ClinVar Submission Portal
ClinVar Submission Portal is the gateway to submit data to ClinVar at NCBI. In Submission Portal, you can:
- Log in or create an account
- Register your organization
- Authorize more than one submitter from your organization
- Submit your data
- Track your submission
- Update a past submission
- Get a cumulative report of your released records
Log in or create an account
Go to ClinVar Submission Portal: https://submit.ncbi.nlm.nih.gov/clinvar/
1. Click the "Log in" button

MyNCBI uses third party logins for account creation and log in. Third party logins are those you use on other sites, including Google, Facebook, ORCID, eRA Commons, and Login.gov.
- If you have an existing MyNCBI account, click the "Log in" button, choose the appropriate third party option and enter your credentials.
- If you don’t have a MyNCBI account, create a new account.
Some tips on third party logins:
- There are many options, and none are wrong (Choosing the Best 3rd-Party Option For You).
- If you have an existing account because you use other NCBI resources (e.g. PubMed), you can use the same credentials.
- Many people use an ORCID account.
- If you wish to use your university/organization login, click the "more login options" button and use the search bar to find it.
- If you have an eRA Commons account for handling NIH grants, you can use that account.
- If you end up with multiple accounts and need to merge them or have other questions about login, reach out to NCBI's service desk.
Security Warning: NCBI strongly discourages sharing of MyNCBI accounts and login information. The security and integrity of your data can be seriously compromised by maintaining a general account for multiple users within an organization, e.g. using a general login name and/or sharing a password. If there are multiple people who will submit from your organization, please see the section below on how to Authorize more than one submitter.
Once logged in, your username will be displayed in the top right hand corner:

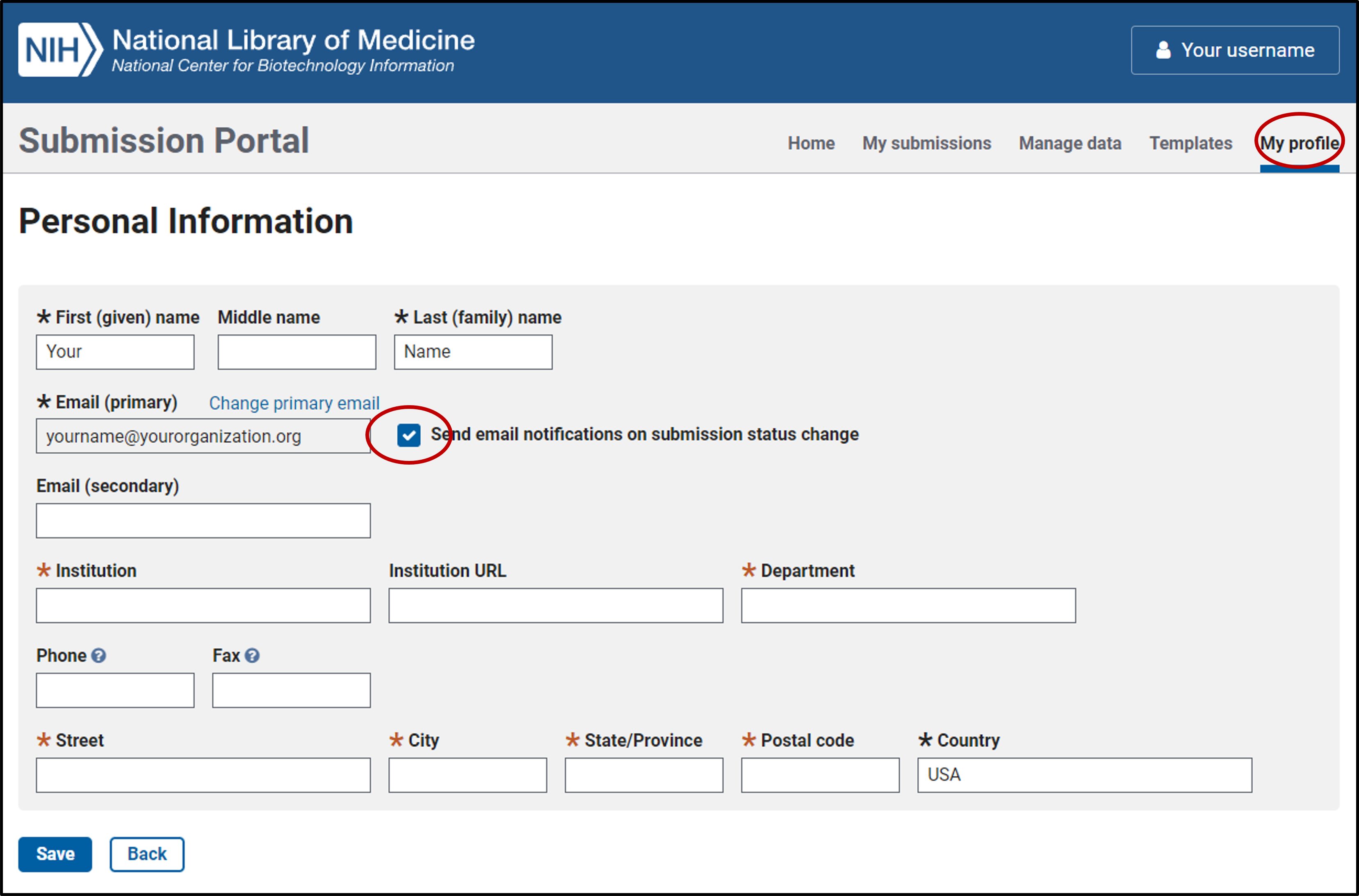
2. Edit your profile
- After your first log in, click on "My Profile" at the upper right of the page to access your profile, and populate the required (*) fields.
