NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Guideline Alliance (UK). Cystic Fibrosis: Diagnosis and management. London: National Institute for Health and Care Excellence (NICE); 2017 Oct 25. (NICE Guideline, No. 78.)

Cystic Fibrosis: Diagnosis and management.
Show details11Prevention of cross infection
Review questions:
- What is the effectiveness of cohorting on the basis of pathogen status versus not cohorting on the basis of pathogen status in reducing transmission of CF pathogens?
- What is the effectiveness of different models of segregating patients in reducing transmission of CF pathogens?
- What is the effectiveness of individual protective equipment in reducing transmission of CF pathogens?
- What is the effectiveness of the combination of cohorting, segregating and protective equipment in reducing transmission of CF pathogens?
11.1. Introduction
Measures to reduce the risk of cross infection with transmissible pathogens are widely accepted as good or optimum clinical practice in the care of people with cystic fibrosis. Prior to the 1990’s it was not unusual for people with cystic fibrosis to attend social events together and share hospital waiting areas. However, landmark evidence of the transmission of bacterial lung infection between people treated at the same hospital, and the emergence of new bacteria, has led to care providers adopting increasingly stringent infection control strategies.
Segregation in single rooms on a ward or in a clinic, cohorting clinics by pathogen status, discouraging social contact and the use of personal protective equipment are all strategies employed in isolation or combination in cystic fibrosis care in the UK. Despite acknowledgement that it is essential to use these measures to reduce the risk of cross infection, people with cystic fibrosis, families and carers can express feelings of anxiety and social isolation as a result, especially where such measures are employed in a varied or inconsistent manner.
11.2. Description of clinical evidence
The aim of this review was to determine the effectiveness of the different strategies (such as cohorting, segregation, or protective equipment) in reducing the transmission of cystic fibrosis pathogens.
The interventions that are reviewed are either cohort segregation by time (for example by clinic schedule), cohort segregation by location (for example separate clinics or separate wards), individual segregation by location (for example patients in separate rooms with en-suite facilities), use of protective equipment, or any combination of these interventions.
One single literature search was run for the review questions. We looked for systematic reviews, RCTs and prospective and retrospective comparative cohort studies that were conducted in Western countries. Studies based on registry and audit data from the UK were also eligible for inclusion. Conference abstracts of RCTs were considered if RCTs were unavailable. However, given that only 1 RCT and no cohort studies were identified for inclusion, before-and-after implementation studies were also considered eligible for inclusion where no data for critical outcomes was available from higher quality studies. Evidence from questionnaires conducted within cross sectional studies was also considered (for example for quality of life or patient satisfaction).
For full details see review protocol in Appendix D.
In total 16 studies were included in this review. There was 1 RCT (Hayes 2010), 3 surveys (Griffiths 2004, Russo 2006, Waine 2007) and 12 retrospective before-and-after studies (Chen 2001, France 2008, Frederiksen 1999, Griffiths 2005, Griffiths 2012, Hoiby & Pedersen 1989, Jones 2005, Lee 2004, McKay 2009, Savant 2014, Thomassen 1986, Whiteford 1995)
Depending on the study, interventions were implemented in the following setting: either outpatient, inpatient or mixed (both outpatient and inpatient) settings. For the purpose of structuring the review, interventions implemented in the same setting are grouped together.
In the outpatient setting, the following comparisons were assessed:
- Comparison 1. Cohort segregation by clinic times versus no cohort segregation (2 studies: Hayes 2010, McKay 2009)
- Comparison 2. Cohort segregation by location versus no cohort segregation (1 study: Lee 2004)
- Comparison 3. Combination of protective equipment + individual segregation versus incomplete protective equipment + incomplete individual segregation (1 study: Savant 2014).
In the inpatient setting, the following comparisons are assessed:
- Comparison 4. Cohort segregation by location versus no cohort segregation (2 studies: Chen 2001, Thomassen 1986)
- Comparison 5. Individual segregation by location versus usual care (1 study: Russo 2006).
When interventions were implemented both in the inpatient and outpatient setting, the following comparisons were assessed:
- Comparison 6. Cohort segregation versus no cohort segregation (7 studies: France 2008, Frederiksen 1999, Griffiths 2005, Griffiths 2012, Hoiby & Pedersen 1989, Jones 2005, Whiteford 1995)
- Comparison 7. Complete cohort segregation versus incomplete cohort segregation (1 study: France 2008)
- Comparison 8. Individual segregation versus usual care (1 study: Waine 2007)
- Comparison 9. Cohort segregation + individual segregation versus cohort segregation (2 studies: Chen 2001, France 2008)
- Comparison 10. Cohort segregation + individual segregation + protective equipment versus usual care. (1 study: Chen 2001)
- Comparison 11. Cohort segregation + individual segregation versus usual care (1 study: Griffiths 2004).
The reported size of the studies ranged from 39 to 232 participants with cystic fibrosis, however for some studies the total size was not reported. 3 studies included adults (France 2008, Jones 2005, Waine 2007), 2 included children and young people and their parents (Griffiths 2004, Russo 2006), 2 included infants and children (Hayes 2010, McKay 2009), 1 included infants, children and young people (Whiteford 1995), 1 included infants, children, young people and adults (Savant 2014). 4 included people receiving paediatric care, however the age range was not reported (Griffiths 2005, Griffiths 2012, Lee 2004, Thomassen 1986). In 3 studies age of the study population was not reported (Chen 2001, Frederiksen 1999, Hoiby & Pedersen 1989).
