Except where otherwise noted, this work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC-BY-NC-ND 4.0). To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
Abstract
In the 1920s, it was recognized that a mucoid or matte colony phenotype of Streptococcus pyogenes was associated with virulence in mice and with resistance to killing by human blood leukocytes. The gelatinous material produced by the mucoid isolates, currently known as capsule, was later characterized as hyaluronic acid, a linear polymer of N-acetylglucosamine and glucuronic acid with a high molecular mass that is structurally identical to hyaluronic acid found in the extracellular matrix of many higher animals, including human beings. It is now recognized that most (but not all) clinical isolates of group A streptococci produce hyaluronic acid, which is associated with the cell surface during exponential growth and shed during stationary phase. This chapter presents a summary of information on the capsule of Streptococcus pyogenes, its biochemistry, genetics, and role in virulence.
For many years, clinical microbiologists and infectious diseases clinicians have noted that group A streptococci freshly isolated from patients with pharyngitis or invasive infection often grow as large, wet-appearing, translucent colonies on blood agar. With prolonged incubation, these “mucoid” colonies collapse and assume an irregular or “matte” appearance. Laboratory passage of such isolates frequently resulted in loss of the mucoid phenotype and conversion to small, compact, or “glossy” colonies (Figure 1). In the 1920s, Lancefield and Todd recognized that the mucoid or matte colony phenotype was associated with virulence in mice and with resistance to killing by human blood leukocytes (Lancefield & Todd, 1928; Todd & Lancefield, 1928). Kendall and co-workers characterized the gelatinous material produced by mucoid isolates as hyaluronic acid, a linear polymer of N-acetylglucosamine and glucuronic acid with a high molecular mass that is structurally identical to hyaluronic acid found in the extracellular matrix of many higher animals, including human beings (Figure 2) (Kendall, Heidelberger, & Dawson, 1937). Wilson demonstrated that the growth of such isolates on media containing hyaluronidase prevented the development of mucoidy, which confirmed that the production of hyaluronic acid was responsible for the mucoid colony phenotype (Wilson, 1959). It is now recognized that most (but not all) clinical isolates of group A streptococci produce hyaluronic acid, which is associated with the cell surface during exponential growth and shed during stationary phase. Presumably because it is recognized by the host immune system as a self-antigen, the group A streptococcal hyaluronic acid capsule is poorly immunogenic in animals, including human beings. In contrast to capsular polysaccharides of Streptococcus pneumoniae and S. agalactiae, hyaluronic acid polymers are not covalently linked to the group A streptococcal cell wall, but rather are associated with the cell surface in a dynamic fashion, as they are synthesized by a cell membrane-associated polymerase. Although its mode of attachment to the bacterial surface is more tenuous than that of covalently bound polysaccharide capsules in other species, there is abundant evidence that the hyaluronic acid capsule is an important virulence determinant as a modulator of multiple interactions between group A streptococci and their human hosts.

Figure 1.
Blood agar plate with typical colonies of a mucoid strain of group A streptococcus (left) and non-mucoid (glossy) colonies of an acapsular mutant (right).
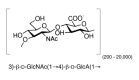
Figure 2.
Repeating unit structure of hyaluronic acid.
Genetics and biochemistry of hyaluronic acid biosynthesis
Studies in the 1990s used transposon mutagenesis to identify a chromosomal locus required for hyaluronic acid production in group A streptococci (DeAngelis, Papaconstantinou, & Weigel, 1993a; Dougherty & van de Rijn, 1992; Wessels, Moses, Goldberg, & DiCesare, 1991). Further characterization of the locus revealed an operon of three genes, hasA, hasB, and hasC, each of which encodes an enzyme involved in hyaluronic acid synthesis (DeAngelis, Papaconstantinou, & Weigel, 1993a; Crater, Dougherty, & van de Rijn, 1995; DeAngelis, Papaconstantinou, & Weigel, 1993b; Dougherty & van de Rijn, 1993; Dougherty & van de Rijn, 1994) (Figure 3). The 4.2 kb has operon is highly conserved among group A streptococcal strains, but is notably absent in isolates of M-types 4 and 22, which do not produce capsules (Henningham, et al., 2014; Flores, Jewell, Fittipaldi, Beres, & Musser, 2012). Notably, strains of M-types 4 and 22 produce hyaluronidase, which degrades hyaluronic acid, while the vast majority of other group A streptococcal isolates harbor an inactivating point mutation in the chromosomal hyaluronidase gene, hylA (Henningham, et al., 2014; Hynes, Johnson, & Stokes, 2009). This association suggests an evolutionary branch point in which group A streptococcal strains developed alternative strategies for adaptation through expression of either the anti-phagocytic hyaluronic acid capsule or hyaluronidase, which facilitates the spread of secreted toxins by degrading the host’s extracellular matrix, but can also digest the group A streptococcus capsule.
