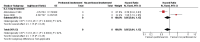Prognostic factors
Prognostic factors for overall survival and predictive factors for treatment response are summarised in to , and in to .
Prognostic factors investigated using multivariate analysis.
Prognostic factors for overall survival, hazard ratio and 95% confidence interval.
Well or moderately well differentiated adenocarcinoma versus other histology, univariate analysis of treatment response.
Well or moderately well differentiated adenocarcinoma versus other histology, univariate analysis of overall survival.
Poorly differentiated adenocarcinoma or carcinoma versus other histology, univariate analysis of treatment response.
Poorly differentiated adenocarcinoma or carcinoma versus other histology, univariate analysis of overall survival.
Undifferentiated carcinoma, univariate analysis of treatment response.
Male versus female, univariate analysis of treatment response.
Male versus female, univariate analysis of overall survival.
Male versus female, multivariate analysis of overall survival.
Liver involvement, univariate analysis of treatment response.
Liver involvement, univariate analysis of overall survival.
Liver involvement, multivariate analysis of overall survival.
Lymph node involvement, univariate analysis of treatment response.
Lymph node involvement, univariate analysis of overall survival.
Lymph node involvement, multivariate analysis of overall survival.
Lung metastases, univariate analysis of treatment response.
Lung metastases, univariate analysis of overall survival.
Elevated serum LDH, univariate analysis of treatment response.
Elevated serum LDH, univariate analysis of overall survival.
Elevated serum LDH, multivariate analysis of overall survival.
Performance status, univariate analysis of treatment response.
Performance status, univariate analysis of overall survival.
Performance status, multivariate analysis of overall survival.
Age, univariate analysis of treatment response.
Age, univariate analysis of overall survival.
Age, multivariate analysis of overall survival.
Number of metastatic sites, univariate analysis of treatment response.
Number of metastatic sites, univariate analysis of overall survival.
Number of metastatic sites, multivariate analysis of overall survival.
Peritoneal involvement, univariate analysis of treatment response.
Peritoneal involvement, univariate analysis of overall survival.
Peritoneal involvement, multivariate analysis of overall survival.
Lactate dehydrogenase (LDH)
Elevated serum LDH was an adverse prognostic factor for overall survival on univariate analysis. Elevated LDH was an independent prognostic factor in five of the nine studies that considered it in multivariate analysis. In these five studies patients with elevated serum LDH had almost twice the risk of death of those with normal serum LDH levels, HR=1.94 [95% C.I. 1.54 to 2.44].
Elevated serum LDH did not significantly affect response to platinum based chemotherapy, RR = 0.98 [0.68 to 1.41], however 95% confidence intervals were wide and included both appreciable benefit and harm.
Serum albumin
Low serum albumin was an independent adverse prognostic factor for overall survival in all three studies that considered it (Assersoh et al 2003; Seve et al 2006a and Munoz et al 2004). Munoz et al 2004 reported that patients with low serum albumin were at greatly increased risk of death, HR = 4.31 [95% C.I. 1.56 to 11.85]. Seve et al (2006a) also found low serum albumin to be an independent risk factor, HR = 2.70 [95% C.I. 1.79 to 4.07]
Serum alkaline phosphatase
Elevated serum alkaline phosphatase was an independent adverse prognostic factor for overall survival in three of the nine studies that examined it in multivariate analysis.
Performance status
Studies of performance status divided people into groups of good performance status and poor performance status. Some studies defined good performance status as 0 on the WHO/ECOG scale, while others defined it as 0 to 1 on the WHO/ECOG scale. Poor PS was everything else. Good performance status (however defined) was a favourable prognostic factor for overall survival in nine of the ten studies that analysed it in multivariate analysis, The pooled hazard ratio in these nine studies was 0.62 [95% C.I. 0.53 to 0.73].
Patients with good performance status were more likely to respond to chemotherapy, RR = 1.60 [1.09 to 2.35] on univariate analysis.
Number of metastatic sites
Studies divided patients into two groups according to the number of metastatic sites. Typically patients with either one or one to two metastatic sites were compared with everyone else. Fewer metastatic sites was a favourable prognostic factor for overall survival, HR = 0.82 [95% C.I. 0.73 to 0.92] on multivariate analysis. Patients with fewer sites were more likely to respond to chemotherapy, RR = 1.64 [95% C.I. 1.18 to 2.29] on univariate analysis.
Age
Studies split patients into two age groups, the cut-point defining older and younger varied between studies from 50 years to 65 years. In chemotherapy series younger age was not a prognostic factor for treatment response or overall survival. In univariate analysis from series of patients not selected by treatment, however, younger age was a favourable prognostic factor for overall survival HR = 0.69 [0.58 to 0.81]. Multivariate analyses suggested age was not an independent prognostic factor.
Histology
Studies were typically restricted to patients with adenocarcinoma, poorly differentiated carcinoma or undifferentiated carcinoma. On univariate analysis adenocarcinoma histology was an adverse prognostic factor for treatment response, RR=0.71 [0.59 to 0.86], and overall survival, HR = 1.32 [1.18 to 1.47]. Multivariate analyses, however, suggested adenocarcinoma histology was not an independent prognostic factor.
