CADTH undertook reanalysis to address limitations in the sponsor’s submission, including adjusting dispensing to the nearest vial size, removing treatment-specific utility estimates, and setting patients’ starting ages and weights to better reflect the HAVEN 3 patient population. CADTH’s findings remained aligned with the sponsor’s: emicizumab is not a cost-effective option at a willingness-to-pay threshold of $50,000 per quality-adjusted life-year (QALY) in patients with severe hemophilia A without inhibitors. In the CADTH base-case reanalysis, emicizumab was associated with an incremental cost-effectiveness ratio (ICER) of $5.53 million per QALY gained compared to factor VIII (FVIII) prophylaxis. The model was highly sensitive to the price of emicizumab and its comparators. To achieve an ICER of $50,000 per QALY, the price of emicizumab would need to be reduced by at least 89%. However, a greater price reduction may be required if the prices of FVIII products are lower than those published by the Patented Medicine Prices Review Board (PMPRB).
While the evidence for bleeding efficacy is robust for the use of emicizumab in Canadian patients not receiving treatment with prophylactic FVIII, the evidence directly comparing emicizumab to prophylactic FVIII is limited. In patients with severe hemophilia A without inhibitors, emicizumab demonstrated statistically and clinically significant improvements in bleeding outcomes (i.e., annualized bleeding ratio for treated bleeds, all bleeds, treated joint bleeds, and treated spontaneous bleeds) compared to on-demand FVIII treatment. Limited comparative evidence exists to establish the comparative effectiveness and safety of emicizumab compared to FVIII prophylaxis, although current evidence suggests that emicizumab showed a reduction in bleeding outcomes compared to no prophylaxis (non-randomized comparison).
The Health Canada indication for emicizumab includes patients with hemophilia A regardless of their disease severity, while the sponsor’s submitted reimbursement request includes only severe patients and mild and moderate patients who meet specific eligibility criteria. The modelled population only investigated patients with severe hemophilia A, as per HAVEN 3’s eligibility criteria. Given that the sponsor’s reimbursement request does not align with the modelled population, uncertainty remains regarding the cost-effectiveness of emicizumab in both the reimbursement-requested population and the full Health Canada indication.
Abbreviations
- ABTR
annualized treated bleed rate
- BDD
B-domain deleted
- BIA
budget impact analysis
- FVIII
factor VIII
- ICER
incremental cost-effectiveness ratio
- NIS
non-interventional study
- PMPRB
Patented Medicine Prices Review Board
- QALY
quality-adjusted life-year
- VWF
von Willebrand factor
Executive Summary
The executive summary is comprised of 2 tables (: Background and : Economic Evaluation) and a conclusion.
Summary of Economic Evaluation.
Conclusions
CADTH undertook reanalysis to address limitations in the sponsor’s submission, including adjusting dispensing to the nearest vial size, removing treatment-specific utility estimates, and setting patients’ starting ages and weights to better reflect the HAVEN 3 patient population. CADTH’s findings remained aligned with the sponsor’s: emicizumab is not a cost-effective option at a willingness-to-pay threshold of $50,000 per quality-adjusted life-year (QALY) in patients with severe hemophilia A without inhibitors. In the CADTH base-case reanalysis, emicizumab was associated with an incremental cost-effectiveness ratio (ICER) of $5.53 million per QALY gained compared to factor VIII (FVIII) prophylaxis. The model was highly sensitive to the price of emicizumab and its comparators. To achieve an ICER of $50,000 per QALY, the price of emicizumab would need to be reduced by at least 89%. However, a greater price reduction may be required if the prices of FVIII products are lower than those published by the Patented Medicine Prices Review Board (PMPRB).
While the evidence for bleeding efficacy is robust for the use of emicizumab in Canadian patients not receiving treatment with prophylactic FVIII, the evidence directly comparing emicizumab to prophylactic FVIII is limited. In patients with severe hemophilia A without inhibitors, emicizumab demonstrated statistically and clinically significant improvements in bleeding outcomes (i.e., annualized bleeding ratio for treated bleeds, all bleeds, treated joint bleeds, and treated spontaneous bleeds) compared to on-demand FVIII treatment. Limited comparative evidence exists to establish the comparative effectiveness and safety of emicizumab compared to FVIII prophylaxis, although current evidence suggests that emicizumab showed a reduction in bleeding outcomes compared to no prophylaxis (non-randomized comparison).
The Health Canada indication for emicizumab includes patients with hemophilia A regardless of their disease severity, while the sponsor’s submitted reimbursement request includes only severe patients and mild and moderate patients who meet specific eligibility criteria. The modelled population only investigated patients with severe hemophilia A, as per HAVEN 3’s eligibility criteria. Given that the sponsor’s reimbursement request does not align with the modelled population, uncertainty remains regarding the cost-effectiveness of emicizumab in both the reimbursement-requested population and the full Health Canada indication.
Stakeholder Input Relevant to the Economic Review
This section is a summary of the feedback received from the patient groups, registered clinicians, and drug plans that participated in the CADTH review process.
Patient input was received from the Canadian Hemophilia Society. According to this input, hemophilia A affects patients’ lives negatively on physical, psychological, and financial levels. The key concerns raised by patients are breakthrough bleeds, venous access challenges, and adherence difficulties due to the complex treatment regimen. Many patients were concerned that the standard treatment for hemophilia A without inhibitors, FVIII replacement therapy, provides insufficient protection given that breakthrough bleeds still occur, leading to a risk of chronic joint damage. The most common challenge reported by patients is difficult venous access, which is particularly challenging in infants or children. A common challenge faced by patients and caregivers is the need to travel long distances to treatment centres for check-ups, treatments, or to pick up factor supplies for home use. This can make it difficult for patients to adhere to the treatment regimen and affect caregivers’ employability. A separate survey of hemophilia A health care providers with patients who have been prescribed emicizumab reported dramatic improvements in their patients’ health outcomes and quality of life. These health care providers stated that patients required fewer treatment administrations, experienced less joint pain and discomfort, and had fewer hospital visits and greater treatment adherence.
In Canada, access to emicizumab is currently restricted to individuals with hemophilia A with inhibitors. Approximately 15 people with hemophilia A without inhibitors were granted compassionate access starting in autumn 2019.
Several of the following concerns were addressed in the sponsor’s model:
Since the health care payer perspective was adopted in this economic submission, patient-borne costs, such as those related to travel and lost potential income, were not considered. The sponsor included a scenario analysis adopting a societal perspective that considered the costs of productivity loss.
Patients receiving emicizumab are expected to require fewer hospitalizations than those treated with FVIII prophylaxis, according to the health care providers surveyed. These costs have been included in the model, with fewer annual days of hospitalization associated with emicizumab compared to FVIII prophylaxis. No feedback was received on how the experience of patients receiving on-demand FVIII may differ from the experiences of those receiving emicizumab.
Difficulties with venous access for patients receiving FVIII were captured indirectly by applying a utility improvement that was associated with subcutaneous administration (0.1112).
Health care providers noted that patients on emicizumab experienced less joint pain and discomfort compared to patients on FVIII prophylaxis. Although the economic model assumed that the same percentage of treated bleeds would be joint bleeds (73%) across treatment options, the ABTRs were lower in patients on emicizumab. Therefore, the model aligned with the clinical experts’ views. The ATBRs in the economic model were informed by HAVEN 3 and non-interventional study (NIS).
Economic Review
The current review is for emicizumab (Hemlibra) for patients with severe hemophilia A (congenital factor VIII deficiency) without factor VIII inhibitors as routine prophylaxis.
Economic Evaluation
Summary of Sponsor’s Economic Evaluation
Overview
The sponsor submitted a cost-utility analysis comparing emicizumab to prophylaxis with factor VIII (FVIII) and on-demand (episodic) use of FVIII. In both cases, recombinant FVIII products included: Antihemophilic Factor (Recombinant) B-domain deleted (BDD), Fc Fusion Protein (Eloctate); Antihemophilic Factor (Recombinant) PEGylated (Adynovate); Antihemophilic Factor (Recombinant) (Kovaltry); Antihemophilic Factor (Recombinant) BDD simoctocog alfa (Nuwiq); and Antihemophilic Factor (Recombinant) BDD recombinant FVIII (Xyntha). The modelled patient population reflected patients recruited in the HAVEN 3 trial, although the model start age was set to 2. This did not align with the Health Canada–indicated population nor the reimbursement-requested population. The sponsor requested reimbursement of emicizumab in eligible patients with severe hemophilia A without FVIII inhibitors, as per the HAVEN 3 trial, and in patients who were candidates for routine prophylaxis if they had limited ability to receive regular IV therapy due to factors such as venous access challenges or geographical treatment access restrictions. It also included patients who were candidates for routine prophylaxis if they were at significant risk of increased bleeding rates due to factors leading to poor adherence or persistence. No scenario analyses were provided to address the populations described by the Health Canada indication or the sponsor’s reimbursement request.
