NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Nursing and Supportive Care (UK). Irritable Bowel Syndrome in Adults: Diagnosis and Management of Irritable Bowel Syndrome in Primary Care [Internet]. London: Royal College of Nursing (UK); 2008 Feb. (NICE Clinical Guidelines, No. 61.)
Update information March 2017 Recommendation 1.1.1.2 in the short version was updated by NICE with more recent guidance on recognition and referral for suspected cancer. Recommendation 1.1.1.3 in the short version was removed as it was no longer needed after the changes to recommendation 1.1.1.2. February 2015 NICE has made new recommendations relating to the clinical management (dietary and lifestyle advice, and pharmacological therapy) of people with IBS. The recommendations and evidence in sections 3, 7.6, 8.4, 8.5.2 and 9.1 of this guideline that have been highlighted in grey have been stood down and replaced. New recommendations on dietary and lifestyle advice, and pharmacological therapy, can be found in the irritable bowel syndrome in adults update CG61.1. September 2012 A recommendation in this guideline (see pages 28 and 37) has been partially updated by recommendation 1.1.2.1 in 'Ovarian cancer' (NICE clinical guideline 122, 2011).

Irritable Bowel Syndrome in Adults: Diagnosis and Management of Irritable Bowel Syndrome in Primary Care [Internet].
Show detailsClinical Questions
- What associations are there between diet and IBS?
- What dietary interventions improve symptoms/quality of life?
- Does Aloe Vera have a role in managing symptoms?
- What associations are there between physical activity and IBS?
- Does physical activity improve IBS or related symptoms?
BACKGROUND
Diet and lifestyle may be factors that trigger or exacerbate symptoms of IBS so they are factors that need to be given due consideration both at the initial and later stages of management. This chapter includes all the reviews in the guideline pertaining to diet and lifestyle interventions.
DIET
A healthy diet, as based on the ‘Balance of Good Health’, is promoted for the UK population. Some aspects of this are appropriate for people with IBS, e.g. regular meals, drinking plenty of fluid (e.g. 8 cups of non-caffeine based fluid per day) and encouraging a wide variety of foods. However, people with IBS often find that following healthy eating advice exacerbates symptoms and, in particular, this may relate to dietary fibre and lactose (milk and dairy foods). Wheat, resistant starch, caffeine, fructose, sorbitol, alcohol and fizzy drinks have also been reported to commonly affect symptoms. Potential beneficial components of the diet include probiotics and prebiotics and water soluble dietary fibre. Diet and nutrition are fundamental in the management of IBS to avoid malnutrition and to contribute to achieving optimal symptom control. Food products have been reported as causing, contributing to and perpetuating Irritable Bowel Syndrome. The term ‘food intolerance’ includes effects of pharmacologically active constituents (e.g. caffeine in coffee); enzyme deficiencies (e.g. lactose intolerance) and demonstrable immunological response (allergy or hypersensitivity to peanut, cow’s milk, gluten, soya bean). The notion of food intolerance and food allergy is not new and many IBS patients give a history of food intolerance, although few clinicians consider food hypersensitivity to be a cause of IBS. There are no objective tests available to identify food intolerance and few to confirm food allergy. Data from dietary elimination and food challenge studies are contradictory.
Dietary intolerance is defined as a non-immunologically mediated response to particular foods, which resolve following dietary elimination and re-occur with food challenge. An exclusion diet is defined as a diet in which specific food products are totally excluded for a specified period of time. The excluded food products are then gradually re-introduced one by one to confirm response.
Diagnostic testing for food intolerance includes hydrogen breath testing and diagnostic testing for Coeliac Disease. Hydrogen breath tests are based on the fact that the only source of hydrogen gas in humans comes from the bacterial metabolism of carbohydrates. Different carbohydrates are given orally to patients and the amount of hydrogen in the expired air is measured. Patients need to be fasted and to have had at least one day of a low fibre diet. Smoking and exercise alters the hydrogen concentrations so are not permitted during the tests.
Potential sources of error are:
- Carbohydrate malabsorption in chronic pancreatitis and Coeliac disease
- False positive for small intestinal bacterial overgrowth due to colonic fermentation
- Delayed gastric emptying may cause false negative
- Oral bacterial flora, failure to follow low fibre diet and rapid transit through small intestine may produce false positive.
Testing for Coeliac disease involves a blood test for immunoglobulin A (IgA) antigliadin antibodies; endomysial antibodies (EMA) and TTG anti-tissue transglutaminase antibodies. The sensitivity and specificity for IgA, EMA and TTG are 95% and 89%; 100% and 97%; and 100% and 97%, respectively in patients with GI symptoms. For general population screening, EMA and TTG have a positive predictive value of 15.7% and 21.8%. A positive blood result requires an endoscopy with duodenal biopsy to confirm a diagnosis of Coeliac disease.
People with IBS may alter their diet to alleviate symptoms of IBS. Guidance may either be sought from inadequately qualified nutritionists or be self directed. Excluding individual foods or complete food groups without appropriate dietetic supervision can lead to inadequate nutrient intake and ultimately malnutrition, e.g. calcium. In addition, symptoms often remain unresolved leading to further inappropriate dietary restriction. The gold standard diagnosis for intolerance to a food is by elimination and reintroduction. Intolerance would be demonstrated if symptoms resolved on elimination and reappeared on reintroduction. Importantly, dietary advice will vary depending on symptoms, e.g. diarrhoea and/or constipation, abdominal bloating and therefore needs to be tailored to the individual to manage symptoms. Expert professional advice on diet and nutrition for IBS should be obtained from a registered dietitian or an appropriately qualified nutritionist.
Dietary Fibre
Fibre is defined as non-starch polysaccharides in agreement with FAO/WHO/DOH measurement methods. Dietary fibre is food material that is not hydrolysed by enzymes secreted by the human gastrointestinal tract. Soluble fibre dissolves in water forming a gel and may be digested by the colonic microbiota increasing bacterial numbers and thus faecal bulk. It includes β-glucans, pectins, gums, mucilages and some hemicelluloses. Dietary sources include oats, psyllium, ispaghula, nuts and seeds, some fruit and vegetables and pectins. Insoluble fibre is not readily broken down by the gastrointestinal microbiota and it increases faecal bulk, shortening colonic transit. It includes celluloses, some hemicelluloses and lignin and is chiefly found in corn (maize) and wheat bran and some fruit and vegetables.
An increase in fibre has often been suggested as an initial treatment for IBS, although more recently there are conflicting data to support its effectiveness and a range of views on its usefulness. The dietary reference value for non-starch polysaccharides (fibre) is 18g per day for adults. A high fibre diet is defined as 18g or more per day in recognition of the fact that many people in the UK eat on average 10 to 12g per day. Dietary manipulation of the fibre content in practice is dependent on the presenting symptom profile (constipation dominant, diarrhoea dominant or alternating symptoms) and whether abdominal bloating and flatus is present.
Wheat
Wheat is a grass and is cultivated worldwide as a food grain, ranking second in total production as a cereal crop behind maize. Whole wheat is made up of 14% bran, 2.5% germ and the rest is starchy endosperm. Wheat bran has a faecal bulking effect, delays gastric emptying and accelerates small bowel transit (McIntyre 1997). Wheat is found in bread, many breakfast cereals, pasta, cakes and biscuits and is one of the major cereals consumed in the UK. In IBS, wheat consumption is often associated with increased symptoms which may be due to the content of fibre, fructans or resisitant starch. Increasing the variety of other cereals and reducing, but not necessarily, excluding wheat may be beneficial in IBS.
Resistant Starches
Resistant starch comprises starch polymers that are not readily digested in the stomach or small intestine Resistant starches are the total amount of starches, and the products of starch degradation that resist digestion in the small intestine of healthy people (Asp 1982) and therefore reach the colon intact. The extent of resistance is influenced by the structure of naturally occurring starch polymers and food processing methods employed, e.g. how starch changes during cooking and cooling. People with IBS may benefit from a reduction of foods high in resistant starch to alleviate symptoms of wind and bloating. Common dietary sources of resistant starch are cold or re-heated potatoes, bread, cereal products containing modified starch (e.g. cakes, biscuits and breakfast cereals.
Lactose
Lactose is a sugar found in milk of all mammalian varieties including cow, goat, sheep and human and it is also used in processed foods, particularly slimming products. Approximately 10% of people with IBS have lactose intolerance (BSG Guidelines). The symptoms of IBS are brought on by undigested lactose passing into the small intestine causing an increase in the secretion of fluid into the small bowel through osmotic mechanisms. It then passes into the colon undigested and is available for colonic fermentation as described above (Mascolo 1998).
Removing lactose from the diet may not lead to complete symptom relief in IBS and exclusion needs careful monitoring due to other nutritional inadequacies in the diet e.g. calcium. Often people with lactose intolerance can manage 10 to 12g lactose per day if spread throughout the day. Milk contains the highest level of lactose (cow’s milk 5g per 100ml), foods that are lower include butter (trace), cheese (cottage cheese: 1g per tablespoon, processed cheese: 1g per slice, Cheddar, edam, brie, Danish blue: trace), yoghurts (trace – 4g per pot) and low lactose milk. It is therefore relatively easy to include a sufficient amount of dairy foods to maintain a balanced diet in diagnosed and self reported people with lactose intolerance.
Fructose
Fructose intake has increased considerably as a result of an increased consumption of high fructose corn syrup, fruits and juices and crystalline fructose. Fructose is almost twice as sweet as normal table sugar (sucrose). Fructose is an important source of energy for humans, but incomplete absorption in the small bowel can lead to colonic fermentation causing diarrhoea, wind and bloating.
Up to 80% of healthy subjects incompletely absorb 50g of fructose (Scoog 2004). In real terms 25g fructose is equivalent to that found in 200ml apple juice or 2 bananas. A regular consumption of dried fruit and high juice squash will easily add another 25g.
Sorbitol
Sorbitol is a natural component of fruits and significant amounts are found in dried apple and apricots, prunes, cherries and pears. Produced from maize it is also used as an artificial, low calorie sweetener for its low cariogenicity, e.g. in sugar-free chewing gum, mints and cough syrups and as a humectant and thickener in confectionary, frozen desserts and toothpaste. It is poorly absorbed in the small bowel and in the colon has a laxative effect if consumed in quantities of around 30g/day, although some individuals, particularly people with IBS may be sensitive to much less (Thomas 1992).
Caffeine
Caffeine is found naturally in many plant-derived foodstuffs and beverages, chiefly coffee, tea, cocoa and chocolate confectionary, cola and other stimulant drinks. It is also found in many pharmacological agents. Caffeine has many reported effects on the body: negative effects include raised blood pressure, increased heart rate, arrhythmias, dehydration, anxiety, insomnia, headaches and heartburn. Caffeine can also stimulate the central nervous system, improve alertness and mental efficiency and improve athletic performance (Thomas 2003). There is a general consensus that a moderate intake of caffeine (up to 300mg/day in adults) is not harmful. Caffeine has stimulatory effects on the digestive system but there is little evidence that it will cause gastrointestinal dysfunction (Thomas 2003). Heartburn is the most commonly reported symptom from drinking coffee. It may promote gastrointestinal reflux and stimulate gastrin release and gastric acid secretion but does not appear to affect gastric emptying or small bowel transit.
Probiotics and Prebiotics
In IBS, the gastrointestinal flora may undergo both qualitative and quantitative changes and the most common finding is a decrease in the population of ‘good bacteria’ such as Bifidobacteria and Lactobacilli and the faecal microflora has increased numbers of facultative organisms (Madden and Hunter 2002; Quigley 2007). Probiotics may be useful in the management of IBS, however dose and specific bacterial strain are important. In vivo studies have identified some of the variables that determine the survival of probiotics through the GI tract, and some have attempted to quantify the degree of survival of the dose administered. This was found to vary from 10 to 50% depending on the probiotic species used and the dose administered.
For the purposes of this guideline probiotics are defined as microbial food supplements which, when administered in adequate amounts, have a beneficial effect on the host. Prebiotics are defined as a non-digestible food ingredient that affects the host by selectively targeting growth and/or activity of one or more bacteria in the colon that can improve health. Synbiotics are defined as a combination of pre and probiotics which beneficially affects the host by improving survival and implantation of live microbial dietary supplements in the gastrointestinal tract.
Fermented milks and yoghurts have been the most commonly used carrier of probiotics. The probiotic organism is added at the end of the milk fermentation process. The range of probiotic products is expanding to include cheese, frozen yoghurt, ice cream and non-dairy foods, liquids, powders, capsules and drinks. It should be noted that many available probiotics have not had their health benefits identified or been scientifically proven to be beneficial to the host (Reid 2001).
In vivo studies have identified some of the variables that determine the survival of probiotics through the GI tract, and some have attempted to quantify the degree of survival of the dose administered. This was found to vary from 10 to 50% depending on the probiotic species used and the dose administered. The GDG defined the minimum acceptable dose to be 1×10 6 (one million) bacteria per day. The duration of the intervention is also considered important. To avoid concerns regarding possible effects during the menstrual cycle, four weeks was thought to be the minimum duration of intervention.
Colonic Fermentation
Some of the symptoms of IBS (e.g. abdominal bloating, flatus and diarrhoea) may be due to colonic fermentation by intestinal microflora of certain dietary constituents to short chain fatty acids (acetate, butyrate and propionate) and gases (hydrogen, carbon dioxide and methane). The short chain fatty acids have been shown to stimulate ileal and colonic smooth muscle contractility (Barbara 2005). Watery diarrhoea may also happen due to the increased osmotic load. The dietary constituents include non absorbed lactose (as in lactose intolerance), dietary fibre/non-starch polysaccharides, resistant starches and oligosacchaides from wheat and other grains.
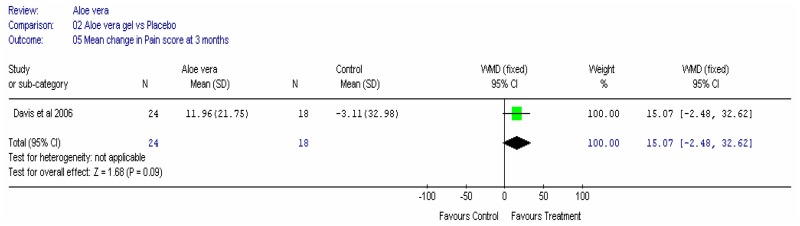
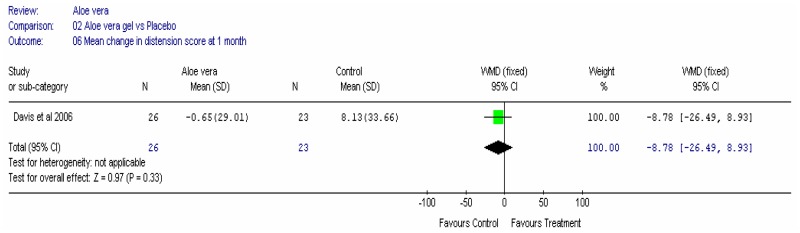
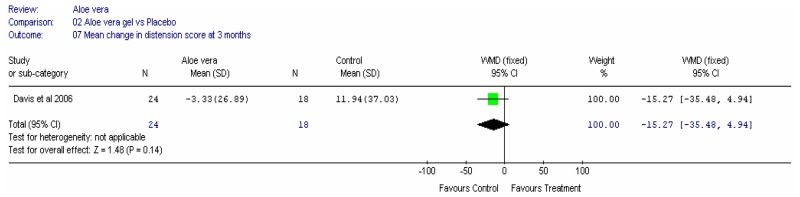
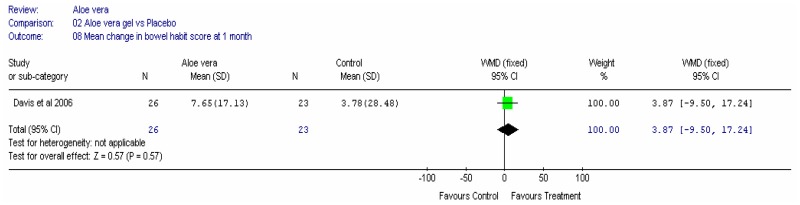
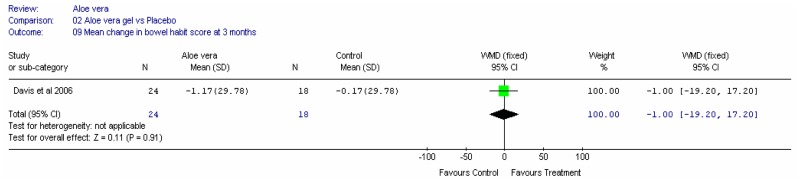
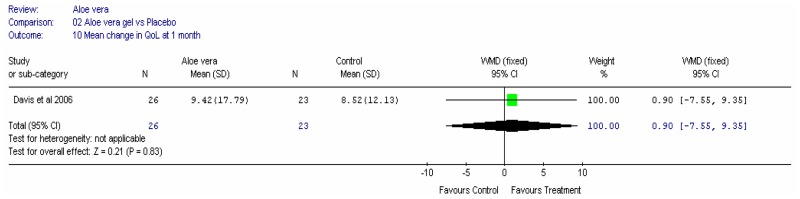
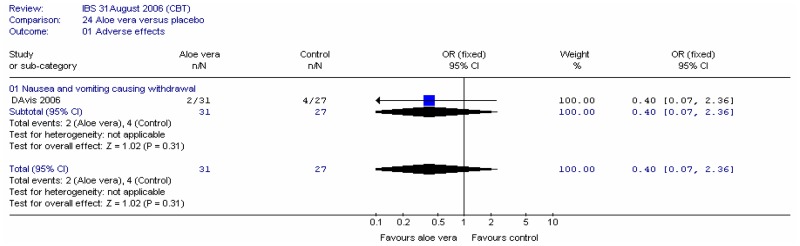
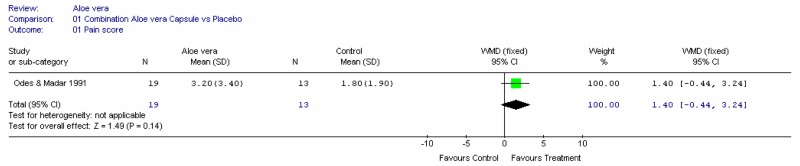
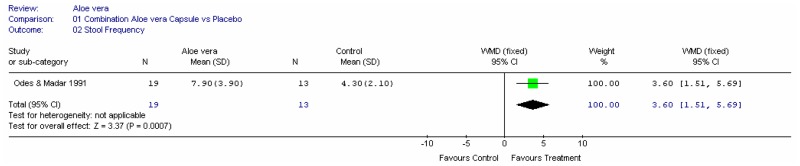
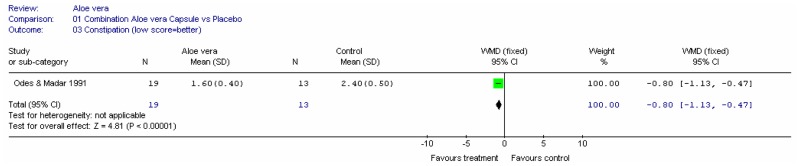
Aloe vera
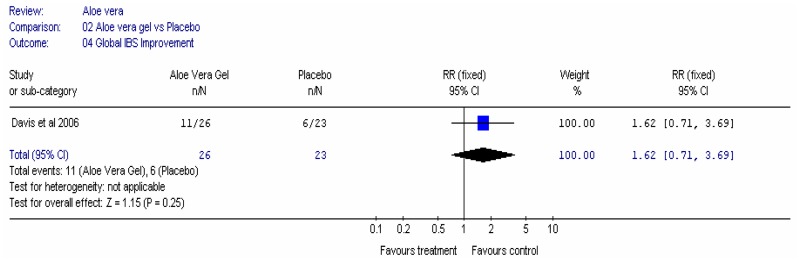
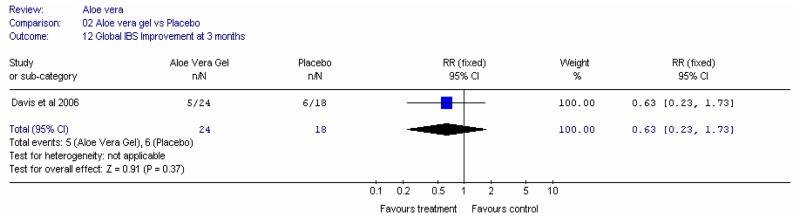
Aloe vera (Aloe barbadensis Miller) belongs to the Liliaceal family of which there are approximately 360 species. Aloe vera is a cactus like plant; cosmetic and medicinal products are derived from the leaf tissue and called aloe vera gel. Aloe sap or juice, often referred to as aloes, are derived from the peripheral bundle sheath cells of aloe vera. Aloe vera sap contains anthraquinones that are known to have laxative effects. These are not found in the gel but may be present in total leaf extracts (Vogler and Ernst 1999). The use of aloe vera is being promoted for many conditions including IBS. Most of the evidence is based on anecdotal, historical use rather than scientific evidence.
