1. Anthrax and the microbiology laboratory; operational safety
With some country-to-country variation in safety level definitions and requirements, recommendations for the manipulation of the causative agent of anthrax, Bacillus anthracis, generally are that BSL (biosafety level) 2 practices, containment equipment and facilities are appropriate for diagnostic tests, but BSL3 standards should be used when the work involves:
- producing quantities of the organism;
- activities with high potential for aerosol production.
and possibly also:
- activities with antibiotic-resistant strains.
In the case of Member States with limited resources and unable to operate at BSL3, it is pertinent to remember that B. anthracis is not highly infectious, and that humans are moderately resistant (see section 4.2.1). For diagnostic test purposes, therefore, good laboratory practice (Table 12) at all times is the important factor in carrying out the necessary tests safely. Large numbers of the organism should not be generated in uncontained laboratory situations, and manipulation of liquid cultures or suspensions should be kept to a minimum. It may be appropriate to distinguish between fully virulent strains and those lacking virulence factors, such as vaccine strains.
TABLE 12
Criteria, equipment and materials for laboratory diagnosis of anthrax.
Only a few confirmatory tests require liquid suspensions, e.g. preparing smears, testing for capsule production in blood and suspension of the organism for PCR (polymerase chain reaction). Small volumes (< 2.5 ml) are needed for these tests.
Work for further tests, such as bacterial counts, sterility tests, etc. involving liquid cultures should be done in biological safety cabinets in laboratories meeting as nearly as is possible the criteria for basic BSL3, or at least BSL2, laboratories. These criteria are readily obtained on the Internet.
2. Principal features of good laboratory practice
- Use and storage of personal protective equipment (PPE):
- Individuals should wear gowns or laboratory coats with elastic cuffing, and disposable gloves.
- Gowns/coats should be hung in a specified place near the entry to the laboratory and should be cleaned or disposed of at suitable intervals or when defective.
- PPE should be removed on leaving the work area and should not be worn outside the laboratory.
- The laboratory door should be kept closed.
- Appropriate disinfectant (usually hypochlorite solution, 10 000 ppm – see Annex 3, section 4.1) should be prepared freshly on a regular basis:
- this should be available, ready for immediate use in the event of a spillage;
- benches should be wiped down with the disinfectant after work is completed.
- Appropriate containers (generally screw-capped, non-breakable) should be used for specimens, cultures, etc., and proper carriers or secondary containers should be used for moving cultures around the laboratory.
- Eating, chewing, drinking, taking medication, smoking, applying cosmetics and mouth pipetting are strictly prohibited in the laboratory.
- Storage of contaminated materials should be done safely:
- “Tools” (pipettes, tips, loops, spreaders, etc.) should be housed safely after use, awaiting autoclaving (e.g. in strong autoclavable bags) or fumigation, or they should be fully immersed in jars of disinfectant (10% bleach or formalin).
- Contaminated items and materials awaiting reuse or disposal should be housed in strong leak-proof containers, preferably within autoclavable bags. Spillage within these should be avoided.
- Infectious disposable waste (see 6 above) should be autoclaved, preferably followed by incineration also. Reusable articles should be autoclaved, fumigated or otherwise sterilized before cleaning.
- Procedures should be performed so as to minimize production of potentially contaminated aerosols or dusts.
- Hands should be thoroughly washed with soap and water before leaving the facility, using disposable towels or an air drier after washing. (Ideally a hand-washing basin should be sited by the laboratory door, adjacent to the hooks for laboratory gowns/coats.)
- An accident and incident emergency plan should be in place.
- Laboratory workers should receive initial and regular revision training on the above.
3. General bacteriology of anthrax
The basic bacteriology of B. anthracis and its identification characteristics have been described in chapter 6. Further practical information is given here. B. anthracis is a non-fastidious, facultatively anaerobic bacterium organism which grows readily on simple laboratory media. The optimum temperature for growth is 35–37 °C.
3.1. Nutrient agar
After overnight incubation at 35–37 °C colonies are large, 2–3 mm in diameter, irregular, raised, dull, opaque and greyish-white with “frosted glass” (ground glass) appearance. Occasionally the colonies may have fringed edges or put out curled protrusions (tailing). This is the so-called “Medusa head appearance” but is not encountered as frequently as textbooks often suggest, and varies from batch-to-batch of media. The colony is notably tacky in consistency.
3.2. Blood agar
After overnight incubation at 35–37 °C on horse or sheep blood agar (BA), colonies of freshly isolated B. anthracis are white, or grey-white and non-haemolytic, 2–4 mm in diameter, again with a slightly moist, matt appearance. Fringed edges or tailing is sometimes seen as with nutrient agar (Fig. 8B).
3.3. Spores
Spores develop at the end of the log phase of multiplication. For diagnostic purposes, they can generally be visualized in smears of standard laboratory agar plate cultures (e.g. blood agar or nutrient agar) after 20–24 hours of incubation at 35 °C to 37 °C. The spores are central/subterminal, ellipsoidal and do not swell the vegetative cell (Fig. 8C). Strain-to-strain differences occur (section 6.3.1.1). In Gram-stained preparations, the developing spores appear as unstained areas within the cell. With malachite green/safranin (or malachite green/basic fuchsin) staining, the spores are stained green and the vegetative forms are pink (Fig. 8C). In the Ziehl-Neelsen staining, spores are pink and the vegetative forms are blue. When fully mature (dormant), the spores can also be seen as refractile egg-shaped bodies under phase contrast. (See also sections 6.3.1.1 and this annex, sections 9.3.4 & 9.3.5.)
3.4. Capsules
Capsules are not formed during normal aerobic in vitro culture. They can be induced either by growth in bicarbonate agar containing serum under a 5%–20% carbon dioxide atmosphere, or in defibrinated blood or in serum (defibrinated horse blood seems to work best) (see also sections 6.3.1.6 and this annex, section 10.7.2).
3.5. Broth cultures
Growth is frequently very floccular, especially in static cultures, due to the tendency of B. anthracis to form long chains in vitro (section 6.3.1.1). Being non-motile, the strands settle as a deposit which comes up as silky strands on shaking the broth gently.
3.6. Selective agars – PLET and TSPBA
Selective media are needed for the isolation of B. anthracis from clinical materials or environmental samples heavily contaminated with other bacteria. Bowen (1999) concluded that the best selective system was the polymyxin, lysozyme, EDTA and thallous acetate (PLET) agar of Knisely (1966) (Fig. 14). PLET agar was also chosen as the most selective and sensitive (3–5 spores per gram of soil) detection medium for all the work leading up to and following decontamination of Gruinard Island (Manchee et al., 1981, 1983). Dragon & Rennie (2001) recorded that, compared with blood agar, as few as 33% of the viable anthrax spores present in a sample germinated and outgrew, but Bowen (1999) and Samaan & Turnbull (unpublished results) found that losses on PLET were normally nil. The single exception was strain LSU 62, a 1962 bovine isolate from Poland, which, uniquely, did not grow on PLET (Turnbull et al., 2004a). Whether this is a laboratory adaptation phenomenon is not known.
While it would seem that PLET agar, prepared well (Annex 2), is an excellent selective isolation medium for B. anthracis, it does generally require at least 36 hours of incubation to read. Its other disadvantage lies in the ingredient, thallous acetate, which is highly toxic and environmentally unfriendly in terms of disposal.
After incubation at 37 °C for 36–48 hours, the colonies of B. anthracis are 2–3 mm, roughly circular, creamy-white with ground-glass texture. Colonies are usually smaller in size on this medium compared to those on nutrient or blood agar, and lack the tackiness. Tailing edges are not seen.
Trimethoprim-sulfamethoxazole agar medium2 has been recommended by some. Polymyxin at the same concentration as used in PLET may give extra selective advantage. This medium is referred to here as TSPBA; instructions for its formulation are given in Annex 2. B. anthracis colonies are recognizable earlier on TSPBA than PLET and at the same rate as conventional blood agar; being a blood agar, it retains the value of showing up haemolysis in the case of any haemolytic species that break through.