- Your email and your first and last name must be visible on this form so we can process your submission.
- We highly recommend that you check the box "Send email notifications on submission status change" so you will be notified by email if we have questions about a submission and when your submission is processed.
3. Review and accept the ClinVar Terms of submission
- You must accept the terms to register your organization and submit to ClinVar.

Register your organization
- Registering your organization in Submission Portal is required before you can submit to ClinVar.
- Registration needs to be done only once; registering the same organization more than once should be avoided.
- If you want to add a new submitter to your organization, don’t register a new one. See how to authorize more than one submitter.
Determine if your organization is registered already
- Search ClinVar's list of submitters to determine if your organization has registered already. Note that this list only includes organizations with at least one submission to ClinVar that is public.
- An additional check for duplicates is performed on the ORGANIZATION INFORMATION tab during the registration process (see the next section).
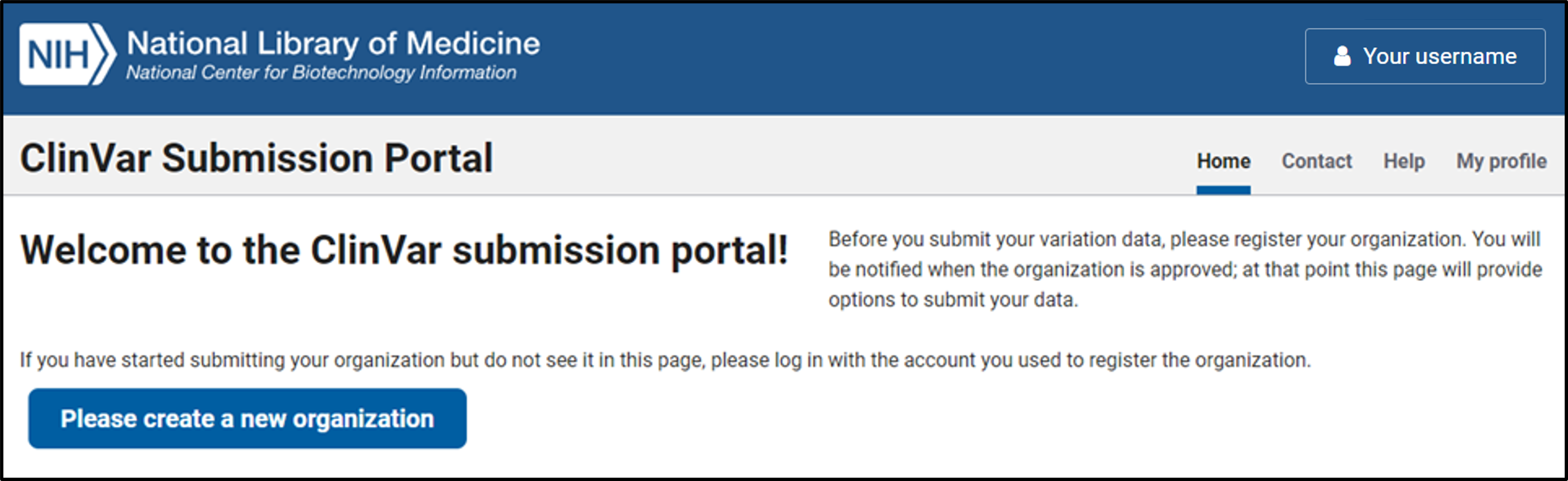
Register a new organization
1. Click the button to “Please create a new organization”
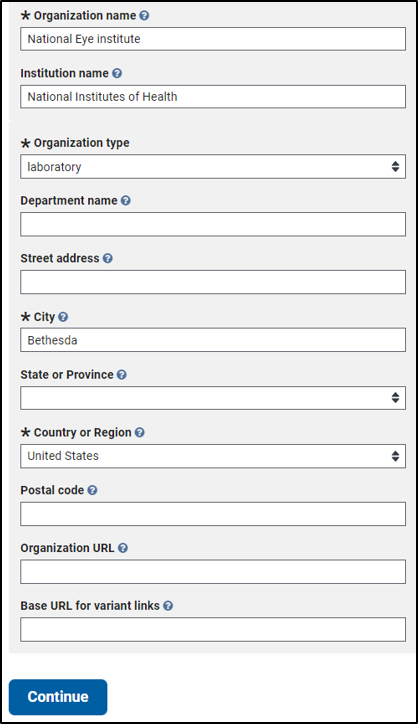
2. Complete the required fields on the ORGANIZATION INFORMATION tab
ClinVar uses the following terms to represent a submitter’s affiliation:
| Term | Definition | Example |
|---|---|---|
| Organization | a smaller entity within the larger Institution | lab, clinic, department, LSDB |
