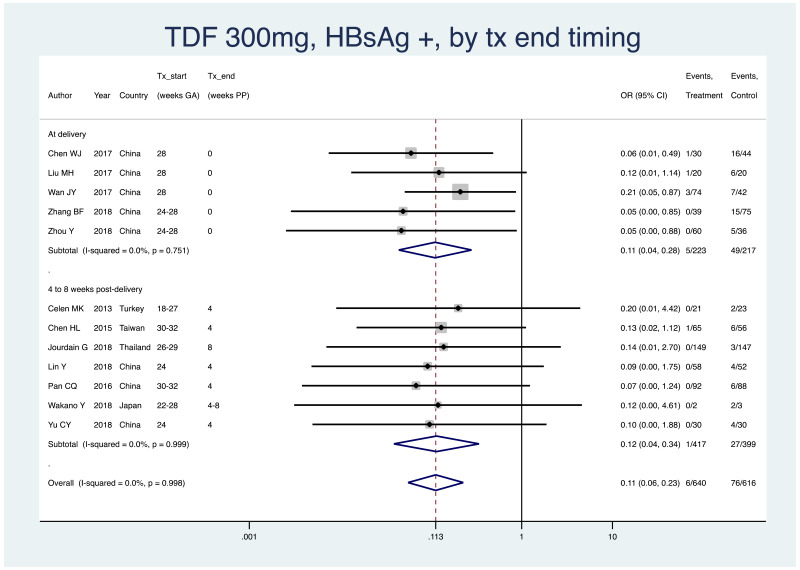
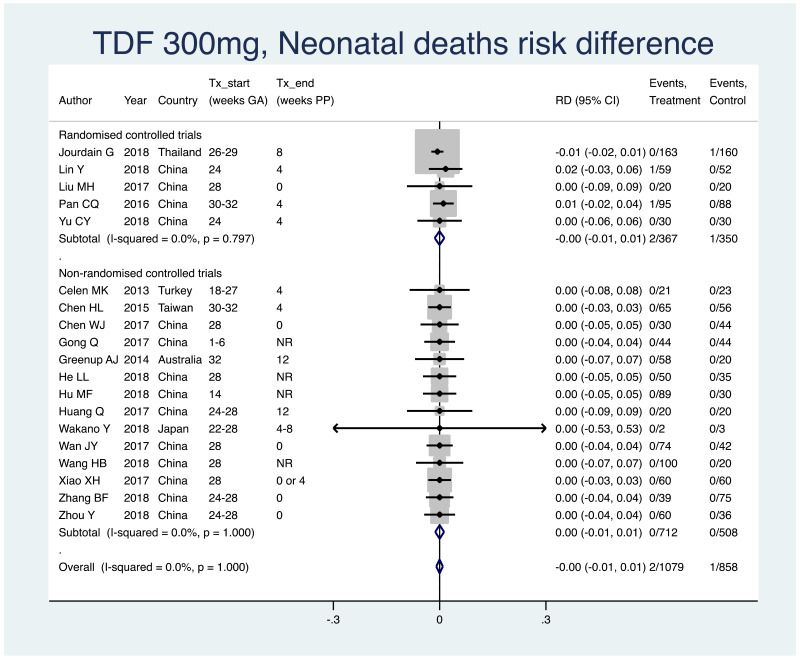
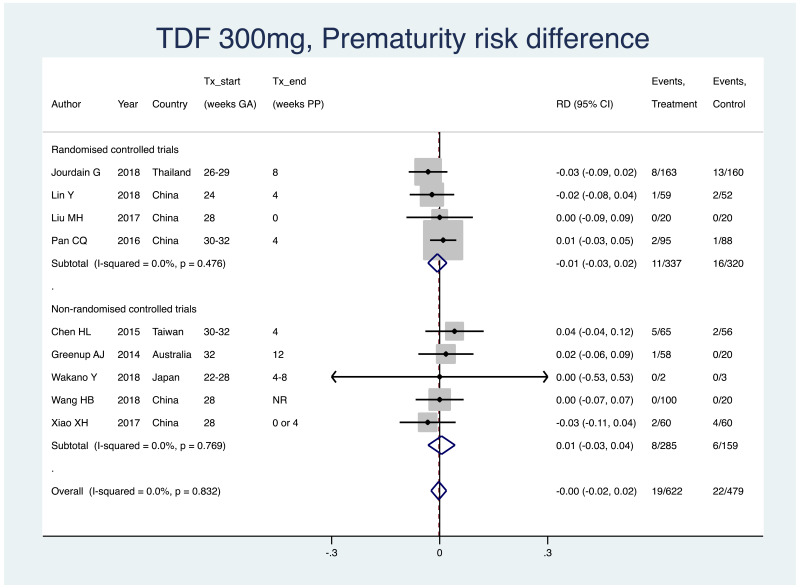
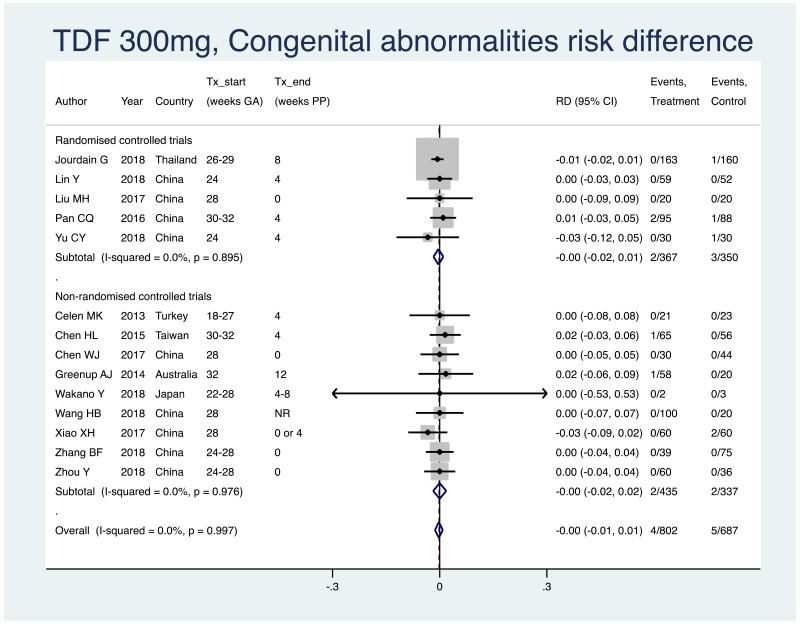
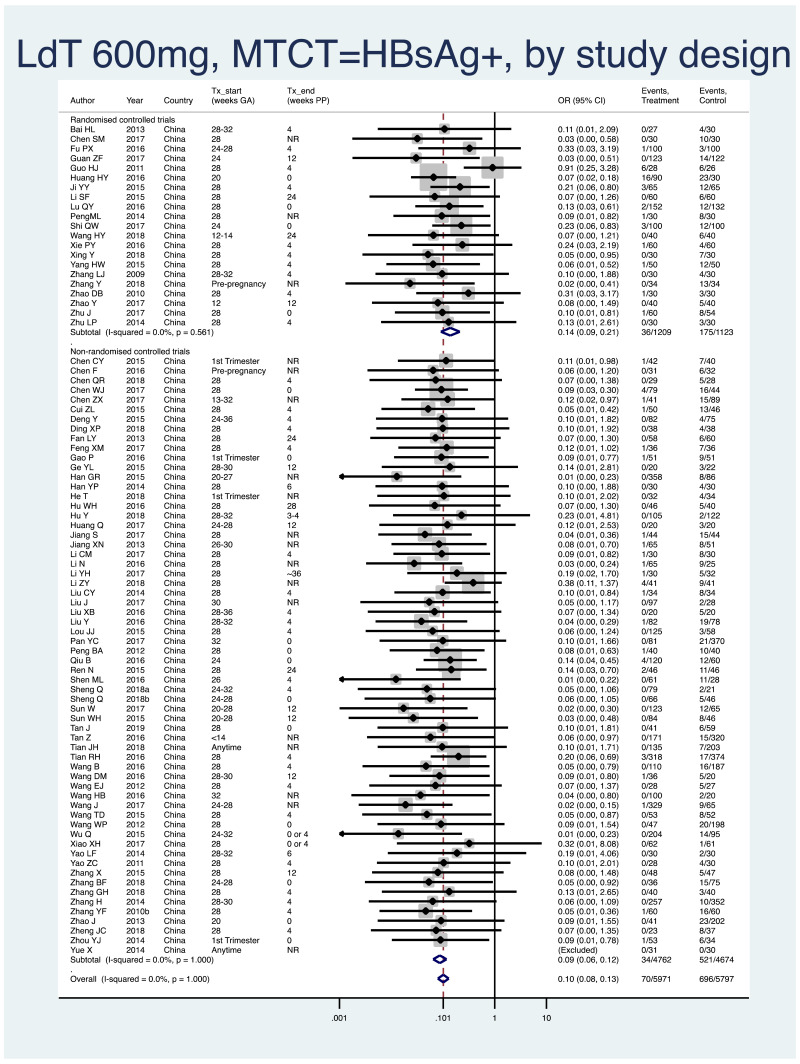
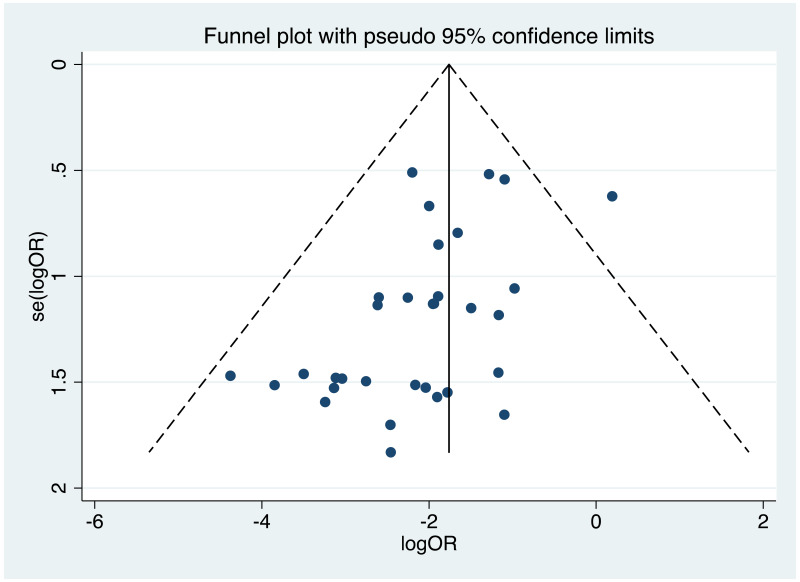
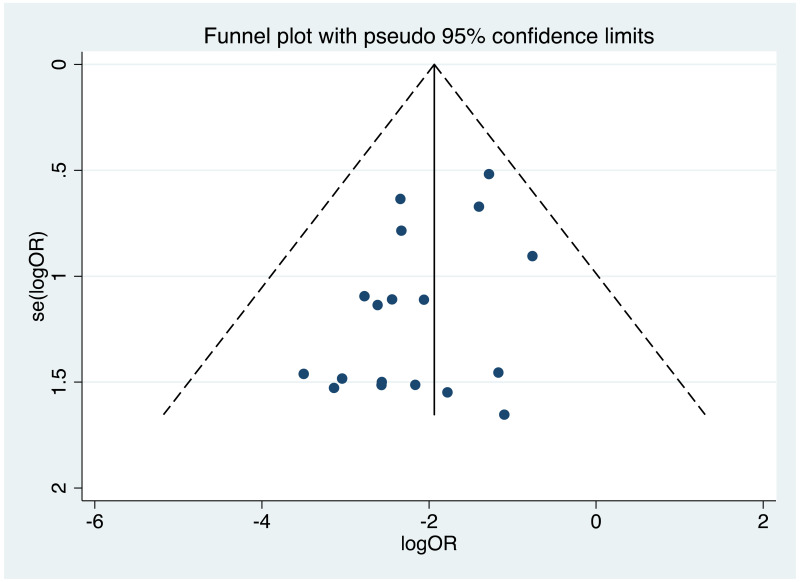
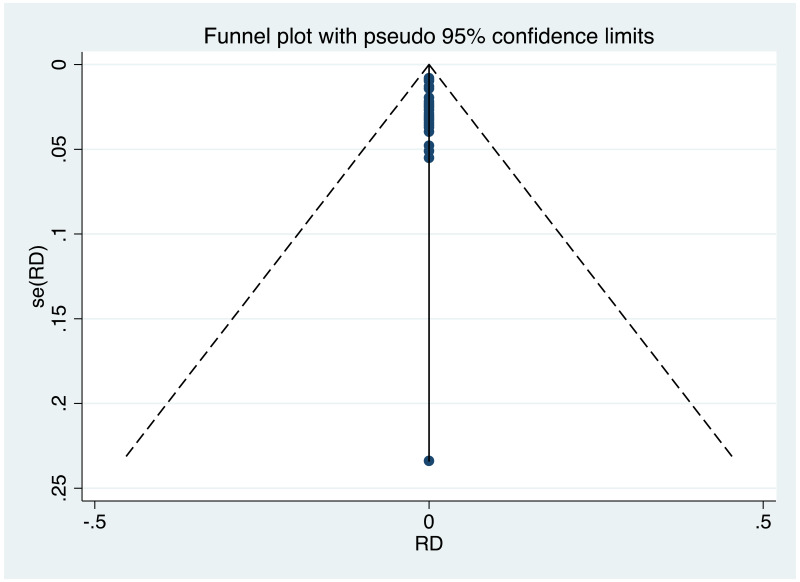
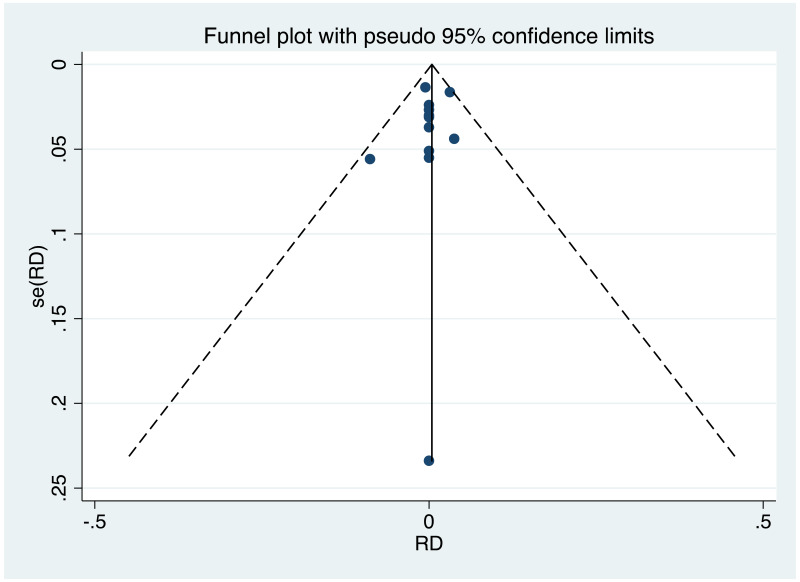
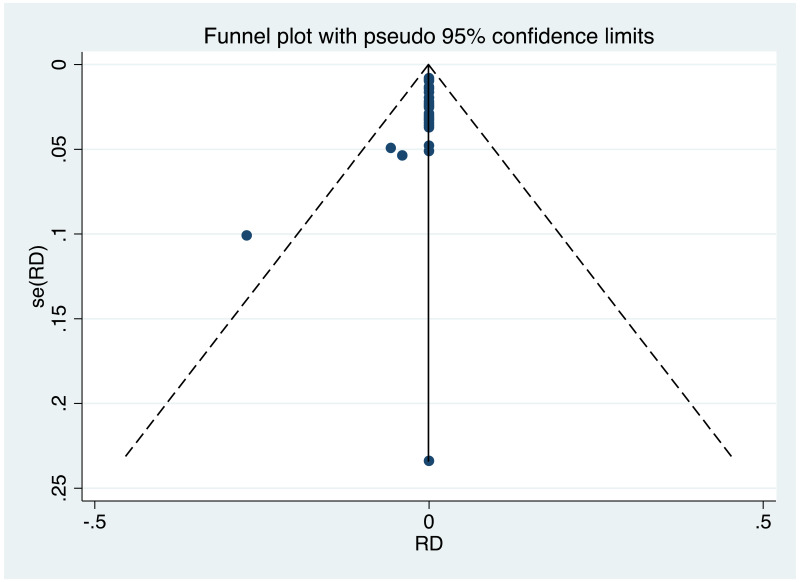
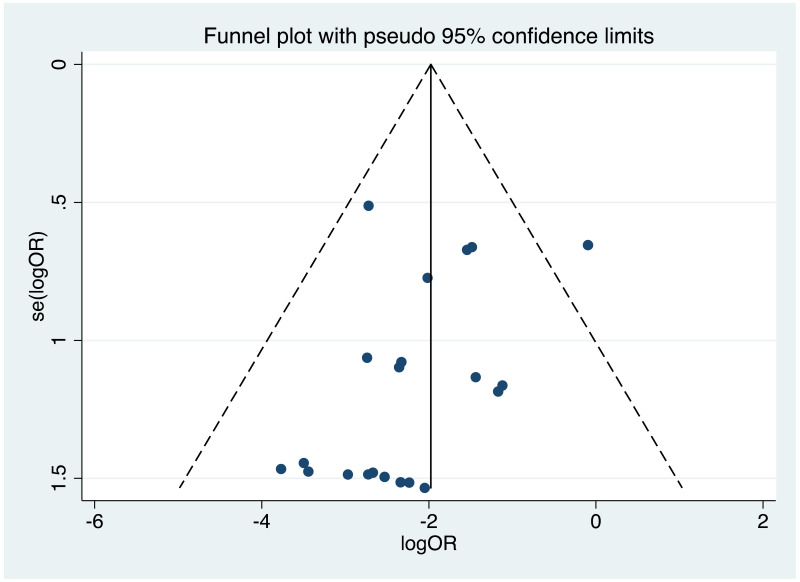
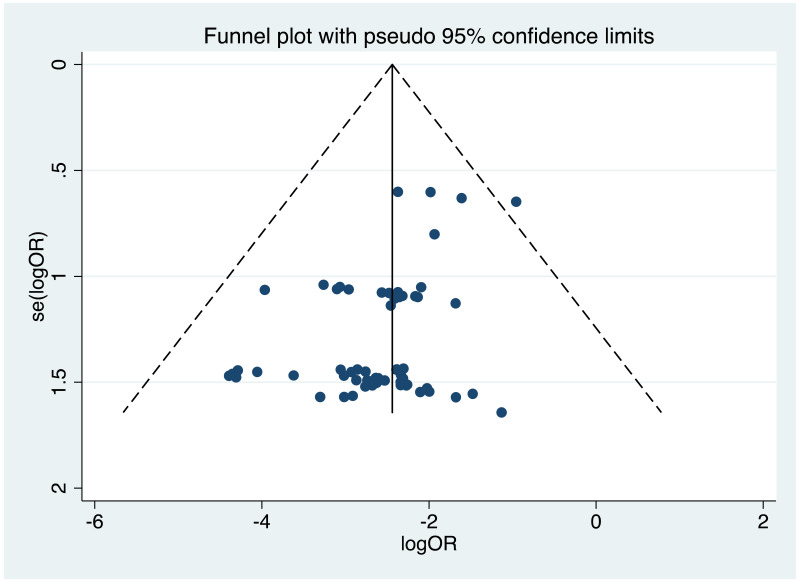
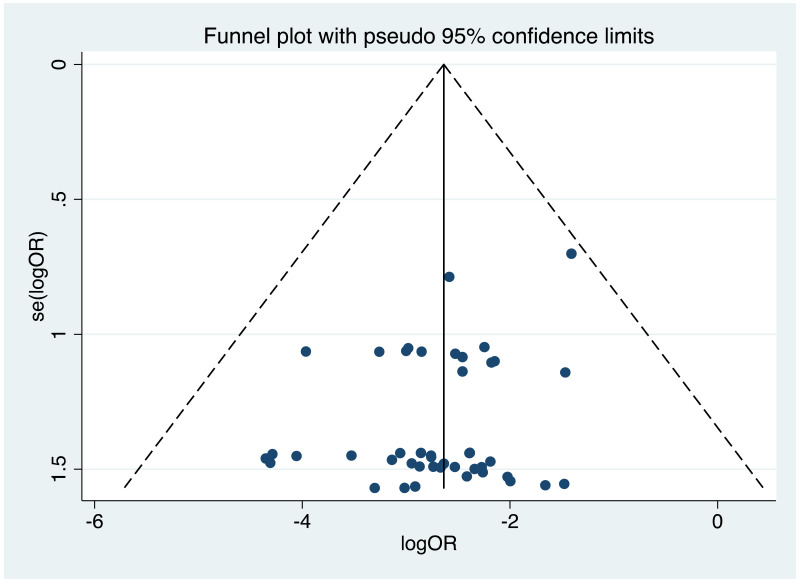
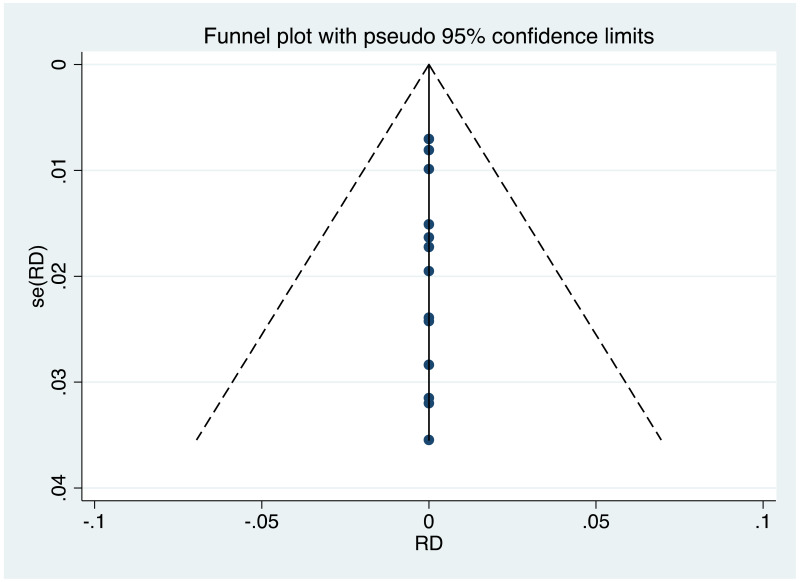
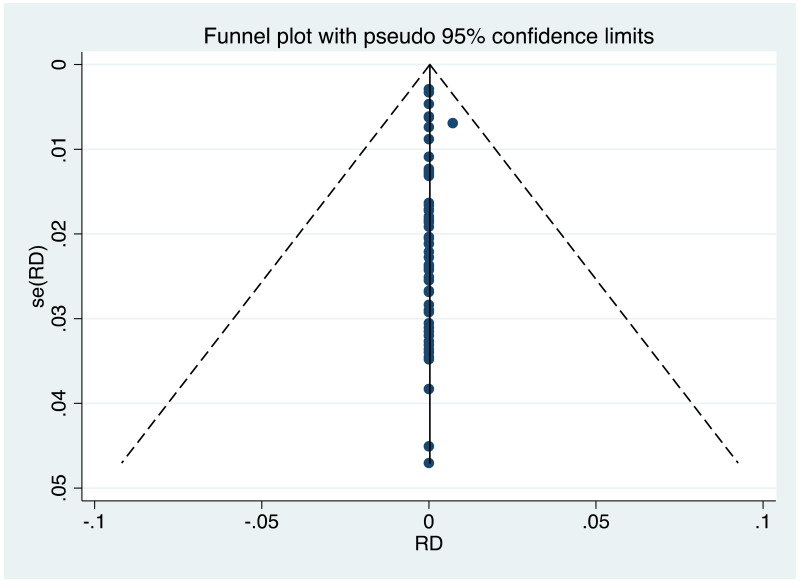
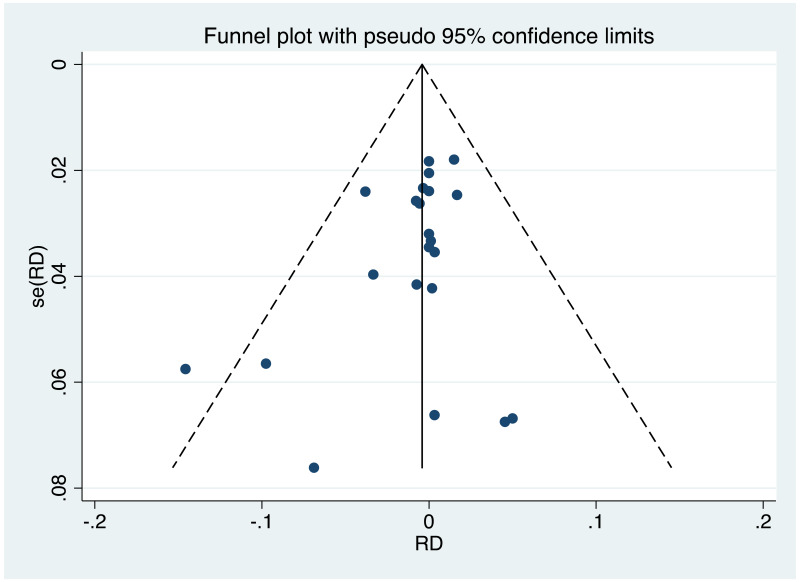
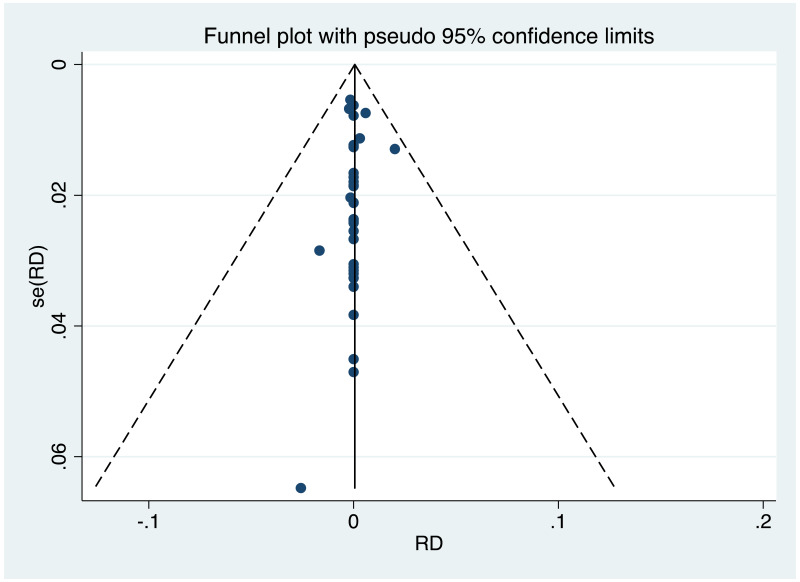
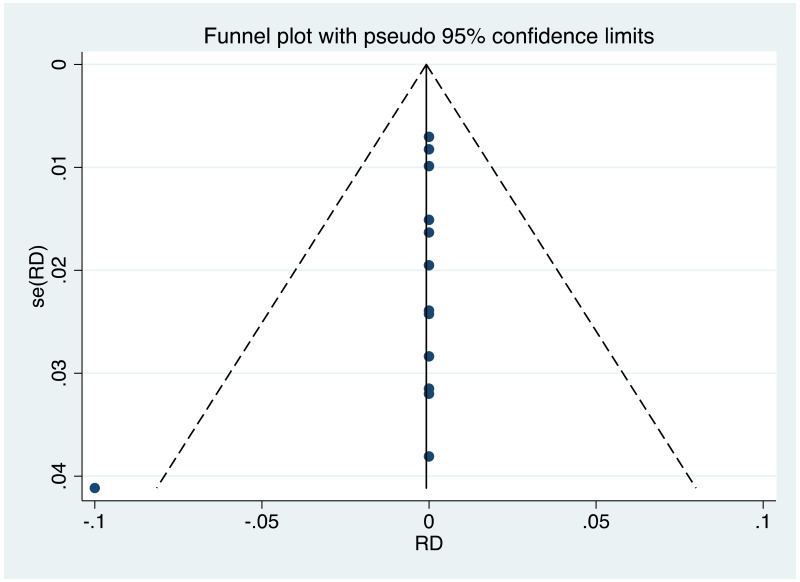
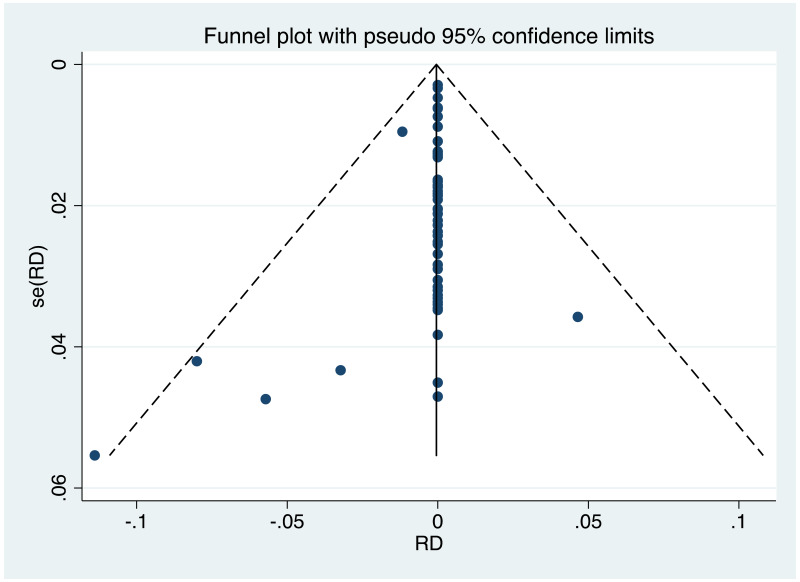
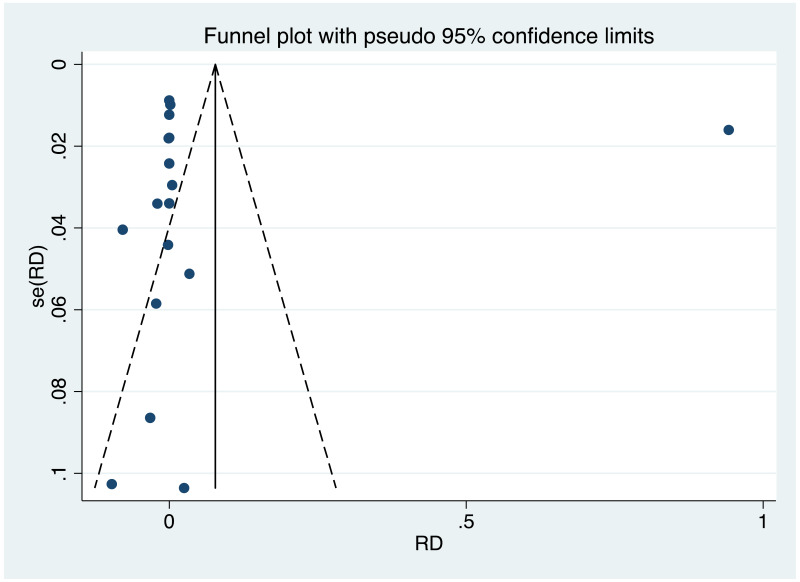
| Tan J (2019) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Comparable for HBV DNA levels at baseline but HBeAg serostatus not described. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 7 (low) |
| Chen QR (2018) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | No description | ☆Always the case | ☆☆Same HBeAg serostatus and comparable HBV DNA levels at baseline. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 6 (high) |
| Ding XP (2018) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and comparable HBV DNA levels at baseline. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 7 (low) |
