NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The complexity of the metastatic cascade has led to the development of a plethora of 2- and 3-dimensional in vitro assays to model the various steps of metastasis under a more controlled environment. These assays have been invaluable in cancer research not only as tools to delineate the molecular events that underpin metastasis but also to enable drug screens and validation of therapeutic targets. Here we review the advantages and limitations of current in vitro platforms used to investigate metastasis. In light of the overwhelming evidence showing that 3-dimentional culture systems better mimic the tumor microenvironment and therapeutic response in vivo, recent advances made toward the development of 3-dimensional culture systems and their applications are discussed in more detail. Relevant information on protocols and resources available to support scientists with an interest in metastasis research is also provided.
Introduction
Given that the majority of cancer-related deaths are due to metastatic disease, the molecular and cellular processes underlying metastasis continue to be a major focus of cancer research. Our understanding of the factors and molecular events regulating the spread of solid tumors to distant organs has improved dramatically over recent years, owing to the development of innovative in vitro assays and in vivo animal models that mimic in part or in its entirety the metastatic cascade. However, limitations still exist within these models in terms of their robustness, tumor specificity as well as physiological and clinical relevance. For instance, it is now evident that the stromal microenvironment surrounding tumor cells, in particular the 3-dimensional (3D) architecture provided by the extracellular matrix (ECM) at both primary and secondary sites, have a profound influence on the functional properties of tumor cells and on their drug sensitivity.1-4 Accordingly, much effort is being devoted to improving the structural and cellular complexity of current in vitro models so that they more faithfully replicate the in vivo tumor microenvironment.
Likewise, owing to the acquisition of drug resistance, it is apparent that the therapeutic response of tumor cells at metastatic sites differs from that of the primary tumor and that targeting metastases will require tailored therapies.5-11 Consequently, the development of effective treatments for advanced disease will necessitate in vitro assays that better model the microenvironment of metastases and animal models that more closely recapitulate tumor dissemination and therapeutic interventions in patients.
This chapter provides a brief overview of in vitro functional assays commonly utilized to investigate metastasis, highlighting their suitability, limitations and potential pitfalls when addressing specific aspects underpinning metastasis. Particular emphasis is given to more recent advances made to improve the in vivo relevance of 3D in vitro culture systems. The accompanying chapter will review the various in vivo platforms developed for the study of metastasis. Since several reviews detailing in vitro and in vivo models are available in the literature12,13 we shall only refer to these publications and online resources for a more in depth description of the methodology. Here, our aim is to outline the strengths and weaknesses of in vitro platforms currently available, to help researchers make a more judicious choice of assays/models to study metastasis and encourage a more critical appraisal of results and conclusions drawn from the use of these models.
The Metastatic Cascade
Metastasis from solid tumors is a complex multistep process whereby proliferating neoplastic cells must breach the basement membrane and migrate away from the primary tumor environment to invade the surrounding stroma and enter the vasculature directly or via the lymphatics (Fig. 1). Once in circulation, tumor cells need to arrest in capillary beds and extravasate into secondary sites, a process involving platelets and direct interaction of metastatic cells with endothelial cells and the sub-endothelial basement membrane.14,15 Subsequent colonization of distant organs relies on the ability of metastatic cells to survive, proliferate and promote vascularization in order to give rise to a clinically relevant secondary tumor.16,17 Each of these steps can be rate-limiting and offers potential opportunities for therapeutic intervention.
The inherent complexity of the metastatic cascade has made it necessary to develop a variety of functional assays in vitro to study metastasis under a more controlled environment. These assays are used most commonly to measure changes in the ability of tumor cells to adhere, migrate, invade, survive or proliferate in response to various stimuli (reviewed in refs. 13,18) for which detailed methodologies are freely available through commercial suppliers and online databases of research protocols (Protocol Online — http://www.protocol-online.org). Perhaps incorrectly, in vitro functional assays are often referred to as "metastasis assays". However, while they offer clear advantages over in vivo models of metastasis most notably, cost, reproducibility and high throughput applications, they remain surrogate assays of metastatic function that merely provide a rapid, simplified system that can be manipulated to investigate factors regulating specific steps of the metastatic cascade or test the efficacy of inhibitors. As such, the results obtained in these assays can provide clues about the potential function of a given gene, protein and/or pathway of interest that may be deregulated in metastatic tumors and guide researchers for subsequent studies. However, it is vital that the results obtained by in vitro approaches be validated in vivo and/or in clinical specimens for their human relevance and therapeutic significance. Practical considerations when performing these assays and their relevance to the metastatic steps in vivo are discussed below.
Two-Dimensional Tumor-Matrix Adhesion Assays
Change in adhesive properties of tumor cells and their interactions with the ECM are fundamental features of cancer progression occurring at each step of the metastatic cascade19,20 (Fig. 1). Indeed, many studies have documented alterations in the expression of ECM proteins in advanced tumors21,22 or changes in the repertoire, level of expression or activation state of adhesion receptors in metastatic tumor cells.23-27 These changes contribute to their spread, survival and subsequent growth at metastatic sites28-30 and therefore have attracted considerable interest as targets for therapy.31-34 For these reasons, adhesion assays are often one of the first assays performed when investigating the functional properties of metastatic tumor cells.4,35,36
Traditionally, tumor-matrix interactions have been studied in 2D in vitro assays whereby tumor cells are seeded into culture plates coated with ECM proteins such as collagen. The receptors involved are typically determined in this assay using function-blocking antibodies or specific adhesion receptor inhibitors37 and adhesion inhibition is quantitated either by cell counting or using a colorimetric (e.g., crystal violet) or fluorometric dye (e.g., SYTOX® green or calcein AM). A variety of ECM preparations can be purchased individually or as pre-coated culture plates through commercial suppliers (e.g., CytoSelect™ or BioPioneer cell adhesion assay kits) and offer good reproducibility. While the range of ECM protein coating available commercially is growing, purification of ECM extracts from tissues such as the placenta38 or from culture conditioned medium36,39,40 may be necessary for adhesive proteins less commonly studied.
Several variations can be introduced to the assay but the key consideration is that the culture conditions chosen, in particular the ECM protein used, must be relevant to the tumor type and the particular step of metastasis being investigated. This will depend largely on the matrix composition encountered by tumor cells at various stages of metastasis. For instance, investigating breast tumor cell adhesion to laminin (LM)-411 or LM-511, two adhesive proteins abundant in the sub-endothelial basement membrane,35,41 would be relevant to the processes of intra- and extravasation whereas adhesion to collagen-I would be more appropriate for studying interactions with the stromal parenchyma of the breast. To add to the complexity, the ECM composition in a given tissue may vary with tumor progression as shown for the composition of LM isoforms in the vascular basement membrane of advanced breast tumors and metastases.42
Other important considerations when performing adhesion assays include the length of the assay and the presence of serum. Short-term adhesion assays (< 30 min) are preferred to minimize potential confounding autocrine effects from matrix proteins often secreted by cancer cell lines in culture.36,43-45 Serum-derived vitronectin can adversely influence the assay results as it was found to efficiently coat the surface of culture plates and promote cell attachment and spreading in vitro.46 Conversely, depletion of vitronectin from serum led to poor cell attachment, spreading and growth.46 Therefore when performing adhesion assays in the presence of serum, one has to be mindful of the potential interference by soluble ECM proteins.
Cell Migration and Metastasis
Cell migration is an integral part of metastasis that is required at virtually every step of the metastatic cascade. Tumor cells migrate either randomly (kinesis) in the presence of a homogeneous concentration of soluble growth factors/chemokines (chemokinesis) or adhesive substrate (haptokinesis) or directionally (taxis) when the external cue is provided in the form of a gradient. Based on the nature of the asymmetric gradient and receptors involved, directed migration can be further defined as chemotaxis (soluble gradient), haptotaxis (gradient of adhesive substrate or ECM-bound factor), electrotaxis (electrical field gradient) or durotaxis (gradient of ECM substrate rigidity).47,48 The distinction between these types of directed migration can be blurred by the presence of multiple signals in vivo or when stimuli from soluble ligands and ECM substrates are combined in in vitro assays but the processes leading to the induction of cell movement are largely similar.
Upon sensing of the extracellular signal by cell surface receptors, coordinated activation of signaling pathways48,49 triggers the formation of adhesive contacts and cell protrusions at the leading edge (filopodia, lamellipodia) or invasive protrusions (invadopodia).50,51 Asymmetric re-organization of the actin cytoskeleton that ensues exerts traction forces facilitating the translocation of the cell body forward and retraction of the tail end of the cell.48,49,52 Readers wishing to learn more about the mechanisms regulating directed cell migration and how the above respones are integrated during tumor invasion and metastatic dissemination are directed to excellent and insightful reviews on the subject.53,54
In Vitro Cell Migration Assays on Solid 2D Substrates
Many pro-migratory factors including chemokines and growth factors have now been identified and shown to contribute functionally to tumor dissemination, by experimental evidence gathered using in vitro assays and/or in vivo animal models.54 These factors or their downstream signaling pathways commonly upregulated in advanced carcinomas,50 constitute attractive therapeutic targets to prevent or delay metastatic progression.54,55 Accordingly, several in vitro assays have been developed to investigate the mechanisms regulating cancer cell migration or test the efficacy of potential therapeutic drugs. These include scratch or wound healing assays, trans-membrane migration assays (Modified Boyden chamber), gap-closure or exclusion zone assays as well as migration assays using microfluidic devices (MFDs). The advantages and drawbacks of these platforms along with examples of commercial kits available are summarized in Table 1.
Table 1.
Advantages and drawbacks of in vitro 2D cell migration assays.
Scratch/Wound Healing Assay
Scratch or wound healing assays refer to the measurement of 2D cell migration into a wound (cell free area) that is created by a central linear scratch across the surface of a tissue culture well containing a confluent monolayer of cells.56,57 Accordingly, this approach is not suitable for chemotaxis measurement or for non-adherent cells but is ideal for cell types that migrate as a collective such as keratinocytes that typically move as epithelial sheets.58 To avoid cell turnover influencing the rate of wound closure, cells are often serum starved 8-24 h prior to wounding, and post-scratch media is typically supplemented with non-toxic doses of Mitomycin C to inhibit proliferation.56 Scratch assays are cheap, straightforward and allow the assessment of cell migration kinetics in real time by time-lapse microscopy.
Migration may be quantitated manually by standard microscope or by using quantitation software such as MetaMorph™ or the IncuCyte™ live-cell imaging software. Automated imaging approaches have the advantage of ensuring that the same location in each well is imaged at each time point. For example, Liu and colleagues59 used an automated scratch assay and quantitation by IncuCyte™ technology to identify potential therapeutic targets for aggressive alveolar rahbdomyosarcoma (ARMS). They found that downregulation or pharmacological inhibition of the pro-metastatic PAX3-FKHR fusion transcription factor or its downstream target carnitidine palmitoyltransferase 1A in ARMS significantly reduces the rate of wound closure in this assay.
A key limitation of the scratch assay is that scratching may physically damage the cells adjacent to the wound and can create inconsistent wound size/area. This can be partially overcome with the assistance of automated technologies and live-cell imaging. For example, the Electric Cell Impendance Sensing assays (ECIS™, Applied BioPhysics) uses electric pulses to prevent cells from growing and migrating over an electrode in the center of a culture well, while a confluent monolayer forms around the wound.60 This technology prevents damage to the cells and the underlying ECM that typically occurs with manual scratching methods.
