NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
The Drosophila light-activated channel TRP is the founding member of a large and diverse family of channel proteins that is conserved throughout evolution. These channels are Ca2+ permeable and have been implicated as an important component of cellular Ca2+ homeostasis in neuronal and nonneuronal cells. The power of the molecular genetics of Drosophila has yielded several mutants in which constitutive activity of TRP leads to a rapid retinal degeneration in the dark. Metabolic stress activates rapidly and reversibly the TRP channels in the dark in a constitutive manner by a still unknown mechanism. The link of TRP gating to the metabolic state of the cell is shared also by mammalian homologues of TRP and makes cells expressing TRP extremely vulnerable to metabolic stress, a mechanism that may underlie retinal degeneration and neuronal cell death.
Introduction
The visual system of Drosophila melanogaster1 has been the subject of genetic analysis through isolation of visual mutants2 and application of molecular genetics combined with powerful electrophysiological and biochemical functional tests (for review see refs. 1–5). One of the key proteins that have been discovered due to the power of the Drosophila genetics is the protein designated by Minke Transient Receptor Potential (TRP) because of the unique phenotype of the trp mutant.6 While genetic evidence has unequivocally demonstrated that phospholipase C is absolutely required for light excitation in Drosophila photoreceptors,2,7 a major unresolved question of Drosophila phototransduction is the gating mechanism of light-sensitive channels TRP and TRP-like (TRPL) and the identity of the second messenger of excitation.1
Photoreceptor degeneration is a phenomenon, which appears in strong alleles of almost every phototransduction-defective mutant of Drosophila. Given the abundance of the signaling molecules in the microvilli (the signaling membranes of the photoreceptor cell) and the crucial roles of these molecules in the assembly and function of the phototransduction machinery, mutants defective in the signaling proteins are likely to show degeneration.1 Because of the great interest in neuronal cell death in general and in human retinal degeneration, a search for mammalian homologues of Drosophila genes has been recently pursued with much effort. Indeed, mammalian homologues of the Drosophila genes rdgB,8,9 rdgC,10,11 ninaC12 and trp13 have been found in mammalian retina. These genes cause photoreceptor degeneration in Drosophila upon malfunction. However, the function of these molecules in vertebrate cells in not clear. Despite extensive investigation, relatively little is known about the molecular mechanisms underlying retinal degeneration due to mutations in the above genes also in invertebrate species.
It has been recently found that the TRP channel is involved in retinal degeneration of several Drosophila mutants in which constitutive activity of TRP seems to cause extremely rapid retinal degeneration.14,15 Therefore, the phenomenon of constitutive activity of TRP and TRPL channels in the dark and the ensuing influx of Ca2+, which possibly lead to retinal degeneration are the main topics of this review.
The Drosophila TRP and TRPL Channels
The Molecular Structure of Drosophila TRP and TRPL
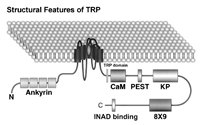
The trp gene was cloned by Montell and Rubin16 and by Wong and colleagues.17 The molecular structure of the TRP protein shows multiple domains that are likely to play an important role in cellular functions.3 TRPL was isolated using a screen to detect calmodulin binding proteins and shared overall 40% identity with TRP with much greater similarity (∼70%) in the putative transmembrane regions.18 The N- and C-termini of TRP and TRPL both contain a number of recognizable motifs, but their function in TRP and TRPL is unknown (Fig. 1). Both TRP and TRPL have weak but significant homologies to a known channel subunit of vertebrate voltage-gated Ca2+ channel (the dihydropyridine receptor). By analogy to voltage-gated K channels and the cyclic nucleotide-gated channels, both trp and trpl gene products represent subunits of putative tetrameric channels. Since null trp or trpl mutants both respond to light, each can clearly function without the other. However, heterologous co-expression studies and co-immunoprecipitation led to the suggestion that in wild-type (WT) flies the light-induced conductance was composed of TRP or TRPL homomers and TRP-TRPL heteromultimers.19 Detailed in situ measurements of biophysical properties including ionic selectivity and single channel conductance questioned this conclusion.20 A recent study has shown that there is an additional subunit(s) called TRPγ,21 which is highly enriched in the photoreceptor cells. The N-terminal domain of TRPγ dominantly suppressed the TRPL-dependent transient receptor potential on a trp mutant background, suggesting that TRPL-TRPγ heteromultimers contribute to the photoresponse. Furthermore, TRPL and TRPγ coimmunoprecipitate, suggesting that physical interaction between these proteins forms heteromeric channels.21
TRP and TRPL Constitute the Light-Sensitive Channels
Whole-cell voltage clamp recordings applied to isolated Drosophila ommatidia22,23 makes it possible to examine the hypothesis that TRP is a Ca2+ channel/transporter.24 The experiments of Hardie and Minke revealed that the fundamental defect in the trp mutant is a change in the ionic selectivity of the light-sensitive conductance. Specifically, the relative Ca2+ permeability of the light-sensitive conductance in trp mutant was reduced by a factor of ten,20,25 along with a significant change in the relative permeability to different monovalent ions. The reduced Ca2+ permeability in the trp mutant has also been corroborated by the demonstration of a reduced light-induced Ca2+ influx using Ca2+ indicator dyes26,27or Ca2+ selective microelectrodes.28
Zuker and colleagues 29 isolated a null mutant of the trpl gene. Under physiological conditions, the trpl mutation has relatively small influence on the light response.29,30 However, unlike in WT flies the response to light in trpl is completely abolished by La3+, which specifically blocks TRP at low concentration25,31,32 or in the double mutant combination (trp;trpl) indicate that TRP and TRPL channels make up all the light-activated channels or are required for their activation (for details see refs. 20,33, rev. 3, 34, 35).
The TRP Family of Channel Proteins
The “TRP-Homologue” Group
On the basis of similarity to Drosophila TRP and TRPL sequences, new mammalian TRP homologues have been cloned using database searches of expressed sequence tags (EST), RT-PCR or expression-cloning strategies. Seven major groups termed TRPC1-7 have been cloned and sequenced (for review see ref. 36). The TRPC homologues have been cloned from human, mouse, rat, rabbit, bovine and Xenopus. Six characteristic features of the Drosophila TRP and TRPL proteins have been found to be common to most members of the “TRP-homologue” group:37 i) The predicted topology of six (but see ref. 38) transmembrane segments (S1S6) including the typical pore region loop between transmembrane regions S5 and S6. ii) The charged residues in the putative S4 helix, which usually underlies voltage gating are replaced by noncharged residues. iii) Three to four ankyrin repeats are found at the N terminal. iv) A proline-rich sequence is found in the C terminal domain.39 v) The TRP domain exists in all members of this group13 vi) Calmodulin binding site is found in the C terminal domain (see Fig. 1).40
Birnbaumer and colleagues and Schultz and colleagues39,41,42 have classified the vertebrate members of the “TRP-homologues” group into four subgroups according to their primary amino acid sequence. Type 1 includes all isoforms of TRPC1. Type 2 includes the TRPC2 homologues, which have the lowest similarity to the other groups. Type 3 includes TRPC3, TRPC6 and TRPC7 channel proteins. Type 4 includes TRPC4 and TRPC5 channels and has a higher similarity to the TRPC1 group relative to the other groups.
