NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-.

PDQ Cancer Information Summaries [Internet].
Show detailsThis PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of adult esophageal cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
General Information About Esophageal Cancer
Two histological types account for most malignant esophageal neoplasms: adenocarcinoma and squamous cell carcinoma. Adenocarcinomas typically start in the lower esophagus, and squamous cell carcinoma can develop throughout the esophagus. The epidemiology of these types varies markedly.
Incidence and Mortality
Estimated new cases and deaths from esophageal cancer in the United States in 2024:[1]
- New cases: 22,370.
- Deaths: 16,130.
The incidence of esophageal cancer has risen in recent decades, coinciding with a shift in histological type and primary tumor location. Worldwide, squamous cell carcinoma is the predominant histology, and was historically more prevalent in the United States. However, the incidence of adenocarcinoma has risen dramatically in the last few decades and is now more prevalent than squamous cell carcinoma in the United States and western Europe.[2-4] The incidence of adenocarcinoma has increased most notably among White men.[5] In the United States, the median age of patients who present with esophageal cancer is 68 years.[6] Most adenocarcinomas are located in the distal esophagus. The cause of the rising incidence and demographic alterations is unknown.
Anatomy
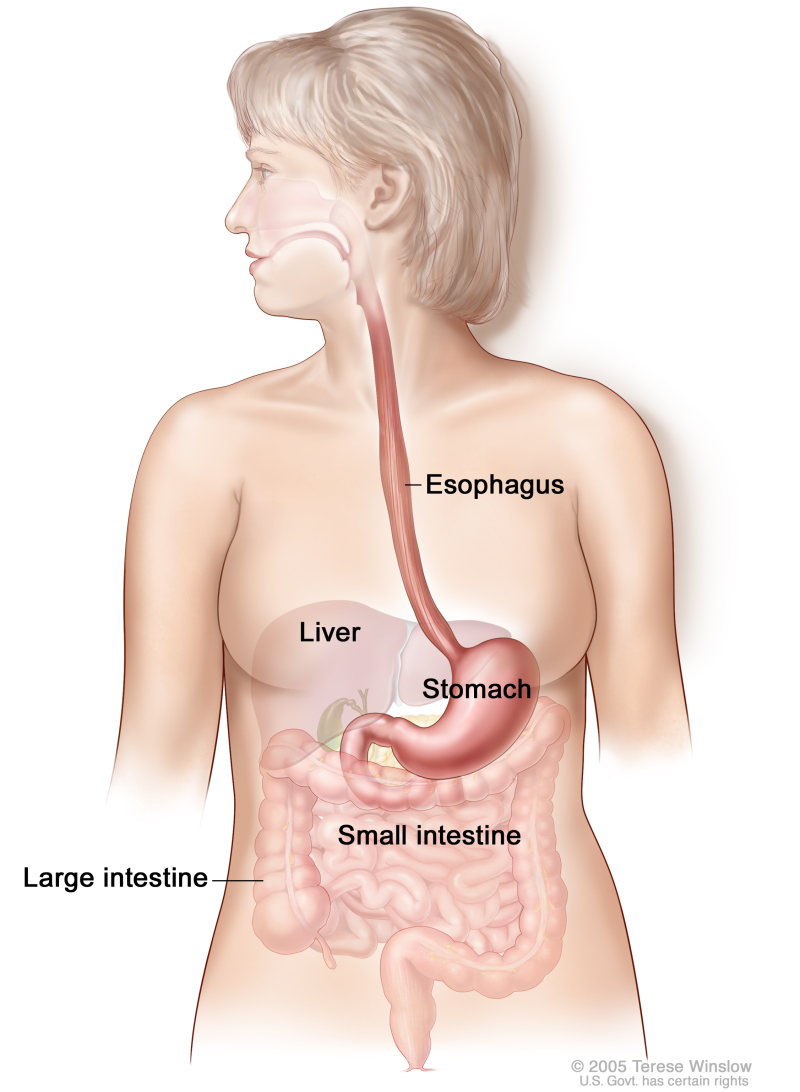
The esophagus serves as a conduit to the gastrointestinal tract for food. The esophagus extends from the larynx to the stomach and lies in the posterior mediastinum within the thorax near the lung pleura, peritoneum, pericardium, and diaphragm. As it travels into the abdominal cavity, the esophagus makes an abrupt turn and enters the stomach. The most muscular segment of the gastrointestinal system, the esophagus is composed of inner circular and outer longitudinal muscle layers. The upper and lower esophagus are controlled by the sphincter function of the cricopharyngeus muscle and gastroesophageal sphincter, respectively. The esophagus has a rich network of lymphatic channels concentrated in the lamina propria and submucosa, which drains longitudinally along the submucosa.
Tumors of the esophagus are conventionally described in terms of distance of the upper border of the tumor to the incisors. When measured from the incisors via endoscopy, the esophagus extends approximately 30 cm to 40 cm. The esophagus is divided into four main segments:
- Cervical esophagus (~15–20 cm from the incisors).
- Upper thoracic esophagus (~20–25 cm from the incisors).
- Middle thoracic esophagus (~25–30 cm from the incisors).
- Lower thoracic esophagus and gastroesophageal junction (~30–40 cm from the incisors).
Risk Factors
Risk factors for squamous cell carcinoma of the esophagus include the following:
- Tobacco use.
- Alcohol use.
Risk factors associated with esophageal adenocarcinoma are less clear.[3] Barrett esophagus is an exception, and its presence is associated with an increased risk of developing adenocarcinoma of the esophagus. Chronic reflux is considered the predominant cause of Barrett metaplasia. The results of a population-based, case-controlled study from Sweden strongly suggest that symptomatic gastroesophageal reflux is a risk factor for esophageal adenocarcinoma. The frequency, severity, and duration of reflux symptoms were positively correlated with increased risk of esophageal adenocarcinoma.[7] For more information, see Esophageal Cancer Prevention.
Prognostic Factors
Favorable prognostic factors include the following:
- Early-stage disease.
- Complete resection.
Patients with severe dysplasia in distal esophageal Barrett mucosa often have in situ or invasive cancer within the dysplastic area. After resection, these patients usually have excellent prognoses.[8]
In most cases, esophageal cancer is a treatable disease, but it is rarely curable. The 5-year relative survival rate is 21.6%. Patients with early-stage disease have a better chance of survival; 18.2% of patients are diagnosed at the local stage and have a 5-year relative survival rate of 48.1%.[6]
References
- American Cancer Society: Cancer Facts and Figures 2024. American Cancer Society, 2024. Available online. Last accessed June 21, 2024.
- Brown LM, Devesa SS, Chow WH: Incidence of adenocarcinoma of the esophagus among white Americans by sex, stage, and age. J Natl Cancer Inst 100 (16): 1184-7, 2008. [PMC free article: PMC2518165] [PubMed: 18695138]
- Blot WJ, McLaughlin JK: The changing epidemiology of esophageal cancer. Semin Oncol 26 (5 Suppl 15): 2-8, 1999. [PubMed: 10566604]
- Schmassmann A, Oldendorf MG, Gebbers JO: Changing incidence of gastric and oesophageal cancer subtypes in central Switzerland between 1982 and 2007. Eur J Epidemiol 24 (10): 603-9, 2009. [PubMed: 19669623]
- Kubo A, Corley DA: Marked multi-ethnic variation of esophageal and gastric cardia carcinomas within the United States. Am J Gastroenterol 99 (4): 582-8, 2004. [PubMed: 15089886]
- National Cancer Institute: SEER Cancer Stat Facts: Esophageal Cancer. Bethesda, Md: National Cancer Institute. Available online. Last accessed August 9, 2024.
- Lagergren J, Bergström R, Lindgren A, et al.: Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 340 (11): 825-31, 1999. [PubMed: 10080844]
- Reed MF, Tolis G, Edil BH, et al.: Surgical treatment of esophageal high-grade dysplasia. Ann Thorac Surg 79 (4): 1110-5; discussion 1110-5, 2005. [PubMed: 15797034]
Cellular Classification of Esophageal Cancer
Adenocarcinomas, typically arising in Barrett esophagus, account for at least 50% of malignant lesions, and the incidence of this histology appears to be rising. Barrett esophagus contains glandular epithelium cephalad to the esophagogastric junction.
Three different types of glandular epithelium can be seen:
- Metaplastic columnar epithelium.
- Metaplastic parietal cell glandular epithelium within the esophageal wall.
- Metaplastic intestinal epithelium with typical goblet cells. Dysplasia is particularly likely to develop in the intestinal-type mucosa.
Approximately 30% of esophageal cancers in the United States are squamous cell carcinomas.[1]
Gastrointestinal stromal tumors can occur in the esophagus and are usually benign. For more information, see Gastrointestinal Stromal Tumors Treatment.
References
- Howlader N, Noone AM, Krapcho M, et al.: SEER Cancer Statistics Review (CSR) 1975-2017. Bethesda, Md: National Cancer Institute, 2020. Available online. Last accessed August 9, 2024.
Stage Information for Esophageal Cancer
One of the major difficulties in allocating and comparing treatment modalities for patients with esophageal cancer is the lack of precise preoperative staging. The stage determines whether the intent of the therapeutic approach will be curative or palliative.
Staging Evaluation
Standard noninvasive staging modalities include the following:
- Endoscopic ultrasonography.
- Computed tomography (CT) scan of the chest and abdomen.
- Positron emission tomography (PET)–CT scan.
The overall tumor depth staging accuracy of endoscopic ultrasonography is 85% to 90%, compared with 50% to 80% for CT. The accuracy of regional nodal staging is 70% to 80% for endoscopic ultrasonography and 50% to 70% for CT.[1,2]
One retrospective series reported 93% sensitivity and 100% specificity of regional nodal staging with endoscopic ultrasound-guided fine-needle aspiration (FNA). Endoscopic ultrasound-guided FNA for lymph node staging is under prospective evaluation.[3]
Thoracoscopy and laparoscopy have been used in esophageal cancer staging at some surgical centers.[4-6] An intergroup trial reported an increase in positive lymph node detection to 56% of 107 evaluable patients with the use of thoracoscopy/laparoscopy, from 41% (with the use of noninvasive staging tests, e.g., CT, magnetic resonance imaging, and endoscopic ultrasound), with no major complications or deaths.[7]
Noninvasive PET scan using the radiolabeled glucose analog fluorine F 18-fludeoxyglucose (18F-FDG) for preoperative staging of esophageal cancer is more sensitive than a CT scan or endoscopic ultrasound in detection of distant metastases. A recent study of 262 patients with potentially resectable esophageal cancer demonstrated the utility of 18F-FDG PET in identifying confirmed distant metastatic disease in at least 4.8% of patients after standard evaluation.[8-12]
AJCC Staging System
The AJCC has designated staging by TNM (tumor, node, metastasis) classification to define cancer of the esophagus and esophagogastric junction.[13] Tumors located in the gastric cardia within 5 cm of the gastroesophageal junction with extension into the esophagus or the gastroesophageal junction are classified as esophageal cancer. Tumors with the epicenter of the tumor located in the gastric cardia beyond 5 cm of the gastroesophageal junction or without extension into the esophagus are classified as gastric cancer.[13] For more information, see the Stage Information for Gastric Cancer section in Gastric Cancer Treatment.
The classification of involved abdominal lymph nodes as M1 disease is controversial. The presence of positive abdominal lymph nodes does not appear to have a prognosis as grave as that for metastases to distant organs.[14] Patients with regional and/or celiac axis lymphadenopathy should not necessarily be considered to have unresectable disease caused by metastases. Complete resection of the primary tumor and appropriate lymphadenectomy is attempted when possible.
Table 1. Definitions of Primary Tumor, Regional Lymph Node, Distant Metastasis, Histological Grade for Squamous Cell Carcinoma and Adenocarcinoma, and Location for Squamous Cell Carcinoma of the Esophagusa
| T Category/Criteria | N Category/Criteria | M Category/Criteria | G Definition | L Category/Criteriab | |
|---|---|---|---|---|---|
| TX = Tumor cannot be assessed. | NX = Regional lymph nodes cannot be assessed. | M0 = No distant metastasis. | GX = Grade cannot be assessed. | X = Location unknown. | |
| T0 = No evidence of primary tumor. | N0 = No regional lymph node metastasis. | M1 = Distant metastasis. | G1 = Well differentiated. | Upper = Cervical esophagus to lower border of azygos vein. | |
| Tis = High-grade dysplasia, defined as malignant cells confined to the epithelium by the basement membrane. | N1 = Metastasis in one or two regional lymph nodes. | G2 = Moderately differentiated. | Middle = Lower border of azygos vein to lower border of inferior pulmonary vein. | ||
| G3 = Poorly differentiated, undifferentiated. | Lower = Lower border of inferior pulmonary vein to stomach, including gastroesophageal junction. | ||||
| T1 = Tumor invades the lamina propria, muscularis mucosae, or submucosa. | N2 = Metastasis in three to six regional lymph nodes. | ||||
| N3 = Metastasis in seven or more regional lymph nodes. | |||||
| T1a = Tumor invades the lamina propria or muscularis mucosae. | |||||
| T1b = Tumor invades the submucosa. | |||||
| T2 = Tumor invades the muscularis propria. | |||||
| T3 = Tumor invades adventitia. | |||||
| T4 = Tumor invades adjacent structures. | |||||
| T4a = Tumor invades the pleura, pericardium, azygos vein, diaphragm, or peritoneum. | |||||
| T4b = Tumor invades other adjacent structures, such as the aorta, vertebral body, or airway. | |||||
T = primary tumor; N = regional lymph nodes; M = distant metastasis; G = grade; L = location.
aReprinted with permission from AJCC: Esophageal and esophagogastric junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 185–202.
bLocation is defined by the position of the epicenter of the tumor in the esophagus.
Staging for squamous cell carcinoma of the esophagus
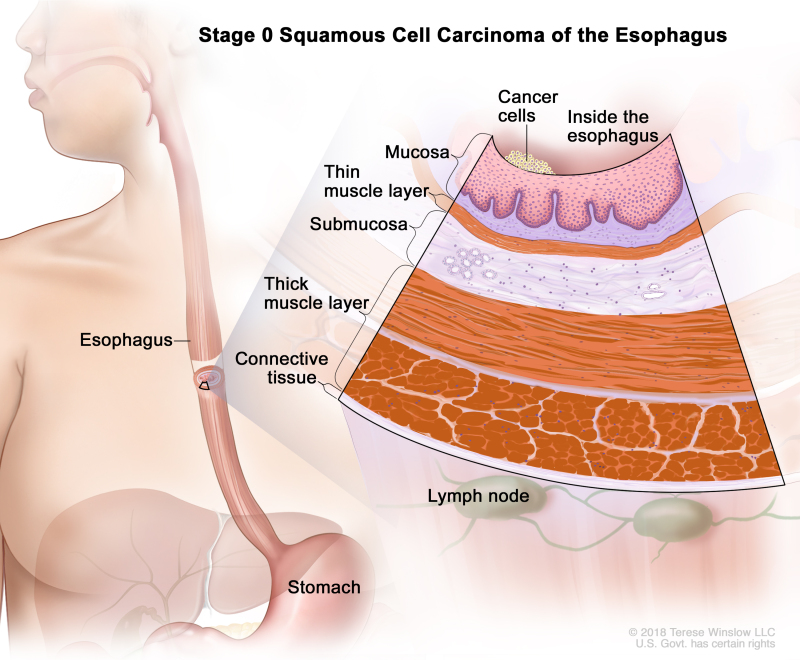
Table 2. Definitions of pTNM Stage 0 for Squamous Cell Carcinoma of the Esophagusa
| Stage | TNM | Grade | Tumor Location | Description | Illustration |
|---|---|---|---|---|---|
| 0 | Tis, N0, M0 | N/A | Any | Tis = High grade dysplasia, defined as malignant cells confined to the epithelium by the basement membrane. | |
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| G1 = N/A. | |||||
| Any L = See Table 1. |
T = primary tumor; N = regional lymph nodes; M = distant metastasis; G = grade; L = location; N/A = not applicable; p = pathological.
aReprinted with permission from AJCC: Esophageal and esophagogastric junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 185–202
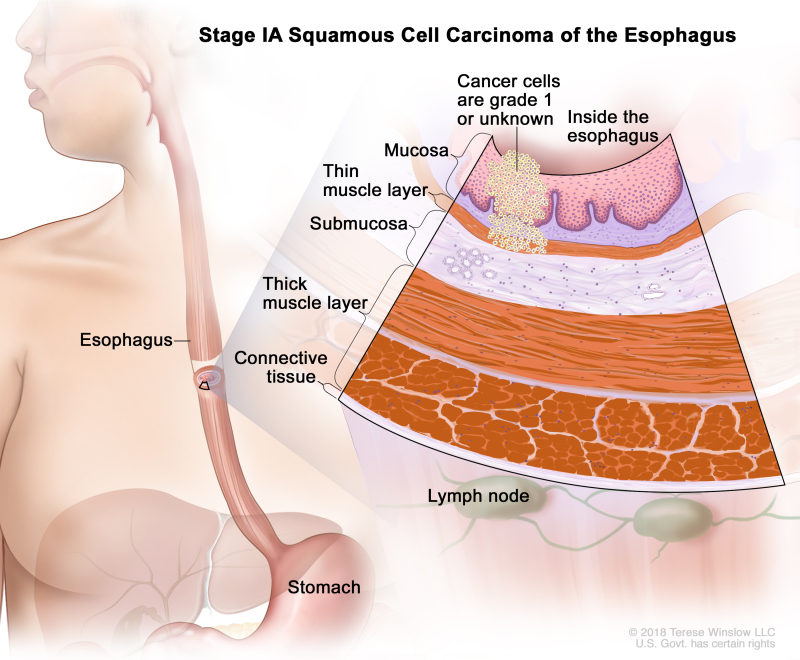
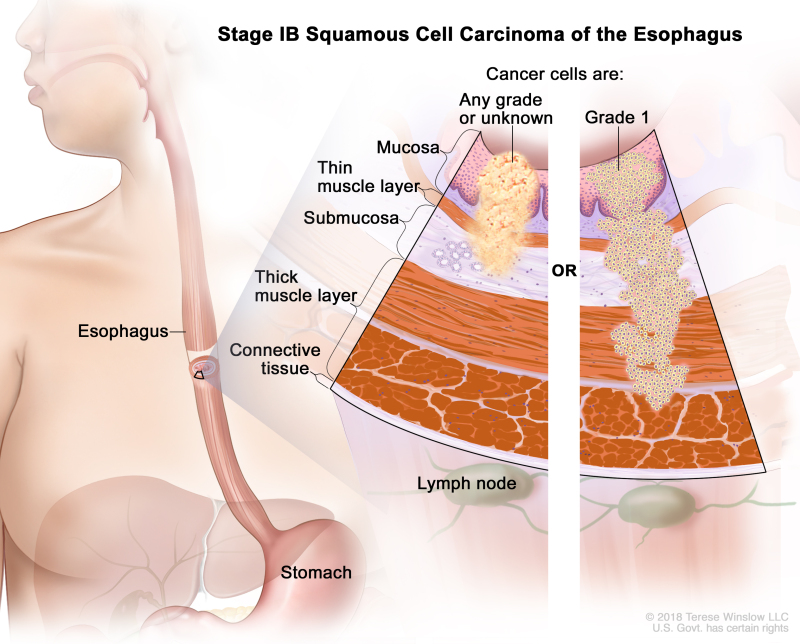
Table 3. Definitions of pTNM Stages IA and IB for Squamous Cell Carcinoma of the Esophagusa
| Stage | TNM | Grade | Tumor Location | Description | Illustration |
|---|---|---|---|---|---|
| IA | T1a, N0, M0 | G1 | Any | –T1a = Tumor invades the lamina propria or muscularis mucosae. | |
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| G1 = Well differentiated. | |||||
| Any L = See Table 1. | |||||
| T1a, N0, M0 | GX | Any | –T1a = Tumor invades the lamina propria or muscularis mucosae. | ||
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| GX = Grade cannot be assessed. | |||||
| Any L = See Table 1. | |||||
| IB | T1a, N0, M0 | G2–G3 | Any | –T1a = Tumor invades the lamina propria or muscularis mucosae. | |
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| G2 = Moderately differentiated. | |||||
| G3 = Poorly differentiated, undifferentiated. | |||||
| Any L = See Table 1. | |||||
| T1b, N0, M0 | G1–G3 | Any | –T1b = Tumor invades the submucosa. | ||
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| G1 = Well differentiated. | |||||
| G2 = Moderately differentiated. | |||||
| G3 = Poorly differentiated, undifferentiated. | |||||
| Any L = See Table 1. | |||||
| T1b, N0, M0 | GX | Any | –T1b = Tumor invades the submucosa. | ||
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| GX = Grade cannot be assessed. | |||||
| Any L = See Table 1. | |||||
| T2, N0, M0 | G1 | Any | T2 = Tumor invades the muscularis propria. | ||
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| G1 = Well differentiated. | |||||
| Any L = See Table 1. |
T = primary tumor; N = regional lymph nodes; M = distant metastasis; G = grade; L = location; p = pathological.
aReprinted with permission from AJCC: Esophageal and esophagogastric junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 185–202.
