This book is licensed under the terms of the Attribution-NonCommercial-NoDerivs 3.0 Unported (CC BY-NC-ND 3.0).
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Purpose and Development of This Guideline
Goals
This guideline on diagnosis and management of acute HIV infection was developed by the Medical Care Criteria Committee of New York State Department of Health AIDS Institute (NYSDOH AI) to guide clinicians in NYS who provide ambulatory, inpatient, and emergency medical care for adults ≥18 years old who present with signs or symptoms of acute HIV infection or report an exposure within the past 4 weeks.
This guideline provides evidence-based clinical recommendations for the diagnosis and treatment of acute HIV infection in adults, with the goals of ensuring that NYS clinicians are able to:
- Recognize the risks for and signs and symptoms of acute HIV, include HIV infection in the differential diagnosis, and consider HIV testing in any person who presents with signs and symptoms suggestive of influenza (“flu”), mononucleosis (“mono”), or other viral syndromes, including suspected COVID-19.
- Perform appropriate diagnostic and confirmatory testing when HIV infection is suspected and manage the treatment of acute HIV.
- Meet the NYS requirements for reporting and partner notification.
- Recommend or offer immediate initiation of antiretroviral therapy (ART) to improve the patient’s health and reduce the risk of HIV transmission; refer and confirm that patients can access optimal HIV care.
- Initiate or refer the patient for prevention services.
TERMINOLOGY
- Acute HIV infection: Describes the period immediately after infection with HIV when an individual is viremic and has detectable p24 antigen or has HIV RNA without diagnostic HIV antibodies. In the medical literature, “primary HIV infection” may describe this same period.
- Recent infection: Generally used to describe the 6-month period after infection occurs.
- Early infection: May refer to acute or recent infection, after which infection is defined as chronic.
Early diagnosis for early treatment: Accumulating evidence supports a decision to begin HIV treatment at the time of diagnosis [Lundgren, et al. 2015]. Initiation of ART during acute infection may have several beneficial clinical outcomes, including improved preservation of immunologic function, significantly reduced time to viral suppression, and reduction of the viral reservoir, which could be important for cure strategies [Pires, et al. 2004; Streeck, et al. 2006; Koegl, et al. 2009; Hocqueloux, et al. 2010; Ananworanich, et al. 2012; Buzon, et al. 2012; Lafeuillade, et al. 2012; Margolick, et al. 2012; Phanuphak, et al. 2012; Le, et al. 2013; Saez-Cirion, et al. 2013; Pilcher C, et al. 2015]. The risk of sexual transmission of HIV during acute or recent infection is significantly higher than during chronic infection [Pilcher CD, et al. 2004; Hollingsworth, et al. 2008; Pinkerton 2008; Hollingsworth, et al. 2015]; this difference likely correlates with high levels of viremia and is consistent with other routes of transmission [Bellan, et al. 2015]. The public health benefit of early ART initiation is well documented, with a significant reduction of HIV transmission among virally suppressed individuals. Further, in September 2017, the NYSDOH endorsed the consensus from the Prevention Access Campaign that Undetectable = Untransmittable (“U = U”), which indicates that individuals with a durable (≥6 months) undetectable viral load will not sexually transmit HIV [NYSDOH 2017; Prevention Access Campaign 2018].
Recognizing and diagnosing acute HIV infection is crucial to linking patients to care early and presents an important opportunity to reduce HIV transmission. Factors that may contribute to the increased risk for transmission during acute infection include:
- Hyperinfectivity associated with both markedly high viral load levels (often much greater than 10 million viral copies/mm3) and increased infectiousness of the virus [Quinn, et al. 2000; Ma, et al. 2009].
- Missed HIV diagnosis [Chin, et al. 2013; Nakao, et al. 2014]. Because the nonspecific flu- or mono-like symptoms are frequently unrecognized as symptoms of acute HIV infection or attributed to a nonspecific viral syndrome, the diagnosis is often missed. Missed diagnosis of acute HIV infection results in a lost opportunity to recommend treatment and risk-reduction counseling that could reduce both viral load levels and high-risk behavior [Colfax, et al. 2002; Steward, et al. 2009; Fonner, et al. 2012; Kroon, et al. 2017; Rutstein, et al. 2017].
