This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008-. doi: 10.3824/stembook.1.30.1
In this chapter I focus on the emergence of endoderm, the origin of these cells and their organization in space. I also discuss the molecular events that lead to endoderm formation and how endoderm can be molecularly defined. Although the molecular control of endoderm formation has initially been deciphered using Xenopus, Zebrafish, sea urchin and several other species many molecular switches have been confirmed in mice. This article preferentially cites references in the mouse model system but data from other model organisms are used when they provide important information missing in mice. Extensive references to other species can be found in Grapin-Botton; Constam, 2007 and Stainier, 2002. This article presents endoderm engineering from ES cells and provides molecular triggers and landmarks that may be used for optimized engineering based on normal development. Due to the similarity of markers between definitive and extraembryonic endoderm and the recent discovery that visceral endoderm can contribute to the digestrive tract, the generation of these lineages is also discussed (Kwon et al., 2008). Although endoderm stem cells, that is stem cells endowed with the ability to give rise to all endodermal derivatives but not ectoderm or mesoderm, have not been reported yet, there are stem cells in specific endodermal organs which will be discussed in the following chapters.
Introduction
The endoderm is classically defined as the inner germ layer of the embryo. The main derivative is the epithelial outlining of the digestive tract but it does also contribute to many other organs detailed in Figure 1. However, the terminology in Amniotes is confusing as different types of cells that contribute to extraembryonic structures have also been called endoderm, such as the primitive (PrE), visceral (VE) and parietal endoderm (PE). Figure 2 depicts the location and lineage of the different types of endoderm. The fact that these extraembryonic structures share many molecular markers with definitive endoderm adds to the confusion.
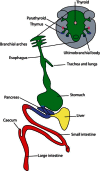
Figure 1
Endoderm derivatives.
Cellular aspects of endoderm emergence
Emergence of definitive endoderm at gastrulation
Definitive endoderm in Amniotes arises at the time of gastrulation, during which endoderm precursors initially located in the epiblast ingress in the anterior primitive streak (see Figure 1). Definitive endoderm cells egress from the primitive streak and insert into the visceral endoderm. VE forms in majority extraembryonic tissues but also contributes some cells to the gut tube (Kwon et al., 2008). Recent obvservations suggest that cells originating from the epiblast visceral endoderm intermingle between VE cells (Kwon et al., 2008) rather than displacing it as a sheet to anterior and lateral regions of the conceptus (Poelmann, 1981)(Lawson et al., 1986; Tam and Beddington, 1992; Tam et al., 2004; Tam et al., 1993). Definitive endoderm movement is accompanied by epithelial-mesenchymal transition and requires Snail or the related gene Slug that transiently repress E-cadherin (Blanco et al., 2007; Nakaya et al., 2008; Thisse et al., 1995). The directionality of movement is controlled by mesoderm-derived Sdf1/Cxcl12b acting on Cxcr4-expressing endoderm in zebrafish and Xenopus (Fukui et al., 2007; Mizoguchi et al., 2008; Nair and Schilling, 2008). Mosaic genetic modifications in Zebrafish have shown that at least in this model, endoderm gastrulation is a combination of active movements of cells and passive movements whereby a cell is mobilized by its neighbours (Carmany-Rampey and Schier, 2001; Pezeron et al., 2008). Sub-populations of animal pole cells forced to express Sox17- an inducer of endoderm- either migrate towards the endodermal layer or die. These observations suggest that there is a feed-back check on the matching between the differentiation status of a cell and its environment (Clements and Woodland, 2000). The latter considerations, in the context of ES cell cultures may allow endodermal cells to aggregate in a heterogeneous culture or die depending on their neighbours.