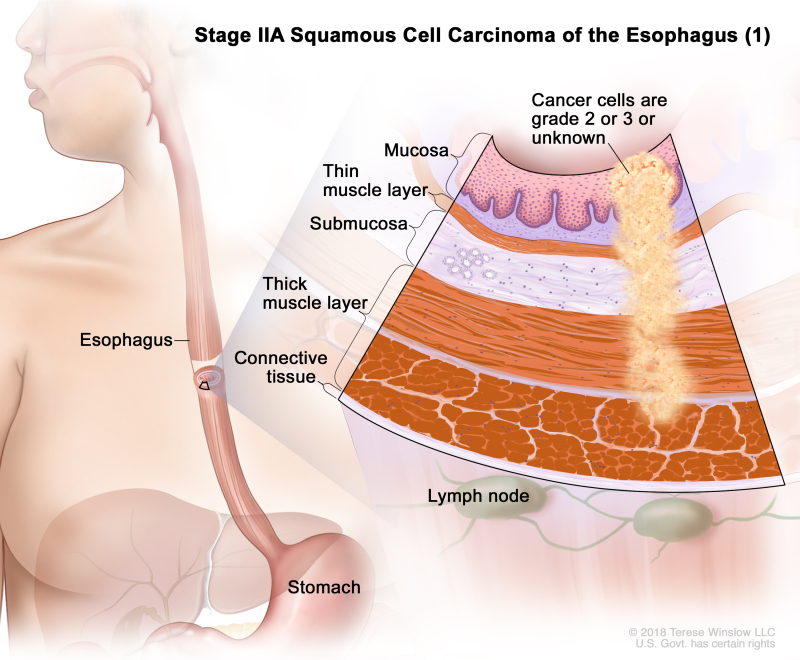
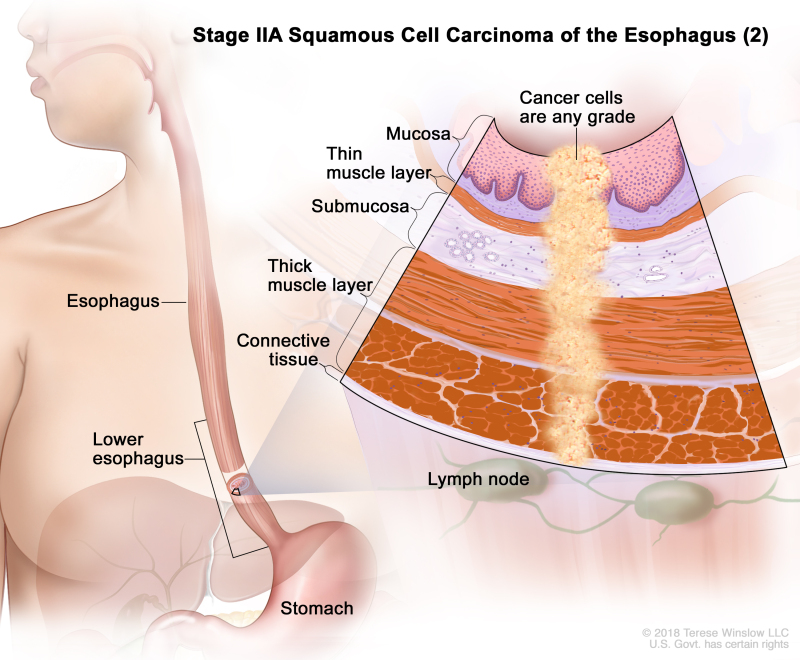
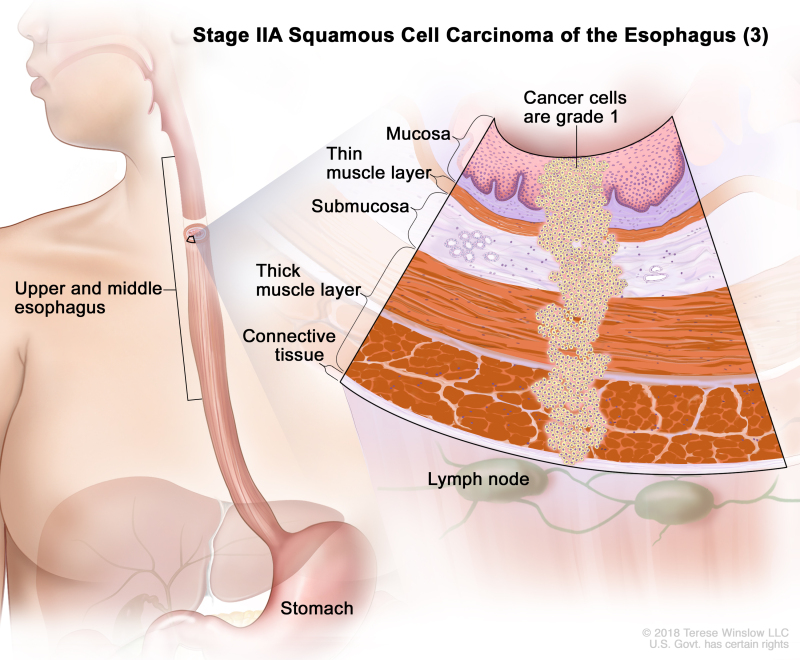
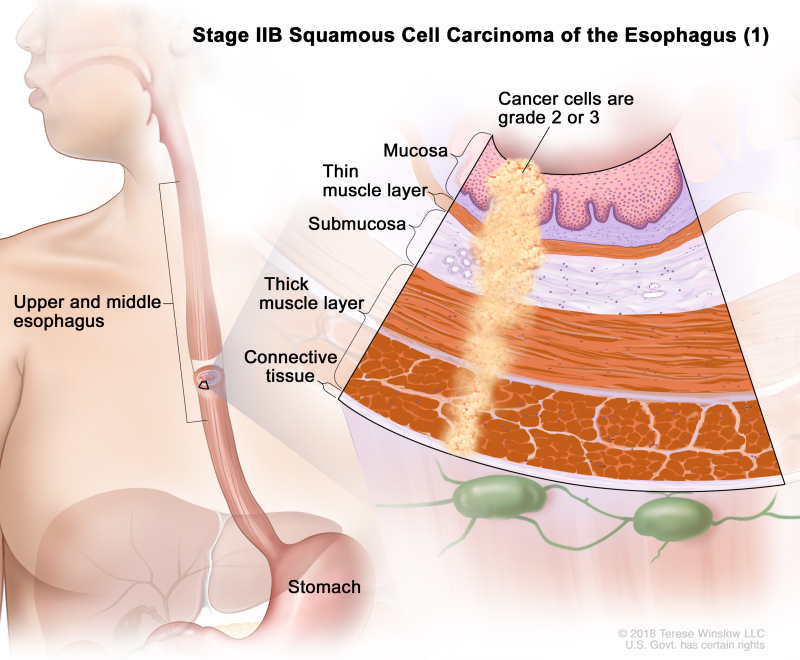
Table 4. Definitions of pTNM Stages IIA and IIB for Squamous Cell Carcinoma of the Esophagusa
| Stage | TNM | Grade | Tumor Locationb | Description | Illustration |
|---|---|---|---|---|---|
| IIA | T2, N0, M0 | GX | Any | T2 = Tumor invades the muscularis propria. | |
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| GX = Grade cannot be assessed. | |||||
| Any L = See Table 1. | |||||
| T2, N0, M0 | G2–G3 | Any | T2 = Tumor invades the muscularis propria. | ||
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| G2 = Moderately differentiated. | |||||
| G3 = Poorly differentiated, undifferentiated. | |||||
| Any L = See Table 1. | |||||
| T3, N0, M0 | Any | Lower | T3 = Tumor invades adventitia. | ||
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Lower = Lower border of inferior pulmonary vein to stomach, including gastroesophageal junction. | |||||
| T3, N0, M0 | G1 | Upper/middle | T3 = Tumor invades adventitia. | ||
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| G1 = Well differentiated. | |||||
| Upper = Cervical esophagus to lower border of azygos vein. | |||||
| Middle = Lower border of azygos vein to lower border of inferior pulmonary vein. | |||||
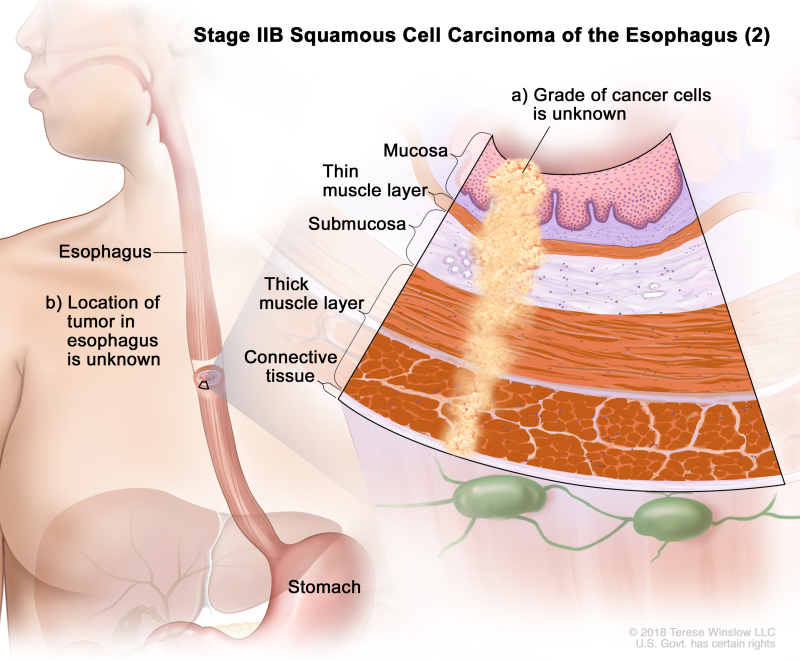
| IIB | T3, N0, M0 | G2–G3 | Upper/middle | T3 = Tumor invades adventitia. | |
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| G2 = Moderately differentiated. | |||||
| G3 = Poorly differentiated, undifferentiated. | |||||
| Upper = Cervical esophagus to lower border of azygos vein. | |||||
| Middle = Lower border of azygos vein to lower border of inferior pulmonary vein. | |||||
| T3, N0, M0 | GX | Any | T3 = Tumor invades adventitia. | ||
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| GX = Grade cannot be assessed. | |||||
| Any L = See Table 1. | |||||
| T3, N0, M0 | Any | Location X | T3 = Tumor invades adventitia. | ||
| N0 = No regional lymph node metastasis. | |||||
| M0 = No distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Location X = Location unknown. | |||||
| T1, N1, M0 | Any | Any | T1 = Tumor invades the lamina propria, muscularis mucosae, or submucosa. | ||
| N1 = Metastasis in one or two regional lymph nodes. | |||||
| M0 = No distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Any L = See Table 1. |
T = primary tumor; N = regional lymph nodes; M = distant metastasis; G = grade; L = location; p = pathological.
aReprinted with permission from AJCC: Esophageal and esophagogastric junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 185–202.
bLocation is defined by the position of the epicenter of the tumor in the esophagus.
Table 5. Definitions of pTNM Stages IIIA and IIIB for Squamous Cell Carcinoma of the Esophagusa
| Stage | TNM | Grade | Tumor Locationb | Description | Illustration |
|---|---|---|---|---|---|
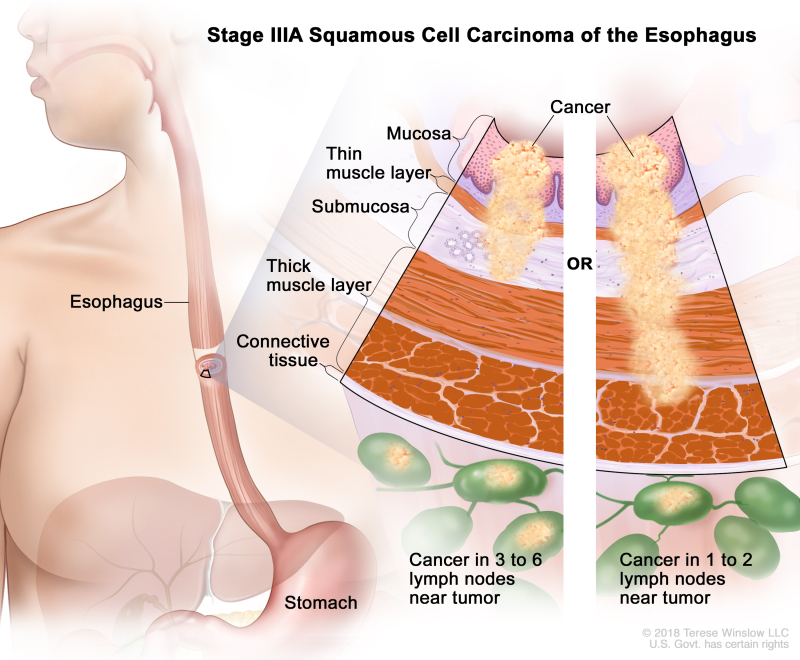
| IIIA | T1, N2, M0 | Any | Any | T1 = Tumor invades the lamina propria, muscularis mucosae, or submucosa. | |
| –T1a = Tumor invades the lamina propria or muscularis mucosae. | |||||
| –T1b = Tumor invades the submucosa. | |||||
| N2 = Metastasis in three to six regional lymph nodes. | |||||
| M0 = No distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Any L = See Table 1. | |||||
| T2, N1, M0 | Any | Any | T2 = Tumor invades the muscularis propria. | ||
| N1 = Metastasis in one or two regional lymph nodes. | |||||
| M0 = No distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Any L = See Table 1. | |||||
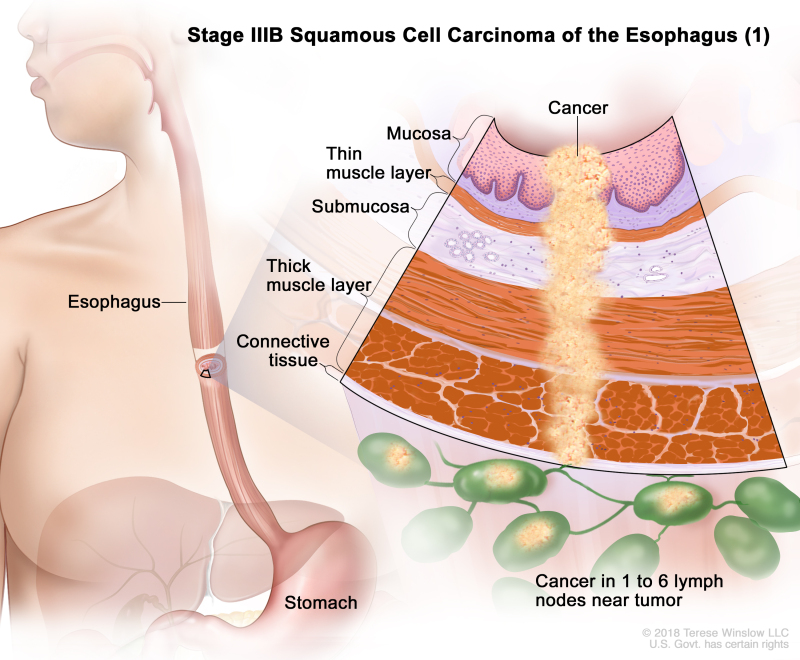
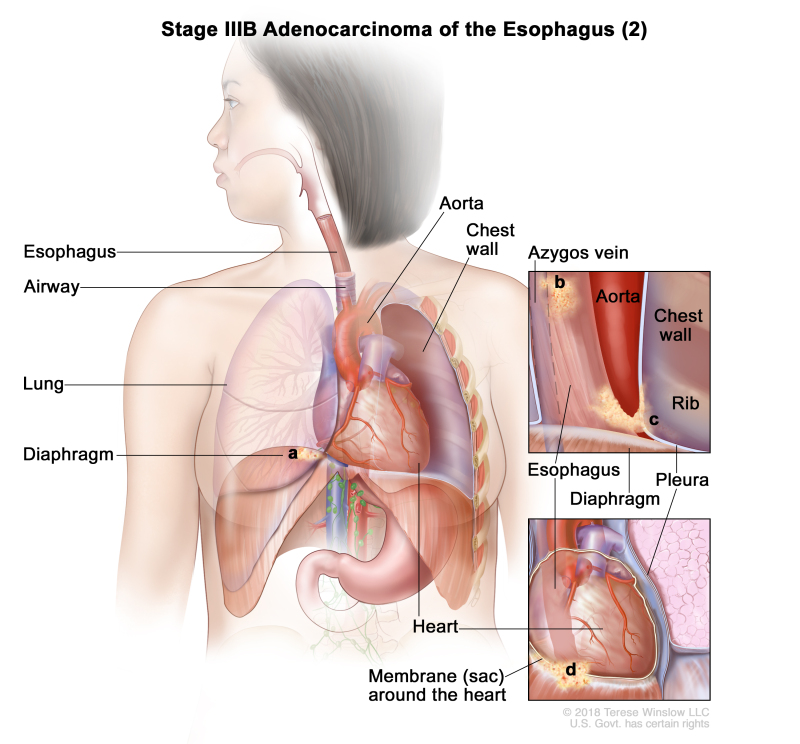
| IIIB | T2, N2, M0 | Any | Any | T2 = Tumor invades the muscularis propria. | |
| N2 = Metastasis in three to six regional lymph nodes. | |||||
| M0 = No distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Any L = See Table 1. | |||||
| T3, N1–N2, M0 | Any | Any | T3 = Tumor invades adventitia. | ||
| N1 = Metastasis in one or two regional lymph nodes. | |||||
| N2 = Metastasis in three to six regional lymph nodes. | |||||
| M0 = No distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Any L = See Table 1. | |||||
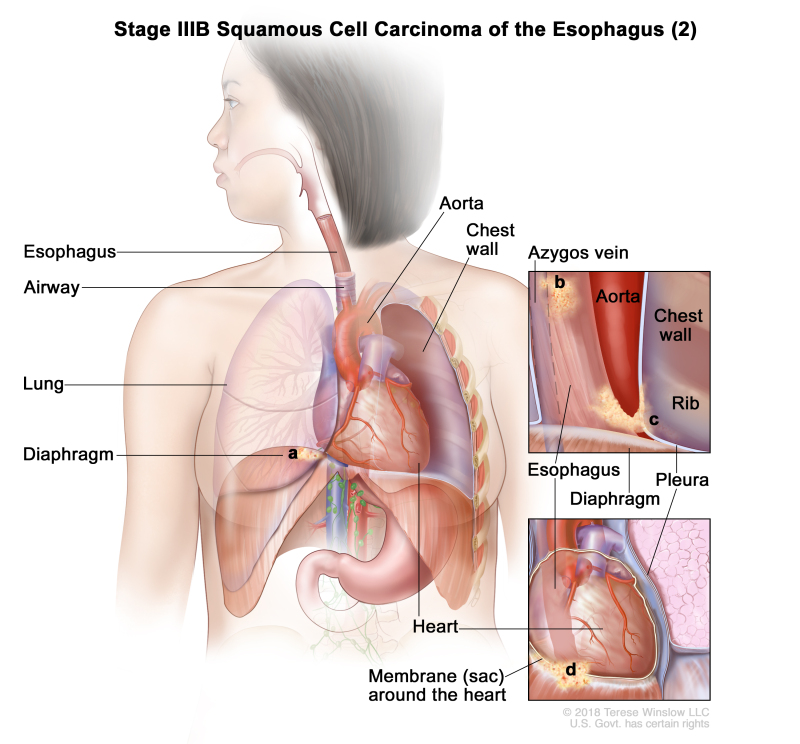
| T4a, N0–1, M0 | Any | Any | –T4a = Tumor invades the pleura, pericardium, azygos vein, diaphragm, or peritoneum. | ||
| N0 = No regional lymph node metastasis. | |||||
| N1 = Metastasis in one or two regional lymph nodes. | |||||
| M0 = No distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Any L = See Table 1. |
T = primary tumor; N = regional lymph nodes; M = distant metastasis; G = grade; L = location; p = pathological.
aReprinted with permission from AJCC: Esophageal and esophagogastric junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 185–202.
bLocation is defined by the position of the epicenter of the tumor in the esophagus.