The dosage regimen recommended by Health Canada for emicizumab is subcutaneous administration, with a loading dose of 3.0 mg/kg for the first 4 weeks.3 This is followed by a maintenance regimen that is age- and weight-specific. Adolescent and adult patients weighing more than 40 kg have the option of 1.5 mg/kg once weekly, 3.0 mg/kg every 2 weeks, or 6.0 mg/kg every 4 weeks. In pediatric patients, and in any patients weighing less than 40 kg, the recommended maintenance regimen is either 1.5 mg/kg once weekly or 3.0 mg/kg every 2 weeks.3 This aligns with the dose used in the economic model, which was based on 3.0 mg/kg as a loading dose for the first 4 weeks of treatment and 1.5 mg/kg once weekly as a maintenance dose for all ages. At the sponsor’s submitted price for emicizumab of $122.05 per mg, the cost for each single-use vial was $3,661.52 (for 30.0 mg/mL), $7,323.04 (for 60.0 mg/ 0.4mL), $12,815.31 (for 105.0 mg/0.7 mL), and $18,307.59 (for 150.0 mg/mL). For an adult patient (70 kg), the sponsor estimated the first-year annual acquisition cost of emicizumab to be $719,946; for subsequent years, $668,685. For FVIII prophylaxis, the annual acquisition cost was calculated by weighting the annual costs for each product by their estimated market shares. This produced an estimate of $472,731 for an adult patient. The sponsor did not factor drug wastage in emicizumab or the FVIII comparators, assuming treatment would be dispensed to the nearest milligram.
The primary clinical outcomes of interest in the model were QALYs. The economic analysis was conducted from the Canadian public payer perspective over a lifetime time horizon (98 years). Both outcomes and costs accrued beyond the first year of the model were discounted at a rate of 1.5%, as per CADTH guidelines.
Model Structure
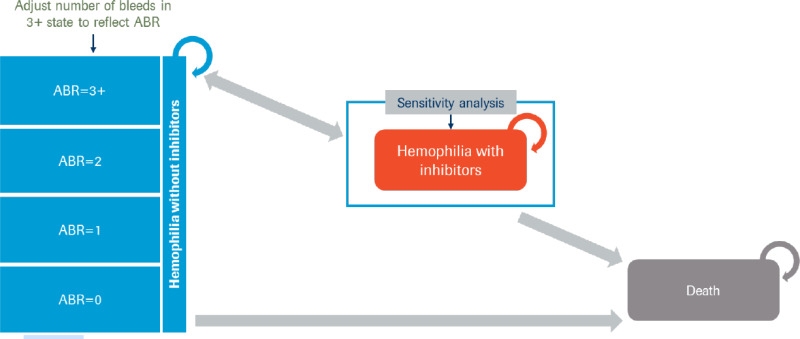
A Markov state-transition model was submitted based on 2 health states: alive with hemophilia A, and death, with the model cycle length defined as 1 year and in which half-cycle corrections were applied ( in Appendix 3). All patients entered the alive health state without inhibitor development. Patients had the risk of dying during each model cycle, depending on their treatment and their age. Alive patients could experience bleed events. Bleed events were stratified by the number of events experienced per year (i.e., 0, 1, 2, or 3 or more bleeds). Patients were at risk of experiencing treatment-related adverse clinical events during the first year of treatment. In addition, the model assumed no patients being treated with emicizumab or with FVIII prophylaxis would require arthroplasty, while patients being treated with on-demand FVIII would require 2 arthroplasties over the course of their lifetimes.
Model Inputs
Patients entered the model at the age of 2 years. Patients’ weight was sourced from the UK population. Pediatric patients were assumed to weigh, on average, 12.5 kg from ages 1 to 4, 28.6 kg from ages 5 to 13, and 60.8 kg from ages 14 to 18. Adults (greater than 18 years of age) were assumed to weigh 75.2 kg on average.
ABTRs were sourced from the HAVEN 3 trial, a 24-week, phase III, randomized controlled trial that investigated adult and adolescent patients (aged greater than 12) with severe congenital hemophilia A without FVIII inhibitors.4 Patients who received episodic treatment with FVIII prior to study entry were randomized in a 2:2:1 ratio to the following treatment arms: emicizumab prophylaxis at 3.0 mg/kg weekly for 4 weeks, followed by 1.5 mg/kg weekly; emicizumab prophylaxis at 3.0 mg/kg weekly for 4 weeks, followed by 3.0 mg/kg every 2 weeks; no prophylaxis (control arm). Patients who received FVIII prophylaxis prior to study entry (derived from the NIS) were enrolled in a separate, non-randomized, single arm where they received treatment with emicizumab prophylaxis at 3.0 mg/kg weekly for 4 weeks, followed by 1.5 mg/kg weekly.4 Tracking of patient trajectories in terms of the number of bleeds over time was not directly modelled; rather, the reported proportions from the trial were applied to the sponsor’s economic model. This meant that the proportion of patients by the number of annual bleeding events was the same across all modelled time horizons. The model further derived the overall ATBR to inform costs and utility impacts from treatment (, Appendix 3). Rates of clinical adverse events were sourced from the HAVEN 1 trial, a phase III, randomized controlled trial that investigated adult and adolescent patients (aged greater than 12) with hemophilia A with FVIII inhibitors.5 Standardized mortality ratios, from a UK study that looked at patients with hemophilia A or B between 1977 and 1998, were applied to UK life tables for the general public.6 The standardized mortality ratio for mild and moderate hemophilia was applied to patients on emicizumab and FVIII prophylaxis, while the standardized mortality ratio for severe hemophilia was applied to on-demand FVIII patients.6
Health-state utilities were derived from a de novo time trade-off vignette study conducted on the Canadian general public. Baseline utility, by treatment, was derived from this study using 2 random-intercept regression models. Differences in treatment-specific utilities were due to adjustments made according to disutilities associated with the annual number of infusions and treated bleed events expected by treatment () and a utility increment associated with subcutaneous administration. No disutility was captured within the economic model for adverse clinical events.
Drug acquisition costs, hospitalization due to bleeding events, and costs to manage adverse events (including arthroplasty) were considered. Dosing information was obtained from the respective Canadian product monographs, with the cost of emicizumab provided by the sponsor; the unit costs for FVIII products were sourced from the PMPRB.7 Vial wastage and drug administration costs were not included. Costs of arthroplasty were taken from the reported cost per surgery from the Canadian Institute for Health Information. Adverse event and hospitalization costs were obtained from the Ontario Case Costing Initiative.7
Summary of Sponsor’s Economic Evaluation Results
The sponsor’s model reported the mean of their probabilistic results, more than 5,000 model iterations. The model also reported deterministic results, which found results that were similar to the probabilistic analysis.
Base-Case Results
The sponsor’s base-case results are presented in . The sponsor reported that emicizumab resulted in greater QALYs than FVIII prophylaxis and on-demand treatment. According to the sponsor’s base-case results, emicizumab was associated with 40.37 QALYs, while prophylaxis and on-demand treatment were associated with 33.01 QALYs and 25.39 QALYs, respectively. However, emicizumab was more costly than its comparators, with a total expected cost of $28,750,976 compared with $20,116,294 and $3,907,944 for prophylaxis and on-demand FVIII, respectively. The ICER for emicizumab was $1,657,813 compared to on-demand FVIII. FVIII prophylaxis was subject to extended dominance through on-demand FVIII and emicizumab (combinations of on-demand FVIII and emicizumab are less costly and more effective than FVIII prophylaxis). At a willingness-to-pay threshold of $50,000 per QALY, emicizumab had a 0% probability of being the optimal therapy.
Summary of the Sponsor’s Economic Evaluation Results.
Sensitivity and Scenario Analysis Results
The sponsor included a scenario analysis in which patients could develop FVIII inhibitors while undergoing treatment. Allowing patients to develop inhibitors in the model led to a sequential ICER of $1,271,160 per QALY compared to on-demand therapy. This change was driven largely by an increase in expected costs for both comparators. Under this scenario, no patients on emicizumab are at risk of developing inhibitors, while patients receiving the comparators were at risk of developing inhibitors and incurred increased costs from immune tolerance induction and switching to bypassing-drug treatments.
