The Hedgehog signalling pathway is crucial for normal vertebrate growth and development. Recent studies would suggest that signalling capability is retained in the post-embryonic organism. Shh signalling has been identified in the adult immune system, participating in CD4+ T lymphocyte activation. Studies on fibrotic pulmonary disorders have demonstrated Shh in both human and mouse lung restricted to areas of active disease. Acute lung injury has also shown upregulated expression and together this data is highly suggestive for a functional role for Shh signalling in adult lung injury and disease. We propose that hedgehog signalling may contribute to epithelial injury and repair and act as an intermediary in cross-talk between damaged epithelium and the immune/inflammatory system.
Introduction
Work within our group has identified the up-regulated expression of the Hedgehog (Hh) signalling pathway in Idiopathic Pulmonary Fibrosis (IPF) and murine models of fibrotic lung disease.1 That Hh signalling may also play a role in lung injury is also suggested by the work of Watkins et al2,3 in their murine model of acute lung injury, and further confirms a role for hedgehog signalling in post embryonic pulmonary tissues.
Another area of interest to our group is the role of Hh signalling in the immune system. Active hedgehog signalling has been identified in cells of the peripheral immune system.1,4-6 Since these immune cells are integral to inflammation and repair in the damaged lung, these observations have led to the emergence of the hedgehog signalling pathway as a possible target for therapeutic intervention in conditions of pulmonary damage and disease. The aim of this chapter is to review divergent avenues of research into the hedgehog signalling pathway and relate them to observations made with this pathway at the pulmonary interface.
The Hedgehog Pathway
The hedgehog signalling pathway has been reviewed extensively elsewhere in this volume and in recent publications,7 thus will be covered only briefly in this review. There are three vertebrate hedgehog signalling molecules, Sonic hedgehog (Shh), Desert hedgehog (Dhh) and Indian hedgehog (Ihh). Of these, most studies have concentrated on the Shh signalling pathway. Shh mRNA generates an inactive 45kD precursor protein which auto-catalytically cleaves, and is post-translationally modified to produce a highly hydrophobic cholesterol/palmitate modified Shh signalling molecule. This signals either as a cell surface molecule or is secreted following association with the Dispatched molecule. Binding of its receptor Patched (Ptc), releases Ptc mediated inhibition of Smoothened (Smo), a G protein coupled receptor-like molecule, allowing Smo to influence gene expression through the GLI zinc finger transcription factors. Signalling upregulates Ptc in an auto-regulatory loop, similar to many developmental pathways.
Studies have shown Shh to be a crucial morphogen in a number of developmental systems, including the limbs, lung, gut, nervous and immune systems (for a review, see ref. 8). It is only relatively recently that a role for the hedgehog signalling pathway has been elucidated in the post embryonic organism.
Hedgehog Signalling in Normal Pulmonary Tissues
Adult lung tissue contains several cell types, including Type I and II epithelial cells, Clara cells, mesenchymal cells (fibroblasts and endothelium), and resident immune system cells including alveolar and interstitial macrophages. Immunolocalisation studies from our group have demonstrated the expression of Shh protein in the lung is restricted to focal patches of epithelium in areas of injury and repair. Ptc is detectable on bronchial and alveolar epithelium and in alveolar macrophages.1 Watkins and colleagues have also identified normal expression of Ptc in the basal layer of bronchial epithelium.3
Continued expression of Ptc in the absence of ligand, might indicate regulation and/or signalling via another pathway.9 However, data emerging from studies in the gastrointestinal tract would suggest that Hh signalling may continue in tissue, in the apparent absence of immunodetectable ligand.
Gastrointestinal tract Shh protein expression is localised to areas of regeneration, such as the fundic glands of the stomach.10-13 Initially protein expression appeared to be absent from many areas of the adult mammalian tract such as the oesophagus, small intestine and colon, although some cells in these regions appeared to retain the ability to generate mRNA.10,11,14 Further to this, many of these areas lacked apparent Ptc expression.12 However, treatment of mice with cyclopamine, a Smo inhibitor, resulted in marked decreases in small intestine epithelial cell proliferation, as evidenced by BrdU incorporation and PCNA studies, suggesting active signalling.11
Of interest is the finding that in the mature colon, cyclopamine inhibition of Hh signalling results in a marked increase in colonic epithelial proliferation and reduced differentiation of the colonocytes. This alternate response to that of the small intestine may represent a Hh specific effect, with variation in Ihh/Shh expression, or may simply reflect the presence or absence of another signalling factor or downstream effector in the tissues of the colon.14
Explanations for signalling in the absence of ligand include the existence of another pathway upstream of Smo (indeed, Ptc-independent pathways have been postulated previously,15) or that Hh signalling continued, but at a concentration below immunohistochemical detection. Recent research would support the latter explanation, for Hh expression has now been immunolocalised to areas of the colon, previously described as negative.10,12 This was achieved through variations in antibody concentration and technique. Thus, future improvement in antibodies, detection methods and specific inhibitors may further clarify this issue for both the gastrointestinal and pulmonary systems.
