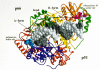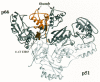NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997.
All retroviral RTs have both DNA polymerase and RNase H activities, and as discussed above, the synthesis of retroviral DNA requires both activities. Early studies demonstrated that retroviral DNA polymerase and RNase H activities copurify (Moelling et al. 1971; Verma 1975). Genetic studies and homology alignments made between various polymerases and RNases H provided evidence that the DNA polymerase and RNase H activities of RT are separate domains of a single polypeptide (Johnson et al. 1986; Tanese and Goff 1988).
Enzymology of Retroviral DNA Synthesis
The first studies of RT were done with material prepared from virions. Large quantities of avian myeloblastosis virus (AMV) can be prepared inexpensively from infected chicks, and more modest quantities of MLV can be recovered from cells infected in culture. Purified virions—usually prepared by simple centrifugation steps—serve as a source of RT. The virions can be lysed with nonionic detergent such as Triton X-100, and the RT can be recovered after one or two column chromatography steps (Hizi and Joklik 1977; Verma 1975). Most RTs are soluble, stable, and well-behaved proteins that can be expressed in bacteria using recombinant DNA methodologies. Enzymes corresponding to the MLV, ASLV, and HIV-1 RTs have all been prepared from Escherichia coli expression systems (Tanese et al. 1985, 1986; Farmerie et al. 1987; Hizi and Hughes 1988; Hizi et al. 1988; Soltis and Skalka 1988; Mizrahi et al. 1989; Mueller et al. 1989, 1991) and are now commercially available in relatively pure forms.
RT is not subject to posttranslational modification. Comparative enzymological analyses of RT isolated from virions and from heterologous expression systems have demonstrated that recombinant RTs possess the properties of the native enzyme. The RT produced in bacteria or other overexpression systems has been used in most of the recent structural and enzymological studies.
The DNA Polymerase Activity of RT
RT can be considered, first and foremost, as a DNA polymerase, which in both structure and function is similar to cellular DNA polymerases. The enzyme shares many of the features normally associated with host DNA polymerases: It requires a primer with a 3′-OH terminus (Shimada et al. 1994), which can be either RNA or DNA, annealed to a DNA or RNA template; it incorporates deoxyribonucleotide triphosphates by elongation of the primer, forming 3′-5′phosphodiester bonds with release of pyrophosphate. The general catalytic scheme involves first, binding of the primer-template by RT, then binding of the appropriate dNTP to the primer-template complex, followed by nucleophilic attack to yield the phosphodiester bond and the release of pyrophosphate. Continued synthesis requires RT to move relative to the template-primer. This movement must reposition the enzyme so that the additional nucleotides have access to the polymerase active site. Under normal processive DNA synthesis, the dissociation rate between RT and the template-primer becomes rate-limiting (Kati et al. 1992; Hsieh et al. 1993). RT incorporates the appropriate bases according to the rules of base pairing with the template, and it is capable of strand displacement, disrupting duplex structures that might block its progression (Boone and Skalka 1981b; Huber et al. 1989; Hottiger et al. 1994; Whiting and Champoux 1994; Wöhrl et al. 1994). RTs can operate over relatively broad ranges of salt and pH and require divalent cations, most commonly Mg++, but MLV RTs display optimal activity in the presence of Mn++ on some templates. RTs show a moderately high affinity for the template-primer (for HIV-1 RT, the dissociation constant of the enzyme-substrate complex is about 5 μM) and for triphosphates (K d is on the order of 5 μM) (Kati et al. 1992). RT can be easily distinguished from host DNA polymerases, primarily because it has a relatively high specific activity on RNA as well as on DNA templates (Hurwitz and Leis 1972; Leis and Hurwitz 1972).