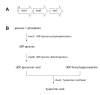
Figure 3.
(A) Schematic of the has operon encoding enzymes involved in hyaluronic acid biosynthesis in group A streptococci. (B) Diagram illustrating the enzymatic function in hyaluronic acid synthesis of the proteins encoded by hasA, hasB, and hasC.
Hyaluronic acid is synthesized from the nucleotide sugar precursors UDP-glucuronic acid and UDP-N-acetylglucosamine by a membrane-associated enzyme, hyaluronan synthase, encoded by hasA. High-Mr hyaluronic acid can be produced by the incubation of cell-free membrane extracts of group A streptococci with the two substrate UDP-sugars in the presence of divalent cations (Markovitz, Cifonelli, & Dorfman, 1959; Stoolmiller & Dorfman, 1969; Sugahara, Schwartz, & Dorfman, 1979). The hasA gene product has a predicted Mr of 47.9 kD and includes at least four predicted membrane-spanning domains, which is consistent with evidence that the enzyme is localized at the cell membrane where it mediates both polymer formation and export (DeAngelis, Papaconstantinou, & Weigel, 1993a; Dougherty & van de Rijn, 1994). The group A streptococcus hyaluronan synthase shares significant similarity with hyaluronan synthases from other microbial and higher animal species (DeAngelis, Yang, & Weigel, 1994; Weigel, Hascall, & Tammi, 1997). The second gene of the has operon, hasB, encodes UDP-glucose dehydrogenase, a 45.5 kD protein that catalyzes the oxidation of UDP-glucose to UDP-glucuronic acid (Dougherty & van de Rijn, 1993). The third gene in the cluster, hasC, encodes a predicted 33.7 kD protein identified as UDP-glucose pyrophosphorylase (Crater, Dougherty, & van de Rijn, 1995). This enzyme catalyzes the condensation of UTP with glucose-1-phosphate to form UDP-glucose. Thus, the reaction catalyzed by the hasC product yields a substrate for UDP-glucose dehydrogenase encoded by hasB, whose reaction product is, in turn, a substrate for hyaluronan synthase encoded by hasA. While the enzyme protein encoded by hasC is enzymatically active, it is not required for hyaluronic acid synthesis by group A streptococci. The inactivation of hasC resulted in no reduction in hyaluronic acid synthesis by a highly encapsulated strain of group A streptococci—a finding that implies that another source of UDP-glucose is available within the cell (Ashbaugh, Alberti, & Wessels, 1998a). Furthermore, expression of recombinant hasA and hasB (without hasC) conferred the capacity to synthesize hyaluronic acid in Escherichia coli and Enterococcus faecalis (DeAngelis, Papaconstantinou, & Weigel, 1993a; DeAngelis, Papaconstantinou, & Weigel, 1993b). A hasB paralog, hasB2, is widely conserved among group A streptococcus isolates. This gene is located at a site remote from the has operon; it encodes a protein with the same enzymatic activity as hasB and can support a reduced level of capsule synthesis in the absence of hasB (Cole, et al., 2012).
