Lentiviruses
The defining features of lentiviruses—virion structure and composition, genetic endowment, and replication strategy—have been described in several chapters in this volume. Lentiviruses are distinguished from other retroviruses by their unusual morphological characteristics (e.g., a cylindrical or cone-shaped nucleoid in the mature virion); by several genes, some known to have regulatory roles (e.g., tat and rev), that are not present in other retroviral genomes; and by a biphasic course of viral gene expression. All of the HIVs and the SIVs exhibit these general characteristics, encode six auxiliary genes (tat, rev, nef, vpr, vif, and vpu, or vpx), and all infect cells using CD4 as the primary cell surface receptor. Primate lentiviruses exhibit strong tropism for CD4+ cells, including lymphocytes and macrophages (as well as some other cell types), and a capacity to persist and replicate in the face of humoral and cellular immune responses.
HIV: Distribution, Grouping, Transmission, and Epidemiology
Distribution and Grouping
Of the two distinct subtypes of HIV, HIV-1 is predominant and found throughout the world, whereas HIV-2 has been isolated primarily in West African countries such as Guinea Bissau, Ivory Coast, and Senegal, with some cases also identified in the Americas and western Europe. Both agents are associated with the development of progressive immunologic deterioration. However, epidemiologic studies suggest that the incubation period for HIV-2 for the development of disease is longer than for HIV-1 (Pepin et al. 1991). In addition, HIV-2 is not as easily transmitted perinatally as HIV-1 (Markovitz 1993). Because HIV-1 has been used as the prototype in the majority of studies on HIV pathogenesis and is the major cause of AIDS (acquired immunodeficiency syndrome) in humans, this chapter focuses primarily on HIV-1 and notes significant differences in the behavior of the two viruses.
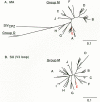
Considerable effort has been spent collecting and comparing nucleotide sequences of HIV isolates from around the world; these sequences have been collected in a specialized database (Myers et al. 1994). This information has been used to infer the molecular epidemiology of the virus. The earliest phylogenetic trees of HIV revealed two clusters of HIV-1: North American/European isolates and African isolates. Later, analyses that included isolates from Thailand revealed yet another HIV-1 cluster spreading rapidly in the northern part of the country (McCutchan et al. 1992). With the sequencing of additional HIV-1 isolates from around the world,two major groups have been identified (Fig. 1). Viruses of group M (for “main”) are responsible for the majority of infections worldwide; group O (for “outgroup”) is a relatively rare group currently found in Cameroon, Gabon, and France. Group M can be divided into at least eight distinct subtypes or clades (A through H) (Myers et al. 1994; Louwagie et al. 1995). Thus, variation in HIV-1 sequences is not continuous over a spectrum, but instead appears to cluster into discrete groupings (Fig. 1). Isolates of HIV-1 from different clades may differ by 30–40% in the amino acid sequence of the gp120 SU protein; isolates within a clade vary from 5% to 20%. It is not clear whether this pattern has resulted from multiple introductions of virus into humans or from discrete pathways of evolution or adaptation after the virus entered the human population. Two chimpanzee isolates occupy a position in the phylogenetic tree intermediate between the M and O group HIVs (Fig. 1), suggesting that the two principal groupings of HIV-1 represent separate introductions (see later). The different clades of HIV-1 are not distributed evenly throughout the world. For example, clade B predominates in North America and Europe and clade E predominates in northern Thailand (Fig. 2). Using criteria that are virtually identical to those for defining HIV-1 group-M sequence subtypes, Gao et al. (1994) have identified five HIV-2 sequence subtypes.
Given the extensive replication of HIV in infected individuals (Ho et al. 1995; Wei et al. 1995) and the substantial number of errors made during retroviral replication (Chapter 4, it is not surprising that extensive viral sequence heterogeneity can emerge within a single individual (Hahn et al. 1986; Saag et al. 1988). Although genetic changes can be observed throughout the genome, specific hypervariable sites exist within the env gene, interspersed among more conserved sequences (Alizon et al. 1986; Starcich et al. 1986; Willey et al. 1986). These hypervariable regions are not likely to reflect sites of frequent errors; rather, they are likely to be regions where change can be tolerated or where mutations confer selective advantage. Variation in env accounts for much of the differences among the isolates (Fisher et al. 1988). These are most likely antigenic variants selected by the immune response of the host and/or variants selected for different cell tropism. As discussed below (see Course of Infection with HIV and SIV, Clinical Latency), HIV isolates that emerge in vivo as a result of selective pressure and genetic variability can also show increased replicative or cytopathic capabilities in certain cell types in vitro.
