NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Smith PB, Smith MJ, Gonzalez D, et al. Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile [Internet]. Bethesda (MD): National Institute of Child Health and Human Development (US); 2015 Oct 15.

Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile [Internet].
Show detailsThe primary objective of this study was to characterize the PK of multiple-dose IV clindamycin in overweight and obese children. The efficacy of clindamycin was not investigated in this study.
11.1. Dosing and PK Sampling
11.1.1. Dosing
Doses are grouped by Standard of Care (SOC) IV doses, Study IV doses and Study PO doses in Table 14.2.1.1 (IV doses) and Table 14.2.1.2 (PO doses). All participants were receiving SOC IV clindamycin at study enrollment. Sites entered a maximum of 6 SOC doses administered prior to the first dose of study drug and all study drug doses administered during the study period (Days 1-14). Doses in this section are presented in mg and mg per kg of participant’s dosing weight.
There were 85 IV SOC doses reported in the 22 participants with a median (range) of 3.5 (1-6) doses per participant. This includes 2 SOC doses used for the PK sampling, an option that was added with Version 6 of the protocol. The median SOC dose was 675 (216-1050) mg. Younger children (2 - <12 years age stratum) had a median dose of 532 (300-780) mg and older children had a median dose of 763 (216 – 1050) mg. Dose per kg of participant dosing weight was similar in all age/BMI strata with median doses of 10.8 mg/kg and 10.0 mg/kg for BMI <95th percentile and BMI ≥95th percentile respectively in the younger age stratum and 10.0 mg/kg and 9.2 mg/kg for BMI <95th percentile and BMI ≥95th percentile respectively in the older age stratum. Doses were scheduled every 8 hours for 72% of the doses and every 6 hours for 27% of the doses. One participant’s first SOC dose was partial because the IV infiltrated during infusion.
One hundred ten study doses were reported in the IV portion of the study with a median of 3 study doses per participant (range 2 – 26). Median study dose was 900 (300-1100) mg; children in the younger age stratum had a median dose of 501 mg and children in the older age strata had a median dose of 900 mg. Median dose per kg of participant dosing weight was 10.0 (3.0-14.3) mg/kg overall and for each strata except 2- <12 years/BMI <95th percentile group which had a median of 13.3 mg/kg. Eighty-six percent of the doses were administered every 8 hours and 14% were administered every 6 hours.
One participant [▬] continued into the PO portion of the study. This participant received 13 PO doses of 300 mg (or 1.3 mg/kg) each, every eight hours. When the participant returned to provide PO PK samples, a review of the dosing diary found that the child did not take the PO doses as instructed and PO PK samples were not collected.
11.1.2. PK Sampling
Twenty-one participants had at least 1 fresh plasma sample and are included in Table 14.2.2.1, Summary of PK Sampling. Ninety-one samples, all on IV doses, were collected during this study with a median (range) of 4 (1-6) PK samples per participant. The 91 samples consisted of 89 timed fresh plasma samples and 2 scavenged plasma samples.
Table 11-1 shows how the 91 collected samples fit into the planned PK sampling schedule. Ninety-six percent (87/91) of the collected samples were during planned sampling times.
Table 11-1
PK Scheduled Sampling Times and PK Samples (PK Population).
As noted above, there were no PO dosing PK samples collected for this study.
11.1.3. PK Drug Concentration
Clindamycin was measured in each sample. Descriptive summary statistics of concentration values (ng/mL) are reported in Table 14.2.3.1. The 89 fresh plasma samples had a median (range) concentration of 6.4 (0.3 – 24.6) ng/mL. The 2 scavanged plasma samples were collected in the same participant [▬] and had values of 1.6 ng/mL and 14.4 ng/mL.
11.2. Data Sets Analyzed
A total of 23 participants were enrolled in this study; 22 participants received study clindamycin and are included in the safety population. Twenty-one participants had at least one fresh timed plasma PK samples drawn and are included in the PK analysis population. One participant transitioned to oral clindamycin, but did not have any oral PK samples drawn.
11.3. Demographic and Other Baseline Characteristics
Baseline demographics for the safety population are presented in Section 12.