6 studies were conducted in the UK (France 2008, Jones 2005, Lee 2004, Russo 2006, Waine 2007, Whiteford 1995), 4 in the USA (Chen 2001, Hayes 2010, Savant 2014, Thomassen 1986), 2 in Denmark (Frederiksen 1999, Hoiby & Pedersen 1989), 4 in Australia (Griffiths 2004, Griffiths 2005, Griffiths 2012, McKay 2009)
A summary of the included studies is presented in Table 159. See study selection flow chart in Appendix F, study evidence tables in Appendix G, list of excluded studies in Appendix H, forest plots in Appendix I, and full GRADE profiles in Appendix J.
11.3. Summary of included studies
A summary of the studies that were included in this review are presented in Table 183.
Table 183
Summary of included studies.
11.4. Clinical evidence profile
The summary clinical evidence profiles for this review question (cross-infection control) are presented in Table 184 to Table 194.
Table 184
Summary clinical evidence profile: Comparison 1. Cohort segregation by clinic times versus no cohort segregation.
Table 194
Summary clinical evidence profile: Comparison 11. Cohort segregation + individual segregation versus usual care.
11.4.1. Outpatient care
Table 185
Summary clinical evidence profile: Comparison 2. Cohort segregation by location versus no cohort segregation.
Table 186
Summary clinical evidence profile: Comparison 3. Combination of protective equipment + individual segregation versus incomplete protective equipment + incomplete individual segregation.
11.4.2. Inpatient care
Table 187
Summary clinical evidence profile: Comparison 4. Cohort segregation by location versus no cohort segregation.
Table 188
Summary clinical evidence profile: Comparison 5. Individual segregation by location versus usual care.
11.4.3. Combined inpatient and outpatient care
Table 189
Summary clinical evidence profile: Comparison 6. Cohort segregation versus no cohort segregation.
Table 190
Summary clinical evidence profile: Comparison 7. Complete cohort segregation versus incomplete cohort segregation.
Table 191
Summary clinical evidence profile: Comparison 8. Individual segregation versus usual care.
Table 192
Summary clinical evidence profile: Comparison 8. Individual segregation versus usual care.
Table 193
Summary clinical evidence profile: Comparison 10. Cohort segregation + individual segregation + protective equipment versus usual care.
11.5. Economic evidence
No economic evaluations of strategies to prevent cross-infection were identified in the literature search conducted for this guideline. Full details of the search and economic article selection flow chart can be found in Appendix E and F, respectively.
This area was prioritised for de novo economic modelling, subsequently a cost-utility model was developed. The decision analytic model took the form of a decision tree (Figure 15) where the outcome (probability of transmissible pathogen) is associated with a treatment cost and utility value. The methods used to construct the model and the results of all analyses are reported in Appendix K. A summary of cost-effectiveness estimates are provided in Table 195 for ease of reference.
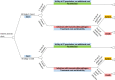
Figure 15
Decision tree.
Table 195
Summary of cost-effectiveness estimates.
11.6. Evidence statements
11.6.1. Outpatient care
11.6.1.1. Cohorting into different pathogens by clinic times
11.6.1.1.1. Comparison 1. Cohort segregation by clinic times versus no cohort segregation
Incidence of patients infected with transmissible pathogens
Low quality evidence from 1 RCT with 39 infants and children with cystic fibrosis showed no clinically significant difference in the incidence of P aeruginosa infections between cohorting patients into different pathogens by clinic times and no cohort segregation at 10 years follow-up.
Prevalence of patients infected with transmissible pathogens
Very low quality evidence from 1 observational study with ≈2,837 sputum cultures showed no significant difference in the prevalence of MRSA and non-mucoid P aeruginosa infections among infants and children with cystic fibrosis after segregation measures (consisting in segregating patients by age using a booking system) were put in place during the 4-year follow-up. However, a significant lower prevalence of mucoid P aeruginosa was observed during the same study period. The uncertainty for these outcomes could not be calculated.
Quality of life
No evidence was found.
Emotional function including anxiety and depression
No evidence was found.
Carer satisfaction
No evidence was found.
Patient satisfaction
No evidence was found.
Staff experience
No evidence was found.
Staff and patient compliance
Very low quality evidence from 1 observational study with staff looking after infants and children with cystic fibrosis, showed that the staff adherence to the segregation programme (using a clinic booking system) was over 90% during the 4-year follow-up period. This result is provided narratively. The total number of people included in the study was not reported.
11.6.1.2. Cohorting into different pathogens by location
11.6.1.2.1. Comparison 2. Cohort segregation by location versus no cohort segregation
Incidence of patients infected with transmissible pathogens
Very low quality evidence from 1 observational study with people with 232 people with cystic fibrosis receiving paediatric care suggested that the annual incidence of new growths of P aeruginosa, while fluctuating, showed no downward trend 9 years after segregation measures (consisting of separate clinics) were put in place, compared to previous usual care. This outcome was reported narratively only.
Prevalence of patients infected with transmissible pathogens
Very low quality evidence from 1 observational study with 2,769 patient months showed a clinically significant lower yearly prevalence of chronic P aeruginosa infections among people with cystic fibrosis receiving paediatric care 9 years after segregation measures (consisting of separate clinics) were put in place, compared to previous usual care. However, a clinically significant higher yearly prevalence of intermittent P aeruginosa infections was observed.
