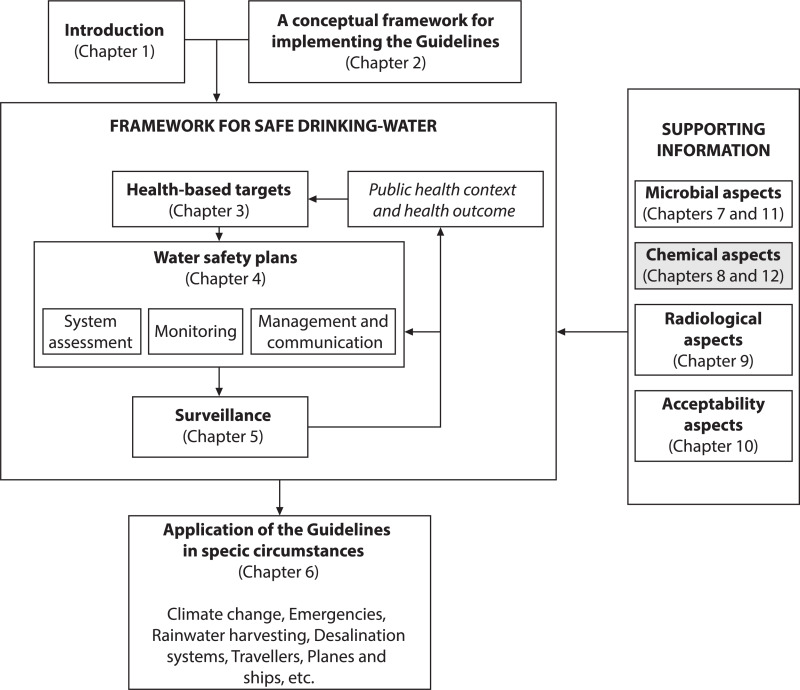
12.1. Chemical contaminants in drinking-water
Acrylamide
Residual acrylamide monomer occurs in polyacrylamide coagulants used in the treatment of drinking-water. In general, the maximum authorized dose of polymer is 1 mg/l. At a monomer content of 0.05%, this corresponds to a maximum theoretical concentration of 0.5 µg/l of the monomer in water. Practical concentrations may be lower by a factor of 2–3. This applies to the anionic and non-ionic polyacrylamides, but residual levels from cationic polyacrylamides may be higher. Polyacrylamides are also used as grouting agents in the construction of drinking-water reservoirs and wells. Human exposure
View in own window
| Guideline value | 0.0005 mg/l (0.5 µg/l) |
|---|
| Occurrence | Concentrations up to a few micrograms per litre occasionally detected in tap water |
|---|
| Basis of guideline value derivation | Combined mammary, thyroid and uterine tumours observed in female rats in a drinking-water study, and using the linearized multistage model |
|---|
| Limit of detection | 0.032 µg/l by gas chromatography (GC); 0.2 µg/l by high-performance liquid chromatography (HPLC); 10 µg/l by HPLC with ultraviolet (UV) detection |
|---|
| Treatment performance | Conventional treatment processes do not remove acrylamide. Acrylamide concentrations in drinking-water are usually controlled by limiting either the acrylamide content of polyacrylamide flocculants or the dose used, or both. Advances in analytical techniques are also beginning to allow control by direct measurement (see background document). |
|---|
| Additional comments | Every effort should be made to limit free acrylamide monomer in polyacrylamide used for water treatment, and water suppliers should also make every effort to ensure that residual acrylamide in drinking-water is kept as low as is technically feasible. In particular, if acrylamide is controlled by limiting the amount dosed, overdosing should always be avoided. |
|---|
| Assessment date | 2011 |
|---|
| Principal references | FAO/WHO (2011)
Evaluation of certain contaminants in food WHO (2011)
Acrylamide in drinking-water |
|---|
is much greater from food than from drinking-water, owing to the formation of acrylamide in foods (e.g. breads, fried and roasted foods) cooked at high temperatures.
Following ingestion, acrylamide is readily absorbed from the gastrointestinal tract and widely distributed in body fluids. Acrylamide can cross the placenta. It is neurotoxic, affects germ cells and impairs reproductive function. In mutagenicity assays, acrylamide was negative in the Ames test but induced gene mutations in mammalian cells and chromosomal aberrations in vitro and in vivo. In a long-term carcinogenicity study in rats exposed via drinking-water, acrylamide induced scrotal, thyroid and adrenal tumours in males and mammary, thyroid and uterine tumours in females. The International Agency for Research on Cancer (IARC) has placed acrylamide in Group 2A (probably carcinogenic to humans). The Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Committee on Food Additives (JECFA) has recently noted concerns regarding the carcinogenicity and neurotoxicity of acrylamide and concluded that dietary exposure should be reduced to as low a level as technically achievable. Recent data have shown that exposure to acrylamide from cooked food is much higher than previously thought. As it is difficult to control the intake of acrylamide from food, it is very important that the acrylamide content of polyacrylamide used as a coagulant aid in water treatment, the most important source of drinking-water contamination by acrylamide, be as low as possible and that polyacrylamide not be overdosed in an attempt to take a shortcut to improving coagulation.
Alachlor
Alachlor (Chemical Abstracts Service [CAS] No. 15972-60-8) is a pre-emergence and post-emergence herbicide used to control annual grasses and many broad-leaved weeds in maize and a number of other crops. It is lost from soil mainly through volatilization, photodegradation and biodegradation. Many alachlor degradation products have been identified in soil. Alachlor was included in the Prior Informed Consent procedure of the Rotterdam Convention on the basis of the final regulatory actions taken by the European Community and by Canada to ban alachlor as a pesticide.
View in own window
| Guideline value | 0.02 mg/l (20 µg/l) |
|---|
| Occurrence | Has been detected in groundwater and surface water; has also been detected in drinking-water at levels below 0.002 mg/l |
|---|
| Basis of guideline value derivation | Calculated by applying the linearized multistage model to data on the incidence of nasal tumours in rats |
|---|
| Limit of detection | 0.1 µg/l by gas–liquid chromatography with electrolytic conductivity detection in the nitrogen mode or by capillary column GC with a nitrogen–phosphorus detector |
|---|
| Treatment performance | 0.001 mg/l should be achievable using granular activated carbon (GAC) |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Alachlor in drinking-water |
|---|
On the basis of available experimental data, evidence for the genotoxicity of alachlor is considered to be equivocal. However, a metabolite of alachlor, 2,6-diethylaniline, has been shown to be mutagenic. Available data from two studies in rats clearly indicate that alachlor is carcinogenic, causing benign and malignant tumours of the nasal turbinate, malignant stomach tumours and benign thyroid tumours.
Aldicarb
Aldicarb (CAS No. 116-06-3) is a systemic pesticide used to control nematodes in soil and insects and mites on a variety of crops. It is very soluble in water and highly mobile in soil. It degrades mainly by biodegradation and hydrolysis, persisting for weeks to months.
View in own window
| Guideline value | 0.01 mg/l (10 µg/l) |
|---|
| Occurrence | Frequently found as a contaminant in groundwater in the vicinity of application areas, particularly when associated with sandy soil; concentrations in well water as high as 500 µg/l have been measured; aldicarb sulfoxide and aldicarb sulfone residues are found in an approximately 1:1 ratio in groundwater |
|---|
| Acceptable daily intake (ADI) | 0–0.003 mg/kg body weight based on cholinesterase depression in a single oral dose study in human volunteers |
|---|
| Limit of detection | 0.001 mg/l by reversed-phase HPLC with fluorescence detection |
|---|
| Treatment performance | 0.001 mg/l should be achievable using GAC or ozonation |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of upper limit of ADI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The guideline value derived from the 1992 assessment of the Joint FAO/WHO Meeting on Pesticide Residues (JMPR) was very similar to the guideline value derived in the second edition, which was therefore retained. |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (1993)
Pesticide residues in food—1992 evaluations
WHO (2003)
Aldicarb in drinking-water
|
|---|
Aldicarb is one of the most acutely toxic pesticides in use, although the only consistently observed toxic effect with both long-term and single-dose administration is acetylcholinesterase inhibition. It is converted to the sulfoxide and sulfone. Aldicarb sulfoxide is a more potent inhibitor of acetylcholinesterase than aldicarb itself, whereas aldicarb sulfone is considerably less toxic than either aldicarb or the sulfoxide. The weight of evidence indicates that aldicarb, aldicarb sulfoxide and aldicarb sulfone are not genotoxic or carcinogenic. IARC has concluded that aldicarb is not classifiable as to its carcinogenicity (Group 3).
Aldrin and dieldrin
Aldrin (CAS No. 309-00-2) and dieldrin (CAS No. 60-57-1) are chlorinated pesticides that are used against soil-dwelling pests, for wood protection and, in the case of dieldrin, against insects of public health importance. Since the early 1970s, many countries have either severely restricted or banned the use of both compounds, particularly in agriculture. The two compounds are closely related with respect to their toxicology and mode of action. Aldrin is rapidly converted to dieldrin under most environmental conditions and in the body. Dieldrin is a highly persistent organochlorine compound that has low mobility in soil, can be lost to the atmosphere and bioaccumulates. Dietary exposure to aldrin/dieldrin is very low and decreasing.
View in own window
| Guideline value | Aldrin and dieldrin (combined): 0.000 03 mg/l (0.03 µg/l) |
|---|
| Occurrence | Seldom detected in drinking water; concentrations of aldrin and dieldrin in drinking-water normally less than 0.01 µg/l; rarely present in groundwater |
|---|
| Provisional tolerable daily intake (PTDI) | 0.1 µg/kg body weight (combined total for aldrin and dieldrin), based on no-observed-adverse-effect levels (NOAELs) of 1 mg/kg diet in the dog and 0.5 mg/kg diet in the rat, which are equivalent to 0.025 mg/kg body weight per day in both species, and applying an uncertainty factor of 250 based on concern about carcinogenicity observed in mice |
|---|
| Limit of detection | 0.003 µg/l for aldrin and 0.002 µg/l for dieldrin by GC with electron capture detector (ECD) |
|---|
| Treatment performance | 0.02 µg/l should be achievable using coagulation, GAC or ozonation |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 1% of PTDI (In view of the reduction in exposure from food this value is probably very conservative.) |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Aldrin and dieldrin are listed under the Stockholm Convention on Persistent Organic Pollutants. Hence, monitoring may occur in addition to that required by drinking-water guidelines. |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (1995)
Pesticide residues in food—1994 evaluations
WHO (2003)
Aldrin and dieldrin in drinking-water
|
|---|
Both compounds are highly toxic in experimental animals, and cases of poisoning in humans have occurred. Aldrin and dieldrin have more than one mechanism of toxicity. The target organs are the central nervous system and the liver. In long-term studies, dieldrin was shown to produce liver tumours in both sexes of two strains of mice. It did not produce an increase in tumours in rats and does not appear to be genotoxic. IARC has classified aldrin and dieldrin in Group 3 (not classifiable as to its carcinogenicity to humans). Exposure through food has decreased significantly with the dramatic reduction in use.
Aluminium
Aluminium is the most abundant metallic element and constitutes about 8% of Earth’s crust. Aluminium salts are widely used in water treatment as coagulants to reduce organic matter, colour, turbidity and microorganism levels. Such use may lead to increased concentrations of aluminium in finished water. Where residual concentrations are high, undesirable colour and turbidity may ensue. Concentrations of aluminium at which such problems may occur are highly dependent on a number of water quality parameters and operational factors at the water treatment plant. Aluminium intake from foods, particularly those containing aluminium compounds used as food additives, represents the major route of aluminium exposure for the general public. The contribution of drinking-water to the total oral exposure to aluminium is usually less than 5% of the total intake.
View in own window
| Reason for not establishing a guideline value | A health-based value of 0.9 mg/l could be derived from the JECFA provisional tolerable weekly intake (PTWI), but this value exceeds practicable levels based on optimization of the coagulation process in drinking-water plants using aluminium-based coagulants: 0.1 mg/l or less in large water treatment facilities and 0.2 mg/l or less in small facilities |
|---|
| Assessment date | 2009 |
|---|
| Principal references |
FAO/WHO (2007) Aluminium (from all sources, including food additives)
IPCS (1997)
Aluminium
WHO (2010)
Aluminium in drinking-water
|
|---|
There is little indication that orally ingested aluminium is acutely toxic to humans despite the widespread occurrence of the element in foods, drinking-water and many antacid preparations. It has been hypothesized that aluminium exposure is a risk factor for the development or acceleration of onset of Alzheimer disease in humans. The 1997 WHO Environmental Health Criteria document for aluminium concludes that:
On the whole, the positive relationship between aluminium in drinking-water and AD [Alzheimer disease], which was demonstrated in several epidemiological studies, cannot be totally dismissed. However, strong reservations about inferring a causal relationship are warranted in view of the failure of these studies to account for demonstrated confounding factors and for total aluminium intake from all sources.
Taken together, the relative risks for AD from exposure to aluminium in drinking-water above 100 µg/l, as determined in these studies, are low (less than 2.0). But, because the risk estimates are imprecise for a variety of methodological reasons, a population-attributable risk cannot be calculated with precision. Such imprecise predictions may, however, be useful in making decisions about the need to control exposures to aluminium in the general population.
In 2007, JECFA developed a PTWI for aluminium from all sources of 1 mg/kg body weight. JECFA concluded the following:
… the available studies have many limitations and are not adequate for defining the dose–response relationships. The Committee therefore based its evaluation on the combined evidence from several studies. The relevance of studies involving administration of aluminium compounds by gavage was unclear because the toxicokinetics after gavage were expected to differ from toxicokinetics after dietary administration, and the gavage studies generally did not report total aluminium exposure including basal levels in the feed. The studies conducted with dietary administration of aluminium compounds were considered most appropriate for the evaluation. The lowest LOELs [lowest-observed-effect levels] for aluminium in a range of different dietary studies in mice, rats and dogs were in the region of 50–75 mg/kg bw [body weight] per day expressed as Al.
The Committee applied an uncertainty factor of 100 to the lower end of this range of LOELs (50 mg/kg bw per day expressed as Al) to allow for inter- and intraspecies differences. There are deficiencies in the database, notably the absence of NOELs [no-observed-effect levels] in the majority of the studies evaluated and the absence of long-term studies on the relevant toxicological end-points. The deficiencies are counterbalanced by the probable lower bioavailability of the less soluble aluminium species present in food. Overall, an additional uncertainty factor of three was considered to be appropriate. The Committee confirmed that the resulting health-based guidance value should be expressed as a PTWI, because of the potential for bioaccumulation. The Committee established a PTWI of 1 mg/kg bw for Al, which applies to all aluminium compounds in food, including additives.
A health-based value derived from the JECFA PTWI would be 0.9 mg/l (rounded value), based on an allocation of 20% of the PTWI to drinking-water and assuming a 60 kg adult drinking 2 litres of water per day. However, there remain uncertainties as to the extent of aluminium absorption from drinking-water, which depends on a number of parameters, such as the aluminium salt administered, pH (for aluminium speciation and solubility), bioavailability and dietary factors.
The beneficial effects of the use of aluminium as a coagulant in water treatment are recognized. Taking this into account, and considering the health concerns about aluminium (i.e. its potential neurotoxicity), a practicable level is derived, based on optimization of the coagulation process in drinking-water plants using aluminium-based coagulants, to minimize aluminium levels in finished water.
Several approaches are available for minimizing residual aluminium concentrations in treated water. These include use of optimum pH in the coagulation process, avoiding excessive aluminium dosage, good mixing at the point of application of the coagulant, optimum paddle speeds for flocculation and efficient filtration of the aluminium floc. Under good operating conditions, concentrations of aluminium of 0.1 mg/l or less are achievable in large water treatment facilities. Small facilities (e.g. those serving fewer than 10 000 people) might experience some difficulties in attaining this level, because the small size of the plant provides little buffering for fluctuation in operation; moreover, such facilities often have limited resources and limited access to the expertise needed to solve specific operational problems. For these small facilities, 0.2 mg/l or less is a practicable level for aluminium in finished water.
As indicated above, a health-based value derived from the JECFA PTWI would be 0.9 mg/l (rounded value) based on an allocation of 20% of the PTWI to drinking-water and assuming a 60 kg adult drinking 2 litres of water per day. However, as also noted above, practicable levels based on optimization of the coagulation process in drinking-water plants using aluminium-based coagulants are less than 0.1 mg/l in large water treatment facilities and less than 0.2 mg/l in small facilities. In view of the importance of optimizing coagulation to prevent microbial contamination and the need to minimize deposition of aluminium floc in distribution systems, it is important to ensure that average residuals do not exceed these values.
Ammonia
The term ammonia includes the non-ionized (NH3) and ionized (NH4+) species. Ammonia in the environment originates from metabolic, agricultural and industrial processes and from disinfection with chloramine. Natural levels in groundwater and surface water are usually below 0.2 mg/l. Anaerobic groundwaters may contain up to 3 mg/l. Intensive rearing of farm animals can give rise to much higher levels in surface water. Ammonia contamination can also arise from cement mortar pipe linings. Ammonia in water is an indicator of possible bacterial, sewage and animal waste pollution.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Ammonia in drinking-water |
|---|
Ammonia is a major component of the metabolism of mammals. Exposure from environmental sources is insignificant in comparison with endogenous synthesis of ammonia. Toxicological effects are observed only at exposures above about 200 mg/kg body weight.
Ammonia in drinking-water is not of immediate health relevance, and therefore no health-based guideline value is proposed. However, ammonia can compromise disinfection efficiency, result in nitrite formation in distribution systems, cause the failure of filters for the removal of manganese and cause taste and odour problems (see also chapter 10).
Anatoxins (cyanobacterial toxins)1
Anatoxin-a, homoanatoxin-a and their dihydro derivatives (ATXs) are naturally occurring alkaloids produced by strains of various species of cyanobacteria, primarily in freshwater environments.2 ATXs have been found in many countries but generally have been reported less often than MCs or CYNs. They have been reported from a number of cyanobacterial genera, including Anabaena, Dolichospermum, Aphanizomenon and Cuspidothrix, many of which are primarily benthic (i.e. grow on sediments or other submerged surfaces) (see also section 11.5). ATXs, like MCs and STXs, usually occur bound to cyanobacterial cells.
Drinking-water is the most likely route of exposure to ATXs where surface water with cyanobacterial blooms is the drinking-water source. Recreational activities in lakes with cyanobacterial blooms may also be a relevant exposure pathway, potentially to high concentrations (see WHO Guidelines on recreational water quality, 2021).
View in own window
| Reason for not establishing a guideline value | Available data inadequate to permit derivation of health-based guideline value |
|---|
| Provisional reference value (short-term)* | Total ATXs (sum of all congeners, free plus cell-bound): 0.03 mg/l The reference value is based on data for anatoxin-a only |
|---|
| Occurrence | Concentrations reported usually range well below 1 mg/l; outside of scum areas, they rarely exceed several µg/l. ATXs largely occur cell-bound unless cell damage causes release. |
|---|
| TDI | A formal TDI could not be derived because of database limitations. However, 98 µg/kg bw per day, based on a 28-day study in mice, was selected as a NOAEL. An uncertainty factor of 100 (10 each for inter- and intra-species variability) was applied. An uncertainty factor for database limitations was not applied because of the conservative assumptions used to select the NOAEL (see text following the table for further detail). |
|---|
| Limit of detection |
0.05 µg/L by LC-MS/MS and <30 µg/L by HPLC coupled with post-derivatization fluorescence. LC-MS/MS requires quantitative reference standards, which are available for anatoxin-a and for dihydro-anatoxin-a. A receptor-binding assay that is commercially available circumvents this problem and provides more reliable results than HPLC. Prior extraction of cells with freeze–thaw cycles and acidified water or acidified mixtures of methanol/water is necessary for cell-bound ATX; neglecting extraction from cells will lead to dramatic underestimation of concentrations.
0.15 µg/L for anatoxin-a and 10 µg/L for homoanatoxin-a by commercially available immunoassay kits (ELISA); although these are less precise than LC with the above-mentioned detection methods, they probably capture all anatoxin-a congeners and thus are useful for most monitoring purposes.
|
|---|
| Monitoring | The likelihood of blooms can be assessed by understanding water body conditions (in particular, nutrient concentrations, water body depth, water retention time, patterns of mixing and stratification; see section 11.5). Where conditions render blooms likely, visual monitoring of source water (including microscopy for potentially ATX-containing genera) for evidence of increasing cyanobacterial biomass (blooms) is important because biomass can increase rapidly. Exceeding alert values of biomass indicators or ATX concentrations should trigger management responses to prevent exposure to elevated toxin concentrations (see the alert level framework in section 11.5). Analysis of cyanotoxins is particularly useful for validating and optimizing the efficacy of control measures such as riverbank filtration or treatment. |
|---|
| Prevention and treatment | Actions to decrease the probability of bloom occurrence include catchment and source water management, such as reducing nutrient loading or changing reservoir stratification and mixing. Filtration is effective for removing intact cyanobacterial cells, but some dissolved ATX may be present. For this dissolved fraction, oxidation with ozone at sufficient concentrations and contact times, as well as GAC and some PAC applications, are effective. Chlorination is not reliably effective for oxidation of ATXs (see chapters 7–10 of Toxic cyanobacteria in water; Annex 1). |
|---|
| Reference value derivation |
|---|
| • allocation to water | 100% |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Total ATXs as gravimetric or molar equivalents should be evaluated against the reference value since these toxins can occur as mixtures. Although the reference value is based on anatoxin-a, limited evidence suggests that all ATXs are similarly toxic. |
|---|
| Because ATX is acutely toxic, avoiding any exposure above the reference value is recommended. |
| It is recommended, as a precautionary measure, that bottle-fed infants and small children be provided with an alternative safe drinking-water source (e.g. bottled water that is certified by the responsible authorities) if concentrations are greater than 6 μg/L, even for short periods. |
| Assessment date | 2020 |
|---|
| Principal references |
WHO (2020)
Cyanobacterial toxins: anatoxin-a and analogues
Chorus & Welker (2021) Toxic cyanobacteria in water
|
|---|
- *
Although a formal guideline value cannot be established, the provisional reference value was derived based on limited available studies, recognizing that a “bounding value” may be useful, to provide guidance to Member States in the event of need. Reference values are too uncertain to be used for developing regulations or standards.
ATXs have been much investigated because of the rapid animal deaths that have been observed upon ingestion of cyanobacterial cells in beached scum material or dislodged benthic mats—in particular, dogs that ingest significant amounts. In contrast, high concentrations of dissolved ATXs near drinking-water abstraction sites have not been reported, presumably because ATX released from the cells in benthic mats or scums is usually rapidly diluted.
The (+) enantiomer of anatoxin-a binds with high affinity to nicotinic acetylcholine receptors of nerve cells, causing chronic overstimulation. This can lead to increases in heart rate and blood pressure, as well as fatigue and eventual paralysis of muscles, which can cause death when it occurs in respiratory muscles. Although anatoxin-a is the best studied analogue, limited evidence suggests that homoanatoxin-a and the dihydro derivatives of these compounds bind to the same receptor and may have similar potency to anatoxin-a when administered orally.
The toxicological database on ATXs is not adequate to support derivation of a formal guideline value since a nonlethal dose that caused significant adverse effects was not identified in the available repeated-dose studies. Nevertheless, a “bounding value” may be useful to risk assessors. Based on the limited available studies of acute and subchronic anatoxin-a toxicity, a health-based reference value is provided that is unlikely to cause adverse effects in exposed adults. The reference value is based on a 28-day mouse study that found no effects that were clearly ATX related in any of the doses tested. However, as a mouse died from unexplained causes at each of two lower doses, the dose below these two has been selected as the NOAEL. Two other studies indicate that this selection is very conservative.
Practical considerations
Where nutrient (phosphorus and nitrogen) concentrations are elevated in lakes, reservoirs or slowly flowing rivers, cyanobacteria occur widely. Where their excessive growth leads to high biomass, sometimes termed “bloom” events, ATXs can reach concentrations in raw water that are potentially hazardous to human health. Such blooms tend to recur in the same water bodies. Cells of some cyanobacterial species (e.g. Anabaena, Cuspidothrix, Dolichspermum) may accumulate at the surface as scums. Such accumulations may develop rapidly and may be of very variable duration (hours to weeks). In many circumstances, blooms and accumulations are seasonal, whereas others occur perennially.
Cyanobacteria are most effectively controlled in the context of developing a WSP (see chapter 4). Control measures to manage potential risks from cyanobacteria, and in particular from their toxins, in drinking-water should include not only adequate treatment, but also measures to control cyanobacterial bloom development. See section 11.5 for more information on cyanobacteria, including further details on monitoring cyanobacterial blooms, the alert level framework, and prevention and management of cyanobacteria in source waters. Effectively minimizing the formation of blooms and locating the raw water intake away from blooms reduce the treatment steps required to remove cyanotoxins.
Drinking-water treatment that removes particles—that is, soil, slow sand or riverbank filtration, conventional water treatment (coagulation, flocculation and filtration) or dissolved air flotation—can remove cell-bound ATXs effectively. Soil, slow sand and riverbank filtration can also remove dissolved cyanotoxins. For all these processes, it is important that they are optimized to target the removal of cells and dissolved toxins. Chlorination is not reliably effective, whereas ozonation at sufficiently high doses and contact times is effective for degrading dissolved ATXs; however, elevated organic carbon in bloom situations will substantially increase the disinfectant demand. Chlorine dioxide and chloramine are ineffective for degrading ATXs. Both for pre-oxidation and conventional treatment, cell rupture and toxin release should be avoided. GAC and PAC can be effective for removing dissolved ATXs, with efficacy dependent on several factors, including the type of activated carbon, contact times (PAC), flow rates (GAC) and water quality. As the challenges that blooms present for treatment are complex, periodic validation of efficacy during bloom situations and under the specific local conditions is particularly important. Avoiding bloom occurrence and intake is therefore the preferred option.
Antimony
Elemental antimony forms very hard alloys with copper, lead and tin. Antimony compounds have various therapeutic uses. Antimony is used in solders as a replacement for lead, but there is little evidence of any significant contribution to drinking-water concentrations from this source. Total exposure from environmental sources, food and drinking-water is very low compared with occupational exposure.
View in own window
| Guideline value | 0.02 mg/l (20 µg/l) |
|---|
| Occurrence | Concentrations in groundwater less than 0.001 µg/l; concentrations in surface water less than 0.2 µg/l; concentrations in drinking-water appear to be less than 5 µg/l |
|---|
| Tolerable daily intake (TDI) | 6 µg/kg body weight, based on a NOAEL of 6.0 mg/kg body weight per day for decreased body weight gain and reduced food and water intake in a 90-day study in which rats were administered potassium antimony tartrate in drinking-water, using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation, 10 for the short duration of the study) |
|---|
| Limit of detection | 0.01 µg/l by electrothermal atomic absorption spectrometry (AAS); 0.1–1 µg/l by inductively coupled plasma mass spectrometry (ICP-MS); 0.8 µg/l by graphite furnace AAS; 5 µg/l by hydride generation AAS |
|---|
| Treatment performance | Conventional treatment processes do not remove antimony. However, antimony is not normally a raw water contaminant. As the most common source of antimony in drinking-water appears to be dissolution from metal plumbing and fittings, control of antimony from such sources would be by product control. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2003)
Antimony in drinking-water |
|---|
There has been a significant increase in the toxicity data available since the previous review, although much of it pertains to the intraperitoneal route of exposure. The form of antimony in drinking-water is a key determinant of the toxicity, and it would appear that antimony leached from antimony-containing materials would be in the form of the antimony(V) oxo-anion, which is the less toxic form. The subchronic toxicity of antimony trioxide is lower than that of potassium antimony tartrate, which is the most soluble form. Antimony trioxide, owing to its low bioavailability, is genotoxic only in some in vitro tests, but not in vivo, whereas soluble antimony(III) salts exert genotoxic effects in vitro and in vivo. Animal experiments from which the carcinogenic potential of soluble or insoluble antimony compounds may be quantified are not available. IARC has concluded that antimony trioxide is possibly carcinogenic to humans (Group 2B) on the basis of an inhalation study in rats, but that antimony trisulfide was not classifiable as to its carcinogenicity to humans (Group 3). However, chronic oral uptake of potassium antimony tartrate may not be associated with an additional carcinogenic risk, as antimony after inhalation exposure was carcinogenic only in the lung but not in other organs and is known to cause direct lung damage following chronic inhalation as a consequence of overload with insoluble particulates. Although there is some evidence for the carcinogenicity of certain antimony compounds by inhalation, there are no data to indicate carcinogenicity by the oral route.
Arsenic3
Arsenic is found widely in Earth’s crust in oxidation states of −3, 0, +3 and +5, often as sulfides or metal arsenides or arsenates. In water, it is mostly present as arsenate (+5), but in anaerobic conditions, it is likely to be present as arsenite (+3). It is usually present in natural waters at concentrations of less than 1–2 µg/l. However, in waters, particularly groundwaters, where there are sulfide mineral deposits and sedimentary deposits deriving from volcanic rocks, the concentrations can be significantly elevated.
Arsenic is found in the diet, particularly in fish and shellfish, in which it is found mainly in the less toxic organic form. There are only limited data on the proportion of inorganic arsenic in food, but these indicate that approximately 25% is present in the inorganic form, depending on the type of food. Apart from occupational exposure, the most important routes of exposure are through food and drinking-water, including beverages that are made from drinking-water. Where the concentration of arsenic in drinking-water is 10 µg/l or greater, this will be the dominant source of intake. In circumstances where soups or similar dishes are a staple part of the diet, the drinking-water contribution through preparation of food will be even greater.
View in own window
| Provisional guideline value | 0.01 mg/l (10 µg/l) |
|---|
| The guideline value is designated as provisional on the basis of treatment performance and analytical achievability. |
| Occurrence | Levels in natural waters generally range between 1 and 2 µg/l, although concentrations may be elevated (up to 12 mg/l) in areas containing natural sources |
|---|
| Basis of guideline value derivation | There remains considerable uncertainty over the actual risks at low concentrations, and available data on mode of action do not provide a biological basis for using either linear or non-linear extrapolation. In view of the practical difficulties in removing arsenic from drinking-water, as well as the practical quantification limit in the region of 1–10 µg/l, the guideline value of 10 µg/l is retained and designated as provisional. |
|---|
| Limit of detection | 0.1 µg/l by ICP-MS; 2 µg/l by hydride generation AAS or flame AAS |
|---|
| Treatment performance | It is technically feasible to achieve arsenic concentrations of 5 µg/l or lower using any of several possible treatment methods. However, this requires careful process optimization and control, and a more reasonable expectation is that 10 µg/l should be achievable by conventional treatment (e.g. coagulation). |
|---|
| Assessment date | 2011 |
|---|
| Principal references |
FAO/WHO (2011)
Evaluation of certain contaminants in food
IARC (1987)
Overall evaluations of carcinogenicity
IPCS (2001)
Arsenic and arsenic compounds
ISO (1982)
Water quality—determination of total arsenic
USNRC (2001)
Arsenic in drinking water, 2001 update
WHO (2011)
Arsenic in drinking-water
|
|---|
Both pentavalent and trivalent soluble arsenic compounds are rapidly and extensively absorbed from the gastrointestinal tract. Metabolism is characterized by 1) reduction of pentavalent to trivalent arsenic and 2) oxidative methylation of trivalent arsenic to form monomethylated, dimethylated and trimethylated products. Methylation of inorganic arsenic facilitates the excretion of inorganic arsenic from the body, as the end-products monomethylarsonic acid and dimethylarsinic acid are readily excreted in urine. There are major qualitative and quantitative interspecies differences in methylation, but in humans and most common laboratory animals, inorganic arsenic is extensively methylated, and the metabolites are excreted primarily in the urine. There is large interindividual variation in arsenic methylation in humans, probably due to a wide difference in the activity of methyltransferases and possible polymorphism. Ingested organoarsenicals are much less extensively metabolized and more rapidly eliminated in urine than inorganic arsenic.
Arsenic has not been demonstrated to be essential in humans. The acute toxicity of arsenic compounds in humans is predominantly a function of their rate of removal from the body. Arsine is considered to be the most toxic form, followed by the arsenites, the arsenates and organic arsenic compounds. Acute arsenic intoxication associated with the ingestion of well water containing very high concentrations (21.0 mg/l) of arsenic has been reported.
Signs of chronic arsenicism, including dermal lesions such as hyperpigmentation and hypo-pigmentation, peripheral neuropathy, skin cancer, bladder and lung cancers and peripheral vascular disease, have been observed in populations ingesting arsenic-contaminated drinking-water. Dermal lesions were the most commonly observed symptom, occurring after minimum exposure periods of approximately 5 years. Effects on the cardiovascular system were observed in children consuming arsenic-contaminated water (mean concentration 0.6 mg/l) for an average of 7 years.
Numerous epidemiological studies have examined the risk of cancers associated with arsenic ingestion through drinking-water. Many are ecological-type studies, and many suffer from methodological flaws, particularly in the measurement of exposure. However, there is overwhelming evidence that consumption of elevated levels of arsenic through drinking-water is causally related to the development of cancer at several sites. Nevertheless, there remain considerable uncertainty and controversy over both the mechanism of carcinogenicity and the shape of the dose–response curve at low intakes. The International Programme on Chemical Safety (IPCS) concluded that long-term exposure to arsenic in drinking-water is causally related to increased risks of cancer in the skin, lungs, bladder and kidney, as well as other skin changes, such as hyperkeratosis and pigmentation changes. These effects have been demonstrated in many studies using different study designs. Exposure–response relationships and high risks have been observed for each of these end-points. The effects have been most thoroughly studied in Taiwan, China, but there is considerable evidence from studies on populations in other countries as well. Increased risks of lung and bladder cancer and of arsenic-associated skin lesions have been reported to be associated with ingestion of drinking-water at concentrations below 50 µg of arsenic per litre. There is a need for more analytical epidemiological studies to determine the dose–time response for skin lesions, as well as cancer, in order to assist in developing suitable interventions and determining practical intervention policies.
Inorganic arsenic compounds are classified by IARC in Group 1 (carcinogenic to humans) on the basis of sufficient evidence for carcinogenicity in humans and limited evidence for carcinogenicity in animals. Although there is a substantial database on the association between both internal and skin cancers and the consumption of arsenic in drinking-water, there remains considerable uncertainty over the actual risks at low concentrations. In its updated evaluation, the United States National Research Council concluded that “the available mode-of-action data on arsenic do not provide a biological basis for using either a linear or nonlinear extrapolation”. The maximum likelihood estimates, using a linear extrapolation, for bladder and lung cancer for populations in the United States of America (USA) exposed to arsenic at concentrations of 10 µg/l in drinking-water are, respectively, 12 and 18 per 10 000 population for females and 23 and 14 per 10 000 population for males. The actual numbers indicated by these estimated risks would be very difficult to detect by current epidemio-logical methods. There is also uncertainty over the contribution of arsenic in food—a higher intake of inorganic arsenic from food would lead to a lower risk estimate for water—and the impact of factors such as variation in the metabolism of arsenic and nutritional status. Some studies in areas with arsenic concentrations somewhat above 50 µg/l have not detected arsenic-related adverse effects in the residents. It remains possible that the estimates of cancer risk associated with various arsenic intakes are overestimates. The concentration of arsenic in drinking-water below which no effects can be observed remains to be determined, and there is an urgent need for identification of the mechanism by which arsenic causes cancer, which appears to be the most sensitive toxicity end-point.
The practical quantification limit for arsenic is in the region of 1–10 µg/l, and removal of arsenic to concentrations below 10 µg/l is difficult in many circumstances. In view of the practical difficulties in removing arsenic from drinking-water, particularly from small supplies, and the practical quantification limit for arsenic, the guideline value of 10 µg/l is retained as a goal and designated as provisional.
The provisional guideline value of 10 µg/l was previously supported by a JECFA PTWI of 15 µg/kg body weight, assuming an allocation of 20% to drinking-water. However, JECFA recently re-evaluated arsenic and concluded that the existing PTWI was very close to the lower confidence limit on the benchmark dose for a 0.5% response (BMDL0.5) calculated from epidemiological studies and was therefore no longer appropriate. The PTWI was therefore withdrawn. Nevertheless, given that, in many countries, even the provisional guideline value may not be attainable, it is retained on the basis of treatment performance and analytical achievability with the proviso that every effort should be made to keep concentrations as low as reasonably possible.
Practical considerations
A silver diethyldithiocarbamate spectrophotometric method (ISO 6595:1982) is available for the determination of arsenic; the detection limit is about 1 µg/l. Graphite furnace AAS, hydride generation AAS and ICP-MS are more sensitive. HPLC in combination with ICP-MS can also be used to determine various arsenic species.
It is technically feasible to achieve arsenic concentrations of 5 µg/l or lower using any of several possible treatment methods. However, this requires careful process optimization and control, and a more reasonable expectation is that 10 µg/l should be achievable by conventional treatment (e.g. coagulation). For local non-piped water supplies, the first option is often substitution by, or dilution with, microbially safe low-arsenic sources. It may also be appropriate to use alternative sources for drinking and cooking but to use the contaminated sources for purposes such as washing and laundry. There are also an increasing number of effective small-scale treatment techniques, usually based around coagulation and precipitation or adsorption, available at relatively low cost for removal of arsenic from small supplies.
For further guidance on identifying and managing arsenic contamination of drinking-water, see Arsenic primer (Annex 1).
Asbestos
Asbestos is introduced into water by the dissolution of asbestos-containing minerals and ores, as well as from industrial effluents and atmospheric pollution. However, the main source of asbestos in drinking-water is asbestos–cement pipes in the distribution system. Rainwater harvesting can contribute to exposure where water runoff is collected from asbestos–cement roofing that is inadequately sealed. Exfoliation of asbestos fibres from asbestos–cement pipes is related to the aggressiveness of the water supply, although, where pipes are already degraded, actions to to control water corrosivity will not prevent asbestos fibre release from the pipes. Exposure to airborne asbestos released from tap water during showers or humidification is unlikely to be significant for human health.
View in own window
| Reason for not establishing a guideline value | No consistent evidence that ingested asbestos is hazardous to health. Further, epidemiological studies have a number of limitations that would preclude their use for deriving a guideline value. |
|---|
| Occurrence | Concentrations in drinking-water are typically less than 1 million fibres per litre (MFL) and rarely exceed 10 MFL. However, much higher concentrations have been reported in drinking-water supplies where asbestos–cement piping is used, although occurrence data are limited. |
|---|
| Limit of detection | Less than 0.1 MFL in water by transmission electron microscopy |
|---|
| Treatment performance | Conventional treatment, including coagulation and filtration, can effectively remove asbestos fibres (>99%) if operation is optimized. |
|---|
| Additional comments | Where existing asbestos–cement pipes are still in active use, suppliers should map and record their location, assess their condition (including related to water aggressiveness) and determine the most appropriate risk reduction strategies. |
|---|
| Degradation of asbestos–cement materials in drinking-water supplies should be minimized. As these materials fail or deteriorate significantly, the asbestos–cement materials should be replaced with non-asbestos-containing materials. No new sources of asbestos fibres in drinking-water should be introduced. |
| Investigative monitoring of asbestos–cement pipes should be considered to provide additional information on the contribution of older asbestos–cement pipes to numbers, types, sizes and shape of fibres in drinking-water. |
| Assessment date | 2021 |
|---|
| Principal reference | WHO (2021)
Asbestos in drinking-water |
|---|
Although asbestos is a known human carcinogen by the inhalation route, findings from epidemiological studies that evaluated the correlation between asbestos exposure via drinking-water and incidence of cancers of the stomach and gastrointestinal tract have been inconsistent, with some studies suggesting a weak positive correlation and others finding no evidence of a correlation. However, in extensive studies in experimental animal species, asbestos has not consistently increased the incidence of tumours of the gastrointestinal tract. Thus, the overall weight of evidence from epidemiological and animal studies does not support the hypothesis that oral exposure to asbestos in drinking-water is associated with an increased risk of developing cancer. Further, uncertainties associated with the epidemiological data preclude the establishment of a health-based guideline value for asbestos in drinking-water.
The primary issue surrounding asbestos–cement in drinking-water is for people working on the outside of the pipes (e.g. cutting pipe) or those repairing roof tiles, because of the risk of inhalation of asbestos dust. Where replacement or repair of pipes is required, it is essential that appropriate measures are taken to prevent any worker exposure to asbestos dust, including during transport and disposal. Similar to asbestos–cement pipes, it is important that appropriate measures are taken to prevent worker and public exposure to asbestos dust generated during work on asbestos–cement roof tiles.
Atrazine and its metabolites
Atrazine is a selective systemic herbicide of the chlorotriazine class, used for the control of annual broadleaf and grassy weeds. Atrazine and its chloro-s-triazine metabolites—deethyl-atrazine, deisopropyl-atrazine and diaminochlorotriazine—have been found in surface water and groundwater as a result of the use of atrazine as a pre-emergent or early post-emergent herbicide. The metabolite hydroxyatrazine is more commonly detected in groundwater than in surface water.
View in own window
| Guideline values | Atrazine and its chloro-s-triazine metabolites: 0.1 mg/l (100 µg/l) |
|---|
| Hydroxyatrazine: 0.2 mg/l (200 µg/l) |
| Occurrence | Concentrations rarely exceed 2 µg/l and are commonly well below 0.1 µg/l |
|---|
| Group ADI for atrazine and its chloro-s-triazine metabolites | 0–0.02 mg/kg body weight based on the NOAEL for atrazine of 1.8 mg/kg body weight per day identified on the basis of luteinizing hormone surge suppression and subsequent disruption of the estrous cycle seen at 3.6 mg/kg body weight per day in a 6-month study in rats, using a safety factor of 100 |
|---|
| ADI for hydroxyatrazine | 0–0.04 mg/kg body weight based on the NOAEL of 1.0 mg/kg body weight per day identified on the basis of kidney toxicity at 7.8 mg/kg body weight per day in a 24-month study in rats, using a safety factor of 25, based on kinetic considerations |
|---|
| Limit of detection | Atrazine: 1 ng/l, isotope dilution MS with solid-phase extraction; 10 ng/l, GC-MS with solid-phase extraction; 50 ng/l, liquid chromatography (LC)–MS with solid-phase extraction; 100 ng/l, GC with nitrogen–phosphorus detection |
|---|
| Metabolites: 5 ng/l, capillary GC with nitrogen thermionic specific detection and HPLC with photodiode array absorption detection following extraction with styrene-divinylbenzene sorbents and elution with acetone |
| Treatment performance | 0.1 µg/l can be achieved using GAC or powdered activated carbon (PAC); bankside filtration and nanofiltration are also effective |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 20% of upper limit of ADI |
|---|
| • body weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments |
JMPR considered that the NOAEL for atrazine is protective for the consequences of neuroendocrine and other adverse effects caused by prolonged exposure to atrazine and its chloro-s-triazine metabolites.
JMPR was not able to assess the source allocation of atrazine to drinking-water. As such, the default 20% allocation was chosen, as it will be very conservative in most countries; in addition, it is expected that exposure of the public will be primarily through drinking-water.
|
|---|
| Assessment date | 2011 |
|---|
| Principal references |
FAO/WHO (2009)
Pesticide residues in food—2007 evaluations
WHO (2011)
Atrazine and its metabolites in drinking-water
|
|---|
JMPR agreed that it is unlikely that atrazine is genotoxic and concluded that atrazine is not likely to pose a carcinogenic risk to humans, as the mode of carcinogenic action in certain susceptible rat strains is not relevant for human risk assessment. The weight of evidence from the epidemiological studies also did not support a causal association between exposure to atrazine and the occurrence of cancer in humans.
In special studies of reproductive toxicity, exposure of rats during early pregnancy (i.e. the luteinizing hormone–dependent period) caused increased pre-implantation or post-implantation losses, including full-litter resorptions. Attenuation of the luteinizing hormone surge and subsequent disruption of the estrous cycle (characterized by an increase in days in estrus) were observed at and above 3.65 mg/kg body weight per day, with a NOAEL of 1.8 mg/kg body weight per day. The effects on the luteinizing hormone surge and disruption of the estrous cycle were further supported by a number of short-term mechanistic studies. Additional experiments suggested that the effects of atrazine on luteinizing hormone and prolactin secretion are mediated via a hypothalamic site of action. JMPR concluded that atrazine was not teratogenic.
Studies using a variety of test systems in vitro and in vivo indicated that modulation of the immune system occurs after exposure to atrazine. However, effects suggestive of impaired function of the immune system were observed only at doses greater than those shown to affect neuroendocrine function, leading to disruption of the estrous cycle or developmental effects.
The toxicity profiles and mode of action of the chloro-s-triazine metabolites are similar to those of atrazine; the potency of these metabolites with regard to their neuroendocrine-disrupting properties appeared to be similar to that of the parent compound.
The metabolite hydroxyatrazine does not have the same mode of action or toxicity profile as atrazine and its chloro-s-triazine metabolites. The main effect of hydroxyatrazine was kidney toxicity (owing to its low solubility in water, resulting in crystal formation and a subsequent inflammatory response), and there was no evidence that hydroxyatrazine has neuroendocrine-disrupting properties. There was no evidence of carcinogenicity, and hydroxyatrazine did not show genotoxicity in an adequate range of tests in vitro and in vivo.