Regulation of capsule expression
While the has operon is highly conserved, there is wide variation in production of the hyaluronic acid capsule among group A streptococcus isolates and under different growth conditions in an individual strain. Transcription of the has operon and synthesis of hyaluronic acid is maximal during exponential phase in liquid cultures, and declines to very low levels during the stationary phase (Crater & van de Rijn, 1995; Unnikrishnan, Cohen, & Sriskandan, 1999). Cessation of capsule synthesis is associated with shedding of hyaluronic acid from the cell surface into the culture medium. Capsule production is highest in a nutrient-rich environment. Expression of the has operon is rapidly induced upon introduction of the bacteria into the peritoneal cavity of mice or into the pharynx of baboons in a non-human primate model of pharyngeal colonization (Gryllos, et al., 2001). Strain-to-strain variation in capsule production may be partially explained by polymorphisms in the promoter region upstream of the has operon (Albertí, Ashbaugh, & Wessels, 1998).
The CsrRS (also known as CovRS) two-component system is a critical regulator of has operon transcription in response to environmental signals. The CsrRS system was discovered by a transposon mutagenesis screen for mutants that formed mucoid colonies; a phenotype that is shown to be the result of inactivation of the csrRS locus (Levin & Wessels, 1998). Subsequent work has shown that CsrRS regulates approximately 10% of group A streptococcal genes, including several virulence factors, in addition to the has operon (Dalton, Collins, Barnett, & Scott, 2006; Federle, McIver, & Scott, 1999; Graham, et al., 2002; Gryllos, et al., 2007; Heath, DiRita, Barg, & Engleberg, 1999). According to our current model of the CsrRS system, CsrS is a cell membrane-associated histidine kinase that is activated by extracellular magnesium, and perhaps also by other unknown environmental signals (Gryllos, et al., 2007; Gryllos, Levin, & Wessels, 2003). Autophosphorylation of the cytoplasmic domain of CsrS is followed by phosphotransfer to CsrR, which increases the affinity of the latter protein for target promoters—including the has operon promoter, where it represses transcription and reduces capsule production. The human antimicrobial peptide LL-37 appears to signal through the CsrRS system in a manner opposite to that of magnesium: exposure of group A streptococci to 100-300 nM LL-37 (concentrations far below those that inhibit bacterial growth) results in stimulation of expression of the has operon (and of other CsrR-repressed genes) (Gryllos, et al., 2008; Tran-Winkler, Love, Gryllos, & Wessels, 2011). LL-37 has been shown to bind to the predicted extracellular domain of CsrS in vitro, and studies of smaller peptides have identified a 10-amino acid internal fragment of LL-37 that is completely devoid of antimicrobial activity against group A streptococci, but retains its CsrS-signaling activity (Velarde, Ashbaugh, & Wessels, 2014). These findings suggest that LL-37 signaling reflects a specific interaction with CsrS rather than a response to a non-specific membrane injury. It has been suggested that the CsrRS system enables group A streptococci to detect the host’s innate immune response to group A streptococcal infection by sensing any subinhibitory concentrations of LL-37 secreted by leukocytes and/or epithelial cells. LL-37 signaling through CsrS results in upregulation of capsule production, as well as that of other antiphagocytic factors that enhance group A streptococcal virulence (Gryllos, et al., 2008; Tran-Winkler, Love, Gryllos, & Wessels, 2011).
RocA is another regulatory protein that affects capsule expression. Inactivating mutations in rocA have been shown to reduce expression of CsrR and are associated with increased capsule production (Biswas & Scott, 2003). In M-type 18 strains that form mucoid colonies as a result of abundant capsule production, the RocA protein is truncated and non-functional. Replacement of the mutated rocA gene with the consensus rocA sequence in a mucoid M18 strain suppressed the mucoid phenotype (Lynskey, et al., 2013).
Role of the capsule in pathogenesis
Early studies by Lancefield, Todd, and others found an association between the presence of the mucoid colony type and virulence in mice (Todd & Lancefield, 1928; Ward & Lyons, 1935). However, because mucoid strains tended to be rich in M protein as well as in hyaluronic acid capsule, it was difficult to confidently ascribe an independent role in virulence to the capsule. Investigators in the mid-twentieth century found that hyaluronidase treatment increased the susceptibility of mucoid strains to killing by human blood phagocytes, supporting a role for the capsule in resistance to phagocytosis (Rothbard, 1948; Foley & Wood, Jr., 1959; Stollerman, Rytel, & Ortiz, 1963). Kass and Seastone showed that hyaluronidase treatment of mice reduced the virulence of group A streptococci during experimental infections (Kass & Seastone, 1944). Epidemiological observations have also suggested a link between capsule expression and virulence. Mucoid strains of group A streptococci have been associated with invasive infections and with outbreaks of acute rheumatic fever (Johnson, Stevens, & Kaplan, 1992; Tamayo, Montes, García-Medina, García-Arenzana, & Pérez-Trallero, 2010; Veasy, et al., 2004).