Compared with other DNA polymerases, RT is a relatively sluggish enzyme in vitro; it has been reported to incorporate perhaps 15 nucleotides per second under optimal conditions on homopolymeric templates and up to 74 nucleotides per second under pre-steady-state conditions. Elongation rates measured under steady-state conditions for different heteropolymeric sequences vary but are significantly slower, suggesting that RT adds about one nucleotide to a growing chain per second in vitro (Majumdar et al. 1988; Huber et al. 1989; Reardon et al. 1991; Kati et al. 1992; Reardon 1993). This rate may be slightly higher in vivo, but the relatively long time required to generate retroviral DNA (∼4 hours from infection to the first appearance of a completed 9-kb DNA duplex) suggests that the in vitro rates are approximately correct and that RT is much slower than host replication machinery.
Although the natural template for RT is a heteropolymeric RNA, the enzyme is significantly more active on certain synthetic homopolymeric RNA templates, and homopolymer templates are commonly used to monitor RT activity, which has been used as a sensitive indication of the presence of retroviruses (Verma 1977; Goff et al. 1981; Johnson et al. 1990).
In reactions with purified enzyme in vitro, retroviral RTs are poorly processive; i.e., they do not remain attached to the primer terminus for a large number of successive nucleotide additions. Whether or not RT also displays low processivity either in vitro in virion cores or in vivo is unclear. In vitro, RT has a pronounced tendency to fall off primer-templates at particular sequences or structures (DeStefano et al. 1992a; Abbotts et al. 1993; Klarmann et al. 1993) and must reassociate before further elongation proceeds. The number of bases incorporated before dissociation varies widely, but in most situations, it is at most a few hundred bases. In vitro reverse transcription can be blocked by strong secondary structures, homopolymeric stretches, and other less well characterized sequence or structural motifs in the templates (Abbotts et al. 1993; Bebenek et al. 1993; Klarmann et al. 1993). Some regions of an RNA template can be particularly difficult for the enzyme to copy. It has been possible to use in vitro selection techniques to identify particular templates—certain RNA pseudoknots, for example— that can block RT. These types of structures can act as RT inhibitors in vitro (Tuerk et al. 1992; Green et al. 1995). The effects of such structures on viral replication have not been reported. Clearly, not all RNA structural features are incompatible with retroviral replication; pseudoknots are an essential feature of the sites of ribosomal frameshifting in some retroviruses (Chamorro et al. 1992; Levin et al. 1993; Chen et al. 1995; see also Chapter 7.
An important feature of RT is its lack of a proofreading function. RT does not contain a 3′exonuclease activity capable of excising mispaired nucleotides (Battula and Loeb 1976) and is, as a result, more error-prone than cellular DNA polymerases that are capable of proofreading (Battula and Loeb 1974). There is no 5′exonuclease activity, so it cannot perform nick-translation reactions. Strand displacement is relatively efficient, however, so that a nicked duplex DNA can serve as a template (Whiting and Champoux 1994).
RT Inhibitors
Since HIV-1 was first associated with AIDS, there have been intense efforts to identify potent and specific inhibitors of HIV-1 RT. Although an RNA-dependent DNA polymerase activity (telomerase) is required for the maintenance of the ends of chromosomes (Blackburn 1993), no retroviral RT-like activities are known to be required in any of the normal functions of mammalian cells. This suggests that it should be possible to identify drugs that specifically interfere with RT but have little or no effect on the host.
So far, most anti-RT drugs fall into two broad categories: nucleoside analogs and nonnucleoside inhibitors. Two problems have limited the efficacy of these drugs: toxicity for the host and the ability of the virus to develop resistant variants. However, when RT inhibitors are used in combinations, in particular in combination with protease inhibitors, clinical benefit is seen, and RT inhibitors will, in all probability, continue to be important in the battle against AIDS. Therapeutic strategies, and RT inhibitors, are discussed more fully in Chapter 12.
The RNase H Activity of RT
All retroviral RTs contain an RNase H activity, which is a nuclease specific for the RNA strand of an RNA-DNA hybrid (Moelling et al. 1971; Baltimore and Smoler 1972; Hansen et al. 1987; Cronn et al. 1992; for review, see Champoux 1993; Hostomsky et al. 1993). This activity is encoded in a discrete domain that is covalently attached to the polymerase domain.