TRPC1
TRPC1 was the first mammalian homologue of TRP that was cloned independently by two laboratories using EST database of human fetal brain cDNA library. The expression pattern of TRPC1 made by several groups43–47 show that TRPC1 is most abundantly expressed in the brain, heart, testes, ovary, bovine aortic endothelial cells and is barely detectable in the liver or adrenal gland. Interestingly, the rat orthologue changes its expression pattern during brain development46 suggesting that it has a role in developmental signaling.
TRPC2
The human TRPC2, which was the first cloned TRPC2 gene,43 is probably a pseudogene since several independent ESTs show mutations introducing early stop codons. A bovine homologue of this pseudogene, which is mainly expressed in the testes, spleen and liver was cloned and sequenced.38 Later, a full-length 1072 amino acids (aa) TRPC2 isoform from mouse48 and 1172 TRPC2 isoform from bovine (GenBank) were cloned. Thus TRPC2 is the longest of all vertebrate “TRP-homologue” group having longer cytoplasmic N-terminal domain. In contrast to the TRPC1, TRPC2 is tissue specific and in the mouse it was found only in the testes and vomeronasal organ (VNO). This organ plays a key role in the detection of pheromones, which are chemicals released by other rats and elicits stereotyped sexual behavior.49 Turning TRPC2 gene into a pseudogene in humans fits well with the hypothesis that the vomeronasal organ is no longer functional in apes. TRPC2 shows only 25% to 30% identity to Drosophila TRP.
Recent studies revealed that InsP3 is a second messenger of vomeronasal receptor neurons of snake.50 This fact fits with TRPC2 as a channel, which is activated by the inositol-lipid signaling cascade.
A TRPC2 isoform is highly enriched in the sperm and seems to have an important function in the fertilization of mice.51
TRPC3, TRPC6 and TRPC7
This subgroup, especially the TRPC3 homologues have been thoroughly studied by a variety of functional tests and, therefore, significantly contribute to our understanding of TRP function and gating mechanism. The molecular structure of TRPC3 shows six features of the TRP family listed above. Human, mouse and rat orthologues of TRPC3 and TRPC6 have been cloned, as well as a mouse orthologue of TRPC7.
TRPC3 homologues are predominantly expressed in the brain and at much lower levels also in ovary, colon, small intestine, lung, prostate, placenta and testes.41,52,53 TRPC6 expression is highest in the brain but it is also expressed in the lungs and ovaries. Interestingly, the development of tumors is associated with down regulation of TRPC6 isoform in a mouse model for an autocrine tumor.54
TRPC7 from mouse turned out to be very similar (81% identity and 89% similarity) to TRPC3 and also to TRPC6 (75% identity and 84% similarity) while only 33% identical (53% similar) to Drosophila TRP and TRPL. TRPC7 is mainly expressed in the heart, lung and eye and at a lower level in the brain, spleen and testes.55
TRPC4 and TRPC5
TRPC4 and TRPC5 channels are similar in structure to TRPC1. The rabbit and mouse orthologues of TRPC5 show 69% sequence identity to bovine TRPC4 and 41% identity to Drosophila TRP. The expression patterns of TRPC4 and TRPC5 are very different. TRPC5 mRNA is expressed predominantly in the brain,56 while TRPC4 is expressed in the brain but also in the adrenal gland and at a much lower level in the heart, lung, liver, spleen kidney testes, thymus, aorta and uterus.57 Of special interest are TRPC4 isoforms, which are expressed in vascular endothelial cells of various species (mouse, human and bovine).58
The “TRP-Related” Group
The “TRP-homologue” group has been discovered due to sequence homology to Drosophila TRP. Additional members of the TRP family with much smaller sequence homology to Drosophila TRP and TRPL have been cloned and sequenced. This group has been designated “TRP related” group.37 The various members of the “TRP-related” group were found following studies aiming to explore specific sensory transduction pathways or specific genetic diseases. These pathways include olfaction and osmolarity in C. elegans,59 defects in mechanosensory transduction in C. elegans and Drosophila 60 defects in pain mechanism in primary afferents of dorsal root ganglia,61 Ca2+ transport in Ca2+ transporting epithelial cells,62 polycystic kidney disease in cells expressing polycystin genes, PKD,63 tumor suppression in the skin (rev. ref. 13) and mucolipidosis type IV in cells expressing defective mucolipin.64,65 The different approaches resulted in a wealth of functional data about “TRP-related” members, in contrast to most mammalian members of the “TRP-homologue” group, whose functions in most of the native tissues are largely unknown. In contrast to mammals, none of the five C. elegans members of the “TRP-related” group or other 4 C. elegans members of the “TRP-homologue” subfamily have been characterized at the cellular level, neither in the native cells nor in heterologous systems.39
In summary, the TRP family of channel proteins reveals a high structural diversity. This family has been divided on the basis of primary amino acid sequence into six subfamilies.13,37 The founding member of this family, the Drosophila TRP shares structure homology with all subfamilies mainly at the region of the ion pore, strongly suggesting that the fundamental function of all members of this family is to constitute cation channels. Additional structural features that are common to most, but not all subfamilies are the ankyrin repeats at the N-terminal and the TRP domain at the C-terminal side of the transmembrane domain. In general, the diverse structural features of members of the TRP family strongly suggest that members of this family are involved in a wide range of cellular functions.
