2.1. The challenge of HCV elimination
WHO estimated that in 2015, 71 million persons were living with chronic HCV infection worldwide (global prevalence: 1%) and that 399 000 had died from cirrhosis or hepatocellular carcinoma (HCC) (9). Aside from the burden of HCV infection secondary to liver-related sequelae, HCV causes an additional burden through comorbidities among persons with HCV infection, including depression, diabetes mellitus and chronic renal disease. A proportion of these morbidities is directly attributable to HCV and is therefore referred to as extrahepatic manifestations. These manifestations are likely to be affected by treatment (see
Chapter 4 and ). The World Health Assembly recognized that viral hepatitis is a major public health problem and passed two initial resolutions in 2010 (10) and 2014 (11).
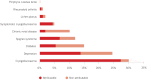
Prevalence of comorbidities among persons with HCV infection, including the fraction that is attributable to HCV infection (calculated on the basis of Younossi et al. 2016, using attributable fractions among those exposed).
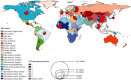
WHO estimated that in 2015, 1.75 million new HCV infections occurred, mostly because of injecting drug use and unsafe health care (9). Worldwide, HCV infection may be caused by one of six major HCV genotypes () (12). However, in many countries, the genotype distribution remains unknown (13).
Worldwide distribution of HCV genotypes. Source: The Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–76. Disclaimer: (more...)
In May 2016, the World Health Assembly endorsed the Global Health Sector Strategy (GHSS) for 2016–2021 on viral hepatitis (HBV and HCV infection), which proposes to eliminate viral hepatitis as a public health threat by 2030. Elimination is defined as a 90% reduction in new chronic infections and a 65% reduction in mortality compared with the 2015 baseline (14). To reach these targets, the GHSS recommends scaling up currently available prevention interventions and introducing newer programmatic components, such as testing and treatment. Elimination of HCV infection as a public health threat requires diagnosing 90% of those infected and treating 80% of those diagnosed. However, in 2015, there were large deficits in achieving these service coverage objectives. Of the 71 million persons with HCV infection, 14 million (20%) had been diagnosed (a 70% gap), and of the 14 million diagnosed, 1.1 million (7%) had been started on treatment (a 73% gap) (9).
2.1.1. Natural history of HCV infection
Hepatitis
HCV infection causes both acute and chronic hepatitis. Incident infection is associated with early symptoms in about 20% of persons. Spontaneous clearance occurs within six months of infection in 15–45% of infected individuals in the absence of treatment. The remaining 55–85% develop chronic infection, which can lead to progressive fibrosis and cirrhosis. The risk of cirrhosis ranges from 15% to 30% after 20 years of infection with HCV (15-17). Initially, cirrhosis may be compensated. Decompensation may occur later, leading to variceal haemorrhages, ascites or encephalopathy (18). Each year, approximately 1–3% of persons with cirrhosis progress to hepatocellular carcinoma (HCC) (19). The risk of progression to cirrhosis and HCC varies according to the person’s characteristics and behaviours. Alcohol use, HBV or HIV coinfection and immunosuppression due to any cause increase the risk of developing cirrhosis or HCC (20).
Extrahepatic manifestations
HCV infection can lead to extrahepatic manifestations (21). Among HCV-infected persons, the three most common comorbidities are depression (24%), diabetes mellitus (15%) and chronic renal disease (10%). A proportion of these morbidities is directly attributable to HCV and is therefore referred to as extrahepatic manifestations (). Extrahepatic manifestations are likely to be affected by treatment (in red in , for example, only 37% of diabetes among HCV-infected persons would be attributable to HCV infection). The prevalence of these extrahepatic manifestations is usually independent of the degree of liver fibrosis (22, 23).
2.1.2. Natural history of HIV/HCV coinfection
Coinfection with HIV adversely affects the course of HCV infection. Coinfected persons, particularly those with advanced immunodeficiency (CD4 count <200 cells/mm3), have significantly accelerated progression to cirrhosis, decompensated cirrhosis and HCC compared to HCV-monoinfected persons (24-26). In high-income countries (HICs), HCV-associated liver disease has become a leading cause of death in people living with HIV, accounting for almost half (47%) of the deaths in the United States (27, 28). It is unclear whether HCV infection accelerates HIV disease progression, but after initiation of antiretroviral therapy (ART), CD4 recovery is impaired in HIV/HCV-coinfected persons when compared to those with HIV monoinfection (29, 30). HIV/HCV-coinfected persons have demonstrated more rapid HIV disease progression compared to those who were HIV-infected alone in some but not all studies (31-33). Assessment of the impact of HCV infection on HIV disease progression may be confounded by the negative health consequences of injecting drug use, which is strongly associated with HCV infection (34, 35). In persons with HIV coinfection, HCC tends to occur at a younger age and within a shorter time period (36, 37).