B. anthracis growth and colony morphology on TSPBA are indistinguishable from those on BA (sections 6.3.1, and this annex, section 3.2; Fig. 8B).
3.7. Bicarbonate agar
Colonies of fully virulent isolates are mucoid in nature on this medium when incubated overnight under CO2 due to capsule formation. Vaccine strains lacking the capsule genes, such as the Sterne strain, are rough (see this annex, section 10.7.2.2; Fig. 8D). The formula for bicarbonate agar is given in Annex 2.
4. Special features
4.1. Susceptibility to penicillin G
Fresh isolates of B. anthracis from cases of anthrax are almost always susceptible to penicillin (see section 7.1.2). In its simplest form, this involves spreading a portion of a nutrient or blood agar plate with the culture under test, and placing a 10U penicillin disc at some point within the area of spread. The zone of susceptibility will be visible after overnight incubation at 35–37 °C (Fig. 8F; see also annex, this section 10.7.1).
4.2. Susceptibility to the diagnostic (“gamma”) bacteriophage
The diagnostic (“gamma”) phage has the ability to lyse B. anthracis grown aerobically on blood or other nutrient agar and rarely lyses any other Bacillus species. Phage-resistant isolates are encountered, but this is rare (see section 6.3.1.5). There are a variety of ways this test can be done, but the simplest is to pick the suspect colony to a segment of a blood agar plate with a 1 µl inoculating loop as shown in Fig. 8F and place a 15 µl drop of phage suspension in the centre of the area over which the loop has been spread. The plate is incubated overnight at 35–37 °C (see also this annex, section 10.8.1).
4.3. Animal pathogenicity test
Definitive identity of a suspect B. anthracis isolate used to be done by inoculating the organism into a mouse or a guinea-pig and confirming the cause of death by smear or isolation. However, for ethical reasons animal inoculations are only done now under exceptional circumstances (see also this annex, section 12).
5. Case definition based on laboratory findings
For the purposes of investigation and control activities, the diagnostic definitions in Table 12 are proposed for anthrax. This is based on the model outlined in the Manual for laboratory diagnosis of anthrax (WHO, 2003) in which laboratories in any country can be broadly subdivided on the basis of their resources and capabilities into: (i) peripheral (district), able to receive clinical samples and carry out basic procedures by which to establish initial “suspect” diagnosis of anthrax so that immediate control measures can be instituted; (ii) intermediate (regional/provincial), which may be equipped to biosafety level 2; and (iii) central (reference) able to reconfirm identifications and perform further tests.
6. Sample processing and containment
6.1. Type of specimen
The approach taken will depend on the type of specimen being examined which, for the purposes of examination, will fall broadly into: (i) fresh specimens from untreated animals or humans; (ii) specimens from treated animals or humans; (iii) specimens from old and decomposed animal carcasses or from animal products; or (iv) environmental specimens, including those from suspected deliberate release events.
6.2. Clinical specimens and materials
Specimens from lesions or from freshly dead humans or animals may be handled at hazard levels lower than level 3 (see this annex, section 2) with the following safety precautions:
- use of adequate protective clothing (gloves, gowns with tight wrists and ties at the back). If the samples are not being processed in a safety cabinet, protective eye-shields and good-quality face masks may be advisable to protect the operator from other (non-anthrax) infectious agents that might be present;
- availability of high-quality, properly positioned facilities for hand-washing;
- careful dressing of skin abrasions.
Old dried-up specimens, such as old hides, that are liable to give off dust during processing, should be handled in a biosafety cabinet, preferably class 3.
6.3. Environmental and suspect deliberate release samples
Environmental samples from sites suspected of having been contaminated naturally (e.g. carcass sites) are best handled in a biological safety cabinet. Samples under suspicion of being artificially contaminated must be handled in a biosafety cabinet, again, preferably a class 3 cabinet. Suspect powders should strictly be processed in a well-constructed class 3 safety cabinet. A deliberately contaminated environmental sample is potentially very dangerous, and the processing of suspect environmental samples should be restricted to a proper hazard-level 3 laboratory with the correct facilities, most likely in the central/reference laboratory.
7. Specimen collection
7.1. Equipment and materials for specimen collection
7.1.1. All-purpose kit
In the case of human specimens (Table 13), these must be collected by the attendant medical professionals.
TABLE 13
Guidelines on appropriate specimens from humans suspected of being infected with B. anthracis.
The following list is for guidance in relation to specimen collection:
- leak-proof specimen containers, wide-mouth in the case of environmental samples;
- secondary containers for “double-bagging”;
- secure carrying containers (e.g. good-quality cool box, metal box, plastic mailing pots, etc.);
- sterile swabs, forceps, scissors, syringes (1 ml) and needles (approx. 19 gauge), spatulas or spoons;
- sterile water and/or saline;
- microscope slides and slide carriers;
- culture plates and inoculating loops (if appropriate to make primary culture at the site);
- “sharps” disposal containers;
- labels and markers or pens;
- adhesive tape;
- autoclavable discard bags for disposables;
- autoclavable discard bags for tools, clothing, boots, etc.;
- tock hypochlorite solution and water to make up working solution (5000–10 000 ppm) and hand-washing facilities (e.g. large water container and basin);
- paper towels.
7.1.2. Personal protective equipment
7.1.2.1. Specimen collection from a human or animal situated indoors
- Laboratory coat, gown or overall, as appropriate to the situation, should be worn. Sleeves should be long with elastic cuff.
- Double disposable gloves and (if appropriate, e.g. large animal dead on floor) overshoes or sterilizable boots should be used. The outer gloves should be changed as necessary to avoid spreading contamination. Skin should not be exposed between the gloves and the sleeves.
- Existing cuts or abrasions should be dressed before putting on personal protective equipment (PPE).
7.1.2.2. Specimen collection from animals in the field
See Table 14 for guidance on appropriate specimens to collect.
TABLE 14
Guidelines on appropriate specimens from animals suspected of having died from anthrax.
Preferably a veterinarian or microbiologist trained in handling disease-causing agents should do the sample collection. This may not always be possible, or only possible with a substantial delay, and farmers/owners/managers may have to collect the samples. The following advice aims at covering either situation:
- You will need an apron or coverall if you anticipate extensive handling of the carcass.
- You will also need disposable covers for your hands and feet (see below) and strong bleach solution (10 000 ppm).
- Dress cuts or abrasions on exposed areas, especially hands and arms.
- The professional approach is to wear apron or coverall, disposable gloves and overboots, or boots that can be disinfected. It may be appropriate to wear two pairs of disposable gloves (double gloving); the outer gloves can then be changed as and when needed without exposing the hands. Minimal alternatives are strong plastic bags as overboots and, for the hands, evert a plastic bag, insert the hand that will touch the carcass into the everted bag and grasp tissue to be sampled; insert swab, or cut off sample with other hand; reverse bag over sample or swab and seal and label the bag. In the case of cutting off a piece of tissue, insert the cutting implement into another plastic bag for transport to where it can be disinfected (strong bleach for 1 hour) or sterilized (boiled for 30 min or pressure cooked for 15–20 min).
- After specimen collection, discard disposable items into disposal bags for subsequent sterilization or incineration. Similarly, non-disposable items should be put into discard containers for subsequent sterilization or disinfection. Care should be taken to ensure that sharp objects are in a container they cannot pierce easily. The containers themselves should be sterilized, incinerated or disinfected.
- Wash hands thoroughly with soap and water.
7.1.2.3. Environmental samples
- Disposable or reusable apron or coverall (as appropriate to the potential hazard of the sample) should be worn.
- Where the possibility exists of aerosolizing and inhaling dust, a respirator is advisable, preferably a quality assurance tested full-face respirator. (Caution: the operator should be trained by a qualified person in correct wearing and use of the respirator.)