Transmission
HIV is transmitted by direct sexual contact, either homosexual or heterosexual; by blood or blood products; and from an infected mother to infant, either intrapartum, perinatally, or via breast milk (Fauci and Lane 1991). There is no evidence that HIV can be transmitted by casual contact or that virus is spread by bites of insects such as mosquitoes.
Although the predominant mode of transmission in the United States and western Europe is via homosexual contact and sharing of needles during drug use, the vast majority of infections worldwide, particularly in developing countries, result from heterosexual contact. HIV has been found in semen both in infected mononuclear cells and as cell-free virus in seminal fluid; in addition, virus is present in cervical smears and vaginal fluid. There is a strong association of HIV transmission with receptive anal intercourse. Only a thin and fragile rectal mucosal membrane separates deposited semen from potential target cells within and beneath the anal mucosa; in addition, there may be trauma associated with anal intercourse. The vaginal mucosa is several layers thicker than the rectal mucosa and is less likely to be traumatized during intercourse; however, it is clear that virus can be transmitted to either partner during vaginal intercourse (de Vicenzi et al. 1994). It has been estimated that the probability of a woman becoming infected by an HIV-positive male partner during vaginal intercourse is probably less than 0.2% per contact. The risk of infection from a woman to a man via vaginal intercourse appears to be less likely (Holmberg 1997). There is also a clear-cut relationship between the presence of ulcerative genital lesions due to sexually transmitted disease and increased susceptibility to HIV infection. This effect may result from an increased chance of virus entering the bloodstream through such a lesion, or a higher frequency of CD4+ target cells in the lesion itself. Although oral sex appears to be a much less efficient means of transmitting HIV, there are rare anecdotal reports of transmission through receptive fellatio and insertive cunnilingus. Prospective studies have clearly demonstrated that condom use decreases transmission of HIV (Weller 1993).
Before the identification of HIV as the causative agent of AIDS, one of the principal modes of transmission was contaminated blood and blood products. Approximately 13,000 cases of AIDS have occurred among hemophiliacs in the United States, and recipients of blood or blood products (together, 2% of the total AIDS cases). Two factors have decreased the numbers of new cases among these risk categories: (1) screening of the blood supply and (2) preventing recognized at-risk individuals from donating blood. In this regard, the estimated rate of HIV infection from undetected HIV-infected, seronegative blood donors ranges from 1 in 40,000 to 1 in 250,000. The risk of infection of health care workers by direct inoculation of blood from an infected individual via a sharp object such as a needle stick is approximately 1 in 300 (Fauci and Lane 1991).
Maternal transmission of HIV accounts for more than 90% of all HIV infections in infants and children. In the absence of intervention, rates of mother-to-infant HIV transmission range from 13% to 42%. In the United States, approximately 20–25% of untreated pregnant HIV-infected women transmit the virus to their infants. An estimated 50–70% of mother-to-child transmissions occurs late in pregnancy or during birth. It has been hypothesized that HIV is transmitted when maternal blood enters the fetal circulation, or by mucosal exposure to virus during labor and delivery. Postnatal HIV infection may occur via breastfeeding, which adds an estimated 14% additional risk over the risk of HIV infections in utero or at delivery. Recently, it has been demonstrated that a specific regimen of 5′azidothymidine (AZT) can markedly reduce the risk of perinatal HIV transmission (Dunn et al. 1992; Connor et al. 1993; Rouzioux et al. 1993; St. Louis et al. 1993; Scarlatti 1996).
The precise mechanisms of transmission of HIV are not completely understood. Virus must enter the body as either cell-free virions or infected cells. The mode of transmission (either through mucosal surfaces or by direct inoculation into the blood) generally determines the initial cells that the virus encounters. Despite this difference, there is at present no reason to believe that the route of transmission alters the subsequent course of HIV infection, although it would be expected (and has been shown experimentally with SIV) that blood-borne transmission would be much more efficient. Likely targets for initial viral infection in the mucosa are antigen-presenting cells, particularly macrophage-related cells of the dendritic cell lineage. Cells residing at the mucosal surfaces are called Langerhans cells; those in the circulation are known as blood dendritic cells. These cells express CD4 as well as an appropriate coreceptor and can be infected by HIV. Infected cells are then transported to the lymphoid tissue where released virus can come into contact with susceptible CD4+ T cells that predominantly reside in the paracortical areas. Infection is thereby established and the subsequent burst of viremia seeds virus throughout the lymphoid tissues of the body (Fauci 1993).
