NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Chou R, Bougatsos C, Blazina I, et al. Treatment of Hepatitis C Virus Infection in Adults: Future Research Needs: Identification of Future Research Needs From Comparative Effectiveness Review No. 76 [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Jan. (Future Research Needs Papers, No. 28.)

Treatment of Hepatitis C Virus Infection in Adults: Future Research Needs: Identification of Future Research Needs From Comparative Effectiveness Review No. 76 [Internet].
Show detailsStakeholders
Of 14 stakeholders invited to participate in the project, eight agreed to participate, four declined, and two did not respond to invitations. Of the eight that agreed to participate, six attended the Webinar and followup discussion, while one stakeholder could not attend the Webinar but participated in followup surveys. Another stakeholder did not contribute to the prioritization process due to inability to participate in the Webinar or in subsequent surveys. No participating stakeholders reported significant conflicts of interest that precluded participation, as determined by AHRQ and our team.
All seven stakeholders who were invited to complete the questionnaires returned the first questionnaire and six returned the second (final) questionnaire. The participating stakeholders identified themselves as representing different perspectives, including clinicians, researchers, policymakers, payers, and consumer advocates. Individuals could represent more than one area. While no stakeholder identified themselves as a “research funder,” we did include one stakeholder from a Federal agency that funds clinical research. Some stakeholders may be hesitant to self-identify as research funders due to concerns that their opinions would be seen as representative of their funding organization.32 The proportions of self-identified stakeholder perspectives that contributed to this report by either providing feedback via the Webinar and/or questionnaires are illustrated in Figure 3.
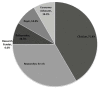
Figure 3
Stakeholder perspectives.
Research Needs
Generated List of Evidence Gaps From Webinar Discussion
As a result of the Webinar discussion, two additional evidence gaps that fell within the scope of the original CER were added to the list of gaps from the CER:
- Lack of studies enrolling patients with advanced age (>65–70 years)
- Lack of studies on effects of using noninvasive methods for assessing liver fibrosis to guide treatment decisions.
The Webinar participants also gave feedback on the list of gaps identified in the original CER. Stakeholders were advised to limit their comments to gaps that would fall within the scope of the CER, so little time was spent discussing gaps outside this scope (e.g., the lack of studies in children).
Initial Prioritization of Evidence Gaps
We circulated 15 research gaps for initial prioritization. All seven stakeholders completed the survey. Results are shown in Table 2.
Table 2
Initial prioritization of evidence gaps.
Final Prioritization of Research Needs
The final, ranked prioritization of the top research needs is shown in Table 3, followed by the second-tier research needs. Six of the seven stakeholders completed the final prioritization questionnaire. There were tie scores for the first and fifth place rankings, so instead of the top five needs, our list includes the top seven needs. In addition, there was not a clear demarcation between top-tier and second-tier research needs, and several of the gaps overlapped. In particular, our research team thought that research need #7 (the lack of studies of clinical outcomes among patients who experience SVR that adequately controlled for potential confounders) and research need #8 (the need for rigorous studies conducted in U.S. applicable settings evaluating the association between SVR and improved clinical outcomes) had considerable overlap in terms of scope. Therefore, even though research need #8 is categorized as second tier, we combined it with research need #7 in our discussion of study designs for top-tier research needs.
Table 3
Final prioritization of research needs.
