NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-.

PDQ Cancer Information Summaries [Internet].
Show detailsThis PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the genetics of endocrine and neuroendocrine neoplasias. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
This summary is reviewed regularly and updated as necessary by the PDQ Cancer Genetics Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Introduction
[Note: Many of the medical and scientific terms used in this summary are found in the NCI Dictionary of Genetics Terms. When a linked term is clicked, the definition will appear in a separate window.]
[Note: Many of the genes described in this summary are found in the Online Mendelian Inheritance in Man (OMIM) database. When OMIM appears after a gene name or the name of a condition, click on OMIM for a link to more information.]
There are several hereditary syndromes that involve endocrine or neuroendocrine glands, such as multiple endocrine neoplasia type 1 (MEN1), multiple endocrine neoplasia type 2 (MEN2), pheochromocytoma, paraganglioma (PGL), Li-Fraumeni syndrome, familial adenomatous polyposis, and von Hippel-Lindau syndrome. This summary currently focuses on MEN1, MEN2, familial PGL syndrome, and Carney-Stratakis syndrome. Li-Fraumeni syndrome, familial adenomatous polyposis, Cowden syndrome, and von Hippel-Lindau syndrome are discussed in the PDQ summaries on Genetics of Breast and Gynecologic Cancers; Genetics of Colorectal Cancer; and Genetics of Kidney Cancer.
The term multiple endocrine neoplasia is used to describe a group of heritable tumors of endocrine tissues that may be benign or malignant. They are typically classified into two main categories: MEN1 and MEN2. The tumors usually manifest themselves by overproduction of hormones, tumor growth, or both.
Comprising varying combinations of more than 20 endocrine and nonendocrine tumors, MEN1 may be a difficult syndrome to define clinically. In general, however, MEN1 is characterized by tumors of the parathyroids, pancreas, and pituitary gland. This syndrome may also include carcinoid tumors, adrenocortical tumors, and nonendocrine tumors, such as facial angiofibromas, collagenomas, lipomas, meningiomas, ependymomas, and leiomyomas.
MEN1 syndrome, also known as Wermer syndrome, results from a mutation in the MEN1 gene. It has a prevalence of about 1 in 30,000 individuals.[1]
MEN2 syndromes are caused by a mutation in the RET proto-oncogene. Historically, MEN2 has been further stratified into the following three subtypes based on the presence or absence of certain endocrine tumors in the individual or family:
All three subtypes of MEN2 (MEN2A, FMTC, and MEN2B) impart a high risk of developing medullary thyroid cancer (MTC). MEN2A has an increased risk of pheochromocytoma and parathyroid adenoma and/or hyperplasia. MEN2B has an increased risk of pheochromocytoma and includes additional clinical features, such as mucosal neuromas of the lips and tongue, distinctive faces with enlarged lips, ganglioneuromatosis of the gastrointestinal tract, and an asthenic Marfanoid body habitus. FMTC has been defined as the presence of at least four individuals with MTC without any other signs or symptoms of pheochromocytoma or hyperparathyroidism in the proband or other family members.[2]
Some families previously classified as having FMTC will go on to develop one or more of the MEN2A-related tumors, suggesting that FMTC is simply a milder variant of MEN2A. Offspring of affected individuals have a 50% chance of inheriting the RET gene mutation.
The age at onset of MTC is different for each subtype of MEN2. MTC typically occurs during early childhood in patients with MEN2B, predominantly during early adulthood in patients with MEN2A, and during middle-age in patients with FMTC.
Germline DNA–based testing of the RET gene (chromosomal region 10q11.2) identifies disease-causing mutations in more than 95% of individuals with MEN2A and MEN2B and in about 88% of individuals with FMTC.[3]
The prevalence of MEN2 has been estimated to be between 1 in 30,000 [4,5] and 1 in 35,000 individuals.[6] The vast majority of MEN2 cases are MEN2A. In the United States, an estimated 468 cases of MEN2-related MTC are diagnosed per year.[7]
PGLs and pheochromocytomas are rare tumors arising from chromaffin cells, which have the ability to synthesize, store, and secrete catecholamines and neuropeptides. In 2004, the World Health Organization characterized pheochromocytomas as tumors arising in the adrenal gland.[6] PGLs may occur sporadically, as a manifestations of a hereditary syndrome, or as the sole tumor in hereditary PGL/pheochromocytoma syndrome.
References
- Agarwal SK, Ozawa A, Mateo CM, et al.: The MEN1 gene and pituitary tumours. Horm Res 71 (Suppl 2): 131-8, 2009. [PMC free article: PMC6413329] [PubMed: 19407509]
- Eng C: Seminars in medicine of the Beth Israel Hospital, Boston. The RET proto-oncogene in multiple endocrine neoplasia type 2 and Hirschsprung's disease. N Engl J Med 335 (13): 943-51, 1996. [PubMed: 8782503]
- Brandi ML, Gagel RF, Angeli A, et al.: Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86 (12): 5658-71, 2001. [PubMed: 11739416]
- Mulligan LM, Kwok JB, Healey CS, et al.: Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363 (6428): 458-60, 1993. [PubMed: 8099202]
- Donis-Keller H, Dou S, Chi D, et al.: Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet 2 (7): 851-6, 1993. [PubMed: 8103403]
- DeLellis RA, Lloyd RV, Heitz PU, et al., eds.: Pathology and Genetics of Tumours of Endocrine Organs. Lyon, France: IARC Press, 2004. World Health Organization classification of tumours, vol. 8.
- American Cancer Society: Cancer Facts and Figures 2015. Atlanta, Ga: American Cancer Society, 2015. Available online. Last accessed July 1, 2015.
Multiple Endocrine Neoplasia Type 1
Clinical Description
Multiple endocrine neoplasia type 1 (MEN1) (OMIM) is an autosomal dominant syndrome, with an estimated incidence in the general population of 1 to 2 cases per 100,000.[1] The major endocrine features of MEN1 include the following:
A diagnosis of MEN1 is made when an individual has two of these three major endocrine tumors. Familial MEN1 is defined as at least one MEN1 case plus at least one first-degree relative with one of these three tumors.[2-4] The age-related penetrance of MEN1 is 45% at age 30 years, 82% at age 50 years, and 96% at age 70 years.[2,5]
Parathyroid Tumors and PHPT
The most common features and often the first presenting signs of MEN1 are parathyroid tumors, which result in PHPT. These tumors occur in 80% to 100% of patients by age 50 years.[2,6-8] Unlike the solitary adenoma seen in sporadic cases, MEN1-associated parathyroid tumors are typically multiglandular and often hyperplastic.[1] The average age at onset of PHPT in MEN1 is 20 to 25 years, in contrast to that in the general population, which is typically age 50 to 59 years. Parathyroid carcinoma in MEN1 is rare but has been described.[9-12]
Individuals with MEN1-associated PHPT will have elevated parathyroid hormone (PTH) and calcium levels in the blood. The clinical manifestations of PHPT are mainly the result of hypercalcemia. Mild hypercalcemia may go undetected and have few or no symptoms. More severe hypercalcemia can result in the following:
- Constipation.
- Nausea and vomiting.
- Dehydration
- Decreased appetite and abdominal pain.
- Anorexia.
- Diuresis.
- Kidney stones.
- Increased bone resorption with resultant increased risk of bone fracture.
- Lethargy.
- Depression.
- Confusion.
- Hypertension.
- Shortened QT interval.
Since MEN1-associated hypercalcemia is directly related to the presence of parathyroid tumors, surgical removal of these tumors may result in normalization of calcium and PTH levels and relief of symptoms; however, high recurrence rates following surgery have been reported in some series.[13-15] (Refer to the Interventions section of this summary for more information.)
Duodenopancreatic NETs
Duodenopancreatic NETs are the second most common endocrine manifestation in MEN1, occurring in 30% to 80% of patients by age 40 years.[2,8] Gastrinomas represent 50% of the gastrointestinal NETs in MEN1 and are the major cause of morbidity and mortality in MEN1 patients.[2,13] Gastrinomas are usually multicentric, with small (<0.5 cm) foci throughout the duodenum.[16] Most result in peptic ulcer disease (Zollinger-Ellison syndrome), and half are malignant at the time of diagnosis.[13,16,17]
Other functioning pancreatic NETs seen in MEN1 include the following:
- Insulinomas (10%–20% penetrance).
- Vasoactive intestinal peptide tumors (VIPomas) (~1% penetrance).
- Glucagonomas (1%–5% penetrance).
- Somatostatinomas (~1% penetrance).
Nonfunctioning pancreatic NETs were originally thought to be relatively uncommon tumors in individuals with MEN1, with early penetrance estimates of 20%.[18,19] With the advent of genetic testing and improved imaging techniques, however, their prevalence in MEN1 has increased, with one study showing a frequency as high as 55% by age 39 years in MEN1 mutation carriers undergoing prospective endoscopic ultrasound of the pancreas.[20,21] These tumors can be metastatic. One study of 108 MEN1 mutation carriers with nonfunctioning pancreatic NETs showed a positive correlation between tumor size and rate of metastasis and death, with tumors larger than 2 cm having significantly higher rates of metastasis than those smaller than 2 cm.[22] (Refer to the Molecular Genetics of MEN1 section of this summary for more information about MEN1 gene mutations.)
Pituitary Tumors
Approximately 15% to 50% of MEN1 patients will develop a pituitary tumor.[2,8] Two-thirds are microadenomas (<1.0 cm in diameter), and the majority are prolactin-secreting.[23] Other functioning pituitary tumors can include somatotropinomas and corticotropinomas.
Other MEN1-associated Tumors
Other manifestations of MEN1 include carcinoids of the foregut (5%–10% of MEN1 patients). These are typically bronchial or thymic and are sometimes gastric. Skin lesions are also common and can include facial angiofibromas (up to 80% of MEN1 patients) and collagenomas (~75% of MEN1 patients).[24] Lipomas (~30% of MEN1 patients) and adrenal cortical lesions (up to 50% of MEN1 patients), including cortical adenomas, diffuse or nodular hyperplasia, or rarely, carcinoma are also common.[25-27] The following manifestations have also been reported:[28-30]
- Thyroid adenomas.
- Pheochromocytoma.
- Spinal ependymoma.
- Meningioma.
- Leiomyoma (e.g., esophageal, lung, and uterine).
Making the Diagnosis of MEN1
MEN1 is often difficult to diagnose in the absence of a significant family history or a positive genetic test for a mutation in the MEN1 gene. One study of 560 individuals with MEN1 showed a significant delay between the time of the first presenting symptom and the diagnosis of MEN1.[31] This may be because some presenting symptoms of MEN1-associated tumors, such as amenorrhea, peptic ulcers, hypoglycemia, and nephrolithiasis, are not specific to MEN1.
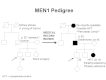
Furthermore, identification of an MEN1-associated tumor is not sufficient to make the clinical diagnosis of MEN1 and may not trigger a referral to an endocrinologist. Other studies have shown similar findings, with median time between the first presenting symptom and diagnosis of MEN1 ranging from 7.6 years to 12 years.[5,26] Genetic testing alleviates some of this delay. Several studies have shown statistically significant differences in the age at MEN1 diagnosis between probands and their family members. In one study, clinically symptomatic probands were diagnosed with MEN1 at a mean age of 47.5 years (standard deviation [SD] +/- 13.5 years), while family members were diagnosed at a mean age of 38.5 years (SD +/- 15.4 years; P < .001).[31] In another study of 154 individuals with MEN1, probands were diagnosed at a mean age of 39.5 years (range: 18–74 years), compared with a mean age 27 years (range: 14–56 years; P < .05) in family members diagnosed by predictive genetic testing.[32] These findings underscore the importance of increased awareness of the signs and symptoms of MEN1-related tumors and the constellation of findings necessary to suspect the diagnosis. It also highlights the importance of genetic counseling and testing and communication among family members once a diagnosis of MEN1 is made. Figure 1 illustrates some of the challenges in identifying MEN1 in a family.
Since many of the tumors in MEN1 are underdiagnosed or misdiagnosed, identifying an MEN1 gene mutation in the proband early in the disease process can allow for early detection and treatment of tumors and earlier identification of at-risk family members. Many studies have been performed to determine the prevalence of MEN1 gene mutations among patients with apparently sporadic MEN1-related tumors. For example, approximately one-third of patients with Zollinger-Ellison syndrome will carry an MEN1 mutation.[33,34] In individuals with apparently isolated PHPT or pituitary adenomas, the mutation prevalence is lower, on the order of 2% to 5%,[23,35,36] but the prevalence is higher in individuals diagnosed with these tumors before age 30 years. Some authors suggest referral for genetics consultation and/or genetic testing for mutations in MEN1 if one of the following conditions is present:[37-39]
- Gastrinoma at any age in the individual or an FDR.
- Multifocal duodenopancreatic NETs at any age.
- PHPT before age 30 or 40 years.
- Multiglandular parathyroid adenomas/hyperplasia or recurrent PHPT.
- Presence of one of the three main MEN1 tumors plus one of the less common tumors/findings.
- Presence of two or more features (e.g., adrenal adenomas and carcinoid tumor).
- Combination of at least two of the following in one individual: parathyroid adenoma; thymic, bronchial, or foregut carcinoid tumor; pancreatic NET; pituitary tumor; adrenal tumor.
- Parathyroid adenoma and a family history of hyperparathyroidism, pituitary adenoma, pancreatic NET, or foregut carcinoid tumor.
- Multiple primary pancreatic NETs in the same person.
Molecular Genetics of MEN1
The MEN1 gene is located on chromosome 11q13 and encodes the protein menin.[3,40,41] Over 1,300 mutations have been identified in the MEN1 gene to date, and these are scattered across the entire coding region.[42] The majority (~65%) of these are nonsense or frameshift mutations. The remainder are missense mutations (20%), which lead to expression of an altered protein, splice-site mutations (9%), or partial- or whole-gene deletions (1%–4%). There is currently no evidence of genotype-phenotype correlations, and inter- and intra-familial variability is common.[43,44]
Genetic Testing and Differential Diagnosis
Genetic testing for MEN1 mutations is recommended for individuals meeting clinical diagnostic criteria and may be considered in a subset of the less common tumors. (Refer to the bulleted list in the Making the diagnosis of MEN1 section of this summary for more information.) For individuals meeting diagnostic criteria, the mutation detection rate is approximately 75% to 90% [43,45] but may be lower in simplex cases.[46] Individuals with isolated parathyroid and/or pituitary tumors are less likely to have an identifiable mutation than those with pancreatic tumors.[46] The majority of commercial laboratories currently offering MEN1 testing use DNA sequencing as their primary method.[47] Several offer additional analysis for partial- or whole-gene deletion and/or duplication, although such mutations are rare and deletion/duplication testing is often reserved for individuals or families in which there is a very high clinical suspicion.
Genetic testing for MEN1 mutations can be used to distinguish between MEN1 and other forms of hereditary hyperparathyroidism, such as familial isolated hyperparathyroidism (FIHP) (OMIM), hyperparathyroidism–jaw tumor syndrome (HPT-JT), and familial hypocalciuric hypercalcemia (FHH). [Note: The hyperparathyroidism in FHH is not primary hyperparathyroidism, which is seen in MEN1, HPT-JT and FIHP.] HPT-JT, which is caused by germline mutations in the HRPT2 gene, is associated with PHPT, ossifying lesions of the maxilla and mandible, and renal lesions, usually bilateral renal cysts, hamartomas, and in some cases, Wilms tumor.[48,49] Unlike MEN1, HPT-JT is associated with an increased risk of parathyroid carcinoma.[50] FIHP, as its name suggests, is characterized by isolated PHPT with no additional endocrine features; in some families, FIHP is the initial diagnosis of what later develops into MEN1, HPT-JT, or FHH.[51-53] Approximately 20% of families with a clinical diagnosis of FIHP carry germline MEN1 mutations.[52,54,55] Mutations in the calcium-sensing receptor (CaSR) gene cause FHH, which can closely mimic the hyperparathyroidism in MEN1. Distinguishing between MEN1 and FHH can be critical in terms of management, as removal of the parathyroid glands in FHH does not correct the patient’s hyperparathyroidism and results in unnecessary surgery without relief of symptoms.[56] Given the differential risks and management of these conditions and the increased risk of parathyroid carcinoma in HPT-JT, genetic diagnosis in a patient presenting with early-onset hyperparathyroidism may play an important role in the management of these patients and their families.[57] Refer to Table 1 for a summary of the clinical features of MEN1 and other forms of hereditary hyperparathyroidism.
Table 1. Major Clinical Features of MEN1, FIHP, HPT-JT, and FHH
| Condition | Gene(s) | Major Clinical Features |
|---|---|---|
| MEN1 | MEN1 | PHPT, pituitary adenomas, pancreatic NETs [1] |
| FIHP | MEN1, HRPT2 | PHPT [51-55] |
| HPT-JT | HRPT2 | PHPT; osteomas of maxilla and mandible; renal cysts or hamartomas; and rarely, Wilms tumor and parathyroid carcinoma [48-50] |
| FHH | CaSR | Hyperparathyroidism (not primary) [56,58] |
CaSR = calcium-sensing receptor gene; FHH = familial hypocalciuric hypercalcemia; FIHP = familial isolated hyperparathyroidism; HPT-JT = hyperparathyroidism–jaw tumor syndrome; HRPT2 = hyperparathyroidism 2 gene; MEN1 = multiple endocrine neoplasia type 1 (gene is italicized); NETs = neuroendocrine tumors; PHPT = primary hyperparathyroidism.
Surveillance
Screening and surveillance for MEN1 may employ a combination of biochemical tests and imaging. Available recommendations are summarized in Table 2.[4,37]
Table 2. Practice Guidelines for Surveillance of Multiple Endocrine Neoplasia Type 1 (MEN1)a
| Biochemical Test or Procedure | Condition Screened For | Age Screening Initiated (y) | Frequency |
|---|---|---|---|
| Serum prolactin and/or insulin-like growth factor 1 | Pituitary tumors | 5 | Every 1 y |
| Fasting total serum calcium and/or ionized calcium and PTH | Parathyroid tumors and PHPT | 8 | Every 1 y |
| Fasting serum gastrin | Duodenopancreatic gastrinoma | 20 | Every 1 y |
| Chromogranin A, pancreatic polypeptide, glucagon, and vasointestinal polypeptide | Pancreatic NETs | <10 | Every 1 y |
| Fasting glucose and insulin | Insulinoma | 5 | Every 1 y |
| Brain MRI | Pituitary tumors | 5 | Every 3–5 y based on biochemical results |
| Abdominal CT or MRIb [4] | Pancreatic NETs | 20 | Every 3–5 y based on biochemical results |
| Abdominal CT, MRI, or endoscopic USb [37] | Pancreatic NETs | <10 | Every 1 y |
CT = computed tomography; MRI = magnetic resonance imaging; NETs = neuroendocrine tumors; PHPT = primary hyperparathyroidism; PTH = parathyroid hormone; US = ultrasound.
bThe recommendations for abdominal imaging differ between two published guidelines for the diagnosis and management of MEN1.[4,37] There is weak evidence at this time to support annual imaging before age 10 years. Imaging before age 10 years does identify disease in a high proportion of patients, but it is not clear whether this impacts prognosis.[20,59]
Interventions
Surgical management of MEN1 is complex and controversial, given the multifocal and multiglandular nature of the disease and the high risk of tumor recurrence even after surgery. Establishing the diagnosis of MEN1 prior to making surgical decisions and referring affected individuals to a surgeon with experience in treating MEN1 can be critical in preventing unnecessary surgeries or inappropriate surgical approaches.
Parathyroid tumors
Once evidence of parathyroid disease is established biochemically, the recommended course of action is surgical removal of the parathyroid glands. The timing and the extent of surgery, however, remain controversial.[60] Preoperative genetic testing helps guide the extent of surgery and can increase the likelihood of successful initial surgery and lower the likelihood of recurrent disease if a mutation is detected.[57] Some groups reserve surgical intervention for symptomatic patients, with continued annual biochemical screening for those who are asymptomatic. Once surgery is necessary, subtotal parathyroidectomy (removal of 3–3.5 glands) is often suggested as the initial treatment.[57] However, the rate of recurrence is quite high (55%–66%), and reoperation is often necessary.[13-15,57] Total parathyroidectomy with autotransplantation of parathyroid tissue to the forearm is also an option. A benefit of this approach is the easier removal of recurrent disease from the forearm than from the neck. Although the likelihood of recurrence is lowered by more extensive surgery, this must be weighed against the risk of rendering the patient hypoparathyroid.[61,62] Studies showing that concomitant bilateral cervical thymectomy decreases the rate of recurrence suggest that the thymus be removed at the initial operation.[61] If the devastating complication of hypocalcemia occurs, management requires oral calcitriol and calcium supplementation. This daily drug dependence can be a major burden on patients.
Duodenopancreatic NETs
The role of surgery for pancreatic NETs in MEN1 is controversial, given postoperative morbidity, long-term complications, and low cure rates. The timing and extent of surgery depend on many factors, including severity of symptoms, extent of disease, type and location of tumor, risk of metastasis, and patient preference. Long-acting somatostatin analogues may have a role in early-stage MEN1 duodenopancreatic NETs. Initial study results suggest that the treatment is safe and that long-term suppression of tumor and hormonal activity can be seen in up to 10% of patients and stability of hormone hyperfunction in 80% of patients.[63] The primary goal of surgery is to improve long-term survival by reducing symptoms associated with hormone excess and lowering the risk of distant metastasis.[17] Surgery is commonly performed for most functional tumors and for nonfunctioning NETs when the tumor exceeds 2 cm, as the likelihood of distant metastases is high.[64] While more extensive surgical approaches (e.g., pancreatoduodenectomy) have been associated with higher cure rates and improved overall survival,[65-67] they also have higher rates of postoperative complications and long-term morbidity.[68] Therefore, the risks and benefits should be carefully considered, and surgical decisions should be made on a case-by-case basis.
Individuals with MEN1 who are diagnosed with NETs often have multiple tumors of various types throughout the pancreas and duodenum, some of which can be identified using magnetic resonance imaging or computed tomography. Many tumors, however, are too small to be detected using standard imaging techniques, and intra-arterial secretin stimulation testing and/or intraoperative ultrasound may be useful.[69,70] Preoperative assessment using various biochemical and imaging modalities, intraoperative assessment of tumor burden, and resolution of hormonal hyper-secretion are critical and, in some series, have been associated with higher cure rates and longer disease-free intervals.[69-71]
In the current era of effective treatment for hyperfunctional hormone excess states, most MEN1-related deaths are due to the malignant nature of pancreatic NETs. A less common but important risk of death is from malignant thymic carcinoid tumors. Indicators of a poor MEN1 prognosis include elevated fasting serum gastrin, the presence of functional hormonal syndromes, liver or distant metastases, aggressive pancreatic NET growth, large pancreatic NET size, or the need for multiple parathyroidectomies. The most common cause of non-MEN1–related death in this patient cohort is from cardiovascular disease.[72]
Insulinomas
Medical management of insulinoma using diet and medication is often unsuccessful; the mainstay of treatment for this tumor is surgery.[37] Insulinomas in MEN1 patients can be located throughout the pancreas, with a preponderance found in the distal gland,[73-75] and have a higher rate of metastasis than sporadic insulinoma.[76] Surgery can range from enucleation of single or multiple large tumors to partial pancreatic resection, or both,[74] to subtotal or total pancreatectomy.[73,74] More extensive surgical approaches are associated with a lower rate of recurrence [65,66,74] but a higher rate of postoperative morbidity. Because insulinoma often occurs in conjunction with nonfunctioning pancreatic tumors, the selective intra-arterial calcium-injection test (SAS test) may be necessary to determine the source of insulin excess.[77] Intraoperative monitoring of insulin/glucose can help determine whether insulin-secreting tumors have been successfully excised.[70,78]
Gastrinomas
The majority of MEN1-associated gastrinomas originate in the duodenum. These tumors are typically multifocal and cause hyper-secretion of gastrin, with resultant peptic ulcer disease (Zollinger-Ellison syndrome). The multifocal nature makes complete surgical resection difficult. It is critical to manage symptoms prior to considering any type of surgical intervention. Historically, some groups have recommended close observation of individuals with smaller tumors (<2.0 cm on imaging) who have relief of symptoms using medications (e.g., proton pump inhibitors or histamine-2 agonists);[79] however, this approach may not be optimal for all patients.
Several published series have shown a positive correlation between primary tumor size and rate of distant metastasis. One retrospective study showed that 61% of patients with tumors larger than 3 cm had liver metastases.[17] In another series, 40% of patients with tumors larger than 3 cm had liver metastases.[80] In contrast, both of these series showed significantly lower rates of liver metastases in individuals with tumors smaller than 3 cm (32% and 4.8%, respectively). On the basis of these and other data, many groups recommend surgery in individuals with nonmetastatic gastrinoma who have tumors larger than 2 cm.[37,67]
The type of surgery for gastrinoma depends on many factors. A Whipple procedure is typically discouraged as an initial surgery, given the high postoperative morbidity and long-term complications, such as diabetes mellitus and malabsorption. Less extensive surgeries have been described with varying results. At a minimum, duodenectomy with intraoperative palpation and/or ultrasound to locate and excise duodenal tumors and peri-pancreatic lymph node dissection are performed.[69,81] Because most patients with gastrinoma will have concomitant NETs throughout the pancreas, some of which may be nonfunctional, some groups recommend resection of the distal pancreas and enucleation of tumors in the pancreatic head in addition to duodenal tumor excision.[69,81,82]
Nonfunctioning NETs
Approximately 50% of individuals with MEN1 will develop nonfunctioning NETs.[20,22] These are often identified incidentally during assessment and exploration for functioning tumors. As with gastrinomas, the metastatic rate is correlated with larger tumor size;[22] the presence of metastatic disease has been associated with earlier age at death than in those without pancreatic NETs.[22,83]
Other pancreatic NETs
Glucagonomas, VIPomas, and somatostatinomas are rare but often have higher rates of malignancy than other pancreatic NETs.[84] These are often treated with aggressive surgery.[76]
Pituitary tumors
Medical therapy to suppress hypersecretion is often the first line of therapy for MEN1-associated pituitary tumors. In one series of 136 patients, medical therapy was successful in approximately one-half of patients with secreting tumors (49 of 116, 42%), and successful suppression was correlated with smaller tumor size.[85] Surgery is often necessary for patients who are resistant to this treatment. Radiation therapy is reserved for patients with incomplete surgical resection.[37,86]
References
- Chandrasekharappa SC, Teh BT: Clinical and molecular aspects of multiple endocrine neoplasia type 1. Front Horm Res 28: 50-80, 2001. [PubMed: 11443853]
- Trump D, Farren B, Wooding C, et al.: Clinical studies of multiple endocrine neoplasia type 1 (MEN1) QJM 89 (9): 653-69, 1996. [PubMed: 8917740]
- Chandrasekharappa SC, Guru SC, Manickam P, et al.: Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science 276 (5311): 404-7, 1997. [PubMed: 9103196]
- Brandi ML, Gagel RF, Angeli A, et al.: Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86 (12): 5658-71, 2001. [PubMed: 11739416]
- Carty SE, Helm AK, Amico JA, et al.: The variable penetrance and spectrum of manifestations of multiple endocrine neoplasia type 1. Surgery 124 (6): 1106-13; discussion 1113-4, 1998. [PubMed: 9854591]
- Benson L, Ljunghall S, Akerström G, et al.: Hyperparathyroidism presenting as the first lesion in multiple endocrine neoplasia type 1. Am J Med 82 (4): 731-7, 1987. [PubMed: 2882676]
- Brandi ML, Marx SJ, Aurbach GD, et al.: Familial multiple endocrine neoplasia type I: a new look at pathophysiology. Endocr Rev 8 (4): 391-405, 1987. [PubMed: 2891500]
- Thakker RV: Multiple endocrine neoplasia type 1. In: DeGroot LJ, Besser M, Burger HG, eds.: Endocrinology. Volume 3. 3rd ed. Philadelphia, Pa: WB Saunders Co, 1995, pp 2815-31.