| Li ZY (2018) | ☆ At least somewhat representative of the average HBV-infected pregnant woman | ☆ Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆ Always the case | ☆☆Comparable for HBeAg serostatus and HBV DNA level. Same regimen for infant immunoprophylaxis | ☆ Indication of record linkage (results viewed retrospectively in medical records) | ☆ Yes (always the case) | None reported (retrospective) | 8 (low) |
| Tian JH (2018) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | No description | ☆Always the case | ☆☆Same threshold for HBV DNA level and same HBeAg serostatus used. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 7 (low) |
| Zhang BF (2018) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Same HBeAg serostatus but different thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 6 (high) |
| Zhang GH (2018) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 7 (low) |
| Zheng JC (2018) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | No description | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 7 (low) |
| Chen WJ (2017) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Feng XM (2017) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Huang Q (2017) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 7 (low) |
| Jiang S (2017) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆ Comparable for HBV DNA level but HBeAg serostatus not described. Same regimen for infant immunoprophylaxis at birth | No description a | ☆Yes | No statement on LFU | 6 (high) |
| Li CM (2017) | ☆ At least somewhat representative of the average HBV-infected pregnant woman | ☆ Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆ Always the case | ☆Comparable for HBV DNA level but HBeAg serostatus not described. Same regimen for infant immunoprophylaxis | Laboratory assays used not well described | ☆ Yes (always the case) | No statement on LFU | 6 (high) |
| Li YH (2017) | ☆At least somewhat representative of the average HBV infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Liu J (2017) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and comparable for HBV DNA levels. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | There is a description of LFU for the exposed but not for the control group | 7 (low) |
| Luo DX (2017) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | Comparable for HBV DNA levels but HBeAg serostatus not described. Regimen for infant immunoprophylaxis at birth not clearly described | No description | ☆Yes | No statement on LFU | 5 (high) |
| Pan YC (2017) | ☆At least somewhat representative of the average HBV infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | No description | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | ☆Subject s lost to follow up unlikely to introduce bias, small number lost | 8 (low) |