For many reasons, detecting acute HIV infection is an essential link in the chain of prevention. Evidence demonstrates that patients with a recent diagnosis of HIV are more likely to reduce risk behaviors if they are given counseling at the time of testing [Steward, et al. 2009; Fonner, et al. 2012] and are linked to primary HIV care [Metsch, et al. 2008]. In addition, for those who elect to initiate ART, their risk of transmission is significantly diminished [Cohen, et al. 2011; Cohen, et al. 2016].
KEY POINTS
- HIV is highly transmissible during acute infection; rapid initiation of antiretroviral therapy (ART) reduces transmission, with significant public health benefits, and early viral suppression preserves immune function, with significant clinical benefits for the individual with HIV.
- Acute HIV often has nonspecific signs and symptoms and often goes unsuspected and undetected. This committee urges a high index of suspicion for acute infection and HIV testing for any individual who reports recent high-risk behavior or presents with signs or symptoms of influenza, mononucleosis, or other viral syndromes.
- When HIV infection is diagnosed, immediate linkage to care is essential; ART dramatically reduces HIV-related morbidity and mortality, and viral suppression prevents HIV transmission.
- The urgency of ART initiation is even greater if the newly diagnosed patient is pregnant, has acute HIV infection, is ≥50 years old, or has advanced disease. For these patients, every effort should be made to initiate ART immediately, ideally on the same day as diagnosis.
- All clinical care settings should be prepared, either on-site or with a confirmed referral, to support patients in initiating ART as rapidly as possible after diagnosis.
- When a diagnosis of acute HIV infection is made, clinicians should discuss the importance of notifying all recent contacts and refer patients to partner notification services, as mandated by New York State Law. The NYSDOH can provide assistance if necessary.
- See NYSDOH Provider Reporting & Partner Services for more information about required reporting.
NEW YORK STATE LAW
- New York State Law mandates that physicians must offer an HIV test to all patients aged 13 years and older (or younger with risk) if a previous test is not documented, even in the absence of symptoms consistent with acute HIV.
- Effective November 28, 2016, amendments to the New York State Public Health Law removed the requirement for written or oral informed consent prior to ordering an HIV-related test, including elimination of written consent for HIV testing in New York State correctional facilities and removing references to consent forms. The objective of the update is to eliminate barriers to HIV testing and make HIV testing comparable to the manner in which other important laboratory tests are conducted. HIV testing remains voluntary, and patients have the right to refuse an HIV test, but obtaining written or oral consent for testing is no longer required in any setting. At a minimum, patients must be advised orally that an HIV test is going to be performed.
- NYS Public Health Law Article 21 (Chapter 163 of the Laws of 1998) requires the reporting of individuals with HIV as well as AIDS to the NYSDOH. The law also requires that reports contain the names of sex or needle-sharing partners known to the medical provider or whom the infected individual wishes to have notified. The Medical Provider Report Form (PRF) (DOH-4189) must be completed within 14 days of diagnosis for individuals with the following diagnoses or with known sex or needle-sharing partners:
- Initial/new HIV diagnosis: First report of HIV antibody positive test results.
- Previously diagnosed HIV infection (non-AIDS): Applies to a medical provider who is seeing the patient for the first time.
- Initial/new diagnosis of AIDS: Including <200 CD4 cells/µL or opportunistic infection (AIDS-defining illness).
- Previously diagnosed AIDS: Applies to a medical provider who is seeing the patient for the first time.
- Known sex or needle-sharing partners of individuals with diagnosed HIV infection.
Guideline Development
This guideline was developed by the NYSDOH AI Clinical Guidelines Program, which is a collaborative effort between the NYSDOH AI Office of the Medical Director and the Johns Hopkins University School of Medicine, Division of Infectious Diseases.
