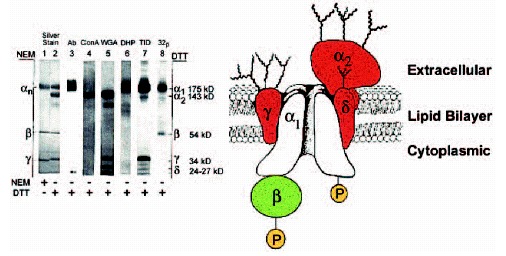
Voltage-gated Ca2+ channels mediate Ca2+ entry into cells in response to membrane depolarization. Electrophysiological studies reveal different Ca2+ currents designated L-, N-, P-, Q-, R-, and T-type. The high-voltage-activated Ca2+ channels that have been characterized biochemically are complexes of a pore-forming α1 subunit of about 190 to 250 kDa, a transmembrane, disulfide-linked complex of α2 and δ subunits, an intracellular β subunit, and in some cases a transmembrane γsubunit.The α1 subunits form the transmembrane pore. The α2 and δ subunits are glycoproteins encoded by the same gene and produced posttranslational proteolytic processing. The γ subunits are transmembrane glycoproteins, whereas the β subunits are hydrophilic subunits located on the cytosolic face of the channel. The CaV1 family of α1 subunits conduct L-type Ca2+ currents, which initiate muscle contraction, endocrine secretion, and gene transcription. The CaV2 family of α1 subunits conduct N-type, P/Q-type, and Rtype Ca2+ currents, which initiate rapid synaptic transmission. Both of these families of Ca2+ channels are regulated by protein phosphorylation and interact with intracellular signal transduction proteins that mediate Ca2+-dependent regulatory events and in turn regulate the channels.
Introduction
Ca2+ channels have been studied intensively by electrophysiological methods since the initial recordings of Ca2+ currents by Reuter in 1967.1 In the early 1980's, stimulated in part by success at purification and reconstitution of sodium channels,2 a new focus of Ca2+ channel research developed aimed at identification of the Ca2+ channel proteins and analysis of their biochemical properties. In this chapter, I briefly review the biochemical studies that led to the first identification of the Ca2+ channel proteins and provided the foundation for the subsequent analysis of the structure and function of the channel subunits by molecular biological methods. I have restricted consideration here primarily to experiments in which biochemical methods were applied to purified protein preparations. For this reason, only the Ca2+ channels of the Cav1 and Cav2 families are considered. Readers are directed to subsequent chapters in this book for consideration of more recent studies that have employed a combination of molecular biological and protein expression methods to further analyze these questions.
Purification and Biochemical Characterization of Skeletal Muscle Ca2+ Channels
The Ca2+ channels in the transverse tubule membranes of skeletal muscle have served as a primary biochemical preparation for studies of Ca2+ channels because of their abundance. These channels serve two critical physiological roles. Like other Ca2+ channels, they mediate Ca2+ entry in response to depolarization. The primary voltage-gated Ca2+ currents in skeletal muscle are L-type,3-5 characterized by slow voltage-dependent inactivation, large single channel conductance (about 25 pS), high voltage of activation, and specific inhibition by dihydropyridine Ca2+ channel antagonists. These channels activate very slowly, and the Ca2+ entering vertebrate skeletal muscle through voltage-gated Ca2+ channels is not required for muscle contraction. It appears to replenish cellular Ca2+ during periods of rapid activity and to increase intracellular Ca2+ in response to tetanic stimulation, leading to increased contractile force. The primary physiological role for the skeletal muscle Ca2+ channel is to serve as a voltage sensor in excitation-contraction coupling. Voltage-gated Ca2+ channels in the transverse tubule membranes are thought to interact physically with the Ca2+ release channels located in the sarcoplasmic reticulum membrane. Voltage-driven conformational changes in the voltage-gated Ca2+ channels then activate the Ca2+ release from the sarcoplasmic reticulum by protein-protein interactions.6-8
Purification of Ca2+ channels from skeletal muscle began with isolation of the transverse tubule membranes, which are highly enriched in Ca2+ channel protein, and specific labeling by high affinity binding to dihydropyridine Ca2+ channel antagonists to identify the channel protein. 9,10 Ca2+ channels were solubilized in the mild detergent digitonin to retain native subunit associations and purified by a combination of ion exchange chromatography, affinity chromatography on wheat germ agglutinin-Sepharose, and sedimentation through sucrose gradients.10 A heterogeous a subunit band9,10 and associated β subunits of 50 kD and γ subunits of 33 kD10 were identified as components of the Ca2+ channel in the initial purification studies, as assessed by comigration during column chromatography and sucrose gradient sedimentation. Subsequent experiments demonstrated that the heterogenous a subunit band contained not only the principal α1 subunits with an apparent molecular mass of 175 kD but also a disulfidelinked dimer of α2-δ subunits with apparent molecular masses of 143 kD and 27 kD, respectively, as illustrated in the SDS-PAGE results in Figure 1A.11-15 The specific association of these proteins as a multisubunit complex was supported by the copurification of each subunit with the dihyropyridine binding activity and Ca2+ conductance activity of the Ca2+ channel,10,14,16,17 by coimmunoprecipitation of all these proteins by antibodies directed against the α1 subunits, 12,14,18 and by co-immunoprecipitation of the Ca2+ channel complex by antibodies against each auxiliary subunit.19,20,21 Estimates of stoichiometry indicated that each mol of Ca2+ channel complex contains approximately 1 mol of each of the 5 subunits. The biochemical and molecular properties of each of the subunits of skeletal muscle Ca2+ channels are considered below.