The Woundmaker™ developed by Essen Bioscience permits rapid and uniform wounds across the surface of 96- and 384-well plates in a matter of seconds. The usefulness of this platform for high throughput screening was demonstrated in a study using the 384-well wound healing assay in combination with a siRNA library targeting up to 5,234 human genes in SKOV-3 ovarian carcinoma to identify potential therapeutic targets such as MAP4K4 (mitogen-activated protein 4 kinase 4).61
Gap Closure or Cell Exclusion Zone Assay
2D gap closure or cell exclusion assays are also referred to as chemokinesis assays, whereby cells are seeded into wells containing inserts that can be removed once a confluent monolayer is formed. The fundamental difference between scratch assays and cell exclusion zone assays is that instead of a wound being scratched onto the surface, a cell-free zone is created by a blockade which can be removed once surrounded by a confluent monolayer, into which cells can migrate. Cell exclusion assays therefore advantageously reduce the presence of residual cell debris associated with scratch assays and markedly increase reproducibility. As for the scratch assays, cell migration kinetics can be detected using digital and fluorescence imaging at multiple time-points in real-time but chemotaxis studies are not possible.
These models of cell migration have also been tailored for 24-well plates and 96-well formats for high throughput screening including the Oris™ cell migration assay (Platypus Technologies) that uses silicone stoppers to exclude cells from the center of the well. Importantly, unlike scratch assays, the removal of the silicone stopper does not damage the underlying surface.
Transwell or Modified Boyden Chamber Assay
Cell migration assays in Transwell chambers involve seeding cells into an upper chamber and monitoring the movement of cells to a lower well separated by a microporous membrane. The chemotactic gradient is created by addition of serum or specific chemotactic factors in the lower well. ECM may also be coated on the underside of the porous membrane to measure haptotaxis.35 An advantage of this approach is that the migratory response of adherent or non-adherent cells can be measured. However, the steepness of the chemotactic gradient is difficult to control and cannot be maintained over extended time. In addition, scoring the number of migrated cells (either manually or by using software packages such as MetaMorph) is time consuming and these assays traditionally have a set end point, preventing kinetic analysis of cell migration.
Advances in Transwell technology include the visualization of cells post-migration using fluorescent dyes that can be added to the media after the assays is completed, preventing potential adverse effects from fluorescent tagging of the cells prior to the assay and eliminating the need to manually count migrated cells (e.g., CytoSelect™). Alternative approaches employed to improve quantitation has been developed by BD biosciences, where the membrane employed blocks the transmission of fluorescence from the upper chamber (FluoroBlock™), thus only cells that have breached the membrane and traversed into the bottom well can be quantitated by a fluorescent plate reader. This approach makes high throughput screening more achievable and has the added advantage of assessing the migration dynamics/kinetics in real-time. A range of different FluoroBlock™ membrane surface coats are also being developed, such as fibronectin to assess endothelial cell migration.
In addition to its use for identifying modulators/inhibitors of cell migration, the Transwell assay has a key advantage over other types of assays in that migrating cells can be recovered. Our group recently took advantage of this property to isolate 4T1 mammary carcinoma variants (4T1BM2) that rapidly migrate toward LM-511.37 4T1BM2 cells exhibited a dramatic increase in spontaneous bone metastatic abilities in vivo compared with parental 4T1 cells. Thus, this approach could be used alone or in combination with standard in vivo selection methods to isolate metastatic variants with enhanced metastatic propensity toward organs that are poorly colonized with current animal models.
Microfluidic Assays
Microfluidic assays involve seeding cells into a chamber that is bridged to a second chamber by an internal channel. In addition to chemokinetic measurements, these assays can also assess chemotaxis of known linear chemotactic gradients, a clear advantage over the other 2D cell migration assays described above. Moreover, the small volumes required for microfluidic assays makes them more suitable for drug testing against rare primary cell populations such as biopsies and decreases the amount of chemoattractant or therapeutic agent needed, reducing the cost of reagents. On the other hand, more frequent media changes are required to maintain cell viability.
Multiple formats and geometric designs are now available commercially. The MilliCell μ-Migration™ assay from Millipore is a slide-based imaging system that enables dynamic determination of chemotaxis that provides data on directionality and velocity in a sustained gradient for up to 48 h. A limitation of many microfluidic assays is their unsuitability for high throughput applications. To overcome this, BellBrook Labs has developed the iuvo™ chemotaxis plates and multiconduit array platform (distributed by Thermo Fisher). A major advantage of this platform is the tubeless passive pumping technology which eliminates the need for complex tubing for fluid replacement. The high throughput multi-conduit array platform also incorporates automated liquid handling. Thus, despite their cost microfluidic assays are rapidly becoming valuable high throughput screening tool for drug development.
To conclude, it should be emphasized that while there is a variety of options to measure migration in vitro, these 2D surrogate metastasis assays represent a simplified view of the metastatic cascade in vivo, and therefore the relevance of data obtained in these assays must be validated in vivo. This point is exemplified by our recent study investigating the anti-metastatic properties of laminin-derived peptides.44 While several peptides with inhibitory activity were identified in in vitro adhesion and Transwell migration assays, the most potent inhibitory peptide actually increased rather than decreased experimental metastasis to lung in vivo.44
Adhesive Interactions and Tumor Growth in 3D
While 2D monolayer cultures provide a rapid and convenient platform to study cell adhesion and migration, they lack the architectural and cellular complexity of tumors in vivo. As a result they do not always faithfully replicate some of the bi-directional signaling that takes place between adhesion and growth factor receptors in 3D cultures or in vivo.62,63 Importantly, the distinct morphology, survival and growth of 3D multicellular tumor spheroids compared with 2D cultures are associated with significant changes in the expression of genes related to ECM-cell adhesion, cell-cell contact and invasion/metastasis. These changes in turn correlate with the activation of signaling pathways documented to play a role in cancer progression and metastasis in vivo and modulate tumor cell resistance to therapeutic drugs.64-69 Thus, 3D multicellular tumor spheroids are considered better suited than 2D monolayer cultures for the identification of relevant therapeutic targets and for predicting drug sensitivity in vivo.
The realization that 3D culture systems provide a more physiological microenvironment has led to an increase in the use of multicellular tumor spheroids to study tumor progression in vitro. The propensity for self-aggregation and the ability of tumor cells, but not non-tumorigenic epithelial cells, to form viable multicellular aggregates in the absence of adhesive substrate68 are distinguishing features of tumor cells that have been proposed to contribute to their metastatic potential in vivo.70-72 Methods that take advantage of these properties to generate multicellular aggregates (or spheroids) have been described and include growth on non-adhesive substrates or suspension type cultures such as the liquid overlay68,73 and the hanging-drop techniques74-76 and spheroids grown on microcarrier beads using rotating-wall vessels.77 Alternatively, tumor spheroids can form when tumor cells are seeded on or embedded in natural matrices such as Matrigel,78-80 a laminin-rich matrix derived from Engelbreth-Holm-Swarm (EHS) mouse tumor extracts,81,82 collagen gels83 or fibroblast-derived matrix.84 These are considered to be more physiologically relevant than multicellular aggregates forming in suspension as the surrounding matrix better simulates the architecture and topography of solid tumors in vivo.
The work pioneered by Mina Bissell's group, clearly showed that unlike monolayer cultures, the disorganized growth that characterizes carcinoma cells in Matrigel is easily distinguished from the well differentiated polarized acinus-like structures formed by normal human mammary epithelial cells.79,80,85 Correct acinar formation was attributed to the ability of normal luminal epithelial cells to respond to LM-111-derived cues through LM-binding integrins whereas this regulation appears to be lost in cancer cells.86-88 These observations are consistent with the role of LM-111 (abundant in Matrigel) in the induction of differentiation and polarization demonstrated in other normal human epithelial cell types.81,89
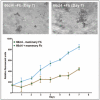
It has been appreciated for some time that signaling cues from resident stromal cells or tumor-infiltrating cells recruited from distant sites also contribute actively to tumor growth and metastatic progression and that these signals can act synergistically with the surrounding matrix to regulate tumor progression.90,91 For example, Matrigel is not sufficient alone to promote the dissemination of non-metastatic lines in vivo but enhances orthotopic growth and subsequent metastasis when co-inoculated with metastatic human MDA-MB-435 breast carcinoma cells in vivo.92 The growth promoting effect of Matrigel in vivo can be potentiated by the inclusion of fibroblasts or fibroblast-derived conditioned medium indicating that it is mediated in part via secreted soluble factors.93 Together, these studies demonstrate the functional synergy that stromal cells and matrix proteins can exert on tumors in vivo. Importantly, the growth promoting effects of stromal fibroblasts can be replicated in 3D in vitro cultures and quantitated by fluorescence as shown for the 66cl4 metastatic mouse mammary carcinoma cell line expressing the tandem repeat (td)Tomato fluorescent marker (Fig. 2).
The stimulatory effect of bone-derived stromal cells on the growth of several human breast tumor lines in 3D in vitro co-cultures has been demonstrated also using a DsRed Express fluorescence-based assay.94 A similar interplay between bone stromal cells and prostate tumor cells appears to operate in vivo and in 3D co-cultures in vitro,95-97 consistent with the propensity of breast and prostate tumors to metastasize to bone. Thus, it is clear that 3D culture systems can be useful tools to investigate heterotypic tumor-stroma interactions at both primary and metastatic sites, thus facilitating the identification and validation of therapeutic targets.
However, while the various 3D platforms used to study tumor-ECM and tumor-host interactions have been highly informative, they have at the same time contributed to conflicting observations concerning the precise nature of adhesive interactions regulating tumor growth and progression. Technical issues and potential pitfalls related to imaging and characterization of adhesion complexes in 3D culture were highlighted in a recent review by Harunaga and Yamada.98 An emerging conclusion from their analysis is that the molecular composition of adhesive structures that form in 3D cultures varies between the matrices to which the cells are exposed and is influenced by the specific substrate composition, its stiffness and topography.
Conceivably, the efficacy of therapies targeting adhesive interactions in metastatic tumors could differ between organs, depending not only on the tumor type but also on the specific matrix composition at each metastatic site colonized by the same tumor. Thus, when using in vitro 3D culture models to predict the efficacy of therapeutic drugs targeting adhesive complexes, it will be particularly important to document and replicate accurately the specific ECM protein composition and physical properties of the matrix of metastatic tumors growing in different target organs.
In this respect, tumor spheroids in reconstituted basement membrane extract Matrigel may not always be the most appropriate in vitro platform to investigate the adhesive interactions regulating tumor progression and metastasis. Matrigel is produced from EHS sarcoma,99 a relatively rare tumor of mesenchymal origin and therefore, its composition is likely to differ from the matrix produced by epithelial carcinomas.81 For instance, while it is rich in basement membrane collagen-IV, it is also abundant in LM-111, a LM isoform whose expression is restricted in adult tissues and absent from many epithelial basement membranes.100,101 Moreover, dissolution of the basement membrane that inevitably occurs in invasive tumors, exposes tumor cells to the interstitial matrix, rich in collagen-I rather than collagen-IV, and is often accompanied by changes in the composition of ECM proteins secreted by epithelial tumor cells themselves. For example, of the three major LM isoforms present in the basement membrane of the normal mammary gland (LM-111, -332 and -511),35,88 the expression of LM-111 and -332 is lost in most advanced breast tumors due to reduced number of laminin-producing myoepithelial cells and/or decreased expression of these isoforms through promoter methylation.35,88,102-105 Production of LM-511 however, a potent adhesive and migratory substrate for breast tumor cells, is often increased in advanced human or mouse mammary tumors and metastases.35,37,42,43
Thus, while the downregulation of LM-111 and LM-332 contributes to the initial disruption of BM integrity and loss of cell polarity and tissue organization in primary tumors, a LM-511-rich matrix may be more relevant to the regulation of late stages of metastasis in breast and other LM-511-producing tumor types such as prostate,106 colorectal22 and melanoma107 than a LM-111-containing matrix such as Matrigel. Importantly, the dynamic changes in the ECM composition of tumors and metastases would be expected to significantly alter their physical and biological properties and as a result, to impact on their drug sensitivity. Consequently, future in vitro 3D culture models will need to take this into account.