- For samples related to known or suspected deliberate release, a quality assurance tested full-face respirator should be regarded as mandatory. (Caution: the operator should be fitted and trained by a qualified person in correct wearing and use of the respirator.)
- Double disposable gloves and overshoes or sterilizable boots should be worn. The outer gloves should be changed as necessary to avoid spreading contamination.
- Existing cuts or abrasions should be dressed before putting on PPE.
7.2. Safety procedures for specimen collection
7.2.1. During specimen collection
- Before specimen collection, put on the chosen clothing, including double gloving. Ensure disinfectant, disposal bags and hand-washing equipment are ready.
- Existing cuts or abrasions should be dressed before putting on PPE.
- After specimen collection, rinse or wipe down gloved hands with 10% hypochlorite solution and discard outer gloves.
- Discard used PPE into disposal bags, separating autoclavable and non-autoclavable items. Inner gloves should be discarded last. Sharps should be placed in a sharps container.
- Wash hands.
7.2.2. Using disinfectants, fumigants, etc
- Prepare hypochlorite solutions (10 000 ppm) freshly every day. Preferably handle sodium hypochlorite wearing gloves and eye protection. Avoid spilling it on clothes. Remember it corrodes ferrous metals.
- Use formalin in well-ventilated areas, wearing gloves and face shield while handling it. If handling it in an enclosed space with little ventilation, or if large volumes are involved, a full-face chemical respirator should be worn (the operator should be fitted and trained by a qualified person in correct wearing and use of the respirator). It is injurious to skin and mucous membranes.
7.3. Labelling
The following information should be recorded:
- a reference code or number marked in indelible ink on the container;
and, either on the container or on a sample documentation sheet:
- the date and time of sampling;
- the location of the sampling point;
- the type of sample;
- the reason for sampling;
- the identity of the person taking the sample.
7.4. Collection of human specimens for anthrax diagnosis
To a great extent, the specimen that can be collected, or that will yield B. anthracis, will depend on the condition of the patient and stage of the disease. For example, it may not be possible to isolate B. anthracis from the vesicular fluid of a cutaneous lesion if the patient has been treated, and vesicular fluid may no longer be available if a cutaneous lesion is older than 3–4 days. As another example, it will not be possible to isolate B. anthracis from blood until the very last few hours of life. With this proviso, Table 13 provides guidelines.
7.5. Collection of animal specimens for anthrax diagnosis
Legislation in most countries forbids postmortem examination of animals that have died of anthrax. Animals that have died suddenly and unexpectedly should not be necropsied unless anthrax has been ruled out as the cause of death (see section 3.5.2).
7.6. Collection of environmental samples for examination for B. anthracis
- Exposed surfaces are swabbed with moistened swabs, which are “double-bagged” (see also this annex, section 7.7) and sent to the laboratory.
- Water is collected by means of a syringe without needle and double-bagged.
- Food samples are collected with sterile spoons or other suitable sterile collecting devices into small sterile containers and double-bagged.
- Soil samples are collected with sterile spoons or other suitable sterilized tools into sterile, sealable containers (e.g. specimen cups with screw-on lids) and double-bagged.
- The cautions outlined in this annex, sections 6.3 and 7.1.2.3, pertain to dust samples, or suspect powders. For most purposes, swabs or sterile gauze “wipes”, premoistened with sterile water, are best. Dry swabs may be used if there is special reason not to use wet ones, such as not damaging evidence, but they will only collect small amounts of sample. The swabs are transferred to an appropriate container and double-bagged. It may also be possible, depending on the circumstances, to transfer dust to a sterile container with a sterile spatula; this should obviously be done carefully so as not to create aerosols. If vacuum collectors, purpose-designed to collect these types of sample into Hepa filter collectors, are available, then these should be the method of choice.
7.7. Containment for transport (“double-bagging”)
- The containers should be wiped down with hypochlorite (10 000 ppm) and, with outer gloves changed first, put into an outer, secondary container (double-bagged). If the secondary container is a plastic bag, then this should be of good quality. It should, in turn, be sealed and, for transport, be put into a good-quality cool box or a strong plastic or metal container with a lid that can be made secure.
- The secondary and outer containers should bear the relevant hazard labels.
Generally, specimens should be stored at 2–8 °C. Preferably they should be transported in cool boxes, especially in hot weather and when the time interval between collection and delivery to the laboratory is likely to be more than 1–2 hours.
The use of dry swabs for samples which cannot be examined immediately is covered in this annex, section 8.1 and Table 14.
For shipping of samples by mail or courier, the appropriate procedures with relevant paperwork must be followed.
7.8. Disinfection, decontamination and discard
Basically all specimens and used disposables should be autoclaved when finished with. Whether in the laboratory or in the field, these should have been collected into autoclavable bags or other suitable containers which are then autoclaved at 121 °C for ≥ 1 hour, preferably followed by incineration.
Contaminated autoclavable non-disposable items should also be deposited in autoclavable containers and ultimately autoclaved.
Microscope slides, coverslips and other sharp items should be placed in autoclavable sharps containers and autoclaved, preferably followed by incineration.
There may be circumstances where it is appropriate to immerse items in hypochlorite solution (10 000 ppm) initially and then to autoclave and incinerate them later.
Disinfect or fumigate non-autoclavable materials (see Annex 3, section 3.3).
Laboratory clothing should be autoclaved before being sent to the laundry. Non-disposable boots should be washed down into an autoclavable basin or bucket, and the washings autoclaved. The boots then should be disinfected by immersion in hypochlorite (10 000 ppm available chlorine) or 10% formalin (see Annex 3, section 6.5) and allowing them to dry for about 30 min before reuse.
7.9. Fumigation/UV
Equipment that cannot be autoclaved, boiled or immersed in disinfectant solutions should be fumigated (see Annex 3, section 3.3). Where fumigation is not readily achieved and a safety cabinet fitted with a UV light is being used, this should be utilized applying the same principles of arranging the items to be sterilized in such a way as to ensure the UV light reaches into and around them to maximum extent (see Annex 3, section 3.3.1). UV should not be relied on alone for decontamination, but should be used in conjunction with wiping the items to be decontaminated with towelling moistened with hypochlorite or possibly formalin, paying attention to the cautions given in Annex 3, section 1.1.
Ideally, cabinets and rooms should be fumigated when suspected of being contaminated. Where this is not possible, they should be given a very thorough floor-to-ceiling wipe-down with hypochlorite solution (10 000 ppm).
8. Types and conditions of specimens versus ease of diagnosis
8.1. Fresh specimens from untreated animals or humans
Few difficulties should be encountered in: (i) identifying B. anthracis in M’Fadyean capsule-stained smears of blood, lymph or oedematous fluid from untreated animals shortly before or within one or two days after death from anthrax; or (ii) isolating B. anthracis from these types of specimen. Similarly, the bacteria should be readily visible in, or isolated from, vesicular fluid before treatment in humans or, so long as no treatment was given, from body fluids near to death or post mortem.
It should be noted, however, as indicated in the footnote to Table 14, that smears and culture should be done within hours of collecting blood. Vegetative cells disintegrate in blood held for much more than a day. If a delay in reaching the laboratory is expected, the smear should be made on a slide immediately after collection, and the blood should be collected on a dry swab. This will encourage sporulation of the B. anthracis on the swab, which is then reliable for culture for long periods
8.2. Specimens from treated animals and humans
Treatment of an animal suffering from anthrax may sterilize the blood and tissues even though the animal may go on to die from the effect of the toxin. Similarly, cutaneous lesions in humans will be quickly sterilized by treatment but will continue to pass through their stages of evolution and resolution (section 7.3.1.5). (See also sections 3.5.3 and 4.4.4.2.)