Hedgehog Signalling in Injury and Disease
Whilst the studies of Watkins et al demonstrated bronchiolar epithelial expression of the Shh signalling components,3 we have demonstrated Shh restricted to type II like cells in the alveoli of patients with IPF.1 Expression was localised to areas of remodelling epithelia, particularly those areas with underlying fibrosis. Interestingly, whilst an irritant induced murine model of fibrosis illustrated similar localisation, a murine model of allergen induced inflammation showed no such upregulation. Whilst both models illustrated evidence of inflammation, the allergen model lacked progressive fibrosis.1,16
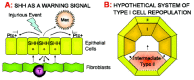
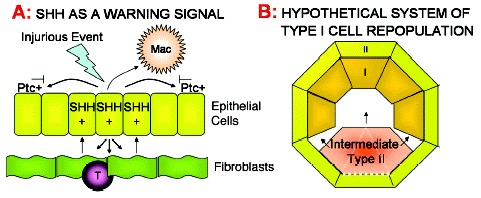
The upregulation of Shh at sites of epithelial injury comes amongst a plethora of other modulating signals and could therefore represent a downstream marker of a much larger process initiated by the underlying mesenchymal/fibroblastic cells, which play a crucial role in epithelial maintenance. Mesenchymal cells produce specific epithelial growth factors such as keratinocyte growth factor (KGF) and fibroblast growth factor 10 (FGF-10). These are upregulated in response to injurious stimuli, resulting in increased epithelial proliferation and rapid reestablishment of an intact epithelial layer (for a review, see ref. 17). Studies using FGF-10, FGFR2b and Shh knockout mice have recently confirmed Shh to be downstream of FGF-10 signalling.18 Shh expression on epithelium at sites of fibroblastic proliferation, such as that observed in IPF, may be a consequence of this repair process rather than an instigator of repair. Thus, Shh could be considered a regulatory factor in this instance, given its ability to downregulate FGF-1019 (see fig. 1A).
Alternatively expression at the alveolar surfaces of IPF patients might relate to a specific element of the disease process, which maybe mirrored in the FITC mouse model, where we observe a similar pattern of expression.1
IPF is a chronic fibrotic condition of the lung, associated with inflammation, where median survival after diagnosis is limited to five years. Although without known cause, progression is believed to have an immunological basis, linked in some way with aberrant epithelial mesenchymal interaction.20 Previous work in this laboratory identified circulating antibodies to a 70-90KD protein in IPF patient serum, which were not observed in normal controls.21 This protein was later localised to type II cells (fig. 2), suggesting an autoantigen.22 Experiments in vivo with a polyclonal antibody raised against the antigen and a human type II epithelial cell line showed upregulated tenascin and TGF-β production.23 Tenascin is an extracellular matrix protein associated with active scar formation, whilst TGF-β can be pro-fibrotic. Both have been well characterised as being present in the lungs of patients with IPF,24,25 and tenascin has been shown to be localised to areas of active fibroplasias or ‘fibroblastic foci’.23 It will be interesting to discover whether Shh is also upregulated by this interaction, as it would explain its localised expression, at areas of putative antibody interaction.
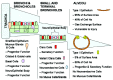
Figure 2
Cell types at the pulmonary surface.
Shh also has the potential to be profibrotic, Shh and FGF signalling are well linked in neural systems both pre and postnatally19,26-28 and hedgehogs have been linked with collagen fibre induction in the neural system.29 Shh also has substantial cell cycle modulating capabilities30,31 and participates in well characterised proliferative mesenchymal interactions in the developing lung. Thus the localisation of Shh at fibrotic foci in IPF may also present a causal relationship with fibrosis in the IPF patient.