| Institution | the larger entity consisting of one or more smaller Organizations | university, hospital, company, government agency |
-
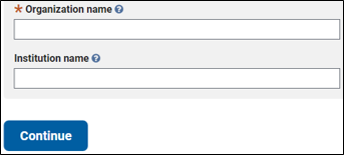
A distinct name for both the Organization and the Institution should be provided so that the proper attribution of a ClinVar submission can be given. In some cases providing only the Organization is sufficient, e.g. for a company (GeneDx) or an LSDB (CFTR2).
-
Provide your organization and institution name:
-
Once complete, click "Continue".
-
If it looks like your organization is already registered to submit to the NIH Genetic Testing Registry (GTR) or ClinVar, a prompt will appear:
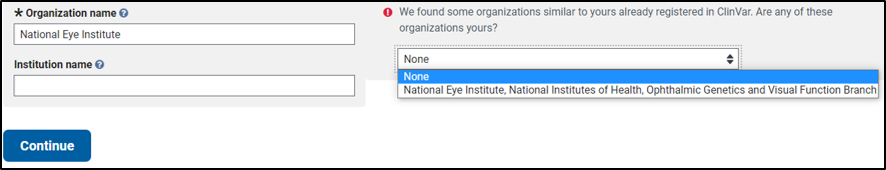
- If the organization is registered in GTR already, select the organization from the dropdown menu. The form will be autopopulated with information from GTR. Click "Continue" to proceed with registration.
- If none of the organizations in the dropdown menu is the same as your organization, select None and click "Continue" again to complete the form.
- If the organization is registered in ClinVar already, the registration process is stopped. Instead, you will be asked to contact the organization's administrator to add to you to the Submitter Group. There is no need to register the organization again.
A completed ORGANIZATION INFORMATION tab follows:
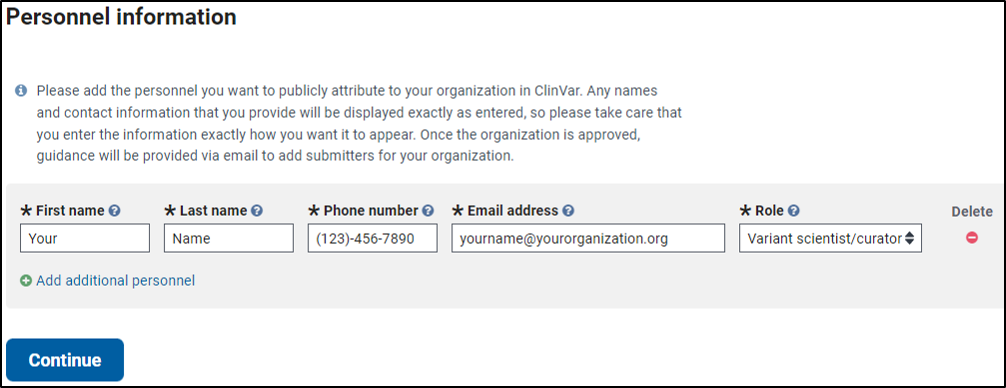
3. Complete information about personnel from your organization on the PERSONNEL INFORMATION tab
- Add any personnel that you want to be displayed on your organization’s page in ClinVar. Note that this section does not authorize other individuals to submit for your organization; to allow this, see the section on how to authorize more than one submitter.