CADTH Appraisal of the Sponsor’s Economic Evaluation
CADTH identified several key limitations to the sponsor’s analysis that have notable implications on the economic analysis:
The model structure does not appropriately capture the clinical disease pathway and the effects of treatment: An appropriate model structure for a given economic evaluation should capture all relevant and meaningful underlying clinical or biological processes.
9 The model submitted by the sponsor consisted primarily of 2 health states — alive and dead — and applied transition probabilities to inform the number of patients who would die during each model cycle.
7 Although within the alive health state, this was further stratified by ATBRs (i.e., 0,1, 2, or 3 or more bleeds), the sponsor did not derive transition probabilities to describe how patients would move between different bleeding strata over time. Rather, a fixed proportion of patients was applied to each bleed strata at each model cycle.
7 This approach is inappropriate for multiple reasons. First, given that transition probabilities were not applied directly to the model, how individuals transition among different bleeding frequency health states over time was not explicitly modelled. The clinical experts consulted by CADTH noted that, in patients with greater ATBRs, they would typically intervene to further optimize their therapy, leading to an expected decline in ATBRs. Without further intervention, these patients would be expected to have an increased risk of bleed-related morbidity and mortality outcomes. Furthermore, patients with higher bleed rates are likely to have bleed-related complications and will be at greater risk of experiencing high frequencies of bleeds in the future. However, the sponsor’s submitted model did not explicitly consider any of these aspects of the condition, the impact of treatment, or its associated costs or utility impacts. Secondly, the sponsor did not incorporate parameter uncertainty. Over each Monte Carlo simulation, the same proportions were applied to the model. This is not appropriate, given that these values were derived from clinical studies and the “true” values remain unknown.
The target population of the model does not reflect the Health Canada indication under review or the sponsor’s reimbursement population. The submitted Health Canada indication is for patients with hemophilia A (congenital factor VIII deficiency) as routine prophylaxis to prevent bleeding or reduce the frequency of bleeding episodes.
3 The sponsor has requested reimbursement in patients with severe hemophilia A (congenital factor VIII deficiency) without FVIII inhibitors, including those who are candidates for routine prophylaxis with FVIII if they are at significant risk of increased bleed rates due to factors leading to poor adherence or persistence, or have limited ability to receive regular IV therapy due to underlying factors. During the review period, the sponsor provided additional information clarifying that the reimbursement-requested indication was for patients with severe, non-inhibitor hemophilia A as well as patients with mild and moderate non-inhibitor hemophilia A who experience geographical treatment access restrictions and venous access challenges.
10 The target population in the model considered only patients with severe, non-inhibitor hemophilia A who met HAVEN 3’s eligibility criteria, given that key model parameters were sourced from this trial. As noted in the CADTH clinical review, the inclusion criteria for patients in HAVEN 3 included previously managed by on-demand FVIII and experiencing 5 or more bleeds in the 24 weeks prior to study entry, which is not representative of patients with hemophilia A in Canadian clinical practice. As the study population in HAVEN 3 had greater uncontrolled bleeding than would be expected in Canadian practice, this may overstate the clinical efficacy of emicizumab compared to what would be expected in the clinical setting. The magnitude of the treatment effect in patients with better control (consistent with the Canadian clinical population) compared to those included in the trials remains unknown. Eligibility criteria further excluded patients who had thromboembolic disease, were at high risk for microangiopathy, or had certain autoimmune diseases, thereby decreasing generalizability to the Canadian clinical population. HAVEN 3 further excluded patients under the age of 12. According to clinical experts consulted by CADTH, patients as young as 1 year of age may be prescribed emicizumab.
Given the clinical evidence base, CADTH was unable to conduct a reanalysis to adequately assess this limitation. To align with the clinical evidence, the age parameter in the model was revised to reflect the distribution reported in HAVEN 3. A scenario analysis was further conducted in which the patient age was set to 2 years, as assumed in the sponsor’s model. Although evidence on the effects of emicizumab on children without inhibitors is lacking, the efficacy and safety of emicizumab should not be age-dependent, according to the clinical experts consulted by CADTH, given the known mechanism of action of emicizumab and the existing clinical evidence for emicizumab in children with inhibitors.
Naive comparison for emicizumab versus FVIII prophylaxis: Treatment efficacy in the sponsor’s model was informed by the HAVEN 3 and the NIS.
7 The NIS preceded HAVEN 3 and was conducted among patients on FVIII prophylaxis. These patients were subsequently eligible for enrolment in HAVEN 3 in a separate, non-randomized arm, and received emicizumab prophylaxis.
4 For the emicizumab and on-demand groups in the economic model, clinical efficacy was informed by the appropriate randomized arms in the HAVEN 3 trial; for FVIII prophylaxis, clinical efficacy was informed by the NIS. It is inappropriate to compare clinical outcomes for FVIII prophylaxis in the NIS with the clinical outcomes for emicizumab in HAVEN 3. As the CADTH clinical report notes, there is no direct comparative evidence to support the efficacy of emicizumab compared to FVIII prophylaxis in patients with severe hemophilia A; the only evidence is limited to an intra-patient analysis (group D in HAVEN 3). The sponsor’s approach reflects a non-randomized, naive comparison; it remains unclear what potential biases are introduced into the economic analysis given this approach. Certain reported baseline characteristics were found to differ between the randomized HAVEN 3 groups and the NIS group: the NIS group was younger and had experienced a lower number of bleeds in the prior 24 weeks. Although the clinical experts consulted by CADTH noted that these baseline differences would be expected in the FVIII prophylaxis population, and that treatment effects are likely independent of patient age, it is unclear whether other biases could have been introduced through this non-randomized comparison. They also commented that while the clinical estimates used in the economic model likely represent the Canadian setting, they may lack precision. The sponsor further commissioned an indirect treatment comparison and incorporated these estimates into a scenario analysis. Although the sponsor’s network meta-analysis suggested that emicizumab prophylaxis was associated with reduced bleed rates compared with FVIII prophylaxis in the treatment of patients with severe hemophilia A without inhibitors, methodological limitations of the indirect treatment comparison affect both the confidence of this finding and the appropriateness of using these estimates to inform the economic model. A small number of trials were included in the analysis, and each trial enrolled a small number of patients. Due to the small evidence base, the results of the analysis were uncertain. A high degree of heterogeneity was further noted across the included studies, including: the severity of hemophilia A; different FVIII products studied; inconsistent or unclear definitions of the bleed outcomes; variable time points for outcome measurement; and differences in study design. Together, these limitations preclude the use of estimates derived from the network meta-analysis to inform the comparative treatment effects of emicizumab relative to FVIII prophylaxis.
Dispensing of treatment does not reflect clinical practice. In the sponsor’s submitted economic model, treatment was dispensed according to the sponsor’s product monographs, with treatment-acquisition costs calculated based on the exact dose (per mg) required. This misaligns with the assumptions stated in the sponsor’s submitted budget impact analysis. The product monograph for emicizumab states that the drug is for single use only and available in pre-set concentrations. According to the CADTH clinical experts, patients on both emicizumab and FVIII would typically have their dose rounded up to the nearest whole vial, with the drug dispensed accordingly to minimize wastage. How treatments are dispensed needs to be accounted for in the cost of treatment. In the sponsor’s approach, the treatment-acquisition costs for both emicizumab and the FVIII comparators were underestimated.
Inappropriate modelling of health-utility estimates. The utility estimates were based on a sponsor’s commissioned utility exercise in which a sample of the Canadian public (n = 82) provided utility estimates based on a time trade-off vignette exercise.
7 Based on the survey responses, regression models were derived. The first model included treatment administration (subcutaneous versus intravenous) and the number of bleeds per year as predictor variables; using the same dataset, a second regression analysis was modelled specific to prophylaxis treatment with a single predictor: the frequency of infusions per year.
The sponsor derived baseline utility for the treatment and comparator arms based on combining the coefficients from both regression analyses. This, in effect, introduces treatment-specific utility values (i.e., emicizumab = 0.908 [based on summing the intercept and the coefficient for subcutaneous treatment from the first regression model]; on-demand FVIII = 0.797 [based on the intercept from the first regression model]; and FVIII prophylaxis = 0.759 [based on the intercept from the first regression model subtracted from the product of the number of infusions and the disutility of infusion from the second regression model]).