Similar hypotheses can be drawn from the observations made in the fibrotic FITC mouse model of IPF pathology.16 Fibrosis and inflammation are believed to be induced in this model through the persistence of the intratracheally instilled irritant FITC, which lodges in the interstitium and binds resident proteins. Antibodies are raised to FITC, and mice develop a pathology similar to that of IPF patients, where Shh expression is localised to sites of damage.1
Interestingly, recent work has illustrated a requirement for cell confluence for Shh responsiveness, in a Ptc expressing Shh bioassay.32,33 Thus, Shh and Ptc expression at a site of proliferative repair, may not automatically indicate that active signalling is occurring. Indeed, the broad expression area of Ptc in the lung might represent a “Shh sponge”, to endocytose, in the absence of signalling, free Shh, as described by Torroja and colleagues.34 However, whilst this may, in part, be true for some expression studies in the lung, work performed in our laboratory and that of others has identified alterations in target gene expression such as Ptc and Gli1, coincident with Shh expression, suggesting that at least some active signalling results from Shh expression.3
Shh would appear to share many of its effects with KGF and FGF-10,30 most notably, stimulation of increased cellular resistance to injury and death.35,36 Shh exhibits this effect in a number of cell types,30,37,38 but has yet to be established at the pulmonary interface. Perhaps the best example of this activity is in Parkinson's disease, where exogenous administration of Shh in both a marmoset and mouse model of the disease results in improved locomotion and function.39,40 Tsuboi and colleagues,40 citing work by Miao et al,38 have suggested that this is likely due to Shh acting as a neuroprotective agent against dopaminogenic neuron cell death, which is central to disease progression.
In support of this Thilbert et al have shown that Shh can induce the anti-apoptotic protein Bcl-2 in keratinocytes, and that its presence prevents the apoptosis of neuroepithelial cells.37 Therefore in a pulmonary situation of injury, such as exposure to environment oxidants, Shh release, along with KGF and FGF-10, may serve to temporarily increase surrounding epithelial cell resilience, and prevent complete denudation of an epithelial surface and the risk of secondary infection (fig. 1A).
Sustaining viability of injured cells carries with it the increased probability of populating the epithelium with cells which have incurred genetic damage. Indeed, this may help to explain the higher incidence of small and nonsmall cell lung cancers following epithelial trauma, and the preponderance for the involvement of Shh signalling in these carcinomas.41
Upregulation of Hh signalling following injury, can be found in a number of model systems Pola et al42,43 have demonstrated the upregulation of Shh and Ptc1 expression in a rodent hind limb ischemia model. Furthermore, the same group demonstrated that the administration of Shh induced robust neovascularisation, enhanced blood flow, and the upregulation of the angiogenic factors, vascular endothelial growth factor (VEGF) and the angiopoietins, Ang1 and Ang2,43 although any direct association is a matter of conjecture.44,45 The findings from other groups have been broadly in keeping with those of Pola et al43 demonstrating that Shh is capable of inducing VEGF expression44 and angiogenesis.45
Such angiogenic modulation could have important implications at the respiratory surfaces of IPF patients, where we have observed Shh expression in association with fibrotic foci.1 Shh expression here may represent an attempt by the lung to revascularise and remodel the fibrotic architecture. Alternatively, it may represent a step in the progression of the fibrosis, where VEGF itself is found upregulated with epithelial injury.46 Perhaps the greatest implication is in the neoangiogenic advancement of pulmonary malignancies, which are associated with the expression of Hh pathway components.3
Shh in the Immune System
A functional role for hedgehog signalling in the adult immune system is perhaps not surprising given the well documented role of Shh in lymphoid development and differentiation.4,47,48 Work in our laboratory has found evidence for expression of Shh and Ptc in both resting and activated murine and human CD4+ and CD8+ T lymphocytes, human macrophages and secondary lymphoid tissue,5,6 both at the mRNA and protein level. This lends support to a possible role for immune-epithelial cell interaction at sites of injury and repair.
It has been postulated that the expression of Hh in a large range of diversified tissues and cell types, might represent a common method of sensing the cellular environment and neighbouring cell health.49 Given the expression of the Ptc molecule on human macrophages, it may be postulated that Shh expression acts as an advertisement of epithelial damage both to the macrophage, to facilitate attention, and to the adjacent cells as a warning signal for defence and repair initiation (fig. 1A).
Further to this, Lowrey and coworkers5,6 have demonstrated that exogenous Shh has the capacity to push sub-optimally activated peripheral CD4+ T lymphocytes through further cell cycles, whilst addition of a blocking antibody to endogenous Shh results in decreased proliferation. Together these results would suggest that exogenous Shh administration enhances an endogenous stimulatory effect of Shh. Indeed, further work from our group has demonstrated that Shh can act as a costimulatory molecule to T-cells activated via the T cell receptor using anti-CD3 antibodies (Lowrey et al, unpublished observations). This explains the upregulation of the activation antigens CD25 and CD69, by exogenous Shh, along with the production of Interleukin 2 (IL-2), a T lymphocyte proliferative factor, interleukin 10 (IL-10) and Interferon gamma (IFN-γ). Thus Shh expression at sites of disease and damage has the potential to sustain T lymphocyte mediated immune responses and even to influence the effector function of immune responses, through an IFN-γ bias.