11.4. Measurement of Treatment Compliance
All participants were inpatients in the hospital. Therefore, each dose of study drug administration was given and monitored by hospital staff, then reviewed by study personnel. The protocol provided requirements for study medication dosing. For those participants who were discharged on oral clindamycin and had not completed the PO PK visit, the participant or parent/guardian kept a drug administration diary and all drugs were accounted for at the outpatient PK study visit.
11.5. Pharmacokinetic Analysis and Results
11.5.1. Analysis of Pharmacokinetics
As previously described, a one compartment PK model described the clindamycin concentration vs. time data well [12]. For the base model, use of WT was compared with FFM, NFM, and LBW. WT resulted in the lowest objective function value: 7157.9, WT; 7173.0, FFM; 7173.0, NFM; and 7164.8, LBW. Thus, all additional covariate models were evaluated after accounting for body size using WT. Consistent with previous findings, after accounting for body size, use of a sigmoidal maturation function with postmenstrual age (PMA) resulted in the largest objective function value drop (-115.6 points). Thereafter, albumin (ALB) and alpha-1 acid glycoprotein (AAG) on V and serum creatinine on CL reached statistical significance; however, the latter was not included in the final model because its retention during the backward elimination step was largely a result of one influential individual (with a value of 3.4 mg/dL). Therefore, the final model included body weight, a sigmoidal maturation relationship between PMA and CL, and exponential relationships between ALB and AAG on V: CL (L/h) = 13.8*(WT/70)0.75*(PMA2.83/(39.52.83+PMA2.83)); V (L)= 63.6*(WT/70)*(ALB/3.3)-0.83*(AAG/2.4)-0.25. Maturation reached 50% adult CL values at ~40 weeks PMA. Diagnostic plots, including visual predictive checks, for the final model are shown in the PK report, see Appendix 16.4.4.
Empirical Bayesian estimates (EBEs) obtained from the final model were stratified by obese status and age (Table 11-2). For the >6-12 years and >12 years age categories, statistically significant differences were observed in the absolute (i.e., non-weight normalized) V estimates (P<0.001). Half-life of elimination was also significantly different, but only for the >6-12 years age group (P=0.01). No other statistically significant differences were observed between obese and non-obese children.
Table 11-2
Comparison of Empirical Bayesian Estimates for the Final Model Using TBW to Correct for Body Size.
Previously recommended clindamycin dosing regimens [12] were simulated using the final population PK model described herein and compared to simulated adult exposure (70 kg) following 600 mg every 8 hours intravenously. The median (2.5th, 97.5th percentiles) simulated steady-state area under the concentration versus time curve from 0 to 8 hours (AUC0-8,SS) when stratified by age-based dosing were: 44.2 mcg*h/mL (14.0-146.0), 12 mg/kg every 8 hours if 2-6 years; 44.8 mcg*h/mL (14.4-144.0), 10 mg/kg every 8 hours if >6-12 years; and 48.6 mcg*h/mL (15.4-156.0), 10 mg/kg every 8 hours if >12 years. Median exposure of these dosing regimens were within 25% of the median observed in a 70 kg simulated adult receiving 600 mg intravenously every 8 hours: 44.7 mcg*h/mL (13.6-134.2), which was comparable to those previously reported in the literature [29]. Patients that would receive greater than 900 mg following weight based dosing (>75 kg for 12 mg/kg and >90 kg for 10 mg/kg), received a maximum dose of 900 mg. Box plots of simulated AUC0-8,SS and maximal drug concentration at steady-state (CMAX,SS) stratified by age is shown in Figures 11-1 and 11-2, respectively.
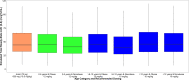
Figure 11-1
Box Plot of AUC0-8,SS Following Age-Based Clindamycin Dosing and Stratified by Obese Status. The upper and lower whiskers extend to the highest and lowest points that are within 1.5*interquartile range.

Figure 11-2
Box plot of CMAX, SS Following Aged-Based Clindamycin Dosing and Stratified by Obese Status.
After correcting for protein binding and using an optimal dosing regimen, the simulated unbound, steady-state clindamycin concentrations were above a minimum inhibitory concentration of 0.12 mcg/mL for at least half the dosing interval in >95% of participants across age groups (Table 11-3).
Table 11-3
Percentage of Participants with Simulated Unbound Concentrations Above a Minimum Inhibitory Concentration (MIC) of 0.12 μg/mL.