PHYSICAL ACTIVITY
The relationship between physical activity and chronic disease
There is strong evidence from observational studies that moderate to high levels of physical activity can have a substantial impact on major non communicable diseases, such as coronary heart disease (CHD), hypertension, diabetes and certain types of cancer (US Department of Health and Human Services, 1996; Department of Health, 2004a; WHO, 2004). People who are physically active typically experience 30 to 50% reductions in relative risk of CHD compared with people who are sedentary, after adjustment for other risk factors (Murphy 2003).
The Chief Medical Officer (CMO) recently published a report stating the importance of physical activity for health (Department of Health, 2004a). As well as linking chronic disease with physical inactivity the report also described how physical activity can reduce the risk of musculoskeletal health conditions, including osteoporosis, back pain and osteoarthritis. It stated that regular physical activity can reduce the risk of depression and promotes many other positive mental health benefits including reducing anxiety and promoting self esteem (Department of Health, 2004a). The CMO’s report also presented a series of recommendations for the amount of physical activity that should be undertaken by different population groups. These recommendations mimicked similar recommendations from other international bodies (Pate et al, 1995; US Department of Health and Human Services, 1996; Department of Health, 2004a). The report advised that adults should undertake at least 30 minutes of moderate intensity physical activity on at least five days of the week (Department of Health, 2004a). In 2002 the cost of physical inactivity was estimated to be £8.2 billion annually in terms of mortality, morbidity and quality of life (Department for Culture Media and Sports and London Strategy Unit, 2002). A more accurate estimate of the direct costs of physical inactivity to the UK health service was £1.06 billion annually (Allender et al, 2006a). Physical activity has been described as a good investment for public health, not only because of the great potential for benefit, but also because ’it is inexpensive and has few side-effects‘ (Morris 1992, in Marmot and Elliot 1992).
In 2006, NICE published guidance (Public Health Intervention Guidance No. 2) on exercise interventions in primary care, pedometers, exercise referral schemes and community-based exercise programmes for walking and cycling to increase physical activity. Two specific recommendations were made for primary health care professionals:
Recommendation 1
Primary care practitioners should take the opportunity, whenever possible, to identify inactive adults and advise them to aim for 30 minutes of moderate activity on 5 days of the week (or more)*. They should use their judgement to determine when this would be inappropriate (for example, because of medical conditions or personal circumstances). They should use a validated tool, such as the Department of Health’s forthcoming general practitioner physical activity questionnaire (GPPAQ), to identify inactive individuals.
* The practitioner may be a GP or another professional with specific responsibility for providing encouragement or advice. This will depend on local conditions, professional interest and resources. Health trainers are likely to have a role in offering brief advice. ‘Inactive’ is used as shorthand for those failing to reach the CMO’s recommendation. ‘Advise’ is used as shorthand for ‘encourage, advise, discuss, negotiate’.
Recommendation 2
When providing physical activity advice, primary care practitioners should take into account the individual’s needs, preferences and circumstances. They should agree goals with them. They should also provide written information about the benefits of activity and the local opportunities to be active. They should follow them up at appropriate intervals over a 3 to 6 month period.
The NICE public health intervention advisory committee determined that there was insufficient evidence to recommend the use of exercise referral schemes to promote physical activity, other than as part of research studies where their effectiveness can be evaluated.
This guidance aims to help practitioners deliver effective interventions that will increase people’s physical activity levels and therefore benefit their health.
The use of physical activity as part of a non-pharmacological therapy for IBS is described as “reasonable” despite the relationship between exercise and gastrointestinal system being unclear (Bi and Triadafilopoulos 2003). For example, moderate physical activity (e.g. brisk walking) is reported to improve gut transit time, whereas vigorous physical activity (e.g. running) can result in “runners trots” (Oettle, 1991). Physical activity has been associated with improved outcomes in uncontrolled studies (Colwell et al, 1998).
7.1. General dietary and lifestyle advice
This section is concerned with the effect of diet and lifestyle on IBS and its management. Five reviews are addressed, fibre, probiotics, aloe vera, exclusion diets and physical activity. In addition, the GDG made some consensus recommendations, partly informed by dietary advice leaflets. These are listed below.
RECOMMENDATION
Healthcare professionals should encourage people with IBS to identify and make the most of their available leisure time and to create relaxation time.
RECOMMENDATION
Diet and nutrition should be assessed for people with IBS and the following general advice given.
- Have regular meals and take time to eat.
- Avoid missing meals or leaving long gaps between eating.
- Drink at least eight cups of fluid per day, especially water or other non-caffeinated drinks, for example herbal teas.
- Restrict tea and coffee to three cups per day.
- Reduce intake of alcohol and fizzy drinks.
- It may be helpful to limit intake of high-fibre food (such as wholemeal or high-fibre flour and breads, cereals high in bran, and whole grains such as brown rice).
- Reduce intake of ‘resistant starch’ (starch that resists digestion in the small intestine and reaches the colon intact), which is often found in processed or re-cooked foods.
- Limit fresh fruit to three portions per day (a portion should be approximately 80g).
- People with diarrhoea should avoid sorbitol, an artificial sweetener found in sugar-free sweets (including chewing gum) and drinks, and in some diabetic and slimming products.
- People with wind and bloating may find it helpful to eat oats (such as oat-based breakfast cereal or porridge) and linseeds (up to one tablespoon per day).
7.2. Physical activity
SELECTION CRITERIA
The selection criteria described in the general methodology section were to be used, but some were specific to the physical activity review and are reported below.
Types of studies
For intervention studies, randomised trials (RCTs) examining the use of physical activity for the treatment or management of IBS were to be included. In the absence of randomised trials, quasi randomised studies were to be considered. Crossover trials with a washout period of less than 2 weeks were to be excluded. All study designs were to be included for adverse effects, but specific searches for adverse effects will not be carried out. Studies were restricted to the English language.
Types of intervention
Studies were included if they had one or more of the following interventions:
- The use of physical activity alone or in combination with other therapies
- 12 weeks minimum length of intervention
A physical activity intervention is defined as the use of physical activity or exercise as a therapeutic and/or preventative medical procedure used to support the management and treatment of IBS. Physical activity is usually defined as any force exerted by skeletal muscles that results in energy expenditure above resting level whereas exercise is defined as a subset of physical activity, which is volitional, planned, structured, repetitive and aimed at improvement or maintenance of any aspects of fitness or health (Casperson et al, 1985). The GDG defined the minimum acceptable dose of physical activity to be at least 30 minutes per week of at least moderate intensity physical activity. The duration of the intervention is also considered important, and the minimum duration of intervention was set at twelve weeks.
Types of comparisons
The following comparisons were to be included
- Physical activity versus attention control
- Combination of physical activity with another non-pharmacological intervention (e.g. diet advice) versus control.
Types of participants
Studies were to be included if the participants were:
- Adults (18 years and over)
- Had symptoms of IBS
- No serious diseases (e.g. cancer, heart disease) other than IBS
- Did not have a single symptom of IBS only (e.g. not constipation only)
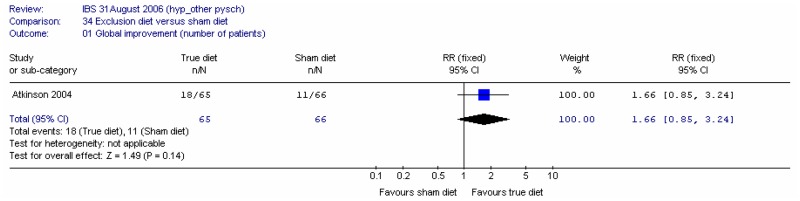
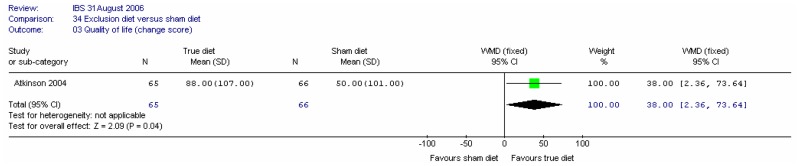
In the absence of studies in patients with IBS, we extended the review to cover studies in people with single symptoms such as constipation or diarrhoea. Studies in these participants were regarded as indirect as far as the population was concerned.
SEARCH STRATEGY FOR IDENTIFICATION OF STUDIES
Searches were performed on the following core databases: MEDLINE, EMBASE, CINAHL and The Cochrane Library (1966 to current day with guidance from the GDG). An additional database searched for this review only was SPORTS DISCUS. The search strategies are listed in Appendix B.
Subgroup analyses
Subgroup analyses were proposed to examine any heterogeneity as follows:
- Dose
- Type of physical activity
- Symptom severity.
Sensitivity analyses
The following sensitivity analyses may be considered:
- Setting (primary/secondary care).
DESCRIPTION OF STUDIES INCLUDED IN THE REVIEW
The search strategy identified 2608 studies. The titles and abstracts of these studies were assessed. Of these, 19 that were potentially relevant to the review were identified on the basis of the title and abstract – these papers were retrieved in full. All reference lists of these studies were inspected for potential papers for inclusion in the review, but none was found.
None of the studies identified met the primary inclusion criteria. Therefore, we included some studies with indirect evidence, and considered other studies to aid GDG discussions. One systematic review was identified (Bi and Triadafilopoulos 2003). This review examined the relationship between exercise and gastrointestinal function for eight disease types.
RESULTS
Evidence from Systematic Reviews
Bi and Triadafilopoulos (2003) reviewed the relationship between exercise and gastrointestinal function for eight disease types. The authors described their review as systematic but provided no methods in the paper.
- Gastroesophageal reflux disease
- Gastric emptying and gastric acid production
- Peptic ulcer disease
- Inflammatory bowel disease
- Constipation and gastrointestinal motility
- Colorectal cancer
- Gastrointestinal bleeding
- Liver disease
The authors attempted to identify if there were any differential effects of physical activity (by intensity or type) on gastrointestinal function. IBS was not identified as a separate class, but it may be possible to extrapolate from the indirect evidence in section 5 of the Bi and Triadafilopoulos (2003) review. Participants in these studies tended to be young, fit and active males, rather than typical clinical populations. The review found that physical activity could improve gastric emptying and lower the risk of bowel cancer. However, there was insufficient evidence to suggest that exercise can relieve chronic constipation. The authors also noted consistent improvements in aerobic fitness and general health for all subjects participating in regular physical activity programmes and that this outcome is a notable behavioural goal for sedentary patients. The majority of risks to gastrointestinal organs relate to very high levels of sustained physical activity (performed at elite levels). However these risks do not outweigh the benefits of light and moderate physical activity.
Evidence from intervention studies
One randomised trial was included as an indirect study as it examined the impact of physical activity upon adults with chronic constipation only (De Schryver 2005). However this study was not included in the Bi and Triadafilopoulos (2003) review.
The aim of the study was to investigate the effect of regular physical activity on colonic transit time and defecation in middle aged inactive patients suffering from chronic idiopathic constipation. Forty three adults aged 51 to 61 were recruited from general practice lists and pharmacies. Using Rome I criteria all were categorised as suffering from constipation, with IBS patients excluded. Participants’ physical activity levels were also assessed using a self report measure and all the participants were categorised as sedentary if they failed to reach the current physical activity recommendation (under 30 minutes or more of moderate physical activity on most days of the week).
Other baseline measures included food consumption, assessed by self report using a diary, in order to determine the average daily fibre and water intake. Defecations patterns were recorded in a 7-day diary, at the start of the study and at 12 weeks follow up. Colonic transit time was measured using radiopaque markers and x-rays. Transit time was calculated based on the number of markers visible in the colon, segmented into three (right colon, left colon and rectosigmoid).
Participants were randomised in to two groups, physical activity versus waiting list control. Group A maintained their normal lifestyle for 12 weeks, and then started their 12 week physical activity programme. Group B started their physical activity programme immediately after randomisation. Both groups were given dietary advice by a dietician concerning the consumption of fluid and fibre at the start of the study. Group A received a second dietary advice after 12 weeks, before starting the physical activity programme. This programme consisted of both aerobic and strength/flexibility exercises. Brisk walking was chosen for aerobic training and strength/ flexibility exercises were chosen for a home based programme. Brisk walking was performed at least twice a week for at least 30 minutes per session, performed at 70 to 80% of the subject’s maximal heart rate. Participants were able to monitor their heart rates using a Polar sports tester (a heart rate monitor worn on the wrist, in conjunction with a chest sensor). Maximal heart rate was assessed for all patients at baseline using a maximal heart test performed on a cycle ergometer. Participants were also asked to perform a walking test on a treadmill at 70% of their heart rate for 5 minutes to establish an average heart rate for their brisk walking.
The number of defecations did not change in either of the study groups (Table 1). However in Group B the percentage of incomplete stools decreased significantly, compared to Group A at 12 weeks (Group A from 58.8% to 39.5% whereas in Group B from 54.3% to 27.4%).
Table 1
Defecation patterns at baseline and after 12 week physical activity programme for 41 adult participants aged 51–61 years old (De Schryver et al, 2005).
Despite randomisation, there were considerable differences between right and total colonic transit times at baseline between groups (Table 2). No significant changes in right or left colonic transit time were observed in either group at the end of the physical activity programme. In Group B there was an observed acceleration in rectosigmoid mean transit time compared to Group A. Total colonic transit time also improved with a significant reduction in Group B. The authors reported that there was no correlation between fibre intake and improvements in defecation patterns and colonic transit times.
Table 2
Colonic transit times (hours) at baseline and after 12 week physical activity programme for 41 adult participants aged 51–61 years old (De Schryver et al, 2005).
The GDG noted that the normal total colonic transit time is 72 hours and concluded that group B was significantly different from group A, so that the study was considered to be at least partially confounded.
The evidence from this study was assessed to be low, using GRADE criteria. Limitations included (i) the study was conducted in secondary care (ii) there were important differences in baseline characteristics, (iii) IBS patients were excluded. This study was also limited because the participants had relatively high levels of baseline physical activity, which equates to over 2 hours of walking per week, and may not be representative. The study did show that moderate physical activity could deliver a consistent reduction in total colonic transit time and improvements in ROME I symptoms amongst older adults with chronic constipation.
Studies used to aid GDG discussions
One pre-post intervention study examined the impact of a lifestyle education programme upon IBS symptoms. This study design was judged inadequate to make recommendations on interventions, but was considered useful to inform GDG discussions, and does illustrate a suitable approach for evaluating a lifestyle intervention for IBS patients.