The development of methods for genetic manipulation of streptococci permitted more direct assessment of the role of the capsule in pathogenesis. Acapsular mutants derived by transposon mutagenesis and subsequently by targeted deletion of the hasA gene were found to have reduced virulence in systemic infection models in mice and in chicken embryos, in a murine model of invasive soft tissue infection, and in airway challenge models in mice (Wessels, Moses, Goldberg, & DiCesare, 1991; Ashbaugh, Warren, Carey, & Wessels, 1998b; Husmann, Yung, Hollingshead, & Scott, 1997; Schmidt, Günther, & Courtney, 1996; Schrager, Rheinwald, & Wessels, 1996; Wessels & Bronze, 1994). Capsule-deficient mutants have increased susceptibility to complement-mediated opsonophagocytic killing by human blood leukocytes, as compared to their respective encapsulated parent strains, and resistance to phagocytosis is thought to be a major mechanism through which the capsule enhances virulence. The presence of the capsule does not inhibit complement activation or deposition of complement fragments on the bacterial cell wall, but rather interferes with access of leukocyte receptors for opsonic complement proteins on the bacterial surface (Dale, Washburn, Marques, & Wessels, 1996).
In a baboon model of group A streptococcal pharyngeal colonization, an acapsular mutant colonized at a similar efficiency as the parent strain, but was cleared more rapidly (Ashbaugh, et al., 2000). This result suggested that the capsule contributed to persistence in the pharynx. However, genomic analysis of serial isolates from the pharynges of experimentally infected macaques showed the development over time of mutations in the has operon and promoter that reduced transcription of the hyaluronic acid biosynthesis genes (Shea, et al., 2011). Similar mutations were detected in sequential group A streptococcal isolates from human pharyngeal samples (Flores, et al., 2014). When taken together, these observations suggest that the down-regulation of capsule production may favor chronic pharyngeal carriage.
In vitro studies of group A streptococcal adherence to epithelial cells have shown that the capsule reduces bacterial attachment (Hollands, et al., 2010; Schrager, Albertí, Cywes, Dougherty, & Wessels, 1998). The capsule itself can act as an adhesin by mediating attachment to the hyaluronic acid binding protein CD44, which is expressed on multiple cell types including oropharyngeal keratinocytes (Schrager, Albertí, Cywes, Dougherty, & Wessels, 1998). The potential role of CD44 as a group A streptococcus receptor was supported by studies showing reduced pharyngeal colonization in mice after intranasal administration of monoclonal antibody to CD44 together with a group A streptococcal challenge or after pretreatment with exogenous hyaluronic acid (Cywes, Stamenkovic, & Wessels, 2000). Mice expressing a CD44 anti-sense transgene targeted to stratified squamous epithelia also were resistant to group A streptococcal pharyngeal colonization. In addition to the role of CD44 in adherence, the binding of encapsulated group A streptococcus to CD44 on human oropharyngeal keratinocytes induces an intracellular signaling cascade that results in disruption of intercellular junctions and enhancement of group A streptococcal translocation across the epithelial barrier. In this way, CD44-mediated signaling by the hyaluronic acid capsule may facilitate group A streptococcus tissue invasion (Cywes & Wessels, 2001).