Ongoing and Recently Published Studies
The authors of the original CER reviewed 2,890 citations for potential inclusion. Of those, 77 studies were ultimately included in the final report. Update searches conducted in August, 2012 identified an additional 433 citations, none of which met criteria for inclusion in the CER. In addition to the updated search of Ovid MEDLINE and other bibliographic databases discussed above, we searched the National Institute of Health’s ClinicalTrials.gov in September 2012 for potentially relevant ongoing studies. As a result of that search, we identified 46 studies that may potentially address a future research need (Appendix D). We searched for studies of currently approved treatments as well of studies of treatments not currently approved, but in phase 3 or 4 of clinical testing. We focused on identifying ongoing studies (rather than completed studies or terminated studies) conducted in treatment-naïve, HCV-infected individuals. We did not look for studies that would fall outside the scope of the treatment CER, such as studies conducted in treatment-experienced patients, patients with HIV or hepatitis B virus coinfection, or children.29
Table 4 lists ongoing trials most relevant to the top-tier future research needs, and a complete list of all identified ongoing trials is shown in Appendix D. One ongoing trial focused on patients with cirrhosis, one enrolled intravenous drug users, four were efficacy trials of new (not yet approved) interferon-free treatment regimens in antiviral-naïve patients, and three evaluated long-term virologic outcomes and harms associated with antiviral treatments. We did not include studies of alisporivir (also known as DEB025), a cyclophilin inhibitor, as research was suspended in April 2012 by the FDA due to safety concerns.33 We identified no ongoing studies evaluating long-term clinical outcomes associated with antiviral treatments or that enrolled screen-detected patients. No study clearly was designed using an effectiveness framework, though details on methods were fairly limited.
Table 4
Selected ongoing studies addressing future research needs.
Although many other ongoing studies enrolled treatment-naïve individuals, they were less relevant to the top-tier research needs. Most studies were short-term (3 to 6 months followup after completion of antiviral therapy), interferon-based efficacy studies with SVR as the primary outcome.
Proposed Study Designs and Research Questions
The following section discusses overall and specific study design considerations and example research questions for the top-tier future research needs (Tables 5–11).
Table 5
PICOT specifications for Future Research Need #1.
Table 6
PICOT specifications for Future Research Need #2.
Table 7
PICOT specifications for Future Research Need #3.
Table 8
PICOT specifications for Future Research Need #4.
Table 9
PICOT specifications for Future Research Need #5.
Table 10
PICOT specifications for Future Research Need #6.
Table 11
PICOT specifications for Future Research Needs #7 and #8.
Overall Study Design Considerations
Randomized Controlled Trials
Advantages of randomized controlled trials (RCTs) for producing a valid result. Appropriately designed and conducted RCTs are highly suitable for evaluating the benefits and harms of antiviral treatments because they are less susceptible to bias and confounding than observational studies. RCTs are often designed using an efficacy paradigm and are the standard for establishing the efficacy of new drug regimens (research need #3). A shortcoming of such efficacy trials is that while results may be valid, they may be poorly generalizable to real-world situations. However, RCTs can also be designed using an effectiveness paradigm, which would help in addressing several top-tier research needs. For example, RCTs conducted in community-based settings that apply broad eligibility criteria could help address future research needs #1 (studies based on an effectiveness paradigm), #2 (patients with comorbidities). RCTs designed with long-term followup and assessing clinical, rather than virologic outcomes, would help address research need #6 (need to assess long-term outcomes of antiviral regimens). In additions, RCTs could focus on evaluation of patients with HCV identified through screening (research need #4) or the effects of noninvasive methods compared with liver biopsy for selecting patients for antiviral therapy (research need #5).
Ability to recruit/availability of data. Comorbidities are common in patients with HCV infection, so this should not be a barrier to recruitment. Also, the possibility of randomization, which can sometimes be a barrier to recruitment, should not be an issue for this research area because RCTs of HCV antiviral treatments always involve the comparison of one antiviral regimen against another; therefore everyone still receives treatment rather than potentially being randomized to placebo. The ability to retain patients in studies could be a barrier to obtaining data on long-term benefits and harms.
Resource use, size, and duration. RCTs are typically more resource-intensive than observational studies. In addition, because differences between treatments may be relatively small, adequately powered RCTs may require large sample sizes. Assessment of long-term outcomes, including important clinical outcomes such as mortality, hepatocellular carcinoma, cirrhosis and related complications, and need for transplantation would likely require followup exceeding 3 to 5 years, depending on the sample size.