Barium
Barium compounds are present in nature as ore deposits and in igneous and sedimentary rocks, and are used in a variety of industrial applications. Barium in water comes primarily from natural sources, although barium also enters the environment from industrial emissions and anthropogenic uses. Food is the primary source of intake for the non-occupationally exposed population. However, where barium concentrations in water are high, drinking-water may contribute significantly to total intake.
View in own window
| Guideline value | 1.3 mg/l (1300 µg/l) |
|---|
| Occurrence | Concentrations in drinking-water are generally below 100 µg/l, although concentrations above 1 mg/l have been measured in drinking-water derived from groundwater |
|---|
| TDI | 0.21 mg/kg bw per day, derived by applying an uncertainty factor of 300 to account for intraspecies variation (10), interspecies variation (10) and database deficiencies (3 for the lack of a developmental toxicity study) to a BMDL05 of 63 mg/kg bw per day for nephropathy in mice in a 2-year study |
|---|
| Limit of detection | 0.004–0.8 µg/l by ICP-MS; 1.0 µg/l by ICP-AES |
|---|
| Treatment performance | Ion exchange, lime softening or direct filtration with chemical precipitation may be able to remove barium to below 1 mg/l |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 20% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | As rounding can have significant practical implications at milligram per litre levels, it was concluded that a guideline value with two significant figures was reasonable in this case. |
|---|
| The guideline value derived based on the long-term mouse study is not inconsistent with health-based values that could be derived from limited human studies. |
| Assessment date | 2016 |
|---|
| Principal references |
IPCS (2001). Barium and barium compounds
USEPA (2005). Toxicological review of barium and compounds. In support of summary information on the Integrated Risk Information System (IRIS).
WHO (2016). Barium in drinking-water
|
|---|
There is no evidence that barium is carcinogenic or genotoxic. Acute hypertension has been observed in case reports, but the effects may be secondary to hypokalaemia. The critical study that had been identified previously for deriving the guideline value has several limitations (e.g. no effect observed at the single dose evaluated, limitations in the exposure methodology and design, no control for important risk factors for hypertension). Another human study that reported no effects on hypertension at 10 mg/l is limited by the small study size and short exposure duration. Barium has been shown to cause nephropathy in laboratory animals, and this was selected as the toxicological end-point of concern for the current guideline.
Bentazone
Bentazone (CAS No. 25057-89-0) is a post-emergence herbicide used for selective control of broadleaf weeds and sedges occurring among a variety of crops. It is highly soluble in water and very resistant to hydrolysis; it is also very mobile in soil. However, photodegradation occurs in both soil and water. Bentazone may leach from soil into groundwater, particularly during heavy rainfall, and may contaminate surface water through effluents from production plants, drainage waters and actual use in the water (rice fields). Exposure from food is likely to be low.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water or drinking-water sources at concentrations well below those of health concern |
|---|
| Health-based value* | 0.5 mg/l |
|---|
| Acute health-based value** | 20 mg/l |
|---|
| Occurrence | Concentrations up to 120 µg/l in groundwater and up to 14 µg/l in surface water have been measured |
|---|
| ADI | 0–0.09 mg/kg bw, based on a NOAEL of 9 mg/kg bw per day for prolonged blood coagulation and clinical chemistry changes indicative of effects on liver and kidney from a 2-year toxicity and carcinogenicity study in rats and application of a safety factor of 100 |
|---|
| ARfD | 0.5 mg/kg bw, based on a NOAEL of 50 mg/kg bw for decreased motor activity observed in male rats on day 0 in an acute neurotoxicity study and application of a safety factor of 100 |
|---|
| Limit of detection | 0.1 µg/l by GC with ECD after liquid–liquid extraction; limit of quantification of 0.01 µg/l by LC-MS/MS |
|---|
| Treatment performance | Conventional treatment, including coagulation and filtration, not effective; activated carbon may be effective under certain circumstances |
|---|
| Health-based value derivation |
|---|
| • allocation to water | 20% of upper bound of ADI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Acute health-based value derivation |
|---|
| • allocation to water | 100% of ARfD (0.5 mg/kg bw) |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The default allocation factor of 20% has been used to account for the fact that the available food exposure data, which suggest that exposure via this route is low, do not generally include information from developing countries, where exposure via this route may be higher |
|---|
| As a general principle, the concentration of pesticides in water, including bentazone, should be kept as low as possible and concentrations should not be allowed to increase up to the health-based value. |
| Further guidance on interpreting the health-based value and deciding when to monitor can be found in section 8.5.3 |
| Assessment date | 2016 and 2020 |
|---|
| Principal references |
WHO (2013). Pesticide residues in food – 2012 evaluations
FAO/WHO (2016)
Pesticide residues in food – 2016 evaluations
WHO (2020). Bentazone in drinking-water
|
|---|
- *
When a formal guideline value is not established, a “health-based value” may be determined in order to provide guidance to Member States when there is reason for local concern. Establishing a formal guideline value for such substances may encourage Member States to incorporate a value into their national standards when this may be unnecessary.
- **
For more information on acute health-based values, see section 8.7.5.
Bentazone is not carcinogenic in rats or mice, and showed no evidence of genotoxicity in a range of in vitro and in vivo assays. Consistent observations in repeated-dose toxicity studies in mice, rats and dogs are effects on haematology and blood coagulation (e.g. prolongation of prothrombin time and partial thromboplastin time).
Benzene
Benzene is used principally in the production of other organic chemicals. It is present in petrol, and vehicular emissions constitute the main source of benzene in the environment. Benzene may be introduced into water by industrial effluents and atmospheric pollution.
View in own window
| Guideline value | 0.01 mg/l (10 µg/l) |
|---|
| Occurrence | Concentrations in drinking-water, when present, generally much less than 5 µg/l |
|---|
| Basis of guideline value derivation | Robust linear extrapolation model (because of statistical lack of fit of some of the data with the linearized multistage model) applied to leukaemia and lymphomas in female mice and oral cavity squamous cell carcinomas in male rats in a 2-year gavage study |
|---|
| Limit of detection | 0.2 µg/l by GC with photoionization detection and confirmation by MS |
|---|
| Treatment performance | 0.01 mg/l should be achievable using GAC or air stripping |
|---|
| Additional comments | Lower end of estimated range of concentrations in drinking-water corresponding to an upper-bound excess lifetime cancer risk of 10−5 (10–80 µg/l) corresponds to the estimate derived from data on leukaemia from epidemiological studies involving inhalation exposure, which formed the basis for the previous guideline value. The previous guideline value is therefore retained. |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Benzene in drinking-water |
|---|
Acute exposure of humans to high concentrations of benzene primarily affects the central nervous system. At lower concentrations, benzene is toxic to the haematopoietic system, causing a continuum of haematological changes, including leukaemia. Because benzene is carcinogenic to humans, IARC has classified it in Group 1. Haematological abnormalities similar to those observed in humans have been observed in experimental animal species exposed to benzene. In animal studies, benzene was shown to be carcinogenic following both inhalation and ingestion. It induced several types of tumours in both rats and mice in a 2-year carcinogenesis bioassay by gavage in corn oil. Benzene has not been found to be mutagenic in bacterial assays, but it has been shown to cause chromosomal aberrations in vivo in a number of species, including humans, and to be positive in the mouse micronucleus test.
Beryllium
The primary source of beryllium compounds in water appears to be release from coal burning and other industries using beryllium. Other sources of beryllium in surface water include deposition of atmospheric beryllium and weathering of rocks and soils containing beryllium. Beryllium is not likely to be found in natural water above trace levels as a result of the insolubility of beryllium oxides and hydroxides in the normal pH range.
View in own window
| Reason for not establishing a guideline value | Rarely found in drinking-water at concentrations of health concern |
|---|
| Assessment date | 2009 |
|---|
| Principal references |
IPCS (2001)
Beryllium and beryllium compounds
WHO (2009)
Beryllium in drinking-water
|
|---|
As beryllium is rarely, if ever, found in drinking-water at concentrations of concern, it is not considered necessary to set a formal guideline value.
A health-based value for beryllium in drinking-water of 12 µg/l can be calculated based on an allocation of 20% of the TDI of 2 µg/kg body weight, derived from a long-term study in which dogs exhibited lesions of the small intestine, to drinking-water and assuming a 60 kg adult drinking 2 litres of water per day. This allocation is probably conservative, as the limited data on food indicate that exposure from this source is likely to be well below the TDI.
Although beryllium appears to be found in drinking-water sources and drinking-water at low concentrations, the database on occurrence is limited, and there may be specific circumstances in which concentrations can be elevated due to natural sources where the pH is either below 5 or above 8 or there is high turbidity.
Boron
Boron compounds are used in the manufacture of glass, soaps and detergents and as flame retardants. Naturally occurring boron is present in groundwater primarily as a result of leaching from rocks and soils containing borates and borosilicates. The borate content of surface water can be increased as a result of wastewater discharges, but this use has decreased significantly, and levels of boron in wastewater discharges continue to fall.
View in own window
| Guideline value | 2.4 mg/l (2400 µg/l) |
|---|
| Occurrence | Concentrations vary widely and depend on the surrounding geology and wastewater discharges; for most of the world, the concentration of boron in drinking-water is judged to be below 0.5 mg/l |
|---|
| TDI | 0.17 mg/kg body weight, based on a BMDL05 of 10.3 mg/kg body weight per day for developmental toxicity (decreased fetal body weight in rats) and an uncertainty factor of 60 (10 for interspecies variation and 6 for intraspecies variation) |
|---|
| Limit of detection | 0.15 µg/l by ICP-MS; 6–10 µg/l by ICP–atomic emission spectrometry (AES) |
|---|
| Treatment performance | Conventional water treatment (coagulation, sedimentation, filtration) does not significantly remove boron, and special methods need to be used in order to remove boron from waters with high boron concentrations. Ion exchange and reverse osmosis processes may enable substantial reduction but are likely to be prohibitively expensive. Blending with low-boron supplies may be the only economical method to reduce boron concentrations in waters where these concentrations are high. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 40% of TDI (because intake from other sources is low) |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Because it will be difficult to achieve the guideline value of 2.4 mg/l in some desalinated supplies and in areas with high natural boron levels, local regulatory and health authorities should consider a value in excess of 2.4 mg/l by assessing exposure from other sources. |
|---|
| Additional comments | 2009 |
|---|
| Principal reference | WHO (2009)
Boron in drinking-water |
|---|
Short- and long-term oral exposures to boric acid or borax in laboratory animals have demonstrated that the male reproductive tract is a consistent target of toxicity. Testicular lesions have been observed in rats, mice and dogs given boric acid or borax in food or drinking-water. Developmental toxicity has been demonstrated experimentally in rats, mice and rabbits. Negative results in a large number of mutagenicity assays indicate that boric acid and borax are not genotoxic. In long-term studies in mice and rats, boric acid and borax caused no increase in tumour incidence.
Bromate
Sodium and potassium bromate are powerful oxidizers used mainly in permanent wave neutralizing solutions and the dyeing of textiles using sulfur dyes. Potassium bromate has also been used as an oxidizer to mature flour during milling, in treating barley in beer making and in fish paste products, although JECFA has concluded that the use of potassium bromate in food processing is not appropriate. Bromate is not normally found in water, but can occur as a result of pollution from industrial sources, sometimes as a consequence of its presence in contaminated soil. However, the main source in drinking-water is its formation during ozonation when the bromide ion is present in water. Bromate may also be formed in hypochlorite solutions produced by electrolysis of bromide-containing salt.
View in own window
| Provisional guideline value | 0.01 mg/l (10 µg/l) |
|---|
| The guideline value is provisional because of limitations in available analytical and treatment methods. |
| Occurrence | Has been reported in drinking-water with a variety of source water characteristics after ozonation at concentrations ranging from less than 2 to 293 µg/l, depending on bromide ion concentration, ozone dosage, pH, alkalinity and dissolved organic carbon; can also be formed in the electrolytic generation of chlorine and hypochlorite from brine with a high level of bromide contamination |
|---|
| Basis of guideline value derivation | Upper-bound estimate of cancer potency for bromate is 0.19 per mg/kg body weight per day, based on low-dose linear extrapolation (a one-stage Weibull time-to-tumour model was applied to the incidence of mesotheli omas, renal tubule tumours and thyroid follicular tumours in male rats given potassium bromate in drinking-water, using the 12-, 26-, 52- and 77-week interim kill data). A health-based value of 2 µg/l is associated with the upper-bound excess cancer risk of 10−5. A similar conclusion may be reached through several other methods of extrapolation, leading to values in the range 2–6 µg/l. |
|---|
| Limit of detection | 0.2 µg/l by ion chromatography with UV/visible absorbance detection; 0.3 µg/l by ion chromatography with detection by ICP-MS; 1.5 µg/l by ion chromatography with suppressed conductivity detection |
|---|
| Treatment performance | Bromate is difficult to remove once formed. By appropriate control of disinfection conditions, it is possible to achieve bromate concentrations below 0.01 mg/l. |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2003)
Bromate in drinking-water |
|---|
IARC has concluded that although there is inadequate evidence of carcinogenicity in humans, there is sufficient evidence for the carcinogenicity of bromate from high-dose studies in experimental animals; IARC has classified bromate in Group 2B (possibly carcinogenic to humans). Bromate is mutagenic both in vitro and in vivo. At this time, there is not sufficient evidence to conclude as to the mode of carcinogenic action for bromate. Observation of tumours at a relatively early time and the positive response of bromate in a variety of genotoxicity assays suggest that the predominant mode of action at low doses is due to oxidative deoxyribonucleic acid (DNA) damage. Although there is evidence to suggest that the DNA reactivity in kidney tumours may have a non-linear dose–response relationship, there is no evidence to suggest that this same dose–response relationship operates in the development of mesotheliomas or thyroid tumours. Oxidative stress may play a role in the formation of kidney tumours, but the evidence is insufficient to establish lipid peroxidation and free radical production as key events responsible for the induction of kidney tumours. However, emerging evidence points to rapid decomposition of bromate in the gastrointestinal tract, blood and liver, which supports a non-linear dose–response relationship at low doses.
Bromide
Bromide is commonly found in nature along with sodium chloride, owing to their similar physical and chemical properties, but in smaller quantities. Bromide concentrations in seawater range from 65 mg/l to well over 80 mg/l, in fresh water from trace amounts to about 0.5 mg/l and in desalinated waters up to 1 mg/l.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2009 |
|---|
| Principal reference | WHO (2009)
Bromide in drinking-water |
|---|
Inorganic bromide was evaluated in 1966 by JMPR, which recommended an ADI of 0–1 mg/kg body weight, based on a minimum pharmacologically effective dosage in humans of about 900 mg of potassium bromide, equivalent to 600 mg of bromide ion. The JMPR ADI was reaffirmed with new data in 1988.
The results of human studies suggest a conservative no-observed-effect level (NOEL) (for marginal effect within normal limits of electroencephalograms in females) of 4 mg/kg body weight per day, giving an ADI of 0–0.4 mg/kg body weight, including a safety factor of 10 for population diversity.
The upper limit of the ADI of 0–0.4 mg/kg body weight yields an acceptable total daily intake of 24 mg/person for a 60 kg person. Assuming a relative source contribution of 50%, the drinking-water value for a 60 kg adult consuming 2 litres/day would be up to 6 mg/l; for a 10 kg child consuming 1 litre/day, the value would be up to 2 mg/l. However, the dietary bromide contribution for a 10 kg child would probably be less than that for an adult. These are reasonably conservative values, and they are unlikely to be encountered in drinking-water supplies.
Bromide can be involved in the reaction between chlorine and naturally occurring organic matter in drinking-water, forming brominated and mixed chloro-bromo by-products, such as trihalomethanes (THMs) and halogenated acetic acids (HAAs), or it can react with ozone to form bromate. The levels of bromide that can result in the formation of these substances are well below the health-based values suggested above. This guidance applies specifically to inorganic bromide ion and not to bromate or organohalogen compounds, for which individual health-based guideline values have been developed.
Brominated acetic acids
Brominated acetic acids are formed during disinfection of water that contains bromide ions and organic matter. Bromide ions occur naturally in surface water and groundwater and exhibit seasonal fluctuations in levels. Bromide ion levels can increase as a result of either saltwater intrusion resulting from drought conditions or pollution. Brominated acetates are generally present in surface water and groundwater distribution systems at mean concentrations below 5 µg/l.
View in own window
| Reason for not establishing guideline values | Available data inadequate to permit derivation of health-based guideline values |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (2000)
Disinfectants and disinfectant by-products
WHO (2004)
Brominated acetic acids in drinking-water
|
|---|
The database for dibromoacetic acid is considered inadequate for the derivation of a guideline value. There are no systemic toxicity studies of subchronic duration or longer. The database also lacks suitable toxicokinetic studies, a carcinogenicity study, a developmental study in a second species and a multigeneration reproductive toxicity study. Available mutagenicity data suggest that dibromoacetate is genotoxic.
Data are also limited on the oral toxicity of monobromoacetic acid and bromochloroacetic acid. Limited mutagenicity and genotoxicity data give mixed results for monobromoacetic acid and generally positive results for bromochloroacetic acid. Data gaps include subchronic or chronic toxicity studies, multigeneration reproductive toxicity studies, standard developmental toxicity studies and carcinogenicity studies. The available data are considered inadequate to establish guideline values for these chemicals.
Cadmium
Cadmium metal is used in the steel industry and in plastics. Cadmium compounds are widely used in batteries. Cadmium is released to the environment in wastewater, and diffuse pollution is caused by contamination from fertilizers and local air pollution. Contamination in drinking-water may also be caused by impurities in the zinc of galvanized pipes and solders and some metal fittings. Food is the main source of daily exposure to cadmium. The daily oral intake is 10–35 µg. Smoking is a significant additional source of cadmium exposure.
View in own window
| Guideline value | 0.003 mg/l (3 µg/l) |
|---|
| Occurrence | Levels in drinking-water usually less than 1 µg/l |
|---|
| PTMI | 25 µg/kg body weight, based on the relationship between β2-microglobulin excretion in urine and cadmium excretion in urine for individuals who are 50 years of age and older |
|---|
| Limit of detection | 0.01 µg/l by ICP-MS; 2 µg/l by flame AAS |
|---|
| Treatment performance | 0.002 mg/l should be achievable using coagulation or precipitation softening |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of provisional tolerable monthly intake (PTMI) because of high intake from food |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Although new information indicates that a proportion of the general population may be at increased risk for tubular dysfunction when exposed at the current PTMI, the risk estimates that can be made at present are imprecise. |
|---|
| It is recognized that the margin between the PTMI and the actual monthly intake of cadmium by the general population is small and that this margin may be even smaller in smokers. |
| Assessment date | 2011 |
|---|
| Principal references |
FAO/WHO (2011)
Evaluation of certain food additives and contaminants
WHO (2003)
Cadmium in drinking-water
|
|---|
Absorption of cadmium compounds is dependent on the solubility of the compounds. Cadmium accumulates primarily in the kidneys and has a long biological half-life in humans of 10–35 years. There is evidence that cadmium is carcinogenic by the inhalation route, and IARC has classified cadmium and cadmium compounds in Group 2A (probably carcinogenic to humans). However, there is no evidence of carcinogenicity by the oral route and no clear evidence for the genotoxicity of cadmium. The kidney is the main target organ for cadmium toxicity.
In its recent evaluation of cadmium, JECFA found that data relating excretion of the biomarker β2-microglobulin in urine to cadmium excretion in urine for individuals who are 50 years of age and older provided the most reliable basis on which to determine a critical concentration of cadmium in the urine. Urinary excretion of less than 5.24 µg of cadmium per gram creatinine was not associated with an increased excretion of β2-microglobulin, and the dietary exposure that would result in a urinary cadmium concentration at the breakpoint of 5.24 µg/g creatinine was estimated to be 0.8 µg/kg body weight per day or about 25 μg/kg body weight per month. Because of cadmium’s exceptionally long half-life, the previous PTWI of 7 µg/kg body weight was withdrawn, and a PTMI of 25 µg/kg body weight was established.
Carbaryl
Carbaryl (CAS No. 63-25-2) is a broad-spectrum carbamate insecticide that is used to control insect pests in crops, trees and ornamental plants. It also has some uses in public health and veterinary practice. Carbaryl has not been reported in drinking-water; however, it could occur following overspraying or spillage into surface water. Exposure through drinking-water is therefore considered to be low unless in exceptional circumstances. The major route of carbaryl intake for the general population is food, but residues are considered to be relatively low.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2006 |
|---|
| Principal references |
FAO/WHO (2002)
Pesticide residues in food—2001 evaluations
WHO (2008)
Carbaryl in drinking-water
|
|---|
Carbaryl acts through inhibition of brain cholinesterase, and this is also its primary mode of toxicity. However, carbaryl is also considered to be a non-genotoxic carcinogen in mice, in which it causes vascular tumours in males. On this basis, JMPR established an ADI of 0–0.008 mg/kg body weight. This was based on a lowest-observed-adverse-effect level (LOAEL) of 15 mg/kg body weight per day and application of a safety factor of 2000 (10 for interspecies variation, 10 for intraspecies variation and 20 to reflect the occurrence of the rare and malignant tumour for which a no-effect level could not be identified).
A health-based value of 50 µg/l (rounded value) can be determined from the JMPR ADI of 0–0.008 mg/kg body weight, assuming a 60 kg adult drinking 2 litres of water per day and allowing 20% of the upper limit of the ADI from drinking-water. However, carbaryl does not appear to be found in drinking-water at significant concentrations, and so it is not considered necessary to propose a formal guideline value.
Carbofuran
Carbofuran (CAS No. 1563-66-2) is used worldwide as a pesticide for many crops. Residues in treated crops are generally very low or not detectable. The physicochemical properties of carbofuran and the few data on occurrence indicate that drinking-water from both groundwater and surface water sources is potentially the major route of exposure.
View in own window
| Guideline value | 0.007 mg/l (7 µg/l) |
|---|
| Occurrence | Has been detected in surface water, groundwater and drinking-water, generally at levels of a few micrograms per litre or lower; highest concentration (30 µg/l) measured in groundwater |
|---|
| ADI | 0–0.002 mg/kg body weight based on a NOAEL of 0.22 mg/kg body weight per day for acute (reversible) effects in dogs in a short-term (4-week) study conducted as an adjunct to a 13-week study in which inhibition of erythrocyte acetylcholinesterase activity was observed, and using an uncertainty factor of 100 |
|---|
| Limit of detection | 0.1 µg/l by GC with a nitrogen–phosphorus detector; 0.9 µg/l by reversed-phase HPLC with a fluorescence detector |
|---|
| Treatment performance | 1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of upper limit of ADI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Use of a 4-week study was considered appropriate because the NOAEL is based on a reversible acute effect; the NOAEL will also be protective for chronic effects. |
|---|
| Assessment date | 1998 |
|---|
| Principal references |
FAO/WHO (1997)
Pesticide residues in food—1996 evaluations
WHO (2004)
Carbofuran in drinking-water
|
|---|
Carbofuran is highly toxic after acute oral administration. The main systemic effect of carbofuran poisoning in short-term and long-term toxicity studies appears to be cholinesterase inhibition. No evidence of teratogenicity has been found in reproductive toxicity studies. On the basis of available studies, carbofuran does not appear to be carcinogenic or genotoxic.
Carbon tetrachloride
Carbon tetrachloride is used mainly in the production of chlorofluorocarbon refrigerants, foam-blowing agents and solvents. However, since the Montreal Protocol on Substances that Deplete the Ozone Layer (1987) and its amendments (1990 and 1992) established a timetable for the phase-out of the production and consumption of carbon tetrachloride, manufacture and use have dropped and will continue to drop. Carbon tetrachloride is released mostly into the atmosphere but also into industrial wastewater. Although it readily migrates from surface water to the atmosphere, levels in anaerobic groundwater may remain elevated for months or even years. Although available data on concentrations in food are limited, the intake from air is expected to be much greater than that from food or drinking-water.
View in own window
| Guideline value | 0.004 mg/l (4 µg/l) |
|---|
| Occurrence | Concentrations in drinking-water generally less than 5 µg/l |
|---|
| TDI | 1.4 µg/kg body weight, based on a NOAEL of 1 mg/kg body weight per day for hepatotoxic effects in a 12-week oral gavage study in rats, adjusting for daily dosing and applying an uncertainty factor of 500 (100 for interspecies and intraspecies variation, 10 for the duration of the study and a modifying factor of 0.5 because it was a bolus study) |
|---|
| Limit of detection | 0.1–0.3 µg/l by GC-ECD or GC-MS |
|---|
| Treatment performance | 0.001 mg/l should be achievable using air stripping |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The guideline value is lower than the range of values associated with upper-bound lifetime excess cancer risks of 10−4, 10−5 and 10−6 calculated by linear extrapolation. |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (1999)
Carbon tetrachloride
WHO (2004)
Carbon tetrachloride in drinking-water
|
|---|
The primary targets for carbon tetrachloride toxicity are liver and kidney. In experiments with mice and rats, carbon tetrachloride proved to be capable of inducing hepatomas and hepatocellular carcinomas. The doses inducing hepatic tumours were higher than those inducing cell toxicity. It is likely that the carcinogenicity of carbon tetrachloride is secondary to its hepatotoxic effects. On the basis of available data, carbon tetrachloride can be considered to be a non-genotoxic compound. Carbon tetrachloride is classified by IARC as being possibly carcinogenic to humans (Group 2B): there is sufficient evidence that carbon tetrachloride is carcinogenic in laboratory animals, but inadequate evidence in humans.
Chloral hydrate
Chloral hydrate, or trichloroacetaldehyde, can be formed as a by-product of the chlorination of water containing organic precursor material, such as fulvic and humic acids. It has been found in drinking-water at concentrations of up to 100 µg/l, but concentrations are usually below 10 µg/l. Concentrations are generally higher in surface water than in groundwater, and concentrations appear to increase during distribution.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2004 |
|---|
| Principal references |
IPCS (2000)
Chloral hydrate
IPCS (2000)
Disinfectants and disinfectant by-products
WHO (2005)
Chloral hydrate in drinking-water
|
|---|
Chloral hydrate is used as an intermediate in the production of insecticides, herbicides and hypnotic drugs. It has also been widely used as a sedative or hypnotic drug in humans at oral doses of up to about 750–1000 mg/day. Although intake from clinical use is considerably higher than intake from drinking-water, clinical exposure is of shorter-term duration.
No epidemiological or carcinogenic studies were found in humans that associated exposure to chloral hydrate with cancer, despite the fact that chloral hydrate has been used for many decades (and still is used) as a sedative and hypnotic drug in adults and children (specifically for dental procedures). IARC classified chloral hydrate as not classifiable as to its carcinogenicity to humans (Group 3), based on inadequate evidence in humans and limited evidence in experimental animals. There is equivocal evidence for the genotoxicity of chloral hydrate.
A health-based value of 0.1 mg/l (rounded figure) can be calculated on the basis of a TDI of 0.0045 mg/kg body weight derived based on an increased incidence of liver histopathology observed in mice in a 2-year drinking-water study, allocating 80% of the TDI to drinking-water (because most exposure to chloral hydrate is from drinking-water) and assuming a 60 kg adult consuming 2 litres of water per day. However, because chloral hydrate usually occurs in drinking-water at concentrations well below those of health concern, it is not considered necessary to derive a guideline value.
Chloral hydrate levels in drinking-water can be controlled by changes to disinfection practice (e.g. enhanced coagulation and softening to remove organic precursor compounds, moving the point of disinfection to reduce the reaction between chlorine and precursor compounds and using chloramines for residual disinfection instead of chlorine) and by GAC treatment.
Chloramines (monochloramine, dichloramine, trichloramine)
Monochloramine, dichloramines and trichloramines are considered by-products of drinking-water chlorination, being formed when chlorine and ammonia are added to water. Monochloramine may also be added to maintain residual disinfection activity in potable water distribution systems. Because higher chloramines are formed only occasionally and cause taste and odour problems at concentrations lower than those at which monochloramine causes taste and odour problems, only monochloramine has been considered for development of a health-based guideline value. Chloramine is rapidly decomposed in the stomach by gastric juice. The use of chloramines for disinfection instead of chlorine reduces the formation of THMs in drinking-water supplies. However, formation of other by-products, such as haloketones, chloropicrin, cyanogen chloride, HAAs, haloacetonitriles, aldehydes and chlorophenols, has been reported. Monochloramine, the most abundant chloramine, is recognized as a less effective disinfectant than chlorine and is used as a secondary disinfectant to maintain a residual in distribution systems.
View in own window
| Guideline value | Monochloramine: 3 mg/l (3000 µg/l) |
|---|
| Occurrence | Typical chloramine concentrations of 0.5–2 mg/l are found in drinking-water supplies where chloramine is used as a primary disinfectant or to provide a chlorine residual in the distribution system |
|---|
| TDI | 94 µg/kg body weight, based on a NOAEL of 9.4 mg/kg body weight per day, the highest dose administered to male rats in a 2-year United States National Toxicology Program (NTP) drinking-water study (although mean body weights of rats given the highest dose were lower than those of their respective control groups, it is probable that the lower body weights were caused by the unpalatability of the drinking-water) |
|---|
| Limit of detection | 10 µg/l by colorimetric methods |
|---|
| Treatment performance | It is possible to reduce the concentration of chloramine effectively to zero (< 0.1 mg/l) by reduction; however, it is normal practice to supply water with a chloramine residual of a few tenths of a milligram per litre to provide some protection against microbial recontamination and limit microbial growth problems during distribution. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 100% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | An additional uncertainty factor for possible carcinogenicity was not applied because equivocal cancer effects reported in the NTP study in only one species and in only one sex were within the range observed in historical controls. |
|---|
| Most individuals are able to taste chloramines at concentrations below 5 mg/l, and some at levels as low as 0.3 mg/l. |
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (2000)
Disinfectants and disinfectant by-products
WHO (2004)
Monochloramine in drinking-water
|
|---|
View in own window
| Reason for not establishing guideline values | Available data inadequate to permit derivation of health-based guideline values for dichloramine and trichloramine |
|---|
| Assessment date | 1993 |
|---|
| Principal references | IPCS (2000)
Disinfectants and disinfectant by-products |
|---|
Monochloramine
Although monochloramine has been shown to be mutagenic in some in vitro studies, it has not been found to be genotoxic in vivo. IARC has classified chloramine in Group 3 (not classifiable as to its carcinogenicity to humans). In the NTP bioassay in two species, the incidence of mononuclear cell leukaemias in female rats was increased, but no other increases in tumour incidence were observed. IPCS did not consider the increase in mononuclear cell leukaemia to be treatment related.
Dichloramine and trichloramine
Dichloramine and trichloramine have not been extensively studied, and available data are inadequate to permit derivation of health-based guideline values for either of these chemicals. However, these substances can cause taste and odour problems (see chapter 10) if formation of monochloramine is not controlled adequately.
Chlordane
Chlordane (CAS No. 57-47-9) is a broad-spectrum insecticide that has been used since 1947. Its use has recently been increasingly restricted in many countries, and it is now used mainly to destroy termites by subsurface injection into soil. Chlordane may be a low-level source of contamination of groundwater when applied by subsurface injection. Technical chlordane is a mixture of compounds, with the cis and trans forms of chlordane predominating. It is very resistant to degradation, highly immobile in soil and unlikely to migrate to groundwater, where it has only rarely been found. It is readily lost to the atmosphere. Although levels of chlordane in food have been decreasing, it is highly persistent and has a high bioaccumulation potential.
View in own window
| Guideline value | 0.0002 mg/l (0.2 µg/l) |
|---|
| Occurrence | Has been detected in both drinking-water and groundwater, usually at levels below 0.1 µg/l |
|---|
| PTDI | 0.5 µg/kg body weight based on a NOAEL of 50 µg/kg body weight per day for increased liver weights, serum bilirubin levels and incidence of hepatocellular swelling, derived from a long-term dietary study in rats, and using an uncertainty factor of 100 (10 each for interspecies and intraspecies variation) |
|---|
| Limit of detection | 0.014 µg/l by GC with ECD |
|---|
| Treatment performance | 0.1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 1% of PTDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Chlordane is listed under the Stockholm Convention on Persistent Organic Pollutants. Hence, monitoring may occur in addition to that required by drinking-water guidelines. |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (1995)
Pesticide residues in food—1994 evaluations
WHO (2003)
Chlordane in drinking-water
|
|---|
In experimental animals, prolonged exposure in the diet causes liver damage. Chlordane produces liver tumours in mice, but the weight of evidence indicates that it is not genotoxic. Chlordane can interfere with cell communication in vitro, a characteristic of many tumour promoters. IARC re-evaluated chlordane in 1991 and concluded that there is inadequate evidence for its carcinogenicity in humans and sufficient evidence for its carcinogenicity in animals, classifying it in Group 2B.
Chloride
Chloride in drinking-water originates from natural sources, sewage and industrial effluents, urban runoff containing de-icing salt and saline intrusion.
The main source of human exposure to chloride is the addition of salt to food, and the intake from this source is usually greatly in excess of that from drinking-water.
View in own window
| Reason for not establishing a guideline value | Not of health concern at levels found in drinking-water |
|---|
| Additional comments | May affect acceptability of drinking-water |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chloride in drinking-water |
|---|
Excessive chloride concentrations increase rates of corrosion of metals in the distribution system, depending on the alkalinity of the water. This can lead to increased concentrations of metals in the supply.
No health-based guideline value is proposed for chloride in drinking-water. However, chloride concentrations in excess of about 250 mg/l can give rise to detectable taste in water (see chapter 10).
Chlorine
Chlorine is produced in large amounts and widely used both industrially and domestically as an important disinfectant and bleach. In particular, it is widely used in the disinfection of swimming pools and is the most commonly used disinfectant and oxidant in drinking-water treatment. In water, chlorine reacts to form hypochlorous acid and hypochlorites. Concentrations of chlorate and some perchlorates increase in hypochlorite solutions upon storage at high ambient temperatures or when new hypochlorite is added to old hypochlorite.
View in own window
| Guideline value | 5 mg/l (5000 µg/l) for free chlorine |
|---|
| Occurrence | Present in most disinfected drinking-water at concentrations of 0.2–1 mg/l |
|---|
| TDI | 150 µg/kg body weight, derived from a NOAEL for the absence of toxicity in rodents ingesting chlorine in drinking-water for 2 years |
|---|
| Limit of detection | 0.01 µg/l following pre-column derivatization to 4-bromoacetanilide by HPLC; 10 µg/l as free chlorine by colorimetry; 200 µg/l by ion chromatography |
|---|
| Treatment performance | It is possible to reduce the concentration of chlorine effectively to zero (< 0.1 mg/l) by reduction. However, it is normal practice to supply water with a chlorine residual of a few tenths of a milligram per litre to provide some protection against microbial recontamination and limit microbial growth problems during distribution. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 100% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The guideline value is conservative, as no adverse effect level was identified in the critical study. |
|---|
| Most individuals are able to taste chlorine at the guideline value. |
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chlorine in drinking-water |
|---|
In humans and experimental animals exposed to chlorine in drinking-water, no specific adverse treatment-related effects have been observed. IARC has classified hypochlorite in Group 3 (not classifiable as to its carcinogenicity to humans).
Chlorine dioxide, chlorite and chlorate
Chlorite and chlorate are DBPs resulting from the use of chlorine dioxide as a disinfectant and for odour and taste control in water. Sodium chlorite and sodium chlorate are both used in the production of chlorine dioxide as well as for other commercial purposes. Chlorite and chlorate are also formed during the decomposition of hypochlorite solutions that are stored for long periods, particularly at warm temperatures. Where hypochlorite or chlorine dioxide is used as a disinfectant, the major route of environmental exposure to chlorite and chlorate is expected to be through drinking-water.
View in own window
| Provisional guideline values | Chlorite: 0.7 mg/l (700 µg/l) |
|---|
| Chlorate: 0.7 mg/l (700 µg/l) |
| The guideline values for chlorite and chlorate are designated as provisional because use of aged hypochlorite or of chlorine dioxide as disinfectants may result in the chlorite and chlorate guideline values being exceeded, and difficulties in meeting the guideline values must never be a reason for compromising adequate disinfection |
| Occurrence | When chlorine dioxide is used as the final disinfectant at typical doses, the resulting chlorite concentration would normally be less than 0.2 mg/l. Chlorate concentrations above 1 mg/l have been reported when hypochlorite was used, but such high concentrations would be unusual unless hypochlorite is stored under adverse conditions. |
|---|
| ADIs |
Chlorite: 0–0.03 mg/kg bw based on a NOAEL of 3 mg/kg bw per day for reduced liver weight of F0 females and F1 males and females in a two-generation reproductive toxicity study in rats and using a safety factor of 100 (10 each for interspecies and intraspecies variability)
Chlorate: 0–0.01 mg/kg bw based on a BMDL10 of 1.1 mg/kg bw per day for non-neoplastic effects on the thyroid of male rats in a carcinogenicity study and using a safety factor of 100 (10 to allow for intraspecies variability and an additional factor of 10 to allow for the deficiencies in the database; a safety factor for interspecies variation was not considered necessary because humans are likely to be less sensitive than rats to these effects)
|
|---|
| Limit of detection | MDLs as low as 0.45 µg/l for chlorite and 0.78 µg/l for chlorate (IC with conductivity detection) and 78 µg/l for chlorine dioxide (UV/visible spectrophotometric method) |
|---|
| Prevention and treatment |
When using hypochlorite, the following control approach is recommended to minimize formation of chlorite and chlorate: purchase fresh solutions that are of an appropriate quality, store them in a cool place and out of direct sunlight, and use the hypochlorite as soon as possible after purchase (e.g. within a month, if possible). Further, new hypochlorite solutions should not be added to containers containing old hypochlorite solutions, as this will accelerate chlorate formation.
It is possible to reduce the concentration of chlorine dioxide and chlorite effectively to zero (<0.1 mg/l) by reduction; however, it is normal practice to supply water with a chlorine dioxide residual of a few tenths of a milligram per litre to provide some protection against microbial regrowth and limit microbial growth problems during distribution. With chlorine dioxide disinfection, the concentrations of chlorate and chlorite depend on process conditions (in both the chlorine dioxide generator and the water treatment plant) and applied dose of chlorine dioxide. As there is no low-cost option for reducing concentrations of chlorate once it is formed, control of chlorate concentration must rely on preventing its addition (from sodium hypochlorite) or formation (from chlorine dioxide). If chlorine dioxide is used as a pre-oxidant, the resulting chlorite concentration may need to be reduced using ferrous iron, sulfur reducing agents or activated carbon.
|
|---|
| Guideline value derivation |
|---|
| • allocation to water | 80% of ADI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Concentrations should be maintained as low as reasonably practical, without compromising adequate disinfection. Although a health-based value of 0.3 mg/l could be derived from the ADI for chlorate, in some circumstances, it may not be possible to adequately disinfect potable water and maintain chlorate concentrations at or below the health-based value as chlorate is a byproduct of hypochlorite. Therefore, the previous provisional guideline value is retained. Moreover, even this provisional guideline value may be exceeded when aged hypochlorite is used and difficulties in meeting the guideline value must never be a reason for compromising adequate disinfection. |
|---|
| Assessment date | 2016 |
|---|
| Principal references |
IPCS (2000). Disinfectants and disinfectant by-products
WHO (2008). Acidified sodium chlorite
WHO (2016). Chlorine dioxide, chlorate and chlorite in drinking-water
|
|---|
Chlorine dioxide
Any chlorine dioxide remaining at the consumer’s tap will be reduced to chlorite and chloride upon ingestion. Consequently, a guideline value for chlorine dioxide has not been established. The provisional guideline values for chlorite and chlorate are adequately protective for potential toxicity from chlorine dioxide. The taste and odour threshold for chlorine dioxide is 0.2–0.4 mg/l.
Chlorite
IARC has concluded that chlorite is not classifiable as to its carcinogenicity to humans. The primary and most consistent finding arising from exposure to chlorite in a number of species was oxidative stress resulting in changes in the red blood cells. This observation was supported by a number of biochemical studies conducted in vitro. Studies with human volunteers for up to 12 weeks did not identify any effect on blood parameters at the highest dose tested, 36 µg/kg bw per day.
Chlorate
Although chlorate has also been reported to have effects on red blood cells, the most sensitive effects observed in rats administered sodium chlorate in drinking-water for 21 or 90 days were changes in thyroid histology (e.g. colloid depletion, hypertrophy, incidence and severity of hyperplasia) and in thyroid hormones. As with chlorite, a chlorate dose of 36 µg/kg bw per day for 12 weeks did not result in any adverse effects in human volunteers.
Chloroacetones
1,1-Dichloroacetone is formed from the reaction between chlorine and organic precursors and has been detected in chlorinated drinking-water. Concentrations are estimated to be less than 10 µg/l and usually less than 1 µg/l.
View in own window
| Reason for not establishing guideline values | Available data inadequate to permit derivation of health-based guideline values for any of the chloroacetones |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chloroacetones in drinking-water |
|---|
The toxicological data on 1,1-dichloroacetone are very limited, although studies with single doses indicate that it affects the liver.
There are insufficient data at present to permit the setting of guideline values for 1,1-dichloroacetone or any of the other chloroacetones.
Chlorophenols (2-chlorophenol, 2,4-dichlorophenol, 2,4,6-trichlorophenol)
Chlorophenols are present in drinking-water as a result of the chlorination of phenols, as by-products of the reaction of hypochlorite with phenolic acids, as biocides or as degradation products of phenoxy herbicides. Those most likely to occur in drinking-water as by-products of chlorination are 2-chlorophenol, 2,4-dichlorophenol and 2,4,6-trichlorophenol. The taste thresholds for chlorophenols in drinking-water are low.
View in own window
| Guideline value | 2,4,6-Trichlorophenol: 0.2 mg/l (200 µg/l) |
|---|
| Occurrence | Concentrations of chlorophenols in drinking-water usually less than 1 µg/l |
|---|
| Basis of guideline value derivation | Applying the linearized multistage model to leukaemias in male rats observed in a 2-year feeding study (hepatic tumours found in this study were not used for risk estimation because of the possible role of contaminants in their induction) |
|---|
| Limit of detection | 0.5–5 µg/l by formation of pentafluorobenzyl ether derivatives; 0.01 µg/l using GC with ECD |
|---|
| Treatment performance | 2,4,6-Trichlorophenol concentrations can be reduced using GAC |
|---|
| Additional comments | The guideline value for 2,4,6-trichlorophenol exceeds its lowest reported taste threshold. |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chlorophenols in drinking-water |
|---|
View in own window
| Reason for not establishing guideline values | Available data inadequate to permit derivation of health-based guideline values for 2-chlorophenol and 2,4-dichlorophenol |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chlorophenols in drinking-water |
|---|
2-Chlorophenol
Data on the toxicity of 2-chlorophenol are limited. Therefore, no health-based guideline value has been derived.
2,4-Dichlorophenol
Data on the toxicity of 2,4-dichlorophenol are limited. Therefore, no health-based guideline value has been derived.
2,4,6-Trichlorophenol
2,4,6-Trichlorophenol has been reported to induce lymphomas and leukaemias in male rats and hepatic tumours in male and female mice. The compound has not been shown to be mutagenic in the Ames test but has shown weak mutagenic activity in other in vitro and in vivo studies. IARC has classified 2,4,6-trichlorophenol in Group 2B (possibly carcinogenic to humans).
Chloropicrin
Chloropicrin, or trichloronitromethane, is formed by the reaction of chlorine with humic and amino acids and with nitrophenols. Its formation is increased in the presence of nitrates. Limited data from the USA indicate that concentrations in drinking-water are usually less than 5 µg/l.
View in own window
| Reason for not establishing a guideline value | Available data inadequate to permit derivation of health-based guideline value |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chloropicrin in drinking-water |
|---|
Decreased survival and body weights have been reported following long-term oral exposure in laboratory animals. Chloropicrin has been shown to be mutagenic in bacterial tests and in in vitro assays in lymphocytes. Because of the high mortality in a carcinogenesis bioassay and the limited number of end-points examined in the 78-week toxicity study, the available data were considered inadequate to permit the establishment of a guideline value for chloropicrin.
Chlorotoluron
Chlorotoluron (CAS No. 15545-48-9) is a pre emergence or early post emergence herbicide that is slowly biodegradable and mobile in soil. There is only very limited exposure to this compound from food.
View in own window
| Guideline value | 0.03 mg/l (30 µg/l) |
|---|
| Occurrence | Detected in drinkingwater at concentrations of less than 1 µg/l |
|---|
| TDI | 11.3 µg/kg body weight, derived from a NOAEL of 11.3 mg/kg body weight per day for systemic effects in a 2-year feeding study in mice using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for evidence of carcinogenicity) |
|---|
| Limit of detection | 0.1 µg/l by separation by reversed-phase HPLC followed by UV and electrochemical detection |
|---|
| Treatment performance | 0.1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chlorotoluron in drinking-water |
|---|
Chlorotoluron is of low toxicity following single, short-term and long-term exposures in experimental animals, but it has been shown to cause an increase in adenomas and carcinomas of the kidneys of male mice given high doses for 2 years. As no carcinogenic effects were reported in a 2-year study in rats, it has been suggested that chlorotoluron has a carcinogenic potential that is both species and sex specific. Chlorotoluron and its metabolites have shown no evidence of genotoxicity.
