. [
PMC free article: PMC1448411] [
PubMed: 15226133]
- 207.
- 208.
- 209.
Patel
V, Weiss
HA, Chowdhary
N, Naik
S, Pednekar
S, Chatterjee
S
et al. Effectiveness of an intervention led by lay health counsellors for depressive and anxiety disorders in primary care in Goa, India (MANAS): a cluster randomised controlled trial. Lancet. 2010;376:2086–95. [
PMC free article: PMC4964905] [
PubMed: 21159375]
- 210.
Jack
H, Wagner
RG, Petersen
I, Thom
R, Newton
CR, Stein
A
et al. Closing the mental health treatment gap in South Africa: a review of costs and cost-effectiveness. Glob Health Action. 2014;7:23431. [
PMC free article: PMC4038770] [
PubMed: 24848654]
- 211.
Wagner
GJ, Ngo
V, Glick
P, Obuku
EA, Musisi
S, Akena
D. INtegration of DEPression Treatment into HIV Care in Uganda (INDEPTH-Uganda): study protocol for a randomized controlled trial. Trials. 2014;15:248. [
PMC free article: PMC4083331] [
PubMed: 24962086]
- 212.
Weaver
MR, Conover
CJ, Proescholdbell
RJ, Arno
PS, Ang
A, Uldall
KK
et al. Costeffectiveness analysis of integrated care for people with HIV, chronic mental illness and substance abuse disorders. J Ment Health Policy Econ. 2009;12:33–46. [
PubMed: 19346565]
- 213.
- 214.
- 215.
Guidelines: prevention and treatment of HIV and other sexually transmitted infections among men who have sex with men and transgender populations: recommendations for a public health approach. World Health Organization: 2011 (
https://apps.who.int/iris/handle/10665/44619, accessed 1 June 2021). [
PubMed: 26158187]
- 216.
- 217.
- 218.
Guidelines for the management of symptomatic sexually transmitted infections. Geneva: World Health Organization; in press.
- 219.
Singer
M, Bulled
N, Ostrach
B, Mendenhall
E. Syndemics and the biosocial conception of health. Lancet. 2017;389:941–50. [
PubMed: 28271845]
- 220.
Detels
R, Green
AM, Klausner
JD, Katzenstein
D, Gaydos
C, Handsfield
H
et al. The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex Transm Dis. 2011;38:503–9. [
PMC free article: PMC3408314] [
PubMed: 22256336]
- 221.
Fleming
DT, Wasserheit
JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. [
PMC free article: PMC1758168] [
PubMed: 10448335]
- 222.
Sexton
J, Garnett
G, Rottingen
JA. Metaanalysis and metaregression in interpreting study variability in the impact of sexually transmitted diseases on susceptibility to HIV infection. Sex Transm Dis. 2005;32:351–7. [
PubMed: 15912081]
- 223.
Cohen
MS. Sexually transmitted diseases enhance HIV transmission: no longer a hypothesis. Lancet. 1998;351(Suppl. 3):5–7. [
PubMed: 9652712]
- 224.
Looker
KJ, Elmes
JAR, Gottlieb
SL, Schiffer
JT, Vickerman
P, Turner
KME
et al. Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. Lancet Infect Dis. 2017;17:1303–16. [
PMC free article: PMC5700807] [
PubMed: 28843576]
- 225.
Glynn
JR, Biraro
S, Weiss
HA. Herpes simplex virus type 2: a key role in HIV incidence. AIDS. 2009;23:1595–8. [
PubMed: 19512858]
- 226.
Naresh
A, Beigi
R, Woc-Colburn
L, Salata
RA. The bidirectional interactions of human immunodeficiency virus-1 and sexually transmitted infections: a review. Infect Dis Clin Pract. 2009;17:362–73.
- 227.
Massad
LS, Xie
X, Burk
RD, D’Souza
G, Darragh
TM, Minkoff
H
et al. Association of cervical precancer with human papillomavirus types other than 16 among HIV coinfected women. Am J Obstet Gynecol. 2016;214:354 e1–6. [
PMC free article: PMC4775397] [
PubMed: 26433170]
- 228.
Cohen
MS. Classical sexually transmitted diseases drive the spread of HIV-1: back to the future. J Infect Dis. 2012;206:1–2. [
PubMed: 22517911]
- 229.