α1 Subunit
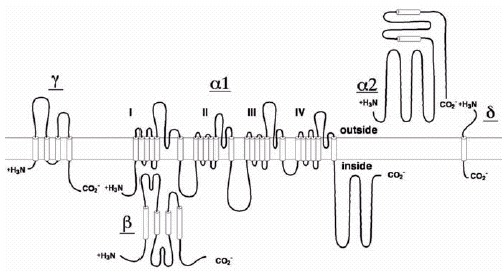
The α1 subunit of skeletal muscle Ca2+ channels was cloned by library screening based on amino acid sequence22 (fig. 2). The cDNA predicts a protein of 1873 amino acids with a molecular weight of 212 kD, considerably larger than the estimate of 175 kD for the α1 subunits of purified Ca2+ channels. Analysis of the α1 subunits of purified Ca2+ channels and Ca2+ channels in transverse tubule membranes using sequence-directed antibodies showed that most (>90%) contained α1 subunits that were truncated in their carboxyl terminal domain between residues 1685 and 1699 resulting in a 190 kD form that runs anomalously in SDS gels at 175 kD.23,24 Only a small fraction (<10%) of skeletal muscle Ca2+ channels contained the full length α1 subunit encoded by the cDNA. Both forms were detected in rat skeletal muscle cells in culture suggesting that both may be present in vivo.25 Since no mRNA encoding the more abundant, truncated form has been identified, the truncated form is probably produced by specific proteolytic processing in vivo. A similar cleavage product can be produced by calpain treatment, suggesting calpain as a candidate for in vivo processing of the a1 subunits.26
α2 Subunit
The α2 subunit of skeletal muscle Ca2+ channels is a hydrophobic glycoprotein with an apparent molecular mass of 143 kD before deglycosylation and 105 kD after deglycosylation (fig.1A, lanes 1 and 2).14,27,28 It contains both high mannose and complex carbohydrate chains (fig. 1A, lanes 4 and 5). Cloning and sequencing cDNAs encoding the α2 subunit defined a protein of 1106 amino acids with a molecular mass of 125 kD, multiple potential transmembrane segments, and multiple consensus sites for N-linked glycosylation29 (fig. 2). The predicted α2 protein was 20 kD larger than the apparent molecular mass of the deglycosylated alpha;2 subunit suggesting that a portion of the protein encoded by the α2 cDNAs may not be present in the mature α2 subunit that had been characterized biochemically. Subsequent studies showed that both α2-δ subunits are encoded by the same mRNA (see below).
β Subunit
The β subunits are hydrophilic proteins that are not glycosylated and therefore are likely to be located on the intracellular side of the membrane (fig. 1).14,20 cDNA cloning and sequencing revealed a protein of 524 amino acids with a predicted molecular mass of 58 kD.30 In agreement with biochemical data, the primary structure does not include any potential transmembrane segments but contains multiple consensus sites for phosphorylation by different protein kinases (fig. 2B).
γ Subunit
The γ subunit of skeletal muscle Ca2+ channels is a hydrophobic glycoprotein with an apparent molecular mass of 30 kD without deglycosylation and 20 kD following deglycosylation.14,21 Cloning and sequencing cDNAs encoding γ subunits revealed a protein of 222 amino acid residues with a molecular mass of 25 kD.31,32 The deduced primary structure contained four predicted hydrophobic transmembrane segments and multiple sites for N-linked glycosylation.
δ Subunit
The δ subunit appears on SDS gels as a doublet of 24 and 27 kD proteins, which are both hydrophobic and glycosylated.14,28 Determination of the amino acid sequences of peptides derived from the δ subunit showed that it was encoded by the same mRNA as the alpha;2 subunit. 33,34 The mature α2 subunit is truncated at alanine 934 of the α2δ precursor protein; residues 935-1106 constitute the disulfide-linked δ subunit. This sequence comprises a protein of 16 kD and contains a single transmembrane segment and three consensus sequences for Nlinked glycosylation. The doublet on SDS gels represents two differently glycosylated forms of the δ subunit.
Membrane Association of Subunits
The transmembrane organization of the Ca2+ channel complex was intially investigated by labeling of potential transmembrane segments with a hydrophobic photoaffinity probe.14 By this criterion, the α1, α2δ complex, and γ subunits were identified as probable integral membrane proteins, with the labeling of the α2 subunits the weakest despite its large size (fig.1A, lane 7, and B).14 Subsequent hydropathy analysis of the primary structures revealed 24 transmembrane segments in the α1 subunit, four in the γ subunit, and one in the δ subunit (fig.2), but the prediction of transmembrane alpha helices in the α2 subunits has been uncertain.29 Analysis of association of the α2 subunit with the membrane by expression and biochemical extraction procedures led to the conclusion that the α2 subunit does not have a true transmembrane segment.35 Instead, it is likely to be associated with the membrane primarily by virtue of its disulfide linkage to the δ subunit and perhaps also by superficial ionic and hydrophobic interactions with the membrane bilayer (fig.1B).
Sites of Protein Phosphorylation
Activation of skeletal muscle L-type Ca2+ channels is enhanced by phosphorylation by cAMPdependent protein kinase.36,37 In cultured skeletal muscle cells, repetitive depolarization causes a dramatic enhancement of Ca2+ currents,38,39 up to 10-fold in the critical membrane potential range near -20 mV. This potentiation of Ca2+ currents is strongly voltage-dependent and also is dependent on the activity of cAMP-dependent protein kinase.39 This may result from interaction between voltage-dependent gating and phosphorylation of the Ca2+ channel itself. This novel regulatory mechanism greatly increases Ca2+ channel activity during tetanic stimulation of skeletal muscle cells and may play a critical role in the regulation of contractile force of skeletal muscle in response to hormones and to the frequency of stimulation of the motor nerve.
The α1 subunit and the β subunit of skeletal muscle Ca2+ channels are substrates for phosphorylation by cAMP-dependent protein kinase and a number of other protein kinases (fig.1A, lane 8).14,40-44 Ca2+ flux through the purified skeletal muscle Ca2+ channel is regulated by cAMP-dependent protein phosphorylation.17,45-47 Ion flux studies in reconstituted phospholipid vesicles show that phosphorylation of the a1 and β subunits can greatly increase the number of functional Ca2+ channels in purified preparations.46,47 Single channel recording experiments in planar bilayer membranes detect both increases in the number of functional Ca2+ channels and increases in the activity of single Ca2+ channels after phosphorylation by cAMP-dependent protein kinase.17,45 Thus, the α1 and β subunits of the purified Ca2+ channel contain the sites at which cAMP-dependent protein phosphorylation modulates channel function in vitro.
Two size forms of the α1 subunit of approximately 190 kDa and 212 kDa are present in purified preparations of skeletal muscle Ca2+ channels, T-tubule membranes, and intact skeletal muscle cells in culture, and both are phosphorylated by PKA in intact cells.23-25 Antibody mapping of the C-terminal region of α1190 placed the C-terminus between residues 1685 and 1699.24 The most rapidly phosphorylated site in the truncated form of the α1 subunit in purified Ca2+ channel preparations is Ser 687, located in the intracellular loop between domains II and III.48,49 In contrast, time course experiments indicated that Ser 1854 near the Cterminal portion of full-length α1212 is the most intensely and rapidly phosphorylated.49,50 To date, the site(s) of phosphorylation that regulate skeletal muscle Ca2+ channel activity have not been directly identified.
Phosphorylation of the β Subunit of Skeletal Muscle Ca2+ Channels
Like the α1 subunit of the skeletal muscle Ca2+ channel, the β subunit is stoichiometrically phosphorylated by cAMP-dependent protein kinase in purified preparations40 and in reconstituted Ca2+ channels that are regulated by cAMP-dependent protein kinase.17,47 Both serine 182 and threonine 205 have been shown to be phosphorylated in vitro.23,30 Since phosphorylation of both α1-β subunits have been shown to be correlated with regulation of the ion conductance activity of skeletal muscle Ca2+ channels, both are candidates for sites of channel modulation by phosphorylation.
