NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Treatment of a new episode of depression
This evidence review contains 2 reviews relating to treatment of a new episode of depression.
- Review question 2.1 For adults with a new episode of less severe depression, what are the relative benefits and harms of psychological, psychosocial, pharmacological and physical interventions alone or in combination?
- Review question 2.2 For adults with a new episode of more severe depression, what are the relative benefits and harms of psychological, psychosocial, pharmacological and physical interventions alone or in combination?
Introduction
There is a wide range of interventions available to treat depression, including pharmacological, psychological, psychosocial and physical interventions. The range of options is further extended as different treatment modalities may be used in combination with each other, leading to a large number of possible permutations.
To inform the choice of intervention, or combination of interventions, knowledge of the relative benefits, harms and costs is essential. It is particularly important to know if combinations of treatments offer any advantages as they are likely to be more resource-intensive and more onerous to patients.
In addition to the complexity introduced by the number of available interventions, the choice of treatment for a new episode of depression may also depend on its severity. In order to address this, the analysis has been sub-divided to identify interventions that are most effective for less severe depression (mild and subthreshold depression), and those that are most effective for more severe depression (moderate and severe depression). The criteria used to define ‘less severe’ and ‘more severe’ depression are described below and in the review protocol (appendix A).
The aim of this review is to compare the effectiveness, acceptability and tolerability of treatments for a new episode of less severe or more severe depression, including a range of pharmacological, psychological, psychosocial and physical interventions.
Summary of the interventions included in this evidence review
Due to the large number of different treatment options considered in this review, they have been grouped into classes to allow comparison between classes of treatment. For example, psychological therapies are grouped according to common theoretical structure and methodological approach, and pharmacological treatments are grouped according to mechanism of action or chemical structure. Further details about the classes and interventions included in each class are provided in Supplement B1 (Interventions and classes).
For inclusion in this review, the committee agreed that pharmacological interventions needed to be licensed in the UK and in routine clinical use for the first-line treatment of depression. The national prescription data for England in 2017 (Prescribing & Medicines Team, Health and Social Care Information Centre, 2017) was used to define routine usage of drugs: if a drug appeared in the top 15 antidepressants prescribed by volume it was included, with the exception of dosulepin which the BNF indicates should be initiated by a specialist.
Some interventions were included in the evidence review to improve connectivity within the network meta-analysis but were not considered as part of the decision problem, so were not considered as candidates for recommendations. If necessary for connectivity in the network, excluded pharmacological interventions were added as ‘any antidepressant’ or ‘any SSRI’ or ‘any TCA’ nodes but only where the pharmacological interventions had been compared against an included psychological or physical intervention and/or combined with an included psychological or physical intervention. This approach is outlined in the review protocol (appendix A).
For psychological interventions, the committee were interested in exploring whether there was a difference in the effects of briefer relative to longer interventions. This differentiation by intensity (number of sessions) was possible for CBT because there was large variation in the number of sessions reported across RCTs, and there was also a large evidence base that allowed formation of 2 separate groups of interventions according to the number of sessions offered. It was not possible to create distinct intervention categories based on intensity for other interventions because there was either no great variation in the number of sessions reported for an intervention in the RCTs included, or the evidence base was too narrow. For each level of severity, for the class of Cognitive and cognitive behavioural therapies, both individual and group, the NMA classification system made a distinction between CBT ≥15 sessions and CBT<15 sessions, which were considered as separate interventions within the class.
Couple interventions, including behavioural couple’s therapy, were considered more appropriate for subgroups of adults with depression, namely for people with problems in the relationship with their partner, and as such these interventions were considered only in pairwise comparisons (and not included in the network meta-analysis).
Summary of the protocol
See Table 1 for a summary of the Population, Intervention, Comparison and Outcome (PICO) characteristics of this review.
Table 1
Summary of the protocol (PICO table).
For further details see the review protocol in appendix A.
Methods and process
This evidence review was developed using the methods and process described in Developing NICE guidelines: the manual. Methods specific to this review question are described in the review protocol in appendix A, and methods specific to the NMA are summarised below, and described in appendix M and in supplement 1 - Methods.
Declarations of interest were recorded according to NICE’s 2014 conflicts of interest policy until 31 March 2018. From 1 April 2018, declarations of interest were recorded according to NICE’s 2018 conflicts of interest policy. Those interests declared until April 2018 were reclassified according to NICE’s 2018 conflicts of interest policy (see Register of Interests).
Summary of methods
Defining less and more severe depression
Baseline mean scores on validated depression scales were used to classify study population severity according to less severe (review question 2.1) or more severe (review question 2.2) using the thresholds outlined in the review protocol (appendix A). These thresholds were derived using standardization of depression measurement crosswalk tables (Carmody 2006; Rush 2003; Uher 2008; Wahl 2014). An anchor point of 16 on the PHQ-9 was selected as the cut-off between less severe and more severe depression, on the basis of alignment with the clinical judgement of the committee and eligibility criteria in published studies. If baseline mean scores were not available, severity was classified according to the inclusion criteria of the study or the description given by the study authors (but only in cases where this is unambiguous, for example ‘severe’ or ‘subthreshold’ or ‘mild’). The category of less severe depression used in this guideline includes the traditional categories of subthreshold symptoms and mild depression, and the category of more severe depression used in this guideline includes the traditional categories of moderate and severe depression.
Evidence synthesis
The main method used to synthesise evidence on pharmacological, psychological, psychosocial, physical and combined interventions included in this review was network meta-analysis (NMA). NMA is a generalisation of standard pairwise meta-analysis for A versus B trials, to data structures that include, for example, A versus B, B versus C, and A versus C trials (Dias 2011a; Lu 2004).
NMA was employed to assess the following outcomes:
- Clinical analysis - critical outcomes:
- Standardised mean difference (SMD) of depression symptom change scores at treatment endpoint; this was selected as the primary critical outcome
- Response in those randomised at treatment endpoint (also known as ‘intention to treat’ or ‘ITT’)
- Remission in those randomised at treatment endpoint (also known as ‘intention to treat’ or ‘ITT’)
- Economic analysis:
- Acceptability: treatment discontinuation for any reason at treatment endpoint in those randomised
- Tolerability: treatment discontinuation due to side effects from medication at treatment endpoint in those who discontinued treatment; this outcome was only relevant to interventions with a pharmacological element.
- Response at treatment endpoint in those who completed treatment (also known as ‘completers’)
- Remission at treatment endpoint in those who completed treatment (also known as ‘completers’)
Pairwise meta-analysis was undertaken to assess the following outcomes, as there was not enough evidence to create a network:
- Quality of life
- Personal, social, and occupational functioning including global functioning, functional impairment, sleeping difficulties, employment, interpersonal problems
- Follow-up data on critical outcomes for the clinical analysis.
In addition, pairwise meta-analysis was employed to synthesise data on all critical outcomes of the clinical analysis (SMD, response in those randomised, remission in those randomised). The aim of this analysis was to compare the results of the NMA with those of pairwise meta-analysis and explore any differences between them and possible reasons for any differences However, results of these pairwise meta-analyses were not considered as a primary source of evidence when formulating recommendations.
SMD was used as a summary statistic as data were synthesised across a number of depression scales. For all scales, the score increased with symptom severity, therefore no transformation was required to correct for differences in the direction of the scales.
Class models
Due to the large number of interventions included in this review, comparing all pairs of interventions individually within the NMA (and also in the pairwise meta-analysis) would not be feasible and would require particularly complex consideration and interpretation of the NMA evidence. Moreover, some interventions included in the systematic review had been tested on small numbers of participants and their effects were characterised by considerable uncertainty. For these reasons, the NMAs utilised class models: each class consisted of interventions with a similar mode of action or similar treatment components or approaches, so that interventions within a class were expected to have similar (but not necessarily identical) effects. Use of class models in the NMA had three benefits:
- strength could be borrowed across interventions in the same class, therefore improving precision of effects
- networks that were otherwise disconnected were possible to connect via interventions belonging to the same class, resulting in a connected network that included all classes and interventions of interest
- relative effects between a more limited number of classes were easier to interpret and thus more helpful for the committee when making recommendations.
Following appropriate tests of fit, random class effect models were used for all outcomes examined in the NMAs, which assume that the effects of interventions in a class are distributed around a common class mean with a within-class variance. Under this approach individual treatment effects are drawn towards a class mean but individual intervention estimates that are more precise can still be estimated.
Bias adjustment NMA models and other sensitivity analyses
A key assumption in NMA is that of transitivity – that is, that the balance of effect modifiers (factors that influence the treatment effect) is similar across all trials in the network. In order to explore the validity of this assumption, several pre-specified sensitivity analyses were conducted.
Publication bias is known to affect results of meta-analyses in several clinical areas, including depression (Driessen 2015; Moreno 2009 & 2011; Trinquart 2012; Turner 2008). Small sample size studies are associated with publication bias as small studies with positive results are more likely to be published compared with small studies with negative results, and may also be associated with lower study quality. Published smaller studies tend to overestimate the relative treatment effect of interventions versus control, compared to larger studies (Chaimani 2013; Moreno 2011). Furthermore, small studies are often of poorer quality, and may be at higher risk of bias, which can lead to inflated estimates of efficacy and violate the transitivity assumption.
As the NMAs included a significant number of small studies, sensitivity analyses were carried out on selected outcomes, which adjusted for bias associated with small study size effects. The analyses, which were based on the assumption that the smaller the study the greater the bias, attempted to estimate the “true” treatment effect that would be obtained in a study of infinite size. The analyses assumed possible bias in comparisons of active interventions versus inactive control and no bias between inactive control comparisons, as well as between active intervention comparisons. The exception to this was in comparisons where non-directive counselling was the control intervention (in which case bias against non-directive counselling was assumed). This exception was based on committee and stakeholder concerns that non-directive counselling when used as a control intervention may be less likely to be manual-based, and to be delivered in a comparable number of sessions by an equivalent healthcare professional as when non-directive counselling is included as an active intervention in trials. Bias adjustment assumptions were supported by empirical evidence of the direction and magnitude of small study bias in meta-analyses of psychological interventions versus control (Driessen 2015) and of antidepressants versus pill placebo (Turner 2008).
Bias adjustment models were developed for the following outcomes synthesised in NMAs:
- SMD of depression symptom change scores (primary critical outcome for clinical analysis)
- Treatment discontinuation for any reason in those randomised
- Response in completers
The latter two outcomes were selected for bias adjustment because they were the main NMA outcomes that informed the economic analysis, with the highest anticipated impact on the results. Subsequently, where bias was identified, an economic probabilistic sensitivity analysis was conducted using the outputs of the bias-adjusted NMAs on these two outcomes, as relevant (see appendix J).
In addition, the validity of the transitivity assumption between participants in pharmacological trials and participants in non-pharmacological trials was explored by a sensitivity analysis on the SMD outcome that included non-pharmacological trials only and examined any differences in magnitude of effects and ranking of non-pharmacological interventions compared to results from the mixed psychological, psychosocial, pharmacological and physical model that utilised the full study dataset.
Moreover, a post-hoc sensitivity analysis that included only RCTs rated as being at low risk of bias according to the Cochrane risk of bias tool version 1.0 for RCTs (see appendix H in Developing NICE guidelines: the manual) was conducted on the SMD outcome, which was the primary critical outcome of the clinical analysis. Such analysis was only possible to conduct for the domain of ‘attrition’ in the risk of bias tool, as this was the only domain that included a sufficient number of RCTs at low risk of bias, and a relatively wide range of treatment classes.
Several other post-hoc sensitivity analyses were also conducted to explore the validity of the transitvity assumption in more detail (see appendix M). These investigated the impact of removing small studies or studies with >5 points contribution to the residual deviance from the analysis, and assuming additivity of treatments combined with TAU.
Presentation of the NMA results
The NMAs undertaken to address the 2 review questions covered in this report (treatments for a new episode of less severe depression and treatments for a new episode of more severe depression) included 676 studies comparing 63 classes of 152 pharmacological, psychological, psychosocial and physical interventions alone or in combination as well as controls; 51 of these classes represented active treatment options that were part of the decision problem, meaning they were candidates for recommendation.
Results of the NMAs are presented in the main report as the posterior mean SMD of depression symptom change scores (continuous data) or log-odds ratios (LORs) (for dichotomous data), as appropriate, with 95% Credible Intervals (CrI) compared with the reference treatment. For the analysis of treatments for less severe depression the selected reference treatment was treatment as usual (TAU), whereas for the analysis of treatments for more severe depression the selected reference treatment was pill placebo. Selection of reference treatments was made following inspection of the size of the evidence and the connectivity of control treatments in each population, and considering control treatments with their own established effects. The committee expressed a preference for pill placebo as it is well-defined across trials. On the other hand, the definition of TAU may vary across trials, although it has been widely used as the control treatment in meta-analyses of psychological trials. The committee considered the comparisons of psychological treatment classes and interventions with pill placebo as an advantage of conducting the NMAs, because psychological therapies are not routinely compared with pill placebo, unless active drug arms are included in the trial. A further advantage of selecting pill placebo is that it provides a more conservative estimate and convincing comparison for clinical effect and addresses treatment expectancy effects for interventions. Nevertheless, pill placebo was tested on a very small number of people in less severe depression and it had limited connectivity (or was completely absent) in most network plots in this population. Therefore, its use as a reference was considered inappropriate and TAU was selected instead as the next best option to serve as reference in NMAs of treatments for less severe depression. No treatment and waitlist were considered to have a minimal effect and to potentially hinder other underlying interventions and therefore were deemed inappropriate baseline comparators.
The main body of the report provides NMA results at the treatment class level for all critical outcomes included in the clinical analysis. Rankings have been calculated only for treatment classes of interest (classes that were part of the decision problem). For the SMD of depression symptom change scores, which was the primary critical efficacy outcome, results of individual interventions are also provided for information.
An overview of the results on outcomes used in the economic analysis are reported in appendix J.
Results of the NMAs on all outcomes that informed the clinical and the economic analysis, including relative effects for all pairs of treatment classes and interventions included in the NMA, are reported in appendix M and supplements B5* and B6**.
Footnotes
- *
Supplement B5 is the Technical Support Unit Report appendices for review questions 2.1-2.2. These are appendices to the economic model. The supplement was made available during consultation but is no longer a publically available document following publication of the guideline.
- **
Supplement B6 is the Technical Support Unit excel files for the network meta-analysis for review questions 2.1-2.2 (the economic model). The supplement was made available during consultation but is no longer a publically available document following publication of the guideline.
Presentation of the pairwise meta-analysis results
In accordance with the data analysis strategy outlined in the review protocol (see appendix A), the NMA results were the primary input for clinical decision-making (and were considered alongside the results from the economic models when developing recommendations). Pairwise meta-analyses were used as follows:
- to analyse important (but not critical) outcomes, and follow-up of critical outcomes, which could not be included in NMA due to a lack of connectivity in the networks for these outcomes and time points
- to compare the results of pairwise meta-analysis with the NMA for critical outcomes
- to analyse interventions that are only appropriate for sub-groups of people with depression (and not included in the NMA), specifically couple interventions for those with problems in the relationship with their partner
- to undertake subgroup analysis of studies included in the NMA. Planned subgroup analyses (provided sufficient data were available) included: older adults (60 years and older) compared to younger adults (younger than 60 years); BME populations; men. Additional subgroup analyses (primary care compared to secondary care; inpatient compared to outpatient settings) were planned to inform the evidence review on settings for care but were not considered for recommendations for first-line treatment of less severe and more severe depression.
For pairwise comparisons, meta-analyses using random-effects models were conducted to combine results from similar studies. An intention to treat (ITT) approach was taken where possible. Continuous outcomes were assessed using standardized mean difference (SMD) and dichotomous outcomes using relative risk (RR) (see supplement 1 - Methods).
The main body of the report presents only statistically significant and clinically important effects for the important (but not critical) outcomes (quality of life and functioning) and follow-up (of at least 6 months post-endpoint) of critical outcomes. Clinically important effects were defined using the default minimally important differences of a RR less than 0.8 or greater than 1.25 or a SMD less than −0.5 or greater than 0.5 or a logOR less than −0.25 or greater than 0.25 [MID for OR calculated as exp[0.52]=1.28]). However, forest plots for all outcomes and all time points are provided in supplements B2 and B3.
Similarly, in the main body of the report, comparisons between pairwise and NMA results for critical outcomes (base-case analysis) are restricted to highlighting comparisons where the difference between the pairwise meta-analysis and NMA results is equal to, or larger than, the minimally important difference (MID, as defined using the values given above). A distinction is also be made between differences where the effect estimate from the NMA is within the 95% confidence interval of the pairwise meta-analysis effect estimate, and differences where the effect estimate from the NMA is not within the 95% confidence interval of the pairwise meta-analysis as the latter (and not the former) may be considered a truly significant difference. The full table of pairwise meta-analysis and NMA comparisons is available in supplement B4. It is important to note that these comparisons have been performed in addition to the NMA inconsistency checks (where direct and indirect evidence is compared) as outlined above.
Evidence from pairwise meta-analyses for interventions that are only appropriate for subgroups of people with depression, specifically, couple interventions are presented in the relevant evidence sections below.
Subgroup analyses were only performed where the comparison and outcome had at least 2 studies in each subgroup. In the main body of the report, only subgroup analyses with statistically significant subgroup differences are presented (see appendix E for forest plots for all subgroup analyses).
References to introductory section
- Carmody TJ, Rush AJ, Bernstein I, Warden D, Brannan S, et al. (2006) The Montgomery Äsberg and the Hamilton ratings of depression: a comparison of measures. European Neuropsychopharmacology, 16(8), 601–611. [PMC free article: PMC2151980] [PubMed: 16769204]
- Chaimani A, Vasiliadis HS, Pandis N, Schmid CH, Welton NJ, Salanti G (2013). Effects of study precision and risk of bias in networks of interventions: a network meta-epidemiological study. International journal of epidemiology, 42(4), 1120–1131. [PubMed: 23811232]
- Dias S, Welton NJ, Sutton AJ, Ades AE (2011a, last updated 2016). NICE DSU Technical Support Document 2: A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. Available from http://www
.nicedsu.org.uk [PubMed: 27466657] - Driessen E, Hollon SD, Bockting CL, Cuijpers P, Turner EH (2015). Does publication bias inflate the apparent efficacy of psychological treatment for major depressive disorder? A systematic review and meta-analysis of US National Institutes of Health-funded trials. PLoS ONE, 10(9), e0137864. [PMC free article: PMC4589340] [PubMed: 26422604]
- Lu G, Ades AE (2004). Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med, 23(20), 3105–3124. [PubMed: 15449338]
- Moreno SG, Sutton AJ, AdesA, Stanley TD, Abrams KR, Peters JL, Cooper NJ (2009). Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC medical research methodology, 9(1), 2. [PMC free article: PMC2649158] [PubMed: 19138428]
- Moreno SG, Sutton AJ, Ades A, Cooper NJ, Abrams KR (2011). Adjusting for publication biases across similar interventions performed well when compared with gold standard data. Journal of clinical epidemiology, 64(11), 1230–1241. [PubMed: 21530169]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, et al. (2003). The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry, 54(5), 573–583. [PubMed: 12946886]
- Trinquart L, Abbé A, Ravaud P (2012). Impact of reporting bias in network meta-analysis of antidepressant placebo-controlled trials. PLoS ONE, 7(4), e35219. [PMC free article: PMC3335054] [PubMed: 22536359]
- Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. (2008). Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal of Medicine, 358(3), 252–260. [PubMed: 18199864]
- Uher R, Farmer A, Maier W, Rietschel M, Hauser J, et al. (2008). Measuring depression: comparison and integration of three scales in the GENDEP study. Psychological Medicine, 38(2), 289–300. [PubMed: 17922940]
- Wahl I, Löwe B, Bjorner JB, Fischer F, Langs G, et al. (2014). Standardization of depression measurement: a common metric was developed for 11 self-report depression measures. Journal of Clinical Epidemiology, 67(1), 73–86. [PubMed: 24262771]
Less severe depression
Review question
For adults with a new episode of less severe depression, what are the relative benefits and harms of psychological, psychosocial, pharmacological and physical interventions alone or in combination?
Clinical evidence
Included studies
A total of 142 randomised controlled trials (RCTs) were included in this evidence review.
See the literature search strategy in appendix B and study selection flow chart in appendix C.
Excluded studies
Studies not included in this review are listed, and reasons for their exclusion are provided in appendix K.
Summary of studies included in the evidence review
The NMAs included 142 RCTs (k=142) representing 20,663 participants (n=20,663).
Of the 142 RCTs included in the NMAs for less severe depression, only 26 studies reported either a HAM-D or MADRS score at baseline, and for these studies the mean depression severity scores were HAM-D=12.99 (SD=7.66; k=23) and MADRS=17.74 (SD=6.87; k=3) respectively. Other commonly reported depression scales at baseline for RCTs within this network included the PHQ-9 (mean severity at baseline=12.78, SD=4.84, k=15), CES-D (mean severity at baseline=23.21, SD=9.30, k=35), BDI (mean severity at baseline=16.73, SD=6.89, k=16), and BDI-II (mean severity at baseline=22.38, SD=7.91, k=45). 10 studies were UK-based RCTs.
According to the interventions assessed and the types of outcomes reported in each RCT, the included RCTs have contributed data to one or more networks of evidence and respective NMAs.
For the SMD of depression symptom change scores outcome, the network of evidence (and the respective NMA) included 127 RCTs, 76 interventions grouped in 34 treatment classes, and 16,829 participants. Of the 127 RCTs, 10 reported change from baseline (CFB) depression symptom score data; 115 reported baseline and endpoint depression symptom score data; and 2 reported dichotomous response data and baseline symptom scores. These data were transformed and synthesised accordingly, allowing estimation of the SMD of depression symptom change scores (see appendix M for details).
For the outcome of response in those randomised, the network of evidence (and the respective NMA) included 75 RCTs, 53 interventions grouped in 26 treatment classes and 12,549 participants. Of the 75 RCTs, 11 reported dichotomous response data, 6 reported CFB depression symptom score data; and 58 reported baseline and endpoint depression symptom score data. These data were transformed and synthesised accordingly, allowing estimation of log-odds ratios of response (see appendix M for details).
For the outcome of remission in those randomised, the network of evidence (and the respective NMA) included 26 RCTs reporting dichotomous remission data, 25 interventions grouped in 16 treatment classes and 3,810 participants.
See the full evidence tables in appendix D.
Relevant information on the networks of evidence and the NMAs that informed the economic analysis are reported in appendix M.
Evidence from the network meta-analysis
Base-case analysis
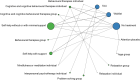
Below is an overview of the treatment class network plots, numbers of people tested on each treatment class and intervention, and NMA findings at the treatment class level (relative effects versus the reference treatment and rankings), for every critical outcome considered in the clinical base-case analysis of treatments for adults with a new episode of less severe depression. For the outcome of the SMD of depressive symptom scores, relative effects of individual interventions versus the reference treatment are also provided in this section.
For each outcome, we present network plots, which depict all treatments considered in each analysis by nodes, and show which treatments have been directly compared in the RCTs included in each NMA, by connecting them with a direct line. In each network plot presented below, the width of lines is proportional to the number of trials that make each direct comparison; the size of each circle (treatment node) is proportional to the number of participants tested on each treatment class.
Full results of the NMA, including network plots and relative effects of individual interventions, as well as relative effects of all pairs of treatment classes and individual interventions, are reported in appendix M and supplements B5 and B6.
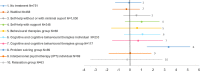
SMD of depression symptom change scores
The network plot at the treatment class level is shown in Figure 1. The numbers of participants tested on each treatment class and each intervention are shown in Table 2. The base-case relative effects (posterior mean SMD with 95% CrI) of all treatment classes versus TAU (reference treatment for less severe depression) are illustrated in Figure 2 (forest plots) and reported in Table 3. The same table also shows the class treatment rankings. Treatment classes in the table have been ordered from lowest to highest ranking (with lower rankings suggesting greater effects).
Table 2
Treatment classes, interventions and numbers of participants tested on each in the NMA of standardised mean difference (SMD) of depression symptom change scores in adults with a new episode of less severe depression.
The base-case relative effects (posterior mean SMD with 95% CrI) of all individual interventions versus TAU (reference treatment for less severe depression) are reported in Table 4. Interventions have been listed by treatment class.
Response in those randomised
The network plot at the treatment class level is shown in Figure 3. The number of participants tested on each treatment class and each intervention are shown in The width of lines is proportional to the number of trials that make each direct comparison; the size of each circle (treatment node) is proportional to the number of participants tested on each treatment class.
SSRIs: selective serotonin uptake inhibitors; TAU: treatment as usual; TCAs: tricyclic antidepressants
Table 5. The base-case relative effects (posterior mean log-odds ratio [LOR] with 95% CrI) of all treatment classes versus TAU (reference treatment for less severe depression) are illustrated in Figure 4 (forest plots) and reported in Table 6. The same table shows also the class treatment rankings. Treatment classes in the table have been ordered from lowest to highest ranking (with lower rankings suggesting greater effects).
Table 5
Treatment classes, interventions and numbers of participants tested on each in the NMA of response in those randomised in adults with a new episode of less severe depression.
Remission in those randomised
The network plot at the treatment class level is shown in Figure 5. The number of participants tested on each treatment class and each intervention are shown in The width of lines is proportional to the number of trials that make each direct comparison; the size of each circle (treatment node) is proportional to the number of participants tested on each treatment class.
TAU: treatment as usual
Table 7. The base-case relative effects (posterior mean log-odds ratio [LOR] with 95% CrI) of all treatment classes versus TAU (reference treatment for less severe depression) are illustrated in Figure 6 (forest plots) and reported in Table 8. The same table shows also the class treatment rankings. Treatment classes in the table have been ordered from lowest to highest ranking (with lower rankings suggesting greater effects).
Table 7
Treatment classes, interventions and numbers of participants tested on each in the NMA of remission in those randomised in adults with a new episode of less severe depression.
Bias-adjusted analysis
Bias models tested on the SMD outcome suggested evidence of bias due to small study size.
Figure 7 shows the bias-adjusted forest plots of relative effects (posterior mean SMD with 95% CrI) of all treatment classes versus TAU (reference treatment for less severe depression). Table 9 shows the relative effects of all treatment classes versus TAU on the SMD and the class treatment rankings. Treatment classes in the table have been ranked from lowest to highest ranking (with lower rankings suggesting greater effects).
Table 10 shows the bias-adjusted relative effects (posterior mean SMD with 95% CrI) of all individual interventions versus TAU (reference treatment for less severe depression). Interventions in this table have been listed by treatment class.
Sensitivity analyses
Effects on the SMD of all treatment classes versus TAU in the post-hoc sensitivity analysis that included only RCTs rated as being at low risk of bias for attrition in the Cochrane Risk of Bias tool are presented in Table 11, alongside the base-case analysis effects, to allow comparison between the two sets of results.
Finally, effects on the SMD of all treatment classes versus TAU in the sensitivity analysis conducted after excluding pharmacological trials are reported in Table 12, presented alongside the base-case analysis effects, to allow comparison between the two sets of results. In each analysis, treatment classes have been ordered from lowest to highest ranking (with lower rankings suggesting higher effects).
Table 12
Comparison of results following exclusion of pharmacological trials from the NMA and results of the NMA base-case analysis: standardised mean difference (SMD) of depression symptom scores in adults with a new episode of less severe depression.
Evidence from the pairwise meta-analyses
Important (but not critical) outcomes
See Table 13 for a summary of the clinically important and statistically significant effects observed for the important (but not critical) outcomes of quality of life and functioning (including personal, social, and occupational functioning and global functioning/functional impairment) at endpoint and longer-term (at least 6 months) follow-up. See supplement B2 for forest plots for all important (but not critical) outcomes.
Table 13
Summary of significant important (but not critical outcomes) at endpoint and longer-term (at least 6 months) follow-up for adults with a new episode of less severe depression.
Follow-up of critical outcomes
See Table 14 for a summary of the clinically important and statistically significant effects observed for critical outcomes at longer-term (at least 6 months) follow-up. See supplement B2 for forest plots for all critical outcomes at all follow-up time points.
Table 14
Summary of significant critical outcomes at longer-term (at least 6 months) follow-up for adults with a new episode of less severe depression.
Comparison of the results of the results of pairwise meta-analysis with the NMA for critical outcomes
See Table 15 for comparisons between pairwise and NMA results (base-case analysis) for critical outcomes where the difference between the pairwise meta-analysis and NMA results is equal to, or larger than, the minimally important difference (MID, defined as SMD −0.5/0.5 or logOR ±0.25 [MID for OR calculated as exp[0.25]=1.28]) and the effect estimate of the NMA is not within the 95% confidence interval of the pairwise effect estimate (considered a significant difference), and see Table 16 for differences between pairwise and NMA results ≥MID but where the NMA effect estimate is within the 95% confidence interval of the pairwise effect estimate (considered a non-significant difference). The full table of pairwise meta-analysis and NMA comparisons is available in supplement B4. Out of a total of 93 comparisons between pairwise and NMA results for less severe depression, 26 differences ≥MID were identified (28% of all comparisons), of these only 11 differences (12% of all comparisons) could be considered significant in that the NMA estimate was not within the 95% confidence interval of the pairwise effect estimate. For most differences identified the difference was in magnitude rather than direction of effect and could probably be accounted for by the smaller evidence base contributing to the pairwise effect estimates. It is important to note that these comparisons have been performed in addition to the NMA inconsistency checks (where direct and indirect evidence is compared). For the NMA inconsistency checks, no evidence of inconsistency was identified in any of the outcomes considered in the clinical analysis.
Table 15
Summary of differences between pairwise and NMA results ≥ MID where NMA effect estimate is not within 95% confidence interval of pairwise effect estimate for adults with a new episode of less severe depression.
Table 16
Summary of differences between pairwise and NMA results ≥ MID where NMA effect estimate is within 95% confidence interval of pairwise effect estimate for adults with a new episode of less severe depression.
Pairwise meta-analysis of couple interventions
No relevant studies were identified for couple interventions for adults with less severe depression and problems in the relationship with their partner.
Subgroup analysis of studies included in the NMA
Subgroup analysis was only possible for older adults (60 years and older) compared to younger adults (younger than 60 years), and not men or BME populations. Subgroup differences were examined for outcomes that had more than 2 studies in each subgroup. Subgroup analysis was only possible for 1 comparison: exercise individual versus waitlist with 2 RCTs included for older adults (Bernard 2014; McNeil 1991) and 3 RCTs included for younger adults (Doyne 1987; Legrand 2014; Nystrom 2017).
There were no significant subgroup differences between older and younger adults for the comparison exercise individual versus waitlist on: depression symptoms endpoint (Test for subgroup differences: Chi2 = 1.40, df = 1, p = 0.24); depression symptoms change score (Test for subgroup differences: Chi2 = 0.14, df = 1, p = 0.71); discontinuation due to any reason (Test for subgroup differences: Chi2 = 0.16, df = 1, p = 0.69).
Quality assessment of studies included in the evidence review and the evidence
A threshold analysis was originally planned to conduct, to test the robustness of treatment recommendations based on the NMA, to potential biases or sampling variation in the included evidence. Threshold analysis has been developed as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analysis (Phillippo 2019). Threshold analysis suggests by how much effects that have been estimated in the NMA need to change before recommendations change, and whether such changes might potentially occur due to bias in the evidence. The NICE Guidelines Technical Support Unit (TSU) attended committee discussions on the rationale for recommendations and noted that, in addition to the results of the NMA, the committee took other pragmatic factors into consideration when making recommendations, including the uncertainty and limitations around the clinical and cost-effectiveness data, and the need to provide a wide range of interventions to take into account individual needs and allow patient choice. The TSU advised that as it was difficult to identify a clear decision rule to link the recommendations directly to the NMA results, it was not feasible or helpful to conduct a threshold analysis. CINeMA was also considered as a method to evaluate the confidence in the results from the NMA (Nikolakopoulou 2020). However, this was not possible to carry out, due to the class models being implemented.
In the absence of undertaking threshold analysis or using CINeMA to evaluate the quality of the evidence and the confidence in the results derived from the NMA that informed this review question, we evaluated and summarise the quality of the evidence narratively, using the domains considered as per a standard GRADE approach (risk of bias, inconsistency, publication bias, indirectness and imprecision).
Risk of bias
The Cochrane risk of bias tool version 1.0 for RCTs (see appendix H in Developing NICE guidelines: the manual) was used to assess potential bias in each study included in the review. Generally the standard of reporting in studies was quite low, as demonstrated by the risk of bias summary diagram (Figure 8). Of the 142 studies included in the NMAs for less severe depression, 56 were at low risk of bias for allocation method and 53 were at low risk of bias for allocation concealment. Trials of psychological therapies were typically considered at high risk of bias for participant and provider blinding, although it is difficult to quantify in risk of bias ratings it is also important to bear in mind that the rate of side effects may also make it difficult to maintain blinding in pharmacological trials. Across interventions, 8 trials were at low risk of bias for blinding participants and providers. Assessor blinding was considered for all trials including those using self-report measures: 14 were at low risk of bias, 127 were unclear, and high risk in 1 trial. For attrition bias, 90 trials were at low risk of bias, unclear risk in 33 trials, and 19 trials were at high risk of bias. Other sources of bias, potential or actual (for instance, potential conflicts of interest associated with funding), were identified in 45 RCTs. See appendix D for full study details, including risk of bias ratings by study.
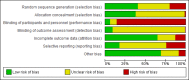
Figure 8
Risk of bias summary for treatments of a new episode in people with less severe depression.
Model goodness of fit and inconsistency
This section reports only findings of goodness of fit and inconsistency checks for the NMAs that informed the clinical evidence. Respective findings for the NMAs that informed the economic analysis are reported in appendix J. Detailed findings of goodness of fit and inconsistency checks for all NMA analyses, including those that informed the guideline economic model, are reported in appendix M and supplements B5 and B6.
For the SMD of depressive symptom scores, relative to the size of the treatment effect estimates, moderate between trial heterogeneity was observed for this outcome, as expressed by the between-studies standard deviation, following bias adjustment, as described below [τ=0.23 (95% CrI 0.10 to 0.47)]. No evidence of inconsistency was identified with the NMA model having a slightly lower DIC, and similar between study heterogeneity. The inconsistency model did not predict the data substantially better for any data points.
For the outcome of response in those randomised, high between trials heterogeneity was found relative to the size of the intervention effect estimates [τ=0.76 (95% CrI 0.55 to 1.01)]. No evidence of inconsistency was identified with the NMA model having a similar posterior mean residual deviance and lower DIC and between study heterogeneity. The inconsistency model did not predict the data substantially better for any data points, although both consistency and inconsistency models provided a poor fit for Zemestani 2016, which compared waitlist, behavioural activation group and third-wave cognitive therapy group.
For the outcome of remission in those randomised, moderate between trials heterogeneity was found relative to the size of the intervention effect estimates, [τ=0.45 (95% CrI 0.05 to 1.03)]. Posterior mean residual deviances and DIC were similar in the NMA random effects consistency model and the inconsistency model, and there was no clear improvement in the prediction of data in individual studies by the inconsistency model. This suggested that there was no evidence of inconsistency. However, both models poorly predicted data from two studies (Yang 2015, Rosso 2017), both of which investigated No treatment compared to an intervention from the Self-help class. The between-study heterogeneity was very similar in consistency and inconsistency models.
Detailed model fit statistics, heterogeneity and results of inconsistency checks for each outcome are provided in supplements B5 and B6. Comparisons between the relative effects of all pairs of treatments obtained from the consistency (NMA) model and those obtained from the inconsistency (pairwise) model are also provided in supplement B6 for all outcomes considered in the NMA.
Selective outcome reporting and publication bias
Bias adjustment models on the SMD of depressive symptom scores were developed to assess potential bias associated with small study size. Between study heterogeneity and posterior mean residual deviance were lower in the bias-adjusted model that accounted for small study effects, suggesting some evidence of small study bias in comparisons between active and inactive interventions in the SMD outcome, in adults with less severe depression.
The bias adjusted model resulted in moderate changes in the relative effects of all treatment classes versus TAU (reference treatment) and had also a moderate impact on some class rankings. Results are presented in the previous section of this evidence review.
Detailed results of all bias models are provided in appendix M and supplements B5 and B6.
Indirectness
In the context of the NMA, indirectness refers to potential differences across the populations, interventions and outcomes of interest, and those included in the relevant studies that informed the NMA.
A key assumption when conducting NMA is that the populations included in all RCTs considered in the NMA are similar. However, participants in pharmacological and non-pharmacological (psychological or physical intervention) trials may differ to the extent that some participants find different interventions more or less acceptable in light of their personal circumstances and preferences (so that they might be willing to participate in a pharmacological trial but not a psychological one and vice versa). Similarly, self-help trials may recruit participants who would not seek or accept face-to-face interventions. However, a number of trials included in the NMA have successfully recruited participants who are willing to be randomised to either pharmacological or psychological intervention and to either self-help or face-to-face treatment. The NMAs have assumed that service users are willing to accept any of the interventions included in the analyses; in practice, treatment decisions may be influenced by individual values and goals, and people’s preferences for different types of interventions. These factors were taken into account when formulating recommendations.
In addition, to explore the transitivity assumption in the context of participants in pharmacological and non-pharmacological trials, a sensitivity analysis on the SMD outcome was conducted after excluding trials with at least one pharmacological or combined intervention arm, where the combined intervention included a pharmacological element. The purpose was to compare the relative effects and rankings of non-psychological treatments between this sensitivity analysis and the base-case analysis. The comparison, which is presented in Table 12, suggested only small changes after exclusion of pharmacological trials, probably because there were not many pharmacological trials included in this dataset (treatments for a new episode of less severe depression).
A post-hoc sensitivity analysis that included only RCTs rated as being at low risk of bias was conducted on the SMD outcome, which was the primary critical outcome of the clinical analysis. Such analysis was only possible to conduct for the domain of ‘attrition’ in the risk of bias tool, as this was the only domain that included a sufficient number of RCTs at low risk of bias, and a relatively wide range of treatment classes.This sub-group analysis showed no substantial difference in treatment effects compared with the base-case analysis, suggesting that bias from attrition was unlikely to be an effect modifier in this population.
Interventions of similar type were grouped in classes following the committee’s advice and considered in class models. These models allowed interventions within each class to have similar, but not identical, effects around a class mean effect. Classes and interventions assessed in the NMAs were directly relevant to the classes and interventions of interest.
Outcomes reported in included studies were also the primary outcomes of interest, as agreed by the committee.
Imprecision
There were wide 95%CrI around mean effects and rankings, for most treatment classes versus the reference treatment (TAU) across all NMA outcomes. For the vast majority of treatment classes, the 95%CrI around relative effects versus TAU crossed the line of no effect.
Overall rating of the quality of the evidence
Based on the narrative assessment of the quality of the evidence using the domains considered as per a standard GRADE approach, the quality of the evidence was considered to be low.
Economic evidence
Included studies
A single economic search was undertaken for all topics included in the scope of this guideline. See the literature search strategy in appendix B and economic study selection flow chart in appendix G. Details on the hierarchy of inclusion criteria for economic studies are provided in supplement 1 - Methods. For this review question, only economic studies conducted in the UK were included.
The systematic search of the economic literature identified 6 studies that assessed the cost effectiveness of interventions for adults with a new episode of less severe depression in the UK (Kendrick 2005/2006a, Kaltenthaler 2006, Peveler 2005/ Kendrick 2006b, Kendrick 2009, Chalder 2012; Hollingworth 2020). Categorisation of the studies according to their population’s severity level of depressive symptoms followed the same criteria used for the categorisation of the clinical studies included in the guideline systematic review. Where study participants’ baseline scores on a depressive symptom scale were not provided, categorisation was based on the description of the participants’ depressive symptom severity in the study.
Economic evidence tables are provided in appendix H. Economic evidence profiles are shown in appendix I.
Excluded studies
A list of excluded economic and utility studies, with reasons for exclusion, is provided in supplement 3 - Economic evidence included & excluded studies.
Summary of studies included in the economic evidence review
All included economic studies were conducted in the UK and adopted a NHS perspective, with some studies including personal social service (PSS) costs as well; in addition, some studies reported separate analyses that adopted a societal perspective. NHS and PSS cost elements included, in the vast majority of studies, intervention, primary and community care, staff time (such as GPs, nurses, psychiatrists, psychologists), medication, inpatient and outpatient care and other hospital care. All studies used national unit costs; in some studies, intervention costs were based on local prices or prices provided by the manufacturers (for example in the case of computerised CBT packages).
Problem solving (individual)
Kendrick 2005/2006a evaluated the cost effectiveness of problem-solving treatment provided by mental health nurses compared with generic community mental health nurse care and usual GP care in adults with a new episode of anxiety, depression or reaction to life difficulties, with duration of symptoms between 4 weeks to 6 months, in the UK. The economic analysis was conducted alongside a RCT (Kendrick 2005/2006a, N=247; analysis based on n=184 with clinical data available; cost data available for n=159). The measure of outcome was the QALY, estimated based on EQ-5D ratings (UK tariff). The time horizon of the analysis was 26 weeks.
Under a NHS perspective, problem solving and generic mental health nurse care were found to be significantly more expensive than GP care. The number of QALYs gained was practically the same across all interventions, meaning that GP care was the dominant option. The study is directly applicable to the NICE decision-making context and is characterised by minor limitations.
Self-help (without or with minimal support): computerised cognitive behavioural therapy
Kaltenthaler 2006 undertook decision-analytic economic modelling to assess the cost-utility of computerised CBT versus treatment as usual in adults with depression attending primary care services in the UK. The study evaluated 3 different computerised CBT packages (Beating the Blues; Cope; Overcoming Depression). Efficacy data were taken from analysis of RCT individualised data, other published RCT data and further assumptions. Resource use data were based on manufacturer submissions, published data and other assumptions. The outcome measure was the QALY, based on EQ-5D ratings (UK tariff). The time horizon of the analysis was 18 months.
Based on a NHS perspective, computerised CBT was more costly and more effective than treatment as usual, with an ICER ranging from £2,678 to £10,614 per QALY (depending on package, uplifted to 2020 prices). The probability of computerised CBT being cost-effective ranged from 0.54 to 0.87 at a cost effectiveness threshold of £44,000 per QALY, suggesting that computerised CBT may overall be a cost-effective intervention. The study is directly applicable to the NICE decision-making context but is characterised by potentially major limitations as a number of input parameters were based on assumptions.
SSRIs
Hollingworth 2020 evaluated the cost effectiveness of sertraline versus placebo in adults presenting to primary care with depression or low mood during the past 2 years. The economic analysis was conducted alongside a RCT (Lewis 2019, N=655; EQ-5D data available for n=505; cost data available for n=381). The measure of outcome was the QALY, estimated based on EQ-5D ratings (UK tariff). The time horizon of the analysis was 12 weeks.
Under a NHS and personal social services perspective, sertraline was found to dominate placebo, as it was both more effective and less costly. Its probability of being cost-effective at the NICE lower cost effectiveness threshold of £20,000/QALY was over 95%. Subgroup analysis showed that sertraline was cost-effective in the treatment of mild, moderate and severe depression. The study is directly applicable to the NICE decision-making context and is characterised by minor limitations.
Kendrick 2009 evaluated the cost effectiveness of provision of SSRIs (fluoxetine, fluvoxamine, sertraline, paroxetine, citalopram or escitalopram) in addition to supportive care provided by GPs compared with GP supportive care alone in adults with mild or moderate depression in the UK. The economic analysis was conducted alongside a RCT (Kendrick 2009, N=220; 12-week completers n=196; 6-month followed-up n=160). The measures of outcome were the change in HAMD17 score and the QALY, estimated based on SF-36/SF-6D ratings (UK tariff). The time horizon of the analysis was 12 and 26 weeks.
Under a NHS and social care perspective, SSRI plus supportive care was dominant over supportive care alone at 12 weeks, as it was more effective and had lower total costs. At 26 weeks, SSRI plus supportive care was still more effective but also more costly than supportive care alone, with an ICER of £115 per unit of improvement on HAMD17 or £18,894 per QALY (2020 prices). SSRI plus supportive care had a probability of being cost-effective of more than 0.50 when the cost effectiveness threshold exceeded £94 per unit reduction on HAMD17. At the NICE cost effectiveness threshold of £20,000-£30,000 /QALY, the probability of SSRI plus supportive care reached 0.65-0.75. The study is directly applicable to the NICE decision-making context and is characterised by minor limitations.
SSRIs versus TCAs
Peveler 2005/Kendrick 2006b evaluated the cost effectiveness of provision of TCAs (amitriptyline, dothiepin or imipramine), SSRIs (fluoxetine, sertraline or paroxetine) and lofepramine (a TCA that was considered in a separate arm) in adults with a new episode of mild-to-moderate depression willing to receive antidepressant treatment in primary care in the UK. The economic analysis was conducted alongside an open-label RCT with a partial preference design: following randomisation, treatment could be prescribed from a different class to the one allocated at random, if participants or their doctor preferred an alternative (N=327; entered preference group n=92; followed-up at 12 months n=171). The measures of outcome were the number of depression-free weeks (DFWs, defined as a HADS-D score <8) and the QALY based on EQ-5D ratings (UK tariff). The time horizon of the analysis was 12 months.
Under a NHS perspective, SSRIs were more costly and more effective than TCAs and lofepramine. Using the number of DFWs as the measure of outcome, TCAs were extendedly dominated (meaning they were less effective and more expensive than a linear combination of the other 2 options). The ICER of SSRI versus lofepramine was £49 per extra DFW. Using the QALY as the measure of outcome, lofepramine was extendedly dominated. The ICER of SSRIs versus TCAs was £4,142/QALY (2020 prices). The probability of SSRIs being cost-effective was approximately 0.6 at the NICE lower cost effectiveness threshold of £20,000/QALY. The study is directly applicable to the NICE decision-making context and is characterised by minor limitations.
Exercise
Chalder 2012 assessed the cost effectiveness of a physical activity intervention delivered by a physical activity facilitator in addition to usual GP care versus usual GP care alone in adults with a recent first or new depressive episode in the UK. The analysis was conducted alongside a RCT, which was excluded from the clinical analysis due to high attrition rates (N=361; at 12 months EQ-5D data n=195; complete resource use data n=156; multiple imputation used in sensitivity analysis). The outcome measure of the analysis was the QALY, estimated based on EQ-5D (UK tariff). The time horizon of the analysis was 12 months.
Under a NHS and PSS perspective and using only completers’ data, the physical activity intervention was found to be more costly and more effective than usual GP care, with an ICER of £24,793/QALY (2020 prices). Its probability of being cost-effective at the NICE lower (£20,000/QALY) and higher (£30,000/QALY) cost effectiveness threshold was 0.49 and 0.57, respectively. Using imputed data, the ICER of the physical activity programme versus usual GP care was £23,079/QALY, while its probability of being cost-effective at the NICE lower and higher cost-effectiveness threshold rose just at 0.50 and 0.60, respectively. The study is directly applicable to the NICE decision-making context but is characterised by potentially serious limitations, mainly its notably high attrition rates.
Economic model
A decision-analytic model was developed to assess the relative cost effectiveness of interventions of adults with a new episode of less severe depression. The objective of economic modelling, the methodology adopted, the results and the conclusions from this economic analysis are described in detail in appendix J. This section provides a summary of the methods employed and the results of the economic analysis.
Overview of economic modelling methods
A hybrid decision-analytic model consisting of a decision-tree followed by a three-state Markov model was constructed to evaluate the relative cost effectiveness of a range of pharmacological, psychological and physical interventions for the treatment of a new episode of less severe depression in adults treated in primary care. The time horizon of the analysis was 12 weeks of acute treatment (decision-tree) plus 2 years of follow-up (Markov model). The interventions assessed were determined by the availability of efficacy and acceptability data obtained from the NMAs that were conducted to inform this guideline. The selection of classes of interventions was made based on the following criteria:
- The economic analysis assessed only classes of interventions that were included in the NMA of standardised mean difference (SMD), which was the main clinical outcome, as the committee wanted to be able to assess their clinical effectiveness prior to assessing cost-effectiveness. Moreover, to be assessed in the economic analysis, classes needed to be included in the NMAs of discontinuation (for any reason) and response in completers, as these two outcomes informed the economic model.
- Only classes of interventions that had been tested on at least 50 participants (across RCTs) in each of the NMAs of SMD, discontinuation (for any reason) and response in completers were included in the economic analysis, as this was the minimum amount of evidence that a treatment class should have in order to be considered for a practice recommendation. The committee looked at the total size of the evidence base in this area and the large volume of evidence for some treatment classes relative to others, and decided not to consider treatment classes with a small size of evidence base (tested on <50 participants) as there were several treatment classes with a much larger volume of evidence. An exception to this rule was made for classes of interventions that are routinely available in the NHS, that is, such classes were included in the analysis even if they had been tested on fewer than 50 participants in the NMAs mentioned above. For some treatment classes, inclusion in the economic model was not possible as no data were available on one or more NMA outcomes that informed economic modelling. For such classes, additional relevant data were sought by contacting authors of studies already included in the guideline systematic review, so as to enable inclusion of the classes in the respective NMAs and, subsequently, in the economic modelling.
- In addition, only classes with a higher mean effect on the SMD outcome compared with the selected reference treatment (TAU) were considered in the economic analysis.
Specific interventions were used as exemplars within each class regarding their intervention costs, so that results of interventions can be extrapolated to other interventions of similar resource intensity within their class. The following interventions [in brackets the classes they belong to] were assessed:
- pharmacological interventions: sertraline [SSRIs]; lofepramine [TCAs]
- psychological interventions: cCBT without or with minimal support [self-help without or with minimal support]; cCBT with support [self-help with support]; individual BA [individual BT]; group BA [group BT]; individual CBT (under 15 sessions) [individual CT/CBT]; group CBT (under 15 sessions) [group CT/CBT]; individual problem solving [individual problem solving]; non-directive/supportive/person-centred counselling [individual counselling]; individual IPT [individual IPT]; individual short-term PDPT [individual short-term PDPT]; group MBCT [mindfulness or meditation group]
- physical interventions: supervised high intensity individual exercise [individual exercise]; supervised high intensity group exercise [group exercise]
- GP care, reflected in the RCT arms of the reference treatment [TAU]
The decision-tree component model structure considered the events of discontinuation for any reason and specifically due to intolerable side effects; treatment completion and response/remission; and treatment completion and inadequate or no response. The Markov component model structure considered the states of remission, depressive episode (due to non-remission or relapse), and death. The specification of the Markov component of the model was based on the relapse prevention model developed for this guideline, details of which are provided in the evidence review C, appendix J.
Efficacy data were derived from the guideline systematic review and NMAs. Bias-adjusted analysis suggested no presence of bias due to small study size in the data. Baseline parameters (baseline risk of discontinuation, discontinuation due to side effects, and response/remission) were estimated based on a review of naturalistic studies. The measure of outcome of the economic analysis was the number of QALYs gained. Utility data were derived from a systematic review of the literature, and were generated using EQ-5D measurements and the UK population tariff. The perspective of the analysis was that of health and personal social care services. Resource use was based on published literature, national statistics and, where evidence was lacking, the committee’s expert opinion. National UK unit costs were used. The cost year was 2020. Model input parameters were synthesised in a probabilistic analysis. This approach allowed more comprehensive consideration of the uncertainty characterising the input parameters and captured the non-linearity characterising the economic model structure. A number of one-way deterministic sensitivity analyses was also carried out.
Results have been expressed in the form of Net Monetary Benefits (NMBs). Incremental mean costs and effects (QALYs) of each intervention versus GP care have been presented in the form of cost effectiveness planes. Results of probabilistic analysis have been summarised in the form of cost-effectiveness acceptability frontiers (CEAFs), which show the treatment option with the highest mean NMB over different cost effectiveness thresholds, and the probability that the option with the highest NMB is the most cost-effective among those assessed.
Overview of economic modelling results and conclusions
Group CBT appeared to be the most cost-effective intervention, followed by group BA, group exercise, sertraline, group MBCT, cCBT without or with minimal support, lofepramine, and cCBT with support. These were followed by individual CBT, individual BA, individual problem solving, IPT, GP care, non-directive counselling, short-term PDPT, and individual exercise. The probability of CBT group being the most cost-effective option was 0.60 at the NICE lower cost effectiveness threshold of £20,000/QALY.
The results of the analysis were characterised by considerable uncertainty, as reflected in the wide 95% credible intervals (CrI) around the rankings of interventions. On the other hand, deterministic sensitivity analysis suggested that the results and the ranking of interventions from the most to the least cost-effective were overall robust under different scenarios explored.
Conclusions from the guideline economic analysis refer mainly to people with depression who are treated in primary care for a new depressive episode; however, they may be relevant to people in secondary care as well, given that clinical evidence was derived from a mixture of primary and secondary care settings (however, it needs to be noted that costs utilised in the guideline economic model were mostly relevant to primary care).
Summary of the evidence
Clinical evidence statements for NMA results
This section reports only NMA results that informed the clinical evidence. Detailed NMA findings on all outcomes, including those that informed the economic analysis, are reported in appendix M and supplements B5 and B6.