Quality of life
No evidence was found.
Emotional function including anxiety and depression
No evidence was found.
Carer satisfaction
No evidence was found.
Patient satisfaction
No evidence was found.
Staff experience
No evidence was found.
Staff and patient compliance
No evidence was found
11.6.1.3. Individual segregation
No studies have been identified.
11.6.1.4. Protective equipment
No studies have been identified.
11.6.1.5. Combination of strategies
11.6.1.5.1. Comparison 3. Combination of protective equipment + individual segregation versus incomplete protective equipment + incomplete individual segregation
Incidence of patients infected with transmissible pathogens
No evidence was found.
Prevalence of patients infected with transmissible pathogens
Very low quality evidence from 1 observational study with people with cystic fibrosis receiving paediatric care and aged 0 to 21 showed a significantly lower prevalence of P aeruginosa and MRSA infections calculated as average prevalence for 4 month intervals over a 5 year period after an infection prevention and control policy (consisting in a combination of individual segregation and protective equipment) was put in place, compared to the previous strategy (that consisted in a combination of incomplete protective equipment + incomplete individual segregation). The uncertainty for these outcomes could not be calculated. The total number of people included in the study was not reported.
Quality of life
No evidence was found.
Emotional function including anxiety and depression
No evidence was found.
Carer satisfaction
No evidence was found.
Patient satisfaction
No evidence was found.
Staff experience
No evidence was found.
Staff and patient compliance
No evidence was found.
11.6.2. Inpatient care
11.6.2.1. Cohorting into pathogen by location
11.6.2.1.1. Comparison 4. Cohort segregation by location versus no cohort segregation
Incidence of patients infected with transmissible pathogens
Very low quality evidence from 1 observational study with people with cystic fibrosis showed a lower annual incidence of B cepacia complex infection over 1 year after infection control measures (consisting in cohorting hospitalised patients on the basis of B cepacia colonisation status) were put in place, compared to the previous situation (no cohorting segregation). The significance and the uncertainty of this result could not be calculated. The age range and the total number of people in the study was not reported.
Very low quality evidence from 1 observational study with people with cystic fibrosis receiving paediatric care showed a clinically significant lower incidence of hospital-associated colonisation of P cepacia over 5 months after precautionary measures were implemented (consisting in admitting all patients with P cepacia to the same ward), compared to the previous situation (where only basic infection prevention measures were in place). The total number of people in the study was not reported
Prevalence of patients infected with transmissible pathogens
No evidence was found.
Quality of life
No evidence was found.
Emotional function including anxiety and depression
No evidence was found.
Carer satisfaction
No evidence was found.
Patient satisfaction
No evidence was found.
Staff experience
No evidence was found.
Staff and patient compliance
No evidence was found.
11.6.2.1.2. Comparison 5. Individual segregation by location versus usual care
Incidence of patients infected with transmissible pathogens
No evidence was found.
Prevalence of patients infected with transmissible pathogens
No evidence was found.
Quality of life
No evidence was found.
Emotional function including anxiety and depression
No evidence was found.
Patient and carer satisfaction
Very low quality from a cross-sectional study with 101 children and young people with cystic fibrosis and their parents suggested that a high percentage of children and parents support having segregated treatment (92% and 91% respectively).
Staff experience
No evidence was found.
Staff and patient compliance
No evidence was found.
11.6.2.2. Inpatient room with ensuite facilities
No studies have been identified.
11.6.2.3. Inpatient recreational facilities
No studies have been identified.
11.6.3. Combined inpatient and outpatient care
11.6.3.1. Cohorting into pathogens
11.6.3.1.1. Comparison 6. Cohort segregation versus no cohort segregation
Incidence of patients infected with transmissible pathogens
Very low quality evidence from 1 observational study with 119 people with cystic fibrosis showed a clinically significant lower monthly incidence of multiply resistant P aeruginosa strain 1 month after control measures (consisting in segregating patients based on PA strains), compared to the previous policy (where patients were separated on the basis of the PA status). The age of the people included in the study was not reported.
Very low quality evidence from 1 observational study with people with cystic fibrosis showed no clinically significant difference in the annual incidence of intermittent and chronic P aeruginosa 1 year after cohort isolation was introduced, compared to previous usual care. The age range and the total number of people in the study was not reported.
Very low quality evidence from 1 observational study with 115 infants, children and young people with cystic fibrosis showed no clinically significant difference in the incidence of B cepacia during the 6-month study period after segregation measures were introduced, compared to previous usual care. The total number of people included in the study was not reported.
Very low evidence from 1 observational study with adults with cystic fibrosis showed a higher annual incidence of Burkholderia species infections after changing infection control practices (consisting in partially segregating patients with Burkholderia species infection), compared to previous usual care. The significance and the uncertainty of this result could not be calculated. The total number of people in the study was not reported.
Prevalence of patients infected with transmissible pathogens
Very low quality evidence from 1 observational study with 119 people with cystic fibrosis showed no clinically significant difference in monthly prevalence of multiply resistant P aeruginosa strain 1 month after control measures (consisting in segregating patients based on PA strains), compared to the previous policy (where patients were separated on the basis of the PA status). The age of the people included in the study was not reported.