Chlorpyrifos
Chlorpyrifos (CAS No. 2921-88-2) is a broad-spectrum organophosphorus insecticide used for the control of mosquitoes, flies, various crop pests in soil and on foliage, household pests and aquatic larvae. Although it is not recommended for addition to water for public health purposes by the WHO Pesticide Evaluation Scheme (WHOPES), it may be used in some countries as an aquatic larvicide for the control of mosquito larvae. Chlorpyrifos is strongly absorbed by soil and does not readily leach from it, degrading slowly by microbial action. It has a low solubility in water and great tendency to partition from aqueous phases into organic phases in the environment.
View in own window
| Guideline value | 0.03 mg/l (30 µg/l) |
|---|
| Occurrence | Detected in surface waters in the USA, usually at concentrations below 0.1 µg/l; also detected in groundwater in less than 1% of the wells tested, usually at concentrations below 0.01 µg/l |
|---|
| ADI | 0–0.01 mg/kg body weight on the basis of a NOAEL of 1 mg/kg body weight per day for inhibition of brain acetylcholinesterase activity in studies in mice, rats and dogs, using a 100-fold uncertainty factor, and on the basis of a NOAEL of 0.1 mg/kg body weight per day for inhibition of erythrocyte acetylcholinesterase activity in a study of human subjects exposed for 9 days, using a 10-fold uncertainty factor |
|---|
| Limit of detection | 1 µg/l by GC using ECD or flame photometric detection |
|---|
| Treatment performance | No data available; should be amenable to treatment by coagulation (10–20% removal), activated carbon adsorption and ozonation |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of upper limit of ADI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (2000)
Pesticide residues in food—1999 evaluations
WHO (2003)
Chlorpyrifos in drinking-water
|
|---|
JMPR concluded that chlorpyrifos is unlikely to pose a carcinogenic risk to humans. Chlorpyrifos was not genotoxic in an adequate range of studies in vitro and in vivo. In long-term studies, inhibition of cholinesterase activity was the main toxicological finding in all species.
Chromium
Chromium is widely distributed in Earth’s crust. It can exist in valences of −2 to +6 but is primarily in its trivalent (III) and hexavalent (VI) forms. In general, with the exception of individuals living close to a point source of contamination, food appears to be the major source of intake.
View in own window
| Guideline value | Total chromium: 0.05 mg/l (50 µg/l) |
|---|
| Occurrence | Total chromium concentrations in drinking-water are usually low (<5 μg/l), although there may be elevated concentrations in areas containing natural sources or as a result of anthropogenic contamination. Few data are available on the speciation of chromium in drinking-water. |
|---|
| Basis of guideline value derivation | The guideline value is based on achievability by available treatment technologies, measurability by analytical methods, and toxicology. The guideline value is for total chromium because of limitations in currently available analytical methods that preclude reliable speciation of chromium in water. Hyperplasia in the small intestine was considered the most sensitive end-point of tumour formation, and is protective of both noncancer (in the case of chromium(III) and chromium(VI)) and cancer (in the case of chromium(VI)) effects. |
|---|
| Limit of detection | 0.08–7 μg/l for total chromium by ICP-AES, ICP-MS, AES or graphite furnace AAS; 0.5 mg/ for total chromium by FAAS. FAAS and electrothermal atomization AAS methods are recommended when concentrations are 0.5–20 mg/l and <0.1 mg/ml, respectively. |
|---|
| 0.0044–0.015 μg/l for chromium(VI) by IC with post-column derivatization and UV-visible spectroscopy; 0.5 μg/l for chromium (III) and chromium(VI) using ion chromatography followed by ICP-MS. |
| Determination of chromium species remains difficult; reliable and validated methods to separate analysis of chromium(III) and chromium(VI) are still required. |
| Treatment performance | Effective central treatment technologies for chromium(III) and chromium(VI) include conventional water treatment (coagulation, sedimentation, filtration—requires chromium(VI) reduction to chromium(III)), adsorption by iron oxides, ion exchange, reverse osmosis and nanofiltration. |
|---|
| Additional comments | As chromium is usually found in drinking-water below the guideline value, in general, monitoring and inclusion in drinking-water standards would only be necessary if there were indications that a problem might exist. Monitoring can usually be limited to treatment works; however, specific pollution events may need to be considered on a case-by-case basis. |
|---|
| Assessment date | 2020 |
|---|
| Principal reference | WHO (2020)
Chromium in drinking-water |
|---|
Chromium toxicity is dependent on its valence state; chromium(VI) is known to be more toxic than chromium(III). Following oral exposure, chromium(VI) is rapidly and efficiently reduced in the gastrointestinal tract to chromium(III), although a proportion of chromium(VI) may remain available for absorption. IARC has classified chromium(VI) compounds as “carcinogenic to humans” (Group 1) by the inhalation route of exposure; however, data on human carcinogenicity via the oral route are lacking. In a 2-year chronic drinking-water study, increased incidence of tumours of the oral cavity squamous epithelium and of the small intestinal epithelium were reported in rats and mice (respectively) exposed to chromium(VI), at doses of 0.77 mg/kg bw per day in rats and 0.38 mg/kg bw/day in mice. These tumours have been attributed to a threshold mode of action, recognizing an absence of mutagenicity in highly proliferative intestinal tissue following drinking-water exposure, lack of concordance of mutagenicity and tumour development, and early onset of crypt cell proliferation, which is unlikely to result from a fixed mutation. Concentrations in drinking-water rarely approach the guideline value, and evaluation of drinking-water carcinogenicity studies indicate that most environmental concentrations of chromium(VI) are orders of magnitude below the lowest concentrations tested in the rodent cancer bioassays; therefore, it was determined that the existing guideline value continues to be adequately health-protective.
Copper
Copper is both an essential nutrient and a drinking-water contaminant. It is used to make pipes, valves and fittings and is present in alloys and coatings. Copper sulfate pentahydrate is sometimes added to surface water for the control of algae. Copper concentrations in drinking-water vary widely, with the primary source most often being the corrosion of interior copper plumbing. Levels in running or fully flushed water tend to be low, whereas those in standing or partially flushed water samples are more variable and can be substantially higher (frequently above 1 mg/l). Copper concentrations in treated water often increase during distribution, especially in systems with an acid pH or high-carbonate waters with an alkaline pH. Food and water are the primary sources of copper exposure in developed countries. Consumption of standing or partially flushed water from a distribution system that includes copper pipes or fittings can considerably increase total daily copper exposure, especially for infants fed formula reconstituted with tap water.
View in own window
| Guideline value | 2 mg/l (2000 µg/l) |
|---|
| Occurrence | Concentrations in drinking-water range from ≤ 0.005 to > 30 mg/l, primarily as a result of the corrosion of interior copper plumbing |
|---|
| Basis of guideline value derivation | To be protective against acute gastrointestinal effects of copper and provide an adequate margin of safety in populations with normal copper homeostasis |
|---|
| Limit of detection | 0.02–0.1 µg/l by ICP-MS; 0.3 µg/l by ICP–optical emission spectroscopy; 0.5 µg/l by flame AAS |
|---|
| Treatment performance | Copper is not removed by conventional treatment processes. However, copper is not normally a raw water contaminant. |
|---|
| Additional comments | For adults with normal copper homeostasis, the guideline value should permit consumption of 2 or 3 litres of water per day, use of a nutritional supplement and copper from foods without exceeding the tolerable upper intake level of 10 mg/day or eliciting an adverse gastrointestinal response. |
|---|
| Staining of laundry and sanitary ware occurs at copper concentrations above 1 mg/l. At levels above 2.5 mg/l, copper imparts an undesirable bitter taste to water; at higher levels, the colour of water is also impacted. |
| In most instances where copper tubing is used as a plumbing material, concentrations of copper will be below the guideline value. However, there are some conditions, such as highly acidic or aggressive waters, that will give rise to much higher copper concentrations, and the use of copper tubing may not be appropriate in such circumstances. |
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (1998)
Copper
WHO (2004)
Copper in drinking-water
|
|---|
IPCS concluded that the upper limit of the acceptable range of oral intake in adults is uncertain but is most likely in the range of several (more than 2 or 3 mg/day), but not many, milligrams per day in adults. This evaluation was based solely on studies of gastrointestinal effects of copper-contaminated drinking-water. The available data on toxicity in experimental animals were not considered helpful in establishing the upper limit of the acceptable range of oral intake owing to uncertainty about an appropriate model for humans, but they help to establish a mode of action for the response. The data on the gastrointestinal effects of copper must be used with caution, as the effects observed are influenced by the concentration of ingested copper to a greater extent than the total mass or dose ingested in a 24-hour period. Recent studies have delineated the threshold for the effects of copper in drinking-water on the gastrointestinal tract, but there is still some uncertainty regarding the long-term effects of copper on sensitive populations, such as carriers of the gene for Wilson disease and other metabolic disorders of copper homeostasis.
Cyanazine
Cyanazine (CAS No. 21725-46-2) is a member of the triazine family of herbicides. It is used as a pre-emergence and post-emergence herbicide for the control of annual grasses and broadleaf weeds. It can be degraded in soil and water by microorganisms and by hydrolysis.
View in own window
| Guideline value | 0.0006 mg/l (0.6 µg/l) |
|---|
| Occurrence | Has been detected in surface water and groundwater, usually at concentrations of a few micrograms per litre, although levels as high as 1.3 and 3.5 mg/l have been measured in surface water and groundwater, respectively |
|---|
| TDI | 0.198 µg/kg body weight based on a NOAEL of 0.198 mg/kg body weight for hyperactivity in male rats in a 2-year toxicity/carcinogenicity study, using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for limited evidence of carcinogenicity) |
|---|
| Limit of detection | 0.01 µg/l by GC-MS |
|---|
| Treatment performance | 0.1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1998 |
|---|
| Principal reference | WHO (2003)
Cyanazine in drinking-water |
|---|
On the basis of the available mutagenicity data on cyanazine, evidence for genotoxicity is equivocal. Cyanazine causes mammary gland tumours in rats but not in mice. The mechanism of mammary gland tumour development in rats is currently under investigation and may prove to be hormonal. Cyanazine is also teratogenic in rats at dose levels of 25 mg/kg body weight per day and higher.
Cyanide
Cyanides can be found in some foods, particularly in some developing countries, and they are occasionally found in drinking-water, but usually only at very low concentrations. However, there are occasions on which large spills of cyanide, associated with industry, occur, and these can give rise to very high concentrations in drinking-water source waters, particularly surface waters.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern, except in emergency situations following a spill to a water source |
|---|
| Assessment date | 2009 |
|---|
| Principal references |
IPCS (2004)
Hydrogen cyanide and cyanides
WHO (2009)
Cyanide in drinking-water
|
|---|
Cyanide is highly acutely toxic. It is detoxified in the liver by first-pass metabolism following oral exposure. As a consequence, exposure to a dose spread over a longer period, through a day, for example, will result in lower toxicity, or higher tolerance, than the same dose given in a single bolus dose. Exposure to high doses can give rise to thyroid toxicity as a secondary effect of exposure due to the inhibition of iodine uptake from the thiocyanate generated through the detoxifying action of rhodanese. It is difficult to interpret human data in view of the difficulty in assessing the actual absorbed dose in humans following acute fatal intoxication and the lack of well-conducted studies on sublethal toxicity.
There is a need for guidance regarding concentrations that would not be of concern for public health following short-term exposure to cyanide. However, because cyanide is unlikely to occur in drinking-water at concentrations of health concern, it is considered unnecessary to derive a formal guideline value for short-term exposure to cyanide.
The data on acute exposure to cyanide are unsuitable for use in deriving a health-based value for short-term exposure because of the high uncertainty surrounding the data. Using the NOAEL for effects on the reproductive organs of male rats in a 13-week study and an uncertainty factor of 100, a TDI of 0.045 mg/kg body weight can be derived. Because this health-based value is intended for short-term use and exposure would not exceed 5 days, it is considered to be acceptable to allocate 40% of the TDI to drinking-water to allow for exposure to cyanogenic glycosides in food. Therefore, assuming a 60 kg adult drinking 2 litres of water per day with an allocation of 40% of the TDI to drinking-water, a health-based value of 0.5 mg/l (rounded value) for short-term exposure can be calculated.
This health-based value is well below the level that is normally considered to be of health concern for humans. Cyanide is rapidly detoxified, and exposure spread throughout the day will further reduce the potential for effects. This health-based value would be suitable for use for a limited period of up to 5 days, which is the longest period likely to be required under the circumstances of such an emergency. However, it is probable that, in most circumstances, this value will be highly conservative for short-term exposure.
It should be noted that the lowest reported odour threshold for cyanide in drinking-water is 0.17 mg/l, which is below the short-term health-based value. It is therefore possible that a small number of individuals will detect cyanide by odour at concentrations below the health-based value.
The health-based value relates to total cyanide concentration at the tap, including cyanide from cyanogen chloride in drinking-water as a by-product of disinfection with chlorine. Cyanogen chloride rapidly breaks down to cyanide in the distribution system or when ingested. As the low levels of cyanide normally found in drinking-water are mostly a consequence of the presence of cyanogen chloride, it is not considered necessary to develop a guideline value for long-term exposure to cyanide.
Cyanogen chloride
Cyanogen chloride may be formed as a by-product of chloramination or chlorination of water. It is also formed by the chlorination of cyanide ion present in raw water.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2009 |
|---|
| Principal references |
IPCS (2004)
Hydrogen cyanide and cyanides
WHO (2009)
Cyanogen chloride in drinking-water
|
|---|
Cyanogen chloride is rapidly metabolized to cyanide in the body. There are few data available on the oral toxicity of cyanogen chloride.
As cyanogen chloride is unlikely to be found in drinking-water at concentrations that are of health concern, it is considered unnecessary to develop a formal guideline value for cyanogen chloride. Instead, for guidance purposes, a health-based value is derived based on cyanide.
Using a NOAEL for cyanide of 4.5 mg/kg body weight per day for minor changes in the testis in a subchronic study in which rats were exposed through their drinking-water and an uncertainty factor of 100, a TDI for cyanide of 0.045 mg/kg body weight (corresponding to a cyanogen chloride dose of 0.11 mg/kg body weight) can be derived. In view of the minor nature of the changes observed and the NOAEL in a previous chronic study, it is not considered necessary to include an additional uncertainty factor to allow for the length of the study. Further, it appears that a dose that may be toxic in acute poisoning would certainly be tolerated under chronic conditions, owing to efficient detoxification. Assuming a 60 kg adult drinking 2 litres of water per day and allowing 20% of the TDI to come from water because of the potential for exposure to cyanogenic glycosides in food, the health-based value for long-term exposure is 0.3 mg/l for cyanide or 0.6 mg/l for cyanogen chloride (rounded values).
Although low concentrations of cyanide in raw waters will be converted to cy anogen chloride by chlorination, cyanogen chloride may also be formed during the production of chloramines in situ as a residual disinfectant to maintain the hygienic condition of the distribution system. It is important that treatment be optimized to minimize the formation of cyanogen chloride while maintaining adequate chloramine residuals where chloramination is practised.
Cylindrospermopsins (cyanobacterial toxins)4
Cylindrospermopsin (CYN) and its four variants are naturally occurring alkaloids produced by strains of various species of cyanobacteria, primarily in freshwater environments. They have been found in cyanobacteria around the globe and have most frequently been reported from the cyanobacterial genera Raphidiopsis (formerly Cylindrospermopsis), Aphanizomenon and Chrysosporum (see also section 11.5). Unlike MCs, a major fraction of CYNs is often found dissolved in water; particularly at lower temperatures, these toxins may persist even after the producing cyanobacteria are no longer present.
Drinking-water is the most likely route of exposure to CYNs where surface water with cyanobacterial blooms is the drinking-water source. Recreational activities in lakes with cyanobacterial blooms may also be a relevant exposure pathway, potentially to high, usually intermittent concentrations (see WHO Guidelines on recreational water quality, 2021). Limited data suggest that CYNs may also accumulate in some food items.
View in own window
| Provisional guideline value (lifetime) |
Total CYNs (sum of all congeners, free plus cell-bound): 0.0007 mg/l (0.7 µg/l)
|
|---|
| The guideline value is provisional because of the high level of uncertainty—it is based on data for only CYN and the database is limited, as reflected in the composite uncertainty factor of 1000 |
| Provisional short-term guideline value | Total CYNs (sum of all congeners, free plus cell-bound): 0.003 mg/l (3 µg/l) |
|---|
| Occurrence | Concentrations reported usually range well below 1 mg/l; outside of scum areas, they rarely exceed several µg/l. Major fractions (up to 90%) can occur dissolved in water |
|---|
| TDI | 0.03 µg/kg bw, based on a NOAEL of 30 µg/kg bw per day for renal pathology observed in an 11-week study in mice and applying an uncertainty factor of 1000 (10 each for inter- and intra-species variability and 10 for database deficiencies,* taking into consideration limitations in the database—in particular, limited data on chronic toxicity, reproductive toxicity and carcinogenicity) |
|---|
| Limit of detection |
0.065 µg/L by LC-MS/MS or LC (including HPLC) followed by UV/PDA detection. LC-MS/MS has the highest specificity and sensitivity but requires quantitative reference standards for each CYN in the sample. For UV/PDA detection, the signal from one can be used to estimate the concentrations of each.
Prior extraction of cells with freeze–thaw cycles and water or methanol/water is necessary for cell-bound CYNs; neglecting extraction from cells will lead to dramatic underestimation of concentrations.
|
|---|
| 0.05 µg/l by commercially available immunoassay kits (ELISA); although these are less precise than LC with the above-mentioned detection methods, they capture all CYNs and thus are useful for most monitoring purposes. |
| Monitoring | The likelihood of blooms can be assessed by understanding water body conditions (in particular, nutrient concentrations, water body depth, water retention time, patterns of mixing and stratification; see section 11.5). Where conditions render blooms likely, visual monitoring of source water (including microscopy for potentially CYN-containing genera) for evidence of increasing cyanobacterial biomass (blooms) is important because biomass can increase rapidly. Exceeding alert values for biomass indicators or CYN concentrations should trigger responses to prevent exposure to elevated toxin concentrations (see the alert level framework in section 11.5). As a major fraction of CYN may occur dissolved in water and be persistent, if blooms of potentially CYN-producing genera have been observed, monitoring should also include toxin analysis for CYNs, if possible. Analysis of cyanotoxins is particularly useful for validating and optimizing the efficacy of control measures such as riverbank filtration or treatment. |
|---|
| Prevention and treatment | Actions to decrease the probability of bloom occurrence include catchment and source water management, such as reducing nutrient loading or changing reservoir stratification and mixing. Filtration is effective for removing intact cyanobacterial cells. For the often large dissolved fraction of CYNs, oxidation with chlorine or ozone at sufficient concentrations and contact times, as well as GAC and some PAC applications, are effective (see chapters 7–10 of Toxic cyanobacteria in water; Annex 1). |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 80% of TDI; for short-term exposure, 100% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Total CYNs as gravimetric or molar equivalents should be evaluated against the guideline values since CYNs usually occur as mixtures. Although the guideline values are based on CYN, limited evidence suggests that other CYN congeners have similar toxicity to CYN. |
|---|
| The provisional short-term drinking-water guideline value is intended to indicate the extent to which the lifetime value can be exceeded for periods of up to about 2 weeks until water treatment can be augmented to bring the concentration of CYNs back under control. It is not intended to allow repeated seasonal exceedances of the lifetime value. |
| It is recommended, as a precautionary measure, that bottle-fed infants and small children be provided with an alternative safe drinking-water source (e.g. bottled water that is certified by the responsible authorities) if concentrations are greater than 0.7 μg/L, even for short periods. |
| Assessment date | 2020 |
|---|
| Principal references |
WHO (2020)
Cyanobacterial toxins: cylindrospermopsins
Chorus & Welker (2021) Toxic cyanobacteria in water
|
|---|
- *
For the short-term guideline value, a database uncertainty factor was applied due to the limited database, including lack of data on reproductive effects after oral exposure, and evidence of potential in vivo genotoxicity of CYNs. An uncertainty factor of 3 was applied because lack of chronic toxicity data does not affect derivation of a guideline value for short-term exposures.
CYN is a potent inhibitor of protein synthesis, and also has cytochrome P450-dependent effects on other processes—for example, DNA damage and induction of cellular stress responses. CYN was the likely cause of a mass human poisoning incident in Australia in 1979.
The provisional guideline values for CYNs are based on a study in male mice using CYN, corroborated by two other studies in both sexes of different strains of mice. The studies demonstrated that a range of organs were adversely affected. The kidneys were identified as the most sensitive organs in these studies, and one study found gender-based differences in the sensitivity of the liver and kidneys. Other studies in mice have demonstrated CYN-induced DNA damage in various organs.
Practical considerations
Where nutrient (phosphorus and nitrogen) concentrations are elevated in lakes, reservoirs or slowly flowing rivers, cyanobacteria occur widely. Where their excessive growth leads to high biomass, sometimes termed “bloom” events, CYNs can reach concentrations in raw water that are potentially hazardous to human health. Such blooms tend to recur in the same water bodies and to be seasonal, whereas others occur perennially. Although some CYN-producing cyanobacteria form scums or accumulate at the thermocline of thermally stratified reservoirs, they tend to not be as pronounced as the scums and accumulations of MC-producing cyanobacteria.
Cyanobacteria are most effectively controlled in the context of developing a WSP (see chapter 4). Control measures to manage potential risks from cyanobacteria, and in particular from their toxins, in drinking-water should include not only adequate treatment, but also measures to control cyanobacterial bloom development. See section 11.5 for more information on cyanobacteria, including further details on monitoring cyanobacterial blooms, the alert level framework, and prevention and management of cyanobacteria in source waters. Effectively minimizing the formation of blooms and locating the raw water intake away from blooms reduce the treatment steps required to remove cyanotoxins.
Drinking-water treatment that removes particles—that is, soil, slow sand or riverbank filtration, conventional water treatment (coagulation, flocculation and filtration or dissolved air flotation) or dissolved air flotation—can remove cell-bound CYNs effectively. Soil, slow sand and riverbank filtration can also remove dissolved cyanotoxins. For all these processes it is important that they are optimized to target the removal of cells and dissolved toxins. While for both pre-oxidation and conventional treatment, cell rupture and toxin release should be avoided, treatment also needs to target the typically large fraction of dissolved CYN. Chlorination and ozonation at sufficiently high doses and contact times are effective for degrading dissolved CYNs; however, elevated organic carbon in bloom situations will substantially increase the disinfectant demand. Chlorine dioxide and chloramine are ineffective for degrading CYNs. GAC and PAC can be effective for removing dissolved CYNs, with efficacy dependent on several factors, including the type of activated carbon, contact times (PAC), flow rates (GAC) and water quality. As the challenges that blooms present for treatment are complex, periodic validation of efficacy during bloom situations and under the specific local conditions is particularly important. Avoiding bloom occurrence and intake is therefore the preferred option.
2,4-D
The term 2,4-D is used here to refer to the free acid, 2,4-dichlorophenoxyacetic acid (CAS No. 94-75-7). Commercial 2,4-D products are marketed as the free acid, alkali and amine salts and ester formulations. 2,4-D itself is chemically stable, but its esters are rapidly hydrolysed to the free acid. 2,4-D is a systemic herbicide used for control of broad-leaved weeds, including aquatic weeds. 2,4-D is rapidly biodegraded in the environment. Residues of 2,4-D in food rarely exceed a few tens of micrograms per kilogram.
View in own window
| Guideline value | 0.03 mg/l (30 µg/l) |
|---|
| Occurrence | Levels in water usually below 0.5 µg/l, although concentrations as high as 30 µg/l have been measured |
|---|
| ADI | 0–0.01 mg/kg body weight for the sum of 2,4-D and its salts and esters, expressed as 2,4-D, on the basis of a NOAEL of 1 mg/kg body weight per day in a 1-year study of toxicity in dogs (for a variety of effects, including histopathological lesions in kidneys and liver) and a 2-year study of toxicity and carcinogenicity in rats (for renal lesions) |
|---|
| Limit of detection | 0.1 µg/l by gas–liquid chromatography with electrolytic conductivity detection |
|---|
| Treatment performance | 1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of upper limit of ADI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The guideline value applies to 2,4-D, as salts and esters of 2,4-D are rapidly hydrolysed to the free acid in water. |
|---|
| Assessment date | 1998 |
|---|
| Principal references |
FAO/WHO (1997)
Pesticide residues in food—1996 evaluations
WHO (2003)
2,4-D in drinking-water
|
|---|
Epidemiological studies have suggested an association between exposure to chlorophenoxy herbicides, including 2,4-D, and two forms of cancer in humans: soft tissue sarcomas and non-Hodgkin lymphoma. The results of these studies, however, are inconsistent; the associations found are weak, and conflicting conclusions have been reached by the investigators. Most of the studies did not provide information on exposure specifically to 2,4-D, and the risk was related to the general category of chlorophenoxy herbicides, a group that includes 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), which was potentially contaminated with dioxins. JMPR concluded that it was not possible to evaluate the carcinogenic potential of 2,4-D on the basis of the available epidemiological studies. JMPR also concluded that 2,4-D and its salts and esters are not genotoxic. The toxicity of the salts and esters of 2,4-D is comparable to that of the acid.
2,4-DB
The half-lives for degradation of chlorophenoxy herbicides, including 2,4-DB, or 2,4-dichlorophenoxybutyric acid (CAS No. 94-82-6), in the environment are in the order of several days. Chlorophenoxy herbicides are not often found in food.
View in own window
| Guideline value | 0.09 mg/l (90 µg/l) |
|---|
| Occurrence | Chlorophenoxy herbicides not frequently found in drinking-water; when detected, concentrations usually no greater than a few micrograms per litre |
|---|
| TDI | 30 µg/kg body weight, based on a NOAEL of 3 mg/kg body weight per day for effects on body and organ weights, blood chemistry and haematological parameters in a 2-year study in rats, with an uncertainty factor of 100 (for interspecies and intraspecies variation) |
|---|
| Limit of detection | 1 µg/l to 1 mg/l for various methods commonly used for the determination of chlorophenoxy herbicides in water, including solvent extraction, separation by GC, gas–liquid chromatography, thin-layer chromatography or HPLC, with ECD or UV detection |
|---|
| Treatment performance | 0.1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The NOAEL used in the guideline value derivation is similar to the NOAEL of 2.5 mg/kg body weight per day obtained in a short-term study in dogs and the NOAEL for hepatocyte hypertrophy of 5 mg/kg body weight per day obtained in a 3-month study in rats. |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chlorophenoxy herbicides (excluding 2,4-D and MCPA) in drinking-water |
|---|
Chlorophenoxy herbicides, as a group, have been classified in Group 2B (possibly carcinogenic to humans) by IARC. However, the available data from studies in exposed populations and experimental animals do not permit assessment of the carcinogenic potential to humans of any specific chlorophenoxy herbicide. Therefore, drinking-water guidelines for these compounds are based on a threshold approach for other toxic effects.
DDT and metabolites
The structure of dichlorodiphenyltrichloroethane, or DDT (CAS No. 107917-42-0), permits several different isomeric forms; commercial products consist predominantly of p,p′-DDT. Its use has been restricted or banned in several countries, although DDT is still used in some countries for the control of vectors that transmit yellow fever, sleeping sickness, typhus, malaria and other insect-transmitted diseases. DDT and its metabolites are persistent in the environment and resistant to complete degradation by microorganisms. Food is the major source of intake of DDT and related compounds for the general population, although exposure has significantly decreased as a consequence of the greatly reduced use of DDT for all except specialist applications.
View in own window
| Guideline value | 0.001 mg/l (1 µg/l) |
|---|
| Occurrence | Detected in surface water at concentrations below 1 µg/l; also detected in drinking-water at 100-fold lower concentrations |
|---|
| PTDI | 0.01 mg/kg body weight based on a NOAEL of 1 mg/kg body weight per day for developmental toxicity in rats, applying an uncertainty factor of 100 (for interspecies and intraspecies variation) |
|---|
| Limit of detection | 0.011 µg/l by GC using ECD |
|---|
| Treatment performance | 0.1 µg/l should be achievable using coagulation or GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 1% of PTDI |
|---|
| • weight | 10 kg child |
|---|
| • consumption | 1 litre/day |
|---|
| Additional comments | DDT is listed under the Stockholm Convention on Persistent Organic Pollutants. Hence, monitoring may occur in addition to that required by drinking-water guidelines. |
|---|
| It should be noted that the level of DDT and its metabolites in food has been falling steadily, and the allocation of 1% of the PTDI may be very conservative. |
| The guideline value is derived on the basis of a 10 kg child consuming 1 litre of drinking-water per day, because infants and children may be exposed to greater amounts of chemicals in relation to their body weight and because of concern over the bioaccumulation of DDT. |
| It should be emphasized that the benefits of DDT use in malaria and other vector control programmes outweigh any health risk from the presence of DDT in drinking-water. |
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (2001)
Pesticide residues in food—2000 evaluations
WHO (2004)
DDT and its derivatives in drinking-water
|
|---|
A working group convened by IARC classified the DDT complex (the mixture of the various isomers of DDT and associated compounds) as a non-genotoxic carcinogen in rodents and a potent promoter of liver tumours. IARC has concluded that there is insufficient evidence in humans and sufficient evidence in experimental animals for the carcinogenicity of DDT (Group 2B) based upon liver tumours observed in rats and mice. The results of epidemiological studies of pancreatic cancer, multiple myeloma, non-Hodgkin lymphoma and uterine cancer did not support the hypothesis of an association with environmental exposure to the DDT complex. Conflicting data were obtained with regard to some genotoxic end-points. In most studies, DDT did not induce genotoxic effects in rodent or human cell systems, nor was it mutagenic to fungi or bacteria. The United States Agency for Toxic Substances and Disease Registry concluded that the DDT complex could impair reproduction and development in several species. Hepatic effects of DDT in rats include increased liver weights, hypertrophy, hyperplasia, induction of microsomal enzymes, including cytochrome P450, cell necrosis, increased activity of serum liver enzymes and mitogenic effects, which might be related to a regenerative liver response to high doses of DDT.
1,2-Dibromo-3-chloropropane
1,2-Dibromo-3-chloropropane (CAS No. 96-12-8), or DBCP, is a soil fumigant that is highly soluble in water. It has a taste and odour threshold in water of 10 µg/l. DBCP was detected in vegetables grown in treated soils, and low levels have been detected in air.
View in own window
| Guideline value | 0.001 mg/l (1 µg/l) |
|---|
| Occurrence | Limited survey found levels of up to a few micrograms per litre in drinking-water |
|---|
| Basis of guideline value derivation | Linearized multistage model was applied to the data on the incidence of stomach, kidney and liver tumours in the male rat in a 104-week dietary study |
|---|
| Limit of detection | 0.02 µg/l by GC with ECD |
|---|
| Treatment performance | 1 µg/l should be achievable using air stripping followed by GAC |
|---|
| Additional comments | The guideline value of 1 µg/l should be protective for the reproductive toxicity of DBCP. |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
1,2-Dibromo-3-chloropropane in drinking-water |
|---|
On the basis of data from different strains of rats and mice, DBCP was determined to be carcinogenic in both sexes by the oral, inhalation and dermal routes. DBCP was also determined to be a reproductive toxicant in humans and several species of laboratory animals. DBCP was found to be genotoxic in a majority of in vitro and in vivo assays. IARC has classified DBCP in Group 2B based upon sufficient evidence of carcinogenicity in animals. Recent epidemiological evidence suggests an increase in cancer mortality in individuals exposed to high levels of DBCP.
1,2-Dibromoethane
1,2-Dibromoethane (CAS No. 106-93-4), or ethylene dibromide, is used as a lead scavenger in tetraalkyl lead petrol and antiknock preparations and as a fumigant for soils, grains and fruits. However, with the phasing out of leaded petrol and of the use of 1,2-dibromoethane in agricultural applications in many countries, use of this substance has declined significantly. In addition to its continued use as a petrol additive in some countries, 1,2-dibromoethane is currently used principally as a solvent and as an intermediate in the chemical industry.
View in own window
| Provisional guideline value | 0.0004 mg/l (0.4 µg/l) |
|---|
| The guideline value is provisional owing to serious limitations of the critical studies. |
| Occurrence | Detected in groundwater following its use as a soil fumigant at concentrations as high as 100 µg/l |
|---|
| Basis of guideline value derivation | Lower end of the range (and thus more conservative estimate) of lifetime low-dose cancer risks calculated by linearized multistage modelling of the incidences of haemangiosarcomas and tumours in the stomach, liver, lung and adrenal cortex (adjusted for the observed high early mortality, where appropriate, and corrected for the expected rate of increase in tumour formation in rodents in a standard bioassay of 104 weeks) of rats and mice exposed by gavage |
|---|
| Limit of detection | 0.01 µg/l by microextraction GC-MS; 0.03 µg/l by purge-and-trap GC with halogen-specific detector; 0.8 µg/l by purge-and-trap capillary column GC with photoionization and electrolytic conductivity detectors in series |
|---|
| Treatment performance | 0.1 µg/l should be achievable using GAC |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (1995)
Report of the 1994 meeting of the Core Assessment Group
IPCS (1996)
1,2-Dibromoethane
WHO (2003)
1,2-Dibromoethane in drinking-water
|
|---|
1,2-Dibromoethane has induced an increased incidence of tumours at several sites in all carcinogenicity bioassays identified in which rats or mice were exposed to the compound by gavage, ingestion in drinking-water, dermal application and inhalation. However, many of these studies were characterized by high early mortality, limited histopathological examination, small group sizes or use of only one exposure level. The substance acted as an initiator of liver foci in an initiation/promotion assay but did not initiate skin tumour development. 1,2-Dibromoethane was consistently genotoxic in in vitro assays, although results of in vivo assays were mixed. Biotransformation to active metabolites, which have been demonstrated to bind to DNA, is probably involved in the induction of tumours. Available data do not support the existence of a non-genotoxic mechanism of tumour induction. The available data thus indicate that 1,2-dibromoethane is a genotoxic carcinogen in rodents. Data on the potential carcinogenicity in humans are inadequate; however, it is likely that 1,2-dibromoethane is metabolized similarly in rodent species and in humans (although there may be varying potential for the production of active metabolites in humans, owing to genetic polymorphism). IARC classified 1,2-dibromoethane in Group 2A (probably carcinogenic to humans).
Dichloroacetic acid
Chlorinated acetic acids, including dichloroacetic acid (DCA), are formed from organic material during water chlorination. DCA has been used as a therapeutic agent to treat lactic acidosis, diabetes and familial hyperlipidaemia in humans.
View in own window
| Provisional guideline value | 0.05 mg/l (50 µg/l) |
|---|
| The guideline value is designated as provisional on the basis of technical achievability. |
| Occurrence | Found in groundwater and surface water distribution systems at concentrations up to about 100 µg/l, with mean concentrations below 20 µg/l |
|---|
| Basis of guideline value derivation | Linear multistage model applied to combined data for carcinomas and adenomas in male mice exposed to doses up to 429 mg/kg body weight per day for up to 2 years |
|---|
| Limit of detection | < 0.1–0.4 µg/l by GC with ECD; practical quantification limit 1 µg/l |
|---|
| Treatment performance | Concentrations may be reduced by installing or optimizing coagulation to remove precursors or by controlling the pH during chlorination. |
|---|
| Additional comments | The concentration associated with a 10−5 upper-bound excess lifetime cancer risk is 40 µg/l. In some circumstances, however, it may not be possible to adequately disinfect potable water and maintain DCA levels below 40 µg/l, so the provisional guideline value of 50 µg/l is retained. |
|---|
| Assessment date | 2004 |
|---|
| Principal reference | WHO (2005)
Dichloroacetic acid in drinking-water |
|---|
IARC reclassified DCA as Group 2B (possibly carcinogenic to humans) in 2002, based on the absence of data on human carcinogenicity and sufficient evidence of its carcinogenicity in experimental animals. This classification was based primarily on findings of liver tumours in rats and mice. Genotoxicity data are considered to be inconclusive, particularly at lower doses. Glycogen deposition, peroxisome proliferation, changes in signal transduction pathways and DNA hypomethylation have all been observed following DCA exposure and have been hypothesized to be involved in its carcinogenicity. However, the available data are not sufficient to establish a cancer mode of action with reasonable certainty, especially at the very low exposure levels expected to apply to humans ingesting chlorinated drinking-water. Recent data suggest that there may be more than one mechanism leading to tumours, as altered hepatic foci from treated mice were found to have three different types of cellular characteristics.
Dichlorobenzenes (1,2-dichlorobenzene, 1,3-dichlorobenzene, 1,4-dichlorobenzene)
The dichlorobenzenes (DCBs) are widely used in industry and in domestic products such as odour-masking agents, chemical dyestuffs and pesticides. Sources of human exposure are predominantly air and food.
View in own window
| Guideline values | 1,2-Dichlorobenzene: 1 mg/l (1000 µg/l) |
|---|
| 1,4-Dichlorobenzene: 0.3 mg/l (300 µg/l) |
| Occurrence | Have been found in raw water sources at levels as high as 10 µg/l and in drinking-water at concentrations up to 3 µg/l; much higher concentrations (up to 7 mg/l) present in contaminated groundwater |
|---|
| TDIs | 1,2-Dichlorobenzene: 429 µg/kg body weight, based on a NOAEL of 60 mg/kg body weight per day for tubular degeneration of the kidney identified in a 2-year mouse gavage study, adjusting for daily dosing and using an uncertainty factor of 100 (for interspecies and intraspecies variation) |
|---|
| 1,4-Dichlorobenzene: 107 µg/kg body weight, based on a LOAEL of 150 mg/kg body weight per day for kidney effects identified in a 2-year rat study, adjusting for daily dosing and using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for the use of a LOAEL instead of a NOAEL and the carcinogenicity end-point) |
| Limit of detection | 0.01–0.25 µg/l by gas–liquid chromatography with ECD; 3.5 µg/l by GC using a photoionization detector |
|---|
| Treatment performance | 0.01 mg/l should be achievable using air stripping |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Guideline values for both 1,2- and 1,4-DCB far exceed their lowest reported taste thresholds in water of 1 and 6 µg/l, respectively. |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Dichlorobenzenes in drinking-water |
|---|
View in own window
| Reason for not establishing a guideline value | Available data inadequate to permit derivation of health-based guideline value for 1,3-dichlorobenzene |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Dichlorobenzenes in drinking-water |
|---|
1,2-Dichlorobenzene
1,2-DCB is of low acute toxicity by the oral route of exposure. Oral exposure to high doses of 1,2-DCB affects mainly the liver and kidneys. The balance of evidence suggests that 1,2-DCB is not genotoxic, and there is no evidence for its carcinogenicity in rodents.
1,3-Dichlorobenzene
There are insufficient toxicological data on this compound to permit a guideline value to be proposed, but it should be noted that it is rarely found in drinking-water.
1,4-Dichlorobenzene
1,4-DCB is of low acute toxicity, but there is evidence that it increases the incidence of renal tumours in rats and of hepatocellular adenomas and carcinomas in mice after long-term exposure. IARC has placed 1,4-DCB in Group 2B (possibly carcinogenic to humans). 1,4-DCB is not considered to be genotoxic, and the relevance for humans of the tumours observed in experimental animals is doubtful.
1,1-Dichloroethane
1,1-Dichloroethane is used as a chemical intermediate and solvent. There are limited data showing that it can be present at concentrations of up to 10 µg/l in drinking-water. It is primarily of concern for groundwater.
View in own window
| Reason for not establishing a guideline value | Available data inadequate to permit derivation of health-based guideline value |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
1,1-Dichloroethane in drinking-water |
|---|
1,1-Dichloroethane is rapidly metabolized by mammals to acetic acid and a variety of chlorinated compounds. It is of relatively low acute toxicity, and limited data are available on its toxicity from short-term and long-term studies. There is limited in vitro evidence of genotoxicity. One carcinogenicity study by gavage in mice and rats provided no conclusive evidence of carcinogenicity, although there was some evidence of an increased incidence of haemangiosarcomas in treated animals.
In view of the very limited database on toxicity and carcinogenicity, it was concluded that no guideline value should be proposed.
1,2-Dichloroethane
1,2-Dichloroethane is used mainly as an intermediate in the production of vinyl chloride and other chemicals and to a lesser extent as a solvent. It was used as a scavenger for tetraethyl lead in gasoline. It may enter surface waters via effluents from industries that manufacture or use the substance. It may also enter groundwater, where it may persist for long periods, following disposal in waste sites. It is found in urban air.
View in own window
| Guideline value | 0.03 mg/l (30 µg/l) |
|---|
| Occurrence | Has been found in drinking-water at levels of up to a few micrograms per litre |
|---|
| Basis of guideline value derivation | Applying the linearized multistage model to haemangiosarcomas observed in male rats in a 78-week gavage study |
|---|
| Limit of detection | 0.03 µg/l by GC with photoionization detection; 0.03–0.2 µg/l by GC with electrolytic conductivity detector; 0.06–2.8 µg/l by GC-MS; 5 µg/l by GC with flame ionization detection (FID) |
|---|
| Treatment performance | 0.0001 mg/l should be achievable using GAC |
|---|
| Additional comments | The guideline value of 0.03 mg/l is consistent with the value derived from IPCS (1998), based on a 10−5 risk level. |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (1995)
1,2-Dichloroethane, 2nd ed.
IPCS (1998)
1,2-Dichloroethane
WHO (2003)
1,2-Dichloroethane in drinking-water
|
|---|
IARC has classified 1,2-dichloroethane in Group 2B (possible human carcinogen). It has been shown to produce statistically significant increases in a number of tumour types in laboratory animals, including the relatively rare haemangiosarcoma, and the balance of evidence indicates that it is potentially genotoxic. Targets of 1,2-dichloroethane toxicity in orally exposed animals included the immune system, central nervous sytem, liver and kidney. Data indicate that 1,2-dichloroethane is less potent when inhaled.
1,1-Dichloroethene
1,1-Dichloroethene, or vinylidene chloride, is used mainly as a monomer in the production of polyvinylidene chloride co-polymers and as an intermediate in the synthesis of other organic chemicals. It is an occasional contaminant of drinking-water, usually being found together with other chlorinated hydrocarbons. There are no data on levels in food, but levels in air are generally less than 40 ng/m3 except at some manufacturing sites. 1,1-Dichloroethene is detected in finished drinking-water taken from groundwater sources at median concentrations of 0.28–1.2 µg/l and in public drinking-water supplies at concentrations up to 0.5 µg/l.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2004 |
|---|
| Principal references |
IPCS (2003)
1,1-Dichloroethene (vinylidene chloride)
WHO (2005)
1,1-Dichloroethene in drinking-water
|
|---|
1,1-Dichloroethene is a central nervous system depressant and may cause liver and kidney toxicity in occupationally exposed humans. It causes liver and kidney damage in laboratory animals. IARC has placed 1,1-dichloroethene in Group 3 (not classifiable as to its carcinogenicity to humans). It was found to be genotoxic in a number of test systems in vitro but was not active in the dominant lethal and micro-nucleus assays in vivo. It induced kidney tumours in mice in one inhalation study but was reported not to be carcinogenic in a number of other studies, including several in which it was given in drinking-water.
A health-based value of 140 μg/l (rounded value) can be calculated on the basis of a TDI of 0.046 mg/kg body weight, derived using the benchmark dose (BMD) approach from a study in which the critical effect was minimal hepatocellular mid-zonal fatty change in female rats. However, this value is significantly higher than the concentrations of 1,1-dichloroethene normally found in drinking-water. It is therefore considered unnecessary to set a formal guideline value for 1,1-dichloroethene in drinking-water.
1,2-Dichloroethene
1,2-Dichloroethene exists in a cis and a trans form. The cis form is more frequently found as a water contaminant. The presence of these two isomers, which are metabolites of other unsaturated halogenated hydrocarbons in wastewater and anaerobic groundwater, may indicate the simultaneous presence of other organochlorine chemicals, such as vinyl chloride. Accordingly, their presence indicates that more intensive monitoring should be conducted. There are no data on exposure from food. Concentrations in air are low, with higher concentrations, in the microgram per cubic metre range, near production sites. The cis isomer was previously used as an anaesthetic.
View in own window
| Guideline value | 0.05 mg/l (50 µg/l) |
|---|
| Occurrence | Has been found in drinking-water supplies derived from groundwater at levels up to 120 µg/l |
|---|
| TDI | 17 µg/kg body weight, based on a NOAEL (for increases in serum alkaline phosphatase levels and increased thymus weight) of 17 mg/kg body weight from a 90-day study in mice administered trans-1,2-dichloroethene in drinking-water, using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for the short duration of the study) |
|---|
| Limit of detection | 0.17 µg/l by GC-MS |
|---|
| Treatment performance | 0.01 mg/l should be achievable using GAC or air stripping |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Data on the trans isomer were used to calculate a joint guideline value for both isomers because toxicity for the trans isomer occurred at a lower dose than for the cis isomer and because data suggest that the mouse is a more sensitive species than the rat. |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
1,2-Dichloroethene in drinking-water |
|---|
There is little information on the absorption, distribution or excretion of 1,2-dichloroethene. However, by analogy with 1,1-dichloroethene, 1,2-dichloroethene would be expected to be readily absorbed, distributed mainly to the liver, kidneys and lungs and rapidly excreted. The cis isomer is more rapidly metabolized than the trans isomer in in vitro systems. Both isomers have been reported to cause increased serum alkaline phosphatase levels in rodents. In a 3-month study in mice given the trans isomer in drinking-water, there was a reported increase in serum alkaline phosphatase and reduced thymus and lung weights. Transient immunological effects were also reported, the toxicological significance of which is unclear. Trans-1,2-dichloroethene also caused reduced kidney weights in rats, but at higher doses. Only one rat toxicity study is available for the cis isomer, which produced toxic effects in rats similar in magnitude to those induced by the trans isomer in mice, but at higher doses. There are limited data to suggest that both isomers may possess some genotoxic activity. There is no information on carcinogenicity.