Shh has also been shown to upregulate Bcl-2 in T lymphocytes, a step linked with memory T lymphocyte generation,4 and thus, Shh at sites of injury might also serve to ensure adaptive immunity to damaging antigens, or an exacerbation of autoimmunity. Given that many of the diseases associated with Shh expression contain elements of autoimmunity or immune dysregulation, this crosstalk could have important implications both for the initiation and maintenance of a disease process.
Hedgehogs and Pulmonary Progenitor Cells
Watkins and colleagues3 depleted Clara cells from mouse airways via their sensitivity to naphthalene injury. This resulted in a transient peak in Shh and Gli expression 3 days post depletion that coincided with the reestablishment of a complete epithelial barrier. It was suggested that Shh signalling might be involved in this repopulation step, since this peak preceded an increase in the number of pulmonary neuroepithelial cells—a cell type identified as a potential epithelial progenitor.41,50 Subsequently, Watkins and colleagues identified clusters of epithelial cells expressing Ptc and the neuroendocrine marker, Calcitonin Gene Related Peptide (CGRP), associated with Shh expressing cells in the developing lung.3 It was suggested that this clustered interaction might continue into the adult pulmonary system, where Shh expression might influence neuroepithelial differentiation.
Presently, progenitor cell phenotypes are characterised via their longevity via nucleotide analogue incorporation, and are defined as label retaining cells. In the mouse these cells are localised to glandular submucosal glands in the distal trachea and bronchi.51 In the absence of these glands, such as in the bronchioles and alveoli, LRCs occur in localised foci,52 referred to as neuroepithelial bodies41 (fig. 2). These foci are populated by Clara Cell secretory protein (CCSP)-expressing epithelial cells, known as variant Clara cells (vCC), which are naphthalene resistant. Associated with these are CGRP-expressing epithelial cells which show intermediate morphology between a pulmonary Neuroendocrine Cells (PNEC) and a Clara cell41,50 (fig. 2). Infiltration of blood borne progenitors in this regenerative process, add a further level of complexity outwith the scope of this chapter, but covered elsewhere.53
Label retaining cells undergo hyperplasia in the event of Clara cell death, such as that induced by naphthalene, resulting in the rapid reestablishment of a functional epithelial cell layer. Selective depletion of vCC using a Herpes simplex virus thymidine kinase linkage system, prior to naphthalene injury, results in a failure to repopulate a differentiated ciliated epithelium, despite PNEC hyperplasia.50 Whether this highlights the CCSP expressing cells as progenitor cells, producers of a signal necessary for PNEC progenitor cell function or as coprogenitors with the PNEC is still an area of conjecture.
As to a potential role for Shh in this process, epithelial coverage is completed at the peak of Shh expression, thus a causative role in nonspecific epithelial proliferation is unlikely, and this is confirmed in our studies where we observe bronchiolar Shh expression restricted to areas of complete epithelial coverage. It is interesting that coverage normally occurs in the absence of differentiation, which typically occurs following reestablishment of epithelial integrity. Given the necessity for confluence for Shh signalling in some cell lines, as mentioned previously, one might speculate that it is in this differentiation step that Shh might play a role.
Reasoning for such speculation arises through the well characterised progenitor modulating role of Hh in a range of post embryonic systems including T lymphocyte development47 and haematopoiesis.54 The work by Bhardwaj in haematopoiesis was notable for it clearly defined the downstream effector of Shh function as BMP-4, where Shh addition to an enriched population of human CD34+Lin-CD38- progenitor cells resulted in increased self renewal, albeit in combination with many other growth factors.
An example of Hh mediated progenitor modulation in injury comes indirectly from several sources.55,56 Pepinsky et al demonstrated that Shh administration can accelerate nerve recovery following sciatic nerve crush injury.55 A Possible explanation for this response comes from studies by Bambakidos et al, using a rat demyelination model.56 These authors observed increased proliferation of stem cell like progenitors in areas of chemical demyelination following direct Shh administration. This suggests that exogenous Shh at a site of injury induces proliferation in stem cell populations, although the specificity of this progenitor proliferation was not addressed in this study.