The RNase H of RT is similar to the RNases H present in both bacterial and eukaryotic cells (Crouch 1990; Wintersberger 1990). The mechanism of cleavage is different from that of conventional ribonucleases: RNase H cleaves phosphodiester bonds to produce a 3′-OH and a 5′-PO4. This property of the enzyme is important to its role in retroviral replication because the oligoribonucleotides formed by RNase H cleavage must be able to prime plus-strand DNA synthesis. Retroviral RNases H are most active at pH values near neutrality and require divalent cations—either Mg++ or Mn++—with the preferred divalent cation depending on the enzyme in question. The RNase H of MLV RT is much more active in Mn++ than in Mg++. The retroviral enzymes can act as either endonucleases or exonucleases (Krug and Berger 1989; Oyama et al. 1989; Schatz et al. 1990).
Although RNA degradation can occur simultaneously with polymerase activity under some experimental conditions, degradation is not obligatorily linked to DNA synthesis (DeStefano et al. 1991b); a preformed RNA-DNA hybrid is cleaved in the absence of triphosphates (Wöhrl and Moelling 1990). Whether DNA synthesis and template degradation are simultaneous or sequential events in vivo is unknown. However, the observation that complementation can occur between retroviruses that encode RTs with defects in RNase H and polymerase does suggest that it is possible to separate these two processes in vivo (Telesnitsky and Goff 1993b). The final products of extended RNase H degradation are heterogeneous, short oligonucleotides 2–15 nucleotides long (Baltimore and Smoler 1972; Leis et al. 1973; Mizrahi 1989). As has already been discussed in the context of generation and removal of the RNA primers, there is considerable specificity in some of the RNase H cleavages; this specificity may result from the structure of the RNA:DNA duplex and its interaction with RT. Heterologous RNases H do not function well in viral replication (Guo et al. 1995). Some of the RNase H specificity appears to derive from specific enzyme-substrate interactions because there are species-specific differences in RNase H cleavage site preference (Mizrahi 1989; Luo et al. 1990). Structural analyses (discussed in more detail later in this chapter) show that, for HIV-1 RT, the primary sites of interaction between RT and the template-primer are in the DNA polymerase domain; RNase H plays, at most, a minor part. This means that binding of the substrate to RNase H, which will determine whether a given position in an RNA:DNA duplex will be cleaved by RNase H may be influenced by the structure adopted by a particular RNA-DNA hybrid region (Jacobo-Molina et al. 1993; Boyer et al. 1994c; Arnold et al. 1995). RNase H cleavage site specificity is most obviously manifest in the formation, from the PPT, of the oligoribonucleotide that is used to initiate plus-strand DNA synthesis (Finston and Champoux 1984; Resnick et al. 1984; Smith et al. 1984a,b; Rattray and Champoux 1987).
Under certain conditions in an in vitro reaction, RTs can also degrade RNA:RNA duplexes (Ben-Artzi et al. 1992; Hostomsky et al. 1992b; Blain and Goff 1993); this activity was originally called RNase D but is now termed RNase H* (Hostomsky et al. 1994). Mutational studies of the RNase H domain of Mo-MLV RT as well as studies of cleavage positions for HIV RT suggest that RNase H* activity is a variant activity of RNase H and reflects a relaxed specificity in substrate recognition (Blain and Goff 1993; Gotte et al. 1995). MLV RT RNase H* activity seems to require an intact polymerase domain; the isolated RNase H domain can cleave only RNA-DNA substrates. Although we cannot be certain, two lines of argument suggest that RNase H* does not have a role in the viral life cycle. First, we know of no place in the viral life cycle where RNase H* activity is required. Second, because the viral RNA genome contains double-stranded RNA (e.g., tRNA/PBS), unregulated RNase H* activity would probably result in significant damage to the genome.