Recent advances in the design of MFDs are beginning to address these issues.108-110 These platforms enable spatial and temporal patterning of multiple cell types embedded into distinct ECM gels in specific 3D configurations. The power of MFDs was demonstrated recently in a study investigating the influence of matrix composition and mammary fibroblasts on the progression of mammary epithelial cells (MCF-DCIS) from non-invasive ductal carcinoma in situ (DCIS) to invasive ductal carcinoma (IDC).110 Of relevance, the study found that optimal invasive transition through the "tumor-stroma interface" was observed when mammary fibroblasts were closely juxtaposed to pre-formed large clusters of MCF-DCIS cells embedded in a matrix combining Matrigel and a low concentration of collagen-I. Interestingly, collagen-I alone did not support the growth of MCF-DCIS 3D clusters in this system whereas Matrigel alone was not sufficient to maintain human mammary fibroblast differentiation and survival or to support invasive transition. Huang and colleagues108 used a similar MFD to demonstrate the paracrine effect of MDA-MB-231 on the migratory/invasive properties of RAW 264.1 macrophages when these cells were embedded side by side in collagen-I and Matrigel 3D matrices respectively. Invasion of RAW 264.1 into the neighboring gel was observed only when MDA-MB-231 cells were present in a manner akin to the stromal infiltration of macrophages observed in mammary tumors in vivo.111 Together, these studies illustrate the importance of accurately mimicking the in vivo tumor-stromal microenvironment in 3D in vitro models.
Biomimetic Synthetic Matrices to Study Tumor-Host Interactions and Metastasis
Other potential issues related to the use of natural matrices such as Matrigel include batch to batch variations and the presence of growth factors and other less well characterized components that can influence tumor responses.81,82,112 Thus, despite the major advances made using the above matrices, there is a need for better reproducibility and models with defined 3D microenvironments amenable to physical and biochemical modifications that better reflect the dynamic changes in matrix composition and properties encountered by metastatic cells in vivo. The limitations of natural matrices are rapidly being overcome through the development of increasingly sophisticated bioengineered 3D platforms113-115 that can be used to study tumor-ECM and heterotypic cellular interactions regulating cancer progression.116,117 In particular, the last decade has seen the development of versatile synthetic or semi-synthetic polyethylene glycol (PEG)-, hyaluronan or alginate-based hydrogels with adjustable stiffness that can be modified to incorporate various biological functionalities including integrin binding motifs (RGD peptides), growth/chemotactic factors, and protease cleavage sites.118-121 Because these platforms allow the investigator to independently alter the biochemical and biophysical properties of the 3D environment, they have been particularly useful to distinguish the effect of 2D to 3D transition and matrix stiffness from that of integrin-mediated adhesion on the growth, long-term viability and secretion of proangiogenic factors by various tumor cell types.122,123 In addition, they have been used to investigate protease-dependent migration of fibroblasts in 3D120 and to demonstrate the enhanced resistance of ovarian cancer cells to common chemotherapy (Paclitaxel) when cultured as 3D spheroids.123
In spite of the clear advantages of 3D culture systems over traditional 2D-substrates, the research community has been somewhat slow to embrace their use and convert to these new in vitro platforms. This is attributed in part to their cost, limited accessibility to the average research laboratory, the difficulty to adapt 3D cultures to high throughput screens and the generally more time consuming, expensive and challenging imaging technology required for data analysis. Fortunately, these issues are beginning to be addressed. There are now a growing number of commercial entities providing an increasing range of 3D matrices and many of these companies have developed a variety of standard protocols to measure cellular responses (i.e., proliferation, survival) and for immunostaining specifically tailored to their products (Table 2).
Table 2.
Examples of commercially available natural and synthetic hydrogels.
Moreover, as discussed above, many specialized MFDs requiring very small volumes of defined matrix (and thus reducing their cost) are being developed to investigate the regulation of tumor growth, migration and invasion by diffusible factors and/or stromal cell populations on 3D substrates. Automation of these platforms is rapidly paving the way toward their use for high throughput studies.124 As 3D platforms are becoming more and more dependent on the use of sophisticated microscopy and complex computational and imaging tools, an ever increasing number of open-source software tools are now available for image acquisition, processing, analysis and storage.125-129 These tools will undoubtedly gain in popularity as 3D cultures become more accessible to most research laboratories.
In Vitro Migration in 3D Matrices and Tumor Cell Invasion
While in vitro migration assays refer to the movement of cells in 2D space, invasion assays involve the migration of cells through a 3D matrix. Many earlier studies made use of tissue explant as an in vitro method to assess the invasive capacity of tumor cells or the effect of growth factor inhibitors on this process (i.e., glioma invasion of fetal brain tissue).130,131 While this approach closely resembles the process observed in vivo, it requires that the tissue of interest be maintained in culture for extended periods, usually several days, limiting its use. As a more convenient alternative, a wide variety of natural basement membrane extracts such as human amniotic membranes132 or chick chorioallantoic (CAM) membranes133 have been used. While these approaches provide a rapid and low cost alternative to the use of animal models and are amenable to intervention not practical in animals or patients, they still suffer from the lack of reproducibility owing to the inherent heterogeneity of tissue preparations. For these reasons, the Transwell assays using more defined 3D matrices like collagen or Matrigel remain the most commonly used to measure tumor cell invasion as the assay is relatively simple, rapid, quantitative and more reproducible than the above methods. Importantly, the invasive behavior of tumor cells in this assay correlates with metastatic potential in animal models.134
The major difference with standard 2D migration assays is that for Transwell invasion assays, the cells are seeded on top or embedded within a thick 3D matrix, typically collagen-I or Matrigel, and invading cells are quantitated after ~18-24 h incubation by counting the number of cells that have invaded the ECM gel and migrated to the underside of the porous membrane. This assay has been described in detail previously.134,135 An alternative asymmetric 3D assay was described more recently where tumor cells are layered in between two collagen gels. Invasion is quantitated by microscopy simply by measuring the distance of migration form the center line in response to specific ECM proteins or fibroblasts embedded in the upper and lower gels.136 An advantage of this approach over the Transwell method is that invading cells are visualized in the 3D matrix without the need for the cells to cross an artificial porous membrane, thus providing additional information on the morphological changes associated with the invasive response.
Our classical view of cell migration during tumor cell invasion and metastasis usually relates to the well documented mesenchymal-type migration whereby single epithelial tumor cells undergo an epithelial to mesenchymal transitions (EMT) to invade the stroma in a process largely dependent on proteolytic degradation of the surrounding matrix by matrix metalloproteinases (MMPs).137 However, it is now well documented that tumor cells can migrate not only as single cells but also as a collective.138 Primary tumor explants derived from squamous cell carcinomas, ductal breast carcinomas, rhabdomyosarcoma and melanoma embedded in a collagen-I lattice frequently migrate toward the adjacent ECM not only as single cells but also as cell clusters of 5 to more than 100 cells.139,140 Since multicellular clusters are rarely seen with normal epithelial cells cultured under the same conditions, these observations suggest that it may be a characteristic of metastatic cells.
Interestingly, a recent study by Matise and colleagues141 showed that the decision to migrate as single cells or as a collective is dictated by TGF-β signaling, a key regulator of EMT. The study employed murine mammary carcinoma cells isolated from MMTV-PyMT mice expressing or lacking expression of TGF-β receptor II. While invasion of both tumor cell types was stimulated by addition of fibroblasts in a Transwell assay, grafting of tumor cells together with fibroblasts onto the chicken embryo CAM showed induction of two distinct migratory phenotypes.
Tumor cells expressing the TGF-β receptor II migrated predominantly as single cells or strands at the tumor-stroma interface and expressed markers of EMT signaling whereas cell lacking the receptor exhibited predominantly collective migration as large cell clusters with no evidence of EMT. Importantly, collective migration was associated with increased experimental metastasis in the CAM assay that was attributed to better extravasation, survival and colonization by epithelial tumor cells lacking TGF-β receptor II. These findings are consistent with previous studies showing that intravenous inoculation of tumor cell clusters in mice gives rise to more metastasis than the same number of cells inoculated as single cells.71 The authors concluded that targeting specific patterns of tumor invasiveness could provide alternative treatment for breast cancer. The use of MFDs designed to investigate differences between collective and single cell migration could provide a cost effective in vitro approach to compare the efficacy of inhibitors against these distinct modes of migration.142
Protease-Dependent and Independent Migration in 3D Matrices and In Vivo
While the role of proteases during invasion has been demonstrated in many studies, current evidence indicates that tumor cells exhibit significant plasticity in their mode of migration and reliance on proteolytic activity in 3D matrices. For example, treatment of primary melanoma explants embedded into 3D collagen matrices with β1 integrin neutralizing antibodies converts their migration into the adjacent gel from predominantly protease-dependent multicellular clusters to protease-independent single cell amoeboid-like migration.140 Similarly, the multicellular migration of HT-1080 sarcoma and MDA-MB-231 breast cancer cells in fibrillar collagen is abrogated by treatment with a broad spectrum inhibitor and converted to protease-independent single cell dissemination.143 The critical role of MMP-14 in this process was later demonstrated by the same group.144 Evidence from other studies and the mechanisms regulating amoeboid migration has been reviewed elsewhere.145 More recently, another type of migration termed lobopodia-based migration was described in fibroblasts migrating in dermal explants and cell-derived matrix.146 This integrin-dependent mode of migration is characterized by the formation of blunt, cylindrical protrusions and appears to be dictated by the elastic properties of the matrix. Whether lobopodia are utilized by invading tumor cells and their relevance in the clinic however remains to be demonstrated.
Similarly, the relevance of amoeboid migration in invading tumor cells in vivo has been debated recently.147 In this study, the authors demonstrated that the invasion of MDA-MB-231 and HT-1080 multicellular spheroids in vitro is dependent on the structural integrity and degree of cross-linking of reconstituted collagens. Amoeboid migration in vitro was only observed in non cross-linked collagen gels. Conversely, migration in gels made from full length covalently cross-linked collagen-I or in human mammary gland explants was strictly dependent on the proteolytic activity of MMP-14. On that basis, the authors cautioned the use of non-covalently cross-linked matrices such as acid-extracted collagen or Matrigel in vitro and proposed that cancer cell amoeboid migration in vivo may only be relevant in tissues devoid of interstitial collagen networks such as the brain or in situations where tumor cells may co-opt pre-existing matrix tunnels generated by tumor-infiltrating fibrobasts, smooth muscle cells or endothelial cells. In light of these observations, future investigations on the role of EMT and proteases in 3D migration might be best addressed using the bioengineered synthetic matrices developed more recently.114,119-123,148
Vascular Dissemination and Homing to Metastatic Sites
Tumor-Endothelial Cell Adhesion
The abundance of ECM proteins such as laminins present in vascular basement membranes26 and the leaky vasculature of tumors compared with normal tissues149-152 facilitate attachment of invasive tumor cells and their subsequent intravasation. Once in circulation, circulating tumour cells (CTCs) need to arrest in the vasculature and extravasate into a secondary organ. This process involves direct interaction of tumor cells with endothelial cells lining the blood vessel and subsequently, with the sub-endothelial basement membrane using two major classes of receptors, the selectins and the integrins.19,153-156 The mechanisms regulating these interactions are complex and have been investigated extensively using both in vitro and in vivo models (reviewed in14,157).