Table 6. Definitions of pTNM Stages IVA and IVB for Squamous Cell Carcinoma of the Esophagusa
| Stage | TNM | Grade | Tumor Locationb | Description | Illustration |
|---|---|---|---|---|---|
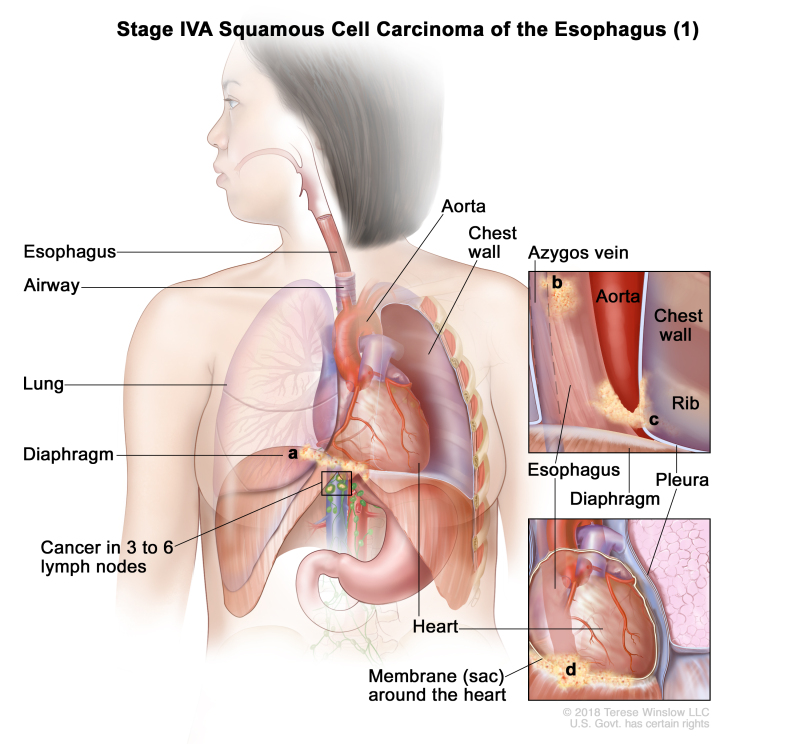
| IVA | T4a, N2, M0 | Any | Any | –T4a = Tumor invades the pleura, pericardium, azygos vein, diaphragm, or peritoneum. | |
| N2 = Metastasis in three to six regional lymph nodes. | |||||
| M0 = No distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Any L = See Table 1. | |||||
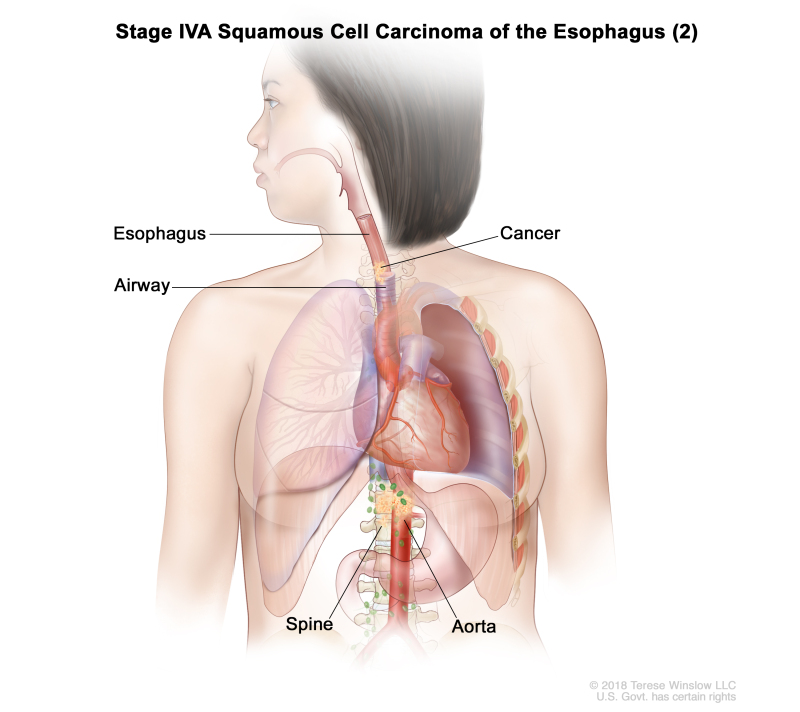
| T4b, N0–2, M0 | Any | Any | –T4b = Tumor invades other adjacent structures, such as the aorta, vertebral body, or airway. | ||
| N0 = No regional lymph node metastasis. | |||||
| N1 = Metastasis in one or two regional lymph nodes. | |||||
| N2 = Metastasis in three to six regional lymph nodes. | |||||
| M0 = No distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Any L = See Table 1. | |||||
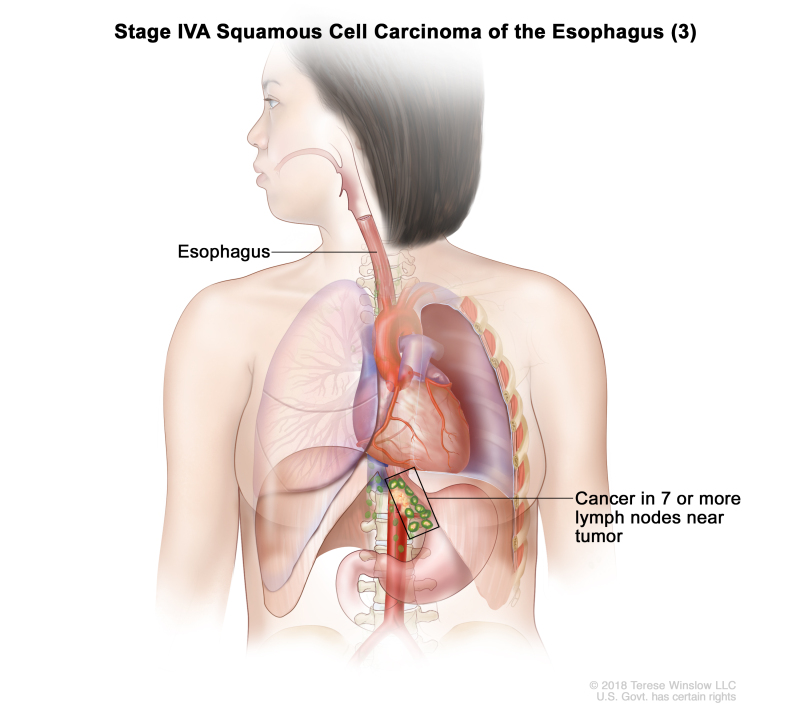
| Any T, N3, M0 | Any | Any | Any T = See Table 1. | ||
| N3 = Metastasis in seven or more regional lymph nodes. | |||||
| M0 = No distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Any L = See Table 1. | |||||
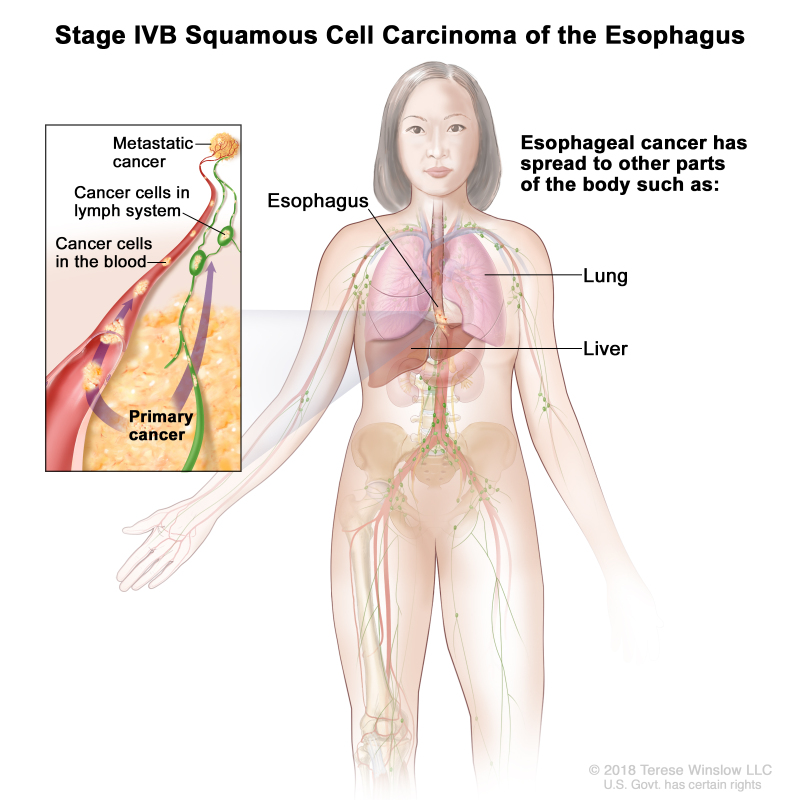
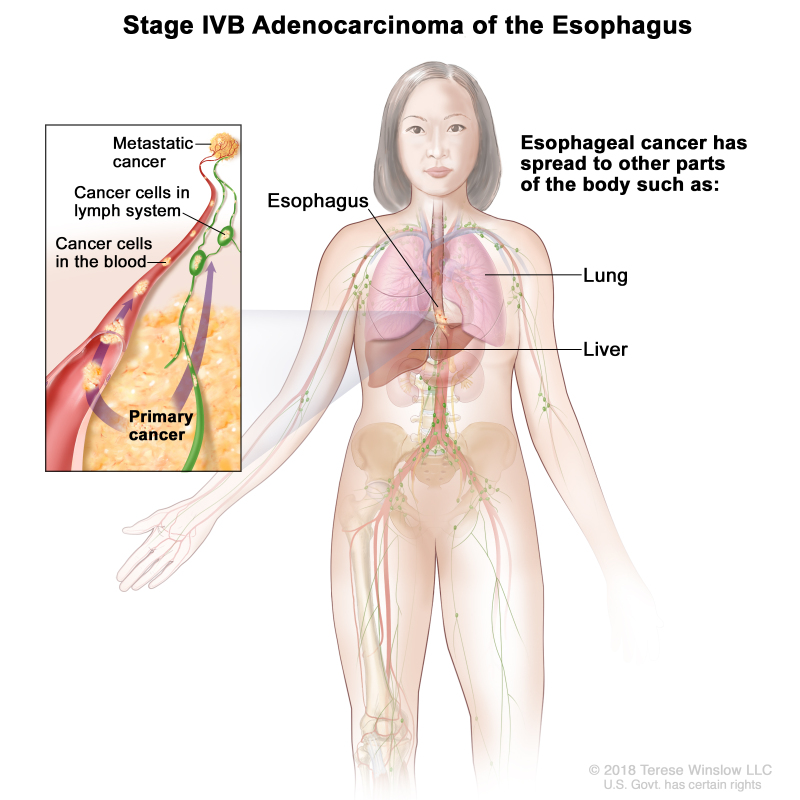
| IVB | Any T, Any N, M1 | Any | Any | Any T = See Table 1. | |
| Any N = See Table 1. | |||||
| M1 = Distant metastasis. | |||||
| Any G = See Table 1. | |||||
| Any L = See Table 1. |
T = primary tumor; N = regional lymph nodes; M = distant metastasis; G = grade; L = location; p = pathological.
aReprinted with permission from AJCC: Esophageal and esophagogastric junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 185–202.
bLocation is defined by the position of the epicenter of the tumor in the esophagus.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Staging for adenocarcinoma of the esophagus
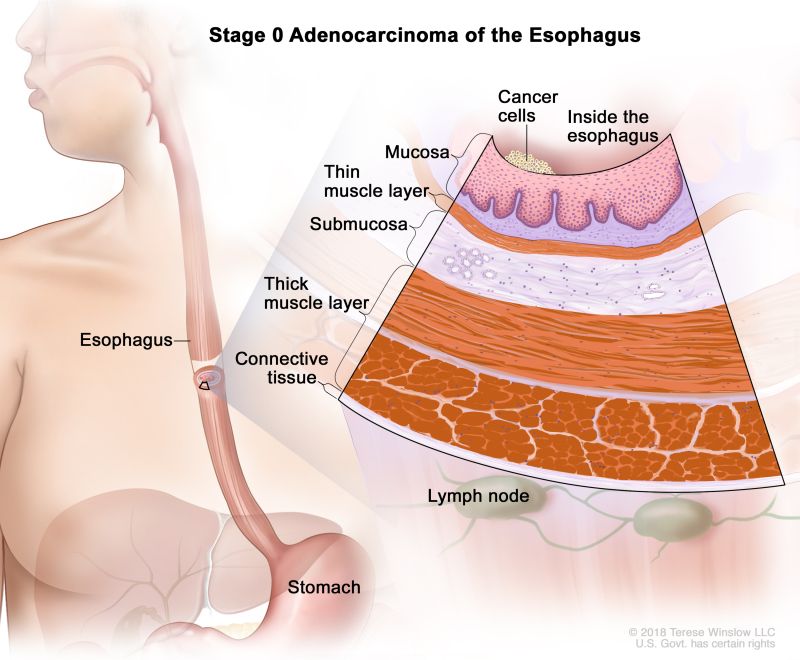
Table 7. Definitions of pTNM Stage 0 for Adenocarcinoma of the Esophagusa
| Stage | TNM | Grade | Description | Illustration |
|---|---|---|---|---|
| 0 | Tis, N0, M0 | N/A | Tis = High-grade dysplasia, defined as malignant cells confined to the epithelium by the basement membrane. | |
| N0 = No regional lymph node metastasis. | ||||
| M0 = No distant metastasis. |
T = primary tumor; N = regional lymph nodes; M = distant metastasis; G = grade; N/A = not applicable; p = pathological.
aReprinted with permission from AJCC: Esophageal and esophagogastric junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 185–202.
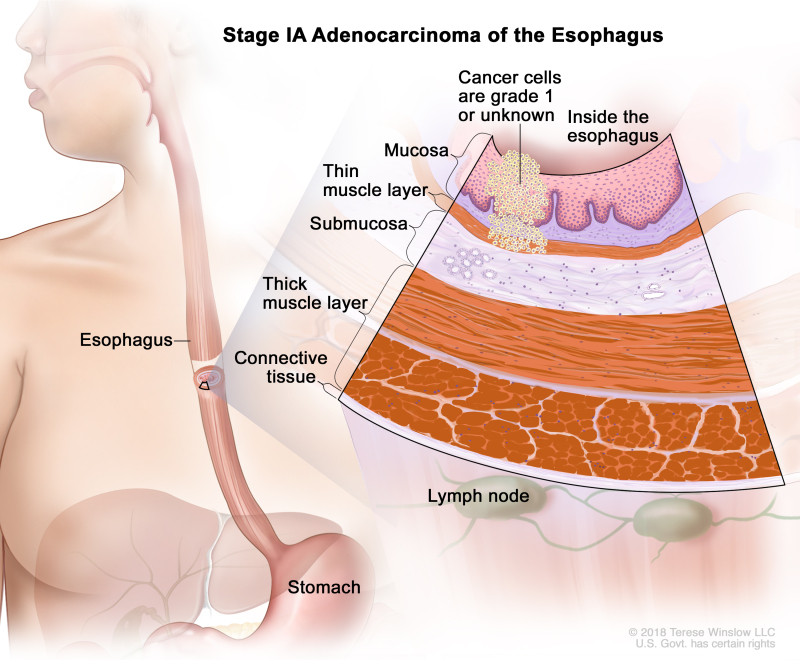
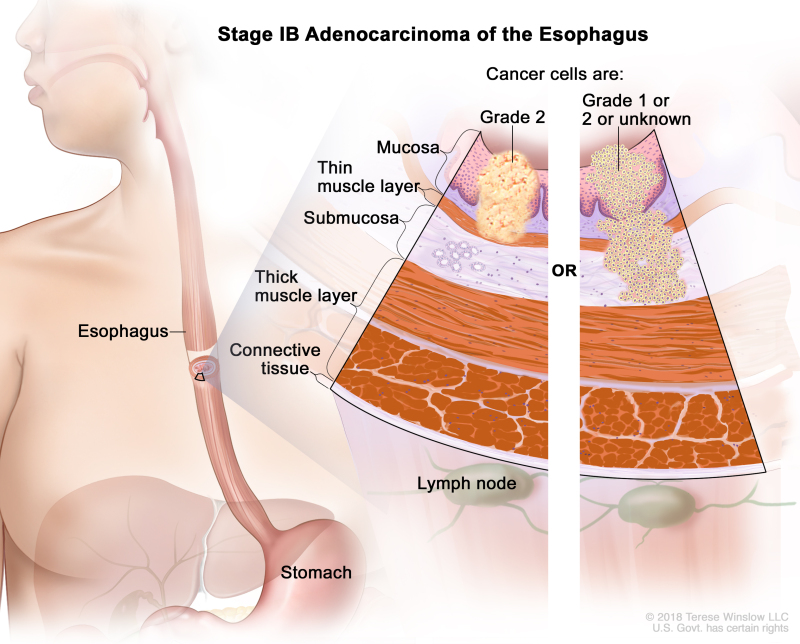
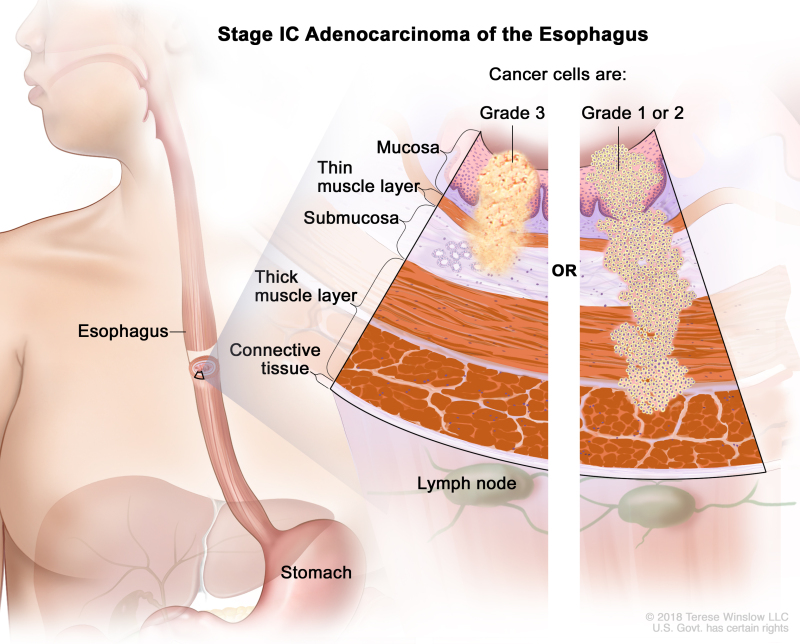
Table 8. Definitions of pTNM Stages IA, IB, and IC for Adenocarcinoma of the Esophagusa
| Stage | TNM | Grade | Description | Illustration |
|---|---|---|---|---|
| IA | T1a, N0, M0 | G1 | –T1a = Tumor invades the lamina propria or muscularis mucosae. | |
| N0 = No regional lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| G1 = Well differentiated. | ||||
| T1a, N0, M0 | GX | –T1a = Tumor invades the lamina propria or muscularis mucosae. | ||
| N0 = No regional lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| GX = Grade cannot be assessed. | ||||
| IB | T1a, N0, M0 | G2 | –T1a = Tumor invades the lamina propria or muscularis mucosae. | |
| N0 = No regional lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| G2 = Moderately differentiated. | ||||
| T1b, N0, M0 | G1–2 | –T1b = Tumor invades the submucosa. | ||
| N0 = No regional lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| G1 = Well differentiated. | ||||
| G2 = Moderately differentiated. | ||||
| T1b, N0, M0 | GX | –T1b = Tumor invades the submucosa. | ||
| N0 = No regional lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| GX = Grade cannot be assessed. | ||||
| IC | T1, N0, M0 | G3 | T1 = Tumor invades the lamina propria, muscularis mucosae, or submucosa. | |
| –T1a = Tumor invades the lamina propria or muscularis mucosae. | ||||
| –T1b = Tumor invades the submucosa. | ||||
| N0 = No regional lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| G3 = Poorly differentiated, undifferentiated. | ||||
| T2, N0, M0 | G1–2 | T2 = Tumor invades the muscularis propria. | ||
| N0 = No regional lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| G1 = Well differentiated. | ||||
| G2 = Moderately differentiated. |
T = primary tumor; N = regional lymph nodes; M = distant metastasis; G = grade; p = pathological.