Very low quality evidence from 1 observational study with people with cystic fibrosis receiving paediatric care who were able to produce sputum showed a clinically significant lower prevalence of P aeruginosa epidemic strain 2 years after cohort segregation measures based on PA status were put in place, compare to previous usual care. The total number of people included in the study was unclear.
Very low quality evidence from 1 observational study with adults with cystic fibrosis showed a clinically significant higher annual prevalence of chronic P aeruginosa infection 1 year after simple segregation measures were put in place, compared to previous usual care. However, no clinically significant differences were found in the annual prevalence of transmissible P aeruginosa infection. In addition, a slightly higher annual prevalence of chronic infection with transmissible P aeruginosa strain was also observed, but the significance and the uncertainty of this outcome could not be calculated. The total number of people included in the study was unclear.
Quality of life
No evidence was found.
Emotional function including anxiety and depression
No evidence was found.
Patient and carer satisfaction
No evidence was found.
Staff experience
No evidence was found.
Staff and patient compliance
No evidence was found
11.6.3.1.2. Comparison 7. Complete cohort segregation versus incomplete cohort segregation
Incidence of patients infected with transmissible pathogens
Very low evidence from 1 observational study with adults with cystic fibrosis showed a lower annual incidence of Burkholderia species infections after changing infection control practices (consisting in cohorting patients with Burkholderia species infection), compared to the previous situation (partial cohort segregation). The significance and the uncertainty of this result could not be calculated. The follow-up and the total number of people included in the study were unclear.
Prevalence of patients infected with transmissible pathogens
Quality of life
No evidence was found.
Emotional function including anxiety and depression
No evidence was found.
Carer satisfaction
No evidence was found.
Patient satisfaction
No evidence was found.
Staff experience
No evidence was found.
Staff and patient compliance
No evidence was found.
11.6.3.1.3. Comparison 8. Individual segregation versus usual care
Incidence of patients infected with transmissible pathogens
No evidence was found.
Prevalence of patients infected with transmissible pathogens
No evidence was found.
Quality of life
No evidence was found.
Emotional function including anxiety and depression
No evidence was found.
Carer satisfaction
No evidence was found.
Patient satisfaction
Very low quality evidence from 1 observational study with 94 adults with cystic fibrosis indicated that the majority of the people (62.5%) who did not mix with others with cystic fibrosis felt that their quality of life did not suffer as a result of this prevention control strategy; whereas almost a quarter of the people (23.3%) who mixed with others with cystic fibrosis were concerned that their quality of life would suffer a ‘significant amount’ or ‘a great deal’ if they were to begin avoiding others. These results are provided narratively only.
Staff experience
No evidence was found.
Staff and patient compliance
No evidence was found.
11.6.3.2. Protective equipment
No studies have been identified.
11.6.3.3. Combination of strategies
11.6.3.3.1. Comparison 9. Cohort segregation + individual segregation versus cohort segregation
Incidence of patients infected with transmissible pathogens
No evidence was found.
Prevalence of patients infected with transmissible pathogens
Very low quality evidence from 1 observational study with people with cystic fibrosis showed a lower annual prevalence of B cepacia complex infection 3 years after infection control measures (consisting in cohorting hospitalised patients on the basis of B cepacia colonisation status and individual isolation) were put in place, compared to the previous situation (cohort segregation on the basis of B cepacia colonisation status only). The significance and the uncertainty of this result could not be calculated. The age and the total number of people included in the study was unclear.
Very low evidence from 1 observational study with adults with cystic fibrosis showed a lower annual prevalence of Burkholderia species infections 5 years after changing infection control practices (consisting in isolating patients with Burkholderia species infection in addition to cohort segregation), compared to the previous situation (cohorting patients with Burkholderia species infection). The significance and the uncertainty of this result could not be calculated. The total number of people included in the study was unclear.
Quality of life
No evidence was found.
Emotional function including anxiety and depression
No evidence was found.
Carer satisfaction
No evidence was found.
Patient satisfaction
No evidence was found.
Staff experience
No evidence was found.
Staff and patient compliance
No evidence was found.
11.6.3.3.2. Comparison 10. Cohort segregation + individual segregation + protective equipment versus usual care
Incidence of patients infected with transmissible pathogens
Very low quality evidence from 1 observational study with people with cystic fibrosis showed a lower annual incidence of B cepacia complex infection after a combination of infection control measures (consisting in cohorting hospitalised patients on the basis of B cepacia colonisation status, in addition to isolation and use of protective equipment) were put in place, compared to the previous situation (usual care). The significance and the uncertainty of this result could not be calculated. The follow-up, age and total number of people included in the study were not reported.
Prevalence of patients infected with transmissible pathogens
No evidence was found.
Quality of life
No evidence was found.
Emotional function including anxiety and depression
No evidence was found.
Carer satisfaction
No evidence was found.
Patient satisfaction
No evidence was found.
Staff experience
No evidence was found.
Staff and patient compliance
No evidence was found.
11.6.3.3.3. Comparison 11. Cohort segregation + individual segregation versus usual care
Incidence of patients infected with transmissible pathogens
No evidence was found
Prevalence of patients infected with transmissible pathogens
No evidence was found.
Quality of life
No evidence was found.
Emotional function including anxiety and depression
No evidence was found.
