1. Introduction
The benefit of the routine use of antimicrobial prophylaxis prior to non-clean and implant surgery has long been recognized. Several experimental and clinical studies demonstrated an effect of the timing of surgical antimicrobial prophylaxis (SAP) on surgical site infections (SSI) (1, 2), but the optimal timing remains to be defined.
Several guidelines issued by professional societies or national authorities, such as the American Society of Health-System Pharmacists (ASHP) (3), the Society for Healthcare Epidemiology of America (SHEA)/Infectious Diseases Society of America (IDSA) (4), the Royal College of Physicians of Ireland (5), or Health Protection Scotland (6), recommend administration within 60 minutes prior to incision. However, these recommendations are not based upon systematic reviews of the literature and meta-analysis or a rigorous evaluation of the quality of available evidence.
2. PICO question
How does the timing of SAP administration impact on the risk of SSI and what is the precise optimal timing?
3. Methods
The following databases were searched: Medline (PubMed); Excerpta Medica Database (EMBASE); Cumulative Index to Nursing and Allied Health Literature (CINAHL); Cochrane Central Register of Controlled Trials (CENTRAL); and WHO regional medical databases. The time limit for the review was between 1 January 1990 and 13 August 2014. Language was restricted to English, French, German and Spanish. A comprehensive list of search terms was used, including Medical Subject Headings (MeSH) (Appendix 1).
Two independent reviewers screened titles and abstracts of retrieved references for potentially relevant studies. The full text of all potentially eligible articles was obtained. Two authors independently reviewed the full text articles for eligibility based on inclusion criteria. Duplicate studies were excluded.
Two authors extracted data in a predefined evidence table (Appendix 2) and critically appraised the retrieved studies using the Newcastle-Ottawa Quality Assessment Scale for cohort studies (7) (Appendix 3). Any disagreements were resolved through discussion or after consultation with the senior author, when necessary.
Meta-analyses of available comparisons according to different SAP timing intervals were performed using Review Manager v5.3 (8) as appropriate (Appendix 4). Adjusted odds ratios (OR) and mean difference with 95% confidence intervals (CI) were extracted and pooled for each comparison with a random effects model. Among the studies investigating the interval between 60 and 0 minutes prior to incision, none reported adjusted ORs and we were unable to compare adjusted outcomes for this interval. However, considering the substantial interest in this specific timing (that is, within 60 minutes prior to incision), we used unadjusted crude data as a surrogate for as recommended in other guidelines. In the other comparisons, crude data yielded the same results as adjusted data, but with a different effect size (Appendix 5).
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (GRADE Pro software) (9, 10) was used to assess the quality of the body of retrieved evidence (Appendix 6).
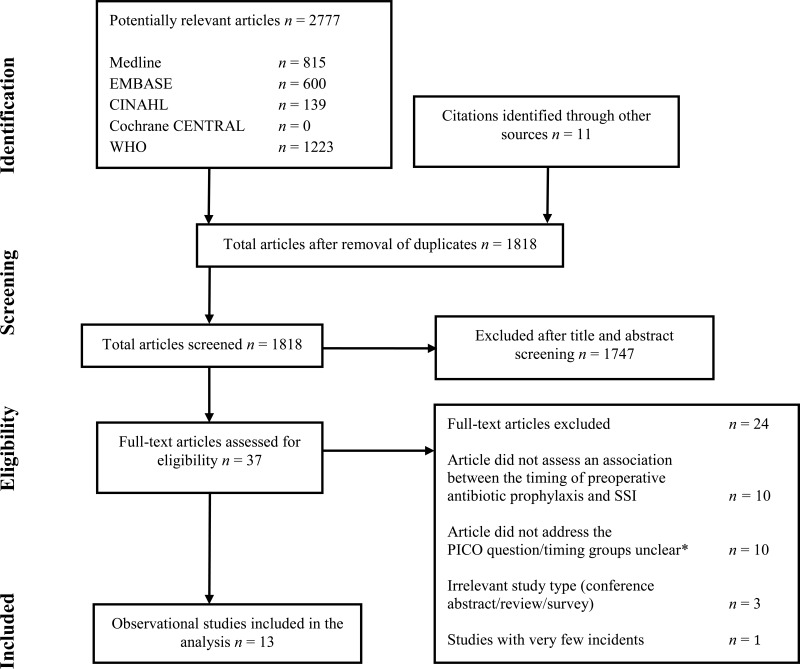
4. Study selection
Flow chart of the study selection process
5. Summary of the findings
Overall, 13 observational studies comparing different timing intervals for SAP with an SSI outcome were identified. All surgical procedures with an indication for SAP were included (that is, clean-contaminated, contaminated and implant surgery). No randomized controlled trials (RCTs) were found related to the topic. The body of retrieved evidence focused on adult patients; no study was available in the paediatric population. The literature search did not identify any studies that reported on SSI-attributable mortality.