Kalichman
SC, Pellowski
J, Turner
C. Prevalence of sexually transmitted coinfections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect. 2011;87:183–90. [
PMC free article: PMC4317792] [
PubMed: 21330572]
- 230.
Jones
J, Weiss
K, Mermin
J, Dietz
P, Rosenberg
ES, Gift
TL
et al. Proportion of incident human immunodeficiency virus cases among men who have sex with men attributable to gonorrhea and chlamydia: a modeling analysis. Sex Transm Dis. 2019;46:357–63. [
PMC free article: PMC6530490] [
PubMed: 31095100]
- 231.
Ong
JJ, Baggaley
RC, Wi
TE, Tucker
JD, Fu
H, Smith
MK
et al. Global epidemiologic characteristics of sexually transmitted infections among individuals using preexposure prophylaxis for the prevention of HIV infection: a systematic review and meta-analysis. JAMA Netw Open. 2019;2:e1917134. [
PMC free article: PMC6991203] [
PubMed: 31825501]
- 232.
Bertagnolio
S, Hermans
L, Jordan
MR, Avila-Rios
S, Iwuji
C, Derache
A
et al. Clinical impact of pretreatment human immunodeficiency virus drug resistance in people initiating nonnucleoside reverse transcriptase inhibitor–containing antiretroviral therapy: a systematic review and meta-analysis. J Infect Dis. 2020;jiaa683. [
PMC free article: PMC8328216] [
PubMed: 33202025]
- 233.
- 234.
- 235.
Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Atlanta: United States Centers for Disease Control and Prevention; 2021 (
http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf, accessed 1 June 2021).
- 236.
Siberry
GK, Abzug
MJ, Nachman
S, Brady
MT, Dominguez
KL, Handelsman
E
et al. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 2013;32(Suppl. 2(0 2)):i–KK4. [
PMC free article: PMC4169043] [
PubMed: 24569199]
- 237.
Geretti
AM, Committee
BIW, Brook
G, Cameron
C, Chadwick
D, Heyderman
RS
et al. British HIV Association guidelines for immunization of HIV-infected adults 2008. HIV Med. 2008;9:795–848. [
PubMed: 18983477]
- 238.
Kolber
MA, Gabr
AH, De La Rosa
A, Glock
JA, Jayaweera
D, Miller
N
et al. Genotypic analysis of plasma HIV-1 RNA after influenza vaccination of patients with previously undetectable viral loads. AIDS. 2002;16:537–42. [
PubMed: 11872996]
- 239.
Lee
PK, Kieffer
TL, Siliciano
RF, Nettles
RE. HIV-1 viral load blips are of limited clinical significance. J Antimicrob Chemother. 2006;57:803–5. [
PubMed: 16533823]
- 240.
- 241.
- 242.
- 243.
- 244.
- 245.
- 246.
- 247.
- 248.
- 249.
- 250.
- 251.
World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age: WHO position paper – February 2019. Wkly Epidemiol Rec. 2019;94:85–104 (
https://apps.who.int/iris/handle/10665/310968, accessed 1 June 2021).
- 252.
- 253.
- 254.
- 255.
Kampiire
L, Archary
M, Elias
L, Marti
M, Penazzato
M, Brusamento
S. Immunization for children living with HIV: a scoping review. In preparation.
- 256.
Osei-Sekyere
B, Karstaedt
AS. Immune reconstitution inflammatory syndrome involving the skin. Clin Exp Dermatol. 2010;35:477–81. [
PubMed: 19874370]
- 257.
- 258.
- 259.
Nutrition counselling, care and support for HIV-infected women: guidelines on HIV-related care, treatment and support for HIV-infected women and their children in resource-limited settings. Geneva: World Health Organization; 2005 (
https://apps.who.int/iris/handle/10665/43023, accessed 1 June 2021).
- 260.
- 261.
Paton
NI, Sangeetha
S, Earnest
A, Bellamy
R. The impact of malnutrition on survival and the CD4 count response in HIV-infected patients starting antiretroviral therapy. HIV Med. 2006;7:323–30. [
PubMed: 16945078]
- 262.
van der Sande
MA, Schim van der Loeff
MF, Aveika
AA, Sabally
S, Togun
T, Sarge-Njie
R
et al. Body mass index at time of HIV diagnosis: a strong and independent predictor of survival. J Acquir Immune Defic Syndr. 2004;37:1288–94. [
PubMed: 15385737]
- 263.
- 264.
WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva: World Health Organization; 2006 (
https://apps.who.int/iris/handle/10665/43413, accessed 1 June 2021).
- 265.
- 266.
- 267.
- 268.
- 269.
Harding
R. Palliative care as an essential component of the HIV care continuum. Lancet HIV. 2018;5:e524–30. [
PubMed: 30025682]
- 270.
Giusti
A, Nkhoma
K, Petrus
R, Petersen
I, Gwyther
L, Farrant
L
et al. The empirical evidence underpinning the concept and practice of person-centred care for serious illness: a systematic review. BMJ Glob Health. 2020;5. [
PMC free article: PMC7733074] [
PubMed: 33303515]
- 271.
Global atlas of palliative care. London: WHPCA/WHO: 2020.
- 272.
- 273.
Knaul
FM, Farmer
PE, Krakauer
EL, De Lima
L, Bhadelia
A, Jiang Kwete
X
et al. Alleviating the access abyss in palliative care and pain relief – an imperative of universal health coverage: the Lancet Commission report. Lancet. 2018;391:1391–454. [
PubMed: 29032993]
- 274.
Lowther
K, Selman
L, Harding
R, Higginson
IJ. Experience of persistent psychological symptoms and perceived stigma among people with HIV on antiretroviral therapy (ART): a systematic review. Int J Nurs Stud. 2014;51:1171–89. [
PubMed: 24602830]
- 275.
Miners
A, Phillips
A, Kreif
N, Rodger
A, Speakman
A, Fisher
M
et al. Health-related quality-of-life of people with HIV in the era of combination antiretroviral treatment: a cross-sectional comparison with the general population. Lancet HIV. 2014;1:e32–40. [
PubMed: 26423814]
- 276.
Moens
K, Higginson
IJ, Harding
R, EURO IMPACT. Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage. 2014;48:18. [
PubMed: 24801658]
- 277.
Baker
V, Nkhoma
K, Trevelion
R, Roach
A, Winston
A, Sabin
C
et al. “I have failed to separate my HIV from this pain”: the challenge of managing chronic pain among people with HIV. AIDS Care. 2021:1–9. [
PubMed: 33443450]
- 278.
Sherr
L, Lampe
F, Norwood
S, Date
HL, Harding
R, Johnson
M
et al. Adherence to antiretroviral treatment in patients with HIV in the UK: a study of complexity. AIDS Care. 2008;20:442–8. [
PubMed: 18449821]
- 279.
Clucas
C, Harding
R, Lampe
FC, Anderson
J, Date
HL, Johnson
M
et al. Doctor-patient concordance during HIV treatment switching decision-making. HIV Med. 2011;12:87–96. [
PubMed: 20561081]
- 280.
Lampe
FC, Harding
R, Smith
CJ, Phillips
AN, Johnson
M, Sherr
L. Physical and psychological symptoms and risk of virologic rebound among patients with virologic suppression on antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;54:500–5. [
PubMed: 20150819]
- 281.
Harding
R, Clucas
C, Lampe
FC, Leake-Date
H, Fisher
M, Johnson
M
et al. What factors are associated with patient self-reported health status among HIV outpatients? A multicentre UK study of biomedical and psychosocial factors. AIDS Care. 2012;24:963–71. [
PubMed: 22519889]
- 282.
Sabin
CA, Harding
R, Bagkeris
E, Nkhoma
K, Post
FA, Sachikonye
M
et al. Pain in people living with HIV and its association with healthcare resource use, well being and functional status. AIDS. 2018;32:2697–706. [
PubMed: 30289809]
- 283.
Selman
LE, Higginson
IJ, Agupio
G, Dinat
N, Downing
J, Gwyther
L
et al. Quality of life among patients receiving palliative care in South Africa and Uganda: a multi-centred study. Health Qual Life Outcomes. 2011;9:21. [
PMC free article: PMC3094195] [
PubMed: 21477274]
- 284.
Selman
L, Harding
R, Higginson
I, Gysels
M, Speck
P, Encompass-Collaborative. Spiritual wellbeing in sub-Saharan Africa: the meaning and prevalence of “feeling at peace”. BMJ Support Palliat Care. 2011;1(Suppl. 1):A22.
- 285.
- 286.
Namisango
E, Bristowe
K, Allsop
MJ, Murtagh
FEM, Abas
M, Higginson
IJ
et al. Symptoms and concerns among children and young people with life-limiting and life-threatening conditions: a systematic review highlighting meaningful health outcomes. Patient. 2019;12:15–55. [
PubMed: 30361884]
- 287.