| Wang J (2017) | ☆At least somewhat representative of the average HBV infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Same thresholds for HBV DNA level but HBeAg serostatus not described. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 6 (high) |
| Xiao XH (2017) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | Same thresholds for HBV DNA level but HBeAg serostatus not described. Regimen for infant immunoprophylaxis at birth not clearly described | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | There is a description of LFU for the exposed but not for the control group | 6 (high) |
| Chen F (2016) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Same HBeAg sero-status and same thresholds for HBV DNA level. Regimen for infant immunoprophylaxis at birth not clearly described | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboaratory and/or record linkage | ☆Yes | No statement of LFU | 7 (low) |
| Gao P (2016) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | Comparable for HBV DNA levels but HBeAg serostatus not described. Regimen for infant immunoprophylaxis at birth not clearly described | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 6 (high) |
| Hu WH (2016) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Comparable for HBV DNA levels but HBeAg serostatus not described. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 7 (low) |
| Li N (2016) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Comparable for HBV DNA levels but HBeAg serostatus not described. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 6 (high) |
| Liu XB (2016) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement of LFU | 7 (low) |
| Qiu B (2016) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Same thresholds for HBV DNA level but HBeAg serostatus not described. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement of LFU | 7 (low) |
| Shen ML (2016) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | Same thresholds for HBV DNA level but HBeAg serostatus not described. Regimen for infant immunoprophylaxis at birth not clearly described | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 6 (high) |
| Tian RH (2016) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | No description | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 6 (high) |
| Wang B (2016) | ☆ At least somewhat representative of the average HBV-infected pregnant woman | ☆ Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆ Always the case | ☆☆Comparable for HBeAg serostatus and HBV DNA level. Same regimen for infant immunoprophylaxis | ☆ Laboratory assays described | ☆ Yes (always the case) | No statement on LFU | 8 (low) |
