Step 1. Identification and Abstraction of Research Gaps from Evidence Report (2008) and Five Published Manuscripts
Our research team abstracted research gaps from the 2008 evidence report on GDM7 and from the five published manuscripts8–12 derived from the report (see Appendix A). We organized these research gaps by key question and within each key question, by gap area using the PICO framework. Concerns with limitations in study designs were also presented by key question. Specific gaps included research in diverse racial/ethnic populations and the type of GDM (diet-controlled, oral medications-requiring, insulin-requiring); the interventions and comparisons that had been the focus on the 2008 report; and many of the same short-term and long-term outcomes included in the 2008 report. In addition, we highlighted some of the challenges related to study design, such as the need for well-designed long-term observational cohort studies to better understand the progression to type 2 diabetes in women with GDM.
In addition to gaps that were specific to a key question, we abstracted general research gaps, also in the PICO and study design format. Examples included interventions to improve compliance with postpartum screening for type 2 diabetes and the comparative effectiveness of strategies (medication, behavioral) to prevent obesity and various forms of glucose intolerance (insulin resistance, impaired fasting glucose, impaired glucose tolerance, type 2 diabetes, etc.) in women with GDM (see Appendix A).
Figure 2 shows the conceptual model depicting the overall location of each of the original report’s key questions and the main types of outcomes. Due to the widespread deficiencies in the literature, this conceptual model only outlines the overall location of the key questions and examples of types of interventions and outcomes. Specific research questions are outlined in individual conceptual models for each key question.
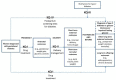
Figure 2
Overall conceptual model for all key questions (KQ-I to KQ-IV) from original (2008) evidence report on gestational diabetes.
Step 2. Feedback from Authors of 2008 Evidence Report
Five out of eight of the other authors of the 2008 report (response rate=62.5 percent) provided feedback on the gaps identified in Step 1. We included all of their comments, clarifications, and additional suggested gaps in Appendix A.
For key question I (see Table 1), the authors highlighted the need for future studies to use consistent definitions of clinically relevant outcomes, such as hypoglycemia, to improve comparisons across studies. One author advised consideration of other medication comparators such as thiazolidinediones and dipeptidyl peptidase-4 (DPP-4) inhibitors. For key question II (see Table 1), authors stated that there is a need for randomized controlled trials (RCTs), but noting the challenges of conducting trials in this population, suggested that large prospective observational studies are needed to address this question. For key question III (see Table 1), authors suggested examining risk factors, such as psychosocial factors (e.g., depression, perceived stress), and their association with developing type 2 diabetes. For key question IV (see Table 1), authors suggested considering cluster randomized trials since they are more effectiveness-based, and may incorporate “real world” screening practices and outcomes.
Step 3. Translation of Research Gaps into Researchable Questions
We incorporated the feedback from the authors of the 2008 report and translated the list of gaps into a list of seventeen research questions (see Appendix B). Of these research questions, six related to key question I (benefits and harms of oral diabetes agents as compared to all types of insulin); three related to key question II (benefits and harms of elective cesarean delivery or the choice of timing of induction); five related to key question III (risk factors associated with short-term and long-term development of type 2 diabetes following a pregnancy with GDM); and three related to key question IV (performance characteristics [sensitivity, specificity, and reproducibility] of tests for diagnosing type 2 diabetes following a pregnancy with GDM).
Step 4. Online and In-Person Feedback on Research Questions from Local Stakeholders
Online Feedback
We sent the seventeen research questions to the six local stakeholders for their online feedback. For each research question, we asked the stakeholders to provide specific comments on the clarity of wording, and rate (on a scale of 1=low to 9=high) the likely clinical benefit/importance and the likely ability to conduct a study that would address the research question. For each research question, Appendix B displays their comments, as well as the mean and range of their ratings of the clinical benefit/importance and the likely ability to conduct studies to address each research question. We received online feedback from each of the six local stakeholders (response rate=100 percent). The mean online feedback completion time was 78 minutes (range 20 to 289 minutes). We have summarized the results by key question below:
Key question I—benefits and harms of oral diabetes agents as compared to all types of insulin for treatment of GDM. We identified six research questions related to key question I. Research question I-1 (comparative effectiveness and safety of any second generation sulfonylurea vs. any insulin) and research question I-2 (comparative effectiveness and safety of metformin vs. any insulin) received the highest ratings for clinical benefit/importance (each with mean 7.8, range 7 to 9). Research question I-3 (comparative effectiveness and safety of any oral hypoglycemic medication [i.e., a second generation sulfonylurea or metformin] vs. any insulin) was rated as the question researchers were most likely to be able to conduct a study to address (mean 7.8, range 7 to 9).