Timing of endoderm specification
In mouse and chick, heterotopic grafting experiments have shown that determination to form endoderm occurs after the cells have left the streak (Kimura et al., 2006; Kinder et al., 2001). Whether the EMT is crucial for endoderm differentiation is unclear at the moment but the ability of endoderm to differentiate prior to gastrulation in several species argues against this hypothesis (Laufer et al., 1980; Leung et al., 1999; Priess and Thomson, 1987; Schroeder and McGhee, 1998). Nevertheless in Amniotes, cells are exposed to signalling centers during their migration. In chick, endoderm progenitors in Koller's sickle undergoing their characteristic ‘Polonaise movement’ are thought to become specified as they receive signals from the posterior marginal zone which activate the Nodal signaling pathway. These signals include Wnt ligands (Skromne and Stern, 2001). At a later stage, mesoderm and endoderm in passing may receive instructive patterning signals from the node (Brennan et al., 2002; Pagan-Westphal and Tabin, 1998). Such signaling centers are formed in ES cell cultures aggregated into embryoid bodies and most likely in dense monolayer cultures (Leahy et al., 1999). By sensing their position relative to signaling center, moving cells thus might coordinate their differentiation.
Mesendoderm
With the notable exception of sea urchin, most species initially segregate ectoderm precursors from progenitors that give rise to endoderm and mesoderm. In C. elegans, sea urchin and zebrafish mesoderm and endoderm derive from bipotential progenitors (Rodaway and Patient, 2001). In Amniotes a similar mesendoderm population has been postulated based on coexpression of endoderm and mesoderm markers in the anterior streak (Rodaway et al., 1999), and the observation that certain signalling cascades induce both types of cells (Lemaire et al., 1998; Reiter et al., 1999; Rodaway and Patient, 2001). In space, endodermal/mesendodermal progenitors tend to be located in the anterior streak whereas mesodermal progenitors extend to the posterior streak. However, single cell lineage tracing has never formally demonstrated the existence of bipotential cells in Aminotes.
Molecular control of definitive endoderm formation
A comprehensive analysis of the regulatory gene network responsible for endoderm differentiation has been carried out in sea urchin and largely corroborated in Xenopus (Davidson et al., 2002; Davidson et al., 2002; Loose and Patient, 2004). Experiments in Zebrafish also pioneered the molecular elucidation of endoderm formation (Stainier, 2002). In non-Amniotes endoderm is initially induced by maternal proteins which will not be discussed here (β-catenin, VegT, Otx). They do not have a maternal activity in Amniotes although β-catenin and eomesodermin (a T-box gene like VegT) have a zygotic activity in endoderm induction. In Amniotes endoderm is initially induced by secreted factors.
The TGFβs Nodal, Gdf1 and Gdf3 are endoderm inducers in vertebrates
Insights into the inductive mechanisms underlying endoderm formation in vertebrates initially came from studies in Xenopus using a dominant-negative activin receptor which blocks secreted TGFß-related activities including Activin, Vg1, and Xenopus nodal-related proteins (Xnr). Vegetal pole endoderm explants of embryos injected with this construct express mesodermal and ectodermal marker genes at the expense of endoderm (Henry et al., 1996). This led to the idea that Vg1 and/or Xnr's are endogenous endoderm inducers. Further analysis of Nodal functions in Xenopus and zebrafish confirmed that mesodermal and endodermal cell fates are specified by different levels of Nodal signaling (Agius et al., 2000; Dougan et al., 2003; Schier et al., 1997; Thisse et al., 2000). Also in the mouse, Nodal induces both mesoderm and endoderm (Brennan et al., 2001; Conlon et al., 1994; Zhou et al., 1993), but endoderm populations appear to be selectively lost in embryos carrying a hypomorphic Nodal allele, mutations in Nodal proteolytic activation site or gradual reductions in the gene dosage of Smad 2 and 3 (Ben-Haim et al., 2006; Dunn et al., 2004; Liu et al., 2004; Lowe et al., 2001; Norris et al., 2002). Conversely, targeted inactivation of the Nodal antagonist Lefty2 leads to excess endoderm formation (Meno et al., 1999). Studies in zebrafish suggest that Nodal proteins establish a morphogen gradient to pattern the marginal zone along the animal-vegetal axis, with peak levels specifying blastomeres closest to the margin to form endoderm. In contrast, cells farther away from the Nodal source respond by expressing mesodermal genes, presumably because they are exposed to lower concentrations of Nodal, and/or a shorter duration of signaling (Chen and Schier, 2001). The regulation of Nodal expression is discussed in the next chapter (see Figure 3). Homologues to the second activin receptor binding protein, Vg1, have been identified in zebrafish, chick and more recently in mouse where the more distant Gdf1 and 3 recapitulate Vg1 activity (Dohrmann et al., 1996; Helde and Grunwald, 1993; Seleiro et al., 1996; Shah et al., 1997: Andersson et al., 2007; Bertocchini et al., 2004; Skromne and Stern, 2001). Gdf1 and 3 are expressed in the node like Nodal and recent experiments show that Gdf1 potentiates Nodal activity by forming heterodimers that signal at a longer range (Tanaka et al., 2007; see Figure 3).