Constitutive Activity of the TRP Channel by Mutations and by Metabolic Stress May Underlie Retinal Degeneration
Mutations in the Transmembrane Domain of TRP Cause Rapid Photoreceptor Degeneration
Our understanding of TRP function and regulation has been augmented in the past by the availability of flies mutated in trp. However, especially informative should be flies that possess a TRP protein altered in its function. unfortunately, the known Drosophila trp mutants either completely lack TRP protein or have a highly reduced amount of functionally normal TRP. Furthermore, attempting to directly mutate the trp gene itself, by way of systematic site-directed mutagenesis, failed to yield any significant information on TRP function or structure. This is because mutated proteins were either unstable or not expressed at all in transgenic flies (leading to a full null phenotypes) or, alternately, the flies exhibited a completely wild-type phenotype, implying that those modifications had no phenotypic consequences (unpublished results). Recently, Pak and colleagues have identified a novel trp mutant (named TrpP365), which genetically mapped to the trp locus and has three mutations in the transmembrane domain of TRP.15 TrpP365 seems to be the first trp allele that expresses a modified (yet presumably active; see below) TRP protein. Phototransduction in TrpP365 mutant flies is aberrant, however; instead of displaying the classical trp null phenotype (a receptor potential that declines to the dark level during illumination) the TrpP365 mutation leads to a total absence of light response, with a constitutive current in the dark (see below). Strikingly, unlike any other trp alleles, these mutants also show extremely rapid retinal degeneration. Thus, the TrpP365 mutant provides us with a first and unique opportunity to characterize essential mechanisms underlying the physiological function of TRP and the TRP-dependent light-activated conductance.15
Functional Analysis at the Single Cell Level by Whole-Cell, Patch-Clamp Recordings Revealed Constitutive Activity of the TRP Channels
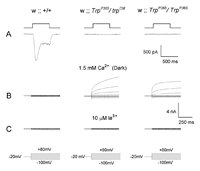
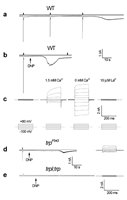
Whole-cell voltage clamp recordings were performed on R1-6 photoreceptors in dissociated ommatidia preparations of TrpP365 homozygotes, TrpP365 heterozygotes, and wild type. The light-induced current (LIC) of the heterozygote TrpP365/+ was indistinguishable from that of wild-type flies. This was not true for most of the ommatidia of the heteroallele TrpP365/TrpCM. In contrast, TrpP365/TrpP365 homozygote did not respond to light of any intensity. Large fraction (20%–70%) of the TrpP365/TrpCM heterozygote ommatidia did not respond to light, like the TrpP365/TrpP365 (Fig. 2A). The LICs of TrpP365/TrpCM flies revealed abnormal features such as highly reduced sensitivity to light and abnormally slow kinetics.
To investigate the reason for the inability of cells to respond to light, after the establishment of whole-cell recordings we stepped the holding voltage to different membrane potentials in the range between +80 mV to −120 mV in steps of 20 mV in the dark. In WT cells no significant currents were observed. In contrast, in the nonresponsive mutants, at positive membrane potentials large outward currents were recorded with strong outward rectification (Fig. 2B). The constitutive currents, which were recorded in a large fraction of TrpP365/TrpCM heterozygote cells and in all TrpP365/TrpP365 cells, were very similar to the current, which reflects activation of the TRP channels of wild-type cells during light. However, in sharp contrast to wild-type photoreceptors, the few TrpP365/TrpP365 cells from which recordings could be made and in all light-insensitive TrpP365/TrpCM cells, voltage steps elicited outward currents in the dark from the moment the recording configuration was established.
The constitutive current of both homozygote and heterozygote TrpP365 cells had all the properties of the TRP-dependent current: In Ca2+-containing medium large outward currents were observed in the homozygote and heterozygote TrpP365 cells, and these currents were very similar to these of WT cells under continuous light conditions. In addition, application of 10 μM La3+ to the extracellular medium blocked the TRP-dependent current and the constitutive currents in a very similar manner (Fig. 2C). Since these currents are mediated by the TRP channel, the TRP channel in these photoreceptors appears to be already open at the time the recording configuration is being established in the nonresponding cells of the TrpP365/TrpCM heterozygote and in all TrpP365/TrpP365 cells but not in WT ommatidia.
The above study thus shows that the TrpP365 mutation highly increased the probability of the TRP channels to open in the dark in correlation to the TrpP365 dosage. Strikingly, constitutively activity of TRP channels was recorded even in cells that do not show any sign of morphological degeneration (i.e., in TrpP365/TrpCM heterozygote cells), thus suggesting that the constitutive activation of the channels precedes the degeneration.15
Constitutive Activity of the TRP and TRPL Channels in the rdgA Mutant Causes Rapid Retinal Degeneration
Mutations in the Drosophila retinal degeneration A ( rdgA) gene causes rapid retinal degeneration as early as at the pupa stage.66 Retinal degeneration in the rdgA mutant is of particular interest because it has been convincingly shown that retinal degeneration in the rdgA mutant is due to mutation in the structural gene, which encodes for eye-specific DAG-kinase.67 The localization of the rdgA DAG-kinase in the Sub Microvillar Cisternae (SMC), an extention of the smooth endoplasmic reticulum, and the need for its product phosphatidic acid for resynthesis of phosphoinositides makes RDGA a key enzyme for synthesis of the PIP2, which is the substrate for PLC activity. In a recent report it has been shown that the rdgA phenotype is rescued by the introduction of the eye-specific DAG-kinase by germ-line transformation.68 Furthermore, construction of a double mutant rdgA;inaC, lacking both DAG-kinase and the eye-specific PKC, which did not affect the induction of retinal degeneration, ruled out the possibility that persistent activation of PKC is responsible for retinal degeneration in the rdgA mutant. Based on previous determination of DAG in WT and rdgA flies showing that DAG content is not increased in rdgA flies,69 the authors suggested that insufficient production of phosphatidic acid rather than excessive accumulation of DAG is responsible for retinal degeneration in rdgA. Hardie and colleagues have recently shown that the TRP and TRPL channels are constitutively active in rdgA photoreceptors. Strikingly, early degeneration and some responsiveness to light were rescued in rdgA;trp double mutants lacking TRP channels.14 Interestingly, it has been recently shown that exogenous polyunsaturated fatty acids (PuFAs), a possible product of DAG, activate the TRP and TRPL channels in the dark.70 Based on the effect of PuFAs on TRP, Hardie and colleagues have suggested that the constitutive activity of the TRP and TRPL channels in the rdgA mutant arise from accumulation of DAG. Accumulation of DAG was suggested to lead to excessive production of PuFA by DAG lipase, although this suggestion has not been supported experimentally.14
Metabolic Stress Constitutively Opens the TRP and TRPL Channels in the Dark at a Late Stage of the Cascade
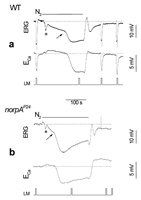
Anoxia is known to rapidly and reversibly depolarize the photoreceptor cells of the fly in the dark, possibly via openings of the light-activated channels. It is well-established that Ca2+ influx into the photoreceptors takes place almost exclusively via the TRP and TRPL channels.25,28 Therefore, Ca2+ influx was measured during anoxia to identify the specific component of the response to anoxia, which arises directly from activation of these channels. Drosophila mutants, which are defective in proteins crucial for the phototransduction cascade, were used to localize the transduction stage that underlies the effects of anoxia. Using Ca2+-selective microelectrodes in WT flies, the well-described reduction in extracellular Ca2+ concentration ([Ca2+]out) was measured during illumination (Fig. 3A, see also refs. 28,71). The reduction of [Ca2+]out during illumination arises from Ca2+ influx into the photoreceptor cells due to openings of TRP and TRPL channels.28 Application of anoxia indeed induced, after a delay, a reduction in [Ca2+]out (Fig. 3A, bottom). This observation indicates that in response to anoxia the large depolarization phase and Ca2+ influx are due to openings of the light-sensitive channels. Fig. 3B shows that illumination of the blind mutant norpAP24, in which light-activated PLC is missing,7,72 did not elicit any response to light as monitored by either voltage or [Ca2+]out changes as expected (Fig. 3B). Application of anoxia in the dark induced a voltage response, similar to that of WT with an initial small phase and a subsequent larger phase. The calcium signal, which accompanied the larger phase of the voltage change, was similar in WT and the mutant except for a faster onset in the mutant and an overshoot after anoxia was turned off (Fig. 3). The effects of anoxia on the ninaEora mutant, which is an opsin Rh1 null mutant,73 and on the Gαq1 mutant, which has a highly reduced level of light-activated G-protein74 were measured. The effects of anoxia on these mutants were similar to those observed in WT flies. The results thus show that anoxia affects a late stage of the phototransduction cascade downstream to PLC activation.