References
- Albertí S., Ashbaugh C. D., Wessels M. R. Structure of the has operon promoter and regulation of hyaluronic acid capsule expression in group A Streptococcus. Molecular Microbiology. 1998;28(2):343–353. [PubMed: 9622359]
- Ashbaugh C. D., Alberti S., Wessels M. R. Molecular analysis of the capsule gene region of group A Streptococcus: the hasAB genes are sufficient for capsule expression. Journal of Bacteriology. 1998a;180(18):4955–4959. [PMC free article: PMC107524] [PubMed: 9733702]
- Ashbaugh C. D., Moser T. J., Shearer M. H., White G. L., Kennedy R. C., Wessels M. R. Bacterial determinants of persistent throat colonization and the associated immune response in a primate model of human group A streptococcal pharyngeal infection. Cellular Microbiology. 2000;2(4):283–292. [PubMed: 11207585]
- Ashbaugh C. D., Warren H. B., Carey V. J., Wessels M. R. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. Journal of Clinical Investigation. 1998b;102(3):550–560. [PMC free article: PMC508916] [PubMed: 9691092]
- Biswas I., Scott J. R. Identification of rocA, a positive regulator of covR expression in the group A streptococcus. The Journal of Bacteriology. 2003;185(10):3081–3090. [PMC free article: PMC154078] [PubMed: 12730168]
- Cole J. N., Aziz R. K., Kuipers K., Timmer A. M., Nizet V., van Sorge N. M. A conserved UDP-glucose dehydrogenase encoded outside the hasABC operon contributes to capsule biogenesis in group A Streptococcus. Journal of Bacteriology. 2012;194(22):6154–6161. [PMC free article: PMC3486382] [PubMed: 22961854]
- Crater D. L., van de Rijn I. Hyaluronic acid synthesis operon (has) expression in group A streptococci. The Journal of Biological Chemistry. 1995;270(31):18452–18458. [PubMed: 7629171]
- Crater D. L., Dougherty B. A., van de Rijn I. Molecular characterization of hasC from an operon required for hyaluronic acid synthesis in group A streptococci. The Journal of Biological Chemistry. 1995;270(48):28676–28680. [PubMed: 7499387]
- Cywes C., Wessels M. R. Group A Streptococcus tissue invasion by CD44-mediated cell signalling. Nature. 2001;414(6864):648–652. [PubMed: 11740562]
- Cywes C., Stamenkovic I., Wessels M. R. CD44 as a receptor for colonization of the pharynx by group A Streptococcus. The Journal of Clinical Investigation. 2000;106(8):995–1002. [PMC free article: PMC314343] [PubMed: 11032859]
- Dale J. B., Washburn R. G., Marques M. B., Wessels M. R. Hyaluronate capsule and surface M protein in resistance to opsonization of group A streptococci. Infection and Immunity. 1996;64(5):1495–1501. [PMC free article: PMC173953] [PubMed: 8613352]
- Dalton T. L., Collins J. T., Barnett T. C., Scott J. R. RscA, a member of the MDR1 family of transporters, is repressed by CovR and required for growth of Streptococcus pyogenes under heat stress. The Journal of Bacteriology. 2006;188(1):77–85. [PMC free article: PMC1317578] [PubMed: 16352823]
- DeAngelis P. L., Papaconstantinou J., Weigel P. H. Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. The Journal of Biological Chemistry. 1993a;268(26):19181–19184. [PubMed: 8366070]
- DeAngelis P. L., Papaconstantinou J., Weigel P. H. Isolation of a Streptococcus pyogenes gene locus that directs hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. The Journal of Biological Chemistry. 1993b;268(20):14568–14571. [PubMed: 8325836]
- DeAngelis P. L., Yang N., Weigel P. H. The Streptococcus pyogenes hyaluronan synthase: sequence comparison and conservation among various group A strains. Biochemical and Biophysical Research Communications. 1994;199(1):1–10. [PubMed: 8122999]
- Dougherty B. A., van de Rijn I. Molecular characterization of a locus required for hyaluronic acid capsule production in group A streptococci. The Journal of Experimental Medicine. 1992;175(5):1291–1299. [PMC free article: PMC2119206] [PubMed: 1569398]
- Dougherty B. A., van de Rijn I. Molecular characterization of hasB from an operon required for hyaluronic acid synthesis in group A streptococci. The Journal of Biological Chemistry. 1993;268(10):7118–7124. [PubMed: 8463246]
- Dougherty B. A., van de Rijn I. Molecular characterization of hasA from an operon required for hyaluronic acid synthesis in group A streptococci. The Journal of Biological Chemistry. 1994;269(1):169–175. [PubMed: 8276791]