Colwell et al (1998) assessed the impact at one and six months of a patient education class, that included exercise, on 52 adult outpatients with IBS (definition of IBS not stated). Patients were advised to increase their physical activity by walking or basic stretching exercises during one 3 hour structured class, delivered by a specialist nurse. Pre-class data was compared with results for physical activity levels at follow up. Exercise scores increased significantly at one month but not at 6 months, compared with baseline, using a self-rating scale. It is difficult to assess if this increase was clinically significant because the physical activity variable was assessed using a categorical scale, and so the physical activity change scores were not adjusted for baseline values. Pain scores at 1 and 6 months reduced significantly (see Table 3). The Manning score also decreased significantly, on a scale of 0 to 6 using Manning criteria.
Table 3
Symptom scores at 1 and 6 months for 57 adult participants in an IBS educational class aged 21–79 years old (Colwell et al, 1998, page 903).
Evidence from Epidemiological studies
Three observational studies reported the prevalence and association between IBS and physical activity. In a case-control study, Kim and Ban (2006) reported a small, non-significant difference in the mean number of hours of exercise per week for students with IBS compared to students without IBS (defined by ROME 2 criteria) (students with IBS 2.38 h/week, SD 3.2 versus students with non-IBS: 2.69 h/week, SD 6.3).
Lustyk et al (2001) compared prevalence and severity of IBS symptoms between active and sedentary women with IBS (defined by ROME I criteria). They found that active and inactive women reported the same level of recalled psychological and somatic symptoms as well as daily reports of GI and psychological distress. Active women (those who took at least 2.5 hours per week of moderate physical activity and meeting recommended physical activity levels) reported significantly less fatigue than sedentary women. This outcome was assessed by combining frequency and severity of fatigue using categorical scale. No differences were observed for other somatic symptoms including backache, headache and insomnia between active and sedentary women with IBS.
Dancey et al (2002) examined gender differences in the prevalence and severity of IBS symptoms. They used a cross sectional survey to compare the prevalence and impact of IBS symptoms between 117 male and female IBS patients. IBS was assessed by self report measure with respondents rating severity of abdominal pain, constipation, diarrhoea, incomplete evacuation after bowel movement, bloating and flatulence on a 7 point severity scale (0 = no symptoms to 7 = extremely severe). Illness intrusiveness ratings were assessed across 13 life domains, using a 7 category Likert scale. Respondents were asked to rate the extent to which their illness interfered with each life domain important to quality of life (e.g. health, diet, financial situation, relationship with partner etc.). One life domain was active recreation (e.g. sports). The authors reported that in response to this item, men and women scored the interference of IBS similarly (i.e. moderate interference), with no significant differences between genders. They found that IBS inference was higher in diet, health and self expression domains than active recreation. Other domains reporting less interference than active recreation were social relations, work, community/civic life, sex life, relationship with spouse, family relations, financial situation, passive recreation and religious expression.
Two studies examined the relationship between physical activity and bowel frequency in the general population. In a cohort study, Sanjoaquin et al (2004) investigated the association between mean number of bowel movements and physical activity, adjusted for other confounding variables (e.g. age, BMI, diet, fibre intake) in 20,630 EPIC-Oxford cohort participants. The EPIC-Oxford cohort is a cohort study forming part of the European Prospective Investigation into Cancer and Nutrition (EPIC). Participants were recruited from general practice surgeries, vegetarian and health food magazines, the Vegetarian Society, the Vegan Society and from friends and relatives of participants. In a follow up study a short questionnaire was sent to all participants and included two questions relating to bowel movements, (i) “About how many bowel movements do you have each week? And (ii) How often do you take laxatives?” The number of bowel movements was counted for each participant. Respondents were then dichotomised into one of two groups, either above of below 7 movements per week.
The authors reported a positive association between increasing amounts of vigorous physical activity and mean number of bowel movements per week for both men and women. However only highly active women (more than 7 hours per week of vigorous physical activity) had a greater likelihood of reporting more than 7 bowel movements per week (OR; 1.70 [95%CI 1.42, 2.03]) compared to women who reported no vigorous physical activity. Curtin et al (1996) conducted a population survey of bowel habits in urban Swiss men but found no relationship between physical activity status and bowel habits.
EVIDENCE STATEMENTS
- There is poor evidence to show that the percentage of incomplete stools decreased significantly in non-IBS constipated people given an exercise programme.
- There is weak evidence that IBS Manning and pain scores at one and six months were reduced significantly in comparison with pre-intervention scores following a patient education class that included exercise for people with IBS.
- There is mixed evidence on whether there is a positive association between physical activity and bowel habits in the general population.
GDG DISCUSSION
The GDG considered the evidence and discussed whether exercise effects were related to stress reduction. It was noted that some people may have increased stress levels because of exercise, depending on their liking for exercise. The GDG thought that exercise would not necessarily be beneficial for people with IBS-D. It was also noted that attendance at exercise classes might prove difficult for patients and a gentle exercise programme that could be carried out at home (e.g. Tai Chi, yoga, stretching) might be more beneficial.
The GDG discussed whether it was useful to recommend taking more fluid after exercise, but concluded that this would not necessarily be appropriate for people with IBS, since many have bladder problems, and taking more fluid does not help constipation.
EVIDENCE TO RECOMMENDATION
The GDG took into consideration the limited evidence and also referred to NICE public health guidance and the Chief Medical Officers report on physical activity. This led to a general recommendation for practice.
Recommendations for active living throughout the lifecourse (DoH 2004)
- For general health benefit, adults should achieve a total of at least 30 minutes a day of at least moderate intensity physical activity on 5 or more days of the week.
- The recommended levels of activity can be achieved either by doing all the daily activity in one session, or through several shorter bouts of activity of 10 minutes or more. The activity can be lifestyle activity* or structured exercise or sport, or a combination of these.
- More specific activity recommendations for adults are made for beneficial effects for individual diseases and conditions. All movement contributes to energy expenditure and is important for weight management. It is likely that for many people, 45–60 minutes of moderate intensity physical activity a day is necessary to prevent obesity. For bone health, activities that produce high physical stresses on the bones are necessary.
- The recommendations for adults are also appropriate for older adults. Older people should take particular care to keep moving and retain their mobility through daily activity. Additionally, specific activities that promote improved strength, co-ordination and balance are particularly beneficial for older people.
- People with disabilities will know their abilities and should modify their physical activity accordingly e.g. chair-based exercises.
The GDG was also interested to know if exercise affects IBS symptoms and quality of life for people with IBS, and whether the type of IBS was important.
RECOMMENDATION
Healthcare professionals should assess the physical activity levels of people with IBS, ideally using the General Practice Physical Activity Questionnaire (GPPAQ; see Appendix J). People with low activity levels should be given brief advice and counselling to encourage them to increase their activity levels.
7.3. Fibre
SELECTION CRITERIA
The selection criteria described in the general methodology section were to be used, but some were specific to the fibre review and are reported below.
Types of studies
The GDG decided that the washout period for crossover studies in this review should be at least 4 weeks. Trials with shorter washout periods were not to be included in the analysis.
Types of intervention
Studies were to include the following interventions:
- Insoluble fibre (corn, wheat, fruit and vegetables)
- Soluble fibre (pectins, fruit and vegetables, oats, nuts and seeds, psyllium, ispaghula)
- Bran.
It was to be noted if the fibre was provided as a food or as a capsule/supplement. In addition, the total amount of fibre in the diet for each intervention was to be recorded where possible.
The following comparisons were to be included:
- Fibre + normal diet versus normal diet (fibre versus nothing)
- Fibre versus low fibre diet or placebo (fibre versus placebo)
- Bran versus placebo
- Insoluble fibre versus soluble fibre
- Insoluble fibre + soluble fibre versus soluble fibre
- Insoluble fibre + soluble fibre versus insoluble fibre
- Fibre level 1 versus fibre level 2
- Duration of treatment 1 versus duration 2
- Fibre versus another type of intervention
- Fibre plus another type of intervention versus another type of intervention.
In spite of the large placebo effect associated with IBS, comparisons with no treatment were to be included.
The fibre review was to be concerned only with longer-term maintenance treatment. The GDG decided that there should be a minimum duration of treatment of four weeks for this review. Studies of shorter durations were to be excluded.
Outcomes
In addition to the outcomes discussed in the general methods section, the GDG were interested in the number of people with global deterioration, other than those who withdrew because of the treatment.
Data extraction
In addition to the items given in the general section, we also extracted information on the total amount of fibre (i.e. the sum of the intervention and the fibre in the diet).
Subgroup analyses
We planned to carry out subgroup analyses by type of fibre (soluble, insoluble, mixed), dose (both intervention and total amount), duration of intervention, and, post-hoc, by means of ingestion (supplement or dietary).
SEARCH STRATEGY FOR IDENTIFICATION OF STUDIES
Searches were performed on the following core databases: MEDLINE, EMBASE, CINAHL and The Cochrane Library (1966 to current day with guidance from the GDG). Additional databases were not searched for this review. The search strategies are given in Appendix B.
The titles and abstracts from the search strategy were assessed. Sixty-four were identified to be potentially relevant to the review and these papers were retrieved in full. Twenty studies met the inclusion criteria for the review. The reference lists of the retrieved studies were inspected for further potential papers, but none were identified. The forty-four excluded studies are listed in the Appendix, along with reasons for exclusion.
DESCRIPTION OF STUDIES INCLUDED IN THE REVIEW
There were 20 included studies (Aller 2004; Arthurs 1983; Chapman 1990; Cook 1990; Dettmar 1999; Fielding 1984; Fowlie 1992; Kruis 1986; Longstreth 1981; Lucey 1987; Manning 1977; Parisi 2002; Parisi 2005; Prior and Whorwell 1987; Rees 2005; Ritchie 1979; Ritchie 1980; Soltoft 1976; Tarpila 2004; Vilagrasa 1991). Nine studies were conducted in the UK (Chapman 1990; Dettmar 1999; Fowlie 1992; Lucey 1987; Manning 1977; Prior and Whorwell 1987; Rees 2005; Ritchie 1979; Ritchie 1980); two in Ireland (Arthurs 1983; Fielding 1984); seven in the rest of Europe, and two in the USA and Canada.
One study (Cook 1990) had fewer than 20 participants (n=14). This was a crossover study so fewer participants were required to achieve adequate power. Five studies had more than 100 participants in total (Chapman 1990; Dettmar 1999; Kruis 1986; Parisi 2002; Villagrasa 1991).
Study Design
Setting: The majority of studies took place in secondary care; one was in primary care (Dettmar 1999) and one study did not report the setting (Tarpila 2004).
There were two crossover studies (Cook 1990; Lucey 1987) in which participants were allocated to receive both the intervention and control treatments during the course of the study, in a random order. The GDG defined the minimum washout period to be four weeks for crossover studies in this review, so the only crossover study eligible was Cook (1990). However, a second crossover study (Lucey 1987) became eligible because individual patient data were reported, allowing calculation of first period results. This gave the study a ‘pseudo-parallel’ design, although the power was reduced. The remaining studies had a parallel design. One study had more than two arms: Kruis (1986) compared bran with mebeverine (anti-spasmodic) and placebo.
Population
The definition of IBS varied between studies: two used the Manning criteria (Chapman 1990; Cook 1990); two used Rome I (Parisi 2002; Rees 2005); two used Rome II (Aller 2004; Parisi 2005) and two met criteria defined by the authors that were similar to the above (Fielding 1984; Tarpila 2004). In five studies, the authors stated that the participants had IBS, with no further explanation (Lucey 1987; Manning 1977; Ritchie 1979; Ritchie 1980; Vilagrasa 1991). The remaining seven studies (Arthurs 1983; Dettmar 1999; Fowlie 1992; Kruis 1986; Longstreth 1981; Prior and Whorwell 1987; Søltoft 1976) did not use a formal definition but described a range of symptoms consistent with IBS.
Most studies included a combination of IBS types. Four specified constipation-predominant IBS (Cook 1990; Fielding 1984, Rees 2005; Tarpila 2004) and three were unclear (Arthurs 1963; Dettmar 1999; Fowlie 1992).
None of the studies stated that any participants had IBS as result of gastrointestinal infection. The majority of studies (13) did not state the number of participants with bloating. Four studies reported that some people had bloating (Aller 2004; Kruis 1986; Longstreth 1981; Vilagrasa 1991). Two studies (Prior and Whorwell 1987; Tarpila 2004) stated that all people had bloating.
Most of the studies did not describe symptom severity. Six studies stated that participants had symptoms of mixed severity (Dettmar 1999; Fowlie 1992; Longstreth 1981; Parisi 2002; Parisi 2005; Prior and Whorwell 1987).
The age range of participants across studies was 14 to 82 years, with the mean age (where given) ranging from 25.8 to 45 years. No study particularly identified elderly people. All studies had more women than men.
Interventions
The studies varied in the type of fibre used: six had insoluble fibre (wheatbran); eight had soluble fibre (six ispaghula, one partially hydrolysed guar gum [‘PHGG’], one psyllium); five had mixed fibres: studies used a combination of fruit, vegetables and cereal.
One study gave the fibre in a capsule form (Fowlie 1992), eight gave the fibre as a supplement (Arthurs 1983; Chapman 1990; Dettmar 1999; Fowlie 1992; Longstreth 1981; Prior and Whorwell 1987; Ritchie 1979; Ritchie 1980); and the rest added fibre to the diet with food (e.g. bran-containing biscuits).
A fibre level of 18g per day is regarded as a threshold dose. When assessing dose we considered both the amount of additional fibre and the amount of total fibre (intervention plus that in the diet). The amount of additional fibre ranged from 7g per day (Dettmar 1999), although a third 3.5g sachet could be added if needed, to 40g per day (Fielding 1984). Ten studies gave additional fibre as amounts of less than 18g (Chapman 1990; Dettmar 1999; Fowlie 1992; Kruis 1986; Lucey 1987; Parisi 2005; Prior and Whorwell 1987; Rees 2005; Ritchie 1979; Ritchie 1980). Nine studies gave more than 18g (Aller 2004; Arthurs 1983; Cook 1990; Fielding 1984; Longstreth 1981; Parisi 2002; Manning 1977; Søltoft 1976; Villagrasa 1991). One study (Tarpila 2004) gave 12 to 24g daily.
Eight studies reported the total fibre in the intervention arm (Aller 2004; Arthurs 1983; Cook 1990; Fielding 1984; Fowlie 1992; Prior and Whorwell 1987; Tarpila 2004; Villagrasa 1991).
The duration of the intervention ranged from four weeks (Arthurs 1983; Dettmar 1999; Fielding 1984; Parisi 2002) to two years (Villagrasa 1991). One study reported follow-up after the end of the trial (Parisi 2005; 3 months follow-up).
Comparisons
The included studies covered the following comparisons:
- Eleven comparisons of fibre versus placebo, including one versus usual diet (Kruis 1986); and one versus reduced fibre (Manning 1977):
- One gave mixed fibre (Fowlie 1992);
- Three studies compared different classes of fibre:
- Two studies compared soluble versus insoluble fibre
- ▪
PHGG versus bran (Parisi 2002)
- ▪
Ispaghula versus bran (Ritchie 1980)
- One study compared mixed versus soluble fibre
- ▪
Ground flax seed (containing 20% flaxseed oil) versus psyllium (Tarpila 2004);
- One study compared different types of fibre in the same class (mixed):
- One study compared different combinations of fruit and cereal fibre (Fielding 1984);
- Two studies compared different doses of fibre:
- One compared 30.5g with 10.4g of mixed fibre. However, the proportion of soluble fibre differed between the two groups (13% versus 19%) (Aller 2004)
- One study compared 5 and 10g of PHGG (Parisi 2005);
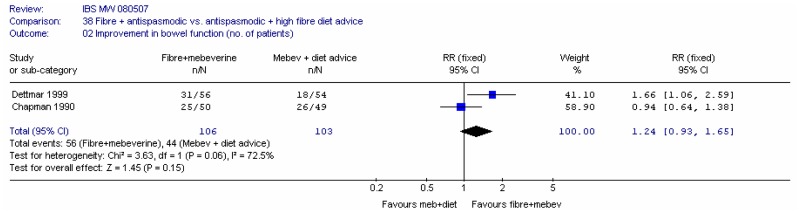
- Two studies compared fibre + mebeverine versus mebeverine + dietary advice (Chapman 1990; Dettmar 1999)
- Two studies compared fibre with an antispasmodic (Kruis 1987; Villagrasa 1991).
OUTCOMES
The studies measured a range of outcomes.
1. Global symptoms
a. Number of people with improvement in global symptoms
Ten studies recorded the participants’ assessment of improvement (Fowlie 1992; Kruis 1986; Longstreth 1981; Lucey 1987; Parisi 2002; Prior and Whorwell 1987; Rees 2005; Ritchie 1979; Ritchie 1980; Søltoft 1976) and one (Arthurs 1983) appeared to record a clinician’s assessment.
b. Number of people with deterioration in global symptoms
Four studies recorded the participants’ assessment of deterioration (Longstreth 1981; Lucey 1987; Parisi 2002; Søltoft 1976).
c. Global symptom score (mean)
Global symptom scores combined pain, bowel habits, flatulence and bloating. This outcome was recorded by five studies (Cook 1990; Fowlie 1992; Longstreth 1981; Lucey 1987; Parisi 2005). Longstreth (1981) recorded how symptoms interfered with normal activity.