7 This approach (i.e., combining values from separate regression equations) is inappropriate because these regressions are conceptually different and not compatible given that each regression equation is estimating a different set of estimates. Therefore, this approach lacks credibility. In effect, it applies 2 separate adjustments for IV infusions (i.e., 1 from each regression model) without consideration that the values from the separate regression equations are, in fact, correlated. As per current guidelines for the conduct of economic evaluations,
9 utilities should reflect the health states within the model and not be specific to treatment. Although an argument could potentially be made on patient preference for the mode of treatment administration, the sponsor’s estimate would indicate that patients are willing to trade off 1.1 years of perfect health every 10 years to avoid treatments involving IV administration, assuming all else is considered equal. This magnitude is greater than the benefit that a patient with mild anemia achieves with treatment that fully resolves their anemia.
11 Of note, the CADTH clinical experts indicated that the effect of treatment on improved quality of life remains inconclusive.
CADTH removed treatment-specific utility values by setting the baseline utility to be identical across all treatments. The utility decrements arising from treated bleeds and infusions were incorporated into the model separately. Given the limitations with the structure of the sponsor’s submitted model, utility decrements could not be applied to adverse events.
Missing comparators. The comparators in the submitted economic model reflected approaches to treating patients with hemophilia A with FVIII products. However, in consultation with the clinical experts, it was noted that plasma-derived von Willebrand factor (VWF) products, such as Antihemophilic Factor/ VWF Complex (Human) (Humate-P) and VWF/Coagulation Factor VIII Complex (Human) (Wilate), may also be used in this patient population. These comparators were not considered in the sponsor’s submitted model.
Underestimation of adult patient weights. Patient weights used in the model were sourced from UK life tables. Hemophilia A primarily affects men; men are, on average, heavier than women.
12 Furthermore, the average weight of patients in the HAVEN 3 trial (79.1kg)
4 was greater than the sponsor’s assumption (75.2 kg). This difference in weight would translate to a different vial size for emicizumab and, thereby, a higher treatment-acquisition cost. Setting the weight at 75.2 kg would have underestimated the expected cost of emicizumab.
One additional limitation was identified, but was considered unlikely to change or to affect the analyses significantly. This limitation is outlined next.
Disutility from arthroplasties was not included in the model. As joint replacement due to severe bleeding events would affect quality of life, a disutility of 0.39 was applied for arthroplasties for the duration across which the model captured their impact.
13
Additionally, the following key assumptions were made by the sponsor and have been appraised by CADTH ().
Key Assumptions of the Submitted Economic Evaluation (Not Noted as Limitations to the Submission).
CADTH Reanalyses of the Economic Evaluation
Base-Case Results
While several limitations with the sponsor’s submission could not be addressed (i.e., model structure, patient population, noncomparative clinical outcomes for FVIII prophylaxis, and missing comparators), other limitations could be explored. CADTH undertook a stepped analysis, incorporating each change detailed in into the sponsor’s corrected base case to highlight the impact of each change. The summary results of the sponsor’s corrected base case and the CADTH reanalyses are presented in .
CADTH Revisions to the Submitted Economic Evaluation.
Summary of the Stepped Analysis of the CADTH Reanalysis Results.
In the CADTH base case, emicizumab was associated with an additional cost of $8,691,419 and 1.57 additional QALYs, for an ICER of 5,530,766 per QALY gained compared to FVIII prophylaxis. Using a willingness-to-pay threshold of $50,000 per QALY, there is a 0% probability that emicizumab would be considered cost-effective in the CADTH base case. This is primarily due to the incorporation of vial wastage and removing treatment-specific utility values.
Detailed results of the CADTH base case are presented in of Appendix 4. Of note, the reanalysis is based on publicly available prices of the comparator treatments.
Scenario Analysis Results
Several scenario and sensitivity analyses were conducted on the CADTH base case. These scenario analyses primarily explored changes in starting age, drug prices, and utility. Furthermore, scenario analyses included testing a structural assumption in which patients on FVIII treatments could be at risk of developing inhibitors and a scenario in which FVIII prophylaxis was removed as a possible comparator in light of the noncomparative evidence available. The model interpretations were found to remain robust () because no scenario brought the sequential ICER of emicizumab close $50,000 per QALY.
The model was most sensitive to the price of the comparator treatment. The public prices for FVIII, reported by PMPRB are ceiling prices (i.e., they reflect the maximum average potential price). Utilizing these prices in the model, a price reduction of at least 89% is required for emicizumab to be considered cost-effective at a willingness-to-pay threshold of $50,000 per QALY, according to the CADTH base case (). Given that PMPRB prices may not reflect the actual price of FVIII products in Canada, 2-way price-reduction analyses were further conducted to highlight the influence of price reduction on the cost-effectiveness results for both emicizumab and FVII ().
CADTH Price-Reduction Analyses.
CADTH 2-Way Price-Reduction Analyses.
Issues for Consideration
Emicizumab is administered subcutaneously, whereas FVIII therapies are administered intravenously. According to the clinical experts consulted, both treatments can be administered at home following adequate patient training.
According to the clinical experts consulted by CADTH and the health care provider input received as part of the patient group’s feedback, adherence is key to reducing breakthrough bleeds that require treatment. The efficacy of treatment is highly dependent on patient adherence. It is plausible that emicizumab may result in better adherence to treatment, given its mode of administration and less frequent administration. Adherence, which would be expected to affect both costs and utilities, was not explicitly captured in the sponsor’s model.
CADTH was unable to adequately assess the impact of potentially lower prices of the comparators on the cost-effectiveness of emicizumab. Reduced effective prices for comparators, arising from the tendering process by Canadian Blood Services
15 (as opposed to PMPRB’s maximum average potential price), may lead to different conclusions than the current analysis, potentially resulting in an even higher ICER for emicizumab.
The development of neutralizing anti-drug antibodies in patients was not reported in the HAVEN 3 trial, although case reports have been observed in practice.
16 The clinical effects of anti-drug antibodies remain unclear, and their impact on both clinical effectiveness and cost-effectiveness remains unknown.
Emicizumab has been reviewed by other Health Technology Assessment agencies. Quebec’s Institut national d’excellence en santé et services sociaux has not recommended the reimbursement of emicizumab for patients without FVIII inhibitors.
17 A draft report from the Institute for Clinical and Economic Review found emicizumab is likely not cost-effective at a threshold of US$200,000 per QALY for patients without FVIII inhibitors.
18According to the clinical experts consulted by CADTH, there are several emerging treatments for patients with hemophilia that are currently under development or emerging. These include extended half-life factor replacement products, non-factor therapies (e.g., anti-tissue pathway inhibitor antibody), and gene therapies (e.g., valoctocogene roxaparvovec).
19With respect to desmopressin’s place in therapy, the clinical experts consulted by CADTH noted that it is primarily used to treat patients with mild or moderate hemophilia A.
Overall Conclusions
Based on the CADTH clinical review of 2 sponsor-submitted trials (i.e., HAVEN 3 and HAVEN 4), emicizumab is associated with a statistically and clinically significantly improvement in bleeding outcomes (i.e., ATBR ratio for treated bleeds, all bleeds, treated joint bleeds, treated spontaneous bleeds) compared to on-demand treatment. Limited comparative evidence exists to establish the comparative effectiveness and safety of emicizumab compared to FVIII prophylaxis; however, current evidence suggests that emicizumab showed a reduction in bleeding outcomes compared to no prophylaxis (non-randomized comparison). No additional studies met the inclusion criteria for the systematic review conducted in the CADTH clinical review, and there is presently no clinical evidence comparing emicizumab to plasma-derived VWF products that could be used in this patient population. These limitations in clinical evidence could not be addressed in the pharmacoeconomic analysis.
Furthermore, CADTH could not address the limitations associated with the model structure and the analysis target population (compared with the intended population). The model target population is specific to patients with severe hemophilia A who meet HAVEN 3’s eligibility criteria. The CADTH reanalysis was able to address only a subset of the limitations in the sponsor’s submission, including: adjusting dispensing to the nearest vial size, removing treatment-specific utility estimates, and setting patients’ starting age and weight to better reflect the patient population in HAVEN 3. CADTH’s findings remained aligned with the sponsor’s: emicizumab is not a cost-effective option at a willingness-to-pay threshold of $50,000 per QALY in patients with severe hemophilia A. In the CADTH base-case reanalysis, the ICER for emicizumab was $5,530,766 per QALY compared with FVIII prophylaxis. A price reduction of at least 89% is necessary for emicizumab to be considered cost-effective at a threshold of $50,000 per QALY. However, a greater price reduction may be required if the price of FVIII products is, in fact, lower than the PMPRB published values.
The cost-effectiveness of emicizumab in both the reimbursement-requested population and the broader Health Canada indication remain unknown.
- a
versus reference category of intravenous infusion
Appendix 1. Cost-Comparison Table
The comparators presented in the following table have been to be deemed appropriate based on feedback from clinical experts. Comparators may be recommended (appropriate) practice or actual practice. Existing Product Listing Agreements are not reflected in the table and as such, the table may not represent the actual costs to public drug plans.