Dichloromethane
Dichloromethane, or methylene chloride, is widely used as a solvent for many purposes, including coffee decaffeination and paint stripping. Exposure from drinking-water is likely to be insignificant compared with that from other sources.
View in own window
| Guideline value | 0.02 mg/l (20 µg/l) |
|---|
| Occurrence | Has been found in surface water samples at concentrations ranging from 0.1 to 743 µg/l; levels usually higher in groundwater because volatilization is restricted, with concentrations as high as 3600 µg/l reported; mean concentrations in drinking-water less than 1 µg/l |
|---|
| TDI | 6 µg/kg body weight, derived from a NOAEL of 6 mg/kg body weight per day for hepatotoxic effects in a 2-year drinking-water study in rats, using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for concern about carcinogenic potential) |
|---|
| Limit of detection | 0.3 µg/l by purge-and-trap GC with MS detection (note that dichloromethane vapour readily penetrates tubing during the procedure) |
|---|
| Treatment performance | 20 µg/l should be achievable using air stripping |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Dichloromethane in drinking-water |
|---|
Dichloromethane is of low acute toxicity. An inhalation study in mice provided conclusive evidence of carcinogenicity, whereas drinking-water studies in rats and mice provided only suggestive evidence. IARC has placed dichloromethane in Group 2B (possible human carcinogen); however, the balance of evidence suggests that it is not a genotoxic carcinogen and that genotoxic metabolites are not formed in relevant amounts in vivo.
1,2-Dichloropropane
1,2-Dichloropropane (CAS No. 78-87-5), or 1,2-DCP, is used as an insecticide fumigant on grain and soil and to control peach tree borers. It is also used as an intermediate in the production of tetrachloroethene and other chlorinated products and as a solvent. 1,2-DCP is relatively resistant to hydrolysis, is poorly adsorbed onto soil and can migrate into groundwater.
View in own window
| Provisional guideline value | 0.04 mg/l (40 µg/l) |
|---|
| The guideline value is provisional owing to limitations of the toxicological database. |
| Occurrence | Detected in groundwater and drinking-water, usually at concentrations below 20 µg/l, although levels as high as 440 µg/l have been measured in well water |
|---|
| TDI | 14 µg/kg body weight based on a LOAEL of 71.4 mg/kg body weight per day (100 mg/kg body weight per day adjusted for daily dosing) for changes in haematological parameters in a 13-week study in male rats, with an uncertainty factor of 5000 (100 for interspecies and intraspecies variation, 10 for use of a LOAEL and 5 to reflect limitations of the database, including the limited data on in vivo genotoxicity and use of a subchronic study) |
|---|
| Limit of detection | 0.02 µg/l by purge-and-trap GC with an electrolytic conductivity detector or GC-MS |
|---|
| Treatment performance | 1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1998 |
|---|
| Principal reference | WHO (2003)
1,2-Dichloropropane (1,2-DCP) in drinking-water |
|---|
1,2-DCP was evaluated by IARC in 1986 and 1987. The substance was classified in Group 3 (not classifiable as to its carcinogenicity to humans) on the basis of limited evidence for its carcinogenicity in experimental animals and insufficient data with which to evaluate its carcinogenicity in humans. Results from in vitro assays for mutagenicity were mixed. The in vivo studies, which were limited in number and design, were negative. In accordance with the IARC evaluation, the evidence from the long-term carcinogenicity studies in mice and rats was considered limited, and it was concluded that the use of a threshold approach for the toxicological evaluation of 1,2-DCP was appropriate.
1,3-Dichloropropane
1,3-Dichloropropane (CAS No. 142-28-9) has several industrial uses and may be found as a contaminant of soil fumigants containing 1,3-dichloropropene. It is rarely found in water.
View in own window
| Reason for not establishing a guideline value | Available data inadequate to permit derivation of health-based guideline value |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
1,3-Dichloropropane in drinking-water |
|---|
1,3-Dichloropropane is of low acute toxicity. There is some indication that it may be genotoxic in bacterial systems. No short-term, long-term, reproductive or developmental toxicity data pertinent to exposure via drinking-water could be located in the literature. The available data are considered insufficient to permit recommendation of a guideline value.
1,3-Dichloropropene
1,3-Dichloropropene (CAS Nos. 542-75-6 isomer mixture; 10061-01-5 cis isomer; 10061-02-6 trans isomer) is a soil fumigant, the commercial product being a mixture of cis and trans isomers. It is used to control a wide variety of soil pests, particularly nematodes in sandy soils. Notwithstanding its high vapour pressure, it is soluble in water at the gram per litre level and can be considered a potential water contaminant.
View in own window
| Guideline value | 0.02 mg/l (20 µg/l) |
|---|
| Occurrence | Has been found in surface water and groundwater at concentrations of a few micrograms per litre |
|---|
| Basis of guideline value derivation | Calculated by applying the linearized multistage model to the observation of lung and bladder tumours in female mice in a 2-year gavage study |
|---|
| Limit of detection | 0.34 and 0.20 µg/l by purge-and-trap packed column GC using an electrolytic conductivity detector or microcoulometric detector for the cis and trans isomers, respectively |
|---|
| Treatment performance | No information found on removal from water |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
1,3-Dichloropropene in drinking-water |
|---|
1,3 Dichloropropene is a direct-acting mutagen that has been shown to produce forestomach tumours following long term oral gavage exposure in rats and mice. Tumours have also been found in the bladder and lungs of female mice and the liver of male rats. Long term inhalation studies in the rat have proved negative, whereas some benign lung tumours have been reported in inhalation studies in mice. IARC has classified 1,3 dichloropropene in Group 2B (possible human carcinogen).
Dichlorprop
The half-lives for degradation of chlorophenoxy herbicides, including dichlorprop (CAS No. 120-36-5), or 2,4-DP, in the environment are in the order of several days. Chlorophenoxy herbicides are not often found in food.
View in own window
| Guideline value | 0.1 mg/l (100 µg/l) |
|---|
| Occurrence | Chlorophenoxy herbicides not frequently found in drinking-water; when detected, concentrations usually no greater than a few micrograms per litre |
|---|
| TDI | 36.4 µg/kg body weight, based on a NOAEL of 3.64 mg/kg body weight per day for renal toxicity in a 2-year dietary study in rats, applying an uncertainty factor of 100 (for intraspecies and interspecies variation) |
|---|
| Limit of detection | 1 µg/l to 1 mg/l for various methods commonly used for the determination of chlorophenoxy herbicides in water, including solvent extraction, separation by GC, gas–liquid chromatography, thin-layer chromatography or HPLC, with ECD or UV detection |
|---|
| Treatment performance | No information found on removal from water |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chlorophenoxy herbicides (excluding 2,4-D and MCPA) in drinking-water |
|---|
Chlorophenoxy herbicides, as a group, have been classified in Group 2B (possible human carcinogen) by IARC. However, the available data from studies in exposed populations and experimental animals do not permit assessment of the carcinogenic potential to humans of any specific chlorophenoxy herbicide. Therefore, drinking-water guidelines for these compounds are based on a threshold approach for other toxic effects. In dietary studies in rats, slight liver hypertrophy was observed in a 3-month study, and effects in a 2-year study included hepatocellular swelling, mild anaemia, increased incidence of brown pigment in the kidneys (possibly indicative of slight degeneration of the tubular epithelium) and decreased urinary specific gravity and protein.
Dichlorvos
Dichlorvos (CAS No. 62-73-7) is a broad-spectrum organophosphorus insecticide used primarily for controlling household pests and for protecting stored products from insects. It is no longer approved for use in some jurisdictions because of concerns over its acute toxicity. Dichlorvos is expected to be very mobile in soils. It is rapidly degraded by microbial activity and hydrolysis in soil, and does not adsorb to sediments. Degradation in water occurs primarily through hydrolysis. There are relatively few studies on its occurrence in source waters. Exposure from food varies widely, depending on local circumstances and usage. Dichlorvos can be inhaled from its use as a domestic insecticide.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water or drinking-water sources at concentrations well below those of health concern |
|---|
| Health-based value* | 0.02 mg/l |
|---|
| Acute health-based value** | 3 mg/l |
|---|
| Occurrence | Concentrations in surface water in the range 10–50 ng/l, but sometimes as high as 1500 ng/l, have been measured |
|---|
| ADI | 0–0.004 mg/kg bw, based on a NOAEL of 0.04 mg/kg bw per day for the inhibition of erythrocyte acetylcholinesterase activity in a 21-day study in male volunteers and application of a safety factor of 10 |
|---|
| ARfD | 0.1 mg/kg bw, based on a NOAEL of 1 mg/kg bw for erythrocyte acetylcholinesterase inhibition in an acute oral study in male volunteers and application of a safety factor of 10 |
|---|
| Limit of detection | 0.01 µg/l (limit of quantification) based on solvent extraction and GC analysis; 0.1 µg/l (reporting limit) based on GC-MS |
|---|
| Treatment performance | Conventional treatment, including coagulation, filtration and chlorination, not effective; removal by membranes depends on membrane type and operational conditions. Removal by nanofiltration membranes has variable effectiveness (removal rates from 4 to 60%). Reverse osmosis would be expected to be effective (removal rates > 85%) based on removal studies and predictions. |
|---|
| Health-based value derivation |
|---|
| • allocation to water | 20% of upper bound of ADI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Acute health-based value derivation |
|---|
| • allocation to water | 100% of ARfD |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The default allocation factor of 20% has been used to account for the fact that the available food exposure data, which suggest that exposure via this route is low, do not generally include information from developing countries, where exposure via this route may be higher, and as potential exposure via inhalation from indoor air resulting from use of dichlorvos as a domestic insecticide is unknown |
|---|
| As a general principle, the concentration of pesticides in water, including dichlorvos, should be kept as low as possible and concentrations should not be allowed to increase up to the health-based value. |
| Further guidance on interpreting the health-based value and deciding when to monitor can be found in section 8.5.3 |
| Assessment date | 2016 |
|---|
| Principal references |
WHO (2012). Pesticide residues in food – 2011 evaluations
WHO (2016). Dichlorvos in drinking-water
|
|---|
- *
When a formal guideline value is not established, a “health-based value” may be determined in order to provide guidance to Member States when there is reason for local concern. Establishing a formal guideline value for such substances may encourage Member States to incorporate a value into their national standards when this may be unnecessary.
- **
For more information on acute health-based values, see section 8.7.5.
As with other organophosphorus insecticides, the inhibition of cholinesterase activity, causing neurotoxicity, is the most sensitive toxicological end-point following acute or repeated exposures to dichlorvos. Dichlorvos is unlikely to be genotoxic in vivo or to pose a carcinogenic risk to humans. Some reproductive toxicity has been observed in rats, but dichlorvos was not found to cause developmental toxicity or to be teratogenic.
Dicofol
Dicofol (CAS No. 115-32-2) is an organochlorine acaricide that has been registered for broad-spectrum contact, non-systemic control of plant-eating mites in cotton, tea and a wide variety of fruit, vegetable and ornamental crops. Products containing dicofol, which is manufactured from DDT, are being phased out in the USA and are no longer approved for use in the European Union. Dicofol is unlikely to reach water, but may do so if bound to particulate matter subject to runoff. Dicofol is only slightly soluble in water and binds strongly to soil. There are few data on the occurrence of dicofol in water. Exposure from food varies widely, depending on local circumstances and usage. Dicofol has been proposed as a persistent organic pollutant under the Stockholm Convention.
View in own window
| Reason for not establishing a guideline value | Unlikely to be found in drinking-water or drinking-water sources* |
|---|
| Health-based value** | 0.01 mg/l |
|---|
| Acute health-based value*** | 6 mg/l |
|---|
| Occurrence | Not detected in limited groundwater monitoring |
|---|
| ADI | 0–0.002 mg/kg bw, based on a NOAEL of 0.22 mg/kg bw per day for histopathological changes in the liver and adrenal gland in a 2-year toxicity and carcinogenicity study in rats and application of a safety factor of 100 |
|---|
| ARfD | 0.2 mg/kg bw, based on a NOAEL of 15 mg/kg bw for decreased body weight and decreased feed intake in an acute neurotoxicity study in rats and application of a safety factor of 100 |
|---|
| Limit of detection | Solvent extraction followed by GC-ECD may be effective (limit of quantification 5 ng/l) |
|---|
| Treatment performance | Should be removed by adsorption onto activated carbon, and any dicofol adsorbed onto particulate matter would likely be removed during coagulation |
|---|
| Health-based value derivation |
|---|
| • allocation to water | 20% of the upper bound of the ADI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Acute health-based value derivation |
|---|
| • allocation to water | 100% of the ARfD |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The default allocation factor of 20% has been used to account for the fact that the available food exposure data, which suggest that exposure via this route is low, do not generally include information from developing countries, where exposure via this route may be higher |
|---|
| As a general principle, the concentration of pesticides in water, including dicofol, should be kept as low as possible and concentrations should not be allowed to increase up to the health-based value. |
| Further guidance on interpreting the health-based value and deciding when to monitor can be found in section 8.5.3 |
| Assessment date | 2016 |
|---|
| Principal references |
WHO (2012). Pesticide residues in food – 2011 evaluations
WHO (2016). Dicofol in drinking-water
|
|---|
- *
Although dicofol does not fulfil one of the three criteria for evaluation in the Guidelines, a background document has been prepared, and a health-based value has been established, in response to a request from Member States for guidance.
- **
When a formal guideline value is not established, a “health-based value” may be determined in order to provide guidance to Member States when there is reason for local concern. Establishing a formal guideline value for such substances may encourage Member States to incorporate a value into their national standards when this may be unnecessary.
- ***
For more information on acute health-based values, see section 8.7.5.
The primary effects of dicofol after short- or long-term exposure of experimental animals were body weight reduction associated with decreased feed intake, and increased liver weight accompanied by changes in liver enzyme activities. Dicofol caused liver tumours in male mice at doses associated with significant enzyme induction and liver hypertrophy. However, on the basis of the absence of genotoxicity in an adequate range of in vitro genotoxicity and in vivo chromosomal aberration tests, the absence of carcinogenic effects in rats and the expectation that the adenomas present in mice will exhibit a threshold, dicofol is unlikely to pose a carcinogenic risk to humans at anticipated dietary exposure levels. There is a margin of 20 000 between the upper bound of the ADI and the LOAEL for liver adenomas in the male mouse.
Di(2-ethylhexyl)adipate
Di(2-ethylhexyl)adipate (DEHA) is used mainly as a plasticizer for synthetic resins such as polyvinyl chloride (PVC). Reports of the presence of DEHA in surface water and drinking-water are scarce, but DEHA has occasionally been identified in drinking-water at levels of a few micrograms per litre. As a consequence of its use in PVC films, food is the most important source of human exposure (up to 20 mg/day).
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2003)
Di(2-ethylhexyl)adipate in drinking-water |
|---|
DEHA is of low short-term toxicity; however, dietary levels above 6000 mg/kg of feed induce peroxisomal proliferation in the liver of rodents. This effect is often associated with the development of liver tumours. DEHA induced liver carcinomas in female mice at very high doses, but not in male mice or rats. It is not genotoxic. IARC has placed DEHA in Group 3 (not classifiable as to its carcinogenicity to humans).
A health-based value of 80 µg/l can be calculated for DEHA on the basis of a TDI of 280 µg/kg body weight, based on fetotoxicity in rats, and allocating 1% of the TDI to drinking-water. However, because DEHA occurs at concentrations well below those of health concern, it is not considered necessary to derive a formal guideline value.
Di(2-ethylhexyl)phthalate
Di(2-ethylhexyl)phthalate (DEHP) is used primarily as a plasticizer. Exposure among individuals may vary considerably because of the broad nature of products into which DEHP is incorporated. In general, food will be the main exposure route.
View in own window
| Guideline value | 0.008 mg/l (8 µg/l) |
|---|
| Occurrence | Found in surface water, groundwater and drinking-water in concentrations of a few micrograms per litre; in polluted surface water and groundwater, concentrations of hundreds of micrograms per litre have been reported |
|---|
| TDI | 25 µg/kg body weight, based on a NOAEL of 2.5 mg/kg body weight per day for peroxisomal proliferation in the liver in rats, using an uncertainty factor of 100 for interspecies and intraspecies variation |
|---|
| Limit of detection | 0.1 µg/l by GC-MS |
|---|
| Treatment performance | No information found on removal from water |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 1% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The reliability of some data on environmental water samples is questionable because of secondary contamination during sampling and working-up procedures. Concentrations that exceed the solubility more than 10-fold have been reported. |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Di(2-ethylhexyl)phthalate in drinking-water |
|---|
In rats, DEHP is readily absorbed from the gastrointestinal tract. In primates (including humans), absorption after ingestion is lower. Species differences are also observed in the metabolic profile. Most species excrete primarily the conjugated mono-ester in urine. Rats, however, predominantly excrete terminal oxidation products. DEHP is widely distributed in the body, with highest levels in liver and adipose tissue, without showing significant accumulation. The acute oral toxicity is low. The most striking effect in short-term toxicity studies is the proliferation of hepatic peroxisomes, indicated by increased peroxisomal enzyme activity and histopathological changes. The available information suggests that primates, including humans, are far less sensitive to this effect than rodents. In long-term oral carcinogenicity studies, hepatocellular carcinomas were found in rats and mice. IARC has concluded that DEHP is possibly carcinogenic to humans (Group 2B). In 1988, JECFA evaluated DEHP and recommended that human exposure to this compound in food be reduced to the lowest level attainable. JECFA considered that this might be achieved by using alternative plasticizers or alternatives to plastic material containing DEHP. In a variety of in vitro and in vivo studies, DEHP and its metabolites have shown no evidence of genotoxicity, with the exception of induction of aneuploidy and cell transformation.
Dimethoate
Dimethoate (CAS No. 60-51-5) is an organophosphorus insecticide used to control a broad range of insects in agriculture, as well as the housefly. It has a half-life of 18 hours to 8 weeks and is not expected to persist in water, although it is relatively stable at pH 2–7. A total daily intake from food of 0.001 µg/kg body weight has been estimated.
View in own window
| Guideline value | 0.006 mg/l (6 µg/l) |
|---|
| Occurrence | Detected at trace levels in a private well in Canada, but not detected in a Canadian survey of surface water or drinking-water supplies |
|---|
| ADI | 0–0.002 mg/kg body weight based on an apparent NOAEL of 1.2 mg/kg body weight per day for reproductive performance in a study of reproductive toxicity in rats, applying an uncertainty factor of 500 (100 for interspecies and intraspecies variation, 5 to take into consideration concern regarding whether the NOAEL could be a LOAEL) |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (1997)
Pesticide residues in food—1996 evaluations
WHO (2004)
Dimethoate in drinking-water
|
|---|
In studies with human volunteers, dimethoate has been shown to be a cholinesterase inhibitor and a skin irritant. Dimethoate is not carcinogenic to rodents. JMPR concluded that although in vitro studies indicate that dimethoate has mutagenic potential, this potential does not appear to be expressed in vivo. In a multi-generation study of reproductive toxicity in rats, the NOAEL appeared to be 1.2 mg/kg body weight per day, but there was some indication that reproductive performance may have been affected at lower doses. No data were available to assess whether the effects on reproductive performance were secondary to inhibition of cholinesterase. JMPR concluded that it was not appropriate to base the ADI on the results of the studies of volunteers, as the crucial end-point (reproductive performance) has not been assessed in humans. It was suggested that there may be a need to re-evaluate the toxicity of dimethoate after the periodic review of the residue and analytical aspects of dimethoate has been completed if it is determined that omethoate is a major residue.
1,4-Dioxane
1,4-Dioxane is used as a stabilizer in chlorinated solvents and as a solvent for resins, oils and waxes, for agricultural and biochemical intermediates and for adhesives, sealants, cosmetics, pharmaceuticals, rubber chemicals and surface coatings.
View in own window
| Guideline value | 0.05 mg/l (50 µg/l) |
|---|
| Occurrence | Has been measured in surface water at concentrations up to 40 µg/l and in groundwater at concentrations up to 80 µg/l |
|---|
| TDI | 16 µg/kg body weight, based on a NOAEL of 16 mg/kg body weight per day for hepatocellular tumours observed in a long-term drinking-water study in rats, using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for non-genotoxic carcinogenicity) |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Basis of guideline value derivation based on carcinogenicity | Linear multistage model applied to data for hepatic tumours from drinking-water studies in rats |
|---|
| Limit of detection | 0.1–50 µg/l by GC-MS |
|---|
| Treatment performance | Not removed using conventional water treatment processes; effectively removed by biological activated carbon treatment |
|---|
| Additional comments | Similar guideline values were derived using the TDI approach (assuming 1,4-dioxane is not genotoxic in humans at low doses) and linear multistage modelling (because the compound clearly induces multiple tumours in various organs). |
|---|
| Assessment date | 2004 |
|---|
| Principal reference | WHO (2005)
1,4-Dioxane in drinking-water |
|---|
1,4-Dioxane caused hepatic and nasal cavity tumours in rodents in most long-term oral studies conducted. Tumours in peritoneum, skin and mammary gland were also observed in rats given a high dose. Lung tumours were specifically detected after intraperitoneal injection. Although cohort studies of workers did not reveal any elevation in the incidence of death by cancer, a significant increase in the incidence of liver cancer was found in a comparative mortality study. However, the evidence is inadequate for human carcinogenicity assessment because of small samples or lack of exposure data. A possibly weak genotoxic potential of 1,4-dioxane has been suggested. IARC has classified 1,4-dioxane in Group 2B (possibly carcinogenic to humans).
Diquat
Diquat (CAS No. 85-00-7; CAS No. 2764-72-9 for diquat ion) is a non-selective, quick-acting contact herbicide that is used for weed control on several food crops, for residential weed control on lawns and ornamental plants, and as an aquatic herbicide for the control of free-floating and submerged aquatic weeds in ponds and irrigation ditches. It is highly soluble in water but is strongly adsorbed to soil and is resistant to degradation in the sorbed state. Photochemical degradation in soil and water occurs in the presence of sunlight. Exposure from food is likely to be low.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water or drinking-water sources at concentrations well below those of health concern |
|---|
| Health-based value* | 0.03 mg/l |
|---|
| Acute health-based value** | 20 mg/l |
|---|
| Occurrence | Rarely detected in surface water |
|---|
| ADI | 0–0.006 mg/kg bw (expressed as the diquat ion), based on a NOAEL of 0.58 mg/kg bw per day for cataracts in a 2-year toxicity and carcinogenicity study in rats and application of a safety factor of 100 |
|---|
| ARfD | 0.8 mg/kg bw (expressed as the diquat ion), based on a NOAEL of 75 mg/kg bw for clinical signs and decreased body weight gain in the 1st week and decreased feed consumption in a neurotoxicity study in rats and application of a safety factor of 100 |
|---|
| Limit of detection | 1 µg/l using HPLC with UV absorbance detection after solid sorbent cartridge extraction; practical quantification limit of 1 µg/l using LC-MS analysis after solid-phase extraction |
|---|
| Treatment performance | Conventional treatment, including coagulation and filtration, not effective; activated carbon may be effective |
|---|
| Health-based value derivation |
|---|
| • allocation to water | 20% of upper bound of unrounded ADI (0.0058 mg/kg bw) |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Acute health-based value derivation |
|---|
| • allocation to water | 100% of unrounded ARfD (0.75 mg/kg bw) |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The default allocation factor of 20% has been used to account for the fact that the available food exposure data, , which suggest that exposure via this route is low, do not generally include information from developing countries, where exposure via this route may be higher |
|---|
| As a general principle, the concentration of pesticides in water, including diquat, should be kept as low as possible and concentrations should not be allowed to increase up to the health-based value. |
| Further guidance on interpreting the health-based value and deciding when to monitor can be found in section 8.5.3 |
| Assessment date | 2016 |
|---|
| Principal references |
WHO (2014). Pesticide residues in food – 2013 evaluations
WHO (2016). Diquat in drinking-water
|
|---|
- *
When a formal guideline value is not established, a “health-based value” may be determined in order to provide guidance to Member States when there is reason for local concern. Establishing a formal guideline value for such substances may encourage Member States to incorporate a value into their national standards when this may be unnecessary.
- **
For more information on acute health-based values, see section 8.7.5.
The eye is the main target organ following short-term repeated exposure in rats and dogs. Effects on kidney, liver and haematological parameters are also observed. Diquat is not carcinogenic in mice or rats. In tests for genotoxicity, diquat gave equivocal or positive responses in the mammalian cell cytogenetic assay, but was negative in the in vivo mouse micronucleus assay and dominant lethal assay. No reproductive effects were observed in a two-generation reproductive toxicity study in rats, and diquat was not teratogenic in rats or rabbits.
Edetic acid
Human exposure to edetic acid, also known as ethylenediaminetetraacetic acid or EDTA, arises directly from its use in food additives, medicines and personal care and hygiene products. Exposure to EDTA from drinking-water will be mostly very low in comparison with that from other sources. Once EDTA is present in the aquatic environment, its speciation will depend on the water quality and the presence of trace metals with which it will combine. The removal of EDTA from communal wastewater by biodegradation in sewage purification plants is very limited.
View in own window
| Guideline value | EDTA (as the free acid): 0.6 mg/l (600 µg/l) |
|---|
| Occurrence | Present in surface waters generally at concentrations below 70 µg/l, although higher concentrations (900 µg/l) have been measured; detected in drinking-water prepared from surface waters at concentrations of 10–30 µg/l |
|---|
| ADI | 0–1.9 mg/kg body weight as the free acid (ADI of 0–2.5 mg/kg body weight proposed by JECFA for calcium disodium edetate as a food additive) |
|---|
| Limit of detection | 1 µg/l by potentiometric stripping analyis |
|---|
| Treatment performance | 0.01 mg/l using GAC plus ozonation |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 1% of upper limit of ADI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Concern has been expressed over the ability of EDTA to complex and therefore reduce the availability of zinc. However, this is of significance only at elevated doses substantially in excess of those encountered in the environment. |
|---|
| Assessment date | 1998 |
|---|
| Principal reference | WHO (2003)
Edetic acid (EDTA) in drinking-water |
|---|
Calcium disodium edetate is poorly absorbed from the gut. The long-term toxicity of EDTA is complicated by its ability to chelate essential and toxic metals. Those toxicological studies that are available indicate that the apparent toxicological effects of EDTA have in fact been due to zinc deficiency as a consequence of complexation. EDTA does not appear to be teratogenic or carcinogenic in experimental animals. The vast clinical experience of the use of EDTA in the treatment of metal poisoning has demonstrated its safety in humans.
Endosulfan
Endosulfan (CAS No. 115-29-7) is an insecticide used in countries throughout the world to control pests on fruit, vegetables and tea and on non-food crops such as tobacco and cotton. In addition to its agricultural use, it is used in the control of the tsetse fly, as a wood preservative and for the control of home garden pests. Endosulfan contamination does not appear to be widespread in the aquatic environment, but the chemical has been found in agricultural runoff and rivers in industrialized areas where it is manufactured or formulated, as well as in surface water and groundwater samples collected from hazardous waste sites in the USA. Surface water samples in the USA generally contain less than 1 µg/l. The main source of exposure of the general population is food, but residues have generally been found to be well below the FAO/WHO maximum residue limits. Another important route of exposure to endosulfan for the general population is the use of tobacco products.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (1999)
Pesticide residues in food—1998 evaluations
WHO (2004)
Endosulfan in drinking-water
|
|---|
JMPR concluded that endosulfan is not genotoxic, and no carcinogenic effects were noted in long-term studies using mice and rats. The kidney is the target organ for toxicity. Several recent studies have shown that endosulfan, alone or in combination with other pesticides, may bind to estrogen receptors and perturb the endocrine system. A health-based value of 20 µg/l can be calculated for endosulfan on the basis of an ADI of 0–0.006 mg/kg body weight, based on results from a 2-year dietary study of toxicity in rats and supported by a 78-week study in mice, a 1-year study in dogs and a developmental toxicity study in rats. However, because endosulfan occurs at concentrations well below those of health concern, it is not considered necessary to derive a formal guideline value.
Endrin
Endrin (CAS No. 72-20-8) is a broad-spectrum foliar insecticide that acts against a wide range of agricultural pests. It is also used as a rodenticide. There is now very little use of endrin. Small amounts of endrin are present in some foods, but the total intake from food has decreased significantly.
View in own window
| Guideline value | 0.0006 mg/l (0.6 µg/l) |
|---|
| Occurrence | Traces of endrin found in the drinking-water supplies of several countries |
|---|
| PTDI | 0.2 µg/kg body weight, based on a NOAEL of 0.025 mg/kg body weight per day in a 2-year study in dogs and applying an uncertainty factor of 100 for interspecies and intraspecies variation |
|---|
| Limit of detection | 0.002 µg/l by GC with ECD |
|---|
| Treatment performance | 0.2 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of PTDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Endrin is listed under the Stockholm Convention on Persistent Organic Pollutants. Hence, monitoring may occur in addition to that required by drinking-water guidelines. |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (1995)
Pesticide residues in food—1994 evaluations
IPCS (1992)
Endrin
WHO (2004)
Endrin in drinking-water
|
|---|
Toxicological data are insufficient to indicate whether endrin is a carcinogenic hazard to humans. The primary site of action of endrin is the central nervous system.
Epichlorohydrin
Epichlorohydrin is used for the manufacture of glycerol, unmodified epoxy resins and water treatment coagulant polymers and some ion exchange resins. No quantitative data are available on its occurrence in food or drinking-water. Epichlorohydrin is slowly hydrolysed in aqueous media.
View in own window
| Provisional guideline value | 0.0004 mg/l (0.4 µg/l) |
|---|
| The guideline value is considered to be provisional because of the uncertainties surrounding the toxicity of epichlorohydrin and the use of a large uncertainty factor in deriving the guideline value. |
| Occurrence | No quantitative data available |
|---|
| TDI | 0.14 µg/kg body weight, on the basis of a LOAEL of 2 mg/kg body weight per day for forestomach hyperplasia observed in a 2-year gavage study in rats, adjusting for daily dosing and using an uncertainty factor of 10 000 to take into consideration interspecies and intraspecies variation (100), the use of a LOAEL instead of a NOAEL (10) and carcinogenicity (10) |
|---|
| Limit of detection | 0.01 µg/l by GC with ECD; 0.1 and 0.5 µg/l by GC-MS; 10 µg/l by GC with FID |
|---|
| Treatment performance | Conventional treatment processes do not remove epichlorohydrin. Epichlorohydrin concentrations in drinking-water are controlled by limiting either the epichlorohydrin content of polyamine flocculants or the dose used, or both. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Although epichlorohydrin is a genotoxic carcinogen, the use of the linearized multistage model for estimating cancer risk was considered inappropriate because tumours are seen only at the site of administration, where epichlorohydrin is highly irritating. |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2004)
Epichlorohydrin in drinking-water |
|---|
Epichlorohydrin is rapidly and extensively absorbed following oral, inhalation or dermal exposure. It binds easily to cellular components. Major toxic effects are local irritation and damage to the central nervous system. It induces squamous cell carcinomas in the nasal cavity by inhalation and forestomach tumours by the oral route. It has been shown to be genotoxic in vitro and in vivo. IARC has placed epichlorohydrin in Group 2A (probably carcinogenic to humans).
Ethylbenzene
The primary sources of ethylbenzene in the environment are the petroleum industry and the use of petroleum products. Because of its physicochemical properties, more than 96% of ethylbenzene in the environment can be expected to be present in air. Values of up to 26 µg/m3 in air have been reported. Ethylbenzene is found in trace amounts in surface water, groundwater, drinking-water and food.
View in own window
| Guideline value | 0.3 mg/l (300 µg/l) |
|---|
| Occurrence | Concentrations in drinking-water generally below 1 µg/l; levels up to 300 µg/l have been reported in groundwater contaminated by point emissions |
|---|
| TDI | 97.1 µg/kg body weight, based on a NOAEL of 136 mg/kg body weight per day for hepatotoxicity and nephrotoxicity observed in a limited 6-month study in rats, adjusting for daily dosing and using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for the limited database and short duration of the study) |
|---|
| Limit of detection | 0.002–0.005 µg/l by GC with photoionization detector; 0.03–0.06 µg/l by GC-MS |
|---|
| Treatment performance | 0.001 mg/l should be achievable using air stripping |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The guideline value exceeds the lowest reported odour threshold for ethylbenzene in drinking-water (0.002 mg/l). |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Ethylbenzene in drinking-water |
|---|
Ethylbenzene is readily absorbed by the oral, inhalation or dermal route. In humans, storage in fat has been reported. Ethylbenzene is almost completely converted to soluble metabolites, which are excreted rapidly in urine. The acute oral toxicity is low. No definite conclusions can be drawn from limited teratogenicity data. No data on reproduction, long-term toxicity or carcinogenicity are available. Ethylbenzene has shown no evidence of genotoxicity in in vitro or in vivo systems.
Fenitrothion
Fenitrothion (CAS No. 122-14-5) is mainly used in agriculture for controlling insects on rice, cereals, fruits, vegetables, stored grains and cotton and in forest areas. It is also used for the control of flies, mosquitoes and cockroaches in public health programmes and indoor use. Fenitrothion is stable in water only in the absence of sunlight or microbial contamination. In soil, biodegradation is the primary route of degradation, although photolysis may also play a role. Fenitrothion residues detected in water were low (maximum 1.30 µg/l) during the spruce budworm spray programme. Following the spraying of forests to control spruce budworm, water samples did not contain detectable amounts of fenitrothion; post-spray samples contained less than 0.01 µg/l. Levels of fenitrothion residues in fruits, vegetables and cereal grains decline rapidly after treatment, with a half-life of 1–2 days. Intake of fenitrothion appears to be primarily (95%) from food.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (2001)
Pesticide residues in food—2000 evaluations
WHO (2004)
Fenitrothion in drinking-water
|
|---|
On the basis of testing in an adequate range of studies in vitro and in vivo, JMPR concluded that fenitrothion is unlikely to be genotoxic. It also concluded that fenitrothion is unlikely to pose a carcinogenic risk to humans. In long-term studies of toxicity, inhibition of cholinesterase activity was the main toxicological finding in all species. A health-based value of 8 µg/l can be calculated for fenitrothion on the basis of an ADI of 0–0.005 mg/kg body weight, based on a NOAEL of 0.5 mg/kg body weight per day for inhibition of brain and erythrocyte cholinesterase activity in a 2-year study of toxicity in rats and supported by a NOAEL of 0.57 mg/kg body weight per day for inhibition of brain and erythrocyte cholinesterase activity in a 3-month study of ocular toxicity in rats and a NOAEL of 0.65 mg/kg body weight per day for reduced food consumption and body weight gain in a study of reproductive toxicity in rats, and allocating 5% of the upper limit of the ADI to drinking-water. However, because fenitrothion occurs at concentrations well below those of health concern, it is not considered necessary to derive a formal guideline value.
Fenoprop
The half-lives for degradation of chlorophenoxy herbicides, including fenoprop (CAS No. 93-72-1), also known as 2,4,5-trichlorophenoxy propionic acid or 2,4,5-TP, in the environment are in the order of several days. Chlorophenoxy herbicides are not often found in food.
View in own window
| Guideline value | 0.009 mg/l (9 µg/l) |
|---|
| Occurrence | Chlorophenoxy herbicides not frequently found in drinking-water; when detected, concentrations usually no greater than a few micrograms per litre |
|---|
| TDI | 3 µg/kg body weight, based on a NOAEL of 0.9 mg/kg body weight for adverse effects on the liver in a study in which dogs were administered fenoprop in the diet for 2 years, with an uncertainty factor of 300 (100 for interspecies and intraspecies variation and 3 for limitations of the database) |
|---|
| Limit of detection | 0.2 µg/l by either packed or capillary column GC with ECD |
|---|
| Treatment performance | 0.001 mg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chlorophenoxy herbicides (excluding 2,4-D and MCPA) in drinking-water |
|---|
Chlorophenoxy herbicides, as a group, have been classified in Group 2B (possibly carcinogenic to humans) by IARC. However, the available data from studies in exposed populations and experimental animals do not permit assessment of the carcinogenic potential to humans of any specific chlorophenoxy herbicide. Therefore, drinking-water guidelines for these compounds are based on a threshold approach for other toxic effects. Effects observed in long-term studies with dogs given fenoprop in the diet include mild degeneration and necrosis of hepatocytes and fibroblastic proliferation in one study and severe liver pathology in another study. In rats, increased kidney weight was observed in two long-term dietary studies.
Fluoride5
Fluorine is a common element that is widely distributed in Earth’s crust and exists in the form of fluorides in a number of minerals, such as fluorspar, cryolite and fluorapatite. Traces of fluorides are present in many waters, with higher concentrations often associated with groundwaters. In some areas rich in fluoride-containing minerals, well water may contain up to about 10 mg of fluoride per litre, although much higher concentrations can be found. High fluoride concentrations can be found in many parts of the world, particularly in parts of India, China, Central Africa and South America, but high concentrations can be encountered locally in most parts of the world. Virtually all foodstuffs contain at least traces of fluorine. All vegetation contains some fluoride, which is absorbed from soil and water. Tea in particular can contain high fluoride concentrations, and levels in dry tea are on average 100 mg/kg.
Fluoride is widely used in dental preparations to combat dental caries, particularly in areas of high sugar intake. These can be in the form of tablets, mouthwashes, toothpaste, varnishes or gels for local application. In some countries, fluoride may also be added to table salt or drinking-water in order to provide protection against dental caries. The amounts added to drinking-water are such that final concentrations are usually between 0.5 and 1 mg/l. The fluoride in final water is always present as fluoride ions, whether from natural sources or from artificial fluoridation.
Total daily fluoride exposure can vary markedly from one region to another. This will depend on the concentration of fluoride in drinking-water and the amount drunk, levels in foodstuffs and the use of fluoridated dental preparations. In addition, fluoride exposure in some areas is considerably higher as a consequence of a range of practices, including the consumption of brick tea and the cooking and drying of food with high-fluoride coal.
View in own window
| Guideline value | 1.5 mg/l (1500 µg/l) |
|---|
| Occurrence | In groundwater, concentrations vary with the type of rock through which the water flows but do not usually exceed 10 mg/l; highest natural level reported is 2800 mg/l |
|---|
| Basis of guideline value derivation | Epidemiological evidence that concentrations above this value carry an increasing risk of dental fluorosis and that progressively higher concentrations lead to increasing risks of skeletal fluorosis. The value is higher than that recommended for artificial fluoridation of water supplies, which is usually 0.5–1.0 mg/l. |
|---|
| Limit of detection | 0.01 mg/l by ion chromatography; 0.1 mg/l by ion-selective electrodes or the sulfo phenyl azo dihydroxy naphthalene disulfonic acid colorimetric method |
|---|
| Treatment performance | 1 mg/l should be achievable using activated alumina (not a “conventional” treatment process, but relatively simple to install filters) |
|---|
| Additional comments | A management guidance document on fluoride is available. |
|---|
| In setting national standards for fluoride or in evaluating the possible health consequences of exposure to fluoride, it is essential to consider the intake of water by the population of interest and the intake of fluoride from other sources (e.g. from food, air and dental preparations). Where the intakes from other sources are likely to approach, or be greater than, 6 mg/day, it would be appropriate to consider setting standards at concentrations lower than the guideline value. |
| In areas with high natural fluoride levels in drinking-water, the guideline value may be difficult to achieve, in some circumstances, with the treatment technology available. |
| Assessment date | 2003 |
|---|
| Principal references |
Fawell et al. (2006)
Fluoride in drinking-water
IPCS (2002)
Fluorides
USNRC (2006)
Fluoride in drinking water
WHO (2004)
Fluoride in drinking-water
|
|---|
After oral uptake, water-soluble fluorides are rapidly and almost completely absorbed from the gastrointestinal tract, although this may be reduced by complex formation with aluminium, phosphorus, magnesium or calcium. There is no difference in absorption between natural or added fluoride in drinking-water. Fluoride in inhaled particles—from high-fluoride coal, for example—is also absorbed, depending on the particle size and solubility of the fluoride compounds present. Absorbed fluoride is rapidly distributed throughout the body, where it is incorporated into teeth and bones, with virtually no storage in soft tissues. Fluoride in teeth and bone can be mobilized after external exposure has ceased or been reduced. Fluoride is excreted via urine, faeces and sweat.
Fluoride may be an essential element for humans; however, essentiality has not been demonstrated unequivocally. Meanwhile, there is evidence of fluoride being a beneficial element with regard to the prevention of dental caries.
To produce signs of acute fluoride intoxication, minimum oral doses of about 1 mg of fluoride per kilogram of body weight were required. Many epidemiological studies of possible adverse effects of the long-term ingestion of fluoride via drinking-water have been carried out. These studies clearly establish that high fluoride intakes primarily produce effects on skeletal tissues (bones and teeth). Low concentrations provide protection against dental caries, both in children and in adults. The protective effects of fluoride increase with concentration up to about 2 mg of fluoride per litre of drinking-water; the minimum concentration of fluoride in drinking-water required to produce it is approximately 0.5 mg/l. However, fluoride can also have an adverse effect on tooth enamel and may give rise to mild dental fluorosis (prevalence: 12–33%) at drinking-water concentrations between 0.9 and 1.2 mg/l, depending on drinking-water intake and exposure to fluoride from other sources. Mild dental fluorosis may not be detectable except by specialist examination. The risk of dental fluorosis will depend on the total intake of fluoride from all sources and not just the concentration in drinking-water.
Elevated fluoride intakes can have more serious effects on skeletal tissues. Skeletal fluorosis (with adverse changes in bone structure) may be observed when drinking-water contains 3–6 mg of fluoride per litre, particularly with high water consumption. Crippling skeletal fluorosis usually develops only where drinking-water contains over 10 mg of fluoride per litre. IPCS concluded that there is clear evidence from India and China that skeletal fluorosis and an increased risk of bone fractures occur at a total intake of 14 mg of fluoride per day. This conclusion was supported by a review by the United States National Research Council in 2006. The relationship between exposure and response for adverse effects in bone is frequently difficult to ascertain because of inadequacies in most of the epidemiological studies. IPCS concluded from estimates based on studies from China and India that for a total intake of 14 mg/day, there is a clear excess risk of skeletal adverse effects; and there is suggestive evidence of an increased risk of effects on the skeleton at total fluoride intakes above about 6 mg/day.
Several epidemiological studies are available on the possible association between fluoride in drinking-water and cancer. IPCS evaluated these studies and concluded that, overall, the evidence of carcinogenicity in laboratory animals is inconclusive and that the available evidence does not support the hypothesis that fluoride causes cancer in humans; however, the data on bone cancer are limited. The results of several epidemio-logical studies on the possible adverse effects of fluoride in drinking-water on pregnancy outcome indicate that there is no relationship between the rates of Down syndrome or congenital malformation and the consumption of fluoridated drinking-water.
There is no evidence to suggest that the guideline value of 1.5 mg/l set in 1984 and reaffirmed in 1993 needs to be revised. Concentrations above this value carry an increasing risk of dental fluorosis, and much higher concentrations lead to skeletal fluorosis. The value is higher than that recommended for artificial fluoridation of water supplies, which is usually 0.5–1.0 mg/l.
In setting national standards or local guidelines for fluoride or in evaluating the possible health consequences of exposure to fluoride, it is essential to consider the average daily intake of water by the population of interest and the intake of fluoride from other sources (e.g. from food and air). Where the intakes are likely to approach, or be greater than, 6 mg/day, it would be appropriate to consider setting a standard or local guideline at a concentration lower than 1.5 mg/l.
Practical considerations
Fluoride is usually determined by means of an ion-selective electrode, which makes it possible to measure the total amount of free and complex-bound fluoride dissolved in water. The method can detect fluoride concentrations in water well below the guideline value. However, appropriate sample preparation is a critical step in the accurate quantification of fluoride, especially where only the free fluoride ion is measured.
A range of treatment technologies are available for both large and small supplies. Different methods for small supplies are favoured in different countries; these are based on bone charcoal, contact precipitation, activated alumina and clay. However, in some areas with high natural fluoride levels in drinking-water, the guideline value may be difficult to achieve in some circumstances with the treatment technology available. Large supplies tend to rely on activated alumina or advanced treatment processes such as reverse osmosis.
Formaldehyde
Formaldehyde occurs in industrial effluents and is emitted into air from plastic materials and resin glues. Formaldehyde in drinking-water results primarily from the oxidation of natural organic matter during ozonation and chlorination. Concentrations of up to 30 µg/l have been found in ozonated drinking-water. Formaldehyde can also be found in drinking-water as a result of release from polyacetal plastic fittings. Formaldehyde’s physicochemical properties suggest that it is unlikely to volatilize from water, so exposure by inhalation during showering is expected to be low.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2004 |
|---|
| Principal references |
IPCS (2002)
Formaldehyde
WHO (2005)
Formaldehyde in drinking-water
|
|---|
Rats and mice exposed to formaldehyde by inhalation exhibited an increased incidence of carcinomas of the nasal cavity at doses that caused irritation of the nasal epithelium. Ingestion of formaldehyde in drinking-water for 2 years caused stomach irritation in rats. Papillomas of the stomach associated with severe tissue irritation were observed in one study. IARC has classified formaldehyde in Group 1 (carcinogenic to humans). The weight of evidence indicates that formaldehyde is not carcinogenic by the oral route.