Sites of Drug Binding
A defining feature of Cav1 channels and the L-type Ca2+ currents they conduct is block by dihydropyridines, phenylalkylamines, and other Ca2+ channel antagonist drugs. Photoaffinity labeling studies established that the binding sites of dihydropyridines,13,14,15,21 phenylalkylamines,51 and benzothiazepines52 are located in the α1 subunits. Detailed mapping of the dihydropyridine receptor site by proteolytic digestion and mapping of the digested fragments with sequence-directed antibodies identified the IIIS6 and IVS6 segments as important components of the binding site.53 These results led to a domain interface model of drug binding of dihydropyridines, in which these drugs bind at the interface between domain III and IV at the point of interaction of the S6 segments.54 Subsequent extensive mapping of the dihydropyridine receptor site by mutagenesis and functional analysis has given a detailed view of this important receptor site (see Chapter 17) and allowed construction of high affinity dihydropyridine receptor in non-L-type Ca2+ channels.55-57 Similarly, the receptor sites of phenylalkylamines and benzothiazepines have been extensively mapped by photoaffininty labeling and peptide mapping58,59 and subsequently by mutagenesis and functional analysis (see Chapter 17). All three receptor sites are close in the expected three-dimensional structure of the channel and have both distinct and overlapping molecular determinants of their binding.
Biochemical Properties of Other Cav1 Channels
Subunits of Purified Cardiac Ca2+ Channels
Like the skeletal muscle Ca2+ channel, the principal cardiac Ca2+ channel conducts L-type Ca2+ currents.60,61 Antibodies against α2d subunits of skeletal muscle Ca2+ channels detect corresponding subunits in cardiac preparations.62,63 Partially purified cardiac Ca2+ channels contain a1, α2δ, and β subunits.64-68 However, the relatively low abundance of Ca2+ channels in cardiac tissue and the difficulty of controlling proteolysis during lengthy purification procedures have frustrated attempts at complete purification of an intact cardiac Ca2+ channel complex. The α1 subunit with an apparent mass of 165 kD to 190 kD has been directly identified by photoaffinity labeling with photoreactive dihydropyridines.64,66,69 As in skeletal muscle, most of the α1 subunits of cardiac Ca2+ channels are truncated at the C-terminus by proteolytic processing.70 The primary site for phosphorylation by PKA in vitro and in cultured cells is Ser1928 (in rabbit Cav1.2 sequence numbering), which is located in the distal C-terminus beyond the site of proteolytic cleavage,70 as observed for the skeletal muscle Ca2+ channel.49,50 Therefore, it is possible that there is a functional relationship between proteolytic cleavage of the C-terminal domain and regulation of the cardiac Ca2+ channel by protein phosphorylation. Consistent with this idea, truncation of the C-terminal domain by expression of cDNAs with appropriate deletions yields increased Ca2+ channel activity,71 and perfusion of C-terminal peptides into the cells expressing truncated channels inhibits the increase in Ca2+ channel activity.72
Molecular Basis for Modulation of Cardiac Ca2+ Channels by PKA
Although regulation of the cardiac Ca2+ channels by the cAMP pathway was the first example of ion channel regulation through second messenger pathways,73,74 the molecular basis for this regulation is still not clearly resolved. Early biochemical studies of the cardiac Ca2+ channel resulted in purification of a short, 160 to 195 kDa form of the α1 subunit that was not a substrate for cAMP-dependent protein kinase.64,66 As for skeletal muscle Ca2+ channels, more recent results on cardiac Ca2+ channels have revealed a full-length a1 subunit form with a molecular mass of approximately 220 kDa, as expected from the cDNA sequence.70 This fulllength a1 subunit is phosphorylated on a single serine residue (Ser1928) in the C-terminal domain.70 Similarly, expression of the C-terminal domain in bacteria yields a protein that is phosphorylated primarily on Ser1928.75 This site is missing in the truncated from of the α1 subunit isolated in early biochemical preparations. Primarily the full-length form of CaV1.2 is present in cardiac tissue.76,77 Both β1 and β2 isoforms are expressed in cardiac tissue76-79 and likely are both associated in CaV1.2 channels.
Ca2+ channels in a CHO cell line that expresses only the full-length form of the CaV1.2 α1 subunit can be regulated by activation of endogenous PKA, by intracellular perfusion of PKA, and by voltage-dependent potentiation, which requires the activity of PKA.80,81 These results indicate that at least part of the regulation of Ca2+ channel activity is due to phosphorylation of the α1 subunit. Since the α1 subunit is only phosphorylated on Ser1928 in vitro or in transfected cells,70 this regulation likely involves phosphorylation of this site. In support of this conclusion, mutation of this serine residue to alanine reduces the regulation of Ca2+ channel activity caused by activation of PKA with 8-Br-cAMP in transiently transfected human embryonic kidney cells from a 35% increase to a 7% increase.76,77 Although these results are promising, the extent of regulation of Ca2+ channel activity in these experiments in transfected cells falls well short of the magnitude recorded in native cardiac cells (2- to 4-fold increase), and regulation of cloned Ca2+ channels analyzed in this way is not observed in some of the major laboratories in the field.82 Therefore, it seems likely that additional regulatory influences that have not yet been reproduced and characterized in transfected cells are important for control of the activity of cardiac Ca2+ channels in vivo.
Phosphorylation of the β Subunit of Cardiac Ca2+ Channels by PKA
The β subunits of cardiac Ca2+ channels are phosphorylated by PKA in intact hearts treated with β-adrenergic agonists.83 Bunemann et al84 have reported substantial (greater than 2-fold) regulation of a C-terminal truncated form of CaV1.2 lacking Ser1928 by intracellular perfusion of activated PKA in human embryonic kidney cells co-transfected with CaV1.2 and β2a subunits. This regulation required phosphorylation of Ser 478 and/or Ser 479, two serine residues in non-classical PKA phosphorylation sites that are specific to the β2a subunit. These results provide the best evidence to date for an important role of the β subunits in regulation of cardiac Ca2+ channels. It will be important to further analyze Ca2+ channel regulation when full-length α1 subunits are co-expressed with β2a and other β subunits expressed in cardiac myocytes and to restore regulation by physiological stimulus procedures rather than intracellular perfusion of activated PKA.
Subunits of Purified Neuronal Cav1 Channels
Because the concentration of Ca2+ channels in skeletal muscle transverse tubules is much higher than in neuronal membranes, the biochemical properties of these channels in neurons are not as well established. Immunoprecipitation of Ca2+ channel complexes labeled with dihydropyridines using specific antibodies against α2δ subunits revealed a complex of polypeptides with sizes corresponding to α1, α2δ, and β subunits of dihydropyridine-sensitive L-type Ca2+ channels in the brain.19,85,86 A potentially novel 100 kD protein was also identified as a specifically associated component of the L-type Ca2+ channel complex from brain.19 Further biochemical characterization of brain Cav1 channels will require development of a method for purification of larger quantities of channel protein.