11.5.2. Statistical and Analytical Issues
11.5.2.1. Adjustments for Covariate
For the base population PK model, to assess whether use of other indirect measures of body size, namely, NFM, FFM, and LBW, resulted in superior model performance, we tested these first in place of WT, and in the absence of accounting for PMA; see Appendix 16.4.3. Additional covariates tested using a forward inclusion (p<0.05 and Δ objective function value [OFV] >3.8) and backward elimination (p<0.001 and ΔOFV >10.8) approach were PMA, ALB, total bilirubin, serum creatinine, obese status, and AAG.
11.5.2.2. Handling of Dropouts or Missing Data
The handling of missing data is described in the PK report, Appendix 16.4.3.
11.5.2.3. Interim Analysis and Data Monitoring
Interim PK analysis was completed on September 18, 2014 and included 11 CLN01 obesity participants with 47 PK samples. The objective of the NICHD-2012-CLN01 study interim analysis was to apply a previously published population PK (PopPK) model to assess the effect of obesity on clindamycin PK in children. The secondary objective was to assess whether the current sample size is sufficient to evaluate the effect of obesity on clindamycin disposition.
A PopPK model was developed using clindamycin PK data from the NICHD-2011-POP01 study. Using merged PK data from three studies namely, NICHD-2011-POP01, NICHD-2012-CLN01 and NICHD-2012-STA01, a total of 39 obese patients were available to assess the effect of obesity on clindamycin PK. The median (range) number of samples and dose in these obese patients was 1 (1–6) sample and 10.0 (1.0–13.7) mg/kg/dose, respectively. Their median (range) age and weight were 13.8 (2.2–20) years and 76.4 (12.8–224) kg, respectively. Although the use of lean body weight resulted in an improved statistical model fit over total body weight, obesity was not identified as a clinically significant covariate on individual clindamycin PK parameters (clearance [CL] or volume [V]). In addition, weight-scaled CL and V values did not clinically differ between obese and non-obese patients of similar age.
After accounting for size-based differences using total body weight and physiologic differences using age, the interim analysis suggested that the PK of clindamycin is not further affected by obesity. Clindamycin may be dosed based on total body weight without dose adjustment based solely on obesity. Based on the lack of significant differences between obese and non-obese subjects with the current sample size and the total number of obese children already enrolled in the NICHD-2012-CLN01 study, it was determined that no additional participants needed to be enrolled in this study and that 22 enrolled participants would be sufficient for the final analyses.
11.5.2.4. Multi-center Studies
The possible effects of study center were not investigated.
11.5.2.5. Multiple Comparison/Multiplicity
Inferential statistical tests were not performed in this study.
11.5.2.6. Examination of Sub-Groups
Subgroups were not analyzed.
11.5.1. By-Participant Displays
By-participant displays (i.e. “patient profiles”) are presented in Figure 14.4.1. For each participant, values over the course of the study from first study procedures through the end of study treatment is presented.
11.6. Other Analyses
11.6.1. Drug-Drug and Drug-Disease Interactions
Drug-drug and drug-disease interactions were not analyzed in this study.
11.7. Conclusions
Statistically significant differences between obese and non-obese children were only observed for volume of distribution (>6 years) and half-life of elimination (2-6 years). After normalized volume of distribution by body weight, these differences were no longer significant. Thus, after accounting for size-based differences using total body weight and physiologic differences using age, the PK of clindamycin are not further affected by obesity. Clindamycin may be dosed based on total body weight (max dose 2.7 g/day) without dose adjustment based solely on obesity.
- PHARMACOKINETIC EVALUATION - Safety and Pharmacokinetics of Multiple-Dose Intrav...PHARMACOKINETIC EVALUATION - Safety and Pharmacokinetics of Multiple-Dose Intravenous and Oral Clindamycin in Pediatric Subjects with BMI ≥ 85th Percentile
- BioSample links for Nucleotide (Select 2712992762) (1)BioSample
- Pseudomonas sp. KBN12P06040 isolated from Tracheal aspitaresPseudomonas sp. KBN12P06040 isolated from Tracheal aspitaresbiosample
Your browsing activity is empty.
Activity recording is turned off.
See more...