- del Pozo C, García-Pascual L, Balsells M, et al.: Parathyroid carcinoma in multiple endocrine neoplasia type 1. Case report and review of the literature. Hormones (Athens) 10 (4): 326-31, 2011 Oct-Dec. [PubMed: 22281890]
- Agha A, Carpenter R, Bhattacharya S, et al.: Parathyroid carcinoma in multiple endocrine neoplasia type 1 (MEN1) syndrome: two case reports of an unrecognised entity. J Endocrinol Invest 30 (2): 145-9, 2007. [PubMed: 17392605]
- Shih RY, Fackler S, Maturo S, et al.: Parathyroid carcinoma in multiple endocrine neoplasia type 1 with a classic germline mutation. Endocr Pract 15 (6): 567-72, 2009 Sep-Oct. [PubMed: 19491073]
- Sato M, Miyauchi A, Namihira H, et al.: A newly recognized germline mutation of MEN1 gene identified in a patient with parathyroid adenoma and carcinoma. Endocrine 12 (3): 223-6, 2000. [PubMed: 10963041]
- Norton JA, Venzon DJ, Berna MJ, et al.: Prospective study of surgery for primary hyperparathyroidism (HPT) in multiple endocrine neoplasia-type 1 and Zollinger-Ellison syndrome: long-term outcome of a more virulent form of HPT. Ann Surg 247 (3): 501-10, 2008. [PMC free article: PMC2717476] [PubMed: 18376196]
- Hellman P, Skogseid B, Oberg K, et al.: Primary and reoperative parathyroid operations in hyperparathyroidism of multiple endocrine neoplasia type 1. Surgery 124 (6): 993-9, 1998. [PubMed: 9854574]
- Schreinemakers JM, Pieterman CR, Scholten A, et al.: The optimal surgical treatment for primary hyperparathyroidism in MEN1 patients: a systematic review. World J Surg 35 (9): 1993-2005, 2011. [PubMed: 21713580]
- Pipeleers-Marichal M, Somers G, Willems G, et al.: Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med 322 (11): 723-7, 1990. [PubMed: 1968616]
- Weber HC, Venzon DJ, Lin JT, et al.: Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology 108 (6): 1637-49, 1995. [PubMed: 7768367]
- Marx SJ: Hyperparathyroid and hypoparathyroid disorders. N Engl J Med 343 (25): 1863-75, 2000. [PubMed: 11117980]
- Skogseid B, Rastad J, Oberg K: Multiple endocrine neoplasia type 1. Clinical features and screening. Endocrinol Metab Clin North Am 23 (1): 1-18, 1994. [PubMed: 7913018]
- Thomas-Marques L, Murat A, Delemer B, et al.: Prospective endoscopic ultrasonographic evaluation of the frequency of nonfunctioning pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. Am J Gastroenterol 101 (2): 266-73, 2006. [PubMed: 16454829]
- Tonelli F, Giudici F, Fratini G, et al.: Pancreatic endocrine tumors in multiple endocrine neoplasia type 1 syndrome: review of literature. Endocr Pract 17 (Suppl 3): 33-40, 2011 Jul-Aug. [PubMed: 21550956]
- Triponez F, Dosseh D, Goudet P, et al.: Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg 243 (2): 265-72, 2006. [PMC free article: PMC1448903] [PubMed: 16432361]
- Corbetta S, Pizzocaro A, Peracchi M, et al.: Multiple endocrine neoplasia type 1 in patients with recognized pituitary tumours of different types. Clin Endocrinol (Oxf) 47 (5): 507-12, 1997. [PubMed: 9425388]
- Darling TN, Skarulis MC, Steinberg SM, et al.: Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch Dermatol 133 (7): 853-7, 1997. [PubMed: 9236523]
- Machens A, Schaaf L, Karges W, et al.: Age-related penetrance of endocrine tumours in multiple endocrine neoplasia type 1 (MEN1): a multicentre study of 258 gene carriers. Clin Endocrinol (Oxf) 67 (4): 613-22, 2007. [PubMed: 17590169]
- Pieterman CR, Schreinemakers JM, Koppeschaar HP, et al.: Multiple endocrine neoplasia type 1 (MEN1): its manifestations and effect of genetic screening on clinical outcome. Clin Endocrinol (Oxf) 70 (4): 575-81, 2009. [PubMed: 18616711]
- Waldmann J, Bartsch DK, Kann PH, et al.: Adrenal involvement in multiple endocrine neoplasia type 1: results of 7 years prospective screening. Langenbecks Arch Surg 392 (4): 437-43, 2007. [PubMed: 17235589]
- Gibril F, Schumann M, Pace A, et al.: Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore) 83 (1): 43-83, 2004. [PubMed: 14747767]
- McKeeby JL, Li X, Zhuang Z, et al.: Multiple leiomyomas of the esophagus, lung, and uterus in multiple endocrine neoplasia type 1. Am J Pathol 159 (3): 1121-7, 2001. [PMC free article: PMC1850469] [PubMed: 11549605]
- Vortmeyer AO, Lubensky IA, Skarulis M, et al.: Multiple endocrine neoplasia type 1: atypical presentation, clinical course, and genetic analysis of multiple tumors. Mod Pathol 12 (9): 919-24, 1999. [PubMed: 10496602]
- Yamazaki M, Suzuki S, Kosugi S, et al.: Delay in the diagnosis of multiple endocrine neoplasia type 1: typical symptoms are frequently overlooked. Endocr J 59 (9): 797-807, 2012. [PubMed: 22673601]
- Lourenço DM Jr, Toledo RA, Coutinho FL, et al.: The impact of clinical and genetic screenings on the management of the multiple endocrine neoplasia type 1. Clinics (Sao Paulo) 62 (4): 465-76, 2007. [PubMed: 17823710]
- Roy PK, Venzon DJ, Shojamanesh H, et al.: Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore) 79 (6): 379-411, 2000. [PubMed: 11144036]
- Bardram L, Stage JG: Frequency of endocrine disorders in patients with the Zollinger-Ellison syndrome. Scand J Gastroenterol 20 (2): 233-8, 1985. [PubMed: 3887554]
- Uchino S, Noguchi S, Sato M, et al.: Screening of the Men1 gene and discovery of germ-line and somatic mutations in apparently sporadic parathyroid tumors. Cancer Res 60 (19): 5553-7, 2000. [PubMed: 11034102]
- Scheithauer BW, Laws ER Jr, Kovacs K, et al.: Pituitary adenomas of the multiple endocrine neoplasia type I syndrome. Semin Diagn Pathol 4 (3): 205-11, 1987. [PubMed: 2890193]
- Thakker RV, Newey PJ, Walls GV, et al.: Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab 97 (9): 2990-3011, 2012. [PubMed: 22723327]
- Newey PJ, Thakker RV: Role of multiple endocrine neoplasia type 1 mutational analysis in clinical practice. Endocr Pract 17 (Suppl 3): 8-17, 2011 Jul-Aug. [PubMed: 21454234]
- Hampel H, Bennett RL, Buchanan A, et al.: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17 (1): 70-87, 2015. [PubMed: 25394175]
- Larsson C, Skogseid B, Oberg K, et al.: Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature 332 (6159): 85-7, 1988. [PubMed: 2894610]
- Bassett JH, Forbes SA, Pannett AA, et al.: Characterization of mutations in patients with multiple endocrine neoplasia type 1. Am J Hum Genet 62 (2): 232-44, 1998. [PMC free article: PMC1376903] [PubMed: 9463336]
- Lemos MC, Thakker RV: Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat 29 (1): 22-32, 2008. [PubMed: 17879353]
- Giraud S, Zhang CX, Serova-Sinilnikova O, et al.: Germ-line mutation analysis in patients with multiple endocrine neoplasia type 1 and related disorders. Am J Hum Genet 63 (2): 455-67, 1998. [PMC free article: PMC1377295] [PubMed: 9683585]
- Wautot V, Vercherat C, Lespinasse J, et al.: Germline mutation profile of MEN1 in multiple endocrine neoplasia type 1: search for correlation between phenotype and the functional domains of the MEN1 protein. Hum Mutat 20 (1): 35-47, 2002. [PubMed: 12112656]
- Agarwal SK, Kester MB, Debelenko LV, et al.: Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. Hum Mol Genet 6 (7): 1169-75, 1997. [PubMed: 9215689]
- Klein RD, Salih S, Bessoni J, et al.: Clinical testing for multiple endocrine neoplasia type 1 in a DNA diagnostic laboratory. Genet Med 7 (2): 131-8, 2005. [PubMed: 15714081]
- GeneTests: Medical Genetics Information Resource [Database]. Seattle, WA: University of Washington, 2010. Available online. Last accessed October 16, 2013.
- Teh BT, Farnebo F, Kristoffersson U, et al.: Autosomal dominant primary hyperparathyroidism and jaw tumor syndrome associated with renal hamartomas and cystic kidney disease: linkage to 1q21-q32 and loss of the wild type allele in renal hamartomas. J Clin Endocrinol Metab 81 (12): 4204-11, 1996. [PubMed: 8954016]
- Carpten JD, Robbins CM, Villablanca A, et al.: HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet 32 (4): 676-80, 2002. [PubMed: 12434154]
- Marx SJ: Multiple endocrine neoplasia type 1. In: Vogelstein B, Kinzler KW, eds.: The Genetic Basis of Human Cancer. New York, NY: McGraw-Hill, 1998, pp 489-506.
- Warner J, Epstein M, Sweet A, et al.: Genetic testing in familial isolated hyperparathyroidism: unexpected results and their implications. J Med Genet 41 (3): 155-60, 2004. [PMC free article: PMC1735699] [PubMed: 14985373]
- Mizusawa N, Uchino S, Iwata T, et al.: Genetic analyses in patients with familial isolated hyperparathyroidism and hyperparathyroidism-jaw tumour syndrome. Clin Endocrinol (Oxf) 65 (1): 9-16, 2006. [PubMed: 16817812]
- Cetani F, Pardi E, Borsari S, et al.: Molecular pathogenesis of primary hyperparathyroidism. J Endocrinol Invest 34 (7 Suppl): 35-9, 2011. [PubMed: 21985978]
- Miedlich S, Lohmann T, Schneyer U, et al.: Familial isolated primary hyperparathyroidism--a multiple endocrine neoplasia type 1 variant? Eur J Endocrinol 145 (2): 155-60, 2001. [PubMed: 11454510]
- Cetani F, Pardi E, Ambrogini E, et al.: Genetic analyses in familial isolated hyperparathyroidism: implication for clinical assessment and surgical management. Clin Endocrinol (Oxf) 64 (2): 146-52, 2006. [PubMed: 16430712]
- Raue F, Frank-Raue K: Primary hyperparathyroidism--what the nephrologist should know--an update. Nephrol Dial Transplant 22 (3): 696-9, 2007. [PubMed: 17138574]
- Romero Arenas MA, Morris LF, Rich TA, et al.: Preoperative multiple endocrine neoplasia type 1 diagnosis improves the surgical outcomes of pediatric patients with primary hyperparathyroidism. J Pediatr Surg 49 (4): 546-50, 2014. [PubMed: 24726110]
- Christensen SE, Nissen PH, Vestergaard P, et al.: Familial hypocalciuric hypercalcaemia: a review. Curr Opin Endocrinol Diabetes Obes 18 (6): 359-70, 2011. [PubMed: 21986511]
- Langer P, Kann PH, Fendrich V, et al.: Prospective evaluation of imaging procedures for the detection of pancreaticoduodenal endocrine tumors in patients with multiple endocrine neoplasia type 1. World J Surg 28 (12): 1317-22, 2004. [PubMed: 15517479]
- Hubbard JG, Sebag F, Maweja S, et al.: Primary hyperparathyroidism in MEN 1--how radical should surgery be? Langenbecks Arch Surg 386 (8): 553-7, 2002. [PubMed: 11914930]
- Pieterman CR, van Hulsteijn LT, den Heijer M, et al.: Primary hyperparathyroidism in MEN1 patients: a cohort study with longterm follow-up on preferred surgical procedure and the relation with genotype. Ann Surg 255 (6): 1171-8, 2012. [PubMed: 22470073]
- Lairmore TC, Govednik CM, Quinn CE, et al.: A randomized, prospective trial of operative treatments for hyperparathyroidism in patients with multiple endocrine neoplasia type 1. Surgery 156 (6): 1326-34; discussion 1334-5, 2014. [PubMed: 25262224]
- Ramundo V, Del Prete M, Marotta V, et al.: Impact of long-acting octreotide in patients with early-stage MEN1-related duodeno-pancreatic neuroendocrine tumours. Clin Endocrinol (Oxf) 80 (6): 850-5, 2014. [PubMed: 24443791]
- Triponez F, Goudet P, Dosseh D, et al.: Is surgery beneficial for MEN1 patients with small (< or = 2 cm), nonfunctioning pancreaticoduodenal endocrine tumor? An analysis of 65 patients from the GTE. World J Surg 30 (5): 654-62; discussion 663-4, 2006. [PubMed: 16680582]
- Bartsch DK, Langer P, Wild A, et al.: Pancreaticoduodenal endocrine tumors in multiple endocrine neoplasia type 1: surgery or surveillance? Surgery 128 (6): 958-66, 2000. [PubMed: 11114630]
- Bartsch DK, Fendrich V, Langer P, et al.: Outcome of duodenopancreatic resections in patients with multiple endocrine neoplasia type 1. Ann Surg 242 (6): 757-64, discussion 764-6, 2005. [PMC free article: PMC1409888] [PubMed: 16327485]
- Norton JA, Jensen RT: Role of surgery in Zollinger-Ellison syndrome. J Am Coll Surg 205 (4 Suppl): S34-7, 2007. [PubMed: 17916516]
- Lopez CL, Waldmann J, Fendrich V, et al.: Long-term results of surgery for pancreatic neuroendocrine neoplasms in patients with MEN1. Langenbecks Arch Surg 396 (8): 1187-96, 2011. [PubMed: 21805182]
- Imamura M, Komoto I, Ota S, et al.: Biochemically curative surgery for gastrinoma in multiple endocrine neoplasia type 1 patients. World J Gastroenterol 17 (10): 1343-53, 2011. [PMC free article: PMC3068271] [PubMed: 21455335]
- Tonelli F, Fratini G, Nesi G, et al.: Pancreatectomy in multiple endocrine neoplasia type 1-related gastrinomas and pancreatic endocrine neoplasias. Ann Surg 244 (1): 61-70, 2006. [PMC free article: PMC1570585] [PubMed: 16794390]
- Lewis MA, Thompson GB, Young WF Jr: Preoperative assessment of the pancreas in multiple endocrine neoplasia type 1. World J Surg 36 (6): 1375-81, 2012. [PubMed: 22382771]
- Ito T, Igarashi H, Uehara H, et al.: Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study: comparison of 106 MEN1/Zollinger-Ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine (Baltimore) 92 (3): 135-81, 2013. [PMC free article: PMC3727638] [PubMed: 23645327]
- O'Riordain DS, O'Brien T, van Heerden JA, et al.: Surgical management of insulinoma associated with multiple endocrine neoplasia type I. World J Surg 18 (4): 488-93; discussion 493-4, 1994 Jul-Aug. [PubMed: 7725733]
- Crippa S, Zerbi A, Boninsegna L, et al.: Surgical management of insulinomas: short- and long-term outcomes after enucleations and pancreatic resections. Arch Surg 147 (3): 261-6, 2012. [PubMed: 22430908]
- Sakurai A, Yamazaki M, Suzuki S, et al.: Clinical features of insulinoma in patients with multiple endocrine neoplasia type 1: analysis of the database of the MEN Consortium of Japan. Endocr J 59 (10): 859-66, 2012. [PubMed: 22785103]
- Akerström G, Stålberg P: Surgical management of MEN-1 and -2: state of the art. Surg Clin North Am 89 (5): 1047-68, 2009. [PubMed: 19836484]
- Grant CS: Insulinoma. Best Pract Res Clin Gastroenterol 19 (5): 783-98, 2005. [PubMed: 16253900]
- Giudici F, Nesi G, Brandi ML, et al.: Surgical management of insulinomas in multiple endocrine neoplasia type 1. Pancreas 41 (4): 547-53, 2012. [PubMed: 22228047]
- Mignon M, Cadiot G: Diagnostic and therapeutic criteria in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. J Intern Med 243 (6): 489-94, 1998. [PubMed: 9681847]
- Cadiot G, Vuagnat A, Doukhan I, et al.: Prognostic factors in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. Groupe d'Etude des Néoplasies Endocriniennes Multiples (GENEM and groupe de Recherche et d'Etude du Syndrome de Zollinger-Ellison (GRESZE). Gastroenterology 116 (2): 286-93, 1999. [PubMed: 9922308]
- Dickson PV, Rich TA, Xing Y, et al.: Achieving eugastrinemia in MEN1 patients: both duodenal inspection and formal lymph node dissection are important. Surgery 150 (6): 1143-52, 2011. [PubMed: 22136834]
- Akerström G, Stålberg P, Hellman P: Surgical management of pancreatico-duodenal tumors in multiple endocrine neoplasia syndrome type 1. Clinics (Sao Paulo) 67 (Suppl 1): 173-8, 2012. [PMC free article: PMC3328819] [PubMed: 22584725]
- Goudet P, Murat A, Binquet C, et al.: Risk factors and causes of death in MEN1 disease. A GTE (Groupe d'Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg 34 (2): 249-55, 2010. [PubMed: 19949948]
- Lévy-Bohbot N, Merle C, Goudet P, et al.: Prevalence, characteristics and prognosis of MEN 1-associated glucagonomas, VIPomas, and somatostatinomas: study from the GTE (Groupe des Tumeurs Endocrines) registry. Gastroenterol Clin Biol 28 (11): 1075-81, 2004. [PubMed: 15657529]
- Vergès B, Boureille F, Goudet P, et al.: Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab 87 (2): 457-65, 2002. [PubMed: 11836268]
- Pieterman CR, Vriens MR, Dreijerink KM, et al.: Care for patients with multiple endocrine neoplasia type 1: the current evidence base. Fam Cancer 10 (1): 157-71, 2011. [PubMed: 21061174]
Multiple Endocrine Neoplasia Type 2
Clinical Description
The endocrine disorders observed in multiple endocrine neoplasia type 2 (MEN2) are medullary thyroid cancer (MTC); its precursor, C-cell hyperplasia (CCH); pheochromocytoma; and parathyroid adenomas and/or hyperplasia. MEN2-associated MTC is often bilateral and/or multifocal and arises in the background of CCH. In contrast, sporadic MTC is typically unilateral and/or unifocal. Since approximately 75% to 80% of sporadic cases also have associated CCH, this histopathologic feature cannot be used as a predictor of familial disease.[1] Metastatic spread of MTC to regional lymph nodes (i.e., parathyroid, paratracheal, jugular chain, and upper mediastinum) or to distant sites, such as the liver, is common in patients who present with a palpable thyroid mass or diarrhea.[2,3] Although pheochromocytomas rarely metastasize, they can be clinically significant in cases of intractable hypertension or anesthesia-induced hypertensive crises. Parathyroid abnormalities in MEN2 can range from benign parathyroid adenomas or multigland hyperplasia to clinically evident hyperparathyroidism with hypercalcemia and renal stones.
Historically, individuals and families with MEN2 were classified into one of the following three clinical subtypes based on the presence or absence of certain endocrine tumors in the individual or family:
Current stratification is moving away from a solely phenotype-based classification and more toward one that is based on genotype (i.e., the mutation) and phenotype.[4]
Clinical findings in the three MEN2 subtypes are summarized in Table 3. All three subtypes confer a high risk of MTC; MEN2A and MEN2B confer an increased risk of pheochromocytoma, and MEN2A has an increased risk of parathyroid hyperplasia and/or adenoma. Classifying a patient or family by MEN2 subtype is useful in determining prognosis and management.
Table 3. Percentage of Patients with Clinical Features of MEN2 by Subtype
| Subtype | Medullary Thyroid Carcinoma (%)a | Pheochromocytoma (%)a | Parathyroid Disease (%)a |
|---|---|---|---|
| MEN2A | 95 | 50 | 15–30 |
| FMTC | ~100 | 0 | 0 |
| MEN2B | 100 | 50 | Uncommon |
MTC and CCH
MTC originates in calcitonin-producing cells (C-cells) of the thyroid gland. MTC is diagnosed when nests of C-cells extend beyond the basement membrane and infiltrate and destroy thyroid follicles. CCH is diagnosed histologically by the presence of an increased number of diffusely scattered or clustered C-cells.[10,11] Individuals with RET (REarranged during Transfection) mutations and CCH are at substantially increased risk of progressing to MTC, although such progression is not universal.[12,13] MTC and CCH are suspected in the presence of an elevated plasma calcitonin concentration.
A study of 10,864 patients with nodular thyroid disease found 44 (1 of every 250) cases of MTC after stimulation with calcitonin, none of which were clinically suspected. Consequently, half of these patients had no evidence of MTC on fine-needle biopsy and thus might not have undergone surgery without the positive calcitonin stimulation test.[14] CCH associated with a positive calcitonin stimulation test occurs in about 5% of the general population; therefore, the plasma calcitonin responses to stimulation do not always distinguish CCH from small MTC and cannot always distinguish between carriers and noncarriers in an MEN2 family.[12,13,15]
MTC accounts for 2% to 3% of new cases of thyroid cancer diagnosed annually in the United States,[16] although this figure may be an underrepresentation of true incidence because of changes in diagnostic techniques. The total number of new cases of MTC diagnosed annually in the United States is between 1,000 and 1,200, about 75% of which are sporadic (i.e., they occur in the absence of a family history of either MTC or other endocrine abnormalities seen in MEN2). The peak incidence of the sporadic form is in the fifth and sixth decades of life.[2,17] A study in the United Kingdom estimated the incidence of MTC at 20 to 25 new cases per year among a population of 55 million.[7]
In the absence of a positive family history, MEN2 may be suspected when MTC occurs at an early age or is bilateral or multifocal. While small series of apparently sporadic MTC cases have suggested a higher prevalence of germline RET mutations,[18,19] larger series indicate a prevalence range of 1% to 7%.[20,21] Based on these data, it is widely recommended that RET gene mutation testing be performed for all cases of MTC.[22-25]
Level of evidence (Screening): 3
Natural history of MTC
Thyroid cancer represents approximately 3% of new malignancies occurring annually in the United States, with an estimated 62,450 cancer diagnoses and 1,950 cancer deaths per year.[26] Of these cancer diagnoses, 2% to 3% are MTC.[16,27]
MTC arises from the parafollicular calcitonin-secreting cells of the thyroid gland. MTC occurs in sporadic and familial forms and may be preceded by CCH, although CCH is a relatively common abnormality in middle-aged adults.[10,11]
Average survival for MTC is lower than that for more common thyroid cancers (e.g., 83% 5-year survival for MTC compared with 90% to 94% 5-year survival for papillary and follicular thyroid cancer).[27,28] Survival is correlated with stage at diagnosis, and decreased survival in MTC can be accounted for in part by a high proportion of late-stage diagnosis.[27-29]
In addition to early stage at diagnosis, other factors associated with improved survival in MTC include smaller tumor size, younger age at diagnosis, familial versus sporadic form, and diagnosis by biochemical screening (i.e., screening for calcitonin elevation) versus symptoms.[29-32]
A Surveillance, Epidemiology, and End Results population-based study of 1,252 MTC patients found that survival varied by extent of local disease. For example, the 10-year survival rates ranged from 95.6% for those with disease confined to the thyroid gland to 40% for those with distant metastases.[30]
Hereditary MTC
While the majority of MTC cases are sporadic, approximately 20% to 25% are hereditary because of mutations in the RET proto-oncogene.[33-35] Mutations in the RET gene cause MEN2, an autosomal dominant disorder associated with a high lifetime risk of MTC. Multiple endocrine neoplasia type 1 (MEN1) (OMIM) is an autosomal dominant endocrinopathy that is genetically and clinically distinct from MEN2; however, the similar nomenclature for MEN1 and MEN2 may cause confusion. There is no increased risk of thyroid cancer for MEN1. (Refer to the MEN1 section of this summary for more information.)
Pheochromocytoma
Pheochromocytomas (OMIM) arise from the catecholamine-producing chromaffin cells of the adrenal medulla. They are a relatively rare tumor and are suspected among patients with refractory hypertension or when biochemical screening reveals elevated excretion of catecholamines and catecholamine metabolites (i.e., norepinephrine, epinephrine, metanephrine, and vanillylmandelic acid) in 24-hour urine collections or plasma. In the past, measurement of urinary catecholamines was considered the preferred biochemical screening method. However, given that catecholamines are only released intermittently and are metabolized in the adrenal medulla into metanephrine and normetanephrine, the measurement of urine or plasma fractionated metanephrines has become the gold standard.[36-41] When biochemical screening in an individual who has or is at risk of MEN2 suggests pheochromocytoma, localization studies, such as magnetic resonance imaging (MRI) or computed tomography, can be performed.[42] Confirmation of the diagnosis can be made using I131-metaiodobenzylguanidine scintigraphy or positron emission tomography imaging.[13,42-44]
A diagnosis of MEN2 is often considered in individuals with bilateral pheochromocytoma, those with an early age of onset (age <35 years), and those with a personal and/or family history of MTC or hyperparathyroidism. However, MEN2 is not the only genetic disorder that includes a predisposition to pheochromocytoma. Other disorders include neurofibromatosis type 1 (NF1), von Hippel-Lindau disease (VHL),[45] and the hereditary paraganglioma syndromes.[46] (Refer to the von Hippel-Lindau Syndrome section in the PDQ summary on the Genetics of Kidney Cancer for more information about VHL.) A large European consortium that included 271 patients from Germany,[47] 314 patients from France,[48] and 57 patients from Italy (total = 642) with apparently sporadic pheochromocytoma analyzed the known pheochromocytoma/functional paraganglioma susceptibility genes (NF1, RET, VHL, SDHB, and SDHD).[49] The diagnosis of NF1 in this series was made clinically, while all other conditions were diagnosed based on the presence of a germline mutation in the causative gene. The disease was associated with a positive family history in 166 (25.9%) patients; germline mutations were detected in RET (n = 31), VHL (n = 56), NF1 (n = 14), SDHB (n = 34), or SDHD (n = 31). Rigorous clinical evaluation and pedigree analysis either before or after testing revealed that of those with a positive family history and/or a syndromic presentation, 58.4% carried a mutation, compared with 12.7% who were nonsyndromic and/or had no family history. Of the 31 individuals with a germline RET mutation, 28 (90.3%) had a positive family history and/or syndromic presentation, suggesting that most individuals with RET mutations and pheochromocytoma will have a positive family history or other manifestations of the disease.