aReprinted with permission from AJCC: Esophageal and esophagogastric junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 185–202.
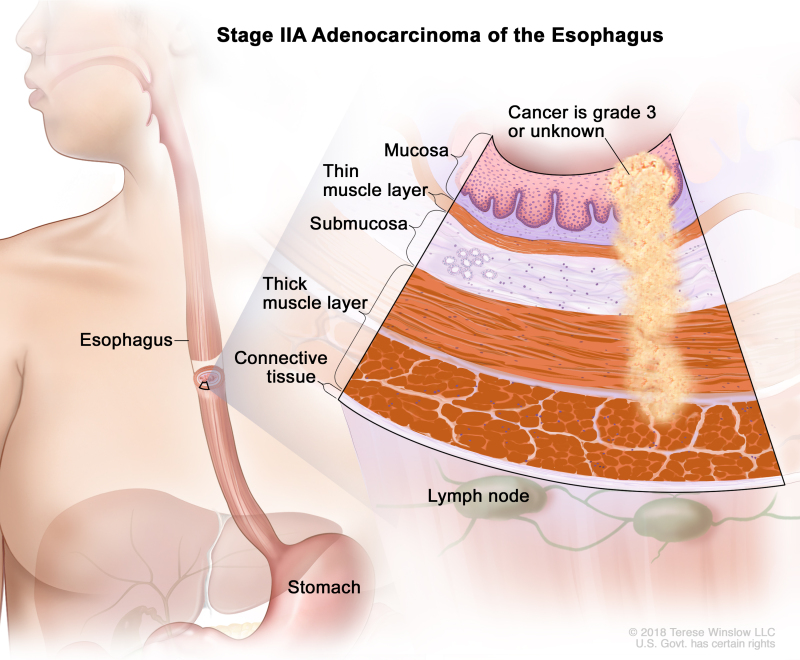
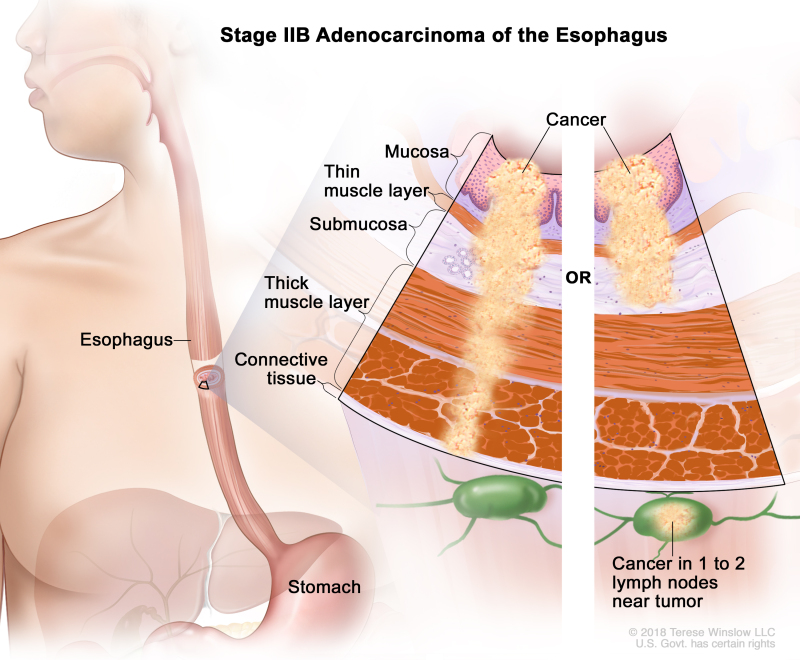
Table 9. Definitions of pTNM Stages IIA and IIB for Adenocarcinoma of the Esophagusa
| Stage | TNM | Grade | Description | Illustration |
|---|---|---|---|---|
| IIA | T2, N0, M0 | G3 | T2 = Tumor invades the muscularis propria. | |
| N0 = No regional lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| G3 = Poorly differentiated, undifferentiated. | ||||
| T2, N0, M0 | GX | T2 = Tumor invades the muscularis propria. | ||
| N0 = No regional lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| GX = Grade cannot be assessed. | ||||
| IIB | T1, N1, M0 | Any | T1 = Tumor invades the lamina propria, muscularis mucosae, or submucosa. | |
| –T1a = Tumor invades the lamina propria or muscularis mucosae. | ||||
| –T1b = Tumor invades the submucosa. | ||||
| N1 = Metastasis in one or two regional lymph nodes. | ||||
| M0 = No distant metastasis. | ||||
| Any G = See Table 1. | ||||
| T3, N0, M0 | Any | T3 = Tumor invades adventitia. | ||
| N0 = No regional lymph node metastasis. | ||||
| M0 = No distant metastasis. | ||||
| Any G = See Table 1. |
T = primary tumor; N = regional lymph nodes; M = distant metastasis; G = grade; p = pathological.
aReprinted with permission from AJCC: Esophageal and esophagogastric junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 185–202.
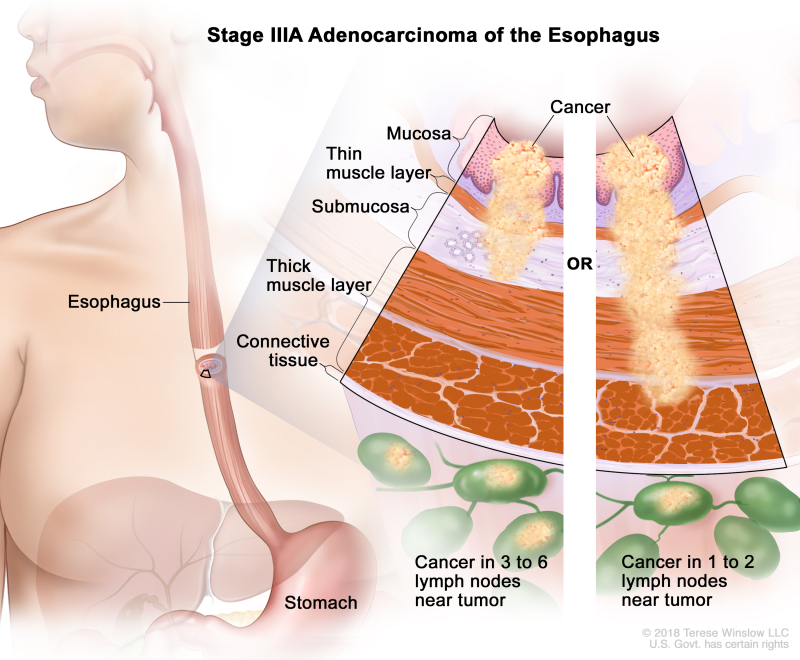
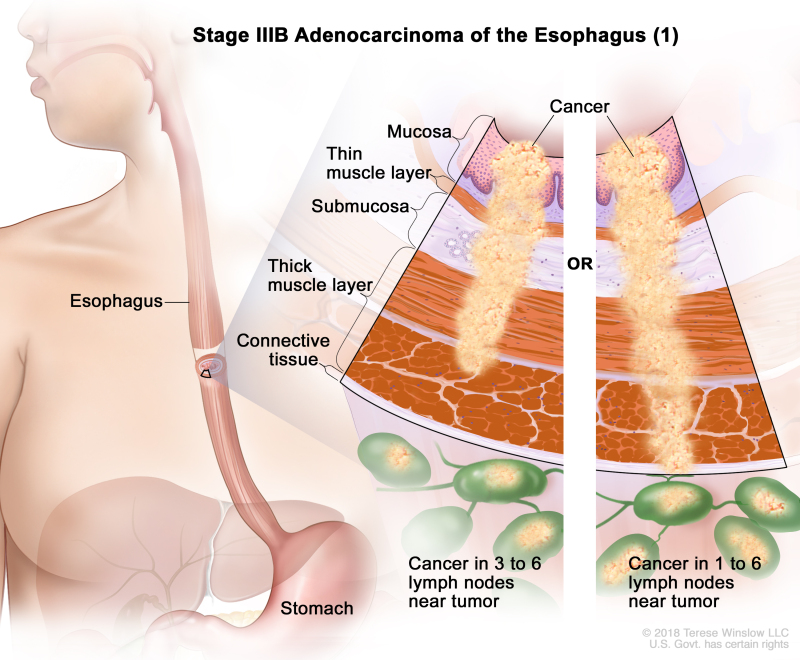
Table 10. Definitions of pTNM Stages IIIA and IIIB for Adenocarcinoma of the Esophagusa
| Stage | TNM | Grade | Description | Illustration |
|---|---|---|---|---|
| IIIA | T1, N2, M0 | Any | T1 = Tumor invades the lamina propria, muscularis mucosae, or submucosa. | |
| –T1a = Tumor invades the lamina propria or muscularis mucosae. | ||||
| –T1b = Tumor invades the submucosa. | ||||
| N2 = Metastasis in three to six regional lymph nodes. | ||||
| M0 = No distant metastasis. | ||||
| Any G = See Table 1. | ||||
| T2, N1, M0 | Any | T2 = Tumor invades the muscularis propria. | ||
| N1 = Metastasis in one or two regional lymph nodes. | ||||
| M0 = No distant metastasis. | ||||
| Any G = See Table 1. | ||||
| IIIB | T2, N2, M0 | Any | T2 = Tumor invades the muscularis propria. | |
| N2 = Metastasis in three to six regional lymph nodes. | ||||
| M0 = No distant metastasis. | ||||
| Any G = See Table 1. | ||||
| T3, N1–2, M0 | Any | T3 = Tumor invades adventitia. | ||
| N1 = Metastasis in one or two regional lymph nodes. | ||||
| N2 = Metastasis in three to six regional lymph nodes. | ||||
| M0 = No distant metastasis. | ||||
| Any G = See Table 1. | ||||
| T4a, N0–1, M0 | Any | –T4a = Tumor invades the pleura, pericardium, azygos vein, diaphragm, or peritoneum. | ||
| N0 = No regional lymph node metastasis. | ||||
| N1 = Metastasis in one or two regional lymph nodes. | ||||
| M0 = No distant metastasis. | ||||
| Any G = See Table 1. |
T = primary tumor; N = regional lymph nodes; M = distant metastasis; G = grade; p = pathological.
aReprinted with permission from AJCC: Esophageal and esophagogastric junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 185–202.
Table 11. Definitions of pTNM Stages IVA and IVB for Adenocarcinoma of the Esophagusa
| Stage | TNM | Grade | Description | Illustration |
|---|---|---|---|---|
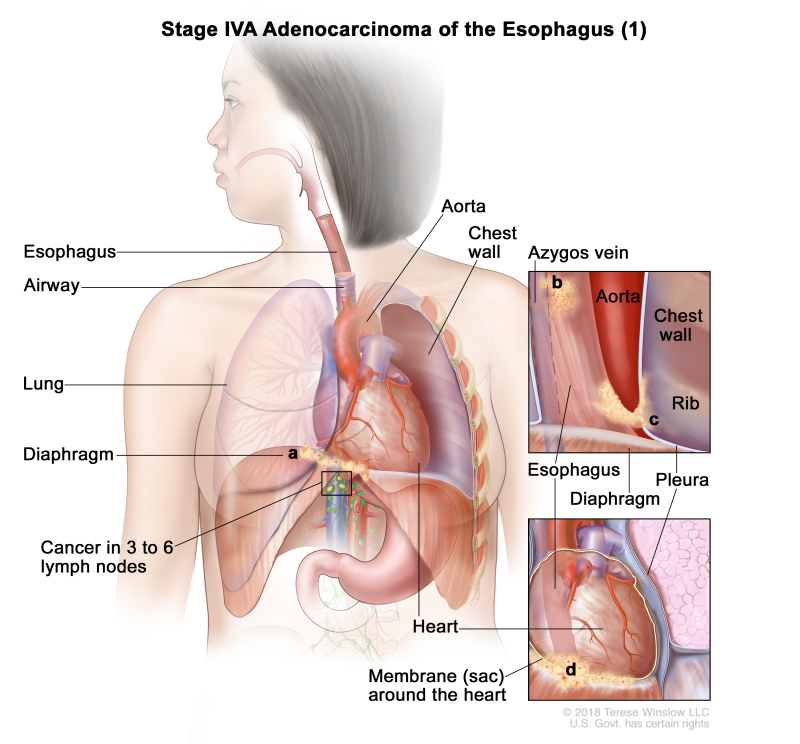
| IVA | T4a, N2, M0 | Any | –T4a = Tumor invades the pleura, pericardium, azygos vein, diaphragm, or peritoneum. | |
| N2 = Metastasis in three to six regional lymph nodes. | ||||
| M0 = No distant metastasis. | ||||
| Any G = See Table 1. | ||||
| T4b, N0–2, M0 | Any | –T4b = Tumor invades other adjacent structures, such as the aorta, vertebral body, or airway. | ||
| N0 = No regional lymph node metastasis. | ||||
| N1 = Metastasis in one or two regional lymph nodes. | ||||
| N2 = Metastasis in three to six regional lymph nodes. | ||||
| M0 = No distant metastasis. | ||||
| Any G = See Table 1. | ||||
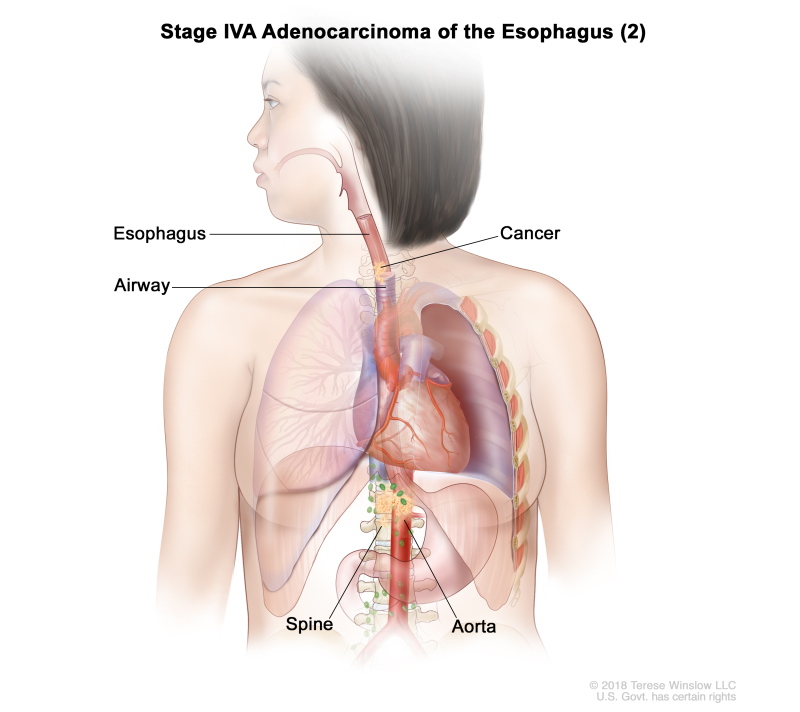
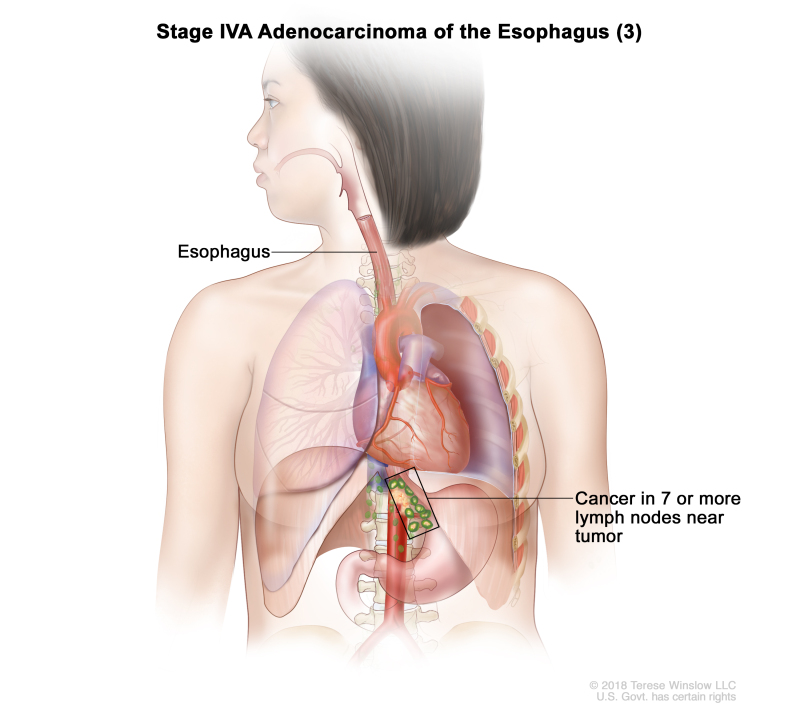
| Any T, N3, M0 | Any | Any T = See Table 1. | ||
| N3 = Metastasis in seven or more regional lymph nodes. | ||||
| M0 = No distant metastasis. | ||||
| Any G = See Table 1. | ||||
| IVB | Any T, Any N, M1 | Any | Any T = See Table 1. | |
| Any N = See Table 1. | ||||
| M1 = Distant metastasis. | ||||
| Any G = See Table 1. |
T = primary tumor; N = regional lymph nodes; M = distant metastasis; G = grade; p = pathological.
aReprinted with permission from AJCC: Esophageal and esophagogastric junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 185–202.