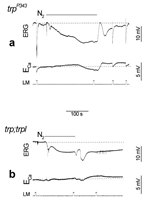
Additional demonstration that anoxia opens both TRP and TRPL channels in the dark was obtained by measuring Ca2+ influx in the trp mutant, in which TRP is missing, and in the trpl;trp double mutant, which lacks both channels.33 The ERG response to light of the trpP343 mutant revealed the typical decline towards baseline during illumination while the Ca2+ signal was transient and relatively small, as previously reported (Fig. 4A).28 Application of anoxia to the trpP343 mutant activated initially the slow and small phase of the voltage response (Fig. 4A). However, in contrast to WT and the other mutants mentioned above, a significantly smaller amplitudes of the second faster phase of the voltage and Ca2+ signal were observed in trpP343 flies. The small Ca2+ signal in response to light and anoxia in the mutant reflects influx of Ca2+ into the photoreceptors via the TRPL channels.28
An interesting characteristic of the response to anoxia of the trpP343 mutant is the existence of a maintained component in both voltage and Ca2+ signals as long as anoxia was applied, as in WT (Fig. 4A). This maintained response to anoxia in the mutant is in sharp contrast to the transient nature of the response to continuous light.
To firmly establish that the Ca2+ influx in response to anoxia is due to activation of TRP and TRPL channels we measured [Ca2+]out in response to anoxia in the double mutant trpl302;trpP343. In this mutant the response to light is completely abolished (Fig. 4B).33 Application of anoxia induced only the initial small voltage change with neither the subsequent larger voltage change nor any significant change in [Ca2+]out during the initial 2 min of anoxia (Fig. 4).
The lack of virtually any Ca2+ influx in the trpl;trp double mutant in response to anoxia (Fig. 4) suggests that the mechanism which controls the opening of the TRP and TRPL channels is the target of anoxia.
Inhibition of Mitochondria Mimicked the Effects of Anoxia in Vivo
Fly photoreceptor cells are known to have a large number of mitochondria.75 To investigate if the effects of anoxia in Drosophila retina are due to impaired function of the mitochondria, 2,4-dinitrophenol (DNP) and carbonyl cyanide m-chlorophenylhydrazone (CCCP) were applied to the intact eye. DNP and CCCP are known uncouplers of the oxidative chain for ATP production in the mitochondria.76 Application of DNP to the intact eyes of wild-type Drosophila induced a negative voltage change and abolished light excitation in a manner similar to the larger phase of the response to anoxia. The effects of DNP were partially reversible ∼20 min after the application. The effects of DNP on wild-type flies were accelerated when combined with either anoxia or illumination, thus suggesting that light stimulation and all forms of metabolic stress were additive. These experiments further suggest that mitochondria uncouplers mimicked the effects of anoxia in wild-type flies through effects on the TRP and TRPL channels. Whole-cell recordings in single photoreceptor cells added more conclusive evidence to the in vivo studies.
Mitochondrial Uncouplers and Depletion of ATP Activate the TRP and TRPL Channels in the Dark in Situ
Impairment of mitochondria function is expected to deplete ATP from the photoreceptor cells. To investigate more directly if depletion of ATP from the photoreceptor cells activates the TRP and TRPL channels, whole-cell patch clamp recordings in isolated ommatidia preparation was used.22,23,25 When ATP and α-nicotinamide adenine dinucleotide (NAD) were omitted from the recording pipette of WT cells, a few light pulses of medium intensity (orange light) induced an inward current in the dark, after a delay of ∼100 s (Fig. 5A). Inclusion of ATP and NAD in the pipette prevented the induction of the inward dark current by repeated illumination, for at least 6 min. This observation suggests that although light pulses are known to reduce the ATP level in photoreceptor cells,77 the supplement of exogenous ATP and NAD probably prevented depletion of ATP by illumination for at least 6 min. Application of 0.1 mM DNP to the bath (Fig. 5B) during recordings with pipettes without ATP and NAD induced the inward current in the dark in WT flies after a delay of only ∼20 s (Fig. 5B). When DNP (0.1 mM) was included in the pipette solution (without ATP and NAD) the inward current was induced in less than 30s from the onset of whole-cell recording.
In either trpP343 or in the double mutant trpl302;trpP343, no dark current was elicited during prolonged recordings (<10 min) using pipettes without ATP and NAD. Application of 0.1 mM DNP either to the bath or into the pipette induced a noisy inward current of small amplitude in trp flies (Fig. 5D). In the double mutant trpl302;trpP343 DNP (applied either to the bath or in the pipette) or CCCP, without ATP and NAD in the pipette had no effect (Fig. 5E), even after incubation of 16 min.