Critical outcomes
Depression symptomatology - standardised mean difference (SMD) of depression symptom change scores (bias-adjusted analysis)
- Evidence from the NMA shows a clinically important and statistically significant benefit of a combined CBT group and exercise group intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −2.51, 95% Crl −4.42 to −0.61; 25 participants randomised to CBT group + exercise group included in this NMA). Combined CBT group and exercise group is the highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 2.92 [out of 32], 95% CrI 1 to 14).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a problem solving group intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −1.52, 95% CrI −3.24 to 0.23; 104 participants randomised to problem solving group included in this NMA). Problem solving group is the second highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 6.61, 95% CrI 1 to 26).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a CBT group intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −1.01, 95% CrI −1.76 to −0.06; 480 participants randomised to CBT group included in this NMA). CBT group is the third highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 9.55, 95% CrI 3 to 22).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined mindfulness or meditation group and antidepressant intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −1.23, 95% CrI −5.14 to 2.80; 15 participants randomised to mindfulness/meditation group + antidepressant included in this NMA). Combined mindfulness or meditation group and antidepressant is the fourth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 12.22, 95% CrI 1 to 32).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a behavioural therapy group intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.73, 95% CrI −1.95 to 0.50; 340 participants randomised to behavioural therapy group included in this NMA). Behavioural therapy group is the fifth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 13.09, 95% CrI 3 to 28).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual CBT intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.73, 95% CrI −1.78 to 0.36; 481 participants randomised to individual CBT included in this NMA). Individual CBT is the sixth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 13.14, 95% CrI 4 to 27).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a TCA relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.83, 95% CrI −2.18 to 0.53; 136 participants randomised to TCAs included in this NMA). TCAs are the seventh highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 13.27, 95% CrI 3 to 29).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined CBT group and antidepressant intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −1.00, 95% CrI −4.47 to 2.61; 32 participants randomised to CBT group + antidepressant included in this NMA). Combined CBT group and antidepressant is the eighth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 13.34, 95% CrI 1 to 32).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined acupuncture and non-directive counselling intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.78, 95% CrI −2.57 to 1.02; 40 participants randomised to acupuncture + counselling included in this NMA). Combined acupuncture and non-directive counselling is the ninth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 13.37, 95% CrI 2 to 31).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a yoga group intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.73, 95% CrI −2.43 to 0.98; 73 participants randomised to yoga group included in this NMA). Yoga group is the tenth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 13.83, 95% CrI 2 to 31).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of acupuncture relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.69, 95% CrI −2.50 to 1.13; 40 participants randomised to acupuncture included in this NMA). Acupuncture is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 14.26, 95% CrI 2 to 31).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a mindfulness or meditation group intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.62, 95% CrI −1.77 to 0.35; 376 participants randomised to mindfulness/meditation group included in this NMA). Mindfulness/meditation group is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 14.47, 95% CrI 4 to 28).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual behavioural therapy intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.63, 95% CrI −2.48 to 1.28; 147 participants randomised to individual behavioural therapy included in this NMA). Individual behavioural therapy is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 14.72, 95% CrI 2 to 31).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an SSRI relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.64, 95% CrI −1.87 to 0.53; 207 participants randomised to SSRIs included in this NMA). SSRIs are outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 15.90, 95% CrI 4 to 30).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual mindfulness or meditation intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.52, 95% CrI −3.10 to 2.22; 20 participants randomised to individual mindfulness/meditation included in this NMA). Individual mindfulness/meditation is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 16.09, 95% CrI 1 to 32).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a short-term psychodynamic psychotherapy intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.48, 95% CrI −2.96 to 2.03; 49 participants randomised to short-term psychodynamic psychotherapy included in this NMA). Short-term psychodynamic psychotherapy is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 16.49, 95% CrI 2 to 32).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual IPT intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.5, 95% CrI −1.94 to 0.83; 153 participants randomised to IPT included in this NMA). IPT is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 16.93, 95% CrI 4 to 30).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a relaxation group intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.42, 95% CrI −2.19 to 1.20; 63 participants randomised to relaxation group included in this NMA). Relaxation group is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 17.84, 95% CrI 3 to 32).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of an exercise group intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.37, 95% CrI −3.56 to 2.79; 199 participants randomised to exercise group included in this NMA). Exercise group is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 17.91, 95% CrI 1 to 32).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of self-help with support relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.33, 95% CrI −0.77 to 0.08; 1286 participants randomised to self-help with support included in this NMA). Self-help with support is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 18.22, 95% CrI 11 to 25).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of an individual relaxation intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.41, 95% CrI −3.07 to 2.23; 13 participants randomised to individual relaxation included in this NMA). Individual relaxation is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 18.39, 95% CrI 1 to 32).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a non-directive counselling intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.20, 95% CrI −2.82 to 2.5; 55 participants randomised to counselling included in this NMA). Non-directive counselling is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 19.20, 95% CrI 2 to 32).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of an individual exercise intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.26, 95% CrI −1.73 to 1.15; 250 participants randomised to individual exercise included in this NMA). Individual exercise is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 19.43, 95% CrI 4 to 31).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a self-help intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.27, 95% CrI −0.66 to 0.09; 4922 participants randomised to self-help included in this NMA). Self-help (with no or minimal support) is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 19.51, 95% CrI 13 to 25).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a combined individual CBT and exercise group intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.18, 95% CrI −2.75 to 2.44; 18 participants randomised to individual CBT + exercise group included in this NMA). Combined individual CBT and exercise group is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 19.78, 95% CrI 2 to 32).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a psychoeducation group intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD −0.09, 95% CrI −2.07 to 1.96; 22 participants randomised to psychoeducation group included in this NMA). Psychoeducation group is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depressive symptom scores (mean rank 20.80, 95% CrI 3 to 32).
- Evidence from the NMA shows no benefit of an individual problem solving intervention relative to TAU on depression symptomatology for adults with less severe depression (SMD 0.17, 95% CrI −1.53 to 1.91; 98 participants randomised to individual problem solving included in this NMA). Individual problem solving is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 24.28, 95% CrI 6 to 32).
Response in those randomised
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a TCA relative to TAU on response (in those randomised) for adults with less severe depression (163 participants randomised to TCAs included in this NMA). TCAs are the highest ranked intervention for response in those randomised (mean rank 4.54 [out of 25], 95% CrI 1 to 20).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a problem solving group intervention relative to TAU on response (in those randomised) for adults with less severe depression (89 participants randomised to problem solving group included in this NMA). Problem solving group is the second highest ranked intervention for response in those randomised (mean rank 4.86, 95% CrI 1 to 18).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an SSRI relative to TAU on response (in those randomised) for adults with less severe depression (159 participants randomised to SSRIs included in this NMA). SSRIs are the third highest ranked intervention for response in those randomised (mean rank 6.27, 95% CrI 1 to 21).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a CBT group intervention relative to TAU on response (in those randomised) for adults with less severe depression (341 participants randomised to CBT group included in this NMA). CBT group is the fourth highest ranked intervention for response in those randomised (mean rank 8.32, 95% CrI 2 to 18).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a behavioural therapy group intervention relative to TAU on response (in those randomised) for adults with less severe depression (184 participants randomised to behavioural therapy group included in this NMA). Behavioural therapy group is the fifth highest ranked intervention for response in those randomised (mean rank 8.86, 95% CrI 2 to 20).
- Evidence from the NMA shows a clinically important and statistically significant benefit of an exercise group intervention relative to TAU on response (in those randomised) for adults with less severe depression (52 participants randomised to exercise group included in this NMA). Exercise group is the sixth highest ranked intervention for response in those randomised (mean rank 9.27, 95% CrI 2 to 20).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined acupuncture and non-directive counselling intervention relative to TAU on response (in those randomised) for adults with less severe depression (40 participants randomised to acupuncture + counselling included in this NMA). Combined acupuncture and non-directive counselling is the seventh highest ranked intervention for response in those randomised (mean rank 10.30, 95% CrI 1 to 24).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual behavioural therapy intervention relative to TAU on response (in those randomised) for adults with less severe depression (65 participants randomised to individual behavioural therapy included in this NMA). Individual behavioural therapy is the eighth highest ranked intervention for response in those randomised (mean rank 10.40, 95% CrI 1 to 23).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a yoga group intervention relative to TAU on response (in those randomised) for adults with less severe depression (65 participants randomised to yoga group included in this NMA). Yoga group is the ninth highest ranked intervention for response in those randomised (mean rank 10.51, 95% CrI 1 to 24).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of acupuncture relative to TAU on response (in those randomised) for adults with less severe depression (40 participants randomised to acupuncture included in this NMA). Acupuncture is the tenth highest ranked intervention for response in those randomised (mean rank 10.81, 95% CrI 1 to 24).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual mindfulness or meditation intervention relative to TAU on response (in those randomised) for adults with less severe depression (20 participants randomised to individual mindfulness/meditation included in this NMA). Individual mindfulness/meditation is outside the top-10 highest ranked interventions for response in those randomised (mean rank 11.06, 95% CrI 1 to 24).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual CBT intervention relative to TAU on response (in those randomised) for adults with less severe depression (121 participants randomised to individual CBT included in this NMA). Individual CBT is outside the top-10 highest ranked interventions for response in those randomised (mean rank 12.16, 95% CrI 1 to 24).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a mindfulness or meditation group intervention relative to TAU on response (in those randomised) for adults with less severe depression (197 participants randomised to mindfulness/meditation group included in this NMA). Mindfulness/meditation group is outside the top-10 highest ranked interventions for response in those randomised (mean rank 12.76, 95% CrI 4 to 22).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual exercise intervention relative to TAU on response (in those randomised) for adults with less severe depression (71 participants randomised to individual exercise included in this NMA). Individual exercise is outside the top-10 highest ranked interventions for response in those randomised (mean rank 14.24, 95% CrI 5 to 23).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a self-help intervention relative to TAU on response (in those randomised) for adults with less severe depression (4373 participants randomised to self-help included in this NMA). Self-help (with no or minimal support) is outside the top-10 highest ranked interventions for response in those randomised (mean rank 15.23, 95% CrI 10 to 19).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a psychoeducation group intervention relative to TAU on response (in those randomised) for adults with less severe depression (22 participants randomised to psychoeducation group included in this NMA). Psychoeducation group is outside the top-10 highest ranked interventions for response in those randomised (mean rank 15.36, 95% CrI 2 to 25).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of self-help with support relative to TAU on response (in those randomised) for adults with less severe depression (849 participants randomised to self-help with support included in this NMA). Self-help with support is outside the top-10 highest ranked interventions for response in those randomised (mean rank 15.62, 95% CrI 10 to 21).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a relaxation group intervention relative to TAU on response (in those randomised) for adults with less severe depression (63 participants randomised to relaxation group included in this NMA). Relaxation group is outside the top-10 highest ranked interventions for response in those randomised (mean rank 15.91, 95% CrI 2 to 25).
- Evidence from the NMA shows no benefit of individual IPT relative to TAU on response (in those randomised) for adults with less severe depression (69 participants randomised to IPT included in this NMA). IPT is outside the top-10 highest ranked interventions for response in those randomised (mean rank 18.48, 95% CrI 4 to 25).
- Evidence from the NMA shows a lower effect of an individual relaxation intervention relative to TAU on response (in those randomised) for adults with less severe depression (15 participants randomised to individual relaxation included in this NMA), although this difference is not statistically significant. Individual relaxation is ranked second from bottom for response in those randomised, and is ranked below attention placebo, TAU and enhanced TAU (mean rank 21.53, 95% CrI 4 to 25).
Remission in those randomised
- Evidence from the NMA shows a clinically important and statistically significant benefit of a problem solving group intervention relative to TAU on remission (in those randomised) for adults with less severe depression (89 participants randomised to problem solving group included in this NMA). Problem solving group is the highest ranked intervention for remission in those randomised (mean rank 1.59 [out of 15], 95% CrI 1 to 5).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a yoga group intervention relative to TAU on remission (in those randomised) for adults with less severe depression (20 participants randomised to yoga group included in this NMA). Yoga group is the second highest ranked intervention for remission in those randomised (mean rank 4.58, 95% CrI 1 to 14).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual CBT intervention relative to TAU on remission (in those randomised) for adults with less severe depression (233 participants randomised to individual CBT included in this NMA). Individual CBT is the third highest ranked intervention for remission in those randomised (mean rank 5.38, 95% CrI 2 to 11).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual behavioural therapy intervention relative to TAU on remission (in those randomised) for adults with less severe depression (16 participants randomised to individual behavioural therapy included in this NMA). Individual behavioural therapy is the fourth highest ranked intervention for remission in those randomised (mean rank 5.45, 95% CrI 1 to 13).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of self-help with support relative to TAU on remission (in those randomised) for adults with less severe depression (348 participants randomised to self-help with support included in this NMA). Self-help with support is the fifth highest ranked intervention for remission in those randomised (mean rank 5.72, 95% CrI 2 to 10).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual mindfulness or meditation intervention relative to TAU on remission (in those randomised) for adults with less severe depression (20 participants randomised to individual mindfulness/meditation included in this NMA). Individual mindfulness/meditation is the sixth highest ranked intervention for remission in those randomised (mean rank 6.57, 95% CrI 2 to 14).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a CBT group intervention relative to TAU on remission (in those randomised) for adults with less severe depression (117 participants randomised to CBT group included in this NMA). CBT group is the seventh highest ranked intervention for remission in those randomised (mean rank 7.02, 95% CrI 2 to 13).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a behavioural therapy group intervention relative to TAU on remission (in those randomised) for adults with less severe depression (68 participants randomised to behavioural therapy group included in this NMA). Behavioural therapy group is the eighth highest ranked intervention for remission in those randomised (mean rank 7.49, 95% CrI 2 to 14).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a self-help intervention relative to TAU on remission (in those randomised) for adults with less severe depression (1050 participants randomised to self-help included in this NMA). Self-help (with no or minimal support) is the ninth highest ranked intervention for remission in those randomised (mean rank 7.74, 95% CrI 4 to 11).
- Evidence from the NMA shows no benefit of individual IPT relative to TAU on remission (in those randomised) for adults with less severe depression (69 participants randomised to IPT included in this NMA). IPT is the tenth highest ranked intervention for remission in those randomised (mean rank 9.81, 95% CrI 3 to 15).
- Evidence from the NMA shows a lower effect of a relaxation group intervention relative to TAU on remission (in those randomised) for adults with less severe depression (63 participants randomised to relaxation group included in this NMA), although this difference is not statistically significant. Relaxation group is ranked fourth from the bottom for remission in those randomised (mean rank 10.48, 95% CrI 2 to 15).
- Evidence from the NMA shows a lower effect of an individual relaxation intervention relative to TAU on remission (in those randomised) for adults with less severe depression (15 participants randomised to individual relaxation included in this NMA), although this difference is not statistically significant. Individual relaxation is ranked bottom for remission in those randomised, and is ranked below TAU, waitlist and attention placebo (mean rank 13.64, 95% CrI 5 to 15).
Clinical evidence statements for pairwise meta-analysis results of studies included in the NMA
Important, but not critical, outcomes
Quality of life
- Single-RCT evidence (N=40) shows a clinically important and statistically significant benefit of an individual behavioural therapy intervention relative to no treatment on quality of life for adults with less severe depression.
- Single-RCT evidence (N=28) shows a clinically important and statistically significant benefit of an individual behavioural therapy intervention relative to waitlist on quality of life for adults with less severe depression.
- Single-RCT evidence (N=62) shows clinically important and statistically significant benefits of a combined CBT group and antidepressant intervention relative to an antidepressant-only on quality of life physical health component and mental health component scores at endpoint and 12-month follow-up for adults with less severe depression.
- Single-RCT evidence (N=204) shows clinically important and statistically significant benefits of self-help relative to waitlist on quality of life physical health component and mental health component scores for adults with less severe depression.
- Single-RCT evidence (N=26) shows a clinically important and statistically significant benefit of an exercise group intervention relative to TAU on quality of life mental health component score for adults with less severe depression.
- Single-RCT evidence (N=60) shows a clinically important and statistically significant benefit of a mindfulness or meditation group intervention relative to waitlist on quality of life for adults with less severe depression.
Personal, social and occupational functioning
- Single-RCT evidence (N=62) shows a clinically important and statistically significant benefit of a combined CBT group and antidepressant intervention relative to an antidepressant-only on functional impairment at 12-month follow-up for adults with less severe depression.
- Single-RCT evidence (N=112) shows a clinically important and statistically significant benefit of a problem solving group intervention relative to TAU on functional impairment for adults with less severe depression.
- Single-RCT evidence (N=90) shows a clinically important and statistically significant benefit of self-help relative to waitlist on interpersonal functioning for adults with less severe depression.
- Single-RCT evidence (N=613) shows a clinically important and statistically significant benefit of self-help with support relative to no treatment on functional impairment for adults with less severe depression.
- Single-RCT evidence (N=54) shows a clinically important and statistically significant benefit of a combined exercise group and CBT group intervention relative to CBT group-only on global functioning for adults with less severe depression.
- Single-RCT evidence (N=30) shows a clinically important and statistically significant benefit of a combined mindfulness or meditation group and antidepressant intervention relative to antidepressant-only on functional impairment for adults with less severe depression.
Economic evidence statements
- Evidence from 1 single UK study conducted alongside a RCT (N = 247) suggests that individual problem solving is unlikely to be cost-effective compared with treatment as usual in adults with a new episode of less severe depression. The evidence is directly applicable to the UK context and is characterised by minor limitations.
- Evidence from 1 UK modelling study suggests that computerised CBT (with minimal support) may be potentially cost-effective compared with treatment as usual in adults with a new episode of less severe depression. The evidence is directly applicable to the UK context and is characterised by potentially serious limitations.
- Evidence from 1 single UK study conducted alongside a RCT (N = 655) suggests that sertraline is very likely to be cost-effective compared with placebo in adults with a new episode of less severe depression. The evidence is directly applicable to the UK context and is characterised by minor limitations.
- Evidence from 1 single UK study conducted alongside a RCT (N = 220) indicates that provision of SSRIs in addition to GP supportive care is likely to be cost-effective compared with GP supportive care alone in adults with a new episode of less severe depression. The evidence is directly applicable to the UK context and is characterised by minor limitations.
- Evidence from 1 single UK study conducted alongside an open label RCT with a partial preference design (N = 327; entering preference group n=92) indicates that provision of SSRIs is likely to be more cost-effective than TCAs or lofepramine in adults with a new episode of less severe depression. The evidence is directly applicable to the UK context and is characterised by minor limitations.
- Evidence from 1 single UK study conducted alongside a RCT (N = 361) suggests that a physical exercise programme is potentially cost-effective compared with treatment as usual in adults with a new episode of less severe depression. The evidence is directly applicable to the UK context but is characterised by potentially serious limitations.
- Evidence from the guideline economic modelling suggests that group CBT is likely to be the most cost-effective option for the treatment of new episodes of less severe depression in adults, followed by group BA, group exercise, sertraline, group MBCT, cCBT without or with minimal support, lofepramine, and cCBT with support. These were followed by individual CBT, individual BA, individual problem solving, IPT, GP care, non-directive counselling, short-term PDPT, and individual exercise. This evidence refers mainly to people treated in primary care for a new depressive episode; however, it may be relevant to people treated in secondary care as well, given that clinical evidence was derived from a mixture of primary and secondary care settings. The economic analysis is directly applicable to the NICE decision-making context and is characterised by minor limitations.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The aim of this review was to identify the most effective and cost-effective treatments for less severe depression and the committee chose depression symptomatology (measured as the standardised mean difference, SMD, of depression symptom change scores at treatment endpoint), remission (in those randomised) and response (in those randomised) as critical outcomes to provide an indication of clinical effectiveness. Discontinuation due to side effects and discontinuation for any reason were also chosen as critical outcomes, as indicators of the tolerability and acceptability of treatments, but results for these outcomes were used as part of the economic modelling (along with remission and response in completers) and were not reviewed by the committee separately.
In addition to the critical, depression-specific, outcomes, the committee prioritised 2 important outcomes – these were quality of life and personal, social and occupational functioning. These were selected to determine if treatments for depression led to improved quality of life, and if they helped overcome other difficulties such as ability to sleep, participate in employment, and carry out activities of daily living. These were selected as important and not critical outcomes as the committee were aware that there was likely to be less evidence for these outcomes. The committee recognised that although these outcomes were very important to people with depression, as they would not be available for all interventions they would be less useful to the committee to make recommendations.
The critical outcomes were assessed at treatment endpoint, but in order to determine if treatments for depression had longer term benefits, follow-up measurements of depression symptomatology, remission and response were analysed. Outcomes at these additional timepoints were also assessed by the committee as part of their decision-making process. However, the committee recognised that although these longer-term outcomes were very important to people with depression, as they would not be available for all interventions they would be less useful to the committee to make recommendations.
For each outcome, the committee decided to consider only treatment classes that had been tested on at least 50 participants across the RCTs included in the respective NMA, after looking at the total size of the evidence base on treatments for a new episode of less severe depression and noticing that there were several treatment classes with a much larger volume of evidence.
The quality of the evidence
The trials included for this evidence review were individually assessed using the Cochrane risk of bias tool (version 1.0), and the summarised quality of the evidence is presented in the evidence review. Overall, the majority of domains were rated as at low risk, or unclear risk, of bias with the exception of blinding of participants and personnel where there was a high risk of bias due to a lack of therapist and patient blinding in the psychological treatment trials.
Regarding the outcomes considered in the clinical analysis, the between-trial heterogeneity relative to the size of the intervention effect estimates was moderate for the SMD of depression symptom scores and for remission in those randomised, and high for response in those randomised. No evidence of inconsistency was identified in any of the outcomes considered in the clinical analysis. In the analysis of the SMD of depression symptom scores there was evidence of bias associated with small study size. The bias adjusted model resulted in moderate changes in the relative effects of all treatment classes versus TAU (reference treatment) and also had a moderate impact on some class rankings. The committee took this information into account when interpreting the results.
Regarding the outcomes that informed the economic analysis, relative to the size of the intervention effect estimates, the between trial heterogeneity was found to be moderate for discontinuation due to any reason and high for response in completers. Some evidence of inconsistency was identified for the response in completers outcome. No evidence of bias associated with small study size was identified for either outcome utilised in the economic analysis.
The sensitivity analysis on the SMD outcome conducted to explore the transitivity assumption of participants in pharmacological and non-pharmacological studies found that there were no substantial differences in the results when the pharmacological trials were excluded from analysis and thus the transitivity assumptions are acceptable in this population. The committee noted that most of the evidence for this population comes from non-pharmacological trials.
The post-hoc sub-group analysis on the SMD outcome that included only studies at low risk for the attrition domain of the Cochrane risk-of-bias tool showed no substantial difference in treatment effects compared with the base-case analysis. This suggested that bias from attrition was unlikely to be an effect modifier in this population.
The committee noted that the effectiveness of psychological interventions may depend on clinicians’ training, expertise and previous experience with specific treatments, as well as patients’ needs, preferences and experiences with previous treatments for depression. The committee acknowledged that these factors may have affected, to some extent, the efficacy of treatments in the RCTs included in the NMAs, and also patient outcomes in clinical practice. These issues were considered when interpreting the available evidence, but also when formulating reocmmendations.
A threshold analysis was originally planned, to assess the robustness of the intervention recommendations to potential limitations in the evidence synthesised in NMAs. Threshold analysis suggests by how much effects that have been estimated in the NMA need to change before recommendations change, and whether such changes might potentially occur due to bias in the evidence. The NICE Guidelines Technical Support Unit (TSU) attended committee discussions on the rationale for recommendations and noted that, in addition to the results of the NMA, the committee took other pragmatic factors into consideration when making recommendations, including the uncertainty and limitations around the clinical and cost-effectiveness data, and the need to provide a wide range of interventions to take into account individual needs and allow patient choice. The TSU advised that as it was difficult to identify a clear decision rule to link the recommendations directly to the NMA results, it was not feasible or helpful to conduct a threshold analysis. The committee agreed with the observation that recommendations were based on a pragmatic approach utilising their clinical experience and the need for inclusivity; and their wish for pragmatic recommendations tailored to individual needs and preferences. Therefore they agreed that threshold analysis would not add value to decision making.
Benefits and harms
In developing the recommendations for the treatment of a new episode of depression the committee were mindful of a number of important factors which underpin the effective delivery of care for people with depression. For example, the need to ensure that progress on treatment is properly monitored and reviewed, and that any potential harms of treatment are minimised. The committee agreed that not addressing these factors could lead to poorer engagement with the service, higher attrition, sub-optimal delivery of treatments and consequent poorer outcomes. The committee therefore carried forward and amended a number of recommendations from the previous guideline and added new recommendations, based on their expertise and experience at providing and receiving treatment for depression. These recommendations included that all interventions should be provided in the context of effective assessment, care planning, liaison and outcome monitoring, and that psychological and psychosocial interventions should be delivered in accordance with appropriate manuals and competence frameworks, and should be supported by effective supervision and audit.
The committee agreed that decisions on treatment should be made in discussion with the person with depression, and recommended that a shared decision should be made. The committee cross-referred to the guideline recommendations on choice of treatment which provided more detailed recommendations on how this shared decision should be made and what should be included in the discussion. It was recognised by the committee that people who have had prior episodes of depression may also have preferences for their treatment based on prior experience or insight into their own depression patterns.
The committee then discussed the results of the clinical and economic analyses and used this information to draft recommendations relating to the use of specific interventions for the treatment of less severe depression. When reviewing the evidence from the network meta-analysis, the committee were aware that a number of important and well-known, often pragmatic, trials were excluded from the NMA, typically because the samples in the trials were <80% first-line treatment or <80% non-chronic depression. These were stipulations of the review protocol in order to create a homogenous data set, but the committee used their knowledge of these studies in the round when interpreting the evidence from the systematic review and making recommendations. The committee were particularly mindful of the UK-based psychological treatment studies that had been excluded on this basis, due to the relevance to the NHS context. For less severe depression, the committee’s knowledge of the results of these trials (Coote & MacLeod, 2012; Cramer et al. 2011; Lambert et al. 2018; Lovell et al. 2008; McClay et al. 2015; Serfaty et al. 2009; Verduyn et al. 2003; Williams et al. 2013, 2018) was brought to bear when interpreting the results of the NMA. The results of these studies were broadly consistent with the evidence from the systematic review, and the committee took this into consideration when making their recommendations.
The committee reviewed the results of the bias-adjusted NMA for less severe depression for the outcome of SMD, compared to treatment as usual. The committee noted that the point estimate for the majority of intervention classes showed an improvement in depression symptoms, but that most also had very wide 95% credible intervals which crossed zero, and therefore there was uncertainty around the effectiveness. The committee noted that the only treatment class for which there was evidence from more than 50 participants, and credible intervals that did not cross zero was group cognitive and cognitive behavioural therapies (CT/CBT). The committee agreed that it would therefore be reasonable to recommend these as treatments of choice in people with less severe depression. The committee also noted that for some other classes of interventions, such as individual CBT, group problem-solving, and group and individual behavioural therapies, the point estimates indicated effectiveness and the credible intervals were narrower (although they crossed zero). There was very litte to differentiate between the other classes based on the bias-adjusted SMD evidence alone.
The committee reviewed the bias-adjusted NMA rankings for the classes of interventions but noted the very wide credible intervals in the ranks provided, and agreed this did not provide any additional information to help them distinguish between the classes. When the SMD for the treatment classes was reviewed by the committee alongside the SMD results for individual interventions within those classes, the committee noted that some individual interventions demonstrated a difference compared to treatment as usual that had not been seen when reviewing the class level data – this included group behavioural activation, individual CBT, group problem-solving and group mindfulness-based cognitive therapy or group mindfulness and meditation.
The committee reviewed the class level NMA results for the outcomes of response and remission in those randomised. For response the results were similar to those seen for SMD, with most treatments showing a point estimate that indicated that they may be effective, but with wide credible intervals that crossed zero. However, group CT/CBT, group problem-solving and group exercise (as well as pill placebo) did not cross the zero line and so the committee agreed this reinforced some of the results seen for SMD. The committee also noted that for the outcome of response, antidepressants (TCAs and SSRIs) appeared to be more effective than seen for the outcome of SMD. For the outcome of remission, there was only data for a smaller number of classes, but again this was in line with the results seen for response, with group problem-solving appearing to be the most effective treatment based on this outcome.
The committee discussed the sensitivity analysis conducted to determine if the inclusion of pharmacological trials impacted on the results seen for psychological, psychosocial and physical therapies. It was noted that exclusion of the pharmacological studies had small effects on some SMDs compared to treatment as usual, but did not affect the overall results, with the only effective treatment for which there were data on more than 50 participants across RCTs remaining as group CBT.
The evidence for the outcomes of quality of life and functioning, and for follow-up of depression outcomes were, as described above, presented as pairwise analyses. The committee reviewed the outcomes where a clinically important and statistically significant difference had been identified, but noted that the results were all from single studies, many of which were small (some with fewer than 50 participants, most with fewer than 100 participants).
In terms of quality of life and functioning there was some evidence of benefit for individual behavioural therapy, group problem-solving, self-help, group exercise and group mindfulness and meditation when compared to no treatment, waitlist or treatment as usual. The committee noted that these were interventions that had been identified as being effective at treating depression symptoms, and so the limited evidence of a benefit on quality of life and functioning could reinforce a decision to recommend these treatments. There was also evidence for these outcomes for combination therapy with CBT and antidepressants compared to antidepressants alone or mindfulness/meditation and antidepressants compared to antidepressants alone, which indicated that CBT and mindfulness/mediation provide additional benefits. Again the committee agreed that this limited evidence was not sufficient to use as a basis for recommendations on its own, but it did suggest that there may be quality of life and functional benefits from some of these treatments which also appeared effective based on the critical outcomes.
There were very few comparisons from the data on follow-up of depression outcomes that showed a clinically important and statistically significant difference. Group CBT and group problem-solving showed benefits on depression symptoms at follow-up compared to treatment as usual, and CBT with antidepressants showed benefits compared to antidepressants alone. The committee agreed that this provided a useful indication that the results seen from the NMA for group CBT and group problem-solving may be maintained over a longer period. A 6-month follow-up of short-term psychodynamic psychotherapy (STPP) compared to non-directive counselling found a benefit for STPP for the outcomes of depression symptoms and remission at 6 months, but the committee noted that this small amount of evidence did not change their view, based on the NMA results, that these treatments had similar levels of effectiveness.
The final piece of clinical evidence the committee reviewed was the summary of the differences between the pairwise analysis and the NMA results. It was noted that the number of comparisons where there was a significant difference was small (12%), and in the majority of cases that difference was in the magnitude of the effect. The committee agreed that these differences did not add any additional information that they needed to take into account when making their recommendations, and that there were not any different treatments that they would recommend based on the pairwise evidence.
Finally, the committee noted that the very limited evidence for the subgroup analysis of older versus younger people showed no difference and so there was no evidence on which to base any specific recommendations for people of different ages.
Based on their overall review of the clinical evidence the committee agreed that some treatment classes and interventions (group CT/CBT class, group BA, individual CBT forms, group problem solving intervention, MBCT and group mindfulness or meditation, and group exercise) appeared to be more effective than others in ranking, but there was otherwise little to choose between treatments. The committee therefore reviewed the results of the health economic modelling (see separate details of this discussion below) which determined which treatments were cost-effective, and used this to develop a suggested prioritisation of which treatments should be offered to people with depression, or considered for use.
The committee agreed that the likely benefits of recommending specific treatments for less severe depression would be improvements in depression symptoms, and in some cases remission and response. However, given the uncertainty associated with the evidence the committee agreed that the relative benefits and harms are likely to vary across individuals, and it was important that a wide range of interventions were available to take into account individual needs and allow patient choice. The potential harms that the committee identified were side effects and withdrawal effects associated with antidepressant treatment. On the basis of safety and tolerability, the committee advised that SSRIs would be the preferred antidepressants to use in people with less severe depression. Given the potential for side effects and/or withdrawal effects and the availability of psychological and physical treatments that were found to be effective (several of which ranked more highly than antidepressants regarding efficacy), the committee made a strong recommendation that medication should not be the default treatment for people with less severe depression, unless it was the person’s preference to take antidepressants rather than engage in a psychological or physical intervention.
As there was limited evidence for the effectiveness of peer support the committee made a research recommendation. As there was uncertainty about the differential effectiveness of psychological treatments, they also made research recommendations about the mode of action of psychological treatments, as this may provide information to support decision-making in the choice of treatments.
A research recommendation about the withdrawal effects of antidepressants was made as there was limited evidence to provide information to patients and support methods of withdrawal. This related to the section of the guideline on starting and stopping antidepressants, which was based on evidence from the NICE guideline on Safe prescribing and so the details of the research recommendation were included in this evidence review.
Cost effectiveness and resource use
According to existing UK economic evidence, computerised CBT (with minimal support) and physical exercise might be potentially cost-effective compared with treatment as usual in adults with a new episode of less severe depression. On the other hand, individual problem solving was unlikely to be cost-effective compared with treatment as usual in this population. Sertraline was likely to be cost-effective compared with placebo, and provision of SSRIs in addition to GP supportive care was likely to be cost-effective compared with GP supportive care alone. SSRIs were also likely to be more cost-effective than TCAs or lofepramine. This evidence was directly applicable to the NICE decision-making context, but methodological limitations ranged from minor to potentially severe.
Existing economic evaluations assessed a limited range of pharmacological, psychological and physical interventions in, mostly, pairwise comparisons, so it was difficult for the committee to draw any robust conclusions on the relative cost effectiveness of the full range of interventions that are available for the treatment of adults with a new episode of less severe depression.
The guideline economic analysis assessed the cost effectiveness of a wide range of pharmacological, psychological and physical interventions, as initial treatments for people with a new episode of less severe depression. The interventions included in the economic analysis were dictated by availability of data and were used as exemplars within their class regarding intervention costs, as for practical reasons it was impossible to model all interventions considered in the guideline NMA. The committee noted that the results of interventions could be extrapolated, with some caution, to other interventions of similar resource intensity within the same class.
Within each of the individual and group CT/CBT classes, there were two separate interventions of CBT≥15 sessions and CBT<15 sessions. Regarding individual CBT, the two interventions were shown to have a similar SMD vs TAU (individual CBT≥15 sessions −0.68, 95% CrI −1.36 to 0.01; individual CBT<15 sessions −0.66, 95% CrI −1.45 to 0.16), and individual CBT<15 sessions had a somewhat larger evidence base across RCTs on the SMD outcome (N=233 vs 123). Individual CBT<15 sessions was considered to have an appropriate intensity for a population with less severe depression by the committee, it had also a wider evidence base than CBT≥15 sessions, and given that individual CBT≥15 sessions and individual CBT<15 sessions had similar effectiveness, individual CBT<15 sessions was selected for consideration as an exemplar of its class in the economic modelling (which ultimately informed guideline recommendations). Regarding group CBT, group CBT<15 sessions had a better SMD vs TAU than group CBT≥15 sessions (group CBT<15 sessions −1.25, 95% CrI −1.72 to −0.83; group CBT≥15 sessions −0.84, 95% CrI −1.91 to 0.78) and also a much wider evidence base (N=316 vs 10). Therefore, as group CBT<15 sessions was shown to have better effects and a much wider evidence base than group CBT≥15 sessions, it was selected for consideration as an exemplar of its class in the economic modelling (which ultimately informed recommendations). The committee considered group CBT<15 sessions to have appropriate intensity for a population with less severe depression.
The economic analysis included only classes that had been tested on at least 50 participants across RCTs included in the NMAs of the SMD, discontinuation for any reason and response in completers, or fewer than 50 participants if the intervention class was one that was already in routine use in the NHS. These criteria meant that some classes of interventions such as group problem-solving were not included in the economic model. To be considered in the economic analysis, treatment classes should have shown a better mean effect than the reference intervention, which was treatment as usual. This was assumed in the model to reflect GP care. The NMAs of discontinuation (for any reason) and response in completers, which informed the economic analysis, were tested for the presence of bias due to small study size. No evidence of bias was identified.
The economic analysis utilised data on the risk of side effects from antidepressants obtained from a large US study that reported claims data. This risk ranged from 4.7% to 9.2%, depending on the antidepressant class. The committee selected these data because they expressed the view that claims for side effects that come up spontaneously, via healthcare service contacts, are more representative of the risk of side effects that have an impact on HRQoL and healthcare costs (which are of interest as they may have an impact on antidepressants’ relative cost-effectiveness) compared with studies asking specifically participants to self-report the presence of side effects choosing from a side-effect checklist. According to the committee’s expert opinion, the latter study design tends to overestimate the prevalence of side effects. There was also a danger of the risk of side effects from antidepressants being overestimated in the economic model, since the risk of common side effects for psychological therapies was conservatively assumed to be zero. Nevertheless, the committee advised that a higher risk of side effects (40%) be tested in a sensitivity analysis. This had some impact on the relative cost-effectiveness of antidepressants, which was considered when making recommendations.
The committee considered the ranking of interventions for adults with a new episode of less severe depression, from the most to the least cost-effective. According to this ranking, group CBT and group behavioural activation appeared to be the most cost-effective therapies. The majority of the other interventions also appeared to be cost-effective compared with GP care, with the exception of non-directive counselling, short-term psychodynamic psychotherapy (PDPT) and individual exercise therapy.
The committee considered the 95% credible intervals (CrI) around the rankings of interventions and noted that these were characterised by considerable uncertainty. For example, the mean ranking of group CBT, which was shown to be the most cost-effective intervention, was 2.61, however its 95% CrI were 1 to 12, suggesting uncertainty around the result for group CBT. On the other hand, group CBT dominated most of the other interventions included in the economic analysis (i.e. it was more effective and less costly), or, regarding the few comparisons where it was more effective and more costly (group exercise, self-help and self-help with support and GP care), the respective ICER never exceeded £3,000/QALY, which is well below the NICE lower cost-effectiveness threshold of £20,000/QALY. Similar uncertainty was shown for the rankings of all interventions included in the analysis. On the other hand, deterministic sensitivity analysis suggested that the results and the ranking of interventions were overall robust under different scenarios explored.
The committee noted that there was evidence of clinical and cost-effectiveness for self-help with support (which, in the economic model, was represented by computerised CBT) and discussed that, in practice, and particularly in the IAPT services, it may be more logical to offer self-help with support (usually known in IAPT as ‘guided self-help’) first. Guided self-help in IAPT services may include materials based on structured CBT, problem solving, psychoeducation and behavioural activation delivered face-to-face or by telephone or online. This is a less intrusive intervention for people with less severe depression, is less resource intensive for IAPT services to deliver, and is likely to be available for people in a timely fashion without the need for a long time on a waiting list. The committee therefore made guided self-help the first suggested option for treatment, before considering a more intensive treatment.
Based on the clinical and cost-effectiveness data, the committee agreed that group CBT or group behavioural activation (BA) were alternative treatments of choice for a new episode of less severe depression in adults, as they had showed a beneficial effect compared to treatment as usual, and appeared to be the most cost-effective classes in the economic analysis. The committee noted that both these treatments were group therapies, and that some people with depression may not wish to attend group treatment. The committee noted that there was evidence of clinical and cost-effectiveness for individual CBT and individual BA and considered offering these as alternatives to people who did not wish to attend group therapy.
The committee did not recommend self-help without support, although this was shown to be more cost-effective than self-help with support, because they acknowledged the importance of building a therapeutic relationship as part of the therapy. They also advised that wider evidence suggests that pure (non-supported) self-help is characterised by lower uptake and adherence compared with self-help with support, which suggests user preference for supported forms of self-help.
The committee agreed that, to allow choice of treatments, a wider range of treatments should be offered – these would provide alternatives to people who did not wish to have guided self-help, CBT or BA, or had tried them for a previous episode of depression and not found them to be effective. The committee discussed that other cost-effective interventions should be included in these alternatives and so recommended group exercise, group mindfulness and meditation, and interpersonal therapy as alternative psychological or physical therapies.
The committee also discussed the role of pharmacological therapy in the treatment of less severe depression. The clinical results for the effect of antidepressants on depression symptoms were similar to those seen for the psychological therapies, showing an improvement in depression symptoms but considerable uncertainty, and the cost-effectiveness results showed both SSRIs and TCAs were likely to be cost-effective (they were placed 4th and 7th in the base-case cost-effectiveness ranking respectively, although they dropped to 10th and 14th place, respectively, in sensitivity analysis that considered a higher risk of side effects). Given the uncertainty and limitations around the clinical and cost-effectiveness data, the committee considered it important to provide a wide range of interventions including psychological, physical and pharmacological options, to take into account individual needs and allow patient choice. The committee considered the fact that there may be people who do not wish or are not able to participate in a psychological or physical therapy, or may prefer a pharmacological treatment. It was also recognised by the committee that people who have had prior episodes of depression may have preferences for their treatment based on prior experience or insight into their own depression patterns. On this basis, antidepressants (specifically SSRIs as these are generally better tolerated and safer than TCAs) were included as a treatment option for people with less severe depression. However, based on the evidence that some psychological interventions may be more effective, and considering safety and tolerability, the committee agreed that SSRIs should only be considered for use after taking into account other recommended treatment options. Although the committee did not want to prohibit the use of antidepressants where these were the patient’s preference, given the potential for side effects and/or withdrawal effects and the availability of effective psychological and physical treatments, the committee made a strong recommendation that medication not be the default treatment for people with less severe depression, unless it was the person’s preference to take antidepressants rather than engage in a psychological or physical intervention.
The committee discussed the 3 treatments that were less cost-effective than other treatment options and did not appear to be cost-effective compared with GP care. They agreed not to recommend individual exercise programs as group exercise had been recommended as a cost-effective option, but agreed that there may be some sub-groups of people in whom supportive empathetic counselling may help, particularly those with psychosocial, relationship or employment problems contributing to their depression, and that in these groups counselling may be more cost-effective than in the wider population of people with depression. Similarly, they agreed that short-term PDPT may be useful (and therefore may be more cost-effective) where developmental difficulties in relationships contributed to depression.
The committee discussed the fact that there had been some evidence of effectiveness for group problem-solving but noted that, due to limited data available and the rules for inclusion in the economic model, this had not been included in the health economic model and so they were not able to determine if this was a cost-effective option. Due to this lack of cost-effectiveness data the committee agreed not to recommend group problem-solving as an intervention. Also, they decided not to recommend individual problem solving as a separate intervention although it was more cost-effective than GP care, because it may form part of guided self-help or individual CBT.
The committee were concerned that psychological interventions are not always implemented consistently – for example audits have suggested that reduced numbers of sessions are used in practice compared with what is recommended, and that commissioners may not be clear how many sessions of a particular therapy are required. It was also important for people with depression to be aware of what was involved in the different types of therapy before making a decision. The committee therefore agreed it was important to specify the focus and structure of the psychological interventions being recommended to ensure consistency, and to highlight any particular advantages or drawbacks so that people could make an informed choice. The recommended structure of all psychological interventions (usual number of sessions, as well as optimal number of therapists and participants for group interventions) was based on the resource use utilised in the economic analysis, which, in turn, was informed by RCT resource use, modified by the committee’s expert advice to represent optimal routine clinical practice in the UK. In this way, the recommended structure of psychological interventions represents cost-effective use of available healthcare resources as implemented in routine clinical practice. The committee were aware that the suggested number of sessions for some high intensity psychological interventions (such as individual BA and individual CBT), which was based on available RCT evindence, was at the lower end of the number of sessions usually delivered in IAPT services, but expressed the opinion that these are high intensity interventions and the suggested number of sessions should be usually adequate to improve outcome in people with less severe depression. Nevertheless, the committee agreed that the recommended structure of all psychological interventions should allow flexibility so that more sessions may be provided according to individual needs. The committee made no recommendation on the duration of sessions of psychological interventions, to allow flexibility in their delivery.
The committee agreed that high intensity group interventions should be optimally delivered by 2 therapists, at least one of whom has therapy-specific training and competence, and actively facilitates and leads the delivery of the intervention, while the other therapist makes observations. However, it was noted that there is evidence that MBCT can be successfully delivered by 1 therapist in RCTs. They also agreed that optimal delivery of group interventions should involve small numbers of participants (usually 8), as reflected in respective RCTs; however, they noted that in some MBCT trials the intervention was delivered to larger numbers of participants (up to 15) per group so the respective recommendation suggested a wider range in the number of participants per group. Nevertheless, the suggested ‘usual’ numbers of participants should only serve as a guide and allow flexibility around the number of participants per group
Other factors the committee took into account
In addition to the results of the network meta-analysis (NMA) the committee took other pragmatic factors into consideration when making recommendations, including the uncertainty and limitations around the clinical and cost-effectiveness data, implementation factors, and the need to provide a wide range of interventions to take into account individual needs and allow patient choice. The recommended first-line treatments for less severe depression were included in a table in the guideline in order to support shared decision-making. The treatment options are arranged in the suggested order in which they should be considered. However, the guideline recommends that all treatments in the table can be used as first-line treatments, and that the least intrusive and least resource intensive treatment should be considered first (guided self-help) unless it is not appropriate based on the person’s clinical needs and preferences.
The committee discussed that the division of the population for this guideline into ‘less severe’ and ‘more severe’ using published cross-walk tables with an anchor score of 16 on the PHQ-9 scale, meant that the less severe population was people with subthreshold symptoms or mild depression only. However, in reality, people with depression are on a continuum, and their feelings and symptoms may vary from day to day, depending on many other factors including what else is happening in their life. Therefore, although the clinical results provided guidance on treatments for depression, the committee agreed that a holistic approach was required with consideration of social causes and available social interventions as well. The committee noted that this was already covered in the guideline in the recommendations on initial assessment of depression, and therefore they did not make any additional recommendations on this in the treatment section of the guideline.
The committee noted that their recommendations for exercise interventions would need to be modified if necessary to ensure that people with disabilities were still able to access this as a treatment option, and they highlighted this in their recommendations.
Recommendations supported by this evidence review
This evidence review supports recommendations 1.5.2 and 1.5.3 and research recommendations in the NICE guideline.
References
Abdollahi 2017
Abdollahi A., LeBouthillier DM, Najafi M, Asmundson GJ, Hosseinian S, et al. (2017) Effect of exercise augmentation of cognitive behavioural therapy for the treatment of suicidal ideation and depression. Journal of Affective Disorders 219: 58–63. [PubMed: 28525821]Addington 2019_phase 1
Addington EL, Cheung EO, Bassett SM, Kwok I, Schuette SA, et al. (2019) The MARIGOLD study: feasibility and enhancement of an online intervention to improve emotion regulation in people with elevated depressive symptoms. Journal of Affective Disorders 257: 352–364. [PMC free article: PMC6711819] [PubMed: 31302525]Addington 2019_phase 2
Addington EL, Cheung EO, Bassett SM, Kwok I, Schuette SA, et al. (2019) The MARIGOLD study: feasibility and enhancement of an online intervention to improve emotion regulation in people with elevated depressive symptoms. Journal of Affective Disorders 257: 352–364. [PMC free article: PMC6711819] [PubMed: 31302525]Ajilchi 2016
Ajilchi B, Nejati V, Town JM, Wilson R, Abbass A (2016) Effects of intensive short-term dynamic psychotherapy on depressive symptoms and executive functioning in major depression. Journal of Nervous and Mental Disease 204:500–505. [PubMed: 27065106]Allart-van Dam 2003/2007
Allart-Van Dam E, Hosman CMH, Hoogduin CAL, Schaap CPDR (2003) The coping with depression course: short-term outcomes and mediating effects of a randomized controlled trial in the treatment of subclinical depression. Behavior Therapy 34: 381–396.
Allart-Van Dam E, Hosman CM, Hoogduin CA, Schaap CP (2007) Prevention of depression in subclinically depressed adults: follow-up effects on the ‘coping with depression’ course. Journal of Affective Disorders 97: 219–228. [PubMed: 16860874]Alsaraireh 2017
Alsaraireh FA, Aloush SM (2017) Mindfulness meditation versus physical exercise in the management of depression among nursing students. Journal of Nursing Education 56: 599–604. [PubMed: 28972629]Altamura 2017
Altamura M, Iuso S, Terrone G, Balzotti A, Carnevale R, et al. (2017) Comparing interpersonal counseling and antidepressant treatment in primary care patients with anxious and nonanxious major depression disorder: a randomized control trial. Clinical Neuropsychiatry 14: 257–262.Andersson 2005
Andersson G, Bergstrom J, Hollandare F, Carlbring P, Kaldo V, Ekselius L. (2005) Internet-based self-help for depression: randomised controlled trial. British Journal of Psychiatry 187: 456–461. [PubMed: 16260822]Arvidsdotter 2013
Arvidsdotter T, Marklund B, Taft C (2013) Effects of an integrative treatment, therapeutic acupuncture and conventional treatment in alleviating psychological distress in primary care patients-a pragmatic randomized controlled trial. BMC Complementary and Alternative Medicine 13: 308. [PMC free article: PMC4226264] [PubMed: 24200100]Asl 2014
Asl NH, Barahmand U (2014) Effectiveness of mindfulness-based cognitive therapy for co-morbid depression in drug-dependent males. Archives of Psychiatric Nursing 28: 314–318. [PubMed: 25439972]Baert 2010_study 1
Baert S, De Raedt R, Schacht R, Koster EH (2010) Attentional bias training in depression: therapeutic effects depend on depression severity. Journal of Behavior Therapy and Experimental Psychiatry 41: 265–274. [PubMed: 20227062]Balchin 2016
Balchin R, Linde J, Blackhurst D, Rauch HL, Schonbachler G (2016) Sweating away depression? the impact of intensive exercise on depression. Journal of Affective Disorders 200: 218–221. [PubMed: 27137088]Bedford 2018
Bedford LA, Dietch JR, Taylor DJ, Boals A, Zayfert C (2018) Computer-guided problem-solving treatment for depression, PTSD, and insomnia symptoms in student veterans: a pilot randomized controlled trial. Behavior Therapy 49: 756–767. [PubMed: 30146142]Beeber 2010
Beeber LS, Holditch-Davis D, Perreira K, Schwartz TA, Lewis V, et al. (2010) Short-term in-home intervention reduces depressive symptoms in early head start Latina mothers of infants and toddlers. Research in Nursing & Health 33:60–76. [PMC free article: PMC4096768] [PubMed: 20043296]Bernard 2014
Bernard P, Ninot G, Bernard PL, Picot MC, Jaussent A, et al. (2015) Effects of a six-month walking intervention on depression in inactive post-menopausal women: a randomized controlled trial. Aging & Mental Health 19: 485–492. [PubMed: 25133492]Bernecker 2016
Bernecker SL, Constantino MJ, Atkinson LR, Bagby RM, Ravitz P, et al. (2016) Attachment style as a moderating influence on the efficacy of cognitive-behavioral and interpersonal psychotherapy for depression: a failure to replicate. Psychotherapy 53: 22–33. [PubMed: 26726822]Bohlmeijer 2011
Bohlmeijer ET, Fledderus M, Rokx TAJJ, Pieterse ME (2011) Efficacy of an early intervention based on acceptance and commitment therapy for adults with depressive symptomatology: evaluation in a randomized controlled trial. Behaviour Research and Therapy 49: 62–67. [PubMed: 21074752]Bolier 2013
Bolier L, Haverman M, Kramer J, Westerhof GJ, Riper H, et al. (2013) An internet-based intervention to promote mental fitness for mildly depressed adults: randomized controlled trial. Journal of Medical Internet Research 15:e200. [PMC free article: PMC3929047] [PubMed: 24041479]Brenes 2007
Brenes GA, Williamson JD, Messier SP, Rejeski WJ, Pahor M, et al. (2007) Treatment of minor depression in older adults: a pilot study comparing sertraline and exercise. Aging and Mental Health 11:61–68. [PMC free article: PMC2885010] [PubMed: 17164159]Buntrock 2015
Buntrock C, Ebert D, Lehr D, Riper H, Smit F, et al. (2015) Effectiveness of a web-based cognitive behavioural intervention for subthreshold depression: pragmatic randomised controlled trial. Psychotherapy and Psychosomatics 84: 348–358. [PubMed: 26398885]Carlbring 2013
Carlbring P, Hägglund M, Luthström A, Dahlin M, Kadowaki Å, et al. Internet-based behavioral activation and acceptance-based treatment for depression: a randomized controlled trial. Journal of Affective Disorders 148:331–337. [PubMed: 23357657]Chalder 2012
Chalder M, Wiles NJ, Campbell J, Hollinghurst SP, Searle A, et al. (2012) A pragmatic randomised controlled trial to evaluate the cost-effectiveness of a physical activity intervention as a treatment for depression: the treating depression with physical activity (TREAD) trial. Health Technology Assessment 16(10). [PubMed: 22398106]Christensen 2004a/Mackinnon 2008
Christensen H, Griffiths KM, Jorm AF (2004) Delivering interventions for depression by using the internet: randomised controlled trial. BMJ 328:265. [PMC free article: PMC324455] [PubMed: 14742346]
Mackinnon A, Griffiths KM, Christensen H (2008) Comparative randomised trial of online cognitive-behavioral therapy and an information website for depression: 12-month outcomes. British Journal of Psychiatry 192:130. [PubMed: 18245031]Chu 2017
Chu IH, Wu WL, Lin IM, Chang YK, Lin YJ, et al. (2017) Effects of yoga on heart rate variability and depressive symptoms in women: a randomized controlled trial. Journal of Alternative and Complementary Medicine 23: 310–316. [PubMed: 28051319]Coote 2012
Coote HM, MacLeod AK (2012) A self-help, positive goal-focused intervention to increase well-being in people with depression. Clinical Psychology & Psychotherapy 19: 305–315. [PubMed: 22610936]Cramer 2011
Cramer H, Salisbury C, Conrad J, Eldred J, Araya R (2011) Group cognitive behavioural therapy for women with depression: pilot and feasibility study for a randomised controlled trial using mixed methods. BMC Psychiatry 11: 1–11. [PMC free article: PMC3119185] [PubMed: 21569488]de Azevedo Cardoso 2014
de Azevedo Cardoso T, Mondin TC, Spessato BC, de Avila Quevedo L, de Mattos Souza LD, et al. (2014) The impact of anxious symptoms in the remission of depressive symptoms in a clinical trial for depression: follow-up of six months. Journal of Affective Disorders 168: 331–336. [PubMed: 25089513]de Graaf 2009/2011
De Graaf LE, Gerhards SA, Arntz A, Riper H, Metsemakers JF, et al. (2009) Clinical effectiveness of online computerised cognitive-behavioural therapy without support for depression in primary care: randomised trial. British Journal of Psychiatry 195: 73–80. [PubMed: 19567900]
De Graaf LE, Gerhards SAH, Arntz A, Riper H, Metsemakers JFM, et al. (2011) One-year follow-up results of unsupported online computerized cognitive behavioural therapy for depression in primary care: a randomized trial. Journal of Behavior Therapy and Experimental Psychiatry 42: 89–95. [PubMed: 20723885]Dear 2018
Dear BF, Fogliati VJ, Fogliati R, Johnson B, Boyle O, et al. (2018) Treating anxiety and depression in young adults: a randomised controlled trial comparing clinician-guided versus self-guided internet-delivered cognitive behavioural therapy. Australian and New Zealand Journal of Psychiatry 52: 668–679. [PubMed: 29064283]DeBerry 1989
DeBerry S, Davis S, Reinhard KE (1989) A comparison of meditation-relaxation and cognitive/behavioral techniques for reducing anxiety and depression in a geriatric population. Journal of Geriatric Psychiatry 22: 231–247. [PubMed: 2701175]Doyne 1987
Doyne EJ, Ossip-Klein DJ, Bowman ED, Osborn KM, McDougall-Wilson IB, et al. (1987) Running versus weight lifting in the treatment of depression. Journal of Consulting and Clinical Psychology 55:748–754. [PubMed: 3454786]Dozeman 2011
Dozeman E, van Schaik DJ, van Marwijk HW, Stek ML, Beekman AT, et al. (2011) Feasibility and effectiveness of activity-scheduling as a guided self-help intervention for the prevention of depression and anxiety in residents in homes for the elderly: a pragmatic randomized controlled trial. International Psychogeriatrics 23: 969–978. [PubMed: 21338550]Ebert 2014
Ebert DD, Lehr D, Boß L, Riper H, Cuijpers P, et al. (2014) Efficacy of an internet-based problem-solving training for teachers: results of a randomized controlled trial. Scandinavian Journal of Work, Environment & Health Nov 1:582–596. [PubMed: 25121986]Ebert 2018
Ebert DD, Buntrock C, Lehr D, Smit F, Riper H, et al. (2018) Effectiveness of web- and mobile-based treatment of subthreshold depression With adherence-focused guidance: a single-blind randomized controlled trial. Behavior Therapy 49: 71–83. [PubMed: 29405923]Fitzpatrick 2017
Fitzpatrick KK, Darcy A, Vierhile M (2017) Delivering cognitive behavior therapy to young adults with symptoms of depression and anxiety using a fully automated conversational agent (woebot): a randomized controlled trial. JMIR Mental Health 4: e19. [PMC free article: PMC5478797] [PubMed: 28588005]Fledderus 2012
Fledderus M, Bohlmeijer ET, Pieterse ME, Schreurs KM (2012) Acceptance and commitment therapy as guided self-help for psychological distress and positive mental health: a randomized controlled trial. Psychological Medicine 42: 485. [PubMed: 21740624]Fletcher 2005
Fletcher J, Lovell K, Bower P, Campbell M (2005) Process and outcome of a non-guided self-help manual for anxiety and depression in primary care: a pilot study. Behavioural & Cognitive Psychotherapy 33: 319–331.Forman 2007
Forman EM, Herbert JD, Moitra E, Yeomans PD, Geller PA (2007) A randomized controlled effectiveness trial of acceptance and commitment therapy and cognitive therapy for anxiety and depression. Behavior Modification 31: 772–799. [PubMed: 17932235]Fremont 1987
Fremont J, Craighead LW. Aerobic exercise and cognitive therapy in the treatment of dysphoric moods. Cognitive therapy and Research 11:241–251.Friedli 1997
Friedlr K, King MB, Lloyd M, Horder J (1997) Randomised controlled assessment of non-directive psychotherapy versus routine general-practitioner care. The Lancet 350: 1662–1665. [PubMed: 9400510]Gallagher-Thompson 2007
Gallagher-Thompson D, Gray HL, Tang PC, Pu CY, Leung LY, et al. (2007) Impact of in-home behavioral management versus telephone support to reduce depressive symptoms and perceived stress in Chinese caregivers: results of a pilot study. American Journal of Geriatric Psychiatry 15: 425–434. [PubMed: 17463192]Gawrysiak 2009
Gawrysiak M, Nicholas C, Hopko DR (2009) Behavioral activation for moderately depressed university students: randomized controlled trial. Journal of Counseling Psychology 56: 468–475.Geisner 2006
Geisner IM, Neighbors C, Larimer ME (2006) A randomized clinical trial of a brief, mailed intervention for symptoms of depression. Journal of Consulting & Clinical Psychology 74: 393–399. [PubMed: 16649884]Geraedts 2014a/2014b
Geraedts AS, Kleiboer AM, Wiezer NM, Cuijpers P, van Mechelen W, et al. (2014) Feasibility of a worker-directed web-based intervention for employees with depressive symptoms. Internet Interventions 1:132–140.
