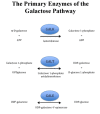
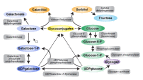
Galactosemia caused by deficiency of the enzyme galactose-1-phosphate uridylyltranserase (GALT) may be divided into three clinical/biochemical phenotypes: (1) classic galactosemia, (2) clinical variant galactosemia, and (3) biochemical variant galactosemia (not covered in this GeneReview; see, for example, Duarte Variant Galactosemia). This categorization is based on residual erythrocyte GALT enzyme activity; the levels of galactose metabolites (e.g., erythrocyte galactose-1-phosphate and urine galactitol) that are observed both off and on a lactose-restricted diet; and, most importantly, the likelihood that the affected individual will develop acute and chronic long-term complications. This categorization allows for proper counseling of the parents of an infant with galactosemia, especially regarding the so-called diet-independent complications.
Classic Galactosemia
Scenario 1 – Abnormal newborn screening (NBS) result and prompt initiation of appropriate management in the neonatal period
Within days of ingesting breast milk or lactose-containing formulas, infants with classic galactosemia develop life-threatening complications including feeding problems, failure to thrive, hypoglycemia, hepatocellular damage, bleeding diathesis, and jaundice (see Table 2).
If a lactose-free diet is provided during the first three to ten days of life, the signs of classic galactosemia resolve quickly and prognosis for prevention of liver failure, E coli sepsis, and neonatal death is good. Failure to implement effective newborn screening may have catastrophic consequences such as liver failure [Malone et al 2011].
Long-term outcome. Even with early and adequate therapy, the long-term outcome in older children and adults with classic galactosemia can include cataracts, speech defects, poor growth, poor intellectual function, neurologic deficits (predominantly extrapyramidal findings with ataxia), and, in females, hypergonadotropic hypogonadism or premature ovarian insufficiency (POI) [Rubio-Gozalbo et al 2019].
Classic galactosemia is associated with extreme variability in chronic complications and/or long-term outcome. Even individuals who have not been sick in the newborn period and who were begun on a lactose-free diet from birth (e.g., those with a prior affected sib in the family) may manifest language delay, speech defects, learning disabilities, cognitive impairment, and in females, POI. These problems may manifest as early as age one to two years, and in almost all instances, no findings that would have predicted eventual brain and ovarian dysfunction were present in early infancy. A minority of individuals may exhibit documented neurologic abnormalities including tremor (postural or intentional), cerebellar ataxia, and dystonia. No findings early in the disease course are good predictors of these long-term complications. Overall, the quality of life is reduced in adults with classic galactosemia, and more so when compared to individuals with phenylketonuria (PKU) [Gubbels et al 2011, ten Hoedt et al 2011, Hoffmann et al 2012].
Scenario 2 – Symptomatic individual with untreated classic galactosemia (resulting from NBS not performed or false negative NBS result)
If classic galactosemia is not treated, sepsis with E coli, shock, and death may occur [Levy et al 1977]. Infants who survive the neonatal period and continue to ingest lactose may develop severe brain damage [Otaduy et al 2006].
Table 2.
Frequency of Specific Findings in Symptomatic Neonates with Classic Galactosemia
View in own window
| Finding | % of Affected
Neonates w/Finding | Additional Details |
|---|
|
Hepatocellular damage
| 89% | Jaundice (74%)
Hepatomegaly (43%)
Abnormal liver function tests (10%)
Coagulation disorders (9%)
Ascites (4%) |
|
Food intolerance
| 76% | Vomiting (47%)
Diarrhea (12%)
Poor feeding (23%) |
|
Failure to thrive
| 29% | |
|
Lethargy
| 16% | |
|
Seizures
| 1% | |
|
Sepsis
| 10% | E coli (26 cases)
Klebsiella (3)
Enterobacter (2)
Staphylococcus (1)
Beta-streptococcus (1)
Streptococcus faecalis (1) |
If the diagnosis of classic galactosemia is not established, the infant who is partially treated with intravenous antibiotics and self-restricted lactose intake demonstrates relapsing and episodic jaundice and bleeding from altered hemostasis concomitant with the introduction of lactose. If treatment is delayed, complications such as growth restriction and progressive liver disease are likely. Rare affected individuals may develop vitreous hemorrhages that may produce blindness [Levy et al 1996, Takci et al 2012].
Long-term outcome. Information on long-term outcomes was initially reported by Waggoner et al [1990] as the result of a retrospective, cross-sectional survey of 270 individuals with classic galactosemia. The largest outcome study involving 507 people with classic galactosemia was published by the international GalNet registry or consortium [Rubio-Gozalbo et al 2019]. To summarize, the data on long-term outcome indicate that complications involving the nervous system and ovarian function do not correlate with any of the well-known biochemical variables (e.g., erythrocyte galactose-1-phosphate levels); furthermore, manifestations of one or more of these complications vary even among individuals with the same genotype associated with classic galactosemia (see Table 3) [Doyle et al 2010, Schadewaldt et al 2010, Hoffmann et al 2011, Krabbi et al 2011, Coss et al 2012, Waisbren et al 2012, Frederick et al 2017].