Biochemical Properties of the Cav2 Family of Ca2+ Channels
Multiple types of Ca2+ channels, which differ in physiological and pharmacological properties, are expressed in neurons. At least three types of high-voltage-activated Ca2+ channels have been distinguished in addition to L-type (see Chapter 3).87-90 N-type, P-type, and Q-type channels all have intermediate single channel conductances (about 15 pS) and can mediate Ca2+ currents with varying rates of voltage-dependent inactivation depending on their subunit composition and on other factors (see below). They are best distinguished by their pharmacological properties: N-type channels are specifically inhibited by ω-conotoxin GVIA whereas P/ Q-type are most sensitive to ω-agatoxin IVA and ω-conotoxin MVIIC. These specific peptide toxins have provided experimental tools for analysis of the protein subunits of neuronal Ca2+ channels.
Cav2.2 Channels
The ω-conotoxin-sensitive N-type Ca2+ channels purified from rat brain contain an a1 subunit, a 140 kD α2-like subunit, and β subunits of 60 kDa to 70 kD as identified by antibodies against the skeletal muscle forms of these subunits.19,91-94 Both L-type and N-type Ca2+ channels from brain appear to lack a γ subunit, but a protein of approximately 100 kD is specifically associated with N-type Ca2+ channels as well as L-type Ca2+ channels from brain and may be an additional, brain-specific associated protein.19,91,92,94 The α1 subunit of N-type Ca2+ channels was identified as Cav2.2 by homology cDNA cloning, co-immunoprecipitation, and functional expression.95,96 As for the Cav1 family channels, analysis of the Cav2.2α1 subunit using sequence-specific antibodies revealed two size forms (240 kD and 210 kD) that differ in their C-termini and in their phosphorylation by specific protein kinases.97,98 It is unknown at present whether these Cav2.2α1 subunits are derived from proteolytic truncation, alternatively spliced mRNAs or both (see Chapter 5).
Cav2.1 Channels
Agatoxin-sensitive P/Q-type Ca2+ channels purified from brain are also composed of α1, α2δ, and β subunits.99,100,101 In addition, recent experiments have unexpectedly revealed a novel γ subunit, which is the target of the stargazer mutation in mice.102 This γ-subunit-like protein can modulate the voltage dependence of expressed Ca2+ channels containing Cav2.1 subunits, so they may be associated with these Ca2+ channels in vivo.102 If this new γ subunit is indeed associated with neuronal Ca2+ channels, their subunit composition would be identical to that of skeletal muscle Ca2+ channels defined in biochemical experiments.14 The cDNA encoding the Cav2.1 subunit was isolated by homology cloning and identified by functional expression.103,104 Analysis of the a1 subunit peptides present in brain has revealed a wide range of size forms, including C-terminal truncations and internal deletions, and these isoforms are differentially phosphorylated by protein kinases.105,106 These results continue the theme that all Ca2+ channels studied to date have multiple forms due to varying C-terminal domains and that they are differentially phosphorylated by protein kinases.
Interactions of Ca2+ Channels with Intracellular Regulatory Proteins
Ca2+ entering cells through voltage-gated Ca2+ channels initiates many intracellular processes through activation of effector mechanisms. Often these effector mechanisms are highly localized to respond to high local concentrations of Ca2+. In addition, local regulation of Ca2+ channel function is also modulated by specifically bound regulatory proteins. The interactions of these intracellular effector and regulatory proteins with Ca2+ channels have been studied by biochemical approaches as outlined here and by molecular biological methods as described in Chapters 8, 9 and 11. These studies show that a functional Ca2+ channel complex not only has four auxiliary subunits but also contains multiple associated regulatory proteins that modify its activity.
Ryanodine Receptors
In skeletal muscle fibers, excitation-contraction coupling involves direct conformational coupling of the voltage-gated Ca2+ channel in the transverse tubule membrane to the ryanodinesensitive Ca2+ release channel (RyR) of the sarcoplasmic reticulum at the transverse tubule/sarcoplasmic reticulum junction.6,7,107 Conformational coupling is mediated by the intracellular loop connecting domains II and III of the α1 subunit.108,109 Depolarization of the transverse tubules by the conducted action potential activates L-type voltage-gated Ca2+ channels. A complex of ryanodine receptor and Ca2+ channels can be identified by detergent extraction and resolution by sucrose gradient sedimentation and antibody labeling.110 This complex is required for excitation/contraction coupling and for feedback regulation of Ca2+ channel function.109, 111 and Chapter 21.
SNARE Proteins
Ca2+ entry through voltage-gated Ca2+ channels is thought to initiate exocytosis by triggering the fusion of secretory vesicle membranes with the plasma membrane through actions on the SNARE protein complex of syntaxin, SNAP-25, and VAMP/synaptobrevin (reviewed in refs. 112,113). The function of the SNARE protein complex is regulated by interactions with numerous proteins, including the synaptic vesicle Ca2+ -binding protein synaptotagmin. The SNARE proteins syntaxin and SNAP-25 are associated with N-type and P/Q-type Ca2+ channels and can be co-purified and co-immunoprecipitated from brain.91,100,114,115 Presynaptic N-type and P/Q-type Ca2+ channels interact directly with the SNARE proteins through a specific synaptic protein interaction (synprint) site in the large intracellular loop connecting domains II and III.116,117 and Chapter 8. This binding interaction is regulated by Ca2+ and protein phosphorylation.117,118 Synaptotagmin also binds to the synprint site of CaV2 Ca2+ channels.119-121 Injection of peptide inhibitors of this SNARE protein interaction into pre-synaptic neurons inhibits synaptic transmission, consistent with the conclusion that this interaction is required to position docked synaptic vesicles near Ca2+ channels for effective fast exocytosis.116,122,123 SNARE protein interaction at this site is also important for G protein regulation of Ca2+ channels (see Chapter 9).