There was a substantial heterogeneity among the included studies. The studies described different arbitrary timing intervals varying from 15 to 120 minutes and not all studies reported the same outcome measures. Despite this heterogeneity in reported time intervals, we were able to make the following comparisons:
SAP administration
Pre- vs. post-incision
Within 120 minutes vs. more than 120 minutes prior to incision
Intervals within 120 minutes prior to incision:
between 120 and 60 minutes prior to incision vs. between 60 and 0 minutes prior to incision
between 60 and 30 minutes prior to incision vs. between 30 and 0 minutes prior to incision
The results of the meta-analyses based on these comparisons are shown in Appendix 5.
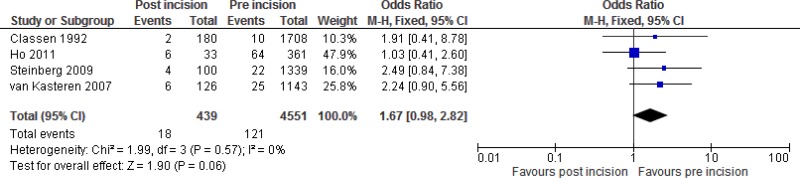
Four
§ studies (
2,
11–
13) comparing the administration of
SAP pre-
vs. post-incision were identified. Three studies (
2,
12,
13) reported an increased
SSI risk when SAP was administered after incision, although none showed a significant effect. The study by Ho and colleagues (
11) reported almost no difference (
Appendix 2).
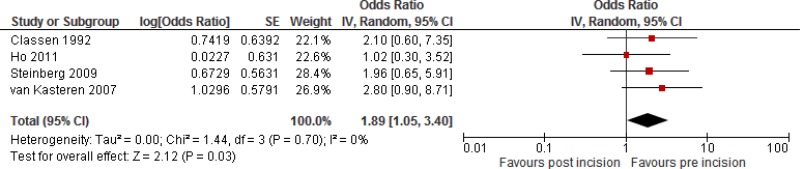
Meta-analysis of these 4 studies showed an increased risk of
SSI following
SAP administration after incision compared to before incision (
OR: 1.89; 95%
CI: 1.05–3.4), which resulted in 25 more infections (from one more to 65 more) per 1000 treated patients. For this comparison, the quality of the evidence was low (
Appendix 6).
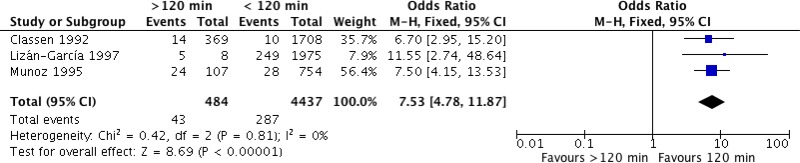
Three
§ studies (
2,
14,
15) comparing the administration of
SAP within 120 minutes vs. more than 120 minutes prior to incision were identified. All studies showed a significantly higher
SSI risk when SAP was administered more than 120 minutes prior to incision (
Appendix 2).
Meta-analysis of these 3 studies showed that administration more than 120 minutes prior to incision increased the risk of
SSI (
OR: 5.26; 95%
CI: 3.29–8.39) and resulted in 250 more infections (from 154 more to 361 more) per 1000 treated patients. Considering the large effect, the quality of evidence was graded as moderate (
Appendix 6).
Seven studies (
2,
11–
13,
16–
18) comparing intervals of
SAP administration within 120 minutes were identified.