The inward current of WT cells, which was induced in the dark following illumination or DNP application in the absence of ATP and NAD, had all the characteristics of the TRP dependent current.78,79 In WT cells in zero external Ca2+ the current showed inward and outward rectification (Fig. 5C) while the amplitude of inward current at negative holding potentials was greatly reduced in the presence of Ca2+ in the medium (Fig. 5C). La3+, a potent Ca2+ channel blocker that mimics the trp phenotype,25,29,31,32,78 completely blocked the TRP-dependent current (Fig. 5C), as previously described.78 In the trp mutant, after application of DNP the resulting inward current had all the characteristics of the TRPL-dependent current of trp mutant flies. It had small amplitude, high noise (Fig. 5C) and nearly absence of inward rectification even at 0 Ca2+ level (see ref. 79).20 The effects of DNP were additive with light or absence of ATP and NAD, thus illumination accelerated the onset of the effect of DNP while inclusion of ATP and NAD in the pipette increased the latency of TRP channels activation to 2–4 min.
In summary, the results show that elimination of ATP and NAD from the recording pipette, combined with conditions that deplete ATP from the mitochondria opened the TRP and TRPL channels in the dark by affecting a late stage of the transduction cascade.
This study thus demonstrates that activation of TRP and TRPL in the dark results from depletion of ATP. The depletion of ATP probably directly activates the channels and not earlier stages of the transduction cascade because of the following reasons: i) Anoxia activated the TRP and TRPL channels at a stage downstream to PLC because anoxia induced Ca2+ influx in the PLC null mutant, norpAP24. ii) Activation of earlier stages results in production of unitary events (quantum bumps)70,80 while depletion of ATP was shown to produce a smooth response with channel noise (see also ref. 78). iii) Application of anoxia to the null trp mutant in vivo and application of DNP in situ produced maintained openings of the TRPL channels.81
The results suggest that the mitochondria are the primary target of anoxia leading to a reduction in cellular ATP. Indeed, previous studies on the large fly Calliphora have demonstrated that application of N2 rapidly and reversibly inhibited a specific transient green fluorescence that normally arises from functional mitochondria in the eye.82
The observation that impaired mitochondia function leads to openings of TRP and TRPL channels has important implications on previous studies. In these studies activation of various TRP channels have been obtained by several means including oxidative stress83 and application of polyunsaturated fatty acids (PuFAs).70 The latter is of special interest because linoleic acid and several other long chain fatty acids have been shown to react as efficient uncouplers of mitochondria in a variety of cells.84,85 The effect of DNP, therefore, suggest that PuFAs action on TRP and TRPL channels is indirect and result from their action as mitochondria uncouplers.81
The mechanism underlying activation of TRP and TRPL channels by depletion of ATP is not clear. However, the need to supply ATP in the dark at a high rate for proper function of the TRP and TRPL channels partially accounts for the well known, but unexplained, phenomenon of a high rate of oxygen consumption of insect retina in the dark.86 The results, thus, suggest that a constitutive ATP dependent process operating at a late stage of the phototransduction cascade is required to keep the TRP and TRPL channels closed in the dark. This makes these channels extremely sensitive to anoxia and may lead to cell death under metabolic stress, not only in Drosophila but also in vertebrate cells, which express TRP channels.83 The present study thus provides a novel target for metabolic stress with possible implications for brain damage since activation of TRP induces massive Ca2+ influx and TRP homologues are expressed in the brain.36,39,52
Taken together, the link of TRP gating to the metabolic state of cells opens up a new avenue of research that may explain many of the seemingly contradictory reports on pharmacological properties and mechanism of activation of TRP channels.36,39
Mammalian TRP Related Channels, Which are Sensitive to the Metabolic State of the Cell
The TRPM subfamily, which belongs to the TRP superfamily, is conserved through evolution and exist in C. elegans (CED11, GON2) Drosophila and humans. Members of this subfamily, share ∼20% amino acid identity with the Drosophila TRP over ∼325 residue region that includes S2-S6 transmembrane segments and the TRP domain.13 The total length of this subfamily is 1000–2000 amino acids and it varies considerably because of large diversity of the C-terminal domain. Members of the TRPM subfamily have a potential role in cell cycle regulation. A very interesting member of this subfamily is the bi-functional channel protein designated PLC-interacting kinase (TRP-PLIK or LTRPC7, or TRPM7), which constitutes the first example of a channel with kinase activity.87,88 Targeted deletion of LTRPC7 in DT-40 B cells was lethal, indicating that LTRPC7 has a fundamental and nonredundant role in cellular physiology. Heterologous expression of LTRPC7 in HEK-293 cells revealed a large outwardly rectifying current like the Drosophila TRPL channel. Further studies showed that the pore of the channel had a high affinity for and is permeant to both Ca2+ and Mg2+. At the same time outward currents are inhibited with increasing Mg2+ concentration. The permeability of the channel to Mg2+ is reminiscent of a similar property of the Drosophila TRP channel.25,89 Interestingly, Mg-ATP at mM concentration suppressed LTRPC7 channel activity. It turned out that Mg-ATP acted as physiological regulator of LTRPC7. Mg-ATPγS (but not Na-ATPγS) effectively and reversibly suppressed LTRPC7-dependent current, suggesting that phosphorylation of the channel in a Mg-ATP-dependent manner directly regulates the channel activity. The studies on LTRPC7 indicate that this channel is an intracellular ligand-gated ion channel whose activation may be linked to cellular energy metabolism through its sensitivity to cytosolic Mg-ATP levels and that it effectively permeates both Ca2+ and Mg2+.88 It is thus striking that similar properties characterize the Drosophila TRP channel which is also permeable (and partially blocked) by Mg2+,25,89 and shows extreme sensitivity to metabolic stress in vivo in a manner that depletion of ATP open the TRP channel in the dark while ATP suppresses channel opening.81
Additional important member of the TRPM subfamily, which is sensitive to the metabolic state of the cell is LTRPC2 (TRPM2) whose gating involves ADP-ribose (ADPR) and β-NAD. Heterologous expression of LTRPC2 in HEK-293 cells forms a current that was induced by application of 100 μM ADPR, showing a linear i-V relationship and reversing at 0 mV, thus, suggesting that this is a non-selective cation channel activated by ADPR.90 The channel was permeable to both monovalent and divalent cations. Excised inside-out patch clamp recordings revealed that the channel is most likely directly opened by ADPR at concentration of 30 μM or higher showing no desensitization.90 These studies show that ADPR can regulate directly the permeability of one member of the TRP family, raising the possibility that the NuD9-H domain of the channel is involved in channel gating.