References
- Ziegler K, Sanft C, Zeitz M, et al.: Evaluation of endosonography in TN staging of oesophageal cancer. Gut 32 (1): 16-20, 1991. [PMC free article: PMC1379206] [PubMed: 1991632]
- Tio TL, Coene PP, den Hartog Jager FC, et al.: Preoperative TNM classification of esophageal carcinoma by endosonography. Hepatogastroenterology 37 (4): 376-81, 1990. [PubMed: 2210603]
- Vazquez-Sequeiros E, Norton ID, Clain JE, et al.: Impact of EUS-guided fine-needle aspiration on lymph node staging in patients with esophageal carcinoma. Gastrointest Endosc 53 (7): 751-7, 2001. [PubMed: 11375583]
- Bonavina L, Incarbone R, Lattuada E, et al.: Preoperative laparoscopy in management of patients with carcinoma of the esophagus and of the esophagogastric junction. J Surg Oncol 65 (3): 171-4, 1997. [PubMed: 9236925]
- Sugarbaker DJ, Jaklitsch MT, Liptay MJ: Thoracoscopic staging and surgical therapy for esophageal cancer. Chest 107 (6 Suppl): 218S-223S, 1995. [PubMed: 7781397]
- Luketich JD, Schauer P, Landreneau R, et al.: Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 114 (5): 817-21; discussion 821-3, 1997. [PubMed: 9375612]
- Krasna MJ, Reed CE, Nedzwiecki D, et al.: CALGB 9380: a prospective trial of the feasibility of thoracoscopy/laparoscopy in staging esophageal cancer. Ann Thorac Surg 71 (4): 1073-9, 2001. [PubMed: 11308139]
- Flamen P, Lerut A, Van Cutsem E, et al.: Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 18 (18): 3202-10, 2000. [PubMed: 10986052]
- Flamen P, Van Cutsem E, Lerut A, et al.: Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol 13 (3): 361-8, 2002. [PubMed: 11996465]
- Weber WA, Ott K, Becker K, et al.: Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 19 (12): 3058-65, 2001. [PubMed: 11408502]
- van Westreenen HL, Westerterp M, Bossuyt PM, et al.: Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol 22 (18): 3805-12, 2004. [PubMed: 15365078]
- Meyers BF, Downey RJ, Decker PA, et al.: The utility of positron emission tomography in staging of potentially operable carcinoma of the thoracic esophagus: results of the American College of Surgeons Oncology Group Z0060 trial. J Thorac Cardiovasc Surg 133 (3): 738-45, 2007. [PubMed: 17320575]
- Rice TW, Kelsen D, Blackstone EH, et al.: Esophagus and Esophagogastric Junction. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. Springer; 2017, pp 185-202.
- Korst RJ, Rusch VW, Venkatraman E, et al.: Proposed revision of the staging classification for esophageal cancer. J Thorac Cardiovasc Surg 115 (3): 660-69; discussion 669-70, 1998. [PubMed: 9535455]
Treatment Option Overview for Esophageal Cancer
For patients with minimally invasive resectable esophageal cancer, surgical resection alone offers the potential for cure. In contrast, therapeutic management for patients with locally advanced resectable esophageal cancer has evolved significantly over the last few decades. Because of the risk of distant metastases and local relapse, multimodality therapy with chemotherapy, radiation therapy, and surgical resection has become the standard of care.
Effective palliation may be obtained in individual cases with various combinations of the following:
- Surgery.
- Chemotherapy.
- Radiation therapy.
Table 12. Treatment Options for Esophageal Cancer
| Stage (TNM Staging Criteria) | Treatment Options |
|---|---|
| Stage 0 Esophageal Cancer | Surgery |
| Endoscopic resection | |
| Stage I Esophageal Cancer | Chemoradiation therapy followed by surgery |
| Surgery alone | |
| Stage II Esophageal Cancer | Chemoradiation therapy followed by surgery |
| Surgery alone | |
| Chemotherapy followed by surgery | |
| Definitive chemoradiation therapy | |
| Stage III Esophageal Cancer | Chemoradiation therapy followed by surgery |
| Preoperative chemotherapy followed by surgery | |
| Definitive chemoradiation therapy | |
| Stage IV Esophageal Cancer | Chemoradiation therapy followed by surgery (for patients with stage IVA disease) |
| Chemotherapy, which has provided partial responses for patients with metastatic distal esophageal adenocarcinomas | |
| Adjuvant therapy for patients with completely resected (negative margins) esophageal adenocarcinoma, esophageal squamous cell carcinoma, or gastroesophageal junction cancer who had residual pathological disease after concurrent chemoradiation therapy | |
| Immunotherapy and chemoimmunotherapy for patients with previously untreated, unresectable, advanced or metastatic esophageal squamous cell carcinoma | |
| Immunotherapy and chemoimmunotherapy for patients with previously untreated advanced or metastatic esophageal adenocarcinoma or gastroesophageal junction cancer | |
| Immunotherapy for patients who relapse after one prior line of standard therapy | |
| Nivolumab and chemotherapy for patients with adenocarcinoma | |
| Nd:YAG endoluminal tumor destruction or electrocoagulation | |
| Endoscopic-placed stents to provide palliation of dysphagia | |
| Radiation therapy with or without intraluminal intubation and dilation | |
| Intraluminal brachytherapy to provide palliation of dysphagia | |
| Clinical trials evaluating single-agent or combination chemotherapy | |
| Recurrent Esophageal Cancer | Palliative use of any of the other therapies, including supportive care |
| Immunotherapy and chemoimmunotherapy for patients with recurrent esophageal squamous cell carcinoma |
Surgery
Surgery (Barrett esophagus)
The prevalence of Barrett metaplasia in adenocarcinoma of the esophagus suggests that Barrett esophagus is a premalignant condition. Endoscopic surveillance of patients with Barrett metaplasia may detect adenocarcinoma at an earlier stage that is more amenable to curative resection. Strong consideration should be given to resection in patients with high-grade dysplasia in the setting of Barrett metaplasia.[7]
Surgery (esophageal cancer)
The survival rate of patients with esophageal cancer is poor. Surgical treatment of resectable esophageal cancers results in 5-year survival rates of 5% to 30%, with higher survival rates in patients with early-stage cancers.[8] Asymptomatic small tumors confined to the esophageal mucosa or submucosa are detected only by chance. Surgery is the treatment of choice for these small tumors. Once symptoms are present (e.g., dysphagia, in most cases), esophageal cancers have usually invaded the muscularis propria or beyond and may have metastasized to lymph nodes or other organs.
In some patients with partial esophageal obstruction, dysphagia may be relieved by placement of an expandable metallic stent [9] or by radiation therapy if the patient has disseminated disease or is not a candidate for surgery. Alternative methods of relieving dysphagia have been reported, including laser therapy and electrocoagulation to destroy intraluminal tumor.[10-12]
In the presence of complete esophageal obstruction without clinical evidence of systemic metastasis, surgical excision of the tumor with mobilization of the stomach to replace the esophagus has been the traditional means of relieving the dysphagia.
The optimal surgical approach for radical resection of esophageal cancer is not known. One approach advocates transhiatal esophagectomy with anastomosis of the stomach to the cervical esophagus. A second approach advocates abdominal mobilization of the stomach and transthoracic excision of the esophagus with anastomosis of the stomach to the upper thoracic esophagus or the cervical esophagus. One study concluded that transhiatal esophagectomy was associated with lower morbidity than was transthoracic esophagectomy with extended en bloc lymphadenectomy; however, median overall disease-free and quality-adjusted survival did not differ significantly.[13] Similarly, no differences in long-term quality of life (QOL) using validated QOL instruments have been reported.[14] More recently, minimally invasive approaches that offer potential advantages of smaller incisions, decreased intraoperative blood loss, fewer postoperative complications, and shorter hospital stays have emerged. However, the ability to obtain negative surgical margins, the adequacy of lymph node dissection, and long-term outcomes have not been fully established with this approach.[15]
In the United States, the median age of patients who present with esophageal cancer is 68 years.[16] The results of a retrospective review of 505 consecutive patients who were operated on by a single surgical team over 17 years found no difference in the perioperative mortality, median survival, or palliative benefit of esophagectomy on dysphagia when the patients older than 70 years were compared with their younger peers.[17][Level of evidence C1] All of the patients in this series were selected for surgery on the basis of potential operative risk. Age alone does not determine therapy for patients with potentially resectable disease.
Preoperative Chemotherapy Plus Anti-Human Epidermal Growth Factor Receptor 2 Therapy and/or Immunotherapy
Fluorouracil, leucovorin, oxaliplatin, and docetaxel chemotherapy plus trastuzumab and pertuzumab
Evidence (fluorouracil [5-FU], leucovorin, oxaliplatin, and docetaxel [FLOT] chemotherapy plus trastuzumab and pertuzumab):
- A multicenter phase II/III trial (NCT02581462) included patients with human epidermal growth factor receptor 2 (HER2)-positive resectable gastric or gastroesophageal junction adenocarcinoma (clinical T2 or larger or clinically node-positive). A total of 81 patients (61 with gastroesophageal cancer, 20 with gastric cancer) were enrolled during the phase II part of the study. Patients were randomly assigned to receive four preoperative and postoperative cycles of either FLOT alone (arm A, n = 41) or FLOT combined with trastuzumab and pertuzumab followed by nine cycles of trastuzumab and pertuzumab (arm B, n = 40). In the phase II part of the study, the primary end point was the rate of pathological complete response. The trial did not transition to phase III. It closed prematurely, after results of the JACOB trial were reported, which demonstrated that adding pertuzumab to trastuzumab and chemotherapy did not significantly improve overall survival (OS) in patients with HER2-positive metastatic gastric or gastroesophageal junction cancer compared with placebo.[18]
- The pathological complete response rate was significantly improved for patients in arm B at 35%, compared with 12% for patients in arm A (P = .02).[18][Level of evidence B3]
- The rate of pathological lymph node negativity was higher for patients who received trastuzumab and pertuzumab (39% for arm A vs. 68% for arm B).
- The R0 resection rate was 90% for patients in arm A and 93% for patients in arm B. Surgical morbidity was also comparable, at 43% for patients in arm A and 44% for patients in arm B.
- The median disease-free survival (DFS) was 26 months for patients in arm A and not yet reached for patients in arm B (hazard ratio [HR], 0.58; P =.14).
- The 24-month DFS rates were 54% (95% confidence interval [CI], 38%–71%) in arm A and 70% (95% CI, 55%–85%) in arm B. The 24-month OS rates were 77% (95% CI, 63%–90%) in arm A and 84% (95% CI, 72%–96%) in arm B.
- More grade 3 or greater adverse events were reported among patients who received trastuzumab and pertuzumab, especially diarrhea (5% of patients in arm A and 41% of patients in arm B) and leukopenia (13% of patients in arm A and 23% of patients in arm B).
Preoperative Chemoradiation Therapy
On the basis of several randomized trial results, chemoradiation followed by surgery is a treatment option for patients with stages IB, II, III, and IVA esophageal cancer.
Phase III trials have compared preoperative concurrent chemoradiation therapy with surgery alone for patients with esophageal cancer.[19-25][Level of evidence A1] The benefit of neoadjuvant chemoradiation has been controversial because of contradictory results of early randomized studies.[19-22] However, the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) has definitively demonstrated a survival benefit for preoperative chemoradiation compared with surgery alone in locally advanced esophageal cancer.[23]
For early-stage tumors, the role of preoperative chemoradiation remains controversial. Although the CROSS study included early-stage patients, the Francophone de Cancérologie Digestive (FFCD) 9901 study (NCT00047112),[25] which included only early-stage (stage I or II) patients, failed to demonstrate a survival advantage in this group of patients.
Evidence (preoperative chemoradiation therapy):
- The CROSS study randomly assigned 366 patients with resectable esophageal or junctional cancers to receive either surgery alone or weekly administration of carboplatin (dose titrated to achieve an AUC [area under the curve] of 2 mg/mL/minute) and paclitaxel (50 mg/m2 of BSA [body surface area]) and concurrent radiation therapy (41.4 Gy in 23 fractions) administered over 5 weeks. Most patients enrolled in the CROSS trial (75%) had adenocarcinoma.[23,26][Level of evidence A1]
- With a median follow-up of 84 months, preoperative chemoradiation was found to improve median OS from 24 months in the surgery-alone group to 48.6 months (HR, 0.68; 95% CI, 0.53–0.88; P = .003). Median OS for patients with squamous cell carcinomas was 81.6 months in the preoperative chemoradiation group, compared with 21.1 months in the surgery-alone group (HR, 0.48; 95% CI, 0.28–0.83; log-rank P = .008); for patients with adenocarcinomas, median OS was 43.2 months in the preoperative chemoradiation group, compared with 27.1 months in the surgery-alone group (HR, 0.73; 95% CI, 0.55–0.98; log-rank P = .038).[26]
- Additionally, preoperative chemoradiation improved the rate of R0 resections (92% vs. 69%; P < .001). R0 is defined as complete resection with no tumor within 1 mm of resection margins.
- A complete pathological response was achieved in 29% of patients who underwent resection after chemoradiation therapy. A pathological complete response was observed in 23% of patients with adenocarcinoma, compared with 49% of patients with squamous cell carcinoma (P = .008).
- Postoperative complications and in-hospital mortality were equivalent in both groups. The most common hematologic side effects in the chemoradiation group were leukopenia (6%) and neutropenia (2%). The most common nonhematologic side effects were anorexia (5%) and fatigue (3%).
- With a median follow-up of 84 months, the 5-year progression-free survival (PFS) rate was 44% in the preoperative chemoradiation group, compared with 27% in the surgery-alone group (HR, 0.61; 95% CI, 0.47–0.78). Preoperative chemoradiation therapy reduced locoregional recurrence from 34% to 14% (P < .001) and peritoneal carcinomatosis from 14% to 4% (P < .001). There was a small but significant effect on hematogenous dissemination in favor of the chemoradiation therapy group (35% vs. 29%; P = .025).[24,26][Level of evidence B1]
- A multicenter, prospective, randomized trial compared preoperative combined chemotherapy (i.e., cisplatin) and radiation therapy (37 Gy in 3.7-Gy fractions) versus surgery alone in patients with squamous cell carcinoma.[19][Level of evidence A1]
- The study showed no improvement in OS and a significantly higher postoperative mortality rate (12% vs. 4%) in the combined-modality arm.
- In patients with adenocarcinoma of the esophagus, a single-institution phase III trial was conducted in patients treated with induction chemoradiation therapy consisting of 5-FU, cisplatin, and 40 Gy (in 2.67-Gy fractions) plus surgery compared with resection alone.[20][Level of evidence A1]
- The results demonstrated a modest survival benefit of 16 months for combined modality therapy versus 11 months for surgery alone.
- A subsequent single-institution trial randomly assigned patients (75% with adenocarcinoma) to 5-FU, cisplatin, vinblastine, and radiation therapy (1.5 Gy twice daily to a total of 45 Gy) plus resection versus esophagectomy alone.[21][Level of evidence A1]
- At a median follow-up of more than 8 years, there was no significant difference between the surgery alone and combined modality therapy with respect to median survival (17.6 months vs. 16.9 months), OS rate (16% vs. 30% at 3 years), or DFS rate (16% vs. 28% at 3 years).
- An intergroup trial (CALGB-9781 [NCT00003118]) planned to randomly assign 475 patients with resectable squamous cell or adenocarcinoma of the thoracic esophagus to treatment with preoperative chemoradiation therapy (5-FU, cisplatin, and 50.4 Gy) followed by esophagectomy and nodal dissection or surgery alone. The trial was closed as a result of poor patient accrual; however, results from the 56 enrolled patients, with a median follow-up of 6 years, were reported.[22][Level of evidence A1]
- The median survival was 4.48 years (95% CI; range, 2.4–not estimable) for trimodality therapy versus 1.79 years (95% CI, 1.41–2.59) for surgery alone (P = .002), with a 5-year OS rate of 39% for trimodality therapy (95% CI, 21%–57%) versus 16% for surgery alone (95% CI, 5%–33%).
- To further evaluate the impact of neoadjuvant chemoradiation therapy for early-stage disease, the FFCD 9901 study randomly assigned 195 patients with stage I or stage II esophageal cancer to receive surgery alone or neoadjuvant chemoradiation therapy (45 Gy in 25 fractions administered with two courses of 5-FU [800 mg/m2] and cisplatin [75 mg/m2]) followed by surgery.[25][Level of evidence A1]
- At interim analysis, accrual to the study was stopped early because of futility.
- With a median follow-up of 94 months, there was no significant improvement in 3-year OS rates with chemoradiation (48% vs. 53%; P = .94); there was a significantly higher postoperative mortality rate of 11.1% versus 3.4% (P = .049).
- The NRG Oncology/RTOG-1010 trial (NCT01196390) evaluated the addition of trastuzumab to trimodality treatment (paclitaxel plus carboplatin and radiotherapy, followed by surgery) in patients with untreated HER2-overexpressing esophageal adenocarcinoma. This phase III trial entered 606 patients for HER2 assessment. A total of 203 patients with HER2-positive disease were randomly assigned to receive chemoradiation therapy plus trastuzumab (n = 102) or chemoradiation therapy alone (n = 101). The primary end point was DFS. The median duration of follow-up was 2.8 years.[27]
- The median DFS was 19.6 months (95% CI, 13.5–26.2) for patients who received chemoradiation therapy plus trastuzumab and 14.2 months (10.5–23.0) for patients who received chemoradiation therapy alone (HR, 0.99; 95% CI, 0.71–1.39; log-rank P = .97).[27][Level of evidence B1]
- Grade 3 treatment-related adverse events occurred in 41 of 95 patients (43%) who received trastuzumab and 52 of 96 patients (54%) of patients who received chemoradiation therapy alone. Grade 4 treatment-related adverse events occurred in 20 patients (21%) who received trastuzumab and 21 patients (22%) who received chemoradiation therapy alone.
- There were five treatment-related deaths in the trastuzumab group (bronchopleural fistula, esophageal anastomotic leak, lung infection, sudden death, and death not otherwise specified), and three deaths in the chemoradiation therapy group (two multiorgan failure and one sepsis).
In conclusion, this trial confirmed that trastuzumab does not have a role in the preoperative treatment of HER2-positive esophageal or gastroesophageal cancer, either with chemotherapy alone or with chemoradiation therapy.