Geraedts AS, Kleiboer AM, Twisk J, Wiezer NM, van Mechelen W, et al. (2014) Long-term results of a web-based guided self-help intervention for employees with depressive symptoms: randomized controlled trial. Journal of Medical Internet Research 16: 14–28. [PMC free article: PMC4115257] [PubMed: 25008127]Gordon 1987
Gordon VC, Gordon EM (1987) Short-term group treatment of depressed women: a replication study in Great Britain. Archives of Psychiatric Nursing 1: 111–124. [PubMed: 3646036]Guo 2017
Guo YF, Zhang X, Plummer V, Lam L, Cross W, et al. (2017) Positive psychotherapy for depression and self-efficacy in undergraduate nursing students: a randomized, controlled trial. International Journal of Mental Health Nursing 26: 375–383. [PubMed: 27633932]Heckendorf 2019
Heckendorf H, Lehr D, Ebert DD, Freund H (2019) Efficacy of an internet and app-based gratitude intervention in reducing repetitive negative thinking and mechanisms of change in the intervention’s effect on anxiety and depression: results from a randomized controlled trial. Behaviour Research and Therapy 119: 103415. [PubMed: 31202003]Hinton 2010
Hinton MJ, Gaynor ST (2010) Cognitive defusion for psychological distress, dysphoria, and low self-esteem: a randomized technique evaluation trial of vocalizing strategies. International Journal of Behavioral Consultation and Therapy 6: 164.Hollyman 1988
Hollyman JA, Freeling P, Paykel ES, Bhat A, Sedgwick P (1988) Double-blind placebo-controlled trial of amitriptyline among depressed patients in general practice. Journal of the Royal College of General Practitioners 38: 393–397. [PMC free article: PMC1711591] [PubMed: 3076905]Hou 2014
Hou RJ, Wong SYS, Yip BHK, Hung AT, Lo HHM, et al. (2014) The effects of mindfulness-based stress reduction program on the mental health of family caregivers: a randomized controlled trial. Psychotherapy and Psychosomatics 83: 45–53. [PubMed: 24281411]Howells 2016
Howells A, Ivtzan I, Eiroa-Orosa FJ (2016) Putting the ‘app’ in happiness: a randomised controlled trial of a smartphone-based mindfulness intervention to enhance wellbeing. Journal of Happiness Studies 17: 163–185.Hsiao 2011
Hsiao FH, Jow GM, Lai YM, Chen YT, Wang KC, et al. (2011) The long-term effects of psychotherapy added to pharmacotherapy on morning to evening diurnal cortisol patterns in outpatients with major depression. Psychotherapy and Psychosomatics 80: 166–172. [PubMed: 21389753]Hunter 2012
Hunter SB, Watkins KE, Hepner KA, Paddock SM, Ewing BA, et al. (2012) Treating depression and substance use: a randomized controlled trial. Journal of Substance Abuse Treatment 43:137–151. [PMC free article: PMC3345298] [PubMed: 22301087]Hur 2018
Hur JW, Kim B, Park D, Choi SW (2018) A scenario-based cognitive behavioral therapy mobile app to reduce dysfunctional beliefs in individuals with depression: a randomized controlled trial. Telemedicine Journal and E-Health 24: 710–716. [PubMed: 29323626]Ince 2013
Ince BÜ, Cuijpers P, van’t Hof E, van Ballegooijen W, Christensen H, et al. (2013) Internet-based, culturally sensitive, problem-solving therapy for Turkish migrants with depression: randomized controlled trial. Journal of Medical Internet Research 15: 248–262. [PMC free article: PMC3849840] [PubMed: 24121307]Jelinek 2020
Jelinek L, Arlt S, Moritz S, Schröder J, Westermann S, et al. (2020) Brief web-based intervention for depression: randomized controlled trial on behavioral activation. Journal of Medical Internet Research 22: e15312. [PMC free article: PMC7146239] [PubMed: 32213470]Joling 2011
Joling KJ, Van Hout HP, van’t Veer-Tazelaar PJ, van der Horst HE, Cuijpers P, et al. (2011) How effective is bibliotherapy for very old adults with subthreshold depression? a randomized controlled trial. American Journal of Geriatric Psychiatry 19: 256–265. [PubMed: 20808151]Jones 2011
Jones LV, Warner LA (2011) Evaluating culturally responsive group work with black women. Research on Social Work Practice 21: 737–746.Judd 2004
Judd LL, Rapaport MH, Yonkers KA, Rush AJ, Frank E, et al. (2004). Randomized, placebo-controlled trial of fluoxetine for acute treatment of minor depressive disorder. American Journal of Psychiatry 161: 1864–1871. [PubMed: 15465984]Kahn 2016
Kahn JR, Collinge W, Soltysik R (2016) Post-9/11 veterans and their partners improve mental health outcomes with a self-directed mobile and Web-based wellness training program: a randomized controlled trial. Journal of Medical Internet Research 18:18–40. [PMC free article: PMC5059485] [PubMed: 27678169]Kaltenthaler 2006
Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, et al. (2006) Computerized cognitive behavior therapy for depression and anxiety update: A systematic review and economic evaluation. Health Technology Assessment 10(33). [PubMed: 16959169]Kasckow 2014
Kasckow J, Klaus J, Morse J, Oslin D, Luther J, et al. (2014) Using problem solving therapy to treat veterans with subsyndromal depression: a pilot study. International Journal of Geriatric Psychiatry 29: 1255–1261. [PMC free article: PMC4216632] [PubMed: 24789736]Kendrick 2005/2006a
Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, et al. (2005) A trial of problem-solving by community mental health nurses for anxiety, depression and life difficulties among general practice patients. The CPN-GP study. Health Technology Assessment 9: iii–59. [PubMed: 16153354]
Kendrick T, Simons L, Mynors-Wallis L, Gray A, Lathlean J, et al. (2006a) Cost-effectiveness of referral for generic care or problem-solving treatment from community mental health nurses, compared with usual general practitioner care for common mental disorders: randomised controlled trial. British Journal of Psychiatry 189: 50–59. [PubMed: 16816306]Kendrick 2009
Kendrick T, Chatwin J, Dowrick C, Tylee A, Morriss R, et al. (2009) Randomised controlled trial to determine the clinical effectiveness and cost-effectiveness of selective serotonin reuptake inhibitors plus supportive care, versus supportive care alone, for mild to moderate depression with somatic symptoms in primary care: the THREAD (THREshold for AntiDepressant response) study. Health Technology Assessment 13(22). [PubMed: 19401066]Khakpoor 2019
Khakpoor S, Saed O, Armani Kian A (2019) Emotion regulation as the mediator of reductions in anxiety and depression in the unified protocol (UP) for transdiagnostic treatment of emotional disorders: double-blind randomized clinical trial. Trends in Psychiatry and Psychotherapy 41: 227–236. [PubMed: 31644691]Khan 2019
Khan MN, Hamdani SU, Chiumento A, Dawson K, Bryant RA, et al. (2019) Evaluating feasibility and acceptability of a group WHO trans-diagnostic intervention for women with common mental disorders in rural Pakistan: a cluster randomised controlled feasibility trial. Epidemiology and Psychiatric Sciences 28: 77–87. [PMC free article: PMC6998939] [PubMed: 28689511]Kitsumban 2009
Kitsumban V, Thapinta D, Sirindharo PB, Anders RL (2009) Effect of cognitive mindfulness practice program on depression among elderly Thai women. Thai Journal of Nursing Research 13: 95–108.Kranzler 2006_Group B
Kranzler HR, Mueller T, Cornelius J, Pettinati HM, Moak D, et al. (2006) Sertraline treatment of co-occurring alcohol dependence and major depression. Journal of Clinical Psychopharmacology 26: 13–20. [PubMed: 16415699]Kvillemo 2016
Kvillemo P, Brandberg Y, Bränström R (2016) Feasibility and outcomes of an internet-based mindfulness training program: a pilot randomized controlled trial. JMIR Mental Health 3: e33. [PMC free article: PMC4975795] [PubMed: 27450466]Kyllonen 2018
Kyllonen HM, Muotka J, Puolakanaho A, Astikainen P, Keinonen K, et al. (2018) A brief acceptance and commitment therapy intervention for depression: a randomized controlled trial with 3-year follow-up for the intervention group. Journal of Contextual Behavioral Science 10: 55–63.Laidlaw 2008
Laidlaw K, Davidson K, Toner H, Jackson G, Clark S, et al. (2008) A randomised controlled trial of cognitive behaviour therapy vs treatment as usual in the treatment of mild to moderate late life depression. International Journal of Geriatric Psychiatry 23: 843–850. [PubMed: 18311844]Lambert 2018
Lambert JD, Greaves CJ, Farrand P, Price L, Haase AM, et al. (2018) Web-based intervention using behavioral activation and physical activity for adults with depression (the eMotion study): pilot randomized controlled trial. Journal of Medical Internet Research 20: e10112. [PMC free article: PMC6066639] [PubMed: 30012547]Lamers 2015
Lamers SM, Bohlmeijer ET, Korte J, Westerhof GJ (2015) The efficacy of life-review as online-guided self-help for adults: a randomized trial. Journals of Gerontology Series B: Psychological Sciences and Social Sciences 70: 24–34. [PubMed: 24691155]Lee 2010
Lee WK, Bang HJ (2010) The effects of mindfulness-based group intervention on the mental health of middle-aged Korean women in community. Stress and Health 26: 341–348.Lee 2018
Lee RA, Jung ME (2018) Evaluation of an mhealth app (destressify) on university students’ mental health: pilot trial. JMIR Mental Health 5: e2. [PMC free article: PMC5801522] [PubMed: 29362209]Legrand 2014
Legrand FD (2014) Effects of exercise on physical self-concept, global self-esteem, and depression in women of low socioeconomic status with elevated depressive symptoms. Journal of Sport and Exercise Psychology 36: 357–365. [PubMed: 25226604]Levesque 2011
Levesque DA, Van Marter DF, Schneider RJ, Bauer MR, Goldberg DN, et al. (2011) Randomized trial of a computer-tailored intervention for patients with depression. American Journal of Health Promotion 26: 77–89. [PubMed: 22040388]Levin 2011
Levin W, Campbell DR, McGovern KB, Gau JM, Kosty DB, et al. (2011) A computer-assisted depression intervention in primary care. Psychological Medicine 41: 1373–1383. [PubMed: 20961474]Lewis 2019 / Hollingworth 2020
Lewis G, Duffy L, Ades A, Amos R, Araya R, et al. (2019) The clinical effectiveness of sertraline in primary care and the role of depression severity and duration (PANDA): a pragmatic, double-blind, placebo-controlled randomised trial. Lancet Psychiatry 6: 903–914. [PMC free article: PMC7029306] [PubMed: 31543474]
Hollingworth W, Fawsitt CG, Dixon P, Duffy L, Araya R, et al. (2020) Cost-effectiveness of sertraline in primary care according to initial severity and duration of depressive symptoms: findings from the PANDA RCT. PharmacoEconomics Open 4: 427–438. [PMC free article: PMC7426336] [PubMed: 31777008]
Duffy L, Lewis G, Ades A, Araya R, Bone J, et al. (2019) Antidepressant treatment with sertraline for adults with depressive symptoms in primary care: the PANDA research programme including RCT. Health Technology Assessment 7(10). [PubMed: 31869013]Lintvedt 2013
Lintvedt OK, Griffiths KM, Sorensen K, Ostvik AR, Wang CE, et al. (2013) Evaluating the effectiveness and efficacy of unguided internet-based self-help intervention for the prevention of depression: a randomized controlled trial. Clinical Psychology & Psychotherapy 20: 10–27. [PubMed: 21887811]Liu 2009a
Liu ET, Chen WL, Li YH, Wang CH, Mok TJ, et al. (2009) Exploring the efficacy of cognitive bibliotherapy and a potential mechanism of change in the treatment of depressive symptoms among the Chinese: a randomized controlled trial. Cognitive Therapy and Research 33: 449–461.Lobner 2018
Lobner M, Pabst A, Stein J, Dorow M, Matschinger H, et al. (2018) Computerized cognitive behavior therapy for patients with mild to moderately severe depression in primary care: a pragmatic cluster randomized controlled trial (@ktiv). Journal of Affective Disorders 238: 317–326. [PubMed: 29902736]Lopez-Maya 2019
Lopez-Maya E, Olmstead R, Irwin MR (2019) Mindfulness meditation and improvement in depressive symptoms among Spanish-and English speaking adults: a randomized, controlled, comparative efficacy trial. PloS One 14: e0219425. [PMC free article: PMC6611613] [PubMed: 31276540]Lopez-Rodriguez 2017
López-Rodríguez MM, Baldrich-Rodríguez I, Ruiz-Muelle A, Cortés-Rodríguez AE, Lopezosa-Estepa T, et al. (2017) Effects of biodanza on stress, depression, and sleep quality in university students. Journal of Alternative and Complementary Medicine 23: 558–565. [PubMed: 28590767]Losada 2015
Losada A, Marquez-Gonzalez M, Romero-Moreno R, Mausbach BT, Lopez J, et al. (2015) Cognitive-behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for dementia family caregivers with significant depressive symptoms: results of a randomized clinical trial. Journal of Consulting and Clinical Psychology 83:760–772. [PubMed: 26075381]Lovell 2008
Lovell K, Bower P, Richards D, Barkham M, Sibbald B, et al. (2008) Developing guided self-help for depression using the Medical Research Council complex interventions framework: a description of the modelling phase and results of an exploratory randomised controlled trial. BMC Psychiatry 8: 91–110. [PMC free article: PMC2596776] [PubMed: 19025646]Luxton 2016
Luxton DD, Pruitt LD, Wagner A, Smolenski DJ, Jenkins-Guarnieri MA, et al. (2016) Home-based telebehavioral health for US military personnel and veterans with depression: a randomized controlled trial. Journal of Consulting and Clinical Psychology 84: 923. [PubMed: 27599225]Lynch 1997
Lynch DJ, Tamburrino M, Nagel R (1997) Telephone counseling for patients with minor depression: preliminary findings in a family practice setting. Journal of Family Practice 44: 293–299. [PubMed: 9071250]Malouff 1988
Malouff JM, Lanyon RI, Schutte NS (1988) Effectiveness of a brief group RET treatment for divorce-related dysphoria. Journal of Rational-Emotive & Cognitive-Behavior Therapy 6: 162–171.Mastikhina 2017
Mastikhina L, Dobson K (2017) Biased attention retraining in dysphoria: a failure to replicate. Cognition and Emotion 31: 625–631. [PubMed: 26799308]McClay 2015
McClay CA, Collins K, Matthews L, Haig C, McConnachie A, et al. (2015) A community-based pilot randomised controlled study of life skills classes for individuals with low mood and depression. BMC Psychiatry 15: 1–9. [PMC free article: PMC4331464] [PubMed: 25884922]McDermott 2019
McDermott R, Dozois DJA (2019) A randomized controlled trial of Internet-delivered CBT and attention bias modification for early intervention of depression. Journal of Experimental Psychopathology 10: 2043808719842502.McGrath 1996
McGrath PJ, Nunes EV, Stewart JW, Goldman D, Agosti V, et al. (1996) Imipramine treatment of alcoholics with primary depression: a placebo- controlled clinical trial. Archives of General Psychiatry 53: 232–240. [PubMed: 8611060]McIndoo 2016
McIndoo CC, File AA, Preddy T, Clark CG, Hopko DR (2016) Mindfulness-based therapy and behavioral activation: a randomized controlled trial with depressed college students. Behaviour Research and Therapy 77: 118–128. [PubMed: 26745622]McMakin 2011
McMakin DL, Siegle GJ, Shirk SR (2011) Positive affect stimulation and sustainment (PASS) module for depressed mood: a preliminary investigation of treatment-related effects. Cognitive Therapy and Research 35: 217–226. [PMC free article: PMC3226735] [PubMed: 22140287]McNeil 1991
McNeil JK, LeBlanc EM, Joyner M (1991) The effect of exercise on depressive symptoms in the moderately depressed elderly. Psychology and Aging 6: 487. [PubMed: 1930766]Melnyk 2015
Melnyk BM, Amaya M, Szalacha LA, Hoying J, Taylor T, et al. (2015) Feasibility, acceptability, and preliminary effects of the COPE online cognitive-behavioral skill-building program on mental health outcomes and academic performance in freshmen college students: a randomized controlled pilot study. Journal of Child and Adolescent Psychiatric Nursing 28: 147–154. [PubMed: 26268362]Mogoaşe 2013
Mogoaşe C, Brăilean A, David D (2013) Can concreteness training alone reduce depressive symptoms? a randomized pilot study using an internet-delivered protocol. Cognitive Therapy and Research 37: 704–712.Mondin 2014/2015
Mondin TC, de Azevedo Cardoso T, Jansen K, Spessato BC, de Mattos Souza LD, et al. (2014) Effects of cognitive psychotherapy on the biological rhythm of patients with depression. Journal of Affective Disorders 155: 142–148. [PubMed: 24268614]
Mondin TC, de Azevedo Cardoso T, Jansen K, da Silva GDG, de Mattos Souza LD, et al. (2015) Long-term effects of cognitive therapy on biological rhythms and depressive symptoms: a randomized clinical trial. Journal of Affective Disorders 187: 1–9. [PubMed: 26300329]Moss 2012
Moss K, Scogin F, Di Napoli E, Presnell A (2012) A self-help behavioral activation treatment for geriatric depressive symptoms. Aging & Mental Health 16: 625–635. [PubMed: 22304676]Murphy 1995
Murphy GE, Carney RM, Knesevitc MA, Wetzel RD, Whitworth P (1995) Cognitive behavior therapy, relaxation training, and tricyclic antidepressant medication in the treatment of depression. Psychological Report 77: 403–420. [PubMed: 8559866]Noguchi 2017
Noguchi R, Sekizawa Y, So M, Yamaguchi S, Shimizu E (2017) Effects of five-minute internet-based cognitive behavioral therapy and simplified emotion-focused mindfulness on depressive symptoms: a randomized controlled trial. BMC Psychiatry 17: 1–4. [PMC free article: PMC5336676] [PubMed: 28259151]Nystrom 2017
Nystrom MBT, Stenling A, Sjostrom E, Neely G, Lindner P, et al. (2017) Behavioral activation versus physical activity via the internet: a randomized controlled trial. Journal of Affective Disorders 215: 85–93. [PubMed: 28319696]Pace 1993
Pace TM, Dixon DN (1993) Changes in depressive self-schemata and depressive symptoms following cognitive therapy. Journal of Counseling Psychology 40:288.Pagni 2020
Pagni BA, Walsh MJ, Foldes E, Sebren A, Dixon MV, et al. (2020) The neural correlates of mindfulness-induced depression reduction in adults with autism spectrum disorder: a pilot study. Journal of Neuroscience Research 98: 1150–1161. [PubMed: 32090389]Peveler 2005/Kendrick 2006b
Peveler R, Kendrick T, Buxton M, Longworth L, Baldwin D, et al. (2005) A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine. Health Technology Assessment 9(16) [PubMed: 15876362]
Kendrick T, Peveler R, Longworth L, Baldwin D, Moore M, et al. (2006) Cost-effectiveness and cost-utility of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine: randomised controlled trial. British Journal of Psychiatry 188: 337–345. [PubMed: 16582060]Pictet 2016
Pictet A, Jermann F, Ceschi G (2016) When less could be more: investigating the effects of a brief internet-based imagery cognitive bias modification intervention in depression. Behaviour Research & Therapy 84: 45–51. [PubMed: 27485252]Pots 2014
Pots WT, Meulenbeek PA, Veehof MM, Klungers J, Bohlmeijer ET (2014) The efficacy of mindfulness-based cognitive therapy as a public mental health intervention for adults with mild to moderate depressive symptomatology: a randomized controlled trial. PloS One 9: e109789. [PMC free article: PMC4198116] [PubMed: 25333885]Pots 2016a
Pots WTM, Fledderus M, Meulenbeek PAM, Ten Klooster PM, Schreurs KMG, et al. (2016) Acceptance and commitment therapy as a web-based intervention for depressive symptoms: randomised controlled trial. British Journal of Psychiatry 208: 69–77. [PubMed: 26250745]Powell 2013
Powell J, Hamborg T, Stallard N, Burls A, McSorley J, et al. (2013) Effectiveness of a web-based cognitive-behavioral tool to improve mental well-being in the general population: randomized controlled trial. Journal of Medical Internet Research 15: e2. [PMC free article: PMC3636304] [PubMed: 23302475]Prathikanti 2017
Prathikanti S, Rivera R, Cochran A, Tungol JG, Fayazmanesh N, et al. (2017) Treating major depression with yoga: a prospective, randomized, controlled pilot trial. Plos One 12: e0173869. [PMC free article: PMC5354384] [PubMed: 28301561]Proyer 2016
Proyer RT, Gander F, Wellenzohn S, Ruch W (2016) Nine beautiful things: a self-administered online positive psychology intervention on the beauty in nature, arts, and behaviors increases happiness and ameliorates depressive symptoms. Personality and Individual Differences 94: 189–193.Quitkin 1990
Quitkin FM, McGrath PJ, Stewart JW, Harrison W, Tricamo E, et al. (1990) Atypical depression, panic attacks, and response to imipramine and phenelzine. A replication. Archives of General Psychiatry 47: 935–941. [PubMed: 2222132]Rapaport 2011
Rapaport MH, Nierenberg AA, Howland R, Dording C, Schettler PJ, et al. (2011) The treatment of minor depression with St. John’s wort or citalopram: failure to show benefit over placebo. Journal of Psychiatric Research 45: 931–941. [PMC free article: PMC3137264] [PubMed: 21632064]Rosen 2018
Rosen D, Engel R, McCall J, Greenhouse J (2018) Using problem-solving therapy to reduce depressive symptom severity among older adult methadone clients: a randomized clinical trial. Research on Social Work Practice 28: 802–809.Rosso 2013
Rosso G, Martini B, Maina G (2013) Brief dynamic therapy and depression severity: a single-blind, randomized study. Journal of Affective Disorders 147: 101–106. [PubMed: 23174499]Rosso 2017
Rosso IM, Killgore WDS, Olson EA, Webb CA, Fukunaga R, et al. (2017) Internet-based cognitive behavior therapy for major depressive disorder: a randomized controlled trial. Depression and Anxiety 34: 236–245. [PMC free article: PMC5540163] [PubMed: 28009467]Ruwaard 2009
Ruwaard J, Schrieken B, Schrijver M, Broeksteeg J, Dekker J, et al. (2009) Standardized web-based cognitive behavioural therapy of mild to moderate depression: a randomized controlled trial with a long-term follow-up. Cognitive Behaviour Therapy 38:206–221. [PubMed: 19221919]Sandoval 2017
Sandoval LR, Buckey JC, Ainslie R, Tombari M, Stone W, et al. (2017) Randomized controlled trial of a computerized interactive media-based problem solving treatment for depression. Behavior Therapy 48: 413–425. [PMC free article: PMC5889085] [PubMed: 28390503]Schuster 2017
Schuster R, Leitner I, Carlbring P, Laireiter AR (2017) Exploring blended group interventions for depression: randomised controlled feasibility study of a blended computer- and multimedia-supported psychoeducational group intervention for adults with depressive symptoms. Internet Interventions 8: 63–71. [PMC free article: PMC6096250] [PubMed: 30135830]Seligman 2006_study 1
Seligman ME, Rashid T, Parks AC (2006) Positive psychotherapy. American Psychology 61: 774–788. [PubMed: 17115810]Serfaty 2009
Serfaty MA, Haworth D, Blanchard M, Buszewicz M, Murad S, et al. (2009) Clinical effectiveness of individual cognitive behavioral therapy for depressed older people in primary care: a randomized controlled trial. Archives of General Psychiatry 66: 1332–1340. [PubMed: 19996038]Sheeber 2012
Sheeber LB, Seeley JR, Feil EG, Davis B, Sorensen E, et al. (2012) Development and pilot evaluation of an Internet-facilitated cognitive-behavioral intervention for maternal depression. Journal of Consulting and Clinical Psychology 80: 739. [PMC free article: PMC3435487] [PubMed: 22663903]Sheeber 2017
Sheeber LB, Feil EG, Seeley JR, Leve C, Gau JM, et al. (2017) Mom-net: evaluation of an internet-facilitated cognitive behavioral intervention for low-income depressed mothers. Journal of Consulting and Clinical Psychology 85: 355–366. [PMC free article: PMC5364807] [PubMed: 28333536]Sims 2006
Sims J, Hill K, Davidson S, Gunn J, Huang N (2006) Exploring the feasibility of a community-based strength training program for older people with depressive symptoms and its impact on depressive symptoms. BMC Geriatrics 6: 1. [PMC free article: PMC1698486] [PubMed: 17134517]Singh 1997a/1997b
Singh NA, Clements KM, Fiatarone MA (1997) A randomized controlled trial of progressive resistance training in depressed elders. Journals of Gerontology Series A: Biological Sciences and Medical Sciences 52: M27–35. [PubMed: 9008666]
Singh NA, Clements KM, Fiatarone MA (1997) A randomized controlled trial of the effect of exercise on sleep. Sleep 20: 95–101. [PubMed: 9143068]Smith 2018
Smith HL, Dillon KH, Cougle JR (2018) Modification of hostile interpretation bias in depression: a randomized controlled trial. Behavior Therapy 49: 198–211. [PubMed: 29530259]Spek 2007/2008a
Spek V, Nyklicek I, Smits N, Cuijpers P, Riper H, et al. (2007) Internet-based cognitive behavioural therapy for subthreshold depression in people over 50 years old: a randomized controlled clinical trial. Psychological Medicine 37: 1797–1806. [PubMed: 17466110]
Spek V, Cuijpers P, Nyklíček I, Smits N, Riper H, et al. (2008) One-year follow-up results of a randomized controlled clinical trial on internet-based cognitive behavioural therapy for subthreshold depression in people over 50 years. Psychological Medicine 38: 635–639. [PubMed: 18205965]Spek 2008b
Spek V, Nyklíček I, Cuijpers P, Pop V (2008) Predictors of outcome of group and internet-based cognitive behavior therapy. Journal of Affective Disorders 105: 137–145. [PubMed: 17543392]Sreevani 2013
Sreevani R, Reddemma K, Chan CL, Leung PPY, Wong V, et al. (2013). Effectiveness of integrated body–mind–spirit group intervention on the well-being of Indian patients with depression: A pilot study. Journal of Nursing Research, 21(3), 179–186. [PubMed: 23958607]Stiles-Shields 2019
Stiles-Shields C, Montague E, Kwasny MJ, Mohr DC (2019) Behavioral and cognitive intervention strategies delivered via coached apps for depression: pilot trial. Psychological Services 16: 233–238. [PMC free article: PMC6499699] [PubMed: 30407055]Streeter 2017/Scott 2019
Streeter CC, Gerbarg PL, Whitfield TH, Owen L, Johnston J, et al. (2017) Treatment of major depressive disorder with Iyengar yoga and coherent breathing: a randomized controlled dosing study. Journal of Alternative and Complementary Medicine 23: 201–207. [PMC free article: PMC5359682] [PubMed: 28296480]
Scott TM, Gerbarg PL, Silveri MM, Nielsen GH, Owen L, et al. (2019) Psychological function, Iyengar yoga, and coherent breathing: a randomized controlled dosing study. Journal of Psychiatric Practice 25: 437–450. [PubMed: 31821220]Strom 2013
Ström M, Uckelstam CJ, Andersson G, Hassmén P, Umefjord G, et al. (2013) Internet-delivered therapist-guided physical activity for mild to moderate depression: a randomized controlled trial. PeerJ 1: e178. [PMC free article: PMC3792189] [PubMed: 24109561]Taghvaienia 2020
Taghvaienia A, Alamdari N (2020) Effect of positive psychotherapy on psychological well-being, happiness, life expectancy and depression among retired teachers with depression: a randomized controlled trial. Community Mental Health Journal 56: 229–237. [PubMed: 31552541]Tan 1994
Tan RS, Barlow RJ, Abel C, Reddy S, Palmer AJ, et al. (1994) The effect of low dose lofepramine in depressed elderly patients in general medical wards. British Journal of Clinical Pharmacology 37: 321–324. [PMC free article: PMC1364731] [PubMed: 8018452]Taylor 2017
Taylor CT, Lyubomirsky S, Stein MB (2017) Upregulating the positive affect system in anxiety and depression: outcomes of a positive activity intervention. Depression & Anxiety 34: 267–280. [PMC free article: PMC7266488] [PubMed: 28060463]Tighe 2017
Tighe J, Shand F, Ridani R, Mackinnon A, De La Mata N, et al. (2017) Ibobbly mobile health intervention for suicide prevention in Australian Indigenous youth: a pilot randomised controlled trial. BMJ Open 7: e013518. [PMC free article: PMC5278278] [PubMed: 28132007]Tol 2020
Tol WA, Leku MR, Lakin DP, Carswell K, Augustinavicius J, et al. (2020) Guided self-help to reduce psychological distress in South Sudanese female refugees in Uganda: a cluster randomised trial. The Lancet Global Health 8: e254–e263. [PMC free article: PMC9899129] [PubMed: 31981556]Van Schaik 2006
Van Schaik A, Van Marwijk H, Adèr H, Van Dyck R, De Haan M, et al. (2006) Interpersonal psychotherapy for elderly patients in primary care. American Journal of Geriatric Psychiatry 14: 777–786. [PubMed: 16943174]Van Straten 2008
Van Straten A, Cuijpers P, Smits N (2008) Effectiveness of a web-based self-help intervention for symptoms of depression, anxiety, and stress: randomized controlled trial. Journal of Medical Internet Research 10: 1. [PMC free article: PMC2483843] [PubMed: 18364344]Vazquez 2012
Vázquez FL, Torres A, Blanco V, Díaz O, Otero P, et al. (2012) Comparison of relaxation training with a cognitive-behavioural intervention for indicated prevention of depression in university students: a randomized controlled trial. Journal of Psychiatric Research 46: 1456–1463. [PubMed: 22939979]Vazquez 2013/Otero 2015/Lopez 2020
Vázquez FL, Otero PO, García EH, Seoane VB, Fernández OD (2013) A brief problem-solving indicated-prevention intervention for prevention of depression in nonprofessional caregivers. Psicothema 25: 87–92. [PubMed: 23336549]
Otero P, Smit F, Cuijpers P, Torres A, Blanco V, et al. (2015). Long-term efficacy of indicated prevention of depression in non-professional caregivers: randomized controlled trial. Psychological Medicine 45: 1401. [PubMed: 25331992]
López L, Smit F, Cuijpers P, Otero P, Blanco V, et al. (2020) Problem-solving intervention to prevent depression in non-professional caregivers: a randomized controlled trial with 8 years of follow-up. Psychological Medicine 50: 1002–1009. [PubMed: 31017076]Vazquez 2016
Vazquez FL, Torres A, Blanco V, Otero P, Diaz O, et al. (2016) Long-term follow-up of a randomized clinical trial assessing the efficacy of a brief cognitive-behavioral depression prevention intervention for caregivers with elevated depressive symptoms. American Journal of Geriatric Psychiatry 24: 421–432. [PubMed: 27067068]Vazquez 2020
Vázquez FL, López L, Torres ÁJ, Otero P, Blanco V, et al. (2020) Analysis of the components of a cognitive-behavioral intervention for the prevention of depression administered via conference call to nonprofessional caregivers: a randomized controlled trial. International Journal of Environmental Research and Public Health 17: 2067. [PMC free article: PMC7143258] [PubMed: 32244970]Verduyn 2003
Verduyn C, Barrowclough C, Roberts J, Tarrier N, Harrington R (2003) Maternal depression and child behaviour problems. British Journal of Psychiatry 183: 342–348. [PubMed: 14519613]Wagner 2014
Wagner B, Horn AB, Maercker A (2014) Internet-based versus face-to-face cognitive-behavioral intervention for depression: a randomized controlled non-inferiority trial. Journal of Affective Disorders 152: 113–121. [PubMed: 23886401]Warmerdam 2008
Warmerdam L, van Straten A, Twisk J, Riper H, Cuijpers P (2008) Internet-based treatment for adults with depressive symptoms: randomized controlled trial. Journal of Medical Internet Research 10: e44. [PMC free article: PMC2629364] [PubMed: 19033149]Watkins 2009
Watkins ER, Baeyens CB, Read R (2009) Concreteness training reduces dysphoria: proof-of-principle for repeated cognitive bias modification in depression. Journal of Abnormal Psychology 118: 55. [PubMed: 19222314]Williams 2013
Williams C, Wilson P, Morrison J, McMahon A, Andrew W, et al. (2013) Guided self-help cognitive behavioural therapy for depression in primary care: a randomised controlled trial. PloS One 8: e52735. [PMC free article: PMC3543408] [PubMed: 23326352]Williams 2018
Williams C, McClay CA, Matthews L, McConnachie A, Haig C, et al. (2018) Community-based group guided self-help intervention for low mood and stress: randomised controlled trial. British Journal of Psychiatry 212: 88–95. [PMC free article: PMC6716980] [PubMed: 29436324]Woolery 2004
Woolery A, Myers H, Sternlieb B, Zeltzer L (2004) A yoga intervention for young adults with elevated symptoms of depression. Alternative Therapies in Health and Medicine 10: 60–63. [PubMed: 15055096]Yang 2015
Yang W, Ding Z, Dai T, Peng F, Zhang JX (2015) Attention bias modification training in individuals with depressive symptoms: a randomized controlled trial. Journal of Behavior Therapy and Experimental Psychiatry 49: 101–111. [PubMed: 25245928]Yang 2018
Yang X, Zhao J, Chen Y, Zu S, Zhao J (2018) Comprehensive self-control training benefits depressed college students: a six-month randomized controlled intervention trial. Journal of Affective Disorders 226: 251–260. [PubMed: 29017069]Yokoyama 2018
Yokoyama S, Okamoto Y, Takagaki K, Okada G, Takamura M, et al. (2018) Effects of behavioral activation on default mode network connectivity in subthreshold depression: a preliminary resting-state fMRI study. Journal of Affective Disorders 227: 156–163. [PubMed: 29065364]Zemestani 2016
Zemestani M, Davoodi I, Honarmand MM, Zargar Y, Ottaviani C (2016) Comparative effects of group metacognitive therapy versus behavioural activation in moderately depressed students. Journal of Mental Health 25: 479–485. [PubMed: 26439473]Zemestani 2017
Zemestani M, Imani M, Ottaviani C (2017) A preliminary investigation on the effectiveness of unified and transdiagnostic cognitive behavior therapy for patients with comorbid depression and anxiety. International Journal of Cognitive Therapy 10: 175–185.Zhang 2016
Zhang B, Ding X, Lu W, Zhao J, Lv Q, et al. (2016). Effect of group cognitive-behavioral therapy on the quality of life and social functioning of patients with mild depression. Shanghai Jingshen Yixue 28: 18–27. [PMC free article: PMC4984610] [PubMed: 27688640]- Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, Salanti G (2020). CINeMA: An approach for assessing confidence in the results of a network meta-analysis PLOS Medicine 17:1–19. [PMC free article: PMC7122720] [PubMed: 32243458]
- Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE (2019) Threshold Analysis as an Alternative to GRADE for Assessing Confidence in Guideline Recommendations Based on Network Meta-analyses. Annals of Internal Medicine 170:538–46. [PMC free article: PMC6739230] [PubMed: 30909295]
Additional references to methodological issues
More severe depression
Review question
For adults with a new episode of more severe depression, what are the relative benefits and harms of psychological, psychosocial, pharmacological and physical interventions alone or in combination?
Clinical evidence
Included studies
A total of 534 randomised controlled trials (RCTs) were included in this evidence review.
In accordance with the review protocol, data from non-English language or unpublished studies was included where it could be extracted from the previous 2009 NICE Depression guideline or from a systematic review, and data was extracted from the following systematic reviews: Cipriani 2018; Geddes 1999; Krogh 2017; Smith 2018.
See the literature search strategy in appendix B and study selection flow chart in appendix C.
Excluded studies
Studies not included in this review are listed, and reasons for their exclusion are provided in appendix K.
Summary of studies included in the evidence review
The NMAs included 534 RCTs (k=534) representing 89,286 participants (n=89,286).
Of the 534 RCTs included in the NMAs for more severe depression, 426 reported either a HAM-D or MADRS score at baseline, and the mean depression severity scores were HAM-D=24.03 (SD=4.68; k=340) and MADRS=30.01 (SD=5.49; k=86) respectively. 34 were UK-based RCTs.
According to the interventions assessed and the types of outcomes reported in each RCT, the included RCTs have contributed data to one or more networks of evidence and respective NMAs.
For the SMD of depression symptom change scores outcome, the network of evidence (and the respective NMA) included 352 RCTs, 99 interventions grouped in 50 treatment classes, and 59,350 participants. Of the 352 RCTs, 146 reported change from baseline (CFB) depression symptom score data; 172 reported baseline and endpoint depression symptom score data; and 34 reported dichotomous response data and baseline symptom scores. These data were transformed and synthesised accordingly, allowing estimation of the SMD of depression symptom scores (see appendix M for details).
For the outcome of response in those randomised, the network of evidence (and the respective NMA) included 364 RCTs, 83 interventions grouped in 43 treatment classes and 68,073 participants. Of the 364 RCTs, 280 reported dichotomous response data, 31 reported CFB depression symptom score data; and 53 reported baseline and endpoint depression symptom score data. These data were transformed and synthesised accordingly, allowing estimation of log-odds ratios of response (see appendix M for details).
For the outcome of remission in those randomised, the network of evidence (and the respective NMA) included 202 RCTs reporting dichotomous remission data, 64 interventions grouped in 38 treatment classes and 40,066 participants.
See the full evidence tables in appendix D.
Relevant information on the networks of evidence and the NMAs that informed the economic analysis are reported in appendix M.
Evidence from the network meta-analysis
Base-case analysis
Below is an overview of the treatment class network plots, numbers of people tested on each treatment class and intervention, and NMA findings at the treatment class level (relative effects versus the reference treatment and rankings), for every critical outcome considered in the clinical base-case analysis of treatments for adults with a new episode of more severe depression. For the outcome of the SMD of depressive symptom scores, relative effects of individual interventions versus the reference treatment are also provided in this section.
For each outcome, we present network plots, which show which treatments have been directly compared in the RCTs included in the NMA, by connecting them with a direct line. In each network plot presented below, the width of lines is proportional to the number of trials that make each direct comparison; the size of each circle (treatment node) is proportional to the number of participants tested on each treatment class.
Full results of the NMA, including network plots and relative effects of individual interventions, as well as relative effects of all pairs of treatment classes and individual interventions, are reported in appendix M and supplements B5 and B6.
SMD of depression symptom change scores
The network plot at the treatment class level is shown in Figure 9. The number of participants tested on each treatment class and each intervention are shown in Table 17. Treatment classes, interventions and numbers of participants tested on each in the NMA of standardised mean difference (SMD) of depression symptom change scores in adults with a new episode of more severe depression. The base-case relative effects (posterior mean SMD with 95% CrI) of all treatment classes versus pill placebo (reference treatment for more severe depression) are illustrated in Figure 10 (forest plots) and reported in Table 18. The same table also shows the class treatment rankings. Treatment classes in the table have been ordered from lowest to highest ranking (with lower rankings suggesting greater effects).
Table 17
Treatment classes, interventions and numbers of participants tested on each in the NMA of standardised mean difference (SMD) of depression symptom change scores in adults with a new episode of more severe depression.
The base-case relative effects (posterior mean SMD with 95% CrI) of all individual interventions versus pill placebo (reference treatment for more severe depression) are reported in Table 19. Interventions have been listed by treatment class.
Response in those randomised
The network plot at the treatment class level is shown in Figure 11. The number of participants tested on each treatment class and each intervention are shown in Table 20. The base-case relative effects (posterior mean log-odds ratio [LOR] with 95% CrI) of all treatment classes versus pill placebo (reference treatment for more severe depression) are illustrated in Figure 12 (forest plots) and reported in Table 21.
Table 21 The same table shows also the class treatment rankings. Treatment classes in the table have been ordered from lowest to highest ranking (with lower rankings suggesting greater effects).
Table 20
Treatment classes, interventions and numbers of participants tested on each in the NMA of response in those randomised in adults with a new episode of more severe depression.
Table 21
Base-case results of the NMA of response in those randomised in adults with a new episode of more severe depression: posterior effects (mean log-odds ratio [LOR], 95%CrI) of all treatment classes versus pill placebo and treatment class rankings.
Remission in those randomised
The network plot at the treatment class level is shown in Figure 13. The number of participants tested on each treatment class and each intervention are shown in Table 22. The base-case relative effects (posterior mean log-odds ratio [LOR] with 95% CrI) of all treatment classes versus pill placebo (reference treatment for more severe depression) are illustrated in Figure 14 (forest plots) and reported in Table 23. The same table shows also the class treatment rankings. Treatment classes in the table have been ordered from lowest to highest ranking (with lower rankings suggesting greater effects).
Table 22
Treatment classes, interventions and numbers of participants tested on each in the NMA of remission in those randomised in adults with a new episode of more severe depression.
Table 23
Base-case results of the NMA of remission in those randomised in adults with a new episode of more severe depression: posterior effects (mean log-odds ratio [LOR], 95%CrI) of all treatment classes versus pill placebo and treatment class rankings.
Bias-adjusted analysis
Bias models tested on the SMD outcome suggested evidence of bias due to small study size.
Figure 15 shows the bias-adjusted forest plots of relative effects (posterior mean SMD with 95% CrI) of all treatment classes versus pill placebo (reference treatment for more severe depression). Table 24 shows the relative effects of all treatment classes versus pill placebo on the SMD and the class treatment rankings. Treatment classes in the table have been ranked from lowest to highest ranking (with lower rankings suggesting greater effects). Table 25 shows the bias-adjusted relative effects (posterior mean SMD with 95% CrI) of all individual interventions versus pill placebo (reference treatment for more severe depression). Interventions in this table have been listed by treatment class.
Sensitivity analyses
Effects on the SMD of all treatment classes versus TAU in the post-hoc sensitivity analysis that included only RCTs rated as being at low risk of bias for attrition in the Cochrane Risk of Bias tool are presented in Table 26, alongside the base-case analysis effects, to allow comparison between the two sets of results.
Finally, effects on the SMD of all treatment classes versus pill placebo in the sensitivity analysis conducted after excluding pharmacological trials are reported in Table 27, presented alongside the base-case analysis effects, to allow comparison between the two sets of results. In each analysis, treatment classes have been ordered from lowest to highest ranking (with lower rankings suggesting higher effects).
Evidence from the pairwise meta-analyses
Important (but not critical) outcomes
See Table 28 for a summary of the clinically important and statistically significant effects observed for the important (but not critical) outcomes of quality of life and functioning (including personal, social, and occupational functioning and global functioning/functional impairment) at endpoint and longer-term (at least 6 months) follow-up. See supplement B3 for forest plots for all important (but not critical) outcomes.
Table 28
Summary of significant important (but not critical outcomes) at endpoint and longer-term (at least 6 months) follow-up for adults with a new episode of more severe depression.
Follow-up of critical outcomes
See Table 29 for a summary of the clinically important and statistically significant effects observed for critical outcomes at longer-term (at least 6 months) follow-up. See supplement B3 for forest plots for all critical outcomes at all follow-up time points.
Table 29
Summary of significant critical outcomes at longer-term (at least 6 months) follow-up for adults with a new episode of more severe depression.
Comparison of the results of pairwise meta-analysis with the NMA for critical outcomes
See Table 30 for comparisons between pairwise and NMA results for critical outcomes where the difference between the pairwise meta-analysis and NMA results is equal to, or larger than, the minimally important difference (default MID, defined as SMD −0.5/0.5 and logOR ±0.25 [MID for OR calculated as exp[0.25]=1.28]) and the effect estimate of the NMA is not within the 95% confidence interval of the pairwise effect estimate (considered a significant difference), and see Table 31 for differences between pairwise and NMA results ≥MID but where the NMA effect estimate is within the 95% confidence interval of the pairwise effect estimate (considered a non-significant difference). The full table of pairwise meta-analysis and NMA comparisons is available in supplement B4. Out of a total of 160 comparisons between pairwise and NMA results for more severe depression, 32 differences ≥MID were identified (20% of all comparisons), and of these only 17 differences (11% of all comparisons) could be considered significant in that the NMA estimate was not within the 95% confidence interval of the pairwise effect estimate. For most differences identified the difference was in magnitude rather than direction of effect and could probably be accounted for by the smaller evidence base contributing to the pairwise effect estimates. It is important to note that these comparisons have been performed in addition to the NMA inconsistency checks (where direct and indirect evidence is compared).
Table 30
Summary of differences between pairwise and NMA results ≥ MID where NMA effect estimate is not within 95% confidence interval of pairwise effect estimate for adults with a new episode of more severe depression.
Table 31
Summary of differences between pairwise and NMA results ≥ MID where NMA effect estimate is within 95% confidence interval of pairwise effect estimate for adults with a new episode of more severe depression.
Pairwise meta-analysis of couple interventions
One RCT was included in pairwise meta-analysis of couple interventions for people with depression and problems in the relationship with their partner (Beach 1992).
The included study is summarised in Table 32.
Studies considered but not included in the pairwise meta-analysis of couple interventions are listed, and reasons for their exclusion are provided in appendix K.
Table 32
Summary of included study for couple interventions for adults with a new episode of more severe depression.
See the full evidence tables in appendix D, the forest plots in appendix E, and clinical evidence profiles in appendix F.
Subgroup analysis of studies included in the NMA
Subgroup analysis of studies included in the NMA was only possible for older adults (60 years and older) compared to younger adults (younger than 60 years), and not men or BME populations. Subgroup differences were examined for outcomes that had more than 2 studies in each subgroup.
Subgroup analysis was possible for 7 comparisons:
- SSRIs versus placebo:
- 7 RCTs included for older adults (Bose 2008; Emsley 2018; Kasper 2005a; Nyth 1992; Rapaport 2009; Roose 2004; Tollefson 1993/1995 [1 RCT reported across 2 papers])
- 99 RCTs included for younger adults (003-048; 29060 07 001; Andreoli 2002/Dubini 1997/Massana 1998_study 1 [1 RCT reported across 3 papers]; Baune 2018; Binnemann 2008; Bjerkenstedt 2005; Blumenthal 2007/Hoffman 2011 [1 RCT reported across 2 papers]; Burke 2002; Byerley 1988; CAGO178A2303; CL3-20098-022; CL3-20098-023; CL3-20098-024; Claghorn 1992a; Claghorn 1992b; Clayton 2006_study 1; Clayton 2006_study 2; Coleman 2001; Corrigan 2000; Detke 2004; Doogan 1994; Dube 2010; Dunbar 1993; Eli Lilly HMAT-A; Fabre 1992; Fabre 1995a; Fava 1998a; Fava 2005; Feighner 1993; Feighner 1999; Forest Laboratories 2000; Forest Laboratories 2010; Forest Research Institute 2003; Forest Research Institute 2005; Godlewska 2012; Golden 2002_448; Golden 2002_449; Goldstein 2002; Goldstein 2004; Griebel 2012_Study DFI5878; Griebel 2012_Study DFI5879; Gual 2003; Higuchi 2009; Higuchi 2011; Hirayasu 2011a; Hirayasu 2011b; Hunter 2010_study 1; Hunter 2011; Jefferson 2000; Kasper 2012; Katz 2004; Keller 2006_Study 059; Keller 2006_Study 061; Keller 2006_Study 062; Komulainen 2018; Kramer 1998; Kranzler 2006_Group A; Lam 2016; Lepola 2003; Loo 2002; Lopez-Rodriguez 2004; M/2020/0046 (Study 046); M/2020/0046 (Study 047); Macias-Cortes 2015; Mathews 2015; Mendels 1999; Miller 1989a; Mundt 2012; MY-1042/BRL-029060/CPMS-251; MY-1045/BRL-029060/1 (PAR 128); NCT01020799; Nemeroff 2007; Nierenberg 2007; NKD20006 (NCT00048204); Olie 1997; PAR 01 001 (GSK & FDA); PAR 279 MDUK; Perahia 2006; Peselow 1989a; Peselow 1989b; Ratti 2011_study 096; Ravindran 1995; Reimherr 1990; Rickels 1992; Rudolph 1999; SER 315 (FDA); Sheehan 2009b; Smith 1992; Sramek 1995; Stark 1985; Study 62b (FDA); Study F1J-MC-HMAQ - Study Group B; Trivedi 2004; Valle-Cabrera 2018; Wade 2002; Wang 2014c; WELL AK1A4006; Wernicke 1987; Wernicke 1988)
- SSRIs versus TCAs:
- 12 RCTs included for older adults (Cohn 1990b; De Ronchi 1998; Forlenza 2001; Geretsegger 1995; GSK_29060/103; Guillibert 1989; Hutchinson 1992; Kyle 1998; MDF/29060/III/070/88/MC; Mulsant 1999; Navarro 2001; Sneed 2014)
- 55 RCTs included for younger adults (29060/299; 29060 07 001; Akhondzadeh 2003; Bascara 1989; Beasley 1993b; Bersani 1994; Bhargava 2012; Bremner 1984; Byerley 1988; Chiu 1996; Christiansen 1996; Cohn 1984b; Danish University Antidepressant Group 1986; Danish University Antidepressant Group 1990; Demyttenaere 1998; Deushle 2003; Fabre 1991; Fabre 1992; Fawcett 1989; Feighner 1993; Freed 1999; Hashemi 2012; Judd 1993; Keegan 1991; Laakmann 1991; Levine 1989; Marchesi 1998; Miura 2000; Moller 1993; Moller 1998; Moller 2000; Moon 1994; Moon 1996; Nielsen 1993; Noguera 1991; Ontiveros Sanchez 1998; PAR 29060/281; PAR MDUK 032; Peselow 1989a; Peselow 1989b; Peters 1990; Preskorn 1991; Reimherr 1990; Ropert 1989; Rosenberg 1994; SER 315 (FDA); SER-CHN-1; Serrano-Blanco 2006; Shaw 1986; Staner 1995; Stark 1985; Suleman 1997; Tollefson 1994; Versiani 1999; Young 1987)
- TCAs versus placebo
- 6 RCTs included for older adults (Cohn 1984a; Georgotas 1986; Katz 1990; Nair 1995; Reynolds 1999a; Schweizer 1998)
- 50 RCTs included for younger adults (29060 07 001; Amsterdam 1986; Barge-Schaapveld 2002; Bakish 1992b; Blashki 1971; Bremner 1995; Byerley 1988; Cassano 1986; Elkin 1989/Imber 1990 [1 RCT reported across 2 papers]; Escobar 1980; Fabre 1992; Feiger 1996; Feighner 1979; Feighner 1982; Feighner 1989b; Feighner 1993; Fontaine 1994; Gelenberg 1990a; Goldberg 1980; Hicks 1988; Kleber 1983; Kusalic 1993; Lecrubier 1997; March 1990; McCallum 1975; MIR 003-020 (FDA); MIR 003-021 (FDA); Mynors-Wallis 1995; Norton 1984; Peselow 1989a; Peselow 1989b; Philipp 1999; Reimherr 1990; Rickels 1982b; Rickels 1982d; Rickels 1982e; Rickels 1987; Rickels 1991; Rickels 1994; Rickels 1995_Study 006-1; Rickels 1995_Study 006-2; Schweizer 1994; SER 315 (FDA); Silverstone 1994; Smith 1990; Stark 1985; Stassen 1993; Thomson 1982; Versiani 1989; White 1984)
- SNRIs versus placebo
- 36 RCTs included for younger adults (Baldwin 2012; Boulenger 2014; Brannan 2005; Cunningham 1994; Cunningham 1997; Cutler 2009; Detke 2002a; Detke 2002b; Detke 2004; Eli Lilly HMAT-A; Goldstein 2002; Goldstein 2004; Guelfi 1995; Hewett 2009; Hewett 2010; Higuchi 2009; Higuchi 2016; Hunter 2010_study 2; Hunter 2010_study 3; Khan 1991; Lecrubier 1997; Levin 2013; Mahableshwarkar 2013; Mahableshwarkar 2015a; Mendels 1993; Nemeroff 2007; Nierenberg 2007; Perahia 2006; Rudolph 1999; Schweizer 1991; Schweizer 1994; Sheehan 2009b; Study F1J-MC-HMAQ - Study Group B; Thase 1997; VEN 600A-303 (FDA); VEN 600A-313 (FDA))
- SNRIs versus TCAs
- 2 RCTs included for older adults (Gasto 2003; Smeraldi 1998b)
- 6 RCTs included for younger adults (Benkert 1996; Dubey 2012; Gentil 2000; Lecrubier 1997; Samuelian 1998; Schweizer 1994)
- SNRIs versus SSRIs
- 36 RCTs included for younger adults (Alves 1999; Basterzi 2009; Bielski 2004; Casabona 2004; Clerc 1994; Costa 1998; DeNayer 2002; Detke 2004; Diaz-Martinez 1998; Dierick 1996; Eli Lilly HMAT-A; Goldstein 2002; Goldstein 2004; Hao 2014; Heller 2009; Higuchi 2009; Jiang 2017; Khan 2007; Kornaat 2000; Lee 2007; Mehtonen 2000; Montgomery 2004; Mowla 2016; Nemeroff 2007; Nierenberg 2007; Owens 2008; Perahia 2006; Rickels 2000; Rudolph 1999; Sheehan 2009b; Shelton 2006; Sir 2005; Study F1J-MC-HMAQ - Study Group B; Tylee 1997; Tzanakaki 2000; Wade 2007)
- Trazodone versus TCAs
Subgroup analysis of older adults (60 years and older) compared to younger adults (younger than 60 years) for the comparison SSRIs versus placebo shows non-significant subgroup differences for: depression symptoms endpoint (Test for subgroup differences: Chi2 = 1.53, df = 1, p = 0.22); depression symptoms change score (Test for subgroup differences: Chi2 = 1.62, df = 1, p = 0.20); remission (Test for subgroup differences: Chi2 = 1.38, df = 1, p = 0.24); response (Test for subgroup differences: Chi2 = 0.24, df = 1, p = 0.63); discontinuation due to side effects (Test for subgroup differences: Chi2 = 0.02, df = 1, p = 0.88); discontinuation due to any reason (Test for subgroup differences: Chi2 = 2.62, df = 1, p = 0.11).