A more recent study has shown that both ADPR and β-NAD activate the LTRPC2 expressed in monocyte cell lines. Biophysical studies confirm LTRPC2 as Ca2+-permeable nonselective cation channel. Interestingly, ATP strongly suppressed NAD- and ADPR-activated LTRPC2 channels.91 Detailed experiments have demonstrated that LTRPC2 mediates Ca2+ influx into immunocytes, where cellular ADPR and NAD directly activate LTRPC2 and enable Ca2+ influx. Importantly, NAD-induced activation was suppressed by ATP, suggesting that NAD activates LTRPC2 when ATP is depleted, while the activated LTRPC2 causes Ca2+ influx and apoptosis in these cells linking apoptosis with the metabolism of ADPR and NAD.91
Conclusion
The family of TRP channel proteins is unusually complex because of the large diversity of the structure and putative function of its members. Although the gating mechanism of any member of this large superfamily is unknown, there are indications that in the Drosophila light-activated channels and in some mammalian members, the gating of TRP is linked to the metabolic state of the cell. Although the function of the linkage between gating and metabolic stress is unknown, it put the cell in a great hazard because TRP channels are permeable to Ca2+. Accordingly, metabolic stress readily leads to toxic increase in cellular Ca2+ and cell death. Such a mechanism may underlie brain damage induced by ischemia, retinal degeneration in Drosophila, and potentially degeneration of retinal neurons expressing TRPs.
Acknowledgments
Experiments described in this paper were supported by the National Institutes of Health (EY 03529), the Israel Science Foundation (ISF), the German Israeli Foundation (GIF), the uS-Israel Binational Science Foundation (BSF), the Minerva Foundation and the Moscona Foundation.
References
- 1.
- Minke B, Hardie RC. Genetic dissection of Drosophila phototransduction In: Stavenga DG, van der Hope DJN, and Pugh E, eds.Molecular mechanisms in visual transduction Elsevier: North Holland,2000449–525.
- 2.
- Pak WL. Drosophila in vision research. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1995;36:2340–57. [PubMed: 7591624]
- 3.
- Minke B, Selinger Z. The roles of trp and calcium in regulating photoreceptor function in Drosophila. Curr Opin Neurobiol. 1996;6:459–66. [PubMed: 8794093]
- 4.
- Montell C. Visual transduction in Drosophila. Annu Rev Cell Dev Biol. 1999;15:231–68. [PubMed: 10611962]
- 5.
- Ranganathan R, Malicki DM, Zuker CS. Signal transduction in Drosophila photoreceptors. Annu Rev Neurosci. 1995;18:283–317. [PubMed: 7605064]
- 6.
- Minke B, Wu C, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–7. [PubMed: 810728]
- 7.
- Bloomquist BT, Shortridge RD, Schneuwly S. et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–33. [PubMed: 2457447]
- 8.
- Chang JT, Milligan S, Li Y. et al. Mammalian homolog of Drosophila retinal degeneration B rescues the mutant fly phenotype. J Neurosci. 1997;17:5881–90. [PMC free article: PMC6573195] [PubMed: 9221785]
- 9.
- Lev S, Hernandez J, Martinez R. et al. Identification of a novel family of targets of PYK2 related to Drosophila retinal degeneration B (rdgB) protein. Mol Cell Biol. 1999;19:2278–88. [PMC free article: PMC84020] [PubMed: 10022914]
- 10.
- Ramulu P, Nathans J. Cellular and subcellular localization, N-terminal acylation, and calcium binding of Caenorhabditis elegans protein phosphatase with EF-hands. J Biol Chem. 2001;276:25127–35. [PubMed: 11312268]
- 11.
- Sherman PM, Sun H, Macke JP. et al. Identification and characterization of a conserved family of protein serine/threonine phosphatase homologous to Drosophila retinal degeneration C (rdgC). Proc Natl Acad Sci uSA. 1997;94:11639–44. [PMC free article: PMC23563] [PubMed: 9326663]
- 12.
- Dose AC, Burnside B. Cloning and chromosomal localization of a human class III myosin. Genomics. 2000;67:333–42. [PubMed: 10936054]
- 13.
- Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channel http://stke.sciencemag.org/cgi/content/full/OC_sigtrans2001/90/rel.20011–17. [PMC free article: PMC57813] [PubMed: 11752662]
- 14.
- Raghu P, Usher K, Jonas S. et al. Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron. 2000;26:169–79. [PubMed: 10798401]
- 15.
- Yoon J, Cohen BenAmi H, Hong YS. et al. Novel mechanism of massive photoreceptor degeneration caused by mutations in the trp gene of Drosophila. J Neurosci. 2000;20:649–59. [PMC free article: PMC6772429] [PubMed: 10632594]
- 16.
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–23. [PubMed: 2516726]
- 17.
- Wong F, Schaefer EL, Roop BC. et al. Proper function of the Drosophila trp gene product during pupal development is important for normal visual transduction in the adult. Neuron. 1989;3:81–94. [PubMed: 2482778]
- 18.
- Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–42. [PubMed: 1314616]
- 19.
- Xu X Z S, Li HS, Guggino WB. et al. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–64. [PubMed: 9215637]
- 20.
- Reuss H, Mojet MH, Chyb S. et al. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron. 1997;19:1249–59. [PubMed: 9427248]
- 21.
- Xu XZ, Chien F, Butler A. et al. TRPγ, a Drosophila TRP-related subunit, forms a regulated cation channel with TRPL. Neuron. 2000;26:647–57. [PubMed: 10896160]
- 22.
- Hardie RC. Whole-cell recordings of the light-induced current in dissociated Drosophila photoreceptors: evidence for feedback by calcium permeating the light-sensitive channels. Proc R Soc Lond B. 1991;245:203–10.
- 23.
- Ranganathan R, Harris GL, Stevens CF. et al. A Drosophila mutant defective in extracellular calcium-dependent photoreceptor deactivation and rapid desensitization. Nature. 1991;354:230–2. [PubMed: 1961249]
- 24.
- Minke B, Selinger Z. Inositol lipid pathway in fly photoreceptors: excitation, calcium mobilization and retinal degeneration In: Osborne NA, Chader GJ, eds.Progress in retinal research Oxford: Pergamon Press,199199–124.
- 25.
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–51. [PubMed: 1314617]
- 26.
- Peretz A, SussToby E, RomGlas A. et al. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron. 1994;12:1257–67. [PubMed: 8011336]
- 27.
- Hardie RC. INDO1 measurements of absolute resting and light-induced Ca2+ concentration in Drosophila photoreceptors. J Neurosci. 1996;16:2924–33. [PMC free article: PMC6579063] [PubMed: 8622123]
- 28.
- Peretz A, Sandler C, Kirschfeld K. et al. Genetic dissection of light-induced Ca2+ influx into Drosophila photoreceptors. J Gen Physiol. 1994;104:1057–77. [PMC free article: PMC2229250] [PubMed: 7699363]
- 29.
- Niemeyer BA, Suzuki E, Scott K. et al. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–9. [PubMed: 8646774]
- 30.