Key question II—benefits and harms of elective cesarean delivery or the choice of timing of induction. We identified three research questions related to key question II. Research question II-1 (comparative effectiveness and safety of elective labor induction vs. expectant management at term) received the highest rating for clinical benefit/importance (mean 7.7, range 7 to 8). Research question II-3 (comparative effectiveness and safety of elective labor induction or cesarean delivery vs. expectant management at term in women with insulin-requiring [class A2] GDM) received the highest rating for likely ability to conduct a study (mean 6.0, range 4 to 7).
Key question III—risk factors associated with short-term and long-term development of type 2 diabetes following a pregnancy with GDM. We identified five research questions related to key question III. Research question III-1 (the effect of maternal lifestyle risk factors [e.g., behavioral risk factors, breastfeeding, physical activity, daily caloric intake]) received the highest rating for clinical benefit/importance (mean 8.2, range 7 to 9). Research question III-4 (the effect of maternal co-morbidities [e.g., obesity, hypertension, hypercholesterolemia]) received the highest rating for likely ability to conduct a study (mean 7.0, range 6 to 9).
Key question IV—performance characteristics (sensitivity, specificity, and reproducibility) of tests for diagnosing type 2 diabetes following a pregnancy with GDM. We identified three research questions related to key question IV. Research question IV-1 (accuracy of a single FBG test compared to the full 2-hour 75-gm OGTT and whether the comparative accuracy varies with the postpartum testing interval) received the highest ratings for clinical benefit/importance (mean 7.7, range 6 to 9) and likely ability to conduct a study (mean 6.8, range 5 to 8).
In-Person Meeting To Provide Additional Feedback
We revised the seventeen questions based on the local stakeholders’ online feedback. We prepared a presentation to provide background and share the results of the 2008 report, and presented the revised questions and changes at the in-person followup meeting. Five of the six local stakeholders attended the meeting (response rate = 83.3 percent) and it lasted 90 minutes. For each research question, Appendix B displays the comments from the in-person meeting in the last column. We have summarized the comments by key question below:
Key question I—benefits and harms of oral diabetes agents as compared to all types of insulin for treatment of GDM. Overall, the stakeholders agreed that the six included research questions were clinically relevant. Some expressed concern about conducting studies of the newer medications, which have little evidence for use in pregnancy. There was agreement of the need to include patient-centered outcomes, such as quality of life, as well as long-term developmental and growth outcomes of the offspring.
Key question II—benefits and harms of elective cesarean delivery or the choice of timing of induction. Stakeholders discussed the use of the term “delivery” vs. “labor.” We subsequently elected to change the questions to include the term “delivery,” as well as to clarify that “term” is considered 40-weeks gestation. The stakeholders also highlighted the need for observational studies to include data with variation in both maternal and estimated fetal weight (EFW) to enable stratification. Stakeholders agreed that designing and conducting RCTs to address key question II would have many challenges, particularly with regards to providers not following random allocation and high levels of crossover between arms.
Key question III—risk factors associated with short-term and long-term development of type 2 diabetes following a pregnancy with GDM. We asked stakeholders to review the list of risk factors included in the research questions in key question III. The stakeholders suggested inclusion of eating disorders and anxiety disorders among the psychosocial factors, interpregnancy interval, and number of pregnancies with GDM.
Key question IV—performance characteristics [sensitivity, specificity, and reproducibility] of tests for screening for type 2 diabetes following a pregnancy with GDM. Stakeholders agreed that patient and provider compliance with postpartum testing are probably of higher importance than assessment of the performance characteristics of the various screening tests.