Patient and carer satisfaction
Very low quality evidence from 190 people with cystic fibrosis receiving paediatric care or their parents or carers indicated that both patients and carers showed an overall positive response to segregation measures. This results are provided narratively only.
Staff experience
No evidence was found.
Staff and patient compliance
No evidence was found.
11.6.4. Economic evidence statements
No published evidence on cost-effectiveness in people with cystic fibrosis was available for this review.
The economic model found that cohort segregation according to chronic P aeruginosa was cost-effective compared to no cohort segregation (3 studies).
The economic model found that cohort segregation according to intermittent P aeruginosa was not cost-effective compared to no cohort segregation (2 studies).
The economic model found that cohort segregation according to intermittent B cepacia complex was cost-effective compared to no cohort segregation in 1 study and cost-ineffective in 1 study.
The economic model found that the addition of protective equipment was cost-effective to prevent cross-infection with intermittent B cepacia complex (1 study) and intermittent P aeruginosa (1 study).
The economic model found that individual inpatient segregation (single inpatient rooms) according to intermittent B cepacia complex would be cost-effective compared to no individual inpatient segregation (shared ward) (2 studies).
11.7. Evidence to recommendations
11.7.1. Relative value placed on the outcomes considered
The aim of this review was to determine the effectiveness of the different strategies, such as cohorting, segregation, or protective equipment, in reducing the transmission of cystic fibrosis pathogens.
The committee identified incidence and prevalence of patients infected with transmissible pathogens as critical outcomes for decision making. Quality of life, emotional function, patient satisfaction, staff experience and staff and patient compliance were rated as important outcomes.
11.7.2. Consideration of clinical benefits and harms
The committee noted that there were existing NICE guidelines on preventing and controlling infection. Therefore, they incorporated a reference to these guidelines and focussed their recommendations on cross-infection concerns specific to people with cystic fibrosis.
The committee noted that the evidence showed mixed results with regards to whether incidence and prevalence of people infected with transmissible pathogens decreased after cohort segregation was implemented across the inpatient and outpatient setting or in the outpatient setting alone. There was some evidence that the incidence of B cepacia complex and hospital-associated P cepacia infections decreased after cohort segregation was implemented in the inpatient setting alone. Moreover, there was some evidence that prevalence of B cepacia complex infection decreased after individual segregation was added to cohort segregation across the inpatient and outpatient setting. The committee noted the lack of evidence assessing individual segregation as an intervention on its own in the outpatient setting alone. The committee noted that feelings of isolation may be a potential disadvantage of individual or cohort segregation. However, there was some evidence that people with cystic fibrosis and carers supported both cohort and individual segregation.
The committee noted that infection control would have to be implemented both in the inpatient and outpatient setting at the same time. Therefore, the committee recommended that arrangements to prevent cross-infection among people with cystic fibrosis should be based on a local infection control strategy that covers both outpatient and inpatient care. This strategy would cover interventions such as cleaning of rooms and equipment, closing the doors of rooms in the hospital or the outpatient clinic and effective ventilation in gyms to remove exposure of airborne cross-infection. Moreover, the committee recommended that arrangements to prevent cross-infection should also be based on a microbiological surveillance programme.
The committee noted that segregation interventions would be ineffective if people did not adhere to segregation rules or they met with each other outside of the health care setting. Therefore, a recommendation was prioritised to inform people with cystic fibrosis, their family members or carers and staff involved in their care about the risk of cross-infection and how to avoid it.
The committee agreed that a combination of cohort segregation and individual segregation is likely to be more effective than cohort segregation alone. Cross-infection can occur between people with cystic fibrosis who are designated as having the same pathogen but have different strains which may have differing virulence characteristics. For example, superinfection with different, more virulent epidemic strains of P aeruginosa, potentially as a result of cross-infection, can occur in people who already have P aeruginosa. Similarly, patients with less virulent species of B cepacia complex, for example Burkholderia multivorans, could become infected with the more virulent B cenocepacia if cohorting is based on B cepacia complex status without species differentiation or if patients have as yet undetected infection. If people were to be cohorted based on different pathogen strains, cohort interventions would become complex due to a relatively high number of different cohorts which may increase as understanding of individual pathogen virulence develops. Moreover, any information on a person’s infection status refers to the last sputum culture that was performed. Therefore, after acquisition of an infection there would be a time interval until the next sputum culture. During this period someone would be cohorted with people who are free of infection. Therefore, the committee concluded that separating all people with cystic fibrosis, regardless of their infection status, from each other was very important both in the outpatient and inpatient setting.
The committee agreed that each specialist cystic fibrosis clinic should be organised as to ensure that contact between people with cystic fibrosis is prevented both during the use of communal areas such as waiting areas, cafes and restrooms, and during attendance at diagnostic, treatment and pharmacy facilities. The committee noted that there were multiple options to separate people; for example, placing the person directly into a room, where the multidisciplinary team can go to conduct the visit.
The committee agreed that even if a clinic separates all people from each other, casual contact may still occur. Therefore, the committee agreed that a combination of cohorting and individual segregation was more effective than individual segregation alone and recommended that the local infection control strategy that covers outpatient and inpatient care should include cohorting. The committee noted casual contact might be more likely to happen in the outpatient setting because the whole patient journey would need to be taken into account. Additionally, it is more common that people attend for the use of diagnostic or treatment facilities without a prior booking in the outpatient setting.