These data indicate that a significant proportion of individuals presenting with apparently sporadic pheochromocytoma are carriers of germline genetic mutations. Of those with apparently sporadic disease, up to 33% have a mutation in one of the susceptibility genes.[47,50-52] Studies have identified additional susceptibility genes that predispose to pheochromocytoma, including TMEM127, MAX, and SDHAF2.[53-56] Mutations in these genes are thought to account for a small proportion of all hereditary pheochromocytoma. Since testing for mutations in multiple genes in every patient may not be feasible or cost-effective, clinical and genetic screening algorithms have been proposed to assist clinicians in deciding which genes to test and in which order.[42,48,49,57-59]
Primary Hyperparathyroidism (PHPT)
PHPT is the third most common endocrine disorder in the general population. The incidence increases with age with the vast majority of cases occurring after the sixth decade of life. Approximately 80% of cases are the results of a single adenoma.[60] PHPT can also be seen as a component tumor in several different hereditary syndromes, including the following:
- MEN1.
- Hyperparathyroidism-Jaw Tumor syndrome.
- Familial Isolated Hyperparathyroidism.
Hereditary PHPT is typically multiglandular, presents earlier in life, and can have histologic evidence of both adenoma and glandular hyperplasia.
Clinical Diagnosis of MEN2 Subtypes
The diagnosis of the three MEN2 clinical subtypes relies on a combination of clinical findings, family history, and molecular genetic testing of the RET gene (chromosomal region 10q11.2).
MEN2A
MEN2A is diagnosed clinically by the occurrence of two or more specific endocrine tumors (MTC, pheochromocytoma, or parathyroid adenoma and/or hyperplasia) in a single individual or in close relatives.
The MEN2A subtype makes up about 60% to 90% of MEN2 cases. The MEN2A subtype was initially called Sipple syndrome.[64] Since genetic testing for RET mutations has become available, it has become apparent that about 95% of individuals with MEN2A will develop MTC; about 50% will develop pheochromocytoma; and about 15% to 30% will develop hyperparathyroidism.[13,65-67]
MTC is generally the first manifestation of MEN2A. In asymptomatic at-risk individuals, stimulation testing may reveal elevated plasma calcitonin levels and the presence of CCH or MTC.[13,66] In families with MEN2A, the biochemical manifestations of MTC generally appear between the ages of 5 and 25 years (mean 15 years).[13] If presymptomatic screening is not performed, MTC typically presents as a neck mass or neck pain at about age 5 to 20 years. More than 50% of such patients have cervical lymph node metastases.[2] Diarrhea, the most frequent systemic symptom, occurs in patients with a plasma calcitonin level of greater than 10 ng/mL and implies a poor prognosis.[2] Up to 30% of patients with MTC present with diarrhea and advanced disease.[68]
MEN2-associated pheochromocytomas are more often bilateral, multifocal, and associated with extratumoral medullary hyperplasia.[69-71] They also have an earlier age of onset and are less likely to be malignant than their sporadic counterparts.[69,72] MEN2-associated pheochromocytomas usually present after MTC, typically with intractable hypertension.[6]
Unlike the PHPT seen in MEN1, hyperparathyroidism in individuals with MEN2 is typically asymptomatic or associated with only mild elevations in calcium.[68,73] A series of 56 patients with MEN2-related hyperparathyroidism has been reported by the French Calcitonin Tumors Study Group.[73] The median age at diagnosis was 38 years, documenting that this disorder is rarely the first manifestation of MEN2. This is in sharp contrast to MEN1, in which the vast majority of patients (87%–99%) initially present with primary hyperparathyroidism.[74-76] Parathyroid abnormalities were found concomitantly with surgery for medullary thyroid carcinoma in 43 patients (77%). Two-thirds of the patients were asymptomatic. Among the 53 parathyroid glands removed surgically, there were 24 single adenomas, four double adenomas, and 25 hyperplastic glands.
A small number of families with MEN2A have pruritic skin lesions known as cutaneous lichen amyloidosis. This lichenoid skin lesion is located over the upper portion of the back and may appear before the onset of MTC.[77,78]
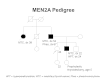
Figure 2 depicts some of the classic manifestations of MEN2A in a family.
In a child, the presence of oral and ocular neuromas and/or a tall and lanky appearance may warrant further investigation.[79] Some authors have recommended referral to genetic counseling for an individual with medullary thyroid cancer or any of the following features:[79,80]
- Benign oral and submucosal neuromas.
- Elongated face and large lips.
- Ganglioneuromatosis.
- Inability to cry tears (biologic mechanism unknown).
Familial medullary thyroid carcinoma (FMTC)
The FMTC subtype makes up 5% to 35% of MEN2 cases and is defined as families with four or more cases of MTC in the absence of pheochromocytoma or parathyroid adenoma/hyperplasia.[65] Families with two or three cases of MTC and incompletely documented screening for pheochromocytoma and parathyroid disease may actually represent MEN2A; it has been suggested that these families should be considered unclassified.[7,81] Misclassification of families with MEN2A as having FMTC (because of too-small family size or later onset of other manifestations of MEN2A) may result in overlooking the risk of pheochromocytoma, a disease with significant morbidity and mortality. For this reason, there is debate about whether FMTC represents a separate entity or is a variation of MEN2A in which there is a lack of or delay in the onset of the other (nonthyroidal) manifestations of the MEN2A syndrome.[82] Some authors recommended,[25] therefore, that patients thought to have pure FMTC also be screened for pheochromocytoma and hyperparathyroidism. (Refer to the Screening of at-risk individuals for pheochromocytoma and Screening of at-risk individuals for hyperparathyroidism sections of this summary for more information.)
MEN2B
MEN2B is diagnosed clinically by the presence of mucosal neuromas of the lips and tongue, medullated corneal nerve fibers, distinctive facies with enlarged lips, an asthenic Marfanoid body habitus, and MTC.[83-85]
The MEN2B subtype makes up about 5% of MEN2 cases. The MEN2B subtype was initially called mucosal neuroma syndrome or Wagenmann-Froboese syndrome.[86] MEN2B is characterized by the early development of an aggressive form of MTC in all patients.[86,87] Patients with MEN2B who do not undergo thyroidectomy at an early age (at approximately age 1 year) are likely to develop metastatic MTC at an early age. Before intervention with early risk-reducing thyroidectomy, the average age at death in patients with MEN2B was 21 years. Pheochromocytomas occur in about 50% of MEN2B cases; about half are multiple and often bilateral. Clinically apparent parathyroid disease is very uncommon.[5,65,88] Patients with MEN2B may be identified in infancy or early childhood by a distinctive facial appearance and the presence of mucosal neuromas on the anterior dorsal surface of the tongue, palate, or pharynx. The lips become prominent over time, and submucosal nodules may be present on the vermilion border of the lips. Neuromas of the eyelids may cause thickening and eversion of the upper eyelid margins. Prominent thickened corneal nerves may be seen by slit lamp examination.
About 40% of patients have diffuse ganglioneuromatosis of the gastrointestinal tract. Associated symptoms include abdominal distension, megacolon, constipation, and diarrhea. About 75% of patients have a Marfanoid habitus, often with kyphoscoliosis or lordosis, joint laxity, and decreased subcutaneous fat. Proximal muscle wasting and weakness can also be seen.[84,85]
Genetically Related Disorder
Hirschsprung disease (HSCR)
HSCR (OMIM), a disorder of the enteric plexus of the colon that typically results in enlargement of the bowel and constipation or obstipation in neonates, is observed in a small number of individuals with MEN2A, FMTC, or very rarely, MEN2B.[89] Up to 40% of familial cases of HSCR and 3% to 7% of sporadic cases are associated with germline mutations in the RET proto-oncogene and are designated HSCR1.[90 ,91] Some of these RET mutations are located in codons that lead to the development of MEN2A or FMTC (i.e., codons 609, 618, and 620).[89,92]
In a study of 44 families, seven families (16%) had cosegregation of MEN2A and HSCR1. The probability that individuals in a family with MEN2A and an exon 10 Cys mutation would manifest HSCR1 was estimated to be 6% in one series.[90] Furthermore, in a multicenter international RET mutation consortium study, 6 of 62 kindreds carrying either the C618R or C620R mutation also had HSCR.[65]
A novel analytic approach employing family-based association studies coupled with comparative and functional genomic analysis revealed that a common RET variant within a conserved enhancer-like sequence in intron 1 makes a 20-fold greater contribution to HSCR compared with all known RET mutations.[93] This mutation has low penetrance and different genetic effects in males and females. Transmission to sons and daughters leads to a 5.7-fold and 2.1-fold increase in susceptibility, respectively. This finding is consistent with the greater incidence of HSCR in males. Demonstrating this strong relationship between a common noncoding mutation in RET and the risk of HSCR also accounts for previous failures to detect coding mutations in RET-linked families.
Molecular Genetics of MEN2
MEN2 syndromes are the result of inherited mutations in the RET gene, located on chromosome region 10q11.2.[94-96] The RET gene is a proto-oncogene composed of 21 exons over 55 kilobase of genomic material.[97,98]
RET encodes a receptor tyrosine kinase with extracellular, transmembrane, and intracellular domains. Details of RET receptor and ligand interaction in this signaling pathway have been reviewed.[99] Briefly, the extracellular domain consists of a calcium-binding cadherin-like region and a cysteine-rich region that interacts with one of four ligands identified to date. These ligands, e.g., glial cell line–derived neurotrophic factor (GDNF), neurturin, persephin, and artemin, also interact with one of four coreceptors in the GDNF-family receptor–alpha family.[99] The tyrosine kinase catalytic core is located in the intracellular domain, which causes downstream signaling events through a variety of second messenger molecules. Normal tissues contain transcripts of several lengths.[100-102]
Genetic testing
MEN2 is a well-defined hereditary cancer syndrome for which genetic testing is considered an important part of the management for at-risk family members. It meets the criteria related to indications for genetic testing for cancer susceptibility outlined by the American Society of Clinical Oncology in its most recent genetic testing policy statement.[103] At-risk individuals are defined as first-degree relatives (parents, siblings, and children) of a person known to have MEN2. Testing allows the identification of people with asymptomatic MEN2 who can be offered risk-reducing thyroidectomy and biochemical screening as preventive measures. A negative mutation analysis in at-risk relatives, however, is informative only after a disease-causing mutation has been identified in an affected relative. (Refer to the PDQ summary on Cancer Genetics Risk Assessment and Counseling for more information.) Because early detection of at-risk individuals affects medical management, testing of children who have no symptoms is considered beneficial.[103,104] (Refer to the Genotype-Phenotype Correlations and Risk Stratification section of this summary for more information about clinical management of at-risk individuals.)
Germline DNA testing for RET mutations is generally recommended to all individuals with a diagnosis of MTC, regardless of whether there is a personal or family history suggestive of MEN2.[23,105] Approximately 95% of patients with MEN2A or MEN2B will have an identifiable germline RET mutation.[65] For FMTC the detection rate is slightly lower at 88%.[65] Importantly, 1% to 7% of apparently sporadic cases of MTC will carry a germline RET mutation, underscoring the importance of testing all cases.[18-21]
There is no evidence for the involvement of other genetic loci, and all mutation-negative families analyzed to date have demonstrated linkage to the RET gene. For families that do not have a detectable mutation, clinical recommendations can be based on the clinical features in the affected individual and in the family.
There is considerable diversity in the techniques used and the approach to RET mutation testing among the various laboratories that perform this procedure. Methods used to detect mutations in RET include polymerase chain reaction (PCR) followed by restriction enzyme digestion of PCR products, heteroduplex analysis, single-stranded conformation polymorphism analysis, denaturing high-performance liquid chromatography, and DNA sequencing.[106-109] Most testing laboratories, at a minimum, offer testing using a targeted exon approach; that is, the laboratories look for mutations in the exons that are most commonly found to carry mutations (exons 10, 11, 13, 14, 15 and 16). Other laboratories offer testing for all exons. If targeted exon testing in a family with a high clinical suspicion for MEN2 is normal, sequencing of the remaining exons can then be performed.
These differences in mutation detection method and targeted versus full gene testing represent important considerations for selecting a laboratory to perform a test and in interpreting the test result. (Refer to the PDQ summary on Cancer Genetics Risk Assessment and Counseling for more information about clinical validity.)
Genotype-Phenotype Correlations and Risk Stratification
Genotype-phenotype correlations in MEN2 are well-established and have long been used to guide clinicians in making medical management recommendations. Several groups have developed mutation-stratification tables based on clinical phenotype, age of onset, and aggressiveness of MTC.[23,25,81] This classification strategy was first put forth after the Seventh International Workshop on MEN in 2001, which provided guidelines for the age of genetic testing and prophylactic thyroidectomy.[23] This stratification was revised by the American Thyroid Association (ATA).[25] The original classification scheme provided three levels of risk based on the genetic mutation of an individual. The new guidelines by the ATA added a fourth category for codon 634 mutations, in recognition of their aggressive clinical course. The specific mutations and their ATA classification are summarized in Table 4 and Table 5 below. The ATA's classification scheme has not been prospectively validated as a basis for clinical decision-making.
ATA-level D mutations are the most aggressive and carry the highest risk of developing MTC.[25] These mutations, which are typically seen in MEN2B, are associated with the youngest age at disease onset and the highest risk of mortality. ATA-level C mutations (codon 634) are associated with a slightly lower risk, yet the MTC in patients with these mutations is still quite aggressive and may present at an early age. ATA-level A and level B mutations are associated with a lower risk of aggressive MTC relative to the risk seen in level C and level D mutation carriers. However, the risk of MTC is still substantially elevated over the general population risk and consideration of risk-reducing thyroidectomy is warranted.[25]
A European multicenter study of 207 RET mutation carriers supported previous suggestions that some mutations are associated with early-onset disease. For example, this study found that individuals with the C634Y mutation developed MTC at a significantly younger age (mean 3.2 years; 95% confidence interval [CI], 1.2–5.4) than individuals with the C634R mutation (mean 6.9 years; 95% CI, 4.9–8.8). In the former group of patients, risk-reducing thyroidectomy warrants consideration before the age of 5 years. Although limited by small numbers, the same study did not support a need for risk-reducing thyroidectomy in asymptomatic carriers of mutations in codons 609, 630, 768, 790, 791, 804, or 891 before the age of 10 years or for central lymph node dissection before the age of 20 years.[110] Some authors suggest using these differences as the basis for decisions on the timing of risk-reducing thyroidectomy and the extent of surgery.[23] Others have advocated using basal and stimulated calcitonin levels as a basis for determining the appropriate timing of thyroidectomy.[111,112]
Mutations 883 and 918 have been seen only in MEN2B and are associated with the earliest age of onset and the most aggressive form of MTC.[113-117] Approximately 95% of individuals with MEN2B will have the M918T mutation.[113-115,118] As discussed above, 50% of individuals with MEN2B will develop pheochromocytoma but PHPT is rare. In addition to mutations at codons 883 and 918, some individuals with an MEN2B-like phenotype have been found to carry two germline mutations.[119-123] It is likely that as testing for RET becomes more common in clinical practice, additional double mutation phenotypes will be described.
Mutations at codon 634 (ATA-level C) are by far the most frequent finding in families with MEN2A. One study of 477 RET carriers showed that 52.1% had the C634R mutation, 26.0% carried the C634Y mutation, and 9.1% had the C634G mutation.[65] In general, mutations at codon 634 are associated with pheochromocytomas and PHPT.[65,124] Until recently, MEN2A with cutaneous lichen amyloidosis had been seen almost exclusively in patients with mutations at codon 634.[65,67,125] However, a recent report described MTC and cutaneous lichen amyloidosis in an individual previously thought to have FMTC due to a codon 804 mutation.[126] Codon 634 mutations have also been described in FMTC but are almost exclusively C634Y.[65]
In summary, ATA-level D and level C mutations confer the highest risk of MTC (about 95% lifetime risk) with a more aggressive disease course. There is an increased risk of pheochromocytoma (up to 50%).[65,127] Individuals with codon 634 mutations (but not codon 883 or 918 mutations) also have an increased risk of PHPT.[65]
ATA-level B mutations, located in exon 10 of the RET gene, include mutations at codons 609, 611, 618, 620, and 630. These mutations involve cysteine residues in the extracellular domain of the RET protein and have been seen in families with MEN2A and those with MTC only (FMTC).[20,65,81,128-132] The risk of MTC in individuals with ATA-level B mutations is approximately 95% to 100%; the risk of pheochromocytoma and hyperparathyroidism is lower than that seen in ATA-level A mutations. In a large series of 518 probands with MTC undergoing RET testing, most individuals with codon 609, 611, 618, 620, or 630 mutations had only MTC and no other features suggestive of MEN2. The authors attributed this to the relatively short follow-up time, incomplete screening of family members, or the method of ascertainment (population-based).[33] Another large study of 390 exon 10 mutation carriers showed an age-related risk of pheochromocytoma for individuals carrying any exon 10 mutation of 23.1% by age 50 years and 33% by age 60 years. Overall prevalence of pheochromocytoma was 17%. This study reported a 3.9% risk of developing hyperparathyroidism by age 60 years.[133]
Individuals with ATA-level A mutations have a lower, albeit still elevated, lifetime risk of MTC. MTC associated with these mutations tends to follow a more indolent course and have a later age at onset, although there are several reports of individuals with ATA-level A mutations who developed MTC before age 20 years.[65,134-138] Although pheochromocytoma and PHPT are not commonly associated with level A mutations, they have been described.[138]
Table 4. Genotype-Phenotype Correlations and American Thyroid Association (ATA) Risk Levelsa,b
| Mutation | ATA Risk Level | Medullary Thyroid Cancer | PHPT | Pheo | References |
|---|---|---|---|---|---|
| R321Gc | A | MA | [139] | ||
| A510V | Unknown | [140] | |||
| E511Kc | Unknown | [140] | |||
| 531/9 base pair duplication | A | MA | [141] | ||
| 532 duplicationc | A | Unknown | [142] | ||
| C515Sc | A | MA | [143] | ||
| C531Rc | MA | [140] | |||
| G533C | A | MA | R | [144-149] | |
| R600Qc | A | MI | [150] | ||
| K603Ec | A | MI | [151] | ||
| Y606Cc | A | Unknown | [152,153] | ||
| C609F/R/G/S/Y | B | MA | MI | R/MI | [34,65,127,133,154-158] |
| C611R/G/F/S/W/Y | B | MA | MI | R/MI | [65,127,133] |
| C618R/G/F/S/Y | B | MA | MI | MI | [65,127,133,159-162] |
| C620R/G/F/S/W/Y | B | MA | MI | MI | [65,127,133,155,161] |
| C630R/F/S/Y | B | MA | R | R | [121,163] |
| D631Y | B | MI | R | MA | [164-166] |
| 633/9 base pair duplication | B | MA | MI | [167] | |
| C634R | C | MA | MI | MA | [65,127,168,169] |
| C634G/F/S/W/Y | C | MA | MI | MA | [65,127,161,168-170] |
| C634Y/Y791F | MA | R | MA | [171] | |
| 634/12 base pair duplication | B | MA | MI | [172] | |
| 635/insertion ELCR;T636P | A | MA | [152] | ||
| S649L | A | MI | R | [34,173-175] | |
| K666E/Nc | A | MI | MI | [140,152,176] | |
| S686Nc | MI | [161] | |||
| E768D | A | MA | R | R | [65,121,164,177] |
| R770Qc | Unknown | [178] | |||
| N777Sc | A | MI | [179] | ||
| L790F | A | MA | R | R/MI | [164,180,181] |
| Y791F | A | MA | MI | MI | [164,180,182] |
| V804L | A | MA | MI | R | [65,180,183] |
| V804M | A | MA | R | R | [65,180,183-185] |
| V804M+V778Ic | B | MA | [186] | ||
| V804M+E805Kd | D | MA | MA | [119] | |
| V804M+Y806Kd | D | MA | MA | [120-122] | |
| V804M+S904Cc,d | D | MA | MI | [123] | |
| G819Kc | A | Unknown | [34] | ||
| R833Cc | A | Unknown | [187] | ||
| R844Qc | A | Unknown | [34,164] | ||
| L881Vc | Unknown | [178] | |||
| A883Fd | D | MA | MA | [116,117,188] | |
| R886Wc | A | MA | [189] | ||
| S891A | A | MA | MI | R | [34,190-193] |
| R912P | A | MI | MI | [34,194] | |
| M918Td | D | MA | MA | [65,161,195] |
Pheo = pheochromocytoma; PHPT = primary hyperparathyroidism.
MA = majority (>50%); MI = minority (10%–50%); R = rare (<10%).
aRefer to Table 5 for more information about the ATA risk levels.
bAdapted from Kloos et al.[25]
cAssociated with multiple endocrine neoplasia type 2 mutations.
dAssociated with mutations based on limited families/case reports and may represent variants of unknown significance.
In addition to the mutations categorized in Table 4, a number of rare or novel RET mutations have been described. Some of these represent mutations that lead to an FMTC or MEN2 phenotype. Others may represent low penetrance alleles or modifying alleles that confer only a modest risk of developing MTC. Still others may be benign polymorphisms of no clinical significance. Research is ongoing into the role of neutral RET sequence variants in modifying the clinical presentation of patients with MEN2A. The presence of certain RET polymorphisms is being analyzed for its impact on the likelihood for development of pheochromocytoma, hyperparathyroidism, and metastatic involvement with MTC.[196-198] A variety of approaches, including segregation analyses, in silico analyses, association studies, and functional assays, can be employed to determine the functional and clinical significance of a given genetic variant. A publicly available RET mutation online database repository was recently developed and includes a complete list of mutations and their associated pathogenicity, phenotype, and other associated clinical information and literature references.[199]
Interventions
Risk-reducing thyroidectomy
Risk-reducing thyroidectomy and parathyroidectomy with reimplantation of one or more parathyroid glands into the neck or nondominant forearm is a preventive option for all subtypes of MEN2. To implement this management strategy, biochemical screening to identify CCH and/or genetic testing to identify persons who carry causative RET mutations is needed to identify candidates for risk-reducing surgery (see below). The optimal timing of surgery, however, is controversial.[3] Current recommendations are based on clinical experience and vary for different MEN2 subtypes, as noted in Table 5.
In contrast, a prospective analysis of 84 carriers of the RET gene mutation found that basal and pentagastrin-stimulated calcitonin levels could be used to determine the timing of total thyroidectomy.[111] When the basal or stimulated calcitonin was greater than 10 pg/mL, total thyroidectomy and central neck dissection were strongly recommended. In this series, a basal calcitonin level lower than 60 pg/mL was always associated with an intrathyroidal MTC; none of the 56 patients who went to surgery had metastatic involvement. These findings suggest that surgery can be safely delayed in gene carriers of a RET mutation until basal or stimulated calcitonin is above 10 pg/mL, while still maintaining the ability to achieve a disease-free state (i.e., an undetectable basal and stimulated calcitonin 6–12 months after surgery). The benefits of this approach are particularly noteworthy in the younger population of gene carriers, as a delay in surgery until the patient is older may reduce the risk of surgical complications. While this approach is promising, pentagastrin is currently not available in the United States for stimulation testing. Although calcium may be used as a substitute for pentagastrin, it has not been widely validated.
One series of 503 at-risk individuals with ATA level A or B mutations (i.e. codons 533, 609, 611, 618, 620, 791, and 804) reported cumulative penetrance rates, median time to MTC, and predictive value of preoperative calcitonin.[112] The risk of developing MTC by age 50 years ranged from 18% to 95%, depending on the codon, with codon 620 having the highest penetrance. Most patients with MTC had node-negative disease, confirming the more indolent disease course that has been previously reported with these mutations. Although an elevated preoperative calcitonin level strongly predicted presence of MTC, relatively high false-negative rates (low normal calcitonin levels with MTC) were noted for many of the codons. This information is useful when counseling mutation carriers regarding surgical decisions.
Another study has confirmed that calcitonin levels could be a useful approach to determine the timing of thyroidectomy.[200] This study utilized preoperative basal calcitonin levels and ultrasound findings to determine timing of prophylactic thyroidectomy in 24 RET mutation carriers, many of whom carried mutations in the highest risk level and had delayed surgery until after age 20 years. All 17 individuals who underwent surgery had elevated preoperative calcitonin levels on the fully-automated chemiluminescence immunoassay. Fifteen of 17 individuals had MTC, but only two had lymph node involvement and/or local tissue invasion, and 16 of 17 were disease free at 22 months. Two patients had CCH. Of note, only 6 of 15 individuals with MTC had elevated calcitonin levels using the radioimmunoassay. The study is limited by a small population of patients with low disease burden but suggests that some calcitonin assays may be more sensitive than others.
Table 5. American Thyroid Association Medullary Thyroid Cancer Risk Stratification and Management Guidelinesa
| Risk level | Mutated Codon(s) | Age of RET Testing | Timing of Prophylactic Thyroidectomy |
|---|---|---|---|
| D | 883, 918, and compound heterozygotes V804M+E805K, V804M+Y806C, and V804M+S904C | ASAP and within the first y of life | ASAP and within the first y of life. |
| C | 634 | <3–5 y | Before age 5 y. |
| B | 609b, 611, 618, 620, 630b, and compound heterozygote V804M+V778I | <3–5 y | Consider surgery before age 5 y. May delay surgery after age 5 y if criteria are met.c |
| A | 768, 790, 791, 804, 891 | <3–5 y | May delay surgery after age 5 y if criteria are met.c |
aAdapted from Kloos et al.[25]
bThese mutations had not been reported at the time of the 7th International Workshop.[23]
cCriteria include a normal annual basal and/or stimulated serum count, normal annual neck ultrasound, less aggressive medullary thyroid cancer family history, and family preference.
In a study of biochemical screening in a large family with MEN2A performed before mutation analysis became available, 22 family members without evidence of clinical disease had elevated calcitonin and underwent thyroidectomy. During a mean follow-up period of 11 years, all remained free of clinical disease, and 3 out of 22 had transient elevation of postoperative calcitonin levels.[9] The use of biochemical screening is limited, however, by the lack of data on age-specific calcitonin levels in children younger than 3 years; caution should be used when interpreting these values in this age group.[25]
A study of 93 patients with MEN2 from a Dutch tumor registry documented the importance of early prophylactic thyroidectomy.[201] This group of patients represented all known Dutch patients with hereditary MTC; the majority of cases (67%) were codon 634 mutations; only 6% were MEN2B cases. Patients in this series were screened with either biochemical testing (pre-RET era) or RET mutation analysis. In both groups, patients underwent surgery at a later age than recommended by current guidelines (see Table 5), but the percentage from the pre-RET era was significantly higher (96% vs. 69%, P = .004). Older age at prophylactic thyroidectomy was significantly associated with a higher risk of postoperative persistent/recurrent disease. Although there is concern that young age at total thyroidectomy is associated with higher risk of surgical complications, this study found no such evidence.