The following questions were removed as separate questions, but the concepts from these questions were included in revised questions that were retained:
- What is the comparative effectiveness and safety of any oral hypoglycemic medication (i.e., a second generation sulfonylurea or metformin) compared with any insulin in the treatment of gestational diabetes with regard to the following maternal and neonatal outcomes? (Key Question I)
- What is the comparative effectiveness and safety of a short-acting insulin compared to diet alone in the treatment of gestational diabetes with regard to maternal and neonatal outcomes? (Key Question I)
- What is the comparative effectiveness and safety of elective labor induction or cesarean delivery compared to expectant management at term in the management of labor in women with insulin-requiring (class A2) gestational diabetes with regard to the following maternal and neonatal outcomes? (Key Question II)
- What is the reproducibility of the 2-hour 75-gram OGTT vs. a single fasting blood glucose test vs. a single HbA1c test in diagnosing type 2 diabetes following a pregnancy with gestational diabetes? (Key Question IV)
The following questions were added based on feedback from local stakeholders:
- What is the evidence that the inter-conception interval is associated with the risk of developing type 2 diabetes or glucose intolerance/prediabetes following a pregnancy with gestational diabetes? (Key Question III)
- What is the evidence that family history, gene mutations, genotypes, gene-environment interactions, epigenetic modifications, or other biomarkers are associated with the risk of developing type 2 diabetes among women with gestational diabetes? Are there differences in these associations by race or ethnic group? (Key Question III)
- What is the comparative effectiveness of various lifestyle interventions for prevention of type 2 diabetes mellitus, glucose intolerance, and obesity in women with a history of gestational diabetes? (Key Question III)
- What is the comparative effectiveness of various innovative strategies and technologies to disseminate educational materials on prevention of type 2 diabetes mellitus and glucose intolerance to women with a history of gestational diabetes? (Key Question III)
- What is the comparative effectiveness of various strategies or interventions to improve clinician compliance with postpartum screening guidelines for type 2 diabetes mellitus and glucose intolerance in women with a history of gestational diabetes? (Key Question IV)
- What is the comparative effectiveness of various innovative strategies or technologies (e.g., electronic health records) to track postpartum screening for the development of type 2 diabetes mellitus and glucose intolerance in women with a history of gestational diabetes? (Key Question IV)
Step 5. Online Feedback, Consensus Development, and Prioritization of Research Questions by External Stakeholders (Delphi Approach)
We further refined the research questions after feedback and suggestions from the local stakeholders at the in-person meeting. This refined list included nineteen research questions: four related to key question I, two related to key question II, nine related to key question III, and four related to key question IV. We sent these nineteen research questions to the nine external stakeholders for their feedback. The stakeholders participated in three Delphi rounds, with the goal of providing constructive feedback about the wording and clarity of each of the research questions and developing consensus on the likely clinical benefit/importance of addressing the research question. Stakeholders rated the latter question on a 9-point Likert scale (1=lowest clinical benefit/importance and 9=highest clinical benefit/importance).
We achieved a response rate of 100 percent for each Delphi round. We have described below the results from each of the Delphi rounds and highlight the sixteen research questions that achieved consensus by the third round (rated as low, medium, or high clinical benefit/importance) and the three that did not achieve consensus by the third round.
Delphi Round 1
In Delphi round 1, we sent the external stakeholders each of the nineteen research questions (see Appendix C). The mean online feedback completion time for Delphi round 1 by external stakeholders was 63 minutes (range 21 to 112 minutes). Among these nineteen research questions, consensus of high clinical benefit/importance was established on eight (42.1 percent) questions.
Two (I-1 and I-2) of four research questions related to key question I, two (II-1 and II-2) of two research questions related to key question II, three (III-1, III-7, and III-8) of nine questions related to key question III, and one (IV-3) of four questions related to key question IV achieved consensus of having high clinical benefit/importance (see Appendix C).