Protocols to study tumor-endothelium adhesion under static or flow conditions have been described previously158,159 or are available from commercial sources (e.g., CytoSelect™ Tumor-Endothelium adhesion assay from Cell Biolabs). Static assays generally involve formation of an endothelial monolayer and activation of endothelial cells with cytokines such as tumor necrosis factor-α for up to 24 h followed by addition of radioactively or fluorescently labeled tumor cells. The time necessary for tumor cell adhesion will depend on the tumor type but usually occurs within 10 to 30 min.
When performing tumor-endothelial adhesion assays however, it is important to realize that adhesion of metastatic tumor cells to the endothelium in vivo is a dynamic process that is influenced by a variety of factors, most notably, the presence of other cell types and the constant shear forces resulting from the blood flow. Thus it is not surprising that several studies have found that the type of adhesion molecules involved varies considerably under static and flow conditions (reviewed in14). In addition, the mechanisms involved in vascular attachment and homing to metastatic sites in vivo may vary between tumor cell types and target organs as demonstrated by the selectin receptors used by mammary, lung and colon carcinoma cells to metastasize to lung and liver in animal models.153,160,161 These differences are most likely attributable to the specific repertoire of adhesion receptors expressed in each cell type and specific properties of the vasculature in host organs. In fact, a correlation between the propensity of some tumors to metastasize to specific organs in vivo and adhesive preference toward endothelial cells isolated from these organs in vitro has been reported.162 Therefore the use of endothelial cells from relevant organs is essential when performing tumor-endothelial adhesion or trans-endothelial migration (TEM) assays in vitro, particularly if the aim is to screen for molecules capable of interfering with these interactions.
In addition to enabling the passive dissemination of circulating tumor cells to distant organs, the tumor vasculature has been proposed also to serve as adhesive conduit for melanoma (angio-tumoral complex) and their spread along the abluminal surface of vessels without evidence of intravasation, a mechanism termed extravascular migratory metastasis.163 An in vitro assay in which human microvascular endothelial cells are induced to form capillary-like structures in Matrigel has been developed to replicate this phenomenon with various tumor lines.164 In this model, melanoma and invasive prostate carcinoma cells but not non-tumorigenic prostate cells were found to attach and become elongated along the endothelial tubules. However, the extent to which this process contributes to local or distant metastatic spread of melanoma and other tumor types has yet to be fully explored. Nevertheless, a better understanding of the mechanisms that regulate these interactions could provide alternative therapeutic targets to prevent metastatic spread.
Trans-Endothelial Migration
Standard in vitro TEM assays using Transwell migration chambers that mimic the crossing of leucocytes or tumor cells through the endothelium in vivo have been described in the literature165,166 or are available from commercial sources (e.g., CytoSelect™ transendothelial migration assay kit from Cell Biolabs or QCM™ tumor cell trans-endothelial migration assay from Millipore). Often these assays are used without much consideration for the difference in functional properties that may exist between endothelial cells lining the blood vessels of different organs. Indeed, there is significant heterogeneity in the structural and functional properties of the endothelium between organs and even between vessels in the same organ.167 Accordingly, the mechanisms by which metastatic cells cross the endothelium to access the host organ have been shown to differ between vessel types.155 Discontinuous endothelia such as those found in the bone marrow sinusoids,168 in contrast to the specialized and complex structure of the capillaries making up the blood-brain barrier,169 are likely to be significantly more permissive to tumor cell entry into the bone parenchyma. This might account in part for the high propensity of many tumor types such as breast, lung and prostate tumors to metastasize to bone.170
Nevertheless, in light of the recent increase in the incidence of advanced cancer patients developing brain metastases,171 the importance of the blood-brain barrier (BBB) in restricting access of CTCs to the brain deserves a special mention. Many studies have used variations of the TEM assay as an in vitro model to mimic the passage of tumor cells through the BBB. Understanding the mechanisms by which tumor cells can overcome the restrictions imposed by the BBB to colonize the brain parenchyma could help identify novel prognostic factors or therapeutic targets for brain metastasis. In a recent study, brain-metastatic tumor variants were isolated from patients with advanced breast cancer and from estasblished breast tumor lines.172 Gene expression profiling of these tumor lines identified several candidate genes whose expression in breast tumors was associated with brain relapse, among which α2,6-sialyltransferase (ST6GALNAC5) was specifically linked to brain metastasis. Importantly, knockdown of ST6GALNAC5 significantly reduced metastasis to brain in a mouse model. Impaired brain metastasis was attributed to a decreased ability to cross the BBB following ST6GALNAC5 silencing as evidenced by a decrease in tumor cell adhesion to primary brain microvascular endothelial cells and transendothelial migration in in vitro assays.
In contrast to the above study, Lorger and colleagues later reported that the ability of various mouse and human breast tumor lines to adhere to human brain microvascular endothelial cells or endothelial-derived matrix and to penetrate the BBB model in vitro correlates poorly with their brain-metastatic potential in animal models of experimental metastasis to brain. The reasons for the apparent discrepancies between these studies are unclear but could be due in part to differences in experimental conditions. Thus, further optimization of the in vitro TEM model may be required to better replicate observations in mouse models. In this regard, more recent studies have established that the properties of the BBB in the TEM model in vitro can be optimized to more accurately replicate the in vivo conditions by altering the glucose concentration in the culture medium,173 coating with astrocyte-derived ECM,174 direct astrocyte-endothelial contacts175 and inclusion of sheer stress.176,177
It is also possible that, while required for tumor cell entry into the brain, penetration of the BBB in vivo may not be the rate-limiting event for successful metastatic colonization. Indeed, earlier studies on metastasis efficiency using either a CAM assay or an experimental mouse model of liver metastasis have shown that the ability to extravasate is not necessarily predictive of subsequent metastasis formation in vivo and that the ability to survive and initiate growth at metastatic site is the critical determinant dictating successful metastasis.178,179 Whether this also holds true for colonization of the brain will require further investigation in both in vitro and in vivo models.
Conclusions and Future Perspectives
Currently, a wide variety of in vitro assays are available to investigate distinct steps of the metastatic cascade and/or for high throughput drug screening. Progress in imaging and bioengineering technologies has fuelled the recent shift from 2D to 3D platforms. Increased interest in the use of 3D culture systems has been motivated also by accumulating evidence that 3D models better reflect the microenvironment of tumors and metastases and more accurately predict therapeutic response in vivo compared with conventional 2D assays. Studies in 3D culture systems have highlighted in particular the importance of selecting an appropriate matrix relevant to the tumor type being investigated when designing research projects incorporating 3D platforms.
Given their simplicity and cost advantage, 2D platforms remain however the preferred option for high throughput drug screens. The need to standardize 3D platforms for better reproducibility is now being recognized180 and this, together with the increased availability and range of 3D bioengineered matrices, MFDs, automated platforms, microscopy and analytical software will undoubtedly facilitate further transition toward 3D platforms. In addition, significant advances are being made toward mimicking organ-specific cell-cell and cell-matrix interactions using microscale engineering technologies to replicate the physical and chemical microenvironment of various organs.109,181 These breakthroughs bring the exciting prospect of developing robust multi-organ integrated systems for evaluation of therapeutic drugs. Other important areas of metastasis research and emerging concepts not addressed in this chapter are likely to lead to the identification of novel therapeutic targets for recurrent metastatic disease. These include the role of lymphangiogenesis in metastatic spread and the regulation of tumor dormancy by the metastatic niche and autophagy. Some of the tools and in vitro 3D platforms to investigate these processes have already been developed and will further enhance our understanding of metastasis in vivo.182-185
References
- 1.
- Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52. http://dx.doi.org/10.1038/nrc2618 . [PMC free article: PMC3251309] [PubMed: 19279573]
- 2.
- Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ. Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008;68:4398–405. http://dx.doi.org/10.1158/0008-5472.CAN-07-6390 . [PMC free article: PMC3719863] [PubMed: 18519702]
- 3.
- Weigelt B, Lo AT, Park CC, Gray JW, Bissell MJ. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res Treat. 2010;122:35–43. http://dx.doi.org/10.1007/s10549-009-0502-2 . [PMC free article: PMC2935800] [PubMed: 19701706]
- 4.
- Furukawa T, Watanabe M, Kubota T, Kase S, Fujita S, Yamamoto T, et al. Significance of in vitro attachment of human colon cancers to extracellular matrix proteins in experimental and clinical liver metastases. J Surg Oncol. 1993;53:10–5. discussion 15-6 http://dx.doi.org/10.1002/jso.2930530105 . [PubMed: 8479191]
- 5.
- Fugmann RA, Anderson JC, Stolfi RL, Martin DS. Comparison of adjuvant chemotherapeutic activity against primary and metastatic spontaneous murine tumors. Cancer Res. 1977;37:496–500. [PubMed: 832274]
- 6.
- Tsuruo T, Fidler IJ. Differences in drug sensitivity among tumor cells from parental tumors, selected variants, and spontaneous metastases. Cancer Res. 1981;41:3058–64. [PubMed: 7248962]
- 7.
- Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–9. http://dx.doi.org/10.1016/j.ccr.2009.01.021 . [PMC free article: PMC4540346] [PubMed: 19249681]
- 8.
- Francia G, Man S, Lee CJ, Lee CR, Xu P, Mossoba ME, et al. Comparative impact of trastuzumab and cyclophosphamide on HER-2-positive human breast cancer xenografts. Clin Cancer Res. 2009;15:635866. [PMC free article: PMC2788792] [PubMed: 19825954]
- 9.
- Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–31. http://dx.doi.org/10.1016/j.ccr.2009.01.027 . [PMC free article: PMC2874829] [PubMed: 19249680]
- 10.
- Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, et al. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. http://dx.doi.org/10.1038/nm.1941 . [PubMed: 19305413]
- 11.
- Mina LA, Sledge GW Jr. Rethinking the metastatic cascade as a therapeutic target. Nature reviews. Clin Oncol. 2011;8:325–32. [PubMed: 21502993]
- 12.
- Box GM, Eccles SA. Simple experimental and spontaneous metastasis assays in mice. Methods Mol Biol. 2011;769:311–29. http://dx.doi.org/10.1007/978-1-61779-207-6_21 . [PubMed: 21748685]
- 13.
- Rusciano D, Welch DR, Burger MM. Ansterdam, The Netherland: Elsevier; 2000. Cancer Metastasis: Experimental Approaches.
- 14.
- Haier J, Nicolson GL. Tumor cell adhesion under hydrodynamic conditions of fluid flow. APMIS. 2001;109:241–62. [PubMed: 11469496]
- 15.