Residual forms of the capsulated bacilli may be visible in fluid smears from such animals or persons. Isolation attempts maybe unsuccessful. Confirmation of diagnosis may be possible with a sensitive antigen-detection device for the toxin. It is hoped this will become more widely available in the future. In cases of extreme need to confirm diagnosis, a last-resort approach may be use of mice or guinea-pigs to isolate B. anthracis (this annex, section 12).
8.3. Specimens from old or decomposed animal specimens, or from animal products or environmental specimens
The problem likely to be encountered with this group of specimens is that detection will frequently involve a search for relatively few B. anthracis among many other Bacillus species, particularly B. cereus (section 6.2). The selective approach covered in this annex, section 10.4 is necessary.
Environmental samples may vary greatly in their composition (different kinds of soil, water and wastewater, biowaste, food and feedstuff), in their content of toxic materials (e.g. organic or inorganic residuals used in tanneries), and the competing bacterial flora. Extensive experience in Germany (Böhm & Beyer, personal communication, 2005) has shown that environmental samples may contain substances which inhibit germination and growth of B. anthracis, and therefore appear negative on culture. Such conditions may result in false negative results if a sample-specific positive control is not included. Therefore in general all sample materials are best divided into two parts before processing, one being the true sample and the other used as the spiked positive control. In order to determine the limit of detection of the culture procedure, further portions of the sample may be spiked with tenfold dilutions of spores. In sufficiently contaminated materials, the effect of the inhibitor may be eliminated and the contaminating B. anthracis detected by suitable dilution of the initial sample suspension.
9. Microscopy for anthrax
9.1. Equipment and materials
The following equipment and materials will be needed:
- binocular microscope with good oil immersion lens
- microscope slides and cover slips
- pen/pencil/diamond pen/label as appropriate to label slides
- sharps container
- wash bottles and water, preferably deionized or distilled
- alcohol, 95%–100%
- immersion oil
- stain tray with slide holder
- inoculating loops
- Bunsen burner or spirit lamp
- Pasteur pipettes, preferably plastic disposable
- paper towel or other absorbent paper
- plasticine
- lens tissue
- autoclavable discard bag
- stock hypochlorite and working bottle for 10 000 ppm solution, preferably a spray bottle
- stains:
- —
Gram stain
- —
polychrome methylene blue (M’Fadyean).
9.2. Safety measures
- Wash the stain off with water into a tray containing hypochlorite solution (10 000 ppm). Leave overnight before discarding, or autoclave.
- Discard the used slides into the sharps container, which is autoclaved.
- Incinerate/autoclave or otherwise decontaminate other used items of equipment.
- Avoid contaminating the microscope, e.g. by changing outer gloves at appropriate times.
9.3. Preparation and staining of smears
9.3.1. Clinical (human or animal material)
- Make two thin smears of clinical/animal material by rolling over the swabs or spreading a small drop on a microscope slide, using a coverslip to do the spreading. The smear should be approximately 1.5 cm square and should not run to the edges or either end of the slide. The thinner the smear, the better (avoid thick smears).
- Air-dry.
- Fix by dipping in 95%–100% alcohol for one minute and redry.
- Stain one smear with Gram stain (this annex, section 9.3.3) and the other with polychrome methylene blue stain for the demonstration of capsule (M’Fadyean stain) (this annex, section 9.3.6).
9.3.2. Smears from cultures
- Transfer some growth from the primary isolation plate to about 0.5 ml of saline and emulsify to give a slightly cloudy suspension. If the culture is already in suspension (e.g a broth culture), transfer one or more loopfuls to about 0.5 ml saline, again to produce a slightly cloudy suspension.
- Using a 1 µl loop, transfer a drop of the suspension to a microscope slide and spread the drop well.
- Allow to dry and fix with heat or dipping in 95%–100% ethanol for one minute.
- Allow the ethanol to evaporate off and stain as required.
9.3.3. Gram stain
- Follow the standard method, washing off into hypochlorite solution (10 000 ppm) at each stage.
- Observe the typical morphology of the bacillus: in clinical material B. anthracis are Gram-positive thick, long, straight bacilli with square or truncated ends with parallel sides found usually single, in pairs or chains of 3 or 4 bacilli. The chain of bacilli with truncated and swollen ends gives a characteristic “bamboo stick” appearance. A further description is given in section 6.3.1.1.
- Remember this is not a suitable stain for demonstration of capsule.
9.3.4. Modified Ziehl-Neelsen stain for spores
- Air-dry and heat or alcohol-fix the smear.
- Cover the smear with carbol fuchsin.
- Heat for 3–5 minutes; do not allow the stain to boil.
- Wash off stain with water using wash bottle (into hypochlorite solution).
- Decolourize with alcohol until all traces of red are removed.
- Wash off stain with water using wash bottle (into hypochlorite solution).
- Counterstain with methylene blue for 1–2 minutes.
- Wash again (into hypochlorite solution) and allow to dry.
- Observe under oil immersion.
Spores will be stained red and vegetative forms blue.
9.3.5. Malachite green stain for spores
- Dry the films and heat or alcohol fix.
Either:
- place the slide over a beaker of boiling water, resting it on the rim with the bacterial smear uppermost;
- cover with 5% aqueous solution of malachite green;
- stain for 5 minutes, adding more stain solution if the stain covering the smear starts to dry;
or
- place the slide in a moist chamber (a petri dish with moistened filter paper will do);
- cover the film with 5% aqueous solution of malachite green;
- leave to act for 60 minutes.
Then, following either procedure:
- wash off stain with water using wash bottle (into hypochlorite solution);
- counterstain with 0.5% safranin or 0.05% basic or carbol fuchsin for 30 seconds;
- wash again (into hypochlorite solution) and allow to dry.
Spores appear green and the vegetative bacilli red (Fig. 8C)
9.3.6. Polychrome methylene blue stain for capsule (M’Fadyean reaction)
This is the ideal method for demonstration of the capsule:
- Put a large drop of polychrome methylene blue on the smear to cover it completely.
- Leave for 30–60 seconds.
- Wash off stain with water using wash bottle (into hypochlorite solution, 10 000 ppm) and allow to dry.
- When dry, examine under the 10x lens. The anthrax bacilli can be seen as tiny short threads. Switch to oil immersion and look for the capsule, which is seen clearly as pink amorphous material surrounding the blue-black bacilli (Fig. 8A).
A positive and negative control should be included with every test. The positive control will require a wild-type isolate, which should be securely stored, or conceivably the Pasteur (pXO1-/2+) if this can be acquired. The Sterne vaccine strain is a good negative control.
9.3.7. India ink method for capsule visualization
This is not a true staining method but highlights the capsule as a transparent halo around the bacillus. It is satisfactory with good capsule preparations, such as blood from a freshly dead animal or smears of bacilli from mucoid colonies on bicarbonate agar grown under CO2 (see this annex, section 10.7.2.2). It may be less sensitive when smaller numbers of anthrax bacilli are present, or when the bacilli are dead and disintegrating as may be the case in specimens from old carcasses, or from animals/humans that were treated before the specimens were collected.
Procedure
Premix a loopful of the blood or other tissue fluid with a small drop of India ink on a clean slide such that a thin layer results when a cover slip is placed on top and pressed down lightly. If the India ink is too dark, dilute appropriately with water. One laboratory supplier of India ink for this purpose is Becton Dickinson Microbiology Systems, Maryland, USA (ref. 261194).
As before, the bacteria can be found by scanning under low power (10x objective) and then examined under oil immersion (100x) for the presence of the capsule.
As with polychrome methylene blue staining, a positive and negative control should be included with every test.
9.3.8. Fluorescent antibody staining for capsule
Mention should be made of the fluorescein-labelled anticapsule system developed by Ezzell & Abshire (1996). This now forms the basis of the capsule visualization tests used by the United States Laboratory Response Network.