Nodal expression is induced by the canonical Wnt pathway and positive feedback signaling
Among the signals which activate Nodal expression is the Wnt pathway. Mouse embryos lacking Nodal, or ß-catenin fail to form a primitive streak (Conlon et al., 1994; Huelsken et al., 2000), suggesting that the canonical Wnt pathway and Nodal act in synergy to specify definitive endoderm. Several Wnt genes are expressed before and during gastrulation (Kemp et al., 2005). Analysis of Wnt null alleles demonstrates that germ layer formation and expression of mesendoderm markers in the mouse depends on Wnt3 (Liu et al., 1999). At the onset of gastrulation, Wnt3 is initially expressed in the posterior visceral endoderm and proximal epiblast region shortly before prospective mesendoderm cells begin to ingress into the primitive streak. Wnt3 and Nodal mutually activate each other (Ben-Haim et al., 2006; Brennan et al., 2001; Liu et al., 1999). Thus, the canonical Wnt pathway may promote endoderm formation in mammals primarily by locally stimulating Nodal feedback signalling (see Figure 3).
Residual Nodal signaling in Wnt3 and ß-catenin mutants indicates that Nodal expression is regulated by additional signals. Peak levels of Nodal expression depend on an autoregulatory loop mediated by the binding of FoxH1 on a FAST binding site in the Nodal regulatory region (Hoodless et al., 2001; Norris et al., 2002; Yamamoto et al., 2001). This autoregulation is potentiated by cripto, an EGF-CFC family GPI-anchored glycoprotein, that can directly associate with Nodal and its signaling receptor Alk4 (Reissmann et al., 2001; Yeo and Whitman, 2001). Although Cripto promotes mesendoderm formation primarily by stimulating Nodal signalling, recent data suggests that Cripto binds Wnt11 and stimulates activation of ß-catenin (Tao et al., 2005).
Similar to other TGFß family members, Nodal is derived from a precursor protein by redundant proteolytic activities of the subtilisin-like proprotein convertases Furin or Pace4 (Constam and Robertson, 1999). Embryo explant experiments and analysis of Furin−/-; Pace4−/- double mutants suggested that Nodal processing is essential to stimulate autoinduction early after implantation, but that uncleaved Nodal during gastrulation may activate at least a subset of mesodermal markers (Beck et al., 2002; Mesnard et al., 2006). Endoderm formation depends on two sequential positive feedback loops mediated by Cripto and Bmp4/Wnt3 that are activated by mature or uncleaved Nodal, respectively, to sustain Nodal signaling from implantation throughout gastrulation (Ben-Haim et al., 2006). According to a recent mathematical model as well as evidence in Zebrafish, the choice between mesodermal and mesendodermal fates depends on how long a particular cell and its ancestors have been exposed to Nodal and its effectors, rather than a concentration gradient (Ben-Haim et al., 2006; Hagos and Dougan, 2007). Recent experiments have demonstrated that the T-box gene Eomesodermin, expressed in the trophectoderm like Furin and Pace 4, synergizes with Nodal (Arnold et al., 2008). It may participate in one of the feed-back loops (see Figure 3).