Two additional case series provide further data supporting early risk-reducing thyroidectomy following testing for RET mutations.[202,203] Cases reported in both series could reflect selection biases: one study reported 71 patients from a national registry who had been treated with thyroidectomy but did not specify how these patients were selected, whereas the other study reported 21 patients seen at a referral center.[202,203] In both studies, a series of children from families with MEN2 or FMTC who were found to have RET mutations were screened for CCH and treated with risk-reducing thyroidectomy. These studies documented MTC in 93% of patients with MEN2 and 77% of patients with FMTC. The larger study found a correlation between age and larger tumor size, nodal metastases, postoperative recurrence of disease, and mean basal calcitonin levels. Surgical complications were rare.[202] No studies have compared the outcome of thyroidectomy based on mutation testing with thyroidectomy based on biochemical screening.
In one large series, 260 MEN2A subjects aged 0 to 20 years were identified as having undergone either an early total thyroidectomy (ages 1–5 years, n = 42), or late thyroidectomy (ages 6–20 years, n = 218).[204] There was a significantly lower rate of invasive or metastatic MTC among those who underwent surgery at an early age (57%) than among those who underwent surgery at a late age (76%). Follow-up information was available on only 28% of the cohort, as a result of the limitations of study design, with a median follow-up of only 2 years for this nonsystematically selected subgroup. Persistent or recurrent disease was reported among 0 of 9 early-surgery subjects, versus 21 of 65 late-surgery subjects. Both findings are consistent with the hypothesis that patients undergoing surgery before age 6 years have a more favorable outcome, but the nature of the data prevents this from being a definitive conclusion. Finally, there was evidence to suggest that subjects carrying codon 634 mutations were much more likely to present with invasive or metastatic MTC and to develop persistent or recurrent disease compared with those harboring mutations in codons 804, 618, or 620.
A study of young, clinically asymptomatic individuals with MEN2A sought to determine if early thyroidectomy could prevent or cure MTC.[205] This study included 50 consecutively identified RET mutation carriers who underwent thyroidectomy at 19 years or younger. Preoperative screening for CCH included basal and stimulated calcitonin levels and postoperative follow-up consisted of annual physical exam and intermittent basal and stimulated calcitonin measurements. All 50 individuals had at least 5 years of follow-up. Although MTC was identified in 33 of 50 patients at the time of surgery, in 44 of 50 (88%) there was no evidence of persistent or recurrent disease at a mean of 7 years follow-up. Six patients had basal or stimulated calcitonin abnormalities thought to represent residual MTC. None of the 22 patients operated on prior to age 8 years had any evidence of MTC. The data suggested that there was a lower incidence of persistent or recurrent disease in patients who had thyroidectomy earlier in life (defined as younger than 8 years) and who had no evidence of lymph node metastases.
Normal preoperative basal calcitonin does not exclude the possibility of the patient having MTC. In one study of 80 RET mutation carriers, 14 carriers had normal calcitonin tests and eight of these patients had small foci of MTC discovered at thyroidectomy.[13] Another study confirmed these findings,[86] as 14 children had total thyroidectomy based on positive genetic testing for MEN2; MTC was present in 11 and only four had elevated stimulated calcitonin levels prior to surgery. Although basal calcitonin levels may not be able to identify all patients with MTC preoperatively, this test has utility as a predictor of postoperative remission, lymph node metastases, and distant metastases.[206] In one study of 224 patients from a single institution, preoperative basal calcitonin levels greater than 500 pg/mL predicted failure to achieve biochemical remission.[206] The authors of this study found that nodal metastases started appearing at basal calcitonin levels of 40 pg/mL (normal, <10 pg/mL). In node-positive patients, distant metastases emerged at basal calcitonin levels of 150 pg/mL to 400 pg/mL. Using current sensitive calcitonin assays, a study of 308 RET carriers found that a normal basal preoperative calcitonin excluded the presence of lymph node metastases (100% negative predictive value).[207] Therefore, the preoperative basal calcitonin level is a useful prognostic indicator and may help guide the surgical approach.
While thyroidectomy prior to biochemical evidence of disease (normal preoperative calcitonin) may reduce the risk of recurrent disease, continued monitoring for residual or recurrent MTC is still recommended.[25,208] One study found that 10% of patients with MEN2A undergoing thyroidectomy developed recurrent disease, based on an initially undetectable basal and stimulated calcitonin levels (<2 pg/mL) that became positive 5 to 10 years after surgery.[205] Only 2% of patients had residual disease after prophylactic surgery as assessed by a persistently elevated basal or stimulated calcitonin.[205]
Questions remain concerning the natural history of MEN2. As more information is acquired, recommendations regarding the optimal age for thyroidectomy and the potential role for genetics and biochemical screening may change. For example, a case report documents MTC before age 5 years in two siblings with MEN2A.[209] Conversely, another case report documents onset of cancer in midlife or later in some families with FMTC and in elderly relatives who carry the FMTC genotype but have not developed cancer.[210] The possibility that certain mutations (e.g., Cys634) might convey a significantly worse prognosis, if confirmed, may permit tailoring intervention based on knowing the specific RET mutation.[204] These clinical observations suggest that the natural history of the MEN2 syndromes is variable and could be subject to modifying effects related to specific RET mutations, other genes, behavioral factors, or environmental exposures.
Screening of at-risk individuals for pheochromocytoma
The presence of a functioning pheochromocytoma should be excluded by appropriate biochemical screening before thyroidectomy in any patient with MEN2A or MEN2B. However, childhood pheochromocytomas are rare in MEN2.[25] The ATA has recommended that annual screening for pheochromocytoma be considered after the age of 8 years in patients with RET mutations in codons 630 and 634 and in patients with RET mutations associated with MEN2B.[25] In carriers of other MEN2A RET mutations, ATA recommends that annual screening begin by age 20 years. Patients with RET mutations associated only with FMTC should have periodic screening for pheochromocytoma beginning at age 20 years.[25] MRI or other imaging tests should be ordered only if the biochemical results are abnormal.[29,211] Studies of individuals with sporadic or hereditary pheochromocytoma (including, but not limited to, individuals with MEN2) have suggested that measurement of catecholamine metabolites, specifically plasma-free metanephrines and/or urinary fractionated metanephrines, provides a higher diagnostic sensitivity than urinary catecholamines because of the episodic nature of catecholamine excretion.[36-42,212] Several reviews provide a succinct summary of the biochemical diagnosis, localization, and management of pheochromocytoma.[42,213] In addition to surgery, there are other clinical situations in which patients with catecholamine excess face special risk. An example is the healthy at-risk female patient who becomes pregnant. Pregnancy, labor, or delivery may precipitate a hypertensive crisis in persons who carry an unrecognized pheochromocytoma. Pregnant patients who are found to have catecholamine excess require appropriate pharmacotherapy before delivery.
Screening of at-risk individuals for hyperparathyroidism
MEN2-related hyperparathyroidism is generally associated with mild, often asymptomatic hypercalcemia early in the natural history of the disease, which, if left untreated, may become symptomatic.[73] Childhood hyperparathyroidism is rare in MEN2. Three studies found the median age at diagnosis was about 38 years.[73,214,215] The ATA provides recommendations for annual screening for hyperparathyroidism.[25] Annual screening should begin at age 8 years in carriers of mutations in codons 630 and 634 and at age 20 years for carriers of other MEN2A RET mutations. Patients with mutations associated only with FMTC should have periodic testing after the age of 20 years. Testing should include albumin-corrected calcium or ionized serum calcium with or without intact parathyroid hormone (PTH) measurement.
Screening of at-risk individuals in kindreds without an identifiable RET mutation
MEN2A: Risk-reducing thyroidectomy is not routinely offered to at-risk individuals if the disorder is unconfirmed. The screening protocol for MTC is an annual calcitonin stimulation test; however, caution must be used in interpreting test results because CCH that is not a precursor to MTC occurs in about 5% of the population.[12,13,216] In addition, there is significant risk of false-negative test results in patients younger than 15 years.[13] Screening for pheochromocytoma and parathyroid disease is the same as described above.
FMTC: Annual screening for MTC, as for MEN2A.
Treatment for those with MTC
Standard treatment for adults with MTC is surgical removal of the entire thyroid gland, including the posterior capsule, and central lymph node dissection. Children with MEN2B having prophylactic thyroidectomy within the first year of life may not require central neck dissection unless there is radiological evidence of nodal disease.[25] Likewise, children with MEN2A or FMTC having prophylactic thyroidectomy before age 3 to 5 years should not have a central neck dissection in the absence of radiological evidence of metastatic lymph node involvement. The ATA also recommends that MEN2A and FMTC patients older than 5 years or asymptomatic MEN2B patients older than 1 year have a preoperative basal calcitonin test and neck ultrasound. A basal calcitonin level over 40 pg/mL or thyroid nodules greater than or equal to 5 mm requires further evaluation, as the patient may have more extensive disease requiring nodal dissection. If an MEN2B patient older than 1 year has nodules smaller than 5 mm or basal calcitonin lower than 40 pg/mL, then total thyroidectomy may be sufficient therapy, but the ATA task force favors prophylactic central neck dissection without lateral compartment dissection in the absence of radiographic evidence of metastatic involvement (level C recommendation).[25] See Table 6 for complete details.
Table 6. American Thyroid Association Management Guidelines for MEN2A/FMTC and MEN2Ba
| Syndrome | Age (y) | Nodal Disease | Basal Calcitonin (pg/mL)b | Nodule ≥ 5mm | Lymph Node Dissection | Strength of Recommendationc |
|---|---|---|---|---|---|---|
| MEN2A/FMTC | <3–5 | No | <40 | No | No | E |
| MEN2A/FMTC | <3–5 | Yes | >40 | Yes | Yes | B |
| MEN2A/FMTC | >5 | No | <40 | No | No | E |
| MEN2A/FMTC | >5 | Yes | >40 | Yes | Yes | B |
| MEN2B | <1 | No | <40 | No | No | E |
| MEN2B | <1 | Yes | >40 | Yes | Yes | B |
| MEN2B | <1 | No | <40 | No | Yes | C |
FMTC = familial medullary thyroid carcinoma; MEN2 = multiple endocrine neoplasia type 2.
aAdapted from Kloos et al.[25]
bBasal calcitonin values are applicable in patients older than 6 months.
cBased on grading definitions established by the U.S. Preventive Services Task Force.
The ATA recommends lymph node dissection for patients meeting any one of the following criteria:[25]
- Radiographic evidence of nodal disease.
- Basal calcitonin level greater than 40 pg/mL.
- A thyroid nodule 5 mm or larger.
Patients who have had total thyroidectomy will require lifelong thyroid hormone replacement therapy. The dosing of medication is age-dependent and treatment should be initiated based on ideal body weight. For healthy adults 60 years and younger with no cardiac disease, a reasonable starting dose is 1.6 to 1.8 µg/kg given once daily.[217] Older patients may require 20% to 30% less thyroid hormone.[218] Children clear T4 more rapidly than adults and consequently require relatively higher replacement by body weight. Depending on the age of the child, replacement should be between 2 to 6 µg/kg.[219] It is important to note, however, that patients should be given replacement, rather than suppressive therapy. Since C-cell tumors are not thyroid-stimulating hormone (TSH)-dependent for growth, the T4 therapy for MTC patients therefore should be adjusted to maintain a TSH within the normal reference range. Thyroglobulin measurement may also be useful for adjusting and maintaining TSH levels within a normal reference range to prevent additional regrowth of remnant thyroid tissue.[220] Further investigation is needed to better interpret how this information should guide management.
There is no difference in survival between familial and sporadic forms of MTC when adjusted for clinicopathologic factors. Chemotherapy and radiation are not effective against this type of cancer,[3,221,222] although clinical trials (phases I–III) of various targeted molecular therapies are ongoing at selected centers. Some of these compounds have shown partial responses in a small percentage of patients, but most studies have demonstrated disease stability as the most favorable response.[223-226] The use of vandetanib and cabozantinib is approved by the U.S. Food and Drug Administration for adult patients with progressive metastatic MTC who are ineligible for surgery. A phase III study found that progression-free survival was longer in adults who received vandetanib than in those who received placebo.[227] A phase I/II study of children with MEN2B found an objective partial response rate of 47% with vandetanib.[228] A double-blind, phase III trial that compared cabozantinib with placebo in 330 patients with progressive MTC showed an improvement in median progression-free survival across all subgroups.[229] To date, neither cabozantinib nor vandetanib has demonstrated improved overall survival.[227,229] Future studies will likely focus on the development of new targeted therapies and the use of combination therapy in MTC. (Refer to NCI's List of Clinical Trials for more information about these trials. Refer to the PDQ summary on Thyroid Cancer Treatment for more information about the treatment of thyroid cancer.)
Treatment for those with pheochromocytoma
Pheochromocytoma may be either unilateral or bilateral in patients with MEN2. Laparoscopic adrenalectomy is the recommended approach by some authorities for the treatment of unilateral pheochromocytoma.[23,25] Two studies examined the value of a posterior retroperitoneoscopic adrenalectomy and found that it was safe and effective, with zero mortality, associated with a low rate of minor complications, and required conversion to open or laparoscopic lateral surgery in only 1.7%.[230,231] This approach appears to be a feasible and safe alternative to open or laparoscopic surgery, but extensive experience is needed.
In one series, 23 patients with a unilateral pheochromocytoma and a macroscopically normal contralateral adrenal gland were treated initially with unilateral adrenalectomy.[232] A pheochromocytoma developed within the retained gland in 12 (52%) of these subjects, occurring a mean of 11.9 years after initial surgery. During follow-up subsequent to unilateral adrenalectomy, no patient experienced a hypertensive crisis or other problems attributable to an undiagnosed pheochromocytoma. In contrast, 10 of 43 patients (23%) treated with bilateral adrenalectomy experienced at least one episode of acute adrenal insufficiency; one of these patients died. Unilateral adrenalectomy appears to represent a reasonable management strategy for unilateral pheochromocytoma in patients with MEN2,[233-235] when coupled with periodic surveillance (serum or urinary catecholamine measurements) for the development of disease in the contralateral adrenal gland.
Synchronous or metachronous bilateral disease is quite common in hereditary pheochromocytoma. In one retrospective series that spanned almost 50 years, 96 patients underwent surgical resection for hereditary pheochromocytoma.[236] Forty-seven patients had bilateral pheochromocytoma and 49 had unilateral pheochromocytoma at presentation. Open and laparoscopic approaches were used, and the extent of initial resection varied. Of the 49 patients who presented with a unilateral pheochromocytoma and who underwent unilateral total adrenalectomy, 15 (30%) developed pheochromocytoma in the contralateral gland. This occurred at a median of 8.2 years (range, 1 to 20 years) after initial diagnosis. Of the 15 patients who developed pheochromocytoma in the contralateral gland, 8 had MEN2A, 2 had MEN2B, 2 had VHL, and 1 had familial pheochromocytoma. The risk of contralateral tumor development increased over time, with 25% of patients developing tumors after a median of 6 years and 43% after a median of 32 years.
This study also analyzed whether cortical-sparing adrenalectomy is a feasible option for patients with bilateral pheochromocytomas or only one viable adrenal gland.[236] Cortical-sparing surgery is an attractive option because it minimizes the risk of adrenal insufficiency and the need for lifelong steroid supplementation. In this same series of 96 patients, 50 underwent cortical-sparing surgery. Twenty-eight of the cortical-sparing surgeries were part of an initial bilateral procedure, 11 were for unilateral disease, and 11 were part of a subsequent procedure on the contralateral gland. There was a 7% recurrence rate after cortical-sparing surgery versus a 3% recurrence rate after total resection (recurrence in the adrenal bed). Interestingly, the rate of recurrence was significantly higher in patients who underwent a laparoscopic procedure (14%) than in patients who underwent an open procedure (6%). The rate of recurrence was also significantly higher in patients who underwent a laparoscopic total adrenalectomy versus an open procedure. The frequency of adrenal insufficiency was lower in patients who underwent cortical-sparing surgery. One of 39 patients (3%) developed adrenal insufficiency after a cortical-sparing procedure; 5 of 25 patients (20%) developed adrenal insufficiency after total adrenalectomy. In summary, cortical-sparing surgery is a viable option for patients with hereditary pheochromocytoma, but ongoing surveillance for new or recurrent disease is necessary, especially in patients who undergo a laparoscopic procedure.
Treatment for those with hyperparathyroidism
Most patients with MEN2-related parathyroid disease are either asymptomatic or diagnosed incidentally at the time of thyroidectomy. Typically, the hypercalcemia (when present) is mild, although it may be associated with increased urinary excretion of calcium and nephrolithiasis. As a consequence, the indications for surgical intervention are generally similar to those recommended for patients with sporadic, primary hyperparathyroidism.[23] In general, fewer than four of the parathyroid glands are involved at the time of detected abnormalities in calcium metabolism.
Cure of hyperparathyroidism was achieved surgically in 89% of one large series of patients;[73] however, 22% of resected patients in this study developed postoperative hypoparathyroidism. Five patients (9%) had recurrent hyperparathyroidism. This series employed various surgical techniques, including total parathyroidectomy with autotransplantation to the nondominant forearm, subtotal parathyroidectomy, and resection only of glands that were macroscopically enlarged. Postoperative hypoparathyroidism developed in 4 of 11 patients (36%), 6 of 12 patients (50%), and 3 of 29 patients (10%), respectively. These data indicate that excision of only those parathyroid glands that are enlarged appears to be sufficient in most cases.
Some investigators have suggested using the MEN2 subtype to decide where to place the parathyroid glands that are identified at the time of thyroid surgery. For patients with MEN2B in whom the risk of parathyroid disease is quite low, the parathyroid glands may be left in the neck. For patients with MEN2A and FMTC, it is suggested that the glands be implanted in the nondominant forearm to minimize the need for further surgery on the neck after risk-reducing thyroidectomy and a central lymph node dissection.[237]
All patients who have undergone parathyroid surgery with autotransplantation of parathyroid tissue should be monitored for hypoparathyroidism.[25,238,239]
Medical therapy of hyperparathyroidism has gained popularity with the advent of calcimimetics, agents that sensitize the calcium-sensing receptors on the parathyroid glands to circulating calcium levels and thereby reduce circulating PTH levels. In a randomized, double-blind, placebo-controlled trial, cinacalcet hydrochloride was shown to induce sustained reduction in circulating calcium and PTH levels in patients with primary hyperparathyroidism.[240] In patients who are high-risk surgical candidates, those with recurrent hyperparathyroidism, or those in whom life expectancy is limited, medical therapy may be a viable alternative to a surgical approach.
Genetic Counseling
Mode of inheritance
All of the MEN2 subtypes are inherited in an autosomal dominant manner. For the child of someone with MEN2, the risk of inheriting the MEN2 mutation is 50%. Some individuals with MEN2, however, carry a de novo gene mutation; that is, they carry a new mutation that was not present in previous generations of their family and thus do not have an affected parent. The proportion of individuals with MEN2 who have an affected parent varies by subtype.
MEN2A: About 95% of affected individuals have an affected parent. It is appropriate to evaluate the parents of an individual with MEN2A for manifestations of the disorder. In the 5% of cases that are not familial, either de novo gene mutations or incomplete penetrance of the mutant allele is possible.[241]
FMTC: Multiple family members are affected; therefore, all affected individuals inherited the mutant gene from a parent.
MEN2B: About 50% of affected individuals have de novo RET gene mutations, and 50% have inherited the mutation from a parent.[242,243] The majority of de novo mutations are paternal in origin, but cases of maternal origin have been reported.[244]
Siblings of a proband: The risk to siblings depends on the genetic status of the parent, which can be clarified by pedigree analysis and/or DNA-based testing. In situations of apparent de novo gene mutations, germline mosaicism in an apparently unaffected parent must be considered, even though such an occurrence has not yet been reported.
Attitudes toward preimplantation genetic diagnosis
One study explored the attitudes of individuals with MEN1 and MEN2 toward preimplantation genetic diagnosis.[245] Ninety-one clinic-based patients from a single U.S. institution who had MEN1 and an MEN1 mutation or MEN2 and a RET mutation were surveyed. The study found that 30% (10 of 33) of those with MEN1 and 37% (21 of 57) of those with MEN2 were aware of PGD; 82% (27 of 33) of those with MEN1 and 61% (34 of 56) of those with MEN2 thought PGD should be offered; and 61% (19 of 31) of those with MEN1 and 43% (23 of 54) of those with MEN2 would consider PGD.
Psychosocial issues
The psychosocial impact of genetic testing for mutations in RET has not been extensively studied. Published studies have had limitations such as small sample size and heterogeneous populations; thus, the clinical relevance of these findings should be interpreted with caution. Identification as the carrier of a deleterious mutation may affect self-esteem, family relationships, and quality of life.[246] In addition, misconceptions about genetic disease may result in familial blame and guilt.[247,248] Several review articles outline both the medical and psychological issues, especially those related to the testing of children.[249-252] The medical value of early screening and risk-reducing treatment are contrasted with the loss of decision-making autonomy for the individual. Lack of agreement between parents about the value and timing of genetic testing and surgery may spur the development of emotional problems within the family.
One study examined levels of psychological distress in the interval between submitting a blood sample and receiving genetic test results. Those individuals who experienced the highest level of distress were younger than 25 years, single, and had a history of responding to distressful situations with anxiety.[253] Mutation-positive parents whose children received negative test results did not seem to be reassured, questioned the reliability of the DNA test, and were eager to continue screening of their noncarrier children.[254]
A small qualitative study (N = 21) evaluated how patients with MEN2A and family members conceptualized participation in lifelong high-risk surveillance.[255] Ongoing surveillance was viewed as a reminder of a health threat. Acceptance and incorporation of lifelong surveillance into routine health care was essential for coping with the implications of this condition. Concern about genetic predisposition to cancer was peripheral to concerns about surveillance. Supportive interventions, such as Internet discussion forums, can serve as an ongoing means of addressing informational and support needs of patients with MTC undergoing lifelong surveillance.[256]
References
- Kaserer K, Scheuba C, Neuhold N, et al.: Sporadic versus familial medullary thyroid microcarcinoma: a histopathologic study of 50 consecutive patients. Am J Surg Pathol 25 (10): 1245-51, 2001. [PubMed: 11688458]
- Robbins J, Merino MJ, Boice JD Jr, et al.: Thyroid cancer: a lethal endocrine neoplasm. Ann Intern Med 115 (2): 133-47, 1991. [PubMed: 2058861]
- Moley JF, Debenedetti MK, Dilley WG, et al.: Surgical management of patients with persistent or recurrent medullary thyroid cancer. J Intern Med 243 (6): 521-6, 1998. [PubMed: 9681853]
- Machens A, Lorenz K, Dralle H: Constitutive RET tyrosine kinase activation in hereditary medullary thyroid cancer: clinical opportunities. J Intern Med 266 (1): 114-25, 2009. [PubMed: 19522830]
- Eng C: Seminars in medicine of the Beth Israel Hospital, Boston. The RET proto-oncogene in multiple endocrine neoplasia type 2 and Hirschsprung's disease. N Engl J Med 335 (13): 943-51, 1996. [PubMed: 8782503]
- Conte-Devolx B, Schuffenecker I, Niccoli P, et al.: Multiple endocrine neoplasia type 2: management of patients and subjects at risk. French Study Group on Calcitonin-Secreting Tumors (GETC). Horm Res 47 (4-6): 221-6, 1997. [PubMed: 9167955]
- Ponder BA: Multiple endocrine neoplasia type 2. In: Vogelstein B, Kinzler KW, eds.: The Genetic Basis of Human Cancer. 2nd ed. New York, NY: McGraw-Hill, 2002, pp 501-513.
- Schuffenecker I, Virally-Monod M, Brohet R, et al.: Risk and penetrance of primary hyperparathyroidism in multiple endocrine neoplasia type 2A families with mutations at codon 634 of the RET proto-oncogene. Groupe D'etude des Tumeurs à Calcitonine. J Clin Endocrinol Metab 83 (2): 487-91, 1998. [PubMed: 9467562]
- Gagel RF, Tashjian AH Jr, Cummings T, et al.: The clinical outcome of prospective screening for multiple endocrine neoplasia type 2a. An 18-year experience. N Engl J Med 318 (8): 478-84, 1988. [PubMed: 2893259]
- Guyétant S, Rousselet MC, Durigon M, et al.: Sex-related C cell hyperplasia in the normal human thyroid: a quantitative autopsy study. J Clin Endocrinol Metab 82 (1): 42-7, 1997. [PubMed: 8989230]
- LiVolsi VA: C cell hyperplasia/neoplasia. J Clin Endocrinol Metab 82 (1): 39-41, 1997. [PubMed: 8989229]
- Landsvater RM, Rombouts AG, te Meerman GJ, et al.: The clinical implications of a positive calcitonin test for C-cell hyperplasia in genetically unaffected members of an MEN2A kindred. Am J Hum Genet 52 (2): 335-42, 1993. [PMC free article: PMC1682203] [PubMed: 8094268]
- Lips CJ, Landsvater RM, Höppener JW, et al.: Clinical screening as compared with DNA analysis in families with multiple endocrine neoplasia type 2A. N Engl J Med 331 (13): 828-35, 1994. [PubMed: 7915822]
- Elisei R, Bottici V, Luchetti F, et al.: Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. J Clin Endocrinol Metab 89 (1): 163-8, 2004. [PubMed: 14715844]
- Kudo T, Miyauchi A, Ito Y, et al.: Serum calcitonin levels with calcium loading tests before and after total thyroidectomy in patients with thyroid diseases other than medullary thyroid carcinoma. Endocr J 58 (3): 217-21, 2011. [PubMed: 21358115]
- Incidence: Thyroid Cancer. Bethesda, Md: National Cancer Institute, SEER, 2004. Available online. Last accessed October 16, 2013.
- Gharib H, McConahey WM, Tiegs RD, et al.: Medullary thyroid carcinoma: clinicopathologic features and long-term follow-up of 65 patients treated during 1946 through 1970. Mayo Clin Proc 67 (10): 934-40, 1992. [PubMed: 1434853]
- Decker RA, Peacock ML, Borst MJ, et al.: Progress in genetic screening of multiple endocrine neoplasia type 2A: is calcitonin testing obsolete? Surgery 118 (2): 257-63; discussion 263-4, 1995. [PubMed: 7638742]
- Kitamura Y, Goodfellow PJ, Shimizu K, et al.: Novel germline RET proto-oncogene mutations associated with medullary thyroid carcinoma (MTC): mutation analysis in Japanese patients with MTC. Oncogene 14 (25): 3103-6, 1997. [PubMed: 9223675]
- Eng C, Mulligan LM, Smith DP, et al.: Low frequency of germline mutations in the RET proto-oncogene in patients with apparently sporadic medullary thyroid carcinoma. Clin Endocrinol (Oxf) 43 (1): 123-7, 1995. [PubMed: 7641404]
- Wohllk N, Cote GJ, Bugalho MM, et al.: Relevance of RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. J Clin Endocrinol Metab 81 (10): 3740-5, 1996. [PubMed: 8855832]
- Lips CJ: Clinical management of the multiple endocrine neoplasia syndromes: results of a computerized opinion poll at the Sixth International Workshop on Multiple Endocrine Neoplasia and von Hippel-Lindau disease. J Intern Med 243 (6): 589-94, 1998. [PubMed: 9681863]
- Brandi ML, Gagel RF, Angeli A, et al.: Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 86 (12): 5658-71, 2001. [PubMed: 11739416]
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma. Version 2.2014. Rockledge, Pa: National Comprehensive Cancer Network, 2014. Available online with free subscription. Last accessed December 04, 2014.