- Woodward J. Crossing the endothelium: E-selectin regulates tumor cell migration under flow conditions. Cell Adh Migr. 2008;2:151–2. http://dx.doi.org/10.4161/cam.2.3.6820 . [PMC free article: PMC2634092] [PubMed: 19262108]
- 16.
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. http://dx.doi.org/10.1038/nrc865 . [PubMed: 12154349]
- 17.
- Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–84. http://dx.doi.org/10.1038/nrc2622 . [PubMed: 19308067]
- 18.
- Methods in molecular medicine, vol II, analysis of cell behavior in vitro and in vivo. Totowa, New Jersey: Humana Press; 2001. Metastasis Research Protocols.
- 19.
- Bendas G, Borsig L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol. 2012;2012:676731. http://dx.doi. org/10.1155/2012/676731 . [PMC free article: PMC3296185] [PubMed: 22505933]
- 20.
- Givant-Horwitz V, Davidson B, Reich R. Laminin-induced signaling in tumor cells. Cancer Lett. 2005;223:1–10. http://dx.doi.org/10.1016/j.canlet.2004.08.030 . [PubMed: 15890231]
- 21.
- Ioachim E, Charchanti A, Briasoulis E, Karavasilis V, Tsanou H, Arvanitis DL, et al. Immunohistochemical expression of extracellular matrix components tenascin, fibronectin, collagen type IV and laminin in breast cancer: their prognostic value and role in tumour invasion and progression. Eur J Cancer. 2002;38:2362–70. http://dx.doi.org/10.1016/S0959-8049(02)00210-1 . [PubMed: 12460779]
- 22.
- Hewitt RE, Powe DG, Morrell K, Balley E, Leach IH, Ellis IO, et al. Laminin and collagen IV subunit distribution in normal and neoplastic tissues of colorectum and breast. Br J Cancer. 1997;75:221–9. http://dx.doi.org/10.1038/bjc.1997.37 . [PMC free article: PMC2063263] [PubMed: 9010030]
- 23.
- Liapis H, Flath A, Kitazawa S. Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol. 1996;5:127–35. [PubMed: 8727100]
- 24.
- Putz E, Witter K, Offner S, Stosiek P, Zippelius A, Johnson J, et al. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res. 1999;59:241–8. [PubMed: 9892213]
- 25.
- Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:9482–7. http://dx.doi.org/10.1073/pnas.1633689100 . [PMC free article: PMC170944] [PubMed: 12874388]
- 26.
- Tagliabue E, Ghirelli C, Squicciarini P, Aiello P, Colnaghi MI, Menard S. Prognostic value of alpha 6 beta 4 integrin expression in breast carcinomas is affected by laminin production from tumor cells. Clin Cancer Res. 1998;4:407–10. [PubMed: 9516929]
- 27.
- Zutter MM, Sun H, Santoro SA. Altered integrin expression and the malignant phenotype: the contribution of multiple integrated integrin receptors. J Mammary Gland Biol Neoplasia. 1998;3:191200. http://dx.doi.org/10.1023/A:1018798907544 . [PubMed: 10819527]
- 28.
- Lipscomb EA, Simpson KJ, Lyle SR, Ring JE, Dugan AS, Mercurio AM. The alpha6beta4 integrin maintains the survival of human breast carcinoma cells in vivo. Cancer Res. 2005;65:10970–6. http://dx.doi.org/10.1158/0008-5472.CAN-05-2327 . [PubMed: 16322245]
- 29.
- Morini M, Mottolese M, Ferrari N, Ghiorzo F, Buglioni S, Mortarini R, et al. The alpha 3 beta 1 integrin is associated with mammary carcinoma cell metastasis, invasion, and gelatinase B (MMP-9) activity. Int J Cancer. 2000;87:336–42. [PubMed: 10897037]
- 30.
- Wewer UM, Shaw LM, Albrechtsen R, Mercurio AM. The integrin alpha 6 beta 1 promotes the survival of metastatic human breast carcinoma cells in mice. Am J Pathol. 1997;151:1191–8. [PMC free article: PMC1858063] [PubMed: 9358743]
- 31.
- Li DM, Feng YM. Signaling mechanism of cell adhesion molecules in breast cancer metastasis: potential therapeutic targets. Breast Cancer Res Treat. 2011;128:7–21. http://dx.doi. org/10.1007/s10549-011-1499-x . [PubMed: 21499686]
- 32.
- Stipp CS. Laminin-binding integrins and their tetraspanin partners as potential antimetastatic targets. Expert Rev Mol Med. 2010;12:e3. http://dx.doi.org/10.1017/S1462399409001355 . [PMC free article: PMC2811424] [PubMed: 20078909]
- 33.
- Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. http://dx.doi.org/10.1038/nrc2748 . [PMC free article: PMC4383089] [PubMed: 20029421]
- 34.
- Millard M, Odde S, Neamati N. Integrin targeted therapeutics. Theranostics. 2011;1:154–88. http://dx.doi.org/10.7150/thno/v01p0154 . [PMC free article: PMC3086618] [PubMed: 21547158]
- 35.
- Chia J, Kusuma N, Anderson R, Parker B, Bidwell B, Zamurs L, et al. Evidence for a role of tumor-derived laminin-511 in the metastatic progression of breast cancer. Am J Pathol. 2007;170:2135–48. http://dx.doi.org/10.2353/ajpath.2007.060709 . [PMC free article: PMC1899445] [PubMed: 17525279]
- 36.
- Pouliot N, Connolly LM, Moritz RL, Simpson RJ, Burgess AW. Colon cancer cells adhesion and spreading on autocrine laminin-10 is mediated by multiple integrin receptors and modulated by EGF receptor stimulation. Exp Cell Res. 2000;261:360–71. http://dx.doi.org/10.1006/ excr.2000.5065 . [PubMed: 11112342]
- 37.
- Kusuma N, Denoyer D, Eble JA, Redvers RP, Parker BS, Pelzer R, et al. Integrin-dependent response to laminin-511 regulates breast tumor cell invasion and metastasis. Int J Cancer. 2012;130:555–66. http://dx.doi.org/10.1002/ijc.26018 . [PubMed: 21387294]
- 38.
- Wewer U, Albrechtsen R, Manthorpe M, Varon S, Engvall E, Ruoslahti E. Human laminin isolated in a nearly intact, biologically active form from placenta by limited proteolysis. J Biol Chem. 1983;258:12654–60. [PubMed: 6415055]
- 39.
- Doi M, Thyboll J, Kortesmaa J, Jansson K, Iivanainen A, Parvardeh M, et al. Recombinant human laminin-10 (alpha5beta1gamma1). Production, purification, and migration-promoting activity on vascular endothelial cells. J Biol Chem. 2002;277:12741–8. http://dx.doi.org/10.1074/jbc. M111228200 . [PubMed: 11821406]
- 40.
- Zamurs L, Pouliot N, Gibson P, Hocking G, Nice E. Strategies for the purification of laminin-10 for studies on colon cancer metastasis. Biomed Chromatogr. 2003;17:201–11. http:// dx.doi.org/10.1002/bmc.248 . [PubMed: 12717810]
- 41.
- Spessotto P, Yin Z, Magro G, Deutzmann R, Chiu A, Colombatti A, et al. Laminin isoforms 8 and 10 are primary components of the subendothelial basement membrane promoting interaction with neoplastic lymphocytes. Cancer Res. 2001;61:339–47. [PubMed: 11196184]
- 42.
- Fujita M, Khazenzon NM, Bose S, Sekiguchi K, Sasaki T, Carter WG, et al. Overexpression of beta1chain-containing laminins in capillary basement membranes of human breast cancer and its metastases. Breast Cancer Res. 2005;7:R411–21. http://dx.doi.org/10.1186/bcr1011 . [PMC free article: PMC1175051] [PubMed: 15987446]
- 43.
- Eckhardt BL, Parker BS, van Laar RK, Restall CM, Natoli AL, Tavaria MD, et al. Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol Cancer Res. 2005;3:1–13. [PubMed: 15671244]
- 44.
- Kusuma N, Anderson RL, Pouliot N. Laminin a5-derived peptides modulate the properties of metastatic breast tumour cells. Clin Exp Metastasis. 2011;28:909–21. http://dx.doi.org/10.1007/ s10585-011-9422-8 . [PubMed: 21938437]
- 45.
- Tani T, Lehto VP, Virtanen I. Expression of laminins 1 and 10 in carcinoma cells and comparison of their roles in cell adhesion. Exp Cell Res. 1999;248:115–21. http://dx.doi.org/10.1006/ excr.1999.4399 . [PubMed: 10094819]
- 46.
- Underwood PA, Bennett FA. A comparison of the biological activities of the cell-adhesive proteins vitronectin and fibronectin. J Cell Sci. 1989;93:641–9. [PubMed: 2481683]
- 47.
- Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–52. http://dx.doi.org/10.1016/S0006-3495(00)76279-5 . [PMC free article: PMC1300921] [PubMed: 10866943]
- 48.
- Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–49. http://dx.doi.org/10.1038/nrm2729 . [PMC free article: PMC2752299] [PubMed: 19603038]
- 49.
- Jiang P, Enomoto A, Takahashi M. Cell biology of the movement of breast cancer cells: intracellular signalling and the actin cytoskeleton. Cancer Lett. 2009;284:122–30. http://dx.doi. org/10.1016/j.canlet.2009.02.034 . [PubMed: 19303207]
- 50.
- Arjonen A, Kaukonen R, Ivaska J. Filopodia and adhesion in cancer cell motility. Cell Adh Migr. 2011;5:421–30. http://dx.doi.org/10.4161/cam.5.5.17723 . [PMC free article: PMC3218609] [PubMed: 21975551]
- 51.
- Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–22. http://dx.doi. org/10.1016/j.cell.2011.06.010 . [PubMed: 21703446]
- 52.
- Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–52. http://dx.doi.org/10.1016/j.bbamcr.2006.07.001 . [PMC free article: PMC4266238] [PubMed: 16926057]
- 53.
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–69. http://dx.doi.org/10.1016/S0092-8674(00)81280-5 . [PubMed: 8608589]
- 54.
- Roussos ET, Condeelis JS, Patsialou A. Chemotaxis in cancer. Nat Rev Cancer. 2011;11:573–87. http://dx.doi.org/10.1038/nrc3078 . [PMC free article: PMC4030706] [PubMed: 21779009]
- 55.
- Palmer TD, Ashby WJ, Lewis JD, Zijlstra A. Targeting tumor cell motility to prevent metastasis. Adv Drug Deliv Rev. 2011;63:568–81. http://dx.doi.org/10.1016/j.addr.2011.04.008 . [PMC free article: PMC3132821] [PubMed: 21664937]
- 56.
- Cory G. Scratch-wound assay. Methods Mol Biol. 2011;769:25–30. http://dx.doi. org/10.1007/978-1-61779-207-6_2 . [PubMed: 21748666]
- 57.
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–33. http://dx.doi.org/10.1038/ nprot.2007.30 . [PubMed: 17406593]
- 58.
- Pouliot N, Saunders NA, Kaur P. Laminin 10/11: an alternative adhesive ligand for epidermal keratinocytes with a functional role in promoting proliferation and migration. Exp Dermatol. 2002;11:387–97. http://dx.doi.org/10.1034/j.1600-0625.2002.110501.x . [PubMed: 12366691]
- 59.