Regarding the two research questions (I-1 and I-2) related to key question I that were rated as high clinical benefit/importance, stakeholders expressed concern that use of oral hypoglycemic agents is now common clinical practice even though their effectiveness and safety has not clearly been established (few published RCTs). There was also interest in examining long-term effects of treatment on offspring, particularly metformin as an insulin-sensitizer. Research question I-3 did not achieve consensus because there was confusion about how the insulin comparisons were phrased. This was clarified based on feedback from the stakeholders. For research question I-4 stakeholders expressed concerns about safety of use of hypoglycemic medications newly approved by the Food and Drug Administration (FDA), both in and outside of pregnancy (see Appendix D).
The two research questions (II-1 and II-2) related to key question II were considered of high clinical benefit/importance because of a dearth of evidence in this clinically important area. Stakeholders emphasized the importance of including patient-oriented outcomes for these questions.
For key question III, stakeholders agreed that the overall question identifying risk factors for type 2 diabetes in women with GDM was of high importance, particularly to guide future preventive interventions. In this Delphi round, they highlighted which risk factors were of the highest clinical importance for future research: maternal lifestyle factors (III-1); family history, gene mutations, genotype and gene-environment interactions (III-7); and lifestyle interventions (III-8).
For key question IV, stakeholders agreed that focusing on low compliance with postpartum screening for type 2 diabetes (IV-3) was more clinically important than the other questions addressing the screening tests’ performance characteristics (see Appendix D).
External stakeholders’ comments about study needs and challenges. In Delphi round 1, in addition to comments about clinical importance, the external stakeholders commented on the study needs and challenges identified during the in-person local stakeholder meeting. These comments are included in Appendix D. For key question I, the external stakeholders emphasized the importance of having a standard GDM diagnosis; designing studies with longer term followup of offspring to assess obesity; and suggested the inclusion of a research question addressing optimal glucose thresholds for starting treatment. For key question II, external stakeholders agreed with the local stakeholders on the practical challenges and barriers to designing an RCT to address these research questions, in particular in women without evidence of elevated EFW. For key question III, stakeholders advised including the intervention of lactation support. They also emphasized the importance of when risk factors were measured—pre, during, and/or post pregnancy (this was subsequently clarified for relevant questions). For key question IV, stakeholders suggested that the ideal study design to assess performance characteristics of screening tests for diabetes would include long-term assessment of complications.
Delphi Round 2
In Delphi round 2, we sent the external stakeholders the eleven research questions that had not achieved consensus in Delphi round 1. The mean online feedback completion time for Delphi round 2 by external stakeholders was 63 minutes (range 11 to 355 minutes).
Consensus was achieved on six (54.6 percent) of these eleven questions. Consensus of high clinical benefit/importance was established on five (45.5 percent) research questions. These included one (I-3) of two questions related to key question I, three (III-3, III-4, and III-9) of six questions related to key question III, and one (IV-2) of three questions related to key question IV. Consensus of medium clinical benefit/importance was established on one research question (III-6) related to key question III (see Appendix C).
After re-wording research question I-3 for clarity of the insulin comparisons, consensus of high clinical benefit/importance was established in Delphi round 2. For research question III-4, we re-structured the question to specify the time periods for risk factor measurement as “prepregnancy,” “antenatal,” and “postpartum.” The question then received consensus of high clinical benefit/importance. During all three Delphi rounds, only one research question achieved consensus of medium clinical benefit/importance. This was research question III-6, focused on inter-conception interval as a risk factor. Stakeholders highlighted the high degree of confounding with this question, such as access to care, contraception, and postpartum weight retention. They thus did not rate it as highly as some of the other risk factors. For key question IV, stakeholders had higher interest in comparing HbA1c as a postpartum screening test (IV-2) than FBG (IV-1) with the gold standard OGTT (see Appendix E).
Delphi Round 3
In Delphi round 3, we sent the external stakeholders the five research questions that had not achieved consensus in Delphi round 2. The mean online feedback completion time for Delphi round 3 by external stakeholders was 16 minutes (range 3 to 31 minutes). Among these five research questions, consensus of high clinical benefit/importance was achieved on two (40 percent) questions. These questions included one research question (I-4) related to key question I and one (IV-1) of two questions related to key question IV (see Appendix C).