Subgroup analysis of older adults (60 years and older) compared to younger adults (younger than 60 years) for the comparison SSRIs versus TCAs shows non-significant subgroup differences for: depression symptoms endpoint (Test for subgroup differences: Chi2 = 0.20, df = 1, p = 0.65); depression symptoms change score (Test for subgroup differences: Chi2 = 0.11, df = 1, p = 0.75); remission (Test for subgroup differences: Chi2 = 1.60, df = 1, p = 0.21); response (Test for subgroup differences: Chi2 = 1.67, df = 1, p = 0.20); discontinuation due to side effects (Test for subgroup differences: Chi2 = 1.85, df = 1, p = 0.17); discontinuation due to any reason (Test for subgroup differences: Chi2 = 0.79, df = 1, p = 0.37).
Subgroup analysis of older adults (60 years and older) compared to younger adults (younger than 60 years) for the comparison TCAs versus placebo shows non-significant subgroup differences for: remission (Test for subgroup differences: Chi2 = 0.41, df = 1, p = 0.52); response (Test for subgroup differences: Chi2 = 0.88, df = 1, p = 0.35); discontinuation due to side effects (Test for subgroup differences: Chi2 = 0.05, df = 1, p = 0.83); discontinuation due to any reason (Test for subgroup differences: Chi2 = 0.02, df = 1, p = 0.88). Subgroup analysis was not possible for the outcomes depression symptoms endpoint, and depression symptoms change score, as there were not at least 2 studies per subgroup for these outcomes.
Subgroup analysis of older adults (60 years and older) compared to younger adults (younger than 60 years) for the comparison SNRIs versus placebo shows non-significant subgroup differences for: depression symptoms change score (Test for subgroup differences: Chi2 = 0.07, df = 1, p = 0.79); remission (Test for subgroup differences: Chi2 = 0.01, df = 1, p = 0.91); response (Test for subgroup differences: Chi2 = 0.04, df = 1, p = 0.85); discontinuation due to side effects (Test for subgroup differences: Chi2 = 0.93, df = 1, p = 0.34); discontinuation due to any reason (Test for subgroup differences: Chi2 = 0.59, df = 1, p = 0.44). Subgroup analysis was not possible for depression symptoms endpoint as there were not at least 2 studies per subgroup for this outcome.
Subgroup analysis of older adults (60 years and older) compared to younger adults (younger than 60 years) for the comparison SNRIs versus TCAs shows non-significant subgroup differences for: discontinuation due to side effects (Test for subgroup differences: Chi2 = 0.10, df = 1, p = 0.75); discontinuation due to any reason (Test for subgroup differences: Chi2 = 1.33, df = 1, p = 0.25). Subgroup analysis was not possible for the outcomes depression symptoms endpoint, depression symptoms change score, remission, and response, as there were not at least 2 studies per subgroup for these outcomes.
Subgroup analysis of older adults (60 years and older) compared to younger adults (younger than 60 years) for the comparison SNRIs versus SSRIs shows non-significant subgroup differences for: remission (Test for subgroup differences: Chi2 = 0.01, df = 1, p = 0.94); response (Test for subgroup differences: Chi2 = 0.87, df = 1, p = 0.35); discontinuation due to side effects (Test for subgroup differences: Chi2 = 0.03, df = 1, p = 0.85); discontinuation due to any reason (Test for subgroup differences: Chi2 = 0.00, df = 1, p = 0.97). Subgroup analysis was not possible for the outcomes depression symptoms endpoint, and depression symptoms change score, as there were not at least 2 studies per subgroup for these outcomes.
Subgroup analysis of older adults (60 years and older) compared to younger adults (younger than 60 years) for the comparison trazodone versus TCAs shows non-significant subgroup differences for: discontinuation due to side effects (Test for subgroup differences: Chi2 = 0.01, df = 1, p = 0.92); discontinuation due to any reason (Test for subgroup differences: Chi2 = 0.89, df = 1, p = 0.35). Subgroup analysis was not possible for the outcomes depression symptoms endpoint, depression symptoms change score, remission, and response, as there were not at least 2 studies per subgroup for these outcomes.
Quality assessment of studies included in the evidence review and the evidence
A threshold analysis was originally planned to conduct, to test the robustness of treatment recommendations based on the NMA, to potential biases or sampling variation in the included evidence. Threshold analysis has been developed as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analysis (Phillippo 2019). Threshold analysis suggests by how much effects that have been estimated in the NMA need to change before recommendations change, and whether such changes might potentially occur due to bias in the evidence. The NICE Guidelines Technical Support Unit (TSU) attended committee discussions on the rationale for recommendations and noted that, in addition to the results of the NMA, the committee took other pragmatic factors into consideration when making recommendations, including the uncertainty and limitations around the clinical and cost-effectiveness data, and the need to provide a wide range of interventions to take into account individual needs and allow patient choice. The TSU advised that as it was difficult to identify a clear decision rule to link the recommendations directly to the NMA results, it was not feasible or helpful to conduct a threshold analysis. CINeMA was also considered as a method to evaluate the confidence in the results from the NMA (Nikolakopoulou 2020). However, this was not possible to carry out, due to the class models being implemented.
In the absence of undertaking threshold analysis or using CINeMA to evaluate the quality of the evidence and the confidence in the results derived from the NMA that informed this review question, we evaluated and summarise the quality of the evidence narratively, using the domains considered as per a standard GRADE approach (risk of bias, inconsistency, publication bias, indirectness and imprecision).
For outcomes analysed only in pairwise meta-analysis (couple interventions), see the clinical evidence profiles in appendix F.
Risk of bias
The Cochrane risk of bias tool version 1.0 for RCTs (see appendix H in Developing NICE guidelines: the manual; NICE 2014) was used to assess potential bias in each study included in the review. Generally the standard of reporting in studies was quite low, as demonstrated by the risk of bias summary diagram (Figure 16). Of the studies included in the NMAs for more severe depression, 106 were at low risk for allocation method, and 86 were at low risk of bias for allocation concealment. Trials of psychological therapies were typically considered at high risk of bias for participant and provider blinding, although it is difficult to quantify in risk of bias ratings it is also important to bear in mind that the rate of side effects may also make it difficult to maintain blinding in pharmacological trials. Across interventions, 364 trials were at low risk of bias for blinding participants and providers. Most reported outcomes were investigator-rated, and assessor blinding was considered for all trials: 82 were at low risk of bias, 423 were unclear, and high risk in 29 trials. For attrition bias, 330 trials were at low risk of bias, unclear risk in 173 trials, and 31 trials were at high risk of bias. For selective reporting bias, 77 trials were at low risk of bias, unclear risk in 143 trials, and 314 trials were at high risk of bias. Other sources of bias, predominantly potential conflict of interest based on the source of funding, were identified in 455 RCTs. See appendix D for full study details, including risk of bias ratings by study.
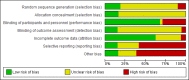
Figure 16
Risk of bias summary for treatments of a new episode in people with more severe depression.
Model goodness of fit and inconsistency
This section reports only findings of goodness of fit and inconsistency checks for the NMAs that informed the clinical evidence. Respective findings for the NMAs that informed the economic analysis are reported in appendix J. Detailed findings of goodness of fit and inconsistency checks for all NMA analyses, including those that informed the guideline economic model, are reported in appendix M and supplements B5 and B6.
For the SMD of depressive symptom scores, relative to the size of the treatment effect estimates, moderate between trial heterogeneity was observed for this outcome, as expressed by the between-studies standard deviation [τ=0.19 (95% CrI 0.15 to 0.23)]. Between-study heterogeneity and posterior mean residual deviance were slightly lower in the inconsistency model than in the random effects consistency model. The inconsistency model notably predicted the data in three studies much better than the consistency model, further adding evidence of inconsistency.
For the outcome of response in those randomised, moderate between trials heterogeneity was found relative to the size of the intervention effect estimates [τ=0.26 (95% CrI 0.21 to 0.31)]. Lower posterior mean residual deviance and between study heterogeneity in the inconsistency model suggested evidence of inconsistency. The inconsistency model notably predicted the data in one study (Sahranavard 2018) much better than the consistency model, further adding evidence of inconsistency. This study compared waitlist, dialectical behavioural therapy (DBT) individual and CBT group (under 15 sessions).
For the outcome of remission in those randomised, moderate between trials heterogeneity was found relative to the size of the intervention effect estimates [τ=0.27 (95% CrI 0.20 to 0.34)]. No meaningful differences were observed in posterior mean residual deviance, though DIC was slightly lower in the random effects consistency model, and between-study heterogeneity slightly lower in the inconsistency model. The prediction of several individual studies was worse in the consistency model, suggesting some evidence of inconsistency. These studies investigated behavioural activation individual, CBT individual (15 sessions or over), sertraline, impiramine and venafalxine.
Detailed model fit statistics, heterogeneity and results of inconsistency checks for each outcome are provided in appendix M and supplements B5 and B6. Comparisons between the relative effects of all pairs of treatments obtained from the consistency (NMA) model and those obtained from the inconsistency (pairwise) model are also provided in supplement B6 for all outcomes considered in the NMA.
Selective outcome reporting and publication bias
Bias adjustment models on the SMD of depressive symptom scores were developed to assess potential bias associated with small study size. The posterior mean residual deviance, DIC and between study heterogeneity was substantially reduced compared to the base-case consistency model suggesting strong evidence of small study bias in comparisons between active and inactive interventions in the SMD outcome, in adults with more severe depression.
The bias adjusted model resulted in small to moderate changes in the relative effects of all treatment classes versus pill placebo (reference treatment) and had also a moderate impact on some class rankings. Results are presented in the previous section of this evidence review.
Detailed results of all bias models are provided in appendix M and supplements B5 and B6.
Indirectness
In the context of the NMA, indirectness refers to potential differences across the populations, interventions and outcomes of interest, and those included in the relevant studies that informed the NMA.
A key assumption when conducting NMA is that the populations included in all RCTs considered in the NMA are similar. However, participants in pharmacological and non-pharmacological (psychological or physical intervention) trials may differ to the extent that some participants find different interventions more or less acceptable in light of their personal circumstances and preferences (so that they might be willing to participate in a pharmacological trial but not a psychological one and vice versa). Similarly, self-help trials may recruit participants who would not seek or accept face-to-face interventions. However, a number of trials included in the NMA have successfully recruited participants who are willing to be randomised to either pharmacological or psychological intervention and to either self-help or face-to-face treatment. The NMAs have assumed that service users are willing to accept any of the interventions included in the analyses; in practice, treatment decisions may be influenced by individual values and goals, and people’s preferences for different types of interventions. These factors were taken into account when formulating recommendations.
In addition, to explore the transitivity assumption in the context of participants in pharmacological and non-pharmacological trials, a sensitivity analysis on the SMD outcome was conducted after excluding trials with at least one pharmacological or combined intervention arm, where the combined intervention included a pharmacological element. The purpose was to compare the relative effects and rankings of non-psychological treatments between this sensitivity analysis and the base-case analysis. The comparison, which is presented in Table 27, suggested some changes in effects and rankings after exclusion of pharmacological trials, and higher uncertainty in the effects, apparently because the majority of the evidence came from pharmacological trials in this dataset (treatments for a new episode of more severe depression).
A post-hoc sensitivity analysis that included only RCTs rated as being at low risk of bias was conducted on the SMD outcome, which was the primary critical outcome of the clinical analysis. Such analysis was only possible to conduct for the domain of ‘attrition’ in the risk of bias tool, as this was the only domain that included a sufficient number of RCTs at low risk of bias, and a relatively wide range of treatment classes.This sub-group analysis showed no substantial difference in treatment effects compared with the base-case analysis, suggesting that bias from attrition was unlikely to be an effect modifier in this population.
Interventions of similar type were grouped in classes following the committee’s advice and considered in class models. These models allowed interventions within each class to have similar, but not identical, effects around a class mean effect. Classes and interventions assessed in the NMAs were directly relevant to the classes and interventions of interest.
Outcomes reported in included studies were also the primary outcomes of interest, as agreed by the committee.
Imprecision
There were wide 95%CrI around mean effects and rankings, for most treatment classes versus the reference treatment (pill placebo) across all NMA outcomes. For several treatment classes, the 95%CrI around relative effects versus pill placebo crossed the line of no effect.
Overall rating of the quality of the evidence
Based on the narrative assessment of the quality of the evidence using the domains considered as per a standard GRADE approach, the quality of the evidence was considered to be low-to-moderate.
Economic evidence
Included studies
A single economic search was undertaken for all topics included in the scope of this guideline. See the literature search strategy in appendix B and economic study selection flow chart in appendix G. Details on the hierarchy of inclusion criteria for economic studies are provided in supplement 1 - Methods. For this review question, only economic studies conducted in the UK were included.
The systematic search of the economic literature identified 11 studies that assessed the cost effectiveness of interventions for adults with a new episode of more severe depression in the UK (Miller 2003, Romeo 2004, Wade 2005a, Wade 2005b, Simon 2006, Wade 200, Lenox-Smith 2009, Benedict 2010, Gilbody 2015/Littlewood 2015, Koeser 2015, Hollingworth 2020). Categorisation of the studies according to their population’s severity level of depressive symptoms followed the same criteria used for the categorisation of the clinical studies included in the guideline systematic review. Where study participants’ baseline scores on a depressive symptom scale were not provided, categorisation was based on the description of the participants’ depressive symptom severity in the study.
Economic evidence tables are provided in appendix H. Economic evidence profiles are shown in appendix I.
Excluded studies
A list of excluded economic and utility studies, with reasons for exclusion, is provided in supplement 3 - Economic evidence included & excluded studies.
Summary of studies included in the economic evidence review
All included economic studies were conducted in the UK and adopted a NHS perspective, with some studies including personal social service (PSS) costs as well; in addition, some studies reported separate analyses that adopted a societal perspective. NHS and PSS cost elements included, in the vast majority of studies, intervention, primary and community care, staff time (such as GPs, nurses, psychiatrists, psychologists), medication, inpatient and outpatient care and other hospital care. All studies used national unit costs; in some studies, intervention costs were based on local prices or prices provided by the manufacturers (for example, in the case of computerised CBT packages).
Self-help with support: computerised cognitive behavioural therapy with support
Gilbody 2015/Littlewood 2015 conducted an economic analysis alongside a RCT (Gilbody 2015/Littlewood 2015, N=691; at 24 months EQ-5D data available for n=416 and NHS cost data available for n=580) to assess the cost effectiveness of 2 computerised CBT programmes with therapist support (the commercially produced package Beating the Blues and the free to use package MoodGYM) versus treatment as usual in adults with depression in the UK. The outcome measure was the QALY estimated based on EQ-5D ratings (UK tariff). The duration of the analysis was 2 years.
Using a NHS and PSS perspective, the commercially produced computerised CBT was more expensive than treatment as usual, and the freely available computerised CBT was less costly than treatment as usual. Treatment as usual produced a higher number of QALYs than either of the 2 computerised CBT packages. Thus, the commercially produced computerised CBT was dominated by treatment as usual. The ICER of treatment as usual versus the free-to-use computerised CBT package was £7,798 per QALY (2020 prices). The probability of treatment as usual being cost-effective across the 3 treatment options was 0.55 at the lower NICE cost effectiveness threshold of £20,000 per QALY. Using QALYs generated based on the SF-6D, the commercially produced computerised CBT programme was still dominated by treatment as usual; in contrast, the freely available computerised CBT programme became the dominant option; under this scenario, the probability of the freely available computerised CBT programme being cost effective at the lower NICE cost effectiveness threshold became 0.76. Results were robust to inclusion of depression-related costs only and to consideration of completers’ data only (instead of imputed data analysis). Moreover, there was little evidence of an interaction effect between preference and treatment allocation on outcomes. These results suggest that computerised CBT with support is unlikely to be cost-effective within the NICE decision-making context (which recommends use of EQ-5D for generation of QALYs). The study is directly applicable to the UK context and is characterised by minor limitations.
Non-directive counselling versus antidepressants
Miller and colleagues (2003) compared the cost effectiveness of non-directive counselling (generic psychological therapy comprising 6 weekly 50-minute sessions) versus routinely prescribed antidepressant drugs (mainly dothiepin, fluoxetine or lofepramine) in adults with moderate to severe depression in the UK. The study was conducted alongside a RCT (Bedi 2000; N=103, at 12 months efficacy data for n=81 and resource data for n=103). People refusing randomisation but agreeing to participate in the patient preference trial were given the treatment of their choice (N=220; at 12 months efficacy data for n=163 and resource use data n=215). The study included only depression-related costs. The measure of outcome was a ‘global outcome’, assessed by a psychiatrist blind to treatment allocation, using the research diagnostic criteria (RDC), the patient’s BDI score and GP notes. The outcome was considered good if the person responded to treatment within 8 weeks and then remained well. The outcome measure of the analysis was 12 months.
In the RCT, antidepressants were more costly and more effective than non-directive counselling, with an ICER of £524 per extra person with a good global outcome (2020 prices). The probability of non-directive counselling being cost-effective was 0.25 and 0.10 at a cost effectiveness threshold of £995 and £3,983 per extra person with a good global outcome, respectively. Sensitivity analysis demonstrated that, assuming missing data reflected good outcomes, the probability of counselling being cost-effective increased at any cost effectiveness threshold; assuming that missing data represented poor outcomes, the probability of non-directive counselling being cost-effective slightly increased for cost effectiveness thresholds lower than £2,987 per good global outcome and decreased for cost effectiveness thresholds higher than £2,987 per good global outcome. In the preference trial, non-directive counselling was more costly and more effective than antidepressants with an ICER of £1,816 per extra person with a good global outcome. The study is partially applicable to the NICE decision-making context as it does not use the QALY as the measure of benefit and is characterised by potentially serious limitations, such as the inclusion of depression-related costs only, the use of local unit costs for counsellors, the small numbers of participants randomised as well as included in the preference trial, and the contradictory results between the RCT and the preference trial which did not allow robust conclusions to be drawn.
Antidepressants (various comparisons between SSRIs, SNRIs, TCAs, mirtazapine)
Sertraline versus placebo
Hollingworth 2020 evaluated the cost effectiveness of sertraline versus placebo in adults presenting to primary care with depression or low mood during the past 2 years. The economic analysis was conducted alongside a RCT (Lewis 2019, N=655; EQ-5D data available for n=505; cost data available for n=381). The measure of outcome was the QALY, estimated based on EQ-5D ratings (UK tariff). The time horizon of the analysis was 12 weeks.
Under a NHS and personal social services perspective, sertraline was found to dominate placebo, as it was both more effective and less costly. Its probability of being cost-effective at the NICE lower cost effectiveness threshold of £20,000/QALY was over 95%. Subgroup analysis showed that sertraline was cost-effective in the treatment of mild, moderate and severe depression. The study is directly applicable to the NICE decision-making context and is characterised by minor limitations.
Escitalopram versus citalopram and venlafaxine
Wade 2005a and 2005b undertook model-based economic analysis to assess the cost effectiveness of escitalopram compared with citalopram and venlafaxine in adults with major depression (Wade 2005a) and escitalopram compared with citalopram in the subgroup of adults with severe major depression (Wade 2005b). The analyses utilised pooled efficacy data from published RCTs. Resource use data were based on information from a general practice research database, published literature and expert opinion. The measure of outcome was the percentage of people with remission in each arm of the model, defined as a MADRS score ≤ 12. The time horizon of the analyses was 26 weeks.
In both models, under a NHS perspective, escitalopram dominated both citalopram and venlafaxine (it was more effective and less costly). Results were robust to changes in clinical and cost model parameters. In adults with severe depression, escitalopram was dominant in more than 99.8% of the probabilistic analysis iterations. The studies are directly applicable to the NICE decision-making context, as, although the QALY was not used as an outcome, results were straightforward to interpret. However, both studies are characterised by potentially serious limitations, such as the lack of consideration of side effects and their impact on costs and outcomes (Wade 2005a), the estimation of resource use based primarily on expert opinion, and the presence of conflicts of interest as both studies were funded by industry.
Escitalopram versus duloxetine
Wade 2008 evaluated the cost effectiveness of escitalopram versus duloxetine in adults with moderate-to-severe depression. The economic analysis was conducted alongside an international RCT (Wade 2007, N=295; health economic data available for n=223). The measures of outcome were the change in Sheehan Disability Scale score, the change in the Montgomery-Asperg Depression Rating Scale (MADRS) score; response and remission. The time horizon of the analysis was 24 weeks.
Under a NHS perspective, escitalopram was found to dominate duloxetine, as it was both more effective and less costly. The study is directly applicable to the NICE decision-making context because although it did not use the QALY as an outcome, the intervention was dominant. The analysis is characterised by potentially serious limitations, mainly lack of probabilistic sensitivity analysis and presentation of cost-effectiveness acceptability curves, and the presence of conflicts of interest as both studies were funded by industry.
Paroxetine versus mirtazapine
Romeo 2004 evaluated the cost effectiveness of paroxetine versus mirtazapine in adults with moderate-to-severe depression. The economic analysis was conducted alongside a RCT (Wade 2003, N=197; data used in economic analysis n=177). The measures of outcome were the % of response defined as at least 50% decrease in HAMD17 and changes in Quality of Life in Depression Scale (QLDS) from baseline to treatment endpoint. The time horizon of the analysis was 24 weeks.
Under a NHS and social care perspective, mirtazapine was found to dominate paroxetine, as it was both more effective and less costly. The study is directly applicable to the NICE decision-making context because although it did not use the QALY as an outcome, the intervention was dominant. The analysis is characterised by potentially serious limitations, mainly that is was based on a relatively small RCT and that results are subject to bias as the study was funded by industry.
Duloxetine versus SSRIs, venlafaxine and mirtazapine
Benedict 2010 constructed an economic model to evaluate the cost effectiveness of SSRIs, duloxetine, venlafaxine and mirtazapine in adults with moderate to severe major depression that had a new treatment episode and were treated in primary care in the UK. Efficacy data were obtained from meta-analyses of RCTs, with randomisation rules possibly being broken. Resource use estimates were based on expert opinion. The outcome measure was the QALY, based on EQ-5D ratings (UK tariff). The duration of the analysis was 48 weeks.
Under the Scottish NHS perspective, duloxetine was the most cost-effective intervention as it dominated venlafaxine and had an ICER versus SSRIs of £9,700/QALY (2020 prices). SSRIs dominated mirtazapine. The probability of duloxetine being cost-effective at the NICE lower cost-effectiveness threshold of £20,000/QALY was approximately 70%. Results were sensitive to the efficacy and utility data used. Although the study is directly applicable to the NICE decision-making context, it is characterised by potentially serious limitations, including the methods for meta-analysis and evidence synthesis (selective use of RCTs and synthesis that appears to have potentially broken randomisation) and the fact that it was funded by industry, which may have introduced bias in the analysis.
Fluoxetine versus amitriptyline versus venlafaxine
Lenox-Smith 2009 updated an economic model developed by the same research team to assess the cost effectiveness of fluoxetine versus amitriptyline and venlafaxine in people with more severe depression in the UK. Efficacy data were taken from synthesis of a meta-analysis of trials (fluoxetine versus venlafaxine) and a single trial (amitriptyline versus venlafaxine). The method of synthesis was unclear, but most likely randomisation was broken. Resource use data were elicited from a Delphi panel. The measure of outcome was the QALY, estimated based on the presumed utilities of a depression-free day and a severely depressed day. The time horizon of the analysis was 24 weeks. Venlafaxine was found to dominate both fluoxetine and amitriptyline, with results being robust to changes in costs but sensitive to the value of the utility gain associated with a depression-free day. The study is partially applicable to the NICE decision-making context (the method of QALY estimation is not consistent with NICE recommendations) and, more importantly, is characterised by very serious limitations, mainly concerning the method of evidence synthesis.
Combined CBT with antidepressant (fluoxetine) versus antidepressant alone
Simon 2006 developed an economic model to assess the cost effectiveness of combination therapy (CBT plus fluoxetine) versus antidepressant (fluoxetine) in adults with moderate or severe depression receiving specialist care in the UK. Efficacy data were derived from a systematic review and meta-analysis of RCTs; resource use data were based on expert opinion and published studies. The outcomes of the analysis were the probability of successful treatment (remission and no relapse over 12 months) with remission defined as HRSD-17 ≤ 6 or HRSD-24 ≤ 8 and the QALY, estimated based on vignettes (descriptions of depression-related health states) valued by service users. The time horizon of the analysis was 15 months.
Using a NHS perspective, combination therapy was found to be more costly and more effective than fluoxetine alone, with an ICER of £6,031 per additional successfully treated person (95% CI £2,081 to £27,209), £21,618/QALY (95% CI £7,136 to £118,054/QALY) for adults with moderate depression, and £8,589/QALY (95% CI £2,825 to 483,873/QALY) for adults with severe depression (2020 prices). Results were sensitive to changes in relative efficacy (in terms of remission and relapse). The authors reported that at the NICE upper cost effectiveness threshold of £30,000/QALY (£44,000/QALY in 2020 price), the probability of combination therapy being cost-effective compared with fluoxetine was 0.88 for adults with moderate depression and 0.97 for adults with severe depression. The study is partially applicable to the NICE decision-making context (as the estimation of QALY was not consistent with NICE recommendations) and is characterised by minor limitations.
Combined CBT with citalopram versus CBT alone versus citalopram alone
Koeser 2015 developed an economic model to assess the cost effectiveness of CBT, citalopram and combined therapy of CBT and citalopram in adults with moderate or severe depression receiving specialist care in the UK. Efficacy data for the analysis were derived from systematic screening of a database of RCTs that compared psychological treatments (single or combined) for adults with depression with a control intervention; data were subsequently synthesised using network meta-analysis. Resource use data were based on published estimates of expert opinion and analysis of RCT data. The measure of outcome was the QALY, estimated based on EQ-5D ratings (UK tariff). The time horizon of the analysis was 27 months.
Using a NHS perspective, combination therapy was found to be dominated by CBT, as it was more costly and less effective. CBT was more costly and more effective than citalopram, with an ICER of £22,538/QALY (2020 prices). The probability of each intervention being cost-effective at a cost effectiveness threshold of £28,000/QALY was 0.43 for CBT, 0.37 for citalopram, and 0.20 for combination therapy. Results were sensitive to changes in inclusion criteria for RCTs for acute and follow-up treatment in the systematic review, and the use of SF-6D values (the ICER of CBT versus citalopram reached £36,646/QALY). The study is directly applicable to the NICE decision-making context and is characterised by minor limitations.
Economic model
A decision-analytic model was developed to assess the relative cost effectiveness of interventions of adults with a new episode of more severe depression. The objective of economic modelling, the methodology adopted, the results and the conclusions from this economic analysis are described in detail in appendix J. This section provides a summary of the methods employed and the results of the economic analysis.
Overview of economic modelling methods
A hybrid decision-analytic model consisting of a decision-tree followed by a three-state Markov model was constructed to evaluate the relative cost effectiveness of a range of pharmacological, psychological, physical and combined interventions for the treatment of a new episode of more severe depression in adults treated in primary care. The time horizon of the analysis was 12 weeks of acute treatment (decision-tree) plus 2 years of follow-up (Markov model). The interventions assessed were determined by the availability of efficacy and acceptability data obtained from the NMAs that were conducted to inform this guideline. The selection of classes of interventions was made based on the following criteria:
- The economic analysis assessed only classes of interventions that were included in the NMA of standardised mean difference (SMD), which was the main clinical outcome, as the committee wanted to be able to assess their clinical effectiveness prior to assessing cost-effectiveness. Moreover, to be assessed in the economic analysis, classes needed to be included in the NMAs of discontinuation (for any reason), response in completers and remission in completers, as these three outcomes informed the economic model.
- Only classes of interventions that had been tested on at least 50 participants (across RCTs) in each of the NMAs of SMD, discontinuation (for any reason), response in completers and remission in completers were included in the economic analysis, as this was the minimum amount of evidence that evidence that a treatment class should have in order to be considered for a practice recommendation. The committee looked at the total size of the evidence base in this area and the large volume of evidence for some treatment classes relative to others, and decided not to consider treatment classes with a small size of evidence base (tested on <50 participants) as there were several treatment classes with a much larger volume of evidence. An exception to this rule was made for classes of interventions that are routinely available in the NHS, that is, such classes were included in the analysis even if they had been tested on fewer than 50 participants in the NMAs mentioned above. For some treatment classes, inclusion in the economic model was not possible as no data were available on one or more NMA outcomes that informed economic modelling. For such classes, additional relevant data were sought by contacting authors of studies already included in the guideline systematic review, so as to enable inclusion of the classes in the respective NMAs and, subsequently, in the economic modelling.
- In addition, only classes with a higher mean effect on the SMD outcome compared with the selected reference treatment (pill placebo) were considered in the economic analysis.
Specific interventions were used as exemplars within each class regarding their intervention costs, so that results of interventions can be extrapolated to other interventions of similar resource intensity within their class. The following interventions [in brackets the classes they belong to] were assessed:
- pharmacological interventions: escitalopram [SSRIs]; lofepramine [TCAs]; duloxetine [SNRIs]; mirtazapine [own class]; trazodone [own class]
- psychological interventions: cCBT without or with minimal support [self-help]; cCBT with support [self-help with support]; individual BA [individual BT]; individual CBT (≥ 15 sessions) [individual CT/CBT]; group CBT (under 15 sessions) [group CT/CBT]; individual problem solving [individual problem solving]; non-directive/supportive/person-centred counselling [individual counselling]; individual IPT [individual IPT]; individual short-term PDPT [individual short-term PDPT]
- physical interventions: supervised high intensity individual exercise [individual exercise]; supervised high intensity group exercise [group exercise]; traditional acupuncture [acupuncture]
- combined interventions: CBT individual (≥ 15 sessions) + escitalopram [combined individual CT/CBT and antidepressant]; traditional acupuncture + escitalopram [combined acupuncture and antidepressant]
- GP care, reflected in the RCT arms of the reference treatment [pill placebo]
The decision-tree component model structure considered the events of discontinuation for any reason and specifically due to intolerable side effects; treatment completion and response reaching remission; treatment completion and response not reaching remission; and treatment completion and inadequate or no response. The Markov component model structure considered the states of remission, depressive episode (due to non-remission or relapse), and death. The specification of the Markov component of the model was based on the relapse prevention model developed for this guideline, details of which are provided in the evidence review C, appendix J.
Efficacy data were derived from the guideline systematic review and NMAs. Data adjusted for bias due to small study size were used in addition to base-case efficacy data, as bias-adjusted analysis suggested the presence of bias due to small study size in the data. Baseline parameters (baseline risk of discontinuation, discontinuation due to side effects, response and remission) were estimated based on a review of naturalistic studies. The measure of outcome of the economic analysis was the number of QALYs gained. Utility data were derived from a systematic review of the literature, and were generated using EQ-5D measurements and the UK population tariff. The perspective of the analysis was that of health and personal social care services. Resource use was based on published literature, national statistics and, where evidence was lacking, the committee’s expert opinion. National UK unit costs were used. The cost year was 2020. Model input parameters were synthesised in a probabilistic analysis. This approach allowed more comprehensive consideration of the uncertainty characterising the input parameters and captured the non-linearity characterising the economic model structure. A number of one-way deterministic sensitivity analyses was also carried out.
Results have been expressed in the form of Net Monetary Benefits (NMBs). Incremental mean costs and effects (QALYs) of each intervention versus GP care have been presented in the form of cost effectiveness planes. Results of probabilistic analysis have been summarised in the form of cost-effectiveness acceptability frontiers (CEAFs), which show the treatment option with the highest mean NMB over different cost effectiveness thresholds, and the probability that the option with the highest NMB is the most cost-effective among those assessed.
Overview of economic modelling results and conclusions
Individual problem solving appeared to be the most cost-effective intervention, followed by combined individual CBT with escitalopram, duloxetine, mirtazapine, individual BA, escitalopram, acupuncture combined with escitalopram, exercise group, lofepramine, trazodone, cCBT with support, individual CBT, group CBT, non-directive counselling, GP care, cCBT without or with minimal support, IPT, short-term PDPT, individual exercise and acupuncture. The probability of individual problem solving being the most cost-effective option was 0.71 at the NICE lower cost effectiveness threshold of £20,000/QALY.
The results of the analysis were characterised by considerable uncertainty, as reflected in the wide 95% credible intervals (CrI) around the rankings of interventions. On the other hand, deterministic sensitivity analysis suggested that the results and the ranking of interventions from the most to the least cost-effective were overall robust under different scenarios explored.
Conclusions from the guideline economic analysis refer mainly to people with depression who are treated in primary care for a new depressive episode; however, they may be relevant to people in secondary care as well, given that clinical evidence was derived from a mixture of primary and secondary care settings (however, it needs to be noted that costs utilised in the guideline economic model were mostly relevant to primary care).
Summary of the evidence
Clinical evidence statements for NMA results
This section reports only NMA results that informed the clinical evidence. Detailed NMA findings on all outcomes, including those that informed the economic analysis, are reported in appendix M and supplements B5 and B6.
Critical outcomes
Depression symptomatology - standardised mean difference (SMD) of depression symptom change scores (bias-adjusted analysis)
- Evidence from the NMA shows a clinically important and statistically significant benefit of a mindfulness or meditation group intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −3.40, 95% Crl −4.77 to −2.03; 15 participants randomised to mindfulness/meditation group included in this NMA). Mindfulness/meditation group is the highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 1.41 [out of 43], 95% CrI 1 to 4).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a problem solving group intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −2.29, 95% Crl −3.49 to −1.10; 47 participants randomised to problem solving group included in this NMA). Problem solving group is the second highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 3.76, 95% CrI 1 to 12).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined yoga group and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −1.89, 95% Crl −3.95 to 0.10; 15 participants randomised to yoga group + antidepressant included in this NMA). Combined yoga group and antidepressant is the third highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 7.82, 95% CrI 1 to 38).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a peer support group intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −1.35, 95% Crl −2.42 to −0.26; 39 participants randomised to peer support group included in this NMA). Peer support group is the fourth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 9.83, 95% CrI 3 to 30).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined peer support group and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −1.47, 95% Crl −3.30 to 0.25; 42 participants randomised to peer support group + antidepressant included in this NMA). Combined peer support group and antidepressant is the fifth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 10.42, 95% CrI 2 to 39).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined exercise group and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −1.37, 95% Crl −2.75 to 0.01; 79 participants randomised to exercise group + antidepressant included in this NMA). Combined exercise group and antidepressant is the sixth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 10.63, 95% CrI 2 to 37).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a combined individual CBT and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −1.18, 95% Crl −2.07 to −0.44; 192 participants randomised to individual CBT + antidepressant included in this NMA). Combined individual CBT and antidepressant is the seventh highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 11.09, 95% CrI 4 to 24).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined CBT group and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −1.23, 95% Crl −2.95 to 0.41; 63 participants randomised to CBT group + antidepressant included in this NMA). Combined CBT group and antidepressant is the eighth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 12.86, 95% CrI 2 to 40).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a psychoeducation group intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −1.01, 95% Crl −2.06 to 0.00; 44 participants randomised to psychoeducation group included in this NMA). Psychoeducation group is the ninth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 14.18, 95% CrI 3 to 36).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a yoga group intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −1.04, 95% Crl −2.25 to 0.17; 65 participants randomised to yoga group included in this NMA). Yoga group is the tenth highest ranked intervention for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 14.26, 95% CrI 3 to 39).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a self-help intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.98, 95% Crl −2.52 to 0.39; 344 participants randomised to self-help included in this NMA). Self-help (with no or minimal support) is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 14.99, 95% CrI 3 to 41).
- Evidence from the NMA shows a clinically important and statistically significant benefit of an individual behavioural therapy intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.86, 95% Crl −1.65 to −0.16; 378 participants randomised to individual behavioural therapy included in this NMA). Individual behavioural therapy is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 15.97, 95% CrI 5 to 33).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined individual exercise and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.96, 95% Crl −2.25 to 0.27; 40 participants randomised to individual exercise + antidepressant included in this NMA). Combined individual exercise and antidepressant is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 15.98, 95% CrI 3 to 40).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a combined bright light therapy and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.86, 95% Crl −1.59 to −0.12; 54 participants randomised to bright light therapy + antidepressant included in this NMA). Combined bright light therapy and antidepressant is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 16.07, 95% CrI 5 to 34).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual problem solving intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.86, 95% Crl −1.75 to 0.01; 367 participants randomised to individual problem solving included in this NMA). Individual problem solving is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 16.22, 95% CrI 5 to 36).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a combined acupuncture and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.78, 95% Crl −1.12 to −0.44; 584 participants randomised to acupuncture + antidepressant included in this NMA). Combined acupuncture and antidepressant is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 16.88, 95% CrI 9 to 26).
- Evidence from the NMA shows a clinically important and statistically significant benefit of an individual CBT intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.78, 95% Crl −1.42 to −0.33; 1044 participants randomised to individual CBT included in this NMA). Individual CBT is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 17.28, 95% CrI 8 to 27).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a non-directive counselling intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.67, 95% Crl −1.53 to 0.15; 404 participants randomised to counselling included in this NMA). Non-directive counselling is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 19.96, 95% CrI 7 to 39).
- Evidence from the NMA suggests a clinically important but not statistically significant benefit of bright light therapy relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.64, 95% Crl −1.60 to 0.29; 32 participants randomised to bright light therapy included in this NMA). Bright light therapy is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 20.89, 95% CrI 6 to 40).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of self-help with support relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.60, 95% Crl −1.61 to 0.54; 267 participants randomised to self-help with support included in this NMA). Self-help with support is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 21.32, 95% CrI 6 to 41).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined IPT and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.66, 95% Crl −2.02 to 0.63; 99 participants randomised to IPT + antidepressant included in this NMA). Combined IPT and antidepressant is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 21.32, 95% CrI 4 to 42).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual short-term psychodynamic psychotherapy intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.58, 95% Crl −1.35 to 0.10; 233 participants randomised to short-term psychodynamic psychotherapy included in this NMA). Individual short-term psychodynamic psychotherapy is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 22.08, 95% CrI 8 to 38).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of IPT relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.45, 95% Crl −1.36 to 0.47; 146 participants randomised to IPT included in this NMA). IPT is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 25.01, 95% CrI 8 to 41).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of acupuncture relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.40, 95% Crl −1.08 to 0.16; 264 participants randomised to acupuncture included in this NMA). Acupuncture is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 26.35, 95% CrI 12 to 39).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a combined individual short-term psychodynamic psychotherapy and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.34, 95% Crl −2.36 to 1.64; 131 participants randomised to short-term psychodynamic psychotherapy + antidepressant included in this NMA). Combined individual short-term psychodynamic psychotherapy and antidepressant is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 26.51, 95% CrI 3 to 43).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a combined psychoeducation group and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.35, 95% Crl −2.13 to 1.35; 27 participants randomised to psychoeducation group + antidepressant included in this NMA). Combined psychoeducation group and antidepressant is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 26.59, 95% CrI 4 to 43).
- Evidence from the NMA shows a statistically significant but not clinically important benefit of mirtazapine relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.35, 95% Crl −0.48 to −0.22; 1884 participants randomised to mirtazapine included in this NMA). Mirtazapine is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 27.04, 95% CrI 20 to 34).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a combined individual behavioural therapy and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.13, 95% Crl −2.82 to 2.71; 22 participants randomised to individual behavioural therapy + antidepressant included in this NMA). Combined individual behavioural therapy and antidepressant is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 28.06, 95% CrI 2 to 43).
- Evidence from the NMA shows a statistically significant but not clinically important benefit of an SNRI relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.32, 95% Crl −0.43 to −0.22; 9538 participants randomised to SNRIs included in this NMA). SNRIs are outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 28.07, 95% CrI 22 to 34).
- Evidence from the NMA shows no benefit of a combined individual relaxation and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD 0.05, 95% Crl −2.82 to 2.96; 10 participants randomised to individual relaxation + antidepressant included in this NMA). Combined individual relaxation and antidepressant is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 29.23, 95% CrI 2 to 43).
- Evidence from the NMA shows a statistically significant but not clinically important benefit of a TCA relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.29, 95% Crl −0.50 to −0.05; 4524 participants randomised to TCAs included in this NMA). TCAs are outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 29.34, 95% CrI 21 to 37).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a music therapy group intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.14, 95% Crl −1.69 to 1.41; 12 participants randomised to music therapy group included in this NMA). Music therapy group is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 29.54, 95% CrI 5 to 43).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a CBT group intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.26, 95% Crl −1.12 to 0.60; 165 participants randomised to CBT group included in this NMA). CBT group is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 29.59, 95% CrI 11 to 42).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of an exercise group intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.19, 95% Crl −1.20 to 0.87; 106 participants randomised to exercise group included in this NMA). Execise group is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 30.60, 95% CrI 10 to 42).
- Evidence from the NMA shows a statistically significant but not clinically important benefit of an SSRI relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.24, 95% Crl −0.32 to −0.16; 22,018 participants randomised to SSRIs included in this NMA). SSRIs are outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 31.21, 95% CrI 25 to 37).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of an individual exercise intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.13, 95% Crl −1.24 to 1.10; 298 participants randomised to individual exercise included in this NMA). Individual exercise is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 31.75, 95% CrI 9 to 43).
- Evidence from the NMA shows no benefit of a combined non-directive counselling and antidepressant intervention relative to pill placebo on depression symptomatology for adults with more severe depression (SMD 0.21, 95% Crl −2.52 to 2.96; 57 participants randomised to counselling + antidepressant included in this NMA). Combined non-directive counselling and antidepressant is outside the top-10 highest ranked interventions for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 32.21, 95% CrI 4 to 43).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of trazodone relative to pill placebo on depression symptomatology for adults with more severe depression (SMD −0.13, 95% Crl −0.29 to 0.04; 1072 participants randomised to trazodone included in this NMA). Trazodone is ranked third from bottom (only above placebo and waitlist) for clinical efficacy as measured by SMD of depression symptom change scores (mean rank 34.14, 95% CrI 27 to 40).
Response in those randomised
- Evidence from the NMA shows a clinically important and statistically significant benefit of a mindfulness or meditation group intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (15 participants randomised to mindfulness/meditation group included in this NMA). Mindfulness/meditation group is the highest ranked intervention for response in those randomised (mean rank 1.48 [out of 38], 95% CrI 1 to 4).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined yoga group and antidepressant intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (15 participants randomised to yoga group + antidepressant included in this NMA). Combined yoga group and antidepressant is the second highest ranked intervention for response in those randomised (mean rank 6.91, 95% CrI 1 to 32).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a combined individual exercise and antidepressant intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (40 participants randomised to individual exercise + antidepressant included in this NMA). Combined individual exercise and antidepressant is the third highest ranked intervention for response in those randomised (mean rank 8.25, 95% CrI 2 to 25).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a combined individual CBT and antidepressant intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (158 participants randomised to individual CBT + antidepressant included in this NMA). Combined individual CBT and antidepressant is the fourth highest ranked intervention for response in those randomised (mean rank 8.39, 95% CrI 2 to 21).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a peer support group intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (39 participants randomised to peer support group included in this NMA). Peer support group is the fifth highest ranked intervention for response in those randomised (mean rank 9.03, 95% CrI 2 to 29).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined peer support group and antidepressant intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (42 participants randomised to peer support group + antidepressant included in this NMA). Combined peer support group and antidepressant is the sixth highest ranked intervention for response in those randomised (mean rank 9.64, 95% CrI 1 to 35).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined exercise group and antidepressant intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (79 participants randomised to exercise group + antidepressant included in this NMA). Combined exercise group and antidepressant is the seventh highest ranked intervention for response in those randomised (mean rank 10.21, 95% CrI 2 to 33).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined CBT group and antidepressant intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (20 participants randomised to CBT group + antidepressant included in this NMA). Combined CBT group and antidepressant is the eighth highest ranked intervention for response in those randomised (mean rank 10.36, 95% CrI 2 to 36).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined individual behavioural therapy and antidepressant intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (10 participants randomised to individual behavioural therapy + antidepressant included in this NMA). Combined individual behavioural therapy and antidepressant is the ninth highest ranked intervention for response in those randomised (mean rank 12.55, 95% CrI 1 to 38).
- Evidence from the NMA shows a clinically important and statistically significant benefit of an individual CBT intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (779 participants randomised to individual CBT included in this NMA). Individual CBT is the tenth highest ranked intervention for response in those randomised (mean rank 13.92, 95% CrI 6 to 24).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined bright light therapy and antidepressant intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (54 participants randomised to bright light therapy + antidepressant included in this NMA). Combined bright light therapy and antidepressant is outside the top-10 highest ranked interventions for response in those randomised (mean rank 14.44, 95% CrI 3 to 36).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual behavioural therapy intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (368 participants randomised to individual behavioural therapy included in this NMA). Individual behavioural therapy is outside the top-10 highest ranked interventions for response in those randomised (mean rank 14.87, 95% CrI 4 to 35).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a self-help intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (168 participants randomised to self-help included in this NMA). Self-help (with no or minimal support) is outside the top-10 highest ranked interventions for response in those randomised (mean rank 15.07, 95% CrI 4 to 34).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual short-term psychodynamic psychotherapy intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (217 participants randomised to short-term psychodynamic psychotherapy included in this NMA). Individual short-term psychodynamic psychotherapy is outside the top-10 highest ranked interventions for response in those randomised (mean rank 16.16, 95% CrI 5 to 32).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a combined acupuncture and antidepressant intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (553 participants randomised to acupuncture + antidepressant included in this NMA). Combined acupuncture and antidepressant is outside the top-10 highest ranked interventions for response in those randomised (mean rank 16.29, 95% CrI 10 to 23).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of self-help with support relative to pill placebo on response (in those randomised) for adults with more severe depression (274 participants randomised to self-help with support included in this NMA). Self-help with support is outside the top-10 highest ranked interventions for response in those randomised (mean rank 17.34, 95% CrI 6 to 33).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined non-directive counselling and antidepressant intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (52 participants randomised to counselling + antidepressant included in this NMA). Combined non-directive counselling and antidepressant outside the top-10 highest ranked interventions for response in those randomised (mean rank 17.97, 95% CrI 3 to 38).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of IPT relative to pill placebo on response (in those randomised) for adults with more severe depression (61 participants randomised to IPT included in this NMA). IPT is outside the top-10 highest ranked interventions for response in those randomised (mean rank 18.9, 95% CrI 5 to 36).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual problem solving intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (338 participants randomised to individual problem solving included in this NMA). Individual problem solving is outside the top-10 highest ranked interventions for response in those randomised (mean rank 19.43, 95% CrI 5 to 36).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of bright light therapy relative to pill placebo on response (in those randomised) for adults with more severe depression (32 participants randomised to bright light therapy included in this NMA). Bright light therapy is outside the top-10 highest ranked interventions for response in those randomised (mean rank 20.52, 95% CrI 2 to 38).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a music therapy group intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (12 participants randomised to music therapy group included in this NMA). Music therapy group is outside the top-10 highest ranked interventions for response in those randomised (mean rank 21.57, 95% CrI 5 to 38).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a non-directive counselling intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (421 participants randomised to counselling included in this NMA). Non-directive counselling is outside the top-10 highest ranked interventions for response in those randomised (mean rank 22.14, 95% CrI 6 to 37).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined self-help and antidepressant intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (79 participants randomised to self-help + antidepressant included in this NMA). Combined self-help and antidepressant is outside the top-10 highest ranked interventions for response in those randomised (mean rank 22.42, 95% CrI 3 to 38).
- Evidence from the NMA shows a clinically important and statistically significant benefit of mirtazapine relative to pill placebo on response (in those randomised) for adults with more severe depression (2629 participants randomised to mirtazapine included in this NMA). Mirtazapine is outside the top-10 highest ranked interventions for response in those randomised (mean rank 22.98, 95% CrI 18 to 28).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a yoga group intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (45 participants randomised to yoga group included in this NMA). Yoga group is outside the top-10 highest ranked interventions for response in those randomised (mean rank 23.32, 95% CrI 5 to 38).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a TCA relative to pill placebo on response (in those randomised) for adults with more severe depression (5437 participants randomised to TCAs included in this NMA). TCAs are outside the top-10 highest ranked interventions for response in those randomised (mean rank 23.45, 95% CrI 18 to 29).
- Evidence from the NMA shows a clinically important and statistically significant benefit of an SNRI relative to pill placebo on response (in those randomised) for adults with more severe depression (10,469 participants randomised to SNRIs are included in this NMA). SNRIs are outside the top-10 highest ranked interventions for response in those randomised (mean rank 24.03, 95% CrI 19 to 29).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a CBT group intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (155 participants randomised to CBT group are included in this NMA). CBT group is outside the top-10 highest ranked interventions for response in those randomised (mean rank 24.44, 95% CrI 7 to 37).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of acupuncture relative to pill placebo on response (in those randomised) for adults with more severe depression (217 participants randomised to acupuncture included in this NMA). Acupuncture is outside the top-10 highest ranked interventions for response in those randomised (mean rank 24.51, 95% CrI 6 to 38).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual exercise intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (273 participants randomised to individual exercise included in this NMA). Individual exercise is outside the top-10 highest ranked interventions for response in those randomised (mean rank 24.77, 95% CrI 10 to 37).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an exercise group intervention relative to pill placebo on response (in those randomised) for adults with more severe depression (126 participants randomised to exercise group included in this NMA). Exercise group is outside the top-10 highest ranked interventions for response in those randomised (mean rank 25.93, 95% CrI 11 to 37).
- Evidence from the NMA shows a clinically important and statistically significant benefit of an SSRI relative to pill placebo on response (in those randomised) for adults with more severe depression (26,961 participants randomised to SSRIs included in this NMA). SSRIs are outside the top-10 highest ranked interventions for response in those randomised (mean rank 26.53, 95% CrI 22 to 31).
- Evidence from the NMA shows a clinically important and statistically significant benefit of trazodone relative to pill placebo on response (in those randomised) for adults with more severe depression (1181 participants randomised to trazodone included in this NMA). Trazodone is outside the top-10 highest ranked interventions for response in those randomised (mean rank 28.71, 95% CrI 24 to 33).
Remission in those randomised
- Evidence from the NMA shows a clinically important and statistically significant benefit of long-term psychodynamic psychotherapy relative to pill placebo on remission (in those randomised) for adults with more severe depression (90 participants randomised to long-term psychodynamic psychotherapy included in this NMA). Long-term psychodynamic psychotherapy is the highest ranked intervention for remission in those randomised (mean rank 3.87 [out of 35], 95% Crl 1 to 17).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a combined long-term psychodynamic psychotherapy and antidepressant intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (91 participants randomised to long-term psychodynamic psychotherapy + antidepressant included in this NMA). Combined long-term psychodynamic psychotherapy and antidepressant is the second highest ranked intervention for remission in those randomised (mean rank 5.54, 95% Crl 1 to 24).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a problem solving group intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (58 participants randomised to problem solving group included in this NMA). Problem solving group is the third highest ranked intervention for remission in those randomised (mean rank 8.18, 95% Crl 1 to 31).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined bright light therapy and antidepressant intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (54 participants randomised to bright light therapy + antidepressant included in this NMA). Combined bright light therapy and antidepressant is the fourth highest ranked intervention for remission in those randomised (mean rank 10.09, 95% Crl 2 to 28).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined IPT and antidepressant intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (16 participants randomised to IPT + antidepressant included in this NMA). Combined IPT and antidepressant is the fifth highest ranked intervention for remission in those randomised (mean rank 11.00, 95% Crl 1 to 32).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a self-help intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (349 participants randomised to self-help included in this NMA). Self-help (with no or minimal support) is the sixth highest ranked intervention for remission in those randomised (mean rank 11.28, 95% Crl 2 to 29).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual short-term psychodynamic psychotherapy intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (129 participants randomised to short-term psychodynamic psychotherapy included in this NMA). Individual short-term psychodynamic psychotherapy is the seventh highest ranked intervention for remission in those randomised (mean rank 12.50, 95% Crl 2 to 30).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined exercise group and antidepressant intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (134 participants randomised to exercise group + antidepressant included in this NMA). Combined exercise group and antidepressant is the eighth highest ranked intervention for remission in those randomised (mean rank 13.42, 95% Crl 3 to 30).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of IPT relative to pill placebo on remission (in those randomised) for adults with more severe depression (63 participants randomised to IPT included in this NMA). IPT is the ninth highest ranked intervention for remission in those randomised (mean rank 13.48, 95% Crl 2 to 32).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual behavioural therapy intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (354 participants randomised to individual behavioural therapy included in this NMA). Individual behavioural therapy is the tenth highest ranked intervention for remission in those randomised (mean rank 13.84, 95% Crl 2 to 32).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual problem solving intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (232 participants randomised to individual problem solving included in this NMA). Individual problem solving is outside the top-10 highest ranked interventions for remission in those randomised (mean rank 13.96, 95% Crl 2 to 33).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined individual CBT and antidepressant intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (117 participants randomised to individual CBT + antidepressant included in this NMA). Combined individual CBT and antidepressant is outside the top-10 highest ranked interventions for remission in those randomised (mean rank 14.17, 95% Crl 3 to 31).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of bright light therapy relative to pill placebo on remission (in those randomised) for adults with more severe depression (32 participants randomised to bright light therapy included in this NMA). Bright light therapy is outside the top-10 highest ranked interventions for remission in those randomised (mean rank 14.77, 95% Crl 2 to 33).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined non-directive counselling and antidepressant intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (13 participants randomised to counselling + antidepressant included in this NMA). Combined non-directive counselling and antidepressant is outside the top-10 highest ranked interventions for remission in those randomised (mean rank 16.43, 95% Crl 1 to 34).