Therefore, in addition to individual segregation, the committee agreed to prioritise a recommendation to keep people with transmissible or chronic P aeruginosa or B cepacia complex infection separate from people who do not have these infections, for example by using separate outpatient clinics. The committee noted that one way of cohorting would be to have separate buildings for separate patients, another way of cohorting would be to book appointments with people with a specific pathogen on one day of the week and for people with a different pathogen on a different day of the week.
Intermittent P aeruginosa infection is harder to diagnose given that tests with positive results would alternate with tests with negative results. Therefore, the committee noted that it would be difficult to cohort people with intermittent P aeruginosa infection from those who do not have this infection. Moreover, some evidence showed a higher prevalence of intermittent P aeruginosa infection after cohort segregation in the outpatient setting. However a lower prevalence of chronic P aeruginosa infection was observed in the same setting. Therefore, these trends may be due to treatment strategies rather than to cohort segregation because treatment may prevent the intermittent pathogen from becoming chronic. With regards to this point, the committee agreed that changes in prevalence and incidence may be due, not only to cross-infection, but also to other factors such as acquisition of infection from environmental sources and advances in treatment. Moreover, prevalence relates to survival. The evidence also showed no clinically significant difference in the incidence of intermittent P aeruginosa after cohort segregation across the inpatient and outpatient setting. Given the lack of evidence supporting segregation and considering the difficulties involved in diagnosing intermittent P aeruginosa, the committee decided not to be overly prescriptive and recommended to consider keeping people who have intermittent isolation of P aeruginosa separate from people who do not have this infection, for example by using separate outpatient clinics.
The committee noted that no evidence was identified on non-tuberculous mycobacteria. Therefore, no recommendation was drafted specific to this pathogen. However, the committee noted that all recommendations on cross-infection except for those two that refer to P aeruginosa and B cepacia complex should apply to all people with cystic fibrosis, irrespective of their pathogen status. Cohorting could be done based on any pathogen that a local cross-infection control strategy may deem relevant.
The committee noted that the inpatient setting is where people would be more likely to come into close contact with each other as they may spend days or weeks in the hospital. Therefore, the committee prioritised a recommendation to give people with cystic fibrosis individual rooms with en-suite facilities. This recommendation is consistent with the NHS service specifications for cystic fibrosis. The committee noted that, in practice, people with cystic fibrosis are sometimes segregated into individual rooms but have contact in communal areas, this is to be avoided. Therefore, the committee recommended to help inpatients with cystic fibrosis plan their attendance to avoid contact with each other, for example when they use hospital restaurants, schools, recreational areas (such as the gym) and diagnostic, treatment and pharmacy facilities. The committee noted that a timetable could be used for school and gym attendance. Moreover, for diagnostic and treatment procedures there could be a computerised system suggesting who should enter specific areas at what time. Finally, systems should be in place to ensure that if someone with cystic fibrosis comes to the hospital, contact with the inpatients is prevented.
There was some evidence that prevalence of people infected with transmissible pathogens decreased after a combination of individual segregation with protective equipment was implemented in the outpatient setting alone. Incidence of infection decreased after a combination of cohort and individual segregation and protective equipment was implemented across the inpatient and outpatient settings. However, there was no evidence on the use of protective equipment alone. The committee noted that the use of protective equipment is difficult to implement. People’s motivation is limited because of stigma and because it protects other people but not themselves, as a result they tend to take it off. Therefore, the committee decided not to make a recommendation on protective equipment.
11.7.3. Consideration of economic benefits and harms
11.7.3.1. Cohort segregation
P aeruginosa
The economic model showed that cohort segregation was cost-effective to reduce the transmission of chronic P aeruginosa compared to no cohort segregation, but was not cost-effective to reduce the transmission of intermittent P aeruginosa. This difference was driven by Lee 2004 who found that a fall in the number of cases with chronic infection was associated with a rise in those classified as intermittent. Following this, the committee noted that it is difficult to define an intermittent infection, adding that the distinction is even more difficult in children who cannot produce sputum.
Based on the evidence presented to them, and their clinical expertise, the committee agreed they could justify a recommendation to cohort people with transmissible P aeruginosa and chronic P aeruginosa as most clinics implement this as a cost-effective measure.
The committee wanted to enable clinics that had the ability to differentiate pathogens for intermittent P aeruginosa to do so, although they agreed that the findings from Lee 2004 were limited. This was because Lee 2004 did not genotype the participants’ P aeruginosa isolates in order to infer if their strains were transmissible and potentially acquired from the clinic. Given that the findings from Lee 2004 can produce an unreliable estimate of cost-effectiveness, and cohort segregation depends on the ability of the clinic to identify intermittent P aeruginosa, the committee reduced the strength of their recommendation to consider cohorting people with cystic fibrosis who have intermittent P aeruginosa to reflect the weaker evidence compared to chronic P aeruginosa.
The committee discussed the potential harms of seeing less people with cystic fibrosis using cohorted clinics. They advised that waiting times could increase in the short term, but in the longer term the number of people with a transmissible pathogen would decrease. This decrease would subsequently increase the number of people that could be seen during “usual” clinics. The committee referred to their recommendations on service delivery. People with cystic fibrosis, and their family members or carers, can contact the MDT at any them when they have urgent enquiries, to minimise delays in identification and management.