Stakeholders achieved consensus on research question I-4, related to the use of newly FDA-approved oral hypoglycemic medications to treat GDM. Although consensus was reached, two stakeholders emphasized the importance of establishing the safety of metformin and glyburide before beginning research on newer agents not currently used in clinical practice. For key question IV, stakeholders reached consensus to include research question IV-1 (comparing a single FBG with the OGTT) as one of high clinical benefit/importance (see Appendix F).
Research Questions for Which No Consensus Was Established After Three Delphi Rounds
Table 3 lists the research questions for which no consensus was established. These included two research questions (III-2 and III-5) related to key question III and one (IV-4) related to key question IV.
Table 3
Research questions where no consensus was established or consensus of medium or low clinical benefit/importance was established.
Research question III-2 addressed the association of maternal psychosocial factors (e.g., mood disorders, substance use disorders, eating disorders, stress) with the risk of developing type 2 diabetes or glucose intolerance/impaired fasting glucose following a pregnancy with GDM. Stakeholders expressed uncertainty about the relevance of these risk factors to pregnancy, compared to the development of diabetes in the general population. Research question III-5 addressed whether contraceptive method (e.g., progestin-only) was associated with the risk of developing type 2 diabetes or glucose intolerance/impaired fasting glucose following a pregnancy with GDM. Some stakeholders determined this question to be of lower clinical importance. This question was complicated by timing of contraceptive use and consistency/compliance with a method. Some stakeholders expressed that the impact of progestin-only contraceptive methods on glucose tolerance was known and that the research question did not address a knowledge gap.
One research question related to key question IV did not achieve consensus. Research question IV-4 addressed the comparative effectiveness of health information technology interventions to track postpartum screening for the development of type 2 diabetes mellitus and glucose intolerance/impaired fasting glucose in women with a history of GDM. This was a newer question that had been added during the in-person discussion with local stakeholders. However, there was no consensus among external stakeholders about the clinical benefit/importance of this question.
Research Question for Which Consensus of Medium Clinical Benefit/Importance Was Established
Table 3 also lists the one research question (III-6) for which consensus of medium clinical benefit/importance was established. This question, related to key question III, addressed whether inter-conception interval is associated with the risk of developing type 2 diabetes or glucose intolerance/impaired fasting glucose following a pregnancy with GDM.
Step 6. Prioritization of Outcomes for Key Questions I and II
Prioritized Outcomes for Research Questions Related to Key Question I
Research questions related to key question I included thirty outcomes of interest (thirteen short-term maternal outcomes, four long-term maternal outcomes, ten neonatal outcomes, and three long-term offspring outcomes). Table 4 lists the prioritized outcomes for research questions related key question I (benefits and harms of oral diabetes agents as compared to all types of insulin). Four of nine stakeholders ranked the long-term offspring outcome of chronic diseases (e.g., obesity, type 2 diabetes) in one of their top three, making it the highest rated outcome. The next most highly rated outcomes were the short-term maternal outcomes of hypertensive disorders of pregnancy (e.g., gestational hypertension, preeclampsia) and medication adherence, and the neonatal outcome of large for gestational age and macrosomia. Three of nine stakeholders rated each of these three outcomes as one of their top three to study.
Table 4
Results from step 6 (prioritization of outcomes for research questions related to key question I (KQ-I)).
Prioritized Outcomes for Research Questions Related to Key Question II
Research questions related to key question II included nineteen outcomes of interest (nine maternal outcomes and ten neonatal outcomes). Table 5 lists the prioritized outcomes for research questions related key question II (benefits and harms of elective cesarean delivery or the choice of timing of induction). The most important outcome was the maternal outcome of cesarean delivery (including primary cesarean and repeat cesarean) and indication for cesarean delivery, which six of nine stakeholders ranked as one of their top three. The next most important outcomes were the neonatal outcomes of birth trauma (e.g., bone fractures, brachial plexus palsy), which four of nine stakeholders ranked as one of their top three, and neonatal intensive care unit (NICU) admission, which three of nine stakeholders ranked as one of their top three.
Table 5
Results from step 6 (prioritization of outcomes for research questions related to key question II (KQ-II).