Other Viral Proteins That May Function in DNA Synthesis
Nucleocapsid
In vivo, the template for reverse transcription is actually a complex of RNA and NC protein, and it has been suggested that viral proteins, in particular NC, might aid strand transfer and processivity during reverse transcription (Weiss et al. 1992; Darlix et al. 1993, 1995; Allain et al. 1994; Peliska et al. 1994; You and McHenry 1994). In reconstituted reactions designed to mimic the process of strand transfer in vitro, NC appears to stimulate both the rate and efficiency of template switching by RT (Darlix et al. 1993; Allain et al. 1994; Peliska et al. 1994; You and McHenry 1994), probably because it can stimulate nucleic acid annealing. The effects of NC on reverse transcription in vivo are more difficult to assess: The principal problem with such experiments is that NC has a key role in viral RNA encapsidation (see Chapters 2 and 7).
dUTPase
Recently, a new gene product with a new enzymatic activity—deoxyuridine triphosphatase (dU)—has been discovered in some lentiviruses and the type-D viruses (Elder et al. 1992; Koppe et al. 1994). For equine infectious anemia virus (EIAV), dU-defective viruses replicate efficiently in certain immortalized cell lines but grow poorly in primary macrophages, which are the natural host cells for this virus. The decreased replication correlates with the presence of uracil in viral DNA (Threadgill et al. 1993). Growth properties of dU-deficient feline immunodeficiency viruses (FIVs) suggest that this activity may only confer replication advantages in cells where the endogenous dU activity is low (Lerner et al. 1995). The role of retroviral dU might be to increase the fidelity of replication by decreasing dUTP pools. Consistent with this hypothesis is the finding that mutation frequencies are increased in dU mutant FIVs (Lerner et al. 1995). Incorporation of dU opposite a template G may be one cause of retroviral G to A hypermutations, which is the predominant mutation seen in dU-deficient FIV.
Molecular Structure of RT
Several different three-dimensional structures of RT have been solved by X-ray crystallography. The implications of these structures can be best appreciated in the context of the biochemical and genetic analyses of the enzyme.
RT Sequences and Subunit Organization
The central portion of the polymerase domain of RT is the most phylogenetically conserved portion of the retroviral genome. Nevertheless, various retroviral RTs differ significantly in primary sequence (Doolittle et al. 1989). The relative position of the polymerase and RNase H domains within RT—DNA polymerase at the amino terminus, followed by RNase H—appears to be conserved. The organization of RT was initially defined on the basis of limited homology with other polymerases and RNases H (Johnson et al. 1986). These domain assignments were confirmed by mutagenesis of MLV RT, which demonstrated that many of the mutations made in the amino-terminal two thirds of RT disrupt DNA polymerase without affecting RNase H, whereas mutations in the carboxy-terminal one third of RT affect RNase H without impairing DNA polymerase activity (Kotewicz et al. 1988; Levin et al. 1988; Tanese and Goff 1988; Repaske et al. 1989). This information was used to divide the region encoding MLV RT into two nonoverlapping segments that were expressed separately in E. coli. The 5′portion encoded an active DNA polymerase without nuclease activity, and the 3′portion encoded an active RNase H with no polymerase, thus demonstrating that the two activities reside in physically separable, independently folded domains (Tanese and Goff 1988). Similar mutagenesis studies of the HIV-1-coding region suggested that HIV-1 RT has a domain organization similar to that of the MLV enzyme but that the two domains were much more interdependent (Hizi et al. 1989, 1990, 1991; Prasad and Goff 1989; Lederer et al. 1992). The three-dimensional structure data presented below can be used to explain the results obtained with HIV-1 RT. Although the HIV-1 RT DNA polymerase and RNase H activities are physically distinct, their physical interconnection and their interactions with the template-primer account for the difficulty in separating the two activities genetically.
The subunit composition and organization of various retroviral RTs are markedly different. MLV RT is, at least in solution, a monomeric enzyme that contains both DNA polymerase and RNase H domains in a single polypeptide. HIV-1 RT is a heterodimer of 66- and 51-kD subunits. Whereas the larger HIV-1 RT subunit, p66, contains both the DNA polymerase and RNase H domains, the smaller HIV-1 subunit is a carboxy-terminal truncation of the larger subunit that lacks essentially all of the RNase H domain. Subunit-selective mutagenesis—i.e., introducing mutations into either the large or the small subunit separately—has demonstrated that both the DNA polymerase and RNase H activities reside within the large subunit of HIV-1 RT (Le Grice et al. 1991; Hostomsky et al. 1992a; Lederer et al. 1992). ASLV RT is also a heterodimer; however, both subunits in ASLV RT contain both DNA polymerase and RNase H, and the larger avian RT subunit contains integrase as well. The subunit compositions of many other RTs have not yet been determined.