The manner in which hedgehog signalling might facilitate this stem cell influence has yet to be characterised, but is likely to lie in its ability to regulate differentiation. Evidence in support of such a role comes from studies of fracture repair, in which Ihh may play a role. Following a fracture, pluripotent mesenchymal cells invade and differentiate into osteoblasts, to initiate the production of hard callus comprising bone matrix, and chondrocytes for the formation of the soft callus. A balanced secondary chondrocyte differentiation step facilitates the remodelling of bone until the bone shape is restored.57,58 Ihh expression is induced by 3 days post fracture persisting past 2 weeks in a murine model, with the greatest level of expression found in chondrocytes undergoing their secondary differentiation step.59,60 Ihh expression here induces further chrondrocyte proliferation, but prevents further chondrocyte differentiation via PTHrP signalling.61-63 Given the osteoblast upregulation of PTHrP in the presence of rShh shown by Jemtland and colleagues64 and the osteoblastic lineage bias induced by Ihh,65 it is likely that in this system, Ihh is central to restricting differential fates of invading pluiripotent mesenchymal cells and facilitates an accurate bone remodelling response.
Central to the stem cell maintenance function of Shh observed in haematopoiesis, is its ability to upregulate BMP-4. These molecules are crucial in early lung morphogenesis and thus have substantial signalling potential in the post embryonic lung. Indeed, studies in a mouse model of allergic inflammation have demonstrated an upregulation of BMP's at sites of inflammation, including BMP-4.66 In-vitro studies would suggest that BMP-4 has anti-proliferative effects in cancer derived cell lines,67 however these effects can often be contradictory and dependant on the cosignals and cell types present in culture.
In-vivo studies into pulmonary BMP functions are limited by the lethality of BMP-4 knockouts. However, innovative in vivo studies in this field from the lab of Brigid Hogan and colleagues have demonstrated a potential progenitor modulating function for BMP's in epithelium. Cells exposed to high levels of BMP-4 retained undifferentiated characteristics.68,69 In light of these studies, were Shh to modulate progenitor function through the BMP's, it would likely be in conjunction with a number of other signals, such as FGF-10. Perhaps in a recapitulation of the process of cell fate designation in the embryonic lung.68
Shh and Type II Epithelial Cells
Studies thus far have focused on roles for Shh expression at the bronchi and bronchiolar levels; however, immunohistochemical data suggest that the hedgehog signal might persist in the larger and smaller airway systems. Watkins and colleagues observed a persistence of Ptc signalling in the progenitor cells of the trachea and bronchi, whilst our lab has identified expression of Shh and Ptc in type II like cells of the terminal bronchioles and alveoli (fig. 2).
Pulmonary epithelial regeneration occurs rapidly at the alveolar level. Here, in response to the removal of contact inhibition and KGF,36 type II cells downregulate specific functional machinery, proliferate and spread out to become thinly spread type I cells, specialised for gaseous exchange. This process occurs almost continually as type I cells are highly susceptible to injury by exogenous factors, i.e., pneumotoxic environmental pollutants and by factors involved in both innate and adaptive pathogen clearance in the lung.
Whilst lack of contact inhibition and KGF are triggers for type II differentiation, no maintenance signal for type II cell number has yet been identified. Perhaps Shh has a modulating function here too. Certainly immunolocalisation in IPF patient biopsies has identified Shh upregulation in individual type II like cells amongst adjacent negative cells, with expression isolated to areas of injury.1
This makes for an attractive hypothesis. Damage upregulates type II cell expression of Shh, this induces type II proliferation and inhibits differentiation. However, type II cells adjacent to areas of denuded basement membrane have incomplete confluence and thus lose responsiveness to the Shh, allowing them to differentiate and cover the exposed area (fig. 1B). However, were Shh a maintenance factor in this continual type II–I transition, it might be expected that normal lung might exhibit some limited immunohistochemical reactivity, and this is not observed. Whether this represents true absence of Shh protein, or the limits of immunohistochemical detection, remains to be determined.
Concluding Comments
The potential for post embryonic recapitulation of developmental signals in injury and disease presents many exciting targets for therapeutic modulation. This is particularly true for the pulmonary system, where the air interface facilitates the direct and rapid delivery of short half life Hh modulating agents to target cells. This will avoid the systemic complications of treatment that might be expected in the treatment of conditions such as multiple sclerosis and Parkinsons disease. Our knowledge of the Hh signalling pathway and its role in development has made great advancements over recent years and has led to the development of many new and exciting ideas which may identify functions for the Hh pathway in post-embryonic systems.
References
- 1.
- Stewart GA, Hoyne GF, Ahmad SA. et al. Expression of the developmental Sonic hedgehog (Shh) signalling pathway is up-regulated in chronic lung fibrosis and the Shh receptor patched 1 is present in circulating T lymphocytes. J Pathol. 2003;199(4):488–495. [PubMed: 12635140]
- 2.