2.1.3. Routes of transmission
Health-care-associated transmission
In countries where infection control measures are insufficient, HCV infection is associated with unsafe injection practices and procedures such as renal dialysis, surgery, dental care and unscreened blood transfusions (38-41). Worldwide, in 2010, 5% of health-care injections were given with unsterilized, reused injection devices (42) and unsafe injections were estimated to lead to 315 000 new HCV infections each year (43). In addition, excessive use of injections to administer medications is a matter of concern (44). Coupled with poor injection practices, overuse of injections further increases HCV transmission. This persisting driver of transmission needs to be addressed through safer health care, introduction of reuse-prevention devices (45) and a reduction in unnecessary health-care injections.
Transmission among people who inject drugs
Globally, injection drug use may account for 23% of new HCV infections; 8% of current HCV infections are among PWID (9). PWID infected with HCV are at increased risk of all-cause mortality, reflecting the combined role of injecting drug use, low socioeconomic status, poor access to health care and environmental factors (46, 47).
Other modes of transmission
Other modes of HCV transmission include mother-to-child transmission, which affects 4–8% of children born to women with HCV infection and 10.8–25% of children born to women with HIV/HCV coinfection (48), other percutaneous procedures, such as tattooing and body piercing (49), and needlestick injuries in health-care workers (50, 51). Sexual transmission of HCV occurs infrequently in heterosexual couples. However, it is more frequent in HIV-positive persons, particularly in men who have sex with men (MSM) (52).
2.2. Direct-acting antivirals
As of May 2018, the FDA or the EMA had approved 13 direct-acting antivirals from four classes (see
), and several fixed-dose combination (FDC) DAAs for the treatment of persons with HCV infection.
Direct-acting antivirals (DAAs) according to class.
2.2.1. Summary of the currently available pangenotypic DAA combinations
DAAs are considered pangenotypic when they achieve high treatment efficacy across all six major HCV genotypes.
Sofosbuvir/velpatasvir
Sofosbuvir/velpatasvir is an FDC of a pangenotypic NS5A inhibitor and sofosbuvir. It was approved both by the FDA and EMA in 2016. In clinical trials, it is associated with good efficacy in infections with genotypes 1–6, HIV/HCV coinfection, persons on opioid substitution therapy (OST) and persons with compensated or decompensated cirrhosis (53-57).
Sofosbuvir/velpatasvir/voxilaprevir
Sofosbuvir/velpatasvir/voxilaprevir is generally considered for use in the retreatment of HCV-infected persons who previously failed a DAA regimen (see also
section 5.2.6 on retreatment of persons with DAA failure); however, in some HICs it is also registered for treatment-naive HCV-infected persons.
Glecaprevir/pibrentasvir
Glecaprevir/pibrentasvir is an FDC containing a pangenotypic NS3/4A protease inhibitor with a pangenotypic NS5A inhibitor that was approved by the FDA and EMA in 2017. In clinical trials, glecaprevir/pibrentasvir suggest good efficacy in infections with genotypes 1–6, compensated cirrhosis, including in persons with renal insufficiency and end-stage renal disease (58-64). It is contraindicated in persons with decompensated cirrhosis (Child–Pugh Class C).
Sofosbuvir/daclatasvir
Daclatasvir, an NS5A inhibitor that has been evaluated with sofosbuvir, was approved by the EMA in 2014 and by the FDA in 2015. Clinical trials reported good efficacy of the combination of daclatasvir and sofosbuvir in infections with genotypes 1–4, persons with decompensated liver disease, liver transplant recipients and those with HIV/HCV coinfection (65-68). Recent data suggest that the combination of sofosbuvir/daclatasvir is also effective in infections with genotypes 5 and 6 (69) (Médecins Sans Frontières [MSF] demonstration project, manuscript in preparation).
Other DAA regimens
Additional evidence being generated may indicate in the future that other DAA regimens (e.g. sofosbuvir/ravidasvir) are pangenotypic or that existing pangenotypic DAA regimens can be used in more populations (e.g. children and adolescents <18 years of age).
2.3. Access to direct-acting antivirals
DAAs for HCV infections have been initially sold at a very high price, limiting access. Opportunities to access low-price generic medicines are increasing, particularly in LMICs (4). (See Strategies for more efficient procurement and supply management of medicines and diagnostics in section 6.7, Table 6.2.)