Homology alignments among RTs identified several regions of the DNA polymerase domain that exhibit sequence similarity (Patarca and Haseltine 1984; Larder et al. 1987; Xiong and Eickbush 1988, 1990; Poch et al. 1989), and alignments that included a more diverse set of polymerases suggested some motifs were conserved among a very broad group of RNA-dependent polymerases (Doolittle et al. 1989; Delarue et al. 1990). The most highly conserved region, which is found in RNA- and DNA-dependent polymerases, contains the motif YXDD (Webster et al. 1989). Mutagenesis has shown that both of the conserved aspartate residues in this motif, Asp-185 and Asp-186 in HIV-1 RT, as well a third conserved residue, Asp-110, are absolutely required for polymerase activity (Larder et al. 1987; Boyer et al. 1992c). These three aspartic acids form the active site of the polymerase. Homology alignments of RNases H from a number of viral and nonviral sources identified four amino acids that are conserved among the RNases H, and the catalytic importance of three of these residues was demonstrated by site-directed mutagenesis of bacterial and retroviral enzymes (Repaske et al. 1989; Kanaya et al. 1990; Mizrahi et al. 1990).
Crystal Structures
Three-dimensional structures have been solved for the intact HIV-1 RT heterodimer (Arnold et al. 1992; Kohlstaedt et al. 1992, 1993; Ding et al. 1995a,b; Esnouf et al. 1995; Ren et al. 1995; Rodgers et al. 1995; Hsiou et al. 1996), the polymerase catalytic cores of HIV-1 and Mo-MLV RTs (Unge et al. 1994; Georgiadis et al. 1995), and the HIV-1 RT RNase H domain (Davies et al. 1991; Chattopadhyay et al. 1993). The picture that emerges is of a flexible molecule that grasps the primer-template so as to simultaneously position the primer terminus near the polymerase active site, and a segment of the template strand, about 15–18 bp away, near the RNase H active site (summarized in Hughes et al. 1996). These structures provide important insights into possible enzyme mechanisms, and it is widely anticipated that they will aid in the design of new anti-RT drugs (Nanni et al. 1993; see also Chapter 12.
HIV-1 RT
The first reported structure was of HIV-1 RT complexed with the nonnucleoside inhibitor, nevirapine (Kohlstaedt et al. 1992, 1993; Smerdon et al. 1994). The structure of HIV-1 RT bound to a short 18/19-mer DNA oligonucleotide duplex and complexed with an antibody Fab portion was reported shortly thereafter (RT-DNA cocrystal) (Jacobo-Molina et al. 1991, 1993; Arnold et al. 1992). There are now three structures of the unliganded enzyme (Esnouf et al. 1995; Rodgers et al. 1995; Hsiou et al. 1996), and recent reports have described additional RT structures that contain other nonnucleoside inhibitors (Ding et al. 1995a,b; Ren et al. 1995).
The structure of the polymerase domain of HIV-1 RT resembles a right hand. On the basis of this similarity, the subdomains have been called the fingers, palm, thumb, and connection subdomains (Fig. 4) (Kohlstaedt et al. 1992). In the palm domain of the large subunit, the conserved Asp-185 and Asp-186 residues of the YXDD motif are adjacent to Asp-110 and form the polymerase active site (Figs. 5 and 6). A “carboxylate-chelated two-metal-ion” catalytic mechanism has been proposed in which two divalent metal ions are coordinated to these conserved residues and to the phosphates of the incoming nucleotide to facilitate nucleophilic attack by the primer 3′-OH and subsequent release of pyrophosphate (Steitz and Steitz 1993; Pelletier et al. 1994; Patel et al. 1995).