A completed PERSONNEL INFORMATION tab follows:
4. Review your organization information and click the "Submit" button on the REVIEW & SUBMIT tab
- Information about your organization registration will be reviewed by ClinVar staff within one to three business days.
- We will contact you to resolve any questions.
- When approved, you will receive an email from clinvar@ncbi.nlm.nih.gov that includes instructions on how to submit to ClinVar.
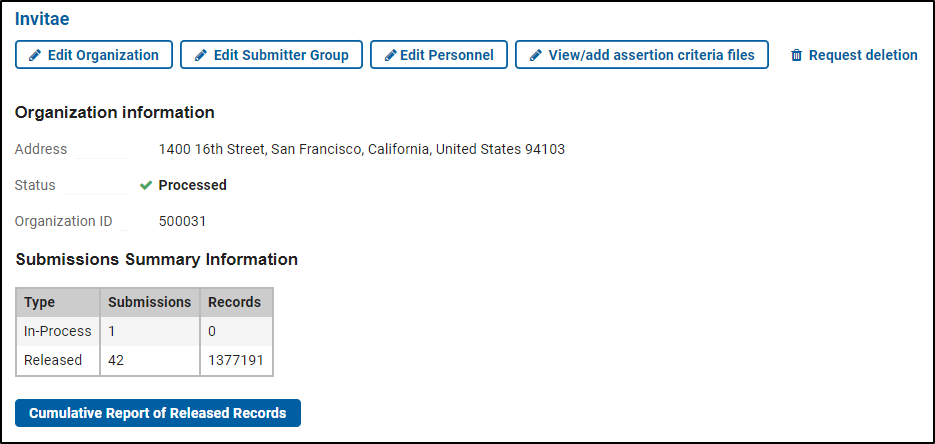
Update an existing organization or personnel
- Log in to the ClinVar Submission Portal and use the "Edit this organization" or "Edit Personnel" button below the Organization name. You must be a member of the organization's Submitter Group (see the next section) and have "Modify" permissions to make any changes.
Authorize more than one submitter from your organization
- Permission to submit to ClinVar, which is distinct from being personnel of an organization, is managed through the organization's Submitter Group. You must be a member of the Submitter Group to be able to submit to ClinVar.
- The Submitter Group is created when your organization registration is approved. At that time, the person who submitted the organization registration is the only Submitter Group member and the only one able to submit to ClinVar. The person is also the Submitter Group administrator and can invite others to join the Submitter Group.
- To send an invitation to join the Submitter Group or make changes, log in to the ClinVar Submission Portal and use the "Edit Submitter Group” button below the Organization name.
- Please see our documentation about Submitter Groups for information on how to access your Submitter Group and manage its membership.
Submit your data
Please refer to our submission instructions on how to Prepare your submission and Send your submission.
Submit on behalf of a different organization
ClinVar also accommodates brokered submissions, i.e. when one organization uploads a submission on behalf of the submitting organization. In this case, only the submitting organization receives attribution for the submission, although ClinVar does track who the brokering organization is. To broker a submission:
- The submitting organization must register in Submission Portal.
- After the organization registration is approved, the submitting organization needs to add to their Submitter Group the person who will broker the submission.
- When ready to submit, the broker logs into Submission Portal and chooses the submitting organization from the list of organizations available.
- On the ORGANIZATION INFORMATION tab, when asked if submitting on behalf of another organization, the broker chooses 'yes' and enters the name of the broker's own organization.
Track your submission
When logged in, ClinVar Submission Portal provides a summary of each submission from your organization. Options for submission status include:
- Unfinished: not yet submitted
- Processing: submitted and under review by ClinVar staff
- Processed: complete and public
- Error: errors were detected that need corrected to proceed with processing
- Deleted: deleted at the submitter’s request or for lack of response
Unfinished submissions include links to edit or delete the submission; once your data is submitted and under review by ClinVar staff these options are not available. Successfully processed submissions include a link to a summary report.

Update a past submission
Please refer to our submission instructions on how to Update a past submission.
Get a cumulative report of your released records
When logged in to the ClinVar Submission Portal a button is provided that will generate a report of the latest version of every one of your records as they appear on the ClinVar web site and FTP site.