- Kloos RT, Eng C, Evans DB, et al.: Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19 (6): 565-612, 2009. [PubMed: 19469690]
- American Cancer Society: Cancer Facts and Figures 2015. Atlanta, Ga: American Cancer Society, 2015. Available online. Last accessed July 1, 2015.
- Hundahl SA, Fleming ID, Fremgen AM, et al.: A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see comments] Cancer 83 (12): 2638-48, 1998. [PubMed: 9874472]
- Bhattacharyya N: A population-based analysis of survival factors in differentiated and medullary thyroid carcinoma. Otolaryngol Head Neck Surg 128 (1): 115-23, 2003. [PubMed: 12574769]
- Modigliani E, Vasen HM, Raue K, et al.: Pheochromocytoma in multiple endocrine neoplasia type 2: European study. The Euromen Study Group. J Intern Med 238 (4): 363-7, 1995. [PubMed: 7595173]
- Roman S, Lin R, Sosa JA: Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 107 (9): 2134-42, 2006. [PubMed: 17019736]
- Bergholm U, Bergström R, Ekbom A: Long-term follow-up of patients with medullary carcinoma of the thyroid. Cancer 79 (1): 132-8, 1997. [PubMed: 8988737]
- Kebebew E, Ituarte PH, Siperstein AE, et al.: Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 88 (5): 1139-48, 2000. [PubMed: 10699905]
- Elisei R, Romei C, Cosci B, et al.: RET genetic screening in patients with medullary thyroid cancer and their relatives: experience with 807 individuals at one center. J Clin Endocrinol Metab 92 (12): 4725-9, 2007. [PubMed: 17895320]
- Paszko Z, Sromek M, Czetwertynska M, et al.: The occurrence and the type of germline mutations in the RET gene in patients with medullary thyroid carcinoma and their unaffected kindred's from Central Poland. Cancer Invest 25 (8): 742-9, 2007. [PubMed: 18058472]
- Pelizzo MR, Boschin IM, Bernante P, et al.: Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur J Surg Oncol 33 (4): 493-7, 2007. [PubMed: 17125960]
- Lenders JW, Pacak K, Walther MM, et al.: Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 287 (11): 1427-34, 2002. [PubMed: 11903030]
- Gerlo EA, Sevens C: Urinary and plasma catecholamines and urinary catecholamine metabolites in pheochromocytoma: diagnostic value in 19 cases. Clin Chem 40 (2): 250-6, 1994. [PubMed: 7906208]
- Guller U, Turek J, Eubanks S, et al.: Detecting pheochromocytoma: defining the most sensitive test. Ann Surg 243 (1): 102-7, 2006. [PMC free article: PMC1449983] [PubMed: 16371743]
- Raber W, Raffesberg W, Bischof M, et al.: Diagnostic efficacy of unconjugated plasma metanephrines for the detection of pheochromocytoma. Arch Intern Med 160 (19): 2957-63, 2000. [PubMed: 11041903]
- Sawka AM, Jaeschke R, Singh RJ, et al.: A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 88 (2): 553-8, 2003. [PubMed: 12574179]
- Unger N, Pitt C, Schmidt IL, et al.: Diagnostic value of various biochemical parameters for the diagnosis of pheochromocytoma in patients with adrenal mass. Eur J Endocrinol 154 (3): 409-17, 2006. [PubMed: 16498054]
- Pacak K, Eisenhofer G, Ahlman H, et al.: Pheochromocytoma: recommendations for clinical practice from the First International Symposium. October 2005. Nat Clin Pract Endocrinol Metab 3 (2): 92-102, 2007. [PubMed: 17237836]
- van der Harst E, de Herder WW, Bruining HA, et al.: [(123)I]metaiodobenzylguanidine and [(111)In]octreotide uptake in begnign and malignant pheochromocytomas. J Clin Endocrinol Metab 86 (2): 685-93, 2001. [PubMed: 11158032]
- Pacak K, Linehan WM, Eisenhofer G, et al.: Recent advances in genetics, diagnosis, localization, and treatment of pheochromocytoma. Ann Intern Med 134 (4): 315-29, 2001. [PubMed: 11182843]
- Kaelin WG Jr: Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer 2 (9): 673-82, 2002. [PubMed: 12209156]
- Maher ER, Eng C: The pressure rises: update on the genetics of phaeochromocytoma. Hum Mol Genet 11 (20): 2347-54, 2002. [PubMed: 12351569]
- Neumann HP, Bausch B, McWhinney SR, et al.: Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med 346 (19): 1459-66, 2002. [PubMed: 12000816]
- Amar L, Bertherat J, Baudin E, et al.: Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol 23 (34): 8812-8, 2005. [PubMed: 16314641]
- Gimenez-Roqueplo AP, Lehnert H, Mannelli M, et al.: Phaeochromocytoma, new genes and screening strategies. Clin Endocrinol (Oxf) 65 (6): 699-705, 2006. [PubMed: 17121518]
- Jafri M, Whitworth J, Rattenberry E, et al.: Evaluation of SDHB, SDHD and VHL gene susceptibility testing in the assessment of individuals with non-syndromic phaeochromocytoma, paraganglioma and head and neck paraganglioma. Clin Endocrinol (Oxf) 78 (6): 898-906, 2013. [PubMed: 23072324]
- Pęczkowska M, Kowalska A, Sygut J, et al.: Testing new susceptibility genes in the cohort of apparently sporadic phaeochromocytoma/paraganglioma patients with clinical characteristics of hereditary syndromes. Clin Endocrinol (Oxf) 79 (6): 817-23, 2013. [PubMed: 23551045]
- Karasek D, Frysak Z, Pacak K: Genetic testing for pheochromocytoma. Curr Hypertens Rep 12 (6): 456-64, 2010. [PMC free article: PMC3061287] [PubMed: 20938758]
- Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, et al.: Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet 43 (7): 663-7, 2011. [PubMed: 21685915]
- Neumann HP, Sullivan M, Winter A, et al.: Germline mutations of the TMEM127 gene in patients with paraganglioma of head and neck and extraadrenal abdominal sites. J Clin Endocrinol Metab 96 (8): E1279-82, 2011. [PubMed: 21613359]
- Burnichon N, Lepoutre-Lussey C, Laffaire J, et al.: A novel TMEM127 mutation in a patient with familial bilateral pheochromocytoma. Eur J Endocrinol 164 (1): 141-5, 2011. [PubMed: 20923864]
- Bayley JP, Kunst HP, Cascon A, et al.: SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol 11 (4): 366-72, 2010. [PubMed: 20071235]
- Neumann HP, Erlic Z, Boedeker CC, et al.: Clinical predictors for germline mutations in head and neck paraganglioma patients: cost reduction strategy in genetic diagnostic process as fall-out. Cancer Res 69 (8): 3650-6, 2009. [PubMed: 19351833]
- Erlic Z, Rybicki L, Peczkowska M, et al.: Clinical predictors and algorithm for the genetic diagnosis of pheochromocytoma patients. Clin Cancer Res 15 (20): 6378-85, 2009. [PubMed: 19825962]
- Mannelli M, Castellano M, Schiavi F, et al.: Clinically guided genetic screening in a large cohort of italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J Clin Endocrinol Metab 94 (5): 1541-7, 2009. [PubMed: 19223516]
- Fraser WD: Hyperparathyroidism. Lancet 374 (9684): 145-58, 2009. [PubMed: 19595349]
- Tonelli F, Marcucci T, Giudici F, et al.: Surgical approach in hereditary hyperparathyroidism. Endocr J 56 (7): 827-41, 2009. [PubMed: 19797826]
- Villablanca A, Calender A, Forsberg L, et al.: Germline and de novo mutations in the HRPT2 tumour suppressor gene in familial isolated hyperparathyroidism (FIHP). J Med Genet 41 (3): e32, 2004. [PMC free article: PMC1735713] [PubMed: 14985403]
- Marx SJ, Simonds WF, Agarwal SK, et al.: Hyperparathyroidism in hereditary syndromes: special expressions and special managements. J Bone Miner Res 17 (Suppl 2): N37-43, 2002. [PubMed: 12412776]
- Sipple JH: The association of pheochromocytoma with carcinoma of the thyroid gland. Am J Med 31 (1): 163-166, 1961.
- Eng C, Clayton D, Schuffenecker I, et al.: The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 276 (19): 1575-9, 1996. [PubMed: 8918855]
- Sanso GE, Domene HM, Garcia R, et al.: Very early detection of RET proto-oncogene mutation is crucial for preventive thyroidectomy in multiple endocrine neoplasia type 2 children: presence of C-cell malignant disease in asymptomatic carriers. Cancer 94 (2): 323-30, 2002. [PubMed: 11900218]
- Yip L, Cote GJ, Shapiro SE, et al.: Multiple endocrine neoplasia type 2: evaluation of the genotype-phenotype relationship. Arch Surg 138 (4): 409-16; discussion 416, 2003. [PubMed: 12686527]
- Raue F, Frank-Raue K, Grauer A: Multiple endocrine neoplasia type 2. Clinical features and screening. Endocrinol Metab Clin North Am 23 (1): 137-56, 1994. [PubMed: 7913021]
- Perren A, Komminoth P: Familial pheochromocytomas and paragangliomas: stories from the sign-out room. Endocr Pathol 17 (4): 337-44, 2006. [PubMed: 17525482]
- Webb TA, Sheps SG, Carney JA: Differences between sporadic pheochromocytoma and pheochromocytoma in multiple endocrime neoplasia, type 2. Am J Surg Pathol 4 (2): 121-6, 1980. [PubMed: 6103678]
- Lips KJ, Van der Sluys Veer J, Struyvenberg A, et al.: Bilateral occurrence of pheochromocytoma in patients with the multiple endocrine neoplasia syndrome type 2A (Sipple's syndrome). Am J Med 70 (5): 1051-60, 1981. [PubMed: 7234871]
- Neumann HP, Berger DP, Sigmund G, et al.: Pheochromocytomas, multiple endocrine neoplasia type 2, and von Hippel-Lindau disease. N Engl J Med 329 (21): 1531-8, 1993. [PubMed: 8105382]
- Kraimps JL, Denizot A, Carnaille B, et al.: Primary hyperparathyroidism in multiple endocrine neoplasia type IIa: retrospective French multicentric study. Groupe d'Etude des Tumeurs á Calcitonine (GETC, French Calcitonin Tumors Study Group), French Association of Endocrine Surgeons. World J Surg 20 (7): 808-12; discussion 812-3, 1996. [PubMed: 8678955]
- Benson L, Ljunghall S, Akerström G, et al.: Hyperparathyroidism presenting as the first lesion in multiple endocrine neoplasia type 1. Am J Med 82 (4): 731-7, 1987. [PubMed: 2882676]
- Trump D, Farren B, Wooding C, et al.: Clinical studies of multiple endocrine neoplasia type 1 (MEN1) QJM 89 (9): 653-69, 1996. [PubMed: 8917740]
- Vasen HF, Lamers CB, Lips CJ: Screening for the multiple endocrine neoplasia syndrome type I. A study of 11 kindreds in The Netherlands. Arch Intern Med 149 (12): 2717-22, 1989. [PubMed: 2574567]
- Bugalho MJ, Limbert E, Sobrinho LG, et al.: A kindred with multiple endocrine neoplasia type 2A associated with pruritic skin lesions. Cancer 70 (11): 2664-7, 1992. [PubMed: 1358428]
- Robinson MF, Furst EJ, Nunziata V, et al.: Characterization of the clinical features of five families with hereditary primary cutaneous lichen amyloidosis and multiple endocrine neoplasia type 2. Henry Ford Hosp Med J 40 (3-4): 249-52, 1992. [PubMed: 1362415]
- Hampel H, Bennett RL, Buchanan A, et al.: A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 17 (1): 70-87, 2015. [PubMed: 25394175]
- Brauckhoff M, Machens A, Hess S, et al.: Premonitory symptoms preceding metastatic medullary thyroid cancer in MEN 2B: An exploratory analysis. Surgery 144 (6): 1044-50; discussion 1050-3, 2008. [PubMed: 19041016]
- Kouvaraki MA, Shapiro SE, Perrier ND, et al.: RET proto-oncogene: a review and update of genotype-phenotype correlations in hereditary medullary thyroid cancer and associated endocrine tumors. Thyroid 15 (6): 531-44, 2005. [PubMed: 16029119]
- Pacini F, Castagna MG, Cipri C, et al.: Medullary thyroid carcinoma. Clin Oncol (R Coll Radiol) 22 (6): 475-85, 2010. [PubMed: 20627492]
- Morrison PJ, Nevin NC: Multiple endocrine neoplasia type 2B (mucosal neuroma syndrome, Wagenmann-Froboese syndrome). J Med Genet 33 (9): 779-82, 1996. [PMC free article: PMC1050735] [PubMed: 8880581]
- Gorlin RJ, Sedano HO, Vickers RA, et al.: Multiple mucosal neuromas, pheochromocytoma and medullary carcinoma of the thyroid--a syndrome. Cancer 22 (2): 293-9 passim, 1968. [PubMed: 5660196]
- Gorlin RJ, Vickers RA: Multiple mucosal neuromas, pheochromocytoma, medullary carcinoma of the thyroid and marfanoid body build with muscle wasting: reexamination of a syndrome of neural crest malmigration. Birth Defects Orig Artic Ser 7 (6): 69-72, 1971. [PubMed: 4950920]
- Skinner MA, DeBenedetti MK, Moley JF, et al.: Medullary thyroid carcinoma in children with multiple endocrine neoplasia types 2A and 2B. J Pediatr Surg 31 (1): 177-81; discussion 181-2, 1996. [PubMed: 8632274]
- O'Riordain DS, O'Brien T, Weaver AL, et al.: Medullary thyroid carcinoma in multiple endocrine neoplasia types 2A and 2B. Surgery 116 (6): 1017-23, 1994. [PubMed: 7985081]
- Vasen HF, van der Feltz M, Raue F, et al.: The natural course of multiple endocrine neoplasia type IIb. A study of 18 cases. Arch Intern Med 152 (6): 1250-2, 1992. [PubMed: 1350898]
- Romeo G, Ceccherini I, Celli J, et al.: Association of multiple endocrine neoplasia type 2 and Hirschsprung disease. J Intern Med 243 (6): 515-20, 1998. [PubMed: 9681852]
- Decker RA, Peacock ML, Watson P: Hirschsprung disease in MEN 2A: increased spectrum of RET exon 10 genotypes and strong genotype-phenotype correlation. Hum Mol Genet 7 (1): 129-34, 1998. [PubMed: 9384613]
- Carrasquillo MM, McCallion AS, Puffenberger EG, et al.: Genome-wide association study and mouse model identify interaction between RET and EDNRB pathways in Hirschsprung disease. Nat Genet 32 (2): 237-44, 2002. [PubMed: 12355085]
- Mulligan LM, Eng C, Attié T, et al.: Diverse phenotypes associated with exon 10 mutations of the RET proto-oncogene. Hum Mol Genet 3 (12): 2163-7, 1994. [PubMed: 7881414]
- Emison ES, McCallion AS, Kashuk CS, et al.: A common sex-dependent mutation in a RET enhancer underlies Hirschsprung disease risk. Nature 434 (7035): 857-63, 2005. [PubMed: 15829955]
- Gardner E, Papi L, Easton DF, et al.: Genetic linkage studies map the multiple endocrine neoplasia type 2 loci to a small interval on chromosome 10q11.2. Hum Mol Genet 2 (3): 241-6, 1993. [PubMed: 8098977]
- Mole SE, Mulligan LM, Healey CS, et al.: Localisation of the gene for multiple endocrine neoplasia type 2A to a 480 kb region in chromosome band 10q11.2. Hum Mol Genet 2 (3): 247-52, 1993. [PubMed: 8098978]
- Takahashi M, Ritz J, Cooper GM: Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 42 (2): 581-8, 1985. [PubMed: 2992805]
- Kwok JB, Gardner E, Warner JP, et al.: Structural analysis of the human ret proto-oncogene using exon trapping. Oncogene 8 (9): 2575-82, 1993. [PubMed: 8361767]
- Myers SM, Eng C, Ponder BA, et al.: Characterization of RET proto-oncogene 3' splicing variants and polyadenylation sites: a novel C-terminus for RET. Oncogene 11 (10): 2039-45, 1995. [PubMed: 7478523]
- Airaksinen MS, Saarma M: The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci 3 (5): 383-94, 2002. [PubMed: 11988777]
- Takaya K, Yoshimasa T, Arai H, et al.: Expression of the RET proto-oncogene in normal human tissues, pheochromocytomas, and other tumors of neural crest origin. J Mol Med 74 (10): 617-21, 1996. [PubMed: 8912182]
- Kurokawa K, Kawai K, Hashimoto M, et al.: Cell signalling and gene expression mediated by RET tyrosine kinase. J Intern Med 253 (6): 627-33, 2003. [PubMed: 12755958]
- Manié S, Santoro M, Fusco A, et al.: The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet 17 (10): 580-9, 2001. [PubMed: 11585664]
- Robson ME, Storm CD, Weitzel J, et al.: American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol 28 (5): 893-901, 2010. [PubMed: 20065170]
- Points to consider: ethical, legal, and psychosocial implications of genetic testing in children and adolescents. American Society of Human Genetics Board of Directors, American College of Medical Genetics Board of Directors. Am J Hum Genet 57 (5): 1233-41, 1995. [PMC free article: PMC1801355] [PubMed: 7485175]
- Cooper DS, Doherty GM, Haugen BR, et al.: Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19 (11): 1167-214, 2009. [PubMed: 19860577]
- Ceccherini I, Hofstra RM, Luo Y, et al.: DNA polymorphisms and conditions for SSCP analysis of the 20 exons of the ret proto-oncogene. Oncogene 9 (10): 3025-9, 1994. [PubMed: 8084609]
- Xue F, Yu H, Maurer LH, et al.: Germline RET mutations in MEN 2A and FMTC and their detection by simple DNA diagnostic tests. Hum Mol Genet 3 (4): 635-8, 1994. [PubMed: 7915165]
- McMahon R, Mulligan LM, Healey CS, et al.: Direct, non-radioactive detection of mutations in multiple endocrine neoplasia type 2A families. Hum Mol Genet 3 (4): 643-6, 1994. [PubMed: 7915166]
- Kambouris M, Jackson CE, Feldman GL: Diagnosis of multiple endocrine neoplasia [MEN] 2A, 2B and familial medullary thyroid cancer [FMTC] by multiplex PCR and heteroduplex analyses of RET proto-oncogene mutations. Hum Mutat 8 (1): 64-70, 1996. [PubMed: 8807338]
- Machens A, Niccoli-Sire P, Hoegel J, et al.: Early malignant progression of hereditary medullary thyroid cancer. N Engl J Med 349 (16): 1517-25, 2003. [PubMed: 14561794]
- Elisei R, Romei C, Renzini G, et al.: The timing of total thyroidectomy in RET gene mutation carriers could be personalized and safely planned on the basis of serum calcitonin: 18 years experience at one single center. J Clin Endocrinol Metab 97 (2): 426-35, 2012. [PubMed: 22162466]
- Rich TA, Feng L, Busaidy N, et al.: Prevalence by age and predictors of medullary thyroid cancer in patients with lower risk germline RET proto-oncogene mutations. Thyroid 24 (7): 1096-106, 2014. [PMC free article: PMC4080849] [PubMed: 24617864]
- Eng C, Smith DP, Mulligan LM, et al.: Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum Mol Genet 3 (2): 237-41, 1994. [PubMed: 7911697]
- Hofstra RM, Landsvater RM, Ceccherini I, et al.: A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 367 (6461): 375-6, 1994. [PubMed: 7906866]
- Carlson KM, Dou S, Chi D, et al.: Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci U S A 91 (4): 1579-83, 1994. [PMC free article: PMC43203] [PubMed: 7906417]
- Gimm O, Marsh DJ, Andrew SD, et al.: Germline dinucleotide mutation in codon 883 of the RET proto-oncogene in multiple endocrine neoplasia type 2B without codon 918 mutation. J Clin Endocrinol Metab 82 (11): 3902-4, 1997. [PubMed: 9360560]
- Smith DP, Houghton C, Ponder BA: Germline mutation of RET codon 883 in two cases of de novo MEN 2B. Oncogene 15 (10): 1213-7, 1997. [PubMed: 9294615]
- Eng C, Mulligan LM, Healey CS, et al.: Heterogeneous mutation of the RET proto-oncogene in subpopulations of medullary thyroid carcinoma. Cancer Res 56 (9): 2167-70, 1996. [PubMed: 8616867]
- Cranston AN, Carniti C, Oakhill K, et al.: RET is constitutively activated by novel tandem mutations that alter the active site resulting in multiple endocrine neoplasia type 2B. Cancer Res 66 (20): 10179-87, 2006. [PubMed: 17047083]
- Miyauchi A, Futami H, Hai N, et al.: Two germline missense mutations at codons 804 and 806 of the RET proto-oncogene in the same allele in a patient with multiple endocrine neoplasia type 2B without codon 918 mutation. Jpn J Cancer Res 90 (1): 1-5, 1999. [PMC free article: PMC5925979] [PubMed: 10076558]
- Kameyama K, Okinaga H, Takami H: RET oncogene mutations in 75 cases of familial medullary thyroid carcinoma in Japan. Biomed Pharmacother 58 (6-7): 345-7, 2004 Jul-Aug. [PubMed: 15271413]
- Iwashita T, Murakami H, Kurokawa K, et al.: A two-hit model for development of multiple endocrine neoplasia type 2B by RET mutations. Biochem Biophys Res Commun 268 (3): 804-8, 2000. [PubMed: 10679286]
- Menko FH, van der Luijt RB, de Valk IA, et al.: Atypical MEN type 2B associated with two germline RET mutations on the same allele not involving codon 918. J Clin Endocrinol Metab 87 (1): 393-7, 2002. [PubMed: 11788682]
- Mulligan LM, Eng C, Healey CS, et al.: Specific mutations of the RET proto-oncogene are related to disease phenotype in MEN 2A and FMTC. Nat Genet 6 (1): 70-4, 1994. [PubMed: 7907913]
- Seri M, Celli I, Betsos N, et al.: A Cys634Gly substitution of the RET proto-oncogene in a family with recurrence of multiple endocrine neoplasia type 2A and cutaneous lichen amyloidosis. Clin Genet 51 (2): 86-90, 1997. [PubMed: 9111993]
- Rothberg AE, Raymond VM, Gruber SB, et al.: Familial medullary thyroid carcinoma associated with cutaneous lichen amyloidosis. Thyroid 19 (6): 651-5, 2009. [PubMed: 19445625]
- Quayle FJ, Fialkowski EA, Benveniste R, et al.: Pheochromocytoma penetrance varies by RET mutation in MEN 2A. Surgery 142 (6): 800-5; discussion 805.e1, 2007. [PubMed: 18063059]
- Bolino A, Schuffenecker I, Luo Y, et al.: RET mutations in exons 13 and 14 of FMTC patients. Oncogene 10 (12): 2415-9, 1995. [PubMed: 7784092]
- Boccia LM, Green JS, Joyce C, et al.: Mutation of RET codon 768 is associated with the FMTC phenotype. Clin Genet 51 (2): 81-5, 1997. [PubMed: 9111992]
- Lesueur F, Cebrian A, Cranston A, et al.: Germline homozygous mutations at codon 804 in the RET protooncogene in medullary thyroid carcinoma/multiple endocrine neoplasia type 2A patients. J Clin Endocrinol Metab 90 (6): 3454-7, 2005. [PubMed: 15741265]
- Shannon KE, Gimm O, Hinze R: Germline V804M mutation in the RET protooncogene in 2 apparently sporadic cases of MTC presenting in the 7th decade of life. The Journal of Endocrine Genetics 1 (1): 39-46, 1999.