10. Bacteriological confirmation
10.1. Equipment and materials
The following equipment and materials will be needed:
- binocular microscope with good oil immersion lens
- microscope slides and cover slips
- pen/pencil/diamond pen/label as appropriate to label slides
- sharps container
- wash bottles and water, preferably deionized or distilled
- alcohol, 95%–100%
- immersion oil
- stain tray with slide holder
- inoculating loops
- Bunsen burner or spirit lamp
- Pasteur pipettes, preferably plastic disposable
- paper towel or other absorbent paper
- plasticine (modelling clay)
- lens tissue
- autoclavable discard bags
- stock hypochlorite and working bottle for 10 000 ppm solution, preferably a spray bottle
- stains:
- —
Gram stain
- —
polychrome methylene blue (M’Fadyean) (quality controlled by reference laboratory for capsule staining), or other capsule stain
- —
malachite green stain, or other spore stain
- culture media (blood agar, nutrient agar, heart infusion agar, brain-heart infusion broth, etc.)
- PLET agar and/or TSPBA ingredients (Annex 2)
- gamma phage (quality controlled by reference laboratory for efficacy)
- penicillin discs
- defribinated horse blood (blood from other species may also be used)
- horse serum (serum from other species may also be used)
- sodium bicarbonate
- incubator (with CO2 facility ideally)
- water bath
- candle jar
- PCR equipment and reagents if appropriate.
10.2. Safety measures
- Place petri dishes (or other culture containers) in a purpose-designed carrier or secondary container, such as a sandwich box, for movement around the laboratory. The carrier or container should be labelled with the agent, the operator’s ID and date.
- Discard the plates/tubes into autoclave bags. Autoclave, preferably followed by incineration.
- Discard used slides and other sharp items into the sharps container which is autoclaved and then, preferably, incinerated also.
- Incinerate/autoclave other used disposable items of equipment.
- Autoclave recyclable item.
- Fumigate or otherwise decontaminate non-disposable items of equipment which cannot be autoclaved.
10.3. Fresh human/animal materials
10.3.1. Smears
- Prepare two smears.
- Gram-stain one (this annex, section 9.3.3), stain the other with polychrome methylene blue (this annex, section 9.3.6) or other appropriate capsule stain.
Gram-positive bacilli in short chains, square-ended and, in the polychrome methylene blue-stained smears exhibiting capsules (Fig. 8A), are definitive. Culture is necessary for further characterization.
10.3.2. Culture
- Inoculate on blood agar (BA).
- Incubate plates at 37 °C for 18–24 hours.
- Read the plates for colony characters.
After overnight incubation, B. anthracis colonies are white with frosted glass appearance and non-haemolytic. They may exhibit some or extensive tailing and are exceptionally tenaceous when teased with an inoculating loop. These can now be tested and checked for penicillin and phage-sensitivity, capsule production confirmed and, where facilities are available, checked by PCR for the presence of toxin and capsule genes.
10.4. Old animal specimens, animal products, environmental samples
Caution: if the sample is associated with suspected deliberate release, the testing should be done by appropriately equipped and trained personnel at the relevant reference laboratory.
Old animal specimens, animal products and environmental samples differ from fresh specimens in that large numbers of other environmental bacteria, especially other Bacillus species, will be present and will outgrow and mask any B. anthracis that may be present, especially if it is present in low numbers. In this case, a selective medium is needed (see this annex, section 3.6 and Annex 2). The procedures given below are summarized in Fig. 15.
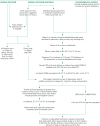
Fig. 15
Flow diagram of suggested procedures for isolation and identification of B. anthracis and confirmation of identity.
It should be noted that opinions differ as to the merit of centrifuging the suspended samples with a view to concentrating any anthrax spores present. Frequently this simply results in the concentration of the competing organisms. Depending on the type of sample, dilution may be more effective in revealing the presence of B. anthracis than concentration.
As pointed out in this annex, section 8.3, inhibitory substances may be present in some environmental samples which could prevent germination and growth of B. anthracis, and lead to false-negative results on culture. Appropriate controls are therefore needed. For example, a portion of the sample may be spiked with approximately 500–1000 cfu of spores of the Sterne 34F2 vaccine strain of B. anthracis.
Fundamental to the search for B. anthracis in environmental samples is a heating phase. This serves the dual purpose of killing non-sporing organisms that are present and heat-activating (heat-shocking) the B. anthracis spores, rendering them more predisposed to germination. Time/temperature combinations for B. anthracis spores found in many publications over the past 60 years range from 60 °C for ≤ 90 min to 80 °C for ≤ 30 min, but appear to have been invariably chosen arbitrarily. A recent study by Turnbull et al. (2006), aimed at determining the limits of flexibility that may be assumed in choosing a time/temperature combination for heat-treating B. anthracis spores, indicated that temperatures are best kept to ≤ 70 °C with no obvious reason for holding times > 15–30 minutes. Turnbull (Turnbull, 1996; Turnbull et al., 1998a) has normally opted for the conservative combination of 62–63 °C for 15–20 minutes, the time depending on the size of container or volume of liquid that has to be brought up to temperature, and the caution based on fear of losing spores in routine samples with low levels of contamination.
10.4.1. Examination of soil, material collected on a swab or filter, etc
- Depending on type and quantity of sample and how collected, make an appropriate w/v suspension of the sample in sterile deionized water (SDW), e.g. 1 g of soil in 10 ml SDW, 10 g soil in 100 ml SDW, 1 ml SDW suspension from a swab sample, a membrane filter suspended in 5–10 ml SDW, etc.
- The same is done in parallel for the artificially-contaminated control.
- Both samples are shaken at 4 °C for several hours. If the samples contain a lot of insoluble matter (e.g. soil samples), they may then be filtered through a plug of gauze and the filtrate processed.
- Make 1:10, 1:100 and possibly 1:1000 dilutions by transferring 1 ml volumes to 9 ml SDW.
- Place in a 62–65 °C water bath for 15–20 minutes to kill all vegetative forms, and heat-shock any spores present.
- Spread 100–200 µl volumes of undiluted and diluted suspensions on 3 plates of predried blood agar or TSPBA plates and 250 µl volumes on 3 pre-dried PLET agar plates.
- Incubate blood agar and TSPBA overnight and PLET agar for 36–48 hours at 37 °C. The suspected colonies are further isolated and identified (see this annex, section 10.3.2).
10.4.2. Muddy/polluted water
If the water sample is highly particulate, treat as a suspension of soil (Fig. 15).
- Place approximately 10 ml of the suspended sample in a 62–65 °C water bath for 15–20 minutes to kill all vegetative forms and heat-shock any spores present.
- Spread 100 µl volumes on 3 plates of blood agar or TSPBA plates and 250 µl volumes on 3 PLET agar plates.
- Incubate blood agar and TSPBA overnight and PLET agar for 36–48 hours at 37 °C. The suspected colonies are further isolated and identified (see this annex, section 10.3.2).
- Include a spiked control as mentioned in this annex, section 10.4.1 if considered advisable.
10.4.3. Drinking-water or apparently clear water
Procedures that have been laid down formally in water quality and public health regulations for enteric pathogens (Anon., 2002b) have not been tested for B. anthracis. However, there is no reason to believe that the membrane filtration methods detailed in Anon. (2002b, parts 3, 8–10) would not be applicable to a search for B. anthracis in water. The suggested procedure based on this would be:
- Pass a known volume up to 100 ml of the sample through a 0.45 µm membrane filter (typically 47 mm in a sterile filter unit).
- Remove the filter unit funnel and transfer the filter to 10 ml of sterile distilled/deionized water and agitate firmly to resuspend organisms trapped by the filter.
- Place the suspension in a water bath at 62–65 °C for 20 minutes.
- Spread 100–200 µl volumes on 3 plates of predried blood agar or TSPBA and 250 µl volumes on 3 pre-dried PLET agar plates. Incubate blood agar overnight and PLET agar for 36–48 hours at 37 °C.