Evidence has been presented which suggests that in sexual transmission, HIV-1 variants present at only low frequency in the donor are passed to the recipient (Zhu et al. 1993). Similar results have been reported for maternal-infant transmission (Wolinsky et al. 1992). Although these results need to be confirmed and the mechanisms elucidated, the mucosa may form a barrier that selects particular HIV variants. It has been suggested that clade-E strains can be transmitted more efficiently by heterosexual intercourse than clade-B strains and that this may correlate with increased infectability of dendritic cells for clade-E strains (Soto-Ramirez et al. 1996). This effect could explain the much more rapid spread of clade-E virus than clade-B virus in Thailand, where transmission is predominantly heterosexual (Gao et al. 1996). However, this conclusion is controversial and needs confirmation.
Epidemiology
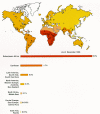
As of January, 1997, approximately 1.5 million cases of AIDS in adults and children had been reported to the World Health Organization(WHO); however, because of the inadequate reporting capabilities in developing countries, WHO estimated that there were more than 8.4 million cumulative cases. Within the same timeframe, there were approximately 580,000 cases of AIDS in the United States. As of January, 1997, WHO estimated that there were approximately 29.4 million cumulative infections with HIV; in the United States, there were approximately 1 million infections. Various estimates of projections of future global prevalence of HIV infection indicate that by the year 2000, between 40 and 100 million people will have been infected. Although the epidemic was first recognized in the United States and western Europe, the focus of the epidemic is currently in sub-Saharan Africa where approximately 14 million people are believed to be infected. It is anticipated that, in the near future, there will be acute problems in Asia, particularly India and Thailand where the numbers of new cases per year are predicted to increase dramatically for the next decade (Quinn 1996).
AIDS was first recognized in the United States in 1981. In the first few years of the epidemic, the vast majority of cases were among homosexual men and injection drug users (IDUs). Although these two risk categories still constitute the majority of total reported cases of AIDS in the United States in 1997, the relative proportion of new cases in which the infection was contracted heterosexually (particularly among women) has increased sharply. In fact, the relative proportion of new cases of AIDS per year among heterosexuals (non-IDUs) increased from less than 2% in the mid-1980s to more than 15% in 1995. This shift toward heterosexual transmission is also seen in developed countries, particularly in western Europe. Among developing countries, particularly in sub-Saharan Africa, more than 90% of infections are transmitted heterosexually.
Distribution, Grouping, and the Origins of Primate Lentiviruses
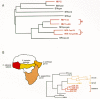
On the basis of genomic sequencing, five discrete groups of primate lentiviruses have been identified (Fig. 3). These five groups are HIV-1/SIVcpz, HIV-2/SIVsmm, SIVmac, SIVagm, SIVmnd, and SIVsyk (cpz, smm, mac, agm, mnd, and syk stand for chimpanzees, sooty mangabey monkeys, macaque monkeys, African green monkeys, mandrill monkeys, and sykes monkeys, respectively). Individual viral isolates from any one group share about a 55–60% amino acid identity in reverse transcriptase (RT) when compared to members of any other group; genetically, each of the five groups is approximately equidistant from the others. Viral isolates from any one group display interisolate variation, but the extent of variation within a group is much less than between groups. All primate lentiviruses are more closely related to one another than to any of the known nonprimate lentiviruses. This implies that the SIVs and HIVs evolved as a group in primates and that they were not recently derived from nonprimates by cross-species transmission.