Ethical, legal, and social issues. Those with ongoing intravenous drug use or major psychological or other serious comorbidities have frequently been excluded from RCTs of antiviral therapies and may pose a challenge in terms of patient recruitment or clinician buy-in. As discussed above, the availability of effective antiviral treatments largely precludes the use of placebo controls in RCTs for ethical reasons.
Cohort Studies
Advantages of cohort studies for producing a valid result. It is not possible to evaluate the association between achieving and not achieving an SVR following antiviral treatment and clinical outcomes with RCTs because patients cannot be randomized to whether or not they experience an SVR (they can only be randomized to a treatment), therefore cohort studies are useful for this purpose. However, cohort studies are more prone to bias and confounding than RCTs since groups are not randomized. Therefore, it is critical for cohort studies evaluating this association to adequately adjust for the key factors known to be associated with poorer prognosis in patients with HCV infection (such as age, race, baseline fibrosis, viral load, genotype, and others).
Cohort studies could also be used to address long-term clinical outcomes such as mortality, cirrhosis, hepatocellular carcinoma, and need for transplantation (future research need #6), as it is often more feasible to analyze long populations with longer followup using a cohort rather than RCT design.
Ability to recruit/availability of data. Large existing registries of patients with HCV infection could be a more efficient source of data than assembling a new cohort, though analysis would necessarily be retrospective. To be most useful, registry data should include clinical information, in addition to information available from administrative databases.
Resource use, size, and duration. Given the large sample sizes and long duration of followup needed to evaluate long-term clinical outcomes, cohort studies would likely be more feasible than RCTs as they generally require fewer resources and could be performed retrospectively; however, studies spanning many years could still be costly.
Ethical, legal, and social issues. Standard ethical issues in the design and conduct of observational studies include maintenance of data security, and participant privacy and confidentiality.
Specific Study Design and Research Question Considerations
We provide the research questions as examples, though the nature of the research needs could yield a number of research questions.
Future Research Need #1. Studies Designed Using Effectiveness Paradigm
The need for studies using an effectiveness paradigm is a general issue relevant across many research questions. RCTs as well as cohort studies that address any of the Key Questions evaluated in the CER would help address this future research need if they are based in community settings, employ broad inclusion criteria, reflect treatment as observed in real-world practice (including lower adherence), evaluate clinical as well as virologic outcomes, and are designed for long-term followup. Example research questions that could address this research need are, “What is the comparative effectiveness of different antiviral regimens in patients recruited from community settings, using broad inclusion criteria?” and “How does the efficacy of antiviral drugs change with lower treatment adherence?”
Future Research Need #2. Studies Enrolling Broader Spectrum of Patients, Including Those With Medical and Psychological Comorbidities Seen in Clinical Practice, Such as Advanced Cirrhosis and IV Drug Users
This need is related to future research need #1, but focused on the patient populations enrolled in the studies. As for future research need #1, RCTs and cohort studies that address any of the Key Questions evaluated in the CER that employ broader inclusion criteria would help address this future research need and help guide treatment decisions in patients commonly encountered in clinical practice but typically excluded from efficacy trials. An example research question that could address this research need is, “How do outcomes of antiviral treatments differ in patients with HCV who are IV drug users versus patients without IV drug use?”
Future Research Need #3. Studies of New Drugs Currently in Clinical Phases of Testing, Including Oral Regimens Without Interferon
Stakeholders emphasized the expected availability within the next few years of interferon-sparing, all-oral antiviral regimens that will represent a major milestone in HCV treatment. In fact, some patients are opting against treatment at this time with the expectation that such regimens will soon be available. Although the CER focused on current FDA-approved antiviral regimens, any new therapy that is approved would become within scope. The standard study design to evaluate new drug regimens and obtain FDA approval is an RCT using an efficacy design, typically focusing on SVR rates (Key Question 2a in the CER). New regimens will likely be compared against pegylated interferon plus ribavirin, with new trials of genotype 1 infection patients comparing effects of new regimens versus telaprevir or boceprevir plus pegylated interferon plus ribavirin. An example research question that could address this research need is, “What is the comparative effectiveness of oral antiviral regimens without interferon for HCV versus interferon-based regimens?”