- Look for typical B. anthracis colonies.
Alternatively:
- Bring two volumes of up to 100 ml of the sample to 62–65 °C and hold them at that temperature for 15–20 minutes.
- Pass each 100 ml through a 0.45 µm membrane filter (typically 47 mm in a sterile filter unit); remove the filter unit funnels and transfer one filter to a blood agar or TSPBA plate and the other to a PLET agar plate.
- Incubate the blood agar or TSPBA overnight and PLET agar for 36–48 hours at 37 °C.
- Look for typical B. anthracis colonies growing on the filters.
10.5. Examination of food
Food should be treated as environmental samples (this annex, section 10.4.1).
10.6. Examination of dusts and powders associated with suspected deliberate release
In a class 3 safety cabinet (see this annex, section 6.3), a sample of the dust or powder should be transferred by means of a dry swab (dry so as not to damage the evidence if follow-up is necessary) to approximately 0.5 ml of buffered saline. This should then be subcultured before and after heat treatment (62–65 °C for 20 minutes) on/in blood agar and other solid and/or broth media as considered appropriate to the circumstances.
In the event of a powder in an envelope or equivalent, the swab should be inserted through a corner of the envelope without opening the envelope wide at its top to minimize unwanted release of the sample.
10.7. Confirmatory tests
10.7.1. Phage and penicillin sensitivity
These tests can be done together in the simple manner illustrated in Fig. 8F. Up to six tests can be done on a BA plate. A control strain should be included in each batch of tests (the Sterne 34F2 vaccine strain, or equivalent, would be suitable).
The colony to be tested is spread over a segment of the plate, right down to the centre. A penicillin disk (2 or 10 U) is placed at the centre of the plate. A 10–15 µl drop of phage suspension is placed in the middle of the spread and allowed to dry in. The plate is incubated at 37 °C.
Phage and penicillin susceptibility can be read from about 6 hours to overnight. Haemolysis can also be checked.
Comments on phage titres and interpretation of zones in section 6.3.1.5 should be noted.
10.7.2. Induction of capsule formation
10.7.2.1. In blood
- Transfer a pinhead quantity of growth from a suspect colony to 2.5 ml defibrinated sheep or horse blood in a sterile test tube or small bottle.
- Incubate 5–18 hours at 35–37 °C.
- Transfer a drop with a 1 µl loop immersed to the bottom of the unshaken bottle or tube to a microscope slide and make a thin smear.
- Stain and examine as described in 9.3.6 above.
- A positive control strain should be included. This could be the Pasteur strain or equivalent if available, but may have to be a virulent wild-type isolate maintained for this purpose.
10.7.2.2. On bicarbonate agar plates
- Plate the suspect colony onto bicarbonate/serum agar (Annex 2).
- Incubate overnight at 35–37 °C under a 10%–20% CO2 atmosphere (or in a candle jar).
10.7.3. Motility
B. anthracis is non-motile. Any of the established tests for motility can be used to check this with an isolate.
10.7.4. PCR
10.7.4.1. Introduction
The section on PCR in the third edition of these guidelines (Turnbull et al., 1998a) was the subject of some criticism. It is known that a number of defence-related laboratories in several countries have designed primers and PCR systems that have a high degree of reliability, but these are generally unavailable to the wider community.
The following protocols are kindly supplied by W. Beyer.3 The methods were also provided in written form to the participants of the Anthrax Wetlab Workshop.4
10.7.4.2. Primers and protocols of choice
Of the primer systems described in the literature, only primers for the protective antigen (pag) and the lethal factor gene (lef) have not yet been shown to cause nonspecific results. Many primers described for the capB and capC genes and currently published primers targeting the chromosome of B. anthracis were shown to produce false positive results with the indigenous soil flora (Ramisse et al., 1999; Beyer et al., 1999; Ellerbrok et al., 2002).
10.7.4.3. DNA preparation
When PCR is simply being used to confirm suspicious colonies, short boiling of resuspended vegetative bacteria in PCR buffer is sufficient to extract DNA. If it is necessary to ensure that there are no viable spores in the DNA preparation, autoclaving of the culture material will also provide DNA suitable for the PCR protocols described here.
To prepare DNA from a non-selective enrichment culture or germinated spore suspensions, a DNA preparation kit is recommended. Depending on the target sequence of the PCR, the DNA preparation should either enrich for plasmid DNA or genomic DNA. In the former, the procedure should be able to isolate large, low copy number plasmids.
For the isolation of PCR compatible DNA from environmental samples, the procedure should also be able to remove polymerase inhibitors. The DNA preparation kits NucleoSpin® Plasmid (Macherey Nagel) and DNeasy Plant (Quiagen) can be used successfully for environmental samples. The DNeasyPlant-Kit of Quiagen is used according to the manufacturer’s recommendations with the following modifications:
- The pellet from 1 ml of the non-selective enrichment broth or germinated spore suspension is used as starting material, resuspended in 400 µl buffer AP1 in the kit.
- After the second washing of the spin column with buffer AW, an additional washing with 500 µl pure ethanol is done. Be sure no residual ethanol is left after the subsequent centrifugation.
- The DNA is eluted in 1 x 50 µl of buffer AE, pre-warmed to 70 °C.
10.7.4.4. Controls to be included in the diagnostic PCR
The following controls should be included in order to verify diagnostic findings:
- DNA prepared from an aliquot, or aliquots, of the original sample material spiked with known concentration(s) of a control strain of B. anthracis. This/these positive control(s) will provide information about any inhibition of germination or growth of B. anthracis during the preculture of the sample materials. Additionally it will provide an indication as to the sensitivity of the diagnostic procedure.
- Addition of 1 pg to 1 ng of purified genomic DNA of B. anthracis to the DNA prepared from the original sample material. This will reveal any inhibition effect that may be occurring on the PCR by polymerase inhibitors. Furthermore, it indicates the sensitivity of the PCR with the particular sample material being tested.
- Purified DNA (1 pg and 1 ng) of a pure culture of B. anthracis. This reaction serves as a positive control for the PCR. In a block cycler PCR, the amplicon should be visible for 1 ng of input DNA after the first round of amplifications, whereas 1 pg of DNA would be detectable after the nested PCR step only. In real-time PCR, 1 pg DNA or less should give a clear signal.
- DNA derived from the “negative in-process” control, e.g. E. coli cells. This control is handled and prepared simultaneously with both the original and the spiked sample materials, and serves as a negative control for contamination occurring throughout the entire process of culture, DNA isolation, and PCR.
- PCR premix without DNA. This reaction serves as a negative control for contamination during the preparation of the PCR premixes only.
10.7.4.5. Protocol for PCR in a block cycler instrument to detect the pag or the cap genes
Prepare a premix consisting of a volume of 50 µl per reaction containing 200 µmol/l dNTPs, 1.5 mmol/l MgCl2, 1 µmol/l of each primer, and 2.5 U polymerase.
Add 1 µg of T4 gene32 protein (Roche) to the standard premix during the first PCR. This single-strand binding protein enhances the efficacy of PCRs by a factor of 100 if the reaction is influenced by polymerase inhibitors (Beyer et al., 1995).
The use of a “hot start” protocol is advisable. Running a second PCR (nested PCR) is necessary: (i) if there is only a weak positive result with the first PCR run, e.g. due to inhibition of growth in the spiked control culture; or (ii) as a control reaction to verify the specificity of a positive result after the first PCR run. The nested PCR step may be omitted if the first PCR yields a negative result for the sample and the inhibition control, and the corresponding positive controls are positive.
The PCR conditions for a thermal block cycler running under block control are:
- 94 °C (83 °C for detection of pag) – 4 min
- 25 cycles (1st PCR); 30 cycles (nested PCR):
- —
94 °C (83 °C for detection of pag) – 1 min
- —
55 °C – 1.5 min
- —
73 °C – 1.5 min
- 72 °C – 9 min
- hold at 8 °C.