- Evidence from the NMA shows a clinically important and statistically significant benefit of a TCA relative to pill placebo on remission (in those randomised) for adults with more severe depression (1747 participants randomised to TCAs included in this NMA). TCAs are outside the top-10 highest ranked interventions for remission in those randomised (mean rank 17.28, 95% Crl 9 to 27).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of acupuncture relative to pill placebo on remission (in those randomised) for adults with more severe depression (122 participants randomised to acupuncture included in this NMA). Acupuncture is outside the top-10 highest ranked interventions for remission in those randomised (mean rank 18.64, 95% Crl 2 to 33).
- Evidence from the NMA shows a clinically important and statistically significant benefit of an SNRI relative to pill placebo on remission (in those randomised) for adults with more severe depression (8727 participants randomised to SNRIs included in this NMA). SNRIs are outside the top-10 highest ranked interventions for remission in those randomised (mean rank 18.76, 95% Crl 12 to 25).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual CBT intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (451 participants randomised to individual CBT included in this NMA). Individual CBT is outside the top-10 highest ranked interventions for remission in those randomised (mean rank 18.84, 95% Crl 5 to 32).
- Evidence from the NMA shows a clinically important and statistically significant benefit of mirtazapine relative to pill placebo on remission (in those randomised) for adults with more severe depression (726 participants randomised to mirtazapine included in this NMA). Mirtazapine is outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU (mean rank 19.15, 95% Crl 12 to 26).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined acupuncture and antidepressant intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (112 participants randomised to acupuncture + antidepressant included in this NMA). Combined acupuncture and antidepressant is outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU (mean rank 19.19, 95% Crl 4 to 33).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of self-help with support relative to pill placebo on remission (in those randomised) for adults with more severe depression (416 participants randomised to self-help with support included in this NMA). Self-help with support is outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU (mean rank 19.56, 95% Crl 5 to 32).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an exercise group intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (104 participants randomised to exercise group included in this NMA). Exercise group is outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU (mean rank 20.59, 95% Crl 4 to 34).
- Evidence from the NMA shows a clinically important and statistically significant benefit of an SSRI relative to pill placebo on remission (in those randomised) for adults with more severe depression (15,203 participants randomised to SSRIs included in this NMA). SSRIs are outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU (mean rank 21.81, 95% Crl 16 to 27).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of a combined individual exercise and antidepressant intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (55 participants randomised to individual exercise + antidepressant included in this NMA). Combined individual exercise and antidepressant is outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU (mean rank 22.13, 95% Crl 4 to 34).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a CBT group intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (65 participants randomised to CBT group included in this NMA). CBT group is outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU (mean rank 22.30, 95% Crl 4 to 34).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a non-directive counselling intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (124 participants randomised to counselling included in this NMA). Non-directive counselling is outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU (mean rank 22.35, 95% Crl 4 to 34).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a yoga group intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (15 participants randomised to yoga group included in this NMA). Yoga group is outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU (mean rank 22.36, 95% Crl 3 to 35).
- Evidence from the NMA shows a clinically important but not statistically significant benefit of an individual exercise intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (336 participants randomised to individual exercise included in this NMA). Individual exercise is outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU and sham acupuncture (mean rank 22.69, 95% Crl 6 to 33).
- Evidence from the NMA shows neither a clinically important nor statistically significant benefit of a combined CBT group and antidepressant intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression (34 participants randomised to CBT group + antidepressant included in this NMA). Combined CBT group and antidepressant is outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU and sham acupuncture (mean rank 22.90, 95% Crl 3 to 34).
- Evidence from the NMA shows a clinically important and statistically significant benefit of trazodone relative to pill placebo on remission (in those randomised) for adults with more severe depression (742 participants randomised to trazodone included in this NMA). Trazodone is outside the top-10 highest ranked interventions for remission in those randomised and is ranked below TAU and sham acupuncture (mean rank 23.11, 95% Crl 16 to 29).
- Evidence from the NMA shows a lower effect of a short-term psychodynamic psychotherapy group intervention relative to pill placebo on remission (in those randomised) for adults with more severe depression, and this difference is clinically important and statistically significant (24 participants randomised to short-term psychodynamic psychotherapy group included in this NMA). Short-term psychodynamic psychotherapy group is ranked bottom for remission in those randomised, and is ranked below TAU, sham acupuncture, pill placebo and waitlist (mean rank 34.32, 95% CrI 28 to 35).
Clinical evidence statements for pairwise meta-analysis results of studies included in the NMA
Important, but not critical, outcomes
Quality of life
- Single-RCT evidence (N=74) shows a clinically important and statistically significant benefit of an individual CBT intervention relative to self-help with support on quality of life for adults with more severe depression.
- Single-RCT evidence (N=38) shows a clinically important and statistically significant benefit of a combined individual CBT and SSRI intervention relative to TAU on quality of life for adults with more severe depression.
- Single-RCT evidence (N=71) shows a clinically important and statistically significant benefit of a self-help intervention relative to no treatment on quality of life for adults with more severe depression.
- Single-RCT evidence (N=127) shows a clinically important and statistically significant benefit of an individual short-term psychodynamic psychotherapy intervention relative to self-help with support on quality of life for adults with more severe depression.
- Single-RCT evidence (N=70) shows a clinically important and statistically significant benefit of an individual exercise intervention relative to no treatment on quality of life for adults with more severe depression.
- Single-RCT evidence (N=43) shows a clinically important and statistically significant benefit of a yoga group intervention relative to waitlist on quality of life for adults with more severe depression.
Personal, social and occupational functioning
- Single-RCT evidence (N=137) shows a clinically important and statistically significant benefit of an individual CBT intervention relative to no treatment on functional impairment for adults with more severe depression.
- Single-RCT evidence (N=121) shows a clinically important and statistically significant benefit of an individual problem solving intervention relative to attention placebo on functional impairment for adults with more severe depression.
- Single-RCT evidence (N=25) shows a clinically important and statistically significant benefit of an individual problem solving intervention relative to non-directive counselling on functional impairment for adults with more severe depression.
- Single-RCT evidence (N=258) shows a clinically important and statistically significant benefit of a non-directive counselling intervention relative to no treatment on functional impairment for adults with more severe depression.
- Single-RCT evidence (N=31) shows a clinically important and statistically significant benefit of a combined IPT and SNRI intervention relative to SNRI-only on global functioning for adults with more severe depression.
- Single-RCT evidence (N=183) shows a clinically important and statistically significant benefit of a self-help intervention relative to waitlist on functional impairment for adults with more severe depression.
- Single-RCT evidence (N=50) shows a clinically important and statistically significant benefit of self-help with support relative to waitlist on sleeping difficulties for adults with more severe depression.
- Single-RCT evidence (N=93) shows a clinically important and statistically significant benefit of an individual short-term psychodynamic psychotherapy intervention relative to individual CBT on interpersonal problems for adults with more severe depression.
- Single-RCT evidence (N=127) shows a clinically important and statistically significant benefit of an individual short-term psychodynamic psychotherapy intervention relative to self-help with support on interpersonal problems for adults with more severe depression.
- Single-RCT evidence (N=210) shows a clinically important and statistically significant benefit of an SSRI relative to placebo on sleeping difficulties for adults with more severe depression.
Clinical evidence statements for pairwise meta-analysis of couple interventions (not included in NMA)
Comparison 1: Behavioural couples therapy versus waitlist
Critical outcomes
Depression symptoms (change score)
- Very low quality evidence from one RCT (N=30) shows a clinically important and statistically significant benefit of behavioural couples therapy relative to waitlist on the change in depression symptoms from baseline to endpoint for adults with more severe depression and with relationship problems.
Important, but not critical, outcomes
Marital adjustment (change score)
- Very low quality evidence from one RCT (N=30) shows a clinically important and statistically significant benefit of behavioural couples therapy relative to waitlist on the change in marital adjustment from baseline to endpoint for adults with more severe depression and with relationship problems.
Comparison 2: Behavioural couples therapy versus CBT individual
Critical outcomes
Depression symptoms (change score)
- Very low quality evidence from one RCT (N=30) shows no significant difference between behavioural couples therapy and an individual CBT intervention on the change in depression symptoms from baseline to endpoint for adults with more severe depression and with relationship problems.
Important, but not critical, outcomes
Marital adjustment (change score)
- Very low quality evidence from one RCT (N=30) shows a clinically important and statistically significant benefit of behavioural couples therapy relative to an individual CBT intervention on the change in marital adjustment from baseline to endpoint for adults with more severe depression and with relationship problems.
Comparison 3: CBT individual versus waitlist
Critical outcomes
Depression symptoms (change score)
- Low quality evidence from one RCT (N=30) shows a clinically important and statistically significant benefit of an individual CBT intervention relative to waitlist on the change in depression symptoms from baseline to endpoint for adults with more severe depression and with relationship problems.
Important, but not critical, outcomes
Marital adjustment (change score)
- Very low quality evidence from one RCT (N=30) shows no benefit of an individual CBT intervention relative to waitlist on the change in marital adjustment from baseline to endpoint for adults with more severe depression and with relationship problems.
Economic evidence statements
- Evidence from 1 single UK study conducted alongside a RCT (N = 691) indicates that computerised CBT with support is unlikely to be cost-effective compared with treatment as usual in adults with a new episode of more severe depression. The evidence is directly applicable to the UK context and is characterised by minor limitations.
- Evidence from 1 single UK study conducted alongside a RCT (N=103) and a preference trial (N= 220) is inconclusive regarding the cost effectiveness of non-directive counselling versus antidepressants in adults with a new episode of more severe depression. The study is partially applicable to the NICE decision-making context and is characterised by potentially serious limitations.
- Evidence from subgroup analysis from a single UK study conducted alongside a RCT (N = 655) suggests that sertraline is very likely to be cost-effective compared with placebo in adults with a new episode of more severe depression. The evidence is directly applicable to the UK context and is characterised by minor limitations.
- Evidence from 2 model-based UK studies suggests that escitalopram is more cost-effective than citalopram and duloxetine (assessed in 1 of the studies) in adults with a new episode of more severe depression. The evidence is directly applicable to the NICE decision-making context but is characterised by potentially serious limitations.
- Evidence from 1 single UK study conducted alongside a RCT (N=295) suggests that sertraline is likely to be cost-effective compared with duloxetine in adults with a new episode of more severe depression. The study is directly applicable to the NICE decision-making context and is characterised by potentially serious limitations.
- Evidence from 1 single UK study conducted alongside a RCT (N=197) suggests that mirtazapine is likely to be cost-effective compared with paroxetine in adults with a new episode of more severe depression. The study is directly applicable to the NICE decision-making context and is characterised by potentially serious limitations.
- Evidence from 1 model-based UK study suggests that duloxetine is likely the most cost-effective option when compared with SSRIs, venlafaxine and mirtazapine in adults with a new episode of more severe depression. The study is directly applicable to the NICE decision-making context but is characterised by potentially serious limitations.
- Evidence from 1 model-based UK study suggests that venlafaxine may be more cost-effective than fluoxetine and amitriptyline in adults with a new episode of more severe depression. However, the study is partially applicable to the NICE decision-making context and is characterised by very serious limitations.
- Evidence from 1 model-based UK study suggests that combination therapy (CBT and fluoxetine) is likely to be more cost-effective versus pharmacological treatment (fluoxetine) alone in adults with a new episode of more severe depression. The evidence is partially applicable to the NICE decision-making context and is characterised by minor limitations.
- Evidence from 1 model-based UK study suggests that CBT is likely to be more cost-effective than combination therapy (CBT and citalopram) in adults with a new episode of more severe depression. The evidence on the cost effectiveness between CBT and pharmacological therapy (citalopram) is inconclusive. The evidence is directly applicable to the NICE decision-making context and is characterised by minor limitations.
- Evidence from the guideline economic modelling suggests that individual problem solving is likely to be the most cost-effective option for the treatment of new episodes of more severe depression in adults, followed by combined individual CBT with escitalopram, duloxetine, mirtazapine, individual BA, escitalopram, acupuncture combined with escitalopram, exercise group, lofepramine, trazodone, cCBT with support, individual CBT, group CBT, non-directive counselling, GP care, cCBT without or with minimal support, IPT, short-term PDPT, individual exercise and acupuncture. This evidence refers mainly to people treated in primary care for a new depressive episode; however, it may be relevant to people treated in secondary care as well, given that clinical evidence was derived from a mixture of primary and secondary care settings. The economic analysis is directly applicable to the NICE decision-making context and is characterised by minor limitations.
The committee’s discussion of the evidence
Interpreting the evidence
The outcomes that matter most
The aim of this review was to identify the most effective and cost-effective treatments for more severe depression and the committee chose depression symptomatology (measured as the standardised mean difference, SMD, of depression symptom change scores at treatment endpoint), remission (in those randomised) and response (in those randomised) as critical outcomes to provide an indication of clinical effectiveness. Discontinuation due to side effects and discontinuation for any reason were also chosen as critical outcomes, as indicators of the tolerability and acceptability of treatments, but results for these outcomes were used as part of the economic modelling (along with remission and response in completers) and were not reviewed by the committee separately.
In addition to the critical, depression-specific, outcomes the committee prioritised 2 important outcomes – these were quality of life and personal, social and occupational functioning. These were selected to determine if treatments for depression led to improved quality of life, and helped overcome difficulties in sleep, participation in employment, and carrying out activities of daily living. These were selected as important and not critical outcomes as the committee were aware that there was likely to be less evidence for these outcomes. The committee recognised that although these outcomes were very important to people with depression, as they would not be available for all interventions they would be less useful to the committee to make recommendations.
The critical outcomes were assessed at treatment endpoint, but in order to determine if treatments for depression had longer term benefits, follow-up measurements of depression symptomatology, remission and response were also analysed. Outcomes at these additional timepoints were also assessed by the committee as part of their decision-making process. However, the committee recognised that although these longer-term outcomes were very important to people with depression, as they would not be available for all interventions they would be less useful to the committee to make recommendations.
For each outcome, the committee decided to consider only treatment classes that had been tested on at least 50 participants across the RCTs included in the respective NMA, after looking at the total size of the evidence base on treatments for a new episode of more severe depression and noticing that there were several treatment classes with a much larger volume of evidence.
The quality of the evidence
The trials included for this evidence review were individually assessed using the Cochrane risk of bias tool (version 1.0), and the summarised quality of the evidence is presented in the evidence review. Overall, the majority of domains were rated as at low risk, or unclear risk of bias, with the exception of selective reporting bias, and other bias (which included potential conflict of interest based on the source of funding).
Regarding the outcomes considered in the clinical analysis, the between-trial heterogeneity relative to the size of the intervention effect estimates was moderate for the SMD of depression symptom scores, response in those randomised, and remission in those randomised. Some evidence of inconsistency was identified in all outcomes considered in the clinical analysis. In the analysis of the SMD of depression symptom scores there was evidence of bias associated with small study size. The bias adjusted model resulted in small to moderate changes in the relative effects of all treatment classes versus pill placebo (reference treatment) and also had a moderate impact on some class rankings. The committee took this information into account when interpreting the results.
Regarding the outcomes that informed the economic analysis, relative to the size of the intervention effect estimates, the between trial heterogeneity was found to be moderate for discontinuation due to any reason, discontinuation due to side effects from medication in those discontinuing treatment, and response in completers, and small for remission in completers. Some evidence of inconsistency was identified for discontinuation due to any reason, discontinuation due to side effects from medication in those discontinuing treatment, and remission in completers. There was also evidence of bias associated with small study size identified for both discontinuation due to any reason and response in completers.
The sensitivity analysis on the SMD outcome conducted to explore the transitivity assumption of participants in pharmacological and non-pharmacological studies found that there were some differences in the results when the pharmacological trials were excluded from analysis, however these were not substantial and thus the transitivity assumptions are acceptable.
The post-hoc sub-group analysis on the SMD outcome that included only studies at low risk for the attrition domain of the Cochrane risk-of-bias tool showed no substantial difference in treatment effects compared with the base-case analysis. This suggested that bias from attrition was unlikely to be an effect modifier in this population.
The committee noted that the effectiveness of psychological interventions may depend on clinicians’ training, expertise and previous experience with specific treatments, as well as patients’ needs, preferences and experiences with previous treatments for depression. The committee acknowledged that these factors may have affected, to some extent, the efficacy of treatments in the RCTs included in the NMAs, and also patient outcomes in clinical practice. These issues were considered when interpreting the available evidence, but also when formulating reocmmendations.
A threshold analysis was originally planned, to assess the robustness of the intervention recommendations to potential limitations in the evidence synthesised in NMAs. Threshold analysis suggests by how much effects that have been estimated in the NMA need to change before recommendations change, and whether such changes might potentially occur due to bias in the evidence. The NICE Guidelines Technical Support Unit (TSU) attended committee discussions on the rationale for recommendations and noted that, in addition to the results of the NMA, the committee took other pragmatic factors into consideration when making recommendations, including the uncertainty and limitations around the clinical and cost-effectiveness data, and the need to provide a wide range of interventions to take into account individual needs and allow patient choice. The TSU advised that as it was difficult to identify a clear decision rule to link the recommendations directly to the NMA results, it was not feasible or helpful to conduct a threshold analysis. The committee agreed with the observation that recommendations were based on a pragmatic approach utilising their clinical experience and the need for inclusivity; and their wish for pragmatic recommendations tailored to individual needs and preferences. Therefore they agreed that threshold analysis would not add value to decision making.
Benefits and harms
The committee discussed the results of the clinical and economic analyses and used this information to draft recommendations relating to the use of specific interventions for the treatment of more severe depression. When reviewing the evidence from the network meta-analysis, the committee were aware that a number of important and well-known, often pragmatic, trials were excluded from the NMA, typically because the samples in the trials were <80% first-line treatment or <80% non-chronic depression. These were stipulations of the review protocol in order to create a homogenous data set, but the committee used their knowledge of these studies in the round when interpreting the evidence from the systematic review and making recommendations. The committee were particularly mindful of the UK-based psychological treatment studies (or multicentre studies that included a UK centre) that had been excluded on this basis, due to the relevance to the NHS context. For more severe depression, the committee’s knowledge of the results of these trials (Blackwell et al. 2015; Brabyn et al. 2016; Delgadillo et al. 2015; Dowrick et al. 2000; Ekers et al. 2011; Kessler et al. 2009; Macaskill & Macaskill, 1996; MacPherson et al. 2013; Martin et al. 2001; Mead et al. 2015; Proudfoot et al. 2003, 2004; Richards et al. 2016; Russell et al. 2019, 2020; Sugg et al. 2018; Teasdale et al. 1984; Watkins et al. 2012) was brought to bear when interpreting the results of the NMA. The results of these studies were broadly consistent with the evidence from the systematic review, and the committee therefore took this into consideration when making their recommendations.
The committee reviewed the results of the bias-adjusted NMA for more severe depression for the outcome of SMD, compared to pill placebo. The committee noted that the point estimate for the majority of intervention classes showed an improvement in depression symptoms, but that most also had very wide 95% credible intervals which crossed zero, and therefore there was uncertainty around the effectiveness. The committee noted that there were some classes for which there was evidence from more than 50 participants, and credible intervals that did not cross zero – these were individual cognitive and cognitive behavioural therapies (CT/CBT), individual behavioural therapy, pharmacological treatments (SSRIs, TCAs, SNRIs, mirtazapine), and combination therapy with individual CT/CBT plus antidepressants, acupuncture plus antidepressants, and light therapy plus antidepressants. The committee noted that the credible intervals for the pharmacological therapies were all very narrow, and that this was due to the fact that these results were based on large populations from multiple studies and therefore there was less uncertainty around these results, whereas the evidence for some of the other interventions was based on far fewer participants. The committee agreed that these results were in-line with their clinical experience that CBT, behavioural therapies and pharmacological therapies were all effective to treat more severe depression, and that it was likely that combination treatments with antidepressants were likely to be effective as well, and might lead to additional benefits, over and above the effect of a single intervention. The committee agreed that there was very litte to differentiate between the other classes based on the bias-adjusted SMD evidence alone. The committee also reviewed the NMA ranking for the classes of interventions but noted the very wide credible intervals in the ranks provided, and agreed this did not provide any additional information to help them distinguish between the classes.
The committee discussed the bias-adjusted SMD results for individual interventions within each class and noted there was evidence that some interventions were effective, even when the class effect did not show a significant difference from pill placebo. For example, self-help (both with and without support) had credible intervals that crossed zero but the individual interventions of cognitive bibliotherapy and computerised CBT (with or without support) showed a significant effect compared to pill placebo. Likewise, the classes of individual problem-solving, non-directive counselling, short-term psychodynamic psychotherapy, combination therapy of group CT/CBT with antidepressants, combination therapy of IPT with antidepressants, and combination therapy of group exercise with antidepressants were non-significant, but individual interventions within these classes showed significant benefit.
The committee next reviewed the results for response and remission in those randomised. For the outcome of response, the committee noted that the results were similar to those seen for the SMD outcome, with most classes of intervention offering some benefits but the majority of the credible intervals crossing zero, and the classes of interventions for which there was evidence from more than 50 participants, and credible intervals that did not cross zero were also similar to the results seen for SMD. These classes were individual CT/CBT and pharmacological treatments (SSRIs, TCAs, SNRIs, mirtazapine, trazodone), and the combinations of CT/CBT with antidepressants and acupuncture with antidepressants. For the outcome of remission, the results were slightly different: all the pharmacological treatments (SSRIs, TCAs, SNRIs, mirtazapine, trazodone) still showed benefits compared to pill placebo, with narrow credible intervals that did not cross zero, but the only psychological intervention that fulfilled this was individual long-term psychodynamic therapy (PDPT), or the combination of long-term PDPT with antidepressants, although the evidence for both these classes was based on a population of 90 people.
The committee discussed the sensitivity analysis conducted to determine if the inclusion of pharmacological trials impacted on the results seen for psychological, psychosocial and physical therapies. It was noted that exclusion of the pharmacological studies had small effects on some SMDs compared to treatment as usual, and that in this analysis the confidence intervals for individual CT/CBT widened so that they crossed zero. However, the committee agreed that these small changes indicated that the NMA analysis including the pharmacological trials was robust and that this would not impact on their recommendations.
The evidence for the outcomes of quality of life and functioning outcomes, and follow-up of depression outcomes were, as described above, presented as pairwise analyses. The committee reviewed the outcomes where a clinically important and statistically significant difference had been identified, but noted that the results were all from single studies, many of which were small (some with fewer than 50 participants). For the studies with more than 50 participants and the outcome of quality of life, the committee noted that there was some evidence of benefit for individual CBT, CBT plus antidepressants, self-help and individual exercise compared to no treatment/treatment as usual/waitlist. For the functional outcomes there was evidence of benefit for individual CBT, individual problem-solving, non-directive counselling, self-help (with or without support) and SSRIs compared to no treatment/attention placebo/waitlist/pill placebo. Comparisons of individual STPP with self-help with support and individual CBT suggested there may be benefits with STPP, and one comparison of individual problem-solving with non-directive counselling, suggested benefits of problem-solving. The committee agreed that these results confirmed that there may be additional benefits on quality of life and functional outcomes with some of the interventions for depression that had shown benefit for the critical outcomes, and this provided reassurance, but there was not enough evidence on these important outcomes to alter their recommendations.
There were very few comparisons from the follow-up data on depression outcomes that showed a clinically important and statistically significant difference. There was some very limited evidence from single studies that individual behavioural therapy led to improved rates of remission at 9 months compared to no treatment and improved rates of response and remission at 8 months compared to SSRIs, and similarly that individual CBT led to an improvement in depression symptoms at 12 months, compared to antidepressants. There was also very limited evidence from small, single studies that self-help may lead to benefits at 6 and 9 months’ follow-up compared to no treatment or treatment as usual. The committee agreed that this very limited evidence provided some reassurance that classes of interventions that had shown beneficial results at endpoint, may have beneficial results at follow-up as well, but that there was not enough evidence to develop recommendations based on follow-up data alone.
The next piece of clinical evidence the committee reviewed was the summary of the differences between the pairwise analysis and the NMA results. It was noted that the number of comparisons where there was a significant difference was small (11%), and in the majority of cases that difference was in the magnitude of the effect. The committee noted that for three interventions, the magnitude was much greater using the pairwise analysis: CBT individual compared to SNRIs, non-directive counselling versus no treatment, and STPP versus self-help with support, but that the confidence intervals for all these comparisons were very wide. The committee agreed that these differences should be considered when making their recommendations.
The committee noted that the evidence for the subgroup analysis of older versus younger people showed no difference between the groups for any of the comparisons and so no specific recommendations were made for people of different ages.
Finally, the committee considered the pairwise analysis of behavioural couples therapy for people with depression and problems in the relationship with their partner. This evidence was based on a small, single study which indicated that compared to waitlist, couples’ therapy demonstrated benefits in terms of depression symptoms and marital adjustment, but when compared to CBT it did not show a benefit in depression sympyoms, but did with marital adjustment. CBT compared to waitlist demonstrated benefits only in terms of depression symptoms. The committee discussed that although this was limited evidence, behavioural couples therapy was included in the range of interventions offered by the IAPT services and that it was useful in the specific population and so recommended its use for this group of people.
Based on their overall review of the clinical evidence the committee agreed that some treatments (such as individual CBT, individual behavioural therapies, antidepressants and combinations of CBT, acupuncture and light therapy with antidepressants) appeared to be more effective than others in ranking, but there was otherwise little to choose between treatments. The committee therefore reviewed the results of the health economic modelling (see separate details of this discussion below) which determined which treatments were cost-effective, and used this to help refine a suggested prioritisation of which treatments should be offered to people with depression, or considered for use.
The committee discussed the fact that acupuncture in combination with antidepressants had been shown to be effective for some outcomes, but noted that the studies had been conducted in China using Chinese acupuncture techniques which were different to Western acupuncture techniques. They therefore agreed that the evidence may not be applicable to the UK population and that acupuncture plus antidepressants should not be recommended, and instead they made a research recommendation.
The committee considered the short-term and long-term harms associated with antidepressants, for example, side effects associated with SSRIs include drowsiness, nausea, insomnia, agitation, restlessness and sexual problems. For the TCAs there is the potential for cardiotoxicity and associated increased risk in overdose, although this is much greater for some TCAs such as amitriptyline and dosulepin. Some antidepressants, including the SNRIs venlafaxine and duloxetine, are also associated with more withdrawal symptoms. On the basis of the safety and tolerability profiles the committee agreed that SSRIs should be considered as the first choice of antidepressant for most people. SNRIs and TCAs were also an option for the treatment of more severe depression, if indicated based on previous clinical and treatment history, although the guideline highlights that TCAs are dangerous in overdose and that of the TCAs lofepramine has the best safety profile. In developing the recommendations, the committee were mindful of the negative consequences of prolonged depressive episodes including not only the impact on the mental health of the individual and their family but also on an individual’s physical health (depression is associated with poorer physical health outcomes) and the impact on employment. The committee agreed that the benefits of improving the outcome of a depressive episode outweighed the potential harms. However, the guideline included detailed recommendations about starting and stopping antidepressants, to enable people with depression and clinicians to make an individualised choice about the suitability of antidepressant treatment, and the choice of a specific antidepressant, based on patient preference and individual needs.
Cost effectiveness and resource use
According to existing UK economic evidence, computerised CBT with support was unlikely to be cost-effective compared with treatment as usual in adults with a new episode of more severe depression. Evidence was inconclusive regarding the cost effectiveness of non-directive counselling versus antidepressants. Sertraline was likely to be cost-effective compared with placebo and duloxetine, while escitalopram appeared to be more cost-effective than citalopram and duloxetine. Existing evidence also suggested that mirtazapine was more cost-effective than paroxetine; venlafaxine might be more cost-effective than fluoxetine and amitriptyline. Other evidence suggested that duloxetine was likely the most cost-effective option when compared with SSRIs, venlafaxine and mirtazapine. Finally, there was evidence that combination therapy (CBT and fluoxetine) was more cost-effective than pharmacological treatment (fluoxetine) alone; other available evidence suggested that CBT was likely to be more cost-effective than combination therapy (CBT and citalopram) and was inconclusive regarding the relative cost effectiveness between CBT and pharmacological therapy (citalopram).
Existing economic evaluations assessed a limited range of psychological interventions and no physical interventions; the range of comparisons made in each study was also limited. Moreover, there was inconsistency across some of the findings or inconclusiveness, so it was difficult for the committee to draw any robust conclusions on the relative cost effectiveness of the full range of interventions that are available for the treatment of adults with a new episode of more severe depression.
The guideline economic analysis assessed the cost effectiveness of a wide range of pharmacological, psychological, physical and combined interventions, as initial treatments for people with a new episode of more severe depression. The interventions included in the economic analysis were dictated by availability of data and were used as exemplars within their class regarding intervention costs as for practical reasons it was impossible to model all interventions considered in the guideline NMA. The committee noted that results of interventions could be extrapolated, with some caution, to other interventions of similar resource intensity within the same class.
Within each of the individual and group CT/CBT classes, there were two separate interventions of CBT≥15 sessions and CBT<15 sessions. Regarding individual CBT, CBT≥15 sessions appeared to have a somewhat smaller effect vs placebo compared with CBT<15 sessions (individual CBT≥15 sessions SMD −0.60, 95% CrI −0.90 to −0.30; individual CBT<15 sessions SMD −0.73, 95% CrI −1.08 to −0.41), but had a larger evidence base across RCTs on the SMD outcome (individual CBT≥15 sessions had N=626, whereas individual CBT<15 sessions had N=369). Individual CBT≥15 sessions was considered to have a more appropriate intensity for a population with more severe depression by the committee, it had also a wider evidence base than individual CBT<15 sessions, and given that individual CBT≥15 sessions and individual CBT<15 sessions had no very different effects versus placebo, individual CBT≥15 sessions was selected for consideration as an exemplar of its class in the economic modelling (which ultimately informed guideline recommendations). Regarding group CBT, for the primary clinical outcome of SMD, there was only evidence on group CBT<15 sessions, therefore it was selected as the only intervention within its class in the economic modelling (which ultimately informed recommendations).
The economic analysis included only classes that had been tested on at least 50 participants across RCTs included in the NMAs of the SMD, discontinuation for any reason, response in completers and remission in completers, or fewer than 50 participants if the intervention class was one that was already in routine use in the NHS. To be considered in the economic analysis, treatment classes should have shown a better mean effect than the reference intervention, which was pill placebo. This was assumed in the model to reflect GP care. The NMAs of discontinuation (for any reason) and response in completers, which informed the economic analysis, were tested for the presence of bias due to small study size. Evidence of bias was identified in both analyses and therefore, in addition to the base-case economic analysis, a bias-adjusted economic analysis was run, using the outputs of the bias-adjusted NMAs on these two outcomes. The results of the bias-adjusted economic analysis were those considered by the committee when making recommendations.
The economic analysis utilised data on the risk of side effects from antidepressants obtained from a large US study that reported claims data. This risk ranged from 4.7% to 9.2%, depending on the antidepressant class. The committee selected these data because they expressed the view that claims for side effects that come up spontaneously, via healthcare service contacts, are more representative of the risk of side effects that have an impact on HRQoL and healthcare costs (which are of interest as they may have an impact on antidepressants’ relative cost-effectiveness) compared with studies asking participants specifically to self-report the presence of side effects, or choose from a side-effect checklist. According to the committee’s expert opinion, the latter study design tends to overestimate the prevalence of side effects. There was also a danger of the risk of side effects from antidepressants being overestimated in the economic model, since the risk of common side effects for psychological therapies was conservatively assumed to be zero. Nevertheless, the committee advised that a higher risk of side effects (40%) be tested in a sensitivity analysis. This had only a small impact on the cost-effectiveness and the ranking of antidepressants and the combination of individual CBT with antidepressants relative to other treatments.
The committee considered the bias-adjusted ranking of interventions for adults with a new episode of more severe depression, from the most to the least cost-effective. According to this ranking, individual problem-solving appeared to be the most cost-effective therapy, followed by the combination of individual CBT with antidepressants. Antidepressants (SSRIs, SNRIs, TCAs, mirtazapine and trazodone) also ranked highly, as did individual behavioural therapy, individual CBT, acupuncture with antidepressants, group exercise and cCBT with support. Other interventions, such as group CBT and non-directive counselling also appeared to be cost-effective compared with GP care. However, 5 interventions did not appear to be cost-effective compared with other cost-effective interventions and with GP care – these were cCBT without or with minimal support, interpersonal therapy, short-term psychodynamic psychotherapy (PDPT), individual exercise therapy and acupuncture.
The committee considered the 95% credible intervals (CrI) around the rankings of interventions and noted that these were characterised by considerable uncertainty. For example, the mean ranking of individual problem solving, which was shown to be the most cost-effective intervention, was 1.98, however its 95% CrI were 1 to 10, suggesting high uncertainty around the result for group CBT. For combined individual CBT and antidepressant, which was the second most cost-effective intervention, the mean ranking was 6.14 with 95% CrI ranging from 1 to 17. Similar uncertainty in the rankings was shown for all interventions included in the analysis. On the other hand, deterministic sensitivity analysis suggested that the results and the ranking of interventions were overall robust under different scenarios explored.
Based on the clinical and cost-effectiveness data, the committee decided to recommend individual CBT alone or combined with an antidepressant or individual behavioural therapies as the treatments of choice for a new episode of more severe depression in adults, as they had showed a beneficial effect compared to pill placebo, and were cost-effective classes in the economic analysis. The committee also recommended antidepressant medication as this had also been shown to be effective and cost-effective, even when using a higher risk of side effects in a sensitivity analysis. Although there was evidence of benefit for SSRIs, SNRIs, TCAs and mirtazapine the committee discussed that the tolerability of SSRIs and SNRIs meant that these would be considered as the preferred antidepressants. However, the committee agreed not to be too prescriptive about the choice of antidepressants as there may be people who had had a favourable response to TCAs in the past and would prefer to receive a TCA. Based on their knowledge and experience the committee added guidance on the safety concerns relating to overdose for TCAs, and advised that lofepramine has the best safety profile.. The committee discussed the role of mirtazapine for first-line treatment and agreed that its use should be reserved as a further-line option. The committee agreed that these treatment options should be discussed with people with depression and a shared decision made on which one was most appropriate for them based on their clinical needs and preferences.
The committee agreed that it was necessary to offer a choice of treatments, and that individual problem-solving and non-directive counselling had also been demonstrated to be cost-effective in more severe depression and so the committee recommended these as alternatives. The committee considered the fact that individual problem-solving was shown to be the most cost-effective treatment option in the economic analysis, but noted that relevant evidence was derived from US studies; problem solving is not available as a stand-alone intervention in the UK and, in some conceptualisations, it is only a variant of CBT, with very similar efficacy with individual CBT but higher uncertainty around the mean effect, as demonstrated by the NMA on the SMD outcome.
The committee noted that there was some evidence that group exercise and computerised CBT with support were both effective and cost-effective for more severe depression. However, the committee were uneasy about recommending these as interventions for more severe depression. This was based on their knowledge and experience, and concerns that these interventions may not be suitable for people with more severe depression as they did not require the development of a therapeutic relationship in the same way that the more intensive psychological therapies did, or that would occur when people were monitored regularly if on antidepressants. However, the committee agreed that as the evidence had shown benefit and cost-effectiveness these interventions could be considered for use in people with more severe depression who wished to try them, or who did not want to consider any other treatment options.
As described above, the committee decided not to recommend the combination of acupuncture with antidepressants because the evidence came from studies conducted in China using Chinese acupuncture techniques which were different to Western acupuncture techniques. They therefore agreed that the evidence may not be applicable to the UK population and instead they made a research recommendation.
The committee discussed the 5 interventions that appeared to be less cost-effective than GP care. They chose not to recommend individual exercise, as group exercise was included as a treatment option, as discussed above, and they did not recommend acupuncture, as acupuncture with SSRIs had been shown to be more effective and cost-effective and had not been recommended as an option. They chose not to recommend cCBT without or with minimal support as they had already recommended cCBT with support. However, the committee identified, based on their knowledge and experience, that there may be specific groups of people in whom STPP or IPT were effective and they therefore recommended these treatments be available as options for these specific groups.
The committee noted that long-term psychodynamic psychotherapy was included in the NMA for more severe depression, and had shown some evidence of effectiveness for the outcome of remission, but as no SMD data were available it was not possible to include it in the economic analysis and to fully consider its clinical effectiveness. Therefore, it was not possible to make any recommendations on this intervention.
The committee were concerned that psychological interventions are not always implemented consistently – for example audits have suggested that reduced numbers of sessions are used in practice compared with what is recommended, and that commissioners may not be clear how many sessions of a particular therapy are required. It was also important for people with depression to be aware of what was involved in the different types of therapy before making a decision. The committee therefore agreed it was important to specify the focus and structure of the psychological interventions being recommended to ensure consistency and that the services were commissioned correctly, and to highlight any particular advantages or drawbacks so that people could make an informed choice. The recommended structure of all psychological interventions (usual number of sessions) was based on the resource use utilised in the economic analysis, which, in turn, was informed by RCT resource use, modified by the committee’s expert advice to represent optimal routine clinical practice in the UK. In this way, the recommended structure of psychological interventions represents cost-effective use of available healthcare resources as implemented in routine clinical practice. Nevertheless, the committee agreed that the recommended structure of psychological interventions should allow flexibility so that more sessions may be provided according to individual needs. The committee made no recommendation on the duration of sessions of psychological interventions, to allow flexibility in their delivery.
Other factors the committee took into account
In addition to the results of the network meta-analysis (NMA) the committee took other pragmatic factors into consideration when making recommendations, including the uncertainty and limitations around the clinical and cost-effectiveness data, and the need to provide a wide range of interventions to take into account individual needs and allow patient choice. The recommended first-line treatments for more severe depression were included in a table in the guideline in order to support shared decision-making. The treatment options are arranged in the suggested order in which they should be considered. However, the guideline recommends that all treatments in the table can be used as first-line treatments.
The committee discussed that the division of the population for this guideline into ‘less severe’ and ‘more severe’ using published cross-walk tables with an anchor score of 16 on the PHQ-9 scale, meant that the more severe population was people with moderate to severe depression and hence a wide range of treatments should be available to allow choice of treatments, and so that treatments could be tailored to individuals and taking into account any previous history of depression and its severity. The committee also discussed that allowing choice from a range of treatments may lead to lower discontinuation rates than had been seen in clinical trials where patients were assigned to a treatment.
The committee were aware of 2 studies that had been published after the cut-off date for inclusion in the evidence review for this guideline, although it was likely that neither would have met the inclusion criteria according to the protocol. However, the committee considered that these were important publications. The first of these was Barkham 2021 which was a pragmatic, randomised non-inferiority trial comparing counselling for depression (in this study called ‘person-centred experiential therapy’, PCET) with cognitive behavioural therapy (CBT) in 510 participants. The primary outcome was depression symptomatology measured using the PHQ-9 score at 6 months, with the secondary outcome of PHQ-9 at 12 months. This study concluded that PCET is non-inferior to CBT at 6 months, but that PCET is inferior to CBT at 12 months. The committee noted that 58% of the participants in this study were already receiving antidepressant medication and as such the study would not have met the protocol criteria for first-line treatment of a new episode of depression. The committee discussed that the PCET used in this study was not the same as non-directive counselling and therefore this study does not provide evidence for the effectiveness of non-directive counselling. However, the committee considered that this study showed that PCET or counselling for depression may be effective, at least in the shorter term, but that CBT may be more beneficial in the longer term and therefore should usually be offered to patients as a preferred option.
The second study was Cuijpers 2021 which was a network meta-analysis of psychotherapies for depression, including CBT, behavioural activation (BA), problem-solving, interpersonal psychotherapy, psychodynamic therapy, life-review therapy, third-wave therapies and non-directive support counselling. The primary outcome was treatment response, and other outcomes were remission and acceptability. This study found that all therapies had significant effects compared to care-as-usual and waiting list, and that the effects of the therapies did not differ significantly from each other, except for non-directive supportive counselling, which was less effective than all the other types of therapy. No differences were found between any of the interventions in terms of acceptability. The committee considered that this study also supported their recommendations made based on their systematic review of the evidence, that all psychological treatments will provide some benefit, so offering a wide choice of treatments is appropriate, but that counselling, although it may be the preferred option for some people with depression, may not provide the same level of treatment response.
The committee noted that their recommendations for exercise interventions would need to be modified if necessary to ensure that people with disabilities were still able to access this as a treatment option, and they highlighted this in their recommendations.
Recommendations supported by this evidence review
This evidence review supports recommendations 1.6.1 and 1.7.1 and research recommendations in the NICE guideline.