A striking structural feature seen in the crystal structures is the degree of asymmetry of the two subunits of the HIV RT heterodimer (Wang et al. 1994). Rather than forming a symmetric dimer interface, the two subunits are nested together such as two cupped hands might fit on top of one another (Fig. 4). Except for the carboxyl terminus of the connection domain, the individual RT subdomains are folded similarly in both the large and small subunits of HIV RT, but the spatial arrangement of the subdomains within each subunit is quite different. Most notably, in the p51 subunit, the relative positions of the connection and thumb subdomains are reversed relative to their positions in p66, with the connection rotated to cover the palm and displace the thumb. As a result, the overall structures of the p66 and p51 subunits are quite different, with the small subunit more tightly packed than the large subunit, even though the amino acid sequence of p51 is identical to the amino-terminal portion of p66 (Fig. 7). Consistent with genetic studies demonstrating that only the large subunit contributes directly to the polymerase activity of the RT heterodimer (Le Grice et al. 1991; Hostomsky et al. 1992a), there is no template binding cleft in p51. The three aspartic acids (110, 185, 186) that form the polymerase active site in p66 are buried in p51: They are occluded by the connection domain which fills the region that forms the nucleic-acid-binding cleft in p66. The p51 subunit of HIV-1 RT appears to have a structural role within the p66/p51 heterodimer; it may also be involved in binding the tRNA primer. In the p66 subunit, the connection subdomain bridges the region between the polymerase and the RNase H domains, and it also is involved in important interactions between the p66 and p51 subunits.
In the HIV-1 RT/nevirapine complex, there is a deep cleft in the large subunit, which genetic and modeling studies suggested comprises the primer-template-binding region (Kohlstaedt et al. 1993). This assignment was confirmed by the RT DNA cocrystal structure (Jacobo-Molina et al. 1993), although the position of the thumb is slightly different in the nevi-rapine complex and the RT-DNA complex (Jacobo-Molina et al. 1993; Nanni et al. 1993).
Taken together, these data suggest that the thumb of p66 is flexible. The extent of this flexibility became clear when unliganded HIV-1 RT structures were solved (Rodgers et al. 1995; Hsiou et al. 1996). In these structures, the thumb has moved approximately 30o relative to its position in the RT-DNA complex; it is in a position where the tip of the thumb touches the tip of the fingers (see Fig. 8). There is a contradictory report in the literature. Esnouf et al. (1995) reported a structure of an unliganded HIV-1 RT with the thumb in a position similar to that seen in the RT-DNA and RT- nevirapine complexes. However, the crystals used in this study were grown in the presence of a weakly binding nonnucleoside inhibitor, which was soaked out after the crystals had been grown. It seems likely that the open configuration of the thumb obtained in these experiments is the result of the method used to prepare the crystals and does not reflect the position the thumb normally occupies in the unliganded enzyme (for discussion, see Hsiou et al. 1996).
A comparison of the structures of the RT-DNA complex with unliganded RT showed more limited structural changes that occurred in other parts of the enzyme upon binding of the primer-template. Most notably, the active site region in the palm appeared to be unchanged in the presence or absence of DNA. The movement of the thumb upon nucleic acid binding extends the analogy of the hand to include a grip. The considerable mobility of the thumb may be a conserved property of polymerases (Sousa et al. 1994). It has been suggested that the small binding cleft still present in unliganded RT may be large enough to accommodate a single-stranded nucleic acid and that RT may initially bind single-stranded template regions and then locate the primer terminus by sliding (Patel et al. 1995).