Future Research Need #4. Studies of Screen-Detected Patients
Screen-detected patients may have less severe disease at baseline than patients identified based on symptoms of liver disease or elevated liver function test. Studies that address any of the Key Questions in the CER that evaluate antiviral treatments in HCV-infected patients identified through screening would be helpful for understanding benefits and harms of treatment in this population and would be helpful for informing screening decisions.34 An example research question that could address this research need is, “How does the efficacy of antiviral treatment for HCV differ in patients identified through screening versus those identified based on symptoms or abnormal liver tests?”
Future Research Need #5. Studies Using Noninvasive Methods for Assessing Liver Fibrosis to Guide Treatment Decisions
Stakeholders emphasized that liver biopsy is no longer performed in all patients who are being considered for antiviral therapy, due to the availability of noninvasive methods for assessing liver fibrosis and more effective treatments. In addition, liver biopsy is associated with a small risk of serious harms (primarily pain and bleeding). However, only one small observational study has evaluated treatment outcomes in patients selected for treatment without a biopsy.35 This future research need was not directly addressed in the CER. An example research question that could address this research need is, “What is the comparative effectiveness of antiviral treatments in patients selected for therapy based on a liver biopsy versus those selected for treatment without undergoing a liver biopsy?”
Future Research Need #6. Studies Assessing Important Long-Term Clinical Outcomes
Evaluating the comparative effectiveness of current antiviral regimens on clinical outcomes in randomized trials or cohort studies is a challenge due to the long lead time and large samples necessary to adequately assess these outcomes. This might be more feasible if the studies were to focus on populations at higher risk for complications from chronic HCV infection (e.g., patients with baseline cirrhosis, high viral load, or other risk factors for progression). RCTs and cohort studies that address Key Questions 1a and 1b in the CER would help address this future research need if they are designed to assess long-term outcomes. An important challenge in carrying out such studies is the potential for high attrition over time. An example research question that could address this research need is, “What are the effects of antiviral therapy on clinical outcomes in patients at higher risk for disease progression?”
Future Research Need #7: Studies That Adequately Control for Potential Confounders Reporting Clinical Outcomes in Patients Who Experience SVR With Those Who Do Not Experience SVR
Future Research Need #8: Need for Methodologically Rigorous Studies Conducted in Settings Applicable to U.S. Populations Evaluating the Association Between Achieving an SVR and Improvements in Clinical Outcomes
The CER identified only one study on the association between achieving an SVR and clinical outcomes that controlled well for confounders.8 Additional large, cohort studies that addressed Key Question 4 from the CER that control for important confounders, including genotype, age, sex, race, viral load, baseline fibrosis, liver function tests, comorbidities, and body weight would help address this future research need. Additionally, many of the studies included in the CER evaluated Asian populations, where the risk of hepatocellular carcinoma and other complications of HCV infection may be higher than in the United States. Therefore, more well-controlled studies in populations applicable to the United States are needed. An example research question that could address these research needs is, “How do outcomes differ among U.S. patients with HCV infection who experience an SVR versus those who do not experience an SVR after antiviral therapy?”
- Results - Treatment of Hepatitis C Virus Infection in Adults: Future Research Ne...Results - Treatment of Hepatitis C Virus Infection in Adults: Future Research Needs
- Acknowledgments - Treatment of Hepatitis C Virus Infection in Adults: Future Res...Acknowledgments - Treatment of Hepatitis C Virus Infection in Adults: Future Research Needs
- Acknowledgments - Future Research Needs for Childhood Obesity Prevention Program...Acknowledgments - Future Research Needs for Childhood Obesity Prevention Programs
- Methodological and Statistical Gaps in Knowledge About PCA3 Testing - PCA3 Testi...Methodological and Statistical Gaps in Knowledge About PCA3 Testing - PCA3 Testing in the Diagnosis and Management of Prostate Cancer: Future Research Needs
Your browsing activity is empty.
Activity recording is turned off.
See more...