2. Individual symptoms
a. Pain
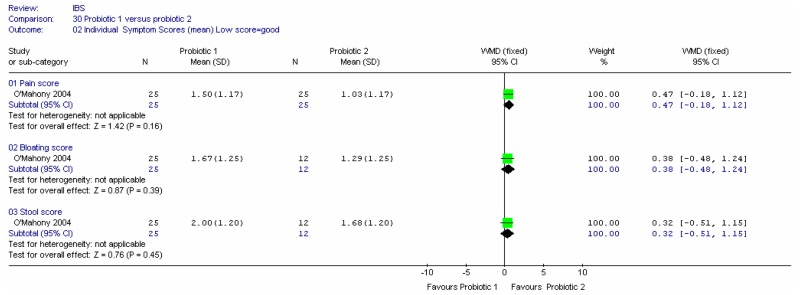
Pain was reported in several ways: the number of people with pain at the end of the study; the number of people whose pain improved or worsened compared with the baseline, and; pain scores. The pain score recorded a range of features, including severity, frequency and duration, or a combination of these. In addition, studies recorded the final scores, mean daily scores or the change from baseline. The studies reporting the following outcomes are listed below:
- Number of people with more pain: one study (Chapman 1990)
- Number of people with no pain: two studies (Prior and Whorwell 1987; Villagrasa 1991)
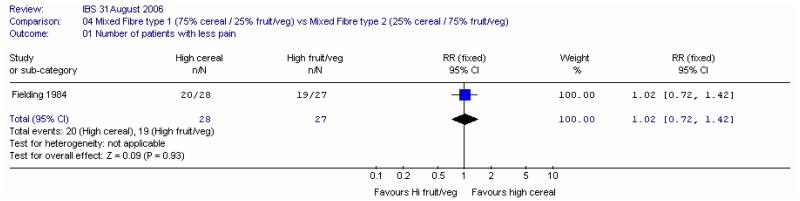
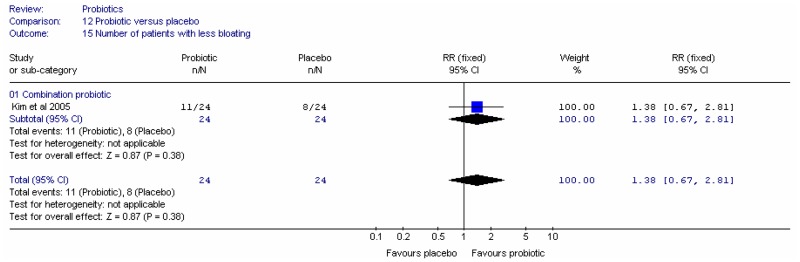
- Number of people with less pain: four studies (Chapman 1990; Dettmar 1999; Fielding 1984; Kruis 1986)
- Pain score (change and final): six studies (Aller 2004; Cook 1990; Fowlie 1992; Longstreth 1981; Manning 1977; Parisi 2005):
- Two studies reported pain severity from daily diary readings (Longstreth 1981; Manning 1977)
- One study reported a combined score for pain frequency and severity (Aller 2004) and this study also reported change scores. In all cases the highest rating meant worst symptoms, although the scales used were not the same.
b. Bloating
- Number of people with bloating: two studies (Prior and Whorwell 1987; Villagrasa 1991)
- Number of people with more bloating: one study (Tarpila 2004)
- Number of people with no bloating: two studies (Prior and Whorwell 1987; Villagrasa 1991)
- Number of people with less bloating: one study (Tarpila 2004)
- Bloating score (change and final): no studies reported this outcome.
c. Combined bloating and flatulence score
Three studies measured end of study scores (Aller 2004; Longstreth 1981; Parisi 2005).
d. Bowel habits
i. Number of people with improved bowel habits
Eight studies recorded the number of people with improved bowel habits (Chapman 1990; Dettmar 1999; Fielding 1984; Kruis 1986; Manning 1977; Parisi 2002; Tarpila 2004; Villagrasa 1991). Of these, two reported normalisation of bowel habits (Parisi 2002; Villagrasa 1991), and the rest reported the patient’s assessment of improvement.
ii. Stool score (aggregate)
Three studies (Aller 2004; Fowlie 1992; Longstreth 1981) measured an aggregate of frequency, consistency and straining. Fowlie (1992) reported the sum of number of stools x consistency score (1=hard; 5=watery), for people whose IBS type was unclear; we regarded this outcome as unhelpful. Longstreth (1981) reported the number of normal stools and this study was included in the analysis.
e. Quality of life
Two studies reported a measure of quality of life (Fielding 1984; Parisi 2005). Parisi (2005) reported the social functioning item on the SF-36 scale.
f. Adverse events
Two studies reported adverse effects (Chapman 1990; Villagrasa 1991).
METHODOLOGICAL QUALITY
The results of the quality assessment for included trials are shown in Appendix D. The method of randomisation was reported in one study, classified as partially adequate (Manning 1977; drawing a randomly numbered card). The other studies did not state the method of randomisation.
Allocation concealment was reported in two studies (Parisi 2002; Parisi 2005), both of which reported a partially adequate method in which randomisation and analysis were said to be ‘supervised by a statistician’.
Nine studies reported that the outcome assessors were blinded to the interventions (Cook 1990; Fielding 1984; Longstreth 1981; Manning 1977; Prior and Whorwell 1987; Ritchie 1979; Ritchie 1980; Søltoft 1976; Tarpila 2004). One study stated that the outcome assessors were not blinded (Parisi 2002). The remaining studies did not report blinding of outcome assessors.
Eleven studies reported that the participants were blinded to the interventions (Arthurs 1983; Cook 1990; Fowlie 1992; Longstreth 1981; Lucey 1987; Prior and Whorwell 1987; Rees 2005; Ritchie 1979; Ritchie 1980; Søltoft 1976; Tarpila 2004). Eight studies stated that the participants were not blinded (or this was deduced from intervention differences) (Aller 2004; Chapman 1990; Dettmar 1999; Fielding 1984; Kruis 1986; Manning 1977; Parisi 2002; Villagrasa 1991). One study (Parisi 2005) was unclear about patient blinding.
Only one study (Cook 1990) described an a-priori power calculation. Several studies included in the review demonstrated baseline comparability of the groups, but eight did not give baseline characteristics (Arthurs 1983; Dettmar 1999; Longstreth 1981; Lucey 1987; Manning 1977; Ritchie 1979; Ritchie 1980; Søltoft 1976).
Six studies reported no withdrawals (Aller 2004; Dettmar 1999; Lucey 1987; Parisi 2002; Ritchie 1979; Ritchie 1980). Four studies reported that more than 20% of people in at least one arm (or overall) were not analysed or were lost to follow-up (attrition bias):
- Cook (1990): 5/14 (36%) of participants withdrew from the study
- Longstreth (1981): 6/40 (15%) on placebo and 11/37 (30%) on psyllium did not complete the study. 3/6 and 7/11 respectively dropped out because of dislike for the study preparation or failure to improve; 1/6 and 1/7 dropped out because their symptoms improved
- Prior and Whorwell (1987): 8/40 (20%) withdrew from ispaghula group; 15/40 (38%) withdrew from placebo group. This study reported most recent data carried forward in the analysis, but this is not an approved method of handling missing data. The study also stated that 4/8 and 10/15 withdrawals, respectively, were because of treatment failure.
- Rees (2005): 2/14 (14%) did not complete the study in the intervention arm and 4/14 (29%) on placebo. There were no further details.
Thus, Cook (1990), Longstreth (1981), Prior and Whorwell (1987) and Rees (2005) were treated with caution and examined in sensitivity analyses.
The risk of bias was assessed for each included study. Four studies were assessed as being at higher risk of bias (Cook 1990; Longstreth 1981; Prior and Whorwell 1987; Rees 2005 – attrition bias) and were treated with caution. The eight studies that reported that the participants were not blinded (Aller 2004; Chapman 1990; Dettmar 1999; Fielding 1984; Kruis 1986; Manning 1977; Parisi 2002; Villagrasa 1991) were also treated more cautiously.
RESULTS
A. Fibre versus Placebo
There were eleven studies that compared fibre with placebo (Arthurs 1983; Cook 1990; Fowlie 1992; Kruis 1986; Longstreth 1981; Lucey 1987 first period only; Manning 1977; Prior and Whorwell 1987; Rees 2005; Ritchie 1979; Søltoft 1976). Two of these studies were in people with constipation-predominant IBS (Cook 1990; Rees 2005); three did not specify the type of IBS (Arthurs 1983; Fowlie 1992; Ritchie 1979) and the remainder had mixed IBS types. Therefore the studies were not stratified by IBS type. Similarly, there was too little information to separate by severity, post-infective cause or bloating status.
Where outcomes were measured at different times during the study, we took the end-study results unless there were significant numbers of withdrawals or problems with compliance. Therefore, for the Kruis (1986) study we took the values at four weeks. The results in Rees (2005) were collected between week 8 and week 12 (11 people were assessed at week 8; six at week 9; three at week 10; one at week 11, and; one at week 12).
1. Global symptoms
a. Number of people with improvement in global symptoms
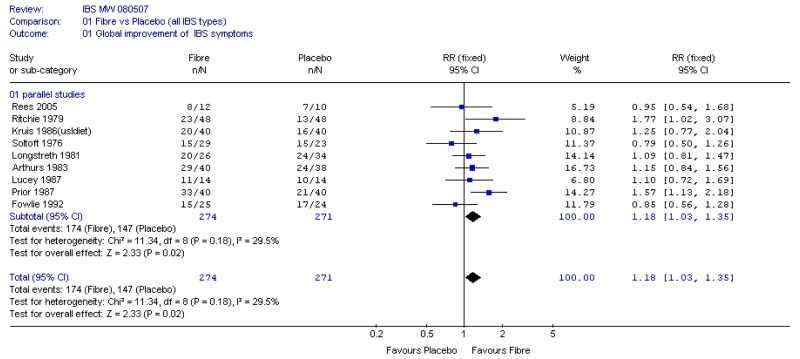
Nine studies with 545 participants reported this outcome. Overall the relative risk was 1.18 (95% CI 1.03 to 1.35), i.e. statistically significant, in favour of fibre.
Subgroup analysis into soluble and insoluble fibres (Figure 2) gave some suggestion that soluble fibre was more effective than insoluble, however, this conclusion was fairly reliant on the Prior and Whorwell (1987) study, which had some attrition bias and was analysed using the last measurement carried forward method. A sensitivity analysis without Prior and Whorwell (1987), Longstreth (1981), Rees (2005 - attrition bias) and Kruis (1986 - which did not have a placebo comparator) showed little difference in global improvement between fibre and placebo overall, although the results for soluble fibre were still significant (Figure 3a).

Figure 3
Sensitivity analysis.
Sensitivity analysis by method of ingestion
A further sensitivity analysis was carried out on the studies that were not at risk of bias, to investigate if there was an effect of supplementary fibre compared with dietary fibre. This was examined in a subgroup analysis (Figure 3b). There was heterogeneity (I2=58%, p=0.09) in the supplement group, which was probably caused by different types of fibre.
b. Number of people with deterioration in global symptoms
Three studies reported this outcome, and included 140 participants (Figure 4). The numbers of events were few and there was too much uncertainty (wide confidence interval) to draw conclusions.
c. Global symptom score (mean)
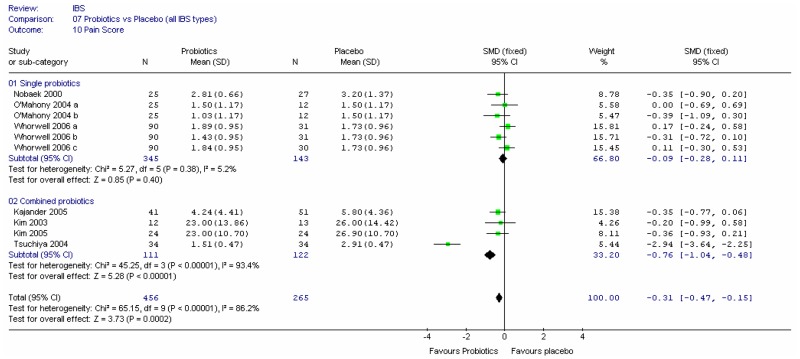
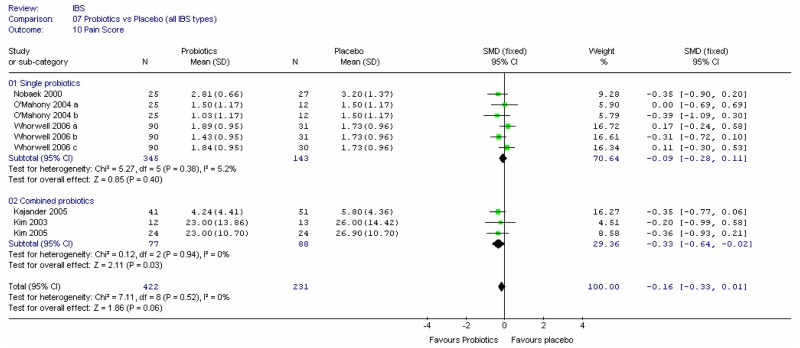
This outcome was recorded by four studies (Cook 1990; Fowlie 1992; Longstreth 1981; Lucey 1987), and different scales were used. Fowlie (1992) did not give scores for the two groups and Cook (1990) was a crossover design (and had some attrition bias). In view of the different scales it was not possible to meta-analyse the parallel and crossover studies using the generic inverse variance method, so the two remaining parallel studies and the crossover study were analysed separately using the standardised mean difference. The results were inconclusive (Figure 5).
2. Individual symptoms
a. Pain
The following studies measured pain:
- Number of people with no pain: one study (Prior and Whorwell 1987)
- Number of people with less pain: three studies (Kruis 1986)
- Pain score (final): four studies (Cook 1990; Fowlie et al 1992; Longstreth et al 1981; Manning 1977);
- Two studies reported pain severity at the end of the study (Cook 1990; Fowlie 1992)
- Two studies reported pain severity from daily diary readings (Longstreth 1981; Manning 1977).
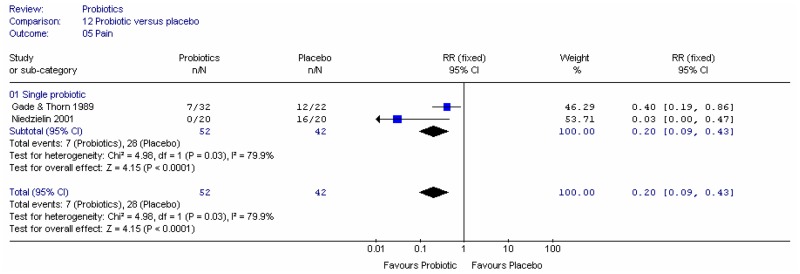
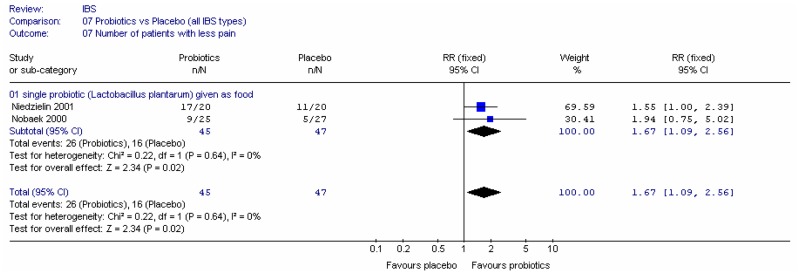
Figure 6 shows the number of people with less pain and the number of people with no pain, in two single studies. The confidence intervals were too wide to draw conclusions.
Fowlie (1992) only gave the difference in the mean change score from baseline and its 95%CI, which was 1 (95%CI −1.5, 4), i.e. not statistically significant.
Combining the other three studies recording pain score, using the standardised mean difference (Figure 7), showed little difference between fibre and placebo, but the data was limited.
b. Bloating
Only one study (Prior and Whorwell 1987) reported bloating (Figure 8). This showed that statistically significantly more people had bloating when they took fibre (soluble) compared with placebo. It should be noted that this was a last measurement carried forward analysis, but that a large proportion withdrew from the study in the ispaghula group.
c. Combined bloating and flatulence score
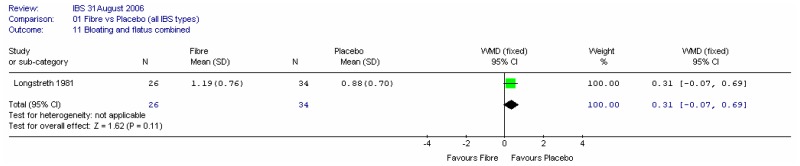
One study reported this outcome (Longstreth 1981). The results showed a small non-significant difference (0.31 on a scale of 0 to 4) in favour of placebo. We noted that this study had attrition bias.
d. Bowel habits
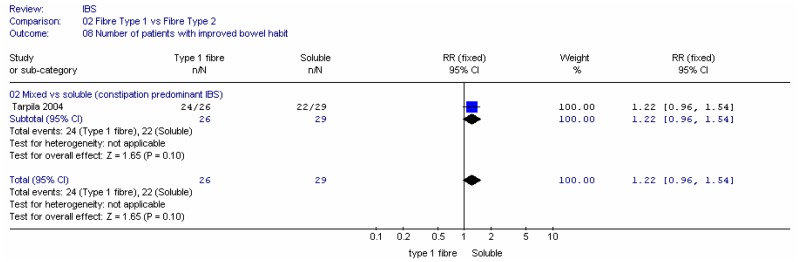
i. Number of people with improved bowel habits
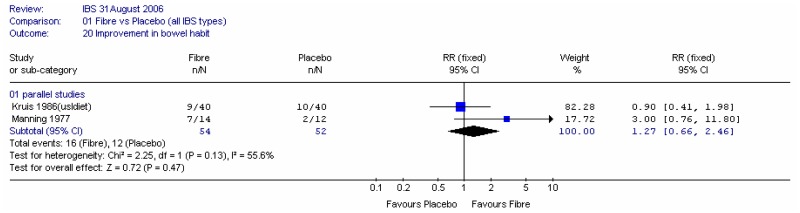
Two studies, with 106 participants, recorded the number of people with improved bowel habits (Kruis 1986; Manning 1977). Meta-analysis showed some heterogeneity between studies and a wide confidence interval. Each study was a comparison with a non-placebo comparator (low fibre or usual diet).
ii. Stool score (aggregate)
Longstreth (1981) reported the number of normal stools per week. The confidence interval was fairly wide (−2.0 to 2.6), but there was little difference between fibre and placebo. We noted that this study had attrition bias.