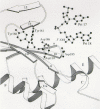
The physical distance between the DNA polymerase and RNase H active sites in HIV-1 RT is consistent with the observed sites of cleavage of an RNA template, 15–18 bases from the 3′end of the primer strand (Wörhl and Moelling 1990; Furfine and Reardon 1991; Fu and Taylor 1992; Gopalakrishnan et al. 1992; Kohlstaedt et al. 1992; Jacobo-Molina et al. 1993). The structural and biochemical data, taken together, suggest that the DNA polymerase and RNase H active sites can be simultaneously engaged on the primer-template. The cocrystals of HIV-1 RT with the duplex DNA showed that the DNA in the vicinity of the polymerase active site is an A-form helix, whereas the DNA near the RNase H site is B-form; at the junction of these two segments is a bend of 40–45o in the helical axis of the DNA (see Fig. 5). The DNA that is relatively near the polymerase active site may be constrained to assume the A form by the numerous contacts between the enzyme and the template-primer. This may also reflect a requirement for the nucleic acid template-primer to have a relatively uniform structure whether the template-primer is RNA:RNA, DNA:RNA, or DNA:DNA. The choice of the A form may be dictated by the fact that although the DNA:DNA duplex can assume an A-form structure, an RNA:RNA duplex cannot assume a B-form structure. The bend in the nucleic acid keeps the double-stranded DNA away from the RNase H active site in the RT-DNA:DNA duplex co- crystal structure. RT must be able to hold RNA-DNA hybrids in a fashion that permits the RNase H to cleave the RNA strand (Fedoroff et al. 1993). It should be remembered, in this context, that the structures of the nucleic acids themselves (the structure of RNA:DNA duplexes are different from both RNA:RNA and DNA:DNA duplexes [Salazar et al. 1993]) may govern whether the template strand is properly positioned for cleavage (Arnold et al. 1995).
Specific regions of HIV-1 RT have been implicated as sites where there are significant interactions with the primer-template (Setlik et al. 1994; Arnold et al. 1995; Wöhrl et al. 1995a). These include the “template grip,” which consists of β4, αß, β5a, and the β8-αE connecting loop portions of the p66 palm and fingers, which are closely associated with the template strand, and the β12-β13 hairpin called the “primer grip,” which contacts the primer strand near its 3′terminus. There are also extensive contacts between the αI and αH helixes of the p66 thumb and the primer-template (Jacobo-Molina et al. 1993). Although the nucleic acid duplex that spans the two sites is approximately 15–18 nucleotides in length, a significantly larger region of the template-primer is protected in a DNase I footprint (Wöhrl et al. 1995b). At least some of the primer-template sequences protected in a footprint reaction are also sites of important RT:primer-template contacts: Notably, portions of β3 and β4 in the finger subdomain appear to contact single-stranded template regions that include the first seven nucleotides “in front” of the primer terminus (Boyer et al. 1994b; Patel et al. 1995).
The structure of HIV-1 RT was initially compared to that of the Klenow fragment of E. coli DNA polymerase I, since this was the only polymerase whose structure was solved before the structure of HIV-1 RT (Kohlstaedt et al. 1992, 1993; Steitz et al. 1994; Arnold et al. 1995). The structural conservation between HIV-1 RT and the Klenow fragment is greatest in the palm region and includes, for both enzymes, the regions that contain the catalytic residues. Structures of both HIV-1 RT and a fragment of MLV RT have implicated several residues, in addition to the three catalytic aspartic acid residues that likely contribute to the nucleotide-binding site (Unge et al. 1994; Georgiadis et al. 1995; Patel et al. 1995). The structures of the catalytic domain of rat DNA polymerase β and T7 RNA polymerase have been reported recently (Sousa et al. 1993; Davies et al. 1994; Pelletier et al. 1994). HIV-1 RT, T7 RNA polymerase, and the Klenow fragment are all quite clearly related in structure; it would appear that polymerase β may have a distinct evolutionary origin and attempts to compare its structure directly to HIV-1 RT may be misleading (Delarue et al. 1990; Steitz et al. 1994; Holm and Sander 1995; Hughes et al. 1996).
MLV RT
A high-resolution structure of the fingers and palm of MLV RT has also been solved (Georgiadis et al. 1995). This structure is quite similar to the corresponding region of HIV RT p66; however, there are some differences. For example, the angle between the fingers and palm subdomains of MLV RT differs slightly from that in p66, and the palm subdomain of MLV RT is larger than that of HIV-1 RT. However, the overall folding and the active site geometry are highly conserved. This suggests that although various RTs differ significantly at the level of primary sequence and quaternary structure, the structures of RT active sites will be highly conserved.