Step 7. Refinement of Final Research Questions and Development of Conceptual Models to Display Research Gaps
Final Research Questions
Through the three Delphi rounds, fifteen of the nineteen research questions achieved consensus of high clinical benefit/importance and one research question achieved consensus of medium clinical benefit/importance, yielding an overall Delphi consensus establishment rate of 84.2 percent.
Research questions of high clinical benefit/importance. Table 6 lists the fifteen final research questions of high clinical benefit/importance. These include four research questions related to key question I, two related to key question II, six related to key question III, and three related to key question IV. The research question that was rated the highest (mean 8.2 on a scale of 1 to 9, range 7 to 9) in terms of clinical benefit/importance was research question I-1. This research question compared the effectiveness and safety of any of the second generation sulfonylureas with any insulin in the treatment of GDM. Research question III-1 was rated the next highest (mean 8.1 on a scale of 1 to 9, range 7 to 9) and addressed whether maternal health behaviors (e.g., breastfeeding, physical activity, diet) are associated with the risk of developing type 2 diabetes or glucose intolerance/impaired fasting glucose following a pregnancy with GDM.
Table 6
Final list of research questions rated as high clinical benefit/importance.
Development of Conceptual Models To Display Research Gaps
For each of the original key questions, we developed a conceptual model to pictorially display the identified research questions, according to the population, and outcomes (see Figures 3, 4, 5, and 6 for conceptual models for research questions related to key questions I, II, III, and IV respectively).

Figure 3
Results from step 7 (conceptual model to display research questions related to key question I (KQ-I)). Abbreviations: DPP-4=dipeptidyl peptides-4, GLP-1=glucagon-like peptide-1.

Figure 4
Results from step 7 (conceptual model to display research questions to key question II (KQ-II)). Abbreviations: EFW=estimated fetal weight.
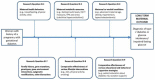
Figure 5
Results from step 7 (conceptual model to display research questions related to key question III (KQ-III). Abbreviations: HPA=hypothalamo-pituitary-adrenal, OGTT=oral glucose tolerance test.
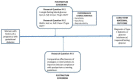
Figure 6
Results from step 7 (conceptual model to display questions related to key question IV (KQ-IV). Abbreviations: HbA1c=hemoglobin A1c, OGTT=oral glucose tolerance test.
Step 8. Evaluation of Entire Process by Evidence Report Authors, Local Stakeholders, and External Stakeholders
We sent an online evaluation form to the twenty contributors to this project, which included five authors of the 2008 evidence report, six local stakeholders, and nine external stakeholders. We received feedback from each of the twenty contributors (response rate=100 percent). The mean feedback completion time for the evaluation form was 12 minutes (range 2 to 72 minutes). Contributors were asked to review the report materials (text, tables, figures, and appendixes) before completing the form. The time taken to review these materials was not recorded.
Responses to the evaluation are listed in Appendix G for the 2008 report authors and the local stakeholders and in Appendix H for the external stakeholders. All twenty contributors felt that they had adequate information to effectively participate and that we had accomplished our objective of identifying important research questions for GDM. A web-based form was the most preferred mode of participation (fifteen contributors, 75 percent). Two (10 percent) contributors (both local stakeholders) preferred in-person participation to a web-based form, while another two (one 2008 evidence report author and one local stakeholder) preferred a combination of a web-based form and in-person participation. One (5 percent) contributor preferred participation via telephone.
Fifteen (79 percent) of nineteen contributors felt that the composition of the local group of stakeholders was comprehensive. One external stakeholder felt unable to comment on the comprehensiveness of the local stakeholder group. Fifteen (75 percent) of twenty contributors felt that the composition of the external group of stakeholders was comprehensive. The following additional perspectives were suggested—endocrinologists/diabetologists managing women with GDM, neonatologists, nephrologists, and patients with current GDM or with a history of GDM.
One (5 percent) contributor felt that we could have abbreviated our process, questioning the need for a local stakeholder group.
Publication Details
Copyright
Publisher
Agency for Healthcare Research and Quality (US), Rockville (MD)
NLM Citation
Bennett WL, Nicholson WK, Saldanha IJ, et al. Future Research Needs for the Management of Gestational Diabetes [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2010 Nov. (Future Research Needs Papers, No. 7.) Results.