B. Fibre type 1 versus Fibre type 2
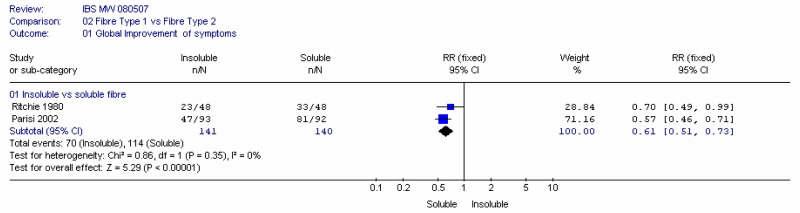
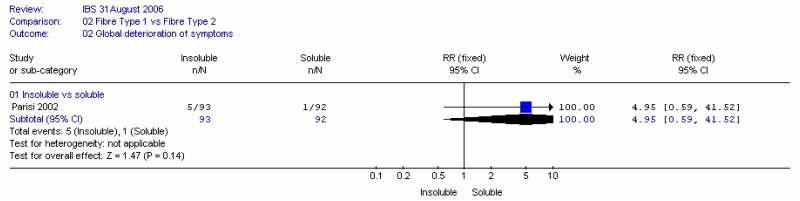
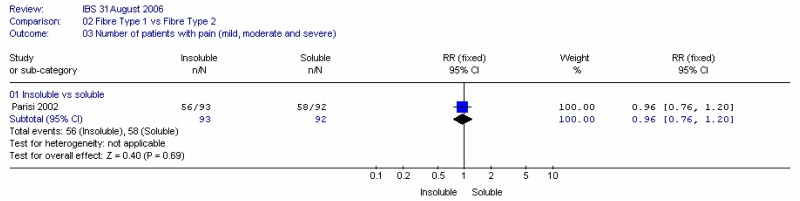
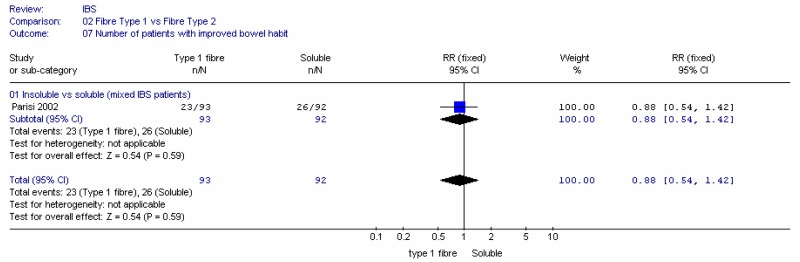
B1. Insoluble versus soluble fibre
Two studies compared insoluble and soluble fibre: Parisi (2002) compared wheat bran (insoluble; 30g/day) with guar gum (soluble; 5g/day) in people with a mixture of IBS types; Ritchie (1980) compared coarse natural bran (insoluble; 20g/day) with ispaghula (soluble; Fibogel 7g/day).
1. Global outcomes
a. Global improvement of symptoms
Meta-analysis of two studies (Parisi 2002; Ritchie 1980) in 281 people, found a statistically significant increase in the number of people reporting improved global symptoms in favour of the soluble fibre (RR 0.61, 95% CI 0.51 to 0.73), with no heterogeneity. This corresponded to a number needed to harm of 3 (95%CI 2, 4), for a soluble group rate of 69 to 88%.
b. Global deterioration in symptoms
One study (Parisi 2002) showed a wide confidence interval for this outcome and conclusions could not be drawn.
2. Individual symptoms
a. Pain
One study (Parisi 2002) showed little difference between the interventions for the number of people with pain.
B2. Mixed fibre versus soluble fibre
Tarpila (2004) compared 6 to 24g/day flax seed (mixed fibre: 33% insoluble, 11% soluble, 20% flaxseed oil) with 6 to 24g/day psyllium (soluble), in 55 people with IBS-C.
a. Bloating
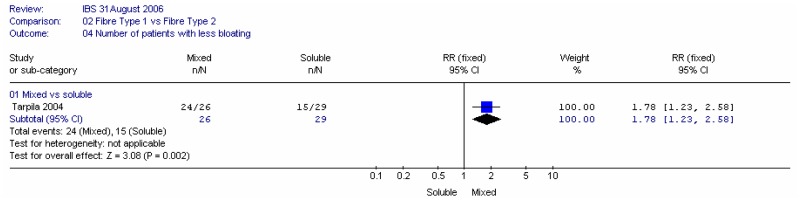
There were significantly more people with a reduction in bloating for the mixed fibres (flax seeds) group, compared to psyllium.
The number of people with more bloating also significantly favoured the mixed fibre, although the confidence interval was very wide.
C. Mixed fibre 1 versus mixed fibre 2
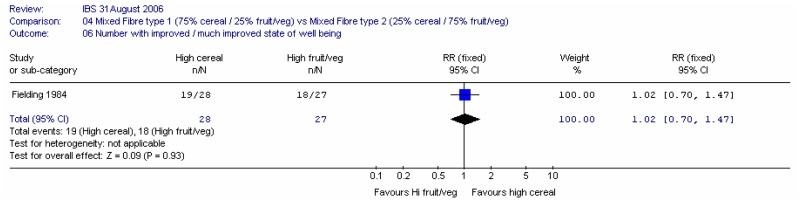
One study (Fielding 1984) compared 40g of mixed fibre diet with different proportions of cereal and fruit/vegetables 75% cereal versus 25% cereal. The study recorded a state of well being score and individual symptom outcomes.
1. Number of people with an improved state of well being
There was little difference between interventions, although the confidence interval was fairly wide.
D. Fibre dose 1 versus fibre dose 2
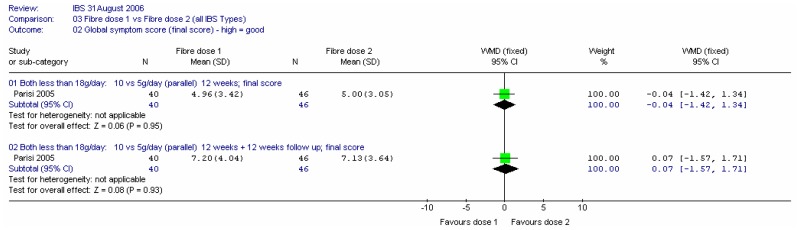
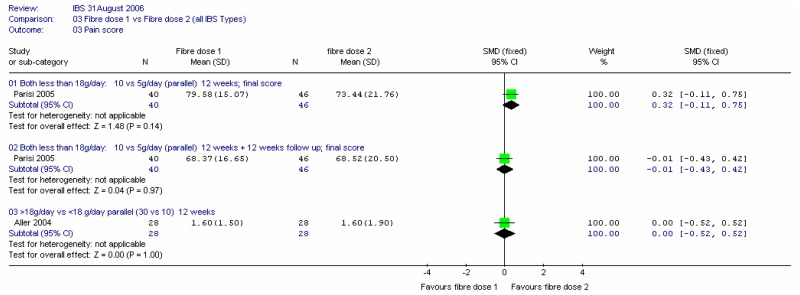
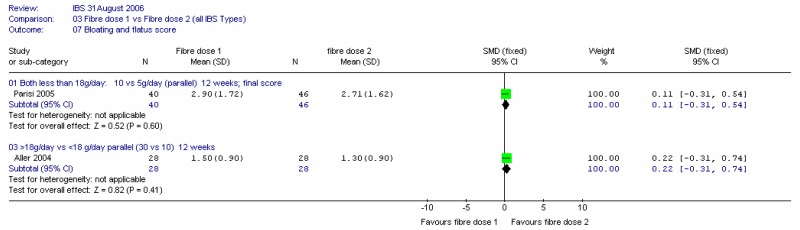
Two studies compared different doses of fibre (Aller 2004; Parisi 2005). In the former, the comparison was 30.5 versus 10.4g/day of mixed fibre over 12 weeks (i.e. above versus below the 18g/day threshold). The latter compared 10 and 5g/day of partially hydrolysed guar gum over 12 weeks, which was then followed up for a further 12 weeks.
a. Global symptom score
There was little difference between interventions in a single study in 96 patients, and the further 12 weeks follow-up did not change this conclusion
b. Pain score
There was a small, non-significant difference between interventions, favouring the lower dose of soluble fibre in Parisi (2005) at 12 weeks, which decreases to zero after a further 12 weeks. There was no significant difference in the two doses (above and below the threshold) for the Aller (2004) study.
c. General bloating and flatus score
There is little difference between dose levels in either study.
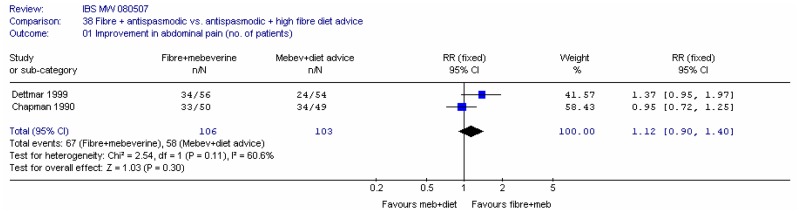
E. Fibre plus another intervention versus another intervention alone
Two studies (Dettmar 1999; Chapman 1990) assessed ispaghula plus mebeverine (antispasmodic) versus mebeverine plus high fibre dietary advice. Each study reported the number of people improved in terms of abdominal pain, and in terms of improvements in bowel habit, at 4 weeks.
F. Protective effects of fibre for the prevention of colorectal adenomas and carcinomas, coronary heart disease and breast cancer
1. Colorectal Cancer
The role of diet in the development of colorectal cancer has long been hypothesised. Although there are many studies investigating the relationship between diet and colorectal cancer, the exact relationship remains unclear.
In the 1970’s epidemiological studies first suggested an inverse relationship between foods rich in dietary fibre and the incidence of colorectal cancer. However, many of these studies were case-control designs, which were subject to selection bias and recall bias. Evidence from two large cohort studies (the Nurses Health Study in 88,757 women and the Health Professionals’ Follow-up Study in 47,325 men) found that dietary fibre had no significant effect on the risk of colorectal cancer. A further cohort study in 61,463 people, however, found a weak association between fruit consumption and reduction in risk, but no association between cereal intake and risk. More recently a Cochrane review (Asano 2002) of five large randomised trials showed no significant protective effect of fibre on the development of colorectal adenomas within two to four years.
2. Coronary Heart Disease (CHD)
Prior to 2000, a number of reviews investigated the relationship between diet and CHD and Stroke. Since 2000 several studies have concentrated on the relationship between wholegrain dietary intake and CHD, and there is a body of evidence to support a 20 to 40% risk reduction of CHD for those who consume a diet rich in wholegrains compared to those who do not. However many studies have not shown an independent effect of fibre alone. The only RCT in secondary prevention of CHD that advised participants to eat more cereal fibre showed no reduction in the reinfarction rate, but there was no data on primary prevention. There was strong evidence to suggest that wheat fibre does not lower cholesterol.
Cereal products provide around 30% of total energy intake in British adults. Several nutrients contained in cereals have the potential to reduce the risk factors for CHD (linoleic acid, fibre vitamin E, selenium and folate, phytoestrogens of the lignan family, phenolic acids with antioxidant properties). It should be noted that some processed cereal foods are high in salt and could contribute to raising blood pressure.
Over 40 human trials have shown that oat fibre tends to lower plasma total and LDL cholesterol but wheat fibre does not. Rice bran and barley may also lower cholesterol but intake of barley tends to be too low to have an effect.
There is no clear association, negative or positive, between total cereal consumption and CHD.
The intake of wholegrain foods may protect against heart disease and stroke but the exact mechanism is not clear. Fibre, magnesium, folate and vitamins B6 and E may be important.
The Joint Health Claims Initiative states that the evidence supports the association between regular consumption of wholegrains and a healthy heart but that it is insufficient to demonstrate cause and effect.
3. Breast Cancer
In the mid 1980’s the role of fibre in breast cancer was suggested. There have been many studies including case control studies in several populations reporting a reduced risk for breast cancer for individuals with a high intake of dietary fibre. Other studies were contradictory and the positive effect of fibre for breast cancer risk reduction was not confirmed by prospective cohort studies in the US (Holmes 2004; Terry 2002). A recent study (Cade 2007) investigated the relationship between dietary fibre intake and breast cancer in a large cohort of British women. The conclusions were that total fibre of more than 30g/day was protective against breast cancer in pre-menopausal women relative to an intake of less than 20g/day, but was not significant in post-menopausal women. After assessing this study we had some reservations.
- The population were highly selected and not necessarily representative
- Lower levels of fibre intake were not protective and subgroup analysis according to fruit, vegetable and cereal fibre showed no significant effect
- There was no data available on the effects of soluble and insoluble fibre (Cade, personal communication to GDG).
A recent large RCT (Pierce 2007) in 3088 women investigated the effect on prognosis, following treatment for breast cancer, of a diet very high in vegetables, fruit and fibre and low in fat, plus telephone counselling, in comparison to dietary guidelines. The trial found no reduction of breast cancer events (recurrence or new primary) or any improvement in survival over a 7.5 year follow-up period.
There is currently insufficient evidence to demonstrate a causal relationship between total cereal consumption and breast cancer prevention. Studies have not investigated the specific effects of soluble and insoluble fibre.
In summary, the protective effects of fibre for the prevention of colorectal adenomas and carcinomas, coronary heart disease and breast cancer remain uncertain.
GDG DISCUSSION
The GDG discussed the use of fibre at some length, also taking into account a survey of the use of bran in people with IBS in primary and secondary care (Miller 2006). This paper suggested that bran was not especially effective in primary care, improving symptoms in 27/100 people with IBS, with 22/100 reporting an exacerbation of symptoms. This was significantly fewer than found in people in secondary care. The effects of soluble fibre were similar in both primary care and secondary care. The study highlighted the issues of extrapolating the response to treatment in IBS from different care settings.
The GDG unanimously agreed that the practice in primary care of recommending high fibre diets to people with IBS should cease. They suggested that GPs should investigate the person’s usual fibre intake with a view to modifying fibre levels to suit the symptom profile and they should monitor the person’s response to dietary modification. GDG consensus was that wheat bran should not be recommended for people with IBS as it is ineffective in the management of symptoms and may even increase symptoms in some people. It may be preferential for the dietary fibre intake to be closer to 12g/day rather than 18g/day. If an increase in fibre were needed, this should be in the form of soluble fibre. Although the RCT evidence for the beneficial effect of soluble fibre was based on trials using supplements such as ispaghula, the GDG wished to give an example of a dietary food that is high in soluble fibre in their recommendation. They also took into consideration the protective effect of oats on cholesterol levels. GDG consensus was that oats should be given as an example of a food high in soluble fibre.
The GDG noted that any protective effect of fibre is from food rich in dietary fibre as opposed to supplemental fibre, because the former contain other nutrients and phytochemicals and the roles these play may be more important than the fibre alone.
HEALTH ECONOMIC EVIDENCE
The cost effectiveness of fibre was not estimated as fibre is not prescribed but purchased by people with IBS as part of their food or as an over the counter food supplement.
EVIDENCE STATEMENTS
- There is a moderate amount of weak evidence to show that significantly more patients have improved global symptoms when taking soluble fibre compared with placebo, and that there is no significant difference for insoluble fibre compared with placebo.
- There is weak evidence to show no significant effect on global symptoms of the means of delivery of fibre, whether given as a food or as a supplement.
- There is good evidence to show that significantly more patients have improved global symptoms when taking soluble fibre compared with insoluble fibre; however there is no significant difference in pain or in improvement in bowel habits.
- There is a fair evidence to show that flax seed containing flaxseed oil gave significantly less bloating than psyllium in people with IBS, but there was no significant difference in the number of people with improved bowel habits.
- There is a moderate amount of fair evidence to show no significant difference in the state of well being and the number of patients with reduced pain, or improved bowel habit, when comparing a mixed diet containing 25 % or 75% cereal.
- There is limited evidence to show little effect of fibre dose on pain, bloating and bowel scores in people with IBS.
- There is inconsistent evidence of a protective effect of fibre on colorectal cancer, breast cancer and coronary heart disease, and a causal protective relationship has not been demonstrated.
EVIDENCE TO RECOMMENDATION
The GDG took into consideration the clinical evidence on the effectiveness of high fibre diets, together with their clinical experience of deleterious effects of a high fibre diet; they balanced these with a consideration of the protective nature of fibre against cancers and heart disease, as determined in the general population. The GDG was unanimous that the practice of recommending that people with IBS eat a diet high in fibre should cease, and recommended that the first stage in improving a person’s diet was to review the fibre intake and adjust accordingly. The improvement in IBS symptoms due to soluble fibre was noted, and its possible protective effect against heart disease, so that the GDG recommended soluble fibre if an increase in fibre was required. Soluble fibre should be either in the form of supplements (as in the RCT evidence) or as foods high in soluble fibre, such as oats (from GDG consensus).
RECOMMENDATION
Healthcare professionals should review the fibre intake of people with IBS, adjusting (usually reducing) it while monitoring the effect on symptoms. People with IBS should be discouraged from eating insoluble fibre (for example, bran). If an increase in dietary fibre is advised, it should be soluble fibre such as ispaghula powder or foods high in soluble fibre (for example, oats).
7.4. Probiotics and prebiotics
SELECTION CRITERIA
The selection criteria described in the general methodology section were used, but some were specific to the probiotics review and are reported below.
Types of studies
The GDG decided that crossover studies should not be included in this review because it was unclear whether probiotics effected longer term changes or how long they were retained in the gut.
Types of intervention
Studies should include the following interventions:
- Single probiotics
- Combination probiotics
- Single prebiotics
- Synbiotics.