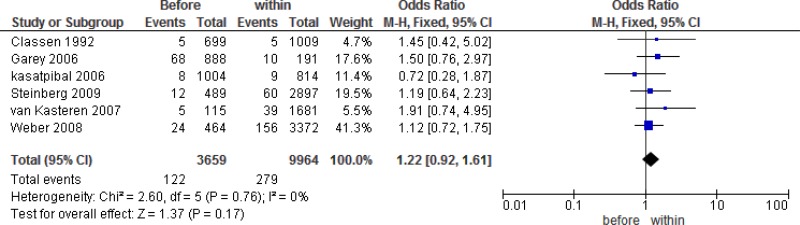
Six
§ studies (
2,
12,
13,
16–
18) compared
SAP administration within 60 to 0 minutes prior to incision with SAP administration within 120 to 60 minutes prior to incision. Five studies favoured SAP administration within 60 to 0 minutes prior to incision, although none of the studies reached significance. One study favoured administration between 120 and 60 minutes prior to incision, but the results were not significantly different from the interval within 60 to 0 minutes (
Appendix 2).
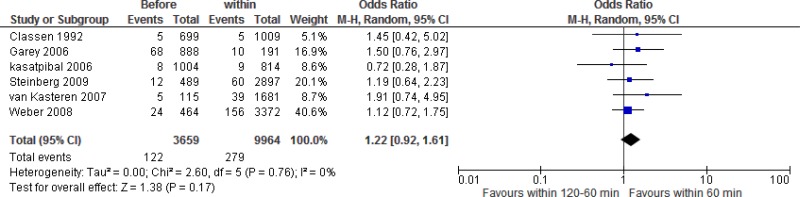
Meta-analysis
* of these 6 studies showed that the administration of
SAP within 60 to 0 minutes prior to incision had no benefit when compared to administration within 120 to 60 minutes prior to incision (
OR: 1.22; 95%
CI: 0.92–1.61) and resulted in 6 more (from 2 fewer to 16 more) infections per 1000 treated patients. The quality of evidence was very low due to serious imprecision (
Appendix 6).
*Crude unadjusted data were used in the meta-analyses
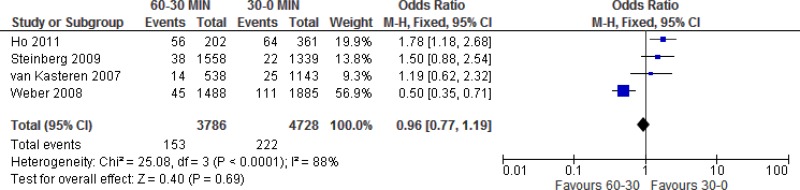
Four
§ studies (
11–
13,
17) comparing
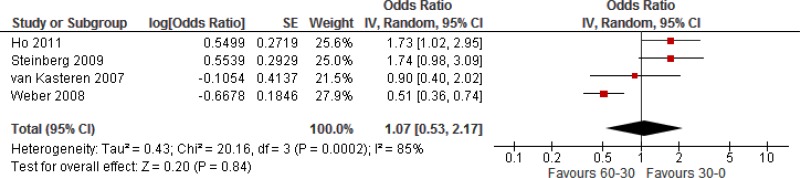
SAP administration within 60 to 30 minutes prior to incision with SAP administration within 30 to 0 minutes prior to incision were identified. Ho showed a significant benefit when SAP was administered within 30 to 0 minutes prior to incision. The results reported by Steinberg also favoured administration within 30 minutes prior to incision, but did not reach significance. Weber reported a significantly lower risk when prophylaxis was administered within 60 to 30 minutes prior to incision and van Kasteren also favoured administration within the same time frame prior to incision, but the results did not reach significance.
Meta-analysis of these 4 studies showed that administration within 30 to 0 minutes prior to incision had neither benefit nor harm when compared to administration within 60 to 30 minutes prior to incision (
OR: 1.07; 95%
CI: 0.53–2.17) and resulted in 3 more (22 fewer to 50 more) infections per 1000 treated patients. The quality of evidence was very low due to serious heterogeneity and very serious imprecision (
Appendix 6).
Four§ studies (19–22) could not be included in any comparison. Trick and colleagues (19) conducted a case-control study in a single centre and used a different methodology than other observational studies included in the meta-analysis. They included 120 coronary artery bypass grafting procedures and compared SAP administration within 120 minutes vs. more than 120 minutes prior to incision. The results showed a significantly increased risk of SSI with SAP administration more than 120 minutes prior to incision (OR: 5; 95% CI: 1.4–17) (Appendix 2).
The study by El-Mahallawy and colleagues (22) and two studies by Koch and colleagues (20, 21) could not be included in the meta-analysis because adjusted ORs could not be derived from their results (Appendix 2). El-Mahallawy and colleagues compared the timing of SAP administration within 30 minutes vs. more than 30 minutes prior to incision in 200 surgical procedures and favoured SAP administration more than 30 minutes prior to incision, but the relation was not statistically significant (P=0.115) (22).
