How to submit information in the Performance characteristics section of the submission form
This page is organized according to the sections you see on the Performance characteristics tab. You may click on the name of the section below to navigate to that section of this document.
Please note that most sections in the form provide tool tips, accessible by this icon (![]() ), that provide hints specific to that area.
), that provide hints specific to that area.
Availability
Information in the Availability section will display in the Performance Characteristics tab of the test record.
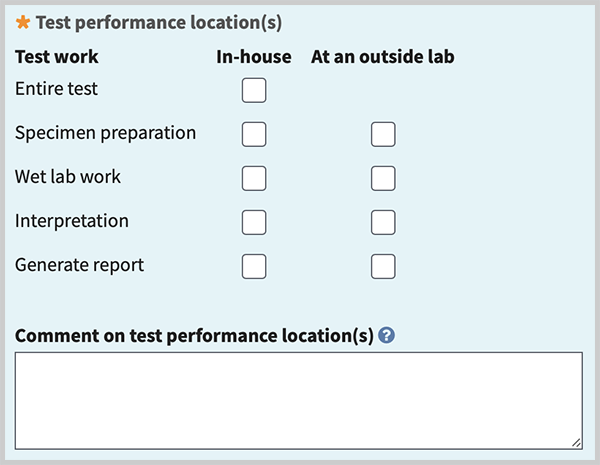
Test performance location(s) (minimal). Describe where test work is performed. You can register tests for which your laboratory performs at least part of the test: specimen preparation, wet lab work, interpretation, and report generation. 'In-house' means within the lab/facility covered by the same CLIA certification number. 'Outside lab' means a different lab/facility than that covered by your CLIA certification, even if both labs have the same parent organization. Tests which are performed entirely at an outside lab/facility (send-out tests) should not be registered in GTR. Send-out tests are expected to be registered by the lab performing them.
Check box(es) to indicate the locations where components of the test work are performed. If 'In-house' is selected for entire test, no other check marks are needed. For other test work choices, you may check one or both for 'In-house' and 'At an outside lab'. If 'In-house' is not selected for 'Entire test', then check marks are needed for each of the remaining components and at least one type of test work must be performed in-house.
Comment on test performance location(s) (minimal if any test work is performed at an outside lab). Briefly describe methods and location for components of test work done at an outside lab.
Analytical validity
How accurately and reliably the test measures the component of interest. This information will display in the Overview and Indication tabs of the test record.
Analytical validity (minimal). Describe the accuracy and reliability of the test for measuring the component of interest. Provide quantitative information as typically available from a validation study required by CAP. Scope includes analytical sensitivity (analytical detection rate) and the number of specimens used to calculate it; analytical specificity (analytical false positive rate); accuracy (how closely the results match those from independent sources known to be the true results); and precision (how closely repeated results match each other).
General statements about methodologies that do not contain specific quantitative information about the test do not satisfy the specifications for this field. Nor do statements such as ‘N/A’, ‘not applicable’, ‘Yes’ or ‘unknown’. Example of an accepted entry: 'The OtoChip is greater than 99% sensitive for detecting substitution variants in the sequence analyzed. In addition, this assay is 95% sensitive for detecting small insertions or deletions (InDels) (100% for 1-2 bp indels, 92.3% for 3-5 bp indels and 87.5% for >10 bp indels)'.
Citations to support analytical validity (recommended). Provide citation(s) to support analytical validity statement. Use search PubMed link to find citation details. Add multiple citations as desired. If no publications are available, provide non-proprietary internal lab data in analytical validity statement, if available.
Assay limitation(s) (recommended). Describe any factors that affect the value of the test for its intended use. Includes limits of detection and validation of test for only specific subpopulations or particular uses.
Citations to support assay limitation (recommended). Provide citations to support assay limitation statement(s). Use search PubMed link to find citation details. Add multiple citations as desired.
Quality Assurance
Describe methods for quality management and quality control. Applicable to preanalytic, analytic, and post-analytic (reporting) phases. Information in the Quality Assurance section will display in the Performance Characteristics tab of the test record.
