NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Smith PB, Smith MJ, Gonzalez D, et al. Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile [Internet]. Bethesda (MD): National Institute of Child Health and Human Development (US); 2015 Oct 15.

Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile [Internet].
Show details10.1. Disposition of Participants
A total of 23 participants were enrolled in the Clindamycin Obesity study between August 8, 2013 and August 12, 2014. One participant [▬] was consented, enrolled and received study procedures but never received study drug. The participant was ineligible due to concomitant medications. This participant was monitored separately and there were no study procedure related deviations or AEs reported. This participant is not included in the safety population. One additional participant [▬] was erroneously enrolled twice and the incorrect enrollment is not included in this report. This subject only received study drug for the correct enrollment.
The safety population comprised of participants who received study clindamycin and included 22 participants. Participant disposition is summarized in Table 10-1, Table 14.1.1.1, and Figure 10-1. Seven participants were enrolled in the 2 - <12 year age stratum; 4 participants had a BMI of 85th - <95th percentile and 3 participants had a BMI of ≥95th percentile. Fifteen participants were enrolled in the 12 - <18 year age stratum of which 4 participants had a BMI of 85th - <95th percentile and 11 participants had a BMI of ≥95th percentile. Nineteen (86%) participants completed the study as planned and 3 (14%) participants early terminated: 2 participants due to investigator decision and 1 participant withdrew consent.
Table 10-1
Participant Disposition.
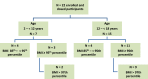
Figure 10-1
Enrollment Flow Chart.
The PK population includes 21 of the 22 safety participants. One participant [▬] stopped treatment with clindamycin before any PK samples could be drawn.
Sites were asked to prioritize enrollment of participants with BMI >97th percentile in each age group. Final enrollment included 2 participants with BMI >97th percentile in the younger age stratum and 9 participants with BMI >97th percentile in the older age stratum.
Participants were enrolled at 4 sites in this study; see Table 14.1.1.2.
10.2. Protocol Deviations
There were 37 protocol deviations (PDs) reported in 12 (55%) of the 22 clindamycin treated participants. Thirteen of the 37 protocol deviations resulted from hospital error and 12 (of 37) resulted from research staff or clinic error. The PDs are summarized by site in Tables 14.1.3.1 and 14.1.3.2; the complete list of protocol deviation by participant is provided in Appendix 16.2.5. PDs are tabulated by deviation classification and indicated reason in Table 14.1.3.3.
PDs were associated with informed consent in 3 participants and with eligibility/enrollment in 2 participants. There were 21 study medication administration PDs in 7 participants and 15 protocol/procedure assessment PDs in 7 participants. One participant had an unreported adverse event deviation.
Two waivers were granted on deviations during this study and both were associated with protocol/procedure assessments. No waivers were granted for PDs associated with eligibility/enrollment.
- STUDY PARTICIPANTS - Safety and Pharmacokinetics of Multiple-Dose Intravenous an...STUDY PARTICIPANTS - Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile
Your browsing activity is empty.
Activity recording is turned off.
See more...