- Watkins DN, Berman DM, Baylin SB. Hedgehog signaling: progenitor phenotype in small-cell lung cancer. Cell Cycle. 2003;2(3):196–198. [PubMed: 12734424]
- 3.
- Watkins DN, Berman DM, Burkholder SG. et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422(6929):313–317. [PubMed: 12629553]
- 4.
- Benson RA, Lowrey JA, Lamb JR. et al. The Notch and Sonic hedgehog signalling pathways in immunity. Mol Immunol. 2004;41(6-7):715–725. [PubMed: 15220006]
- 5.
- Lowrey JA, Stewart GA, Lindey S. et al. Sonic hedgehog promotes cell cycle progression in activated peripheral CD4(+) T lymphocytes. J Immunol. 2002;169(4):1869–1875. [PubMed: 12165511]
- 6.
- Stewart GA, Lowrey JA, Wakelin SJ. et al. Sonic hedgehog signaling modulates activation of and cytokine production by human peripheral CD4+ T cells. J Immunol. 2002;169(10):5451–5457. [PubMed: 12421920]
- 7.
- Nybakken K, Perrimon N. Hedgehog signal transduction: Recent findings. Curr Opin Genet Dev. 2002;12(5):503–511. [PubMed: 12200154]
- 8.
- McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. [PubMed: 12509125]
- 9.
- Agren M, Kogerman P, Kleman MI. et al. Expression of the PTCH1 tumor suppressor gene is regulated by alternative promoters and a single functional Gli-binding site. Gene. 2004;330:101–114. [PubMed: 15087129]
- 10.
- van den Brink GR, Hardwick JC, Nielsen C. et al. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut. 2002;51(5):628–633. [PMC free article: PMC1773421] [PubMed: 12377798]
- 11.
- van den Brink GR, Hardwick JC, Tytgat GN. et al. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology. 2001;121(2):317–328. [PubMed: 11487541]
- 12.
- Dimmler A, Brabletz T, Hlubek F. et al. Transcription of sonic hedgehog, a potential factor for gastric morphogenesis and gastric mucosa maintenance, is up-regulated in acidic conditions. Lab Invest. 2003;83(12):1829–1837. [PubMed: 14691301]
- 13.
- Ishizuya-Oka A, Ueda S, Inokuchi T. et al. Thyroid hormone-induced expression of sonic hedgehog correlates with adult epithelial development during remodeling of the Xenopus stomach and intestine. Differentiation. 2001;69(1):27–37. [PubMed: 11776392]
- 14.
- van den Brink GR, Bleuming SA, Hardwick JC. et al. Indian Hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat Genet. 2004;36(3):277–282. [PubMed: 14770182]
- 15.
- Testaz S, Jarov A, Williams KP. et al. Sonic hedgehog restricts adhesion and migration of neural crest cells independently of the Patched- Smoothened-Gli signaling pathway. Proc Natl Acad Sci USA. 2001;98(22):12521–12526. [PMC free article: PMC60086] [PubMed: 11592978]
- 16.
- Roberts SN, Howie SE, Wallace WA. et al. A novel model for human interstitial lung disease: Hapten-driven lung fibrosis in rodents. J Pathol. 1995;176(3):309–318. [PubMed: 7674093]
- 17.
- Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: Roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002;282(5):L924–L940. [PubMed: 11943656]
- 18.
- Rice R, Spencer-Dene B, Connor EC. et al. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J Clin Invest. 2004;113(12):1692–1700. [PMC free article: PMC420504] [PubMed: 15199404]
- 19.
- Chuang PT, Kawcak T, McMahon AP. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 2003;17(3):342–347. [PMC free article: PMC195990] [PubMed: 12569124]
- 20.
- Thannickal VJ, Toews GB, White ES. et al. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. [PubMed: 14746528]
- 21.
- Wallace WA, Roberts SN, Caldwell H. et al. Circulating antibodies to lung protein(s) in patients with cryptogenic fibrosing alveolitis. Thorax. 1994;49(3):218–224. [PMC free article: PMC1021149] [PubMed: 8202877]
- 22.
- Wallace WA, Schofield JA, Lamb D. et al. Localisation of a pulmonary autoantigen in cryptogenic fibrosing alveolitis. Thorax. 1994;49(11):1139–1145. [PMC free article: PMC475277] [PubMed: 7831631]
- 23.
- Wallace WA, Howie SE. Upregulation of tenascin and TGFbeta production in a type II alveolar epithelial cell line by antibody against a pulmonary auto-antigen. J Pathol. 2001;195(2):251–256. [PubMed: 11592106]
- 24.