- Federle M. J., McIver K. S., Scott J. R. A response regulator that represses transcription of several virulence operons in the group A streptococcus. The Journal of Bacteriology. 1999;181(12):3649–3657. [PMC free article: PMC93840] [PubMed: 10368137]
- Flores A. R., Jewell B. E., Fittipaldi N., Beres S. B., Musser J. M. Human disease isolates of serotype m4 and m22 group a streptococcus lack genes required for hyaluronic acid capsule biosynthesis. mBio. 2012;3(6):e00413–e12. [PMC free article: PMC3487777] [PubMed: 23131832]
- Flores A. R., Jewell B. E., Olsen R. J., Shelburne S. A. 3rd, Fittipaldi N., Beres S. B., et al. Asymptomatic carriage of group A streptococcus is associated with elimination of capsule production. Infection and Immunity. 2014;82(9):3958–3967. [PMC free article: PMC4187823] [PubMed: 25024363]
- Foley M. J., Wood W. B. Jr. Studies on the pathogenicity of group A streptococci II. The antiphagocytic effects of the M protein and the capsular gel. The Journal of Experimental Medicine. 1959;110:617–628. [PMC free article: PMC2137000] [PubMed: 13823728]
- Graham M. R., Smoot L. M., Lux Migliaccio C. A., Virtaneva K., Sturdevant D. E., Porcella S. F., et al. Virulence control in group A Streptococcus by a two-component gene regulatory system: Global expression profiling and in vivo infection modeling. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13855–13860. [PMC free article: PMC129787] [PubMed: 12370433]
- Gryllos I., Cywes C., Shearer M. H., Cary M., Kennedy R. C., Wessels M. R. Regulation of capsule gene expression by group A Streptococcus during pharyngeal colonization and invasive infection. Molecular Microbiology. 2001;42(1):61–74. [PubMed: 11679067]
- Gryllos I., Grifantini R., Colaprico A., Jiang S., Deforce E., Hakansson A., et al. Mg(2+) signalling defines the group A streptococcal CsrRS (CovRS) regulon. Molecular Microbiology. 2007;65(3):671–683. [PubMed: 17608796]
- Gryllos I., Levin J. C., Wessels M. R. The CsrR/CsrS two-component system of group A Streptococcus responds to environmental Mg2+. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):4227–4232. [PMC free article: PMC153075] [PubMed: 12646707]
- Gryllos I., Tran-Winkler H. J., Cheng M.-F., Chung H., Bolcome R. III, Lu W., et al. Induction of group A Streptococcus virulence by a human antimicrobial peptide. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(43):16755–16760. [PMC free article: PMC2575492] [PubMed: 18936485]
- Heath A., DiRita V. J., Barg N. L., Engleberg N. C. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infection and Immunity. 1999;67(10):5298–5305. [PMC free article: PMC96884] [PubMed: 10496909]
- Henningham A., Yamaguchi M., Aziz R. K., Kuipers K., Buffalo C. Z., Dahesh S., et al. Mutual exclusivity of hyaluronan and hyaluronidase in invasive group a streptococcus. The Journal of Biological Chemistry. 2014;289(46):32303–32315. [PMC free article: PMC4231703] [PubMed: 25266727]
- Hollands A., Pence M. A., Timmer A. M., Osvath S. R., Turnbull L., Whitchurch C. B., et al. Genetic switch to hypervirulence reduces colonization phenotypes of the globally disseminated group A streptococcus M1T1 clone. The Journal of Infectious Diseases. 2010;202(1):11–19. [PMC free article: PMC2880657] [PubMed: 20507231]
- Husmann L. K., Yung D. L., Hollingshead S. K., Scott J. R. Role of putative virulence factors of Streptococcus pyogenes in mouse models of long-term throat colonization and pneumonia. Infection and Immunity. 1997;65(4):1422–1430. [PMC free article: PMC175149] [PubMed: 9119483]
- Hynes W., Johnson C., Stokes M. A single nucleotide mutation results in loss of enzymatic activity in the hyaluronate lyase gene of Streptococcus pyogenes. Microbial Pathogenesis. 2009;47(6):308–313. [PubMed: 19778599]
- Johnson D. R., Stevens D. L., Kaplan E. L. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. The Journal of Infectious Diseases. 1992;166(2):374–382. [PubMed: 1634809]
- Kass E. H., Seastone C. V. The role of the mucoid polysaccharide (hyaluronic acid) in the virulence of group A hemolytic streptococci. The Journal of Experimental Medicine. 1944;79(3):319–330. [PMC free article: PMC2135371] [PubMed: 19871373]
- Kendall F. E., Heidelberger M., Dawson M. H. A serologically inactive polysaccharide elaborated by mucoid strains of group A hemolytic streptococcus. The Journal of Biological Chemistry. 1937;118:61–69.