- Liu L, Wang YD, Wu J, Cui J, Chen T. Carnitine palmitoyltransferase 1A (CPT1A): a transcriptional target of PAX3-FKHR and mediates PAX3-FKHR-dependent motility in alveolar rhabdomyosarcoma cells. BMC Cancer. 2012;12:154. http://dx.doi.org/10.1186/1471-2407-12-154 . [PMC free article: PMC3453510] [PubMed: 22533991]
- 60.
- Jiang WG, Martin TA, Lewis-Russell JM, Douglas-Jones A, Ye L, Mansel RE. Eplin-alpha expression in human breast cancer, the impact on cellular migration and clinical outcome. Mol Cancer. 2008;7:71. http://dx.doi.org/10.1186/1476-4598-7-71 . [PMC free article: PMC2553413] [PubMed: 18796137]
- 61.
- Collins CS, Hong J, Sapinoso L, Zhou Y, Liu Z, Micklash K, et al. A small interfering RNA screen for modulators of tumor cell motility identifies MAP4K4 as a promigratory kinase. Proc Natl Acad Sci U S A. 2006;103:3775–80. http://dx.doi.org/10.1073/pnas.0600040103 . [PMC free article: PMC1383649] [PubMed: 16537454]
- 62.
- Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling : the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–51. http://dx.doi.org/10.1530/JOE-10-0377 . [PubMed: 21307119]
- 63.
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–6. http://dx.doi.org/10.1073/pnas.95.25.14821 . [PMC free article: PMC24533] [PubMed: 9843973]
- 64.
- Ghosh S, Spagnoli GC, Martin I, Ploegert S, Demougin P, Heberer M, et al. Three-dimensional culture of melanoma cells profoundly affects gene expression profile: a high density oligonucleotide array study. J Cell Physiol. 2005;204:522–31. http://dx.doi.org/10.1002/jcp.20320 . [PubMed: 15744745]
- 65.
- Härmä V, Virtanen J, Mäkelä R, Happonen A, Mpindi JP, Knuuttila M, et al. A comprehensive panel of three-dimensional models for studies of prostate cancer growth, invasion and drug responses. PLoS One. 2010;5:e10431. http://dx.doi.org/10.1371/journal.pone.0010431 . [PMC free article: PMC2862707] [PubMed: 20454659]
- 66.
- Kenny PA, Lee GY, Myers CA, Neve RM, Semeiks JR, Spellman PT, et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol Oncol. 2007;1:84–96. http://dx.doi.org/10.1016/j.molonc.2007.02.004 . [PMC free article: PMC2391005] [PubMed: 18516279]
- 67.
- Kobayashi H, Man S, Graham CH, Kapitain SJ, Teicher BA, Kerbel RS. Acquired multicellular-mediated resistance to alkylating agents in cancer. Proc Natl Acad Sci U S A. 1993;90:3294–8. http://dx.doi.org/10.1073/pnas.90.8.3294 . [PMC free article: PMC46286] [PubMed: 8475071]
- 68.
- Rak J, Mitsuhashi Y, Erdos V, Huang SN, Filmus J, Kerbel RS. Massive programmed cell death in intestinal epithelial cells induced by three-dimensional growth conditions: suppression by mutant c-Hras oncogene expression. J Cell Biol. 1995;131:1587–98. http://dx.doi.org/10.1083/ jcb.131.6.1587 . [PMC free article: PMC2120690] [PubMed: 8522614]
- 69.
- Zschenker O, Streichert T, Hehlgans S, Cordes N. Genome-wide gene expression analysis in cancer cells reveals 3D growth to affect ECM and processes associated with cell adhesion but not DNA repair. PLoS One. 2012;7:e34279. http://dx.doi.org/10.1371/journal.pone.0034279 . [PMC free article: PMC3324525] [PubMed: 22509286]
- 70.
- Fidler IJ. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer. 1973;9:223–7. http://dx.doi.org/10.1016/ S0014-2964(73)80022-2 . [PubMed: 4787857]
- 71.
- Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889–94. [PubMed: 1253177]
- 72.
- Updyke TV, Nicolson GL. Malignant melanoma cell lines selected in vitro for increased homotypic adhesion properties have increased experimental metastatic potential. Clin Exp Metastasis. 1986;4:27384. http://dx.doi.org/10.1007/BF00133592 . [PubMed: 3791726]
- 73.
- Rofstad EK, Wahl A, Davies CdeL, Brustad T. Growth characteristics of human melanoma multicellular spheroids in liquid-overlay culture: comparisons with the parent tumour xenografts. Cell Tissue Kinet. 1986;19:205–16. [PubMed: 3698078]
- 74.
- Del Duca D, Werbowetski T, Del Maestro RF. Spheroid preparation from hanging drops: characterization of a model of brain tumor invasion. J Neurooncol. 2004;67:295–303. http://dx.doi. org/10.1023/B:NEON.0000024220.07063.70 . [PubMed: 15164985]
- 75.
- Kelm JM, Timmins NE, Brown CJ, Fussenegger M, Nielsen LK. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol Bioeng. 2003;83:17380. http://dx.doi.org/10.1002/bit.10655 . [PubMed: 12768623]
- 76.
- Timmins NE, Nielsen LK. Generation of multicellular tumor spheroids by the hanging-drop method. Methods Mol Med. 2007;140:141–51. http://dx.doi.org/10.1007/978-1-59745-4438_8 . [PubMed: 18085207]
- 77.
- Becker JL, Prewett TL, Spaulding GF, Goodwin TJ. Three-dimensional growth and differentiation of ovarian tumor cell line in high aspect rotating-wall vessel: morphologic and embryologic considerations. J Cell Biochem. 1993;51:283–9. http://dx.doi.org/10.1002/jcb.240510307 . [PubMed: 8501130]
- 78.
- Bergstraesser LM, Weitzman SA. Culture of normal and malignant primary human mammary epithelial cells in a physiological manner simulates in vivo growth patterns and allows discrimination of cell type. Cancer Res. 1993;53:2644–54. [PubMed: 8495428]
- 79.
- Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–8. http://dx.doi.org/10.1073/ pnas.89.19.9064 . [PMC free article: PMC50065] [PubMed: 1384042]
- 80.
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–95. http://dx.doi.org/10.1083/jcb.115.5.1383 . [PMC free article: PMC2289247] [PubMed: 1955479]
- 81.
- Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–86. http://dx.doi.org/10.1016/j.semcancer.2005.05.004 . [PubMed: 15975825]
- 82.
- Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1–8. http://dx.doi. org/10.1016/0014-4827(92)90397-Q . [PubMed: 1511725]
- 83.
- Miller BE, Miller FR, Heppner GH. Factors affecting growth and drug sensitivity of mouse mammary tumor lines in collagen gel cultures. Cancer Res. 1985;45:4200–5. [PubMed: 4028010]
- 84.
- Serebriiskii I, Castello-Cros R, Lamb A, Golemis EA, Cukierman E. Fibroblast-derived 3D matrix differentially regulates the growth and drug-responsiveness of human cancer cells. Matrix Biol. 2008;27:573–85. [PMC free article: PMC2603546] [PubMed: 18411046]
- 85.
- Park CC, Zhang H, Pallavicini M, Gray JW, Baehner F, Park CJ, et al. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006;66:1526–35. http://dx.doi.org/10.1158/0008-5472.CAN-05-3071 . [PMC free article: PMC2933188] [PubMed: 16452209]
- 86.
- Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ. Cellular growth and survival are mediated by beta 1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995;108:1945–57. [PubMed: 7544798]
- 87.
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, et al. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. http://dx.doi.org/10.1083/jcb.129.3.591 . [PMC free article: PMC2120432] [PubMed: 7730398]
- 88.
- Gudjonsson T, Rønnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002;115:39–50. [PMC free article: PMC2933194] [PubMed: 11801722]
- 89.
- Turck N, Lefebvre O, Gross I, Gendry P, Kedinger M, Simon-Assmann P, et al. Effect of laminin-1 on intestinal cell differentiation involves inhibition of nuclear nucleolin. J Cell Physiol. 2006;206:545–55. http://dx.doi.org/10.1002/jcp.20501 . [PubMed: 16245305]
- 90.
- Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. http://dx.doi.org/10.1016/j. ccr.2012.02.022 . [PubMed: 22439926]
- 91.
- Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol. 2001;11:97–104. http://dx.doi.org/10.1006/scbi.2000.0361 . [PubMed: 11322829]
- 92.
- Bao L, Matsumura Y, Baban D, Sun Y, Tarin D. Effects of inoculation site and Matrigel on growth and metastasis of human breast cancer cells. Br J Cancer. 1994;70:228–32. http://dx.doi. org/10.1038/bjc.1994.284 . [PMC free article: PMC2033496] [PubMed: 8054270]
- 93.
- Nol A, De Pauw-Gillet MC, Purnell G, Nusgens B, Lapiere CM, Foidart JM. Enhancement of tumorigenicity of human breast adenocarcinoma cells in nude mice by matrigel and fibroblasts. Br J Cancer. 1993;68:909–15. http://dx.doi.org/10.1038/bjc.1993.453 . [PMC free article: PMC1968733] [PubMed: 8217606]
- 94.
- Sasser AK, Mundy BL, Smith KM, Studebaker AW, Axel AE, Haidet AM, et al. Human bone marrow stromal cells enhance breast cancer cell growth rates in a cell line-dependent manner when evaluated in 3D tumor environments. Cancer Lett. 2007;254:255–64. http://dx.doi.org/10.1016/j. canlet.2007.03.012 . [PubMed: 17467167]
- 95.
- Gleave ME, Hsieh JT, von Eschenbach AC, Chung LW. Prostate and bone fibroblasts induce human prostate cancer growth in vivo: implications for bidirectional tumor-stromal cell interaction in prostate carcinoma growth and metastasis. J Urol. 1992;147:1151–9. [PubMed: 1372662]
- 96.
- Hsiao AY, Torisawa YS, Tung YC, Sud S, Taichman RS, Pienta KJ, et al. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials. 2009;30:3020–7. http:// dx.doi.org/10.1016/j.biomaterials.2009.02.047 . [PMC free article: PMC2675053] [PubMed: 19304321]
- 97.
- Sieh S, Lubik AA, Clements JA, Nelson CC, Hutmacher DW. Interactions between human osteoblasts and prostate cancer cells in a novel 3D in vitro model. Organogenesis. 2010;6:181–8. http://dx.doi.org/10.4161/org.6.3.12041 . [PMC free article: PMC2946051] [PubMed: 21197221]
- 98.
- Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix Biol. 2011;30:363–8. [PMC free article: PMC3191245] [PubMed: 21723391]
- 99.
- Kleinman HK. Preparation of basement membrane components from EHS tumors. In: Bonifacino JS, et al. eds. Current Protocols in Cell Biology. Wiley; 2001. Chapter 10: Unit 10 2. [PubMed: 18228296]
- 100.
- Falk M, Ferletta M, Forsberg E, Ekblom P. Restricted distribution of laminin alpha1 chain in normal adult mouse tissues. Matrix Biol. 1999;18:557–68. [PubMed: 10607917]
- 101.
- Virtanen I, Gullberg D, Rissanen J, Kivilaakso E, Kiviluoto T, Laitinen LA, et al. Laminin alpha1-chain shows a restricted distribution in epithelial basement membranes of fetal and adult human tissues. Exp Cell Res. 2000;257:298–309. http://dx.doi.org/10.1006/excr.2000.4883 . [PubMed: 10837144]
- 102.