References
003-008 (FDA)
http://digitalcommons .ohsu.edu/fdadrug/20/ (data extracted from Cipriani 2018). 003-042
http://digitalcommons .ohsu.edu/fdadrug/20/ (data extracted from Cipriani 2018). 003-048
http://digitalcommons .ohsu.edu/fdadrug/20/ (data extracted from Cipriani 2018). 29060 07 001
http://www.gsk-clinicalstudyregister .com /study/29060/07/001#rs (data extracted from Cipriani 2018). 29060/299
https://www.gsk-clinicalstudyregister .com/study/29060/299#rs (data extracted from Cipriani 2018). 29060/356
https://www.gsk-clinicalstudyregister .com/study/29060/356#rs (data extracted from Cipriani 2018). Aguglia 1993
Aguglia E, Casacchia M, Cassano GB, Faravelli C, Ferrari G, et al. (1993). Double-blind study of the efficacy and safety of sertraline versus fluoxetine in major depression. International Clinical Psychopharmacology 8: 197–202. [PubMed: 8263318]Ai 2018
Ai CQ, Wang QB, Wang X, Wang Y, Chen SM, et al. (2018). Therapeutic observation of cranial suture acupuncture in treating depression. Journal of Acupuncture and Tuina Science 16: 161–166.Akhondzadeh 2003
Akhondzadeh S, Faraji H, Sadeghi M, Afkham K, Fakhrzadeh H, et al. (2003). Double-blind comparison of fluoxetine and nortriptyline in the treatment of moderate to severe major depression. Journal of Clinical Pharmacy and Therapeutics 28: 379–384. [PubMed: 14632962]Alavi 2016
Alavi N, Hirji A, Sutton C, Naeem F (2016) Online CBT is effective in overcoming cultural and language barriers in patients with depression. Journal of Psychiatric Practice 22: 2–8. [PubMed: 26813483]Albornoz 2011
Albornoz Y (2011) The effects of group improvisational music therapy on depression in adolescents and adults with substance abuse: a randomized controlled trial. Nordic Journal of Music Therapy 20: 208–224.Alexopoulos 2003b
Alexopoulos GS, Raue P, Areán P (2003) Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. American Journal of Geriatric Psychiatry 11:46–52. [PubMed: 12527539]Allard 2004
Allard P, Gram L, Timdahl K, Behnke K, Hanson M, et al. (2004) Efficacy and tolerability of venlafaxine in geriatric outpatients with major depression: a double-blind, randomised 6-month comparative trial with citalopram. International Journal of Geriatric Psychiatry 19:1123–1130. [PubMed: 15526307]Allen 2006
Allen JJ, Schnyer RN, Chambers AS, Hitt SK, Moreno FA, et al. (2006) Acupuncture for depression: a randomized controlled trial. Journal of Clinical Psychiatry 67: 1665–1673. [PubMed: 17196044]Altamura 1989a
Altamura AC, Mauri MC, Rudas N, Carpiniello B, Montanini R, et al. (1989) Clinical activity and tolerability of trazodone, mianserin, and amitriptyline in elderly subjects with major depression: a controlled multicenter trial. Clinical Neuropharmacology 12(suppl. 1): s25–s33. [PubMed: 2663151]Alves 1999
Alves C, Cachola I, Brandao J (1999) Efficacy and tolerability of venlafaxine and fluoxetine in outpatients with major depression. Primary Care Psychiatry 5: 57–63.Amini 2005
Amini H, Aghayan S, Jalili SA, Akhondzadeh S, Yahyazadeh O, et al. (2005) Comparison of mirtazapine and fluoxetine in the treatment of major depressive disorder: a double-blind, randomized trial. Journal of Clinical Pharmacy and Therapeutics 30: 133–138 (data extracted from Cipriani 2018). [PubMed: 15811165]Amsterdam 1986
Amsterdam JD, Case WG, Csanalosi E, Singer M, Rickels K (1986) A double-blind comparative trial of zimelidine, amitriptyline, and placebo in patients with mixed anxiety and depression. Pharmacopsychiatry 19: 115–119. [PubMed: 2941771]Andreescu 2011
Andreescu C, Glick RM, Emeremni CA, Houck PR, Mulsant BH (2011) Acupuncture for the treatment of major depressive disorder: a randomized controlled trial. Journal of Clinical Psychiatry 72: 1129–1135. [PMC free article: PMC10536993] [PubMed: 21672495]Andreoli 2002/Dubini 1997/Massana 1998_study 1
Andreoli V, Caillard V, Deo RS, Rybakowski JK, Versiani M (2002) Reboxetine, a new noradrenaline selective antidepressant, is at least as effective as fluoxetine in the treatment of depression. Journal of Clinical Psychopharmacology 22: 393–399. [PubMed: 12172339]
Dubini A (1997) Do noradrenaline and serotonin differentially affect social motivation and behaviour? European Neuropsychopharmacology 7(suppl. 1): S49–55. [PubMed: 9169310]
Massana J (1998) Reboxetine versus fluoxetine: an overview of efficacy and tolerability. Journal of Clinical Psychiatry 59(suppl. 14): 8–10. [PubMed: 9818624]Arean 1993
Arean PA, Perri MG, Nezu AM, Schein RL, Christopher F, et al. (1993) Comparative effectiveness of social problem-solving therapy and reminiscence therapy as treatments for depression in older adults. Journal of Consulting and Clinical Psycholology 61: 1003–1010. [PubMed: 8113478]Arean 2010
Arean PA, Raue P, Mackin RS, Kanellopoulos D, McCulloch C, et al. (2010) Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. American Journal of Psychiatry 167: 1391–1398. [PMC free article: PMC2998516] [PubMed: 20516155]Arjadi 2018
Arjadi R, Nauta MH, Scholte WF, Hollon SD, Chowdhary N, et al. (2018) Internet-based behavioural activation with lay counsellor support versus online minimal psychoeducation without support for treatment of depression: a randomised controlled trial in Indonesia. Lancet Psychiatry 5: 707–716. [PubMed: 30006262]Arminen 1992
Arminen SL, Ikonen U, Pulkkinen M, Leinonen E, Mahlanen A, et al. (1992) Paroxetine and imipramine: a 12-week, double-blind multicentre study in hospitalized depressed patients. Nordic Journal of Psychiatry 46: 27–31.Ashouri 2013
Ashouri A, Atef-Vahid M-K, Gharaee B, Rasoulian M (2013) Effectiveness of meta-cognitive and cognitive-behavioral therapy in patients with major depressive disorder. Iranian Journal of Psychiatry and Behavioral Sciences 7: 24–34. [PMC free article: PMC3939994] [PubMed: 24644507]Ather 1985
Ather SA, Ankier SI, Middleton RS (1985) A double-blind evaluation of trazodone in the treatment of depression in the elderly. British Journal of Clinical Practice 39: 192–199. [PubMed: 3907684]Badyal 2005
Badyal DK, Khosla PP, Deswal RS, Matreja PS (2005) Safety and efficacy of duloxetine versus venlafaxine in major depression in Indian patients. JK Science 8: 95–99.Bakish 1992b
Bakish D, Bradwejn J, Nair N, McClure J, Remick R, et al. (1992) A comparison of moclobemide, amitriptyline and placebo in depression: a Canadian multicentre study. Psychopharmacology 106(suppl. 1): S98–S101. [PubMed: 1546154]Baldwin 2006b
Baldwin DS, Cooper JA, Huusom AK, Hindmarch I (2006) A double-blind, randomized, parallel-group, flexible-dose study to evaluate the tolerability, efficacy and effects of treatment discontinuation with escitalopram and paroxetine in patients with major depressive disorder. International Clinical Psychopharmacology 21: 159–169. [PubMed: 16528138]Baldwin 2012
Baldwin DS, Loft H, Dragheim M (2012) A randomised, double-blind, placebo controlled, duloxetine-referenced, fixed-dose study of three dosages of Lu AA21004 in acute treatment of major depressive disorder (MDD). European Neuropsychopharmacology 22: 482–491. [PubMed: 22209361]Balestrieri 1971
Balestrieri A, Benassi P, Cassano GB, Castrogiovanni P, Catalano A, et al. (1971) Clinical comparative evaluation of maprotiline, a new antidepressant drug. International Pharmacopsychiatry 6: 236–248. [PubMed: 4950566]Barge-Schaapveld 2002
Barge-Schaapveld DQ, Nicolson NA (2002) Effects of antidepressant treatment on the quality of daily life: an experience sampling study. Journal of Clinical Psychiatry 63: 477–485. [PubMed: 12088158]Bascara 1989
Bascara L (1989) A double-blind study to compare the effectiveness and tolerability of paroxetine and amitriptyline in depressed patients. Acta Psychiatrica Scandinavica 350:141–142. [PubMed: 2530771]Basterzi 2009
Başterzi AD, Yazici K, Aslan E, Delialioğlu N, Taşdelen B, et al. (2009) Effects of fluoxetine and venlafaxine on serum brain derived neurotrophic factor levels in depressed patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry 33: 281–285. [PubMed: 19110026]Bastos 2015
Bastos AG, Guimaraes LS, Trentini CM (2015) The efficacy of long-term psychodynamic psychotherapy, fluoxetine and their combination in the outpatient treatment of depression. Psychotherapy Research 25: 612–624. [PubMed: 25041333]Baune 2018
Baune BT, Sluth LB, Olsen CK (2018) The effects of vortioxetine on cognitive performance in working patients with major depressive disorder: a short-term, randomized, double-blind, exploratory study. Journal of Affective Disorders 229: 421–428. [PubMed: 29331703]Beach 1992
Beach S, O’Leary K (1992) Treating depression in the context of marital discord: outcome and predictors of response of marital therapy versus cognitive therapy. Behavior Therapy 23: 507–528.Beasley 1991a
Beasley CM Jr, Dornseif BE, Pultz JA, Bosomworth JC, Sayler ME (1991) Fluoxetine versus trazodone: efficacy and activating-sedating effects. Journal of Clinical Psychiatry 52: 294–299. [PubMed: 2071559]Beasley 1993b
Beasley CM Jr, Sayler ME, Potvin JH (1993) Fluoxetine versus amitriptyline in the treatment of major depression: a multicenter trial. International Clinical Psychopharmacology 8:143–149. [PubMed: 8263311]Bedi 2000/Chilvers 2001 / Miller 2003
Bedi N, Chilvers C, Churchill R, Dewey M, Duggan C, et al. (2000) Assessing effectiveness of treatment of depression in primary care. Partially randomised preference trial. British Journal of Psychiatry 177: 312–318. [PubMed: 11116771]
Chilvers C, Dewey M, Fielding K, Gretton V, Miller P, et al. (2001) Antidepressant drugs and generic counselling for treatment of major depression in primary care: randomised trial with patient preference arms. BMJ 322: 772–775. [PMC free article: PMC30555] [PubMed: 11282864]
Miller P, Chilvers C, Dewey M, Fielding K, Gretton V, et al. (2003) Counseling versus antidepressant therapy for the treatment of mild to moderate depression in primary care economic analysis. International Journal of Technology Assessment in Health Care 19: 80–90. [PubMed: 12701941]Behnke 2003
Behnke K, Sogaard J, Martin S, Bauml J, Ravindran AV, et al. (2003) Mirtazapine orally disintegrating tablet versus sertraline: a prospective onset of action study. Journal of Clinical Psychopharmacology 23: 358–364. [PubMed: 12920411]Benedict 2010
Benedict A, Arellano J, De Cock E, Baird J (2010) Economic evaluation of duloxetine versus serotonin selective reuptake inhibitors and venlafaxine XR in treating major depressive disorder in Scotland. Journal of Affective Disorders 120: 94–104. [PubMed: 19497623]Benkert 1996
Benkert O, Grunder G, Wetzel H, Hackett D (1996) A randomized, double-blind comparison of a rapidly escalating dose of venlafaxine and imipramine in inpatients with major depression and melancholia. Journal of Psychiatric Research 30: 441–451. [PubMed: 9023787]Benkert 2000
Benkert O (2000) Mirtazapine compared with paroxetine in major depression. Journal of Clinical Psychiatry 61: 656–663. [PubMed: 11030486]Benkert 2006
Benkert O, Szegedi A, Philipp M, Kohnen R, Heinrich C, et al. (2006) Mirtazapine orally disintegrating tablets versus venlafaxine extended release. Journal of Clinical Psychopharmacology 26: 75–78. [PubMed: 16415711]Bennie 1995
Bennie EH, Mullin JM, Martindale JJ (1995) A double-blind multicentre trial comparing sertraline and fluoxetine in outpatients with major depression. Journal of Clinical Psychiatry 56: 229–237. [PubMed: 7775364]Bersani 1994
Bersani G, Rapisarda V, Ciani N, Bertolino A, Sorge G (1994) A double-blind comparative study of sertraline and amitriptyline in outpatients with major depressive episodes. Human Psychopharmacology 9: 63–68.Bhargava 2012
Bhargava J, Khan ZY (2012) Comparative evaluation of the effcacy and side effects of imipramine, sertraline and an ayurvedic formulation in patients of depression. Journal of Clinical and Diagnostic Research 6: 220–225.Bielski 2004
Bielski RJ, Ventura D, Chang CC (2004) A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major dperessive disorder. Journal of Clinical Psychiatry 65: 1190–1196. [PubMed: 15367045]Binnemann 2008
Binneman B, Feltner D, Kolluri S, Shi Y, Qiu R, et al. (2008) A 6-week randomized, placebo-controlled trial of CP-316,311 (a selective CRH1 antagonist) in the treatment of major depression. American Journal of Psychiatry 165: 617–620. [PubMed: 18413705]Bjerkenstedt 2005
Bjerkenstedt L, Edman GV, Alken RG, Mannel M (2005) Hypericum extract LI 160 and fluoxetine in mild to moderate depression: a randomized, placebo-controlled multi-center study in outpatients. European Archives of Psychiatry and Clinical Neuroscience 255: 40–47. [PubMed: 15538592]Blackburn 1981
Blackburn IM, Bishop S, Glen AI, Whalley LJ, Christie JE (1981) The efficacy of cognitive therapy in depression: a treatment trial using cognitive therapy and pharmacotherapy, each alone and in combination. British Journal of Psychiatry 139: 181–189. [PubMed: 7317698]Blackburn 1997
Blackburn IM, Moore RM (1997) Controlled acute and follow-up trial of cognitive therapy and pharmacotherapy in out-patients with recurrent depression. British Journal of Psychiatry 171: 328–334. [PubMed: 9373420]Blackwell 2015
Blackwell SE, Browning M, Mathews A, Pictet A, Welch J, et al. (2015) Positive imagery-based cognitive bias modification as a web-based treatment tool for depressed adults: a randomized controlled trial. Clinical Psychological Science 3: 91–111. [PMC free article: PMC4359210] [PubMed: 25984421]Blashki 1971
Blashki TG, Mowbray R, Davies B (1971) Controlled trial of amitriptyline in general practice. British Medical Journal 5741:133–138. [PMC free article: PMC1795147] [PubMed: 4923928]Blom 2007
Blom MB, Jonker K, Dusseldorp E, Spinhoven P, Hoencamp E, et al. (2007) Combination treatment for acute depression is superior only when psychotherapy is added to medication. Psychotherapy and Psychosomatics 76: 289–297. [PubMed: 17700049]Blumenthal 1999/Babyak 2000
Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, et al. (1999) Effects of exercise training on older patients with major depression. Archives of Internal Medicine 159: 2349–2356. [PubMed: 10547175]
Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, et al. (2000). Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosomatic Medicine 62: 633–638. [PubMed: 11020092]Blumenthal 2007/Hoffman 2011
Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, et al. (2007) Exercise and pharmacotherapy in the treatment of major depressive disorder. Pychosomatic Medicine 69:587–596. [PMC free article: PMC2702700] [PubMed: 17846259]
Hoffman BM, Babyak MA, Craighead WE, Sherwood A, Doraiswamy PM, et al. (2011) Exercise and pharmacotherapy in patients with major depression: one-year follow-up of the SMILE study. Psychosomatic Medicine 73: 127–133. [PMC free article: PMC3671874] [PubMed: 21148807]Bose 2008
Bose A, Li D, Gandhi C (2008) Escitalopram in the acute treatment of depressed patients aged 60 years or older. American Journal of Geriatric Psychiatry 16: 14–20. [PubMed: 18165459]Boulenger 2006
Boulenger JP, Huusom AKT, Florea I, Baekdal T, Sarchiapone M (2006) A comparative study of the efficacy of long-term treatment with escitalopram and paroxetine in severely depressed patients. Current Medical Research and Opinion 22: 1331–1341. [PubMed: 16834832]Boulenger 2014
Boulenger JP, Loft H, Olsen CK (2014) Efficacy and safety of vortioxetine (Lu AA21004), 15 and 20 mg/day: a randomized, double-blind, placebo-controlled, duloxetine-referenced study in the acute treatment of adult patients with major depressive disorder. International Clinical Psychopharmacology 29: 138. [PMC free article: PMC3979887] [PubMed: 24257717]Bowman 1995
Bowman D, Scogin F, Lyrene B (1995) The efficacy of self-examination therapy and cognitive bibliotherapy in the treatment of mild to moderate depression. Psychotherapy Research 5: 95–140.Boyer 1998
Boyer P, Danion JM, Bisserbe JC, Hotton JM, Troy S (1998) Clinical and economic comparison of sertraline and fluoxetine in the treatment of depression. Pharmacoeconomics 13: 157–169. [PubMed: 10184835]Brabyn 2016
Brabyn S, Araya R, Barkham M, Bower P, Cooper C, et al. (2016) The second randomised evaluation of the effectiveness, cost-effectiveness and acceptability of computerised therapy (REEACT-2) trial: does the provision of telephone support enhance the effectiveness of computer-delivered cognitive behaviour therapy? a randomised controlled trial. Health Technology Assessment: 1–92. [PMC free article: PMC5165284] [PubMed: 27922448]Brannan 2005
Brannan SK, Mallinckrodt CH, Brown EB, Wohlreich MM, Watkin JG, et al. (2005) Duloxetine 60 mg once-daily in the treatment of painful physical symptoms in patients with major depressive disorder. Journal of Psychiatric Research 39: 43–53. [PubMed: 15504423]Bremner 1984
Bremner JD (1984) Fluoxetine in depressed patients: a comparison with imipramine. Journal of Clinical Psychiatry 45: 414–419. [PubMed: 6384203]Bremner 1995
Bremner JD (1995) A double-blind comparison of Org 3770, amitriptyline, and placebo in major depression. Journal of Clinical Psychiatry 56: 519–525. [PubMed: 7592505]Bulmash 2009
Bulmash E, Harkness KL, Stewart JG, Bagby RM (2009) Personality, stressful life events, and treatment response in major depression. Journal of Consulting and Clinical Psychology 77: 1067–1077. [PubMed: 19968383]Burke 2002
Burke WJ, Gergel I, Bose A (2002) Fixed-dose trial of the single isomer SSRI escitalopram in depressed outpatients. Journal of Clinical Psychiatry 63: 331–336. [PubMed: 12000207]Byerley 1988
Byerley WF, Reimherr FW, Wood DR, Grosser BI (1988) Fluoxetine, a selective serotonin uptake inhibitor, for the treatment of outpatients with major depression. Journal of Clinical Psychopharmacology 8: 112–115. [PubMed: 3286684]CAGO178A2303
CAGO178A2303. A placebo- and paroxetine-controlled study of the efficacy, safety and tolerability of agomelatine (25 or 50 mg) in the treatment of major depressive disorder (MDD). https://clinicaltrials.gov/ct2/show/record/NCT00463242 [accessed August 2019] (data extracted from Cipriani 2018).Carman 1991
Carman JS, Ahdieh H, Wyatt-Knowles E, Warga E, Panagides J (1991) A controlled study of mianserin in moderately to severely depressed outpatients. Psychopharmacology Bulletin 27: 135–139. [PubMed: 1924659]Casabona 2004
Casabona G, Guazzelli M, Silenzi V (2004) Study 428: A randomized, double-blind, comparison of venlafaxine XR and paroxetine in outpatients with moderate to severe major depression. Unpublished (data extracted from 2009 Depression NICE guideline).Cassano 1986
Cassano GB, Conti L, Massimetti G, Mengali F, Waekelin JS, et al. (1986) Use of a standardized documentation system (BLIPS/BDP) in the conduct of a multicenter international trial comparing fluvoxamine, imipramine, and placebo. Psychopharmacology Bulletin 22: 52–58. [PubMed: 3088668]Chang 2015
Chang HH, Chou CH, Yang YK, Lee IH, Chen PS (2015) Association between ABCB1 polymorphisms and antidepressant treatment response in Taiwanese major depressive patients. Clinical Psychopharmacology and Neuroscience 13: 250. [PMC free article: PMC4662166] [PubMed: 26598582]Chiu 1996
Chiu HJ, Hong CJ, Chan CH (1996) Paroxetine in the treatment of Chinese patients with depressive episode: a double-blind randomized comparison with imipramine. Chinese Medical Journal 57: 418–423. [PubMed: 8803304]Choi 2012
Choi I, Zou J, Titov N, Dear BF, Li S, et al. (2012) Culturally attuned Internet treatment for depression amongst Chinese Australians: a randomised controlled trial. Journal of Affective Disorders 136: 459–468. [PubMed: 22177742]Choi 2014a/Choi 2014b
Choi NG, Hegel MT, Marti CN, Marinucci ML, Sirrianni L, et al. (2014) Telehealth problem-solving therapy for depressed low-income homebound older adults. American Journal of Geriatric Psychiatry 22: 263–271. [PMC free article: PMC3519946] [PubMed: 23567376]
Choi NG, Marti CN, Bruce ML, Hegel MT, Wilson NL, et al. (2014) Six-month postintervention depression and disability outcomes of in-home telehealth problem-solving therapy for depressed, low-income homebound older adults. Depression and Anxiety 31: 653–661. [PMC free article: PMC4122624] [PubMed: 24501015]Chouinard 1999
Chouinard G, Saxena B, Bélanger MC, Ravindran A, Bakish D, et al. (1999) A Canadian multicenter, double-blind study of paroxetine and fluoxetine in major depressive disorder. Journal of Affective Disorders 54: 39–48. [PubMed: 10403145]Christiansen 1996
Christiansen PE, Behnke K, Black CH, Ohrstrom JK, Bork RH, et al. (1996) Paroxetine and amitriptyline in the treatment of depression in general practice. Acta Psychiatrica Scandinavica 93: 158–163. [PubMed: 8739658]Cipriani 2018
Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, et al. (2018) Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Focus 16: 420–429. [PMC free article: PMC6996085] [PubMed: 32021580]CL3-20098-022
CL3-20098-022. CMHP assessment report for Thymanax. Procedure No. EMEA/H/C/000916. https://www.ema.europa .eu/en/documents/assessment-report /thymanax-epar-public-assessment-report_en .pdf [accessed Augist 2019] (data extracted from Cipriani 2018). CL3-20098-023
CL3-20098-023. CMHP assessment report for valdoxan. Procedure No. EMEA/H/C/000915. https://www.ema.europa .eu/en/documents/assessment-report /valdoxan-epar-public-assessment-report_en .pdf [accessed August 2019] (data extracted from Cipriani 2018). CL3-20098-024
CL3-20098-024. CMHP assessment report for thymanax. Procedure No. EMEA/H/C/000916. https://www.ema.europa .eu/en/documents/assessment-report /thymanax-epar-public-assessment-report_en .pdf [accessed August 2019] (data extracted from Cipriani 2018). Claghorn 1992a
Claghorn J (1992) A double-blind comparison of paroxetine and placebo in the treatment of depressed outpatients. International Clinical Psychopharmacology 6(suppl. 4): 25–30. [PubMed: 1431007]Claghorn 1992b
Claghorn JL (1992) The safety and efficacy of paroxetine compared with placebo in a double-blind trial of depressed outpatients. Journal of Clinical Psychiatry 53(suppl.): 33–35. [PubMed: 1531821]Clayton 2006_study 1
Clayton AH, Croft HA, Horrigan JP, Wightman DS, Krishen A, et al. (2006) Bupropion extended release compared with escitalopram. Effects on sexual functioning and antidepressant efficacy in 2 randomised, double-blind, placebo-controlled studies. Journal of Clinical Psychiatry 67: 736–746. [PubMed: 16841623]Clayton 2006_study 2
Clayton AH, Croft HA, Horrigan JP, Wightman DS, Krishen A, et al. (2006) Bupropion extended release compared with escitalopram. Effects on sexual functioning and antidepressant efficacy in 2 randomised, double-blind, placebo-controlled studies. Journal of Clinical Psychiatry 67: 736–746. [PubMed: 16841623]Clerc 1994
Clerc GE, Ruimy P, Verdeau-Palles J (1994) A double-blind comparison of venlafaxine and fluoxetine in patients hospitalized for major depression and melancholia. International Clinical Psychopharmacology 9: 139–143. [PubMed: 7814822]Cohn 1984a
Cohn JB, Varga L, Lyford A (1984) A two-center double-blind study of nomifensine, imipramine, and placebo in depressed geriatric outpatients. Journal of Clinical Psychiatry 45: 68–72 (data extracted from 2009 Depression NICE guideline). [PubMed: 6370980]Cohn 1984b
Cohn JB, Wilcox C (1984) A comparison of fluoxetine, imipramine and placebo in patients with depressive disorder. Journal of Clinical Psychiatry 45: 414–419. [PubMed: 3882677]Cohn 1990b
Cohn CK, Shrivastava R, Mendels J, Cohn JB, Fabre LF, et al. (1990) Double-blind, mulitcenter comparison of sertraline and amitriptyline in elderly depressed patients. Journal of Clinical Psychiatry 51(suppl. B): 28–33 (data extracted from Cipriani 2018). [PubMed: 2258379]Cohn 1996
Cohn CK, Robinson DS, Roberts DL, Schwiderski UE, O’Brien K, et al. (1996) Responders to antidepressant drug treatment: a study comparing nefazodone, imipramine, and placebo in patients with major depression. Journal of Clinical Psychiatry 57(suppl. 2): 15–18. [PubMed: 8626358]Coleman 2001
Coleman CC, King BR, Bolden-Watson C, Book MJ, Segraves RT, et al. (2001) A placebo-controlled comparison of the effects on sexual functioning of bupropion sustained release and fluoxetine. Clinical Therapeutics 23:1040–1058. [PubMed: 11519769]Colonna 2005
Colonna L, Andersen HF, Reines EH (2005) A randomised, double-blind, 24 -week study of escitalopram (10 mg/day), versus citalopram (20 mg/day) in primary care patients with major depressive disorder. Current Medical Research and Opinion 21: 1659–1668. [PubMed: 16238906]Connolly Gibbons 2012
Gibbons MB, Thompson SM, Scott K, Schauble LA, Mooney T, et al. (2012) Supportive-expressive dynamic psychotherapy in the community mental health system: a pilot effectiveness trial for the treatment of depression. Psychotherapy 49: 303. [PMC free article: PMC3784980] [PubMed: 22962971]Connolly Gibbons 2016
Gibbons MB, Gallop R, Thompson D, Luther D, Crits-Christoph K, et al. (2016) Comparative effectiveness of cognitive therapy and dynamic psychotherapy for major depressive disorder in a community mental health setting: a randomized clinical noninferiority trial. JAMA Psychiatry 73: 904–911. [PMC free article: PMC5627972] [PubMed: 27487573]Cook 1999
Cook IA, Leuchter AF, Witte E, Abrams M, Uijtdehaage SH, et al. (1999) Neurophysiologic predictors of treatment response to fluoxetine in major depression. Psychiatry Research 85: 263–273. [PubMed: 10333379]Cornelius 1997
Cornelius JR, Salloum IM, Ehler JG, Jarrett PJ, Cornelius MD, et al. (1997) Fluoxetine in depressed alcoholics: a double-blind, placebo-controlled trial. Archives of General Psychiatry 54: 700–705. [PubMed: 9283504]Corrigan 2000
Corrigan MH, Denahan AQ, Wright CE, Ragual RJ, Evans DL (2000) Comparison of pramipexole, fluoxetine, and placebo in patients with major depression. Depression & Anxiety 11: 58–65. [PubMed: 10812530]Costa 1998
Costa e Silva (1998) Randomized, double-blind comparison of venlafaxine and fluoxetine in outpatients with major depression. Journal of Clinical Psychiatry 59: 352–357. [PubMed: 9714263]Costa 2016
Costa A, Barnhofer T (2016) Turning towards or turning away: a comparison of mindfulness meditation and guided imagery relaxation in patients with acute depression. Behavioural & Cognitive Psychotherapy 44: 410–419. [PubMed: 26190664]Covi 1987
Covi L, Lipman RS (1987) Cognitive behavioral group psychotherapy combined with imipramine in major depression. Psychopharmacology Bulletin 23: 173–176. [PubMed: 3602315]Cunningham 1994
Cunningham LA, Borison RL, Carman JS, Chouinard G, Crowder JE, et al. (1984) A comparison of venlafaxine, trazodone, and placebo in major depression. Journal of Clinical Psychopharmacology 14: 99–106. [PubMed: 8195464]Cunningham 1997
Cunningham LA (1997) Once-daily venlafaxine extended release (XR) and venlafaxine immediate release (IR) in outpatients with major depression. Annals of Clinical Psychiatry 9: 157–164. [PubMed: 9339881]Cutler 2009
Cutler AJ, Montgomery SA, Feifel D, Lazarus A, Astrom M, et al. (2009) Extended release quetiapine fumarate monotherapy in major depressive disorder: a placebo- and duloxetine-controlled study. Journal of Clinical Psychiatry 70: 526–539. [PubMed: 19358790]Danish University Antidepressant Group 1986
Danish University Antidepressant Group (1986) Citalopram: clinical effect profile in comparison with clomipramine: a controlled multicenter study. Psychopharmacology 90: 131–138. [PubMed: 2876451]Danish University Antidepressant Group 1990
Danish University Antidepressant Group (1990) Paroxetine: a selective serotonin reuptake inhibitor showing better tolerance, but weaker antidepressant effect than clomipramine in a controlled multicenter study. Journal of Affective Disorders 18: 289–299. [PubMed: 2140382]Danish University Antidepressant Group 1999
Danish University Antidepressant Group (1999) Clomipramine dose-effect study in patients with depression: clinical end points and pharmacokinetics. Clinical Pharmacology & Therapeutics 66:152–165. [PubMed: 10460069]De Ronchi 1998
De Ronchi D, Rucci P, Lodi M, Ravaglia G, Forti P, et al. (1998) Fluoxetine and amitriptyline in elderly depressed patients. A 10-week, double-blind study on course of neurocognitive adverse events and depressive symptoms. Archives Gerontology Geriatrics 6(suppl.): 125–140.DeJonghe 2001/Kool 2003
De Jonghe FE, Kool S, Van Aalst G, Dekker J, Peen J (2001) Combining psychotherapy and antidepressants in the treatment of depression. Journal of Affective disorders 64: 217–229. [PubMed: 11313088]
Kool S, Dekker J, Duijsens IJ, de Jonghe F, Puite B (2003) Efficacy of combined therapy and pharmacotherapy for depressed patients with or without personality disorders. Harvard Review of Psychiatry 11: 133–141. [PubMed: 12893503]Dekker 2005
Dekker J, Molenaar PJ, Kool S, Van Aalst G, Peen J, et al. (2005) Dose–effect relations in time-limited combined psycho-pharmacological treatment for depression. Psychological Medicine 35: 47–58. [PubMed: 15842028]Delgadillo 2015
Delgadillo J, Gore S, Ali S, Ekers D, Gilbody S, et al. (2015) Feasibility randomized controlled trial of cognitive and behavioral interventions for depression symptoms in patients accessing drug and alcohol treatment. Journal of Substance Abuse Treatment 55: 6–14. [PubMed: 25819701]Demyttenaere 1998
Demyttenaere K, Van Ganse E, Gregoire J, Gaens E, Mesters P (1998) Compliance in depressed patients treated with fluoxetine or amitriptyline. International Clinical Psychopharmacology 13: 11–17. [PubMed: 9988362]DeNayer 2002
De Nayer A, Geerts S, Ruelens L, Schittecatte M, De Bleeker E, et al. (2002) Venlafaxine compared with fluoxetine in outpatients with depression and concomitant anxiety. International Journal of Neuropsychopharmacology 5: 115–120. [PubMed: 12135535]Detke 2002a
Detke MJ, Lu Y, Goldstein DJ, McNamara RK, Demitrack MA (2002) Duloxetine 60 mg once daily dosing versus placebo in the acute treatment of major depression. Journal of Psychiatric Research 36: 383–390. [PubMed: 12393307]Detke 2002b
Detke MJ, Lu Y, Goldstein DJ, Hayes JR, Demitrack MA (2002) Duloxetine, 60 mg once daily, for major depressive disorder: a randomized double-blind placebo-controlled trial. Journal of Clinical Psychiatry 63: 308–315. [PubMed: 12000204]Detke 2004
Detke MJ, Wiltse CG, Mallinckrodt CH, McNamara RK, Demitrack MA, et al. (2004) Duloxetine in the acute and long-term treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. European Neuropsychopharmacology 14: 457–470. [PubMed: 15589385]Deushle 2003
Deuschle M, Hamann B, Meichel C, Krumm B, Lederbogen F, et al. (2003) Antidepressive treatment with amitriptyline and paroxetine: effects on saliva cortisol concentrations. Journal of Clinical Psychopharmacology 23: 201–205. [PubMed: 12640223]DeWilde 1993
De Wilde J, Spiers R, Mertens C, Bartholome F, Schotte G, et al. (1993) A double-blind, comparative, multicentre study comparing paroxetine with fluoxetine in depressed patients. Acta Psychiatrica Scandinavica 87: 141–145. [PubMed: 8447241]Diaz-Martinez 1998
Diaz-Martinez A, Benassinni O, Ontiveros A, Gonzalez S, Salin R, et al. (1998) A randomized, open-label comparison of venlafaxine and fluoxetine in depressed outpatients. Clinical Therapeutics 20: 467–476. [PubMed: 9663362]Dierick 1996
Dierick M, Ravizza L, Realini R, Martin A (1996) A double-blind comparison of venlafaxine and fluoxetine for treatment of major depression in outpatients. Progress in NeuroPsychopharmacology & Biological Psychiatry 20: 57–71. [PubMed: 8861177]Doogan 1994
Doogan DP, Langdon CJ (1994) A double-blind, placebo-controlled comparison of sertraline and dothiepin in the treatment of major depression in general practice. International Clinical Psychopharmacology 9: 95–100. [PubMed: 8057000]Doongaji 1993
Doongaji DR, Bal S, Rajkumar S, Seth AS (1993) Multicentre double-blind comparison of lofepramine and amitriptyline in the treatment of major depressive disorders in Indian patients. British Journal Clinical Research 4: 45–53.Dowrick 2000
Dowrick C, Dunn G, Ayuso-Mateos JL, Dalgard OS, Page H, et al. (2000) Problem solving treatment and group psychoeducation for depression: multicentre randomised controlled trial. BMJ 321: 1450. [PMC free article: PMC27549] [PubMed: 11110739]Driessen 2013
Driessen E, Van HL, Don FJ, Peen J, Kool S, et al. (2013) The efficacy of cognitive-behavioral therapy and psychodynamic therapy in the outpatient treatment of major depression: a randomized clinical trial. American Journal of Psychiatry 170: 1041–1050. [PubMed: 24030613]Du 2005
Du YH, Li GP, Yan H, Zhang XJ, Huang LF (2005) Clinical study on needling method for regulating mental activities and soothing liver for treatment of melancholia. Chinese Acupuncture & Moxibustion 25: 151–154. [PubMed: 16312916]Duan 2009
Duan DM, Tu Y, Chen LP, Wu ZJ (2009) Efficacy evaluation for depression with somatic symptoms treated by electroacupuncture combined with fluoxetine. Journal of Traditional Chinese Medicine 29: 167–173. [PubMed: 19894377]Dube 2010
Dubé S, Dellva MA, Jones M, Kielbasa W, Padich R, et al. (2010) A study of the effects of LY2216684, a selective norepinephrine reuptake inhibitor, in the treatment of major depression. Journal of Psychiatric Research 44: 356–363. [PubMed: 19909980]Dubey 2012
Dubey AK, Raichandani O, Pandey SP (2012) Efficacy of duloxetine compared with imipramine in the treatment of major depressive disorder in Indian patients. International Journal of Pharmacy and Bio Sciences 3: 133–141.Dunbar 1993
Dunbar GC, Claghorn JL, Kiev A, Rickels K, Smith WT (1993) A comparison of paroxetine and placebo in depressed outpatients. Acta Psychiatrica Scandinavica 87: 302–305. [PubMed: 8517168]Dunn 2005
Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO (2005) Exercise treatment for depression: efficacy and dose response. American Journal of Preventive Medicine 28: 1–8. [PubMed: 15626549]E-1569
E-1569. https://www.iqwig.de /download/A05-20C_Abschlussbericht _1-1_Bupropion _Mirtazapin_und _Reboxetin_bei_Depressionen.pdf (data extracted from Cipriani 2018). Egede 2015
Egede LE, Acierno R, Knapp RG, Lejuez C, Hernandez-Tejada M, et al. (2015) Psychotherapy for depression in older veterans via telemedicine: a randomised, open-label, non-inferiority trial. Lancet Psychiatry 2: 693–701. [PubMed: 26249300]Ekers 2011
Ekers D, Richards D, McMillan D, Bland JM, Gilbody S (2011) Behavioural activation delivered by the non-specialist: phase II randomised controlled trial. British Journal of Psychiatry 198: 66–72. [PubMed: 21200079]Ekselius 1997
Ekselius L, Eberhard G (1997) A double-blind multicenter trial comparing sertraline and citalopram in patients with major depression treated in general practice. International Clinical Psychopharmacology 12: 323–331. [PubMed: 9547134]Eli Lilly HMAT-A
Eli Lilly study F1J-MC-HMAT-A, CT Registry ID# 4091. Duloxetine versus placebo and paroxetine in the acute treatment of major depression. Study Group A. Clinicaltrialresults.org [date site accessed 13.06.08] (data extracted from 2009 Depression NICE guideline).Elkin 1989/Imber 1990
Elkin I, Shea M, Watkins JT, Imber SD, Sotsky SM, et al. (1989) National institute of mental health treatment of depression collaborative research program: general effectiveness of treatments. Archives of General Psychiatry 46: 971–982. [PubMed: 2684085]
Imber SD, Pilkonis PA, Sotsky SM, Elkin I, Watkins JT, et al. (1990) Mode-specific effects among three treatments for depression. Journal of Consulting & Clinical Psychology 58: 352–359. [PubMed: 2195085]Emsley 2018
Emsley R, Ahokas A, Suarez A, Marinescu D, Dóci I, et al. (2018) Efficacy of tianeptine 25–50 mg in elderly patients with recurrent major depressive disorder: an 8-week placebo-and escitalopram-controlled study. Journal of Clinical Psychiatry 79: doi: 10.4088/jcp.17m11741. [PubMed: 29995359] [CrossRef]Escobar 1980
Escobar JI, Gomez J, Constain C, Rey J, Santacruz H (1980) Controlled clinical trial with trazodone, a novel antidepressant: a South American experience. Journal of Clinical Pharmacology 20: 124–130. [PubMed: 6991533]Fabre 1980
Fabre L, Mclendon D (1980) A double-blind study comparing the efficacy and safety of alprazolam with imipramine and placebo in primary depression. Current Therapeutic Research 27: 474–482.Fabre 1985
Fabre L, Crismon L (1985) Efficacy of fluoxetine in outpatients with major depression. Current Therapeutic Research 37: 115–123.Fabre 1991
Fabre LF, Scharf MB, Itil TM (1991) Comparative efficacy and safety of nortriptyline and fluoxetine in the treatment of major depression: a clinical study. Journal of Clinical Psychiatry 52(suppl.): 62–67. [PubMed: 2050651]Fabre 1992
Fabre LF (1992) A 6-week, double-blind trial of paroxetine, imipramine, and placebo in depressed outpatients. Journal of Clinical Psychiatry 53(suppl.): 40–43. [PubMed: 1531823]Fabre 1995a
Fabre L, Abuzzahab F, Amin M, Claghorn J, Mendels J, et al. (1995) Sertraline in major depression: double-blind comparison with placebo. Biological Psychiatry 38: 592–602. [PubMed: 8573661]Fagiolini 2020
Fagiolini A, Albert U, Ferrando L, Herman E, Muntean C, et al. (2020) A randomized, double-blind study comparing the efficacy and safety of trazodone once-a-day and venlafaxine extended-release for the treatment of patients with major depressive disorder. International Clinical Psychopharmacology 35: 137. [PMC free article: PMC7099841] [PubMed: 31972628]Farabaugh 2015
Farabaugh A, Fisher L, Nyer M, Holt D, Cohen M, et al. (2015) Similar changes in cognitions following cognitive-behavioral therapy or escitalopram for major depressive disorder: implications for mechanisms of change. Annals of Clinical Psychiatry 27: 118–126. [PubMed: 25954938]Farrer 2011/Farrer 2012
Farrer L, Christensen H, Griffiths KM, Mackinnon A (2011) Internet-based CBT for depression with and without telephone tracking in a national helpline: randomised controlled trial. PloS One 6: e28099. [PMC free article: PMC3227639] [PubMed: 22140514]
Farrer L, Christensen H, Griffiths KM, Mackinnon A (2012) Web-based cognitive behavior therapy for depression with and without telephone tracking in a national helpline: secondary outcomes from a randomized controlled trial. Journal of Medical Internet Research 14: 60–69. [PMC free article: PMC3414870] [PubMed: 22738715]Fava 1998a
Fava M, Amsterdam JD, Deltito JA, Salzman C, Schwaller M, et al. (1998) A double-blind study of paroxetine, fluoxetine, and placebo in outpatients with major depression. Annals of Clinical Psychiatry 10: 145–150. [PubMed: 9988054]Fava 2002a
Fava M, Hoog SL, Judge RA, Kopp JB, Nilsson ME, et al. (2002) Acute efficacy of fluoxetine versus sertraline and paroxetine in major depressive disorder including effects of baseline insomnia. Journal of Clinical Psychopharmacology 22: 137–147. [PubMed: 11910258]Fava 2005
Fava M, Alpert J, Nierenberg AA, Mischoulon D, Otto MW, et al. (2005) A double-blind, randomized trial of St John’s wort, fluoxetine, and placebo in major depressive disorder. Journal of Clinical Psychopharmacology 25: 441–447. [PubMed: 16160619]Fawcett 1989
Fawcett J, Zajecka J, Kravitz H (1989) Fluoxetine vs amitriptyline in adult inpatients with major depression. Current Therapeutic Research 45: 821–832.FDA 244 (EMD 68 843-009)
http://www.accessdata .fda.gov/drugsatfda_docs /nda/2011/022567Orig1s000MedR.pdf (data extracted from Cipriani 2018). FDA 245 (EMD 68 843-010)
http://www.accessdata .fda.gov/drugsatfda_docs /nda/2011/022567Orig1s000MedR.pdf (data extracted from Cipriani 2018). FDA 246 (SB 659746-003)
http://www.accessdata .fda.gov/drugsatfda_docs /nda/2011/022567Orig1s000MedR.pdf (data extracted from Cipriani 2018). Feiger 1996
Feiger AD (1996) A double-blind comparison of gepirone extended release, imipramine, and placebo in the treatment of outpatient major depression. Psychopharmacology Bulletin 32: 659–665. [PubMed: 8993088]Feighner 1979
Feighner JP, Brauzer B, Gelenberg AJ, Gomez E, Kiev A, et al. (1979) A placebo-controlled multicenter trial of limbitrol versus its components (amitriptyline and chlordiazepoxide) in the symptomatic treatment of depressive illness. Psychopharmacology 61: 217–225. [PubMed: 108739]Feighner 1982
Feighner JP, Meridith CH, Dutt JE, Hendrickson GG (1982) A double blind comparison of lofepramine, imipramine and placebo in patients with depression. Acta Psychiatrica Scandinavica 66: 100–108. [PubMed: 6753500]Feighner 1983c
Feighner JP, Meredith CH, Frost NR, Chammas S, Hendrickson G (1983) A double-blind comparison of alprazolam vs. imipramine and placebo in the treatment of major depressive disorder. Acta Psychiatrica Scandinavica 68: 223–233. [PubMed: 6138925]Feighner 1989b
Feighner JP, Pambakian R, Fowler RC, Boyer WF, D’Amico MF (1989) A comparison of nefazodone, imipramine, and placebo in patients with moderate to severe depression. Psychopharmacology Bulletin 25: 219–221. [PubMed: 2690165]Feighner 1993
Feighner JP, Cohn JB, Fabre LFJ, Fieve RR, Mendels J, et al. (1993) A study comparing paroxetine placebo and imipramine in depressed patients. Journal of Affective Disorders 28: 71–79. [PubMed: 8354771]Feighner 1999
Feighner JP, Overo K (1999) Multicenter, placebo-controlled, fixed-dose study of citalopram in moderate-to-severe depression. Journal of Clinical Psychiatry 60: 824–830. [PubMed: 10665628]Finkel 1999
Finkel SI, Richter EM, Clary CM, Batzar E (1999) Comparative efficacy of sertraline vs. fluoxetine in patients age 70 or over with major depression. American Journal of Geriatric Psychiatry 7: 221–227. [PubMed: 10438693]Fonagy 2020
Fonagy P, Lemma A, Target M, O’Keeffe S, Constantinou MP, et al. (2020) Dynamic interpersonal therapy for moderate to severe depression: a pilot randomized controlled and feasibility trial. Psychological Medicine 50:1010–1019. [PubMed: 31084635]Fontaine 1994
Fontaine R, Ontiveros A, Elie R, Kensler TT, Roberts DL, et al. (1994) A double-blind comparison of nefazodone, imipramine, and placebo in major depression. Journal of Clinical Psychiatry 55: 234–241. [PubMed: 8071277]Forest Laboratories 2000
Forest Laboratories Inc. Flexible dose comparison of the saftey and efficacy of lu 26-054, citalopram, and placebo in the treatment of major depressive disorder (SCT-MD-02). Report date: December 5, 2000 (data extracted from 2009 Depression NICE guideline).Forest Laboratories 2010
https://clinicaltrials.gov/ct2/show/NCT00668525 (data extracted from Cipriani 2018).Forest Research Institute 2003
Forest Research Institute. Two-week double-blind placebo controlled study of escitalopram in the treatment of severe major depression. Report date: November 26, 2003 (data extracted from 2009 Depression NICE guideline).Forest Research Institute 2004a
Forest Research Institute. Flexible dose comparison of the safety and efficacy of escitalopram and fluoxetine in the treatment of major depressive disorder (SCT-MD-16). Report date: July 26, 2004 (data extracted from 2009 Depression NICE guideline).Forest Research Institute 2005
Forest Research Institute. A double-blind flexible dose comparison of escitalopram sertraline and placebo in the treatment of major depressive disorder. Report date: February 7, 2005 (data extracted from 2009 Depression NICE guideline).Forlenza 2001
Forlenza OV, Almeida OP, Stoppe A, Hirata ES, Ferreira RC (2001) Antidepressant efficacy and safety of low-dose sertraline and standard-dose imipramine for the treatment of depression in older adults: results from a double-blind, randomized, controlled clinical trial. International Psychogeriatrics 13: 75–84. [PubMed: 11352337]Freed 1999
Freed E, Goldney R, Lambert TT (1999) A double-blind, multicentre study to assess the tolerability and efficacy of paroxetine compared with amitriptyline in the treatment of depressed patients in Australian general practice. Australian and New Zealand Journal of Psychiatry 33: 416–421. [PubMed: 10442799]Fu 2003
Fu WB (2003) Observations on the curative effect of acupuncture on depressive neurosis. Journal of Acupuncture and Tuina Science 1: 15–17.Fu 2008
Fu WB, Fan L, Zhu XP, He Q, Wang L, et al. (2008) Acupuncture for treatment of depressive neurosis: a multi-center randomized controlled study. Chinese Acupuncture & Moxibustion 28: 3–6 (data extracted from Smith 2018). [PubMed: 18257177]Fudge 1990
Fudge JL, Perry PJ, Garvey MJ, Kelly MW (1990) A comparison of the effect of fluoxetine and trazodone on the cognitive functioning of depressed outpatients. Journal of Affective Disorders 18: 275–280. [PubMed: 2140380]Gagiano 1993
Gagiano CA (1993) A double blind comparison of paroxetine and fluoxetine in patients with major depression. British Journal of Clinical Research 4: 145–152.Gallagher-Thompson 1994
Gallagher-Thompson DE, Steffen AM (1994) Comparative effects of cognitive behavioral therapy and brief psychodynamic psychotherapies for depressed family caregivers. Journal of Consulting and Clinical Psychology 62: 543–549. [PubMed: 8063980]Gasto 2003
Gastó C, Navarro V, Marcos T, Portella MJ, Torra M, et al. (2003) Single-blind comparison of venlafaxine and nortriptyline in elderly major depression. Journal of Clinical Psychopharmacology 23: 21–26. [PubMed: 12544371]Gater 2010
Gater R, Waheed W, Husain N, Tomenson B, Aseem S, et al. (2010) Social intervention for British Pakistani women with depression: randomised controlled trial. British Journal of Psychiatry 197: 227–233. [PubMed: 20807969]Geddes 1999
Geddes JR, Freemantle N, Mason J, Eccles M, Boynton J (1999) Selective serotonin reuptake inhibitors (SSRIs) versus other antidepressants for depression. Cochrane Database of Systematic Reviews 4: Art. No. CD001851. [PubMed: 17636689]Gelenberg 1990a
Gelenberg AJ, Wojcik JD, Falk WE, Spring B, Brotman AW, et al. (1990) Clovoxamine in the treatment of depressed outpatients: a double-blind, parallel-group comparison against amitriptyline and placebo. Comprehensive Psychiatry 31: 307–314. [PubMed: 2201481]Gentil 2000
Gentil V, Kerr-Correa F, Moreno R, Busnello ED, de Campos JA, et al. (2000) Double-blind comparison of venlafaxine and amitriptyline in outpatients with major depression with or without melancholia. Journal of Psychopharmacology 14: 61–66. [PubMed: 10757255]Georgotas 1982
Georgotas A, Krakowski M, Gershon S (1982) Controlled trial of zimelidine, a 5-HT reuptake inhibitor, for treatment of depression. American Journal of Psychiatry 139: 1057–1058. [PubMed: 6211994]Georgotas 1986
Georgotas A (1986) Comparative efficacy and safety of MAOIs versus TCAs in treating depression in the elderly. Biological Psychiatry 21:1155–1166. [PubMed: 3756264]Gerber 2020
Gerber M, Imboden C, Beck J, Brand S, Colledge F, et al. (2020) Effects of aerobic exercise on cortisol stress reactivity in response to the trier social stress test in inpatients with major depressive disorders: a randomized controlled trial. Journal of Clinical Medicine 9: 1419. [PMC free article: PMC7291068] [PubMed: 32403243]Geretsegger 1994
Geretsegger C, Böhmer F, Ludwig M (1994) Paroxetine in the elderly depressed patient: randomized comparison with fluoxetine of efficacy, cognitive and behavioural effects. International Clinical Psychopharmacology 9: 25–29. [PubMed: 8195578]Geretsegger 1995
Geretsegger C, Stuppaeck CH, Mair M, Platz T, Fartacek R, et al. (1995) Multicenter double blind study of paroxetine and amitriptyline in elderly depressed inpatients. Psychopharmacology 119: 277–281. [PubMed: 7675961]Gershon 1981
Gershon S, Mann J, Newton R, Gunther BJ (1981) Evaluation of trazodone in the treatment of endogenous depression: results of a multicenter double-blind study. Journal of Clinical Psychopharmacology 1: 39S–44S. [PubMed: 7028798]Gilbody 2015/Littlewood 2015
Gilbody S, Littlewood E, Hewitt C, Brierley G, Tharmanathan P, et al. (2015) Computerised cognitive behaviour therapy (cCBT) as treatment for depression in primary care (REEACT trial): large scale pragmatic randomised controlled trial. BMJ 351: h5627. [PMC free article: PMC4641883] [PubMed: 26559241]
Littlewood E, Duarte A, Hewitt C, Knowles S, Palmer S, et al. (2015) A randomised controlled trial of computerised cognitive behaviour therapy for the treatment of depression in primary care: the randomised evaluation of the effectiveness and acceptability of computerised therapy (REEACT) trial. Health Technology Assessment 19(101). [PMC free article: PMC4781165] [PubMed: 26685904]
Duarte A, Walker S, Littlewood E, Brabyn S, Hewitt C, et al. (2017) Cost-effectiveness of computerized cognitive-behavioural therapy for the treatment of depression in primary care: findings from the randomised evaluation of the effectiveness and acceptability of computerised therapy (REEACT) trial. Psychological Medicine 47: 1825–1835. [PubMed: 28228182]Godlewska 2012
Godlewska BR, Norbury R, Selvaraj S, Cowen PJ, Harmer CJ (2012) Short-term SSRI treatment normalises amygdala hyperactivity in depressed patients. Psychological Medicine 42: 2609–2617. [PMC free article: PMC3488813] [PubMed: 22716999]Goldberg 1980
Goldberg HL, Finnerty RJ (1980) Trazodone in the treatment of neurotic depression. Journal of Clinical Psychiatry 41: 430–434. [PubMed: 7440519]Golden 2002_448
Golden RN, Nemeroff CB, McSorley P, Pitts CD, Dube EM (2002) Efficacy and tolerability of controlled-release and immediate-release paroxetine in the treatment of depression. Journal of Clinical Psychiatry 63: 577–584 (data extracted from Cipriani 2018). [PubMed: 12143913]Golden 2002_449
Golden RN, Nemeroff CB, McSorley P, Pitts CD, Dube EM (2002) Efficacy and tolerability of controlled-release and immediate-release paroxetine in the treatment of depression. Journal of Clinical Psychiatry 63: 577–584 (data extracted from Cipriani 2018). [PubMed: 12143913]Goldstein 2002
Goldstein DJ, Mallinckrodt C, Lu Y, Demitrack MA (2002) Duloxetine in the treatment of major depressive disorder: a double-blind clinical trial. Journal of Clinical Psychiatry 63: 225–231. [PubMed: 11926722]Goldstein 2004
Goldstein DJ, Lu Y, Detke MJ, Wiltse C, Mallinckrodt C, Demitrack MA (2004) Duloxetine in the treatment of depression: a double-blind placebo-controlled comparison with paroxetine. Journal of Clinical Psychopharmacology 24: 389–399. [PubMed: 15232330]Goodarzi 2015
Goodarzi K, Astaneh N, Mazinani R, Alibeigi N, Mousavi Z (2015) Comparison of therapeutic effects of mirtazaine and citalopram on outpatients with major depressive disorder and anxiety symptoms. World Journal of Pharmaceutical Research 4: 346–357 (data extracted from Cipriani 2018).Griebel 2012_Study DFI5878
Griebel G, Beeské S, Stahl SM (2012) The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: results from 4 randomized, double-blind, placebo-controlled studies. Journal of Clinical Psychiatry 73: 1403–1411. [PubMed: 23146246]Griebel 2012_Study DFI5879
Griebel G, Beeské S, Stahl SM (2012) The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: results from 4 randomized, double-blind, placebo-controlled studies. Journal of Clinical Psychiatry 73: 1403–1411. [PubMed: 23146246]GSK_29060/103
GlaxoSmithKline (1993) A double-blind, multicentre study comparing the efficacy, tolerability and effects on cognitive function of paroxetine with those of lofepramine in elderly depressed hospital in- or out-patients. GSK - Clinical Study Register 29060/103.Gual 2003
Gual A, Balcells M, Torres M, Madrigal M, Diez T, et al. (2003) Sertraline for the prevention of relapse in detoxicated alcohol dependent patients with a comorbid depressive disorder: a randomized controlled trial. Alcohol and Alcoholism 38: 619–625. [PubMed: 14633652]Guelfi 1995
Guelfi JD, White C, Hackett D, Guichoux JY, Magni G (1995) Effectiveness of venlafaxine in patients hospitalized for major depression and melancholia. Journal of Clinical Psychiatry 56: 450–458. [PubMed: 7559370]Guelfi 2001
Guelfi JD, Ansseau M, Timmerman L, Korsgaard S, Mirtazapine-Venlafaxine Study Group (2001) Mirtazapine versus venlafaxine in hospitalized severely depressed patients with melancholic features. Journal of Clinical Psychopharmacology 21: 425–431. [PubMed: 11476127]Guifeng 2015
Guifeng S (2015) The role of setting - up exercise on rehabilitation in patients with depressive episode. Chinese Journal of Health Psychology 23: 184–186 (data extracted from Krogh 2017).Guillibert 1989
Guillibert E, Pelicier Y, Archambault JC, Chabannes JP, Clerc G, et al. (1989) A double-blind, multicentre study of paroxetine versus clomipramine in depressed elderly patients. Acta Psychiatrica Scandinavica 350: 132–134. [PubMed: 2530766]Hackett 1996
Hackett D (1996) A randomized double blind placebo controlled fixed dose study of the efficacy and safety of venlafaxine extended release and paroxetine in depressed outpatients. Unpublished (data extracted from 2009 Depression NICE guideline).Halikas 1995
Halikas JA (1995) Org 3770 (mirtazapine) versus trazodone: a placebo controlled trial in depressed elderly patients. Human Psychopharmacology: Clinical and Experimental 10(suppl. 2): S125–S133.Hamamci 2006
Hamamci Z (2006) Integrating psychodrama and cognitive behavioral therapy to treat moderate depression. The Arts in Psychotherapy 33: 199–207.Hamdan-Mansour 2009
Hamdan-Mansour AM, Puskar K, Bandak AG (2009) Effectiveness of cognitive-behavioral therapy on depressive symptomatology, stress and coping strategies among Jordanian university students. Issues in Mental Health Nursing 30: 188–196. [PubMed: 19291496]Hao 2014
Hao X, Yang F, Kuang H, Shi J, Du B, et al. (2014) Duloxetine hydrochloride enteric tablets in the treatment of major depressive disorder: a multicentre, randomised, double-blind and positive drug parallel controlled clinical trial. Chinese Journal of New Drugs 23: 2767–2780 (data extracted from Cipriani 2018).Hashemi 2012
Hashemi SN, Shirazi HG, Mohammadi A, Zadeh-Bagheri GH, Noorian KH, et al. (2012) Nortriptyline versus fluoxetine in the treatment of major depressive disorder: a six-month, double-blind clinical trial. Clinical Pharmacology: Advances and Applications 4: 1. [PMC free article: PMC3284259] [PubMed: 22359466]Hatcher 2018
Hatcher S, Whittaker R, Patton M, Miles WS, Ralph N, et al. (2018) Web-based therapy plus support by a coach in depressed patients referred to secondary mental health care: randomized controlled trial. JMIR Mental Health 5: e5. [PMC free article: PMC5801511] [PubMed: 29362207]Hautzinger 1996
Hautzinger M, Jong-Meyer R (1996) Two multi-center studies of behavior therapy and pharmacotherapy for unipolar depression. Starting point, research questions, procedure, methods, and interventions. Zeitschrift fur Klinische Psychologie 25: 83–92 (data extracted from 2009 Depression NICE guideline).Heller 2009
Heller AS (2009) Brain imaging techniques that predict antidepressant responsiveness (WyethKolden). NCT00909155. Available from: https://clinicaltrials.gov/ct2/show/results/NCT00909155 [accessed Augist 2019] (data extracted from Cipriani 2018).Hemat-Far 2012
Hemat-Far A, Shahsavari A, Roholla Mousavi S (2012) Effects of selected aerobic exercises on the depression and concentrations of plasma serotonin in the depressed female students aged 18 to 25. Journal of Applied Research in Clinical and Experimental Therapeutics 12: 47.Herman 2002
Herman S, Blumenthal JA, Babyak M, Khatri P, Craighead WE, et al. (2002) Exercise therapy for depression in middle-aged and older adults: predictors of early dropout and treatment failure. Health Psychology 21: 553. [PubMed: 12433007]Hermens 2007
Hermens ML, Van Hout HP, Terluin B, Adèr HJ, Penninx BW, et al. (2007) Clinical effectiveness of usual care with or without antidepressant medication for primary care patients with minor or mild-major depression: a randomized equivalence trial. BMC Medicine 5: 1–11. [PMC free article: PMC2234409] [PubMed: 18067659]Hersen 1984
Hersen M, Himmelhoch JM, Thase ME, Bellack AS (1984) Effects of social skill training, amitriptyline, and psychotherapy in unipolar depressed women. Behavior Therapy 15: 21–40.Hewett 2009
Hewett K, Chrzanowski W, Schmitz M, Savela A, Milanova V, et al. (2009) Eight-week, placebo-controlled, double-blind comparison of the antidepressant efficacy and tolerability of bupropion XR and venlafaxine XR. Journal of Psychopharmacology 23: 531–538. [PubMed: 18635695]Hewett 2010
Hewett K, Gee MD, Krishen A, Wunderlich HP, Le Clus A, et al. (2010) Double-blind, placebo-controlled comparison of the antidepressant efficacy and tolerability of bupropion XR and venlafaxine XR. Journal of Psychopharmacology 24: 1209–1216. [PubMed: 19939870]Hicks 1988
Hicks F, Robins E, Murphy GE (1988) Comparison of adinazolam, amitriptyline, and placebo in the treatment of melancholic depression. Psychiatry Research 23: 221–227. [PubMed: 3283804]Hieronymus 2016_PZ/109
Hieronymus F, Emilsson JF, Nilsson S, Eriksson E (2016) Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Molecular Psychiatry 21: 523–530. [PMC free article: PMC4804177] [PubMed: 25917369]Hieronymus 2016_PZ/111
Hieronymus F, Emilsson JF, Nilsson S, Eriksson E (2016) Consistent superiority of selective serotonin reuptake inhibitors over placebo in reducing depressed mood in patients with major depression. Molecular Psychiatry 21: 523–530. [PMC free article: PMC4804177] [PubMed: 25917369]Higuchi 2009
