Traditionally, the metabolic phenotype of maple syrup urine disease (MSUD) is termed classic or intermediate on the basis of residual branched-chain alpha-ketoacid dehydrogenase (BCKD) enzyme activity. Rarely, affected individuals have partial BCKD enzyme deficiency that manifests only intermittently or responds to dietary thiamine therapy (see Table 2). Phenotypic distinctions are not absolute: individuals with intermediate or intermittent forms of MSUD can experience severe metabolic intoxication and encephalopathy if physiologic stress is sufficient to overwhelm residual BCKD activity or this activity is reduced by transient changes in the phosphorylation state of the enzyme complex. Even in persons with relatively high baseline residual BCKD enzyme activity, episodes of metabolic intoxication can be fatal.
Classic MSUD Phenotype
Maple syrup odor is evident in cerumen soon after birth and in urine by age five to seven days. In untreated neonates, ketonuria, irritability, and poor feeding occur within 48 hours of delivery. Lethargy, intermittent apnea, opisthotonus, and stereotyped movements such as "fencing" and "bicycling" are evident by age four to five days and are followed by coma and central respiratory failure. Preemptive detection of affected newborns, before they exhibit neurologic signs of MSUD, significantly reduces lifetime risk of intellectual disability, mental illness, and global functional impairment [Strauss et al 2012, Muelly et al 2013, Strauss et al 2020].
Following the neonatal period, acute metabolic intoxication (leucinosis) and neurologic deterioration can develop rapidly at any age as a result of net protein degradation precipitated by infection, surgery, injury, or psychological stress (see ). In infants and toddlers, leucinosis causes nausea, anorexia, altered level of consciousness, acute dystonia, and ataxia. Neurologic signs of intoxication in older individuals vary and can include cognitive impairment, hyperactivity, sleep disturbances, hallucinations, mood swings, focal dystonia, choreoathetosis, and ataxia. As plasma concentrations of leucine and alpha-ketoisocaproic acid (aKIC) increase, individuals become increasingly stuporous and may progress to coma. In persons of all ages with MSUD, nausea and vomiting are common during crisis and often necessitate hospitalization [Morton et al 2002].
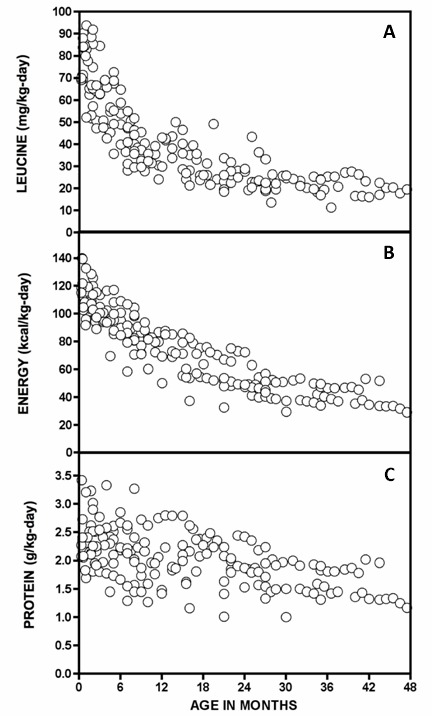
Serial plasma leucine measurements over a 62-day NICU course in a Mennonite newborn with trisomy 21 and classic MSUD. Plasma leucine levels rise predictably as a result of net protein catabolism provoked by a variety of physiologic stresses, including (more...)
Each episode of acute leucinosis is associated with a risk for cerebral edema (see ) [Levin et al 1993] and death [Strauss et al 2020]. Mechanisms of brain edema in MSUD are not completely understood. Plasma leucine concentration correlates only indirectly with the degree of swelling; severe cerebral edema and neurologic impairment are more directly related to the rate of change of plasma leucine and concomitant decreases in blood osmolarity. During the evolution of leucinosis, cerebral vasopressin release may be provoked by both acute hyperosmolarity (from the accumulation of BCAAs, ketoacids, ketone bodies, and free fatty acids in the circulation) and vomiting. Renal excretion of branched-chain alpha-ketoacids (BCKAs) is accompanied by obligate urine sodium loss, and when this coincides with renal free water retention (antidiuresis), administration of hypotonic or even isotonic fluids can result in hyponatremia and critical brain edema [Strauss & Morton 2003].
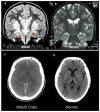
A. Coronal T2-weighted MRI from a Mennonite boy age five years during an acute metabolic crisis. Diffuse gray matter swelling and signal hyperintensity (on T2-weighted and FLAIR images) involve the cortical mantle, basal ganglia nuclei, hippocampus, and (more...)
Transient periods of MSUD encephalopathy appear fully reversible, provided no global or focal ischemic brain damage occurs. In contrast, prolonged amino acid imbalances, particularly if they occur during the early years of brain development, lead to structural and functional neurologic abnormalities that have morbid long-term psychomotor consequences [Carecchio et al 2011, Shellmer et al 2011, Muelly et al 2013, Strauss et al 2020].