Kinase Anchoring Proteins
cAMP-dependent protein kinase is often anchored to specific subcellular compartments or specific kinase substrates by A Kinase Anchoring Proteins (AKAPs).124,125 These proteins contain a targeting domain that directs the AKAP to a specific cellular site and a kinase anchoring domain containing an amphipathic alpha helix that binds the regulatory subunit dimer of cAMP-dependent protein kinase. Because regulation of the skeletal muscle Ca2+ channel by membrane depolarization and cAMP-dependent protein kinase is very rapid, with observable effects in 50 ms, it was an attractive candidate for regulation through PKA bound to AKAPs. Biochemical studies of skeletal muscle Ca2+ channels revealed a novel 15-kDa AKAP (AKAP- 15) associated with purified Ca2+ channels and with specifically immunoprecipitated Ca2+ channels.126 This AKAP is an 81-residue protein with N-terminal palmitoyl and myristoyl moieties that serve as membrane anchors and an amphipathic helix that binds PKA 127 (also designated AKAP-18).128 AKAP-15, PKA, and Ca2+ channels are co-localized in the specialized junctions formed between sarcoplasmic reticulum and transverse tubule membranes, where excitation-contraction coupling takes place.127,129 AKAP-15 binds to the skeletal muscle Ca2+ channel through a specific modified leucine zipper motif in the distal C-terminal domain.130 In this way, the bound AKAP directly targets PKA to its sites of phosphorylation in the C-terminal of the full-length α1 subunit.
Ca2+ Binding Proteins
Ca2+ entering cells through Ca2+ channels is involved in initiation of intracellular signaling processes, and these often involve Ca2+ binding proteins like calmodulin, which is expressed ubiquitously, and neuro-specific Ca2+ binding proteins, which are expressed in specific neurons and in distinct subcellular locations. Research reviewed in Chapter 11 shows that both calmodulin and neuro-specific Ca2+ binding proteins interact with Ca2+ channels at specific binding sites in the C-terminal domains and modulate their function.
Conclusion
Voltage-gated Ca2+ channels are complex proteins containing five distinct protein subunits, α, α, β, γ, and δ, that specifically associate with each other. In addition to subunit assembly, their biosynthesis involves extensive glycosylation as well as proteolytic processing and disulfide linkage of subunits. Each α1 subunit that has been studied is present in multiple isoforms that differ in their C-terminal domains and in phosphorylation by specific kinases. Ca2+ channels provide intracellular Ca2+ to initiate local signaling events, and they are directly associated with both effector proteins that initiate Ca2+ -dependent processes and with regulatory proteins that control their activity. Ca2+ channel signaling complexes are a crucial element of local regulation of cellular events.
References
- 1.
- Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calciumconcentration. J Physiol (Lond). 1967;192:479–492. [PMC free article: PMC1365567] [PubMed: 6050160]
- 2.
- Catterall WA. The molecular basis of neuronal excitability. Science. 1984;223:653–661. [PubMed: 6320365]
- 3.
- Almers W, Fink R, Palade PT. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol (Lond). 1981;312:177–217. [PMC free article: PMC1275548] [PubMed: 6267262]
- 4.
- Rosenberg RL, Hess P, Reeves JP. et al. Calcium channels in planar lipid bilayers: Insights into mechanisms of ion permeation and gating. Science. 1986;231:1564–1566. [PubMed: 2420007]
- 5.
- Sanchez JA, Stefani E. Inward calcium current in twitch muscle fibers of the frog. J Physiol. 1978;283:197–209. [PMC free article: PMC1282773] [PubMed: 309941]
- 6.
- Adams BA, Beam KG. Muscular dysgenesis in mice: A model system for studying excitation-contraction coupling. FASEB J. 1990;4:2809–2816. [PubMed: 2165014]
- 7.
- Catterall WA. Excitation-contraction coupling in vertebrate skeletal muscle: A tale of two calcium channels. Cell. 1991;64:871–874. [PubMed: 1848157]
- 8.
- Rios E, Brum G. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature (London). 1987;325:717–720. [PubMed: 2434854]
- 9.
- Borsotto M, Barhanin J, Fosset M. et al. The 1,4-dihydropyridine receptor associated with the skeletal muscle voltage-dependent Ca++ J Biol. Chem1985;260:14255–14263. [PubMed: 2997201]
- 10.
- Curtis BM, Catterall WA. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochem. 1984;23:2113–2118. [PubMed: 6329263]
- 11.
- Hosey MM, Barhanin J, Schmid A. et al. Photoaffinity labelling and phosphorylation of a 165 kilodalton peptide associated with dihydropyridine and phenylalkylamine-sensitive calcium channels. Biochem Biophys Res Commun. 1987;147:1137–1145. [PubMed: 2444223]
- 12.
- Leung AT, Imagawa T, Campbell KP. Structural characterization of the 1,4-dihydropyridine receptor of the voltage-dependent Ca2+ channel from rabbit skeletal muscle. Evidence for two distinct high molecular weight subunits. J Biol Chem. 1987;262:7943–7946. [PubMed: 2439496]
- 13.
- Sieber M, Nastainczyk W, Zubor V. et al. The 165-kDa peptide of the purified skeletal muscle dihydropyridine receptor contains the known regulatory sites of the calcium channel. Eur J Biochem. 1987;167:117–122. [PubMed: 2441986]
- 14.
- Takahashi M, Seagar MJ, Jones JF. et al. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci USA. 1987;84:5478–5482. [PMC free article: PMC298881] [PubMed: 2440051]
- 15.
- Vaghy PL, Striessnig J, Miwa K. et al. Identification of a novel 1,4-dihydropyridine- and phenylalkylamine-binding polypeptide in calcium channel preparations. J Biol Chem. 1987;262:14337–14342. [PubMed: 2443504]
- 16.
- Curtis BM, Catterall WA. Reconstitution of the voltage-sensitive calcium channel purified from skeletal muscle transverse tubules. Biochemistry. 1986;25:3077–3083. [PubMed: 2425846]
- 17.
- Flockerzi V, Oeken H-J, Hofmann F. et al. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986;323:66–68. [PubMed: 2427959]
- 18.
- Morton ME, Froehner SC. Monoclonal antibody identifies a 200-kDa subunit of the dihydropyridine-sensitive calcium channel. J Biol Chem. 1987;262:11904–11907. [PubMed: 2442152]
- 19.
- Ahlijanian MK, Westenbroek RE, Catterall WA. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990;4:819–832. [PubMed: 2163262]
- 20.
- Leung AT, Imagawa T, Block B. et al. Biochemical and ultrastructural characterization of the 1,4- dihydropyridine receptor from rabbit skeletal muscle Evidence for a 52,000 Da subunit. J Biol Chem. 1988;263:994–1001. [PubMed: 2826471]
- 21.
- Sharp AH, Campbell KP. Characterization of the 1,4-dihydropyridine receptor using subunit-specific polyclonal antibodies. Evidence for a 32,000-Da subunit. J Biol Chem. 1989;264:2816–2825. [PubMed: 2536724]
- 22.
- Tanabe T, Takeshima H, Mikami A. et al. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. [PubMed: 3037387]
- 23.
- De JonghKS, Merrick DK, Catterall WA. Subunits of purified calcium channels: a 212-kDa form of α1 and partial amino acid sequence of a phosphorylation site of an independent β subunit. Proc Natl Acad Sci USA. 1989;86:8585–8589. [PMC free article: PMC298327] [PubMed: 2554320]
- 24.