Owing to formaldehyde’s high reactivity, effects in the tissue of first contact following ingestion are more likely to be related to the concentration of the formaldehyde consumed than to its total intake. A tolerable concentration of 2.6 mg/l for ingested formaldehyde has been established based on a NOEL of 260 mg/l for histopathological effects in the oral and gastric mucosa of rats administered formaldehyde in their drinking-water for 2 years, using an uncertainty factor of 100 (for interspecies and intraspecies variation). In view of the significant difference between the expected concentrations of formaldehyde in drinking-water and the tolerable concentration, it is not considered necessary to set a formal guideline value for formaldehyde.
Glyphosate and AMPA
Glyphosate (CAS No. 1071-83-6) is a broad-spectrum herbicide used in both agriculture and forestry and for aquatic weed control. Microbial biodegradation of glyphosate occurs in soil, aquatic sediment and water, the major metabolite being aminomethylphosphonic acid (AMPA) (CAS No. 1066-51-9). Glyphosate is chemically stable in water and is not subject to photochemical degradation. The low mobility of glyphosate in soil indicates minimal potential for the contamination of groundwater. Glyphosate can, however, enter surface and subsurface waters after direct use near aquatic environments or by runoff or leaching from terrestrial applications.
View in own window
| Reason for not establishing guideline values | Occur in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (1998)
Pesticide residues in food—1997 evaluations
IPCS (1994)
Glyphosate
WHO (2005)
Glyphosate and AMPA in drinking-water
|
|---|
Glyphosate and AMPA have similar toxicological profiles, and both are considered to exhibit low toxicity. A health-based value of 0.9 mg/l can be derived based on the group ADI for AMPA alone or in combination with glyphosate of 0–0.3 mg/kg body weight, based upon a NOAEL of 32 mg/kg body weight per day, the highest dose tested, identified in a 26-month study of toxicity in rats fed technical-grade glyphosate and using an uncertainty factor of 100 (for interspecies and intraspecies variation).
Because of their low toxicity, the health-based value derived for AMPA alone or in combination with glyphosate is orders of magnitude higher than concentrations of glyphosate or AMPA normally found in drinking-water. Under usual conditions, therefore, the presence of glyphosate and AMPA in drinking-water does not represent a hazard to human health. For this reason, the establishment of a formal guideline value for glyphosate and AMPA is not deemed necessary.
Halogenated acetonitriles (dichloroacetonitrile, dibromoacetonitrile, bromochloroacetonitrile, trichloroacetonitrile)
Halogenated acetonitriles are produced during water chlorination or chloramination from naturally occurring substances, including algae, fulvic acid and proteinaceous material. In general, increasing temperature or decreasing pH is associated with increasing concentrations of halogenated acetonitriles. Ambient bromide levels appear to influence, to some degree, the speciation of halogenated acetonitrile compounds. Dichloroacetonitrile is by far the most predominant halogenated acetonitrile species detected in drinking-water.
View in own window
| Provisional guideline value | Dichloroacetonitrile: 0.02 mg/l (20 µg/l) |
|---|
| The guideline value for dichloroacetonitrile is provisional owing to limitations of the toxicological database. |
| Guideline value | Dibromoacetonitrile: 0.07 mg/l (70 µg/l) |
|---|
| Occurrence | Concentrations of individual halogenated acetonitriles can exceed 0.01 mg/l, although levels of 0.002 mg/l or less are more usual |
|---|
| TDIs | Dichloroacetonitrile: 2.7 µg/kg body weight based on a LOAEL of 8 mg/kg body weight per day for increased relative liver weight in male and female rats in a 90-day study, using an uncertainty factor of 3000 (taking into consideration intraspecies and interspecies variation, the short duration of the study, the use of a minimal LOAEL and database deficiencies) |
|---|
| Dibromoacetonitrile: 11 µg/kg body weight, based on a NOAEL of 11.3 mg/kg body weight per day for decreased body weight in male rats in a 90-day drinking-water study and an uncertainty factor of 1000 (accounting for interspecies and intraspecies variation, subchronic to chronic extrapolation and database insufficiencies) |
| Limit of detection | 0.03 µg/l by GC with ECD |
|---|
| Treatment performance | Reduction of organic precursors will reduce the formation of halogenated acetonitriles. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 20% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (2000)
Disinfectants and disinfectant by-products
WHO (2004)
Halogenated acetonitriles in drinking-water
|
|---|
View in own window
| Reason for not establishing guideline values | Available data inadequate to permit derivation of health-based guideline values for bromochloroacetonitrile and trichloroacetonitrile |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (2000)
Disinfectants and disinfectant by-products
WHO (2004)
Halogenated acetonitriles in drinking-water
|
|---|
IARC has concluded that dichloroacetonitrile, dibromoacetonitrile, bromochloroacetonitrile and trichloroacetonitrile are not classifiable as to their carcinogenicity in humans. Dichloroacetonitrile and bromochloroacetonitrile have been shown to be mutagenic in bacterial assays, whereas results for dibromoacetonitrile and trichloroacetonitrile were negative. All four of these halogenated acetonitriles induced sister chromatid exchange and DNA strand breaks and adducts in mammalian cells in vitro but were negative in the mouse micronucleus test.
The majority of reproductive and developmental toxicity studies of the halogenated acetonitriles were conducted using tricaprylin as a vehicle for gavage administration of the compound under study. As tricaprylin was subsequently demonstrated to be a developmental toxicant that potentiated the effects of trichloroacetonitrile and, presumably, other halogenated acetonitriles, results reported for developmental studies using tricaprylin as the gavage vehicle are likely to overestimate the developmental toxicity of these halogenated acetonitriles.
Dichloroacetonitrile
Dichloroacetonitrile induced decreases in body weight and increases in relative liver weight in short-term studies. Although developmental toxicity has been demonstrated, the studies used tricaprylin as the vehicle for gavage administration.
Dibromoacetonitrile
Dibromoacetonitrile is currently under analysis for chronic toxicity in mice and rats. None of the available reproductive or developmental studies were adequate to use in the quantitative dose–response assessment. The data gap may be particularly relevant because cyanide, a metabolite of dibromoacetonitrile, induces male reproductive system toxicity and because of uncertainty regarding the significance of the testes effects observed in a 14-day NTP rat study.
Bromochloroacetonitrile
Available data are insufficient to serve as a basis for derivation of a guideline value for bromochloroacetonitrile.
Trichloroacetonitrile
Available data are also insufficient to serve as a basis for derivation of a guideline value for trichloroacetonitrile. The previous provisional guideline value of 1 µg/l was based on a developmental toxicity study in which trichloroacetonitrile was administered by gavage in tricaprylin vehicle, and a re-evaluation judged this study to be unreliable in light of the finding in a more recent study that tricaprylin potentiates the developmental and teratogenic effects of halogenated acetonitriles and alters the spectrum of malformations in the fetuses of treated dams.
Hardness
Hardness in water is caused by a variety of dissolved polyvalent metallic ions, predominantly calcium and magnesium cations. It is usually expressed as milligrams of calcium carbonate per litre. Hardness is the traditional measure of the capacity of water to react with soap, hard water requiring considerably more soap to produce a lather.
View in own window
| Reason for not establishing a guideline value | Not of health concern at levels found in drinking-water |
|---|
| Additional comments | May affect acceptability of drinking-water |
|---|
| Assessment date | 1993, revised in 2011 |
|---|
| Principal reference | WHO (2011)
Hardness in drinking-water |
|---|
Natural and treated waters have a wide range of mineral content, from very low levels in rainwater and naturally soft and softened water to higher levels in naturally hard waters. Bottled and packaged waters can be naturally mineralized or naturally soft or demineralized. Thus, the mineral consumption from drinking-water and cooking water will vary widely, depending upon location, treatment and water source.
The degree of hardness of drinking-water is important for aesthetic acceptability by consumers (see chapter 10) and for economic and operational considerations. Many hard waters are softened for those reasons using several applicable technologies. The choice of the most appropriate conditioning technology will depend on local circumstances (e.g. water quality issues, piping materials, corrosion) and will be applied either centrally or in individual homes as a consumer preference.
Consumers should be informed of the mineral composition of their water, whether or not it is modified. The contribution of drinking-water minerals to mineral nutrition should be considered where changes in supply are proposed or where less traditional sources, such as recycled water, seawater or brackish water, are processed and exploited for drinking-water. The treatments used remove most minerals, and stabilization of water is always necessary prior to distribution.
Drinking-water can be a contributor to calcium and magnesium intake and could be important for those who are marginal for calcium and magnesium. Where drinking-water supplies are supplemented with or replaced by demineralized water that requires conditioning, consideration should be given to adding calcium and magnesium salts to achieve concentrations similar to those that the population received from the original supply. Modification of calcium and magnesium concentrations in drinking-water for health reasons should comply with the technical requirements to provide water suitable for distribution.
Although there is evidence from epidemiological studies for a protective effect of magnesium or hardness on cardiovascular mortality, the evidence is being debated and does not prove causality. Further studies are being conducted. There are insufficient data to suggest either minimum or maximum concentrations of minerals at this time, as adequate intake will depend on a range of other factors. Therefore, no guideline values are proposed.
Heptachlor and heptachlor epoxide
Heptachlor (CAS No. 76-44-8) is a broad-spectrum insecticide, the use of which has been banned or restricted in many countries. At present, the major use of heptachlor is for termite control by subsurface injection into soil. Heptachlor is quite persistent in soil, where it is mainly transformed to its epoxide. Heptachlor epoxide (CAS No. 1024-57-3) is very resistant to further degradation. Heptachlor and heptachlor epoxide bind to soil particles and migrate very slowly. Heptachlor and heptachlor epoxide have been found in drinking-water at nanogram per litre levels. Diet is considered to represent the major source of exposure to heptachlor, although intake is decreasing significantly, as its use has substantially declined.
View in own window
| Reason for not establishing a guideline value | Occur in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (1992)
Pesticide residues in food—1991 evaluations
FAO/WHO (1995)
Pesticide residues in food—1994 evaluations
WHO (2003)
Heptachlor and heptachlor epoxide in drinking-water
|
|---|
Prolonged exposure to heptachlor has been associated with damage to the liver and central nervous system toxicity. In 1991, IARC reviewed the data on heptachlor and concluded that the evidence for carcinogenicity was sufficient in animals and inadequate in humans, classifying it in Group 2B (possibly carcinogenic to humans). A health-based value of 0.03 µg/l can be calculated for heptachlor and heptachlor epoxide on the basis of a PTDI of 0.1 µg/kg body weight, based on a NOAEL for heptachlor of 0.025 mg/kg body weight per day from two studies in the dog, taking into consideration inadequacies of the database and allocating 1% of the PTDI to drinking-water. However, because heptachlor and heptachlor epoxide occur at concentrations well below those of health concern, it is not considered necessary to derive a formal guideline value. It should also be noted that concentrations below 0.1 µg/l are generally not achievable using conventional treatment technology.
Hexachlorobenzene
The major agricultural application for hexachlorobenzene (CAS No. 118-74-1), or HCB, was as a seed dressing for crops to prevent the growth of fungi, but its use is now uncommon. At present, it appears mainly as a by-product of several chemical processes or an impurity in some pesticides. HCB is distributed throughout the environment because it is mobile and resistant to degradation. It bioaccumulates in organisms because of its physicochemical properties and its slow elimination. HCB is commonly detected at low levels in food, and it is generally present at low concentrations in ambient air. It has been detected only infrequently, and at very low concentrations (below 0.1 µg/l), in drinking-water supplies.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (1997)
Hexachlorobenzene
WHO (2004)
Hexachlorobenzene in drinking-water
|
|---|
IARC has evaluated the evidence for the carcinogenicity of HCB in animals and humans and assigned it to Group 2B (possibly carcinogenic to humans). HCB has been shown to induce tumours in three animal species and at a variety of sites. A health-based value of 1 µg/l can be derived for HCB by applying the linearized multistage low-dose extrapolation model to liver tumours observed in female rats in a 2-year dietary study. Using an alternative (tumorigenic dose05, or TD05) approach, a TDI of 0.16 µg/kg body weight can be calculated, which corresponds to a health-based value of approximately 0.05 µg/l, if one assumes a 1% allocation of the TDI to drinking-water. It should be noted that concentrations in food have been falling steadily, and this allocation factor may be considered very conservative.
Because the health-based values derived from both of these approaches are considerably higher than the concentrations at which HCB is detected in drinking-water (i.e. sub-nanograms per litre), when it is detected, it is not considered necessary to establish a formal guideline value for HCB in drinking-water. HCB is listed under the Stockholm Convention on Persistent Organic Pollutants.
Hexachlorobutadiene
Hexachlorobutadiene, or HCBD, is used as a solvent in chlorine gas production, a pesticide, an intermediate in the manufacture of rubber compounds and a lubricant. Concentrations of up to 6 µg/l have been reported in the effluents from chemical manufacturing plants. HCBD is also found in air and food.
View in own window
| Guideline value | 0.0006 mg/l (0.6 µg/l) |
|---|
| Occurrence | Has been detected in surface water at concentrations of a few micrograms per litre and in drinking-water at concentrations below 0.5 µg/l |
|---|
| TDI | 0.2 µg/kg body weight, based on a NOAEL of 0.2 mg/kg body weight per day for renal toxicity in a 2-year feeding study in rats, using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for limited evidence of carcinogenicity and genotoxicity of some metabolites) |
|---|
| Limit of detection | 0.01 µg/l by GC-MS; 0.18 µg/l by GC with ECD |
|---|
| Treatment performance | 0.001 mg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The practical quantification limit for HCBD is of the order of 2 µg/l, but concentrations in drinking-water can be controlled by specifying the HCBD content of products coming into contact with it. |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (1994)
Hexachlorobutadiene
WHO (2003)
Hexachlorobutadiene in drinking-water
|
|---|
HCBD is easily absorbed and metabolized via conjugation with glutathione. This conjugate can be further metabolized to a nephrotoxic derivative. Kidney tumours were observed in a long-term oral study in rats. HCBD has not been shown to be carcinogenic by other routes of exposure. IARC has placed HCBD in Group 3 (not classifiable as to its carcinogenicity to humans). Positive and negative results for HCBD have been obtained in bacterial assays for point mutation; however, several metabolites have given positive results.
Hydrogen sulfide
Hydrogen sulfide is a gas with an offensive “rotten eggs” odour that is detectable at very low concentrations, below 0.8 µg/m3 in air. It is formed when sulfides are hydrolysed in water. However, the level of hydrogen sulfide found in drinking-water will usually be low, because sulfides are readily oxidized in well-aerated or chlorinated water.
View in own window
| Reason for not establishing a guideline value | Not of health concern at levels found in drinking-water |
|---|
| Additional comments | May affect acceptability of drinking-water |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Hydrogen sulfide in drinking-water |
|---|
The acute toxicity to humans of hydrogen sulfide following inhalation of the gas is high; eye irritation can be observed at concentrations of 15–30 mg/m3. Although oral toxicity data are lacking, it is unlikely that a person could consume a harmful dose of hydrogen sulfide from drinking-water. Consequently, no guideline value is proposed. However, hydrogen sulfide can be easily detected in drinking-water by taste or odour (see chapter 10).
Inorganic tin
Tin is used principally in the production of coatings used in the food industry. Food, particularly canned food, therefore represents the major route of human exposure to tin. For the general population, drinking-water is not a significant source of tin, and levels in drinking-water greater than 1–2 µg/l are exceptional. However, there is increasing use of tin in solder, which may be used in domestic plumbing, and tin has been proposed for use as a corrosion inhibitor.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2004)
Inorganic tin in drinking-water |
|---|
Tin and inorganic tin compounds are poorly absorbed from the gastrointestinal tract, do not accumulate in tissues and are rapidly excreted, primarily in faeces.
No increased incidence of tumours was observed in long-term carcinogenicity studies conducted in mice and rats fed tin(II) chloride. Tin has not been shown to be teratogenic or fetotoxic in mice, rats or hamsters. In rats, the NOAEL in a long-term feeding study was 20 mg/kg body weight per day.
The main adverse effect on humans of excessive levels of tin in canned beverages (above 150 mg/kg) or other canned foods (above 250 mg/kg) has been acute gastric irritation. There is no evidence of adverse effects in humans associated with chronic exposure to tin.
In 1989, JECFA established a PTWI of 14 mg/kg body weight from a TDI of 2 mg/kg body weight on the basis that the problem with tin is associated with acute gastrointestinal irritancy, the threshold for which is about 200 mg/kg in food. This was reaffirmed by JECFA in 2000. In view of its low toxicity, the presence of tin in drinking-water does not, therefore, represent a hazard to human health. For this reason, the establishment of a guideline value for inorganic tin is not deemed necessary.
Iodine
Iodine occurs naturally in water in the form of iodide. Traces of iodine are produced by oxidation of iodide during water treatment. Iodine is occasionally used for water disinfection in the field or in emergency situations. The diet is the major source of exposure to iodine for the general human population; the contribution to total exposure from drinking-water is assumed to be low (around 5%).
View in own window
| Reason for not establishing a guideline value | Available data inadequate to permit derivation of health-based guideline value. Additionally, occurrence in drinking-water is usually low. Although higher levels of exposure may occur when iodine is used as a drinking-water disinfectant at the point of use, extended periods of exposure to iodine through water disinfection are unlikely. |
|---|
| Occurrence | Average concentrations have ranged from 0.5 to 20 µg/l in rivers and lakes |
|---|
| Limit of detection | 10 µg/l by a leuco crystal violet method |
|---|
| Treatment performance | Not applicable as occurrence in drinking-water is usually low |
|---|
| Additional comments | Iodine is not recommended for use as a primary disinfectant of drinking-water but it can be used as a point-of-use disinfectant. Caution should be exercised for longer-term point-of-use disinfection in susceptible individuals (see Part II of the supporting document Alternative drinking-water disinfectants: Bromine, iodine and silver; Annex 1). |
|---|
| Assessment date | 2020 |
|---|
| Principal reference | WHO (2020)
Iodine in drinking-water |
|---|
Iodine is an essential element for the synthesis of thyroid hormones. Various national and international organizations have established recommended daily intakes, with recommendations by WHO/FAO set in 2004 at 90 μg/day for infants, 120 μg/day for children, 150 μg/day for adults and 200 μg/day for pregnant or lactating women. In many parts of the world, dietary deficiencies in iodine are a health issue, which can adversely affect neurological development.
In 1989, JECFA set a provisional maximum tolerable daily intake (PMTDI) for iodine of 1000 μg/day (17 µg/kg body weight per day) from all sources, based primarily on data on the effects of iodide. Various national and international organizations have also established recommended upper intake levels for iodine, ranging from 500 to 1100 μg/day. Exposure to excess iodine can lead to hypothyroidism (with or without goitre—enlargement of the thyroid), hyperthyroidism, and changes in the incidence and types of thyroid malignancies.
However, available data are inadequate to establish a dose–response relationship between iodine exposure and changes in thyroid levels, and to clearly identify a threshold associated with thyrotoxicosis. Further, a guideline value cannot be derived using the more robust toxicological dataset for iodide because the effects of iodine and iodide on thyroid hormone concentrations in the blood may differ. Because iodine is generally not recommended for long-term disinfection, lifetime exposure to iodine concentrations such as might occur from water disinfection is unlikely. For these reasons, a guideline value for iodine has not been established at this time.
Disinfection of iodide-containing water by adding chlorine or chloramine can result in the production of iodinated DBPs; most often, these are formed during chloramination when complete oxidation is prevented. Iodinated DBPs are occasionally detected in drinking-water from treatment plants located in coastal saltwater areas. As with all DBPs, their concentrations in drinking-water can be reduced at the treatment plant by removing the natural organic matter from the water before the disinfection process occurs. It is critical that any method used to control DBP levels does not compromise the effectiveness of disinfection.
Iron
Iron is one of the most abundant metals in Earth’s crust. It is found in natural fresh waters at levels ranging from 0.5 to 50 mg/l. Iron may also be present in drinking-water as a result of the use of iron coagulants or the corrosion of steel and cast iron pipes during water distribution.
View in own window
| Reason for not establishing a guideline value | Not of health concern at levels found in drinking-water |
|---|
| Additional comments | May affect acceptability of drinking-water |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003) Iron in drinking-water |
|---|
Iron is an essential element in human nutrition, particularly in the iron(II) oxidation state. Estimates of the minimum daily requirement for iron depend on age, sex, physiological status and iron bioavailability and range from about 10 to 50 mg/day.
As a precaution against storage in the body of excessive iron, in 1983, JECFA established a PMTDI of 0.8 mg/kg body weight, which applies to iron from all sources except for iron oxides used as colouring agents and iron supplements taken during pregnancy and lactation or for specific clinical requirements. An allocation of 10% of this PMTDI to drinking-water gives a value of about 2 mg/l, which does not present a hazard to health. The taste and appearance of drinking-water will usually be affected below this level (see chapter 10).
No guideline value for iron in drinking-water is proposed.
Isoproturon
Isoproturon (CAS No. 34123-59-6) is a selective, systemic herbicide used in the control of annual grasses and broad-leaved weeds in cereals. It can be photodegraded, hydro-lysed and biodegraded and persists for periods ranging from days to weeks. It is mobile in soil. There is evidence that exposure to this compound through food is low.
Isoproturon is of low acute toxicity and low to moderate toxicity following short-term
View in own window
| Guideline value | 0.009 mg/l (9 µg/l) |
|---|
| Occurrence | Has been detected in surface water and groundwater, usually at concentrations below 0.1 µg/l; levels above 0.1 µg/l have occasionally been detected in drinking-water |
|---|
| TDI | 3 µg/kg body weight based on a NOAEL of approximately 3 mg/kg body weight in a 90-day study in dogs and a 2-year feeding study in rats, with an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for evidence of non-genotoxic carcinogenicity in rats) |
|---|
| Limit of detection | 10–100 ng/l by reversed-phase HPLC followed by UV or electrochemical detection |
|---|
| Treatment performance | 0.1 µg/l should be achievable using ozonation |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Isoproturon in drinking-water |
|---|
and long-term exposures. It does not possess significant genotoxic activity, but it causes marked enzyme induction and liver enlargement. Isoproturon caused an increase in hepatocellular tumours in male and female rats, but this was apparent only at doses that also caused liver toxicity. Isoproturon appears to be a tumour promoter rather than a complete carcinogen.
Lead
Lead is used principally in the production of lead-acid batteries, solder and alloys. The organolead compounds tetraethyl and tetramethyl lead have also been used extensively as antiknock and lubricating agents in petrol, although their use for these purposes in many countries has largely been phased out. Owing to the decreasing use of lead-containing additives in petrol and of lead-containing solder in the food processing industry, concentrations in air and food are declining; in most countries, lead levels in blood are also declining unless there are specific sources, such as dust from leaded paint or occupational/household recycling of lead-containing materials. Lead is rarely present in tap water as a result of its dissolution from natural sources; rather, its presence is primarily from corrosive water effects on household plumbing systems containing lead in pipes, solder or fittings (including alloy fittings with high lead content), or from the service connections to homes. The amount of lead dissolved from the plumbing system depends on several factors, including pH, temperature, alkalinity, scale in pipe and standing time of the water, with soft, acidic water being the most plumbosolvent. Free chlorine residuals in drinking-water tend to form more insoluble lead-containing deposits, whereas chloramine residuals may form more soluble sediments in lead pipe. Accordingly, significant changes in the water quality of a supply, resulting from, for example, changes in treatment or changes of source, can result in changes in plumbosolvency or solubilization of lead deposits, or both.
View in own window
| Provisional guideline value | 0.01 mg/l (10 µg/l) |
|---|
| The guideline value is designated as provisional on the basis of treatment performance and analytical achievability. As this is no longer a health-based guideline value, concentrations should be maintained as low as reasonably practical. New sources of lead, such as service connections and lead solder, should not be introduced into any system, and low lead alloy fittings should be used in repairs and new installations. |
| Occurrence | Concentrations in drinking-water are generally below 5 µg/l, although much higher concentrations (above 100 µg/l) have been measured where lead service connections or fittings are present. The primary source of lead is from service connections and plumbing in buildings; therefore, lead should be measured at the tap. Lead concentrations can also vary according to the period in which the water has been in contact with the lead-containing materials. |
|---|
| Basis of guideline derivation | The guideline value was previously based on a JECFA PTWI, which has since been withdrawn, and no new PTWI has been established, on the basis that there does not appear to be a threshold for the key effects of lead. However, substantial efforts have been made to reduce lead exposure from a range of sources, including drinking-water. The guideline value is maintained at 10 µg/l but is designated as provisional on the basis of treatment performance and analytical achievabiilty because it is extremely difficult to achieve a lower concentration than this by central conditioning, such as phosphate dosing. |
|---|
| Limit of detection | 1 µg/l by AAS; practical quantification limit in the region of 1–10 µg/l |
|---|
| Treatment performance | Not a raw water contaminant; treatment not applicable |
|---|
| Additional comments | Infants and children are considered to be the most sensitive subgroups of the population |
|---|
|
Lead is exceptional compared with other chemical hazards, in that most lead in drinking-water arises from lead service connections and plumbing in buildings, and the remedy consists principally of removing service connections, plumbing and fittings containing lead. This requires much time and money, and it is recognized that not all water will meet the guideline value immediately. Meanwhile, all other practical measures to reduce total exposure to lead, including corrosion control, should be implemented. In new installations or repairs, lead-free service connections and solder and low lead alloy fittings should be used to prevent the introduction of contamination.
The sampling protocol adopted – e.g. first draw, random daytime sampling or flushed – will depend on the objective of taking the samples. Where there is a need to verify that lead solder and/or high-lead fittings have not been installed in new or repaired systems, the approach used is to take a worst-case sample that reflects an extended period of stagnation, to maximize the chance of identifying the presence of lead.
|
| Assessment date | 2011, revised 2016 |
|---|
| Principal references |
FAO/WHO (2011)
Evaluation of certain food additives and contaminants
WHO (2016)
Lead in drinking-water
|
|---|
Exposure to lead is associated with a wide range of effects, including various neurodevelopmental effects, mortality (mainly due to cardiovascular diseases), impaired renal function, hypertension, impaired fertility and adverse pregnancy outcomes. Impaired neurodevelopment in children is generally associated with lower blood lead concentrations than the other effects, the weight of evidence is greater for neurodevelopmental effects than for other health effects and the results across studies are more consistent than those for other effects. For adults, the adverse effect associated with lowest blood lead concentrations for which the weight of evidence is greatest and most consistent is a lead-associated increase in systolic blood pressure. JECFA concluded that the effects on neurodevelopment and systolic blood pressure provided the appropriate bases for dose–response analyses.
Based on the dose–response analyses, JECFA estimated that the previously established PTWI of 25 µg/kg body weight is associated with a decrease of at least 3 intelligence quotient (IQ) points in children and an increase in systolic blood pressure of approximately 3 mmHg (0.4 kPa) in adults. These changes are important when viewed as a shift in the distribution of IQ or blood pressure within a population. JECFA therefore concluded that the PTWI could no longer be considered health protective, and it was withdrawn.
Because the dose–response analyses do not provide any indication of a threshold for the key effects of lead, JECFA concluded that it was not possible to establish a new PTWI that would be considered to be health protective. JECFA reaffirmed that because of the neurodevelopmental effects, fetuses, infants and children are the subgroups that are most sensitive to lead.
It needs to be recognized that lead is exceptional compared with other chemical hazards, in that most lead in drinking-water arises from lead service connections and plumbing in buildings, and the remedy consists principally of removing plumbing and fittings containing lead, which requires much time and money. It is therefore emphasized that all other practical measures to reduce total exposure to lead, including corrosion control, should be implemented. New sources of lead, such as lead service connections and solder, should not be introduced into any system, and low lead alloy fittings should be used in repairs and new installations.
In terms of monitoring, if the monitoring objective is to identify the presence of lead in the internal plumbing of a building, then the sample should be from the tap. The sampling protocols also depend on the objective of taking the samples. First-draw samples typically will have the highest lead concentrations, but this may not be reflected in normal use if the same system provides water for toilet flushing, etc. Flushed samples, in contrast, give consistent values, but reflect the minimum contact time between the water and the lead-containing material. The random daytime samples, although most truly reflecting the water that the consumer drinks, give the most variable levels; hence, it is necessary to collect more samples to determine the mean level of exposure. Where there is a need to verify that lead service connections, lead solder and/or high-lead fittings have not been installed in new or repaired systems, the approach used is to take a worst-case sample that reflects an extended period of stagnation and to maximize the chance of identifying the presence of lead. Extended stagnation with sequential volume can also be used to identify sources or locations of lead as an investigative activity.
Lindane
Lindane (γ-hexachlorocyclohexane; γ-HCH) (CAS No. 58-89-9) is used as an insecticide on fruit and vegetable crops, for seed treatment and in forestry. It is also used as a therapeutic pesticide in humans and animals. Several countries have restricted the use of lindane. Lindane can be degraded in soil and rarely leaches to groundwater. In surface waters, it can be removed by evaporation. Exposure of humans occurs mainly via food, but this is decreasing. There may also be exposure from its use in public health and as a wood preservative.
View in own window
| Guideline value | 0.002 mg/l (2 µg/l) |
|---|
| Occurrence | Has been detected in both surface water and groundwater, usually at concentrations below 0.1 µg/l, although concentrations as high as 12 µg/l have been measured in wastewater-contaminated rivers |
|---|
| ADI | 0–0.005 mg/kg body weight on the basis of a NOAEL of 0.47 mg/kg body weight per day in a 2-year toxicity/carcinogenicity study in rats in which an increased incidence of periacinar hepatocellular hypertrophy, increased liver and spleen weights and increased mortality occurred at higher doses, using an uncertainty factor of 100 (for interspecies and intraspecies variation) |
|---|
| Limit of detection | 0.01 µg/l using GC |
|---|
| Treatment performance | 0.1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 1% of upper limit of ADI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | It should be noted that concentrations in food have been falling steadily, and the 1% allocation factor may be considered very conservative. |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (2003)
Pesticide residues in food—2002 evaluations
WHO (2003)
Lindane in drinking-water
|
|---|
Lindane was toxic to the kidney and liver after administration orally, dermally or by inhalation in short-term and long-term studies of toxicity and reproductive toxicity in rats. The renal toxicity of lindane was specific to male rats and was considered not to be relevant to human risk assessment, as it is a consequence of accumulation of α2u-globulin, a protein that is not found in humans. Hepatocellular hypertrophy was observed in a number of studies in mice, rats and rabbits and was reversed only partially after recovery periods of up to 6 weeks. Lindane did not induce a carcinogenic response in rats or dogs, but it caused an increased incidence of adenomas and carcinomas of the liver in agouti and pseudoagouti mice, but not in black or any other strains of mice, in a study of the role of genetic background in the latency and incidence of tumorigenesis. JMPR concluded that there was no evidence of genotoxicity. In the absence of genotoxicity and on the basis of the weight of the evidence from the studies of carcinogenicity, JMPR concluded that lindane is not likely to pose a carcinogenic risk to humans. Further, in an epidemiological study designed to assess the potential association between breast cancer and exposure to chlorinated pesticides, no correlation with lindane was found.
Malathion
Malathion (CAS No. 121-75-5) is commonly used to control mosquitoes and a variety of insects that attack fruits, vegetables, landscaping plants and shrubs. It can also be found in other pesticide products used indoors, on pets to control ticks and insects and to control human head and body lice. Under least favourable conditions (i.e. low pH and little organic content), malathion may persist in water with a half-life of months or even years. However, under most conditions, the half-life appears to be roughly 7–14 days. Malathion has been detected in surface water and drinking-water at concentrations below 2 µg/l.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (1998)
Pesticide residues in food—1997 evaluations
WHO (2003)
Malathion in drinking-water
|
|---|
Malathion inhibits cholinesterase activity in mice, rats and human volunteers. It increased the incidence of liver adenomas in mice when administered in the diet. Most of the evidence indicates that malathion is not genotoxic, although some studies indicate that it can produce chromosomal aberrations and sister chromatid exchange in vitro. JMPR has concluded that malathion is not genotoxic.
A health-based value of 0.9 mg/l can be calculated for malathion based on an allocation of 10% of the upper limit of the JMPR ADI—based on a NOAEL of 29 mg/kg body weight per day in a 2-year study of toxicity and carcinogenicity in rats, using an uncertainty factor of 100 for interspecies and intraspecies variation and supported by a NOAEL of 25 mg/kg body weight per day in a developmental toxicity study in rabbits—to drinking-water. However, intake of malathion from all sources is generally low and well below the upper limit of the ADI. As the chemical occurs in drinking-water at concentrations much lower than the health-based value, the presence of malathion in drinking-water under usual conditions is unlikely to represent a hazard to human health. For this reason, it is considered unnecessary to derive a formal guideline value for malathion in drinking-water.
Manganese6
Manganese is one of the most abundant metals in Earth’s crust, usually occurring with iron. It can exist in 11 oxidation states, often as chloride, oxides and sulfates. The most common oxidation states for manganese in natural water are manganese(II) and manganese(IV). Manganese is used principally in the manufacture of iron and steel alloys, and manganese compounds such as potassium and sodium permanganate are ingredients in various products used for cleaning, bleaching and disinfection. Manganese compounds are additionally used in some locations for potable water treatment and can also be an impurity in coagulants used during water treatment. Manganese occurs naturally in many surface water and groundwater sources; although naturally occurring manganese is usually the most important source for drinking-water, anthropogenic activities can also contribute to high levels of manganese in water. Manganese also occurs naturally in many food sources, and the greatest exposure to manganese is usually from food.
View in own window
| Provisional guideline value | Total manganese: 0.08 mg/l (80 µg/l), to be protective against neurological effects in the most sensitive subpopulation—bottle-fed infants—and consequently the general population |
|---|
| This guideline value is provisional because of the high level of uncertainty, as reflected in the composite uncertainty factor of 1000 |
| Occurrence | Levels in fresh waters vary widely. They are typically in the range 1–200 µg/l. Higher levels are usually associated with groundwater, lakes and reservoirs under acidic or reducing conditions, or in aerobic waters with industrial pollution. Very high concentrations (up to 10 mg/l) have been reported in acidic groundwater. In treated drinking-water, concentrations are typically less than 50 µg/l. |
|---|
| TDI | 0.025 mg/kg bw, derived by applying an uncertainty factor of 1000 to a LOAEL of 25 mg/kg bw per day identified from studies that reported neurological effects in rats exposed to manganese from birth to postnatal day 21. The uncertainty factor takes into account interpecies variation (10), intraspecies variation (10) and database uncertainties (10, including use of a LOAEL). |
|---|
| Limit of detection | 0.002 µg/l by ICP-MS; 0.005–50 µg/l by ICP-AES and GFAA spectrometry; and 10–70 µg/l by colorimetric methods. None of these methods distinguish between the different oxidation states of manganese. |
|---|
| Treatment performance | Manganese concentrations in drinking-water can be easily lowered to less than 0.05 mg/l using several treatment methods, including oxidation/filtration, adsorption/oxidation, softening/ion exchange and biological filtration. Selection of the appropriate treatment system for manganese removal depends on the form of manganese (dissolved or particulate) in the source water. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 50% of TDI |
|---|
| • weight | 5 kg body weight for a bottle-fed infant |
|---|
| • consumption | 0.75 litres per day for a bottle-fed infant |
|---|
| Additional comments | Risks to infants arising from exceedance of the provisional guideline value may be mitigated by following the WHO recommendation for exclusive breastfeeding, or by using an alternative safe source of drinking-water (e.g. bottled water that is certified by the responsible authorities) to prepare formula. |
|---|
| The presence of particulate manganese in drinking-water systems can cause acceptability problems; concentrations above 0.02 mg/l have caused complaints about discoloured water and staining of plumbing fixtures and laundry. Therefore, aesthetic as well as health aspects should be considered in the management of manganese in drinking-water, and when setting regulations and standards for drinking-water quality. |
| Assessment date | 2020 |
|---|
| Principal references | WHO (2021)
Manganese in drinking-water |
|---|
Manganese is an essential trace element. It is a necessary component of a number of enzymes and activates several others. The central nervous system is the primary concern for manganese toxicity in mammals, including humans. Neurodevelopmental toxicity studies in manganese-exposed juvenile rats revealed behavioural and sensorimotor effects, and corresponding neurostructural and neurochemical changes. Several epidemiological studies have also reported neurological effects (including reduced cognitive ability) in adult populations and children following ingestion of manganese-contaminated water. The epidemiological studies have limited utility in risk assessment due to uncertain manganese exposure levels, unclear temporality of effects and other potential confounding factors. However, collectively, they provide qualitative support that the neurological effects reported in animal studies are relevant in humans. Existing studies and reports do not provide adequate evidence to assess potential carcinogenicity from oral exposure to manganese in humans.
Absorption of manganese from the gastrointestinal (GI) tract has been suggested to take place through both an active transport mechanism and passive diffusion. GI absorption is influenced by several factors, including dietary factors: absorption is negatively correlated with intake of dietary fibre, oxalic acids and phytic acids. Some studies and reports have suggested that absorption and bioavailability of manganese are greater from drinking-water than from food, although other studies have reported no differences. However, absorption from drinking-water may be influenced by fasting conditions and the chemical form of manganese.
Following GI tract absorption, manganese is distributed via the systemic circulation to all tissues. Levels of manganese can increase in several tissues following oral exposure, including some regions of the brain in infants and adults. The main route of elimination of manganese from the body is faecal elimination via hepatobiliary excretion.
Manganese absorption from the GI tract may be higher in infants than in adults. Infants also retain higher levels of manganese than adults during the early neonatal period, possibly because of the incomplete development of the biliary excretion system. Along with the important neurodevelopmental processes occurring in neonates, this may render them particularly susceptible to toxicity from exposure to manganese. Further, there is potential for increased exposure to manganese in bottle-fed infants compared with breastfed infants—from the concentrated or powdered formula itself as well as the tap water used to prepare the formula.
Practical considerations
Manganese levels in drinking-water can be an issue in both high- and low-income countries, and should be considered in establishing national standards and local guidance. Resource-limited suppliers, in particular, may have difficulty in achieving the provisional guideline value; in such cases, incremental improvements towards meeting the provisional guideline value are encouraged. This is a particular problem for groundwater, for which treatment may be minimal and prohibitively expensive. In such instances, benefits from a reliable, microbiologically safe groundwater source should be assessed against the risks posed by an alternative source that may be subject to faecal contamination. Issues of acceptability of the drinking-water (which varies between different populations) should also be considered, since reduced acceptability may lead consumers to turn to more aesthetically acceptable but less microbiologically safe water supplies. It is vital that a sufficient supply of acceptable, microbiologically safe water is always available, even if some guidelines or standards for chemicals such as manganese cannot be immediately met.
Manganese should be evaluated and managed in the context of developing a WSP (see chapter 4). Surface waters prone to high and variable concentrations of manganese may require more frequent and targeted monitoring than groundwater. Where manganese is present at concentrations close to the provisional guideline value or the water is treated to remove manganese, routine monitoring should be conducted after treatment. If manganese is detected at the point of collection or use, or aesthetic issues related to manganese are reported by consumers, this indicates that treatment for manganese removal is not optimized or that the distribution system is not appropriately managed.
Options for controlling levels in groundwater include drilling a new well or blending water from different wells. For lake and reservoir sources where there is a thermocline and lower water levels become anoxic, management of the sources to prevent release of manganese from sediment is important.
Selection of the appropriate treatment system for manganese removal depends on the form of manganese (dissolved or particulate) in the source water. Dissolved manganese(II) is most often the predominant form present in anoxic and acidic groundwater or lakes. However, depending on the pH and the dissolved oxygen content of the water, a combination of dissolved and particulate manganese can be present. In general, treatment methods used for manganese rely on a combination of processes (e.g. oxidation, adsorption, filtration) to remove both the dissolved and particulate forms. At the point of use, reverse osmosis is the most effective and reliable treatment technology; however, point-of-use units using ion exchange media are also moderately effective. To reduce water discoloration and staining of laundry and fixtures, ion exchange and greensand filtration with careful operation and maintenance can be used at the point of entry.
Low levels of manganese in source or treated water can accumulate in the distribution system. Periodic release of manganese can then occur, resulting in high levels at the tap. Releases can occur as a result of physical or hydraulic disturbances to the system (e.g. mains breaks, hydrant flushing) or changes in water chemistry (e.g. changes in pH, temperature, chlorine residual, source water type/blending). Physical and hydraulic disturbances most often release particulate manganese and can cause discoloured water and consumer complaints. Chemical releases can go unnoticed if manganese occurs predominantly in the dissolved form. Other contaminants (e.g. arsenic, barium, chromium, lead, uranium) that deposit with manganese oxides in the distribution system may also be released into the water and reach consumers’ taps. Control measures to minimize manganese release events include maintaining stable water chemistry and minimizing manganese levels entering the distribution system, the amount of manganese oxide deposits in the distribution system (through best practices for water mains cleaning), and physical or hydraulic disturbances.
MCPA
MCPA is a phenoxyacetic acid herbicide that is found in various formulations: as the free acid (CAS No. 94-74-6), as a dimethylamine salt (CAS No. 2039-46-5), as a sodium salt (CAS No. 3653-48-3) and as a 2-ethylhexyl ester (CAS No. 29450-45-1). It is a post-emergence herbicide that is widely used against broadleaf weeds in agriculture and horticulture and on grassland and lawns. All forms of MCPA will dissociate in water to the acid (anion) form. MCPA is highly soluble in water. Biological degradation is an important process in determining MCPA’s environmental fate. Chlorophenols and chlorocresols are potential soil metabolites and may, if present in water, give rise to unacceptable tastes. Surface water may be contaminated via spray drift and runoff, whereas groundwater may be contaminated via leaching from soil. Exposure from food is likely to be low.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water or drinking-water sources at concentrations well below those of health concern |
|---|
| Health-based value* | 0.7 mg/l |
|---|
| Acute health-based value** | 20 mg/l |
|---|
| Occurrence | Concentrations in surface water usually less than 1 µg/l; concentrations in drinking-water usually below 0.1 µg/l |
|---|
| ADI |
0–0.1 mg/kg bw for MCPA ion, based on an overall NOAEL of 12 mg/kg bw per day for changes in clinical chemistry parameters indicative of effects on the kidneys from four subchronic studies in rats and application of a safety factor of 100
ADI established for the sum of MCPA and its salts and esters, expressed as MCPA acid equivalents
|
|---|
| ARfD |
0.6 mg/kg bw for MCPA ion, based on the overall NOAEL of 60 mg/kg bw for maternal and developmental toxicity in rats and application of a safety factor of 100
ARfD established for the sum of MCPA and its salts and esters, expressed as MCPA acid equivalents
|
|---|
| Limit of detection | 0.8 µg/L using HPLC with a photodiode array UV detector; 0.09 µg/l using derivatization and GC with ECD; limit of quantification of 0.0005 µg/l for LC-MS/MS |
|---|
| Treatment performance | Conventional treatment not effective; activated carbon adsorption and/or ozonation and advanced oxidation processes (e.g. UV with hydrogen peroxide) are effective; membrane filtration processes (e.g. reverse osmosis) may be effective |
|---|
| Health-based value derivation |
|---|
| • allocation to water | 20% of upper bound of unrounded ADI (0.12 mg/kg bw) |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Acute health-based value derivation |
|---|
| • allocation to water | 100% of ARfD |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The default allocation factor of 20% has been used to account for the fact that the available food exposure data, which suggest that exposure via this route is low, do not generally include information from developing countries, where exposure via this route may be higher. |
|---|
| As a general principle, the concentration of pesticides in water, including MCPA, should be kept as low as possible and concentrations should not be allowed to increase up to the health-based value. |
| Further guidance on interpreting the health-based value and deciding when to monitor can be found in section 8.5.3. |
| Assessment date | 2016 |
|---|
| Principal references |
WHO (2013). Pesticide residues in food – 2012 evaluations
WHO (2016). MCPA in drinking-water
|
|---|
- *
When a formal guideline value is not established, a “health-based value” may be determined in order to provide guidance to Member States when there is reason for local concern. Establishing a formal guideline value for such substances may encourage Member States to incorporate a value into their national standards when this may be unnecessary.
- **
For more information on acute health-based values, see section 8.7.5.
The target organs for the MCPA ion are the kidney, liver and blood. MCPA is not carcinogenic in mice or rats, and the MCPA ion exhibits no genotoxic potential. In multigeneration studies in rats, there was no evidence of reproductive toxicity up to the highest dose tested. The MCPA ion was not teratogenic in rats or rabbits.
Mecoprop
The half-lives for degradation of chlorophenoxy herbicides, including mecoprop (CAS No. 93-65-2; 7085-19-0 racemic mixture), also known as 2(2-methyl-chlorophenoxy) propionic acid or MCPP, in the environment are in the order of several days. Chlorophenoxy herbicides are not often found in food.