- Raue F, Frank-Raue K: Genotype-phenotype relationship in multiple endocrine neoplasia type 2. Implications for clinical management. Hormones (Athens) 8 (1): 23-8, 2009 Jan-Mar. [PubMed: 19269918]
- Frank-Raue K, Rybicki LA, Erlic Z, et al.: Risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germline RET mutations located in exon 10. Hum Mutat 32 (1): 51-8, 2011. [PubMed: 20979234]
- Mulligan LM, Marsh DJ, Robinson BG, et al.: Genotype-phenotype correlation in multiple endocrine neoplasia type 2: report of the International RET Mutation Consortium. J Intern Med 238 (4): 343-6, 1995. [PubMed: 7595170]
- Moers AM, Landsvater RM, Schaap C, et al.: Familial medullary thyroid carcinoma: not a distinct entity? Genotype-phenotype correlation in a large family. Am J Med 101 (6): 635-41, 1996. [PubMed: 9003111]
- Niccoli-Sire P, Murat A, Rohmer V, et al.: Familial medullary thyroid carcinoma with noncysteine ret mutations: phenotype-genotype relationship in a large series of patients. J Clin Endocrinol Metab 86 (8): 3746-53, 2001. [PubMed: 11502806]
- Machens A, Ukkat J, Brauckhoff M, et al.: Advances in the management of hereditary medullary thyroid cancer. J Intern Med 257 (1): 50-9, 2005. [PubMed: 15606376]
- Mukherjee S, Zakalik D: RET codon 804 mutations in multiple endocrine neoplasia 2: genotype-phenotype correlations and implications in clinical management. Clin Genet 79 (1): 1-16, 2011. [PubMed: 20497437]
- Dvorakova S, Vaclavikova E, Duskova J, et al.: Exon 5 of the RET proto-oncogene: a newly detected risk exon for familial medullary thyroid carcinoma, a novel germ-line mutation Gly321Arg. J Endocrinol Invest 28 (10): 905-9, 2005. [PubMed: 16419493]
- Muzza M, Cordella D, Bombled J, et al.: Four novel RET germline variants in exons 8 and 11 display an oncogenic potential in vitro. Eur J Endocrinol 162 (4): 771-7, 2010. [PubMed: 20103606]
- Pigny P, Bauters C, Wemeau JL, et al.: A novel 9-base pair duplication in RET exon 8 in familial medullary thyroid carcinoma. J Clin Endocrinol Metab 84 (5): 1700-4, 1999. [PubMed: 10323403]
- Niccoli-Sire P, Murat A, Rohmer V, et al.: When should thyroidectomy be performed in familial medullary thyroid carcinoma gene carriers with non-cysteine RET mutations? Surgery 134 (6): 1029-36; discussion 1036-7, 2003. [PubMed: 14668737]
- Fazioli F, Piccinini G, Appolloni G, et al.: A new germline point mutation in Ret exon 8 (cys515ser) in a family with medullary thyroid carcinoma. Thyroid 18 (7): 775-82, 2008. [PubMed: 18631007]
- Da Silva AM, Maciel RM, Da Silva MR, et al.: A novel germ-line point mutation in RET exon 8 (Gly(533)Cys) in a large kindred with familial medullary thyroid carcinoma. J Clin Endocrinol Metab 88 (11): 5438-43, 2003. [PubMed: 14602786]
- Bethanis S, Koutsodontis G, Palouka T, et al.: A newly detected mutation of the RET protooncogene in exon 8 as a cause of multiple endocrine neoplasia type 2A. Hormones (Athens) 6 (2): 152-6, 2007 Apr-Jun. [PubMed: 17704047]
- Kaldrymides P, Mytakidis N, Anagnostopoulos T, et al.: A rare RET gene exon 8 mutation is found in two Greek kindreds with familial medullary thyroid carcinoma: implications for screening. Clin Endocrinol (Oxf) 64 (5): 561-6, 2006. [PubMed: 16649977]
- Peppa M, Boutati E, Kamakari S, et al.: Multiple endocrine neoplasia type 2A in two families with the familial medullary thyroid carcinoma associated G533C mutation of the RET proto-oncogene. Eur J Endocrinol 159 (6): 767-71, 2008. [PubMed: 18805915]
- Oliveira MN, Hemerly JP, Bastos AU, et al.: The RET p.G533C mutation confers predisposition to multiple endocrine neoplasia type 2A in a Brazilian kindred and is able to induce a malignant phenotype in vitro and in vivo. Thyroid 21 (9): 975-85, 2011. [PubMed: 21834681]
- Signorini PS, França MI, Camacho CP, et al.: A ten-year clinical update of a large RET p.Gly533Cys kindred with medullary thyroid carcinoma emphasizes the need for an individualized assessment of affected relatives. Clin Endocrinol (Oxf) 80 (2): 235-45, 2014. [PubMed: 23745650]
- Sáez ME, Ruiz A, Cebrián A, et al.: A new germline mutation, R600Q, within the coding region of RET proto-oncogene: a rare polymorphism or a MEN 2 causing mutation? Hum Mutat 15 (1): 122, 2000. [PubMed: 10612852]
- Rey JM, Brouillet JP, Fonteneau-Allaire J, et al.: Novel germline RET mutation segregating with papillary thyroid carcinomas. Genes Chromosomes Cancer 32 (4): 390-1, 2001. [PubMed: 11746981]
- Ahmed SA, Snow-Bailey K, Highsmith WE, et al.: Nine novel germline gene variants in the RET proto-oncogene identified in twelve unrelated cases. J Mol Diagn 7 (2): 283-8, 2005. [PMC free article: PMC1867532] [PubMed: 15858153]
- Ercolino T, Lombardi A, Becherini L, et al.: The Y606C RET mutation causes a receptor gain of function. Clin Endocrinol (Oxf) 69 (2): 253-8, 2008. [PubMed: 18248647]
- Igaz P, Patócs A, Rácz K, et al.: Occurrence of pheochromocytoma in a MEN2A family with codon 609 mutation of the RET proto-oncogene. J Clin Endocrinol Metab 87 (6): 2994, 2002. [PubMed: 12050290]
- Fialkowski EA, DeBenedetti MK, Moley JF, et al.: RET proto-oncogene testing in infants presenting with Hirschsprung disease identifies 2 new multiple endocrine neoplasia 2A kindreds. J Pediatr Surg 43 (1): 188-90, 2008. [PubMed: 18206480]
- Mian C, Barollo S, Zambonin L, et al.: Characterization of the largest kindred with MEN2A due to a Cys609Ser RET mutation. Fam Cancer 8 (4): 379-82, 2009. [PubMed: 19475497]
- Kinlaw WB, Scott SM, Maue RA, et al.: Multiple endocrine neoplasia 2A due to a unique C609S RET mutation presents with pheochromocytoma and reduced penetrance of medullary thyroid carcinoma. Clin Endocrinol (Oxf) 63 (6): 676-82, 2005. [PubMed: 16343103]
- Calva D, O'Dorisio TM, Sue O'Dorisio M, et al.: When is prophylactic thyroidectomy indicated for patients with the RET codon 609 mutation? Ann Surg Oncol 16 (8): 2237-44, 2009. [PubMed: 19472011]
- Jung J, Uchino S, Lee Y, et al.: A Korean family of familial medullary thyroid cancer with Cys618Ser RET germline mutation. J Korean Med Sci 25 (2): 226-9, 2010. [PMC free article: PMC2811288] [PubMed: 20119574]
- Qi XP, Ying RB, Ma JM, et al.: Case report: a p.C618S RET proto-oncogene germline mutation in a large Chinese pedigree with familial medullary thyroid carcinoma. Fam Cancer 11 (1): 131-6, 2012. [PubMed: 22068382]
- Hedayati M, Zarif Yeganeh M, Sheikhol Eslami S, et al.: Predominant RET Germline Mutations in Exons 10, 11, and 16 in Iranian Patients with Hereditary Medullary Thyroid Carcinoma. J Thyroid Res 2011: 264248, 2011. [PMC free article: PMC3134203] [PubMed: 21765987]
- Neocleous V, Skordis N, Portides G, et al.: RET proto-oncogene mutations are restricted to codon 618 in Cypriot families with multiple endocrine neoplasia 2. J Endocrinol Invest 34 (10): 764-9, 2011. [PubMed: 21422799]
- Chu CS, Lee KT, Lee ST, et al.: Effects of atorvastatin on ventricular late potentials and repolarization dispersion in patients with hypercholesterolemia. Kaohsiung J Med Sci 23 (5): 217-24, 2007. [PubMed: 17525003]
- Berndt I, Reuter M, Saller B, et al.: A new hot spot for mutations in the ret protooncogene causing familial medullary thyroid carcinoma and multiple endocrine neoplasia type 2A. J Clin Endocrinol Metab 83 (3): 770-4, 1998. [PubMed: 9506724]
- Elston MS, Meyer-Rochow GY, Holdaway I, et al.: Patients with RET D631Y mutations most commonly present with pheochromocytoma and not medullary thyroid carcinoma. Horm Metab Res 44 (5): 339-42, 2012. [PubMed: 22274720]
- Asai N, Iwashita T, Murakami H, et al.: Mechanism of Ret activation by a mutation at aspartic acid 631 identified in sporadic pheochromocytoma. Biochem Biophys Res Commun 255 (3): 587-90, 1999. [PubMed: 10049754]
- Höppner W, Dralle H, Brabant G: Duplication of 9 base pairs in the critical cysteine-rich domain of the RET proto-oncogene causes multiple endocrine neoplasia type 2A. Hum Mutat Suppl (1): S128-30, 1998. [PubMed: 9452064]
- Puñales MK, Graf H, Gross JL, et al.: RET codon 634 mutations in multiple endocrine neoplasia type 2: variable clinical features and clinical outcome. J Clin Endocrinol Metab 88 (6): 2644-9, 2003. [PubMed: 12788868]
- Zhou YL, Zhu SX, Li JJ, et al.: [The clinical patterns and RET proto-oncogene in fifteen multiple endocrine neoplasia type 2A pedigrees]. Zhonghua Nei Ke Za Zhi 46 (6): 466-70, 2007. [PubMed: 17663821]
- Lemos MC, Carrilho F, Rodrigues FJ, et al.: Early onset of medullary thyroid carcinoma in a kindred with multiple endocrine neoplasia type iia associated with cutaneous lichen amyloidosis. Endocr Pract 8 (1): 19-22, 2002 Jan-Feb. [PubMed: 11939755]
- Toledo RA, Wagner SM, Coutinho FL, et al.: High penetrance of pheochromocytoma associated with the novel C634Y/Y791F double germline mutation in the RET protooncogene. J Clin Endocrinol Metab 95 (3): 1318-27, 2010. [PubMed: 20080836]
- Höppner W, Ritter MM: A duplication of 12 bp in the critical cysteine rich domain of the RET proto-oncogene results in a distinct phenotype of multiple endocrine neoplasia type 2A. Hum Mol Genet 6 (4): 587-90, 1997. [PubMed: 9097963]
- Wiench M, Wygoda Z, Gubala E, et al.: Estimation of risk of inherited medullary thyroid carcinoma in apparent sporadic patients. J Clin Oncol 19 (5): 1374-80, 2001. [PubMed: 11230481]
- Colombo-Benkmann M, Li Z, Riemann B, et al.: Characterization of the RET protooncogene transmembrane domain mutation S649L associated with nonaggressive medullary thyroid carcinoma. Eur J Endocrinol 158 (6): 811-6, 2008. [PubMed: 18322301]
- Vierhapper H, Bieglmayer C, Heinze G, et al.: Frequency of RET proto-oncogene mutations in patients with normal and with moderately elevated pentagastrin-stimulated serum concentrations of calcitonin. Thyroid 14 (8): 580-3, 2004. [PubMed: 15320968]
- Borrello MG, Aiello A, Peissel B, et al.: Functional characterization of the MTC-associated germline RET-K666E mutation: evidence of oncogenic potential enhanced by the G691S polymorphism. Endocr Relat Cancer 18 (4): 519-27, 2011. [PubMed: 21690267]
- Dabir T, Hunter SJ, Russell CF, et al.: The RET mutation E768D confers a late-onset familial medullary thyroid carcinoma -- only phenotype with incomplete penetrance: implications for screening and management of carrier status. Fam Cancer 5 (2): 201-4, 2006. [PubMed: 16736292]
- Frank-Raue K, Döhring J, Scheumann G, et al.: New mutations in the RET protooncogene-L881V - associated with medullary thyroid carcinoma and -R770Q - in a patient with mixed medullar/follicular thyroid tumour. Exp Clin Endocrinol Diabetes 118 (8): 550-3, 2010. [PubMed: 20013610]
- D'Aloiso L, Carlomagno F, Bisceglia M, et al.: Clinical case seminar: in vivo and in vitro characterization of a novel germline RET mutation associated with low-penetrant nonaggressive familial medullary thyroid carcinoma. J Clin Endocrinol Metab 91 (3): 754-9, 2006. [PubMed: 16384843]
- Frank-Raue K, Machens A, Scheuba C, et al.: Difference in development of medullary thyroid carcinoma among carriers of RET mutations in codons 790 and 791. Clin Endocrinol (Oxf) 69 (2): 259-63, 2008. [PubMed: 18248648]
- Bihan H, Baudin E, Meas T, et al.: Role of prophylactic thyroidectomy in RET 790 familial medullary thyroid carcinoma. Head Neck 34 (4): 493-8, 2012. [PubMed: 21688339]
- Vestergaard P, Vestergaard EM, Brockstedt H, et al.: Codon Y791F mutations in a large kindred: is prophylactic thyroidectomy always indicated? World J Surg 31 (5): 996-1001; discussion 1002-4, 2007. [PubMed: 17483988]
- Learoyd DL, Gosnell J, Elston MS, et al.: Experience of prophylactic thyroidectomy in multiple endocrine neoplasia type 2A kindreds with RET codon 804 mutations. Clin Endocrinol (Oxf) 63 (6): 636-41, 2005. [PubMed: 16343097]
- Lal G, Nowell AG, Liao J, et al.: Determinants of survival in patients with calciphylaxis: a multivariate analysis. Surgery 146 (6): 1028-34, 2009. [PubMed: 19958929]
- Shifrin AL, Ogilvie JB, Stang MT, et al.: Single nucleotide polymorphisms act as modifiers and correlate with the development of medullary and simultaneous medullary/papillary thyroid carcinomas in 2 large, non-related families with the RET V804M proto-oncogene mutation. Surgery 148 (6): 1274-80; discussion 1280-1, 2010. [PubMed: 21134561]
- Kasprzak L, Nolet S, Gaboury L, et al.: Familial medullary thyroid carcinoma and prominent corneal nerves associated with the germline V804M and V778I mutations on the same allele of RET. J Med Genet 38 (11): 784-7, 2001. [PMC free article: PMC1734769] [PubMed: 11732489]
- Cranston A, Carniti C, Martin S, et al.: A novel activating mutation in the RET tyrosine kinase domain mediates neoplastic transformation. Mol Endocrinol 20 (7): 1633-43, 2006. [PubMed: 16469774]
- Jasim S, Ying AK, Waguespack SG, et al.: Multiple endocrine neoplasia type 2B with a RET proto-oncogene A883F mutation displays a more indolent form of medullary thyroid carcinoma compared with a RET M918T mutation. Thyroid 21 (2): 189-92, 2011. [PMC free article: PMC3025175] [PubMed: 21186952]
- Prazeres HJ, Rodrigues F, Figueiredo P, et al.: Occurrence of the Cys611Tyr mutation and a novel Arg886Trp substitution in the RET proto-oncogene in multiple endocrine neoplasia type 2 families and sporadic medullary thyroid carcinoma cases originating from the central region of Portugal. Clin Endocrinol (Oxf) 64 (6): 659-66, 2006. [PubMed: 16712668]
- Schulte KM, Machens A, Fugazzola L, et al.: The clinical spectrum of multiple endocrine neoplasia type 2a caused by the rare intracellular RET mutation S891A. J Clin Endocrinol Metab 95 (9): E92-7, 2010. [PubMed: 20554711]
- Hofstra RM, Fattoruso O, Quadro L, et al.: A novel point mutation in the intracellular domain of the ret protooncogene in a family with medullary thyroid carcinoma. J Clin Endocrinol Metab 82 (12): 4176-8, 1997. [PubMed: 9398735]
- Dang GT, Cote GJ, Schultz PN, et al.: A codon 891 exon 15 RET proto-oncogene mutation in familial medullary thyroid carcinoma: a detection strategy. Mol Cell Probes 13 (1): 77-9, 1999. [PubMed: 10024437]
- Jimenez C, Habra MA, Huang SC, et al.: Pheochromocytoma and medullary thyroid carcinoma: a new genotype-phenotype correlation of the RET protooncogene 891 germline mutation. J Clin Endocrinol Metab 89 (8): 4142-5, 2004. [PubMed: 15292360]
- Jimenez C, Dang GT, Schultz PN, et al.: A novel point mutation of the RET protooncogene involving the second intracellular tyrosine kinase domain in a family with medullary thyroid carcinoma. J Clin Endocrinol Metab 89 (7): 3521-6, 2004. [PubMed: 15240641]
- Zenaty D, Aigrain Y, Peuchmaur M, et al.: Medullary thyroid carcinoma identified within the first year of life in children with hereditary multiple endocrine neoplasia type 2A (codon 634) and 2B. Eur J Endocrinol 160 (5): 807-13, 2009. [PubMed: 19240193]
- Siqueira DR, Ceolin L, Ferreira CV, et al.: Role of RET genetic variants in MEN2-associated pheochromocytoma. Eur J Endocrinol 170 (6): 821-8, 2014. [PubMed: 24616415]
- Ceolin L, Siqueira DR, Romitti M, et al.: Molecular basis of medullary thyroid carcinoma: the role of RET polymorphisms. Int J Mol Sci 13 (1): 221-39, 2012. [PMC free article: PMC3269683] [PubMed: 22312249]
- Robledo M, Gil L, Pollán M, et al.: Polymorphisms G691S/S904S of RET as genetic modifiers of MEN 2A. Cancer Res 63 (8): 1814-7, 2003. [PubMed: 12702567]
- Margraf RL, Crockett DK, Krautscheid PM, et al.: Multiple endocrine neoplasia type 2 RET protooncogene database: repository of MEN2-associated RET sequence variation and reference for genotype/phenotype correlations. Hum Mutat 30 (4): 548-56, 2009. [PubMed: 19177457]
- Qi XP, Zhao JQ, Du ZF, et al.: Prophylactic thyroidectomy for MEN 2-related medullary thyroid carcinoma based on predictive testing for RET proto-oncogene mutation and basal serum calcitonin in China. Eur J Surg Oncol 39 (9): 1007-12, 2013. [PubMed: 23849459]
- Schreinemakers JM, Vriens MR, Valk GD, et al.: Factors predicting outcome of total thyroidectomy in young patients with multiple endocrine neoplasia type 2: a nationwide long-term follow-up study. World J Surg 34 (4): 852-60, 2010. [PMC free article: PMC2832884] [PubMed: 20063095]
- Niccoli-Sire P, Murat A, Baudin E, et al.: Early or prophylactic thyroidectomy in MEN 2/FMTC gene carriers: results in 71 thyroidectomized patients. The French Calcitonin Tumours Study Group (GETC). Eur J Endocrinol 141 (5): 468-74, 1999. [PubMed: 10576762]
- Wells SA Jr, Skinner MA: Prophylactic thyroidectomy, based on direct genetic testing, in patients at risk for the multiple endocrine neoplasia type 2 syndromes. Exp Clin Endocrinol Diabetes 106 (1): 29-34, 1998. [PubMed: 9516056]
- Szinnai G, Meier C, Komminoth P, et al.: Review of multiple endocrine neoplasia type 2A in children: therapeutic results of early thyroidectomy and prognostic value of codon analysis. Pediatrics 111 (2): E132-9, 2003. [PubMed: 12563086]
- Skinner MA, Moley JA, Dilley WG, et al.: Prophylactic thyroidectomy in multiple endocrine neoplasia type 2A. N Engl J Med 353 (11): 1105-13, 2005. [PubMed: 16162881]
- Machens A, Schneyer U, Holzhausen HJ, et al.: Prospects of remission in medullary thyroid carcinoma according to basal calcitonin level. J Clin Endocrinol Metab 90 (4): 2029-34, 2005. [PubMed: 15634717]
- Machens A, Lorenz K, Dralle H: Individualization of lymph node dissection in RET (rearranged during transfection) carriers at risk for medullary thyroid cancer: value of pretherapeutic calcitonin levels. Ann Surg 250 (2): 305-10, 2009. [PubMed: 19638924]
- Franc S, Niccoli-Sire P, Cohen R, et al.: Complete surgical lymph node resection does not prevent authentic recurrences of medullary thyroid carcinoma. Clin Endocrinol (Oxf) 55 (3): 403-9, 2001. [PubMed: 11589685]
- van Heurn LW, Schaap C, Sie G, et al.: Predictive DNA testing for multiple endocrine neoplasia 2: a therapeutic challenge of prophylactic thyroidectomy in very young children. J Pediatr Surg 34 (4): 568-71, 1999. [PubMed: 10235324]
- Hansen HS, Torring H, Godballe C, et al.: Is thyroidectomy necessary in RET mutations carriers of the familial medullary thyroid carcinoma syndrome? Cancer 89 (4): 863-7, 2000. [PubMed: 10951350]
- Wells SA Jr, Donis-Keller H: Current perspectives on the diagnosis and management of patients with multiple endocrine neoplasia type 2 syndromes. Endocrinol Metab Clin North Am 23 (1): 215-28, 1994. [PubMed: 7913027]
- Gardet V, Gatta B, Simonnet G, et al.: Lessons from an unpleasant surprise: a biochemical strategy for the diagnosis of pheochromocytoma. J Hypertens 19 (6): 1029-35, 2001. [PubMed: 11403350]
- Pacak K, Ilias I, Adams KT, et al.: Biochemical diagnosis, localization and management of pheochromocytoma: focus on multiple endocrine neoplasia type 2 in relation to other hereditary syndromes and sporadic forms of the tumour. J Intern Med 257 (1): 60-8, 2005. [PubMed: 15606377]
- Raue F, Kraimps JL, Dralle H, et al.: Primary hyperparathyroidism in multiple endocrine neoplasia type 2A. J Intern Med 238 (4): 369-73, 1995. [PubMed: 7595174]
- Milos IN, Frank-Raue K, Wohllk N, et al.: Age-related neoplastic risk profiles and penetrance estimations in multiple endocrine neoplasia type 2A caused by germ line RET Cys634Trp (TGC>TGG) mutation. Endocr Relat Cancer 15 (4): 1035-41, 2008. [PubMed: 18794325]
- Marsh DJ, McDowall D, Hyland VJ, et al.: The identification of false positive responses to the pentagastrin stimulation test in RET mutation negative members of MEN 2A families. Clin Endocrinol (Oxf) 44 (2): 213-20, 1996. [PubMed: 8849577]
- Mandel SJ, Brent GA, Larsen PR: Levothyroxine therapy in patients with thyroid disease. Ann Intern Med 119 (6): 492-502, 1993. [PubMed: 8357116]
- Sawin CT, Geller A, Hershman JM, et al.: The aging thyroid. The use of thyroid hormone in older persons. JAMA 261 (18): 2653-5, 1989. [PubMed: 2709545]
- Baloch Z, Carayon P, Conte-Devolx B, et al.: Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13 (1): 3-126, 2003. [PubMed: 12625976]
- Seib CD, Harari A, Conte FA, et al.: Utility of serum thyroglobulin measurements after prophylactic thyroidectomy in patients with hereditary medullary thyroid cancer. Surgery 156 (2): 394-8, 2014. [PMC free article: PMC4099273] [PubMed: 24882762]
- Samaan NA, Schultz PN, Hickey RC: Medullary thyroid carcinoma: prognosis of familial versus nonfamilial disease and the role of radiotherapy. Horm Metab Res Suppl 21: 21-5, 1989. [PubMed: 2807151]
- Scherübl H, Raue F, Ziegler R: Combination chemotherapy of advanced medullary and differentiated thyroid cancer. Phase II study. J Cancer Res Clin Oncol 116 (1): 21-3, 1990. [PubMed: 2312602]
- Cohen EE, Rosen LS, Vokes EE, et al.: Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol 26 (29): 4708-13, 2008. [PMC free article: PMC4859206] [PubMed: 18541897]
- Lam ET, Ringel MD, Kloos RT, et al.: Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 28 (14): 2323-30, 2010. [PMC free article: PMC2881718] [PubMed: 20368568]
- Carr LL, Mankoff DA, Goulart BH, et al.: Phase II study of daily sunitinib in FDG-PET-positive, iodine-refractory differentiated thyroid cancer and metastatic medullary carcinoma of the thyroid with functional imaging correlation. Clin Cancer Res 16 (21): 5260-8, 2010. [PMC free article: PMC3063514] [PubMed: 20847059]
- Kurzrock R, Sherman SI, Ball DW, et al.: Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 29 (19): 2660-6, 2011. [PMC free article: PMC3646303] [PubMed: 21606412]
- Wells SA Jr, Robinson BG, Gagel RF, et al.: Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30 (2): 134-41, 2012. [PMC free article: PMC3675689] [PubMed: 22025146]
- Fox E, Widemann BC, Chuk MK, et al.: Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res 19 (15): 4239-48, 2013. [PMC free article: PMC4274128] [PubMed: 23766359]
- Elisei R, Schlumberger MJ, Müller SP, et al.: Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31 (29): 3639-46, 2013. [PMC free article: PMC4164813] [PubMed: 24002501]
- Walz MK, Alesina PF, Wenger FA, et al.: Posterior retroperitoneoscopic adrenalectomy--results of 560 procedures in 520 patients. Surgery 140 (6): 943-8; discussion 948-50, 2006. [PubMed: 17188142]
- Walz MK, Alesina PF, Wenger FA, et al.: Laparoscopic and retroperitoneoscopic treatment of pheochromocytomas and retroperitoneal paragangliomas: results of 161 tumors in 126 patients. World J Surg 30 (5): 899-908, 2006. [PubMed: 16617419]
- Lairmore TC, Ball DW, Baylin SB, et al.: Management of pheochromocytomas in patients with multiple endocrine neoplasia type 2 syndromes. Ann Surg 217 (6): 595-601; discussion 601-3, 1993. [PMC free article: PMC1242859] [PubMed: 8099474]
- Okamoto T, Obara T, Ito Y, et al.: Bilateral adrenalectomy with autotransplantation of adrenocortical tissue or unilateral adrenalectomy: treatment options for pheochromocytomas in multiple endocrine neoplasia type 2A. Endocr J 43 (2): 169-75, 1996. [PubMed: 8793332]
- Inabnet WB, Caragliano P, Pertsemlidis D: Pheochromocytoma: inherited associations, bilaterality, and cortex preservation. Surgery 128 (6): 1007-11;discussion 1011-2, 2000. [PubMed: 11114636]
- Scholten A, Valk GD, Ulfman D, et al.: Unilateral subtotal adrenalectomy for pheochromocytoma in multiple endocrine neoplasia type 2 patients: a feasible surgical strategy. Ann Surg 254 (6): 1022-7, 2011. [PubMed: 22107743]
- Grubbs EG, Rich TA, Ng C, et al.: Long-term outcomes of surgical treatment for hereditary pheochromocytoma. J Am Coll Surg 216 (2): 280-9, 2013. [PubMed: 23317575]
- Norton JA, Brennan MF, Wells SA Jr: Surgical Management of Hyperparathyroidism. In: Bilezikian JP, Marcus R, Levine MA: The Parathyroids: Basic and Clinical Concepts. New York: Raven Press, 1994, pp 531-551.