- De JonghKS, Warner C, Colvin AA. et al. Characterization of the two size forms of the α1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci USA. 1991;88:10778–10782. [PMC free article: PMC53014] [PubMed: 1720551]
- 25.
- Lai Y, Seagar MJ, Takahashi M. et al. Cyclic AMP-dependent phosphorylation of two size forms of α1 subunits of L-type calcium channels in rat skeletal muscle cells. J Biol Chem. 1990;265:20839–20848. [PubMed: 2174428]
- 26.
- De JonghKS, Colvin AA, Wang KKW. et al. Differential proteolysis of the full-length form of the L-type calcium channel α1 subunit by calpain. J Neurochem. 1994;63:1558–1564. [PubMed: 7931310]
- 27.
- Burgess AJ, Norman RI. The large glycoprotein subunit of the skeletal muscle voltage-sensitive calcium channel. Eur J Biochem. 1988;178:527–533. [PubMed: 2850183]
- 28.
- Vandaele S, Fosset M, Galizzi J-P. et al. Monoclonal antibodies that coimmunoprecipitate the 1,4- dihydropyridine and phenylalkylamine receptors and reveal the Ca2+ channel structure. Biochemistry. 1987;26:5–9. [PubMed: 2435317]
- 29.
- Ellis SB, Williams ME, Ways NR. et al. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. [PubMed: 2458626]
- 30.
- Ruth P, Röhrkasten A, Biel M. et al. Primary structure of the beta subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989;245:1115–1118. [PubMed: 2549640]
- 31.
- Bosse E, Regulla S, Biel M. et al. The cDNA and deduced amino acid sequence of the gamma subunit of the L-type calcium channel from rabbit skeletal muscle. FEBS Lett. 1990;267:153–156. [PubMed: 2163895]
- 32.
- Jay SD, Ellis SB, McCue AF. et al. Primary structure of the gamma subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1990;248:490–492. [PubMed: 2158672]
- 33.
- De JonghKS, Warner C, Catterall WA. Subunits of purified calcium channels. α2 and δ J Biol Chem. 1990;are encoded by the same gene265:14738–14741. [PubMed: 2168391]
- 34.
- Jay SD, Sharp AH, Kahl SD. et al. Structural characterization of the dihydropyridine-sensitive calcium channel α2-subunit and the associated δ peptides. J Biol Chem. 1991;266:3287–3293. [PubMed: 1847144]
- 35.
- Gurnett CA, De WaardM, Campbell KP. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron. 1996;16:431–440. [PubMed: 8789958]
- 36.
- Arreola J, Calvo J, Garcia MC. et al. Modulation of calcium channels of twitch skeletal muscle fibres of the frog by adrenaline and cyclic adenosine monophosphate. J Physiol (Lond). 1987;393:307–330. [PMC free article: PMC1192395] [PubMed: 2451739]
- 37.
- Schmid A, Renaud J, Lazdunski M. Short term and long term effects of beta-adrenergic effectors and cyclic AMP on nitrendipine-sensitive voltage-dependent Ca2+ channels of skeletal muscle. J Biol Chem. 1985;260:13041–13046. [PubMed: 2414273]
- 38.
- Fleig A, Penner R. Silent calcium channels generate excessive tail currents and facilitation of calcium currents in rat skeletal myoballs. J Physiol (Lond). 1996;494:141–153. [PMC free article: PMC1160620] [PubMed: 8814612]
- 39.
- Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993a;364:240–243. [PubMed: 8391648]
- 40.
- Curtis BM, Catterall WA. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1985;82:2528–2532. [PMC free article: PMC397592] [PubMed: 2581248]
- 41.
- Haase H, Podzuweit T, Lutsch G. et al. Signaling from beta-adrenoceptor to L-type calcium channel: identification of a novel cardiac protein kinase A target possessing similarities to AHNAK. FASEB J. 1999;13:2161–2172. [PubMed: 10593863]
- 42.
- Jahn H, Nastainczyk W, Röhrkasten A. et al. Site-specific phosphorylation of the purified receptor for calcium-channel blockers by cAMP- and cGMP-dependent protein kinases, protein kinase C, calmodulin-dependent protein kinase II and casein kinase II. Eur J Biochem. 1988;178:535–542. [PubMed: 2850184]
- 43.
- Nastainczyk W, Röhrkasten A, Sieber M. et al. Phosphorylation of the purified receptor for calcium channel blockers by cAMP kinase and protein kinase C. Eur J Biochem. 1987;169:137–142. [PubMed: 2824197]
- 44.
- O'Callahan CM, Hosey MM. Multiple phosphorylation sites in the 165-kilodalton peptide associated with dihydropyridine-sensitive calcium channels. Biochemistry. 1988;27:6071–6077. [PubMed: 2847783]
- 45.
- Hymel L, Striessnig J, Glossmann H. et al. Purified skeletal muscle 1,4-dihydropyridine receptor forms phosphorylation-dependent oligomeric calcium channels in planar bilayers. Proc Natl Acad Sci USA. 1988;85:4290–4294. [PMC free article: PMC280414] [PubMed: 2454467]
- 46.
- Mundiñna-Weilenmann C, Chang CF, Gutierrez LM. et al. Demonstration of the phosphorylation of dihydropyridine-sensitive calcium channels in chick skeletal muscle and the resultant activation of the channels after reconstitution. J Biol Chem. 1991;266:4067–4073. [PubMed: 1847914]
- 47.
- Nunoki K, Florio V, Catterall WA. Activation of purified calcium channels by stoichiometric protein phosphorylation. Proc Natl Acad Sci USA. 1989;86:6816–6820. [PMC free article: PMC297937] [PubMed: 2549550]
- 48.
- Röhrkasten A, Meyer HE, Nastainczyk W. et al. cAMP-dependent protein kinase rapidly phosphorylates serine- 687 of the skeletal muscle receptor for calcium channel blockers. J Biol Chem. 1988;263:15325–15329. [PubMed: 2844809]
- 49.
- Rotman EI, De JonghKS, Florio V. et al. Specific phosphorylation of a COOH-terminal site on the full-length form of the a1 subunit of the skeletal muscle calcium channel by cAMP-dependent protein kinase. J Biol Chem. 1992;267:16100–16105. [PubMed: 1322891]
- 50.
- Rotman EI, Murphy BJ, Catterall WA. Sites of selective cAMP-dependent phosphorylation of the L-type calcium channel α1 subunit from intact rabbit skeletal muscle myotubes. J Biol Chem. 1995;270:16371–16377. [PubMed: 7608207]
- 51.