The committee stated that genotyping is currently used by cystic fibrosis clinics to keep a track on the number of epidemic strains. This identification can prompt further investigation when there is a significant increase in numbers. Cystic fibrosis clinics currently follow CF Trust laboratory standards and infection control guidelines that advise surveillance should be performed on all new isolates and annually on those from people infected with P aeruginosa. Therefore, genotyping all isolates of P aeruginosa, in order to provide more up to date information for accurate clinic segregation, as a means to reduce cross-infections with transmissible pathogens could not be achieved with existing laboratory resources. The committee advised that it would be relatively easy to set up in-house polymerase chain reaction (PCR) methods for detecting the presence, or otherwise, of known epidemic strains. However, this work would require dedicated project budget and recruitment of technical and scientific staff to deliver it. Moreover, to achieve the required level of discriminatory power, more complex typing methodologies such as variable number of tandem repeats (VNTR) typing or whole genome sequencing (WGS) would be necessary, but would raise costs and workloads even further. Overall, the committee considered a recommendation was needed to ensure microbiological surveillance and local infection control strategies were in place. The committee were reluctant to specify how such arrangements should be achieved as this would depend on the expertise and prevalence of pathogens within each clinic.
In addition, there is insufficient evidence to enable full understanding of the results regarding detailed genotyping. This is especially so as there is data suggesting significant intra-patient, and intra-isolate, variability when detailed methods are used referring to the study by Darch 2015. Therefore, a recommendation in favour of regular, detailed genotyping was not prioritised by the committee. As a result, the committee wanted to make a research recommendation to define optimal microbiological methods and their role to inform infection control strategies, to assess if the benefits of regular genotyping could justify the additional resources. However, this was later deprioritised as it was considered a public health issue for local areas to devise their own policies.
B cepacia complex
One study (Whitford 1995) found cohort segregation according to B cepacia complex to be cost-effective compared to no cohort segregation. Conversely, the second study (France 2008) found no cohort segregation to dominate cohort segregation according to B cepacia complex, as no cohort segregation was less expensive and more effective than cohort segregation. However, given that complete cohort segregation, with the addition of single inpatient rooms, dominated (less expensive and more effective) incomplete cohort segregation. The committee agreed that people infected with B cepacia complex also should be cohorted at outpatient clinics and inpatient care. Following this, the committee noted that the species of B cepacia complex must be determined otherwise those with B cenocepacia could be cohorted with multivorans, which would be inappropriate and hazardous. The committee noted that the prevalence of B cepacia complex is decreasing (circa 3%) according to UK registry data, indicating that current infection control measures to cohort these people with cystic fibrosis are working.
11.7.3.2. Individual segregation
The committee advised that, unlike cohort segregation, individual segregation acts as a preventative measure as there is less need to worry about the specific pathogen. The committee added that individual segregation is a strategy currently applied in cystic fibrosis clinics in order to reduce the risk of cross-infection with transmissible pathogens.
The committee agreed that the additional cost of single rooms, compared to beds in shared rooms, was best reflected by NHS Estates 2005 (an additional £10.61/day in 2016 prices) and potentially overestimated when an additional 25% was assumed.
The clinical evidence was not meta-analysed as the studies were too heterogeneous. Consequently, the committee considered the cost-effective results produced by each of the studies and concluded that France 2008 was the study most reflective of UK clinical practice today. Based on France 2008, the dominant strategy (less expensive and more effective strategy) was to admit people with cystic fibrosis to single rooms when an additional £10.61/bed/day or 25%/bed/day compared to beds in shared rooms was assumed. As a result, the committee prioritised a recommendation for all inpatients to have their own single room as the economic model provided additional justifications for current practice to be followed.
The committee considered the acceptable additional cost given the additional QALY gain, based on the study by Jones 2005 who compared incomplete cohort segregation to no cohort segregation. Incomplete cohort segregation provided inpatient rooms with en-suite facilities, whereas only 2 of the 11 rooms in no cohort segregation strategy included en-suite facilities. Incomplete cohort segregation also meant people without chronic P aeruginosa attended different outpatient clinic appointments to other people with cystic fibrosis. The committee acknowledged that the incidence of superinfection by transmissible strains among trial participants (Jones 2005) already infected with chronic P aeruginosa was sporadic, potentially resulting in a dominated (more expensive and less effective) strategy.
However, incomplete cohort segregation produced more QALYs than no cohort segregation, with a fall in the incidence of intermittent P aeruginosa from 9.7% to 0% each year, following the new strategy. Based on this benefit, the committee believed that incomplete cohort segregation could be cost-effective. The committee added that the majority of single rooms include en-suite facilities and referred to the NHS Commissioning document on service specifications that states “Every CF patient will be in their own room, with en-suite facilities to minimise the risk of cross infection and to enable them to continue life as normally as possible”. Given that the majority of centres follow this, the committee agreed a recommendation in favour of en-suite facilities that would not lead to substantial increase from current resource use.
11.7.3.3. Protective equipment
The new strategies implemented by Savant 2014 and Chen 2001 that included protective equipment to prevent intermittent infections were cost-effective (dominant). However, it is important to note that the effect of protective equipment alone could not be disaggregated into a separate effect measure from the combination of strategies applied. Following this, the committee agreed that a dominant result was unlikely to be driven by the protective equipment as Chen 2001 also cohorted their participants according to B cepacia complex infection and Savant 2014 applied a “no-waiting” room policy. The committee believed this cohorting to be the true cost-effective strategies.
