Lymphoid Organs Are Sites of Immune Activation, Reservoirs of Viral Burden, and Centers for Viral Replication
Most of the early work on the pathogenesis of HIV infection focused on HIV in PBMCs since this compartment is readily accessible. However, the majority of the body's lymphocyte pool resides in lymphoid organs: lymph nodes, spleen, and gut-associated lymphoid tissue and not in the peripheral blood (for review, see Fauci 1993; Pantaleo et al. 1993a,b). Lymphocytes and macrophages in tissues may differ from those in peripheral blood in important ways, such as the level of activation differentiation, cytokine milieu and in their exposure to virus. As such, studies of HIV infection in the peripheral blood may not accurately reflect events in the lymphoid organs. It is possible that the initial depletion of CD4+ T cells in the peripheral blood that occurs during the acute phase of HIV infection is a result of a redistribution of cells in the lymphoid organs as the host begins to mount an anti-HIV immune response. Although it is not usually convenient to study these sites in human patients, the behaviors of the virus and lymphoid organs in SIV-macaque models are quite similar to HIV-1 infection in human lymphoid tissue (Ringler et al. 1989; Wyand et al. 1989; Joling et al. 1992).
Since the function of the lymphoid organs is to filter and trap invading pathogens and present them to immune competent cells, the high levels of viremia seen following primary infection presumably lead to an efficient infection of the lymphoid tissue with HIV or SIV. Virus is trapped within the germinal centers of the lymphoid organs (see below), which causes cellular recruitment, cellular activation, and germinal center hyperplasia that are part of the normal immune response to an invading pathogen. This process of cellular activation could make the resident and recruited CD4+ T cells susceptible to infection with HIV or SIV and permissive for viral replication. As discussed above, infection of CD4+ T cells in the lymphoid tissue is associated with rapid viral turnover and cell death (Ho et al. 1995; Wei et al. 1995). Infected cells accumulate within the lymphoid organs; however, the ratio of infected cells that express detectable viral mRNA to infected cells in which viral mRNA is not detectable is quite low. The latter population includes both cells that contain defective proviruses and any latently infected cells. It is difficult to culture virus from plasma in the presence of an anti-HIV-1 immune response. Despite the fact that virus-infected cells can be readily demonstrated in PBMCs by DNA-based PCR, cells that are actively expressing virus are only rarely detectable in PBMCs. In contrast, the lymphoid organs contain a much larger percentage of infected cells compared to PBMCs, and viral replication (although present in variable amounts) can be detected in the lymphoid organs at all stages of HIV disease, including the period of prolonged clinical latency. As disease progresses, the relative numbers of cells in lymphoid organs that express viral mRNA increase concomitant with a decrease in the antiviral immune response (Fauci 1993; Pantaleo et al. 1991 , 1993a,b,c).
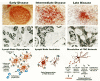
The large majority of HIV or SIV genomes present in lymphoid tissue are in extracellular virions that are trapped within the germinal centers of the lymph node. The germinal center is a network of follicular dendritic cells (FDC) which contain long, interdigitating processes that surround lymphocytes (Fig. 10). FDCs trap antigen, antibody, and complement on their extended cellular processes and in so doing present antigens to B cells. Extracellular HIV/SIV particles adhere to processes of the FDCs and, at the early stage of disease, do not appear to cause any obvious deleterious effects to them. Initially, the structure of the lymphoid tissue, with discrete, functional germinal centers, remains intact; however, there is hyperplasia because of both in situ proliferation of activated immune cells and retrafficking of cells from the peripheral blood and/or lymphatics (Embretson et al. 1993a; Fauci 1993; Pantaleo et al. 1993a,c,c).
Deterioration of Lymphoid Organs
Lymph nodes from patients at an intermediate stage of disease (CD4+ T-cell levels between 200 and 500 cells/ μl) show obvious damage. The node begins to disintegrate and the trapping efficiency of the FDC begins to decline (Pantaleo et al. 1993c). At the cellular level, electron microscopy shows that organelles within the FDC become swollen and cell death occurs. At a more advanced stage of disease (below 200 cells/μl), there is an increase in both viral load and HIV expression in PBMCs. This change may reflect either increasing viral replication or inability of the lymphoid system to limit the amount of virus or infected cells reaching the peripheral blood, allowing the two compartments to equilibrate (Pantaleo et al. 1993c).
During advanced stages of disease, the architecture of lymphoid tissue is virtually completely destroyed (“burnt out”) with a concomitant disruption of the FDC network and the disappearance of FDC. The precise mechanism for the loss of FDC is unclear since the degree of destruction of the FDC is much greater than the level of infection of these cells. As a result of the dissolution of the germinal center, the lymph node is no longer able to trap pathogens. The virus that was previously trapped in the node is now able to enter the peripheral blood, allowing the proportion of virus-infected cells in the peripheral blood and lymphoid organs to approach equilibrium. Because the lymphoid tissue is unable to mount an effective immune response, there is increased risk of opportunistic infections (Fauci 1993; Pantaleo et al. 1993a,b; Centers for Disease Control and Prevention 1997).
Depletion of CD4+ T cells occurs throughout the course of disease and can be at least partially explained by the effects of HIV infection on lymphoid tissue. Susceptible, activated CD4+ T cells that are continually recruited into the lymphoid tissue during the course of infection are readily infected by cell-to-cell transmission of HIV. The progressive destruction of the lymph node architecture and diminution of function contribute to the escalating immunosuppression, followed by the complete collapse of the immune system (for review, see Embretson et al. 1993a; Fauci 1993; Pantaleo et al. 1993a,c,c).
Publication Details
Copyright
Publisher
Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY)
NLM Citation
Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997. Role of Lymphoid Organs in Immunopathogenesis.