Table 9CADTH Cost-Comparison Table for Prophylaxis of Bleeding in Patients With Hemophilia A Without Factor VIII Inhibitors
View in own window
| Treatment | Strength | Form | Price per unit ($) | Recommended dosage | Daily cost ($) | Course or annual cost ($) |
|---|
| Emicizumab (Hemlibra) | 30.0 mg/1.0 mL
60.0 mg/0.4.0 mL
105.0 mg/0.7 mL
150.0 mg/1.0 mL | Vial for SC injection | 122.0506 per mga | Loading dose: 3.0 mg/kg once weekly for 4 weeks
Maintenance dose:
1.5 mg/kg once weekly or
3.0 mg/kg every 2 weeks or
6.0 mg/kg every 4 weeks, starting week 5 for patients ≥ 12 years of age who weigh ≥ 40 kg. Patients under 12 years or those who weigh < 40kg should receive
1.5 mg/kg every week or
3.0 mg/kg every 2 weeks starting week 5. | 40 kg patient:
Loading: 2,092.30
Maintenance: 1,046.15
80 kg patient:
Loading: 4,184.59
Maintenance: 2,092.30 | 40 kg patient:
Year 1: 411,136
Thereafter: 381,841
80 kg patient:
Year 1: 822,272
Thereafter: 763,688 |
| Factor VIII treatments available through Canadian Blood Services |
|---|
| Antihemophilic Factor (Recombinant), PEGylated (Adynovate) | 250 IU
500 IU
750 IU
1,000 IU
1,500 IU
2,000 IU
3,000 IU | Powder for IV injection | 1.8862 per IUb | Patients < 12 years:
40 IU/kg to 60 IU/kg twice weekly
Patients ≥ 12 years:
40 IU/kg to 50 IU/kg twice weekly | 40 kg patient:
943.10 to 1,077.83
80 kg patient:
1,751.47 to 2,694.57 | 40 kg patient:
344,232 to 393,407
80 kg patient:
639,287 to 983,519 |
| Antihemophilic Factor (Recombinant) BDD, Fc Fusion Protein (Eloctate) | 250 IU
500 IU
750 IU
1,000 IU
1,500 IU
2,000 IU
3,000 IU | Powder for IV injection | 1.8862 per IUc | 50 IU/kg every 3 to 5 days | 40 kg patient:
754.48 to 1,257.47
80 kg patient:
1,508.96 to 2,514.93 | 40 kg patient
275,385 to 458,975
80 kg patient:
550,770 to 917,951 |
| Antihemophilic Factor (Recombinant) BDD, PEGylated (Jivi) | 250 IU
500 IU
1,000 IU
2,000 IU
3,000 IU | Vial | 1.8862 per IUb | Patients ≥ 12 years:
30 IU/kg to 40 IU/kg twice weekly | 40 kg patient:
673.64 to 943.10
80 kg patient
1,347.29 to 1,751.47 | 40 kg patient:
245,880 to 344,232
80 kg patient:
491,759 to 639,287 |
| Antihemophilic Factor (Recombinant) (Kovaltry) | 250 IU
500 IU
1,000 IU
2,000 IU
3,000 IU | Vial | 1.4592 per IUc | Patients ≤ 12 years:
20 IU/kg to 50 IU/kg, 2 to 3 times weekly or every other day
Patients > 12 years:
20 IU/kg to 40 IU/kg, 2 to 3 times weekly | 40 kg patient:
(≤ 12) 416.91 to 1,459.20
80 kg patient:
729.60 to 2,032.46 | 40 kg patient:
(≤ 12) 152,174 to 532,608
80 kg patient:
266,304 to 741,847 |
| Antihemophilic Factor (Recombinant) BDD, (Nuwiq) | 250 IU
500 IU
1,000 IU
2,000 IU
2,500 IU
3,000 IU
4,000 IU | Powder and solvent for IV injection | 287.2426c
559.0977c
Unknown
Unknown
Unknown
3,823.5677c
5,098.0903c | Childrend:
30 IU/kg to 40 IU/kg every other day or 3 times weekly
Adults:
30 IU/kg to 40 IU/kg every other day | 40 kg patient (child):
399.36 to 838.65e
80 kg patient:
798.71 to 1,038.32e | 40 kg patient (child):
145,765 to 306,106e
80 kg patient:
291,530 to 378,988e |
| Antihemophilic Factor (Recombinant)BDD rFVIII (Xyntha) | 250 IU
500 IU
1,000 IU
2,000 IU | Powder in vial | 1.1182 per IUf | Adults and adolescentsg:
30 IU/kg ± 5 IU/kg 3 times weekly | 40 kg patient:
479.23 to 718.84
80 kg patient:
958.45 to 1,437.68 | 40 kg patient:
174,918 to 262,377
80 kg patient:
349,835 to 524,753 |
| Factor VIII and von Willebrand factor treatments available through Canadian Blood Services |
|---|
| Antihemophilic Factor/VWF Complex (Human) (Humate-P) | 250/600 IU
500/1,200 IU
1,000/2,400 IU | Vial for IV injection | 1.1182 per IUf | Not indicated for prophylaxis. Depends on severity of bleed. Loading doses may be 15 IU/kg to 50 IU/kg, followed by additional doses of approximately half the loading dose, 1 to 3 times daily for up to 14 days. | 40 kg patient:
Loading dose: 838.65 to 2,236.39
80 kg patient:
Loading dose: 1,397.74 to 3,913.68 | NA |
| VWF/Coagulation Factor VIII Complex (Human) (Wilate) | 500/500 IU
1,000/1,000 IU | Powder and solvent for IV injection | 1.1182 per IUf | 20 IU/kg every 2 to 3 days | 40 kg patient:
372.73 to 559.10
80 kg patient:
745.47 to 1,118.20 | 40 kg patient:
136,048 to 204,072
80 kg patient:
272,095 to 408,143 |
| Desmopressin |
|---|
| Desmopressin (Deamino D-arginine Vasopressin) | 4 mcg/mL | Vial for injection | 10.9800 per mLh | Not indicated for prophylaxis. 0.3 mcg/kg by IV infusion; maximum dose 20 mcg | 40 kg patient:
32.94 per use
80 kg patient:
54.90 per use | NA |
BDD = B-domain deleted; FVIII = factor VIII; IU = international unit; IV = intravenous; NA = not applicable; PMPRB = Patented Medicine Prices Review Board; FVIII = factor VIII; SC = subcutaneous; VWF = von Willebrand factor.
Note: Daily and annual costs are rounded up to the nearest available vial size; patients typically administer the full amount in the vial for factor VIII products, according to the clinical experts consulted by CADTH. PMPRB pricing represents the maximum average potential price in Canada; it is likely that due to the Canadian Blood Services tendering system, actual costs per international unit are substantially lower.
- a
Sponsor’s submitted price.
- b
The price for Jivi was unavailable from PMPRB. The price for Eloctate was used as the lowest available long-acting proxy.
- c
PMPRB maximum average potential price, accessed September 2020.20
- d
The term “children” is not defined in the dosage and administration section of the Nuwiq product monograph; however, other sections specify that pediatric studies were conducted in patients ≤ 12 years of age.21
- e
The lowest cost per international unit across available strengths of Nuwiq of $1.1182 was assumed in daily and annual cost calculations.20
- f
Price unavailable from PMPRB. Nuwiq price was used as a proxy price.20
- g
Xyntha is deemed appropriate for children of all ages, including newborns, in the product monograph; however, no dosing information is provided for prophylactic use in pre-adolescent children.22
- h
The Saskatchewan Formulary (accessed August 2020).23
Appendix 3. Additional Information on the Submitted Economic Evaluation
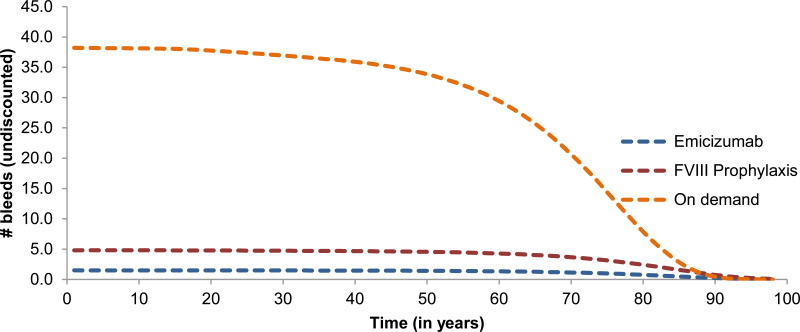
The model estimates that patients receiving on-demand treatment will experience 30 to 40 bleed events, while patients receiving emicizumab will experience nearly 0 bleed events on average ().