- Khan MI, Waguespack SG, Hu MI: Medical management of postsurgical hypoparathyroidism. Endocr Pract 17 (Suppl 1): 18-25, 2011 Mar-Apr. [PubMed: 21134871]
- Stålberg P, Carling T: Familial parathyroid tumors: diagnosis and management. World J Surg 33 (11): 2234-43, 2009. [PubMed: 19184636]
- Peacock M, Bilezikian JP, Klassen PS, et al.: Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 90 (1): 135-41, 2005. [PubMed: 15522938]
- Schuffenecker I, Ginet N, Goldgar D, et al.: Prevalence and parental origin of de novo RET mutations in multiple endocrine neoplasia type 2A and familial medullary thyroid carcinoma. Le Groupe d'Etude des Tumeurs a Calcitonine. Am J Hum Genet 60 (1): 233-7, 1997. [PMC free article: PMC1712555] [PubMed: 8981969]
- Norum RA, Lafreniere RG, O'Neal LW, et al.: Linkage of the multiple endocrine neoplasia type 2B gene (MEN2B) to chromosome 10 markers linked to MEN2A. Genomics 8 (2): 313-7, 1990. [PubMed: 1979053]
- Carlson KM, Bracamontes J, Jackson CE, et al.: Parent-of-origin effects in multiple endocrine neoplasia type 2B. Am J Hum Genet 55 (6): 1076-82, 1994. [PMC free article: PMC1918453] [PubMed: 7977365]
- Kitamura Y, Scavarda N, Wells SA Jr, et al.: Two maternally derived missense mutations in the tyrosine kinase domain of the RET protooncogene in a patient with de novo MEN 2B. Hum Mol Genet 4 (10): 1987-8, 1995. [PubMed: 8595427]
- Rich TA, Liu M, Etzel CJ, et al.: Comparison of attitudes regarding preimplantation genetic diagnosis among patients with hereditary cancer syndromes. Fam Cancer 13 (2): 291-9, 2014. [PMC free article: PMC4159051] [PubMed: 24072553]
- Freyer G, Ligneau B, Schlumberger M, et al.: Quality of life in patients at risk of medullary thyroid carcinoma and followed by a comprehensive medical network: trends for future evaluations. Ann Oncol 12 (10): 1461-5, 2001. [PubMed: 11762820]
- Freyer G, Dazord A, Schlumberger M, et al.: Psychosocial impact of genetic testing in familial medullary-thyroid carcinoma: a multicentric pilot-evaluation. Ann Oncol 10 (1): 87-95, 1999. [PubMed: 10076727]
- Grosfeld FJ, Lips CJ, Ten Kroode HF, et al.: Psychosocial consequences of DNA analysis for MEN type 2. Oncology (Huntingt) 10 (2): 141-6; discussion 146, 152, 157, 1996. [PubMed: 8838257]
- Johnston LB, Chew SL, Trainer PJ, et al.: Screening children at risk of developing inherited endocrine neoplasia syndromes. Clin Endocrinol (Oxf) 52 (2): 127-36, 2000. [PubMed: 10671936]
- MacDonald DJ, Lessick M: Hereditary cancers in children and ethical and psychosocial implications. J Pediatr Nurs 15 (4): 217-25, 2000. [PubMed: 10969494]
- Grosfeld FJ, Lips CJ, Beemer FA, et al.: Psychological risks of genetically testing children for a hereditary cancer syndrome. Patient Educ Couns 32 (1-2): 63-7, 1997 Sep-Oct. [PubMed: 9355573]
- Giarelli E: Multiple endocrine neoplasia type 2a (MEN2a): a call for psycho-social research. Psychooncology 11 (1): 59-73, 2002 Jan-Feb. [PubMed: 11835593]
- Grosfeld FJ, Lips CJ, Beemer FA, et al.: Distress in MEN 2 family members and partners prior to DNA test disclosure. Multiple endocrine neoplasia type 2. Am J Med Genet 91 (1): 1-7, 2000. [PubMed: 10751081]
- Grosfeld FJ, Beemer FA, Lips CJ, et al.: Parents' responses to disclosure of genetic test results of their children. Am J Med Genet 94 (4): 316-23, 2000. [PubMed: 11038446]
- Giarelli E: Bringing threat to the fore: participating in lifelong surveillance for genetic risk of cancer. Oncol Nurs Forum 30 (6): 945-55, 2003 Nov-Dec. [PubMed: 14603352]
- Schultz PN: Providing information to patients with a rare cancer: using Internet discussion forums to address the needs of patients with medullary thyroid carcinoma. Clin J Oncol Nurs 6 (4): 219-22, 2002 Jul-Aug. [PubMed: 12087618]
Familial Paraganglioma Syndrome
Introduction
Paragangliomas (PGLs) and pheochromocytomas are rare tumors arising from chromaffin cells, which have the ability to synthesize, store, and secrete catecholamines and neuropeptides. In 2004, the World Health Organization characterized pheochromocytomas as tumors arising in the adrenal gland.[1] (Refer to the Pheochromocytoma section of this summary for more information). Extra-adrenal neoplasms, referred to as PGLs, may arise in various sites from the glomera along the parasympathetic nerves or the paraganglia in the sympathetic trunk. PGLs may be found in the head and neck region, abdomen, or pelvis. PGLs found in the skull base or head and neck region are usually from parasympathetic paraganglia and rarely secrete catecholamines. Representing 3% of all PGLs,[2] the tumors in the cervical region typically arise in the glomus cells, near the aortic body, along the vagal nerve, in the temporal bone, in the jugular fossa (middle ear space), or in the nose and parasinus.[3] Tumors below the neck are most commonly located in the upper mediastinum, adrenal medulla (pheochromocytoma), or the urinary bladder.[3] The reported incidence of these tumors in the general population is variable because they may be asymptomatic but ranges from 1 in 30,000 to 1 in 100,000 individuals.[3] One autopsy study found a much greater incidence of 1 in 2,000 individuals, suggesting a high frequency of occult tumors.[4] These tumors have an equal sex distribution.[5,6] PGLs can occur at any age but have the highest incidence between the ages of 40 and 50 years.[5,6]
Clinical Description
PGLs may occur sporadically, as a manifestation of a hereditary syndrome, or as the sole tumor in one of several hereditary PGL/pheochromocytoma syndromes. Up to 40% of patients presenting with apparently sporadic PGLs actually have a recognizable germline mutation in one of the known PGL/pheochromocytoma susceptibility genes.[7,8] One study found that in individuals with a single tumor and a negative family history, the likelihood of an inherited mutation was 11.6%,[7] whereas other groups detected mutations in 41% of such patients.[8,9]
PGLs are typically slow-growing tumors, and some may be present for many years before coming to clinical attention. Conversely, a minority of these tumors may be malignant and present with a more aggressive clinical course. PGL and pheochromocytoma malignancy is defined by the presence of metastases at sites distant from the primary tumor in nonchromaffin tissue. Some experts view local invasion into surrounding tissue as an additional marker of malignancy.[10,11] Others have disagreed with this classification because locally invasive tumors tend to follow a more indolent course than tumors with distant metastatic involvement.[12] There are no reliable molecular, immunohistochemical, or genetic predictors to distinguish benign and malignant tumors,[13] although some studies have shown a higher rate in SDHB carriers [14] and in individuals with larger tumors.[15] Consequently, estimation of the rate of malignancy in PGLs is difficult; rates from 5% to 20% have been reported.[7,16,17] Common sites of metastases include bone, liver, and lungs.[1]
PGLs arise from cells involved in the metabolism of catecholamines, but approximately 95% of tumors located in the head and neck region are parasympathetic and nonsecretory.[11] Only tumors arising from the sympathetic neural chain have secretory capacity. The most recognizable PGL of the head and neck is the tumor arising from the carotid body. These tumors (glomus caroticum) present a significant challenge for surgeons because of their proximity to critical vessels and cranial nerves. The presence of the neoplasm in this critical location frequently results in the compromise of nearby structures; removal of the tumor may cause permanent impairment.
Clinical Diagnosis of PGL
A PGL may cause a variety of symptoms depending on the location of the tumor and whether the tumor has secretory capacity. PGLs of the head and neck are rarely associated with elevated catecholamines. Secretory PGLs may cause hypertension, headache, tachycardia, sweating, and flushing. Typically, nonsecretory tumors are painless, coming to attention only when growth of the lesion into surrounding structures causes a mass effect. Patients with a head or neck PGL may present with an enlarging lateral neck mass, hoarseness, Horner syndrome, pulsatile tinnitus, dizziness, facial droop, or blurred vision.[1]
Imaging is the mainstay of diagnosis; the initial evaluation includes computed tomography of the neck and chest. PGLs typically appear homogeneous with intense enhancement after administration of intravenous contrast. Magnetic resonance imaging may also be used to distinguish the tumor from adjacent vascular and skeletal structures. On T2-weighted images, a tumor that is larger than 2 cm is likely to display a classic "salt and pepper" appearance, a reflection of scattered areas of signal void mingled with areas of high signal intensity from increased vascularity.[18]
Nuclear imaging in combination with anatomic imaging may be useful for localization and determination of the extent of disease (multifocality vs. distant metastatic deposits). Functional imaging with 18F-dihydroxyphenylalanine (18F-DOPA), 18F-fluorodopamine, or positron emission tomography–computed tomography (PET-CT) may be particularly helpful in localizing head and neck tumors. Additionally, 123I-metaiodobenzylguanidine plus PET-CT is very specific for PGLs. Data suggest that the selection of PET tracer utilized for tumor localization should be centered on the patient’s genetic status, based on the metabolic activity of the various tumors.[14] It has been suggested that patients with SDH and VHL mutations are more likely to have higher 18F-fluorodeoxyglucose activity, which is related to gene activation in response to hypoxia.[14,19] Some SDHB tumors only weakly concentrate 18F-DOPA, and patients with SDHx mutations may have false-negative results with such scans. Tumors with VHL mutations may likewise be missed with metaiodobenzylguanidine scans.[14] (Refer to Table 7 for a list of various mutations and their optimal imaging modality.)
Table 7. Gene-specific Imaging and Paraganglioma (PGL) Malignancy Rates
| Gene | Tumor Location | PGL Malignancy Rate | First-line Imaging Modality | Second-line Imaging Modality | |
|---|---|---|---|---|---|
| Head/Neck | Thoracic/ Retroperitoneal | ||||
| SDHB | MI | MA | 30%–70% | 18F-FDG | 18F-DOPA |
| SDHC | MA | MI | NR | 18F-DOPA | NR |
| SDHD | MA | MI | <5% | 18F-DOPA | FDG-MIBG |
| SDHAF2a | MA | NR | NR | 18F-DOPA | NR |
| SDHAa | MA | R | <10% | 18F-FDG | 18F-FDAb |
| RET | R | MA | <5% | 18F-FDOPA | 123I-MIBG |
| VHL | R | MA | 5% | 18F-FDG | 18F-FDOPA |
| NF1 | NR | MA | 12% | 18F-FDOPA | 123I-MIBG |
| TMEM127a | MI | MA | NR | NR | NR |
| MAXa | NR | MA | NR | NR | NR |
FDG-MIBG = fluorodeoxyglucose-metaiodobenzylguanidine; 18F-DOPA = 18F-dihydroxyphenylalanine; 18F-FDA = 18F-fluorodopamine; 18F-FDG = 18F-fluorodeoxyglucose; 18F-FDOPA = 18F-fluoro-L-dihydroxyphenylalanine; 123I-MIBG = 123I-metaiodobenzylguanidine.
MA = majority (>50%); MI = minority (10%–50%); NR = not reported; R = rare (<10%).
aThese mutations are rare. There is very little information available about the best imaging modality for these mutations. This table will be updated as more information becomes available.
b18F-FDA is currently only available at the National Institutes of Health in Bethesda, MD, as an experimental tracer.
Genetics, Inheritance, and Genetic Testing
PGLs and pheochromocytomas can be seen as part of several well-described tumor susceptibility syndromes including von Hippel-Lindau, multiple endocrine neoplasia type 2, neurofibromatosis type 1, Carney-Stratakis syndrome, and familial paraganglioma (FPGL) syndrome. FPGL is most commonly caused by mutations in one of the following four genes: SDHA, SDHB, SDHC, and SDHD (collectively referred to as SDHx). The SDHx proteins form part of the succinate dehydrogenase (SDH) complex, which is located on the inner mitochondrial membrane and plays a critical role in cellular energy metabolism.[24] Mutations in SDHB are most common, followed by SDHD and rarely SDHC and SDHA. More recently, mutations in the SDHAF2 (also called SDH5), TMEM127, and MAX genes have been described in FPGL, but these mutations are rare. The mechanism of tumor formation has remained elusive. One study suggests that SDHx-associated tumors display a hypermethylator phenotype that is associated with downregulation of important genes involved in the differentiation of neuroendocrine tissues.[25]
The inheritance pattern of FPGL depends on the gene involved. While most families show traditional autosomal dominant inheritance, those with mutations in SDHAF2 and SDHD show almost exclusive paternal transmission of the phenotype. In other words, while the mutation can be passed down from mother or father, tumors will develop only if the mutation is inherited from the father.[26,27] In cases of FPGL not caused by SDHD or SDHAF2 mutations, first-degree relatives of an affected individual have a 50% chance of carrying the mutation and are at increased risk of developing PGLs. Because the family history can appear negative in families with lower penetrance mutations, it is important to offer genetic testing to all unaffected first-degree relatives once the mutation in the family has been identified. All patients with pheochromocytoma or PGL, even those with apparently sporadic tumors, may be considered for genetic testing because of the high frequency of genetic mutations associated with these conditions.[28]
Genetic testing for hereditary pheochromocytoma and PGL syndromes is largely based on published algorithms,[28] whereby testing is performed stepwise based on factors such as tumor type and location, age at diagnosis, family history, and presence of malignancy.[7,29,30] This approach has allowed for cost-effective, targeted testing based on clinical features. Within the last several years, however, next-generation sequencing (NGS) technology has led to a dramatic decrease in the cost of genetic testing, and it is now possible to test for mutations in 10 to 30 genes for the same cost of testing two or three genes. These tests may be more appropriate for individuals and families who have an atypical presentation or overlapping clinical features. If the cost associated with multigene testing panels continues to decrease, it is likely that the testing algorithms may soon be obsolete for PGL and pheochromocytoma. A recent series analyzed 85 PGL and pheochromocytoma samples using an NGS panel test for the ten known PGL susceptibility genes and showed a sensitivity of 98.7%.[31]
Genotype-Phenotype Correlations
In FPGL, the type and location of tumors, age at onset, and lifetime penetrance vary depending on the gene that is mutated. While these correlations can help guide genetic testing and screening decisions, caution must be used given the high degree of variability seen in this condition. FPGL syndromes are among the rare inherited diseases in which genomic imprinting occurs. For example, the SDHD mutation is normally not activated when inherited from the mother, and the risk of FPGL syndromes is not increased. However, the mutation is turned on when the gene is inherited from the father, and the risk is increased.
SDHD mutations are mainly associated with an increased risk of parasympathetic PGLs. These are more commonly multifocal and located in the head and neck, while tumors in SDHB carriers are more often located in the abdomen.[32,33] One series showed a risk of 71% for a head and neck tumor in SDHD carriers, as opposed to a 29% risk in SDHB carriers. The lifetime risk for any PGL in any location in SDHD carriers was estimated to be as high as 77% by age 50 years in one series [32] and 90% by age 70 years in a second series.[33] A review of more than 1,700 cases reported in the literature provided similar estimates, suggesting a lifetime penetrance of 86%.[34] The rate of malignancy in SDHD carriers is lower than 5%.[34]
Mutations in the SDHB gene are associated with sympathetic PGLs, although pheochromocytoma and parasympathetic PGLs also have been described. SDHB PGLs are more commonly located in the abdomen and mediastinum than in the head and neck. A review of 1,700 cases suggested a lifetime penetrance of 77%.[34] The rate of malignancy is higher with SDHB than with the other SDH genes, with up to one-third of patients having malignant tumors in most series.[32,33] Mutations in SDHB have also been associated with several other tumors and malignancies, including gastrointestinal stromal tumors (GISTs), renal cell carcinoma, and papillary thyroid cancer.[32,33]
SDHC mutations are rare, accounting for an estimated 0.5% of all PGLs.[34] In one series of 153 patients with multiple PGLs or a single PGL diagnosed before age 40 years, three (2%) had an SDHC mutation.[35] Another series of 121 index cases from a head and neck PGL registry showed a mutation rate of 4% (5 of 121).[36] SDHC mutations most commonly cause head and neck PGLs but have been seen in a small number of patients with abdominal PGLs.[7,37] Mutations in SDHB, SDHC, and SDHD can also cause Carney-Stratakis syndrome, which is characterized by the dyad of PGLs and GISTs.[38]
Mutations in SDHA, SDAHF2, MAX, and TMEM127 have been described in a small number of cases. Collectively, they account for less than 2% to 3% of all cases. Although biallelic mutations in SDHA have long been known to cause the autosomal recessive condition inherited juvenile encephalopathy/Leigh syndrome,[39] it was not until recently that monoallelic mutations were linked to an increased risk of developing PGL. Only a handful of cases have been described. Tumors can develop in the head and neck, the adrenal, or in the abdomen (extra-adrenal).[40,41] The SDHAF2 gene encodes a protein that is responsible for flavination of SDHA and proper functioning of the SDHA subunit of the SDH complex. To date, mutations in SDHAF2 have been described in fewer than 20 cases and only with head and neck PGLs.[42] The MAX gene was first described as a pheochromocytoma susceptibility gene in 2011 through exome sequencing of three unrelated cases.[43] Three different germline mutations were identified, and a follow-up series of 59 cases by the same group identified an additional five mutations. The MAX protein is part of MYC-MAX-MXD1 network, which plays a key role in the development and progression of neural crest cell tumors.[44] The TMEM127 gene is located on chromosome 2q11.2 and encodes a transmembrane protein known to be a negative regulator of mTOR, which regulates multiple cellular processes. A review of 23 patients with TMEM127 mutations showed that 96% (22 of 23) had a pheochromocytoma and 9% (2 of 23) had a PGL.[34]
Interventions
Preoperative management
Before surgical removal of a PGL, all patients, including those without clinically apparent catecholamine excess, generally undergo biochemical testing to evaluate the secretory capacity of the tumor(s).[45] This evaluation is best performed by measuring urine and/or plasma fractionated metanephrines (normetanephrine and metanephrine), which yields a higher sensitivity and specificity than directly measuring catecholamines (norepinephrine, dopamine, and epinephrine).[46-48] For patients whose plasma metanephrines levels are measured, blood is collected after an intravenous catheter has been inserted and the patient has been in a supine position for 15 to 20 minutes.[49] Additionally, the patient should not have food or caffeinated beverages, smoke cigarettes, or engage in strenuous physical activity in the 8 to 12 hours before the blood draw.[49]
Preoperative medical therapy is not essential for patients without evidence of catecholamine hypersecretion, although some advocate its use regardless of the results of hormonal testing.[49] However, patients with catecholamine-secreting tumors unquestionably require some form of pharmacologic therapy to control hypertension for at least 10 to 14 days before surgery.[50] Failure to adequately block the catecholamine excess can dramatically increase the risk of perioperative mortality from hypertensive crisis and lethal arrhythmias.[51,52]
The preoperative medical regimen may incorporate one of several drugs; in the absence of a randomized controlled trial comparing the various regimens, there is no universally recommended approach. The alpha-adrenoreceptor blocker phenoxybenzamine (Dibenzyline) is most frequently used to control blood pressure and expand the blood volume.[49] Other alpha-blocking drugs have also been used with success, including prazosin, terazosin, or doxazosin; these drugs are more specific alpha-1 adrenergic competitive antagonists and have a shorter half-life than phenoxybenzamine.[53,54] The noncompetitive binding of phenoxybenzamine to the alpha receptors, coupled with its longer half-life, may result in a sustained effect of the drug, with some patients experiencing postoperative hypotension.[49,55] One study found that patients treated with sustained-release doxazosin had more stable perioperative hemodynamic changes and a shorter time interval to preoperative blood pressure control than did patients who received phenoxybenzamine.[55]
Once the alpha blockade is initiated, expansion of the blood volume is often necessary, as these patients are typically volume contracted.[56,57] In addition to the vasodilatory effects from alpha blockade, volume expansion may be achieved by consuming a high-sodium diet and high fluid intake or a preoperative saline infusion. A clinical manifestation of adequate blockade is the symptom of nasal stuffiness or lightheadedness.
If heart-rate control is needed, a beta blocker may be used in conjunction with the alpha blocker 2 to 3 days before surgery. Beta blockers are not generally used alone, as unopposed alpha stimulation can result in exacerbation of hypertension. It is also important to note that some patients may have undiagnosed cardiac insufficiency from the chronic stimulation by catecholamines; expansion of the blood volume and use of beta blockers may precipitate acute heart failure.
Calcium channel blockers such as nicardipine or nifedipine also have been employed to control the hypertension preoperatively.[58] A calcium channel blocker may be used in conjunction with alpha and beta blockade for refractory hypertension or used alone as a second-line agent for patients with intolerable side effects from alpha blockade.[49]
Surgical interventions
Surgical resection is the treatment of choice for PGLs. Genetic testing is best performed before the initial surgery to inform the risk of recurrent or contralateral disease and to guide the extent of resection (e.g., whether to preserve the cortex). After appropriate preoperative pharmacologic preparation (see above), a successful resection involves several key elements. A thorough medical history and physical exam includes obtaining information about the patient's autonomic nervous system, including the presence of tachyarrhythmias or elevated blood pressure. Inappropriate preoperative preparation, induction of anesthesia, tumor manipulation, or other stimulation can result in massive intraoperative outpouring of catecholamines with subsequent hypertensive crisis and possible stroke, arrhythmia, or myocardial infarction.[59]
Accurate preoperative imaging identifies vascularity and enables assessment of peritumoral invasion. En bloc resection with possible vascular reconstruction may be part of the preoperative plan. The arterial supply frequently includes small tributaries from the aorta, renal artery, and the inferior phrenic artery or iliac arteries. The arterial supply should be studied preoperatively to inform the surgical planning strategy. This is particularly critical because unexpected intraoperative vascular findings can be life threatening.
Central line access is critical so that intravenous fluids and medications can be rapidly administered. Communication between the operating surgeon and the anesthesiologist is necessary during the procedure. Before the tumor is removed, blood pressure is controlled with vasodilators, nitroprusside, and/or calcium channel blockers. Upon ligation of the vessels, pressor support may be required.
Adequate exposure of the tumor is important. PGLs are commonly located in the para-aortic retroperitoneal sympathetic chain above the aortic bifurcation, below the takeoff of the inferior mesenteric artery (organ of Zuckerkandl), or near the dome of the bladder.[59,60] Means of exposure using the transperitoneal approach are based on the anatomic location of the tumor. Proximal and distal control of blood vessels is critical. Vascular reconstruction can be considered in selected patients with local, large-vessel invasion, if such reconstruction will result in complete tumor extirpation. Dissection around the tumor without capsular rupture or tumor manipulation is important. Ideally, the adrenal vein is ligated early in the dissection to prevent release of catecholamines if the tumor is manipulated. However, this may not be possible in the case of a large tumor. When direct visualization of the tumor is apparent, clear differentiation between the surrounding tissue planes is necessary. Commonly, malignant PGLs have a dense fibrous capsule that is adherent to surrounding vascularity, which can make a complete resection difficult.[59] Regional lymph nodes are commonly involved with the tumor, and if suspected preoperatively or noted intraoperatively, a regional lymphadenectomy should be performed. En bloc resection of surrounding organs is rarely necessary.
Open resection and laparoscopic approaches both are safe.[61] There is no evidence that the carbon dioxide insufflation triggers a catecholamine crisis.[61] Open resection is recommended for tumors larger than 6 cm because of the increased risk of technical difficulty within the confined and closed space created with only carbon dioxide insufflation during laparoscopy. Additionally, there is an increased risk of tumor rupture with laparoscopic approaches, and they are inferior regarding nodal sampling.[62] However, patients experience faster resolution of postoperative ileus; decreased analgesic requirements; a shorter hospital stay; and shorter convalescence, with a quicker return to normal activity with laparoscopic approaches.[16] Direct access to the para-aortic region can be achieved with the posterior approach. Robotic assistance has improved the technique by offering a 3-dimensional, magnified view of the anatomy.[63] For an anterior open resection, incisions are based on the tumor location. Cardiopulmonary bypass may be required for tumors in the middle mediastinum because they usually involve the left atrium.[64] Posterior mediastinal tumors may require posterior thoracotomy. Tumors located in the bladder may require partial cystectomy. Regardless of tumor size, all patients should undergo evaluation for metastasis.
In summary, surgical resection is the treatment of choice for patients with PGL. If the disease is limited at the time of diagnosis, surgical resection can be undertaken with curative intent. If disease is more extensive or locally recurrent, then surgical intervention is undertaken for tumor debulking and palliation.[59] In patients with noncurable disease, surgery of the primary tumor and/or metastases could reduce hormone secretion and may be appropriate to prevent complications related to a critical anatomical location.[65] Surgery should be performed in referral centers and by expert hands. Long-term follow-up may identify metastases years after the initial diagnosis.
References
- DeLellis RA, Lloyd RV, Heitz PU, et al., eds.: Pathology and Genetics of Tumours of Endocrine Organs. Lyon, France: IARC Press, 2004. World Health Organization classification of tumours, vol. 8.