View in own window
| Guideline value | 0.01 mg/l (10 µg/l) |
|---|
| Occurrence | Chlorophenoxy herbicides not frequently found in drinking-water; when detected, concentrations usually no greater than a few micrograms per litre |
|---|
| TDI | 3.33 µg/kg body weight, based on a NOAEL of 1 mg/kg body weight for effects on kidney weight in 1- and 2-year studies in rats, with an uncertainty factor of 300 (100 for interspecies and intraspecies variation and 3 for limitations in the database) |
|---|
| Limit of detection | 0.01 µg/l by GC-MS; 0.01–0.02 µg/l by GC with ECD |
|---|
| Treatment performance | 0.1 µg/l should be achievable using GAC or ozonation |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chlorophenoxy herbicides (excluding 2,4-D and MCPA) in drinking-water |
|---|
Chlorophenoxy herbicides, as a group, have been classified in Group 2B (possibly carcinogenic to humans) by IARC. However, the available data from studies in exposed populations and experimental animals do not permit assessment of the carcinogenic potential to humans of any specific chlorophenoxy herbicide. Therefore, drinking-water guidelines for these compounds are based on a threshold approach for other toxic effects. Effects of dietary administration of mecoprop in short-term and long-term studies include decreased relative kidney weight (rats and dogs), increased relative liver weight (rats), effects on blood parameters (rats and dogs) and depressed body weight gain (dogs).
Mercury
Mercury is used in the electrolytic production of chlorine, in electrical appliances, in dental amalgams and as a raw material for various mercury compounds. Methylation of inorganic mercury has been shown to occur in fresh water and in seawater, although almost all mercury in uncontaminated drinking-water is thought to be in the form of Hg2+. Thus, it is unlikely that there is any direct risk of the intake of organic mercury compounds, especially of alkylmercurials, as a result of the ingestion of drinking-water. However, there is a possibility that methylmercury will be converted into inorganic mercury. Food is the main source of mercury in non-occupationally exposed populations; the mean dietary intake of mercury in various countries ranges from 2 to 20 µg/day per person.
View in own window
| Guideline value | 0.006 mg/l (6 µg/l) for inorganic mercury |
|---|
| Occurrence | Mercury is present in the inorganic form in surface water and groundwater at concentrations usually below 0.5 µg/l, although local mineral deposits may produce higher levels in groundwater |
|---|
| TDI | 2 µg/kg body weight for inorganic mercury based on a NOAEL of 0.23 mg/kg body weight per day for kidney effects in a 26-week study in rats and applying an uncertainty factor of 100 (for interspecies and intraspecies variation) after adjusting for daily dosing |
|---|
| Limit of detection | 0.05 µg/l by cold vapour AAS; 0.6 µg/l by ICP; 5 µg/l by flame AAS |
|---|
| Treatment performance | It should be possible to achieve a concentration below 1 µg/l by treatment of raw waters that are not grossly contaminated with mercury using methods that include coagulation/sedimentation/filtration, PAC and ion exchange. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | A similar TDI may be obtained by applying an uncertainty factor of 1000 (an additional uncertainty factor of 10 for adjustment from a LOAEL to a NOAEL) to the LOAEL for renal effects of 1.9 mg/kg body weight per day in a 2-year NTP study in rats. |
|---|
| The current guideline value applies to inorganic mercury, which is the form found in drinking-water, whereas the previous guideline value applied to total (inorganic and organic) mercury. |
| Assessment date | 2004 |
|---|
| Principal references |
IPCS (2003)
Elemental mercury and inorganic mercury compounds
WHO (2005)
Mercury in drinking-water
|
|---|
The toxic effects of inorganic mercury compounds are seen mainly in the kidney in both humans and laboratory animals following short-term and long-term exposure. In rats, effects include increased absolute and relative kidney weights, tubular necrosis, proteinuria and hypoalbuminaemia. In humans, acute oral poisoning results primarily in haemorrhagic gastritis and colitis; the ultimate damage is to the kidney. The overall weight of evidence is that mercury(II) chloride has the potential to increase the incidence of some benign tumours at sites where tissue damage is apparent and that it possesses weak genotoxic activity but does not cause point mutations.
Methoxychlor
Methoxychlor (CAS No. 72-43-5) is an insecticide used on vegetables, fruit, trees, fodder and farm animals. It is poorly soluble in water and highly immobile in most agricultural soils. Under normal conditions of use, methoxychlor does not seem to be of environmental concern. Daily intake from food and air is expected to be below 1 µg per person. Environmental metabolites are formed preferentially under anaerobic rather than aerobic conditions and include mainly the dechlorinated and demethylated products. There is some potential for the accumulation of the parent compound and its metabolites in surface water sediments.
View in own window
| Guideline value | 0.02 mg/l (20 µg/l) |
|---|
| Occurrence | Detected occasionally in drinking-water, at concentrations as high as 300 µg/l in rural areas |
|---|
| TDI | 5 µg/kg body weight, based on a systemic NOAEL of 5 mg/kg body weight in a teratology study in rabbits, with an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 reflecting concern for threshold carcinogenicity and the limited database) |
|---|
| Limit of detection | 0.001–0.01 µg/l by GC |
|---|
| Treatment performance | 0.1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2004)
Methoxychlor in drinking-water |
|---|
The genotoxic potential of methoxychlor appears to be negligible. In 1979, IARC assigned methoxychlor to Group 3. Subsequent data suggest a carcinogenic potential of methoxychlor for liver and testes in mice. This may be due to the hormonal activity of proestrogenic mammalian metabolites of methoxychlor and may therefore have a threshold. The study, however, was inadequate, because only one dose was used and because this dose may have been above the maximum tolerated dose. The database for studies on long-term, short-term and reproductive toxicity is inadequate. A teratology study in rabbits reported a systemic NOAEL of 5 mg/kg body weight per day, which is lower than the LOAELs and NOAELs from other studies. This NOAEL was therefore selected for use in the derivation of a TDI.
Methyl parathion
Methyl parathion (CAS No. 298-00-0) is a non-systemic insecticide and acaricide that is produced throughout the world and has been registered for use on many crops, in particular cotton. It partitions mainly to air and soil in the environment. There is virtually no movement through soil, and neither the parent compound nor its breakdown products will reach groundwater. By far the most important route for the environmental degradation of methyl parathion is microbial degradation. Half-lives of methyl parathion in water are in the order of weeks to months. Concentrations of methyl parathion in natural waters of agricultural areas in the USA ranged up to 0.46 µg/l, with highest levels in summer. The general population can come into contact with methyl parathion via air, water or food.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (1996)
Pesticide residues in food—1995 evaluations.
IPCS (1992)
Methyl parathion
WHO (2004)
Methyl parathion in drinking-water
|
|---|
A NOAEL of 0.3 mg/kg body weight per day was derived from the combined results of several studies conducted in humans, based on the depression of erythrocyte and plasma cholinesterase activities. Methyl parathion decreased cholinesterase activities in long-term studies in mice and rats, but did not induce carcinogenic effects. Methyl parathion was mutagenic in bacteria, but there was no evidence of genotoxicity in a limited range of studies in mammalian systems.
A health-based value of 9 µg/l can be calculated for methyl parathion on the basis of an ADI of 0–0.003 mg/kg body weight, based on a NOAEL of 0.25 mg/kg body weight per day in a 2-year study in rats for retinal degeneration, sciatic nerve demyelination, reduced body weight, anaemia and decreased brain acetylcholinesterase activity, using an uncertainty factor of 100 for interspecies and intraspecies variation. As the toxicological end-points seen in experimental animals were other than acetylcholinesterase inhibition, it was considered more appropriate to use these data rather than the NOAEL derived for cholinesterase inhibition in humans.
Intake of methyl parathion from all sources is generally low and well below the upper limit of the ADI. As the health-based value is much higher than concentrations of methyl parathion likely to be found in drinking-water, the presence of methyl parathion in drinking-water under usual conditions is unlikely to represent a hazard to human health. For this reason, the establishment of a formal guideline value for methyl parathion is not deemed necessary.
Methyl tertiary-butyl ether
The major use of methyl tert-butyl ether, or MTBE, is as a gasoline additive. Surface water can be contaminated by gasoline spills; however, owing to the high volatility of MTBE, most is lost to evaporation. Spills and leaking storage tanks can cause more serious problems in groundwater, where MTBE is more persistent. MTBE has been detected in groundwater and drinking-water at concentrations in the nanogram to microgram per litre range.
View in own window
| Reason for not establishing a guideline value | Any guideline that would be derived would be significantly higher than concentrations at which MTBE would be detected by odour |
|---|
| Assessment date | 2004 |
|---|
| Principal references |
IPCS (1998)
Methyl tertiary-butyl ether
WHO (2005)
Methyl tertiary-butyl ether (MTBE) in drinking-water
|
|---|
No human cancer studies have been published for either the general population or occupationally exposed cohorts. There have been a number of human studies of neurological and clinical effects of exposure to MTBE by inhalation, with mixed results. In general, no objective changes could be seen at levels of MTBE normally found, even in such microenvironments as gasoline filling stations.
The weight of evidence suggests that MTBE is not genotoxic. A large number of studies using in vitro and in vivo mammalian and non-mammalian systems have been conducted to assess the mutagenicity of MTBE, almost all of which have produced negative results. These results suggest that the mechanism of action of MTBE is more likely to be non-genotoxic than genotoxic, although no one mechanism appears to explain all of the observed effects.
It has been concluded that MTBE should be considered a rodent carcinogen but that it is not genotoxic, and the carcinogenic response is evident only at high levels of exposure that also induce other adverse effects. The available data are therefore considered inconclusive and prohibit their use for human carcinogenic risk assessment. A health-based guideline value has not been derived for MTBE, owing to the fact that any guideline value that would be derived would be significantly higher than the concentration at which it would be detected by odour (15 µg/l is the lowest level eliciting a response in a study using taste- and odour-sensitive participants).
Metolachlor
Metolachlor (CAS No. 51218-45-2) is a selective pre-emergence herbicide used on a number of crops. It can be lost from the soil through biodegradation, photodegradation and volatilization. It is fairly mobile and under certain conditions can contaminate groundwater, but it is mostly found in surface water.
View in own window
| Guideline value | 0.01 mg/l (10 µg/l) |
|---|
| Occurrence | Detected in surface water and groundwater at concentrations that can exceed 10 µg/l |
|---|
| TDI | 3.5 µg/kg body weight, based on a NOAEL of 3.5 mg/kg body weight for an apparent decrease in kidney weight at the two highest dose levels in a 1-year dog study, with an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 reflecting some concern regarding carcinogenicity) |
|---|
| Limit of detection | 0.75–0.01 µg/l by GC with nitrogen–phosphorus detection |
|---|
| Treatment performance | 0.1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Metolachlor in drinking-water |
|---|
In a 1-year study in dogs, administration of metolachlor resulted in decreased kidney weight at the two highest dose levels. In 2-year studies with rodents fed metolachlor in the diet, the only toxicological effects observed in mice were decreased body weight gain and decreased survival in females at the highest dose level, whereas rats showed decreased body weight gain and food consumption at the highest dose level. There is no evidence from available studies that metolachlor is carcinogenic in mice. In rats, an increase in liver tumours in females as well as a few nasal tumours in males have been observed. Metolachlor is not genotoxic.
Microcystins (cyanobacterial toxins)7
Microcystins (MCs) are naturally occurring heptapeptides produced by strains of various species of cyanobacteria, primarily in freshwater environments. They occur widely around the globe, particularly with strains of the frequently occurring cyanobacterial genera Microcystis, Planktothrix and Dolichspermum (see also section 11.5). Among the more than 250 MCs identified to date, only a few occur frequently and in high concentrations. MCs are usually cell-bound, and substantial amounts are released to the surrounding water only during cell rupture (i.e. lysis). One of the most common MCs, and the one most studied, is microcystin-LR (MC-LR).
Drinking-water is the most likely route of exposure to MCs where surface water with cyanobacterial blooms is the drinking-water source. Recreational activities in lakes with cyanobacterial blooms may also be a relevant exposure pathway, potentially to high, usually intermittent concentrations (see WHO Guidelines on recreational water quality, 2021). Limited data suggest that MCs may also accumulate in some food items.
View in own window
| Provisional guideline value (lifetime) | Total MCs (sum of all congeners, free plus cell-bound): 0.001 mg/l (1 µg/l) |
|---|
| The guideline value is provisional because of the high level of uncertainty—it is based on data for only MC-LR, and the database is limited, as reflected in the composite uncertainty factor of 1000 |
| Provisional short-term guideline value | Total MCs (sum of all congeners, free plus cell-bound): 0.012 mg/l (12 µg/l) |
|---|
| Occurrence | Although concentrations in scums can reach the range of mg/l (even up to 100 mg/L), outside of scum areas they rarely exceed several tens of µg/l. MCs largely occur cell-bound unless cell damage causes release |
|---|
| TDI | 0.04 µg/kg bw, based on a NOAEL of 40 µg/kg bw per day for liver pathology observed in a 13-week study in mice and applying an uncertainty factor of 1000 (10 each for inter- and intra-species variability and 10 for database deficiencies,* taking into consideration limitations in the database—in particular, limited data on chronic toxicity, reproductive toxicity and carcinogenicity) |
|---|
| Limit of detection |
<1 µg/l by LC-MS/MS, or LC (including HPLC) followed by UV/PDA detection. LC-MS/MS has the highest specificity and sensitivity but requires quantitative reference standards for all relevant MCs in the sample. For UV/PDA detection, standards for a few MCs whose signals are representative of all are sufficient.
Prior extraction of cells with freeze–thaw cycles and 75% aqueous methanol is necessary for cell-bound MCs; neglecting extraction from cells will lead to dramatic underestimation of concentrations.
<1 µg/l by commercially available immunoassay (ELISA) or enzyme assay (PPA) kits; although these are less precise than LC with the above-mentioned detection methods, they capture all MCs and thus are useful for most monitoring purposes.
|
|---|
| Monitoring | The likelihood of blooms can be assessed by understanding water body conditions (in particular, nutrient concentrations, water body depth, water retention time, patterns of mixing and stratification; see section 11.5). Where conditions render blooms likely, visual monitoring of source water (including microscopy for potentially MC-containing genera) for evidence of increasing cyanobacterial biomass (blooms) is important because biomass can increase rapidly. Exceeding alert values for biomass indicators or MC concentrations should trigger management responses to prevent exposure to elevated toxin concentrations (see the alert level framework in section 11.5). Analysis of cyanotoxins is particularly useful for validating and optimizing the efficacy of control measures such as riverbank filtration or treatment. |
|---|
| Prevention and treatment | Actions to decrease the probability of bloom occurrence include catchment and source water management, such as reducing nutrient loading or changing reservoir stratification and mixing. Filtration is effective for removing intact cyanobacterial cells, and in most cases this removes the major fraction of MCs. For dissolved MCs, oxidation with chlorine or ozone at sufficient concentrations and contact times, as well as GAC and some PAC applications, are effective (see chapters 7–10 of Toxic cyanobacteria in water; Annex 1). |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 80% of TDI; for short-term exposure, 100% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Total MCs as gravimetric or molar equivalents should be evaluated against the guideline values since MCs usually occur as mixtures. The guideline values are based on MC-LR, which is one of the most toxic and common MCs. |
|---|
| The provisional short-term drinking-water guideline value is intended to indicate the extent to which the lifetime value can be exceeded for periods of up to about 2 weeks until water treatment can be augmented to bring the concentration of MCs back under control. It is not intended to allow repeated seasonal exceedances of the lifetime value. |
| It is recommended, as a precautionary measure, that bottle-fed infants and small children be provided with an alternative safe drinking-water source (e.g. bottled water that is certified by the responsible authorities) if concentrations are greater than 3 μg/L, even for short periods. |
| Assessment date | 2020 |
|---|
| Principal references |
WHO (2020)
Cyanobacterial toxins: microcystins
Chorus & Welker (2021) Toxic cyanobacteria in water
|
|---|
- *
For the short-term guideline value, a database uncertainty factor was not applied because chronic toxicity, carcinogenicity (tumour promotion) and the limited evidence for reproductive toxicity following subchronic dosing are not relevant to short-term exposures.
MCs are actively transported into cells by specific organic anion transport proteins (OATPs). Because the liver has high expression of OATPs, MCs are considered to be primarily hepatotoxins, although distribution to other organs and tissues expressing OATPs does occur. Once in the cell, MCs bind with high affinity to certain protein phosphatases that are involved in a wide range of regulatory pathways, including those responsible for cytoskeletal structure, cell replication, stress responses and DNA repair. Acute effects of MC poisoning include intrahepatic haemorrhage. Chronic effects include a reduced efficacy of proliferation control mechanisms within tumour cells. Cases of liver damage have been reported from MCs in drinking-water that was inadequately treated. As well, human fatalities clearly attributable to MCs have occurred through renal dialysis using inadequately treated water, including drinking-water that contained high MC levels. In 2006, IARC classified MC-LR as a possible human carcinogen (Group 2B) based on evidence for tumour promotion.
The provisional guideline values for MCs are based on a study in mice using MC-LR (the only congener with sufficiently comprehensive oral toxicity data for guideline value derivation), corroborated by a similar study in rats. The liver was identified as the most sensitive organ in these studies. Although some recent research has suggested that certain reproductive organs may be affected by prolonged exposure to lower concentrations, these reports suffer from a number of methodological and reporting deficiencies and, further, are contradicted by earlier studies. Therefore, these findings of adverse effects require corroboration.
Practical considerations
Where nutrient (phosphorus and nitrogen) concentra¬tions are elevated in lakes, reservoirs or slowly flowing rivers, cyanobacteria occur widely. Where their excessive growth leads to high biomass, sometimes termed “bloom” events, MCs can reach concentrations in raw water that are potentially hazardous to human health. Such blooms tend to recur in the same water bodies. Cells of some cyanobacterial species may accumulate at the surface as scums (particularly of Microcystis) or at the thermocline of thermally stratified reservoirs (i.e. Planktothrix rubescens). Such accumulations may develop rapidly and may be of very variable duration (hours to weeks). In many circumstances, blooms and accumulations are seasonal, whereas others occur perennially.
Cyanobacteria are most effectively controlled in the context of developing a WSP (see chapter 4). Control measures to manage potential risks from cyanobacteria, and in particular from their toxins, in drinking-water should include not only adequate treatment, but also measures to control cyanobacterial bloom development. See section 11.5 for more information on cyanobacteria, including further details on monitoring cyanobacterial blooms, the alert level framework, and prevention and management of cyanobacteria in source waters. Effectively minimizing the formation of blooms and locating the raw water intake away from blooms reduce the treatment steps required to remove cyanotoxins.
Drinking-water treatment that removes particles—that is, soil, slow sand or riverbank filtration, conventional water treatment (coagulation, flocculation and filtration or dissolved air flotation)—can remove cell-bound MCs effectively. Soil, slow sand and riverbank filtration can also remove dissolved cyanotoxins. For all these processes, it is important that they are optimized to target the removal of cells and dissolved toxins. Chlorination and ozonation at sufficiently high doses and contact times are effective for degrading dissolved MCs; however, elevated organic carbon in bloom situations will substantially increase the disinfectant demand. Chlorine dioxide and chloramine are ineffective for degrading MCs. Both for pre-oxidation and conventional treatment, cell rupture and toxin release should be avoided. GAC and PAC can be effective for removing dissolved MCs, with efficacy dependent on several factors, including the type of activated carbon, contact times (PAC), flow rates (GAC) and water quality. As the challenges that blooms present for treatment are complex, periodic validation of efficacy during bloom situations and under the specific local conditions is particularly important. Avoiding bloom occurrence and intake is therefore the preferred option.
Molinate
Molinate (CAS No. 2212-67-1) is a herbicide used to control broad-leaved and grassy weeds in rice. The available data suggest that groundwater pollution by molinate is restricted to some rice growing regions. Data on the occurrence of molinate in the environment are limited. Molinate is of low persistence in water and soil, with a half life of about 5 days.
View in own window
| Guideline value | 0.006 mg/l (6 µg/l) |
|---|
| Occurrence | Concentrations in water rarely exceed 1 µg/l |
|---|
| TDI | 2 µg/kg body weight, based on a NOAEL for reproductive toxicity in the rat of 0.2 mg/kg body weight, with an uncertainty factor of 100 (for interspecies and intraspecies variation) |
|---|
| Limit of detection | 0.01 µg/l by GC-MS |
|---|
| Treatment performance | 0.001 mg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Molinate in drinking-water |
|---|
On the basis of the limited information available, molinate does not seem to be carcinogenic or mutagenic in experimental animals. Evidence suggests that impairment of the reproductive performance of the male rat represents the most sensitive indicator of molinate exposure. However, epidemiological data based on the examination of workers involved in molinate production do not indicate any effect on human fertility.
Molybdenum
Molybdenum is found naturally in soil and is used in the manufacture of special steels and in the production of tungsten and pigments, and molybdenum compounds are used as lubricant additives and in agriculture to prevent molybdenum deficiency in crops. Concentrations in drinking-water are usually less than 0.01 mg/l, although concentrations as high as 200 µg/l have been reported in areas near mining sites.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 1993, revised in 2011 |
|---|
| Principal references | WHO (2011)
Molybdenum in drinking-water |
|---|
Molybdenum is considered to be an essential element, with an estimated daily requirement of 0.1–0.3 mg for adults.
As molybdenum occurs at very low concentrations in drinking-water, it is not considered necessary to set a formal guideline value. For guidance purposes, a health-based value can be derived.
In a 2-year study of humans exposed via drinking-water, the NOAEL was found to be 0.2 mg/l, but there are some concerns about the quality of this study. As molybdenum is an essential element, a factor of 3 is considered to be adequate to reflect intraspecies variation. This gives a health-based value of 0.07 mg/l (rounded figure), which is in the same range as that derived on the basis of the results of toxicological studies in experimental animals and is consistent with the essential daily requirement for molybdenum.
Monochloroacetic acid
Chlorinated acetic acids are formed from organic material during water chlorination.
View in own window
| Guideline value | 0.02 mg/l (20 µg/l) |
|---|
| Occurrence | Present in surface water–derived drinking-water at concentrations up to 82 µg/l (mean 2.1 µg/l) |
|---|
| TDI | 3.5 µg/kg body weight, based on a LOAEL of 3.5 mg/kg body weight per day from a study in which increased absolute and relative spleen weights were observed in male rats exposed to monochloroacetic acid in drinking-water for 2 years, using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for use of a minimal LOAEL instead of a NOAEL and database deficiencies, including the lack of a multigeneration reproductive toxicity study) |
|---|
| Limit of detection | 2 µg/l by GC with ECD; 5 µg/l by GC-MS |
|---|
| Treatment performance | No information available |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 20% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2004)
Monochloroacetic acid in drinking-water |
|---|
No evidence of carcinogenicity of monochloroacetate was found in 2-year gavage bioassays with rats and mice. Monochloroacetate has given mixed results in a limited number of mutagenicity assays and has been negative for clastogenicity in genotoxicity studies. IARC has not classified the carcinogenicity of monochloroacetic acid.
Monochlorobenzene
Releases of monochlorobenzene (MCB) to the environment are thought to be mainly due to volatilization losses associated with its use as a solvent in pesticide formulations, as a degreasing agent and from other industrial applications. MCB has been detected in surface water, groundwater and drinking-water; mean concentrations were less than 1 µg/l in some potable water sources (maximum 5 µg/l) in Canada. The major source of human exposure is probably air.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern, and health-based value would far exceed lowest reported taste and odour threshold |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2004)
Monochlorobenzene in drinking-water |
|---|
MCB is of low acute toxicity. Oral exposure to high doses of MCB results in effects mainly on the liver, kidneys and haematopoietic system. There is limited evidence of carcinogenicity in male rats, with high doses increasing the occurrence of neoplastic nodules in the liver. The majority of evidence suggests that MCB is not mutagenic; although it binds to DNA in vivo, the level of binding is low.
A health-based value of 300 µg/l can be calculated for MCB on the basis of a TDI of 85.7 µg/kg body weight, based on neoplastic nodules identified in a 2-year rat study with dosing by gavage, and taking into consideration the limited evidence of carcinogenicity. However, because MCB occurs at concentrations well below those of health concern, it is not considered necessary to derive a formal guideline value. It should also be noted that the health-based value far exceeds the lowest reported taste and odour threshold for MCB in water.
MX
MX, which is the common name for 3-chloro-4-dichloromethyl-5-hydroxy-2-(5H)-furanone, is formed by the reaction of chlorine with complex organic matter in drinking-water. It has been identified in chlorinated humic acid solutions and drinking-water in Finland, the United Kingdom and the USA and was found to be present in 37 water sources at levels of 2–67 ng/l. Five drinking-water samples from different Japanese cities contained MX at concentrations ranging from less than 3 to 9 ng/l.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (2000)
Disinfectants and disinfectant by-products
WHO (2003)
MX in drinking-water
|
|---|
MX is a potent mutagen in bacteria and in cells in vitro and has undergone a lifetime study in rats in which some tumorigenic responses were observed. These data indicate that MX induces thyroid and bile duct tumours. IARC has classified MX in Group 2B (possibly carcinogenic to humans) on the basis of rat tumorigenicity and its strong mutagenicity.
A health-based value of 1.8 µg/l can be calculated for MX on the basis of the increase in cholangiomas and cholangiocarcinomas in female rats using the linearized multistage model (without a body surface area correction). However, this is significantly above the concentrations that would be found in drinking-water, and, in view of the analytical difficulties in measuring this compound at such low concentrations, it is considered unnecessary to propose a formal guideline value for MX in drinking-water.
Nickel
Nickel is a naturally occurring element, which is used mainly in the production of stainless steel and nickel alloys. Food is the dominant source of nickel exposure in the non-smoking, non-occupationally exposed population; water is generally a minor contributor to the total daily oral intake. However, the nickel contribution from water may be significant where there is heavy pollution, where nickel that occurs naturally in groundwater is mobilized, or where nickel leaches from nickel- or chromium-plated taps or stainless steel devices or materials that are in contact with water. The primary source of nickel in drinking-water is leaching from metals that are in contact with drinking-water.
View in own window
| Guideline value | 0.07 mg/l (70 µg/l) |
|---|
| Occurrence | Concentration in drinking-water are typically less than 25 μg/l, although concentrations may be elevated (up to 5 mg/l) where nickel is released from metal alloys that are in contact with drinking-water, such as fittings, including taps, or as a result of anthropogenic contamination or mobilization from natural deposits |
|---|
| Basis of guideline value derivation | The guideline value is based on achievability by available source control measures and treatment technologies, measurability by analytical methods and toxicology. Reproductive toxicity (increased incidence of rat litters with post-implantation loss) was considered the most sensitive end-point and is therefore the basis for the TDI derivation of 13 µg/kg body weight. An uncertainty factor of 100 was applied to account for interspecies differences (10) and intraspecies variation (10) to the BDML10 of 1.3 mg/kg bw per day, from a two-generation study in rats. Although the 2021 risk assessment supports a health-based value of 80 μg/l based on this TDI, the guideline value was retained at 70 µg/l considering the above factors. Further, 80 μg/l is only slightly higher than the previous guideline value of 70 µg/l (established in 2014); factoring in the imprecision inherent in risk assessment procedures, this difference is not judged significant enough to warrant a revised, minimally relaxed guideline value. |
|---|
| Limit of detection | 0.5–5 μg/l for ICP-MS, ICP-AES and graphite furnace AAS; 0.1 mg/l for flame AAS |
|---|
| Prevention and treatment |
For surface water, conventional water treatment (coagulation, sedimentation, filtration) may be effective under certain circumstances, depending on a number of factors, including the coagulant dosage and pH. For groundwater, ion-exchange resins have been shown to be effective.
The most important means of control is by product specifications through an appropriate certification scheme for materials in contact with drinking-water. Consumers, particularly nickel-sensitive people, should flush chromium- or nickel-plated taps before using the water, particularly after periods of stagnation.
|
|---|
| Health-based value derivation |
|---|
| • allocation to water | 20% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The guideline value is also protective of any possible acute effects, including systemic contact dermatitis |
|---|
| As nickel is usually found in drinking-water below the guideline value, monitoring and inclusion in drinking-water regulations and standards would usually only be necessary if there were indications that a specific pollution or problem might exist |
| Assessment date | 2021 |
|---|
| Principal reference |
WHO (2021)
Nickel in drinking-water
EFSA (2020)
Update of the risk assessment of nickel in food
|
|---|
IARC concluded that inhaled nickel compounds are carcinogenic to humans (Group 1) and that metallic nickel is possibly carcinogenic (Group 2B). However, there is a lack of evidence of a carcinogenic risk from oral exposure to nickel. In a well-conducted two-generation reproductive study in rats administered nickel by gavage, an increase in the frequency of litters with post-implantation loss was reported; thus, reproductive and developmental toxicity was identified as the relevant and sensitive end-point for derivation of the health-based value.
Exposure to nickel through the skin or by inhalation may lead to nickel sensitization. Whereas oral ingestion of nickel is not known to lead to systemic contact dermatitis (SCD) in the general population, it can elicit SCD in nickel-sensitized individuals. However, the guideline value of 70 µg/L is considered adequately protective of SCD that may result from drinking-water exposure based on a margin of exposure (MOE) assessment. Acute consumption of water containing nickel at the chronic health-based value of 80 µg/l would result in an MOE of approximately 16. The MOE of 16 was calculated from an acute LOAEL for SCD of 4.3 µg/kg bw and an acute exposure of 0.27 µg/kg bw (based on a scenario of a person weighing 60 kg, drinking a glass of tap water (about 200 ml) containing nickel at the health-based value of 80 µg/l). The guideline value is further considered adequately protective considering that SCD elicitation was associated with a bolus exposure, in contrast to the intermittent nature of a drinking-water exposure scenario.
Nitrate and nitrite8
Nitrate (NO3−) is found naturally in the environment and is an important plant nutrient. It is present at varying concentrations in all plants and is a part of the nitrogen cycle. Nitrite (NO2−) is not usually present in significant concentrations except in a reducing environment, because nitrate is the more stable oxidation state. It can be formed by the microbial reduction of nitrate and in vivo by reduction from ingested nitrate. Nitrite can also be formed chemically in distribution pipes by Nitrosomonas bacteria during stagnation of nitrate-containing and oxygen-poor drinking-water in galvanized steel pipes, or if chloramination is used to provide a residual disinfectant. An excess of free ammonia entering the distribution system can lead to nitrification and the potential increase of nitrate and nitrite in drinking-water. Nitrate can reach both surface water and groundwater as a consequence of agricultural activity (including excess application of inorganic nitrogenous fertilizers and manures), from waste-water disposal and from oxidation of nitrogenous waste products in human and other animal excreta, including septic tanks. Nitrate can also occasionally reach ground-water as a consequence of natural vegetation. Surface water nitrate concentrations can change rapidly owing to surface runoff of fertilizer, uptake by phytoplankton and denitrification by bacteria, but groundwater concentrations generally show relatively slow changes. Nitrate and nitrite can also be produced as a result of nitrification in source water or distribution systems.
In general, the most important source of human exposure to nitrate and nitrite is through vegetables (nitrate and nitrite) and through meat in the diet (nitrite is used as a preservative in many cured meats). In some circumstances, however, drinking-water can make a significant contribution to nitrate and, occasionally, nitrite intake. In the case of bottle-fed infants, drinking-water can be the major external source of exposure to nitrate and nitrite.
View in own window
| Guideline values9 | Nitrate: 50 mg/l as nitrate ion, to be protective against methaemoglobinaemia and thyroid effects in the most sensitive subpopulation, bottle-fed infants, and, consequently, other population subgroups |
|---|
| Nitrite: 3 mg/l as nitrite ion, to be protective against methaemoglobinaemia induced by nitrite from both endogenous and exogenous sources in bottle-fed infants, the most sensitive subpopulation, and, consequently, the general population |
| Combined nitrate plus nitrite: The sum of the ratios of the concentrations of each of nitrate and nitrite to its guideline value should not exceed 1 |
| Occurrence | Nitrate levels vary significantly, but levels in well water are often higher than those in surface water and, unless heavily influenced by surface water, are less likely to fluctuate. Concentrations often approach or exceed 50 mg/l where there are significant sources of contamination. Nitrite levels are normally lower, less than a few milligrams per litre. |
|---|
| Basis of guideline value | Nitrate (bottle-fed infants): In epidemiological studies, no adverse health derivation effects (methaemoglobinaemia or thyroid effects) were reported in infants in areas where drinking-water consistently contained nitrate at concentrations below 50 mg/l |
|---|
| Nitrite (bottle-fed infants): Based on: 1) no incidence of methaemoglobinaemia at nitrate concentrations below 50 mg/l (as nitrate ion) in drinking-water for bottle-fed infants less than 6 months of age (assuming body weight of 2 kg); 2) converting 50 mg/l as nitrate to corresponding molar concentration for nitrite; 3) multiplying by a factor of 0.1 to account for the estimated conversion rate of nitrate to nitrite in infants where nitrite is formed endogenously from nitrate at a rate of 5–10%; and 4) multiplying by a source allocation factor for drinking-water of 100% or 1, as a bottle-fed infant’s primary exposure to nitrite is through consumption of formula reconstituted with drinking-water that contains nitrate or nitrite. As the guideline value is based on the most sensitive subgroup of the population (bottle-fed infants less than 6 months of age), application of an uncertainty factor is not deemed necessary. |
| Combined nitrate plus nitrite: To account for the possibility of the simultaneous occurrence of nitrate and nitrite in drinking-water |
| Limit of detection | MDLs of 0.009 mg/l as nitrate ion and 0.013 mg/l as nitrite ion by IC; MDL of 0.04–4.4 mg/l as nitrate ion by automated cadmium reduction with colorimetry (recommended for the analysis of nitrate at concentrations below 0.4 mg/l) |
|---|
| Treatment performance | Nitrate: Effective central treatment technologies involve the physical/chemical and biological removal of nitrate and include ion exchange, reverse osmosis, biological denitrification and electrodialysis, which are capable of removing over 80% of nitrate from water to achieve effluent nitrate concentrations as low as 13 mg/l; conventional treatment processes (coagulation, sedimentation, filtration and chlorination) are not effective |
|---|
| Nitrite: Treatment usually focuses on nitrate, because nitrite is readily converted to nitrate by many disinfectants |
| Additional comments | The guideline values for both nitrate and nitrite are based on short-term effects; however, they are also considered protective for any possible long-term effects. |
|---|
| Methaemoglobinaemia is complicated by the presence of microbial contamination and subsequent gastrointestinal infection, which can increase the risk for bottle-fed infants significantly. Authorities should therefore be all the more vigilant that water to be used for bottle-fed infants is microbiologically safe when nitrate is present at concentrations near or above the guideline value. It is particularly important to ensure that these infants are not currently exhibiting symptoms of gastrointestinal infection (diarrhoea). Also, as excessive boiling of water to ensure microbiological safety can concentrate levels of nitrate in the water, care should be taken to ensure that water is heated only until it reaches a rolling boil. In extreme situations, alternative sources of water (e.g. bottled water) can be used. |
|
Nitrite is relatively unstable and can be rapidly oxidized to nitrate. Nitrite can occur in the distribution system at higher concentrations when chloramination is used, but the occurrence is almost invariably intermittent. Methaemoglobinaemia is therefore the most important consideration, and the guideline value derived for protection against methaemoglobinaemia would be the most appropriate under these circumstances, allowing for any nitrate that may also be present.
All water systems that practise chloramination should closely and regularly monitor their systems to verify disinfectant levels, microbiological quality and nitrite levels. If nitrification is detected (e.g. reduced disinfectant residuals and increased nitrite levels), steps can be taken to modify the treatment train or water chemistry in order to minimize nitrite formation. Effective disinfection must never be compromised. Excessively high levels may occur in small supplies; where this is suspected from the risk assessment, testing may be appropriate.
|
| Assessment date | 2016 |
|---|
| Principal references |
Health Canada (2013). Guidelines for Canadian Drinking Water Quality:
Guideline Technical Document – Nitrate and nitrite
WHO (2016). Nitrate and nitrite in drinking-water
|
|---|
Absorption of nitrate ingested from vegetables, meat or water is rapid and in excess of 90%; final excretion is in the urine. In humans, about 25% of ingested nitrate is recirculated in saliva, of which about 20% is converted to nitrite by the action of bacteria in the mouth. There is also endogenous formation of nitrate from nitric oxide and protein breakdown as part of normal metabolism. In normal healthy adults, this endogenous synthesis leads to the excretion of about 62 mg of nitrate ion per day in the urine. Endogenous formation of nitrate or nitrite can be significantly increased in the presence of infections, particularly gastrointestinal infections. When nitrate intake is low, endogenous formation may be the major source of nitrate in the body. Nitrate metabolism is different in humans and rats, as rats may not actively secrete nitrate in their saliva.
Nitrate probably has a role in protecting the gastrointestinal tract against a variety of gastrointestinal pathogens, as nitrous oxide and acidified nitrite have antibacterial properties. It may have other beneficial physiological roles. Hence, there may be a benefit from exogenous nitrate uptake, and there remains a need to balance the potential risks with the potential benefits.
Significant bacterial reduction of nitrate to nitrite does not normally take place in the stomach, except in individuals with low gastric acidity or with gastrointestinal infections. These may include individuals using antacids, particularly those that block acid secretion. In humans, methaemoglobinaemia is a consequence of the reaction of nitrite with haemoglobin in the red blood cells to form methaemoglobin, which binds oxygen tightly and does not release it, thus blocking oxygen transport. Although most absorbed nitrite is oxidized to nitrate in the blood, residual nitrite can react with haemoglobin. High levels of methaemoglobin (>10%) formation in infants can give rise to cyanosis, referred to as blue-baby syndrome. Although clinically significant methaemoglobinaemia can occur as a result of extremely high nitrate intake in adults and children, the most familiar situation is its occurrence in bottle-fed infants. This was considered to be primarily a consequence of high levels of nitrate in water, although there have been cases of methaemoglobinaemia in weaned infants, associated with high nitrate intake from vegetables. Bottle-fed infants are considered to be at greater risk because the intake of water in relation to body weight is high and, in infants, the development of repair enzymes is limited. In clinical epidemiological studies of methaemoglobinaemia and subclinical increases in methaemoglobin levels associated with drinking-water nitrate, 97% of cases occurred at concentrations in excess of 44.3 mg/l, with clinical symptoms associated with the higher concentrations. The affected individuals were almost exclusively under 3 months of age.
Although drinking-water nitrate may be an important risk factor for methaemoglobinaemia in bottle-fed infants, there is compelling evidence that the risk of methaemoglobinaemia is primarily increased in the presence of simultaneous gastrointestinal infections, which increase endogenous nitrite formation, may increase reduction of nitrate to nitrite and may also increase the intake of water in combatting dehydration. Cases have been described in which gastrointestinal infection seems to have been the primary cause of methaemoglobinaemia. Most cases of methaemoglobinaemia reported in the literature are associated with contaminated private wells (predominantly when the drinking-water is anaerobic) that also have a high probability of microbial contamination, which should not occur if it is properly disinfected.
Although numerous epidemiological studies have investigated the relationship between exposure to nitrate or nitrite in drinking-water and cancer occurrence, the weight of evidence does not support an association between cancer and exposure to nitrate or nitrite per se. Nitrite can react with nitrosatable compounds, primarily secondary amines, in the body to form N-nitroso compounds. A number of these are considered to be carcinogenic to humans, whereas others, such as N-nitrosoproline, are not. Several studies have been carried out on the formation of N-nitroso compounds in relation to nitrate intake in humans, but there is large variation in the intake of nitrosatable compounds and in gastric physiology. Higher mean levels of N-nitroso compounds, along with high nitrate levels, have been found in the gastric juice of individuals who are achlorhydric (i.e. have very low levels of hydrochloric acid in the stomach). However, other studies have been largely inconclusive, and there appears to be no clear relationship with drinking-water nitrate compared with overall nitrate intake in relation to formation of N-nitroso compounds. Moderate consumption of a number of dietary antioxidant components, such as ascorbic acid and green tea, appears to reduce endogenous N-nitrosamine formation.
A significant number of epidemiological studies have been carried out on the association of nitrate intake with primarily gastric cancers. Although the epidemio-logical data are considered to be inadequate to allow definitive conclusions to be drawn regarding all cancers, there is no convincing evidence of a causal association with any cancer site. The weight of evidence indicates that there is unlikely to be a causal association between gastric cancer and nitrate in drinking-water. This is consistent with the conclusion by IARC that ingested nitrate or nitrite under conditions that result in endogenous nitrosation is probably carcinogenic to humans (Group 2A), but not nitrate alone.
There have been suggestions that nitrate in drinking-water could be associated with congenital malformations, but the overall weight of evidence does not support this.
Nitrate appears to competitively inhibit iodine uptake, with the potential for an adverse effect on the thyroid. Current evidence also suggests that exposure to nitrate in drinking-water may alter human thyroid gland function by competitively inhibiting thyroidal iodide uptake, leading to altered thyroid hormone concentrations and functions. Although studies found that exposure to nitrate concentrations above 50 mg/l are weakly associated with altered thyroid function, the evidence is limited, conflicting and based on studies with important methodological limitations. Mode of action data suggest that pregnant women and infants are the most sensitive populations, owing primarily to the importance of adequate thyroid hormones for normal neurodevelopment in the fetus and infant, but also to increased thyroid hormone turnover and low intrathyroidal stores in fetal and early life.
There have been suggestions of an association between nitrate in drinking-water and the incidence of childhood diabetes mellitus. However, subsequent studies have not found a significant relationship, and no mechanism has been identified.
In some studies on rats treated with high doses of nitrite, a dose-related hyper-trophy of the zona glomerulosa of the adrenal was seen; one strain of rats appeared to be more sensitive than others. However, this minimal hyperplasia was considered to be due to physiological adaptation to small fluctuations in blood pressure in response to high nitrite doses.
Nitrate is not carcinogenic in laboratory animals. Nitrite has been frequently studied, and there have been suggestions of carcinogenic activity, but only at very high doses. The most recent long-term studies have shown only equivocal evidence of carcinogenicity in the forestomach of female mice, but not in rats or male mice. In view of the lack of evidence for genotoxicity, this led to the conclusion that sodium nitrite was not carcinogenic in mice and rats. In addition, as humans do not possess a forestomach and the doses were high, the significance of these data for humans is very doubtful.
The guideline value for nitrate of 50 mg/l, as nitrate ion, is based on an absence of health effects (methaemoglobinaemia and thyroid effects) in epidemiological studies and is protective for bottle-fed infants and, consequently, other parts of the population. Methaemoglobinaemia is complicated by the presence of microbial contamination and subsequent gastrointestinal infection, which can increase the risk for this group significantly. Authorities should therefore be all the more vigilant that water to be used for bottle-fed infants is microbiologically safe when nitrate is present at concentrations near the guideline value. It is particularly important to ensure that these infants are not currently exhibiting symptoms of significant gastrointestinal infection (diarrhoea). Also, as excessive boiling of water to ensure microbiological safety can concentrate levels of nitrate in the water, care should be taken to ensure that water is heated only until it reaches a rolling boil. In extreme situations, alternative sources of water (e.g. bottled water) can be used.
The guideline for nitrite of 3 mg/l, as nitrite ion, is based on: 1) no incidence of methaemoglobinaemia at nitrate concentrations below 50 mg/l in drinking-water for bottle-fed infants less than 6 months of age (assuming body weight of 2 kg), 2) converting 50 mg/l nitrate to the corresponding molar concentration for nitrite, 3) multiplying by a factor of 0.1 to account for the estimated conversion rate of nitrate to nitrite in infants where nitrite is formed endogenously from nitrate at a rate of 5–10% and 4) multiplying by a source allocation factor for drinking water of 100% or 1, as a bottle-fed infant’s primary exposure to nitrite is through consumption of formula reconstituted with nitrate- or nitrite-containing drinking-water. As the health-based value is based on the most sensitive subgroup of the population (bottle-fed infants less than 6 months of age), application of an uncertainty factor is not deemed necessary.
Because of the possibility of the simultaneous occurrence of nitrate and nitrite in drinking-water, the sum of the ratios of the concentration (C) of each to its guideline value (GV) should not exceed 1:
The guideline values are based on short-term effects; however, they are also considered protective for long-term effects.
Practical considerations
The most appropriate means of controlling nitrate concentrations, particularly in groundwater, is the prevention of contamination. This may take the form of appropriate management of agricultural practices (e.g. management of fertilizer and manure application and storage of animal manures) and sanitation practices (e.g. the careful siting of pit latrines and septic tanks, sewer leakage control).
Methaemoglobinaemia has most frequently been associated with private wells. It is particularly important to ensure that septic tanks and pit latrines are not sited near a well or where a well is to be dug and to ensure that animal manure is kept at a sufficient distance to ensure that runoff cannot enter the well or the ground near the well. It is particularly important that the household use of manures and fertilizers on small plots near wells should be managed with care to avoid potential contamination. The well should be sufficiently protected to prevent runoff from entering the well. Where there are elevated concentrations of nitrate or where inspection of the well indicated that there are sources of nitrate close by that could be causing contamination, particularly where there are also indications that microbiological quality might also be poor, a number of actions can be taken. As noted above, water should be heated only until the water reaches a rolling boil or disinfected by an appropriate means before consumption. Where alternative supplies are available for bottle-fed infants, these can be used, taking care to ensure that they are microbiologically safe. Steps should then be taken to protect the well and ensure that sources of both nitrate and microbial contamination are removed from the vicinity of the well.