Table 11Number of Bleeds Experienced in a Year per Treatment Arm
View in own window
| Number of bleeds per year | Emicizumab | FVIII prophylaxis | On-demand therapy |
|---|
| 0 bleeds (%) | 41.70 | 31.30 | 0.00 |
| 1 bleed (%) | 14.60 | 12.50 | 0.00 |
| 2 bleeds (%) | 8.30 | 4.20 | 0.00 |
| 3 bleeds (%) | 35.40 | 52.10 | 100.00 |
| Overall annual bleed rate | 1.50 | 4.80 | 38.20 |
| Number of bleeds for ≥ 3 bleeds | 3.356 | 8.812 | 38.20 |
Source: Sponsor’s pharmacoeconomic submission ().8
Appendix 5. Submitted Budget Impact Analysis and CADTH Appraisal
View in own window
| Key take-aways of the BIA |
|---|
CADTH identified the following key limitations with the sponsor’s analysis:
Because the actual cost of FVIII products is confidential, there is significant uncertainty in the price of FVIII comparators. This uncertainty is likely to favour the adoption of emicizumab. The adult weights used were not representative of the Canadian or trial populations. ATBRs in the severe, on-demand population are unlikely to be representative of the Canadian context. The uptake of emicizumab across patient groups is uncertain. The distribution of patients currently receiving FVIIII prophylaxis versus on-demand treatment is unknown.
The CADTH reanalyses included: assuming patients with severe hemophilia A on on-demand therapy have an ATBR of 24.1; and changing the adult weight to be consistent with the average weight observed in the HAVEN 3 trial (79.1 kg). 24The sponsor’s budget impact analysis (BIA) population was aligned with the sponsor’s reimbursement request: patients with severe hemophilia A without FVIII inhibitors; patients at significant risk for increased bleeding rates due to factors that lead to poor adherence or persistence (despite being candidates for routine prophylaxis with FVIII); and patients who have limited ability to receive regular IV therapy. Based on the CADTH reanalyses, the budget impact from the introduction of emicizumab for the sponsor’s reimbursement request is expected to be $73,091,035 in year 1, $77,522,044 in year 2, and $88,989,541 in year 3, with a 3-year total budget impact of $239,602,620. Uncertainty remains regarding comparator pricing, the expected uptake of emicizumab, and the distribution of patients across currently available therapies. Results appear to be driven mostly by the price of FVIII and the price of emicizumab.
|
Summary of Sponsor’s Business Impact Analysis
In the submitted BIA, the sponsor assessed the introduction of prophylactic emicizumab for the treatment of patients with hemophilia A without factor VIII (FVIII) inhibitors compared to prophylactic or on-demand treatment with FVIII replacement therapy, consistent with the requested reimbursement criteria (see ). The BIA was undertaken from the perspective of a Canadian public payer over a 3-year time horizon using an epidemiological approach. The sponsor included the acquisition costs associated with plasma protein products, including wastage, but excluded mark-ups and dispensing fees (). Data for the model were obtained from various sources, including the HAVEN 3 and 4 trials,14,24 the Canadian Blood Disorders Registry,25 published literature, CADTH’s 2015 budget impact analysis for Eloctate,26 and expert opinion.8
Table 14Summary of Key Model Parameters
View in own window
| Parameter | Sponsor’s estimate (reported as year 1, year 2, and year 3, if appropriate) |
|---|
| Target population |
|---|
Patients with hemophilia A (congenital FVIII deficiency) across Canada 25Without inhibitors (97%) 25
| 3,105/ 3,138/ 3,171 3,012/ 3,044/ 3,075 |
| Number of patients eligible for emicizumab (i.e., excluding Quebec) | 2,332/ 2,357/ 2,381 |
| Market uptake (3 years) |
|---|
| Uptake (reference scenario) | See Source: Sponsor’s pharmacoeconomic submission, BIA report, and model.8 for patient proportions by severity and reference scenario treatment regimen. |
| Uptake (new drug scenario)a | |
| Adults: | |
|
| ▬%/ ▬%/ ▬% |
| ▬%/ ▬%/ ▬% |
| ▬%/ ▬%/ ▬% |
| ▬%/ ▬%/ ▬% |
| Pediatrics: | |
|
| ▬%/ ▬%/ ▬% |
| ▬%/ ▬%/ ▬% |
| ▬%/ ▬%/ ▬% |
| Costs |
|---|
| Drugsb: | |
Emicizumab, cost per mg Long-acting FVIII, cost per IU cShort-acting FVIII, cost per IU d
| $122.0506 |
| $1.8900 |
| $1.1182 |
| Annual treated bleeds, by product: | |
Emicizumab FVIII prophylaxis On-demand
| 1.7 |
| 1.9 |
| 40 (severe hemophilia); 10 (moderate hemophilia) |
BIA = budget impact analysis; FVIII = factor VIII; IU = international unit.
- a
An additional 4% of all eligible non-inhibitor patients (4% × 2,333 = 86.63 in year 1) were assumed to switch to emicizumab due to issues with venous access, regional access, or adherence/persistence. However, these patients were all assumed to be distributed across severe and moderate pediatric patients and moderate adult patients, regardless of whether they were receiving FVIII prophylaxis or on-demand therapy.
- b
Drug prices in the BIA were implemented per IU. To account for wastage, the sponsor assumed a wastage of 7%, 8%, and 6% for pediatric patients receiving emicizumab, long-acting FVIII, and short-acting FVIII, respectively. For adults, this was 4%, 4%, and 3%, respectively.8
- c
The ELOCATE cost was used to represent the price for all long-acting FVIII drugs.20
- d
The Nuwiq cost was used to represent the price for all short-acting FVIII drugs.20
Source: Sponsor’s pharmacoeconomic submission, BIA report, and model.8
Table 15Sponsor’s Estimations and Assumptions on Population Size, Disease Severity, and Treatment Regimen (Reference Scenario)
View in own window
| Patient flow | Patient numbers |
|---|
| Age | Severity | Treatment | Approach to therapy | Year 1 | Year 2 | Year 3 |
|---|
| Hemophilia A (without FVIII inhibitors) | Pediatric (< 12) 14% | Severe 60% | Long-acting FVIII 12% | Prophylaxis 95% | 36 | 36 | 37 |
|---|
| On-demand 5% | 2 | 2 | 2 |
| Short-acting FVIII 88% | Prophylaxis 95% | 144 | 145 | 147 |
| On-demand 5% | 8 | 8 | 8 |
| Moderate 10% | Long-acting FVIII 12% | Prophylaxis 70% | 5 | 5 | 5 |
| On-demand 30% | 1 | 1 | 1 |
| Short-acting FVIII 88% | Prophylaxis 85% | 21 | 21 | 22 |
| On-demand 15% | 4 | 4 | 4 |
| Mild 31% | Long-acting FVIII 0% | Prophylaxis 0% | 0 | 0 | 0 |
| On-demand 100% | 0 | 0 | 0 |
| Short-acting FVIII 100% | Prophylaxis 0% | 0 | 0 | 0 |
| On-demand 100% | 97 | 98 | 99 |
| Adult (≥ 12) 86% | Severe 29% | Long-acting FVIII 12% | Prophylaxis 70% | 100 | 102 | 103 |
| On-demand 30% | 18 | 18 | 18 |
| Short-acting FVIII 88% | Prophylaxis 85% | 401 | 406 | 410 |
| On-demand 15% | 72 | 72 | 73 |
| Moderate 10% | Long-acting FVIII 12% | Prophylaxis 70% | 31 | 31 | 31 |
| On-demand 30% | 10 | 10 | 10 |
| Short-acting FVIII 88% | Prophylaxis 85% | 122 | 124 | 125 |
| On-demand 15% | 41 | 41 | 42 |
| Mild 61% | Long-acting FVIII 0% | Prophylaxis 0% | 0 | 0 | 0 |
| On-demand 100% | 0 | 0 | 0 |
| Short-acting FVIII 100% | Prophylaxis 0% | 0 | 0 | 0 |
| On-demand 100% | 1,220 | 1,232 | 1,245 |
| Total number of patients eligible for emicizumab, per year | 2,332 | 2,357 | 2,381 |
BIA = budge impact analysis; FVIII = factor VIII.
Note: Figures may not add up to 100% due to rounding.
- a
Proportion switching by year 3 indicates the proportion of patients in each severity and regimen category who are assumed to switch to emicizumab prophylaxis in the new drug scenario. See .