Preoperative Chemotherapy
The effects of preoperative chemotherapy are being evaluated in randomized trials. Several studies have demonstrated a survival benefit with preoperative chemotherapy compared with surgery alone.[28-30] However, one large randomized study failed to confirm a survival benefit with preoperative chemotherapy.[31] Compared with preoperative chemotherapy alone, preoperative chemoradiation therapy improves pathological response and may improve outcomes.[32]
Evidence (preoperative chemotherapy):
- An intergroup trial (NCT00525785) randomly assigned 440 patients with local and operable esophageal cancer of any cell type to three cycles of preoperative 5-FU and cisplatin followed by surgery and two additional cycles of chemotherapy versus surgery alone.[31][Level of evidence A1]
- After a median follow-up of 55 months, there were no significant differences in median survival between the chemotherapy-plus-surgery group (14.9 months) and the surgery-alone group (16.1 months). The median 2-year survival rate was 35% in the chemotherapy-plus-surgery group and 37% in the surgery-alone group.
- The addition of chemotherapy did not increase the morbidity associated with surgery.
- The Medical Research Council Oesophageal Cancer Working Party randomly assigned 802 patients with resectable esophageal cancer, also of any cell type, to two cycles of preoperative 5-FU and cisplatin followed by surgery versus surgery alone.[28][Level of evidence A1]
- At a median follow-up of 37 months, median survival was significantly improved in the preoperative chemotherapy arm (16.8 months vs. 13.3 months with surgery alone; 95% CI), as was the 2-year OS rate (43% in the preoperative chemotherapy arm and 34% in the surgery-alone arm; 95% CI).
The interpretation of the results from the intergroup and preoperative chemotherapy trials is challenging because T or N staging was not reported, and prerandomization and radiation could be offered at the discretion of the treating oncologist. - The Japanese Clinical Oncology Group randomly assigned 330 patients with clinical stage II or III, excluding T4, squamous cell carcinomas to receive either two cycles of preoperative cisplatin and 5-FU followed by surgery or surgery followed by postoperative chemotherapy of the same regimen. A planned interim analysis was conducted after patient accrual. Although the primary end point of PFS was not met, there was a significant benefit in OS among patients treated with preoperative chemotherapy (P = .01). As a result of these findings, the Data and Safety Monitoring Committee recommended early closure of the study.[29][Level of evidence A3]
- With a median follow-up of 61 months, the 5-year OS rate was 55% among patients treated with preoperative chemotherapy, compared with 43% among patients treated with postoperative chemotherapy (P = .04). However, there was no significant difference between groups with respect to PFS (5-year PFS rate, 39% vs. 44%; P = .22).
- Additionally, there were no significant differences between the two groups with respect to postoperative complications or treatment-related toxicities.
- The Fédération Nationale des Centres de Lutte contre le Cancer and the FFCD randomly assigned 224 patients with resectable adenocarcinoma of the lower esophagus, gastroesophageal junction, or stomach to receive either perioperative chemotherapy and surgery (n = 113) or surgery alone (n = 111). Chemotherapy consisted of two or three preoperative cycles of intravenous (IV) cisplatin (100 mg/m2) on day 1 and continuous IV infusion of 5-FU (800 mg/m2) for 5 consecutive days (day 1–5) every 28 days, and three or four postoperative cycles of the same regimen.[30][Level of evidence A1]
- Perioperative chemotherapy was associated with an improved 5-year OS rate (38% vs. 24%; HR, 0.69; P = .02).
- Grade 3 and 4 toxicity occurred in 38% of patients treated with perioperative chemotherapy, but there was no increase in postoperative morbidity.
- The Preoperative Chemotherapy or Radiochemotherapy in Esophago-gastric Adenocarcinoma Trial (POET) sought to evaluate the additional benefit of radiation therapy to preoperative chemotherapy. Patients were randomly assigned to receive either induction chemotherapy (15 weeks) followed by surgery or chemotherapy (12 weeks) followed by chemoradiation therapy (3 weeks) and surgery.[32][Level of evidence A1]
- The study was closed early because of poor accrual. In total, 126 patients were randomly assigned.
- Preoperative radiation therapy yielded 3-year survival rates of 27% to 47% (log-rank P = .07). The postoperative mortality rate was nonsignificantly increased in the chemoradiation therapy group (10.2% vs. 3.8%; P = .26).
Perioperative Chemotherapy
- A trial that included patients with resectable adenocarcinoma of the stomach, esophagogastric junction, or lower esophagus randomly assigned 250 patients to receive perioperative chemotherapy and surgery and 253 patients to receive surgery alone. Chemotherapy consisted of three preoperative and three postoperative cycles of IV epirubicin (50 mg/m2) and cisplatin (60 mg/m2) on day 1, and a continuous IV infusion of 5-FU (200 mg/m2 per day) for 21 days. The primary end point was OS.[33]
- After a median follow-up of 4 years, 149 patients in the perioperative chemotherapy group and 170 patients in the surgery-alone group had died.
- Compared with the surgery-alone group, the perioperative chemotherapy group had a higher likelihood of OS (HRdeath, 0.75; 95% CI, 0.60–0.93; P = .009). The 5-year survival rate was 36.3% in the perioperative chemotherapy group (95% CI, 29.5%–43.0%) and 23% (95% CI, 16.6%–29.4%) in the surgery-alone group.[33][Level of evidence A1]
- The PFS rate was also improved in the perioperative chemotherapy group (HRprogression, 0.66; 95% CI, 0.53–0.81; P < .001) compared with the surgery-alone group.
- The rate of postoperative complications was similar in the two groups; 46% in the perioperative chemotherapy group and 45% in the surgery-alone group. The number of deaths within 30 days after surgery was also similar between the two groups.
- An open-label, randomized, phase II/III trial compared the efficacy and safety of the docetaxel-based triplet FLOT regimen with the combination of epirubicin and cisplatin plus either 5-FU given as a continuous infusion or capecitabine given orally. The trial included 716 patients with adenocarcinoma in clinical stage cT2 or higher, node-positive stage (cN+), or both, or resectable tumors with no evidence of distant metastases. Patients were randomly assigned to receive either: (1) ECF/ECX (three preoperative and three postoperative 3-week cycles of epirubicin [50 mg/m2] and cisplatin [60 mg/m2] on day 1 plus either 5-FU [200 mg/m2] given as a continuous infusion or capecitabine [1,250 mg/m2] orally on days 1 to 21), or (2) FLOT (four preoperative and four postoperative 2-week cycles of docetaxel [50 mg/m2], oxaliplatin [85 mg/m2], leucovorin [200 mg/m2], and 5-FU [2,600 mg/m2] as a 24-hour infusion on day 1). The primary end point of the trial was OS (superiority) in the intention-to-treat population.[34]
- OS was longer in the FLOT group than the ECF/ECX group (HR, 0.77; 95% CI, 0.63–0.94). The median OS was 50 months in the FLOT group (38.33–not reached) and 35 months in the ECF/ECX group (27.35–46.26).[34][Level of evidence A1]
- The number of patients with related serious adverse events (including those occurring during a hospital stay for surgery) was similar between the two groups: 96 patients (27%) in the ECF/ECX group and 97 patients (27%) in the FLOT group.
- The number of deaths due to toxicity (two [<1%]) was similar in both groups. Hospitalization for toxicity occurred in 94 patients (26%) in the ECF/ECX group and 89 patients (25%) in the FLOT group.
- The ESOPEC trial (NCT02509286), reported in abstract form, compared perioperative FLOT and surgery with neoadjuvant chemoradiation therapy with the CROSS regimen (41.4 Gy plus carboplatin and docetaxel) followed by surgery. The trial enrolled 438 patients with clinical (c)T1, cN+, cM0 or cT2–4a, any cN, cM0 resectable esophageal adenocarcinoma. The primary end point was OS.[35]
- After a median follow-up of 55 months, 218 patients had died: 97 patients in the FLOT group and 121 patients in the CROSS group. The median OS was 66 months (95% CI, 36–not estimable) for patients in the FLOT group and 37 months (95% CI, 28–43) for patients in the CROSS group.
- The 3-year OS rates were 57.4% (95% CI, 50.1%–64.0%) for patients in the FLOT group and 50.7% (95% CI, 43.5%–57.5%) for patients in the CROSS group (HR, 0.70; 95% CI, 0.53–0.92; P = .012).[35][Level of evidence A1]
- Neoadjuvant treatment was given to 403 patients: 207 patients in the FLOT group and 196 patients in the CROSS group.
- Surgery was performed in 371 patients: 191 patients in the FLOT group and 180 patients in the CROSS group. R0 resection was achieved in 351 patients, including 180 patients in the FLOT group and 171 patients in the CROSS group.
- More patients who received FLOT (87.3%) than CROSS (67.7%) were able to complete all planned neoadjuvant therapy.
- The 90-day postsurgical mortality was 4.3%: 3.2% in the FLOT group and 5.6% in the CROSS group.
In the ESOPEC trial, patients did not receive adjuvant nivolumab; thus, it is not possible to conclude that perioperative FLOT is superior to preoperative CROSS and adjuvant nivolumab.
Definitive Chemoradiation Therapy
For patients who are deemed either medically inoperable or have tumors that are unresectable, the efficacy of definitive chemoradiation has been established in numerous randomized controlled trials.[36,37] For patients with squamous cell carcinomas of the esophagus, definitive chemoradiation may offer equivalent outcomes, compared with preoperative chemoradiation followed by surgical resection.[38,39]
Evidence (definitive chemoradiation):
- A Radiation Therapy Oncology Group trial (RTOG-8501) randomly assigned patients to receive radiation therapy alone (64 Gy in 32 fractions) or chemoradiation (50 Gy in 25 fractions) with concurrent cisplatin (75 mg/m2) and continuous-infusion 5-FU (1,000 mg/m2 on days 1 to 4 in weeks 1 and 5 followed by two additional cycles of chemotherapy administered 3 weeks apart).[36][Level of evidence A1]
- There was an improvement in the 5-year survival rate for the combined modality group (27% vs. 0%).
- An 8-year follow-up of this trial demonstrated an OS rate of 22% for patients who received chemoradiation therapy.
- Intergroup-0123 (RTOG-9405 [NCT00002631]) was conducted in an attempt to improve upon the results of RTOG-8501. Intergroup-0123 randomly assigned 236 patients with localized esophageal tumors to undergo chemoradiation with high-dose radiation therapy (64.8 Gy) and four monthly cycles of 5-FU and cisplatin versus conventional-dose radiation therapy (50.4 Gy) and the same chemotherapy schedule.[37][Level of evidence A1]
- Although originally designed to accrue 298 patients, this trial was closed in 1999 after a planned interim analysis showed that it was statistically unlikely that there would be any advantage to using high-dose radiation.
- At a 2-year median follow-up, no statistically significant differences in median survival were observed between the high-dose and conventional-dose radiation therapy arms (13 months vs. 18 months), 2-year survival rates (31% vs. 40%), or local and regional failure rates (56% vs. 52%).
- There was a higher treatment mortality rate in the higher-dose arm (9% vs. 2%). However, 7 of 11 deaths in the high-dose arm occurred in patients who had received 50.4 Gy or less.
- An Eastern Cooperative Oncology Group trial (EST-1282) evaluated 135 patients.[40][Level of evidence A1]
- This trial showed that chemotherapy plus radiation therapy provided a better 2-year survival rate than did radiation therapy alone, similar to results from the intergroup trial.
- The PRODIGE5/ACCORD17 trial (NCTE) compared the efficacy and safety of oxaliplatin, 5-FU, and leucovorin calcium (FOLFOX) versus 5-FU and cisplatin as the chemotherapy backbone among patients treated with definitive chemoradiation for localized esophageal cancer. In this multicenter, randomized, phase II and III trial, 267 patients were randomly assigned to receive either six cycles of FOLFOX (three cycles concomitant with radiation therapy), oxaliplatin (85 mg/m2), leucovorin (200 mg/m2), bolus 5-FU (400 mg/m2), and infusion 5-FU (1,600 mg/m2 over 46 hours) or four cycles of 5-FU (1,000 mg/m2 for 4 days) and cisplatin (75 mg/m2 on day 1). All patients received radiation therapy (50 Gy in 25 fractions).[41][Level of evidence B1]
- With a median follow-up of 25.3 months, there was no significant difference in PFS (9.7 months with FOLFOX vs. 9.4 months with 5-FU and cisplatin; P = .64).
- There was one death caused by toxicity in the FOLFOX group versus six deaths in the 5-FU and cisplatin arm (P = .066).
- There were no significant differences in grade 3 or 4 adverse events between treatment groups. Among toxicities of all grades, paresthesia, sensory neuropathy, and increases in aspartate transaminase and alanine transaminase were more common in the FOLFOX group; increases in serum creatinine, mucositis, and alopecia were more common in the 5-FU and cisplatin group.
- A phase III German trial also compared induction chemotherapy (three courses of bolus 5-FU, leucovorin, etoposide, and cisplatin) followed by chemoradiation therapy (cisplatin, etoposide, and 40 Gy) followed by surgery (arm A), or the same induction chemotherapy followed by chemoradiation therapy (at least 65 Gy) without surgery (arm B) for patients with T3 or T4 squamous cell carcinoma of the esophagus. OS was the primary outcome.[38][Level of evidence A1]
- The analysis of 172 eligible randomly assigned patients showed that OS rates at 2 years were not statistically significantly different between the two treatment groups (arm A, 39.9%; 95% CI, 29.4%–50.4%; arm B, 35.4%; 95% CI, 25.2%–45.6%; log-rank test for equivalence with 0.15, P < .007).
- The local 2-year PFS rate was higher in the surgery group (64.3%; 95% CI, 52.1%–76.5%) than in the chemoradiation therapy group (40.7%; 95% CI, 28.9%–52.5%; HR for arm B vs. arm A, 2.1; 95% CI, 1.3–3.5; P < .003).
- The treatment-related mortality rate was higher in the surgery group (12.8%) than in the chemoradiation therapy group (3.5%) (P < .03).
- FFCD 9102 (NCTE) randomly assigned 259 patients with T3N0–1M0 thoracic esophageal cancer to receive either two cycles of 5-FU and cisplatin (days 1–5 and 22–26) and either conventional radiation therapy (46 Gy in 4.5 weeks) or split course (15 Gy, days 1–5 and 22–26). Patients with response were then randomly assigned to receive either surgical resection (arm A) or continuation of chemoradiation (arm B: three cycles of 5-FU plus cisplatin and either conventional 20 Gy or split-course 15 Gy radiation therapy).[39][Level of evidence A1]
- Of the 259 randomly assigned patients, 230 (89%) had squamous cell carcinoma, and 29 patients (11%) had adenocarcinomas.
- The 2-year OS rate was 34% in patients randomly assigned to receive surgery versus 40% in patients randomly assigned to receive definitive chemoradiation (HR, 0.90; P = .44). Median survival was 17.7 months for surgery and 19.3 months for definitive chemoradiation.
- The 3-month mortality rate was 9.3% in the surgery arm, compared with 0.8% in the chemoradiation arm (P = .002).
Adjuvant Therapy
Evidence (adjuvant therapy):
- A global, randomized, double-blind, placebo-controlled, phase III trial evaluated a checkpoint inhibitor as adjuvant therapy in 794 patients with esophageal or gastroesophageal junction cancer. Adults with a performance status of 0 or 1 and R0 stage II or III disease who had received neoadjuvant chemoradiation therapy and had residual pathological disease were included. Patients were randomly assigned in a 2:1 ratio to receive either nivolumab (240 mg every 2 weeks for 16 weeks) followed by nivolumab (480 mg every 4 weeks) (532 patients) or matching placebo (262 patients). Patients were enrolled regardless of programmed death-ligand 1 (PD-L1) expression. The maximum duration of the trial intervention period was 1 year. The primary end point was DFS. The median follow-up was 24.4 months.[42][Level of evidence B1]
- The median DFS was 22.4 months (95% CI, 16.6–34.0) among patients who received nivolumab, compared with 11.0 months (95% CI, 8.3–14.3) among patients who received placebo. The HRdisease recurrence or death was 0.69 (96.4% CI, 0.56–0.86; P < .001).
- Median DFS was evaluated across a prespecified subgroup according to histological type. Among patients with adenocarcinoma, the median DFS was 19.4 months in the 376 patients who received nivolumab (95% CI, 15.9–29.4), compared with 11.1 months in the 187 patients who received the placebo (95% CI, 8.3–16.8); the HRdisease recurrence or death was 0.75 (95% CI, 0.59–0.96). Among patients with squamous cell carcinoma, the median DFS was 29.7 months (95% CI, 14.4 to not estimable) in the 155 patients who received nivolumab, compared with 11 months (95% CI, 7.6–17.8) in the 75 patients who received the placebo; the HRdisease recurrence or death was 0.61 (95% CI, 0.42–0.88).
- HRs for disease recurrence or death were remarkably close between the 570 patients whose tumors expressed less than 1% of PD-L1 (HR, 0.73; 95% CI, 0.57–0.92) and the 129 patients whose tumors expressed 1% or more of PD-L1 (HR, 0.75; 95% CI, 0.45–1.24).
- Grade 3 or 4 adverse events of any cause occurred in 183 of 532 patients (34%) in the nivolumab group and 84 of 260 patients (32%) in the placebo group, and serious adverse events of any grade occurred in 30% of the patients in each group (nivolumab: 158 of 532; placebo: 78 of 260). Adverse events that were considered by the investigators to be related to the trial regimen were more common with nivolumab than with placebo, including grade 3 or 4 events (nivolumab: 71 of 532 patients [13%]; placebo: 15 of 260 patients [6%]) and events leading to discontinuation of therapy (nivolumab: 48 of 532 patients [9%]; placebo: 8 of 260 patients [3%]).
- OS data have not been reported for this study.
Given the positive results for the use of nivolumab after chemoradiation therapy and surgery in patients with esophageal cancer, an ongoing study will determine whether the adjuvant use of checkpoint inhibitor therapy improves outcomes in patients undergoing definitive chemoradiation therapy without surgery (KEYNOTE-975 [NCT04210115]). Studies are also evaluating potential benefits in patients undergoing perioperative chemotherapy without radiation therapy (e.g., KEYNOTE-585 [NCT03221426]).[43]
Immunotherapy and Chemoimmunotherapy
Immunotherapy and chemoimmunotherapy for patients with squamous cell carcinoma
Phase III randomized trials have compared chemotherapy with chemoimmunotherapy as first-line treatment for patients with advanced squamous cell carcinoma.[44-46]
Evidence (immunotherapy and chemoimmunotherapy for patients with squamous cell carcinoma):
- In the CheckMate 648 trial (NCT03143153), 970 adults with previously untreated, unresectable advanced, recurrent, or metastatic squamous cell carcinoma were enrolled, regardless of tumor cell PD-L1 expression. Patients were randomly assigned to receive either nivolumab (240 mg every 2 weeks) plus chemotherapy with 5-FU and cisplatin (every 4 weeks), nivolumab (3 mg/kg every 2 weeks) plus ipilimumab (1 mg/kg every 6 weeks), or chemotherapy alone. Primary end points for all groups were OS and PFS per blinded independent central review in patients with tumor cell PD-L1 expression of 1% or more (observed in 49% of patients). Further analyses included all patients regardless of PD-L1 status.[44]
- In patients with PD-L1-positive disease, the median OS was 15.4 months for patients who received nivolumab plus chemotherapy (95% CI, 11.9–19.5; n = 158), 13.7 months for patients who received nivolumab plus ipilimumab (95% CI, 11.2–17.0; n = 158), and 9.1 months for patients who received chemotherapy alone (95% CI, 7.7–10.0; n = 157). The corresponding HR (vs. chemotherapy) was 0.54 (99.5% CI, 0.37–0.8; P < .0001) for patients who received nivolumab plus chemotherapy and 0.64 (98.6% CI, 0.46–0.9; P < .001) for patients who received nivolumab plus ipilimumab.[44][Level of evidence A1]
- A statistically significant improvement in median OS, compared with chemotherapy, was also observed in all randomized patients, irrespective of PD-L1 status. Median OS was 13.2 months for patients who received nivolumab plus chemotherapy (95% CI, 11.1–15.7; n = 321), 12.8 months for patients who received nivolumab plus ipilimumab (95% CI, 11.3–15.5; n = 325), and 10.7 months for patients who received chemotherapy alone (95% CI, 9.4–11.9; n = 324). The corresponding HR (vs. chemotherapy) was 0.74 (99.1% CI, 0.58–0.96; P = .0021) for patients who received nivolumab plus chemotherapy and 0.78 (98.2% CI, 0.62–0.98; P = .011) for patients who received nivolumab plus ipilimumab.[44][Level of evidence A1]
- However, among patients whose tumors expressed less than 1% of PD-L1, OS was not significantly changed by the addition of nivolumab to chemotherapy (n = 329; median OS, 12.2 months with chemotherapy and 12 months with nivolumab plus chemotherapy) or by the combination of nivolumab with ipilimumab (n = 330; median OS, 12.2 months with chemotherapy and 12 months with nivolumab plus ipilimumab).