Evidence is quite good that some monkey species have been natural hosts for their cognate strains of SIV for long periods of time (Nathanson et al. 1993). For instance, infection of green monkeys (Cercopithecus genus) in captivity and in the wild in Africa is widespread, with the prevalence of infection in most surveys ranging from 10% to 40% of all green monkeys. Infected animals are distributed across most of sub-Saharan Africa, including east, west, central, and south. The extent of genetic variation among SIVagm isolates is quite large, apparently even larger than the variation among HIV-1 isolates. Furthermore, natural infection of green monkeys with SIVagm does not appear to cause any disease despite a lifelong persistent infection and despite the pathogenicity of this virus in other species, such as rhesus monkeys. This pattern is expected if a virus and host have had sufficient time to adapt to each other. Similar arguments can be made for SIVsmm in mangabey monkeys (Cercocebus genus) in western Africa. Only one or a few isolates of SIVmnd and SIVsyk have been identified (Hirsch et al. 1993) and characterized so that relatively little is known about the extent of infection of the host species.
The Cercopithecus genus is complex, with at least 27 individual subspecies (Allan 1992). Four subspecies of Cercopithecus aethiops have been identified: the grivet, vervet, sabaeus, and tantalus monkeys. These subspecies have distinctive markings and distinct but sometimes overlapping habitat ranges; all four harbor SIV. Although the SIVs that they carry are more closely related to each other than to any of the other four primate lentivirus groupings, the SIVagm isolates can be subgrouped according to the subspecies of monkey from which they were derived and not according to geographic distributions (Fig. 3B) (Allan et al. 1991; M.C. Müller et al. 1993). Again, this is the expected pattern if the virus has been associated with green monkeys for a relatively long period of time, so that the viruses would have coevolved with their respective hosts.
In other situations, SIV infections appear to have resulted from recent, or relatively recent, transmission from one species to another. SIV was first isolated from a captive rhesus monkey (Macaca mulatta) at the New England Regional Primate Research Center (Daniel et al. 1985). Detailed analyses showed that infection of rhesus and other macaques in captivity is quite rare and that the SIV isolates obtained from rhesus and other macaques are, at the genetic level, members of the SIVsmm group of viruses. Infection of macaque monkeys with this SIV is pathogenic, resulting in an AIDS-like disease (Daniel et al. 1985; Letvin et al. 1985; Desrosiers 1990a). In their native habitats in Asia, rhesus monkeys and other macaques do not appear to be naturally infected with SIV. These and other results suggest that, on rare occasions, macaque monkeys were infected with SIV in captivity by cross-species transmission from mangabey monkeys.
Similarly, HIV-2 infection of humans in western Africa may have arisen, and may still be occurring, by cross-species transmission from mangabey monkeys (Desrosiers 1990a; Gao et al. 1992; Nathanson et al. 1993). The natural habitat of mangabey monkeys (the forested regions of western Africa) is nearly coincident with the region where human infection with HIV-2 is endemic, and the sequences of HIV-2 isolates are within the range of variation of known SIVsmm isolates (Hirsch et al. 1989; Marx et al. 1991; Gao et al. 1992). Cross-species transmission of SIVs has also occurred between monkey species in Africa: For example, there have been isolated examples of isolates from baboons that closely resemble SIVs from African green monkeys; the ranges of these two primates overlap (Allan et al. 1991).
Implications for Origins of HIV-1
The recent history of HIV-1 is much less clear than that of HIV-2 and most SIVs (Desrosiers 1990b). Two possibilities for the origin of HIV have been widely discussed. One possibility is that the virus has always been present in the human population, in one form or another, but may have gone unrecognized due to an extremely low prevalence and/or its confinement to isolated populations. In this model, features of modern society, urbanization of populations in developing countries, and extensive world travel would have introduced the virus into developed countries. The virus would then be spread by sharing of drug paraphernalia and sexual promiscuity. Alternatively, HIV-1 may have entered the human population from another species relatively recently. Increasing prevalence and high mortality would then be consequences of infection of the new host to which the virus was not fully adapted. The most likely possibility is a combination of these two: that the virus has been entering the human population from an animal reservoir (through the butchering of monkeys, for example) but has been unable until recently to spread from initial limited, foci of infection. Which theory is correct has not been resolved and may never be. The most significant clue has been the discovery that a few captive chimpanzees are infected with SIVcpz, a virus in the HIV-1 group (Fig. 1) (Huet et al. 1990). However, wild chimpanzee populations are difficult to study, and the vast majority of chimpanzees studied to date are not infected with SIVcpz. A major question is whether the few SIVcpz isolates represent a natural infectious agent of chimpanzees.
Publication Details
Copyright
Publisher
Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY)
NLM Citation
Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997. Etiologic Agents.