Sources: Sponsor’s pharmacoeconomic submission;8 Budget Impact Analysis Report, and and ; sponsor’s submitted BIA model. Discrepancies between the sponsor’s BIA report and model were corrected to reflect those reported in the model.
Summary of the Sponsor’s Budget Impact Analysis Results
Results of the sponsor’s base case suggested an incremental cost of $67,028,982 in year 1, $70,861,365 in year 2, and $81,247,668 in year 3, for a total incremental cost of $219,138,016 over the 3-year time horizon when emicizumab is reimbursed for adult patients with severe hemophilia A (congenital FVIII deficiency) without FVIII inhibitors; for adult and pediatric patients with limited ability to receive regular IV therapy due to underlying factors, such as venous access challenges or geographical treatment access restrictions; or for adult and pediatric patients at significant risk for increased bleeding rates due to factors that lead to poor adherence or persistence.
CADTH Appraisal of the Sponsor’s BIA
CADTH identified several key limitations to the sponsor’s analysis that have notable implications on the results of the BIA:
Uncertainty in the cost of FVIII comparators. The price of long- and short-acting FVIII was estimated by the sponsor from the PMPRB, which provides the maximum average potential price of a new patented medicine.
20 Public procurement of FVIII products is based on a tendering process in which the reimbursed price is likely to be lower.
8 Despite these prices being confidential, using the maximum price for the comparators introduces significant uncertainty because a higher comparator price will favour the adoption of emicizumab.
Uncertainty in annualized treated bleed rates for patients receiving on-demand therapy. The sponsor estimated an ATBR of 40 for adult and pediatric patients with severe disease receiving on-demand therapy.
26 The clinical experts consulted by CADTH for this review noted that a patient would be unlikely to experience such a high frequency of bleeds without intervention.
In CADTH reanalyses, the ATBR for severe pediatric and adult patients was changed to 24.1, reflecting the on-demand values reported in patients with severe disease in the PROTEC VIII trial.
27
Inappropriate adult weight used in the model. The sponsor used an adult weight of 72.47 kg.
4 CADTH was unable to validate this weight in the cited publication. According to the clinical experts consulted by CADTH for this review, this weight may not be representative of Canadian adults; they noted that the average weight may be higher.
Uncertainty regarding the uptake of emicizumab among eligible patients. The BIA investigated the impact of the sponsor’s reimbursement request, which included patients with both severe and non-severe disease who met specific criteria. In the sponsor’s new drug scenario, a proportion of adults with severe disease who are currently receiving both prophylaxis and on-demand therapies were assumed to switch to emicizumab, whereas 0% of pediatric patients who have severe disease were assumed to switch. Separately, the sponsor assumed that ▬% of all patients eligible for emicizumab would switch to emicizumab, given that they would meet the specific sponsor’s requested criteria. However, these patients were redistributed to the adult moderate population and pediatric severe and moderate populations only (). Estimates of market share are uncertain. Furthermore, this mathematical approach to deriving market share estimates is inconsistent and lacks transparency. The clinical experts consulted by CADTH for this review expected that uptake among adult patients with moderate disease would be similar to adult patients with severe disease. Experts also noted that adult patients receiving on-demand therapy may be less likely to switch to emicizumab than those treated with prophylaxis, which contradicts the sponsor’s assumption that uptake among moderate patients would be equal across both treatment groups. Lastly, the CADTH clinical review report notes that the area of greatest unmet need is in the pediatric population. Therefore, uptake among pediatric patients may be higher than assumed in the sponsor’s base-case analysis.
Uncertainty in the proportion of patients across current FVIII treatment paradigms. The sponsor estimated the proportions of patients, by age group and severity, who would be currently receiving FVIII prophylaxis versus on-demand therapy based, on clinical expert opinion. These estimates are uncertain, given that the distribution of the current treatment mix is not publicly available. Because experts expect that adult patients currently receiving prophylaxis would be more likely to switch to emicizumab than those receiving on-demand therapy alone, these values in the reference scenario have the potential to influence the BIA results.
Comparator product missing. According to the clinical experts consulted by CADTH for this review, some patients may use plasma-derived VWF products for treatment and prophylaxis of bleeds.
28
CADTH Reanalyses of the Budget Impact Analysis
CADTH revised the sponsor’s submission by changing the ATBR for on-demand patients with severe disease and adjusting the adult weight to align with the HAVEN 3 trial. compares the assumption and values used by the sponsor with those used by CADTH in its reanalysis.
Table 16CADTH Revisions to the Submitted Budget Impact Analysis
View in own window
| Stepped analysis | Sponsor’s value or assumption | CADTH value or assumption |
|---|
| Corrections to sponsor’s base case (none) |
|---|
| Changes to derive the CADTH base case |
|---|
| 1. Uncertain ATBRs for on-demand patients | 40 | 24.1 |
| 2. Inappropriate adult weight used in the model | 72.47 kg | 79.1 kg |
| CADTH base case | | 1 + 2 |
ATBR = annualized treated bleed rate.
The results of the CADTH step-wise reanalysis are presented in summary format in . A more detailed breakdown is presented in . Applying these changes increased the 3-year total budget impact to $239,602,620.
Table 17Summary of the CADTH Reanalyses of the Budget Impact Analysis
View in own window
| Stepped analysis | 3-year total |
|---|
| Submitted base case | $219,138,016 |
| CADTH reanalysis 1: on-demand ATBR for patients with severe disease | $220,610,606 |
| CADTH reanalysis 2: adult patient weight | $238,004,585 |
| CADTH base case | $239,602,620 |
ATBR = annualized bleed rate.
Note: The reanalyses are based on the sponsor’s assumed prices for FVIII comparators. The true cost of these products is not publicly available.
CADTH also conducted additional scenario analyses to address remaining uncertainties:
Assume uptake among pediatric patients with severe disease will be equal to that of adults with severe disease, in addition to the ▬% uptake assumed by the sponsor (revised uptake among pediatric patients with severe disease = 40%, 45%, and 50% and 25%, 28%, and 30% for year 1, year 2, and year 3 for pediatric patients on prophylactic and on-demand treatment, respectively).
Revise uptake such that identical uptake rates were assumed by the approach to management, irrespective of hemophilia A severity and age:
View in own window
| Year 1 | Year 2 | Year 3 |
|---|
| Severe adult and pediatric, prophylaxis Moderate adult, prophylaxis | ▬% | ▬% | ▬% |
|---|
| Severe adult and pediatric, on-demand Moderate adult, on-demand | ▬% | ▬% | ▬% |
|---|
Assume reimbursement in the population of adults with severe disease only (to align with the population studied in the HAVEN-3 trial and the target population modelled in the cost-effectiveness analysis).
Revise on-demand ATBR to reflect the sponsor’s pharmacoeconomic submission (38.1).
Reduce the price of emicizumab to the value at which it would be cost-effective at a threshold of $50,000 per QALY (89%).
The results of CADTH’s scenario analyses demonstrate that the model is highly sensitive to comparator prices and the price of emicizumab. When the price of emicizumab was reduced by 89% (and FVIII comparator pricing remained unchanged), adopting emicizumab became cost-saving. Given that the true price of FVIII products is unknown, a 2-way price-reduction analysis between emicizumab and FVIII was conducted. The results are presented in .