Afolabi
O, Abboah-Offei
M, Namisango
E, Chukwusa
E, Oluyase
A, Luyirika
E
et al. Do the clinical management guidelines for Covid-19 in African countries reflect the African quality palliative care standards? A review of current guidelines. J Pain Symptom Manage. 2021;61:e17–23. [
PMC free article: PMC7894087] [
PubMed: 33617951]
- 288.
Namisango
E, Bristowe
K, Allsop
MJ, Murtagh
FEM, Abas
M, Higginson
IJ
et al. Symptoms and concerns among children and young people with life-limiting and life-threatening conditions: a systematic review highlighting meaningful health outcomes. Patient. 2019;12:15–55. [
PubMed: 30361884]
- 289.
Frigati
LJ, Ameyan
W, Cotton
MF, Gregson
CL, Hoare
J, Jao
J
et al. Chronic comorbidities in children and adolescents with perinatally acquired HIV infection in sub-Saharan Africa in the era of antiretroviral therapy. Lancet Child Adolesc Health. 2020;4:688–98. [
PubMed: 32359507]
- 290.
Sleeman
KE, de Brito
M, Etkind
S, Nkhoma
K, Guo
P, Higginson
IJ
et al. The escalating global burden of serious health-related suffering: projections to 2060 by world regions, age groups, and health conditions. Lancet Glob Health. 2019;7:e883–92. [
PMC free article: PMC6560023] [
PubMed: 31129125]
- 291.
Smit
M, Brinkman
K, Geerlings
S, Smit
C, Thyagarajan
K, Sighem
A
et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15:810–8. [
PMC free article: PMC4528076] [
PubMed: 26070969]
- 292.
Croxford
S, Kitching
A, Desai
S, Kall
M, Edelstein
M, Skingsley
A
et al. Mortality and causes of death in people diagnosed with HIV in the era of highly active antiretroviral therapy compared with the general population: an analysis of a national observational cohort. Lancet Public Health. 2017;2:e35–46. [
PubMed: 29249478]
- 293.
Sohn
AH, Lumbiganon
P, Kurniati
N, Lapphra
K, Law
M, Do
VC
et al. Determining standardized causes of death of infants, children, and adolescents living with HIV in Asia. AIDS. 2020;34:152737. [
PMC free article: PMC7487212] [
PubMed: 32443064]
- 294.
- 295.
Gwyther
L, Brennan
F, Harding
R. Advancing palliative care as a human right. J Pain Symptom Manage. 2009;38:767–74. [
PubMed: 19783399]
- 296.
- 297.
- 298.
Downing
J, Gomes
B, Gikaara
N, Munene
G, Daveson
BA, Powell
RA
et al. Public preferences and priorities for end-of-life care in Kenya: a population-based street survey. BMC Palliat Care. 2014;13:4. [
PMC free article: PMC3936799] [
PubMed: 24529217]
- 299.
Powell
RA, Namisango
E, Gikaara
N, Moyo
S, Mwangi-Powell
FN, Gomes
B
et al. Public priorities and preferences for end-of-life care in Namibia. J Pain Symptom Manage. 2014;47:620–30. [
PubMed: 23870841]
- 300.
Gomes
B, Higginson
IJ, Calanzani
N, Cohen
J, Deliens
L, Daveson
BA
et al. Preferences for place of death if faced with advanced cancer: a population survey in England, Flanders, Germany, Italy, the Netherlands, Portugal and Spain. Ann Oncol. 2012;23:2006–15. [
PubMed: 22345118]
- 301.
Harding
R, Marchetti
S, Onwuteaka-Philipsen
BD, Wilson
DM, Ruiz-Ramos
M, Cardenas-Turanzas
M
et al. Place of death for people with HIV: a population-level comparison of eleven countries across three continents using death certificate data. BMC Infect Dis. 2018;18:55. [
PMC free article: PMC5785855] [
PubMed: 29370765]
- 302.
Harding
R, Epiphaniou
E, Chidgey-Clark
J. Needs, experiences, and preferences of sexual minorities for end-of-life care and palliative care: a systematic review. J Palliat Med. 2012;15:602–11. [
PubMed: 22401314]
- 303.