Is proficiency testing performed for this test? (recommended). Select Yes or No to indicate whether the test is subject to periodic internal or external evaluation of the accuracy of test results.
Proficiency testing method (recommended; minimal if Yes is selected for proficiency testing). Specify which type of proficiency testing is performed. Choices include Alternative Assessment, Formal Proficiency Testing (PT) program, Intra-Laboratory, Inter-Laboratory, and Other (specify). Proficiency testing is a determination of laboratory testing performance by means of interlaboratory comparisons whereby a group of laboratories receive multiple specimens for analysis and/or identification and the program compares the results among laboratories and/or with an assigned value. Alternative assessment is the determination of laboratory testing performance by means other than PT such as split-sample testing or testing by a different method.
Provider for proficiency testing (recommended). Specify the agency or society that administers the proficiency testing program for the test. If Other than listed, please specify. To see the complete, current list of choices for this field, click here.
Major CAP category (recommended). If College of American Pathologists (CAP) is chosen as Provider for proficiency testing, select Major CAP category from list. Add multiple as needed by selecting 'add another CAP test'. If category is not available, specify under 'Category for my test is not listed here'.
CAP category (recommended). If Major CAP category is selected, specify relevant sub-category. Add multiple as needed by selecting 'add another CAP test'.
Description of proficiency testing method (recommended). Explain proficiency testing (PT) method and provide PT scores and/or results, the PT reportable range, the PT interval and the number of specimens tested.
Citations to support the above statement (recommended). Provide citations to support proficiency testing method statement. Use search PubMed link to find citation details. Add multiple citations as desired.
Description of internal test validation method (recommended). Explain how the laboratory validates the test.
Citations to support the above statement (recommended). Provide citations to support internal test validation method statement. Use search PubMed link to find citation details. Add multiple citations as desired.
Clinical Validity
How consistently and accurately the test detects or predicts the intermediate or final outcomes of interest. This information will display in the Overview and Indication tabs of the test record.
Statement of clinical validity (recommended). Describe clinical sensitivity and specificity, positive and negative predictive values, the population(s) assessed, and the number of specimens used to calculate clinical validity. Clinical sensitivity is the proportion of positive test results among patients with the defined clinical presentation. Clinical specificity is the proportion of negative test results among patients without the defined clinical presentation. Positive predictive value is the chance of having the marker among those that test positive. Negative predictive value is the chance of not having the marker among those that test negative.
Citations to support the above statement (recommended). Provide citations to support clinical validity statement. Use search PubMed link to find citation details. Add multiple citations as desired.
Clinical Utility
How likely the test is to significantly improve patient outcomes. This information will display in the Overview and Indication tabs of the test record.
Category of clinical utility (recommended). Select a category from the list and support it with a URL and/or citations. You can enter multiple category sets of information by clicking the "add another clinical utility" link below the box.
Choose from the following options:
Avoidance of invasive testing
Establish or confirm diagnosis
Guidance for management
Guidance for selecting a drug therapy and/or dose
Lifestyle planning
Predictive risk information for patient and/or family members
Reproductive decision-making
Other (specify)
Sufficient research has not been conducted to establish utility of the test
To see the complete, current list of choices for this field, click here.
URL to explain the clinical utility (minimal for each category of clinical utility selected; at least one URL or citation). Cite recommendations or practice guidelines for the test that have been issued by authoritative groups. Some practice guidelines may be available via a URL but not a citation. URL to the laboratory web page with information on the clinical utility of the test may be used.
Citations to support the clinical utility (minimal for each category of clinical utility selected; at least one URL or citation). Cite recommendations or practice guidelines for the test that have been issued by authoritative groups (e.g., U.S. Preventive Services Task Force, professional societies such as the American College of Medical Genetics and Genomics). Use search PubMed link to find citation details. Add multiple citations as desired.
Save your work
Don't forget to save your work by clicking the Save & Continue button at the bottom of the page! If you need to interrupt or delay data entry, you can return at a later time and you will be taken to the last tab you saved.
If any required fields or data inconsistencies are detected on this page, an error message will display after you click Save & Continue indicating what the issue is. The field(s) that need attention will be outlined in red. Please correct your data and click "Save & Continue" again.