- Sathyanarayana UG, Padar A, Huang CX, Suzuki M, Shigematsu H, Bekele BN, et al. Aberrant promoter methylation and silencing of laminin-5-encoding genes in breast carcinoma. Clin Cancer Res. 2003;9:6389–94. [PubMed: 14695139]
- 103.
- Gusterson BA, Warburton MJ, Mitchell D, Ellison M, Neville AM, Rudland PS. Distribution of myoepithelial cells and basement membrane proteins in the normal breast and in benign and malignant breast diseases. Cancer Res. 1982;42:4763–70. [PubMed: 6290045]
- 104.
- Henning K, Berndt A, Katenkamp D, Kosmehl H. Loss of laminin-5 in the epithelium-stroma interface: an immunohistochemical marker of malignancy in epithelial lesions of the breast. Histopathology. 1999;34:305–9. http://dx.doi.org/10.1046/j.1365-2559.1999.00634.x . [PubMed: 10231397]
- 105.
- Martin KJ, Kwan CP, Nagasaki K, Zhang X, OHare MJ, Kaelin CM, et al. Down-regulation of laminin-5 in breast carcinoma cells. Mol Med. 1998;4:602–13. [PMC free article: PMC2230317] [PubMed: 9848077]
- 106.
- Bair EL, Chen ML, McDaniel K, Sekiguchi K, Cress AE, Nagle RB, et al. Membrane type 1 matrix metalloprotease cleaves laminin-10 and promotes prostate cancer cell migration. Neoplasia. 2005;7:3809. http://dx.doi.org/10.1593/neo.04619 . [PMC free article: PMC1501144] [PubMed: 15967115]
- 107.
- Oikawa Y, Hansson J, Sasaki T, Rousselle P, Domogatskaya A, Rodin S, et al. Melanoma cells produce multiple laminin isoforms and strongly migrate on a5 laminin(s) via several integrin receptors. Exp Cell Res. 2011;317:1119–33. http://dx.doi.org/10.1016/j.yexcr.2010.12.019 . [PubMed: 21195710]
- 108.
- Huang CP, Lu J, Seon H, Lee AP, Flanagan LA, Kim HY, et al. Engineering microscale cellular niches for three-dimensional multicellular co-cultures. Lab Chip. 2009;9:1740–8. http:// dx.doi.org/10.1039/b818401a . [PMC free article: PMC3758562] [PubMed: 19495458]
- 109.
- Huh D, Torisawa YS, Hamilton GA, Kim HJ, Ingber DE. Microengineered physiological biomimicry: organs-on-chips. Lab Chip. 2012;12:2156–64. http://dx.doi.org/10.1039/c2lc40089h . [PubMed: 22555377]
- 110.
- Sung KE, Yang N, Pehlke C, Keely PJ, Eliceiri KW, Friedl A, et al. Transition to invasion in breast cancer: a microfluidic in vitro model enables examination of spatial and temporal effects. Integr Biol (Camb). 2011;3:439–50. [PMC free article: PMC3094750] [PubMed: 21135965]
- 111.
- Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65:159–63. http://dx.doi. org/10.1136/jclinpath-2011-200355 . [PubMed: 22049225]
- 112.
- Benton G, Kleinman HK, George J, Arnaoutova I. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int J Cancer. 2011;128:1751–7. http://dx.doi.org/10.1002/ijc.25781 . [PubMed: 21344372]
- 113.
- Cushing MC, Anseth KS. Materials science. Hydrogel cell cultures. Science. 2007;316:1133–4. http://dx.doi.org/10.1126/science.1140171 . [PubMed: 17525324]
- 114.
- Lutolf MP. Biomaterials: Spotlight on hydrogels. Nat Mater. 2009;8:451–3. http:// dx.doi.org/10.1038/nmat2458 . [PubMed: 19458644]
- 115.
- Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124–8. http://dx.doi.org/10.1126/science.1214804 . [PubMed: 22654050]
- 116.
- Hutmacher DW, Horch RE, Loessner D, Rizzi S, Sieh S, Reichert JC, et al. Translating tissue engineering technology platforms into cancer research. J Cell Mol Med. 2009;13:1417–27. http:// dx.doi.org/10.1111/j.1582-4934.2009.00853.x . [PMC free article: PMC3828855] [PubMed: 19627398]
- 117.
- Kim JB. Three-dimensional tissue culture models in cancer biology. Semin Cancer Biol. 2005;15:36577. http://dx.doi.org/10.1016/j.semcancer.2005.05.002 . [PubMed: 15975824]
- 118.
- Ehrbar M, Rizzi SC, Schoenmakers RG, Miguel BS, Hubbell JA, Weber FE, et al. Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions. Biomacromolecules. 2007;8:3000–7. http://dx.doi.org/10.1021/bm070228f . [PubMed: 17883273]
- 119.
- Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. http://dx.doi.org/10.1126/ science.1169494 . [PMC free article: PMC2756032] [PubMed: 19342581]
- 120.
- Raeber GP, Lutolf MP, Hubbell JA. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J. 2005;89:1374–88. http://dx.doi. org/10.1529/biophysj.104.050682 . [PMC free article: PMC1366622] [PubMed: 15923238]
- 121.
- Rizzi SC, Ehrbar M, Halstenberg S, Raeber GP, Schmoekel HG, Hagenmüller H, et al. Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part II: biofunctional characteristics. Biomacromolecules. 2006;7:3019–29. http://dx.doi. org/10.1021/bm060504a . [PubMed: 17096527]
- 122.
- Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci U S A. 2009;106:399404. http://dx.doi.org/10.1073/pnas.0808932106 . [PMC free article: PMC2626714] [PubMed: 19126683]
- 123.
- Loessner D, Stok KS, Lutolf MP, Hutmacher DW, Clements JA, Rizzi SC. Bioengineered 3D platform to explore cell-ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials. 2010;31:8494–506. http://dx.doi.org/10.1016/j.biomaterials.2010.07.064 . [PubMed: 20709389]
- 124.
- Montanez-Sauri SI, Sung KE, Puccinelli JP, Pehlke C, Beebe DJ. Automation of three-dimensional cell culture in arrayed microfluidic devices. J Lab Autom. 2011;16:171–85. [PMC free article: PMC3104941] [PubMed: 21609700]
- 125.
- Cardona A, Tomancak P. Current challenges in open-source bioimage informatics. Nat Methods. 2012;9:661–5. http://dx.doi.org/10.1038/nmeth.2082 . [PubMed: 22743770]
- 126.
- de Chaumont F, Dallongeville S, Chenouard N, Hervé N, Pop S, Provoost T, et al. Icy: an open bioimage informatics platform for extended reproducible research. Nat Methods. 2012;9:690–6. http://dx.doi.org/10.1038/nmeth.2075 . [PubMed: 22743774]
- 127.
- Eliceiri KW, Berthold MR, Goldberg IG, Ibáñez L, Manjunath BS, Martone ME, et al. Biological imaging software tools. Nat Methods. 2012;9:697–710. http://dx.doi.org/10.1038/ nmeth.2084 . [PMC free article: PMC3659807] [PubMed: 22743775]
- 128.
- Kankaanpää P, Paavolainen L, Tiitta S, Karjalainen M, Päivärinne J, Nieminen J, et al. BioImageXD: an open, general-purpose and high-throughput image-processing platform. Nat Methods. 2012;9:683–9. http://dx.doi.org/10.1038/nmeth.2047 . [PubMed: 22743773]
- 129.
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–82. http://dx.doi. org/10.1038/nmeth.2019 . [PMC free article: PMC3855844] [PubMed: 22743772]
- 130.
- Engebraaten O, Bjerkvig R, Pedersen PH, Laerum OD. Effects of EGF, bFGF, NGF and PDGF(bb) on cell proliferative, migratory and invasive capacities of human brain-tumour biopsies in vitro. Int J Cancer. 1993;53:209–14. [PubMed: 8381111]
- 131.
- Penar PL, Khoshyomn S, Bhushan A, Tritton TR. Inhibition of glioma invasion of fetal brain aggregates. In Vivo. 1998;12:75–84. [PubMed: 9575429]
- 132.
- Liotta LA, Lee CW, Morakis DJ. New method for preparing large surfaces of intact human basement membrane for tumor invasion studies. Cancer Lett. 1980;11:141–52. http://dx.doi. org/10.1016/0304-3835(80)90105-6 . [PubMed: 7459842]
- 133.
- Armstrong PB, Quigley JP, Sidebottom E. Transepithelial invasion and intramesenchymal infiltration of the chick embryo chorioallantois by tumor cell lines. Cancer Res. 1982;42:1826–37. [PubMed: 7066899]
- 134.
- Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, et al. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–45. [PubMed: 2438036]
- 135.
- Kleinman HK, Jacob K. Invasion assays. In: Bonifacino JS et al., eds. Current Protocols in Cell Biology. Wiley; 2001. Chapter 12: Unit 12 2.
- 136.
- Brekhman V, Neufeld G. A novel asymmetric 3D in-vitro assay for the study of tumor cell invasion. BMC Cancer. 2009;9:415. http://dx.doi.org/10.1186/1471-2407-9-415 . [PMC free article: PMC2791776] [PubMed: 19948022]
- 137.
- Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:201–12. http://dx.doi.org/10.1007/ s10911-010-9177-x . [PMC free article: PMC2886087] [PubMed: 20440544]
- 138.
- Rørth P. Collective cell migration. Annu Rev Cell Dev Biol. 2009;25:407–29. http:// dx.doi.org/10.1146/annurev.cellbio.042308.113231 . [PubMed: 19575657]
- 139.
- Friedl P, Noble PB, Walton PA, Laird DW, Chauvin PJ, Tabah RJ, et al. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res. 1995;55:4557–60. [PubMed: 7553628]
- 140.
- Hegerfeldt Y, Tusch M, Bröcker EB, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, beta1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–30. [PubMed: 11929834]
- 141.
- Matise LA, Palmer TD, Ashby WJ, Nashabi A, Chytil A, Aakre M, et al. Lack of transforming growth factor-beta signaling promotes collective cancer cell invasion through tumor-stromal crosstalk. Breast Cancer Res. 2012;14:R98. http://dx.doi.org/10.1186/bcr3217 . [PMC free article: PMC3680921] [PubMed: 22748014]
- 142.
- Mills RJ, Frith JE, Hudson JE, Cooper-White JJ. Effect of geometric challenges on cell migration. Tissue Eng Part C Methods. 2011;17:999–1010. http://dx.doi.org/10.1089/ten. tec.2011.0138 . [PubMed: 21631399]
- 143.
- Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–77. http://dx.doi.org/10.1083/jcb.200209006 . [PMC free article: PMC2172637] [PubMed: 12527751]
- 144.
- Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9:893–904. http://dx.doi.org/10.1038/ncb1616 . [PubMed: 17618273]
- 145.
- Guck J, Lautenschlager F, Paschke S, Beil M. Critical review: cellular mechanobiology and amoeboid migration. Integr Biol (Camb). 2010;2:575–83. [PubMed: 20871906]
- 146.
- Petrie RJ, Gavara N, Chadwick RS, Yamada KM. Nonpolarized signaling reveals two distinct modes of 3D cell migration. J Cell Biol. 2012;197:439–55. http://dx.doi.org/10.1083/ jcb.201201124 . [PMC free article: PMC3341168] [PubMed: 22547408]
- 147.
- Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–9. http://dx.doi.org/10.1083/jcb.200807195 . [PMC free article: PMC2700505] [PubMed: 19332889]
- 148.
- Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413–8. http://dx.doi. org/10.1073/pnas.0737381100 . [PMC free article: PMC154359] [PubMed: 12686696]
- 149.
- Baluk P, Morikawa S, Haskell A, Mancuso M, McDonald DM. Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2003;163:1801–15. http://dx.doi.org/10.1016/S0002-9440(10)63540-7 . [PMC free article: PMC1892429] [PubMed: 14578181]
- 150.
- Dudley AC. Tumor endothelial cells. Cold Spring Harb Perspect Med. 2012;2:a006536. [PMC free article: PMC3282494] [PubMed: 22393533]
- 151.
- Dvorak HF. Leaky tumor vessels: consequences for tumor stroma generation and for solid tumor therapy. Prog Clin Biol Res. 1990;354A:317–30. [PubMed: 2247496]
- 152.
- Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156:1363–80. http://dx.doi.org/10.1016/S0002-9440(10)65006-7 . [PMC free article: PMC1876882] [PubMed: 10751361]
- 153.
- Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer. 1997;71:612–9. [PubMed: 9178816]
- 154.
- Laferrire J, Houle F, Huot J. Adhesion of HT-29 colon carcinoma cells to endothelial cells requires sequential events involving E-selectin and integrin beta4. Clin Exp Metastasis. 2004;21:257–64. http://dx.doi.org/10.1023/B:CLIN.0000037708.09420.9a . [PubMed: 15387376]
- 155.
- Lapis K, Paku S, Liotta LA. Endothelialization of embolized tumor cells during metastasis formation. Clin Exp Metastasis. 1988;6:73–89. http://dx.doi.org/10.1007/BF01580408 . [PubMed: 3335082]
- 156.
- Shen S, Fan J, Cai B, Lv Y, Zeng M, Hao Y, et al. Vascular endothelial growth factor enhances cancer cell adhesion to microvascular endothelium in vivo. Exp Physiol. 2010;95:369–79. http://dx.doi.org/10.1113/expphysiol.2009.050260 . [PMC free article: PMC2859456] [PubMed: 19880535]
- 157.
- Gout S, Tremblay PL, Huot J. Selectins and selectin ligands in extravasation of cancer cells and organ selectivity of metastasis. Clin Exp Metastasis. 2008;25:335–44. http://dx.doi. org/10.1007/s10585-007-9096-4 . [PubMed: 17891461]
- 158.
- Rainger GE. Adhesion of tumor cells to matrices and endothelium. Methods Mol Med. 2001;58:103–17. [PubMed: 21340852]
- 159.
- Remuzzi A, Giavazzi R. Adhesion of tumor cells under flow. Methods Mol Biol. 1999;96:153–7. [PubMed: 10098133]
- 160.
- Hiratsuka S, Goel S, Kamoun WS, Maru Y, Fukumura D, Duda DG, et al. Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci U S A. 2011;108:3725–30. http://dx.doi.org/10.1073/pnas.1100446108 . [PMC free article: PMC3048115] [PubMed: 21321210]
- 161.
- Laubli H, Borsig L. Selectins as mediators of lung metastasis. Cancer Microenviron. 2010;3:97–105. [PMC free article: PMC2990482] [PubMed: 21209777]
- 162.
- Auerbach R, Lu WC, Pardon E, Gumkowski F, Kaminska G, Kaminski M. Specificity of adhesion between murine tumor cells and capillary endothelium: an in vitro correlate of preferential metastasis in vivo. Cancer Res. 1987;47:1492–6. [PubMed: 3815350]
- 163.
- Lugassy C, Dickersin GR, Christensen L, Karaoli T, LeCharpentier M, Escande JP, et al. Ultrastructural and immunohistochemical studies of the periendothelial matrix in human melanoma: evidence for an amorphous matrix containing laminin. J Cutan Pathol. 1999;26:78–83. http://dx.doi. org/10.1111/j.1600-0560.1999.tb01806.x . [PubMed: 10082397]
- 164.
- Lugassy C, Kleinman HK, Fernandez PM, Patierno SR, Webber MM, Ghanem G, et al. Human melanoma cell migration along capillary-like structures in vitro: a new dynamic model for studying extravascular migratory metastasis. J Invest Dermatol. 2002;119:703–4. http://dx.doi. org/10.1046/j.1523-1747.2002.01857.x . [PubMed: 12230517]
- 165.
- Muller WA, Luscinskas FW. Assays of transendothelial migration in vitro. Methods Enzymol. 2008;443:155–76. http://dx.doi.org/10.1016/S0076-6879(08)02009-0 . [PMC free article: PMC2759874] [PubMed: 18772016]
- 166.
- Li YH, Zhu C. A modified Boyden chamber assay for tumor cell transendothelial migration in vitro. Clin Exp Metastasis. 1999;17:423–9. http://dx.doi.org/10.1023/A:1006614232388 . [PubMed: 10651309]
- 167.
- Pries AR, Kuebler WM. 176 Pt 1. 2006. Normal endothelium. Handbook of experimental pharmacology; pp. 1–40. [PubMed: 16999215]
- 168.
- Yoshino K, Tanabe M, Ohnuma N, Takahashi H. Histopathologic analysis of bone marrow and bone metastasis in murine neuroblastoma. Clin Exp Metastasis. 1996;14:459–65. http:// dx.doi.org/10.1007/BF00128962 . [PubMed: 8871540]
- 169.
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. http://dx.doi.org/10.1038/nrn1824 . [PubMed: 16371949]
- 170.
- Lote K, Walløe A, Bjersand A. Bone metastasis. Prognosis, diagnosis and treatment. Acta Radiol Oncol. 1986;25:227–32. http://dx.doi.org/10.3109/02841868609136410 . [PubMed: 2435109]
- 171.
- Steeg PS, Camphausen KA, Smith QR. Brain metastases as preventive and therapeutic targets. Nat Rev Cancer. 2011;11:352–63. http://dx.doi.org/10.1038/nrc3053 . [PMC free article: PMC7351203] [PubMed: 21472002]
- 172.
- Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. http://dx.doi.org/10.1038/ nature08021 . [PMC free article: PMC2698953] [PubMed: 19421193]
- 173.
- Chronopoulos A, Trudeau K, Roy S, Huang H, Vinores SA, Roy S. High glucose-induced altered basement membrane composition and structure increases trans-endothelial permeability: implications for diabetic retinopathy. Curr Eye Res. 2011;36:747–53. http://dx.doi.org/10. 3109/02713683.2011.585735 . [PubMed: 21780924]
- 174.
- Zhang Z, McGoron AJ, Crumpler ET, Li CZ. Co-culture based blood-brain barrier in vitro model, a tissue engineering approach using immortalized cell lines for drug transport study. Appl Biochem Biotechnol. 2011;163:278–95. http://dx.doi.org/10.1007/s12010-010-9037-6 . [PubMed: 20652765]
- 175.
- Hatherell K, Couraud PO, Romero IA, Weksler B, Pilkington GJ. Development of a three-dimensional, all-human in vitro model of the blood-brain barrier using mono-, co-, and tri-cultivation Transwell models. J Neurosci Methods. 2011;199:223–9. http://dx.doi.org/10.1016/j. jneumeth.2011.05.012 . [PubMed: 21609734]
- 176.
- Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (mBBB). Lab Chip. 2012;12:1784–92. http://dx.doi.org/10.1039/c2lc40094d . [PubMed: 22422217]
- 177.
- Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress in Blood-Brain Barrier endothelial physiology. BMC Neurosci. 2011;12:40. http://dx.doi.org/10.1186/14712202-12-40 . [PMC free article: PMC3103473] [PubMed: 21569296]
- 178.
- Koop S, Schmidt EE, MacDonald IC, Morris VL, Khokha R, Grattan M, et al. Independence of metastatic ability and extravasation: metastatic ras-transformed and control fibroblasts extravasate equally well. Proc Natl Acad Sci U S A. 1996;93:11080–4. http://dx.doi.org/10.1073/ pnas.93.20.11080 . [PMC free article: PMC38287] [PubMed: 8855312]
- 179.
- Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol. 1998;153:865–73. http://dx.doi.org/10.1016/ S0002-9440(10)65628-3 . [PMC free article: PMC1853000] [PubMed: 9736035]
- 180.
- Vinci M, Gowan S, Boxall F, Patterson L, Zimmermann M, Court W, et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biol. 2012;10:29. http://dx.doi.org/10.1186/1741-7007-10-29 . [PMC free article: PMC3349530] [PubMed: 22439642]
- 181.
- Grafton MM, Wang L, Vidi PA, Leary J, Lelievre SA. Breast on-a-chip: mimicry of the channeling system of the breast for development of theranostics. Integr Biol (Camb). 2011;3:451–9. [PubMed: 21234506]
- 182.
- Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–16. http://dx.doi.org/10.1158/0008-5472.CAN-09-2356 . [PMC free article: PMC3436125] [PubMed: 20570886]
- 182.
- Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol. 2010;221:117–24. http://dx.doi.org/10.1002/path.2694 . [PMC free article: PMC2989884] [PubMed: 20225337]
- 184.
- Bielenberg DR. Metastasis: two assays explore the two roads traveled. Nat Methods. 2008;5:384–5. http://dx.doi.org/10.1038/nmeth0508-384 . [PubMed: 18446156]
- 185.
- Bruyére F, Melen-Lamalle L, Blacher S, Roland G, Thiry M, Moons L, et al. Modeling lymphangiogenesis in a three-dimensional culture system. Nat Methods. 2008;5:431–7. http://dx.doi.org/10.1038/nmeth.1205 . [PubMed: 18425139]
- Introduction
- The Metastatic Cascade
- Two-Dimensional Tumor-Matrix Adhesion Assays
- Cell Migration and Metastasis
- In Vitro Cell Migration Assays on Solid 2D Substrates
- Adhesive Interactions and Tumor Growth in 3D
- Biomimetic Synthetic Matrices to Study Tumor-Host Interactions and Metastasis
- In Vitro Migration in 3D Matrices and Tumor Cell Invasion
- Protease-Dependent and Independent Migration in 3D Matrices and In Vivo
- Vascular Dissemination and Homing to Metastatic Sites
- Conclusions and Future Perspectives
- References
- Investigating Metastasis Using In Vitro Platforms - Madame Curie Bioscience Data...Investigating Metastasis Using In Vitro Platforms - Madame Curie Bioscience Database
- Molecular Basis of Oncogenesis by NF-κB: From a Bird's Eye View to a RELevant Ro...Molecular Basis of Oncogenesis by NF-κB: From a Bird's Eye View to a RELevant Role in Cancer - Madame Curie Bioscience Database
- Hepatocyte Transplantation and Liver-Directed Gene Therapy - Madame Curie Biosci...Hepatocyte Transplantation and Liver-Directed Gene Therapy - Madame Curie Bioscience Database
- Laminopathies: One Gene, Two Proteins, Five Diseases.. - Madame Curie Bioscience...Laminopathies: One Gene, Two Proteins, Five Diseases.. - Madame Curie Bioscience Database
- Structural and Functional Relation of Neuropilins - Madame Curie Bioscience Data...Structural and Functional Relation of Neuropilins - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...