Despite the low cost of protective equipment, the committee agreed, based on their knowledge and expertise, that there was not enough evidence to support the use of protective equipment as compliance outside of a trial setting would be low, due to the negative impact the equipment has on social interaction.
11.7.3.4. Other considerations
The committee agreed that communal areas in cystic fibrosis clinics promote patient contact and increase the risk of infection from transmissible pathogens. The acknowledged the opportunity cost of the space would be of greater value in other areas of the hospital. For these reasons, the committee made a recommendation to manage the use of communal waiting areas in outpatient clinics. The committee considered the benefits from closing clinic room doors. This is a very simple, quick and costless way to prevent transmissions that can be overlooked, or stopped, by reluctant parents. However, a recommendation was not prioritised.
11.7.4. Quality of evidence
All the evidence included in the review was of very low quality as assessed by GRADE.
One RCT was included. This study had unclear risk of selection bias, performance bias, detection bias, attrition bias and outcome reporting bias.
Most included studies were retrospective uncontrolled before-after studies. In some of these studies, the intervention and comparison groups were drawn from years, or time intervals, that were considerably distant in time. These time intervals increased the likelihood that the intervention group may have been exposed to additional interventions affecting incidence or prevalence. Moreover, in some studies the frequency of cultures performed to detect relevant infections was unclear or only annual. Therefore, people that were considered “at risk” when the intervention started may have already had the infection. Consequently, there was a high risk of selection bias. Most studies did not control for any factor, therefore, there was a high risk of comparability bias. Furthermore, many studies looked at changes in prevalence and incidence without making a distinction between transmissible strains and unique strains. Most studies did not use genotyping in order to understand whether changes in incidence or prevalence were related to cross-infection. Therefore, there was high risk of bias in relation to the outcomes of incidence and prevalence.
Three surveys were included. Only 1 study assessed comparability between respondents and non-respondents and all 3 studies had high risk of selection bias. None of the 3 studies controlled the analysis for possible confounders, therefore, there was high risk of comparability bias.
11.7.5. Other considerations
There was some evidence that staff adherence to cohort segregation was high. This indicated that cohort segregation was feasible.
No equality issues were identified by the committee for this review question.
The committee discussed the need for a research recommendation in this area, but they agreed it was not needed. They noted it is the responsibility of local areas to devise their own policies and is primarily a public health issue. They acknowledged there is a NICE guideline on infection.
11.7.6. Key conclusions
The committee concluded that people with cystic fibrosis should avoid contact with each other irrespective of their infection status. Moreover, cohorting should be implemented in addition to individual segregation, to reduce chances of casual contact between people with different infection status. The minimum requirement in terms of cohorting is that people with transmissible or chronic P aeruginosa or B cepacia complex infection should be kept separate from people who do not have these infections, for example by using separate outpatient clinics. Additionally, outpatient and inpatient care facilities should follow their local infection control strategy to prevent cross-infection.
11.8. Recommendations
- 133.
For recommendations on preventing and controlling infection, see the NICE guidelines on infection control in primary and community care and healthcare-associated infections, and the NICE quality standard on infection prevention and control.
- 134.
To prevent cross-infection among people with cystic fibrosis in outpatient and inpatient care, use microbiological surveillance and a local infection control strategy that includes cohorting.
- 135.
Inform people with cystic fibrosis, their family members or carers (as appropriate) and staff involved in their care about the risk of cross-infection and how to avoid it.
- 136.
Each specialist cystic fibrosis clinic should be organised to prevent cross-infection. Separate people individually during the clinic, including by organising:
- the use of communal areas
- attendance at diagnostic, treatment and pharmacy facilities.
- 137.
Keep people with transmissible or chronic Pseudomonas aeruginosa or Burkholderia cepacia complex infection separate from people who do not have these infections, for example by using separate outpatient clinics.
- 138.
Consider keeping people with cystic fibrosis who have intermittent isolation of Pseudomonas aeruginosa separate from people who do not have this infection, for example by using separate outpatient clinics. Help people with cystic fibrosis plan their inpatient attendance to avoid contact with each other, for example when they use:
- hospital restaurants, schools and recreation areas
- diagnostic, treatment and pharmacy facilities (see Information and Support).
- 139.
During inpatient care, give people with cystic fibrosis individual rooms with en-suite facilities.
- Prevention of cross infection - Cystic FibrosisPrevention of cross infection - Cystic Fibrosis
- Homo sapiens isolate CHM13 chromosome 11, alternate assembly T2T-CHM13v2.0Homo sapiens isolate CHM13 chromosome 11, alternate assembly T2T-CHM13v2.0gi|2194973393|gnl|ASM:GCF_009914825 ef|NC_060935.1||gpp|GPC_000012750.1||gnl|NCBI_GENOMES|119571Nucleotide
- Taxonomy Links for Nucleotide (Select 2462527291) (1)Taxonomy
- tubby protein homolog isoform X2 [Homo sapiens]tubby protein homolog isoform X2 [Homo sapiens]gi|2462527292|ref|XP_054225783.1|Protein
- Homo sapiens isolate:CHM13Homo sapiens isolate:CHM13Homo sapiens isolate:CHM13 RefSeq Genome sequencing and assemblyBioProject
Your browsing activity is empty.
Activity recording is turned off.
See more...