- Wallace WA, Howie SE, Lamb D. et al. Tenascin immunoreactivity in cryptogenic fibrosing alveolitis. J Pathol. 1995;175(4):415–420. [PubMed: 7540684]
- 25.
- Khalil N, O'Connor RN, Unruh HW. et al. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5(2):155–162. [PubMed: 1892646]
- 26.
- Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22(1):103–114. [PubMed: 10027293]
- 27.
- Ruiz i Altaba A, Palma V, Dahmane N. Hedgehog-Gli signalling and the growth of the brain. Nat Rev Neurosci. 2002;3(1):24–33. [PubMed: 11823802]
- 28.
- Brewster R, Mullor JL, Ruiz i Altaba A. Gli2 functions in FGF signaling during antero-posterior patterning. Development. 2000;127(20):4395–4405. [PubMed: 11003839]
- 29.
- Parmantier E, Lynn B, Lawson D. et al. Schwann cell-derived Desert hedgehog controls the development of peripheral nerve sheaths. Neuron. 1999;23(4):713–724. [PubMed: 10482238]
- 30.
- Fan H, Khavari PA. Sonic hedgehog opposes epithelial cell cycle arrest. J Cell Biol. 1999;147(1):71–76. [PMC free article: PMC2164980] [PubMed: 10508856]
- 31.
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13(12):1501–1512. [PubMed: 10385618]
- 32.
- Ingram WJ, Wicking CA, Grimmond SM. et al. Novel genes regulated by Sonic Hedgehog in pluripotent mesenchymal cells. Oncogene. 2002;21(53):8196–8205. [PubMed: 12444557]
- 33.
- Taipale J, Chen JK, Cooper MK. et al. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature. 2000;406(6799):1005–1009. [PubMed: 10984056]
- 34.
- Torroja C, Gorfinkiel N, Guerrero I. Patched controls the Hedgehog gradient by endocytosis in a dynamin-dependent manner, but this internalization does not play a major role in signal transduction. Development. 2004;131(10):2395–2408. [PubMed: 15102702]
- 35.
- Upadhyay D, Bundesmann M, Panduri V. et al. Fibroblast growth factor-10 attenuates H2O2-induced alveolar epithelial cell DNA damage: Role of MAPK activation and DNA repair. Am J Respir Cell Mol Biol. 2004;31(1):107–113. [PubMed: 14975937]
- 36.
- Oswari J, Matthay MA, Margulies SS. Keratinocyte growth factor reduces alveolar epithelial susceptibility to in vitro mechanical deformation. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1068–L1077. [PubMed: 11597897]
- 37.
- Thibert C, Teillet MA, Lapointe F. et al. Inhibition of neuroepithelial patched-induced apoptosis by sonic hedgehog. Science. 2003;301(5634):843–846. [PubMed: 12907805]
- 38.
- Miao N, Wang M, Ott JA. et al. Sonic hedgehog promotes the survival of specific CNS neuron populations and protects these cells from toxic insult In vitro. J Neurosci. 1997;17(15):5891–5899. [PMC free article: PMC6573190] [PubMed: 9221786]
- 39.
- Dass B, Iravani MM, Jackson MJ. et al. Behavioural and immunohistochemical changes following supranigral administration of sonic hedgehog in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated common marmosets. Neuroscience. 2002;114(1):99–109. [PubMed: 12207958]
- 40.
- Tsuboi K, Shults CW. Intrastriatal injection of sonic hedgehog reduces behavioral impairment in a rat model of Parkinson's disease. Exp Neurol. 2002;173(1):95–104. [PubMed: 11771942]
- 41.
- Reynolds SD, Giangreco A, Power JH. et al. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156(1):269–278. [PMC free article: PMC1868636] [PubMed: 10623675]
- 42.
- Pola R, Ling LE, Aprahamian TR. et al. Postnatal recapitulation of embryonic hedgehog pathway in response to skeletal muscle ischemia. Circulation. 2003;108(4):479–485. [PubMed: 12860919]
- 43.
- Pola R, Ling LE, Silver M. et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7(6):706–711. [PubMed: 11385508]
- 44.
- Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3(1):127–136. [PubMed: 12110173]
- 45.
- Kanda S, Mochizuki Y, Suematsu T. et al. Sonic hedgehog induces capillary morphogenesis by endothelial cells through phosphoinositide 3-kinase. J Biol Chem. 2003;278(10):8244–8249. [PubMed: 12514186]
- 46.
- Pham I, Uchida T, Planes C. et al. Hypoxia upregulates VEGF expression in alveolar epithelial cells in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):L1133–L1142. [PubMed: 12376368]
- 47.