- Leung HT, Geng C, Pak WL. Phenotypes of trpl mutants and interactions between the transient receptor potential (TRP) and TRPlike channels in Drosophila. J Neurosci. 2000;20:6797–803. [PMC free article: PMC6772831] [PubMed: 10995823]
- 31.
- Hochstrate P. Lanthanum mimicks the trp photoreceptor mutant of Drosophila in the blowfly Calliphora. J Comp Physiol A. 1989;166:179–87. [PubMed: 2514264]
- 32.
- Suss Toby E, Selinger Z, Minke B. Lanthanum reduces the excitation efficiency in fly photoreceptors. J Gen Physiol. 1991;98:849–68. [PMC free article: PMC2229083] [PubMed: 1960531]
- 33.
- Scott K, Sun Y, Beckingham K. et al. Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell. 1997;91:375–83. [PubMed: 9363946]
- 34.
- Scott K, Zuker C. TRP, TRPL and trouble in photoreceptor cells. Curr Opin Neurobiol. 1998;8:383–8. [PubMed: 9687362]
- 35.
- Montell C. New light on TRP and TRPL. Mol Pharmacol. 1997;52:755–63. [PubMed: 9351965]
- 36.
- PutneyJW J, McKay RR. Capacitative calcium entry channels. Bioessays. 1999;21:38–46. [PubMed: 10070252]
- 37.
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–272. [PubMed: 11917094]
- 38.
- Wissenbach U, Schroth G, Philipp S. et al. Structure and mRNA expression of a bovine trp homologue related to mammalian trp2 transcripts. FEBS Lett. 1998;429:61–6. [PubMed: 9657384]
- 39.
- Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–66. [PubMed: 10717675]
- 40.
- Tang J, Lin Y, Zhang Z. et al. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of Trp channels. J Biol Chem. 2001;276:21303–10. [PMC free article: PMC1847329] [PubMed: 11290752]
- 41.
- Zhu X, Jiang MS, Peyton M. et al. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca 2+ entry. Cell. 1996;85:661–71. [PubMed: 8646775]
- 42.
- Birnbaumer L, Zhu X, Jiang MS. et al. On the molecular basis and regulation of cellular capacitative calcium entry: Roles for Trp proteins. Proc Natl Acad Sci USA. 1996;93:15195–202. [PMC free article: PMC26380] [PubMed: 8986787]
- 43.
- Wes PD, Chevesich J, Jeromin A. et al. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA. 1995;92:9652–6. [PMC free article: PMC40860] [PubMed: 7568191]
- 44.
- Sinkins WG, Estacion M, Schilling WP. Functional expression of TrpC1: a human homologue of the Drosophila Trp channel. Biochem J. 1998;331:331–9. [PMC free article: PMC1219356] [PubMed: 9512497]
- 45.
- Zhu X, Chu PB, Peyton M. et al. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–8. [PubMed: 7589464]
- 46.
- Funayama M, Goto K, Kondo H. Cloning and expression localization of cDNA for rat homolog of TRP protein, a possible store-operated Ca2+ channel. Brain Res Mol Brain Res. 1996;43:259–66. [PubMed: 9037541]
- 47.
- Chang AS, Chang SM, Garcia RL. et al. Concomitant and hormonally regulated expression of trp genes in bovine aortic endothelial cells. FEBS Lett. 1997;415:335–40. [PubMed: 9357995]
- 48.
- Vannier B, Peyton M, Boulay G. et al. Mouse trp2, the homologue of the human trpc2 pseudogene, encodes mTrp2, a store depletion-activated capacitative Ca2+ entry channel. Proc Natl Acad Sci USA. 1999;96:2060–4. [PMC free article: PMC26736] [PubMed: 10051594]
- 49.
- Liman ER, Corey DP, Dulac C. TRP2: a candidate transduction channel for mammalian pheromone sensory signaling. Proc Natl Acad Sci USA. 1999;96:5791–6. [PMC free article: PMC21939] [PubMed: 10318963]
- 50.
- Taniguchi M, Wang D, Halpern M. Chemosensitive conductance and inositol 1,4,5-trisphosphate-induced conductance in snake vomeronasal receptor neurons. Chem Senses. 2000;25:67–76. [PubMed: 10667996]
- 51.
- Jungnickel MK, Marrero H, Birnbaumer L. et al. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol. 2001;3:499–502. [PubMed: 11331878]
- 52.
- Garcia RL, Schilling WP. Differential expression of mammalian TRP homologues across tissues and cell lines. Biochem Biophys Res Commun. 1997;239:279–83. [PubMed: 9345310]
- 53.
- Mizuno N, Kitayama S, Saishin Y. et al. Molecular cloning and characterization of rat trp homologues from brain. Brain Res Mol Brain Res. 1999;64:41–51. [PubMed: 9889314]
- 54.
- Buess M, Engler O, Hirsch HH. et al. Search for oncogenic regulators in an autocrine tumor model using differential display PCR: identification of novel candidate genes including the calcium channel mtrp6. Oncogene. 1999;18:1487–94. [PubMed: 10050885]
- 55.
- Okada T, Inoue R, Yamazaki K. et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca2+-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J Biol Chem. 1999;274:27359–70. [PubMed: 10488066]
- 56.
- Philipp S, Hambrecht J, Braslavski L. et al. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274–82. [PMC free article: PMC1170761] [PubMed: 9687496]
- 57.
- Okada T, Shimizu S, Wakamori M. et al. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J Biol Chem. 1998;273:10279–87. [PubMed: 9553080]
- 58.
- Freichel M, Suh SH, Pfeifer A. et al. Lack of endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3:121–127. [PubMed: 11175743]
- 59.
- Colbert HA, Smith TL, Bargmann CI. OSM9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–69. [PMC free article: PMC6573730] [PubMed: 9334401]
- 60.
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–34. [PubMed: 10744543]
- 61.
- Caterina MJ, Schumacher MA, Tominaga M. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. [PubMed: 9349813]
- 62.
- Hoenderop JG, van der Kemp AW. et al. Molecular identification of the apical Ca2+ channel in 1,25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem. 1999;274:8375–8378. [PubMed: 10085067]
- 63.
- Chen XZ, Vassilev PM, Basora N. et al. Polycystin-L is a calcium-regulated cation channel permeable to calcium ions. Nature. 1999;401:383–6. [PubMed: 10517637]
- 64.
- Bargal R, Avidan N, BenAsher E. et al. Identification of the gene causing mucolipidosis type IV. Nat Genet. 2000;26:118–23. [PubMed: 10973263]
- 65.
- Sun M, Goldin E, Stahl S. et al. Mucolipidosis type VI is caused by mutations in a gene encoding a novel transient receptor potential channel. Human Molecular Genetics. 2000;9:2471–8. [PubMed: 11030752]
- 66.