Probiotics may be given as a food or as an enteric coated capsule. Prebiotics should fulfil three criteria: (a) resistance to gastric acidity, hydrolysis by mammalian enzymes and gastrointestinal absorption; (b) fermentation by intestinal microflora; (c) selective stimulation of the growth and/or activity of intestinal bacteria associated with health and well-being. Acceptable prebiotics are mainly fructo-oligosaccharides, galacto-oligosaccharides and lactulose.
The following comparisons were included:
- Single probiotic versus placebo
- Combination probiotic versus placebo
- Single prebiotic versus placebo
- Synbiotics versus placebo
- Probiotic 1 versus probiotic 2
- Probiotic dose 1 versus dose 2
- Intervention duration 1 versus duration 2.
The probiotics review was concerned only with longer-term maintenance treatment.
In spite of the large placebo effect associated with IBS, comparisons with no treatment were included, and the minimum duration of treatment was four weeks.
Stratification and Subgroup analyses
Pre and probiotics were to be treated separately. We planned to carry out subgroup analyses as follows:
- Type of probiotic (single, combination)
- Nature of bacteria, including the strain (e.g. Lactobacillus salivarius, Bifidobacterium infantis, Streptococcus faecium)
- Dose (above and below 1 x 106 bacteria per day; this was later revised to 106, 108, 1010 subgroups and the GDG later excluded studies with levels below 1 x 106)
- Duration of intervention (5–8, 9–12, 13–16, 16+ weeks).
We also planned to investigate the effect of enteric coated capsules compared with the addition of probiotics as a food.
SEARCH STRATEGY FOR IDENTIFICATION OF STUDIES
Searches were performed on the following core databases: MEDLINE, EMBASE, CINAHL and The Cochrane Library (1966 to current day with guidance from the GDG). The search strategies are listed in Appendix B.
The titles and abstracts from the search strategy were assessed. Thirty-seven were identified to be potentially relevant to the review and these papers were retrieved in full. Thirteen studies met the inclusion criteria for the review. The reference lists of these were inspected for further potential papers, but none were identified. The excluded studies are listed in Appendix E, along with reasons for exclusion.
DESCRIPTION OF STUDIES INCLUDED IN THE REVIEW
Thirteen studies met the inclusion criteria for the review (Bittner 2005; Gade 1989; Kajander 2005; Kim 2003; Kim 2005; Niedzielin 2001; Niv 2005; Nobaek 2000; Olesen 2000; O’Mahony 2004; Saggioro 2004; Tsuchiya 2004; Whorwell 2006). One study was conducted in the UK (Whorwell 2006) and one was carried out in Ireland (O’Mahony 2004). Two were conducted in Italy, three in the USA, two in Denmark and one each in Finland, Sweden, Poland and Israel.
The majority of studies had fewer than 100 patients. Two studies (Kajander 2005; Whorwell 2006) had more than 100 patients in total.
Study Design
Setting: The majority of studies took place in secondary care, but three were carried out in primary care (Bittner 2005; Gade 1989; Whorwell 2006) and one was assumed to be primary care (Nobaek 2000; recruited by newspaper advertisement). One study had patients from both primary and secondary care (O’Mahony 2004; patients from gastroenterology clinics and newspaper advertisement).
All the studies included in the review had a parallel design.
One study (O’Mahony 2004) had 3 arms comparing Lactobacillus salivarius UCC4431, Bifidobacterium infantis 35624 and placebo malted milk. Whorwell (2006) compared three different doses of encapsulated Bifidobacterium infantis 35624 with placebo in women with IBS. This gave a total of 20 comparisons in the review.
Population
The definition of IBS varied between studies: two used the Manning criteria (Niedzielin 2001; Olesen 2000); one used the Rome I criteria (Kajander 2005); one met criteria defined by the authors that were similar to the above (Gade 1989); and the rest used the Rome II criteria.
All studies but one included patients who had a range of IBS types; the other study specified diarrhoea predominant IBS symptoms (Kim 2003). Only one study (Niv 2005) stated that the participants had IBS as result of gastrointestinal infection.
The majority of studies (12) did not state the number of participants with bloating. Five studies had some patients with bloating measured as an outcome (Kajander 2005; Kim 2003; Olesen 2000; Tsuchiya 2004; Whorwell 2006). Two studies (Kim 2005; Niedzielin 2001) identified all patients as having bloating.
Most of the studies described symptom severity as mixed; one study described the symptoms as mild (Nobaek 2000). Two studies did not state symptom severity (Kajander 2005; O’Mahony 2004). Three studies suggested that the patients had refractory IBS: Saggioro (2004) reported that the patients had been treated with drugs without success; Niedzielin (2001) stated that all patients had been referred to secondary care because of problems with management; Tsuchiya (2004) reported that all patients had undergone a number of treatments without significant and lasting benefit.
The age range of participants across studies was 19 to 78 years, with the mean age (where given) ranging from 34 to 48 years. No study particularly identified elderly participants. All studies had a ratio of women to men greater than one. Whorwell (2006) included only women participants.
Interventions
The studies varied in the type of probiotics used:
- Six used a single probiotic (Gade 1989; Niedzielin 2001; Niv 2005; Nobaek 2000; O’Mahony 2004; Whorwell 2006)
- Five used a combination of probiotics (Kajander 2005; Kim 2003; Kim 2005; Saggioro 2004; Tsuchiya 2004)
- One study used a prebiotic (Olesen 2000)
- One gave a pre/probiotic combination (Bittner 2005).
A range of different bacteria was used, and included various strains of Lactobacillus, Bifidobacterium and Streptococcus. Further details are available in the Appendix.
Three studies gave the probiotic in a capsule form (Kajander 2005; Whorwell 2006; Bittner 2005), two gave a tablet (Gade and Thorn 1989; Niv 2005) and the remainder used a food source or solution as the means of ingestion. The food sources used included milk or milk products, yoghurt, oatmeal soup and fruit drinks. The GDG considered the medium in which the probiotics were ingested to be an important difference and decided to consider, as subgroups, capsules versus other delivery routes. Whether the intervention was given with food was also considered important because of the increased levels of bile salts, as a result of digestive process, which are a serious obstacle to probiotic survival. However only three studies gave details as to when the probiotics were taken: Niedzielin (2001) directed that they should be taken before breakfast and two hours after the evening meal; in Gade (1989) the dose was given in the morning and evening with meals; and Olesen (2000) required the dose of prebiotics be taken with breakfast.
The GDG defined the minimum dose of probiotic as 1 x106. The doses of probiotic varied considerably, and ranged from 8x106 (Gade 1989) to 4x1010 (Niedzielin 2001) for single probiotics, and 5x109 to 5x1011 for combination probiotics. It is noted that the activity of the probiotics vary according to strain.
The duration of the intervention ranged from four weeks (Gade 1989; Niedzielin 2001; Nobaek 2000; Saggioro 2004; Whorwell 2006) to six months (Kajander 2005; Niv 2005). Three studies had durations of eight weeks (Kim 2003; Kim 2005; O’Mahony 2004), and two studies had interventions lasting 12 weeks (Olesen 2000; Tsuchiya 2004). One study followed the patients for 12 months (Nobaek 2000).
Comparisons
The included studies covered the following comparisons:
- Nine comparisons of a single probiotic versus placebo (Gade 1989; Niedzielin 2001; Niv 2005; Nobaek 2000; O’Mahony 2004 x 2; Whorwell 2006 x3)
- Lactobacillus salivarius UCC4331 (1x1010) in malted milk drink (O’Mahony 2004)
- Lactobacillus plantarum DSM 9843 (5x107 CFU) in oatmeal soup (Nobaek 2000)
- Lactobacillus plantarum 299V (5x107) in oatmeal soup (Niedzielin 2001)
- Lactobacillus reuteri ATCC 55730 (1x108) tablet (Niv 2005)
- Two used Bifidobacterium infantis 35624 (O’Mahony 2004 (1x1010) in malted milk drink; Whorwell (2006) 1x106, 1x108, 1x1010 CFU in capsule)
- Streptococcus faecium (dose estimated as 8x106) tablet (Gade 1989);
- Five comparisons of a combination of probiotics versus placebo:
- SCM-III solution (Lactobacillus acidophilus1.25x106 CFU; Lactobacillus helveticus 1.3x109; bifidobacterium 4.95x10^9) (Tsuchiya 2004)
- Lactobacillus rhamnosus GG, L. rhamnosus LC705, Bacillus breve Bb99, P.freudenreichii ssp.shermanii JS capsule (Kajander 2005)
- Lactobacillus plantarum LPO1 & Bifidobacterium Breve BRO 5x109 CFU sachet dissolved in water (Saggioro 2004);
- One study compared two different probiotics (Lactobacillus salivarius UCC4331 versus Bifidobacterium infantis 35624) (O’Mahony 2004)
- One study compared three doses of probiotics (Whorwell 2006; 3 comparisons)
- One comparison of Prebiotic versus placebo (Oleson and Hoyer 2000)
- One comparison of a pre/probiotic capsule versus placebo, but this study contained no analysable data (Bittner 2005).
OUTCOMES
The studies measured a range of outcomes.
1. Global symptoms
a. Number of patients with an improvement in global symptoms
Seven studies recorded the patients’ assessment of improvement (Gade 1989; Kajander 2005; Kim 2003; Niedzielin 2001; Olesen 2000; Tsuchiya 2004 – overall clinical effectiveness; Whorwell 2006 – adequate symptom relief) and two (Gade 1989; Tsuchiya 2004) also recorded a clinician assessment.
b. Global symptom score (mean)
The global symptom score was recorded by seven studies (Kajander 2005; Kim 2003; Niv 2005; Nobaek 2000; O’Mahony 2004; Saggioro 2004; Whorwell 2006), but Saggioro (2004) recorded the percentage change in global symptom score.
c. Global improvement in symptoms score (mean)
This outcome was recorded by two studies (Kim 2003; Olesen 2000).
d. Number of patients with deterioration in global symptoms
This outcome was recorded by three studies (Gade 1989; Olesen 2000; Tsuchiya 2004).
2. Individual symptoms
a. Pain
Pain was reported in several ways, either giving the number of patients with pain at the end of the study, the number of patients whose pain improved or worsened compared with the baseline, and pain scores. The latter recorded a range of features, including severity, frequency and duration, or a combination of these. In addition, studies recorded the final scores, mean daily scores or the change from baseline. The studies reporting the following outcomes are listed below:
- Pain score: eight studies recorded a pain score (Kajander 2005; Kim 2003; Kim 2005; Nobaek 2000; O’Mahony 2004; Saggioro 2004; Tsuchiya 2004; Whorwell 2006), although Saggioro (2004) recorded the percentage change in pain score.In all cases the highest rating meant worst symptoms, although the scales used were not the same.
- Number of patients with less pain: one study (Niedzielin 2001).
b. Bloating
- Number of patients with more bloating (Olesen 2000)
- Number of patients with less bloating (Kim 2005; Olesen 2000)
c. Bowel habits
- Stool frequency (change and final) (Kajander 2005; Kim 2003; Kim 2005; Tsuchiya 2004). We decided that stool frequency was an unreliable measure of improvement if the type of IBS was not given. Only one of these studies specified the type of IBS (Kim 2003, which was in patients with diarrhoea predominant IBS), and the other studies were disregarded for this outcome.
- Stool score which was an aggregate score including stool frequency, consistency, ease of passage and completeness of evacuation (Kim 2003; Kim 2005; Nobaek 2000; O’Mahony 2004).
3. Quality of Life
- Two studies reported quality of life as an outcome (Niv 2005; Whorwell 2006).
METHODOLOGICAL QUALITY
The quality assessment for included trials is shown in Appendix D. The method of randomisation was reported in four studies, all of which gave an adequate method: computer generated numbers (Gade 1989; Kajander 2005; Olesen 2000) and one picking a card from a pack (O’Mahony 2004). The other studies did not state the method of randomisation (Kim 2003; Kim 2005; Niedzielin 2001; Niv 2005; Nobaek 2000; Tsuchiya 2004; Whorwell 2006).
Allocation concealment was reported in three studies (Kim 2005; Olesen 2000; O’Mahony 2004), one of which reported a partially adequate method (O’Mahony 2004), in which randomisation and analysis were said to be ‘supervised by a person independent from the study’. The other two were classified as having adequate concealment because the sequence was retained by a third party.
All the studies reported that the outcome assessors and the patients were blinded to the interventions. All described in detail the appearance and taste of the placebo and active intervention.
Four studies (Kajander 2005; Kim 2003; Kim 2005; Tsuchiya 2004) described an a-priori power calculation. Five studies used an intention to treat analysis (Kim 2003; Kim 2005; Olesen 2000; O’Mahony 2004; Whorwell 2006). All studies included in the review demonstrated some level of baseline comparability of the groups, but two provided limited data regarding baseline characteristics (Gade 1989; Nobaek 2000).
One study had no loss to follow-up (Niedzielin 2001). Three studies reported that more than 20% of patients in at least one arm (or overall) were not analysed or were lost to follow-up (Kim 2005; Niv 2005; Olesen 2000). For the Kim (2005) study we used four week data instead. In Niv (2005), 9/27 (33%) in the control group withdrew; and in Olesen (2000), 14/52 (27%) did not complete the 12 week comparative phase in FOS group.
The risk of bias was assessed for each included study and only Niv (2005) and Olesen (2000) were considered to be at higher risk of bias. These were considered, where possible, in sensitivity analyses.
RESULTS
A. Probiotics versus placebo
Ten studies compared probiotics (singly or in combination) with placebo (Gade 1989; Kajander 2005; Kim 2003; Kim 2005; Nobaek 2000; Niedzielin 2001; Niv 2005; O’Mahony 2004; Tsuchiya 2004; Whorwell 2006).
1. Global symptoms
a. Number of patients with an improvement in global symptoms
Six studies (eight comparisons), with 629 patients, recorded the patients’ assessment of global improvement (Gade 1989; Kajander 2005, Kim 2003, Niedzielin 2001, Tsuchiya 2004, Whorwell 2007). Meta-analysis showed significant heterogeneity (I2=65%; p=0.006).
This heterogeneity was investigated in terms of the pre-specified subgroup analyses: by type of probiotic (single, combination), by duration and by dose and strain of bacterium (Figures 2 to 3).

Figure 2
Subgroup analysis by type of probiotic.

Figure 3
By type and dose of bacterium.
Type of probiotic (Figure 2)
There was still significant heterogeneity in the single probiotic group, but it was not significant in the combination probiotic group (I2=31%, p=0.23). Meta-analysis of three studies in 173 patients showed a statistically significant improvement in global symptoms. This corresponded to an NNT of 3 (95%CI 3, 5), for a control group rate of 42 to 47%. We noted there was significant heterogeneity for the risk difference (I2=74%, p=0.02), which may have been an indication that the particular combination of probiotics was important.
Duration
The Kajander (2005) study was six months duration, Tsuchiya (2004) was 12 weeks and the others were four or eight weeks. This did not account for the heterogeneity amongst studies.
Strain and dose of probiotic (Figure 3)
All the studies had different strains and/or doses, and the confidence intervals are wide in some cases. The heterogeneity may be indicative of different efficacies of the different probiotics; most of the probiotics tested in the trials gave a greater improvement in symptoms than placebo, but there were some exceptions. Whorwell (2006) showed a maximum in the improvement of global symptoms with increasing dose, with only the 108 dose of Bifidobacterium infantis being significant at four weeks. The authors attributed this effect to dissolution problems of the capsule for particular concentrations (see GDG discussion at the end of this review).
b. Number of patients with deterioration in global symptoms
This outcome was recorded by two studies (Gade 1989; Tsuchiya 2004). The confidence intervals were very wide, although Tsuchiya (2004) was statistically significantly in favour of probiotics.
c. Global symptom score (mean)
This outcome was recorded by seven studies (Kajander 2005; Kim 2003; Niv 2005; Nobaek 2000; O’Mahony 2004; Saggioro 2004; Whorwell 2006). One study (Saggioro 2004) reported percentage change scores, with p values, so these results are given separately. The other studies reported the global symptom score, but on different scales, so the standardised mean difference was used to analyse the data (Figure 5). The Niv (2005) values were taken from a graph and it was assumed that the standard error was given. This study also had some attrition bias, so a sensitivity analysis was repeated excluding this study (Figure 6).

Figure 6
Sensitivity analysis without Niv (2005).
Meta-analysis of eight comparisons in 624 patients showed no significant difference between probiotics and placebo overall, with little heterogeneity (I2=4%, p=0.41). However, there was a statistically significant difference for the combined probiotic subgroup in 105 patients. Meta-analysis of seven single probiotics showed no significant difference between probiotic and placebo, with no heterogeneity. Sensitivity analysis without Niv (2005) made a small difference.
The Saggioro (2004) study in 40 patients reported a statistically significant difference in the percentage change in IBS symptom severity (−44% versus −8.5% after 28 days; p<0.001 for the combined probiotic versus placebo).
Subgroup analyses were carried out by strain and dose of bacterium because the GDG was uncertain whether studies using different bacteria should be combined. Figure 7 shows the studies by bacterium and dose. Most comparisons showed no significant difference compared with placebo, including the meta-analysis of two studies in 232 patients, receiving Bifidobacterium infantis at a dose of 1x1010 CFU; there was no significant heterogeneity for these two studies (I2=6%, p=0.30). There were only two statistically significant comparisons:

Figure 7a
Global symptom score.
- Encapsulated Bifidobacterium infantis at a dose of 1x108 CFU versus placebo, in 182 patients. This had a mean difference of −0.33 (95%CI −0.59, −0.07) on a scale of 0 to 15 (i.e. a fairly small effect)
- Encapsulated combined probiotic versus placebo in 81 patients. This had a mean difference of −6.48 (95%CI −12.56, −0.40) on a scale of 0 to 112.
This may, however, be a size effect; most of the non-significant studies had around 50 patients or fewer.