The first report by Koch and colleagues was a prospective study evaluating 28 250 cardiac surgical procedures involving median sternotomy and investigated different timings of SAP related to SSI rate reduction. Cefuroxime and vancomycin were administered 15 and 30 minutes prior to incision, respectively (20). The second study compared SAP administration within 30 to 0 minutes prior to incision with SAP administration within 60 to 30 minutes prior to incision in 4453 general surgery procedures. SAP administration within 60 to 30 minutes prior to incision had a 30% higher risk compared to administration within 30 to 0 minutes prior to incision (11.7% vs. 9%) (21). This difference was statistically significant (P=0.01).
§ Numbers do not add up to 13 because some studies were included in multiple analyses
In conclusion, the retrieved evidence can be summarized as follows.
Overall, low quality evidence shows that the administration of
SAP after incision causes significant harm due to an increase of the
SSI risk when compared to administration prior to incision.
Overall, a moderate quality of evidence shows that
SAP administration before 120 minutes prior to incision causes significant harm due to an increase of the
SSI risk compared to administration within 120 minutes.
It is not possible to establish more precisely the optimal timing within the 120-minute interval. No significant difference was found between the different time intervals within this period, that is, within 120 to 60 minutes prior to incision vs. within 60 to 0 minutes prior to incision or within 60 to 30 minutes prior to incision vs. within 30 to 0 minutes prior to incision.
Overall, a very low quality of evidence shows that administration within 60 minutes prior to incision has neither benefit nor harm related to the reduction of the
SSI rate when compared to administration within 60 to 120 minutes prior to incision.
Overall, a very low quality of evidence shows that administration within 30 minutes prior to incision has neither benefit nor harm related to the reduction of the
SSI rate when compared to administration within 60 to 30 minutes prior to incision.
Several limitations can be observed among the available studies. All reported studies are observational. No randomized prospective studies have been done on this topic. Several aspects of the antibiotic regimen differed between studies or were unclear: (a) all studies used multiple agents with varying half-lives; (b) all studies reported the time of administration, but information on infusion time was lacking in many; (c) the duration of the procedure and redosing protocol varied; when a redosing protocol was applied, it was based on the duration of the procedure rather than on the time after the first dose, thus leading to a high risk of inadequate redosing; and (d) postoperative antibiotic duration was not the same. All these aspects influence the effect of timing and also SSI rates.
6. Future research priorities
The systematic review team identified the following key uncertainties and future research priorities.
Future research should focus on an optimal interval within 120 minutes, preferably through randomized prospective trials. The above-mentioned methodological aspects should be well described and standardized in future studies.
Appendices
Appendix 1. Search terms
Medline
“antibiotic prophylaxis”[MeSH]
OR antibiotic prophylaxis [tiab] OR antimicrobial agent*[tiab] OR antimicrobial [tiab] OR antibiotic therapy [tiab] OR antibiotic*[tiab]
“surgical wound Infection”[MeSH]
OR surgical site infection*[tiab] OR
SSI [tiab] OR SSIs [tiab] OR surgical wound infection*[tiab] OR surgical infection*[tiab] OR postoperative wound infection*[tiab] OR postoperative wound infection*[tiab]
“time factors”[MeSH]
OR timing [tiab]
EMBASE
surgical infection/or (surgical site infection* or
SSI or SSIs or surgical wound infection* or surgical infection* or post-operative wound infection* or postoperative wound infection*).ti,ab,kw.
exp prophylaxis/or exp antibiotic prophylaxis/or exp antibiotic agent/or antibiotic*.mp. or antiinfective agent/ct, ad, iv, su
exp time/or timing.ti,ab,kw.