Bristowe
K, Hodson
M, Wee
B, Almack
K, Johnson
K, Daveson
BA
et al. Recommendations to reduce inequalities for LGBT people facing advanced illness: ACCESSCare national qualitative interview study. Palliat Med. 2018;32:23–35. [
PMC free article: PMC5758934] [
PubMed: 28502218]
- 304.
Bristowe
K, Marshall
S, Harding
R. The bereavement experiences of lesbian, gay, bisexual and/or trans* people who have lost a partner: a systematic review, thematic synthesis and modelling of the literature. Palliat Med. 2016;30:730–44. [
PMC free article: PMC4984311] [
PubMed: 26944532]
- 305.
Hunt
J, Bristowe
K, Chidyamatare
S, Harding
R. “So isolation comes in, discrimination and you find many people dying quietly without any family support”: accessing palliative care for key populations – an in-depth qualitative study. Palliat Med. 2019;33:685–92. [
PMC free article: PMC6535799] [
PubMed: 30859906]
- 306.
Hunt
J, Bristowe
K, Chidyamatare
S, Harding
R. “They will be afraid to touch you”: LGBTI people and sex workers’ experiences of accessing healthcare in Zimbabwe-an in-depth qualitative study. BMJ Glob Health. 2017;2:e000168. [
PMC free article: PMC5435254] [
PubMed: 28589012]
- 307.
Namisango
E, Bristowe
K, Murtagh
FE, Downing
J, Powell
RA, Abas
M
et al. Towards person-centred quality care for children with life-limiting and life-threatening illness: self-reported symptoms, concerns and priority outcomes from a multi-country qualitative study. Palliat Med. 2020;34:319–35. [
PubMed: 32081084]
- 308.
Harding
R, Karus
D, Easterbrook
P, Raveis
V, Higginson
I, Marconi
K. Does palliative care improve outcomes for patients with HIV/AIDS? A systematic review of the evidence. Sex Transm Infect. 2005;81:5–14. [
PMC free article: PMC1763726] [
PubMed: 15681714]
- 309.
Lowther
K, Harding
R, Simms
V, Ahmed
A, Ali
Z, Gikaara
N
et al. Active ingredients of a person-centred intervention for people on HIV treatment: analysis of mixed methods trial data. BMC Infect Dis. 2018;18:27. [
PMC free article: PMC5761128] [
PubMed: 29316883]
- 310.
Lowther
K, Harding
R, Simms
V, Gikaara
N, Ahmed
A, Ali
Z
et al. Effect of participation in a randomised controlled trial of an integrated palliative care intervention on HIV-associated stigma. AIDS Care. 2018;30:1180–8. [
PubMed: 29663828]
- 311.
Lowther
K, Harding
R, Ahmed
A, Gikaara
N, Ali
Z, Kariuki
H
et al. Conducting experimental research in marginalised populations: clinical and methodological implications from a mixed-methods randomised controlled trial in Kenya. AIDS Care. 2016;28(Suppl. 1):60–3. [
PMC free article: PMC4828598] [
PubMed: 26916738]
- 312.
Lowther
K, Selman
L, Simms
V, Gikaara
N, Ahmed
A, Ali
Z
et al. Nurse-led palliative care for HIV-positive patients taking antiretroviral therapy in Kenya: a randomised controlled trial. Lancet HIV. 2015;2:e328–34. [
PubMed: 26423375]
- 313.
- 314.
Reid
EA, Kovalerchik
O, Jubanyik
K, Brown
S, Hersey
D, Grant
L. Is palliative care costeffective in low-income and middle-income countries? A mixed-methods systematic review. BMJ Support Palliat Care. 2019;9:120–9. [
PubMed: 30274970]
- 315.
Desrosiers
T, Cupido
C, Pitout
E, van Niekerk
L, Badri
M, Gwyther
L
et al. A hospital-based palliative care service for patients with advanced organ failure in sub-Saharan Africa reduces admissions and increases home death rates. J Pain Symptom Manage. 2014;47:786–92. [
PubMed: 23969328]
- 316.
Integrating palliative care and symptom relief into primary health care: a WHO guide for planners, implementers and managers. Geneva: World Health Organization; 2018 (
https://apps.who.int/iris/handle/10665/274559, accessed 1 June 2021).
- 317.
Krakauer
EL, Kwete
X, Verguet
S, Arreola-Ornelas
H, Bhadelia
A, Mendez
O
et al. Palliative care and pain control. In: Jamison
DT, Gelband
H, Horton
S, Jha
P, Laxminarayan
R, Mock
CN
et al., editors. Disease control priorities: improving health and reducing poverty. Washington (DC): World Bank; 2017;9:235–46.