Primers used in the diagnostic PCR are shown in Table 15. Additional primer systems are provided in Table 16.
TABLE 15
Primers for nested PCR to detect the pag-gene.
TABLE 16
Additional published primer systems.
10.7.4.6. Protocols for real-time PCR in a LightCycler instrument
The LightCycler (LC) instrument (Roche) is designed for high-speed thermal cycling using air instead of thermal blocks. A capillary sample tube system ensures efficient heat transfer to the PCR samples. As a result, the time needed for each PCR cycle, including measurement of the sample fluorescence, is minimized to approximately 15–20 seconds. A 30–40 cycle PCR run can be completed within 20–30 minutes. The formation of amplification products can be monitored in real time.
The hybridization probe format is used for DNA detection and quantification. Two specially designed, sequence-specific oligonucleotides labelled with fluorescent dyes are applied for this detection method. This allows highly specific detection of the amplification product. Oligo 1 carries a fluorescein label at its 3’ end, whereas oligo 2 carries another label (LC Red 640) at its 5’ end. The sequences of the two oligonucleotides are selected so that they hybridize to the amplified DNA fragment in a head-to-tail arrangement. When the oligonucleotides hybridize in this orientation, the two fluorescence dyes are positioned in close proximity to each other. The first dye (fluorescein) is excited by the LightCycler’s LED (light emitting diode) filtered light source, and emits green fluorescent light at a slightly longer wavelength. When the two dyes are in close proximity, the emitted energy excites the LC Red 640 attached to the second hybridization probe that subsequently emits red fluorescent light at an even longer wavelength. This energy transfer, referred to as FRET (fluorescence resonance energy transfer) is highly dependent on the spacing between the two dye molecules. Only if the molecules are in close proximity (a distance between 1 and 5 nucleotides) is the energy transferred at high efficiency. The intensity of the light emitted by the LightCycler Red 640 is filtered and measured by the LightCycler instrument’s optics. The increasing amount of measured fluorescence is proportional to the increasing amount of DNA generated during the ongoing PCR process.
a. Detection of the pag gene
The premix (20 µl) consists of:
- 4 mM MgCl2
- 0.5 µM of each primer (Ellerbrok et al., 2002):
- —
BAPA-S: 5’-CGGATCAAGTATATGGGAATATAGCAA-3’
- —
BAPA-R: 5-CCGGTTTAGTCGTTTCTAATGGAT-3’
- 0.2 µM of probe
- —
BAPA-FL: 5’-TGCGGTAACACTTCACTCCAGTTCGA-X
- 0.2 µM of probe BAPA-LCRed 6405’-CCTGTATCCACCCTCACTCTTCCATTTTC-P
- 1/10 vol. of FastStart mastermix (Roche)
- 5 µl DNA.
b. Detection of the capC gene
The premix (20 µl) consists of:
- 4 mM MgCl2
- 0.5 µM of each primer:
- —
CapS: 5’-ACGTATGGTGTTTCAAGATTCATG-3’ (Ellerbrok et al., 2002)
- —
CapA**: 5-GATTGCAAATGTTGCACCACTTA-3’
- 0.2 µM of probe
- —
CapC-FL+: 5’-TATTGTTATCCTGTTATGCCATTTGAGATTTTT-X
- 0.2 µM of probe
- —
CapC-LC Red640: 5’-AATTCCGTGGTATTGGAGTTATTGTTCC-P
- 1/10 vol. of FastStart mastermix
- 5 µl DNA.
c. Experimental protocol
- Pre-incubation step: 95 °C for 10 min, slope at 20 °C/sec.
- Amplification (45 cycles): 95 °C for 10 sec; 55 °C for 20 sec; 72 °C for 30 sec, slope 20 °C/sec; one single signal acquisition at the end of annealing.
- Denaturation: 95 °C for 0 sec, slope 20 °C/sec; 40 °C for 30 sec, slope 20 °C/sec; 80 °C for 0 sec, slope 0.1 °C/sec with continuous acquisition of the signal.
- Cooling to 40 °C for 30 sec, slope 20 °C/sec.
10.7.4.7. Real-time PCR protocol for the specific detection of B. anthracis chromosome
a. Sequence alignment and PCR design
The sequences of the B-type sasp gene of B. cereus (Gene bank No. M16813, gi: 143507) and of B. anthracis, (NCBI Ref. Seq. NC003995, Gene bank: AAAC01000001), contributed by TIGR, were aligned by the appropriate tool of the NTI8 software package.5
The PCR reaction mixture of 20 µl consists of:
- 4 mM MgCl2
- 0.5 µM of each primer
- —
ANT-F: 5’-GCTAGTTATGGTACAGAGTTTGCGAC-3’
- —
ANT-Amt: 5’-CCATAACTGACATTTGTGCTTTGAAT-3’
- 0.2 µM of probe
- —
ANT-FL: 5’-CAAGCAAACGCACAATCAGAAGCTAAG-X
- 0.2 µM of probe
- —
ANT-LC: Red640: 5’-GCGCAAGCTTCTGGTGCTAGC-P
- 1/10 vol. of FastStart mastermix
- 5 µl DNA.
b. Experimental protocol
- Pre-incubation step: 95 °C for 10 min, slope at 20 v°C/sec.
- Amplification (45 cycles): 95 °C for 10 sec; 57 °C for 20 sec; 72 °C for 30 sec, slope 20 °C/sec; one single signal acquisition at the end of annealing.
- Denaturation: 95 v°C for 0 sec, slope 20 °C/sec; 40 °C for 30 sec, slope 20 °C/sec; 80 °C for 0 sec, slope 0.1 °C/sec with continuous acquisition of the signal.
- Cooling to 40 °C for 30 sec, slope 20 °C/sec.
10.7.4.8. Commercial kit
The LightCycler Bacillus anthracis Detection Kit for the detection of capsule (capB) and PA (pagA) genes is available commercially from Roche Applied Sciences for the detection of both virulence plasmids of B. anthracis. Kits for a range of sample types, both clinical and environmental, are now available, for instance, from Idaho Technology.6
11. Antigen detection tests
11.1. Ascoli precipitin test (thermostable antigen test)
The purpose of this very old test dating from 1911 (Ascoli, 1911) is to supply rapid retrospective evidence of anthrax infection in an animal. It was designed to detect B. anthracis antigens in the tissues of animals being utilized in animal by-products, and thereby to reveal when these products contained ingredients originating from animals that had died of anthrax. Over the years, it has been one of the most valuable tools for controlling anthrax in most European countries and it remains in use, particularly in eastern Europe. Regular or occasional use of the test was indicated on returns of a survey by OIE in 2002 from Croatia, Germany, the Philippines, the Republic of Moldova and The former Yugoslav Republic of Macedonia. There may be other countries that use the test but did not reply.
It needs to be borne in mind that this test is not rigorously specific for B. anthracis. The thermostable antigens involved are common to other Bacillus species so the test depends on the fact that the only Bacillus likely to have proliferated within and throughout an animal depositing extensive precipitating antigens in the tissues is B. anthracis.
The test is not suitable for detection of B. anthracis in environmental specimens; numerous other Bacillus species can be expected to occur in these. It is hoped that immunochromatographic, on-site tests (6.2) will become widely available as the replacements of the future.
11.1.1. Procedure
- Chop or slice the specimen into fine pieces or strips.
- Boil approximately 2 g of the specimen for 5 minutes in 5 ml saline containing 1:100 (final concentration) acetic acid. Alternatively, soak in saline containing 0.5% phenol for 24–48 hours in a refrigerator.
- After cooling, filter through filter paper until completely clear.
- Insert a few drops of antiserum (11.1.2 below) in the bottom of a small test-tube and carefully add some of the filtrate down the side of the tube to form a layer of antigen above the antiserum. (As an alternative to using a test-tube, and more economical on the antisera, capillary tubes can be used as in the Lancefield test for streptococcal grouping.)