- Hotta Y, Benzer S. Genetic dissection of the Drosophila nervous system by means of mosaics. Proc Natl Acad Sci USA. 1970;67:1156–63. [PMC free article: PMC283331] [PubMed: 5274445]
- 67.
- Masai I, Okazaki A, Hosoya T. et al. Drosophila retinal degeneration A gene encodes an eye-specific diacylglycerol kinase with cysteine-rich zinc-finger motifs and ankyrin repeats. Proc Natl Acad Sci USA. 1993;90:11157–61. [PMC free article: PMC47941] [PubMed: 8248222]
- 68.
- Masai I, Suzuki E, Yoon CS. et al. Immunolocalization of Drosophila eye-specific diacylgylcerol kinase, rdgA, which is essential for the maintenance of the photoreceptor. J Neurobiol. 1997;32:695–706. [PubMed: 9183747]
- 69.
- Inoue H, Yoshioka T, Hotta Y. Diacylglycerol kinase defect in a Drosophila retinal degeneration mutant rdgA. J Biol Chem. 1989;264:5996–6000. [PubMed: 2538432]
- 70.
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397:255–259. [PubMed: 9930700]
- 71.
- Sandler C, Kirschfeld K. Light-induced extracellular Ca2+ and Na concentration changes in the retinal of Calliphora: involvement in the mechanism of light adaptation. J Comp Physiol A. 1991;169:229–311.
- 72.
- Pearn MT, Randall LL, Shortridge RD. et al. Molecular, biochemical, and electrophysiological characterization of Drosophila norpA mutannts. J Biol Chem. 1996;271:4937–45. [PubMed: 8617767]
- 73.
- O'Tousa JE, Leonard DS, Pak WL. Morphological defects in oraJK84 photoreceptors caused by mutation in R1-6 opsin gene of Drosophila. J Neurogenet. 1989;6:41–52. [PubMed: 2528612]
- 74.
- Scott K, Becker A, Sun Y. et al. Gqa protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron. 1995;15:919–27. [PubMed: 7576640]
- 75.
- Boschek BC. On the fine structure of the peripheral retina and lamina ganglionaris of the fly, Musca domestica . Z Zellforsc. 1971;118:369–409. [PubMed: 5566322]
- 76.
- McLaughlin SG, Dilger JP. Transport of protons across membranes by weak acids. Physiol Rev. 1980;60:825–63. [PubMed: 6248908]
- 77.
- Dimitracos SA, Tsacopoulos M. The recovery from a transient inhibition of the oxidative metabolism of the photoreceptors of the drone (Apis mellifera). J Exp Biol. 1985;119:165–181.
- 78.
- Hardie RC, Minke B. Spontaneous activation of lightsensitive channels in Drosophila photoreceptors. J Gen Physiol. 1994;103:389–407. [PMC free article: PMC2216849] [PubMed: 8195780]
- 79.
- Hardie RC, Minke B. Calcium-dependent inactivation of light-sensitive channels in Drosophila photoreceptors. J Gen Physiol. 1994;103:409–427. [PMC free article: PMC2216844] [PubMed: 8195781]
- 80.
- Scott K, Zuker CS. Assembly of the Drosophila phototransduction cascade into a signalling complex shapes elementary responses. Nature. 1998;395:805–808. [PubMed: 9796815]
- 81.
- Agam K, vonCampenhausen M, Levy S. et al. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J Neurosci. 2000;20:5748–5755. [PMC free article: PMC6772559] [PubMed: 10908615]
- 82.
- Stavenga DG, Tinbergen J. Light dependence of oxidative metabolism in fly compound eyes studied in vivo by micospectrophotometry. Naturwissenschaften. 1983;70S:618–620.
- 83.
- Balzer M, Lintschinger B, Groschner K. Evidence for a role of Trp proteins in the oxidative stress-induced membrane conductances of porcine aortic endothelial cells. Cardiovascular Research. 1999;42:543–549. [PubMed: 10533589]
- 84.
- Hermesh O, Kalderon B, Bar TJ. Mitochondria uncoupling by a long chain fatty acyl analogue. J Biol Chem. 1998;273:3937–42. [PubMed: 9461579]
- 85.
- Arslan P, Corps AN, Hesketh TR. et al. cis-Unsaturated fatty acids uncouple mitochondria and stimulate glycolysis in intact lymphocytes. Biochem J. 1984;217:419–25. [PMC free article: PMC1153232] [PubMed: 6696740]
- 86.
- Tsacopoulos M, Poitry S, Borsellino A. Diffusion and consumption of oxygen in the superfused retina of the drone (Apis mellifera) in darkness. J Gen Physiol. 1981;77:6012–28. [PMC free article: PMC2215446] [PubMed: 7264598]
- 87.
- Runnels LW, Yue L, Clapham DE. TRPPLIK, a bifunctional protein with kinase and ion channel activities. Science. 2001;291:1043–1047. [PubMed: 11161216]
- 88.
- Nadler MJ, Hermosura MC, Inabe K. et al. LTRPC7 is a Mg-ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. [PubMed: 11385574]
- 89.
- Hardie RC, Mojet MH. Magnesium-dependent block of the light-activated and trp-dependent conductance in Drosophila photoreceptors. J Neurosci. 1995;74:2590–2599. [PubMed: 8747216]
- 90.
- Perraud AL, Fleig A, Dunn CA. et al. ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature. 2001;411:595–599. [PubMed: 11385575]
- 91.
- Sano Y, Inamura K, Miyake A. et al. Immunocyte Ca2+ influx system mediated by LTRPC2. Science. 2001;293:1327–1330. [PubMed: 11509734]
- The TRP Calcium Channel and Retinal Degeneration - Madame Curie Bioscience Datab...The TRP Calcium Channel and Retinal Degeneration - Madame Curie Bioscience Database
- PNA and Oligonucleotide Inhibitors of Human Telomerase - Madame Curie Bioscience...PNA and Oligonucleotide Inhibitors of Human Telomerase - Madame Curie Bioscience Database
- The Role of Serine/Threonine Protein Phosphatases in Ceramide Signaling - Madame...The Role of Serine/Threonine Protein Phosphatases in Ceramide Signaling - Madame Curie Bioscience Database
- Target Recognition of Guanylate Cyclase by Guanylate Cyclase-Activating Proteins...Target Recognition of Guanylate Cyclase by Guanylate Cyclase-Activating Proteins - Madame Curie Bioscience Database
- Vascular Endothelial Growth Factor - Madame Curie Bioscience DatabaseVascular Endothelial Growth Factor - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...