Established in 1986, the goal of the Clinical Guidelines Program is to develop and disseminate evidence-based, state-of-the-art clinical practice guidelines to improve the quality of care provided to people who have HIV, HCV, or sexually transmitted infections; people with substance use issues; and members of the LGBTQ community. NYSDOH AI guidelines are developed by committees of clinical experts through a consensus-driven process.
The NYSDOH AI charged the Medical Care Criteria Committee (adult HIV and related guidelines) with developing evidence-based clinical recommendations for the diagnosis and management of acute HIV infection. The resulting recommendations are based on an extensive review of the medical literature and reflect a consensus among this panel of experts. Each recommendation is rated for strength and quality of the evidence (see below). If recommendations are based on expert opinion, the rationale for the opinion is included.
| NYSDOH AI Clinical Guidelines Program Ratings Scheme, Updated June 26, 2019 [a] | |
|---|---|
| Strength of Recommendation Ratings | |
A B C | Strong recommendation Moderate recommendation Optional |
| Quality of Supporting Evidence Ratings | |
|
1 |
Indicates that the evidence supporting a recommendation is derived from published results of at least one randomized trial with clinical outcomes or validated laboratory endpoints. |
|
* |
Indicates that the evidence supporting a recommendation is strong because it is based on a self-evident conclusion(s) or conclusive, published in vitro data, or because the recommendation articulates well-established, accepted practice that cannot be tested because ethics would preclude a clinical trial. |
|
2 |
Indicates that the evidence supporting a recommendation is derived from published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
|
2† |
Indicates that the evidence supporting a recommendation has been extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. When this rating is assigned to a recommendation, the source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. |
|
3 |
Indicates that a recommendation is based on the expert opinion of the committee members. The rationale for the recommendation is provided in the guideline text. |
a. With the June 2019 update, the ratings for quality of supporting evidence were expanded to add the * rating and the 2† rating.
Presentation and Diagnosis
| RECOMMENDATIONS |
|---|
Presentation
When Acute HIV Infection Is Suspected
Diagnosis
ART Initiation
Partner Notification
|
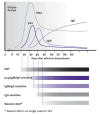
The time from HIV infection to detection of the virus depends on the test that is used. Figure 1: Window of Detection for HIV, Based on Test Used illustrates the window of detection of HIV infection according to antibody, antibody/antigen combination, and HIV RNA tests.
Presentation
Patients acutely infected with HIV will often experience at least some symptoms of acute retroviral syndrome (ARS). Fever and influenza- or mononucleosis-like symptoms are common in acute HIV infection but are nonspecific. Rash, mucocutaneous ulcers, oropharyngeal candidiasis, and meningismus are more specific and should raise the index of suspicion (see below for a more extensive list of signs and symptoms). The mean time from exposure to onset of symptoms is generally 2 to 4 weeks, with a range of 5 to 29 days; however, some cases have presented with symptoms up to 3 months after exposure [Apoola, et al. 2002]. Theoretically, this time course may be prolonged in patients who become infected while on PEP or PrEP.
| Box 1: Acute Retroviral Syndrome [a] | ||
|---|---|---|
|
Signs and symptoms of ARS with the expected frequency among symptomatic patients are listed below [a]. The most specific symptoms in this study were oral ulcers and weight loss; the best predictors were fever and rash. The index of suspicion should be high when these symptoms are present. | ||
|
|
|
a. Data are from Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS 2002;16(8):1119-1129. [PMID: 12004270] https://pubmed
.ncbi.nlm.nih.gov/12004270
Diagnosis
Acute HIV infection is often not recognized in the primary care setting because the symptom profile is similar to that of influenza, mononucleosis, and other common illnesses. Furthermore, patients often do not recognize that they may have recently been exposed to HIV. Therefore, the clinician should have a high index of suspicion for acute HIV infection in a patient who may have recently engaged in behavior involving sexual or parenteral exposure to another individual’s blood or body fluids and who is presenting with a febrile, influenza-, or mononucleosis-like illness. Identifying acute HIV infection during pregnancy is particularly important because effective intervention can prevent mother-to-child transmission [Patterson, et al. 2007].