- Grade 3 or higher adverse events occurred in 147 patients (47%) who received nivolumab plus chemotherapy, 102 patients (32%) who received nivolumab plus ipilimumab, and 108 patients (36%) who received chemotherapy alone. No new safety signals were observed.
- The results of ESCORT-1st (NCT03691090), a randomized, double-blind, multicenter, placebo-controlled trial conducted in China, corroborated the positive impact of checkpoint inhibitors combined with first-line chemotherapy on patient survival. In this trial, 596 patients with previously untreated locally advanced or metastatic esophageal squamous cell cancer were randomly assigned (1:1) to receive either the humanized anti-PD-1 monoclonal antibody camrelizumab (200 mg; n = 298) or placebo (n = 298). Patients in both groups also received up to 6 cycles of paclitaxel (175 mg/m2) and cisplatin (75 mg/m2). All drugs were given IV every 3 weeks. Co-primary end points were OS and independent review committee (IRC)-assessed PFS.[45]
- With a median follow-up of 10.8 months, patients who received camrelizumab plus chemotherapy had significantly improved OS compared with patients who received placebo plus chemotherapy (median OS, 15.3 months [95% CI, 12.8–17.3] vs. 12.0 months [11.0–13.3]; HR, 0.70; 95% CI, 0.56–0.88; one-sided P = .0010).
- Grade 3 or higher treatment-related adverse events occurred in 189 patients (63.4%) in the camrelizumab group and 201 patients (67.7%) in the placebo group. Treatment-related deaths occurred in 9 patients (3.0%) in the camrelizumab group and 11 patients (3.7%) in the placebo group.
Camrelizumab is only approved for use in China. - In KEYNOTE-590 (NCT03189719), a double-blind, placebo-controlled, randomized, phase III trial, 1,020 patients with previously untreated, locally advanced, unresectable, or metastatic esophageal cancer or Siewert type 1 gastroesophageal junction cancer (regardless of PD-L1 status) were screened.[46] A total of 749 patients were randomly assigned (1:1) to receive either pembrolizumab (200 mg) or placebo plus chemotherapy (5-FU at 800 mg/m2 on days 1–5 and cisplatin 80 mg/m2 on day 1 [for up to 6 cycles]). Treatment was repeated once every 3 weeks for up to 35 cycles. The primary end points were: (1) OS in patients with esophageal squamous cell carcinoma and a PD-L1 combined positive score (CPS) of 10 or more, and (2) OS and PFS in patients with esophageal squamous cell carcinoma, patients with a PD-L1 CPS of 10 or more irrespective of the histology, and all randomized patients. A total of 548 enrolled patients (73%) had squamous cell histology, 286 of whom (52%) had a CPS of 10 or more.
- At the first interim analysis (after a median follow-up of 22.6 months), patients who received pembrolizumab plus chemotherapy had superior OS compared with patients who received placebo plus chemotherapy for each of the following subgroups:[46][Level of evidence A1]
- Patients with esophageal squamous cell carcinoma and a PD-L1 CPS of 10 or more (median OS, 13.9 months vs. 8.8 months; HR, 0.57; 95% CI, 0.43–0.75; P < .0001).
- Patients with esophageal squamous cell carcinoma (OS, 12.6 months vs. 9.8 months; HR, 0.72; 95% CI, 0.60–0.88; P = .0006).
- Patients with a PD-L1 CPS of 10 or more (OS, 13.5 months vs. 9.4 months; HR, 0.62; 95% CI, 0.49–0.78; P < .0001).
- All randomized patients (OS, 12.4 months vs. 9.8 months; HR, 0.73; 95% CI, 0.62–0.86; P < .0001).
- The 24-month OS rate in each of the above subgroups was double in the pembrolizumab and chemotherapy group compared with the placebo and chemotherapy group (approximately 30% vs. 15%).
- Grade 3 or higher treatment-related adverse events occurred in 266 patients (72%) in the pembrolizumab group and 250 patients (68%) in the placebo group.
Notably, in an exploratory analysis in patients with a PD-L1 CPS of less than 10, median OS was 10.5 months in the pembrolizumab and chemotherapy group versus 10.6 months in the placebo and chemotherapy group (HR, 0.86; 95% CI, 0.68–1.10).
Immunotherapy and chemoimmunotherapy for patients with adenocarcinoma
In April 2021, the U.S. Food and Drug Administration approved nivolumab in combination with fluoropyrimidine- and platinum-containing chemotherapy for patients with advanced or metastatic gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma after the publication of the results of the CheckMate-649 trial.[47]
Evidence (immunotherapy and chemoimmunotherapy for patients with adenocarcinoma):
- In a randomized, open-label, international, phase III study (CheckMate-649 [NCT02872116]), 1,581 patients (including 955 with a PD-L1 CPS ≥5) with HER2-negative gastric, gastroesophageal junction, or esophageal adenocarcinomas were randomly assigned (1:1:1) (1:1 after enrollment in the nivolumab-plus-ipilimumab group was closed). A total of 789 patients (473 with a PD-L1 CPS ≥5) were assigned to receive nivolumab and chemotherapy (nivolumab 360 mg with capecitabine and oxaliplatin every 3 weeks, or nivolumab 240 mg with FOLFOX every 2 weeks). A total of 792 patients (482 with a PD-L1 CPS ≥5) were assigned to receive chemotherapy alone (capecitabine and oxaliplatin every 3 weeks or FOLFOX every 2 weeks). Disease site of origin was as follows: 1,100 patients (70%) had gastric adenocarcinoma, 260 patients (17%) had gastroesophageal junction carcinoma, and 211 patients (13%) had esophageal adenocarcinoma.[47]
- The median follow-up for OS was 13.1 months (interquartile range [IQR], 6.7–19.1) for the nivolumab-plus-chemotherapy group and 11.1 months (IQR, 5.8–16.1) for the chemotherapy-alone group. Nivolumab plus chemotherapy resulted in improvements in OS (14.4 vs. 11.1 months; HR, 0.71; 98.4% CI, 0.59–0.86; P < .0001) and PFS (HR, 0.68; 98% CI, 0.56–0.81; P < .0001) compared with chemotherapy alone in patients with a PD-L1 CPS of 5 or more (minimum follow-up, 12.1 months).[47][Level of evidence A1]
- The unstratified HROS for patients with a PD-L1 CPS of less than 1 who received nivolumab plus chemotherapy versus chemotherapy alone was 0.92 (95% CI, 0.70–1.23). For patients with a PD-L1 CPS of less than 5, the HR was 0.94 (0.78–1.13).
- Grades 3 and 4 adverse events occurred in 462 patients (59%) in the nivolumab group and in 341 patients (44%) in the chemotherapy-alone group.
- In KEYNOTE-590 (NCT03189719), patients with esophageal cancer or gastroesophageal junction cancer received either pembrolizumab or placebo plus chemotherapy. A total of 221 patients (27%) had adenocarcinoma histology. For study details, see the Immunotherapy and chemoimmunotherapy for patients with squamous cell carcinoma section.[46]
- The role of nivolumab in combination with chemotherapy was also assessed in ATTRACTION-4 (NCT02746796), a randomized, multicenter, double-blind, placebo-controlled, phase III trial. The study enrolled patients with previously untreated (except for neoadjuvant or adjuvant chemotherapy completed ≥180 days before recurrence), HER2-negative, unresectable, advanced or recurrent gastric or gastroesophageal junction cancer, regardless of PD-L1 expression. Only 9% of enrolled patients had gastroesophageal junction cancer. Patients were randomly assigned (1:1) to receive chemotherapy every 3 weeks (oxaliplatin 130 mg/m² IV on day 1 plus either oral S-1 40 mg/m² or oral capecitabine 1,000 mg/m², twice daily on days 1–14), in addition to either 360 mg nivolumab IV or placebo. The trial was conducted in Japan, South Korea, and Taiwan, and assigned 724 patients to treatment: 362 patients to the nivolumab plus chemotherapy group and 362 to the placebo plus chemotherapy group. The primary end points were centrally assessed PFS and OS in the intention-to-treat population.[48]
- At the time of data cutoff, with a median follow-up of 11.6 months, the median PFS was 10.45 months (95% CI, 8.44–14.75) in the nivolumab plus-chemotherapy group and 8.34 months (6.97–9.40) in the placebo plus-chemotherapy group (HR, 0.68; 98.51% CI, 0.51–0.90; P = .0007).[48][Level of evidence B1]
- At the time of data cutoff, with a median follow-up of 26.6 months, the median OS at the final analysis was 17.45 months (95% CI, 15.67–20.83) in the nivolumab-plus-chemotherapy group and 17.15 months (15.18–19.65) in the placebo-plus-chemotherapy group (HR, 0.90; 95% CI, 0.75–1.08; P = .26).[48][Level of evidence A1]
- The most common treatment-related grade 3 or 4 adverse events were decreased neutrophil count (observed in 20% of patients in the nivolumab-plus-chemotherapy group vs. 16% of patients in the placebo-plus-chemotherapy group) and decreased platelet count (observed in 9% of patients in both groups).
- Six treatment-related deaths occurred: three in the nivolumab-plus-chemotherapy group (one each of febrile neutropenia, hepatic failure, and sudden death) and three in the placebo-plus-chemotherapy group (one each of sepsis, hemolytic anemia, and interstitial lung disease).
- Tumor PD-L1 expression was ≥1% in 16% of patients randomized to the nivolumab arm and 15% of patients randomized to the placebo arm.
Immunotherapy for patients who relapse after one prior line of standard therapy
Pembrolizumab or nivolumab can be given to patients with esophageal cancer who were previously treated with a chemotherapy regimen that did not include an immune checkpoint inhibitor. Pembrolizumab is appropriate for patients with squamous or adenocarcinoma histology and a CPS of 10 or more. Nivolumab can be given to patients with squamous or adenosquamous histology, regardless of PD-L1 expression.
Evidence (immunotherapy for patients who relapse after one prior line of standard therapy):
- The open-label phase II KEYNOTE-181 study (NCT02564263) included 628 patients with advanced or metastatic squamous cell carcinoma (n = 401) or adenocarcinoma (n = 227) of the esophagus that progressed after one prior therapy. Patients were randomly assigned (1:1) to receive either pembrolizumab (200 mg every 3 weeks for up to 2 years) or chemotherapy (investigator’s choice of paclitaxel, docetaxel, or irinotecan). Primary end points were OS in patients with a PD-L1 CPS of 10 or more, OS in patients with squamous cell carcinoma, and OS in all patients (one-sided alpha, 0.9%, 0.8%, and 0.8%, respectively).[49]
- At final analysis, conducted 16 months after the last patient was randomly assigned, OS was prolonged in patients with a CPS of 10 or more who received pembrolizumab versus chemotherapy (median OS, 9.3 vs. 6.7 months; HR, 0.69; 95% CI, 0.52–0.93; P = .0074).[49][Level of evidence A1]
- Among patients with squamous cell carcinoma, the median OS was 8.2 months for patients who received pembrolizumab and 7.1 months for patients who received chemotherapy (HR, 0.78; 95% CI, 0.63–0.96; P = .0095). Among all patients, the median OS was 7.1 months for patients who received pembrolizumab and 7.1 months for patients who received chemotherapy (HR, 0.89; 95% CI, 0.75–1.05; P = .0560).
- Grade 3 to 5 treatment-related adverse events occurred in 18.2% of patients who received pembrolizumab and 40.9% of patients who received chemotherapy.
- Patients in this study had not received prior immunotherapy.
- The ATTRACTION-3 study (NCT02569242), a multicenter, randomized, open-label, phase III trial, included 419 patients with squamous or adenosquamous esophageal cancer refractory or intolerant to one previous fluoropyrimidine-based and platinum-based chemotherapy. Patients were randomly assigned (1:1) to receive either nivolumab (240 mg every 2 weeks) (n = 210) or investigator's choice of chemotherapy (weekly paclitaxel or docetaxel every 3 weeks) (n = 209). The primary end point was OS.[50]
- At a follow-up time of 17.6 months, OS was significantly improved in the nivolumab group compared with the chemotherapy group (median OS, 10.9 months [95% CI, 9.2–13.3] vs. 8.4 months [95% CI, 7.2–9.9]) (HRdeath, 0.77; 95% CI, 0.62–0.96; P = .019).[50][Level of evidence A1]
- Grade 3 or 4 treatment-related adverse events occurred in 38 of 209 patients (18%) in the nivolumab group and 131 of 208 patients (63%) in the chemotherapy group.
- The most frequent grade 3 or 4 treatment-related adverse events were anemia (4 patients [2%]) in the nivolumab group and decreased neutrophil count (59 patients [28%]) in the chemotherapy group. Five deaths were treatment-related: two in the nivolumab group (one of interstitial lung disease and one of pneumonitis) and three in the chemotherapy group (one of pneumonia, one of spinal cord abscess, and one of interstitial lung disease).
Postoperative Radiation Therapy
Two randomized trials have shown no significant OS benefit for postoperative radiation therapy compared with surgery alone.[51,52][Level of evidence A1] All newly diagnosed patients should be considered candidates for therapies and clinical trials comparing various treatment modalities. Information about ongoing clinical trials is available from the NCI website.
Capecitabine and Fluorouracil Dosing
The DPYD gene encodes an enzyme that catabolizes pyrimidines and fluoropyrimidines, like capecitabine and fluorouracil. An estimated 1% to 2% of the population has germline pathogenic variants in DPYD, which lead to reduced DPD protein function and an accumulation of pyrimidines and fluoropyrimidines in the body.[53,54] Patients with the DPYD*2A variant who receive fluoropyrimidines may experience severe, life-threatening toxicities that are sometimes fatal. Many other DPYD variants have been identified, with a range of clinical effects.[53-55] Fluoropyrimidine avoidance or a dose reduction of 50% may be recommended based on the patient's DPYD genotype and number of functioning DPYD alleles.[56-58] DPYD genetic testing costs less than $200, but insurance coverage varies due to a lack of national guidelines.[59] In addition, testing may delay therapy by 2 weeks, which would not be advisable in urgent situations. This controversial issue requires further evaluation.[60]References
- Baron TH: Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 344 (22): 1681-7, 2001. [PubMed: 11386268]
- Javle M, Ailawadhi S, Yang GY, et al.: Palliation of malignant dysphagia in esophageal cancer: a literature-based review. J Support Oncol 4 (8): 365-73, 379, 2006. [PubMed: 17004508]
- Doosti-Irani A, Mansournia MA, Rahimi-Foroushani A, et al.: Complications of stent placement in patients with esophageal cancer: A systematic review and network meta-analysis. PLoS One 12 (10): e0184784, 2017. [PMC free article: PMC5624586] [PubMed: 28968416]
- Fuccio L, Scagliarini M, Frazzoni L, et al.: Development of a prediction model of adverse events after stent placement for esophageal cancer. Gastrointest Endosc 83 (4): 746-52, 2016. [PubMed: 26344881]
- Litle VR, Luketich JD, Christie NA, et al.: Photodynamic therapy as palliation for esophageal cancer: experience in 215 patients. Ann Thorac Surg 76 (5): 1687-92; discussion 1692-3, 2003. [PubMed: 14602313]
- Katsoulis IE, Karoon A, Mylvaganam S, et al.: Endoscopic palliation of malignant dysphagia: a challenging task in inoperable oesophageal cancer. World J Surg Oncol 4: 38, 2006. [PMC free article: PMC1540418] [PubMed: 16820062]
- Lerut T, Coosemans W, Van Raemdonck D, et al.: Surgical treatment of Barrett's carcinoma. Correlations between morphologic findings and prognosis. J Thorac Cardiovasc Surg 107 (4): 1059-65; discussion 1065-6, 1994. [PubMed: 8159027]
- Kelsen DP, Bains M, Burt M: Neoadjuvant chemotherapy and surgery of cancer of the esophagus. Semin Surg Oncol 6 (5): 268-73, 1990. [PubMed: 2237085]
- Saxon RR, Morrison KE, Lakin PC, et al.: Malignant esophageal obstruction and esophagorespiratory fistula: palliation with a polyethylene-covered Z-stent. Radiology 202 (2): 349-54, 1997. [PubMed: 9015055]
- Campbell WR, Taylor SA, Pierce GE, et al.: Therapeutic alternatives in patients with esophageal cancer. Am J Surg 150 (6): 665-8, 1985. [PubMed: 4073357]
- Mellow MH, Pinkas H: Endoscopic therapy for esophageal carcinoma with Nd:YAG laser: prospective evaluation of efficacy, complications, and survival. Gastrointest Endosc 30 (6): 334-9, 1984. [PubMed: 6210226]
- Karlin DA, Fisher RS, Krevsky B: Prolonged survival and effective palliation in patients with squamous cell carcinoma of the esophagus following endoscopic laser therapy. Cancer 59 (11): 1969-72, 1987. [PubMed: 2436743]
- Hulscher JB, van Sandick JW, de Boer AG, et al.: Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 347 (21): 1662-9, 2002. [PubMed: 12444180]
- de Boer AG, van Lanschot JJ, van Sandick JW, et al.: Quality of life after transhiatal compared with extended transthoracic resection for adenocarcinoma of the esophagus. J Clin Oncol 22 (20): 4202-8, 2004. [PubMed: 15483031]
- Santillan AA, Farma JM, Meredith KL, et al.: Minimally invasive surgery for esophageal cancer. J Natl Compr Canc Netw 6 (9): 879-84, 2008. [PubMed: 18926097]
- National Cancer Institute: SEER Cancer Stat Facts: Esophageal Cancer. Bethesda, Md: National Cancer Institute. Available online. Last accessed August 9, 2024.