- 318.
- 319.
- 320.
Strengthening of palliative care as a component of comprehensive care throughout the life course: report by the Secretariat. Sixty-Seventh World Health Assembly. Geneva: World Health Organization; 2014 (
https://apps.who.int/iris/handle/10665/158962, accessed 1 June 2021).
- 321.
- 322.
Nkhoma
K, Norton
C, Sabin
C, Winston
A, Merlin
J, Harding
R. Self-management interventions for pain and physical symptoms among people living with HIV: a systematic review of the evidence. J Acquir Immune Defic Syndr. 2018;79:206–25. [
PubMed: 30212435]
- 323.
Harding
R, Carrasco
JM, Serrano-Pons
J, Lemaire
J, Namisango
E, Luyirika
E
et al. Design and evaluation of a novel mobile phone application to improve palliative home-care in resource-limited settings. J Pain Symptom Manage. 2021;62:1–9. [
PubMed: 33246073]
- 324.
Abboah-Offei
M, Bristowe
K, Harding
R. Are patient outcomes improved by models of professionally-led community HIV management which aim to be person-centred? A systematic review of the evidence. AIDS Care. 2020:1–11. [
PubMed: 32741201]
- 325.
Bamberger
J. Reducing homelessness by embracing housing as a Medicaid benefit. JAMA Intern Med. 2016;176:1051–2. [
PubMed: 27322820]
- 326.
Carrillo
JE, Carrillo
VA, Perez
HR, Salas-Lopez
D, Natale-Pereira
A, Byron
AT. Defining and targeting health care access barriers. J Health Care Poor Underserved. 2011;22:562–75. [
PubMed: 21551934]
- 327.
- 328.
Slogrove
AL, Powis
KM, Johnson
LF, Stover
J, Mahy
M. Estimates of the global population of children who are HIV-exposed and uninfected, 2000–18: a modelling study. Lancet Glob Health. 2020;8:e67–75. [
PMC free article: PMC6981259] [
PubMed: 31791800]
- 329.
Black
MM, Walker
SP, Fernald
LCH, Andersen
CT, DiGirolamo
AM, Lu
C
et al. Early childhood development coming of age: science through the life course. Lancet. 2017;389:77–90. [
PMC free article: PMC5884058] [
PubMed: 27717614]
- 330.
le Roux
SM, Abrams
EJ, Donald
KA, Brittain
K, Phillips
TK, Nguyen
KK
et al. Growth trajectories of breastfed HIV-exposed uninfected and HIV-unexposed children under conditions of universal maternal antiretroviral therapy: a prospective study. Lancet Child Adolesc Health. 2019;3:234–44. [
PubMed: 30773459]
- 331.
le Roux
SM, Abrams
EJ, Donald
KA, Brittain
K, Phillips
TK, Zerbe
A
et al. Infectious morbidity of breastfed, HIV-exposed uninfected infants under conditions of universal antiretroviral therapy in South Africa: a prospective cohort study. Lancet Child Adolesc Health. 2020;4:220–31. [
PMC free article: PMC7235356] [
PubMed: 31932246]
- 332.
- 333.
- 334.
- 335.
Frigati
LJ, Ameyan
W, Cotton
MF, Gregson
CL, Hoare
J, Jao
J
et al. Chronic comorbidities in children and adolescents with perinatally acquired HIV infection in sub-Saharan Africa in the era of antiretroviral therapy. Lancet Child Adolesc Health. 2020;4:688–98. [
PubMed: 32359507]
- 336.
Bartlett
AW, Mohamed
TJ, Sudjaritruk
T, Kurniati
N, Nallusamy
R, Hansudewechakul
R
et al. Disease-and treatment-related morbidity in adolescents with perinatal HIV infection in Asia. Pediatr Infect Dis J. 2019;38:287–92. [
PMC free article: PMC6375771] [
PubMed: 30281549]
- 337.
Feucht
UD, Van Bruwaene
L, Becker
PJ, Kruger
M. Growth in HIV-infected children on long-term antiretroviral therapy. Trop Med Int Health. 2016;21:619–29. [
PubMed: 26914715]
- 338.