| Wang DM (2016) | ☆At least somewhat representative of the average HBV infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboaratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Wang HB (2016) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Comparable for HBV DNA level but HBeAg serostatus not described. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 6 (high) |
| Zhang R (2016) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | No description | ☆Always the case | HBeAg serostatus and threshold for HBV DNA level not described. Regimen for infant immunoprophylaxis at birth not clearly described | No description | ☆Yes | No statement on LFU | 4 (high) |
| Chen CY (2015) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Same HBeAg serostatus and same thresholds for HBV DNA level. Regimen for infant immunoprophylaxis at birth not clearly described | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 7 (low) |
| Cui ZL (2015) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Deng Y (2015) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Same thresholds for HBV DNA level but HBeAg serostatus not described. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 6 (high) |
| Ge YL (2015) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg sero-status and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 7 (low) |
| Lou JJ (2015) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Ren N (2015) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Sun WH (2015) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Wang TD (2015) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Zhang X (2015) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Chen YL (2014) | No description of the derivation of the cohort | No description of the derivation of the non-exposed cohort | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Comparablefor HBV DNA levels but HBeAg serostatus not described. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 4 (high) |
| Han YP (2014) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 7 (low) |
| Liu CY (2014) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Yao LF (2014) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Same HBeAg serostatus and same thresholds for HBV DNA level. Regimen for infant immunoprophylaxis at birth not clearly described | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 7 (low) |
| Yue X (2014) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | ☆Complete follow up | 8 (low) |
| Zhou YJ (2014) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | No description | ☆Always the case | ☆Comparable HBeAg serostatus and same thresholds for HBV DNA level. Regimen for infant immunoprophylaxis at birth not described clearly | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 6 (high) |
| Fan LY (2013) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 7 (low) |
| Jiang XN (2013) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Same HBeAg serostatus and same thresholds for HBV DNA level. Regimen for infant immunoprophylaxis at birth not described clearly | No description | ☆Yes | ☆Complete follow up | 7 (low) |
| Zhao J (2013) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | No description | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 7 (low) |
| Peng BA (2012) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 7 (low) |
| Wang EJ (2012) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Wang WP (2012) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 8 (low) |
| Yao ZC (2011) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆Same thresholds for HBV DNA level but HBeAg serostatus not described. Same regimen for infant immunoprophylaxis at birth | ☆Laboratory methods described in detail (which assay used), indicating use of a central laboratory and/or record linkage | ☆Yes | No statement on LFU | 7 (low) |
| Zhang YF (2010) | ☆At least somewhat representative of the average HBV-infected pregnant woman | ☆Drawn from the same community (same inclusion and exclusion criteria also) | ☆Valid method was used to ascertain adherence to the antiviral therapy (decrease in viral load levels subsequent to the treatment) | ☆Always the case | ☆☆Same HBeAg serostatus and same thresholds for HBV DNA level. Same regimen for infant immunoprophylaxis at birth | No description | ☆Yes | No statement on LFU | 7 (low) |