Higuchi T, Murasaki M, Kamijima K (2009) Clinical evaluation of duloxetine in the treatment of major depressive disorder placebo and paroxetine controlled double-blind comparative study. Japanese Journal of Clinical Psychopharmacology 12: 1613–1634 (data extracted from Cipriani 2018).Higuchi 2011
Higuchi T, Hong JP, Jung HY, Watanabe Y, Kunitomi T, et al. (2011) Paroxetine controlled-release formulation in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled study in Japan and Korea. Psychiatry and Clinical Neurosciences 65: 655–663. [PubMed: 21895859]Higuchi 2016
Higuchi T, Kamijima K, Nakagome K, Itamura R, Asami Y, et al. (2016) A randomized, double-blinded, placebo-controlled study to evaluate the efficacy and safety of venlafaxine extended release and a long-term extension study for patients with major depressive disorder in Japan. International Clinical Psychopharmacology 31: 8. [PMC free article: PMC4667751] [PubMed: 26513202]Hirayasu 2011a
Hirayasu Y (2011) A dose-response study of escitalopram in patients with major depressive disorder: a placebo-controlled, double-blind study. Japanese Journal of Clinical Psychopharmacology 14: 871–882 (data extracted from Cipriani 2018).Hirayasu 2011b
Hirayasu Y (2011) A dose-response and non-inferiority study evaluating the efficacy and safety of escitalopram in patients with major depressive disorder. Japanese Journal of Clinical Psychopharmacology 14: 883–899 (data extracted from Cipriani 2018).Hojatifar 2017
Hojatifar Y, Zadeh MH, Dortaj F (2017) The effectiveness of mindfulness on the reduction of anxiety and depression of divorced women. Indian Journal of Public Health Research and Development 8: 23–27.Hollon 1992
Hollon SD, DeRubeis RJ, Evans MD, Wiemer MJ, Garvey MJ, et al. (1992) Cognitive therapy and pharmacotherapy for depression. Singly and in combination. Archives of General Psychiatry 49: 774–781. [PubMed: 1417429]Hong 2003
Hong CJ, Hu WH, Chen CC, Hsiao CC, Tsai SJ, et al. (2003) A double-blind, randomized, group-comparative study of the tolerability and efficacy of 6 weeks’ treatment with mirtazapine or fluoxetine in depressed Chinese patients. Journal of Clinical Psychiatry 64: 921–926. [PubMed: 12927007]Hopko 2003
Hopko DR, Lejuez CW, Lepage JP, Hopko SD, McNeil DW (2003) A brief behavioral activation treatment for depression: A randomized pilot trial within an inpatient psychiatric hospital. Behavior Modification 27: 458–469. [PubMed: 12971122]Hoyberg 1996
Hoyberg OJ, Maragakis B, Mullin J, Norum D, Stordall E, et al. (1996) A double-blind multicentre comparison of mirtazapine and amitriptyline in elderly depressed patients. Acta Psychiatrica Scandinavica 93: 184–190. [PubMed: 8739664]Hsu 2011
Hsu J-W, Su T-P, Huang C-Y, Chen Y-S, Chou Y-H (2011) Faster onset of antidepressant effects of citalopram compared with sertraline in drug-naive first-episode major depressive disorder in a Chinese population: A 6-week double-blind, randomized comparative study. Journal of Clinical Psychopharmacology 31: 577–581. [PubMed: 21869697]Hu 2009
Hu MR, Li LH, Lu XZ, Xun GL, Chen JD (2010) Escitalopram vs citalopram for depressioni: a randomized, double-blind, double-dummy, multicenter, parallel controlled study. Central South Pharmacy 8: 67–69 (data extracted from Cipriani 2018).Huipeng 2013
Huipeng F, Xiaohui Z (2013) Curative effect and observation of exercise on patients with depression. Chinese Medicine and Pharmacy 3: 187–188 (data extracted from Krogh 2017).Hunter 2010_study 1
Hunter AM, Muthén BO, Cook IA, Leuchter AF (2010) Antidepressant response trajectories and quantitative electroencephalography (QEEG) biomarkers in major depressive disorder. Journal of Psychiatric Research 44: 90–98. [PMC free article: PMC2925497] [PubMed: 19631948]Hunter 2010_study 2
Hunter AM, Muthén BO, Cook IA, Leuchter AF (2010) Antidepressant response trajectories and quantitative electroencephalography (QEEG) biomarkers in major depressive disorder. Journal of Psychiatric Research 44: 90–98. [PMC free article: PMC2925497] [PubMed: 19631948]Hunter 2010_study 3
Hunter AM, Muthén BO, Cook IA, Leuchter AF (2010) Antidepressant response trajectories and quantitative electroencephalography (QEEG) biomarkers in major depressive disorder. Journal of Psychiatric Research 44: 90–98. [PMC free article: PMC2925497] [PubMed: 19631948]Hunter 2011
Hunter AM, Cook IA, Greenwald S, Tran ML, Miyamoto KN, et al. (2011) The antidepressant treatment response (ATR) index and treatment outcomes in a placebo-controlled trial of fluoxetine. Journal of Clinical Neurophysiology 28: 478. [PMC free article: PMC3188349] [PubMed: 21946361]Husain 2014
Husain N, Chaudhry N, Fatima B, Husain M, Amin R, et al. (2014) Antidepressant and group psychosocial treatment for depression: a rater blind exploratory RCT from a low income country. Behavioural and Cognitive Psychotherapy 42: 693–705. [PubMed: 23867053]Hutchinson 1992
Hutchinson DR, Tong S, Moon CA, Vince M, Clarke A (1992) Paroxetine in the treatment of elderly depressed patients in general practice: a double-blind comparison with amitriptyline. International Clinical Psychopharmacology 6 (suppl. 4): 43–51. [PubMed: 1431010]Hwang 2004
Hwang J-P, Yang C-H, Tsai S-J (2004) Comparison study of venlafaxine and paroxetine for the treatment of depression in elderly Chinese inpatients. International Journal of Geriatric Psychiatry 19: 189–190. [PubMed: 14758586]Hwang 2015
Hwang WC, Myers HF, Chiu E, Mak E, Butner JE, et al. (2015) Culturally adapted cognitive-behavioral therapy for Chinese Americans with depression: a randomized controlled trial. Psychiatric Services 66: 1035–1042. [PMC free article: PMC4591116] [PubMed: 26129996]Indu 2018
Indu PS, Anilkumar TV, Vijayakumar K, Kumar KA, Sarma PS, et al. (2018) Effectiveness of community-based depression intervention programme (ComDIP) to manage women with depression in primary care- randomised control trial. Asian Journal of Psychiatry 34: 87–92. [PubMed: 29677524]Jacobson 1996
Jacobson NS, Dobson KS, Truax PA, Addis ME, Koerner K, et al. (1996) A component analysis of cognitive-behavioral treatment for depression. Journal of Consulting and Clinical Psychology 64: 295–304. [PubMed: 8871414]Jamison 1995
Jamison C (1995) The outcome of cognitive bibliotherapy with depressed adults. Journal of Consulting & Clinical Psychology 63: 644–650. [PubMed: 7673542]Janakiramaiah 2000
Janakiramaiah N, Gangadhar BN, Murthy PNV, Harish MG, Subbakrishna DK, et al. (2000) Antidepressant efficacy of Sudarshan Kriya Yoga (SKY) in melancholia: a randomized comparison with electroconvulsive therapy (ECT) and imipramine. Journal of Affective Disorders 57: 255–259. [PubMed: 10708840]Jefferson 2000
Jefferson J, Griest J (2000) A double-blind, placebo controlled, fixed-dosage study comparing the efficacy and tolerability of paroxetine CR and citalopram to placebo in the treatment of major depressive disorder with anxiety. American College of Neuropsychopharmacology, 39th Annual Meeting 12/11/2000 San Juan, Puerto Rico (data extracted from Cipriani 2018).Jeong 2015
Jeong JH, Bahk WM, Woo YS, Lee KU, Kim DH, et al. (2015) Efficacy and safety of generic escitalopram (lexacure®) in patients with major depressive disorder: a 6-week multicenter, randomized, rater-blinded, escitalopram-comparative, non-inferiority study. Neuropsychiatric Disease and Treatment 11: 2557–2564. [PMC free article: PMC4605251] [PubMed: 26504387]Jiahui 2006
Jiahui ZHAO, & Peng SANG (2006) Clinical observation on acupuncture treatment of depressive neurosis in 30 cases. Journal of Traditional Chinese Medicine 26: 191. [PubMed: 17078447]Jiang 2009
Jiang X, Ren K (2009) A comparative study on escitalopram and citalopram hydrobromide in the treatment of depression. Journal of Qiqihar Medical College 30: 1–2 (data extracted from Cipriani 2018).Jiang 2017
Jiang H, Chen S, Lu N, Yue Y, Yin Y, et al. (2017) Reduced serum VGF levels were reversed by antidepressant treatment in depressed patients. World Journal of Biological Psychiatry 18: 586–591. [PubMed: 28635540]Jinchun 2015
Jinchun W, Bo Y, Rong X, et al. (2015) Effect of exercise therapy in improving social function and quality of life in patients with depression. Nursing Journal of China 32: 24 (data extracted from Krogh 2017).Jordan 2014
Jordan J, Carter JD, McIntosh VV, Fernando K, Frampton CM, et al. (2014) Metacognitive therapy versus cognitive behavioural therapy for depression: a randomized pilot study. Australian and New Zealand Journal of Psychiatry 48: 932–943. [PubMed: 24810871]Judd 1993
Judd FK, Moore K, Norman TR, Burrows GD, Gupta RK, et al. (1993) A multicentre double blind trial of fluoxetine versus amitriptyline in the treatment of depressive illness. Australian & New Zealand Journal of Psychiatry 27: 49–55. [PubMed: 8481170]Judd 2001
Judd FK, Piterman L, Cockram AM, McCall L, Weissman MM (2001) A comparative study of venlafaxine with a focused education and psychotherapy program versus venlafaxine alone in the treatment of depression in general practice. Human Psychopharmacology 16: 423–428. [PubMed: 12404563]Kampman 1978
Kampman R, Nummikko-Pelkonen A, Kuha S (1978) Tricyclic antidepressants in the treatment of depression. Acta Psychiatrica Scandinavica 58: 142–148. [PubMed: 358754]Kang 2009
Kang EH, Lee IS, Chung SK, Lee SY, Kim EJ, et al. (2009) Mirtazapine versus venlafaxine for the treatment of somatic symptoms associated with major depressive disorder: a randomized, open-labeled trial. Psychiatry Research 169: 118–123. [PubMed: 19695711]Kanter 2015
Kanter JW, Santiago-Rivera AL, Santos MM, Nagy G, López M, et al. (2015) A randomized hybrid efficacy and effectiveness trial of behavioral activation for Latinos with depression. Behavior Therapy 46: 177–192. [PubMed: 25645167]Kasper 2005a
Kasper S, Swart H, Andersen HF (2005) Escitalopram in the treatment of depressed elderly patients. American Journal of Geriatric Psychiatry 13: 884–891. [PubMed: 16223967]Kasper 2005b
Kasper S, Olivieri L, Di loreto G, Dionisio P (2005) A comparative, randomised, double-blind study of trazodone prolonged-release and paroxetine in the treatment of patients with major depressive disorder. Current Medical Research and Opinion 21: 1139–1146. [PubMed: 16083521]Kasper 2012
Kasper S, Ebert B, Larsen K, Tonnoir B (2012) Combining escitalopram with gaboxadol provides no additional benefit in the treatment of patients with severe major depressive disorder. International Journal of Neuropsychopharmacoloy 15: 715–725. [PubMed: 22008735]Katona 2012
Katona C, Hansen T, Olsen CK (2012) A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. International Clinical Psychopharmacology 27: 215–223. [PubMed: 22572889]Katz 1990
Katz IR, Simpson GM, Curlik SM, Parmelee PA (1990) Pharmacologic treatment of major depression for elderly patients in residential care settings. Journal of Clinical Psychiatry 51(suppl.): 41–47. [PubMed: 2195013]Katz 1993_Protocol 01
Katz RJ, Lott M, Landau P, Waldmeier P (1993) A clinical test of noradrenergic involvement in the therapeutic mode of action of an experimental antidepressant. Biological Psychiatry 33: 261–266. [PubMed: 8471679]Katz 1993_Protocol 03
Katz RJ, Lott M, Landau P, Waldmeier P (1993) A clinical test of noradrenergic involvement in the therapeutic mode of action of an experimental antidepressant. Biological Psychiatry 33: 261–266. [PubMed: 8471679]Katz 2004
Katz MM, Tekell JL, Bowden CL, Brannan S, Houston JP, et al. (2004) Onset and early behavioral effects of pharmacologically different antidepressants and placebo in depression. Neuropsychopharmacology 29: 566–579. [PubMed: 14627997]Kay-Lambkin 2009
Kay-Lambkin FJ, Baker AL, Lewin TJ, Carr VJ (2009) Computer-based psychological treatment for comorbid depression and problematic alcohol and/or cannabis use: a randomized controlled trial of clinical efficacy. Addiction 104: 378–388. [PubMed: 19207345]Kay-Lambkin 2011/2017
Kay-Lambkin FJ, Baker AL, Kelly B, Lewin TJ (2011) Clinician-assisted computerised versus therapist-delivered treatment for depressive and addictive disorders: a randomised controlled trial. Medical Journal of Australia 195: S44–S50. [PubMed: 21806518]
Kay-Lambkin FJ, Baker AL, Palazzi K, Lewin TJ, Kelly BJ (2017) Therapeutic alliance, client need for approval, and perfectionism as differential moderators of response to eHealth and traditionally delivered treatments for comorbid depression and substance use problems. International Journal of Behavioral Medicine 24: 728–739. [PubMed: 28819922]Keegan 1991
Keegan D, Bowen RC, Blackshaw S, Saleh S, Dayal N, et al. A comparison of fluoxetine and amitriptyline in the treatment of major depression. International Clinical Psychopharmacology 6: 117–124. [PubMed: 1960381]Keller 2006_Study 059
Keller M, Montgomery S, Ball W, Morrison M, Snavely D, et al. (2006) Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biological Psychiatry 59: 216–223. [PubMed: 16248986]Keller 2006_Study 061
Keller M, Montgomery S, Ball W, Morrison M, Snavely D, et al. (2006) Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biological Psychiatry 59: 216–223. [PubMed: 16248986]Keller 2006_Study 062
Keller M, Montgomery S, Ball W, Morrison M, Snavely D, et al. (2006) Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biological Psychiatry 59: 216–223. [PubMed: 16248986]Kennedy 2005
Kennedy SH, Anderson HF, Lam RW (2005) A pooled analysis of selective serotonin reuptake inhibitors and venlafaxine. Poster presented at the American Psychiatric Association, May 2005 (data extracted from Cipriani 2018).Kennedy 2007
Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, et al. (2007) Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. American Journal of Psychiatry 164; 778–788. [PubMed: 17475737]Kerr 1984
Kerr TA, McClelland HA, Stephens DA, Ankier SI (1984) Trazodone. A comparative clinical and predictive study. Acta Psychiatrica Scandinavica 70: 573–577. [PubMed: 6395636]Kessler 2009
Kessler D, Lewis G, Kaur S, Wiles N, King M, et al. (2009) Therapist-delivered internet psychotherapy for depression in primary care: a randomised controlled trial. The Lancet 374: 628–634. [PubMed: 19700005]Khan 1991
Khan A, Fabre LF, Rudolph R (1991) Venlafaxine in depressed outpatients. Psychopharmacology Bulletin 27: 141–144. [PubMed: 1924660]Khan 1995
Khan MC (1995) A randomised, double-blind, placebo-controlled, 5-weeks’ study of org 3770 (mirtazapine) in major depression. Human Psychopharmacology: Clinical and Experimental 10(suppl. 2): S119–S124.Khan 1998
Khan A, Upton GV, Rudolph RL, Entsuah R, Leventer SM (1998) The use of venlafaxine in the treatment of major depression and major depression associated with anxiety: a dose-response study. Journal of Clin Psychopharmacology 18: 19–25. [PubMed: 9472838]Khan 2007
Khan A, Bose A, Alexopoulos GS, Gommoll C, Li D, et al. (2007) Double-blind comparison of escitalopram and duloxetine in the acute treatment of major depressive disorder. Clinical Drug Investigation 27: 481–492. [PubMed: 17563128]Kheirabadi 2020
Kheirabadi GR, Yousefian Z, Zargar F, Bahrami M, Maracy MR (2020) Citalopram and metacognitive therapy for depressive symptoms and cognitive emotion regulation in patients with major depressive disorder: a randomized controlled trial. Journal of Education and Health Promotion 9: 6. [PMC free article: PMC7032026] [PubMed: 32154301]Khoshnab 2017
Khoshnab LP, Nikseresht A (2017) Comparison of the effect of aerobic exercise and antidepressant medications on depression and sexual desire of depressed middle-aged women. International Journal of Women’s Health and Reproduction Sciences 5: 119–122.Kiev 1992
Kiev A (1992) A double-blind, placebo-controlled study of paroxetine in depressed outpatients. Journal of Clinical Psychiatry 53 (suppl.): 27–29. [PubMed: 1531818]Kim 2011
Kim JE, Yoon SJ, Kim J, Jung JY, Jeong HS, et al. (2011) Efficacy and tolerability of mirtazapine in treating major depressive disorder with anxiety symptoms: an 8-week open-label randomised paroxetine-controlled trial. International Journal of Clinical Practice 65: 323–329. [PubMed: 21314870]Kinoshita 2009
Kinoshita T (2009) A double-blind, placebo controlled study of a new antidepressant, mirtazapine, in depressed patients. Japanese Journal of Clinical Psychopharmacology 12: 289–306 (data extracted from Cipriani 2018).Kleber 1983
Kleber HD, Weissman MM, Rounsaville BJ, Wilber CH, Prusoff BA, et al. (1983) Imipramine as treatment for depression in addicts. Archives of General Psychiatry 40: 649–653. [PubMed: 6342564]Klieser 1988
Klieser E, Lehmann E (1988) Experimental comparison between the effect of standardized trazodone-amitriptyline and placebo treatment in vitalized depressive patients. Psychopharmacology 95(suppl.): S3–S5. [PubMed: 3133710]Komulainen 2018
Komulainen E, Heikkila R, Nummenmaa L, Raij TT, Harmer CJ, et al. (2018) Short-term escitalopram treatment normalizes aberrant self-referential processing in major depressive disorder. Journal of Affective Disorders 236: 222–229. [PubMed: 29747140]Koeser 2015
Koeser L, Donisi V, Goldberg DP, McCrone P (2015) Modelling the cost-effectiveness of pharmacotherapy compared with cognitive-behavioural therapy and combination therapy for the treatment of moderate to severe depression in the UK. Psychological Medicine 45: 3019–3031. [PubMed: 26040631]Kornaat 2000
Kornaat H (2000) Study 626: randomized, double-blind comparison of venlafaxine and fluoxetine in moderately depressed outpatients. Unpublished (data extracted from 2009 Depression NICE guideline).Kramer 1998
Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, et al. (1998) Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 281: 1640–1645. [PubMed: 9733503]Kramer 2014
Kramer J, Conijn B, Oijevaar P, Riper H (2014) Effectiveness of a web-based solution-focused brief chat treatment for depressed adolescents and young adults: randomized controlled trial. Journal of Medical Internet Research 16: e141. [PMC free article: PMC4062279] [PubMed: 24874006]Kranzler 2006_Group A
Kranzler HR, Mueller T, Cornelius J, Pettinati HM, Moak D, et al. (2006) Sertraline treatment of co-occurring alcohol dependence and major depression. Journal of Clinical Psychopharmacology 26: 13–20. [PubMed: 16415699]Krejtz 2018
Krejtz I, Holas P, Rusanowska M, Nezlek JB (2018) Positive online attentional training as a means of modifying attentional and interpretational biases among the clinically depressed: an experimental study using eye tracking. Journal of Clinical Psychology 74: 1594–1606. [PubMed: 29542816]Krogh 2012
Krogh J, Videbech P, Thomsen C, Gluud C, Nordentoft M (2012) DEMO-II trial. Aerobic exercise versus stretching exercise in patients with major depression-a randomised clinical trial. PloS One 7: e48316. [PMC free article: PMC3485141] [PubMed: 23118981]Krogh 2017
Krogh J, Hjorthøj C, Speyer H, Gluud C, Nordentoft M (2017) Exercise for patients with major depression: a systematic review with meta-analysis and trial sequential analysis. BMJ Open 7: e014820. [PMC free article: PMC5623558] [PubMed: 28928174]Kusalic 1993
Kusalic M, Engelsmann F, Bradwejn J (1993) Thyroid functioning during treatment for depression. Journal of Psychiatry and Neuroscience 18: 260. [PMC free article: PMC1188546] [PubMed: 8297924]Kyle 1998
Kyle CJ, Petersen HEH, Overo KF (1998) Comparison of the tolerability and efficacy of citalopram and amitriptyline in elderly depressed patients treated in general practice. Depression & Anxiety 8: 147–153. [PubMed: 9871816]Laakmann 1988
Laakmann G, Blaschke D, Engel R, Schwarz A (1988) Fluoxetine vs amitriptyline in the treatment of depressed out-patients. British Journal of Psychiatry 153(suppl. 3): 64–68. [PubMed: 3074868]Laakmann 1991
Laakmann G, Pögelt A, Kriszio B, Breull A, Blaschke D, et al. (1991) Behandlungsergebnisse mit Fluoxetin im Vergleich zu Amitriptylin bei ambulanten und stationären Patienten im Rahmen von Doppelblindstudien (Gesamtanalysen). InSelektive Re-uptake-Hemmung und ihre Bedeutung für die Depression 1991 (pp. 23–46). Springer, Berlin, Heidelberg (data extracted from 2009 Depression NICE guideline).Lam 2013
Lam RW, Parikh SV, Ramasubbu R, Michalak EE, Tam EM, et al. (2013) Effects of combined pharmacotherapy and psychotherapy for improving work functioning in major depressive disorder. British Journal of Psychiatry 203: 358–365. [PubMed: 24029535]Lam 2016
Lam RW, Levitt AJ, Levitan RD, Michalak EE, Cheung AH, et al. (2016) Efficacy of bright light treatment, fluoxetine, and the combination in patients with nonseasonal major depressive disorder: a randomized clinical trial. JAMA Psychiatry 73: 56–63. [PubMed: 26580307]Lecrubier 1997
Lecrubier Y, Bourin M, Moon CA, Schifano F, Blanchard C, et al. (1997) Efficacy of venlafaxine in depressive illness in general practice. Acta Psychiatrica Scandinavica 95: 485–493. [PubMed: 9242843]Lee 2007
Lee P, Shu L, Xu X, Wang CY, Lee MS, et al. (2007) Once-daily duloxetine 60 mg in the treatment of major depressive disorder: multicenter, double-blind, randomized, paroxetine-controlled, non-inferiority trial in China, Korea, Taiwan and Brazil. Psychiatry & Clinical Neurosciences 61: 295–307. [PubMed: 17472599]Legrand 2016
Legrand FD, Neff EM (2016) Efficacy of exercise as an adjunct treatment for clinically depressed inpatients during the initial stages of antidepressant pharmacotherapy: an open randomized controlled trial. Journal of Affective Disorders 191: 139–144. [PubMed: 26655860]Leinonen 1999
Leinonen E, Skarstein J, Behnke K, Agren H, Helsdingen JT, et al. (1999) Efficacy and tolerability of mirtazapine versus citalopram: a double-blind, randomized study in patients with major depressive disorder. International Clinical Psychopharmacology 14: 329–337. [PubMed: 10565799]Leinonen 2007
Leinonen E, Niemi H (2007) The influence of educational information on depressed outpatients treated with escitalopram: a semi-naturalistic study. Nordic Journal of Psychiatry 61: 109–114. [PubMed: 17454725]Lenox-Smith 2009
Lenox-Smith A, Greenstreet L, Burslem K, Knight C (2009) Cost effectiveness of venlafaxine compared with generic fluoxetine or generic amitriptyline in major depressive disorder in the UK. Clinical Drug Investigation 29: 173–184. [PubMed: 19243210]
Lenox-Smith A, Conway P, Knight C (2004) Cost effectiveness of representatives of three classes of antidepressants used in major depression in the UK. PharmacoEconomics 22: 311–319 (updated by Lenox 2009). [PubMed: 15061681]Lepola 2003
Lepola UM, Loft H, Reines H (2003) Escitalopram (10-20 mg/day) is effective and well tolerated in a placebo-controlled study in depression in primary care. International Clinical Psychopharmacology 18: 211–217. [PubMed: 12817155]Levin 2013
Levin FR, Mariani J, Brooks DJ, Pavlicova M, Nunes EV, et al. (2013) A randomized double-blind, placebo-controlled trial of venlafaxine-extended release for co-occurring cannabis dependence and depressive disorders. Addiction 108: 1084–1094. [PMC free article: PMC3636166] [PubMed: 23297841]Levine 1989
Levine S, Deo R, Mahadevan K (1987) A comparative trial of a new antidepressant, fluoxetine. British Journal of Psychiatry 150: 653–655. [PubMed: 3307982]Lewis 2019 / Hollingworth 2020
Lewis G, Duffy L, Ades A, Amos R, Araya R, et al. (2019) The clinical effectiveness of sertraline in primary care and the role of depression severity and duration (PANDA): a pragmatic, double-blind, placebo-controlled randomised trial. Lancet Psychiatry 6: 903–914. [PMC free article: PMC7029306] [PubMed: 31543474]
Hollingworth W, Fawsitt CG, Dixon P, Duffy L, Araya R, et al. (2020) Cost-effectiveness of sertraline in primary care according to initial severity and duration of depressive symptoms: findings from the PANDA RCT. PharmacoEconomics Open 4:427–438. [PMC free article: PMC7426336] [PubMed: 31777008]
Duffy L, Lewis G, Ades A, Araya R, Bone J, et al. (2019) Antidepressant treatment with sertraline for adults with depressive symptoms in primary care: the PANDA research programme including RCT. Health Technology Assessment 7(10). [PubMed: 31869013]Li 2004b
Li GP, Du YH, Yan H, Zhang XJ, Huang LF (2004) Clinical effect of Tiaoshen Shugan acupuncture on depression. Tianjin Journal of Traditional Chinese Medicine 21: 382–385 (data extracted from Smith 2018).Li 2006
Li J, Shen WW, Liu Y, Xu L, Liu SM, et al. (2006) The effectiveness and safety of escitalopram in the treatment of major depression: a randomized double-blind active-drug controlled trial. Chinese Journal of Evidence-Based Medicine 6: 552–556 (data extracted from Cipriani 2018).Lindegaard 2019
Lindegaard T, Brohede D, Koshnaw K, Osman SS, Johansson R, et al. (2019) Internet-based treatment of depressive symptoms in a Kurdish population: a randomized controlled trial. Journal of Clinical Psychology 75: 985–998. [PubMed: 30702758]Liu 2018a
Liu B, Tan Y, Cai D, Liang Y, He R, et al. (2018) A study of the clinical effect and dropout rate of drugs combined with group integrated psychotherapy on elderly patients with depression. Shanghai Jingshen Yixue 30: 39–46. [PMC free article: PMC5925597] [PubMed: 29719357]Loo 2002
Lôo H, Hale A, D’haenen H (2002) Determination of the dose of agomelatine, a melatoninergic agonist and selective 5-HT(2C) antagonist, in the treatment of major depressive disorder: a placebo-controlled dose range study. International Clinical Psychopharmacology 17: 239–247. [PubMed: 12177586]Lopez-Rodriguez 2004
Rodriguez JL, Lopez Butron MA, Vargas Terrez BE, Villamil Salcedo V. Estudio doble ciego con antidepressivo, psicoterapia breve y placebo en pacientes con depression leve a moderada. Salud Mental 27: 53–61 (data extracted from Cipriani 2018).Luo 1985
Luo HC, Yunkui J, Zhan L (1985) Electro-acupuncture versus amitriptyline in the treatment of depressive states. Journal of Traditional Chinese Medicine 5: 3–8. [PubMed: 3849629]Luo 1988
Luo HC, Shen YC, Jia YK, Zhou D (1988) Clinical study of electro-acupuncture on 133 patients with depression in comparison with tricyclic amitriptyline. Chinese Journal of Modern Developments in Traditional Medicine 8: 77–80 (data extracted from Smith 2018). [PubMed: 3261644]Lv 2013
Lv Z, Zhang Y, Qi S (2013) A randomized double-blind study of escitalopram and citalopram in treating major depression. Journal of Clinical Psychiatry 23: 1–3 (data extracted from Cipriani 2018).Lydiard 1989
Lydiard RB, Laird LK, Morton WA Jr, Steele TE, Kellner C, et al. Fluvoxamine, imipramine, and placebo in the treatment of depressed outpatients: effects on depression. Psychopharmacology Bulletin 25: 68–70. [PubMed: 2505304]M/2020/0046 (Study 046)
https://www.iqwig.de /de/projekte-ergebnisse /studieninformationen-zu-reboxetin .3304.html (data extracted from Cipriani 2018). M/2020/0046 (Study 047)
https://www.iqwig.de /de/projekte-ergebnisse /studieninformationen-zu-reboxetin .3304.html (data extracted from Cipriani 2018). Macaskill 1996
Macaskill ND, Macaskill A (1996) Rational-emotive therapy plus pharmacotherapy versus pharmacotherapy alone in the treatment of high cognitive dysfunction depression. Cognitive Therapy and Research 20: 575–592.Macias-Cortes 2015
Macías-Cortés EdC, Llanes-González L, Aguilar-Faisal L, Asbun-Bojalil J (2015) Individualized homeopathic treatment and fluoxetine for moderate to severe depression in peri-and postmenopausal women (HOMDEP-MENOP study): a randomized, double-dummy, double-blind, placebo-controlled trial. PloS One 10: e0118440. [PMC free article: PMC4359147] [PubMed: 25768800]MacPherson 2013
MacPherson H, Richmond S, Bland M, Brealey S, Gabe R, et al. (2013) Acupuncture and counselling for depression in primary care: a randomised controlled trial. PLoS Medicine 10: e1001518. [PMC free article: PMC3782410] [PubMed: 24086114]Mahableshwarkar 2013
Mahableshwarkar AR, Jacobsen PL, Chen Y (2013) A randomized, double-blind trial of 2.5 mg and 5 mg vortioxetine (Lu AA21004) versus placebo for 8 weeks in adults with major depressive disorder. Current Medical Research and Opinion 29: 217–226. [PubMed: 23252878]Mahableshwarkar 2015a
Mahableshwarkar AR, Jacobsen PL, Chen Y, Serenko M, Trivedi MH (2015) A randomized, double-blind, duloxetine-referenced study comparing efficacy and tolerability of 2 fixed doses of vortioxetine in the acute treatment of adults with MDD. Psychopharmacology 232: 2061–2070. [PMC free article: PMC4432084] [PubMed: 25575488]Maina 2007
Maina G, Rosso G, Crespi C, Bogetto F (2007) Combined brief dynamic therapy and pharmacotherapy in the treatment of major depressive disorder: a pilot study. Psychotherapy and Psychosomatics 76: 298–305. [PubMed: 17700050]Mao 2008
Mao PX, Tang YL, Jiang F, Shu L, Gu X, et al. (2008) Escitalopram in major depressive disorder: a multicenter, randomized, double-blind, fixed-dose, parallel trial in a Chinese population. Depression and Anxiety 25: 46–54. [PubMed: 17149753]March 1990
March JS, Kobak KA, Jefferson JW, Mazza J, Greist JH (1990) A double-blind, placebo-controlled trial of fluvoxamine versus imipramine in outpatients with major depression. Journal of Clinical Psychiatry 51: 200–202. [PubMed: 2110560]Marchesi 1998
Marchesi C, Ceccherininelli A, Rossi A, Maggini C (1998) Is anxious-agitated major depression responsive to fluoxetine? A double-blind comparison with amitriptyline. Pharmacopsychiatry 31: 216–221. [PubMed: 9930635]Markkula 2019
Markkula N, Lehti V, Adhikari P, Peña S, Heliste J, et al. (2019) Effectiveness of non-medical health worker-led counselling on psychological distress: a randomized controlled trial in rural Nepal. Global Mental Health 6: e15. [PMC free article: PMC6669965] [PubMed: 31391947]Marshall 2008
Marshall MB, Zuroff DC, McBride C, Bagby RM (2008) Self-criticism predicts differential response to treatment for major depression. Journal of Clinical Psychology 64: 231–244. [PubMed: 18302208]Martin 2001
Martin SD, Martin E, Rai SS, Richardson MA, Royall R (2001) Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride: preliminary findings. Archives of General Psychiatry 58: 641–648. [PubMed: 11448369]Mathews 2015
Mathews M, Gommoll C, Chen D, Nunez R, Khan A (2015) Efficacy and safety of vilazodone 20 and 40 mg in major depressive disorder: a randomized, double-blind, placebo-controlled trial. International Clinical Psychopharmacology 30: 67–74. [PMC free article: PMC4314105] [PubMed: 25500685]Matreja 2007
Matreja PS, Badyal DK, Khosla P, Deswal RS (2007) Effectiveness and acceptability of sertraline and citalopram in major depressive disorder: pragmatic randomized open-label comparison. Human Psychopharmacology: Clinical and Experimental 22: 477–482. [PubMed: 17647298]McCallum 1975
McCallum P, Meares R (1975) A controlled trial of maprotiline (Ludiomil) in depressed outpatients. Medical Journal Australia 2: 392–394. [PubMed: 1102877]McKnight 1992
McKnight DL, Nelson-Gray RO, Barnhill J (1992) Dexamethasone suppression test and response to cognitive therapy and antidepressant medication. Behavior Therapy 23: 99–111.McNamara 1986
McNamara K, Horan JJ (1986) Experimental construct validity in the evaluation of cognitive and behavioral treatments for depression. Journal of Counseling Psychology 33: 23.MDF/29060/III/070/88/MC
https://www.gsk-clinicalstudyregister .com/study/29060/070#rs (data extracted from Cipriani 2018). Mead 2015
Mead N, MacDonald W, Bower P, Lovell K, Richards D, et al. (2005) The clinical effectiveness of guided self-help versus waiting-list control in the management of anxiety and depression: a randomized controlled trial. Psychological Medicine 35: 1633–1643. [PubMed: 16219121]Mehtonen 2000
Mehtonen OP, Sogaard J, Roponen P, Behnke K (2000) Randomized, double-blind comparison of venlafaxine and sertraline in outpatients with major depressive disorder. Journal of Clinical Psychiatry 61: 95–100. [PubMed: 10732656]Menchetti 2014
Menchetti M, Rucci P, Bortolotti B, Bombi A, Scocco P, et al. (2014) Moderators of remission with interpersonal counselling or drug treatment in primary care patients with depression: randomised controlled trial. British Journal of Psychiatry 204: 144–150. [PubMed: 24311553]Mendels 1968
Mendels J (1968) Comparative trial of nortriptyline and amitriptyline in 100 depressed patients. American Journal Psychiatry 124: 59–62. [PubMed: 4865733]Mendels 1993
Mendels J, Johnston R, Mattes J, Riesenberg R (1993) Efficacy and safety of b.i.d. doses of venlafaxine in a dose-response study. Psychopharmacology Bulletin 29: 169–174. [PubMed: 8290661]Mendels 1999
Mendels J (1999) Double-blind comparison of citalopram and placebo in depressed outpatients with melancholia. Depression & Anxiety 9: 54–60. [PubMed: 10207659]Merideth 1983
Merideth CH, Feighner JP (1983) A double-blind, controlled evaluation of zimeldine, imipramine and placebo in patients with primary affective disorders. Acta Psychiatrica Scandinavica 308(suppl.): 70–79. [PubMed: 6230897]Miller 1989a
Miller SM, Naylor GJ, Murtagh M, Winslow G (1989) A double-blind comparison of paroxetine and placebo in the treatment of depressed patients in a psychiatric outpatient clinic. Acta Neurologica Scandinavica 80(suppl.): 143–144. [PubMed: 2530772]MIR 003-003 (FDA)
http://digitalcommons .ohsu.edu/fdadrug/20/ (data extracted from Cipriani 2018). MIR 003-020 (FDA)
http://digitalcommons .ohsu.edu/fdadrug/20/ (data extracted from Cipriani 2018). MIR 003-021 (FDA)
http://digitalcommons .ohsu.edu/fdadrug/20/ (data extracted from Cipriani 2018). Miranda 2003/2006
Miranda J, Chung JY, Green BL, Krupnick J, Siddique J, et al. (2003) Treating depression in predominantly low-income young minority women: a randomized controlled trial. JAMA 290: 57–65. [PubMed: 12837712]
Miranda J, Green BL, Krupnick JL, Chung J, Siddique J, et al. (2006) One-year outcomes of a randomized clinical trial treating depression in low-income minority women. Journal of Consulting and Clinical Psychology 74: 99–111. [PubMed: 16551147]Miura 2000
Miura S, Koyama T, Asai M, Endo S, Kamijima K, et al. (2000) Clinical evaluation of paroxetine HCl, a selective serotonin reuptake inhibitor, in the treatment of depression or depressive spisodes: double-blind, parallel group study with amitriptyline HCl. Yakuri To Chiro (Japanese Pharmacology & Therapeutics) 28(suppl.): 187–210 (data extracted from Cipriani 2018).Moak 2003
Moak DH, Anton RF, Latham PK, Voronin KE, Waid RL, et al. (2003) Sertraline and cognitive behavioral therapy for depressed alcoholics: results of a placebo-controlled trial. Journal of Clinical Psychopharmacology 23: 553–562. [PubMed: 14624185]Mohr 2011
Mohr DC, Carmody T, Erickson L, Jin L, Leader J (2011) Telephone-administered cognitive behavioral therapy for veterans served by community-based outpatient clinics. Journal of Consulting and Clinical Psychology 79: 261–265. [PubMed: 21299274]Moises 1981
Moises HW, Kasper S, Beckmann H (1981) Trazodone and amitriptyline in treatment of depressed inpatients. A double-blind study. Pharmacopsychiatry 14: 167–171. [PubMed: 7027279]Moller 1993
Moller HJ, Berzewski H, Eckmann F, Gonzalves N, Kissling W, et al. (1993) Double-blind multicenter study of paroxetine and amitriptyline in depressed inpatients. Pharmacopsychiatry 26: 75–78. [PubMed: 8415897]Moller 1998
Möller HJ, Gallinat J, Hegerl U, Arato M, Janka Z, et al. (1998) Double-blind, multicenter comparative study of sertraline and amitriptyline in hospitalized patients with major depression. Pharmacopsychiatry 31: 170–177. [PubMed: 9832348]Moller 2000
Möller HJ, Glaser K, Leverkus F, Göbel C (2000) Double-blind, multicenter comparative study of sertraline versus amitriptyline in outpatients with major depression. Pharmacopsychiatry 33: 206–212. [PubMed: 11147927]Montgomery 1992
Montgomery SA, Rasmussen JG, Lyby K, Connor P (1992) Dose response relationship of citalopram 20 mg, citalopram 40 mg and placebo in the treatment of moderate and severe depression. International Clinical Psychopharmacology 6(suppl. 5): 65–70. [PubMed: 1431024]Montgomery 2004
Montgomery SA, Huusom AKT, Bothmer J (2004) A randomised study comparing escitalopram with venlafaxine XR in primary care patients with major depressive disorder. Neuropsychobiology 50: 57–64. [PubMed: 15179022]Moon 1994
Moon CAL, Jago LW, Wood K, Doogan DP (1994) A double-blind comparison of sertraline and clomipramine in the treatment of major depressive disorder and associated anxiety in general practice. Journal of Psychopharmacology 8: 171–176. [PubMed: 22298585]Moon 1996
Moon CA, Vince M (1996) Treatment of major depression in general practice: a double-blind comparison of paroxetine and lofepramine. British Journal of Clinical Practice 50: 240–244. [PubMed: 8794599]Moore 2005
Moore N, Verdoux H, Fantino B (2005) Prospective, multicentre, randomized, double-blind study of the efficacy of escitalopram versus citalopram in outpatient treatment of major depressive disorder. International Clinical Psychopharmacology 20: 131–137. [PubMed: 15812262]Moradveisi 2013
Moradveisi L, Huibers MJ, Renner F, Arasteh M, Arntz A (2013) Behavioural activation v. antidepressant medication for treating depression in Iran: randomised trial. British Journal of Psychiatry 202: 204–211. [PubMed: 23391727]Mowla 2016
Mowla A, Dastgheib SA, Razeghian Jahromi L (2016) Comparing the effects of sertraline with duloxetine for depression severity and symptoms: a double-blind, randomized controlled trial. Clinical Drug Investigation 36: 539–543. [PubMed: 27071759]Mullin 1996
Mullin J (1996) A multicentre, double-blind, amitriptyline-controIled study of mirtazapine in patients with major depression. Journal of Psychopharmacology 10: 235–240. [PubMed: 22302951]Mulsant 1999
Mulsant BH, Pollock BG, Nebes RD, Miller MD, Little JT, et al. (1999) A double-blind randomized comparison of nortriptyline and paroxetine in the treatment of late-life depression: 6-week outcome. Journal of Clinical Psychiatry 60(suppl. 20): 16–20. [PubMed: 10513853]Mundt 2012
Mundt JC, Vogel AP, Feltner DE, Lenderking WR (2012) Vocal acoustic biomarkers of depression severity and treatment response. Biological Psychiatry 72: 580–587. [PMC free article: PMC3409931] [PubMed: 22541039]Munizza 2006
Munizza C, Olivieri L, Di Loreto G, Dionisio P (2006) A comparative, randomized, double-blind study of trazodone prolonged-release and sertraline in the treatment of major depressive disorder. Current Medical Research & Opinion 22: 1703–1713. [PubMed: 16968574]Murphy 1984
Murphy GE, Simons AD, Wetzel RD, Lustman PJ (1984) Cognitive therapy and pharmacotherapy singly and together in the treatment of depression. Archives of General Psychiatry 41: 33–41. [PubMed: 6691783]Murri 2015
Murri MB, Amore M, Menchetti M, Toni G, Neviani F, et al. (2015) Physical exercise for late-life major depression. British Journal of Psychiatry 207: 235–242. [PubMed: 26206864]MY-1042/BRL-029060/CPMS-251
http://www.gsk-clinicalstudyregister .com /study/29060/251?search =compound&compound =paroxetine#rs (data extracted from Cipriani 2018). MY-1045/BRL-029060/1 (PAR 128)
http://www.gsk-clinicalstudyregister .com/study/29060/128#rs (data extracted from Cipriani 2018). Mynors-Wallis 1995
Mynors-Wallis LM, Gath DH, Lloyd-Thomas AR, Tomlinson D (1995) Randomised controlled trial comparing problem solving treatment with amitriptyline and placebo for major depression in primary care. BMJ 310: 441–445. [PMC free article: PMC2548821] [PubMed: 7873952]Mynors-Wallis 2000
Mynors-Wallis LM, Gath DH, Day A, Baker F (2000) Randomised controlled trial of problem solving treatment, antidepressant medication, and combined treatment for major depression in primary care. BMJ 320: 26–30. [PMC free article: PMC27250] [PubMed: 10617523]Naeem 2011
Naeem F, Waheed W, Gobbi M, Ayub M, Kingdon D (2011) Preliminary evaluation of culturally sensitive CBT for depression in Pakistan: findings from developing culturally-sensitive CBT project (DCCP). Behavioural and Cognitive Psychotherapy 39: 165–173. [PubMed: 21092353]Naeem 2014
Naeem F, Sarhandi I, Gul M, Khalid M, Aslam M, et al. (2014) A multicentre randomised controlled trial of a carer supervised culturally adapted CBT (CaCBT) based self-help for depression in Pakistan. Journal of Affective Disorders 156: 224–227. [PubMed: 24274963]Naeem 2015
Naeem F, Gul M, Irfan M, Munshi T, Asif A, et al. (2015) Brief culturally adapted CBT (CaCBT) for depression: a randomized controlled trial from Pakistan. Journal of Affective Disorders 177: 101–107. [PubMed: 25766269]Nair 1995
Nair NP, Amin M, Holm P, Katona C, Klitgaard N, et al. (1995) Moclobemide and nortriptyline in elderly depressed patients. A randomized, multicentre trial against placebo. Journal of Affective Disorders 33: 1–9. [PubMed: 7714303]Navarro 2001
Navarro V, Gasto C, Torres X, Marcos T, Pintor L (2001) Citalopram versus nortriptyline in late-life depression: a 12-week randomized single-blind study. Acta Psychiatrica Scandinavica 103: 435–440. [PubMed: 11401657]- https://clinicaltrials.gov/ct2/show/NCT01020799 (data extracted from Cipriani 2018).
Nemeroff 2007
Nemeroff CB, Thase ME, EPIC 014 Study Group (2007) A double-blind, placebo-controlled comparison of venlafaxine and fluoxetine treatment in depressed outpatients. Journal of Psychiatric Research 41: 351–359. [PubMed: 16165158]Nezu 1989
Nezu AM, Perri MG (1989) Social problem-solving therapy for unipolar depression: an initial dismantling investigation. Journal of Consulting and Clinical Psychology 57: 408–413. [PubMed: 2738213]Nielsen 1993
Nielsen BM, Behnke K, Arup P, Christiansen PE, Geisler A, et al. (1993) A comparison of fluoxetine and imipramine in the treatment of outpatients with major depressive disorder. Acta Psychiatrica Scandinavica 87: 269–272. [PubMed: 8488748]Nierenberg 2007
Nierenberg AA, Greist JH, Mallinckrodt CH, Prakash A, Sambunaris A, et al. (2007) Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority study. Current Medical Research & Opinion 23: 401–416. [PubMed: 17288694]NKD20006 (NCT00048204)
http://www.gsk-clinicalstudyregister .com/study/NKD20006#ps (data extracted from Cipriani 2018). Noguera 1991
Noguera R, Altuna R, Alvarez E, Ayuso JL, Casais L, et al. (1991) Fluoxetine vs. clomipramine in depressed patients: a controlled multicentre trial. Journal of Affective Disorders 22: 119–124. [PubMed: 1918655]Norton 1984
Norton KRW, Sireling LI, Bhat AV, Rao B, Paykel ES (1984) A double blind comparison of fluvoxamine, imipramine and placebo in depressed patients. Journal of Affective Disorders 7: 297–308. [PubMed: 6241211]Nyth 1992
Nyth AL, Gottfries CG, Lyby K, Smedegaard-Andersen L, Gylding-Sabroe J, et al. (1992) A controlled multicenter clinical study of citalopram and placebo in elderly depressed patients with and without concomitant dementia. Acta Psychiatrica Scandinavica 86: 138–145. [PubMed: 1529737]Olie 1997
Olie JP, Gunn KP, Katz E. (1997) A double-blind placebo-controlled multicentre study of sertraline in the acute and continuation treatment of major depression. European Psychiatry 12: 34–41. [PubMed: 19698503]Ontiveros 1997
Ontiveros A, Garcia-Barriga C (1997) A double-blind, comparative study of paroxetine and fluoxetine in out-patients with depression. British Journal of Clinical Research 8: 23–32.Ontiveros Sanchez 1998
Ontiveros Sanchez JA, Brandi F, Brunner E (1998) Double-blind study of fluoxetine vs. amitriptyline in depressive and anxiety symptoms and life quality in adults with major depression. Salud Mental 21: 58–63 (data extracted from Cipriani 2018).Ou 2011
Ou JJ, Xun GL, Wu RR, Li LH, Fang MS, et al. (2011) Efficacy and safety of escitalopram versus citalopram in major depressive disorder: a 6-week, multicenter, randomized, double-blind, flexible-dose study. Psychopharmacology 213: 639–646. [PubMed: 20340011]Owens 2008
Owens MJ, Krulewicz S, Simon JS, Sheehan DV, Thase ME, et al. (2008) Estimates of serotonin and norepinephrine transporter inhibition in depressed patients treated with paroxetine or venlafaxine. Neuropsychopharmacology 33: 3201–3212. [PubMed: 18418363]Ozdemir 2015
Ozdemir PG, Boysan M, Smolensky MH, Selvi Y, Aydin A, et al. (2015) Comparison of venlafaxine alone versus venlafaxine plus bright light therapy combination for severe major depressive disorder. Journal of Clinical Psychiatry 76: E645–E654. [PubMed: 26035199]PAR 01 001 (GSK & FDA)
http://digitalcommons .ohsu.edu/fdadrug/26/ (data extracted from Cipriani 2018). PAR 279 MDUK
http://www.gsk-clinicalstudyregister .com /study/29060/012_3?search =compound&compound =paroxetine#rs (data extracted from Cipriani 2018). PAR 29060/281
https://www.gsk-clinicalstudyregister .com/study/29060/281#rs (data extracted from Cipriani 2018). PAR MDUK 032
https://www.gsk-clinicalstudyregister .com/study/29060/032#rs (data extracted from Cipriani 2018). Paslakis 2010
Paslakis G, Luppa P, Gilles M, Kopf D, Hamann-Weber B, et al. (2010) Venlafaxine and mirtazapine treatment lowers serum concentrations of dehydroepiandrosterone-sulfate in depressed patients remitting during the course of treatment. Journal of Psychiatric Research 44: 556–560. [PubMed: 20022345]Patel 2017/Weobong 2017
Patel V, Weobong B, Weiss HA, Anand A, Bhat B, et al. (2017) The healthy activity program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: a randomised controlled trial. The Lancet 389: 176–185. [PMC free article: PMC5236064] [PubMed: 27988143]
Weobong B, Weiss HA, McDaid D, Singla DR, Hollon SD, et al. (2017) Sustained effectiveness and cost-effectiveness of the Healthy Activity Programme, a brief psychological treatment for depression delivered by lay counsellors in primary care: 12-month follow-up of a randomised controlled trial. PLoS Medicine 14: e1002385. [PMC free article: PMC5595303] [PubMed: 28898283]Patris 1996
Patris M, Bouchard JM, Bougerol T, Charbonnier JF, Chevalier JF, et al. (1996) Citalopram versus fluoxetine: a double-blind, controlled, multicentre, phase III trial in patients with unipolar major depression treated in general practice. International Clinical Psychopharmacology 11: 129–136. [PubMed: 8803650]Pei 2006
Pei Y, Zhang J, Chen J, Qiao J (2006) Clinical observation of acupuncture in treating depression on Wang-Shi Back-shu points of five-zang. Chinese Journal of Information on TCM 13: 62–63 (data extracted from Smith 2018).Perahia 2006
Perahia DG, Wang F, Mallinckrodt CH, Walker DJ, Detke MJ (2006) Duloxetine in the treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. European Psychiatry 21: 367–378. [PubMed: 16697153]Perahia 2008a_study 1
Perahia DGS, Pritchett Y, Kajdasz D, Bauer M, Jain R, et al. (2008) A randomized, double-blind comparison of duloxetine and venlafaxine in the treatment of patients with major depressive disorder. Journal of Psychiatric Research 42: 22–34 (data extracted from Cipriani 2018). [PubMed: 17445831]Perahia 2008a_study 2
Perahia DGS, Pritchett Y, Kajdasz D, Bauer M, Jain R, et al. (2008) A randomized, double-blind comparison of duloxetine and venlafaxine in the treatment of patients with major depressive disorder. Journal of Psychiatric Research 42: 22–34 (data extracted from Cipriani 2018). [PubMed: 17445831]Perahia 2008b
Perahia DGS, Quail D, Gandhi P, Walker DJ, Peveler RC (2008) A randomized, controlled trial of duloxetine alone vs. duloxetine plus a telephone intervention in the treatment of depression. Journal of Affective Disorders 108: 33–41. [PubMed: 17905442]Peselow 1989a
Peselow ED, Stanley M, Filippi AM, Barouche F, Goodnick P, et al. (1989) The predictive value of the dexamethasone suppression test. A placebo-controlled study. British Journal of Psychiatry 155: 667–672. [PubMed: 2532946]Peselow 1989b
Peselow ED, Filippi AM, Goodnick P, Barouche F, Fieve RR (1989) The short- and long-term efficacy of paroxetine HCl: A. Data from a 6-week double-blind parallel design trial vs. imipramine and placebo. Psychopharmacology Bulletin 25: 267–271. [PubMed: 2532373]Peters 1990
Peters UH, Lenhard P, Metz M (1990) Therapy of depression in the psychiatrist’s office - a doubleblind multicenter study. Nervenheilkunde 9: 28–31 (data extracted from 2009 Depression NICE guideline).Pettinati 2010
Pettinati HM, Oslin DW, Kampman KM, Dundon WD, Xie H, et al. (2010) A double-blind, placebo-controlled trial combining sertraline and naltrexone for treating co-occurring depression and alcohol dependence. American Journal of Psychiatry 167: 668–675. [PMC free article: PMC3121313] [PubMed: 20231324]Philipp 1999
Philipp M, Kohnen R, Hiller KO (1999) Hypericum extract versus imipramine or placebo in patients with moderate depression: randomised multicentre study of treatment for eight weeks. British Medical Journal 319: 1534–1538. [PMC free article: PMC28296] [PubMed: 10591711]Preskorn 1991
Preskorn SH, Silkey B, Beber J, Dorey C (1991) Antidepressant response and plasma concentrations of fluoxetine. Annals of Clinical Psychiatry 3: 147–151.Proudfoot 2003
Proudfoot J, Goldberg D, Mann A, Everitt B, Marks I, et al. (2003). Computerized, interactive, multimedia cognitive-behavioural program for anxiety and depression in general practice. Psychological Medicine 33: 217–227. [PubMed: 12622301]Proudfoot 2004
Proudfoot J, Ryden C, Everitt B, Shapiro DA, Goldberg D, et al. (2004) Clinical efficacy of computerised cognitive-behavioural therapy for anxiety and depression in primary care: randomised controlled trial. British Journal of Psychiatry 185: 46–54. [PubMed: 15231555]Qu 2013
Qu SS, Huang Y, Zhang ZJ, Chen JQ, Lin RY, et al. (2013) A 6-week randomized controlled trial with 4-week follow-up of acupuncture combined with paroxetine in patients with major depressive disorder. Journal of Psychiatric Research 47: 726–732. [PubMed: 23498306]Quah-Smith 2005
Quah-Smith JI, Tang WM, Russell J (2005) Laser acupuncture for mild to moderate depression in a primary care setting–a randomised controlled trial. Acupuncture in Medicine 23: 103–111. [PubMed: 16259308]Quah-Smith 2013
Quah-Smith I, Smith C, Crawford JD, Russell J (2013) Laser acupuncture for depression: a randomised double blind controlled trial using low intensity laser intervention. Journal of Affective Disorders 148: 179–187. [PubMed: 23337655]Quilty 2014
Quilty LC, Dozois DJ, Lobo DS, Ravindran LN, Bagby RM (2014) Cognitive structure and processing during cognitive behavioral therapy vs. pharmacotherapy for depression. International Journal of Cognitive Therapy 7: 235–250.Rapaport 2009
Rapaport MH, Lydiard MH, Edward CD, Carfagno IB, Lipschitz A (2009) Low doses of controlled-release paroxetine in the treatment of late-life depression: a randomized, placebo-controlled trial. Journal of Clinical Psychiatry 70: 46–57. [PubMed: 19026248]Raskin 2007
Raskin J, Wiltse CG, Siegal A, Sheikh J, Xu J, et al. (2007) Efficacy of duloxetine on cognition, depression, and pain in elderly patients with major depressive disorder: an 8-week, double-blind, placebo-controlled trial. American Journal of Psychiatry 164: 900–909. [PubMed: 17541049]Ratti 2011_study 096
Ratti E, Bellew K, Bettica P, Bryson H, Zamuner S, et al. (2011) Results from 2 randomized, double-blind, placebo-controlled studies of the novel NK1 receptor antagonist casopitant in patients with major depressive disorder. Journal of Clinical Psychopharmacology 31: 727–733. [PubMed: 22020354]Ravindran 1995
Ravindran AV, Teehan MD, Bakish D, Yatham L, O-Reilly R, et al. (1995) The impact of sertraline, desipramine, and placebo on psychomotor functioning in depression. Human Psychopharmacology 10: 273–281.Reimherr 1990
Reimherr FW, Chouinard G, Cohn CK, Cole JO, Itil TM, et al. (1990) Antidepressant efficacy of sertraline: a double-blind, placebo- and amitriptyline-controlled, multicenter comparison study in outpatients with major depression. Journal of Clinical Psychiatry 51(suppl. B): 18–27. [PubMed: 2258378]Reynolds 1999a
Reynolds III CF, Miller MD, Pasternak RE, Frank E, Perel JM, et al. (1999) Treatment of bereavement-related major depressive episodes in later life: a controlled study of acute and continuation treatment with nortriptyline and interpersonal psychotherapy. American Journal of Psychiatry 156: 202–208. [PubMed: 9989555]Richards 2016
Richards DA, Ekers D, McMillan D, Taylor RS, Byford S, et al. (2016) Cost and outcome of behavioural activation versus cognitive behavioural therapy for depression (COBRA): a randomised, controlled, non-inferiority trial. The Lancet 388: 871–880. [PMC free article: PMC5007415] [PubMed: 27461440]Richou 1995
Richou H (1995) A multicentre, double-blind, clomipramine-controlled efficacy and safety study of Org 3770. Human Psychopharmacology 10: 263–271.Rickels 1982b
Rickels K, Weise CC, Zal HM, Csanalosi I, Werblowsky J (1982) Lofepramine and imipramine in unipolar depressed outpatients. Acta Psychiatrica Scandinavica 66: 109–120. [PubMed: 6753501]Rickels 1982d
Rickels K, Cohen D, Csanalosi I, et al. (1982) Alprazolam and imipramine in depressed outpatients: a controlled study. Current Therapeutic Research, Clinical & Experimental 32.Rickels 1982e
Rickels K, Weise CC, Sandler K, Schless A, Zal M, et al. (1982) Nomifensine, imipramine and placebo in depressed outpatients. International Pharmacopsychiatry 17 (suppl. 1): 73–88. [PubMed: 6752080]Rickels 1985
Rickels K, Feighner JP, Smith WT (1985) Alprazolam, amitriptyline, doxepin, and placebo in the treatment of depression. Archives of General Psychiatry 42: 134–141. [PubMed: 2858187]Rickels 1987
Rickels K, Chung HR, Csanalosi IB, Hurowitz AM, London J, et al. (1987) Alprazolam, diazepam, imipramine, and placebo in outpatients with major depression. Archives of General Psychiatry 44: 862–866. [PubMed: 3310952]Rickels 1991
Rickels K, London J, Fox I, Hassman H, Csanalosi I, et al. (1991) Adinazolam, diazepam, imipramine, and placebo in major depressive disorder: a controlled study. Pharmacopsychiatry 24: 127–131. [PubMed: 1754608]Rickels 1992
Rickels K (1992) The efficacy and safety of paroxetine compared with placebo in outpatients with major depression. Journal of Clinical Psychiatry 53(suppl.): 30–32 (data extracted from 2009 Depression NICE guideline). [PubMed: 1531820]Rickels 1994
Rickels K, Schweizer E, Clary C, Fox I, Weise C (1994) Nefazodone and imipramine in major depression: a placebo-controlled trial. British Journal of Psychiatry 164: 802–805. [PubMed: 7952987]Rickels 1995_Study 006-1
Rickels K, Robinson DS, Schweizer E, Marcus RN, Roberts DL (1995) Nefazodone: aspects of efficacy. Journal of Clinical Psychiatry 56(suppl. 6): 43–46. [PubMed: 7649973]Rickels 1995_Study 006-2
Rickels K, Robinson DS, Schweizer E, Marcus RN, Roberts DL (1995) Nefazodone: aspects of efficacy. Journal of Clinical Psychiatry 56(suppl. 6): 43–46. [PubMed: 7649973]Rickels 2000
Rickels K (2000) Study 332: a randomized double-blind comparison of fluoxetine in outpatients with major depression. Unpublished (data extracted from 2009 Depression NICE guideline).Rickhi 2011
Rickhi B, Moritz S, Reesal R, Xu TJ, Paccagnan P, et al. (2011) A spirituality teaching program for depression: a randomized controlled trial. International Journal of Psychiatry in Medicine 42: 315–329. [PubMed: 22439299]Robinson 2014
Robinson M, Oakes TM, Raskin J, Liu P, Shoemaker S, et al. (2014) Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. American Journal of Geriatric Psychiatry 22: 34–45. [PubMed: 24314888]Rohini 2000
Rohini V, Pandey RS, Janakiramaiah N, Gangadhar BN, Vedamurthachar A (2000) A comparative study of full and partial Sudarshan Kriya Yoga (SKY) in major depressive disorder. Nimhans Journal 18: 53–57.Romeo 2004
Romeo R, Patel A, Knapp M, Thomas C (2004) The cost-effectiveness of mirtazapine versus paroxetine in treating people with depression in primary care. International Clinical Psychopharmacology 19: 125–134. [PubMed: 15107654]Roose 2004
Roose SP, Sackeim HA, Krishnan KRR, Pollock BG, Alexopoulos G, et al. (2004) Antidepressant pharmacotherapy in the treatment of depression in the very old: a randomized, placebo-controlled trial. American Journal of Psychiatry 161: 2050–2059. [PubMed: 15514406]Ropert 1989
Ropert R (1989) Fluoxetine versus clomipramine in major depressive disorders. International Clinical Psychopharmacology 4(suppl. 1): 89–95. [PubMed: 2644343]Rosenberg 1994
Rosenberg C, Damsbo N, Fuglum E, Jacobsen LV, Horsgard S (1994) Citalopram and imipramine in the treatment of depressive patients in general practice. A Nordic multicentre clinical study. International Clinical Psychopharmacology 9(suppl. 1): 41–48. [PubMed: 8021437]Rosner 1999
Rosner R, Frick U, Beutler LE, Daldrup R (1999) Course of depression in different psychotherapies - an application of hierarchical linear models. Zeitschrift fur Klinische Psychologie 28: 112–120 (data extracted from 2009 Depression NICE guideline).Roy 2018
Roy A, Govindan R, Muralidharan K (2018) The impact of an add-on video assisted structured aerobic exercise module on mood and somatic symptoms among women with depressive disorders: study from a tertiary care centre in India. Asian Journal of Psychiatry 32: 118–122. [PubMed: 29227962]Rudolph 1999
Rudolph RL, Feiger AD (1999) A double-blind, randomized, placebo-controlled trial of once-daily venlafaxine extended release (XR) and fluoxetine for the treatment of depression. Journal of Affective Disorders 56: 171–181. [PubMed: 10701474]Rush 1977/Kovacs 1981
Rush A, Beck A, Kovacs M, Hollon S (1977) Comparative efficacy of cognitive therapy and pharmacotherapy in the treatment of depressed outpatients. Cognitive Therapy and Research 1: 17–37.