Nobaek (2000) also reported 12 month follow-up, but it was unclear if the patients continued to modify their diet after the trial had ended. There was a borderline significant effect of probiotics at 12 months, but not at 5 to 6 weeks. The scale was 0 to 10.
e. Global improvement in symptoms score (mean)
This outcome was recorded by one study (Kim 2003), which showed too much uncertainty to draw conclusions. It is unclear what scale is used.
2. Individual symptoms
a. Pain
i. Number of patients with pain
Two studies measured this outcome (Gade 1989; Niedzielin 2001) and both separately were statistically significantly in favour of probiotic. The relative risk ranged from 0.03 to 0.2 (i.e. 5 to 33 times less risk of pain with the probiotic). However, combining the studies gave heterogeneity (I2=80%; p=0.03).
ii. Pain score
Eight studies (Kajander 2005; Kim 2003; Kim 2005; Nobaek 2000; O’Mahony 2004; Saggioro 2004; Tsuchiya 2004; Whorwell 2006) reported a pain score. Saggioro (2004) reported percentage change scores, with p values, so these results are given separately.
This outcome showed no significant difference between interventions for the single probiotic group (although it is difficult to estimate the width of the confidence interval because the standardised mean difference was used) and a highly heterogeneous result for the combined probiotics group (I2=93%, p=0.00001), attributable to the Tsuchiya (2004) study, from which data were extracted from a graph, which may not have been to scale for the standard deviations. In the absence of this study the meta-analysis of three studies gave a statistically significant reduction in pain for the combined probiotic group, and no heterogeneity (I2=0%; p=0.94).
Saggioro (2004) reported a statistically significant difference in the percentage change in pain score (−38% versus −18% after 28 days; p<0.05 for the combined probiotic versus placebo).
Figure 10bWithout Tsuchiya 2004
Nobaek (2000) also reported 12 month follow-up data, shown in Figure 10c. The scale is a visual analogue scale of 0 to 10. There was no significant difference at 5 to 6 weeks, but a statistically significant difference after 12 months. It was unclear if the patients in the intervention group changed their dietary habits following the trial or if there was a long-term effect.
iii. Number of patients with less pain
Two studies compared Lactobacillus planetarum, given in food, with placebo (Niedzielin 2001; Nobaek 2000) and reported the number of patients with reduced pain. There was a statistically significant reduction in pain; RR 1.67 (95% CI 1.09, 2.56), with no heterogeneity (I2=0, p=0.64). This corresponded to a number needed to treat of 5 (95% CI 3, 20) for a control group rate of 19 to 55%.
b. Bloating
i. Number of patients with less bloating (Kim 2005)
In a single study in 48 patients, there was no significant difference between probiotics and placebo in the number of patients with less bloating, although the confidence interval was fairly wide.
ii. Bloating score (Kajander 2005; Kim 2003; Kim 2005; O’Mahony 2004; Tsuchiya 2004; Whorwell 2006)
This outcome showed no significant difference between interventions for the single probiotic group and a highly heterogeneous result for the combined probiotics group, attributable to the Tsuchiya (2004) study, from which data were extracted from a graph, which may not be to scale for the standard deviations. The authors of this study reported that there was no significant difference between groups, which belies the data on the graph, suggesting that the standard deviations on the graph were inaccurate. In the absence of this study, meta-analysis of the three studies in 73 patients gave a statistically significant reduction in bloating score for the combined probiotic group, MD −0.42 (95% CI −0.73, −0.10), with no heterogeneity (I2=0%; p=0.87).
Figure 13bSensitivity analysis without Tsuchiya (2004)
c. Bowel habits
i. Stool frequency
Only one study specified the type of IBS (Kim 2003), which was in patients with diarrhoea predominant IBS. For this study, the frequency was seen as a negative outcome and there was no significant difference between probiotic and placebo.
ii. Stool score
This was aggregated to include stool frequency, consistency, ease of passage and completeness of evacuation (Kim 2003; Kim 2005; O’Mahony 2004; Nobaek 2000; Whorwell 2006 – bowel habit satisfaction). Tsuchiya (2004) also reported assessment of bowel habits, but these values were not included in the meta-analysis in view of the uncertainties in the standard deviation described above.
In the meta-analysis of eight comparisons (562 patients) there was no significant difference between probiotics and placebo for this outcome, either overall, or for single or combined probiotics, and there was no significant heterogeneity.
3. Quality of Life
Only one study reported quality of life as an outcome (Niv 2005). This showed no significant difference in the quality of life score. The scale used was unclear, however: the study reported that there were 26 questions, each rated from mild (1) to severe (7), and the sum of all of them yielded the total QoL score, but the baseline scores for the total were similar to the individual components and were about 4 to 5 points.
Examination of the two studies using Bifidobacterium infantis 35624
Two studies compared Bifidobacterium infantis 35624, at a dose of 1×1010 CFU per day, versus placebo. One study (Whorwell 2006) gave the probiotic in a capsule and the other (O’Mahony 2004) in a malted drink.
The outcomes are summarised in Figure 17. There was some heterogeneity between studies for the outcomes of pain and stool score, with the encapsulated probiotic having less effect. This is discussed further in the next section.
B. Probiotic dose 1 versus probiotic dose 2
One study (Whorwell 2006) compared three doses of Bifidobacterium infantis 35624, 1×106, 1×108, 1×1010, with approximately 90 patients in each arm. The outcomes compared are reported in Figure 18.
Head-to-head comparison of the doses 1×108 and 1×106 showed there was a significant difference in global symptoms, pain and bloating scores and a borderline difference in stool score, favouring the 1×108 dose. However, there was no significant difference between the 1×1010 and 1×106 doses, an unexpected dose effect.
Whorwell (2006) explained this using in-vitro dissolution experiments, showing that the highest concentration of probiotic coagulated on exposure to moisture, making dissolution very difficult, such that the probiotic was not bioavailable to the patient.
This effect also explains the differences between Whorwell (2006) and O’Mahony (2004); in the latter, the probiotic was bioavailable because it was present in a drink.
C. Probiotic 1 versus probiotic 2
One study compared two strains of bacteria, Lactobacillus salivarius UCC4331 versus Bifidobacterium infantis 35624 (O’Mahony 2004) directly in a randomised trial of 50 patients. The results are presented below for the different outcome measures.
1. Global symptom score
There was no significant difference in global symptoms at eight weeks, but the Bifidobacterium is favoured. A 10 cm visual analogue scale was used for individual symptoms and combined to give a global score (maximum 30).
D. Prebiotics versus placebo
One study (Olesen 2000) compared a prebiotic (Fructooliogsaccharide given as a 10g sachet for 2 weeks then 20g for 10 weeks) with placebo in 98 patients. The results are given below and generally showed no significant differences between prebiotics and placebo, in either global symptoms or bloating (although the confidence interval was fairly wide in the latter). The confidence interval was too wide to determine if there was a difference for the pain outcome. We noted that there was some attrition bias for this study.
HEALTH ECONOMIC EVIDENCE
The cost effectiveness of pre and probiotics was not estimated as they are not prescribed, but currently purchased by patients as a food supplement.
GDG DISCUSSION
The GDG discussed the use of pre and probiotics at some length. They were unanimous in their view that different types and doses of probiotic should not be combined together in an analysis because they all have different effects. The main issues raised for discussion were dose, method of ingestion and quality of products available to patients. Probiotics are not generally prescribed by GPs. Patients purchase them and there was concern that sources are not always reliable or safe. There was agreement that there is insufficient information for patients about the quality of products and insufficient information on packaging regarding dose and quality of individual products.
The studies that investigated Bifidobacterium infantis 35624 (Whorwell 2006; O’Mahony 2004) were discussed with regard to the observed maximum in the dose response in Whorwell (2006) and the inconsistencies between the two studies. This was explained by the method of ingestion. In Whorwell (2006), a capsule was used as the means of ingesting the different doses of probiotic. For the 1×1010 CFU concentration of probiotic, contact with water led to the probiotic coagulating so that it was no longer bioavailable to the patient. The same dose of probiotic was found to be effective in O’Mahony (2004) because the probiotic was ingested in the form of a milk based drink so the concentration of probiotic was evenly dispersed through the fluid and therefore bioavailable to the patient.
EVIDENCE STATEMENTS
- There is fair evidence to show that some probiotics (single or combination) give a significantly greater improvement in global symptoms of IBS than placebo. However, this is bacterium dependent, in terms of both dose and strain.
- There is good evidence to show a significant difference in global symptom score for combined probiotics compared with placebo, favouring probiotics, but no significant difference for single probiotics as a group in people with IBS.
- There is fair evidence to show a significant reduction in the number of people with pain for those taking single probiotics compared with placebo; there is weak evidence to suggest the extent of this depends on the bacterium strain and/or dose.
- There is good evidence to show no significant difference in pain score or bloating score for single probiotics, both as a group and individually, compared with placebo. There is a significant difference for combined probiotics, with the probiotic giving significantly less pain and bloating.
- There is weak evidence to show no significant difference in the number of people with bloating for combined probiotics compared with placebo.
- There is good evidence to show that the use of probiotics (single or combination) resulted in participants reporting no significant difference in bowel habit.
- There is good evidence to show that high doses of Bifidobacterium infantis (1010 CFU) in capsule form are significantly less effective than moderate doses (108 CFU); moderate doses are more effective than low doses (106). There is weak indirect evidence to show that this reduction in effect at high doses does not occur when probiotics are delivered in a drink.
- There is fair evidence to show no significant difference between Lactobacillus salivarius UCC4331 and Bifidobacterium infantis 35624, in global symptoms, pain, bloating or stool scores.
- There is a moderate amount of weak evidence to show no significant difference in the number of people with improvement in global symptoms or with bloating, between those given the prebiotic, Fructooliogsaccharide, in comparison with placebo.
EVIDENCE TO RECOMMENDATION
The review evidence suggests that some probiotics are effective in people with IBS, but others are not. The effect is dose and strain dependent, and the method of ingestion is also important. Although, there is some evidence from single trials, the GDG did not feel able to recommend named bacteria or probiotic products. On the other hand, it was the view of the GDG that probiotics were not harmful (unless they came from an unreliable source), they were widely available and it might benefit people with IBS if they experimented with probiotics as part of their diet. The GDG agreed there was insufficient evidence to make a recommendation on prebiotics.
RECOMMENDATION
People with IBS who choose to try probiotics should be advised to take the product for at least 4 weeks while monitoring the effect. Probiotics should be taken at the dose recommended by the manufacturer.
7.5. Aloe vera
SELECTION CRITERIA
The selection criteria described in the general methodology section were used.
DESCRIPTION OF STUDIES
Types of Studies
Two randomised trials were included (Odes and Madar 1991; Davis 2006) and two excluded studies are listed in Appendix E, along with reasons for exclusion.
Types of participants
All participants in Davis (2006) had IBS (28% IBS-C, 52% IBS-D, 20% IBS-A); participants had to be between 18 and 65 years, have no other co-morbidities and had to have previously failed conventional management of IBS defined as antispasmodics, bulking agents and dietary interventions. Constipation was defined as per Rome II criteria.
Odes and Madar (1991) had 11/32 people with IBS-C (the rest had simple constipation); people with IBS-D or IBS-A were excluded; participants had to have been receiving laxative therapy for constipation for a minimum of two years as an indication of severity. Participants had previously received other treatments including diet and enemas, but it is not stated if they were refractory to treatment.
Types of intervention
Davis (2006) used aloe vera gel made up in a pink mango flavoured syrup. The dose was 50 ml taken four times a day for one month. The placebo was a matching pink mango flavoured inert syrup.
Odes and Madar (1991) used a capsule laxative preparation made up of celandin, aloe vera and psyllium in ratio of 6:3:1 (total fibre content 47%) given for 28 days. The aloe vera fibre was derived from leaves of Aloe socotrine and contains anthraquinone. The dose was one 500 mg capsule per day taken with water at bedtime increasing to a maximum of three capsules a day. The placebo capsule was of identical appearance but contained no active ingredient. Participants in Odes and Madar (1991) were given no dietary modification advice. No additional medication was prescribed throughout the treatment period but people could continue with prescribed laxative medication, provided that the dose and frequency were recorded in the study data sheet. Davis (2006) did not state if other medications could be continued.
METHODOLOGICAL QUALITY
Davis (2006) used a computerised random numbers table to generate the randomisation schedule. Allocation concealment was implemented in this study; the pharmacist held the randomisation code. Both studies were double blind. Davis (2006) carried out an a-priori sample size calculation.
In Davis (2006), 58 people were randomised. 49 completed the protocol to one month and 41 to three months (i.e. data missing for 17/58 (29%) overall; 33% in the placebo group and 26% in the active group). In Odes and Madar (1991), 35 people were randomised. Three people (placebo) withdrew citing lack of benefit as reason and were excluded from the analysis because of incomplete data.
The groups in both trials were comparable at baseline as regards age, gender, duration and severity of condition, but Odes and Madar (1991) reported that the treatment group had significantly higher pain scores at baseline.
Overall, neither study was considered to have higher potential for bias.
RESULTS
In view of the differences in population and interventions, these two studies were reported separately.
A. Aloe vera gel versus placebo
One study (Davis 2006) in compared aloe vera gel with placebo in 58 people with IBS.
1. Global improvement of symptoms
The primary outcome was the number of people with an improvement in global symptom score (pain; distension; bowel habit, and; quality of life). The symptom score was derived by adding the scores of individual symptoms and the proportion of days symptoms occurred with a maximum score of 500. A reduction of 50 points was defined as improvement. Participants were assessed at one month and at three months post-intervention. The forest plots below illustrate that there was no significant difference between the active and placebo treatment for global improvement of symptoms, although the confidence interval was fairly wide.
2. Individual symptoms
a. Pain
There was no significant difference, either at 1 month or at 3 months, in the change in pain score, on a scale of 0 to 100%, for which a positive change represented an improvement over baseline.
b. Bloating
There was no significant difference either at 1 month or at 3 months, in the change in distension score, on a scale of 0 to 100%, for which a positive change represented an improvement over baseline.
3. Quality of Life
There was no effect on quality of life at one month or at three months. The scale used was not stated.
B. Combined capsule of celandin, aloe vera and psyllium versus placebo
1. Global improvement of symptoms
Odes and Madar (1991) did not report global symptoms.
2. Individual symptoms
One study with 32 participants reported differences in bowel habits (including frequency and consistency) and pain scores for the final two weeks of treatment compared with those in the 14 day pre-intervention run in period (Odes and Madar 1991). Eleven participants (34%) were identified as having IBS-C; the rest had simple constipation.
a. Pain
There was no significant difference in pain scores (number of episodes of pain per week) between groups.
b. Bowel Habits
Compared to the placebo group, people in the intervention arm of the trial experienced a significant increase of 3.6 (95% CI 1.51, 5.69) in the mean number of bowel movements per week.
The consistency of the stools also improved. There was a statistically significant decrease in laxative use in the intervention group of −0.8 (95% CI −1.12, −0.47) on a scale of 1 to 3 and 16/19 people considered their bowel symptoms improved compared to 4/13 of the control group.
No adverse effects were reported and all participants reported that the capsules were easy and convenient to use.
Comment
Whilst this study shows a significant positive effect for bowel habit, we noted that this was a small study (35 patients) in a population of which only one-third had IBS. In addition, the intervention used a combination of aloe vera (30%), celandin and psyllium (soluble fibre). Therefore, it was not possible to attribute the effect to aloe vera alone, and this study was not included in the evidence statements.
SAFETY DATA
The following safety data is based on a systematic review of the scientific literature (case reports and systematic reviews) edited and peer reviewed by contributors to the US Natural Standard Research Collaboration (2006).
Adverse effects
The use of aloe by mouth for laxative effects can cause abdominal cramps and diarrhoea. Adverse effects, reported in a small number of studies, include low blood sugar levels and electrolyte imbalance, particularly lowered potassium levels.
Drug interaction
Use of aloe with laxative drugs may increase the risk of dehydration, electrolyte imbalance, potassium depletion and changes in blood pH.
Oral preparations of aloe have been reported to lower potassium levels, which may impact on the effectiveness of drugs used to manage heart rhythm disturbances, heart disease and renal disease.
Oral preparations of aloe may lower blood sugar, so have the potential to interact with drugs used in the management of diabetes.
Aloe vera should not be used by individuals who may be at increased risk from the aforementioned adverse effects, particularly people with heart disease, kidney disease, diabetes and blood disorders.
GDG DISCUSSION
The GDG expressed concerns that people with IBS purchase aloe vera products at considerable expense without evidence of effectiveness. The GDG also expressed concerns about the adverse effects of oral preparations of aloe vera, about which there was little awareness.
HEALTH ECONOMIC EVIDENCE
The cost-effectiveness of aloe vera was not estimated as it is not prescribed, but purchased by patients as a food supplement.
EVIDENCE STATEMENTS
- There is fair evidence to show no significant effect of aloe vera, in comparison with placebo, in global improvement of symptoms, pain, bloating, bowel score or quality of life.
- There is limited evidence of potentially serious adverse effects associated with oral aloe preparations.
EVIDENCE TO RECOMMENDATION
There is only one trial of aloe vera in people with IBS, but this gave fair evidence to show a lack of effectiveness. The GDG took this into account, together with aloe vera’s potentially serious adverse effects, especially for people with comorbidities. Since aloe vera is a commercially available product that people with IBS pay for at considerable expense, the GDG wished to highlight these points by discouraging its use. Clinicians and people with IBS should be made aware of the lack of effectiveness and potential adverse effects.
RECOMMENDATION
Healthcare professionals should discourage the use of aloe vera in the treatment of IBS.
7.6. Exclusion Diets
SELECTION CRITERIA
The selection criteria described in the general methodology section were used, but some were specific to this review and are reported below.