CINAHL
(MH “surgical wound infection”)
OR (TI (surgical site infection* OR
SSI OR SSIs OR surgical wound infection* OR surgical infection* OR post-operative wound infection* OR postoperative wound infection*) OR AB (surgical site infection* OR SSI OR SSIs OR surgical wound infection* OR surgical infection* OR post-operative wound infection* OR postoperative wound infection*))
(MH “antibiotic prophylaxis”)
OR TI (antimicrobial OR antibiotic*) OR AB (antimicrobial OR antibiotic*)
(MH “time factors”)
OR TI (timing) OR AB (timing)
Cochrane CENTRAL
wound infection:ti,ab,kw
OR surgical wound infection:ti,ab,kw
time:ti,ab,kw
OR timing:ti,ab,kw
prophylaxis:ti,ab,kw
antibiotic:ti,ab,kw
WHO global regional medical databases
(surgical site infection)
(surgical site infections)
(wound infection)
(wound infections)
(postoperative wound infection)
(prophylaxis)(prophylactic)
(antibiotic)
(antimicrobial)
(anti infective)
(time)
(timing)
- ti:
title;
- ab:
abstract;
- kw:
keyword
Appendix 3. Newcastle-Ottawa risk of bias table
View in own window
Observational cohort studies
Author, year, reference | Representativeness of cohort | Selection of non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start | Comparability of cohorts | Assessment of outcome | Follow-up long enough | Adequacy of follow-up of cohorts | Total |
|---|
| Classen 1992 (2) | B* | A* | A* | Yes* | * | B* | Discharge | A* | 7 |
| Munoz 1995 (15) | B* | A* | A* | Yes* | * | A* | Discharge | B* | 7 |
| Lizán-García 1997 (14) | B* | A* | A* | Yes* | * | A* | Discharge | A* | 7 |
| Garey 2006 (18) | C | A* | A* | Yes* | * | A* | 30 days* | A* | 7 |
| Kasatpibal 2006 (16) | C | A* | A* | Yes* | * | A* | 30 days* | B* | 7 |
| van Kasteren 2007 (13) | C | A* | A* | Yes* | * | A* | 30 days/1 year* | A* | 7 |
| Weber 2008 (17) | B* | A* | A* | Yes* | * | A* | 30 days/1 year* | B* | 8 |
| Steinberg 2009 (12) | A* | A* | A* | Yes* | * | A* | 30 days-1 year* | A* | 8 |
| Ho 2011 (11) | C | A* | A* | Yes* | * | A* | 30 days* | A* | 7 |
| Koch 2012 (20) | C | A* | A* | Yes* | | B* | Discharge | A* | 5 |
| El-Mahallawy (22) | C | A* | A* | Yes* | * | D | Until removal of stitches | A* | 5 |
| Koch 2013 (21) | B* | A* | A* | Yes* | | A* | 30 days* | B* | 7 |
Case control study
Author, year, reference | Is the case definition adequate? | Representiveness of cases | Selection of controls | Definition of controls | Comparability of cases and controls | Ascertainment of exposure | Same method of ascertainment of | Non-response rate | Total |
|---|
| Trick 2000 (19) | A* | A* | B | A* | ** | A* | Yes* | B | 7 |
Newcastle Ottawa scale was used. For each * a rating point was added to the total per study
Appendix 4. Meta-analyses using adjusted odds ratios
Appendix 5. Meta-analyses with crude data
References
- 1.
Burke
JF. The effective period of preventive antibiotic action in experimental incisions and dermal lesions. Surgery. 1961;50:387–97. [
PubMed: 16722001]
- 2.
Classen
DC, Evans
RS, Pestotnik
SL, Horn
SD, Menlove
RL, Burke
JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326(5):281–6. [
PubMed: 1728731]
- 3.
Bratzler
DW, Dellinger
EP, Olsen
KM, Perl
TM, Auwaerter
PG, Bolon
MK, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg Infect (Larchmt). 2013;14(1):73–156. [
PubMed: 23461695]
- 4.
Anderson
DJ, Podgorny
K, Berrios-Torres
SI, Bratzler
DW, Dellinger
EP, Greene
L, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35 (Suppl. 2):S66–88. [
PubMed: 25376070]
- 5.
Preventing surgical site infections. Key recommendations for practice. 2012, updated 2014. Dublin: Joint Royal College of Surgeons in Ireland/Royal College of Physicians of Ireland Working Group on the Prevention of Surgical Site Infection, 2014 (
http://www.rcpi.ie/content/docs/000001/1005_5_media.pdf, accessed 10 May 2016).
- 6.
Scottish Intercollegiate Guidelines Network. Antibiotic prophylaxis in surgery. July
2008, updated April 2014. Edinburgh: Healthcare Improvement Scotland (
http://www.sign.ac.uk/pdf/sign104.pdf, accessed 10 May 2016).
- 7.
Wells
GA, Shea
B, O’Connell
D, Peterson
J, Welch
V, Losos
M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Toronto: The Ottawa Hospital Research Institute; 2014 (
http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp, accessed 13 May 2016).
- 8.
The Nordic Cochrane Centre TCC. Review Manager (RevMan). Version 5.3. Copenhagen: The Cochrane Collaboration; 2014.