- Lancefield R. C., Todd E. W. Antigenic differences between matt haemolytic streptococci and their glossy variants. The Journal of Experimental Medicine. 1928;48(6):769–790. [PMC free article: PMC2131507] [PubMed: 19869520]
- Levin J. C., Wessels M. R. Identification of csrR/csrS, a genetic locus that regulates hyaluronic acid capsule synthesis in group A Streptococcus. Molecular Microbiology. 1998;30(1):209–219. [PubMed: 9786197]
- Lynskey N. N., Goulding D., Gierula M., Turner C. E., Dougan G., Edwards R. J., et al. RocA truncation underpins hyper-encapsulation, carriage longevity and transmissibility of serotype M18 group A streptococci. PLoS Pathogens. 2013;9(12):e1003842. [PMC free article: PMC3868526] [PubMed: 24367267]
- Markovitz A., Cifonelli J. A., Dorfman A. The biosynthesis of hyaluronic acid by group A Streptococcus. VI. Biosynthesis from uridine nucleotides in cell-free extracts. The Journal of Biological Chemistry. 1959;234:2343–2350. [PubMed: 14421284]
- Rothbard S. Protective effect of hyaluronidase and type-specific anti-M serum on experimental group A Streptococcus infections in mice. The Journal of Experimental Medicine. 1948;88(3):325–342. [PMC free article: PMC2135829] [PubMed: 18881491]
- Schmidt K. H., Günther E., Courtney H. S. Expression of both M protein and hyaluronic acid capsule by group A streptococcal strains results in a high virulence for chicken embryos. Medical Microbiology and Immunology. 1996;184(4):169–173. [PubMed: 8811648]
- Schrager H. M., Albertí S., Cywes C., Dougherty G. J., Wessels M. R. Hyaluronic acid capsule modulates M protein-mediated adherence and acts as a ligand for attachment of group A Streptococcus to CD44 on human keratinocytes. The Journal of Clinical Investigation. 1998;101(8):1708–1716. [PMC free article: PMC508753] [PubMed: 9541502]
- Schrager H. M., Rheinwald J. G., Wessels M. R. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. The Journal of Clinical Investigation. 1996;98(9):1954–1958. [PMC free article: PMC507637] [PubMed: 8903312]
- Shea P. R., Beres S. B., Flores A. R., Ewbank A. L., Gonzalez-Lugo J. H., Martagon-Rosado A. J., et al. Distinct signatures of diversifying selection revealed by genome analysis of respiratory tract and invasive bacterial populations. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):5039–5044. [PMC free article: PMC3064369] [PubMed: 21383167]
- Stollerman G. H., Rytel M., Ortiz J. Accessory plasma factors involved in the bactericidal test for type-specific antibody to group A streptococci. II. Human plasma cofactor)s) enhancing opsonization of encapsulated organisms. The Journal of Experimental Medicine. 1963;117(1):1–17. [PMC free article: PMC2180439] [PubMed: 13984363]
- Stoolmiller A. C., Dorfman A. The biosynthesis of hyaluronic acid by Streptococcus. The Journal of Biological Chemistry. 1969;244(2):236–246. [PubMed: 5773295]
- Sugahara K., Schwartz N. B., Dorfman A. Biosynthesis of hyaluronic acid by Streptococcus. The Journal of Biological Chemistry. 1979;254(14):6252–6261. [PubMed: 376529]
- Tamayo E., Montes M., García-Medina G., García-Arenzana J. M., Pérez-Trallero E. Spread of a highly mucoid Streptococcus pyogenes emm3/ST15 clone. BMC Infectious Diseases. 2010;10:233. [PMC free article: PMC2921389] [PubMed: 20687911]
- Todd E. W., Lancefield R. C. Variants of hemolytic streptococci; their relation to type specific substance, virulence, and toxin. The Journal of Experimental Medicine. 1928;48(6):751–767. [PMC free article: PMC2131511] [PubMed: 19869519]