In areas where household wells are common, health authorities may wish to take a number of steps to ensure that nitrate contamination is not or does not become a problem. Such steps could include targeting mothers, particularly expectant mothers, with appropriate information about water safety, assisting with visual inspection of wells to determine whether a problem may exist, providing testing facilities where a problem is suspected, providing guidance on disinfecting water or, where nitrate levels are particularly high, providing bottled water from safe sources or providing advice as to where such water can be obtained.
With regard to piped supplies, where nitrate is present, the first potential approach to treatment of drinking-water supplies, if source substitution is not feasible, is to dilute the contaminated water with a low-nitrate source. Where blending is not feasible, a number of treatment techniques are available for drinking-water. The first is disinfection, which may serve to oxidize nitrite to the less toxic nitrate as well as minimize the pathogenic and non-pathogenic reducing bacterial population in the water. Nitrate removal methods include ion exchange, biological denitrification, reverse osmosis and electrodialysis. However, there are disadvantages associated with all of these approaches, including cost, operational complexities and the need for disposal of resin, brine or reject water. Conventional municipal water treatment processes (coagulation, sedimentation, filtration and chlorination) are not effective for nitrate removal, as nitrate is a stable and highly soluble ion with low potential for co-precipitation and adsorption.
In systems with a water source containing naturally occurring ammonia or that add ammonia for chloramination, free ammonia entering the distribution system can be one of the causative factors of nitrification and the potential increase of nitrate and nitrite in the distribution system. Care should be taken with the use of chloramination for providing a residual disinfectant in the distribution system. It is important to manage this to minimize nitrite formation, either in the main distribution system or in the distribution systems of buildings.
Nitrilotriacetic acid
Nitrilotriacetic acid, or NTA, is used primarily in laundry detergents as a replacement for phosphates and in the treatment of boiler water to prevent accumulation of mineral scale.
View in own window
| Guideline value | 0.2 mg/l (200 µg/l) |
|---|
| Occurrence | Concentrations in drinking-water usually do not exceed a few micrograms per litre, although concentrations as high as 35 µg/l have been measured |
|---|
| TDI | 10 µg/kg body weight, based on nephritis and nephrosis in a 2-year study in rats and using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for carcinogenic potential at high doses) |
|---|
| Limit of detection | 0.2 µg/l using GC with a nitrogen-specific detector |
|---|
| Treatment performance | No information found on removal from water |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 50% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Nitrilotriacetic acid in drinking-water |
|---|
NTA is not metabolized in experimental animals and is rapidly eliminated, although some may be briefly retained in bone. It is of low acute toxicity to experimental animals, but it has been shown to produce kidney tumours in rodents following long-term exposure to doses higher than those required to produce nephrotoxicity. IARC has placed NTA in Group 2B (possibly carcinogenic to humans). It is not genotoxic, and the reported induction of tumours is believed to be due to cytotoxicity resulting from the chelation of divalent cations such as zinc and calcium in the urinary tract, leading to the development of hyperplasia and subsequently neoplasia.
Nitrobenzene
Nitrobenzene is used primarily in the production of aniline, but it is also used as a solvent, as an ingredient of metal polishes and soaps and in the synthesis of other organic compounds, including acetaminophen. Nitrobenzene can be released to water during these production processes.
Concentrations of nitrobenzene in environmental samples, such as surface water, groundwater and air, are generally low, except in areas with industrial pollution. Based on limited data, it appears that the potential for contamination is greater for groundwater than for surface water.
The general population can be exposed to variable concentrations of nitrobenzene in air and possibly drinking-water. Only populations in the vicinity of manufacturing activities and petroleum refining plants are likely to have any significant exposure to nitrobenzene; however, people living in and around abandoned hazardous waste sites may also have potential for higher exposure, due to possible groundwater and soil contamination and uptake of nitrobenzene by plants.
View in own window
| Reason for not establishing a guideline value | Rarely found in drinking-water at concentrations of health concern |
|---|
| Assessment date | 2009 |
|---|
| Principal reference | WHO (2009)
Nitrobenzene in drinking-water |
|---|
Nitrobenzene is toxic to humans by the inhalation, dermal and oral routes of exposure. The main systemic effect associated with human exposure to nitrobenzene is methaemoglobinaemia. Although some recent studies have reported positive results in mutagenicity tests, it cannot be excluded that nitrobenzene is a non-genotoxic chemical. No long-term oral administration studies are available. Based on inhalation studies, IARC concluded that there was inadequate evidence in humans but sufficient evidence in experimental animals for the carcinogenicity of nitrobenzene and classified nitrobenzene in Group 2B (possibly carcinogenic to humans).
Because nitrobenzene occurrence in drinking-water at concentrations above trace levels is infrequent, it is not considered necessary to derive a formal guideline value. However, health-based values can be calculated to provide guidance in the event of spills and where there are higher concentrations in industrial areas. Two health-based values are derived based on the limited available information: one for short-term exposure (30 µg/l) and the other for long-term exposure (8–63 µg/l, depending on end-point and approach used). It should be emphasized that the derivation of the long-term health-based values includes large uncertainties because of the dose metric conversion from inhalation studies and the possibility of increased metabolism to aniline in the gastrointestinal tract.
It should be emphasized that nitrobenzene is a potent methaemoglobinaemic agent in humans, which is of particular concern for bottle-fed infants. Currently, data are not adequate to determine a separate health-based value for this end-point.
It should also be noted that the reported odour threshold for nitrobenzene in water is 30–110 µg/l.
N-Nitrosodimethylamine
N-Nitrosodimethylamine, or NDMA, can occur in drinking-water through the degradation of dimethylhydrazine (a component of rocket fuel) as well as from several other industrial processes. It is also a contaminant of certain pesticides. NDMA has recently been identified as a disinfection by-product of chloramination (by the reaction of monochloramine with dimethylamine, a ubiquitous component of waters affected by wastewater discharges) and, to some extent, chlorination. NDMA can also be formed as a by-product of anion exchange treatment of water.
View in own window
| Guideline value | 0.0001 mg/l (0.1 µg/l) |
|---|
| Occurrence | Where chloramination is used, distribution system samples can have much higher levels of NDMA than the finished water at the treatment plant; levels as high as 0.16 µg/l have been measured in the distribution system, but concentrations in water at the treatment plant are generally less than 0.01 µg/l |
|---|
| Basis of guideline value derivation | Hepatic biliary cystadenomas in female rats, the most sensitive carcinogenic end-point, observed in a drinking-water study, using a multistage model |
|---|
| Limit of detection | 0.028 ng/l by capillary column GC and chemical ionization tandem MS; 0.4 ng/l by capillary column GC and high-resolution MS; 0.7–1.6 ng/l by GC-MS and ammonia positive chemical ionization detection |
|---|
| Treatment performance | The most common process for NDMA removal is UV irradiation. A concentration below 0.005 µg/l should be achievable by UV irradiation provided that the water is not grossly contaminated. NDMA is not removable by air stripping, activated carbon adsorption, reverse osmosis or biodegradation. |
|---|
| Additional comments | Potential methods for reducing the formation of NDMA during disinfection include avoiding the use of chloramination, use of breakpoint chlorination and removal of ammonia prior to chlorination. |
|---|
| Assessment date | 2006 |
|---|
| Principal references |
IPCS (2002)
N-Nitrosodimethylamine
WHO (2008)
N-Nitrosodimethylamine in drinking-water
|
|---|
There is conclusive evidence that NDMA is a potent carcinogen in experimental animals by several routes of exposure, including through ingestion of drinking-water. NDMA has been classified by IARC as probably carcinogenic to humans. The mechanism by which NDMA produces cancer is well understood to involve biotransformation by liver microsomal enzymes, generating the methyldiazonium ion. This reactive metabolite forms DNA adducts, with most evidence pointing to O6-methylguanine as the likely proximal carcinogenic agent. As a consequence of the clear evidence of carcinogenicity, there have been few studies of other possible toxicity end-points.
There is also ample evidence that NDMA is genotoxic both in vivo and in vitro. Activation by liver microsomal S9 fractions is necessary for a positive in vitro result. The recent observation that human S9 fractions are much more active in promoting genotoxicity in the Ames test than rat S9 fractions suggests that humans may be especially sensitive to the carcinogenicity of NDMA.
Although there have been several case–control studies and one cohort study of NDMA in humans, none of them can be used to derive a quantitative risk of cancer. The results are supportive of the assumption that NDMA consumption is positively associated with either gastric or colorectal cancer. However, none of the studies focused on drinking-water as the route of exposure; instead, they used estimations of total dietary intake of NDMA.
Organotins
The group of chemicals known as the organotins is composed of a large number of compounds with differing properties and applications. Uses for the mono- and di-substituted compounds include as heat stabilizers in plastics, including PVC and chlorinated PVC (CPVC) water pipes. The tri-substituted compounds have been widely used as biocides and as antifouling agents in paint.
View in own window
| Reason for not establishing a guideline value | TBT, TPT, DBT and DOT occur in drinking-water or drinking-water sources at concentrations well below those of health concern |
|---|
| Establishing a guideline value for MMT, DMT and DMTC is unnecessary because their use as stabilizers in PVC and CPVC is normally controlled by product specifications |
| For other organotins, available data inadequate to permit derivation of health-based guideline values |
| Health-based value* | Sum of TBT, TPT, DBT and DOT: 1.5 µg/l (equivalent to approximately 0.6 µg/l tin) |
|---|
| Occurrence | Concentrations in drinking-water from PVC pipes are usually below a few hundred nanograms. However, many organotins have been detected as contaminants in environmental waters, including fresh waters that are possible drinking-water sources; the concentrations were mostly between the detection limits and hundreds of nanograms per litre. |
|---|
| TDI | Sum of TBT, TPT, DBT and DOT: 0.25 µg/kg bw (0.1 µg/kg bw as tin) derived by applying an uncertainty factor of 100 to account for interspecies (10) and intraspecies (10) variation to the NOAEL for tributyltin oxide (the reference organotin), based on chronic immunotoxicity studies in rats |
|---|
| Limit of detection | 24–51 pg as tin for six organotin compounds (chlorides of DMT, DBT, TMT, TBT, DPT and TPT) by ICP-MS; 0.2–0.4 ng/l for DBT, TBT, DPT and TPT; and 2 ng/l for MPT, by derivatization–liquid extraction followed by GC and MS |
|---|
| Prevention and treatment |
The effectiveness of water treatment appears to be significantly different for different compounds. For example, conventional water treatment (coagulation, sedimentation, filtration) has been shown to be effective in removing TBT in shipyard waters under optimal conditions, whereas it was ineffective in removing DPT and TPT. In contrast, advanced treatment processes were effective in removing these compounds but ineffective in removing other organotin compounds. However, available data and quantification information are still limited.
Where the organotins originate from plastic service water pipes and fittings, particularly monoalkyltins and dialkyltins in PVC and CPVC pipes and fittings, the most important means of control is by product specifications through an appropriate certification scheme.
|
|---|
| Health-based value derivation |
|---|
| • allocation to water | 20% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 2020 |
|---|
| Principal reference | WHO (2020)
Organotins in drinking-water |
|---|
DBT, dibutyltin; DMT, dimethyltin; DMTC, dimethyltin dichloride; DOT, di-n-octyltin; DPT, diphenyltin; MMT, monomethyltin; MPT, monophenyltin; TBT, tributyltin; TMT, trimethyltin; TPT, triphenyltin
- *
When a formal guideline value is not established, a “health-based value” may be determined in order to provide guidance to Member States when there is reason for local concern. Establishing a formal guideline value for such substances may encourage Member States to incorporate a value into their national standards when this may be unnecessary.
Reliable lifetime TDI values for monomethyltin, dimethyltin and dimethyltin dichloride could not be derived because of a lack of long-term studies with systematic experimental data. However, there is no need to establish a guideline value for these organotins because their use as stabilizers in PVC and CPVC is normally controlled by product specifications. The data available are insufficient to permit the proposal of guideline or health-based values for other organotins, including trimethyltin, tetrabutyltin, mono-n-octyltin, tetraocyltin, monophenyltin, diphenyltin and tetraphenyltin.
Parathion
Parathion (CAS No. 56-38-2) is a non-systemic insecticide that is used in many countries throughout the world. It is used as a fumigant and acaricide and as a pre-harvest soil and foliage treatment on a wide variety of crops, both outdoors and in greenhouses. Parathion released to the environment will adsorb strongly to the top layer of soil and is not likely to leach significantly. Parathion disappears from surface waters in about a week. The general population is not usually exposed to parathion from air or water. Parathion residues in food are the main source of exposure.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (1996)
Pesticide residues in food—1995 evaluations
WHO (2004)
Parathion in drinking-water
|
|---|
Parathion inhibits cholinesterase activity in all species tested. There has been no evidence of carcinogenicity in 2-year rat studies. JMPR concluded that parathion is not genotoxic.
A health-based value of 10 µg/l can be calculated for parathion on the basis of an ADI of 0–0.004 mg/kg body weight based on a NOAEL of 0.4 mg/kg body weight per day in a 2-year study in rats for retinal atrophy and inhibition of brain acetylcholinesterase at the next higher dose, and using an uncertainty factor of 100 for interspecies and intraspecies variation. Lower NOAELs in experimental animals, based only on inhibition of erythrocyte or brain acetylcholinesterase, were not considered relevant because of the availability of a NOAEL for erythrocyte acetylcholinesterase inhibition in humans, which was 0.1 mg/kg body weight per day.
Intake of parathion from all sources is generally low and well below the upper limit of the ADI. As the health-based value is much higher than concentrations of parathion likely to be found in drinking-water, the presence of parathion in drinking-water under usual conditions is unlikely to represent a hazard to human health. For this reason, the establishment of a formal guideline value for parathion is not deemed necessary.
Pendimethalin
Pendimethalin (CAS No. 40487-42-1) is a pre-emergence herbicide that is fairly immobile and persistent in soil. It is used in large amounts in Japan (5000 tonnes per year). It is lost through photodegradation, biodegradation and volatilization. The leaching potential of pendimethalin appears to be very low, but little is known about its more polar degradation products.
View in own window
| Guideline value | 0.02 mg/l (20 µg/l) |
|---|
| Occurrence | Rarely found in drinking-water in the limited studies available |
|---|
| TDI | 5 µg/kg body weight, based on evidence of slight liver toxicity even at the lowest dose tested (5 mg/kg body weight) in a long-term rat feeding study, with an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for a combination of the use of a LOAEL instead of a NOAEL and limitations of the database) |
|---|
| Limit of detection | 0.01 µg/l by GC-MS |
|---|
| Treatment performance | 1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Pendimethalin in drinking-water |
|---|
In a short-term dietary study in rats, a variety of indications of hepatotoxicity as well as increased kidney weights in males were observed at the highest dose level. In a long-term dietary study, some toxic effects (hyperglycaemia in the mouse and hepatotoxicity in the rat) were present even at the lowest dose level. On the basis of available data, pendimethalin does not appear to have significant mutagenic activity. Long-term studies in mice and rats have not provided evidence of carcinogenicity; however, these studies have some important methodological limitations.
Pentachlorophenol
Pentachlorophenol (CAS No. 87-86-5), or PCP, and other chlorophenols are used primarily for protecting wood from fungal growth. Food is usually the major source of exposure to PCP unless there is a specific local contamination of drinking-water by PCP or exposure from log homes treated with PCP.
View in own window
| Provisional guideline value | 0.009 mg/l (9 µg/l) |
|---|
| The guideline value is considered provisional because of the variations in metabolism between experimental animals and humans. |
| Occurrence | Concentrations in water samples are usually below 10 µg/l, although much higher concentrations in groundwater may be measured under certain conditions |
|---|
| Basis of guideline value derivation | Multistage modelling of tumour incidence in an NTP bioassay without incorporation of a body surface area correction, recognizing that there are interspecies differences in metabolism between experimental animals and humans, with an important metabolite formed in rats being only a minor metabolite in humans |
|---|
| Limit of detection | 0.005–0.01 µg/l by GC with ECD |
|---|
| Treatment performance | 0.4 µg/l should be achievable using GAC |
|---|
| Additional comments | The concentration of PCP associated with a 10−5 upper-bound excess lifetime cancer risk is similar to the guideline value established in the second edition, so that guideline value is retained. |
|---|
| Assessment date | 1998 |
|---|
| Principal reference | WHO (2003)
Pentachlorophenol in drinking-water |
|---|
IARC classified PCP in Group 2B (possibly carcinogenic to humans) on the basis of inadequate evidence of carcinogenicity in humans but sufficient evidence in experimental animals. There is suggestive, although inconclusive, evidence of the carcinogenicity of PCP from epidemiological studies of populations exposed to mixtures that include PCP. Conclusive evidence of carcinogenicity has been obtained in one animal species (mice). Although there are notable variations in metabolism between experimental animals and humans, it was considered prudent to treat PCP as a potential carcinogen.
Perchlorate
Perchlorate is a naturally occurring anion that is frequently detected in the environment. It is used primarily as an oxidizer for solid rocket fuels, automotive airbags, fireworks and road flares. Perchlorate is found in water due to contamination from perchlorate manufacturing or use, natural deposits of perchlorate, use of fertilizers containing natural deposits of perchlorate, and natural formation of perchlorate in the atmosphere and its deposition during rain or snow events. It also forms in hypochlorite solutions to varying degrees, depending on the hypochlorite concentration, age and storage conditions.
View in own window
| Guideline value | 0.07 mg/l (70 µg/l) |
|---|
| Occurrence | Generally found in drinking-water at concentrations below 10 µg/l, although concentrations above 40 µg/l have been measured |
|---|
| PMTDI | 0.01 mg/kg bw, based on a BMDL50 of 0.11 mg/kg bw per day for 50% inhibition of iodide uptake, derived from a human clinical study on healthy adult volunteers administered perchlorate in drinking-water, and using an uncertainty factor of 10 to account for inter-individual differences |
|---|
| Limit of detection | 20–50 ng/l (method reporting limits) by LC-MS; 4 µg/l (method reporting limit) by IC with suppressed conductivity detection |
|---|
| Treatment performance | The perchlorate anion is highly stable in water and is difficult to remove using conventional water treatment technologies. Treatment technologies that have been shown to effectively remove perchlorate from water include nanofiltration and reverse osmosis membranes, anaerobic biodegradation and ion exchange. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 20% of unrounded PMTDI (0.011 mg/kg bw) |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 2016 |
|---|
| Principal references |
EFSA (2014). Scientific opinion on the risks to public health related to the presence of perchlorate in food, in particular fruits and vegetables
FAO/WHO (2011). Safety evaluation of certain contaminants in food
WHO (2016). Perchlorate in drinking-water
|
|---|
The primary effect of perchlorate is its ability to competitively inhibit uptake of iodide by the thyroid gland. Inhibition of iodide uptake by perchlorate reduces the amount of iodide available for the synthesis of thyroid hormones. Sustained reduction in iodide uptake by the thyroid may result in hypothyroidism, which has adverse implications for structural and functional brain development in the fetus, infant and child, and for metabolism and the functioning of the cardiovascular, gastrointestinal, skeletal, neuromuscular and reproductive systems in adults. As the rat is not a good model for humans for substances known to affect the thyroid and having a mode of action involving inhibition of the uptake of iodide, the guideline value was derived from human studies.
Petroleum products
Petroleum products are used in large quantities, primarily as fuels. They are complex mixtures of chemicals derived from crude oil by distillation and fractionation. They consist primarily of a wide range of aliphatic and aromatic hydrocarbons, many of which are of extremely low solubility in water. Petroleum products are widely stored and handled and are often spilt. The primary concern for drinking-water is the potential for spills into source water, penetration of distribution systems and contamination of drinking-water treatment works.
View in own window
| Reason for not establishing a guideline value | Taste and odour will in most cases be detectable at concentrations below those of health concern, particularly with short-term exposure |
|---|
| Assessment date | 2004 |
|---|
| Principal reference | WHO (2008)
Petroleum products in drinking-water |
|---|
Exposure to the constituents of petroleum products through drinking-water is frequently short term, as the result of an accidental spill or short-term incident. Such incidents may lead to high concentrations of total petroleum hydrocarbons. However, a number of the most soluble aromatic hydrocarbons will be detectable by taste or odour at concentrations below those concentrations of concern for health, particularly for short-term exposure. Substances such as the alkyl benzenes and the alkyl naphthalenes have taste and odour thresholds of a few micrograms per litre. In view of the above, it is not considered appropriate to set a formal health-based guideline value for petroleum products in drinking-water.
In the event of a spill, it may be necessary to carry out a context-specific assessment of the risk to health. The fact that petroleum products are complex mixtures of many individual hydrocarbons is a complicating factor in determining the potential risks to consumers. The traditional approach of evaluating individual chemicals in assessing the risks from drinking-water is therefore largely inappropriate. In order to overcome this difficulty, it is more practical to consider a series of hydrocarbon fractions and to determine appropriate tolerable concentrations for those fractions. The most widely accepted approach is that developed by the Total Petroleum Hydrocarbons Criteria Working Group in the USA, which divided total petroleum hydrocarbons into a series of aliphatic and aromatic fractions based on the number of carbon atoms and the boiling point, to give equivalent carbon numbers.
This pragmatic approach provides a suitable basis for assessing the potential health risks associated with larger-scale contamination of drinking-water by petroleum products. The allocation of 10% of each of the reference doses, equivalent to TDIs, for the various fractions to drinking-water provides a conservative assessment of the risks. Although the approach is based on the analysis of hydrocarbon fractions, most are of low solubility, and the most soluble fractions, consisting largely of lower molecular weight aromatic hydrocarbons, will be present in the greatest concentration.
pH
No health-based guideline value is proposed for pH. Although pH usually has no direct impact on consumers, it is one of the most important operational water quality parameters (see chapter 10).
View in own window
| Reason for not establishing a guideline value | Not of health concern at levels found in drinking-water |
|---|
| Additional comments | An important operational water quality parameter |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2007)
pH in drinking-water |
|---|
2-Phenylphenol and its sodium salt
2-Phenylphenol (CAS No. 90-43-7) is used as a disinfectant, bactericide and virucide. In agriculture, it is used in disinfecting fruits, vegetables and eggs. It is also used as a general surface disinfectant in hospitals, nursing homes, veterinary hospitals, poultry farms, dairy farms, commercial laundries, barbershops and food processing plants. 2-Phenylphenol is readily degraded in surface waters, with a half-life of about 1 week in river water.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal references |
FAO/WHO (2000)
Pesticide residues in food—1999 evaluations
WHO (2003)
2-Phenylphenol and its sodium salt in drinking-water
|
|---|
2-Phenylphenol has been determined to be of low toxicity. Both 2-phenylphenol and its sodium salt are carcinogenic in male rats, and 2-phenylphenol is carcinogenic in male mice. However, urinary bladder tumours observed in male rats and liver tumours observed in male mice exposed to 2-phenylphenol appear to be threshold phenomena that are species and sex specific. JMPR concluded that 2-phenylphenol is unlikely to represent a carcinogenic risk to humans. Although a working group convened by IARC classified 2-phenylphenol, sodium salt, in Group 2B (possibly carcinogenic to humans) and 2-phenylphenol in Group 3 (not classifiable as to its carcinogenicity to humans), JMPR noted that the IARC classification is based on hazard identification, not risk assessment, and is furthermore limited to published literature, excluding unpublished studies on toxicity and carcinogenicity. JMPR also concluded that there are unresolved questions about the genotoxic potential of 2-phenylphenol.
A health-based value of 1 mg/l can be calculated for 2-phenylphenol on the basis of an ADI of 0–0.4 mg/kg body weight, based on a NOAEL of 39 mg/kg body weight per day in a 2-year toxicity study on the basis of decreased body weight gain and hyperplasia of the urinary bladder and carcinogenicity of the urinary bladder in male rats, using an uncertainty factor of 100 for interspecies and intraspecies variation. Because of its low toxicity, however, the health-based value derived for 2-phenylphenol is much higher than concentrations of 2-phenylphenol likely to be found in drinking-water. Under usual conditions, therefore, the presence of 2-phenylphenol in drinking-water is unlikely to represent a hazard to human health. For this reason, the establishment of a formal guideline value for 2-phenylphenol is not deemed necessary.
Polynuclear aromatic hydrocarbons
Polynuclear aromatic hydrocarbons, or PAHs, form a class of diverse organic compounds each containing two or more fused aromatic rings of carbon and hydrogen atoms. Most PAHs enter the environment via the atmosphere from a variety of combustion processes and pyrolysis sources. Owing to their low solubility and high affinity for particulate matter, they are not usually found in water in notable concentrations. The main source of PAH contamination in drinking-water is usually the coal tar coating of drinking-water distribution pipes, used to protect the pipes from corrosion. Fluoranthene is the most commonly detected PAH in drinking-water and is associated primarily with coal tar linings of cast iron or ductile iron distribution pipes. PAHs have been detected in a variety of foods as a result of the deposition of airborne PAHs and in fish from contaminated waters. PAHs are also formed during some methods of food preparation, such as char-broiling, grilling, roasting, frying or baking. For the general population, the major routes of exposure to PAHs are from food and ambient and indoor air. The use of open fires for heating and cooking, which is common especially in developing countries, may increase PAH exposure. Where there are elevated levels of contamination by coal tar coatings of water pipes, PAH intake from drinking-water could equal or even exceed that from food.
View in own window
| Guideline value | Benzo[a]pyrene: 0.0007 mg/l (0.7 µg/l) |
|---|
| Occurrence | PAH levels in uncontaminated groundwater usually in range 0–5 ng/l; concentrations in contaminated groundwater may exceed 10 µg/l; typical concentration range for sum of selected PAHs in drinking-water is from about 1 ng/l to 11 µg/l |
|---|
| Basis of guideline value derivation | Based on an oral carcinogenicity study in mice and calculated using a two-stage birth–death mutation model, which incorporates variable dosing patterns and time of killing; quantification of dose–response for tumours, on the basis of new studies in which the carcinogenicity of benzo[a]pyrene was examined following oral administration in mice, but for which the number of dose groups was smaller, confirms this value |
|---|
| Limit of detection | 0.01 µg/l by GC-MS and reversed-phase HPLC with a fluorescence detector |
|---|
| Treatment performance | 0.05 µg/l should be achievable using coagulation |
|---|
| Additional comments | The presence of significant concentrations of benzo[a]pyrene in drinking-water in the absence of very high concentrations of fluoranthene indicates the presence of coal tar particles, which may arise from seriously deteriorating coal tar pipe linings. |
|---|
| It is recommended that the use of coal tar–based and similar materials for pipe linings and coatings on storage tanks be discontinued. |
| Assessment date | 1998 |
|---|
| Principal reference | WHO (2003)
Polynuclear aromatic hydrocarbons in drinking-water |
|---|
View in own window
| Reason for not establishing a guideline value | Fluoranthene: Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 1998 |
|---|
| Principal reference | WHO (2003)
Polynuclear aromatic hydrocarbons in drinking-water |
|---|
Evidence that mixtures of PAHs are carcinogenic to humans comes primarily from occupational studies of workers following inhalation and dermal exposure. No data are available for humans for the oral route of exposure. There are few data on the oral toxicity of PAHs other than benzo[a]pyrene, particularly in drinking-water. Relative potencies of carcinogenic PAHs have been determined by comparison of data from dermal and other studies. The order of potencies is consistent, and this scheme therefore provides a useful indicator of PAH potency relative to that of benzo[a]pyrene.
A health-based value of 4 µg/l can be calculated for fluoranthene on the basis of a NOAEL of 125 mg/kg body weight per day for increased serum glutamate–pyruvate transaminase levels, kidney and liver pathology, and clinical and haematological changes in a 13-week oral gavage study in mice, using an uncertainty factor of 10 000 (100 for interspecies and intraspecies variation, 10 for the use of a subchronic study and inadequate database and 10 because of clear evidence of co-carcinogenicity with benzo[a]pyrene in mouse skin painting studies). However, this health-based value is significantly above the concentrations normally found in drinking-water. Under usual conditions, therefore, the presence of fluoranthene in drinking-water does not represent a hazard to human health. For this reason, the establishment of a formal guideline value for fluoranthene is not deemed necessary.
Potassium
Potassium is an essential element in humans and is seldom, if ever, found in drinking-water at levels that could be a concern for healthy humans. The recommended daily requirement is greater than 3000 mg. Potassium occurs widely in the environment, including all natural waters. It can also occur in drinking-water as a consequence of the use of potassium permanganate as an oxidant in water treatment. In some countries, potassium chloride is being used in ion exchange for household water softening in place of, or mixed with, sodium chloride, so potassium ions would exchange with calcium and magnesium ions. Possible replacement or partial replacement of sodium salts with potassium salts for conditioning desalinated water has been suggested. The latter seems to be an unlikely development at this stage, in view of the cost difference.
View in own window
| Reason for not establishing a guideline value | Occurs in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2009 |
|---|
| Principal reference | WHO (2009)
Potassium in drinking-water |
|---|
Currently, there is no evidence that potassium levels in municipally treated drinking-water, even water treated with potassium permanganate, are likely to pose any risk for the health of consumers. It is not considered necessary to establish a health-based guideline value for potassium in drinking-water.
Although potassium may cause some health effects in susceptible individuals, potassium intake from drinking-water is well below the level at which adverse health effects may occur. Health concerns would be related to the consumption of drinking-water treated by potassium-based water treatment (principally potassium chloride for regeneration of ion exchange water softeners), affecting only individuals in high-risk groups (i.e. individuals with kidney dysfunction or other diseases, such as heart disease, coronary artery disease, hypertension, diabetes, adrenal insufficiency, pre-existing hyperkalaemia; people taking medications that interfere with normal potassium-dependent functions in the body; and older individuals or infants). It is recommended that susceptible individuals seek medical advice to determine whether they should avoid the consumption of water (for drinking or cooking) treated by water softeners using potassium chloride.
When high-risk individuals have been advised by a physician to avoid elevated potassium intake from water, the recommended strategy is to limit the addition of potassium to water that will be ingested or to avoid ingesting such water. This can be done by having a proportion of the water bypass the softener altogether; this approach is recommended by several countries. Although technologies are available to remove potassium, they are generally more expensive and redundant when combined with the softening treatment.
Propanil
Propanil (CAS No. 709-98-8) is a contact post-emergence herbicide used to control broad-leaved and grassy weeds, mainly in rice. It is a mobile compound with affinity for the water compartment. Propanil is not, however, persistent, being easily transformed under natural conditions to several metabolites. Two of these metabolites, 3,4-dichloroaniline and 3,3′,4,4′-tetrachloroazobenzene, are more toxic and more persistent than the parent compound. Although used in a number of countries, propanil has only occasionally been detected in groundwater.
View in own window
| Reason for not establishing a guideline value | Readily transformed into metabolites that are more toxic; a guideline value for the parent compound is considered inappropriate, and there are inadequate data to enable the derivation of guideline values for the metabolites |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2003)
Propanil in drinking-water |
|---|
Although a health-based value for propanil can be derived, this has not been done, because propanil is readily transformed into metabolites that are more toxic. Therefore, a guideline value for the parent compound is considered inappropriate, and there are inadequate data on the metabolites to allow the derivation of guideline values for them. Authorities should consider the possible presence in water of more toxic environmental metabolites.
Saxitoxins (cyanobacterial toxins)10
Saxitoxins (STXs) are naturally occurring alkaloids produced by some marine dinoflagellates and by strains of various species of freshwater cyanobacteria. They have been found in cyanobacterial blooms around the globe and from a number of cyanobacterial genera, including Dolichospermum, Raphidiopsis and Aphanizomenon (see also section 11.5).
Whereas STX-contaminated marine shellfish are the most likely cause of the severe illness known as paralytic shellfish poisoning, drinking-water is the most likely exposure route where surface water with cyanobacterial blooms is the drinking-water source. However, in locations where marine shellfish are contaminated with STXs, they are likely to be the greater source of exposure. Limited data suggest that STXs may also accumulate in some freshwater food items. Recreational activities may also be a relevant exposure pathway, potentially to high concentrations (see WHO Guidelines on recreational water quality, 2021).
View in own window
| Acute guideline value | Total STXs (sum of all congeners, free plus cell-bound): 0.003 mg/l (3 µg/l) |
|---|
| Occurrence | Although concentrations usually range well below 1 µg/l, several µg/l are occasionally reported, and up to almost 200 µg/l has been found in scums. STXs largely occur cell-bound unless cell damage causes release |
|---|
| ARfD | FAO (2004) and EFSA (2009) reviewed a large dataset of human STX poisoning cases. Exposure was to a range of STX analogues. An ARfD for saxitoxins of 0.5 µg/kg bw was derived based on a LOAEL for mild symptoms of 1.5 µg/kg bw of STX equivalents (EFSA, 2009). A database uncertainty factor of 3 was applied for use of a LOAEL. |
|---|
| Limit of detection |
<3 µg/l by LC-MS or HPLC methods with either pre- or post-column derivatisation and fluorescence detection; LC-MS methods require quantitative reference standards, which are available for only some congeners. A commercially available receptor-binding assay circumvents this problem and provides more reliable results than HPLC.
Prior extraction of cells with freeze–thaw cycles and acetic acid, hydrochloric acid or acidified aqueous methanol is necessary for cell-bound STX; neglecting extraction from cells will lead to dramatic underestimation of concentrations.
STX analysis requires particular care in the laboratory because congeners may interconvert; furthermore, the chemical variability of congeners presents challenges.
|
|---|
| 0.02 µg/l for STX alone by commercially available immunoassay kits (ELISA), but cross-reactivity with other congeners is highly variable. |
| Monitoring | The likelihood of blooms can be assessed by understanding water body conditions (in particular, nutrient concentrations, water body depth, water retention time, patterns of mixing and stratification; see section 11.5). Where conditions render blooms likely, visual monitoring of source water (including microscopy for potentially STX-containing genera) is important because biomass can increase rapidly. Exceeding alert values for biomass indicators or STX concentrations should trigger management responses to prevent exposure to elevated toxin concentrations (see the alert level framework in section 11.5). Analysis of cyanotoxins is particularly useful for validating and optimizing the efficacy of control measures such as riverbank filtration or treatment. |
|---|
| Prevention and treatment | Actions to decrease the probability of bloom occurrence include catchment and source water management, such as reducing nutrient loading or changing reservoir stratification and mixing. Filtration is effective for removing intact cyanobacterial cells, but some dissolved STX may be present. For this dissolved fraction, oxidation with chlorine or ozone at sufficient concentrations and contact times, as well as GAC and some PAC applications, are effective (see chapters 7–10 of Toxic cyanobacteria in water; Annex 1). |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 100% of ARfD |
|---|
| • allocation to water | 5 kg infant |
|---|
| • consumption | 0.75 litres/day |
|---|
| Additional comments | The total amount of STX and its structural analogues as gravimetric or molar equivalents should be evaluated against the guideline value. |
|---|
|
The drinking-water guideline value was derived to protect bottle-fed infants because the guideline value is for acute exposure, and a guideline value based on adult exposure could allow exposure of infants to a concentration of STXs close to the LOAEL. For a 60 kg adult consuming 2 litres of drinking-water per day, a 5-fold higher concentration than the acute guideline value would be tolerable.
In locations where exposure to STXs may occur through both marine shellfish and drinking-water, health authorities may need to consider the combined exposure in their risk assessments.
|
| Assessment date | 2020 |
|---|
| Principal references |
FAO (2004)
Marine biotoxins
EFSA (2009)
Marine biotoxins in shellfish: saxitoxin group
WHO (2020)
Cyanobacterial toxins: saxitoxins
Chorus & Welker (2021) Toxic cyanobacteria in water
|
|---|
STXs act by blocking sodium channels in nerve cell axons, which inhibits propagation of an action potential along the axon. When this occurs in sensory neurons, symptoms such as tingling and numbness are induced; in motor neurons, muscle weakness or paralysis ensues. The effects of human intoxication by STXs have been well described from numerous cases of paralytic shellfish poisoning after consumption of marine shellfish. They range from numbness and tingling in the tongue and mouth through muscular weakness in the limbs to, in severe cases, respiratory failure and death. If a person recovers from the acute toxicity, they appear to make a full recovery and no long-term adverse effects are known. STXs were the cause of massive animal deaths along 1000 km of the Murray–Darling river system in Australia during a heavy bloom of Dolichospermum in 1991. Unlike the other cyanotoxins considered in these Guidelines (MCs, CYNs and ATXs), a guideline value for STXs could be derived from human data that are available from incidents of shellfish poisoning.
Practical considerations
Where nutrient (phosphorus and nitrogen) concentrations are elevated in lakes, reservoirs or slowly flowing rivers, cyanobacteria occur widely. Where their excessive growth leads to high biomass, sometimes termed “bloom” events, STXs can reach concentrations in raw water that are potentially hazardous to human health. Such blooms tend to recur in the same water bodies. Cells of some cyanobacterial species (e.g. Raphidiopsis, Aphanizomenon, Dolichospermum) may accumulate at the surface as scums. Such accumulations may develop rapidly and may be of very variable duration (hours to weeks). In many circumstances, blooms and accumulations are seasonal, whereas others occur perennially.
Cyanobacteria are most effectively controlled in the context of developing a WSP (see chapter 4). Control measures to manage potential risks from cyanobacteria, and in particular from their toxins, in drinking-water should include not only adequate treatment, but also measures to control cyanobacterial bloom development. See section 11.5 for more information on cyanobacteria, including further details on monitoring cyanobacterial blooms, the alert level framework, and prevention and management of cyanobacteria in source waters. Effectively minimizing the formation of blooms and locating the raw water intake away from blooms reduce the treatment steps required to remove cyanotoxins.
Drinking-water treatment that removes particles—that is, soil, slow sand or riverbank filtration, conventional water treatment (coagulation, flocculation and filtration) or dissolved air flotation—can remove cell-bound STXs effectively. Soil, slow sand and riverbank filtration can also remove dissolved cyanotoxins. For all these processes, it important that they are optimized to target the removal of cells and dissolved toxins. Chlorination and ozonation at sufficiently high doses and contact times are effective for degrading dissolved STXs; however, elevated organic carbon in bloom situations will substantially increase the disinfectant demand. Chlorine dioxide and chloramine are ineffective for degrading STXs. Both for pre-oxidaton and conventional treatment, cell rupture and toxin release should be avoided. GAC and PAC can be effective for removing dissolved STXs, with efficacy dependent on several factors, including the type of activated carbon, contact times (PAC), flow rates (GAC) and water quality. As the challenges that blooms present for treatment are complex, periodic validation of efficacy during bloom situations and under the specific local conditions is particularly important. Avoiding bloom occurrence and intake is therefore the preferred option.
Selenium
Selenium is present in Earth’s crust, often in association with sulfur-containing minerals. Selenium is an essential trace element, and foodstuffs such as cereals, meat and fish are the principal source of selenium for the general population. Levels in food also vary greatly according to geographical area of production. However, even in high-selenium areas, the relative contribution of selenium from drinking-water is likely to be small in comparison with that from locally produced food.
View in own window
| Provisional guideline value | 0.04 mg/l (40 µg/l) |
|---|
| The guideline value is designated as provisional because of the uncertainties inherent in the scientific database. |
| Occurrence | Most drinking-water contains concentrations of selenium that are much lower than 10 µg/l, except in certain seleniferous areas |
|---|
| Basis of guideline value derivation | An allocation of 20% of the upper tolerable intake of 400 µg/day to drinking-water provides a sensible balance that will assist regulators and suppliers in making decisions about whether further action is needed |
|---|
| Limit of detection | 0.5 µg/l by hydride generation AAS |
|---|
| Treatment performance | Selenium is not removed by conventional treatment processes; significant removals of selenium from water using activated alumina adsorption, ion exchange, reverse osmosis and nanofiltration have been reported. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 20% of upper tolerable intake |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | It is important that a proper balance be achieved between recommended intakes and undesirable intakes in determining an appropriate guideline value for selenium in drinking-water. While for most parts of the world, the concentration of selenium in drinking-water will not exceed 10 µg/l, there are circumstances in which selenium may be elevated significantly above normal concentrations, and guidance may be required. Where selenium intake from the diet is known, this should be used in determining a concentration that ensures that intake is safe and sufficient. Where selenium intake from the diet is not known, guidance may be required. |
|---|
| For most Member States, a drinking-water guideline for selenium is unnecessary. Where there are regions of high intake from a number of sources, of which drinking-water may be one, then Member States should take into consideration exposure from all sources in determining actions to reduce exposure. For drinking-water, this may include using alternative sources, blending low-selenium sources with high-selenium sources as well as considering selenium removal. |
| Assessment date | 2010 |
|---|
| Principal references |
FAO/WHO (2004)
Vitamin and mineral requirements in human nutrition
WHO (2011)
Selenium in drinking-water
|
|---|
Selenium is an essential element for humans, and there are indications that selenium status may be marginal in many parts of the world, including western Europe. The potential for adverse effects from selenium deficiency appears to be dependent on a number of factors, including overall health and nutritional status. Very low selenium status in humans has been associated with a juvenile, multifocal myocarditis called Keshan disease and a chondrodystrophy called Kaschin-Beck disease. Several studies have also found blood selenium levels to be inversely associated with the prevalence of several types of cancer.
High intakes of selenium are also associated with a number of specific diseases and the potential for adverse effects, but, again, this seems to be strongly influenced by other factors. Symptoms in people with high urinary selenium levels included gastrointestinal disturbances, discoloration of the skin, decayed teeth, hair or nail loss, nail abnormalities and changes in peripheral nerves. Slight biochemical changes have also been observed. One case of selenium toxicity directly attributable to a water source (well water containing selenium at a concentration of 9 mg/l) has been reported. The average dietary intake that is associated with selenosis has been found to be in excess of 900 µg/day.
As selenium is an essential element, various national and international organizations have established recommended daily intakes of selenium. A joint FAO/WHO consultation recommended intakes of 6–21 µg of selenium per day for infants and children, according to age, 26 and 30 µg of selenium per day for adolescent females and males, respectively, and 26 and 35 µg of selenium per day for adult females and males, respectively.
Because of concern about the adverse effects resulting from exposure to excessive levels of selenium, various national and international organizations have established upper limits of exposure for selenium. FAO/WHO established an upper tolerable limit for selenium of 400 µg/day.
Silver
Silver occurs naturally, mainly in the form of its very insoluble and immobile sulfide, oxides and some salts. Silver ions are primarily found in the +1 oxidation state, and the ionic compounds silver nitrate (soluble) and silver chloride (relatively insoluble) are the most important forms of silver for drinking-water. Silver can also occur as nanoparticles, which can be present in water bodies as a result of release into the environment from wastewater or industrial discharges. When silver is used in point-of-use water treatment devices, drinking-water is expected to be the major source of exposure to silver.
View in own window
| Reason for not establishing a guideline value | Available data inadequate to permit derivation of health-based guideline value, and silver usually occurs in drinking-water at concentrations well below those of health concern |
|---|
| Provisional reference value* | 0.1 mg/l |
|---|
| Occurrence | In surface water and groundwater, silver concentrations are usually below 5 µg/l; however, detections >100 µg/l, although rare, have been reported |
|---|
| TDI | A formal TDI could not be derived because of database limitations. However, 0.6 mg/kg bw of colloidal silver per day was considered a LOAEL based on a case report of argyria in a woman who ingested this dose of silver for 16 months. An uncertainty factor of 100 (10 for intraspecies variability and 10 for database limitations) was applied. The database limitations include the short duration of the study, uncertainty associated with a dose level derived from human recall and use of a LOAEL instead of a NOAEL. |
|---|
| Limit of detection | 2 ng/ by neutron activation analysis; 5 ng/l by ICP-MS; 2 µg/l by graphite furnace AAS; 10 µg/l by a spectrographic and colorimetric method with dithizone for a 20 ml sample. In addition, asymmetric flow field-flow fractionation in combination with single particle ICP-MS can differentiate between silver nanoparticles and dissolved silver, although this method has not yet been standardized. Since ionic silver can be released from silver nanoparticles, it may be difficult to determine whether silver dispersed in water has originated from the ionic or the particulate fraction. |
|---|
| Treatment performance | Conventional treatment (coagulation, sedimentation, filtration) and lime softening are effective for removing ionic silver; conventional treatment is also expected to be effective for removing coated silver nanoparticles |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 80% |
|---|
| • allocation to water | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The provisional reference value is applicable particularly where silver is used in point-of-use water treatment devices. However, silver is not recommended for disinfection of drinking-water including in point-of-use water treatment devices (see Part III of the supporting document Alternative drinking-water disinfectants: Bromine, iodine and silver; Annex 1). It is also noted that silver with copper may be necessary to control Legionella in the distribution systems of buildings, and the risks of legionellosis outweigh the risks from low levels of silver ions (usually in the low μg/l range) that may be detected in drinking-water as a result of such use. See Silver in drinking-water, Annex 2, for further information, including on Legionella control measures. |
|---|
| Assessment date | 2021 |
|---|
| Principal reference | WHO (2021)
Silver in drinking-water |
|---|
- *
Although a formal guideline value cannot be established, the provisional reference value was derived based on limited available studies, recognizing that a “bounding value” may be useful, to provide guidance to Member States in the event of need. Reference values are too uncertain to be used for developing regulations or standards.