- Include appropriate positive and negative specimen controls.
11.1.2. Antiserum for the Ascoli test
Commercially prepared serum is available from:
- Bioveta plc, Komenského 212, 683 23 Ivanovice na Hané, Czech Republic; fax +42 507 932 84
- The National Institute of Animal Health, 3–1 Kannondai 3-chome, Tsukuba-shi, Ibaraki-pref, 305–0856 Japan; tel: +81 298 38 7713; fax: +81 298 38 7880
- DD “Vet Zavod Zemun”, Batajnicki drum 4, Belgrade, Serbia
- National Anthrax Reference Laboratory, Friedrich-Loeffler-Institut, Bundesforschungsinstitut für Tiergesundheit, Institut für Bakterielle Infektionen und Zoonosen, Nationales Referenzlaboratorium für Milzbrand, Naumburger Straße 96a, D-07743 Jena, Germany; tel. +49 3641 804-0; fax +49 3641 804-228; web site: http://www.fli.bund.de/66+M52087573ab0.html
- C-C Pro: Gesellschaft für Herstellung und Vertrieb von Produkten für Cellculturen GmbH, Am Bahnhof 1, D-99986 Oberdorla; tel. +49 (700) 22 77 63 66; fax. +49 (700) 22 77 63 29; web site: http://www.c-c-pro.com/
It should be recalled that the following procedure was designed decades before the current era of fear of bioterrorism and associated strict control in many developed countries on access to virulent B. anthracis.
On days 1 and 14, rabbits are inoculated subcutaneously with animal anthrax vaccine (Sterne strain 34F2) (Annex 5, section 2). On days 28 and 35, further subcutaneous injections of 0.05 ml of a suspension in physiological saline of a mixture of several strains of virulent B. anthracis are administered. The viable count of this suspension should not exceed 100 000 colony-forming units/ml. After a further 10 days, a test-bleed will reveal the activity of the antiserum; if not adequate, further injections of the virulent B. anthracis suspension should be administered at 7–10 day intervals.
If considerations of safety prevent the use of live virulent B. anthracis, the mixture of several strains of B. anthracis can be suspended to a final count of 108–109/ml in physiological saline containing 0.2% formalin. This is held until sterile (at least 2 weeks). After the vaccine strain inoculations on days 1 and 14 as before, increasing doses of 0.1, 0.5, 1 and 2 ml of the killed suspension should be administered intravenously at approximately 4–5 day intervals. A test bleed should be done 10 days after the last injection. Further 2 ml doses can be administered if the titre is not adequate initially.
11.3. Other antigen detection tests
The rapid, hand-held, on-site, immunochromatographic detection devices that have been developed in recent years are discussed in section 6.2.
12. Isolation in animals
On account of increasing concern to eliminate the use of laboratory animals wherever possible, and of the increasing reliability and sophistication of alternative in vitro methods, the use of animals for isolation or confirmation of identity of B. anthracis can and should generally be avoided. It should be noted that EC Directive 86/609/EEC relating to protection of animals used for experimental and other scientific purposes pertains to members of the European Union. Several other countries have strict laws which make ad hoc use of an animal for isolation or confirmation of identification of B. anthracis virtually impossible nowadays.
There still are occasions, however, such as those where potential legal disputes may be involved, when confirmation of the presence or the virulence of B. anthracis is necessary. In the absence of a selective enrichment system (see section 6.2), inoculation of mice or guinea-pigs, essentially as done more than a century ago by Pasteur, is still the most sensitive isolation method. Pending the development of equally sensitive conventional immunological or DNA-based techniques, animal tests may offer the only chance of (i) confirmation of diagnosis in certain situations such as in the case of individuals or animals that were treated before specimens were taken, or (ii) detection of the organism when present in very low numbers in environmental samples, or in environmental samples containing sporostatic chemicals.
Confirmation of identity or of virulence can be done by injecting light suspensions (approximately 10 000 colony-forming units/ml) into mice (0.05–0.1 ml subcutaneously) or guinea-pigs (0.1–0.2 ml intramuscularly). Virulent B. anthracis will kill the animals after about 42–48 hours; M’Fadyean-stained blood smears examined at death will reveal large numbers of the capsulated bacilli which can also be isolated and confirmed bacteriologically.
In the rare situation in which it is necessary to use animals to isolate B. anthracis from soil or other environmental samples, the animals should be inoculated the day before with subcutaneous doses of mixed gas-gangrene antisera (extremely difficult to obtain, however) and antitetanus serum. Heated (62 °C to 65 C for 15–20 min) soil extracts, as prepared for plating on selective or non-selective agar (see 10.4 above; Fig. 15), are then injected (0.05–0.1 ml subcutaneously in a mouse or up to 0.4 ml intramuscularly in a guinea-pig – 0.2 ml in each thigh muscle). M’Fadyean-stained blood smears from any animals that die are examined for the presence of the typical capsulated B. anthracis which can also be isolated and confirmed bacteriologically.
13. Retrospective confirmation; serology and delayed type hypersensitivity testing
Effective serological enzyme immunoassays (EIA) for confirmation of the diagnosis of anthrax have been designed and have proved to be useful diagnostic, epidemiological and research aids (Turnbull et al., 1992a; Quinn et al., 2004) (see section 4.4.2.2). The usual provisos for any serological confirmatory test apply, namely that: (i) two or more serum samples taken 2–4 weeks apart will give greater diagnostic reliability; (ii) if only one serum sample is collected, it will be of greater diagnostic value if collected more than a week after onset of symptoms; and (iii) negative or weak results be interpreted in the light of treatment the patient or animal may have received early on in the course of the infection. The last is particularly important in anthrax since antibiotic therapy rapidly kills infecting B. anthracis and, if carried out early enough in an infected individual, may prevent the elaboration of sufficient antigen to induce a detectable immune response (section 4.4.2.2).
Available immunoassays for anthrax are mostly based on antibodies to the toxin antigens, primarily the protective antigen component of the toxin (see section 5.5.3). Although not difficult to perform (any standardized EIA methodology may be used), they are, at present, confined to a few specialist laboratories capable of preparing the necessary purified toxin antigens or with the resources to purchase them.7
The AnthraxinT delayed type hypersensitivity test is described in section 4.4.2.2 and involves intradermal injection of 0.1 ml of AnthraxinT. A positive test is defined as erythema of ≥ 8 mm with induration persisting for 48 hours.
An immunochromatographic assay for detecting anti-PA antibody, analagous to the assay for detecting PA (section 6.2), might be valuable for retrospective confirmation of diagnosis.
The value of immunohistochemical staining of tissues for retrospective confirmation in the anthrax letter events in the USA is discussed in section 4.4.4.2.
Footnotes
- 1
Significant use has been made in this annex of the operating procedures drawn up for the Manual for laboratory diagnosis of anthrax (WHO, 2003) recently produced by the WHO Regional Office for South-East Asia.
- 2
As found on http://www
.ourfood.com/Anthrax.html. - 3
Institute for Environmental and Animal Health, University of Hohenheim, Stuttgart, Germany (initially published in Tierärztliche Umschau, 58:653–62).
- 4
Global Health Security Action Group (GHSAG) Laboratory Network, 2004, HPA, Porton Down, United Kingdom.
- 5
Informax, Inc. Bethesda, United States. Primers and probes synthesized by TIB MolBiol, Berlin, Germany.
- 6
- 7
Publication Details
Copyright
All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: tni.ohw@sredrokoob). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: tni.ohw@snoissimrep).
Publisher
World Health Organization, Geneva
NLM Citation
Anthrax in Humans and Animals. 4th edition. Geneva: World Health Organization; 2008. Annex 1, Laboratory procedures for diagnosis of anthrax, and isolation and identification of Bacillus anthracis.
