NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997.
Reverse transcription begins when the viral particle enters the cytoplasm of a target cell. The viral RNA genome enters the cytoplasm as part of a nucleoprotein complex that has not been well characterized. The process of reverse transcription generates, in the cytoplasm, a linear DNA duplex via an intricate series of steps. This DNA is colinear with its RNA template, but it contains terminal duplications known as the long terminal repeats (LTRs) that are not present in viral RNA (Fig. 1). Extant models for reverse transcription propose that two specialized template switches known as strand-transfer reactions or “jumps” are required to generate the LTRs.
Retroviral DNA synthesis is absolutely dependent on the two distinct enzymatic activities of RT: a DNA polymerase that can use either RNA or DNA as a template, and a nuclease, termed ribonuclease H (RNase H), that is specific for the RNA strand of RNA:DNA duplexes. Although a role for other proteins cannot be ruled out, and it is likely that certain viral proteins (e.g., nucleocapsid, NC) increase the efficiency of reverse transcription, all of the enzymatic functions required to complete the series of steps involved in the generation of a retroviral DNA can be attributed to either the DNA polymerase or the RNase H of RT. The process of retroviral DNA synthesis is believed to follow the scheme outlined in Figure 2:
- 1.
Minus-strand DNA synthesis is initiated using the 3′end of a partially unwound transfer RNA which is annealed to the primer-binding site (PBS) in genomic RNA, as a primer. Minus-strand DNA synthesis proceeds until the 5′end of genomic RNA is reached, generating a DNA intermediate of discrete length termed minus-strand strong-stop DNA (–sssDNA). Since the binding site for the tRNA primer is near the 5′ end of viral RNA, –sssDNA is relatively short, on the order of 100–150 bases
- 2.
Following RNase-H-mediated degradation of the RNA strand of the RNA:–sssDNA duplex, the first strand transfer causes –sssDNA to be annealed to the 3′end of a viral genomic RNA. This transfer is mediated by identical sequences known as the repeated (R) sequences, which are present at the 5′ and 3′ends of the RNA genome. The 3′end of –sssDNA was copied from the R sequences at the 5′end of the viral genome and therefore contains sequences complementary to R. After the RNA template has been removed, –sssDNA can anneal to the R sequences at the 3′end of the RNA genome. The annealing reaction appears to be facilitated by the NC.
- 3.
Once the –sssDNA has been transferred to the 3′R segment on viral RNA, minus-strand DNA synthesis resumes, accompanied by RNase H digestion of the template strand. This degradation is not complete, however.
- 4.
The RNA genome contains a short polypurine tract (PPT) that is relatively resistant to RNase H degradation. A defined RNA segment derived from the PPT primes plus-strand DNA synthesis. Plus-strand synthesis is halted after a portion of the primer tRNA is reverse-transcribed, yielding a DNA called plus-strand strong-stop DNA (+sssDNA). Although all strains of retroviruses generate a defined plus-strand primer from the PPT, some viruses generate additional plus-strand primers from the RNA genome.
- 5.
RNase H removes the primer tRNA, exposing sequences in +sssDNA that are complementary to sequences at or near the 3′end of plus-strand DNA.
- 6.
Annealing of the complementary PBS segments in +sssDNA and minus-strand DNA constitutes the second strand transfer.
- 7.
Plus- and minus-strand syntheses are then completed, with the plus and minus strands of DNA each serving as a template for the other strand.
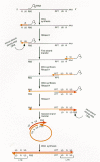
Figure 2
Process of reverse transcription of the retroviral genome. (Black line) RNA; (light color) minus-strand DNAs; (dark color) plus-strand DNA. See text for a description of this process.
A more detailed description of these steps is presented below.
- Overview of Reverse Transcription - RetrovirusesOverview of Reverse Transcription - Retroviruses
Your browsing activity is empty.
Activity recording is turned off.
See more...