Table 18Detailed Breakdown of the CADTH Reanalyses of the Budget Impact Analysis
View in own window
| Stepped analysis | Scenario | Year 1 | Year 2 | Year 3 | 3-year total |
|---|
| Submitted base case | Reference | $308,178,071 | $311,954,451 | $315,024,937 | $935,157,460 |
| New drug | $375,207,054 | $382,815,816 | $396,272,606 | $1,154,295,476 |
| Budget impact | $67,028,982 | $70,861,365 | $81,247,668 | $219,138,016 |
| CADTH base case | Reference | $327,837,313 | $331,951,020 | $335,194,972 | $994,983,305 |
| New drug | $400,928,348 | $409,473,064 | $424,184,513 | $1,234,585,925 |
| Budget impact | $73,091,035 | $77,522,044 | $88,989,541 | $239,602,620 |
| CADTH scenario analysis 1 | Reference | $327,837,313 | $331,951,020 | $335,194,972 | $994,983,305 |
| New drug | $404,589,328 | $413,602,923 | $429,198,783 | $1,247,391,034 |
| Budget impact | $76,752,015 | $81,651,903 | $94,003,811 | $252,407,729 |
| CADTH scenario analysis 2 | Reference | $327,837,313 | $331,951,020 | $335,194,972 | $994,983,305 |
| New drug | $393,315,687 | $407,931,212 | $427,110,214 | $1,228,357,113 |
| Budget impact | $65,478,374 | $75,980,192 | $91,915,242 | $233,373,808 |
| CADTH scenario analysis 3 | Reference | $327,837,313 | $331,951,020 | $335,194,972 | $994,983,305 |
| New drug | $374,418,227 | $385,785,421 | $400,271,159 | $1,160,474,808 |
| Budget impact | $46,580,914 | $53,834,401 | $65,076,188 | $165,491,503 |
| CADTH scenario analysis 4 | Reference | $333,685,586 | $337,799,293 | $341,105,591 | $1,012,590,470 |
| New drug | $406,461,763 | $414,835,325 | $429,488,926 | $1,250,786,015 |
| Budget impact | $72,776,177 | $77,036,032 | $88,383,335 | $238,195,545 |
| CADTH scenario analysis 5 | Reference | $327,837,313 | $331,951,020 | $335,194,972 | $994,983,305 |
| New drug | $283,531,513 | $277,878,136 | $272,282,938 | $833,692,587 |
| Budget impact | –$44,305,801 | –$54,072,884 | –$62,912,033 | –$161,290,718 |
ATBR = annualized bleed rate; BIA = budget impact analysis.
Table 19Two-Way Price-Reduction Analyses: 3-Year Total Budget Impact Analysis
View in own window
| Price of emicizumab |
|---|
| No reduction | 25% reduction | 50% reduction | 75% reduction | 90% reduction |
|---|
| Price of FVIII products | No reduction | $239,602,620 | $126,992,131 | $14,381,643 | –$98,228,845 | –$165,795,138 |
|---|
| 25% reduction | $292,312,453 | $179,701,965 | $67,091,477 | –$45,519,012 | –$113,085,304 |
|---|
| 50% reduction | $345,022,286 | $232,411,798 | $119,801,310 | –$7,190,822 | –$60,375,471 |
|---|
| 75% reduction | $397,732,119 | $285,121,631 | $172,511,143 | $59,900,655 | –$7,665,638 |
|---|
| 90% reduction | $429,358,019 | $316,747,531 | $204,137,043 | $91,526,555 | $23,960,262 |
|---|
References
- 1.
- 2.
- 3.
Hemlibra (emicizumab): 30 mg/mL, 60 mg/0.4 mL (150 mg/mL), 105 mg/0.7 mL (150 mg/mL), 150 mg/mL subcutaneous [product monograph]. Mississauga (ON): Hoffmann-La Roche Limited; 2019
Jun
14.
- 4.
Mahlangu
J, Oldenburg
J, Paz-Priel
I, et al. Emicizumab prophylaxis in patients who have hemophilia A without inhibitors.
N Engl J Med. 2018;379(9):811–822. [
PubMed: 30157389]
- 5.
Oldenburg
J, Mahlangu
JN, Kim
B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors.
N Engl J Med. 2017;377(9):809–818. [
PubMed: 28691557]
- 6.
Darby
SC, Kan
SW, Spooner
RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV.
Blood. 2007;110(3):815–825. [
PubMed: 17446349]
- 7.
CDR submission: Hemlibra (emicizumab), 30 mg/mL, 60 mg/0.4 mL (150 mg/mL), 105 mg/0.7 mL (150 mg/mL), 150 mg/mL subcutaneous [CONFIDENTIAL sponsor’s submission]. Mississauga (ON): Hoffmann-La Roche Limited; 2020
Jun
30.
- 8.
Pharmacoeconomic evaluation. In: CDR submission: Hemlibra (emicizumab), 30 mg/mL, 60 mg/0.4 mL (150 mg/mL), 105 mg/0.7 mL (150 mg/mL), 150 mg/mL subcutaneous [CONFIDENTIAL sponsor’s submission]. Mississauga (ON): Hoffmann-La Roche Limited; 2020
Jun
30.
- 9.
- 10.
Hoffmann-La Roche Limited response to August 27, 2020 CDR request for additional information regarding Hemlibra (emicizumab) CDR review: clarification on reimbursement criteria [CONFIDENTIAL additional sponsor’s information]. Mississauga (ON). Mississauga (ON): Hoffmann-La Roche Limited; 2020
Aug
27.
- 11.
Klarenbach
S, Manns
B, Reiman
T, et al. Economic evaluation of erythropoiesis-stimulating agents for anemia related to cancer.
Cancer. 2010;116(13):3224–3232. [
PubMed: 20564645]
- 12.
- 13.
Ballal
RD, Botteman
MF, Foley
I, et al. Economic evaluation of major knee surgery with recombinant activated factor VII in hemophilia patients with high titer inhibitors and advanced knee arthropathy: exploratory results via literature-based modeling.
Curr Med Res Opin. 2008;24(3):753–768. [
PubMed: 18234151]
- 14.
Clinical Study Report: BO39182 (HAVEN 4). A multicenter, open-label, phase III study to evaluate the efficacy, safety, pharmacokinetics, and pharmacodynamics of emicizumab given every 4 weeks (Q4W) in patients with hemophilia A [CONFIDENTIAL internal sponsor’s report]. Basel (CH); Tokyo (JP): F. Hoffmann-La Roche Ltd., Chugai Pharmaceutical Co. Ltd; 2018
May
30.
- 15.
- 16.
- 17.
- 18.
- 19.
Arruda
VR, Doshi
BS, Samelson-Jones
BJ. Emerging therapies for hemophilia: controversies and unanswered questions. F1000Res. 2018;7(F1000):Faculty Rev–489.
- 20.
- 21.
Nuwiq (antihemophilic factor (recombinant, B-domain deleted)): powder and solvent for solution for intravenous injection 250 IU FViii/vial reconstituted with 2.5 mL of solvent, 500 IU FVIII/vial reconstituted with 2.5 mL of solvent, 1000 IU FVIII/vial reconstituted with 2.5 mL of solvent, 2000 IU FVIII/vial reconstituted with 2.5 mL of solvent, 2500 IU FVIII/vial reconstituted with 2.5 mL of solvent, 3000 IU FVIII/vial reconstituted with 2.5 mL of solvent, 4000 IU FVIII/vial reconstituted with 2.5 mL of solvent [product monograph]. Toronto (ON): Octapharma Canada Inc.; 2018
Dec
14:
https://pdf.hres.ca/dpd_pm/00050487.PDF. Accessed 2020 Sep 22.
- 22.
Xyntha (lyophilized powder for reconstitution in a vial): 250, 500, 1000, or 2000 IU in single-use vials and one pre-filled diluent syringe containing 4 mL 0.9% sodium chloride for reconstitution; Xyntha Solufuse (lyophilized powder for reconstitution in a prefilled dual-chamber syringe): 250, 500, 1000, 2000, or 3000 IU and 4 mL 0.9% sodium chloride solution for reconstitution in a prefilled dula-chamber syringe [product monograph]. Kirkland (QC): T.M. Wyeth LLC, Pfizer Canada Inc., Licensee; 2016
May
11:
https://pdf.hres.ca/dpd_pm/00034847.PDF. Accessed 2020 Sep 22.
- 23.
- 24.
Clinical Study Report: BH30071 (HAVEN 3). A randomized, multicenter, open-label, phase III clinical trial to evaluate the efficacy, safety, and pharmacokinetics of prophylactic emicizumab versus no prophylaxis in hemophilia A patients without inhibitors [CONFIDENTIAL internal sponsor’s report]. Basel (CH), Tokyo (JP): F. Hoffmann-La Roche Ltd., Chugai Pharmaceutical Co. Ltd; 2018
Mar
26.
- 25.
- 26.
- 27.
Jivi (antihemophilic factor (recombinant, B-domain deleted, PEGylated)): IV injection 250, 500, 1000, 2000, 3000 IU/vial [product monograph]. Mississauga (ON): Bayer Inc; 2018
Oct
18:
https://pdf.hres.ca/dpd_pm/00047833.PDF. Accessed 2020 Sep 17.
- 28.
Wilate (human von Willebrand factor (VWF) and human coagulation factor VIII (FVIII): 500 IU VWF and 500 IU FVIII reconstituted with 5 mL of diluent, 1000 IU VWF and 1000 IU FVIII reconstituted with 10 mL of diluent [product monograph]. Toronto (ON): Octapharma Canada, Inc; 2018
May
8:
https://pdf.hres.ca/dpd_pm/00045156.PDF. Accessed 2020 Sep 17.
Funding: CADTH receives funding from Canada’s federal, provincial, and territorial governments, with the exception of Quebec.
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third-party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.