High levels of HIV RNA detected in plasma through sensitive NAAT, combined with a negative or indeterminate HIV screening or type-differentiation test, support the presumptive diagnosis of acute HIV infection [Robb, et al. 2016; DHHS 2019].
When low-level viremia is reported by HIV RNA testing (<5,000 copies/mL) in the absence of serologic confirmation of HIV infection, HIV RNA testing should be repeated to exclude a false-positive result [Hecht, et al. 2002]. Repeat HIV RNA testing with a result that indicates the presence of low-level viremia may represent true HIV infection, warranting appropriate counseling regarding transmission risk and initiation of ART.
HIV RNA levels tend to be very high in acute infection; however, a low value may represent any point on the upward or downward slope of the viremia associated with acute infection or could simply represent chronic infection. HIV RNA can also be suppressed during acute infection in patients who are taking PrEP. Plasma HIV RNA levels during acute infection do not appear significantly different in patients who are and are not symptomatic [Patterson, et al. 2007]. Viremia occurs approximately 1 to 2 weeks before the detection of a specific immune response. Patients diagnosed with acute infection by HIV RNA testing should always receive follow-up diagnostic testing 3 weeks later to confirm infection (see the CDC HIV testing algorithm) [CDC 2013, 2014]. Figure 2: Diagnostic Testing for HIV Infection illustrates diagnostic testing for acute HIV infection.
KEY POINTS
- The diagnosis of acute HIV infection requires a high degree of clinical awareness. The nonspecific signs and symptoms of acute HIV infection are often not recognized or attributed to another viral illness.
- Diagnostic HIV RNA testing should be considered for all patients who present with compatible symptoms (see signs and symptoms of ARS, above), particularly in the context of a sexually transmitted infection [Patel, et al. 2006] or a recent sexual or parenteral exposure with a partner known to have HIV or a partner whose HIV serostatus is not known.
- Individual laboratories have internal protocols for reporting HIV tests with preliminary results. The terms used when preliminary results cannot be classified include indeterminate, inconclusive, nondiagnostic, and pending validation. Clinicians can contact the appropriate laboratory authority to determine the significance of nondefinitive results and the recommended supplemental testing, particularly when acute HIV infection is suspected. Clinicians are advised to become familiar with the internal test-reporting policies of their institutions.
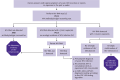
Figure 2.
Diagnostic Testing for Acute HIV Infection
Management, Including While on PEP or PrEP
| RECOMMENDATIONS |
|---|
Management
|
Patients are at greatest risk for transmitting HIV during periods of high viremia early in infection. Clinicians should counsel patients with acute HIV about the increased risk of transmission during the 6 months after infection. Partner notification [Golden, et al. 2004], counseling on safer sex, and screening for other sexually transmitted infections are all essential in the management of any new HIV diagnosis.
Consultation: When choosing an ART regimen for a patient with acute HIV infection, clinicians should consult a care provider experienced in treating acute HIV infection.
- Data are insufficient to support a specific ART regimen(s) for the treatment of acute HIV infection; instead, the choice of regimen should be made based on recommendations for selecting an initial ART regimen.
- The risks of transmitted resistance should be considered when prescribing ART while awaiting HIV resistance results.
- The risks of acquired mutations should be considered in those who acquire HIV while on PrEP.
Clinicians who do not have access to experienced HIV care providers should call the CEI Line at 1-866-637-2342.