Intellectual development. Of 177 individuals age six years or older with no obvious medical causes for developmental delay other than galactosemia, 45% were described as developmentally delayed.
Speech problems were reported in 56% (136/243) of individuals age three years or older.
More than 90% of individuals with speech problems were described as having delayed vocabulary and articulation problems. The speech problems resolved in only 24%.
Speech defects are heterogeneous (involving both central defects and motor abnormalities) and evolve with time [
Potter et al 2013].
The developmental quotients and IQ scores observed in individuals with speech disorders as a group were significantly lower than those of individuals with normal speech, although some individuals with speech problems tested in the average range.
Motor function. Among individuals older than age five years, 18% had fine motor tremors and problems with coordination, gait, and balance.
Gonadal function in females. Of 47 girls and women, 81% had signs of POI.
The mean age at menarche was 14 years with a range from ten to 18 years.
Eight of 34 women older than age 17 years (including 2 with "streak gonads") had primary amenorrhea.
Most women developed oligomenorrhea and secondary amenorrhea within a few years of menarche.
Only five of 17 women older than age 22 years had normal menstruation. Two, who gave birth at ages 18 and 26 years, had never experienced normal menstrual periods.
Guerrero et al [2000] determined that the development of POI in females with galactosemia is more likely if the following are true:
Gonadal function in males
Normal serum concentrations of testosterone and/or follicle-stimulating hormone and luteinizing hormone were reported for males. However, the literature has few reports of males with classic galactosemia who have fathered a child [
Panis et al 2006a,
Waisbren et al 2012,
Gubbels et al 2013].
There have been no data to support structural abnormalities in the male reproductive tract that would lead to infertility; preliminary data suggest an increased prevalence of cryptorchidism and low semen volume [
Gubbels et al 2013].
Growth. In many individuals, growth was severely delayed during childhood and early adolescence; when puberty was delayed and growth continued through the late teens, final adult heights were within the normal range. Decreased height over mean parental height was related to low insulin-like growth factor-I [
Panis et al 2007].
Cataracts were reported in 30% of 314 individuals.
Nearly half the cataracts were described as "mild," "transient," or "neonatal" and resolved with dietary treatment; only eight were treated surgically.
Dietary treatment had begun at a mean age of 77 days for those with cataracts compared to 20 days for those without cataracts. However, one of the eight individuals who required cataract surgery was an infant who had been treated from birth.
Relationship between treatment and outcome. No significant associations were found between treatment and outcome except for a greater incidence of developmental delay among individuals who were not treated until after age two months. However, IQ scores were not highly correlated with the age at which treatment began. The effect of early treatment on outcome was also studied in 27 sibships, three of which had three affected sibs. The older sibs were diagnosed and treated after clinical symptoms occurred or newborn screening results had been reported, whereas the younger sibs were treated within two days of birth. Although the younger sibs were treated early and only one developed neonatal symptoms, the differences in IQ scores among the sibs were not statistically significant, and the speech and ovarian function of the younger sibs were no better than those of their older sibs.
Restriction of milk in the mother's diet during pregnancy was reported for 21 of the 38 infants who were treated from birth. The long-term outcome of these 21 was no better than that of the 17 individuals whose intake of mother's milk was not restricted during the pregnancy.
No significant differences could be observed in the rate of complications between the individuals with residual enzyme activity and those with no measurable enzyme activity, except that individuals with some enzyme activity tended to be taller for age.
Individuals with or without neurologic complications. No differences were observed in treatment or biochemical factors between the 56 individuals with normal intellect, speech, and motor function and the 25 individuals with developmental delay and speech and motor problems.
Relationships of complications. Developmental delay and low IQ scores were associated with speech problems, motor problems, and delayed growth, but not with abnormal ovarian function.
Sex differences. Females had lower mean IQ scores than males after age ten years (p<0.05) and had lower mean heights for age at five to 12 years (p<0.05), but did not differ in frequency of speech or motor problems or in the treatment variables, including age at which treatment began, neonatal illness, or galactose-1-phosphate erythrocyte concentration. However, the association of problems with intellectual development, speech, and motor function could also indicate a specific neurologic abnormality in some cases of galactosemia [Schadewaldt et al 2010].
Clinical Variant Galactosemia
Individuals with variant forms of galactosemia may have some aspects of classic galactosemia, including early cataracts, liver disease, mild intellectual disability with ataxia, and growth restriction [Fridovich-Keil et al 2011]. Clinical variant galactosemia can result in life-threatening complications in untreated infants, including feeding problems, failure to thrive, hepatocellular damage (including cirrhosis), and bleeding.
Clinical variant galactosemia is exemplified by the disease that occurs in African Americans and native Africans in South Africa with a p.Ser135Leu/Ser135Leu genotype. Neonates with clinical variant galactosemia may be missed with NBS because the hypergalactosemia is not as marked as in classic galactosemia and breath testing is normal [Crushell et al 2009].
If a lactose-restricted diet is provided during the first ten days of life, the severe acute neonatal complications usually do not occur.
To the best of current knowledge, African Americans with clinical variant galactosemia and adequate early treatment do not develop long-term complications, including POI.