- Offergeld C, Brase C, Yaremchuk S, et al.: Head and neck paragangliomas: clinical and molecular genetic classification. Clinics (Sao Paulo) 67 (Suppl 1): 19-28, 2012. [PMC free article: PMC3328838] [PubMed: 22584701]
- Raygada M, Pasini B, Stratakis CA: Hereditary paragangliomas. Adv Otorhinolaryngol 70: 99-106, 2011. [PMC free article: PMC4221053] [PubMed: 21358191]
- McNeil AR, Blok BH, Koelmeyer TD, et al.: Phaeochromocytomas discovered during coronial autopsies in Sydney, Melbourne and Auckland. Aust N Z J Med 30 (6): 648-52, 2000. [PubMed: 11198571]
- O'Riordain DS, Young WF Jr, Grant CS, et al.: Clinical spectrum and outcome of functional extraadrenal paraganglioma. World J Surg 20 (7): 916-21; discussion 922, 1996. [PubMed: 8678971]
- Erickson D, Kudva YC, Ebersold MJ, et al.: Benign paragangliomas: clinical presentation and treatment outcomes in 236 patients. J Clin Endocrinol Metab 86 (11): 5210-6, 2001. [PubMed: 11701678]
- Mannelli M, Castellano M, Schiavi F, et al.: Clinically guided genetic screening in a large cohort of italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J Clin Endocrinol Metab 94 (5): 1541-7, 2009. [PubMed: 19223516]
- Fishbein L, Merrill S, Fraker DL, et al.: Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol 20 (5): 1444-50, 2013. [PMC free article: PMC4291281] [PubMed: 23512077]
- Bacca A, Sellari Franceschini S, Carrara D, et al.: Sporadic or familial head neck paragangliomas enrolled in a single center: clinical presentation and genotype/phenotype correlations. Head Neck 35 (1): 23-7, 2013. [PubMed: 22290790]
- Eisenhofer G, Bornstein SR, Brouwers FM, et al.: Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer 11 (3): 423-36, 2004. [PubMed: 15369446]
- Zarnegar R, Kebebew E, Duh QY, et al.: Malignant pheochromocytoma. Surg Oncol Clin N Am 15 (3): 555-71, 2006. [PubMed: 16882497]
- Medeiros LJ, Wolf BC, Balogh K, et al.: Adrenal pheochromocytoma: a clinicopathologic review of 60 cases. Hum Pathol 16 (6): 580-9, 1985. [PubMed: 3997135]
- Jovanovic R, Kostadinova-Kunovska S, Bogoeva B, et al.: Histological features, Ki-67 and Bcl-2 immunohistochemical expression and their correlation with the aggressiveness of pheochromocytomas. Prilozi 33 (2): 23-40, 2012. [PubMed: 23425867]
- Taïeb D, Neumann H, Rubello D, et al.: Modern nuclear imaging for paragangliomas: beyond SPECT. J Nucl Med 53 (2): 264-74, 2012. [PubMed: 22302963]
- Eisenhofer G, Lenders JW, Siegert G, et al.: Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer 48 (11): 1739-49, 2012. [PMC free article: PMC3372624] [PubMed: 22036874]
- Walz MK, Alesina PF, Wenger FA, et al.: Laparoscopic and retroperitoneoscopic treatment of pheochromocytomas and retroperitoneal paragangliomas: results of 161 tumors in 126 patients. World J Surg 30 (5): 899-908, 2006. [PubMed: 16617419]
- Lee JH, Barich F, Karnell LH, et al.: National Cancer Data Base report on malignant paragangliomas of the head and neck. Cancer 94 (3): 730-7, 2002. [PubMed: 11857306]
- Olsen WL, Dillon WP, Kelly WM, et al.: MR imaging of paragangliomas. AJR Am J Roentgenol 148 (1): 201-4, 1987. [PubMed: 3024473]
- Span PN, Rao JU, Oude Ophuis SB, et al.: Overexpression of the natural antisense hypoxia-inducible factor-1alpha transcript is associated with malignant pheochromocytoma/paraganglioma. Endocr Relat Cancer 18 (3): 323-31, 2011. [PubMed: 21422080]
- Fishbein L, Nathanson KL: Pheochromocytoma and paraganglioma: understanding the complexities of the genetic background. Cancer Genet 205 (1-2): 1-11, 2012 Jan-Feb. [PMC free article: PMC3311650] [PubMed: 22429592]
- Gimenez-Roqueplo AP, Dahia PL, Robledo M: An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res 44 (5): 328-33, 2012. [PubMed: 22328163]
- Bausch B, Malinoc A, Maruschke L, et al.: [Genetics of pheochromocytoma]. Chirurg 83 (6): 511-8, 2012. [PubMed: 22481546]
- Taïeb D, Timmers HJ, Hindié E, et al.: EANM 2012 guidelines for radionuclide imaging of phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging 39 (12): 1977-95, 2012. [PMC free article: PMC4714772] [PubMed: 22926712]
- Hussain I, Husain Q, Baredes S, et al.: Molecular genetics of paragangliomas of the skull base and head and neck region: implications for medical and surgical management. J Neurosurg 120 (2): 321-30, 2014. [PubMed: 24236653]
- Letouzé E, Martinelli C, Loriot C, et al.: SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 23 (6): 739-52, 2013. [PubMed: 23707781]
- van der Mey AG, Maaswinkel-Mooy PD, Cornelisse CJ, et al.: Genomic imprinting in hereditary glomus tumours: evidence for new genetic theory. Lancet 2 (8675): 1291-4, 1989. [PubMed: 2574254]
- Baysal BE: Mitochondrial complex II and genomic imprinting in inheritance of paraganglioma tumors. Biochim Biophys Acta 1827 (5): 573-7, 2013. [PubMed: 23291190]
- Lenders JW, Duh QY, Eisenhofer G, et al.: Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99 (6): 1915-42, 2014. [PubMed: 24893135]
- Amar L, Bertherat J, Baudin E, et al.: Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol 23 (34): 8812-8, 2005. [PubMed: 16314641]
- Neumann HP, Erlic Z, Boedeker CC, et al.: Clinical predictors for germline mutations in head and neck paraganglioma patients: cost reduction strategy in genetic diagnostic process as fall-out. Cancer Res 69 (8): 3650-6, 2009. [PubMed: 19351833]
- Rattenberry E, Vialard L, Yeung A, et al.: A comprehensive next generation sequencing-based genetic testing strategy to improve diagnosis of inherited pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 98 (7): E1248-56, 2013. [PubMed: 23666964]
- Neumann HP, Pawlu C, Peczkowska M, et al.: Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 292 (8): 943-51, 2004. [PubMed: 15328326]
- Ricketts CJ, Forman JR, Rattenberry E, et al.: Tumor risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Hum Mutat 31 (1): 41-51, 2010. [PubMed: 19802898]
- Welander J, Söderkvist P, Gimm O: Genetics and clinical characteristics of hereditary pheochromocytomas and paragangliomas. Endocr Relat Cancer 18 (6): R253-76, 2011. [PubMed: 22041710]
- Pęczkowska M, Kowalska A, Sygut J, et al.: Testing new susceptibility genes in the cohort of apparently sporadic phaeochromocytoma/paraganglioma patients with clinical characteristics of hereditary syndromes. Clin Endocrinol (Oxf) 79 (6): 817-23, 2013. [PubMed: 23551045]
- Schiavi F, Boedeker CC, Bausch B, et al.: Predictors and prevalence of paraganglioma syndrome associated with mutations of the SDHC gene. JAMA 294 (16): 2057-63, 2005. [PubMed: 16249420]
- Peczkowska M, Cascon A, Prejbisz A, et al.: Extra-adrenal and adrenal pheochromocytomas associated with a germline SDHC mutation. Nat Clin Pract Endocrinol Metab 4 (2): 111-5, 2008. [PubMed: 18212813]
- Pasini B, McWhinney SR, Bei T, et al.: Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet 16 (1): 79-88, 2008. [PubMed: 17667967]
- Horváth R, Abicht A, Holinski-Feder E, et al.: Leigh syndrome caused by mutations in the flavoprotein (Fp) subunit of succinate dehydrogenase (SDHA). J Neurol Neurosurg Psychiatry 77 (1): 74-6, 2006. [PMC free article: PMC2117401] [PubMed: 16361598]
- Burnichon N, Brière JJ, Libé R, et al.: SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet 19 (15): 3011-20, 2010. [PMC free article: PMC2901140] [PubMed: 20484225]
- Korpershoek E, Favier J, Gaal J, et al.: SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab 96 (9): E1472-6, 2011. [PubMed: 21752896]
- Bayley JP, Kunst HP, Cascon A, et al.: SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol 11 (4): 366-72, 2010. [PubMed: 20071235]
- Comino-Méndez I, Gracia-Aznárez FJ, Schiavi F, et al.: Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet 43 (7): 663-7, 2011. [PubMed: 21685915]
- Grandori C, Cowley SM, James LP, et al.: The Myc/Max/Mad network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol 16: 653-99, 2000. [PubMed: 11031250]
- Grossman A, Pacak K, Sawka A, et al.: Biochemical diagnosis and localization of pheochromocytoma: can we reach a consensus? Ann N Y Acad Sci 1073: 332-47, 2006. [PubMed: 17102103]
- Lenders JW, Pacak K, Walther MM, et al.: Biochemical diagnosis of pheochromocytoma: which test is best? JAMA 287 (11): 1427-34, 2002. [PubMed: 11903030]
- Eisenhofer G, Lenders JW, Linehan WM, et al.: Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel-Lindau disease and multiple endocrine neoplasia type 2. N Engl J Med 340 (24): 1872-9, 1999. [PubMed: 10369850]
- Sawka AM, Jaeschke R, Singh RJ, et al.: A comparison of biochemical tests for pheochromocytoma: measurement of fractionated plasma metanephrines compared with the combination of 24-hour urinary metanephrines and catecholamines. J Clin Endocrinol Metab 88 (2): 553-8, 2003. [PubMed: 12574179]
- Chen H, Sippel RS, O'Dorisio MS, et al.: The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas 39 (6): 775-83, 2010. [PMC free article: PMC3419007] [PubMed: 20664475]
- Pacak K: Preoperative management of the pheochromocytoma patient. J Clin Endocrinol Metab 92 (11): 4069-79, 2007. [PubMed: 17989126]
- GRAHAM JB: Pheochromocytoma and hypertension; an analysis of 207 cases. Int Abstr Surg 92 (2): 105-21, 1951. [PubMed: 14823753]
- Goldstein RE, O'Neill JA Jr, Holcomb GW 3rd, et al.: Clinical experience over 48 years with pheochromocytoma. Ann Surg 229 (6): 755-64; discussion 764-6, 1999. [PMC free article: PMC1420821] [PubMed: 10363888]
- Miura Y, Yoshinaga K: Doxazosin: a newly developed, selective alpha 1-inhibitor in the management of patients with pheochromocytoma. Am Heart J 116 (6 Pt 2): 1785-9, 1988. [PubMed: 2904751]
- Prys-Roberts C, Farndon JR: Efficacy and safety of doxazosin for perioperative management of patients with pheochromocytoma. World J Surg 26 (8): 1037-42, 2002. [PubMed: 12192533]
- Zhu Y, He HC, Su TW, et al.: Selective α1-adrenoceptor antagonist (controlled release tablets) in preoperative management of pheochromocytoma. Endocrine 38 (2): 254-9, 2010. [PubMed: 21046486]
- Ross EJ, Prichard BN, Kaufman L, et al.: Preoperative and operative management of patients with phaeochromocytoma. Br Med J 1 (5534): 191-8, 1967. [PMC free article: PMC1840518] [PubMed: 4381157]
- Hack HA: The perioperative management of children with phaeochromocytoma. Paediatr Anaesth 10 (5): 463-76, 2000. [PubMed: 11012949]
- Proye C, Thevenin D, Cecat P, et al.: Exclusive use of calcium channel blockers in preoperative and intraoperative control of pheochromocytomas: hemodynamics and free catecholamine assays in ten consecutive patients. Surgery 106 (6): 1149-54, 1989. [PubMed: 2588118]
- Mundschenk J, Lehnert H: Malignant pheochromocytoma. Exp Clin Endocrinol Diabetes 106 (5): 373-6, 1998. [PubMed: 9831301]
- Ober WB: Emil Zuckerkandl and his delightful little organ. Pathol Annu 18 Pt 1: 103-19, 1983. [PubMed: 6348671]
- Vargas HI, Kavoussi LR, Bartlett DL, et al.: Laparoscopic adrenalectomy: a new standard of care. Urology 49 (5): 673-8, 1997. [PubMed: 9145969]
- Mazzaglia PJ, Vezeridis MP: Laparoscopic adrenalectomy: balancing the operative indications with the technical advances. J Surg Oncol 101 (8): 739-44, 2010. [PubMed: 20512951]
- Dickson PV, Jimenez C, Chisholm GB, et al.: Posterior retroperitoneoscopic adrenalectomy: a contemporary American experience. J Am Coll Surg 212 (4): 659-65; discussion 665-7, 2011. [PubMed: 21463807]
- Nonaka K, Makuuchi H, Naruse Y, et al.: Surgical excision of malignant pheochromocytoma in the left atrium. Jpn J Thorac Cardiovasc Surg 48 (2): 126-8, 2000. [PubMed: 10769996]
- Adjallé R, Plouin PF, Pacak K, et al.: Treatment of malignant pheochromocytoma. Horm Metab Res 41 (9): 687-96, 2009. [PMC free article: PMC3658628] [PubMed: 19672813]
Carney-Stratakis Syndrome
Clinical Description
Carney-Stratakis syndrome (CSS; also known as Carney-Stratakis dyad) was first described in 2002. Although similarly named, this syndrome is distinctly different from Carney Complex and Carney Triad (see Table 8). CSS is characterized by an autosomal dominant germline mutation in the succinate dehydrogenase (SDH) subunit B, C, or D (SDHx) genes that demonstrates incomplete penetrance. Affected individuals develop multifocal, locally aggressive gastrointestinal stromal tumors (GISTs) and multiple neck, intrathoracic, and intra-abdominal paragangliomas (PGLs) at relatively early ages.[1-3] CSS-associated GISTs and PGLs display phenotypes that differ from their sporadically occurring, more-common counterparts; as a result, it is important to understand the unique features of imaging, treatment, and surveillance in patients with CSS.
Table 8. Comparison of Carney-Stratakis Syndrome, Carney Triad, and Carney Complex
| Syndrome | Inheritance Pattern | Mean Age at Onset (y) | Affected Sex | Associated Lesions | Mutations | Tumor Behavior |
|---|---|---|---|---|---|---|
| Carney-Stratakis Syndrome [1,3,4] | AD | 23 | M, F | Paraganglioma, stomach epithelioid GIST | Germline SDH mutations; no KIT or PDGFRA mutations | GIST metastasis but protracted course; paraganglioma aggressive |
| Carney Triad [4-6] | None | <30 | >95% F | Lung chondroma, paraganglioma, stomach epithelioid GIST | No KIT, PDGFRA, or SDH mutations | GIST metastasis but protracted course |
| Carney Complex [7,8] | AD | 20 | M, F | Lentigines, myxomas, schwannoma, thyroid follicular adenomas or carcinoma, primary pigmented nodular adrenocortical disease, pituitary adenomas | Germline PRKAR1A mutations | N/A |
AD = autosomal dominant; GIST = gastrointestinal stromal tumor; F = female; M = male.
Genetics, Inheritance, and Genetic Testing
The tumorigenesis of CSS-associated GISTs appears to involve succinate dehydrogenase deficiency rather than gain-of-function mutations in the KIT or PDGFRA gene, as is seen in the vast majority of GISTs.[9] SDH deficiency is also a characteristic finding of pediatric-type GISTs; CSS-associated GISTs display clinical findings similar to these tumors, including young age at onset (median age, 19 years), specificity to the stomach, multifocality, and resistance to imatinib.[3,10-12] Furthermore, tumor size and mitotic rate do not accurately predict metastatic potential or survival, as SDH-deficient GISTs frequently metastasize to regional lymph nodes, the peritoneal cavity, and the liver; however, long-term survival is common.[6,13]
Refer to the Genetics, Inheritance, and Genetic Testing section in the Familial PGL section of this summary for more information about genetic testing for the genes involved in CSS.
Interventions
Because multiple primary GISTs and PGLs are common with CSS, preoperative imaging is paramount to accurately identify the extent of disease before surgical planning. Most patients will present having already undergone imaging with computed tomography (CT) or magnetic resonance imaging (MRI). Both methods have excellent sensitivity for identifying PGLs, but additional functional imaging is recommended because of the diffuse nature of these tumors. 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET)/CT is superior to 123I-metaiodobenzylguanidine (123I-MIBG) at identifying SDHx-associated PGLs and, because of the high metabolic activity of GISTs, has excellent sensitivity in identifying them..[14,15] Thus, in patients with SDHx mutations, including those with CSS, 18F-FDG PET/CT is the preferred functional imaging modality to optimally detect and stage all GISTs and PGLs.[16] Some evidence suggests that 18F-fluoro-L-dihydroxyphenylalanine (18F-FDOPA) PET/CT is superior at identifying the primary PGL, while 18F-FDG PET/CT is superior at identifying metastases.
There are no prospective treatment studies involving patients with CSS; therefore, recommendations are based on limited clinical experience, single case series, and extrapolations from genetically-similar tumors with similar clinical behavior. The mainstay of treatment for CSS-associated GISTs and PGLs is complete surgical resection of the tumor. The timing of the operation correlates with the presentation of the tumor. Surgical resection can be accomplished with laparoscopic or open techniques. For PGLs, vascular reconstruction is uncommon. Although PGLs are commonly present in the paraaortic region, the need for major vascular reconstruction is uncommon. GIST tumors can be resected with wedge resection and primary closure and re-anastomosis. Ensuring negative margins is important, as patients for whom a complete resection is accomplished experience the longest survival.[17] In the rare setting of synchronous disease, combined resection is appropriate if tolerable by the patient. More commonly, tumors develop metachronously, with GISTs arising first; individual resection occurs at the time of diagnosis of each tumor.
A thorough preoperative endoscopy and complete surgical exploration of the stomach are essential, as multiple separate GISTs are frequently encountered. The high frequency of multifocality and the likelihood of tumor recurrence do not justify a prophylactic total gastrectomy because of its substantial associated morbidity. Furthermore, a total gastrectomy is generally only performed when the current disease burden precludes a lesser resection. To this end, gastric wedge resection with gross negative margins is the surgical goal.[18] Sampling of any suspicious nodes at the time of resection is commonly performed. Evidence suggests that locally advanced CSS-associated GISTs demonstrate a rather indolent course;[19] thus, the concern for nodal involvement based on preoperative imaging or abdominal exploration need not deter resection of the primary tumor. While a role for neoadjuvant imatinib in locally advanced adult-type GISTs has been widely described to improve resectability or reduce the burden of resection, it is unlikely to have any effect in locally advanced SDH-deficient GISTs.[20] Evidence suggests that for these tumors, the second-line targeted agents, including sorafenib, sunitinib, dasatinib, and nilotinib, may be beneficial in the adjuvant setting.[21,22] No data support using these agents in the neoadjuvant setting at this time.
Regarding treatment of CSS-associated PGLs, patients are commonly initiated on alpha-blockade preoperatively to minimize perioperative cardiac morbidity and mortality. PGLs typically occur in the para-aortic chain from the urinary bladder and the aortic bifurcation to the superior mediastinum and head and neck. As in the treatment of GISTs, the operative goal is resection of all known disease. Preoperative imaging and intra-operative exploration are essential to achieving this goal. Multiple tumors are common; when disease is present in the bilateral adrenal glands, the surgeon faces the possibility of rendering a patient steroid dependent with a lifelong risk of a fatal Addisonian crisis. In this setting, a surgeon proficient in performing a cortical-sparing adrenalectomy should be consulted.
Surveillance
Although the natural history of CSS is poorly understood, experts recommend that ongoing surveillance include the following: close patient follow-up with annual history that focuses on symptoms of anemia and catecholamine excess, physical exam, biochemical analysis with plasma metanephrine level and chromogranin A to detect recurrent PGLs, and radial imaging. Although many PGLs do not secrete catecholamines, chromogranin A has been found to be elevated in PGLs and may be a useful marker for tumor recurrence. The appropriate screening imaging modality is unknown at this time, but as stated above, 18F-FDG PET/CT is highly sensitive at identifying extra-adrenal PGLs and GISTs. Because of the risks of ionizing radiation exposure from CT, some suggest using MRI for annual surveillance.[14,16]
References
- Carney JA, Stratakis CA: Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am J Med Genet 108 (2): 132-9, 2002. [PubMed: 11857563]
- McWhinney SR, Pasini B, Stratakis CA, et al.: Familial gastrointestinal stromal tumors and germ-line mutations. N Engl J Med 357 (10): 1054-6, 2007. [PubMed: 17804857]
- Pasini B, McWhinney SR, Bei T, et al.: Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet 16 (1): 79-88, 2008. [PubMed: 17667967]
- Gaal J, Stratakis CA, Carney JA, et al.: SDHB immunohistochemistry: a useful tool in the diagnosis of Carney-Stratakis and Carney triad gastrointestinal stromal tumors. Mod Pathol 24 (1): 147-51, 2011. [PMC free article: PMC3415983] [PubMed: 20890271]
- Agaimy A, Pelz AF, Corless CL, et al.: Epithelioid gastric stromal tumours of the antrum in young females with the Carney triad: a report of three new cases with mutational analysis and comparative genomic hybridization. Oncol Rep 18 (1): 9-15, 2007. [PubMed: 17549339]
- Zhang L, Smyrk TC, Young WF Jr, et al.: Gastric stromal tumors in Carney triad are different clinically, pathologically, and behaviorally from sporadic gastric gastrointestinal stromal tumors: findings in 104 cases. Am J Surg Pathol 34 (1): 53-64, 2010. [PMC free article: PMC3652406] [PubMed: 19935059]
- Boikos SA, Stratakis CA: Carney complex: pathology and molecular genetics. Neuroendocrinology 83 (3-4): 189-99, 2006. [PubMed: 17047382]
- Correa R, Salpea P, Stratakis CA: Carney complex: an update. Eur J Endocrinol 173 (4): M85-97, 2015. [PMC free article: PMC4553126] [PubMed: 26130139]
- Hensen EF, Bayley JP: Recent advances in the genetics of SDH-related paraganglioma and pheochromocytoma. Fam Cancer 10 (2): 355-63, 2011. [PMC free article: PMC3100491] [PubMed: 21082267]
- Agaram NP, Laquaglia MP, Ustun B, et al.: Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res 14 (10): 3204-15, 2008. [PMC free article: PMC3805121] [PubMed: 18483389]
- Miettinen M, Wang ZF, Sarlomo-Rikala M, et al.: Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol 35 (11): 1712-21, 2011. [PMC free article: PMC3193596] [PubMed: 21997692]
- Sawhney SA, Chapman AD, Carney JA, et al.: Incomplete Carney triad--a review of two cases. QJM 102 (9): 649-53, 2009. [PubMed: 19561114]
- Rege TA, Wagner AJ, Corless CL, et al.: "Pediatric-type" gastrointestinal stromal tumors in adults: distinctive histology predicts genotype and clinical behavior. Am J Surg Pathol 35 (4): 495-504, 2011. [PubMed: 21358303]
- Ayala-Ramirez M, Callender GG, Kupferman ME, et al.: Paraganglioma syndrome type 1 in a patient with Carney-Stratakis syndrome. Nat Rev Endocrinol 6 (2): 110-5, 2010. [PubMed: 20098451]
- Timmers HJ, Chen CC, Carrasquillo JA, et al.: Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 94 (12): 4757-67, 2009. [PMC free article: PMC2795662] [PubMed: 19864450]
- Timmers HJ, Kozupa A, Chen CC, et al.: Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol 25 (16): 2262-9, 2007. [PubMed: 17538171]
- Abadin SS, Ayala-Ramirez M, Jimenez C, et al.: Impact of surgical resection for subdiaphragmatic paragangliomas. World J Surg 38 (3): 733-41, 2014. [PubMed: 24390286]
- Demetri GD, Benjamin RS, Blanke CD, et al.: NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 5 (Suppl 2): S1-29; quiz S30, 2007. [PubMed: 17624289]
- Maki RG, Blay JY, Demetri GD, et al.: Key Issues in the Clinical Management of Gastrointestinal Stromal Tumors: An Expert Discussion. Oncologist 20 (7): 823-30, 2015. [PMC free article: PMC4492234] [PubMed: 26070915]
- Ganjoo KN, Villalobos VM, Kamaya A, et al.: A multicenter phase II study of pazopanib in patients with advanced gastrointestinal stromal tumors (GIST) following failure of at least imatinib and sunitinib. Ann Oncol 25 (1): 236-40, 2014. [PMC free article: PMC4271129] [PubMed: 24356634]
- Gill AJ, Chou A, Vilain R, et al.: Immunohistochemistry for SDHB divides gastrointestinal stromal tumors (GISTs) into 2 distinct types. Am J Surg Pathol 34 (5): 636-44, 2010. [PubMed: 20305538]
- Janeway KA, Albritton KH, Van Den Abbeele AD, et al.: Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer 52 (7): 767-71, 2009. [PubMed: 19326424]
Changes to This Summary (09/25/2015)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Multiple Endocrine Neoplasia Type 1 (MEN1)
Revised text about the conditions that some authors suggest warrant referral for genetics consultation and/or genetic testing for mutations in MEN1 (cited Hampel et al. as reference 39).
Added Romero Arenas et al. as reference 57.
The Parathyroid tumors subsection was comprehensively reviewed and extensively revised.
Added text to state that long-acting somatostatin analogues may have a role in early-stage MEN1 duodenopancreatic neuroendocrine tumors (NETs); initial study results suggest that the treatment is safe and that long-term suppression of tumor and hormonal activity can be seen in up to 10% of patients and stability of hormone hyperfunction in 80% of patients (cited Ramundo et al. as reference 63). Also added text to state that surgery is commonly performed for most functional tumors and for nonfunctioning NETs when the tumor exceeds 2 cm, as the likelihood of distant metastases is high (cited Triponez et al. as reference 64).
Multiple Endocrine Neoplasia Type 2
Added text to state that of individuals with apparently sporadic pheochromocytoma, up to 33% have a mutation in one of the susceptibility genes (cited Pęczkowska et al. and Karasek et al. as references 51 and 52, respectively).
Added Attitudes toward preimplantation genetic diagnosis as a new subsection.
Familial Paraganglioma Syndrome
Revised text to state that up to 40% of patients presenting with apparently sporadic paragangliomas (PGLs) actually have a recognizable germline mutation in one of the known PGL/pheochromocytoma susceptibility genes (cited Fishbein et al. as reference 8).
Added text to state that with a high frequency of genetic mutations, even in apparently sporadic tumors, genetic testing may be considered for all patients with pheochromocytoma or PGL.
Added text to state that genetic testing is best performed before the initial surgical resection of PGLs to inform the risk of recurrent or contralateral disease and to guide the extent of resection.
Added this new section.
This summary is written and maintained by the PDQ Cancer Genetics Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the genetics of endocrine and neuroendocrine neoplasias. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Cancer Genetics Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Genetics of Endocrine and Neuroendocrine Neoplasias are:
- Kathleen A. Calzone, PhD, RN, APNG, FAAN (National Cancer Institute)
- Jennifer Lynn Hay, PhD (Memorial Sloan-Kettering Cancer Center)
- Sarah Nielsen, MS, LCGC (The University of Chicago)
- Suzanne M. O'Neill, MS, PhD, CGC (Northwestern University)
- Nancy D. Perrier, MD, FACS (University of Texas, M.D. Anderson Cancer Center)
- Beth N. Peshkin, MS, CGC (Lombardi Comprehensive Cancer Center at Georgetown University Medical Center)
- Susan K. Peterson, PhD, MPH (University of Texas, M.D. Anderson Cancer Center)
- Jennifer Sipos, MD (The Ohio State University)
- Susan T. Vadaparampil, PhD, MPH (H. Lee Moffitt Cancer Center & Research Institute)
- Catharine Wang, PhD, MSc (Boston University School of Public Health)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Cancer Genetics Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
National Cancer Institute: PDQ® Genetics of Endocrine and Neuroendocrine Neoplasias. Bethesda, MD: National Cancer Institute. Date last modified <MM/DD/YYYY>. Available at: http://www.cancer.gov/types/thyroid/hp/medullary-thyroid-genetics-pdq. Accessed <MM/DD/YYYY>.
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Get More Information From NCI
Call 1-800-4-CANCER
For more information, U.S. residents may call the National Cancer Institute's (NCI's) Cancer Information Service toll-free at 1-800-4-CANCER (1-800-422-6237) Monday through Friday from 8:00 a.m. to 8:00 p.m., Eastern Time. A trained Cancer Information Specialist is available to answer your questions.
Chat online
The NCI's LiveHelp® online chat service provides Internet users with the ability to chat online with an Information Specialist. The service is available from 8:00 a.m. to 11:00 p.m. Eastern time, Monday through Friday. Information Specialists can help Internet users find information on NCI websites and answer questions about cancer.
Write to us
For more information from the NCI, please write to this address:
- NCI Public Inquiries Office
- 9609 Medical Center Dr.
- Room 2E532 MSC 9760
- Bethesda, MD 20892-9760
Search the NCI websites
The NCI website provides online access to information on cancer, clinical trials, and other websites and organizations that offer support and resources for cancer patients and their families. For a quick search, use the search box in the upper right corner of each web page. The results for a wide range of search terms will include a list of "Best Bets," editorially chosen web pages that are most closely related to the search term entered.
There are also many other places to get materials and information about cancer treatment and services. Hospitals in your area may have information about local and regional agencies that have information on finances, getting to and from treatment, receiving care at home, and dealing with problems related to cancer treatment.
Find Publications
The NCI has booklets and other materials for patients, health professionals, and the public. These publications discuss types of cancer, methods of cancer treatment, coping with cancer, and clinical trials. Some publications provide information on tests for cancer, cancer causes and prevention, cancer statistics, and NCI research activities. NCI materials on these and other topics may be ordered online or printed directly from the NCI Publications Locator. These materials can also be ordered by telephone from the Cancer Information Service toll-free at 1-800-4-CANCER (1-800-422-6237).
- Review Gastrointestinal Neuroendocrine Tumors Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Gastrointestinal Neuroendocrine Tumors Treatment (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Cancer Genetics Overview (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Cancer Genetics Overview (PDQ®): Health Professional Version.PDQ Cancer Genetics Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Genetics of Prostate Cancer (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Genetics of Prostate Cancer (PDQ®): Health Professional Version.PDQ Cancer Genetics Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Genetics of Colorectal Cancer (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Genetics of Colorectal Cancer (PDQ®): Health Professional Version.PDQ Cancer Genetics Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Genetics of Skin Cancer (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Genetics of Skin Cancer (PDQ®): Health Professional Version.PDQ Cancer Genetics Editorial Board. PDQ Cancer Information Summaries. 2002
- Genetics of Endocrine and Neuroendocrine Neoplasias (PDQ®) - PDQ Cancer Informat...Genetics of Endocrine and Neuroendocrine Neoplasias (PDQ®) - PDQ Cancer Information Summaries
Your browsing activity is empty.
Activity recording is turned off.
See more...