RNase H
The structure of the HIV-1 RNase H domain was determined before that of the RT holoenzyme and shortly after the E. coli RNase H structure was solved (Katayanagi et al. 1990; Yang et al. 1990; Davies et al. 1991). Although the bacterial and the retroviral proteins are only about 20% identical in primary sequence, the three-dimensional structures of the RNases H of HIV-1 RT and E. coli are strikingly similar, especially in their catalytic cores.
The presence of three acidic residues suggests that RNase H binds two metal ions, and a two-metal mechanism, similar to the general two-metal-ion transesterification that has been proposed for polymerases, has also been proposed for HIV-1 RNase H (Joyce and Steitz 1994; Yang and Steitz 1995). However, a one-metal-ion mechanism has also been proposed for E. coli RNase H (Nakamura et al. 1991; Oda et al. 1993). The similarity in structure of E. coli RNase H and RNase H of HIV-1 RT suggests that two enzymes use the same enzymatic mechanism, and the issue of the precise mechanism of cleavage has not, despite additional experimental effort, been resolved. RNase H has significant structural similarity both to the integration proteins of HIV-1 and ASLV and to the bacteriophage Mu transposase and to the E. coli endonuclease RuvC (see Chapter 5. It may well be that a resolution to the question of the number of metals and the exact mechanism of RNase H cleavage will have implications for these other enzymes as well.
The similarity of the isolated RNase H domain of HIV-1 RT and the free RNase H from E. coli raises an interesting question. Although there are contradictory claims in the literature, most reports suggest that the isolated RNase H domain of HIV-1 RT has considerably less enzymatic activity than it does in the context of the intact enzyme (Evans et al. 1991; Smith and Roth 1993). Close examination of the sequence of these proteins shows that E. coli RNase H has a basic loop, which appears to have a critical role in binding the substrate. The RNase H domain of HIV-1 RT lacks this basic extension; it is possible to convert the inactive HIV-1 RT RNase H domain to an active form by inserting the basic loop (Stahl et al. 1994; Keck and Marqusee 1995). This suggests that the problem with the isolated RNase H domain of HIV-1 RT is an inability to bind the nucleic acid substrate, which implies that in intact HIV-1 RT, the RNase H substrate is bound primarily by the polymerase domain. This is consistent with what is known about the behavior of mutants in the polymerase domain that reduce RNase H activity (Hizi et al. 1988, 1990; Prasad and Goff 1989; Boyer et al. 1992a,b) and with the observation that mixing the isolated HIV-1 RT RNase H domain and polymerase domain restores RNase H activity (Hostomsky et al. 1991; Smith et al. 1994).
Mutational Studies
RT has been the subject of extensive mutational analysis—so extensive, that even a simple cataloging of the mutants and their phenotypes is beyond the scope of this chapter. Most of the efforts in mutagenesis have focused on HIV-1 RT. RT mutants that have been selected in the presence of drugs or other inhibitory agents are discussed in Chapter 12 and they have recently been extensively reviewed (De Clercq 1992; Tantillo et al. 1994; Kimberlin et al. 1995; Mellors et al. 1995). This section cannot do justice to the wealth of biophysical data—much of which have been validated by crystallographic data—that have implicated specific regions of the enzyme in such functions as substrate binding, nor does this section describe mutants with alterations larger than single-residue substitutions. Instead, Table 1 presents a sampling of some of the many point mutations that have been introduced into HIV-1 RT and the effects that have been reported. The descriptions provided are inescapably simplistic, both reflecting the brevity of this discussion and the complex nature of the effects of the mutations that can affect enzyme function either directly or indirectly. It should be noted that the majority of these mutations have not been introduced into viruses and that additional experiments to measure the effects of the mutations on viral replication need to be done.
Table 1
Substitution Mutations Introduced into HIV-1 RT.
- Biochemistry of Reverse Transcription - RetrovirusesBiochemistry of Reverse Transcription - Retroviruses
- (("clinical guidelines"[Resource Type]) OR "practice guideline"[P... (51)(("clinical guidelines"[Resource Type]) OR "practice guideline"[Publication Type]) AND ("Cranial nerve paralysis")SearchBooks
Your browsing activity is empty.
Activity recording is turned off.
See more...