- Ellis FH, Williamson WA, Heatley GJ: Cancer of the esophagus and cardia: does age influence treatment selection and surgical outcomes? J Am Coll Surg 187 (4): 345-51, 1998. [PubMed: 9783779]
- Hofheinz RD, Merx K, Haag GM, et al.: FLOT Versus FLOT/Trastuzumab/Pertuzumab Perioperative Therapy of Human Epidermal Growth Factor Receptor 2-Positive Resectable Esophagogastric Adenocarcinoma: A Randomized Phase II Trial of the AIO EGA Study Group. J Clin Oncol 40 (32): 3750-3761, 2022. [PubMed: 35709415]
- Bosset JF, Gignoux M, Triboulet JP, et al.: Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 337 (3): 161-7, 1997. [PubMed: 9219702]
- Walsh TN, Noonan N, Hollywood D, et al.: A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 335 (7): 462-7, 1996. [PubMed: 8672151]
- Urba SG, Orringer MB, Turrisi A, et al.: Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 19 (2): 305-13, 2001. [PubMed: 11208820]
- Tepper J, Krasna MJ, Niedzwiecki D, et al.: Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 26 (7): 1086-92, 2008. [PMC free article: PMC5126644] [PubMed: 18309943]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al.: Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366 (22): 2074-84, 2012. [PubMed: 22646630]
- Oppedijk V, van der Gaast A, van Lanschot JJ, et al.: Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 32 (5): 385-91, 2014. [PubMed: 24419108]
- Mariette C, Dahan L, Mornex F, et al.: Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 32 (23): 2416-22, 2014. [PubMed: 24982463]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al.: Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 16 (9): 1090-8, 2015. [PubMed: 26254683]
- Safran HP, Winter K, Ilson DH, et al.: Trastuzumab with trimodality treatment for oesophageal adenocarcinoma with HER2 overexpression (NRG Oncology/RTOG 1010): a multicentre, randomised, phase 3 trial. Lancet Oncol 23 (2): 259-269, 2022. [PMC free article: PMC8903071] [PubMed: 35038433]
- Medical Research Council Oesophageal Cancer Working Group: Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359 (9319): 1727-33, 2002. [PubMed: 12049861]
- Ando N, Kato H, Igaki H, et al.: A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19 (1): 68-74, 2012. [PubMed: 21879261]
- Ychou M, Boige V, Pignon JP, et al.: Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29 (13): 1715-21, 2011. [PubMed: 21444866]
- Kelsen DP, Ginsberg R, Pajak TF, et al.: Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 339 (27): 1979-84, 1998. [PubMed: 9869669]
- Stahl M, Walz MK, Stuschke M, et al.: Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 27 (6): 851-6, 2009. [PubMed: 19139439]
- Cunningham D, Allum WH, Stenning SP, et al.: Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355 (1): 11-20, 2006. [PubMed: 16822992]
- Al-Batran SE, Homann N, Pauligk C, et al.: Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 393 (10184): 1948-1957, 2019. [PubMed: 30982686]
- Hoeppner J, Brunner T, Lordick F, et al.: Prospective randomized multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (ESOPEC trial). [Abstract] J Clin Oncol 42 (Suppl 17): A-LBA1, 2024. [PMC free article: PMC4952147] [PubMed: 27435280]
- Cooper JS, Guo MD, Herskovic A, et al.: Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 281 (17): 1623-7, 1999. [PubMed: 10235156]
- Minsky BD, Pajak TF, Ginsberg RJ, et al.: INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 20 (5): 1167-74, 2002. [PubMed: 11870157]
- Stahl M, Stuschke M, Lehmann N, et al.: Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 23 (10): 2310-7, 2005. [PubMed: 15800321]
- Bedenne L, Michel P, Bouché O, et al.: Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 25 (10): 1160-8, 2007. [PubMed: 17401004]
- Smith TJ, Ryan LM, Douglass HO, et al.: Combined chemoradiotherapy vs. radiotherapy alone for early stage squamous cell carcinoma of the esophagus: a study of the Eastern Cooperative Oncology Group. Int J Radiat Oncol Biol Phys 42 (2): 269-76, 1998. [PubMed: 9788404]
- Conroy T, Galais MP, Raoul JL, et al.: Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol 15 (3): 305-14, 2014. [PubMed: 24556041]
- Kelly RJ, Ajani JA, Kuzdzal J, et al.: Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 384 (13): 1191-1203, 2021. [PubMed: 33789008]
- Ilson DH: Adjuvant Nivolumab in Esophageal Cancer - A New Standard of Care. N Engl J Med 384 (13): 1269-1271, 2021. [PubMed: 33789017]
- Doki Y, Ajani JA, Kato K, et al.: Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 386 (5): 449-462, 2022. [PubMed: 35108470]
- Luo H, Lu J, Bai Y, et al.: Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 326 (10): 916-925, 2021. [PMC free article: PMC8441593] [PubMed: 34519801]
- Sun JM, Shen L, Shah MA, et al.: Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398 (10302): 759-771, 2021. [PubMed: 34454674]
- Janjigian YY, Shitara K, Moehler M, et al.: First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398 (10294): 27-40, 2021. [PMC free article: PMC8436782] [PubMed: 34102137]
- Kang YK, Chen LT, Ryu MH, et al.: Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 23 (2): 234-247, 2022. [PubMed: 35030335]
- Kojima T, Shah MA, Muro K, et al.: Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 38 (35): 4138-4148, 2020. [PubMed: 33026938]
- Kato K, Cho BC, Takahashi M, et al.: Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20 (11): 1506-1517, 2019. [PubMed: 31582355]
- Ténière P, Hay JM, Fingerhut A, et al.: Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet 173 (2): 123-30, 1991. [PubMed: 1925862]
- Fok M, Sham JS, Choy D, et al.: Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 113 (2): 138-47, 1993. [PubMed: 8430362]
- Sharma BB, Rai K, Blunt H, et al.: Pathogenic DPYD Variants and Treatment-Related Mortality in Patients Receiving Fluoropyrimidine Chemotherapy: A Systematic Review and Meta-Analysis. Oncologist 26 (12): 1008-1016, 2021. [PMC free article: PMC8649021] [PubMed: 34506675]
- Lam SW, Guchelaar HJ, Boven E: The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat Rev 50: 9-22, 2016. [PubMed: 27569869]
- Shakeel F, Fang F, Kwon JW, et al.: Patients carrying DPYD variant alleles have increased risk of severe toxicity and related treatment modifications during fluoropyrimidine chemotherapy. Pharmacogenomics 22 (3): 145-155, 2021. [PubMed: 33410339]
- Amstutz U, Henricks LM, Offer SM, et al.: Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103 (2): 210-216, 2018. [PMC free article: PMC5760397] [PubMed: 29152729]
- Henricks LM, Lunenburg CATC, de Man FM, et al.: DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: a prospective safety analysis. Lancet Oncol 19 (11): 1459-1467, 2018. [PubMed: 30348537]
- Lau-Min KS, Varughese LA, Nelson MN, et al.: Preemptive pharmacogenetic testing to guide chemotherapy dosing in patients with gastrointestinal malignancies: a qualitative study of barriers to implementation. BMC Cancer 22 (1): 47, 2022. [PMC free article: PMC8742388] [PubMed: 34996412]
- Brooks GA, Tapp S, Daly AT, et al.: Cost-effectiveness of DPYD Genotyping Prior to Fluoropyrimidine-based Adjuvant Chemotherapy for Colon Cancer. Clin Colorectal Cancer 21 (3): e189-e195, 2022. [PMC free article: PMC10496767] [PubMed: 35668003]
- Baker SD, Bates SE, Brooks GA, et al.: DPYD Testing: Time to Put Patient Safety First. J Clin Oncol 41 (15): 2701-2705, 2023. [PMC free article: PMC10414691] [PubMed: 36821823]
Treatment of Stage 0 Esophageal Cancer
Treatment Options for Stage 0 Esophageal Cancer
Stage 0 squamous cell esophageal cancer is rarely seen in the United States, but surgery has been used. For early-stage minimally invasive esophageal cancer, surgical and endoscopic techniques offer high rates of cure.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Rusch VW, Levine DS, Haggitt R, et al.: The management of high grade dysplasia and early cancer in Barrett's esophagus. A multidisciplinary problem. Cancer 74 (4): 1225-9, 1994. [PubMed: 8055442]
- Heitmiller RF, Redmond M, Hamilton SR: Barrett's esophagus with high-grade dysplasia. An indication for prophylactic esophagectomy. Ann Surg 224 (1): 66-71, 1996. [PMC free article: PMC1235248] [PubMed: 8678620]
- Pech O, Bollschweiler E, Manner H, et al.: Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett's esophagus at two high-volume centers. Ann Surg 254 (1): 67-72, 2011. [PubMed: 21532466]
- Prasad GA, Wu TT, Wigle DA, et al.: Endoscopic and surgical treatment of mucosal (T1a) esophageal adenocarcinoma in Barrett's esophagus. Gastroenterology 137 (3): 815-23, 2009. [PMC free article: PMC3815672] [PubMed: 19524578]
Treatment of Stage I Esophageal Cancer
Treatment Options for Stage I Esophageal Cancer
Treatment options for stage I esophageal cancer include the following:
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Bosset JF, Gignoux M, Triboulet JP, et al.: Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 337 (3): 161-7, 1997. [PubMed: 9219702]
- Walsh TN, Noonan N, Hollywood D, et al.: A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 335 (7): 462-7, 1996. [PubMed: 8672151]
- Urba SG, Orringer MB, Turrisi A, et al.: Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 19 (2): 305-13, 2001. [PubMed: 11208820]
- Tepper J, Krasna MJ, Niedzwiecki D, et al.: Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 26 (7): 1086-92, 2008. [PMC free article: PMC5126644] [PubMed: 18309943]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al.: Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366 (22): 2074-84, 2012. [PubMed: 22646630]
- Conroy T, Galais MP, Raoul JL, et al.: Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol 15 (3): 305-14, 2014. [PubMed: 24556041]
- Mariette C, Dahan L, Mornex F, et al.: Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 32 (23): 2416-22, 2014. [PubMed: 24982463]
Treatment of Stage II Esophageal Cancer
Treatment Options for Stage II Esophageal Cancer
Treatment options for stage II esophageal cancer include the following:
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Walsh TN, Noonan N, Hollywood D, et al.: A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 335 (7): 462-7, 1996. [PubMed: 8672151]
- Tepper J, Krasna MJ, Niedzwiecki D, et al.: Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 26 (7): 1086-92, 2008. [PMC free article: PMC5126644] [PubMed: 18309943]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al.: Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366 (22): 2074-84, 2012. [PubMed: 22646630]
- Urba SG, Orringer MB, Turrisi A, et al.: Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 19 (2): 305-13, 2001. [PubMed: 11208820]
- Bosset JF, Gignoux M, Triboulet JP, et al.: Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 337 (3): 161-7, 1997. [PubMed: 9219702]
- Conroy T, Galais MP, Raoul JL, et al.: Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol 15 (3): 305-14, 2014. [PubMed: 24556041]
- Mariette C, Dahan L, Mornex F, et al.: Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 32 (23): 2416-22, 2014. [PubMed: 24982463]
- Medical Research Council Oesophageal Cancer Working Group: Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359 (9319): 1727-33, 2002. [PubMed: 12049861]
- Ando N, Kato H, Igaki H, et al.: A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19 (1): 68-74, 2012. [PubMed: 21879261]
- Ychou M, Boige V, Pignon JP, et al.: Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29 (13): 1715-21, 2011. [PubMed: 21444866]
- Stahl M, Stuschke M, Lehmann N, et al.: Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 23 (10): 2310-7, 2005. [PubMed: 15800321]
- Bedenne L, Michel P, Bouché O, et al.: Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 25 (10): 1160-8, 2007. [PubMed: 17401004]
Treatment of Stage III Esophageal Cancer
Treatment Options for Stage III Esophageal Cancer
Treatment options for stage III esophageal cancer include the following:
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Walsh TN, Noonan N, Hollywood D, et al.: A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 335 (7): 462-7, 1996. [PubMed: 8672151]
- Tepper J, Krasna MJ, Niedzwiecki D, et al.: Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 26 (7): 1086-92, 2008. [PMC free article: PMC5126644] [PubMed: 18309943]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al.: Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 366 (22): 2074-84, 2012. [PubMed: 22646630]
- Medical Research Council Oesophageal Cancer Working Group: Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359 (9319): 1727-33, 2002. [PubMed: 12049861]
- Ando N, Kato H, Igaki H, et al.: A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19 (1): 68-74, 2012. [PubMed: 21879261]
- Ychou M, Boige V, Pignon JP, et al.: Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 29 (13): 1715-21, 2011. [PubMed: 21444866]
- Conroy T, Galais MP, Raoul JL, et al.: Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol 15 (3): 305-14, 2014. [PubMed: 24556041]
- Stahl M, Stuschke M, Lehmann N, et al.: Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 23 (10): 2310-7, 2005. [PubMed: 15800321]
- Bedenne L, Michel P, Bouché O, et al.: Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 25 (10): 1160-8, 2007. [PubMed: 17401004]
Treatment of Stage IV Esophageal Cancer
Treatment Options for Stage IV Esophageal Cancer
At diagnosis, approximately 50% of patients with esophageal cancer have metastatic disease and are candidates for palliative therapy.[1]
Treatment options for stage IV esophageal cancer include the following:
- Chemoradiation therapy followed by surgery (for patients with stage IVA disease).
- Endoscopic-placed stents to provide palliation of dysphagia.[15]
- Radiation therapy with or without intraluminal intubation and dilation.
- Clinical trials evaluating single-agent or combination chemotherapy.
The treatment of patients with human epidermal growth factor receptor 2 (HER2)-negative, locally advanced, inoperable, recurrent, or metastatic esophageal cancer has improved with the introduction of chemoimmunotherapy as front-line therapy and immunotherapy for patients who relapse following one prior line of chemotherapy. First-line chemoimmunotherapy can now be considered the standard of care for patients with advanced esophageal cancer of squamous or adenocarcinoma histology and programmed death-ligand 1 (PD-L1) expression (although the optimal cutoff still needs to be defined). For more information, see the Immunotherapy and Chemoimmunotherapy section.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Enzinger PC, Ilson DH, Kelsen DP: Chemotherapy in esophageal cancer. Semin Oncol 26 (5 Suppl 15): 12-20, 1999. [PubMed: 10566606]
- Waters JS, Norman A, Cunningham D, et al.: Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: results of a randomized trial. Br J Cancer 80 (1-2): 269-72, 1999. [PMC free article: PMC2363002] [PubMed: 10390007]
- Ross P, Nicolson M, Cunningham D, et al.: Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) With epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 20 (8): 1996-2004, 2002. [PubMed: 11956258]
- Taïeb J, Artru P, Baujat B, et al.: Optimisation of 5-fluorouracil (5-FU)/cisplatin combination chemotherapy with a new schedule of hydroxyurea, leucovorin, 5-FU and cisplatin (HLFP regimen) for metastatic oesophageal cancer. Eur J Cancer 38 (5): 661-6, 2002. [PubMed: 11916548]
- Kelly RJ, Ajani JA, Kuzdzal J, et al.: Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N Engl J Med 384 (13): 1191-1203, 2021. [PubMed: 33789008]
- Doki Y, Ajani JA, Kato K, et al.: Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 386 (5): 449-462, 2022. [PubMed: 35108470]
- Luo H, Lu J, Bai Y, et al.: Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 326 (10): 916-925, 2021. [PMC free article: PMC8441593] [PubMed: 34519801]
- Sun JM, Shen L, Shah MA, et al.: Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398 (10302): 759-771, 2021. [PubMed: 34454674]
- Janjigian YY, Shitara K, Moehler M, et al.: First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398 (10294): 27-40, 2021. [PMC free article: PMC8436782] [PubMed: 34102137]
- Kang YK, Chen LT, Ryu MH, et al.: Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 23 (2): 234-247, 2022. [PubMed: 35030335]
- Kojima T, Shah MA, Muro K, et al.: Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol 38 (35): 4138-4148, 2020. [PubMed: 33026938]
- Kato K, Cho BC, Takahashi M, et al.: Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 20 (11): 1506-1517, 2019. [PubMed: 31582355]
- Javle M, Ailawadhi S, Yang GY, et al.: Palliation of malignant dysphagia in esophageal cancer: a literature-based review. J Support Oncol 4 (8): 365-73, 379, 2006. [PubMed: 17004508]
- Katsoulis IE, Karoon A, Mylvaganam S, et al.: Endoscopic palliation of malignant dysphagia: a challenging task in inoperable oesophageal cancer. World J Surg Oncol 4: 38, 2006. [PMC free article: PMC1540418] [PubMed: 16820062]
- Baron TH: Expandable metal stents for the treatment of cancerous obstruction of the gastrointestinal tract. N Engl J Med 344 (22): 1681-7, 2001. [PubMed: 11386268]
- Sur RK, Levin CV, Donde B, et al.: Prospective randomized trial of HDR brachytherapy as a sole modality in palliation of advanced esophageal carcinoma--an International Atomic Energy Agency study. Int J Radiat Oncol Biol Phys 53 (1): 127-33, 2002. [PubMed: 12007950]
- Gaspar LE, Nag S, Herskovic A, et al.: American Brachytherapy Society (ABS) consensus guidelines for brachytherapy of esophageal cancer. Clinical Research Committee, American Brachytherapy Society, Philadelphia, PA. Int J Radiat Oncol Biol Phys 38 (1): 127-32, 1997. [PubMed: 9212013]
Treatment of Recurrent Esophageal Cancer
Treatment Options for Recurrent Esophageal Cancer
Palliation presents difficult problems for all patients with recurrent esophageal cancer. All patients should be considered candidates for clinical trials as outlined in the Treatment Option Overview for Esophageal Cancer section of this summary.
Treatment options for recurrent esophageal cancer include the following:
- Palliative use of any of the other therapies, including supportive care.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Doki Y, Ajani JA, Kato K, et al.: Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N Engl J Med 386 (5): 449-462, 2022. [PubMed: 35108470]
- Luo H, Lu J, Bai Y, et al.: Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA 326 (10): 916-925, 2021. [PMC free article: PMC8441593] [PubMed: 34519801]
- Sun JM, Shen L, Shah MA, et al.: Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet 398 (10302): 759-771, 2021. [PubMed: 34454674]
Latest Updates to This Summary (08/09/2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
General Information About Esophageal Cancer
Revised text about the 5-year relative survival rate of patients with esophageal cancer.
Treatment Option Overview for Esophageal Cancer
Added Perioperative Chemotherapy as a new subsection.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about treatment of adult esophageal cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Esophageal Cancer Treatment are:
- Andrea Bonetti, MD (Azienda ULSS 9 of the Veneto Region)
- Amit Chowdhry, MD, PhD (University of Rochester Medical Center)
- Leon Pappas, MD, PhD (Dana-Farber Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Esophageal Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/esophageal/hp/esophageal-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389338]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
- General Information About Esophageal Cancer
- Cellular Classification of Esophageal Cancer
- Stage Information for Esophageal Cancer
- Treatment Option Overview for Esophageal Cancer
- Treatment of Stage 0 Esophageal Cancer
- Treatment of Stage I Esophageal Cancer
- Treatment of Stage II Esophageal Cancer
- Treatment of Stage III Esophageal Cancer
- Treatment of Stage IV Esophageal Cancer
- Treatment of Recurrent Esophageal Cancer
- Latest Updates to This Summary (08/09/2024)
- About This PDQ Summary
- Review Childhood Esophageal Cancer Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Childhood Esophageal Cancer Treatment (PDQ®): Health Professional Version.PDQ Pediatric Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Esophageal Cancer Prevention (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Esophageal Cancer Prevention (PDQ®): Health Professional Version.PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Esophageal Cancer Screening (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Esophageal Cancer Screening (PDQ®): Health Professional Version.PDQ Screening and Prevention Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Prostate Cancer Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Prostate Cancer Treatment (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Review Bladder Cancer Treatment (PDQ®): Health Professional Version.[PDQ Cancer Information Summari...]Review Bladder Cancer Treatment (PDQ®): Health Professional Version.PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. 2002
- Esophageal Cancer Treatment (PDQ®) - PDQ Cancer Information SummariesEsophageal Cancer Treatment (PDQ®) - PDQ Cancer Information Summaries
- Streptococcus sp. CSL10205-OR2Streptococcus sp. CSL10205-OR2Streptococcus sp. CSL10205-OR2 Genome sequencing and assemblyBioProject
Your browsing activity is empty.
Activity recording is turned off.
See more...