Kovacs M, Rush AJ, Beck AT, Hollon SD (1981) Depressed outpatients treated with CT or pharmacotherapy: a one-year follow-up. Archives of General Psychiatry 38: 33–39. [PubMed: 7006557]Rush 1988
Rush AJ, Weissenburger J, Vasavada N, Orsulak PJ, Fairchild CJ (1988) Dexamethasone suppression test status does not predict differential response to nortriptyline versus amitriptyline. Journal Clinical Psychopharmacology 8: 421–425. [PubMed: 3069882]Russell 2019
Russell A, Gaunt D, Cooper K, Horwood J, Barton S, et al. (2019) Guided self-help for depression in autistic adults: the ADEPT feasibility RCT. Health Technology Assessment (Winchester, England) 23(68): 1. [PMC free article: PMC6943380] [PubMed: 31856942]Russell 2020
Russell A, Gaunt DM, Cooper K, Barton S, Horwood J, et al. (2020) The feasibility of low-intensity psychological therapy for depression co-occurring with autism in adults: the autism depression trial (ADEPT)–a pilot randomised controlled trial. Autism 24: 1360–1372. [PMC free article: PMC8645299] [PubMed: 31782656]Sahranavard 2018
Sahranavard S, Miri MR (2018) A comparative study of the effectiveness of group-based cognitive behavioral therapy and dialectical behavioral therapy in reducing depressive symptoms in Iranian women substance abusers. Psicologia: Reflexão e Crítica 31. [PMC free article: PMC6966741] [PubMed: 32025975]Salminen 2008
Salminen JK, Karlsson H, Hietala J, Kajander J, Aalto S, et al. Short-term psychodynamic psychotherapy and fluoxetine in major depressive disorder: a randomized comparative study. Psychotherapy and Psychosomatics 77: 351–357. [PubMed: 18701831]Saloheimo 2016
Saloheimo HP, Markowitz J, Saloheimo TH, Laitinen JJ, Sundell J, et al. (2016) Psychotherapy effectiveness for major depression: a randomized trial in a Finnish community. BMC Psychiatry 16: 1–9. [PMC free article: PMC4859990] [PubMed: 27153942]Samuelian 1998
Samuelian JC, Hackett D (1998) A randomized, double-blind, parallel-group comparison of venlafaxine and clomipramine in outpatients with major depression. Journal of Psychopharmacology 12: 273–278. [PubMed: 10958254]Sauer 2003
Sauer H, Huppertz-Helmhold S, Dierkes W (2003) Efficacy and safety of venlafaxine ER vs. amitriptyline ER in patients with major depression of moderate severity. Pharmacopsychiatry 36: 169–175. [PubMed: 14571350]Schatzberg 2000
Schatzberg AF, Cantillon M (2000) Study 015: antidepressant early response and remission with venlafaxine or fluoxetine in geriatric outpatients. Unpublished (data extracted from 2009 Depression NICE guideline).Schatzberg 2002
Schatzberg AF, Kremer C, Rodrigues HE, Murphy JG Jr, The Mirtazapine Vs Paroxetine Study Group (2002) Double-blind, randomized comparison of mirtazapine and paroxetine in elderly depressed patients. American Journal of Geriatric Psychiatry 10: 541–550. [PubMed: 12213688]Schmidt 1983
Schmidt MM, Miller WR (1983) Amount of therapist contact and outcome in a multidimensional depression treatment program. Acta Psychiatrica Scandinavica 67: 319–332. [PubMed: 6869040]Schone 1993
Schöne W, Ludwig M (1993) A double-blind study of paroxetine compared with fluoxetine in geriatric patients with major depression. Journal of Clinical Psychopharmacology 13(suppl. 2): 34S–39S (data extracted from Cipriani 2018). [PubMed: 8106654]Schweizer 1991
Schweizer E, Weise C, Clary C, Fox I, Rickels K (1991) Placebo-controlled trial of venlafaxine for the treatment of major depression. Journal of Clinical Psychopharmacology 11: 233–236. [PubMed: 1918421]Schweizer 1994
Schweizer E, Feighner J, Mandos LA, Rickels K (1994) Comparison of venlafaxine and imipramine in the acute treatment of major depression in outpatients. Journal of Clinical Psychiatry 55: 104–108. [PubMed: 8071246]Schweizer 1998
Schweizer E, Rickels K, Hassman H, Garcia-Espana F (1998) Buspirone and imipramine for the treatment of major depression in the elderly. Journal of Clinical Psychiatry 59: 175–183. [PubMed: 9590668]Scott 1992
Scott AIF, Freeman CPL (1992) Edinburgh primary care depression study: treatment outcome, patient satisfaction and cost after 16 weeks. British Medical Journal 304: 883–887. [PMC free article: PMC1882822] [PubMed: 1392754]Scott 1997
Scott C, Tacchi MJ, Jones R, Scott J (1997) Acute and one-year outcome of a randomised controlled trial of brief cognitive therapy for major depressive disorder in primary care. British Journal of Psychiatry 171: 131–134. [PubMed: 9337947]Sechter 1999
Sechter D, Troy S, Paternetti S, Boyer P (1999) A double-blind comparison of sertraline and fluoxetine in the treatment of major depressive episode in outpatients. European Psychiatry 14: 41–48. [PubMed: 10572324]SER 315 (FDA)
http://digitalcommons .ohsu.edu/fdadrug/27/ (data extracted from Cipriani 2018). SER-CHN-1
https://www.gsk-clinicalstudyregister .com/study/29060/403#rs (data extracted from Cipriani 2018). Serrano-Blanco 2006
Serrano-Blanco A, Gabarron E, Garcia-Bayo I, Soler-Vila M, Carames E, et al. (2006) Effectiveness and cost-effectiveness of antidepressant treatment in primary health care: a six-month randomised study comparing fluoxetine to imipramine. Journal of Affective Disorders 91: 153–163. [PubMed: 16458976]Sharma 2005
Sharma VK, Das S, Mondal S, Goswami U, Gandhi A (2005) Effect of Sahaj yoga on depressive disorders. Indian Journal of Physiology and Pharmacology 49: 462. [PubMed: 16579401]Shaw 1986
Shaw DM, Thomas DR, Briscoe MH, Watkins SE, Crimmins R, et al. (1986) A comparison of the antidepressant action of citalopram and amitriptyline. British Journal of Psychiatry 149: 515–517. [PubMed: 3545354]Sheehan 2009a
Sheehan DV, Croft HA, Gossen ER, Levitt RJ, Brullé C, et al. (2009) Extended-release trazodone in major depressive disorder: a randomized, double-blind, placebo-controlled study. Psychiatry 6: 20–33. [PMC free article: PMC2719441] [PubMed: 19724732]Sheehan 2009b
Sheehan DV, Nemeroff CB, Thase ME, Entsuah R, EPIC 016 Study Group (2009) Placebo-controlled inpatient comparison of venlafaxine and fluoxetine for the treatment of major depression with melancholic features. International clinical psychopharmacology 24: 61–86. [PubMed: 19238088]Shelton 2006
Shelton RC, Haman KL, Rapaport MH, Kiev A, Smith WT, et al. (2006) A randomized, double-blind, active-control study of sertraline versus venlafaxine XR in major depressive disorder. Journal of Clinical Psychiatry 67: 1674–1681. [PubMed: 17196045]Silverstone 1994
Silverstone T, bou-Saleh MT, Pathak R, Lock T, Bennie E, et al. (1994) A multicentre comparative trial of moclobemide, imipramine and placebo in major depressive disorder. UK moclobemide study group. International Clinical Psychopharmacology 9: 109–113. [PubMed: 8056992]Simon 2006
Simon J, Pilling S, Burbeck R, Goldberg D (2006) Treatment options in moderate and severe depression: Decision analysis supporting a clinical guideline. British Journal of Psychiatry 189: 494–501. [PubMed: 17139032]Singh 2005
Singh NA, Stavrinos TM, Scarbek Y, Galambos G, Liber C, et al. (2005) A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. Journals of Gerontology Series A: Biological Sciences and Medical Sciences 60: 768–776. [PubMed: 15983181]Siqueira 2016
Siqueira CC, Valiengo LL, Carvalho AF, Santos-Silva PR, Missio G, et al. (2016) Antidepressant efficacy of adjunctive aerobic activity and associated biomarkers in major depression: a 4-week, randomized, single-blind, controlled clinical trial. Plos One 11: e0154195. [PMC free article: PMC4859497] [PubMed: 27152523]Sir 2005
Sir A, D’Souza RF, Uguz S, George T, Vahip S, et al. (2005) Randomized trial of sertraline versus venlafaxine XR in major depression: efficacy and discontinuation symptoms. Journal of Clinical Psychiatry 66: 1312–1320. [PubMed: 16259546]Smeraldi 1998b
Smeraldi E, Rizzo F, Crespi G (1998) Double-blind, randomised study of venlafaxine, clomipramine and trazodone in geriatric patients with major depression. Primary Care Psychiatry 4: 189–195.Smith 1990
Smith WT, Glaudin V, Panagides J, Gilvary E (1990) Mirtazapine vs. amitriptyline vs. placebo in the treatment of major depressive disorder. Psychopharmacology Bulletin 26: 191–196. [PubMed: 2236455]Smith 1992
Smith WT, Glaudin V (1992) A placebo-controlled trial of paroxetine in the treatment of major depression. Journal of Clinical Psychiatry 53(suppl. 9). [PubMed: 1531822]Smith 2018
Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ (2018) Acupuncture for depression. Cochrane Database of Systematic Reviews 3: Art. No. CD004046. [PMC free article: PMC6494180] [PubMed: 29502347]Sneed 2014
Sneed JR, Reinlieb ME, Rutherford BR, Miyazaki M, Fitzsimons L, et al. (2014) Antidepressant treatment of melancholia in older adults. American Journal of Geriatric Psychiatry 22: 46–52. [PubMed: 24119858]Solati 2016
Solati K (2016) Effectiveness of cognitive-behavior group therapy, psycho-education family, and drug therapy in reducing and preventing recurrence of symptoms in patients with major depressive disorder. Group 2: 3–55.Spring 1992
Spring C, Gelenberg AJ, Garvin R, Thompson S (1992) Amitryptyline, clovoxamine and cognitive function: a placebo-controlled comparison in depressed outpatients. Psychopharmacology 108: 327–332. [PubMed: 1523282]Sramek 1995
Sramek JJ, Kashkin K, Jasinsky O, Kardatzke D, Kennedy S, et al. (1995) Placebo-controlled study of ABT-200 versus fluoxetine in the treatment of major depressive disorder. Depression 3: 199–203.Staner 1995
Staner L, Kerkhofs M, Detroux D, Leyman S, Linkowski P, et al. (1995) Acute, subchronic and withdrawal sleep EEG changes during treatment with paroxetine and amitriptyline: a double-blind randomized trial in major depression. Sleep 18: 470–477. [PubMed: 7481419]Stark 1985
Stark P, Hardison CD (1985) A review of multicenter controlled studies of fluoxetine vs imipramine and placebo in out patients with major depressive disorder. Journal of Clinical Psychiatry 46: 53–58. [PubMed: 3882682]Stassen 1993
Stassen HH, Delini-Stula A, Angst J (1993) Time course of improvement under antidepressant treatment: a survival-analytical approach. European Neuropsychopharmacology 3: 127–135. [PubMed: 8364348]Study 62b (FDA)
http://digitalcommons .ohsu.edu/fdadrug/22/ (data extracted from Cipriani 2018). Study 89306 (FDA)
http://www.accessdata .fda.gov/drugsatfda_docs /nda/98/020822a_medr_P2.pdf (data extracted from Cipriani 2018). Study F1J-MC-HMAQ - Study Group B
http://www.lillytrials .com/results/Cymbalta.pdf (data extracted from Cipriani 2018). Sugg 2018
Sugg HVR, Richards DA, Frost J (2018) Morita therapy for depression (morita trial): a pilot randomised controlled trial. BMJ Open 8: e021605. [PMC free article: PMC6089263] [PubMed: 30099395]Suleman 1997
Suleman MI, Sebit MB, Acuda SW, Siziya S (1997) Fluoxetine and moclobemide versus amitriptyline in major depression: a single blind randomised clinical trial in Zimbabwe. Central-African Journal of Medicine 43: 38–40.Sun 2010
Sun H, Zhao H, Zhang J, Bao F, Wei J, et al. (2010) Effect of acupuncture at Baihui (GV 20) and Zusanli (ST36) on the level of serum inflammatory cytokines in patients with depression. Chinese Acupuncture and Moxibustion 30: 195–199 (extracted from Smith 2018). [PubMed: 20496732]Sun 2013
Sun H, Zhao H, Ma C, Bao F, Zhang J, et al. (2013) Effects of electroacupuncture on depression and the production of glial cell line-derived neurotrophic factor compared with fluoxetine: a randomized controlled pilot study. Journal of Alternative and Complementary Medicine 19: 733–739. [PMC free article: PMC3768227] [PubMed: 23647408]Teasdale 1984
Teasdale JD, Fennell MJ, Hibbert GA, Amies PL (1984) Cognitive therapy for major depressive disorder in primary care. British Journal of Psychiatry 144: 400–406. [PubMed: 6372925]Thase 1997
Thase ME (1997) Efficacy and tolerability of once-daily venlafaxine extended release (XR) in outpatients with major depression. Journal of Clinical Psychiatry 58: 393–398. [PubMed: 9378690]Thase 2018
Thase ME, Wright JH, Eells TD, Barrett MS, Wisniewski SR, et al. (2018) Improving the efficiency of psychotherapy for depression: Computer-assisted versus standard CBT. American Journal of Psychiatry 175: 242–250. [PMC free article: PMC5848497] [PubMed: 28969439]Thomas 1987
Thomas JR, Petry RA, Goldman JR (1987) Comparison of cognitive and behavioral self-control treatments of depression. Psychological Reports 60: 975–982. [PubMed: 3615741]Thomson 1982
Thomson J, Rankin H, Ashcroft GW, Yates CM, McQueen JK, et al. (1982) The treatment of depression in general practice: a comparison of L-tryptophan, amitriptyline, and a combination of L-tryptophan and amitriptyline with placebo. Psychological Medicine 12: 741–751. [PubMed: 7156248]Tignol 1993
Tignol J (1993) A double-blind, randomized, fluoxetine-controlled, multicenter study of paroxetine in the treatment of depression. Journal of Clinical Psychopharmacology 13(suppl. 2): 18S–22S. [PubMed: 8106650]Tollefson 1993/1995
Tollefson GD, Holman SL (1993) Analysis of the Hamilton depression rating scale factors from a double-blind, placebo-controlled trial of fluoxetine in geriatric major depression. International Clinical Psychopharmacology 8: 253–259. [PubMed: 8277144]
Tollefson GD, Bosomworth JC, Heiligenstein JH, Potvin JH, Holman S, et al. (1995) A double-blind, placebo-controlled clinical trial of fluoxetine in geriatric patients with major depression. International Psychogeriatrics 7: 89–104. [PubMed: 7579025]Tollefson 1994
Tollefson GD, Greist JH, Jefferson JW, Heiligenstein JH, Sayler ME, et al. (1994) Is baseline agitation a relative contraindication for a selective serotonin reuptake inhibitor: a comparative trial of fluoxetine versus imipramine. Journal of Clinical Psychopharmacology 14: 385–391. [PubMed: 7884018]Tong 2020
Tong P, Bu P, Yang Y, Dong L, Sun T, et al. (2020) Group cognitive behavioural therapy can reduce stigma and improve treatment compliance in major depressive disorder patients. Early Intervention in Psychiatry 14: 172–178. [PMC free article: PMC7065070] [PubMed: 31264787]Trivedi 2004
Trivedi MH, Pigotti TA, Perera P, Dillingham KE, Carfagno ML, et al. (2004) Effectiveness of low doses of paroxetine controlled release in the treatment of major depressive disorder. Journal of Clinical Psychiatry 65: 1356–1364. [PubMed: 15491239]Tsai 2000
Tsai S-J, Hong C-J (2000) Study 102: a double-blind, randomised 8-week study of the safety and efficacy of venlafaxine extended release (ER) compared to fluoxetine in patients with moderate to severe depression. Unpublished (data extracted from 2009 Depression NICE guideline).Tsutsui 2000
Tsutsui S, Okuse T, Sasaki D, et al. (2000) Clinical evaluation of paroxetine HCl, a selective serotonin reuptake inhibitor, in the treatment of depression or depressive episodes: double-blind, parallel group study with trazodone HCl. Yakuri-To-Chiryo (Japanese Pharmacology & Therapeutics) suppl.: 161–185 (data extracted from Cipriani 2018).Tural 2003
Tural U, Onder E (2003) Fluoxetine once every third day in the treatment of major depressive disorder. European Archives of Psychiatry and Clinical Neuroscience 253: 307–312. [PubMed: 14714120]Tylee 1997
Tylee A, Beaumont G, Bowden MW (1997) A double-blind, randomised, 12-week comparison study of the safety and efficacy of venlafaxine and fluoxetine in moderate to severe depression in general practice. Primary Care Psychiatry 3: 51–58.Tzanakaki 2000
Tzanakaki M, Guazzelli M, Nimatoudis I, Zissis NP, Smeraldi E, et al. (2000) Increased remission rates with venlafaxine compared with fluoxetine in hospitalized patients with major depression and melancholia. International Clinical Psychopharmacology 15: 29–34. [PubMed: 10836283]Umadevi 2013
Umadevi P, Ramachandra SV, Philip M, Gangadhar BN (2013) Effect of yoga therapy on anxiety and depressive symptoms and quality-of-life among caregivers of in-patients with neurological disorders at a tertiary care center in India: a randomized controlled trial. Indian Journal of Psychiatry 55(suppl. 3): S385. [PMC free article: PMC3768217] [PubMed: 24049204]Usaf 1990
Usaf S, Kavanagh DJ (1990) Mechanisms of improvement in treatment for depression: test of a self-efficacy and performance model. Journal of Cognitive Psychotherapy 4: 51–70.Valle-Cabrera 2018
Valle-Cabrera R, Mendoza-Rodriguez Y, Robaina-Garcia M, Ballesteros J, Cordero-Jimenez JR, et al. (2018) Efficacy of sertraline in patients with major depressive disorder naive to selective serotonin reuptake inhibitors. Journal of Clinical Psychopharmacology 38: 454–459. [PubMed: 30106883]Van Moffaert 1995a
Van Moffaert M, Bartholome F, Cosyns P, De Nayer AR, Mertens C (1995) A controlled comparison of sertraline and fluoxetine in acute and continuation treatment of major depression. Human Psychopharmacology 10: 393–405.Van Moffaert 1995b
Van Moffaert M (1995) Mirtazapine is more effective than trazodone: a double-blind controlled study in hospitalized patients with major depression. International Clinical Psychopharmacology 10: 3–9. [PubMed: 7622801]Vartiainen 1994
Vartiainen H (1994) Double-blind study of mirtazapine and placebo in hospitalized patients with major depression. European Neuropsychopharmacology 4: 145–50. [PubMed: 7919944]VEN 600A-303 (FDA)
http://digitalcommons .ohsu.edu/fdadrug/24/ (data extracted from Cipriani 2018). VEN 600A-313 (FDA)
http://digitalcommons .ohsu.edu/fdadrug/24/ (data extracted from Cipriani 2018). VEN XR 367 (FDA)
http://digitalcommons .ohsu.edu/fdadrug/25/ (data extracted from Cipriani 2018). Ventura 2007
Ventura D, Armstrong EP, Skrepnek GH, Erder MH (2007) Escitalopram versus sertraline in the treatment of major depressive disorder: a randomized clinical trial. Current Medical Research and Opinion 23: 245–250. [PubMed: 17288677]Vernmark 2010
Vernmark K, Lenndin J, Bjärehed J, Carlsson M, Karlsson J, et al. (2010) Internet administered guided self-help versus individualized e-mail therapy: a randomized trial of two versions of CBT for major depression. Behaviour Research and Therapy 48: 368–376. [PubMed: 20152960]Versiani 1989
Versiani M, Oggero U, Alterwain P, Capponi R, Dajas F, et al. (1989) A double-blind comparative trial of moclobemide v. imipramine and placebo in major depressive episodes. British Journal of Psychiatry 6(suppl.): 72–77. [PubMed: 2695129]Versiani 1990a
Versiani M, Nardi AE, Mundim FD, Alves A, Schmid-Burgk W (1990) Moclobemide, imipramine and placebo in the treatment of major depression. Acta Psychiatrica Scandinavica 360(suppl.): 57–58. [PubMed: 2248073]Versiani 1999
Versiani M, Ontiveros A, Mazzotti GG (1999) Fluoxetine versus amittriptyline in the treatment of major depression with associated anxiety (anxious depression): a double-blind comparison. International Clinical Psychopharmacology 14: 321–327. [PubMed: 10565798]Versiani 2005
Versiani M, Moreno R, Ramakers-van Moorsel CJ, Schutte AJ (2005) Comparison of the effects of mirtazapine and fluoxetine in severely depressed patients. CNS Drugs 19: 137–146. [PubMed: 15697327]Vitriol 2009
Vitriol VG, Ballesteros ST, Florenzano RU, Weil KP, Benadof DF (2009) Evaluation of an outpatient intervention for women with severe depression and a history of childhood trauma. Psychiatric Services 60: 936–942. [PubMed: 19564224]Wade 2002
Wade A, Lemming O, Hedegaard KB (2002) Escitalopram 10 mg/day is effective and well tolerated in a placebo-controlled study in depression in primary care. International Clinical Psychopharmacology 17: 95–102. [PubMed: 11981349]Wade 2005a
Wade AG, Toumi I, Hemels MEH (2005a) A probabilistic cost-effectiveness analysis of escitalopram, generic citalopram and venlafaxine as a first-line treatment of major depressive disorder in the UK. Current Medical Research and Opinion 21: 631–641. [PubMed: 15899113]Wade 2005b
Wade AG, Toumi I, Hemels MEH (2005b) A pharmacoeconomic evaluation of escitalopram versus citalopram in the treatment of severe depression in the United Kingdom. Clinical Therapeutics 27: 486–496. [PubMed: 15922821]Wade 2007 / Wade 2008
Wade A, Gembert K, Florea I (2007) A comparative study of the efficacy of acute and continuation treatment with escitalopram versus duloxetine in patients with major depressive disorder. Current Medical Research & Opinion 23: 1605–1614. [PubMed: 17559755]
Wade AG, Fernandez JL, Francois C, Hansen K, Danchenko N, et al. (2008) Escitalopram and duloxetine in major depressive disorder: A pharmacoeconomic comparison using UK cost data. PharmacoEconomics 26: 969–981. [PubMed: 18850765]Wang 2013
Wang WD, Lu XY, Ng SM, Hong L, Zhao Y, et al. (2013) Effects of electro-acupuncture on personality traits in depression: a randomized controlled study. Chinese Journal of Integrative Medicine 19: 777–782. [PubMed: 24092242]Wang 2014a
Wang T, Wang L, Tao W, Chen L (2014) Acupuncture combined with an antidepressant for patients with depression in hospital: a pragmatic randomised controlled trial. Acupuncture in Medicine 32: 308–312. [PubMed: 24781054]Wang 2014c
Wang G, McIntyre A, Earley WR, Raines SR, Eriksson H (2014) A randomized, double-blind study of the efficacy and tolerability of extended-release quetiapine fumarate (quetiapine XR) monotherapy in patients with major depressive disorder. Neuropsychiatric Disease Treatment 10: 201–216. [PMC free article: PMC3916085] [PubMed: 24511235]Ward 2000/King 2000
Ward E, King M, Lloyd M, Bower P, Sibbald B, et al. (2000) Randomised controlled trial of non-directive counselling, cognitive-behaviour therapy, and usual general practitioner care for patients with depression. I: clinical effectiveness. BMJ 321: 1383–1388. [PMC free article: PMC27542] [PubMed: 11099284]
King M, Sibbald B, Ward E, Bower P, Lloyd M, et al. (2000) Randomised controlled trial of non-directive counselling, cognitive-behaviour therapy and usual general practitioner care in the management of depression as well as mixed anxiety and depression in primary care. Health Technology Assessment (Winchester, England) 4: 1–83. [PubMed: 11086269]Watkins 2012
Watkins ER, Taylor RS, Byng R, Baeyens C, Read R, et al. (2012) Guided self-help concreteness training as an intervention for major depression in primary care: a Phase II randomized controlled trial. Psychological Medicine 42:1359–1371. [PMC free article: PMC3359637] [PubMed: 22085757]WELL AK1A4006
http://digitalcommons .ohsu.edu/fdadrug/21/ (data extracted from Cipriani 2018). Wernicke 1987
Wernicke JF, Dunlop SR, Dornseif BE, Zerbe RL (1987) Fixed-dose fluoxetine therapy for depression. Psychopharmacology Bulletin 23: 164–168. [PubMed: 3496625]Wernicke 1988
Wernicke JF, Dunlop SR, Dornseif BE, Bosomworth JC, Humbert M (1988) Low-dose fluoxetine therapy for depression. Psychopharmacology Bulletin 24: 183–188. [PubMed: 3290940]Wheatley 1998
Wheatley DP, Van Moffaert M, Timmerman L, Kremer CME (1998) Mirtazapine: efficacy and tolerability in comparison with fluoxetine in patients with moderate to severe major depressive disorder. Journal of Clinical Psychiatry 59: 306–312. [PubMed: 9671343]White 1984
White K, Razani J, Cadow B, Gelfand R, Palmer R, et al. (1984) Tranylcypromine vs nortriptyline vs placebo in depressed outpatients: a controlled trial. Psychopharmacology 82: 258–262. [PubMed: 6425910]Whitmyer 2007
Whitmyer VG, Dunner DL, Kornstein SG, Meyers AL, Mallinckrodt CH, et al. (2007) A comparison of initial duloxetine dosing strategies in patients with major depressive disorder. Journal of Clinical Psychiatry 68: 1921–1930. [PubMed: 18162024]Wilson 1982
Wilson P (1982) Combined pharmacological and behavioural treatment of depression. Behavior Research and Therapy 20: 173–184. [PubMed: 7073647]Wright 2005
Wright JH, Wright AS, Albano AM, Basco MR, Goldsmith LJ, et al. (2005) Computer-assisted cognitive therapy for depression: maintaining efficacy while reducing therapist time. American Journal of Psychiatry 162: 1158–1164. [PubMed: 15930065]Xiaoling 2018
Xiaoling Z, Yunping H, Yingdong L (2018) Analysis of curative effect of fluoxetine and escitalopram in the depression treatment based on clinical observation. Pakistan Journal of Pharmaceutical Sciences: 1115–1118. [PubMed: 29737292]Xu 2011
Xu L, Wang LL (2011) Clinical observation on depression treated by electroacupuncture combined with western medicine. Chinese Acupuncture & Moxibustion 31: 779–782 (data extracted from Smith 2018). [PubMed: 21972619]Yan 2004
Yan M, Mao X, Wu JL (2004) Comparison of electroacupuncture and amitriptyline in treating depression. Chinese Journal of Clinical Rehabilitation 18: 3548–3549 (data extracted from Smith 2018).Yevtushenko 2007
Yevtunshenko VY, Belous AI, Yevtushenko YG, Gusinin SE, Buzik OJ, et al. (2007) Efficacy and tolerability of escitalopram versus citalopram in major depressive disorder: a 6-week, multicentre, prospective, randomized, double-blind, active-controlled study in adult outpatients. Clinical Therapeutics 29: 2319–2332. [PubMed: 18158074]Young 1987
Young JP, Coleman A, Lader MH (1987) A controlled comparison of fluoxetine and amitriptyline in depressed out-patients. British Journal of Psychiatry 151: 337–340. [PubMed: 3322468]Zhang 2005
Zhang DL (2005) Application of acupuncture in patients with depression. Chinese Journal of Clinical Rehabilitation 9: 217–218 (data extracted from Smith 2018).Zhang 2007a
Zhang GJ, Shi ZY, Liu S, Gong SH, Liu JQ (2007) Clinical observation of treatment of depression by electro-acupuncture combined with paroxetine: clinical observations. Chinese Journal of Integrative Medicine 13: 228–230. [PubMed: 17898957]Zhang 2007b
Zhang LJ, Zhao H (2007) Clinical observation of acupuncture in treating depression and effect on serum cell factors. Chinese Journal of Information on TCM 14: 15–17 (data extracted from Smith 2018).Zhang 2014
Zhang L, Xie WW, Li LH (2014) Efficacy and safety of prolonged-release trazodone in major depressive disorder. Pharmacology 94: 199–206. [PubMed: 25376160]Zhang 2019
Zhang L, Long M, Xu L (2019) Comparative studies on the therapeutic and adverse effects of mirtazapine and fluoxetine in the treatment of adult depression. Tropical Journal of Pharmaceutical Research 18: 135–139.Zhao 2006
Zhao J, Ang Q, Wang J, Sun X, Wei J, et al. (2006) A comparative efficacy of fluoxetine and trazodone in depression with remarkable retardation and loss of energy: a randomised openlabel trial. Internal Medicine Journal 13: 25–30.Zhao 2019a
Zhao B, Li Z, Wang Y, Ma X, Wang X, et al. (2019) Manual or electroacupuncture as an add-on therapy to SSRIs for depression: a randomized controlled trial. Journal of Psychiatric Research 114: 24–33. [PubMed: 31015098]Ziegler 1977
Ziegler V, Clayton P, Biggs J (1977) A comparison study of amitriptyline and nortiptyline with plasma levels. Archives of General Psychiatry 34: 607–612. [PubMed: 860894]Zivkov 1995
Zivkov M (1995) Org 3770 versus amitriptyline: A 6-week randomized double-blind multicentre trial in hospitalized depressed patients. Human Psychopharmacology 10: 173–180.Zu 2014
Zu S, Xiang YT, Liu J, Zhang L, Wang G, et al. (2014) A comparison of cognitive-behavioral therapy, antidepressants, their combination and standard treatment for Chinese patients with moderate–severe major depressive disorders. Journal of Affective Disorders 152: 262–267. [PubMed: 24140226]Barkham 2021
Barkham M, Saxon, D, Hardy G, et al (2021). Person-centred experiential therapy versus cognitive behavioural therapy delivered in the English Improving Access to Psychological Therapies service for the treatment of moderate or severe depression (PRaCTICED): a pragmatic randomised, non-inferiority trial. Lancet Psychiatry 8:487–499. [PubMed: 34000240]Cuijpers 2021
Cuijpers P, Quero S, Noma H, et al. (2021). Psychotherapies for depression: a network meta-analysis covering efficacy, acceptability and long-term outcomes of all main treatment types. World Psychiatry 20:283–293 [PMC free article: PMC8129869] [PubMed: 34002502]- Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, Salanti G (2020). CINeMA: An approach for assessing confidence in the results of a network meta-analysis PLOS Medicine 17:1–19. [PMC free article: PMC7122720] [PubMed: 32243458]
- Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE (2019) Threshold Analysis as an Alternative to GRADE for Assessing Confidence in Guideline Recommendations Based on Network Meta-analyses. Annals of Internal Medicine 170:538–46. [PMC free article: PMC6739230] [PubMed: 30909295]
Additional references discussed by the committee
Additional references to methodological issues
Appendices
Appendix A. Review protocol
Appendix B. Literature search strategies
Appendix C. Clinical evidence study selection
Appendix D. Clinical evidence tables
Clinical evidence table for review questions: For adults with a new episode of less severe depression or more severe depression, what are the relative benefits and harms of psychological, psychosocial, pharmacological and physical interventions alone or in combination?
Please refer to supplement B1 - Clinical evidence tables for treatment of a new episode of depression
Appendix E. Forest plots
Forest plots for review questions: For adults with a new episode of less severe depression or more severe depression, what are the relative benefits and harms of psychological, psychosocial, pharmacological and physical interventions alone or in combination?
Please refer to supplements B2 and B3 for forest plots for studies included in the NMA treatment of a new episode of less severe depression and more severe depression, respectively
Forest plots for review questions: For adults with a new episode of less severe depression, what are the relative benefits and harms of psychological, psychosocial, pharmacological and physical interventions alone or in combination?
Download PDF (380K)
Forest plots for review question: For adults with a new episode of more severe depression, what are the relative benefits and harms of psychological, psychosocial, pharmacological and physical interventions alone or in combination?
Download PDF (4.0M)
Appendix F. GRADE tables
To evaluate the quality of the evidence of the NMAs undertaken to inform this review question, we report information about the factors considered in a GRADE profile (risk of bias, publication bias, imprecision, inconsistency, and indirectness) – see under ‘Quality assessment of studies included in the evidence review’.
GRADE table for pairwise meta-analysis of couple interventions (not included in NMA) (PDF, 171K)
Appendix G. Economic evidence study selection
Appendix H. Economic evidence tables
Appendix I. Economic evidence profiles
Appendix J. Economic analysis
References
- Akhtar W, Chung Y (2014). Saving the NHS one blood test at a time. BMJ Qual Improv Rep 2(2), u204012.w1749. [PMC free article: PMC4663853] [PubMed: 26734249]
- Anderson HD, Pace WD, Libby AM, West DR, Valuck RJ (2012). Rates of 5 common antidepressant side effects among new adult and adolescent cases of depression: a retrospective US claims study. Clin Ther, 34(1), 113–123. [PubMed: 22177545]
- Andrade L, Caraveo-Anduaga JJ, Berglund P, Bijl RV, De Graaf R, Vollebergh W, Dragomirecka E, Kohn R, Keller M, Kessler RC, Kawakami N, Kiliç C, Offord D, Ustun TB, Wittchen HU (2003). The epidemiology of major depressive episodes: results from the International Consortium of Psychiatric Epidemiology (ICPE) Surveys. Int J Methods Psychiatr Res, 12(1), 3–21. Erratum in: Int J Methods Psychiatr Res (2003), 12(3), 165. [PMC free article: PMC6878531] [PubMed: 12830306]
- Briggs A, Sculpher M, Claxton K (2006) Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press
- British Association for Behavioural & Cognitive Psychotherapies (2016). Criteria and Guidelines for re-accreditation as a Behavioural and/or Cognitive Psychotherapist. British Association for Behavioural & Cognitive Psychotherapies.
- British National Formulary (January 2021). London: BMJ Group and the Royal Pharmaceutical Society of Great Britain. https://bnf
.nice.org.uk [Accessed 7 January 2021] - Bull SA, Hunkeler EM, Lee JY, Rowland CR, Williamson TE, Schwab JR, Hurt SW (2002). Discontinuing or switching selective serotonin-reuptake inhibitors. Ann Pharmacother, 36(4), 578–584. [PubMed: 11918502]
- Burton C, Anderson N, Wilde K, Simpson CR (2012). Factors associated with duration of new antidepressant treatment: analysis of a large primary care database. Br J Gen Pract, 62(595), e104–112. [PMC free article: PMC3268489] [PubMed: 22520784]
- Byford S, Barrett B, Despiégel N, Wade A (2011). Impact of treatment success on health service use and cost in depression. PharmacoEconomics, 29(2), 157–170. [PubMed: 21142289]
- Caldwell DM, Ades AE, Higgins JP (2005) Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331(7521), 897–900 [PMC free article: PMC1255806] [PubMed: 16223826]
- Coupland C, Dhiman P, Morriss R, Arthur A, Barton G, Hippisley-Cox J (2011). Antidepressant use and risk of adverse outcomes in older people: population based cohort study. BMJ, 343, d4551. [PMC free article: PMC3149102] [PubMed: 21810886]
- Curtis L, Burns A (2020) Unit Costs of Health & Social Care 2020. Canterbury: PSSRU, University of Kent.
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW (2014). Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry, 171(4), 453–62. [PubMed: 24434956]
- Dias S, Welton NJ, Sutton AJ, Ades AE (2011, last updated 2016). NICE DSU Technical Support Document 2: A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. Available from http://www
.nicedsu.org.uk [PubMed: 27466657] - Eaton WW, Shao H, Nestadt G, Lee HB, Bienvenu OJ, Zandi P (2008). Population-based study of first onset and chronicity in major depressive disorder. Arch Gen Psychiatry, 65(5), 513–520. [PMC free article: PMC2761826] [PubMed: 18458203]
- Fenwick E, Claxton K, Sculpher M (2001) Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Economics 10(8), 779–87. [PubMed: 11747057]
- Fernandez-Pujals AM, Adams MJ, Thomson P, McKechanie AG, Blackwood DH, Smith BH, Dominiczak AF, Morris AD, Matthews K, Campbell A, Linksted P, Haley CS, Deary IJ, Porteous DJ, MacIntyre DJ, McIntosh AM (2015). Epidemiology and Heritability of Major Depressive Disorder, Stratified by Age of Onset, Sex, and Illness Course in Generation Scotland: Scottish Family Health Study (GS:SFHS). PLoS One, 10(11), e0142197. [PMC free article: PMC4646689] [PubMed: 26571028]
- Gilbody S, Richards D, Barkham M (2007a). Diagnosing depression in primary care using self-completed instruments: UK validation of PHQ-9 and CORE-OM. Br J Gen Pract, 57(541), 650–652. [PMC free article: PMC2099671] [PubMed: 17688760]
- Gilbody S, Richards D, Brealey S, Hewitt C (2007b). Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med, 22(11), 1596–1602. [PMC free article: PMC2219806] [PubMed: 17874169]
- Goethe JW, Woolley SB, Cardoni AA, Woznicki BA, Piez DA (2007). Selective serotonin reuptake inhibitor discontinuation: side effects and other factors that influence medication adherence. J Clin Psychopharmacol, 27(5), 451–458. [PubMed: 17873676]
- Gonzales LR, Lewinsohn PM, Clarke GN (1985). Longitudinal follow-up of unipolar depressives: an investigation of predictors of relapse. J Consult Clin Psychol, 53(4), 461–469. [PubMed: 4031201]
- Hansen DG, Vach W, Rosholm JU, Sondergaard J, Gram LF, Kragstrup J (2004). Early discontinuation of antidepressants in general practice: association with patient and prescriber characteristics. Fam Pract, 21(6), 623–629. [PubMed: 15520034]
- Holma KM, Holma IA, Melartin TK, Rytsala HJ, Isometsa ET (2008). Long-term outcome of major depressive disorder in psychiatric patients is variable. J Clin Psychiatry, 69(2), 196–205. [PubMed: 18251627]
- Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Moller K, Iversen M, Banke MB, Petersen IJ, Klingenberg SL, Krogh J, Ebert SE, Timm A, Lindschou J, Gluud C (2017). Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and Trial Sequential Analysis. BMC Psychiatry, 17(1), 58. [PMC free article: PMC5299662] [PubMed: 28178949]
- Kaltenthaler E, Brazier J, De Nigris E, Tumur I, Ferriter M, Beverley C, Parry G, Rooney G, Sutcliffe P (2006). Computerised cognitive behaviour therapy for depression and anxiety update: a systematic review and economic evaluation. Health Technol Assess, 10(33). [PubMed: 16959169]
- Keller MB, Klerman GL, Lavori PW, Coryell W, Endicott J, Taylor J (1984). Long-term outcome of episodes of major depression. Clinical and public health significance. JAMA, 252(6), 788–792. [PubMed: 6748178]
- Keller MB, Lavori PW, Mueller TI, Endicott J, Coryell W, Hirschfeld RM, Shea T (1992). Time to recovery, chronicity, and levels of psychopathology in major depression. A 5-year prospective follow-up of 431 subjects. Arch Gen Psychiatry, 49(10), 809–816. [PubMed: 1417434]
- Keller MB, Shapiro RW (1981). Major depressive disorder. Initial results from a one-year prospective naturalistic follow-up study. J Nerv Ment Dis, 169(12), 761–768. [PubMed: 7310387]
- Kessing LV, Andersen PK (1999). The effect of episodes on recurrence in affective disorder: a case register study. J Affect Disord, 53(3), 225–231. [PubMed: 10404708]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 62(6), 593–602. [PubMed: 15939837]
- Kind P, Hardman G, Macran S (1999). UK population norms for EQ-5D. Centre for Health Economics, University of York.
- Koeser L, Donisi V, Goldberg DP, McCrone P (2015). Modelling the cost-effectiveness of pharmacotherapy compared with cognitive-behavioural therapy and combination therapy for the treatment of moderate to severe depression in the UK. Psychological Medicine, 45, 3019–3031. [PubMed: 26040631]
- Kolovos S, Bosmans JE, van Dongen JM, van Esveld B, Magai D, van Straten A, van der Feltz-Cornelis C, van Steenbergen-Weijenburg KM, Huijbregts KM, van Marwijk H, Riper H, van Tulder MW (2017). Utility scores for different health states related to depression: individual participant data analysis. Qual Life Res, 26(7), 1649–1658. [PMC free article: PMC5486895] [PubMed: 28260149]
- Kuyken W, Byford S, Taylor RS, Watkins E, Holden E, White K, Barrett B, Byng R, Evans A, Mullan E, Teasdale JD (2008). Mindfulness-based cognitive therapy to prevent relapse in recurrent depression. J Consult Clin Psychol, 76(6), 966–78. [PubMed: 19045965]
- Lewis, E, Marcus SC, Olfson M, Druss BG, Pincus HA (2004). Patients’ early discontinuation of antidepressant prescriptions. Psychiatr Serv, 55(5), 494. [PubMed: 15128956]
- Lu G, Ades AE (2004) Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 23(20), 3105–24 [PubMed: 15449338]
- Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000). WinBUGS-A Bayesian modelling framework: Concepts, structure, and extensibility. Statistics and Computing 10, 325–337
- Mann R, Gilbody S, Richards D (2009). Putting the ‘Q’ in depression QALYs: A comparison of utility measurement using EQ-5D and SF-6D health related quality of life measures. Social psychiatry and psychiatric epidemiology, 44(7), 569–578. [PubMed: 19011721]
- Mattisson C, Bogren M, Horstmann V, Munk-Jörgensen P, Nettelbladt P (2007). The long-term course of depressive disorders in the Lundby Study. Psychol Med, 37(6), 883–91. [PubMed: 17306047]
- McManus S, Bebbington P, Jenkins R, Brugha T (eds) (2016). Mental health and wellbeing in England: Adult Psychiatric Morbidity Survey 2014. Leeds: NHS Digital.
- Montgomery SA, Nielsen RZ, Poulsen LH, Häggström L (2014). A randomised, double-blind study in adults with major depressive disorder with an inadequate response to a single course of selective serotonin reuptake inhibitor or serotonin-noradrenaline reuptake inhibitor treatment switched to vortioxetine or agomelatine. Hum Psychopharmacol, 29(5), 470–82. [PMC free article: PMC4265248] [PubMed: 25087600]
- Mueller TI, Keller MB, Leon AC, Solomon DA, Shea MT, Coryell W, Endicott J (1996). Recovery after 5 years of unremitting major depressive disorder. Arch Gen Psychiatry, 53(9), 794–799. [PubMed: 8792756]
- National Clinical Guidelines Centre (2016). Routine preoperative tests for elective surgery. NICE guideline [NG45]. Appendix M: Economic considerations for Delphi. Available from: https://www
.nice.org .uk/guidance/NG45/documents /guideline-appendices-13 [Accessed 29 January 2021] - National Institute for Health and Care Excellence (2013) Guide to the Methods of Technology Appraisal 2013 (PMG 9). Available from: www
.nice.org.uk/pmg9 [PubMed: 27905712] - National Institute for Health and Care Excellence (2014, last updated October 2020) Developing NICE guidelines: the manual (PMG 20). Available from: www
.nice.org.uk/process/pmg20 [PubMed: 26677490] - Netten A, Knight J, Dennett J, Cooley R, Slight A (1998). Development of a ready reckoner for staff costs in the NHS, Vols 1 & 2. Canterbury: PSSRU, University of Kent.
- NHS Business Services Authority (2020) Prescription Cost Analysis - England 2019. NHS Business Services Authority. Available from: https://www
.nhsbsa.nhs .uk/statistical-collections /prescription-cost-analysis-england /prescription-cost-analysis-england-2019 [Accessed 20 January 2021] - NHS Business Services Authority, NHS Prescription Services (2021). NHS England and Wales. Electronic Drug Tariff. Issue: August 2021. Compiled on the behalf of the Department of Health and Social Care. Available from: https://www
.nhsbsa.nhs .uk/pharmacies-gp-practices-and-appliance-contractors /drug-tariff [Accessed 5 August 2021] - NHS England and Health Education England (2016a). 2015 Adult IAPT workforce census report. NHS England and Health Education England.
- NHS England and Health Education England (2016b). National College for Teaching and Leadership. Review of clinical and educational psychology training arrangements. NHS England and Health Education England.
- NHS England (18 September 2016). More people than ever receiving psychological therapies and recovering. Available from: https://www
.england.nhs .uk/2016/07/psychological-therapies/ [Accessed 3 February 2021] - NHS Improvement (2020). National Schedule of NHS costs - Year 2018-19 - NHS trusts and NHS foundation trusts. Available from: https://improvement
.nhs .uk/resources/national-cost-collection/ - NHS The Information Centre (2007). Prescription Cost Analysis: England 2006. NHS The Information Centre, Prescribing Support Unit.
- Office for National Statistics (2020). National life tables – life expectancy in the UK: 2017 to 2019. Available from: https://www
.ons.gov.uk /peoplepopulationandcommunity /birthsdeathsandmarriages /lifeexpectancies /bulletins /nationallifetablesunitedkingdom /2017to2019 [accessed 15 January 2021] - Olfson M, Marcus SC, Tedeschi M, Wan GJ (2006). Continuity of antidepressant treatment for adults with depression in the United States. Am J Psychiatry, 163(1), 101–108. [PubMed: 16390896]
- Richards A, Barkham M, Cahill J, Richards D, Williams C, Heywood P (2003). PHASE: a randomised, controlled trial of supervised self-help cognitive behavioural therapy in primary care. Br J Gen Pract, 53(495), 764–770. [PMC free article: PMC1314708] [PubMed: 14601351]
- Radhakrishnan M, Hammond G, Jones PB, Watson A, McMillan-Shields F, Lafortune L (2013). Cost of improving Access to Psychological Therapies (IAPT) programme: an analysis of cost of session, treatment and recovery in selected Primary Care Trusts in the East of England region. Behav Res Ther, 51(1), 37–45. [PubMed: 23178677]
- Richards DA, Lovell K, Gilbody S, Gask L, Torgerson D, Barkham M, Bland M, Bower P, Lankshear AJ, Simpson A, Fletcher J, Escott D, Hennessy S, Richardson R (2008). Collaborative care for depression in UK primary care: a randomized controlled trial. Psychol Med, 38(2), 279–87. [PubMed: 17803837]
- Sapin C, Fantino B, Nowicki ML, Kind P (2004). Usefulness of EQ-5D in assessing health status in primary care patients with major depressive disorder. Health and Quality of Life Outcomes, 2(20). [PMC free article: PMC416488] [PubMed: 15128456]
- Skodol AE, Grilo CM, Keyes KM, Geier T, Grant BF, Hasin DS (2011). Relationship of personality disorders to the course of major depressive disorder in a nationally representative sample. Am J Psychiatry, 168(3), 257–264. [PMC free article: PMC3202962] [PubMed: 21245088]
- Simon GE, Goldberg D, Tiemens BG, Ustun TB (1999). Outcomes of recognized and unrecognized depression in an international primary care study. Gen Hosp Psychiatry, 21(2), 97–105. [PubMed: 10228889]
- Sobocki P, Ekman M, Agren H, Krakau I, Runeson B, Martensson B, Jonsson B (2007). Health-related quality of life measured with EQ-5D in patients treated for depression in primary care. Value in Health, 10(2), 153–160. [PubMed: 17391424]
- Sobocki P, Ekman M, Agren H, Runeson B, Jonsson B (2006). The mission is remission: Health economic consequences of achieving full remission with antidepressant treatment for depression. International Journal of Clinical Practice, 60(7), 791–798. [PubMed: 16846399]
- Soini E, Hallinen T, Brignone M, Campbell R, Diamand F, Cure S, Aalto-Setälä M, Danchenko N, Koponen H, Kolasa K (2017). Cost-utility analysis of vortioxetine versus agomelatine, bupropion SR, sertraline and venlafaxine XR after treatment switch in major depressive disorder in Finland. Expert Rev Pharmacoecon Outcomes Res, 17(3), 293–302. [PubMed: 27680105]
- Spiegelhalter D, Thomas A, Best N, Lunn DJ (2003). WinBUGS user manual: Version 1.4. Cambridge: MRC Biostatistics Unit.
- Spijker J, de Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA (2002). Duration of major depressive episodes in the general population: results from The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Br J Psychiatry, 181, 208–213. [PubMed: 12204924]
- Stegenga BT, Kamphuis MH, King M, Nazareth I, Geerlings MI (2012). The natural course and outcome of major depressive disorder in primary care: the PREDICT-NL study. Soc Psychiatry Psychiatr Epidemiol, 47(1), 87–95. [PMC free article: PMC3249585] [PubMed: 21057769]
- Sullivan PW, Valuck R, Saseen J, MacFall HM (2004). A comparison of the direct costs and cost effectiveness of serotonin reuptake inhibitors and associated adverse drug reactions. CNS Drugs, 18, 911–932. [PubMed: 15521793]
Appendix K. Excluded studies
Excluded studies for review questions: For adults with a new episode of less severe depression or more severe depression, what are the relative benefits and harms of psychological, psychosocial, pharmacological and physical interventions alone or in combination?
Clinical studies
Please refer to supplement B1 - Clinical evidence tables for treatment of a new episode of depression
Economic studies
Please refer to Supporting documentation - Economic evidence included & excluded studies.
Appendix L. Research recommendations
Appendix M. Network meta-analysis report from the NICE Guidelines Technical Support Unit (TSU)
References
- Brooks S, Gelman A (1998). Alternative methods for monitoring convergence of iterative simulations. Journal of Computatinal and Graphical Statistics 7,434–55.
- Caldwell DM, Ades AE, Higgins JPT (2005). Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331,897–900. [PMC free article: PMC1255806] [PubMed: 16223826]
- Chinn S (2000). A simple method for converting an odds ratio to effect size for use in metaanalysis. Statistics in Medicine 19,3127–31 [PubMed: 11113947]
- Cohen, J (1969). Statistical power analysis for the behavioral sciences. Academic Press, New York.
- Cooper H, Hedges LV, Valentine JC (2009). The Handbook of Research Synthesis and Meta-analysis. New York, Russel Sage Foundation.
- Daly C, Welton NJ, Dias S, Anwer S, Ades AE (2021). Meta-Analysis of Continuous Outcomes. Guideline Methodology Document 2. Version 1. NICE Guidelines Technical Support Unit. Available from: http://www
.bristol.ac .uk/media-library/sites /social-community-medicine /documents /mpes/gmd-2-continuous-jan2021.pdf - Dias S, Ades A, Sutton A, Welton N (2013). Evidence Synthesis for Decision Making 2: A Generalized Linear Modeling Framework for Pairwise and Network Meta-analysis of Randomized Controlled Trials. Medical Decision Making 33,607–17. [PMC free article: PMC3704203] [PubMed: 23104435]
- Dias S, Welton NJ, Marinho V, Salanti G, Higgins JP, Ades A (2010). Estimation and adjustment of bias in randomised evidence by using Mixed Treatment Comparison Meta-analysis. Journal of the Royal Statistical Society Series A 173(3),613–629.
- Dias S, Welton N, Sutton A, Ades A (2011). A generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials NICE Decision Support Unit Evidence Synthesis Technical Support Document 2 (last updated September 2016) Available from: http://nicedsu
.org.uk /technical-support-documents /evidence-synthesis-tsd-series/ - Gelman A, Rubin D (1992). Inferences from iterative simulation using multiple sequences. Statistical Science 7,457–72.
- Higgins JPT, Green S (eds) (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from: www
.cochrane-handbook.org - Higgins JPT, Whitehead A (1996). Borrowing Strength From External Trials In A Meta-Analysis. Statistics in Medicine 15,2733–2749. [PubMed: 8981683]
- Lu G, Ades A (2004). Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 23,3105–3124. [PubMed: 15449338]
- Lunn D, Jackson C, Best N, Thomas A, Spiegelhalter D (2013). The BUGS book. Boca Raton, FL: CRC Press.
- Lunn DJ, Thomas A, Best N, Spiegelhalter D (2000). WinBUGS -- a Bayesian modelling framework: concepts, structure, and extensibility. Statistics and Computing 10,325–337.
- Salanti G (2012). Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 3(2). [PubMed: 26062083]
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A (2002). Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society B 64,583–616.
- Sweeting M, Sutton A, Lambert P (2004). What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Statistics in Medicine 23(9),1351–75. [PubMed: 15116347]
- Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JPT (2015). Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Statistics in Medicine 34,984–998. [PMC free article: PMC4383649] [PubMed: 25475839]
- Welton N, Sutton A, Cooper N, Abrams K, Ades A (2012). Evidence synthesis for decision making in healthcare. Wiley
Appendix N. Resource use reported in the RCTs included in the network meta-analysis that informed the guideline economic analysis
Supplemental Data
b1: Clinical evidence tables for review questions 2.12.2 (Excel)
b2: Forest plots for treatment of a new episode of less severe depression (PDF)
b3: Forest plots for firstline treatment of more severe depression (PDF)
b4: Pairwise and nma comparisons for review questions 2.12.2 (Excel)
Final
Evidence reviews underpinning recommendations 1.5.2 to 1.5.3, 1.6.1, 1.7.1 and research recommendations in the NICE guideline
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- Review Further-line treatment: Depression in adults: Evidence review D[ 2022]Review Further-line treatment: Depression in adults: Evidence review D. 2022 Jun
- Review The clinical effectiveness and cost-effectiveness of low-intensity psychological interventions for the secondary prevention of relapse after depression: a systematic review.[Health Technol Assess. 2012]Review The clinical effectiveness and cost-effectiveness of low-intensity psychological interventions for the secondary prevention of relapse after depression: a systematic review.Rodgers M, Asaria M, Walker S, McMillan D, Lucock M, Harden M, Palmer S, Eastwood A. Health Technol Assess. 2012 May; 16(28):1-130.
- Review Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force[ 2020]Review Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task ForcePatnode CD, Perdue LA, Rossom RC, Rushkin MC, Redmond N, Thomas RG, Lin JS. 2020 Feb
- Review Nonsomatic treatment of depression.[Child Adolesc Psychiatr Clin N...]Review Nonsomatic treatment of depression.Sherrill JT, Kovacs M. Child Adolesc Psychiatr Clin N Am. 2002 Jul; 11(3):579-93.
- Treatment of a new episode of depressionTreatment of a new episode of depression
Your browsing activity is empty.
Activity recording is turned off.
See more...