References
- Ananworanich J, Schuetz A, Vandergeeten C, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One. 2012;7(3):e33948. [PMC free article: PMC3316511] [PubMed: 22479485]
- Apoola A, Ahmad S, Radcliffe K. Primary HIV infection. Int J STD AIDS. 2002;13(2):71–78. [PubMed: 11839160]
- Bellan SE, Dushoff J, Galvani AP, et al. Reassessment of HIV-1 acute phase infectivity: accounting for heterogeneity and study design with simulated cohorts. PLoS Med. 2015;12(3):e1001801. [PMC free article: PMC4363602] [PubMed: 25781323]
- Buzon M, Siess K, Sone A. Treatment of early HIV infection reduces viral reservoir to levels found in elite controllers. Abstract 151. CROI; 2012 Mar 5-8; Seattle, WA.
- CDC. Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013;62(24):489–494. [PMC free article: PMC4604890] [PubMed: 23784012]
- CDC. Laboratory testing for the diagnosis of HIV infection: updated recommendations. 2014 Jun 27. https://stacks
.cdc.gov/view/cdc/23447 [accessed 2018 Aug 21] - Chin T, Hicks C, Samsa G, et al. Diagnosing HIV infection in primary care settings: missed opportunities. AIDS Patient Care STDS. 2013;27(7):392–397. [PMC free article: PMC3704080] [PubMed: 23802143]
- Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–839. [PMC free article: PMC5049503] [PubMed: 27424812]
- Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [PMC free article: PMC3200068] [PubMed: 21767103]
- Colfax GN, Buchbinder SP, Cornelisse PG, et al. Sexual risk behaviors and implications for secondary HIV transmission during and after HIV seroconversion. AIDS. 2002;16(11):1529–1535. [PubMed: 12131191]
- DHHS. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. 2019 Dec 18. https:
//clinicalinfo .hiv.gov/en/guidelines /adult-and-adolescent-arv /acute-and-recent-early-hiv-infection [accessed 2021 Jun 29] - Fonner VA, Denison J, Kennedy CE, et al. Voluntary counseling and testing (VCT) for changing HIV-related risk behavior in developing countries. Cochrane Database Syst Rev. 2012;(9):Cd001224. [PMC free article: PMC3931252] [PubMed: 22972050]
- Golden MR, Hogben M, Potterat JJ, et al. HIV partner notification in the United States: a national survey of program coverage and outcomes. Sex Transm Dis. 2004;31(12):709–712. [PubMed: 15608584]
- Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS. 2002;16(8):1119–1129. [PubMed: 12004270]
- Hocqueloux L, Prazuck T, Avettand-Fenoel V, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS. 2010;24(10):1598–1601. [PubMed: 20549847]
- Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198(5):687–693. [PubMed: 18662132]
- Hollingsworth TD, Pilcher CD, Hecht FM, et al. High transmissibility during early HIV infection among men who have sex with men-San Francisco, California. J Infect Dis. 2015;211(11):1757–1760. [PMC free article: PMC4425938] [PubMed: 25542958]
- Koegl C, Wolf E, Hanhoff N, et al. Treatment during primary HIV infection does not lower viral set point but improves CD4 lymphocytes in an observational cohort. Eur J Med Res. 2009;14(7):277–283. [PMC free article: PMC3458637] [PubMed: 19661009]
- Kroon E, Phanuphak N, Shattock AJ, et al. Acute HIV infection detection and immediate treatment estimated to reduce transmission by 89% among men who have sex with men in Bangkok. J Int AIDS Soc. 2017;20(1):21708. [PMC free article: PMC5515043] [PubMed: 28691441]
- Lafeuillade A, Hittinger G, Lambry V. Long-term control of HIV reservoir after a 2-year ART course at acute infection. Abstract 358. CROI; 2012 Mar 5-8; Seattle, WA.
- Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368(3):218–230. [PMC free article: PMC3657555] [PubMed: 23323898]
- Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. [PMC free article: PMC4569751] [PubMed: 26192873]
- Ma ZM, Stone M, Piatak M Jr, et al. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol. 2009;83(7):3288–3297. [PMC free article: PMC2655556] [PubMed: 19129448]
- Margolick J, Apuzzo L, Tossonian H. Effect of randomized HAART on viral suppression off therapy in patients with acute/early HIV infection. Abstract 356. CROI; 2012 Mar 5-8; Seattle, WA.