- Tran-Winkler H. J., Love J. F., Gryllos I., Wessels M. R. Signal transduction through CsrRS confers an invasive phenotype in group A Streptococcus. PLoS Pathogens. 2011;7(10):e1002361. [PMC free article: PMC3203184] [PubMed: 22046138]
- Unnikrishnan M., Cohen J., Sriskandan S. Growth-phase-dependent expression of virulence factors in an M1T1 clinical isolate of Streptococcus pyogenes. Infection and Immunity. 1999;67(10):5495–5499. [PMC free article: PMC96913] [PubMed: 10496938]
- Veasy L. G., Tani L. Y., Daly J. A., Korgenski K., Miner L., Bale J., et al. Temporal association of the appearance of mucoid strains of Streptococcus pyogenes with a continuing high incidence of rheumatic fever in Utah. Pediatrics. 2004;113(3 Pt 1):e168–e172. [PubMed: 14993572]
- Velarde J. J., Ashbaugh M., Wessels M. R. The human antimicrobial peptide LL-37 binds directly to CsrS, a sensor histidine kinase of group A Streptococcus, to activate expression of virulence factors. The Journal of Biological Chemmistry. 2014;289(52):36315–36324. [PMC free article: PMC4276891] [PubMed: 25378408]
- Ward H. K., Lyons C. Studies on the Hemolytic Streptococcus of Human Origin : I. Observations on the Virulent, Attenuated, and Avirulent Variants. The Journal of Experimental Medicine. 1935;61(4):515–530. [PMC free article: PMC2133238] [PubMed: 19870376]
- Weigel P. H., Hascall V. C., Tammi M. Hyaluronan synthases. The Journal of Biological Chemistry. 1997;272(22):13997–14000. [PubMed: 9206724]
- Wessels M. R., Bronze M. S. Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(25):12238–12242. [PMC free article: PMC45412] [PubMed: 7991612]
- Wessels M. R., Moses A. E., Goldberg J. B., DiCesare T. J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(19):8317–8321. [PMC free article: PMC52499] [PubMed: 1656437]
- Wilson A. T. The relative importance of the capsule and the M antigen in determining colony form of group A streptococci. The Journal of Experimental Medicine. 1959;109(3):257–270. [PMC free article: PMC2136946] [PubMed: 13620853]
- Review Cell Wall and Surface Molecules of Streptococcus pyogenes: Capsule.[Streptococcus pyogenes: Basic ...]Review Cell Wall and Surface Molecules of Streptococcus pyogenes: Capsule.Wessels MR. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. 2022 Oct 8
- Capsular Polysaccharide of Group A Streptococcus.[Microbiol Spectr. 2019]Capsular Polysaccharide of Group A Streptococcus.Wessels MR. Microbiol Spectr. 2019 Jan; 7(1).
- Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci.[Proc Natl Acad Sci U S A. 1991]Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci.Wessels MR, Moses AE, Goldberg JB, DiCesare TJ. Proc Natl Acad Sci U S A. 1991 Oct 1; 88(19):8317-21.
- Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus.[Infect Immun. 1997]Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus.Moses AE, Wessels MR, Zalcman K, Albertí S, Natanson-Yaron S, Menes T, Hanski E. Infect Immun. 1997 Jan; 65(1):64-71.
- Review Human disease isolates of serotype m4 and m22 group a streptococcus lack genes required for hyaluronic acid capsule biosynthesis.[mBio. 2012]Review Human disease isolates of serotype m4 and m22 group a streptococcus lack genes required for hyaluronic acid capsule biosynthesis.Flores AR, Jewell BE, Fittipaldi N, Beres SB, Musser JM. mBio. 2012 Nov 6; 3(6):e00413-12. Epub 2012 Nov 6.
- Cell Wall and Surface Molecules: Capsule - Streptococcus pyogenesCell Wall and Surface Molecules: Capsule - Streptococcus pyogenes
Your browsing activity is empty.
Activity recording is turned off.
See more...