- Striessnig J, Knaus HG, Grabner M. et al. Photoaffinity labelling of the phenylalkylamine receptor of the skeletal muscle transverse-tubule calcium channel. FEBS Lett. 1987;212:247–253. [PubMed: 2434359]
- 52.
- Striessnig J, Scheffauer F, Mitterdorfer J. et al. Identification of the benzothiazepine-binding polypeptide of skeletal muscle calcium channels with (+)-cis-azidodiltiazem and anti-ligand antibodies. J Biol Chem. 1990b;265:363–370. [PubMed: 2152924]
- 53.
- Striessnig J, Murphy BJ, Catterall WA. The dihydropyridine receptor of L-type Ca2+ channels: Identification of binding domains for (+)-[3H]PN200-110 and [3H]azidopine within the α-1 subunit. Proc Natl Acad Sci USA. 1991;88:10769–10773. [PMC free article: PMC53012] [PubMed: 1660150]
- 54.
- Catterall WA, Striessnig J. Receptor sites for Ca2+ channel antagonists. Trends Pharmacol Sci. 1992;13:256–262. [PubMed: 1321525]
- 55.
- Hockerman GH, Peterson BZ, Sharp E. et al. Construction of a high-affinity receptor site for dihydropyridine agonists and antagonists by single amino acid substitutions in a non-L-type Ca2+ channel. Proc Natl Acad Sci USA. 1997;94:14906–14911. [PMC free article: PMC25136] [PubMed: 9405712]
- 56.
- Ito H, Klugbauer N, Hofmann F. Transfer of the high affinity dihydropyridine sensitivity from L-type to non-L-type calcium channel. Mol Pharmacol. 1997;52:735–740. [PubMed: 9380037]
- 57.
- Sinnegger MJ, Wang ZY, Grabner M. et al. Nine L-type amino acid residues confer full 1,4- dihydropyridine sensitivity to the neuronal calcium channel α1A subunit - Role of L-type MET. J Biol Chem. 1997;272:27686–27693. [PubMed: 9346909]
- 58.
- Kraus R, Reichl B, Kimball SD. et al. Identification of benz(othi)azepine-binding regions within L-type calcium channel α1 subunits. J Biol Chem. 1996;271:20113–20118. [PubMed: 8702733]
- 59.
- Striessnig J, Glossmann H, Catterall WA. Identification of a phenylalkylamine binding region within the α1 subunit of skeletal muscle Ca2+ channels. Proc Natl Acad Sci USA. 1990a;87:9108–9112. [PMC free article: PMC55113] [PubMed: 2174553]
- 60.
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301:569–574. [PubMed: 6131381]
- 61.
- Tsien RW. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. [PubMed: 6303205]
- 62.
- Schmid A, Barhanin J, Coppola T. et al. Immunochemical analysis of subunit structures of 1,4- dihydropyridine receptors associated with voltage dependent Ca++ channels in skeletal, cardiac and smooth muscle. Biochemistry. 1986;25:3492–3495. [PubMed: 2424495]
- 63.
- Takahashi M, Catterall WA. Dihydropyridine-sensitive calcium channels in cardiac and skeletal muscle membranes: studies with antibodies against the alpha subunits. Biochemistry. 1987a;26:5518–5526. [PubMed: 2445374]
- 64.
- Chang FC, Hosey MM. Dihydropyridine and phenylalkylamine receptors associated with cardiac and skeletal muscle calcium channels are structurally different. J Biol Chem. 1988;263:18929–18937. [PubMed: 2848812]
- 65.
- Kuniyasu A, Oka K, Ide-Yamada T. et al. Structural characterization of the dihydropyridine receptor- linked calcium channel from porcine heart. J Biochem(Tokyo). 1992;112:235–242. [PubMed: 1328169]
- 66.
- Schneider T, Hofmann F. The bovine cardiac receptor for calcium channel blockers is a 195-kDa protein. Eur J Biochem. 1988;174:369–375. [PubMed: 2838274]
- 67.
- Tokumaru H, Anzai K, Abe T. et al. Purification of the cardiac 1,4-dihydropyridine receptor using immunoaffinity chromatography with a monoclonal antibody against the α2δ subunit of the skeletal muscle dihydropyridine receptor. Eur J Pharmacol Mol Pharmacol. 1992;227:363–370. [PubMed: 1332871]
- 68.
- Tuana BS, Murphy BJ, Yi Q. Subcellular distribution and isolation of the Ca2+ antagonist receptor associated with the voltage regulated Ca2+ channel from rabbit heart muscle. Mol Cell Biochem. 1987;76:173–184. [PubMed: 2444872]
- 69.
- Ferry DR, Goll A, Glossmann H. Photoaffinity labelling of the cardiac calcium channel. (-)- [3H]azidopine labels a 165 kDa polypeptide, and evidence against a [3H]=1,4-dihydropyridineisothiocyanate being a calcium-channel-specific affinity ligand. Biochem J. 1987;243:127–135. [PMC free article: PMC1147823] [PubMed: 2440422]
- 70.
- De JonghKS, Murphy BJ, Colvin AA. et al. Specific phosphorylation of a site in the full-length form of the a1 subunit of the cardiac L-type calcium channel by cAMP-dependent protein kinase. Biochemistry. 1996;35:10392–10402. [PubMed: 8756695]
- 71.
- Wei XNA, Lacerda AE, Olcese R. et al. Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac α1 subunit. J Biol Chem. 1994;269:1635–1640. [PubMed: 7507480]
- 72.
- Gao T, Cuadra AE, Ma H. et al. C-terminal fragments of the alpha 1C (Cav1.2) subunit associate with and regulate L-type calcium channels containing C-terminal-truncated alpha 1C subunits. J Biol Chem. 2001;276:21089–21097. [PubMed: 11274161]
- 73.
- Armstrong DL, Rossier MF, Scherbatko AD. et al. Enzymatic gating of voltage-activated calcium channels. Ann NY Acad Sci. 1991;635:26–34. [PubMed: 1660238]
- 74.
- McDonald TF, Pelzer S, Trautwein W. et al. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. [PubMed: 8171118]
- 75.
- Mitterdorfer J, Froschmayr M, Grabner M. et al. Identification of PK-A phosphorylation sites in the carboxyl terminus of L-type calcium channel a1 subunits. Biochemistry. 1996;35:9400–9406. [PubMed: 8755718]
- 76.
- Gao T, Yatani A, Dell'Acqua ML. et al. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. [PubMed: 9247274]
- 77.
- Gao TY, Puri TS, Gerhardstein BL. et al. Identification and subcellular localization of the subunits of L-type calcium channels and adenylyl cyclase in cardiac myocytes. J Biol Chem. 1997;272:19401–19407. [PubMed: 9235939]
- 78.