Poorly differentiated adenocarcinoma or poorly differentiated carcinoma histology was an positive prognostic factor for treatment response, RR = 1.44 [1.16 to 1.78], and overall survival, HR = 0.78 [0.67 to 0.91].
Evidence from two studies (Van der Gaast el al 1990; Pavlidis et al, 1992), suggests that patients with undifferentiated carcinoma are more than twice as likely to respond to platinum based chemotherapy than patients with other histology. The relative risk for response to treatment was 2.10 [95% C.I. 1.21 to 3.66]
Liver metastases
People with liver metastases tended to have poorer overall survival than people without. On multivariate analysis seven of the twelve studies that considered it found liver metastases to be an adverse prognostic factor for survival. The pooled hazard ratio in these seven studies was 1.40 [95% C.I. 1.24 to 1.57].
The presence of liver metastases was the factor most strongly associated with lack of response to chemotherapy, RR = 0.56 [0.45 to 0.69]
Lung metastases
The presence of lung metastases was an adverse prognostic factor for overall survival, HR=1.40 [1.24 to 1.57] on univariate analysis. It was unlikely that presence of lung metastases was an independent prognostic factor, however, as no studies retained this factor in their multivariate models.
People with lung metastases were also less likely to respond to chemotherapy, RR = 0.70 [0.53 to 0.93].
Peritoneal metastases
Presentation with peritoneal metastases was a favourable prognostic factor for treatment response, RR = 1.45 [95% C.I. 1,12 to 1.88]. There was imprecision and inconsistency in the estimate of the effect on peritoneal metastases on overall survival, and it was unclear whether peritoneal metastasis was a prognostic factor for overall survival.
Lymph node metastases
Lymph node metastases were a independent favourable prognostic factor for overall survival in only two of the nine studies that considered it. The presence of lymph node metastases was the factor most strongly associated with response to chemotherapy, RR = 2.68 [1.94 to 3.70].
Prognostic models
Prognostic models aim to classify patients into risk groups for overall survival and could be used as decision aids in treatment decisions (see ). These models are developed using clinical data from group of patients (the development cohort) but need to be tested in an independent set of patients to confirm their validity.
Multivariate prognostic models for overall survival.
Culine et al (2002) developed a prognostic model to classify patients with CUP into high and low risk groups for death from any cause, using two prognostic factors: performance status and serum LDH. In the group of patients used to develop the model the median survival in high and low risk groups was 4 months and 12 months respectively. In an independent set of patients used to validate the model the median survival in high and low risk groups was 7 and 12 months respectively. The model of Culine et al was validated by Van de Wouw et al (2004) who reported median survival of 1 month and 6.5 months median survival for the high and low risk groups in their cohort. Similarly Yonemori et al (2006) reported median survivals of 10 and 21 months for the high and low risk groups using the Culine model (P=0.003). Munoz et al (2008), however, failed to demonstrate a significant difference in the overall survival of the three risk groups in their cohort of patients with CUP.
Van der Gaast et al (1995) developed a model for patients with undifferentiated cancer of unknown primary using two prognostic factors: performance status and serum alkaline phosphatase. The median survival of high and intermediate risk groups was 4 and 10 months respectively. Median survival was not reached in the low risk group. Yonemori et al (2006) validated the model of Van der Gast, reporting median survival in the high, intermediate and low risk groups of 20, 12 and 7 months respectively (P not reported).
Ponce Lorenzo (2007) developed a prognostic model to classify patients into three risk groups on the basis of performance status and presence of liver metastases. Munoz et al (2008) challenged this model, after testing it in their CUP cohort, claiming that it failed to discriminate between low and intermediate risk groups well enough. Unsuprisingly the model of Munoz et al (2008), using serum albumin and performance status, performed better in their own cohort (probably because it was developed using the same patients).
Seve et al (2006a) reported a prognostic model to divide patients with CUP into high and low risk groups for death from any cause using serum albumin and the presence of liver metastases. The model was validated by the authors in an independent set of patients, with median survival of 3 months and 13 months in the high and low risk groups respectively (P<0.0001). Seve et al (2006a) suggested that the model of Culine et al (2002) was less powerful than their own, in this validation set: using Culine’s model median survival in the high and low risk groups was 4 and 13 months respectively (P=0.07).
Trivanovic et al (2009) reported a prognostic model to classify patients into three risk groups using the following adverse prognostic factors: elevated LDH, QTc prolongation, liver mets, PS 2 or more, anaemia, age 63 years or more. The model has not been validated.
Hess et al (1999) used classification and regression tree (CART) analysis to put patients into one of ten risk groups. Their CART model incorporates: presence of liver, bone, adrenal, lymph node and pleural metastases, neuroendocrine histology, age, number of metastatic sites and adenocarcinoma histology. The authors note that validation studies are particularly important for CART models as their structure is highly dependent on the development cohort. No validation studies were found for this model.