- 9.
Guyatt
GH, Oxman
AD, Vist
GE, Kunz
R, Falck-Ytter
Y, Alonso-Coello
P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. [
PMC free article: PMC2335261] [
PubMed: 18436948]
- 10.
GRADEpro Guideline Development Tool. Summary of findings tables, health technology assessment and guidelines. GRADE Working Group, Ontario: McMaster University and Evidence Prime Inc.; 2015 (
http://www.gradepro.org, accessed 5 May 2016).
- 11.
Ho
VP, Barie
PS, Stein
SL, Trencheva
K, Milsom
JW, Lee
SW, et al. Antibiotic regimen and the timing of prophylaxis are important for reducing surgical site infection after elective abdominal colorectal surgery. Surg Infect (Larchmt). 2011;12(4):255–60. [
PubMed: 21790479]
- 12.
Steinberg
JP, Braun
BI, Hellinger
WC, Kusek
L, Bozikis
MR, Bush
AJ, et al. Timing of antimicrobial prophylaxis and the risk of surgical site infections: results from the Trial to Reduce Antimicrobial Prophylaxis Errors. Ann Surg. 2009;250(1):10–6. [
PubMed: 19561486]
- 13.
van Kasteren
ME, Mannien
J, Ott
A, Kullberg
BJ, de Boer
AS, Gyssens
IC. Antibiotic prophylaxis and the risk of surgical site infections following total hip arthroplasty: timely administration is the most important factor. Clin Infect Dis. 2007;44(7):921–7. [
PubMed: 17342642]
- 14.
Lizan-Garcia
M, Garcia-Caballero
J, Asensio-Vegas
A. Risk factors for surgical-wound infection in general surgery: a prospective study. Infect Control Hosp Epidemiol. 1997;18(5):310–5. [
PubMed: 9154472]
- 15.
Munoz
PE, Jimenez Antolin
JA, Brea
ZS, Bravo
GP. [The effect of surgical antibiotic prophylaxis and the timing of its administration on the risk of surgical wound infection.] [Article in Spanish] Rev Clin Esp. 1995;195(10):669–73. [
PubMed: 8532921]
- 16.
Kasatpibal
N, Norgaard
M, Sorensen
H, Schonheyder
H, Jamulitrat
S, Chongsuvivatwong
V. Risk of surgical site infection and efficacy of antibiotic prophylaxis: a cohort study of appendectomy patients in Thailand. BMC Infect Dis. 2006;6(1):111. [
PMC free article: PMC1553447] [
PubMed: 16836755]
- 17.
Weber
WP, Marti
WR, Zwahlen
M, Misteli
H, Rosenthal
R, Reck
S, et al. The timing of surgical antimicrobial prophylaxis. Ann Surg. 2008;247(6):918–26. [
PubMed: 18520217]
- 18.
Garey
KW, Dao
T, Chen
H, Amrutkar
P, Kumar
N, Reiter
M, et al. Timing of vancomycin prophylaxis for cardiac surgery patients and the risk of surgical site infections. J Antimicrob Chemother. 2006;58(3):645–50. [
PubMed: 16807254]
- 19.
Trick
WE, Scheckler
WE, Tokars
JI, Jones
KC, Reppen
ML, Smith
EM, et al. Modifiable risk factors associated with deep sternal site infection after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2000;119(1):108–14. [
PubMed: 10612768]
- 20.
Koch
CG, Nowicki
ER, Rajeswaran
J, Gordon
SM, Sabik
JF
III, Blackstone
EH. When the timing is right: Antibiotic timing and infection after cardiac surgery. J Thorac Cardiovasc Surg. 2012;144(4):931–7. [
PubMed: 22608676]
- 21.
Koch
CG, Li
L, Hixson
E, Tang
A, Gordon
S, Longworth
D, et al. Is it time to refine? An exploration and simulation of optimal antibiotic timing in general surgery. J Am Coll Surg. 2013;217(4):628–35. [
PubMed: 23849901]
- 22.
El-Mahallawy
HA, Hassan
SS, Khalifa
HI, El-Sayed Safa
MM, Khafagy
MM. Comparing a combination of penicillin G and gentamicin to a combination of clindamycin and amikacin as prophylactic antibiotic regimens in prevention of clean contaminated wound infections in cancer surgery. J Egypt Natl Canc Inst. 2013;25(1):31–5. [
PubMed: 23499204]