The only obvious sign of silver overload is argyria, a condition in which skin and hair are heavily discoloured by silver in the tissues. In argyria, silver is deposited in various organs (e.g. skin, kidney, liver) after oral ingestion of silver in its ionic form or as silver nanoparticles, oxidizing to insoluble silver sulfide, which is responsible for the discolouring effects. It is difficult to determine the lowest dose that may lead to development of argyria, and the toxicological database on silver is not adequate to support derivation of a formal guideline value. Furthermore, a formal guideline value is considered unnecessary since silver usually occurs naturally in drinking-water at concentrations well below those of health concern. Nevertheless, a bounding value (provisional reference value) may provide a useful benchmark where elevated concentrations of silver in drinking-water may be expected. The provisional reference value of 0.1 mg/l is supported by the prior assessments that concluded that 10 g of ingested silver can be considered a human NOAEL (WHO, 1984a, b, 1993; US EPA 1992). Over a 70-year period, assuming a drinking-water intake of 2 litres/day, 0.1 mg/l is a concentration in drinking-water that would give a total dose of half this NOAEL.
Simazine
Simazine (CAS No. 122-34-9) is a pre-emergence herbicide used on a number of crops as well as in non-crop areas. It is fairly resistant to physical and chemical dissipation processes in the soil. It is persistent and mobile in the environment.
View in own window
| Guideline value | 0.002 mg/l (2 µg/l) |
|---|
| Occurrence | Frequently detected in groundwater and surface water at concentrations of up to a few micrograms per litre |
|---|
| TDI | 0.52 µg/kg body weight, based on a NOAEL of 0.52 mg/kg body weight from a long-term study in the rat (based on weight changes, effects on haematological parameters and an increase in mammary tumours) and an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for possible non-genotoxic carcinogenicity) |
|---|
| Limit of detection | 0.01 µg/l by GC-MS; 0.1–0.2 µg/l by GC with flame thermionic detection |
|---|
| Treatment performance | 0.1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Simazine in drinking-water |
|---|
Simazine does not appear to be genotoxic in mammalian systems. Recent studies have shown an increase in mammary tumours in the female rat but no effects in the mouse. IARC has classified simazine in Group 3 (not classifiable as to its carcinogenicity to humans).
Sodium
Sodium salts (e.g. sodium chloride) are found in virtually all food (the main source of daily exposure) and drinking-water. Although concentrations of sodium in potable water are typically less than 20 mg/l, they can greatly exceed this in some countries. The levels of sodium salts in air are normally low in relation to those in food or water. It should be noted that some water softeners can add significantly to the sodium content of drinking-water.
View in own window
| Reason for not establishing a guideline value | Not of health concern at levels found in drinking-water |
|---|
| Additional comments | May affect acceptability of drinking-water |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Sodium in drinking-water |
|---|
No firm conclusions can be drawn concerning the possible association between sodium in drinking-water and the occurrence of hypertension. Therefore, no health-based guideline value is proposed. However, concentrations in excess of 200 mg/l may give rise to unacceptable taste (see chapter 10).
Sodium dichloroisocyanurate
Sodium dichloroisocyanurate is the sodium salt of a chlorinated hydroxytriazine and is used as a source of free available chlorine, in the form of hypochlorous acid, for the disinfection of water. It is widely used as a stable source of chlorine for the disinfection of swimming pools and in the food industry. It is also used as a means of disinfecting drinking-water, primarily in emergencies, when it provides an easy-to-use source of free chlorine, and, more recently, as the form of chlorine for household point-of-use water treatment.
View in own window
| Guideline values | Sodium dichloroisocyanurate: 50 mg/l (50 000 µg/l) |
|---|
| Cyanuric acid: 40 mg/l (40 000 µg/l) |
| Occurrence | Where sodium dichloroisocyanurate is used for the disinfection of drinking-water, exposure will be to both the chlorinated species and residual cyanuric acid. The concentrations will relate directly to the quantities added to achieve adequate disinfection. |
|---|
| TDI | 2.2 mg/kg body weight for anhydrous sodium dichloroisocyanurate and 1.54 mg/kg body weight for cyanuric acid, based on a NOEL of 154 mg/kg body weight per day (equivalent to 220 mg/kg body weight per day as anhydrous sodium dichloroisocyanurate) for urinary tract and cardiac lesions from a 2-year study of rats exposed to sodium cyanurate and using an uncertainty factor of 100 for interspecies and intraspecies variation |
|---|
| Limit of detection | 0.001 mg/l by GC with flame thermionic specific detection; 0.05 mg/l by reversed-phase LC with UV detection; 0.09 mg/l by GC with MS selective ion monitoring |
|---|
| Treatment performance | At very high chlorine doses (up to 10 mg/l), the sodium cyanurate concentration would be below 11 mg/l. In emergency situations, “topping up” might be done in an attempt to maintain a free chlorine residual, but this practice should be discouraged. In this case, it would be possible for the sodium cyanurate concentration to build up to undesirable levels. In such cases, it would be very desirable to monitor the concentration of sodium cyanurate. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 80% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The controlling factors are the level of free chlorine and the residue of cyanuric acid, particularly if there is topping up of chlorine in a static system under emergency conditions. The concentration of free chlorine should normally be such that it should not give rise to unacceptable tastes and should not normally exceed the guideline value of 5 mg/l for free chlorine. |
|---|
| Sodium dichloroisocyanurate used for disinfecting drinking-water should be of adequate purity so that there is no increase in any inorganic or organic contaminants in the drinking-water. The amounts of sodium dichloroisocyanurate used should be the lowest consistent with adequate disinfection, and the concentrations of cyanuric acid should be managed to be kept as low as is reasonably possible. |
| Assessment date | 2007 |
|---|
| Principal references |
FAO/WHO (2004)
Evaluation of certain food additives and contaminants
WHO (2008)
Sodium dichloroisocyanurate in drinking-water
|
|---|
Studies of the toxicity of sodium cyanurate are appropriate for assessing the safety of sodium dichloroisocyanurate, because any residues of intact sodium dichloroisocyanurate in drinking-water would be rapidly converted to cyanuric acid on contact with saliva. Both sodium dichloroisocyanurate and sodium cyanurate have low acute oral toxicity. Sodium cyanurate does not induce any genotoxic, carcinogenic or teratogenic effects. The NOEL from which the guideline value was derived was based on multiple lesions of the urinary tract (calculi and hyperplasia, bleeding and inflammation of the bladder epithelium, dilated and inflamed ureters and renal tubular nephrosis) and cardiac lesions (acute myocarditis, necrosis and vascular mineralization) in male rats exposed at the next higher dose.
Styrene
Styrene, which is used primarily for the production of plastics and resins, is found in trace amounts in surface water, drinking-water and food. In industrial areas, exposure via air can result in intake of a few hundred micrograms per day. Smoking may increase daily exposure by up to 10-fold.
View in own window
| Guideline value | 0.02 mg/l (20 µg/l) |
|---|
| Occurrence | Has been detected in drinking-water and surface water at concentrations below 1 µg/l |
|---|
| TDI | 7.7 µg/kg body weight, based on a NOAEL of 7.7 mg/kg body weight per day for decreased body weight observed in a 2-year drinking-water study in rats, and using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for the carcinogenicity and genotoxicity of the reactive intermediate styrene-7,8-oxide) |
|---|
| Limit of detection | 0.3 µg/l by GC with photoionization detection and confirmation by MS |
|---|
| Treatment performance | 0.02 mg/l may be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | May affect the acceptability of drinking-water at the guideline value |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Styrene in drinking-water |
|---|
Following oral or inhalation exposure, styrene is rapidly absorbed and widely distributed in the body, with a preference for lipid depots. It is metabolized to the active intermediate styrene-7,8-oxide, which is conjugated with glutathione or further metabolized. Metabolites are rapidly and almost completely excreted in urine. Styrene has a low acute toxicity. In short-term toxicity studies in rats, impairment of glutathione transferase activity and reduced glutathione concentrations were observed. In in vitro tests, styrene has been shown to be mutagenic in the presence of metabolic activation only. In in vitro as well as in vivo studies, chromosomal aberrations have been observed, mostly at high doses of styrene. The reactive intermediate styrene-7,8-oxide is a direct-acting mutagen. In long-term studies, orally administered styrene increased the incidence of lung tumours in mice at high dose levels but had no carcinogenic effect in rats. Styrene-7,8-oxide was carcinogenic in rats after oral administration. IARC has classified styrene in Group 2B (possibly carcinogenic to humans). The available data suggest that the carcinogenicity of styrene is due to overloading of the detoxification mechanism for styrene-7,8-oxide (e.g. glutathione depletion).
Sulfate
Sulfates occur naturally in numerous minerals and are used commercially, principally in the chemical industry. They are discharged into water in industrial wastes and through atmospheric deposition; however, the highest levels usually occur in ground-water and are from natural sources. In general, the average daily intake of sulfate from drinking-water, air and food is approximately 500 mg, food being the major source. However, in areas with drinking-water supplies containing high levels of sulfate, drinking-water may constitute the principal source of intake.
View in own window
| Reason for not establishing a guideline value | Not of health concern at levels found in drinking-water |
|---|
| Additional comments | May affect acceptability of drinking-water |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2004)
Sulfate in drinking-water |
|---|
The existing data do not identify a level of sulfate in drinking-water that is likely to cause adverse human health effects. The data from a liquid diet study with piglets and from tap water studies with human volunteers indicate a laxative effect at concentrations of 1000–1200 mg/l, but no increase in diarrhoea, dehydration or weight loss.
No health-based guideline is proposed for sulfate. However, because of the gastrointestinal effects resulting from ingestion of drinking-water containing high sulfate levels, it is recommended that health authorities be notified of sources of drinking-water that contain sulfate concentrations in excess of 500 mg/l. The presence of sulfate in drinking-water may also cause noticeable taste (see chapter 10) and may contribute to the corrosion of distribution systems.
2,4,5-T
The half-lives for degradation of chlorophenoxy herbicides, including 2,4,5-T (CAS No. 93-76-5), also known as 2,4,5-trichlorophenoxyacetic acid, in the environment are in the order of several days. Chlorophenoxy herbicides are not often found in food.
View in own window
| Guideline value | 0.009 mg/l (9 µg/l) |
|---|
| Occurrence | Chlorophenoxy herbicides not frequently found in drinking-water; when detected, concentrations usually no greater than a few micrograms per litre |
|---|
| TDI | 3 µg/kg body weight, based on a NOAEL of 3 mg/kg body weight for reduced body weight gain, increased liver and kidney weights and renal toxicity in a 2-year study in rats, with an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 to take into consideration the suggested association between 2,4,5-T and soft tissue sarcoma and non-Hodgkin lymphoma in epidemiological studies) |
|---|
| Limit of detection | 0.02 µg/l by GC with ECD |
|---|
| Treatment performance | 1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Chlorophenoxy herbicides (excluding 2,4-D and MCPA) in drinking-water |
|---|
Chlorophenoxy herbicides, as a group, have been classified in Group 2B (possibly carcinogenic to humans) by IARC. However, the available data from studies in exposed populations and experimental animals do not permit assessment of the carcinogenic potential to humans of any specific chlorophenoxy herbicide. Therefore, drinking-water guidelines for these compounds are based on a threshold approach for other toxic effects. The NOAEL for reproductive effects (reduced neonatal survival, decreased fertility, reduced relative liver weights and thymus weights in litters) of dioxin-free (< 0.03 µg/kg) 2,4,5-T in a three-generation reproduction study in rats is the same as the NOAEL for reduced body weight gain, increased liver and kidney weights and renal toxicity in a toxicity study in which rats were fed 2,4,5-T (practically free from dioxin contamination) in the diet for 2 years.
Terbuthylazine
Terbuthylazine (CAS No. 5915-41-3), or TBA, a herbicide that belongs to the chlorotriazine family, is used in both pre-emergence and post-emergence treatment of a variety of agricultural crops and in forestry. Degradation of TBA in natural water depends on the presence of sediments and biological activity.
View in own window
| Guideline value | 0.007 mg/l (7 µg/l) |
|---|
| Occurrence | Concentrations in water seldom exceed 0.2 µg/l, although higher concentrations have been observed. |
|---|
| TDI | 2.2 µg/kg body weight, based on a NOAEL of 0.22 mg/kg body weight for decreased body weight gain at the next higher dose in a 2-year toxicity/carcinogenicity study in rats, with an uncertainty factor of 100 (for interspecies and intraspecies variation) |
|---|
| Limit of detection | 0.1 µg/l by HPLC with UV detection |
|---|
| Treatment performance | 0.1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Assessment date | 1998 |
|---|
| Principal reference | WHO (2003)
Terbuthylazine in drinking-water |
|---|
There is no evidence that TBA is carcinogenic or mutagenic. In long-term dietary studies in rats, effects on red blood cell parameters in females, an increased incidence of non-neoplastic lesions in the liver, lung, thyroid and testis and a slight decrease in body weight gain were observed.
Tetrachloroethene
Tetrachloroethene (PCE) has been used primarily as a solvent in dry-cleaning industries, and to a lesser extent as a degreasing solvent. Since the 1980s, as a result of regulations in North America, Europe and elsewhere, its use has substantially decreased. PCE is widespread in the environment and is found in trace amounts in water, aquatic organisms, air, foodstuffs and human tissue. The most relevant routes of exposure are considered to be inhalation of contaminated air and ingestion of contaminated drinking-water, particularly from groundwater sources. Poor handling and improper disposal of PCE in landfills have been the main causes of water contamination. Higher levels of PCE are expected in groundwater than in surface water because of the lack of volatilization that occursfrom groundwater. However, drinking-water is not a major source of exposure, unless in the vicinity of a contaminated site.
View in own window
| Guideline value | 0.1 mg/l (100 µg/l) |
|---|
| Occurrence | Concentrations in drinking-water are generally below 10 µg/l, although much higher concentrations have been detected in well water (23 mg/l) and in contaminated groundwater (1.5 mg/l) |
|---|
| TDI | 16 µg/kg bw, based on a BMDL10 of 4.7 mg/kg bw per day for neurological effects (decreased colour vision) observed in an occupational study and applying an uncertainty factor of 300 (10 each for inter- and intra-species variability and 3 for extrapolation from an occupational study with intermittent exposure) |
|---|
| Limit of detection | 0.002–0.008 µg/l by GC with ECD after liquid–liquid extraction; 0.02–0.05 µg/l by purge-and-trap capillary GC with PD and ECD in series; 0.036 µg/l by volatile organic compound analysis with GC-MS; and 0.05–0.14 µg/l by purge-and-trap capillary GC-MS |
|---|
| Prevention and treatment |
Source control, by improving handling and disposal practices, should be the priority action since PCE can persist in waters where volatilization cannot occur.
Treatment of surface water sources is not needed because PCE volatilizes to the atmosphere. GAC, packed tower aeration and diffused aeration are effective central treatment technologies, although diffused aeration achieves lower removal efficiencies than packed tower aeration systems. Advanced oxidation processes may also be effective, but effectiveness depends on the physical and chemical properties of the water.
|
|---|
| Guideline value derivation |
|---|
| • allocation to water | 20% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The guideline value is considered protective against both cancer and noncancer effects. |
|---|
| In developing national standards, authorities may take into consideration the additional exposures through the dermal and inhalation routes from showering and bathing, especially in countries with low rates of ventilation in houses.Authorities may also consider overall exposure in developing national standards, noting the continued decline in human exposure to PCE by all probable exposure routes. |
| Requirements for monitoring PCE in drinking-water regulations and standards should be limited to groundwater sources, where a possibility of PCE contamination is indicated. Monitoring is not needed for surface water sources because PCE volatilizes to the atmosphere. |
| Assessment date | 2020 |
|---|
| Principal reference | WHO (2020)
Tetrachloroethene in drinking-water |
|---|
IARC has classified PCE in Group 2A (probably carcinogenic to humans). PCE has been reported to produce liver tumours in male and female mice following inhalation, and there is some evidence of mononuclear cell leukaemia in male and female rats and kidney tumours in male rats. However, absorption, metabolic pathways, excretion and pattern of effects are similar for inhalation and oral exposure. The weight of evidence for PCE suggests that a nonlinear, nonmutagenic mode of action is predominant for liver tumours, and this effect is considered the most relevant end-point for cancer risk assessment of PCE exposure. Additionally, noncancer effects resulting from inhalation exposure, including evidence of neurotoxicity, were observed in human occupational studies and in laboratory animal studies. The nervous system is the most sensitive organ for noncancer effects. To protect against both cancer and noncancer effects, comparative points of departure were identified using benchmark dose modelling and physiologically based pharmacokinetic modelling (to account for the first-pass effects and inhalation-to-ingestion extrapolation). The most sensitive human-relevant effects were determined to be reductions in colour vision, and changes in cognitive function and reaction time in exposed workers; neurological effects were therefore the basis for the TDI derivation.
Toluene
Most toluene (in the form of benzene–toluene–ethylbenzene–xylene mixtures) is used in the blending of petrol. It is also used as a solvent and as a raw material in chemical production. The main exposure is via air. Exposure is increased by smoking and in traffic.
View in own window
| Guideline value | 0.7 mg/l (700 µg/l) |
|---|
| Occurrence | Concentrations of a few micrograms per litre have been found in surface water, groundwater and drinking-water; point emissions can lead to higher concentrations in groundwater (up to 1 mg/l); it may also penetrate plastic pipes from contaminated soil |
|---|
| TDI | 223 µg/kg body weight, based on a LOAEL of 312 mg/kg body weight per day for marginal hepatotoxic effects observed in a 13-week gavage study in mice, adjusting for daily dosing and using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for the short duration of the study and use of a LOAEL instead of a NOAEL) |
|---|
| Limit of detection | 0.13 µg/l by GC with FID; 6 µg/l by GC-MS |
|---|
| Treatment performance | 0.001 mg/l should be achievable using air stripping |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The guideline value exceeds the lowest reported odour threshold for toluene in water. |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2003)
Toluene in drinking-water |
|---|
Toluene is absorbed completely from the gastrointestinal tract and rapidly distributed in the body, with a preference for adipose tissue. Toluene is rapidly metabolized and, following conjugation, excreted predominantly in urine. With occupational exposure to toluene by inhalation, impairment of the central nervous system and irritation of mucous membranes are observed. The acute oral toxicity is low. Toluene exerts embryotoxic and fetotoxic effects, but there is no clear evidence of teratogenic activity in laboratory animals and humans. In long-term inhalation studies in rats and mice, there is no evidence for carcinogenicity of toluene. Genotoxicity tests in vitro were negative, whereas in vivo assays showed conflicting results with respect to chromosomal aberrations. IARC has concluded that there is inadequate evidence for the carcinogenicity of toluene in both experimental animals and humans and classified it as Group 3 (not classifiable as to its carcinogenicity to humans).
Total dissolved solids
Total dissolved solids (TDS) comprise inorganic salts (principally calcium, magnesium, potassium, sodium, bicarbonates, chlorides and sulfates) and small amounts of organic matter that are dissolved in water. TDS in drinking-water originates from natural sources, sewage, urban runoff and industrial wastewater. Salts used for road de-icing in some countries may also contribute to the TDS content of drinking-water. Concentrations of TDS in water vary considerably in different geological regions owing to differences in the solubilities of minerals.
View in own window
| Reason for not establishing a guideline value | Not of health concern at levels found in drinking-water |
|---|
| Additional comments | May affect acceptability of drinking-water |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Total dissolved solids in drinking-water |
|---|
Reliable data on possible health effects associated with the ingestion of TDS in drinking-water are not available, and no health-based guideline value is proposed. However, the presence of high levels of TDS in drinking-water may be objectionable to consumers (see chapter 10).
Trichloroacetic acid
Chlorinated acetic acids are formed from organic material during water chlorination.
View in own window
| Guideline value | 0.2 mg/l (200 µg/l) |
|---|
| Occurrence | Detected in groundwater and surface water distribution systems in the USA at mean concentrations of 5.3 µg/l (up to a maximum of 80 µg/l) and 16 µg/l (up to a maximum of 174 µg/l), respectively; maximum concentration (200 µg/l) measured in chlorinated water in Australia |
|---|
| TDI | 32.5 µg/kg body weight, based on a NOAEL of 32.5 mg/kg body weight per day from a study in which decreased body weight, increased liver serum enzyme activity and liver histopathology were seen in rats exposed to trichloroacetate in drinking-water for 2 years, incorporating an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for database deficiencies, including the absence of a multigeneration reproductive study, the lack of a developmental study in a second species and the absence of full histopathological data in a second species) |
|---|
| Limit of detection | 1 µg/l by GC-MS or GC-ECD |
|---|
| Treatment performance | Concentrations may be reduced by installing or optimizing coagulation to remove precursors or by controlling the pH during chlorination. |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 20% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | A similar TDI for trichloroacetate was established by IPCS based on a NOAEL for hepatic toxicity in a long-term study in mice. |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2003)
Trichloroacetic acid in drinking-water |
|---|
Trichloroacetic acid has been shown to induce tumours in the liver of mice. It has given mixed results in in vitro assays for mutations and chromosomal aberrations and has been reported to cause chromosomal aberrations in in vivo studies. IARC has classified trichloroacetic acid in Group 3, not classifiable as to its carcinogenicity to humans. The weight of evidence indicates that trichloroacetic acid is not a genotoxic carcinogen.
Trichlorobenzenes (total)
Releases of trichlorobenzenes (TCBs) into the environment occur through their manufacture and use as industrial chemicals, chemical intermediates and solvents. TCBs are found in drinking-water, but rarely at levels above 1 µg/l. General population exposure will primarily result from air and food.
View in own window
| Reason for not establishing a guideline value | Occur in drinking-water at concentrations well below those of health concern, and health-based value would exceed lowest reported odour threshold |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2003)
Trichlorobenzenes in drinking-water |
|---|
The TCBs are of moderate acute toxicity. After short-term oral exposure, all three isomers show similar toxic effects, predominantly on the liver. Long-term toxicity and carcinogenicity studies via the oral route have not been carried out, but the data available suggest that all three isomers are non-genotoxic.
A health-based value of 20 µg/l can be calculated for total TCBs on the basis of a TDI of 7.7 µg/kg body weight, based on liver toxicity identified in a 13-week rat study, taking into consideration the short duration of the study. However, because TCBs occur at concentrations well below those of health concern, it is not considered necessary to derive a formal guideline value. It should be noted that the health-based value exceeds the lowest reported odour threshold in water.
1,1,1-Trichloroethane
1,1,1-Trichloroethane is widely used as a cleaning solvent for electrical equipment, as a solvent for adhesives, coatings and textile dyes and as a coolant and lubricant. It is found mainly in the atmosphere, although it is mobile in soils and readily migrates to groundwaters. 1,1,1-Trichloroethane has been found in only a small proportion of surface waters and groundwaters, usually at concentrations of less than 20 µg/l; higher concentrations (up to 150 µg/l) have been observed in a few instances. There appears to be increasing exposure to 1,1,1-trichloroethane from other sources.
View in own window
| Reason for not establishing a guideline value | Occur in drinking-water at concentrations well below those of health concern |
|---|
| Assessment date | 2003 |
|---|
| Principal reference | WHO (2003)
1,1,1-Trichloroethane in drinking-water |
|---|
1,1,1-Trichloroethane is rapidly absorbed from the lungs and gastrointestinal tract, but only small amounts—about 6% in humans and 3% in experimental animals—are metabolized. Exposure to high concentrations can lead to hepatic steatosis (fatty liver) in both humans and laboratory animals. In a well-conducted oral study in mice and rats, effects included reduced liver weight and changes in the kidney consistent with hyaline droplet neuropathy. IARC has placed 1,1,1-trichloroethane in Group 3. 1,1,1-Trichloroethane does not appear to be mutagenic.
A health-based value of 2 mg/l can be calculated for 1,1,1-trichloroethane on the basis of a TDI of 0.6 mg/kg body weight, based on changes in the kidney that were consistent with hyaline droplet nephropathy observed in a 13-week oral study in male rats, and taking into account the short duration of the study. However, because 1,1,1-trichloroethane occurs at concentrations well below those of health concern, it is not considered necessary to derive a formal guideline value.
Trichloroethene
Trichloroethene (TCE) is used primarily in metal degreasing. However, its use has been substantially declining since the 1990s as a result of increased environmental regulations on TCE emissions. It is emitted mainly to the atmosphere, but it may also be introduced into groundwater and, to a lesser extent, surface water in industrial effluents. Poor handling and improper disposal of TCE in landfills have been the main causes of water contamination. Higher levels of TCE are expected in groundwater than in surface water because of the lack of volatilization that occurs from ground-water. Therefore, the most relevant routes of exposure are considered inhalation of contaminated air and ingestion of contaminated drinking-water, particularly from groundwater sources.
View in own window
| Guideline value | 0.008 mg/l (8 µg/l) |
|---|
| Occurrence | Typically present at low or undetectable concentrations in surface water (≤1 μg/l) due to high volatility and continued decline in TCE production. Concentrations may be higher (usually below 100 µg/l) in groundwater systems where volatilization and biodegradation are limited. |
|---|
| TDI | 0.5 µg/kg bw, based on the TDI values derived from three key studies showing decreased thymus weight in female mice, increased incidence of developmental immunotoxicity in mice and increased incidence of fetal cardiac malformations in rats. The narrow range of TDIs among the three key studies was further supported by two other studies in rats evidencing renal effects. Where applicable, uncertainty factors were applied to the points of departure from each of the three studies to account for interspecies differences, intraspecies variation and use of a LOAEL instead of a NOAEL. |
|---|
| Limit of detection | 0.01 µg/l by GC with ECD after liquid–liquid extraction; 0.01–3.0 µg/l by purge-and-trap capillary GC with PD or with PD and ECD in series; and 0.5 µg/l by purge-and-trap capillary GC-MS |
|---|
| Prevention and treatment |
Source control is by improved handling and disposal practices.
Treatment of surface water sources is not needed because TCE volatilizes to the atmosphere. For groundwater sources, aeration (packed tower aeration and air stripping) and GAC are effective central treatment techonologies. Ozone and advanced oxidation processes may also be effective.
|
|---|
| Guideline value derivation |
|---|
| • allocation to water | 50% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The guideline value is considered protective against both cancer and noncancer effects. |
|---|
| In developing national standards, authorities may take into consideration the additional exposures through the dermal and inhalation routes from showering and bathing, especially in countries with low rates of ventilation in houses. |
| Requirements for monitoring TCE in drinking-water regulations and standards should be limited to groundwater sources where a possibility of TCE contamination is indicated. Monitoring is not needed for surface water sources because TCE volatilizes to the atmosphere. |
| Assessment date | 2020 |
|---|
| Principal reference | WHO (2020)
Trichloroethene in drinking-water |
|---|
Available human and animal data after repeated exposure to TCE identify the kidney, liver, immune system, male reproductive system and developing fetus as potential targets of TCE toxicity and/or carcinogenicity. IARC has classified TCE as carcinogenic to humans (Group 1), concluding that sufficient epidemiological data are available for an association between exposure to TCE and human kidney cancer. Associations reported for liver cancer and non-Hodgkin lymphoma are characterized as limited, and evidence for other tumours is classified as inadequate. Multiple points of departure from different studies, rather than a single key study, were included in the derivation of the TDI. The overall TDI is based on three key studies with TDIs ranging from 0.3 to 0.6 µg/kg bw per day, and supported by other two studies in rats. Critical effects include increased incidence of heart malformations in rats (TDI of 0.6 µg/kg bw per day), decreased thymus weights in mice (TDI of 0.6 µg/kg bw per day) and developmental immunotoxicity (TDI of 0.37 µg/kg bw per day). The further supporting data in the database include studies reporting toxic nephropathy in rats (TDI of 0.3 µg/kg bw per day) and increased kidney weight in rats (TDI of 0.8 µg/kg bw per day by using route-to-route extrapolation from the inhalation study). Whenever possible, benchmark dose modelling and physiologically based pharmacokinetic modelling (to account for first-pass effects and inhalation-to-ingestion extrapolation) were applied.
Trifluralin
Trifluralin (CAS No. 1582-09-8) is a pre-emergence herbicide used in a number of crops. It has low water solubility and a high affinity for soil. However, biodegradation and photodegradation processes may give rise to polar metabolites that may contaminate drinking water sources. Although this compound is used in many countries, relatively few data are available concerning contamination of drinking water.
View in own window
| Guideline value | 0.02 mg/l (20 µg/l) |
|---|
| Occurrence | Not detected in the small number of drinking-water samples analysed; has been detected in surface water at concentrations above 0.5 µg/l and rarely in groundwater |
|---|
| TDI | 7.5 µg/kg body weight, based on a NOAEL of 0.75 mg/kg body weight for mild hepatic effects in a 1year feeding study in dogs, with an uncertainty factor of 100 (for interspecies and intraspecies variation) |
|---|
| Limit of detection | 0.05 µg/l by GC with nitrogen–phosphorus detection |
|---|
| Treatment performance | 1 µg/l should be achievable using GAC |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Authorities should note that some impure technical grades of trifluralin could contain potent carcinogenic compounds and therefore should not be used. |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Trifluralin in drinking-water |
|---|
Trifluralin of high purity does not possess mutagenic properties. Technical trifluralin of low purity may contain nitroso contaminants and has been found to be mutagenic. No evidence of carcinogenicity was demonstrated in a number of long-term toxicity/carcinogenicity studies with pure (99%) test material. IARC has assigned technical-grade trifluralin to Group 3 (not classifiable as to its carcinogenicity to humans).
Trihalomethanes (bromoform, bromodichloromethane, chloroform, dibromochloromethane)
THMs are formed in drinking-water primarily as a result of chlorination of organic matter present naturally in raw water supplies. The rate and degree of THM formation increase as a function of the chlorine and humic acid concentration, temperature, pH and bromide ion concentration. Chloroform is the most common THM and the principal disinfection by-product in chlorinated drinking-water. In the presence of bromides, brominated THMs are formed preferentially, and chloroform concentrations decrease proportionally. It is assumed that most THMs present in water are ultimately transferred to air as a result of their volatility. For chloroform, for example, individuals may be exposed during showering to elevated concentrations from chlorinated tap water. For the volatile THMs, approximately equal contributions to total exposure come from four areas: ingestion of drinking-water, inhalation of indoor air largely due to volatilization from drinking-water, inhalation and dermal exposure during showering or bathing and ingestion of food, with all but food exposure arising primarily from drinking-water. Indoor air exposure to the volatile THMs is particularly important in countries with low rates of ventilation in houses and high rates of showering and bathing.
View in own window
| Guideline values | Chloroform: 0.3 mg/l (300 µg/l) |
|---|
| Bromoform: 0.1 mg/l (100 µg/l) |
| Dibromochloromethane (DBCM): 0.1 mg/l (100 µg/l) |
| Bromodichloromethane (BDCM): 0.06 mg/l (60 µg/l) |
| Occurrence | THMs are not expected to be found in raw water (unless near a pollution source), but are usually present in finished or chlorinated water; concentrations are generally below 100 µg/l; in most circumstances, chloroform is the dominant compound |
|---|
| TDIs | Chloroform: 15 µg/kg body weight, derived from the lower 95% confidence limit for 5% incidence of hepatic cysts, generated by physiologically based pharmacokinetic modelling, in dogs that ingested chloroform in toothpaste for 7.5 years, using an uncertainty factor of 25 (10 for intraspecies differences in toxicokinetics and toxicodynamics and 2.5 for differences in interspecies toxicodynamics) |
|---|
| Bromoform: 17.9 µg/kg body weight, based on the absence of histopathological lesions in the liver in a well-conducted and well-documented 90-day study in rats, using an uncertainty factor of 1000 (100 for intraspecies and interspecies variation and 10 for possible carcinogenicity and short duration of exposure) |
| DBCM: 21.4 µg/kg body weight, based on the absence of histopathological effects in the liver in a well-conducted and well-documented 90-day study in rats, using an uncertainty factor of 1000 (100 for intra-species and interspecies variation and 10 for the short duration of the study); an additional uncertainty factor for potential carcinogenicity was not applied because of the questions regarding mouse liver tumours from corn oil vehicles and inconclusive evidence of genotoxicity |
| Basis of guideline value derivation | BDCM: Application of the linearized multistage model for the observed increases in incidence of kidney tumours in male mice observed in an NTP bioassay |
|---|
| Limit of detection | 0.1–0.2 µg/l (method detection limits) by purge-and-trap and liquid–liquid extraction and direct aqueous injection in combination with a chromatographic system; 0.1 µg/l by GC-ECD; 2.2 µg/l by GC-MS |
|---|
| Treatment performance | Concentrations can be reduced by changes to disinfection practice (e.g. reducing organic THM precursors) or using air stripping. |
|---|
| Guideline value derivation |
|---|
| • allocation to water |
20% of TDI for bromoform and DBCM
75% of TDI for chloroform
|
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments on THMs |
For authorities wishing to establish a total THM standard to account for additive toxicity, the following fractionation approach could be taken:
where C = concentration and GV = guideline value.
Authorities wishing to use a guideline value for total THMs should not simply add up the guideline values for the individual compounds in order to arrive at a standard.
|
|---|
| It is emphasized that adequate disinfection should never be compromised in attempting to meet guidelines for THMs. Nevertheless, in view of the potential link between adverse reproductive outcomes and THMs, particularly brominated THMs, it is recommended that THM levels in drinking-water be kept as low as practicable. |
| Additional comments on chloroform | In countries with low rates of ventilation in houses and high rates of showering and bathing, the guideline value could be lowered to account for the additional exposures from inhalation of indoor air largely due to volatilization from drinking-water and inhalation and dermal exposure during showering or bathing. |
|---|
| The guideline value is based on the same study as in the third edition; the increase in value is primarily a result of an increase in the allocation of exposure in drinking-water from 50% to 75% to account for the fact that chloroform is used less now than it was in 1993 when the original guideline was developed. |
| Additional comments on BDCM |
Although a health-based value of 21 µg/l is derived, the previous guideline value of 60 µg/l has been retained for two reasons: 1) both calculations were based on the same study, the only differences being the model and model assumptions used to derive the guideline value; there is therefore no scientific basis on which to justify a change in the guideline value; and 2) BDCM concentrations below 50 µg/l may be difficult to achieve using currently available technology without compromising the effectiveness of disinfection.
As with chloroform, countries with low rates of ventilation and high rates of showering and bathing may wish to lower the guideline value to account for dermal and inhalation exposures, although, as noted above, concentrations below 50 µg/l may be difficult to achieve using currently available technology without compromising the effectiveness of disinfection.
|
|---|
| As BDCM was negative for carcinogenicity in a recent NTP bioassay in which it was dosed in drinking-water, exceedances of the guideline value are not likely to result in an increased risk of cancer. |
| Assessment date | 2004 |
|---|
| Principal references | IPCS (2000)
Disinfectants and disinfectant by-products IPCS (2004)
Chloroform USNTP (1987). Toxicology and carcinogenesis studies of
bromodichloromethane in F344/N rats and B6C3F1 mice (gavage studies) WHO (2005)
Trihalomethanes in drinking-water
|
|---|
Chloroform
The weight of evidence for genotoxicity of chloroform is considered negative. IARC has classified chloroform as possibly carcinogenic to humans (Group 2B) based on limited evidence of carcinogenicity in humans but sufficient evidence of carcinogenicity in experimental animals. The weight of evidence for liver tumours in mice is consistent with a threshold mechanism of induction. Although it is plausible that kidney tumours in rats may similarly be associated with a threshold mechanism, there are some limitations of the database in this regard. The most universally observed toxic effect of chloroform is damage to the centrilobular region of the liver. The severity of these effects per unit dose administered depends on the species, vehicle and method by which the chloroform is administered.
Bromoform
In an NTP bioassay, bromoform induced a small increase in relatively rare tumours of the large intestine in rats of both sexes but did not induce tumours in mice. Data from a variety of assays on the genotoxicity of bromoform are equivocal. IARC has classified bromoform in Group 3 (not classifiable as to its carcinogenicity to humans).
Dibromochloromethane
In an NTP bioassay, DBCM induced hepatic tumours in female mice and possibly in male mice but not in rats. The genotoxicity of DBCM has been studied in a number of assays, but the available data are considered inconclusive. IARC has classified DBCM in Group 3 (not classifiable as to its carcinogenicity to humans).
Bromodichloromethane
IARC has classified BDCM in Group 2B (possibly carcinogenic to humans). BDCM gave both positive and negative results in a variety of in vitro and in vivo genotoxicity assays. In an NTP bioassay, BDCM induced renal adenomas and adenocarcinomas in both sexes of rats and male mice, rare tumours of the large intestine (adenomatous polyps and adenocarcinomas) in both sexes of rats and hepatocellular adenomas and adenocarcinomas in female mice. However, BDCM was negative for carcinogenicity in a recent NTP bioassay in which it was dosed in drinking-water. Exposure to BDCM has also been linked to a possible increase in reproductive effects (increased risk for spontaneous abortion or stillbirth).
Uranium
Uranium is widespread in nature, occurring in granites and various other mineral deposits. It is used mainly as fuel in nuclear power stations. Uranium is present in the environment as a result of leaching from natural deposits, release in mill tailings, emissions from the nuclear industry, the combustion of coal and other fuels and the use of phosphate fertilizers that contain uranium. Intake of uranium through air is low, and it appears that intake through food is between 1 and 4 µg/day. Intake through drinking-water is normally extremely low; however, in circumstances in which uranium is present in a drinking-water source, the majority of intake can be through drinking-water.
View in own window
| Provisional guideline value | 0.03 mg/l (30 µg/l) |
|---|
| The guideline value is designated as provisional because of scientific uncertainties surrounding uranium toxicity. |
| Occurrence | Levels in drinking-water are generally less than 1 µg/l, although concentrations as high as 700 µg/l have been measured in private supplies. |
|---|
| TDI | 60 µg, derived from the lower 95% confidence limit on the 95th percentile uranium exposure distribution in a study from Finland, using an uncertainty factor of 10 for intraspecies variation |
|---|
| Limit of detection | 0.01 µg/l by ICP-MS; 0.1 µg/l by solid fluorimetry with either laser excitation or UV light; 0.2 µg/l by ICP using adsorption with chelating resin |
|---|
| Treatment performance | 1 µg/l should be achievable using conventional treatment (e.g. coagulation or ion exchange) |
|---|
| Guideline value derivation |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | Where supplies exceed 30 µg/l, it is important that precipitate action be avoided. Consideration should first be given to exposure from all sources and the availability of alternative safe sources. |
|---|
| Only chemical, not radiological, aspects of uranium toxicity have been addressed here. |
| Assessment date | 2003, revised in 2011 |
|---|
| Principal reference | WHO (2012)
Uranium in drinking-water |
|---|
There are insufficient data regarding the carcinogenicity of uranium in humans and experimental animals. Nephritis is the primary chemically induced effect of uranium in humans. Little information is available on the chronic health effects of exposure to environmental uranium in humans. A number of epidemiological studies of populations exposed to uranium in drinking-water have shown a correlation with alkaline phosphatase and β-microglobulin in urine along with modest alterations in proximal tubular function. However, the actual measurements were still within the normal physiological range, and these findings are not consistent between studies.
No clear no-effect concentration has emerged from the human studies to date. This is not surprising, as most of the study populations are quite small, and there is substantial normal variation in the measured parameters in the human population. However, the overall indications are that there is no clear evidence of effects below an exposure concentration of 30 µg/l. In fact, the evidence for effects on the kidney, which appears to be the most sensitive organ, is equivocal until much higher exposure concentrations.
The provisional guideline value of 30 μg/l, which is derived from new epidemio-logical studies on populations exposed to high uranium concentrations, replaces the previous value derived from experimental animal studies and designated as provisional on the basis of uncertainties regarding the toxicology and epidemiology of uranium as well as difficulties concerning its technical achievability in smaller supplies. It is noted that studies on human populations, when available and of good quality, are the preferred source of health-related information to be used in deriving guideline values.
Vinyl chloride
Vinyl chloride is used primarily for the production of PVC. Owing to its high volatility, vinyl chloride has rarely been detected in surface waters, except in contaminated areas. Unplasticized PVC is increasingly being used in some countries for water mains supplies. Migration of vinyl chloride monomer from unplasticized PVC is a possible source of vinyl chloride in drinking-water. It appears that inhalation is the most important route of vinyl chloride intake, although drinking-water may contribute a substantial portion of daily intake where PVC piping with a high residual content of vinyl chloride monomer is used in the distribution network. Vinyl chloride has been reported in groundwater as a degradation product of the chlorinated solvents trichloroethene and tetrachloroethene.
View in own window
| Guideline value | 0.0003 mg/l (0.3 µg/l) |
|---|
| Occurrence | Rarely detected in surface waters, the concentrations measured generally not exceeding 10 µg/l; much higher concentrations found in groundwater and well water in contaminated areas; concentrations up to 10 µg/l detected in drinking-water |
|---|
| Basis of guideline value derivation | Application of a linear extrapolation by drawing a straight line between the dose, determined using a pharmocokinetic model, resulting in tumours in 10% of animals in rat bioassays involving oral exposure and the origin (zero dose), determining the value associated with the upper-bound risk of 10−5 and assuming a doubling of the risk for exposure from birth |
|---|
| Limit of detection | 0.01 µg/l by GC-ECD or GC-FID with MS for confirmation |
|---|
| Treatment performance | 0.001 mg/l should be achievable using air stripping |
|---|
| Additional comments | The results of the linear extrapolation are nearly identical to those derived using the linearized multistage model. |
|---|
| As vinyl chloride is a known human carcinogen, exposure to this compound should be avoided as far as practicable, and levels should be kept as low as technically feasible. |
| Vinyl chloride is primarily of concern as a potential contaminant from some grades of PVC pipe and is best controlled by specification of material quality. |
| Assessment date | 2003 |
|---|
| Principal references |
IPCS (1999)
Vinyl chloride
WHO (2004)
Vinyl chloride in drinking-water
|
|---|
There is sufficient evidence of the carcinogenicity of vinyl chloride in humans from industrial populations exposed to high concentrations via the inhalation route, and IARC has classified vinyl chloride in Group 1 (carcinogenic to humans). Studies of workers employed in the vinyl chloride industry showed a marked exposure–response for all liver cancers, angiosarcomas and hepatocellular carcinoma, but no strong relationship between cumulative vinyl chloride exposure and other cancers. Experimental animal data show vinyl chloride to be a multisite carcinogen. When administered orally or by inhalation to mice, rats and hamsters, it produced tumours in the mammary gland, lungs, Zymbal gland and skin, as well as angiosarcomas of the liver and other sites. Evidence indicates that vinyl chloride metabolites are genotoxic, interacting directly with DNA. DNA adducts formed by the reaction of DNA with a vinyl chloride metabolite have also been identified. Occupational exposure has resulted in chromosomal aberrations, micronuclei and sister chromatid exchanges; response levels were correlated with exposure levels.
Xylenes
Xylenes are used in blending petrol, as a solvent and as a chemical intermediate. They are released to the environment largely via air. Exposure to xylenes is mainly from air, and exposure is increased by smoking.
View in own window
| Guideline value | 0.5 mg/l (500 µg/l) |
|---|
| Occurrence | Concentrations of up to 8 µg/l have been reported in surface water, groundwater and drinking-water; levels of a few milligrams per litre were found in groundwater polluted by point emissions; xylenes can also penetrate plastic pipe from contaminated soil |
|---|
| TDI | 179 µg/kg body weight, based on a NOAEL of 250 mg/kg body weight per day for decreased body weight in a 103-week gavage study in rats, adjusting for daily dosing and using an uncertainty factor of 1000 (100 for interspecies and intraspecies variation and 10 for the limited toxicological end-points) |
|---|
| Limit of detection | 0.1 µg/l by GC-MS; 1 µg/l by GC-FID |
|---|
| Treatment performance | 0.005 mg/l should be achievable using GAC or air stripping |
|---|
| Guideline value derivation |
|---|
| • allocation to water | 10% of TDI |
|---|
| • weight | 60 kg adult |
|---|
| • consumption | 2 litres/day |
|---|
| Additional comments | The guideline value exceeds the lowest reported odour threshold for xylenes in drinking-water. |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Xylenes in drinking-water |
|---|
Xylenes are rapidly absorbed by inhalation. Data on oral exposure are lacking. Xylenes are rapidly distributed in the body, predominantly in adipose tissue. They are almost completely metabolized and excreted in urine. The acute oral toxicity of xylenes is low. No convincing evidence for teratogenicity has been found. Long-term carcinogenicity studies have shown no evidence for carcinogenicity. In vitro as well as in vivo mutagenicity tests have proved negative.
Zinc
Zinc is an essential trace element found in virtually all food and potable water in the form of salts or organic complexes. The diet is normally the principal source of zinc. Although levels of zinc in surface water and groundwater normally do not exceed 0.01 and 0.05 mg/l, respectively, concentrations in tap water can be much higher as a result of dissolution of zinc from pipes.
View in own window
| Reason for not establishing a guideline value | Not of health concern at levels found in drinking-water |
|---|
| Additional comments | May affect acceptability of drinking-water |
|---|
| Assessment date | 1993 |
|---|
| Principal reference | WHO (2003)
Zinc in drinking-water |
|---|
In 1982, JECFA proposed a PMTDI for zinc of 1 mg/kg body weight. The daily requirement for adult men is 15–20 mg/day. It was considered that, taking into account recent studies on humans, the derivation of a formal guideline value is not required at this time. However, drinking-water containing zinc at levels above 3 mg/l may not be acceptable to consumers (see chapter 10).