Types of studies
For intervention studies, randomised trials (RCTs) and quasi-randomised studies, examining the use of dietary manipulation/exclusion for the treatment of IBS were preferred. Crossover trials with a washout period of less than 2 weeks were included but treated with caution. Double-blind placebo controlled studies are technically difficult and most elimination diet studies use a non-randomised open dietary elimination and re-challenge design. This has the potential to introduce bias due to the large placebo effect identified in IBS patients. For this review non-randomised studies were also permitted. Studies were restricted to the English language, but the date was not restricted.
Types of intervention
Interventions were to be included if they referred to an exclusion diet (excluding certain foods) or an elimination diet (only allowing certain foods):
- Lactose restricted diet
- Elimination diet based on foods with IgG4 titres >250 μg/l
- Elimination diet based on food challenge and re-challenge
- Elimination diet based on patient-reported intolerance and re-challenge
- Elimination diet using lamb, rice and pears
- Fasting therapy.
Types of comparisons
The following types of comparisons were to be included:
- True diet versus sham diet
- Elimination diet and food challenge with foods that had been identified as potential causes of intolerance
- Fasting therapy versus usual treatment.
Sensitivity analyses
The following sensitivity analyses may be considered:
- Setting (primary/secondary care)
- Blinding of patients.
SEARCH STRATEGY FOR IDENTIFICATION OF STUDIES
Searches were performed on the following core databases: MEDLINE, EMBASE, CINAHL and The Cochrane Library (1966 to current day with guidance from the GDG). Additional databases were not searched for this review. The search strategies are listed in Appendix B.
The search strategy identified 957 studies. The titles and abstracts of these studies were assessed. Of these, 33 that were potentially relevant to the review were retrieved in full. The reference lists of the retrieved studies were inspected for potential papers for inclusion in the review but none were identified. Sixteen studies were included in the review. The excluded studies are listed in Appendix E, along with reasons for exclusion.
DESCRIPTION OF STUDIES INCLUDED IN THE REVIEW
There were two randomised trials included (Atkinson 2004; Symons 1992). There were three reviews: one of which examined the evidence for the role of food hypersensitivity in IBS (Zar 2001) and the others were systematic reviews of non-randomised evidence for the dietary treatment of IBS (Niec 1998; Burden 2001). The remaining fourteen included studies were non-randomised studies (Bentley 1983; Böhmer and Tuynman 1996; Drisco 2006; Hawthorn 1991; Hunter 1985; Jones 1982; Kanazawa and Fukudo 2006; McKee 1987; Nanda 1989; Parker 1995; Petitpierre 1985; Smith 1985; Zar 2005; Zwetchkenbaum and Burakoff 1988).
Study Design
One study had a crossover design: Symons (1992) stated that the patients were randomised to interventions on two days, following a 12 hour fast, but the washout period was not clear.
Setting: all the studies included patients in secondary care, many of whom had not responded to previous treatment for IBS symptom management.
The majority of studies took place in the UK (Atkinson 2004; Bentley 1983; Hawthorn 1991; Hunter 1985; Jones 1982; McKee 1987; Nanda 1989; Parker 1995; Smith 1985; Zar 2005; Zwetchkenbaum and Burakoff 1988). One took place in Japan (Kanazawa and Fukudo 2006), two in Europe (Böhmer and Tuynman 1996; Petitpierre 1985), two in the US (Drisko 2006; Zwetchkenbaum and Burakoff 1988) and one in Australia (Symons 1992).
Population
All studies included patients with a diagnosis of IBS, although the definition varied between studies. Four studies used Rome criteria: Rome I (Hawthorne 1991); Rome II (Atkinson 2004, mean duration of IBS over 10 years; Drisko 2006; Zar 2006); Zar (2006) also predefined the IBS type. Two studies used the Manning Criteria (Kanazawa and Fukudo 2006; Symons 1992). Two studies used a definition described by the author (Böhmer and Tuynman 1996; Parker 1995). Seven studies simply said the patients ‘had IBS’ (Jones 1982; Bentley 1983; McKee 1987; Petitpierre 1985; Smith 1985; Nanda 1989; Zwetchkenbaum and Burakoff 1988). None of the studies stated that any patients had IBS as result of gastrointestinal infection.
Atkinson (2004) did not state whether patients had bloating and/or pain but described the symptom severity to be severe. Another study described the duration and frequency of symptom episodes (Nanda 1989), and one study reported duration of symptoms and the percentage of patients with pain, bloating and urgency (Hawthorne 1991). The remaining studies did not state the severity of symptoms. The age range of patients was 18 to 80 years with the average mean age being approximately 28 to 44 years. None of the studies particularly identified elderly patients. All studies had more women than men.
Interventions
Fructose-Sorbitol Dose 1 versus Dose 2
The RCT, Symons (1992), compared the difference in symptom provocation in IBS patients using two different doses of fructose–sorbitol solution. The lower dose solution was made up of 20g fructose and 3.5g sorbitol in 200 ml water; the higher dose contained 25g fructose and 5g sorbitol in 250ml water, i.e. a comparison of 17.5 and 20g/litre of sorbitol, for a constant concentration of fructose 100g/litre. Thirty-nine patients (15 IBS patients and 24 healthy controls) were randomised to receive the higher or lower dose on different days, and results were reported separately for the two population groups.
Exclusion diets
The other RCT, Atkinson (2004), tested patients’ blood for IgG antibodies against 29 foods. A true and a sham diet sheet were then prepared for each patient. The true intervention diet excluded those foods to which the patient had antibodies; the sham diet excluded an equal number of foods but not those to which the patients had antibodies. The sham diet also included an equally difficult-to-exclude staple food as the true diet (for example, cow’ milk was replaced by potato, wheat with rice, yeast with whole egg, etc.).
Exclusion diets (non-randomised studies)
The majority of studies used a low allergenic diet, and initially excluded a range of foods, including dairy products, wheat, corn, yeast, eggs, rye, potatoes, onions, cocoa, citrus, coffee, tea spices, alcohol, peas, banana, additives, preservatives and tomatoes. Then an open or single-blinded food challenge re-introduced foods 2 to 7 days apart.
- One study used a diet of one meat, one fruit and distilled water (Jones 1982)
- One study used only lamb, rice and pears (Bentley 1983)
- One study used lamb, white fish, cabbage, carrots, peas, ‘Ryvita’, weak black tea and dairy free margarine (Smith 1985)
- Two studies used IgG4 antibody and mould guided exclusion diets (Drisko 2006; Zar 2006)
- One study used a lactose restricted diet, but gave no further details. Low lactose consumption was defined as less than 9 g per day (Böhmer and Tuynman 1996)
- One study used starvation followed by 5 days of re-feeding in hospitalised IBS patients (Kanazawa and Fukudo 2006).
Comparisons
One RCT compared true diet with sham diet (Atkinson 2004).
One RCT compared two different doses of fructose-sorbitol solution (Symons 1992).
The remaining studies used diet and food challenge in all patients. The duration of the exclusion diet ranged from seven days (Jones 1982) to six months (Zar 2006). The challenge tests used in the studies involved patients being placed on a diet excluding foods believed to provoke symptoms and then re-introducing the foods in a double-blind or controlled way.
OUTCOMES
I. RANDOMISED TRIALS
1. Global symptoms score
a. Global improvement of IBS score
Atkinson (2004) used a validated IBS symptom severity score with a range from 0 to 500. The scale took into consideration scores for pain, distension, bowel function and general well-being, with mild, moderate and severe cases indicated by scores of 75–175, 175–300 and >300 respectively. A reduction in score of 50 or more was regarded as a clinically significant improvement.
Atkinson (2004) also reported a global rating of IBS using the question: ‘Compared with your IBS before you started the food elimination diet, are you now: terrible, worse, slightly worse, no change, slightly better, better or excellent?’ Significant improvement was defined as ‘better’ or ‘excellent’.
Symons (1992) used a symptom score composite: abdominal pain/discomfort, bloating, distension, belching, nausea, bowel frequency, flatulence and borborygmi were each scored on a scale of 0 to 3 with 0 = absent and 3 = severe.
2. Quality of Life
Atkinson (2004) assessed the patients using a validated quality of life scale that is sensitive to change in IBS (range 0 to 500).
3. Mental health
Atkinson (2004) assessed the patients using the Hospital Anxiety and Depression (HAD). This instrument scores anxiety and depression up to a maximum score of 21 for each parameter, and a score above 9 indicates significant psychopathology.
Symons (1992) did not record other outcomes.
METHODOLOGICAL QUALITY OF RANDOMISED TRIALS
The quality assessment for included studies is shown in Appendix D.
The methods of randomisation and allocation concealment were reported in one randomised study, both of which were classified as adequate (computer generated and the sequence was retained by a central telephone centre: Atkinson 2004). Symons (1992) gave no details of the methods of randomisation or allocation concealment.
Atkinson (2004) described an a-priori power calculation and used an intention to treat analysis. The groups were mainly comparable, except that the baseline IBS symptom score was higher in the intervention group (331.9 (SD 70.8) versus 309.0 (SD 78.5), which is not a significant difference (p=0.06)). The number of patients who withdrew from the studies or were lost to follow-up was minimal. Atkinson (2004) reported data from 131 of the original 150 patients (65/75 true and 66/75 sham diet groups) at 12 weeks (87%).
Symons (1992) did not describe an a-priori power calculation. All patients completed the study. The study reported no baseline data so it was not possible to judge whether the groups were comparable.
RESULTS
A. True diet versus sham diet
1. Global symptoms
a. Number of patients with improvement in global symptoms
Atkinson (2004) randomised 150 patients to true and sham exclusion diets. They reported the number of patients with improvement in global symptoms. There was no significant difference between true and sham diets; however, the confidence interval was fairly wide.
b. Global improvement of symptoms score
Atkinson (2004) reported the final global symptom score on a scale from 0 to 500 (where lower scores are better). There was no significant difference between true and sham diets, although the true diet was favoured.
2. Quality of life
Atkinson (2004) reported a significant improvement in quality of life change-from-baseline scores for the true diet compared with sham diet, but the confidence interval was fairly wide. The mean difference was 38.0 (95%CI 2.36, 73.64), for a sham diet change from baseline of 50 points. The scale was 0 to 500.
3. Mental health
There was a small significant difference between the sham and true diet groups in the HAD anxiety scores (scale 0 to 21), but no significant difference in the depression scores.
Symptoms on reintroduction of excluded foods at end of trial period
Of the 131 patients who gave data at the end of the trial period in Atkinson (2004), 93 (41 in the true diet group and 52 in the sham diet group) agreed to attempt reintroduction of eliminated foods. The mean IBS symptom score significantly increased (i.e. worsened) more in the true diet group (83.3 points) than in the sham diet group (31 points, p=0.003; standard deviations not given).
The change in global symptom score also showed that significantly more patients in the true diet group worsened on reintroduction of foods to which they had IgG antibodies (i.e. those that had been excluded during the diet): 41.5% of these patients worsened on reintroduction of these foods, versus 25% worsening in the sham diet group on reintroduction of similar foods (to which they had not been shown to have antibodies), p=0.047. We noted though that the self-selecting group taking part in this section of the trial may not have been representative of the randomised groups.
B. Fructose-sorbitol solution dose 1 versus fructose-sorbitol dose 2
Fructose and sorbitol, when ingested together, are thought to provoke symptoms of IBS. Sorbitol is found naturally in fruits, particularly peaches, pears, and plums. It is also added to soft drinks and diet products. One study (Symons 1992) compared two concentrations of sorbitol in a mixed solution of fructose and sorbitol. Concentrations compared were 17.5 and 20 g/litre sorbitol (the fructose concentration was kept constant). We noted that the duration of the study was very short – two days in each phase.
1. Global symptoms
Symons (1992) used a symptom score composite: abdominal pain/discomfort, bloating, distension, belching, nausea, bowel frequency, flatulence and borborygmi were each scored on a scale of 0 to 3 with 0 = absent and 3 = severe. It is unclear what the maximum score is, but it could be 21. The data were expressed as median (interquartile range). For the IBS patients the total symptom score was significantly greater (i.e. more severe) following consumption of the higher concentration solution, compared to the lower concentration solution (p=0.04).
| Intervention | Median symptom score (range) (n=15) |
|---|---|
| Lower F-S dose | 1.5 (0 to 4) |
| Higher F-S dose | 3.5 (1 to 9) * |
- *
p = 0.04
II. NON-RANDOMISED STUDIES
All thirteen studies identified food intolerance with varying degrees of response to exclusion diets (Bentley 1983, Böhmer and Tuynman 1996, Drisco 2006, Hawthorn 1991, Hunter 1985, Jones 1982, Kanazawa and Fukudo 2006, McKee 1987, Nanda 1989, Parker 1995, Petitpierre 1985, Zar 2005, Zwetchkenbaum and Burakoff 1988), illustrated in Table 1.
Table 1
Non-randomised studies; exclusion diets and results.
The non-randomised studies have been grouped according to the type of diet, and the following general conclusions can be drawn:
- There is some evidence to suggest that a simple diet of lamb, pears and rice or one meat and one fruit may improve symptoms
- A low allergenic diet does not appear to give remission in IBS symptoms
- Food exclusion diets based on IgG antibody testing appear to be effective in improving symptoms
- A starvation diet significantly decreased symptoms, but this was in a group of hospitalised patients, and is not applicable to primary care
- A lactose restricted diet gave a significant decrease in symptom score for lactose intolerant patients, but not for the lactose tolerant group.
It should be remembered that this evidence is from non-randomised studies, so its overall quality is reduced.
GDG DISCUSSION
The GDG discussed this review at length. They noted that lifestyle change and adjustment of diet according to symptoms can offer relief to people with IBS. Further dietary manipulation in the form of avoidance of specific foods offers improvement for up to two-thirds of people suffering from IBS. However, the GDG was concerned that exclusion diets undertaken without the advice of a dietician could lead to malnourishment and deficiencies.
Diet and nutrition are fundamental in the management of IBS to avoid malnutrition and to achieve optimal symptom control. The gold standard diagnosis for intolerance to a food is by elimination and reintroduction. Intolerance is demonstrated if symptoms resolve on elimination and reappear on reintroduction. Importantly, dietary advice will vary depending on symptoms, e.g. diarrhoea and/or constipation, abdominal bloating and therefore needs to be tailored to the individual to manage symptoms. Consequently, the GDG did not wish to produce a list of possible suspect foods, or to encourage patients to adopt a trial-and-error approach.
The GDG also emphasised that the dietitian should be registered and therefore trained to work in clinical settings and able to advise on all aspects of diet. The GDG noted that, currently, anyone can call themselves a nutritionist, regardless of qualifications. The Nutrition Society is the professional organisation for nutritionists; registration can be checked at www.hpcheck.org.
The GDG commented that an implementable dietary assessment tool would be useful, but accepted that such a tool had to be validated before it could be recommended.
Finally, the GDG recommended that the term, ‘balanced diet’ should be avoided because it was not specific. They commented that the 5-a-day public health recommendation could be problematic, especially for IBS-D patients.
The consensus was as follows:
- Patients should have a dietary assessment at initial consultation, and this should include examining eating patterns and when patients are eating
- Regular eating patterns should be encouraged
- Exclusion diets should be reserved for severe cases of IBS and should be carried out only under the advice of a dietician
- Dietary referral would be a useful option for mild IBS.
Several of these consensus points have been included in recommendations in the general dietary lifestyle and advice section.
EVIDENCE STATEMENTS
- There is fair evidence to show no significant difference in global symptoms, between true and sham exclusion diets (i.e. foods excluded for which the patient had or had not IgG antibodies).
- There is fair evidence to show a significant difference in quality of life, favouring a true exclusion diet, in comparison with a sham diet.
- There is weak evidence to show that reintroduction of excluded foods to patients previously given a true exclusion diet resulted in significant worsening of global symptoms in comparison with those given a sham diet.
- There is weak evidence to suggest that food exclusion diets based on IgG antibody testing are effective in improving symptoms, but a low allergenic diet does not appear to be effective.
- There is weak evidence to suggest that a simple diet of lamb, pears and rice or 1 meat and 1 fruit may improve symptoms.
- There is weak evidence that a lactose restricted diet gave a significant decrease in symptom score for lactose intolerant patients, but not for lactose tolerant patients.
- There is limited evidence to show significantly more severe symptoms following consumption of a solution containing 20 g/litre sorbitol, compared to one with 17.5 g/litre, in the presence of fructose.
EVIDENCE TO RECOMMENDATIONS
The GDG took into consideration the clinical effectiveness evidence. Although there was some evidence to support the use of exclusion diets, the GDG believed that such diets should only be undertaken with the specialist help of a dietitian to ensure the diet remains well balanced.
RECOMMENDATION
If diet continues to be considered a major factor in a person’ symptoms and they are following general lifestyle/dietary advice, they should be referred to a dietitian for advice and treatment, including single food avoidance and exclusion diets. Such advice should only be given by a dietitian.
Footnotes
- *
Lifestyle activity: activities that are performed as part of everyday life, such as climbing stairs, walking (for example to work, school or shops) and cycling. They are normally contrasted with ‘programmed’ activities such as attending a dance class or fitness training session.
- Diet and lifestyle - Irritable Bowel Syndrome in AdultsDiet and lifestyle - Irritable Bowel Syndrome in Adults
- glial fibrillary acidic protein [Danio rerio]glial fibrillary acidic protein [Danio rerio]gi|66393075|ref|NP_571448.2|Protein
- peripheral myelin protein 22 isoform 2 [Mus musculus]peripheral myelin protein 22 isoform 2 [Mus musculus]gi|2220175420|ref|NP_001391209.1|Protein
- Homo sapiens proenkephalin (PENK), mRNAHomo sapiens proenkephalin (PENK), mRNAgi|1519473622|ref|NM_001135690.3|Nucleotide
- UNVERIFIED: Parapetalocephala sp. 2 XYW-2023a mitochondrion sequenceUNVERIFIED: Parapetalocephala sp. 2 XYW-2023a mitochondrion sequencegi|2701068775|gb|OK075163.1|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...