- Outram SV, Varas A, Pepicelli CV. et al. Hedgehog signaling regulates differentiation from double-negative to double-positive thymocyte. Immunity. 2000;13(2):187–197. [PubMed: 10981962]
- 48.
- Varas A, Hager-Theodorides AL, Sacedon R. et al. The role of morphogens in T-cell development. Trends Immunol. 2003;24(4):197–206. [PubMed: 12697452]
- 49.
- Ruiz i Altaba A, Stecca B, Sanchez P. Hedgehog—Gli signaling in brain tumors: Stem cells and paradevelopmental programs in cancer. Cancer Lett. 2004;204(2):145–157. [PubMed: 15013214]
- 50.
- Reynolds SD, Hong KU, Giangreco A. et al. Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol. 2000;278(6):L1256–L1263. [PubMed: 10835332]
- 51.
- Borthwick DW, Shahbazian M, Krantz QT. et al. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24(6):662–670. [PubMed: 11415930]
- 52.
- Hong KU, Reynolds SD, Giangreco A. et al. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24(6):671–681. [PubMed: 11415931]
- 53.
- Giangreco A, Shen H, Reynolds SD. et al. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L624–L630. [PubMed: 12909587]
- 54.
- Bhardwaj G, Murdoch B, Wu D. et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2(2):172–180. [PubMed: 11175816]
- 55.
- Pepinsky RB, Shapiro RI, Wang S. et al. Long-acting forms of Sonic hedgehog with improved pharmacokinetic and pharmacodynamic properties are efficacious in a nerve injury model. J Pharm Sci. 2002;91(2):371–387. [PubMed: 11835197]
- 56.
- Bambakidis NC, Wang RZ, Franic L. et al. Sonic hedgehog-induced neural precursor proliferation after adult rodent spinal cord injury. J Neurosurg. 2003;99(1 Suppl):70–75. [PubMed: 12859063]
- 57.
- Bolander ME. Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 1992;200(2):165–170. [PubMed: 1374563]
- 58.
- Sandberg MM, Aro HT, Vuorio EI. Gene expression during bone repair. Clin Orthop. 1993;289:292–312. [PubMed: 8472429]
- 59.
- Ferguson CM, Miclau T, Hu D. et al. Common molecular pathways in skeletal morphogenesis and repair. Ann NY Acad Sci. 1998;857:33–42. [PubMed: 9917830]
- 60.
- Vortkamp A, Pathi S, Peretti GM. et al. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev. 1998;71(1-2):65–76. [PubMed: 9507067]
- 61.
- St JacquesB, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13(16):2072–2086. [PMC free article: PMC316949] [PubMed: 10465785]
- 62.
- Lanske B, Karaplis AC, Lee K. et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273(5275):663–666. [PubMed: 8662561]
- 63.
- Zou H, Wieser R, Massague J. et al. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 1997;11(17):2191–2203. [PMC free article: PMC275391] [PubMed: 9303535]
- 64.
- Jemtland R, Divieti P, Lee K. et al. Hedgehog promotes primary osteoblast differentiation and increases PTHrP mRNA expression and iPTHrP secretion. Bone. 2003;32(6):611–620. [PubMed: 12810168]
- 65.
- Spinella-Jaegle S, Rawadi G, Kawai S. et al. Sonic hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci. 2001;114(Pt 11):2085–2094. [PubMed: 11493644]
- 66.
- Rosendahl A, Pardali E, Speletas M. et al. Activation of bone morphogenetic protein/Smad signaling in bronchial epithelial cells during airway inflammation. Am J Respir Cell Mol Biol. 2002;27(2):160–169. [PubMed: 12151307]
- 67.
- Buckley S, Shi W, Driscoll B. et al. BMP4 signaling induces senescence and modulates the oncogenic phenotype of A549 lung adenocarcinoma cells. Am J Physiol Lung Cell Mol Physiol. 2004;286(1):L81–L86. [PubMed: 12959928]
- 68.
- Weaver M, Yingling JM, Dunn NR. et al. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126(18):4005–4015. [PubMed: 10457010]
- 69.
- Bellusci S, Henderson R, Winnier G. et al. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122(6):1693–1702. [PubMed: 8674409]
Publication Details
Author Information and Affiliations
Authors
Paul M. Fitch, Sonia J. Wakelin, Jacqueline A. Lowrey, William A.H. Wallace, and Sarah E.M Howie*.Affiliations
Copyright
Publisher
Landes Bioscience, Austin (TX)
NLM Citation
Fitch PM, Wakelin SJ, Lowrey JA, et al. Shh Expression in Pulmonary Injury and Disease. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.