- Metsch LR, Pereyra M, Messinger S, et al. HIV transmission risk behaviors among HIV-infected persons who are successfully linked to care. Clin Infect Dis. 2008;47(4):577–584. [PubMed: 18624629]
- Nakao JH, Wiener DE, Newman DH, et al. Falling through the cracks? Missed opportunities for earlier HIV diagnosis in a New York City Hospital. Int J STD AIDS. 2014;25(12):887–893. [PubMed: 24535693]
- NYSDOH. Dear colleague letter. 2017 Sep. https://www
.health.ny .gov/diseases/aids/ending_the_epidemic /docs /september_physician_letter.pdf [accessed 2018 Aug 21] - Patel P, Klausner JD, Bacon OM, et al. Detection of acute HIV infections in high-risk patients in California. J Acquir Immune Defic Syndr. 2006;42(1):75–79. [PMC free article: PMC6764592] [PubMed: 16763493]
- Patterson KB, Leone PA, Fiscus SA, et al. Frequent detection of acute HIV infection in pregnant women. AIDS. 2007;21(17):2303–2308. [PubMed: 18090278]
- Phanuphak N, Teeratakulpisarn N, Mungyu P. Time to undetectable HIV RNA in ano-genital compartment of acute HIV Thai male subjects treated with 5- and 3-drug HAART. Abstract 555. CROI; 2012 Mar 5-8; Seattle, WA.
- Pilcher C, Hatano H, Dasgupta A, et al. Providing same-day, observed ART to newly diagnosed HIV+ outpatients is associated with improved virologic suppression. 8th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; 2015 Jul 19-22; Vancouver, Canada.
- Pilcher CD, Tien HC, Eron JJ Jr, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189(10):1785–1792. [PubMed: 15122514]
- Pinkerton SD. Probability of HIV transmission during acute infection in Rakai, Uganda. AIDS Behav. 2008;12(5):677–684. [PMC free article: PMC2614120] [PubMed: 18064559]
- Pires A, Hardy G, Gazzard B, et al. Initiation of antiretroviral therapy during recent HIV-1 infection results in lower residual viral reservoirs. J Acquir Immune Defic Syndr. 2004;36(3):783–790. [PubMed: 15213561]
- Prevention Access Campaign. Prevention Access Campaign consensus statement: Risk of sexual transmission of HIV from a person living with HIV who has an undetectable viral load. 2018 Jan 28. https://www
.preventionaccess .org/consensus [accessed 2018 Aug 21] - Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929. [PubMed: 10738050]
- Robb ML, Eller LA, Kibuuka H, et al. Prospective study of acute HIV-1 infection in adults in East Africa and Thailand. N Engl J Med. 2016;374(22):2120–2130. [PMC free article: PMC5111628] [PubMed: 27192360]
- Rutstein SE, Ananworanich J, Fidler S, et al. Clinical and public health implications of acute and early HIV detection and treatment: a scoping review. J Int AIDS Soc. 2017;20(1):21579. [PMC free article: PMC5515019] [PubMed: 28691435]
- Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211. [PMC free article: PMC3597518] [PubMed: 23516360]
- Steward WT, Remien RH, Higgins JA, et al. Behavior change following diagnosis with acute/early HIV infection-a move to serosorting with other HIV-infected individuals. The NIMH Multisite Acute HIV Infection Study: III. AIDS Behav. 2009;13(6):1054–1060. [PMC free article: PMC2785897] [PubMed: 19504178]
- Streeck H, Jessen H, Alter G, et al. Immunological and virological impact of highly active antiretroviral therapy initiated during acute HIV-1 infection. J Infect Dis. 2006;194(6):734–739. [PubMed: 16941338]
Supplementary Material
Created: September 2020; Last Update: July 2021.
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Diagnosis and Management of Acute HIVDiagnosis and Management of Acute HIV
Your browsing activity is empty.
Activity recording is turned off.
See more...