Neonatal screening and sophisticated enteral and parenteral treatment protocols (see Management) have significantly improved neurologic outcomes for persons with classic MSUD [Strauss et al 2010, Muelly et al 2013, Strauss et al 2020], but risks of acute brain injury or death are always present, and the long-term neuropsychiatric prognosis is guarded. In two longitudinal studies of individuals with classic MSUD [Muelly et al 2013, Strauss et al 2020], an asymptomatic neonatal course and stringent longitudinal biochemical control proved fundamental to optimizing long-term cognitive outcome and mental health.
Early developmental milestones. Children with MSUD who are diagnosed during the neonatal period and managed prospectively under stringent dietary control can achieve major developmental milestones along time courses similar to their unaffected sibs [
Strauss et al 2020].
Cognitive function. Among individuals with classic MSUD (n = 81, ages 3.6-51.1 years), full scale intelligence quotient (FSIQ) correlates with birthdate (r
s = 0.39, p = 0.0044) and is on average 20%-40% lower in affected individuals as compared to their unaffected sibs [
Strauss et al 2020]. This difference is most striking for affected individuals born before the advent of newborn screening (NBS) (FSIQ of 62 ± 17, range 40-99). FSIQ correlates directly with the frequency of amino acid monitoring and inversely with both average lifetime plasma leucine and its concentration ratio to valine [
Muelly et al 2013]. Prolonged neonatal encephalopathy is the single strongest predictor of neurocognitive disability and global functional impairment [
Muelly et al 2013,
Strauss et al 2020].
Mood and anxiety. Among individuals with classic MSUD who complete appropriate objective testing, the probability of affective illness (depression, anxiety, and panic disorder) is between 83% and 100% by age 35 years [
Muelly et al 2013,
Strauss et al 2020]. Newborns who were encephalopathic at the time of diagnosis are five and ten times more likely, respectively, to later suffer from anxiety and depression (see
Table 3) [
Muelly et al 2013].
Attention and hyperactivity. Cumulative lifetime incidence of attention-deficit/hyperactivity disorder (ADHD) exceeds 50% among individuals with MSUD on dietary therapy and may be even higher among those who underwent liver transplantation [
Muelly et al 2013].
Movement disorders. Among 17 adults with MSUD (mean age 27.5 years), 12 (70.6%) had a movement disorder (primarily tremor, dystonia, or a combination of both) on clinical examination [
Carecchio et al 2011]. Parkinsonism and simple motor tics were also observed. Pyramidal signs were present in 11 affected individuals (64.7%), and a spastic-dystonic gait was observed in six (35.2%). In the authors' experience, such motor disabilities are rare in individuals with MSUD who are managed appropriately from the neonatal period but common among those who did not have the advantage of NBS [
Strauss et al 2020].
Table 3.
Lifetime Relative Risk of Each Finding Based on Condition at the Time of Diagnosis
View in own window
| Ill vs Well at Diagnosis | Relative Risk | Fisher's Exact p |
|---|
| Depression | 10.3 | 0.001 |
| Anxiety | 5.1 | 0.007 |
| Global assessment of functioning <70 | 4.0 | 0.05 |
| Full scale intelligence quotient <70 | 2.9 | 0.20 |
| Attention-deficit/hyperactivity | 1.4 | 0.28 |
The relative risk in this table compares the likelihood of developing the finding if the affected individual was ill at the time of diagnosis versus if the affected individual was well at the time of diagnosis.
Liver transplantation appears to prevent catastrophic brain injuries that can occur during metabolic intoxication [Mazariegos et al 2012] and arrests the progression of neurocognitive impairment [Shellmer et al 2011], but does not reverse preexisting cognitive disability or psychiatric illness [Strauss et al 2020]. Neuropsychiatric morbidity and neurochemistry are similar among individuals with MSUD who have and have not undergone liver transplantation [Muelly et al 2013, Strauss et al 2020].
Non-central nervous system involvement in MSUD can include:
Iatrogenic essential amino acid deficiency. Anemia, acrodermatitis, hair loss, growth failure, arrested head growth, anorexia, and lassitude are complications of chronic deficiency of leucine, isoleucine, or valine [
Puzenat et al 2004]. Iatrogenic cerebral essential amino acid deficiency can be a cause of significant neurologic morbidity in any individual ingesting a diet low in natural protein and high in prescription medical protein [
Strauss et al 2010,
Manoli et al 2016].
Recurrent oroesophageal candidiasis.
Candida infections are common in hospitalized persons with MSUD and may result from T-cell inhibitory effects of elevated plasma leucine [
Hidayat et al 2003] or iatrogenic immunodeficiency as a result of inadequate BCAA intake.
Thiamine-Responsive MSUD
It is not known with certainty if individuals with true thiamine-responsive MSUD exist. In general, such putative individuals have residual ex vivo BCKD enzyme activity of up to 40% normal and are not ill in the neonatal period, but present later in life with a clinical course similar to intermediate MSUD. To date, no person with "thiamine-responsive" MSUD has been treated solely with thiamine. Rather, they are treated with a combination of thiamine (doses ranging from 10 to 1,000 mg/day) and dietary BCAA restriction, making the in vivo contribution of thiamine impossible to discern [Chuang et al 2004]. Based on in vitro data, Chuang et al [2006] provided a biochemical model of thiamine responsiveness linked to specific pathogenic variants in the E2 subunit of BCKD. It is therefore reasonable to try thiamine supplementation under controlled dietary conditions in any individual with MSUD who has verified BCKDHB pathogenic variants.