- Biel M, Hullin R, Freundner S. et al. Tissue-specific expression of high-voltage-activated dihydropyridine-sensitive L-type calcium channels. Eur J Biochem. 1991;200:81–88. [PubMed: 1652442]
- 79.
- Hullin R, Singer-Lahat D, Freichel M. et al. Calcium channel beta subunit heterogeneity: Functional expression of cloned cDNA from heart, aorta and brain. EMBO J. 1992;11:885–890. [PMC free article: PMC556528] [PubMed: 1312465]
- 80.
- Sculptoreanu A, Rotman E, Takahashi M. et al. Voltage-dependent potentiation of the activity of cardiac L-type calcium channel α1 subunits due to phosphorylation by cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1993;90:10135–10139. [PMC free article: PMC47728] [PubMed: 7694283]
- 81.
- Yoshida A, Takahashi M, Nishimura S. et al. Cyclic AMP-dependent phosphorylation and regulation of the cardiac dihydropyridine-sensitive Ca channel. FEBS Lett. 1992a;309:343–349. [PubMed: 1325377]
- 82.
- Zong X, Schreieck J, Mehrke G. et al. On the regulation of the expressed L-type calcium channel by cAMP-dependent phosphorylation. Pflugers Arch. 1995;430:340–347. [PubMed: 7491257]
- 83.
- Haase H, Bartel S, Karczewski P. et al. In-vivo phosphorylation of the cardiac L-type calcium channel beta-subunit in response to catecholamines. Mol Cell Biochem. 1996;163-164:99–106. [PubMed: 8974044]
- 84.
- Bunemann M, Gerhardstein BL, Gao TY. et al. Functional regulation of L-type calcium channels via protein kianse A-mediated phosphorylation of the beta-2 subunit. J Biol Chem. 1999;274:33851–33854. [PubMed: 10567342]
- 85.
- Takahashi M, Catterall WA. Identification of an alpha subunit of dihydropyridine-sensitive brain calcium channels. Science. 1987b;236:88–91. [PubMed: 2436296]
- 86.
- Takahashi M, Fujimoto Y. Identification of a dihydropyridine-sensitive calcium channel in chick brain by a monoclonal antibody. Biochem Biophys Res Comm. 1989;163:1182–1188. [PubMed: 2551270]
- 87.
- Tsien RW, Lipscombe D, Madison DV. et al. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. [PubMed: 2469160]
- 88.
- Bean BP. Classes of calcium channels in vertebrate cells. Annu Rev Physiol. 1989;51:367–384. [PubMed: 2540697]
- 89.
- Llinas R, Sugimori M, Hillman DE. et al. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992;15:351–355. [PubMed: 1382335]
- 90.
- Zhang J-F, Randall AD, Ellinor PT. et al. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurons. Neuropharmacology. 1993;32:1075–1088. [PubMed: 8107963]
- 91.
- Leveque C, El FarO, Martin-Moutot N. et al. Purification of the N-type calcium channel associated with syntaxin and synaptotagmin: a complex implicated in synaptic vesicle exocytosis. J Biol Chem. 1994;269:6306–6312. [PubMed: 8119979]
- 92.
- McEnery MW, Snowman AM, Sharp AH. et al. Purified ω-conotoxin GVIA receptor of rat brain resembles a dihydropyridine-sensitive L-type calcium channel. Proc Natl Acad Sci USA. 1991;88:11095–11099. [PMC free article: PMC53080] [PubMed: 1662383]
- 93.
- Sakamoto J, Campbell KP. A monoclonal antibody to the β subunit of the skeletal muscle dihydropyridine receptor immunoprecipitates the brain ?-conotoxin GVIA receptor. J Biol Chem. 1991;266:18914–18919. [PubMed: 1655767]
- 94.
- Witcher DR, De WaardM, Sakamoto J. et al. Subunit identification and reconstitution of the Ntype Ca2+ channel complex purified from brain. Science. 1993;261:486–489. [PubMed: 8392754]
- 95.
- Dubel SJ, Starr TVB, Hell J. et al. Molecular cloning of the α-1 subunit of an ω-conotoxinsensitive calcium channel. Proc Natl Acad Sci USA. 1992;89:5058–5062. [PMC free article: PMC49228] [PubMed: 1317580]
- 96.
- Williams ME, Brust PF, Feldman DH. et al. Structure and functional expression of an omegaconotoxin- sensitive human N-type calcium channel. Science. 1992;257:389–395. [PubMed: 1321501]
- 97.
- Hell JW, Appleyard SM, Yokoyama CT. et al. Differential phosphorylation of two size forms of the N-type calcium channel α1 subunit which have different COOH-termini. J Biol Chem. 1993;269:7390–7396. [PubMed: 8125957]
- 98.
- Westenbroek RE, Hell JW, Warner C. et al. Biochemical properties and subcellular distribution of an N-type calcium channel α1 subunit. Neuron. 1992;9:1099–1115. [PubMed: 1334419]
- 99.
- Liu H, De WaardM, Scott VES. et al. Identification of three subunits of the high affinity ω- conotoxin MVIIC-sensitive Ca2+ channel. J Biol Chem. 1996;271:13804–13810. [PubMed: 8662888]
- 100.
- Martin-Moutot N, Charvin N, Leveque C. et al. Interaction of SNARE complexes with P/Q-type calcium channels in rat cerebellar synaptosomes. J Biol Chem. 1996;271:6567–6570. [PubMed: 8636067]
- 101.
- Martin-Moutot N, Leveque C, Sato K. et al. Properties of omega conotoxin MVIIC receptors associated with α1A calcium channel subunits in rat brain. FEBS Lett. 1995;366:21–25. [PubMed: 7789508]
- 102.
- Letts VA, Felix R, Biddlecome GH. et al. The mouse stargazer gene encodes a neuronal Ca2+- channel γ subunit. Nature Genet. 1998;19:340–347. [PubMed: 9697694]
- 103.
- Mori Y, Friedrich T, Kim M-S. et al. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. [PubMed: 1849233]
- 104.
- Starr TVB, Prystay W, Snutch TP. Primary structure of a calcium channel that is highly expressed in the rat cerebellum. Proc Natl Acad Sci USA. 1991;88:5621–5625. [PMC free article: PMC51929] [PubMed: 1648226]
- 105.
- Sakurai T, Hell JW, Woppmann A. et al. Immunochemical identification and differential phosphorylation of alternatively spliced forms of the α1A subunit of brain calcium channels. J Biol Chem. 1995;270:21234–21242. [PubMed: 7673157]
Publication Details
Author Information and Affiliations
Authors
William A. Catterall*.Affiliations
Notes
Copyright
Publisher
Landes Bioscience, Austin (TX)
NLM Citation
Catterall WA. Biochemical Studies of Voltage-Gated Ca2+ Channels. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.