Jesson
J, Koumakpaï
S, Diagne
NR, Amorissani-Folquet
M, Aka
A, Lawson-Evi
K
et al. Effect of age at antiretroviral therapy initiation on catch-up growth within the first 24 months among HIV-infected children in the IeDEA West African Pediatric Cohort. Pediatr Infect Dis J. 2015;34:e159. [
PMC free article: PMC4466006] [
PubMed: 25955835]
- 339.
- 340.
Gregson
CL, Hartley
A, Majonga
E, McHugh
G, Crabtree
N, Rukuni
R
et al. Older age at initiation of antiretroviral therapy predicts low bone mineral density in children with perinatally-infected HIV in Zimbabwe. Bone. 2019;125:96–102. [
PMC free article: PMC6599174] [
PubMed: 31082498]
- 341.
- 342.
Attia
EF, Obimbo
EM, West
TE, Ndukwe-Wambutsi
L, Kiptinness
C, Cagle
A
et al. Adolescent age is an independent risk factor for abnormal spirometry among people living with HIV in Kenya. AIDS. 2018;32:1353. [
PMC free article: PMC6834296] [
PubMed: 29794491]
- 343.
Arigliani
M, Canciani
MC, Mottini
G, Altomare
M, Magnolato
A, Loa Clemente
SV
et al. Evaluation of the Global Lung Initiative 2012 reference values for spirometry in African children. Am J Respir Crit Care Med. 2017;195:229–36. [
PubMed: 27564235]
- 344.
Githinji
LN, Gray
DM, Hlengwa
S, Myer
L, Zar
HJ. Lung function in South African adolescents infected perinatally with HIV and treated long-term with antiretroviral therapy. Ann Am Thorac Soc. 2017;14:722–9. [
PMC free article: PMC5427744] [
PubMed: 28248548]
- 345.
Crowell
CS, Malee
KM, Yogev
R, Muller
WJ. Neurologic disease in HIV-infected children and the impact of combination antiretroviral therapy. Rev Med Virol. 2014;24:316–31. [
PubMed: 24806816]
- 346.
Phillips
NJ, Thomas
KG, Myer
L, Sacktor
N, Zar
HJ, Stein
DJ
et al. Screening for HIV-associated neurocognitive disorders in perinatally infected adolescents: youthInternational HIV Dementia Scale validation. AIDS. 2019;33:815–24. [
PubMed: 30649059]
- 347.
Kinyanda
E, Salisbury
TT, Levin
J, Nakasujja
N, Mpango
RS, Abbo
C
et al. Rates, types and co-occurrence of emotional and behavioural disorders among perinatally HIV-infected youth in Uganda: the CHAKA study. Soc Psychiatry Psychiatr Epidemiol. 209;54:415–25. [
PubMed: 30788554]
- 348.
Hoare
J, Phillips
N, Brittain
K, Myer
L, Zar
HJ, Stein
DJ. Mental health and functional competence in the Cape Town Adolescent Antiretroviral Cohort. J Acquir Immune Defic Syndr. 2019;81:e109–16. [
PMC free article: PMC6597184] [
PubMed: 31241543]
- 349.
- 350.
- 351.
Bohlius
J, Maxwell
N, Spoerri
A, Wainwright
R, Sawry
S, Poole
J
et al. Incidence of AIDS-defining and other cancers in HIV-positive children in South Africa: record linkage study. Pediatr Infect Dis J. 2016;35:e164. [
PMC free article: PMC4865449] [
PubMed: 26906162]
- 352.
- 353.
Oni
T, Youngblood
E, Boulle
A, McGrath
N, Wilkinson
RJ, Levitt
NS. Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa – a cross sectional study. BMC Infect Dis. 2015;15:20. [
PMC free article: PMC4300166] [
PubMed: 25595711]
- 354.
Njuguna
I, Beima-Sofie
K, Mburu
C, Black
D, Evans
Y, Guthrie
B
et al. What happens at adolescent and young adult HIV clinics? A national survey of models of care, transition and disclosure practices in Kenya. Trop Med Int Health. 2020;25:558–65. [
PMC free article: PMC7274774] [
PubMed: 31984597]
- 1
Site-specific cancers: bladder, breast, colon, endometrial, oesophageal adenocarcinoma, gastric and renal.
- 2
A person living with HIV is classified as seriously ill if one or more of the following danger signs are present: unable to walk unaided; respiratory rate over 30 per minute; fever of more than 39°C; or pulse rate exceeding 120 per minute.