This important role of Nodal emerged recently as it has different functions in Echinoderms, Ascidian and possibly Amphioxus (Duboc et al., 2004; Hudson and Yasuo, 2006; Yu et al., 2002). The role of Nodal in endoderm and mesoderm induction in vertebrates may have derived from its original function in dorso-ventral axis specification. The role of β-catenin and Wnt signalling in endoderm induction is prominent in C.elegans, Echinoderms and all vertebrates (Reviewed in (Grapin-Botton and Constam, 2007). Nodal autoregulation and induction by Wnts/β-catenin functions in echinoderms and must predate chordates. Clearance of FoxQ2, a Nodal repressor, by β-catenin maintains Nodal expression in Sea Urchin (Yaguchi et al., 2008). Although FoxQ2 identification in Vertebrates is awaiting, its expression in Chordates suggests that this function may be evolutionary conserved (Yu et al., 2003).
GATA factors are expressed in mesendoderm and required for endoderm differentiation
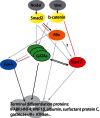
Several transcription factors are acting downstream of secreted factors responsible for endoderm induction in a network tentatively represented in Figure 4 although the direct regulatory mechanisms are still largely unknown. Several Gata family members are acting downstream of Nodal although the proteins leading to Gata activation are different in several model organisms (Grapin-Botton and Constam, 2007).
Forkhead transcription factors of the FoxA family and GATA factors are key players of the endodermal network of transcription factors in all triploblasts studied so far. Several family members are expressed in endoderm or mesoderm in most species. Inactivation of Serpent, one of the Drosophila Gatas precludes endoderm formation (Rehorn et al., 1996; Reuter, 1994). Among the 6 vertebrate Gata genes, Gata4, 5 and 6 play partially redundant roles in endoderm development. Zebrafish faust mutants lacking Gata5, contain about 60% of the wild type number of endodermal cells (Reiter et al., 1999). Gata4 (Jacobsen et al., 2002; Soudais et al., 1995) and 6 (Koutsourakis et al., 1999; Morrisey et al., 1998), but not Gata5 (Molkentin et al., 2000) knockout mice show impaired visceral and definitive endoderm development. In Xenopus, Gata4, 5 and 6 are expressed in endoderm and convert ectomesoderm into endoderm in a redundant manner (Afouda et al., 2005; Gao et al., 1998; Jiang and Evans, 1996; Weber et al., 2000). In vertebrates, GATA factors have been shown to activate genes involved in adult endodermal cell function (Intestinal fatty acid binding protein, hepatic nuclear factor 4/HNF4, gastric H+/K+ ATPase) and in some cases such as albumin bind their promoter (Bossard and Zaret, 1998; Gao et al., 1998; Maeda et al., 1996; Morrisey et al., 1998).
Forkhead factor expression and requirement for endoderm differentiation
Forkhead genes of the FoxA class are also expressed in endoderm all triploblasts (Harada et al., 1996; Ang et al., 1993; Horner et al., 1998; Kaestner et al., 2000; Kalb et al., 1998; Monaghan et al., 1993; Odenthal and Nusslein-Volhard, 1998; Sasaki and Hogan, 1993; Schier et al., 1997; Strahle et al., 1993; Weigel et al., 1989). Their inactivation perturbs but does not abolish endoderm development. Interestingly, they are often expressed in a subpopulation of endodermal cells and their inactivation usually inhibits the development of parts of the gut. In mouse, neither FoxA1- nor FoxA3-inactivated mutants exhibit any early phenotype (Kaestner et al., 1998). By contrast, FoxA2, which is expressed at the onset of gastrulation, is required for fore- and midgut formation (Ang et al., 1993; Dufort et al., 1998; Sasaki and Hogan, 1993; Weinstein et al., 1994).
In zebrafish, Gata5/fau is expressed before FoxA2, suggesting that it is upstream of this forkhead transcription factor, as also described in sea urchin, C. elegans and Drosophila (Azzaria et al., 1996; Casanova, 1990; Horner et al., 1998; Kalb et al., 1998; Mango et al., 1994; Weigel et al., 1989). Direct regulation by Tcfs and T-boxes have been characterized in sea urchin and Ciona but remain to be investigated in other species (Davidson et al., 2002; Di Gregorio et al., 2001). Furthermore, the presence of Smad2 binding elements in the Xenopus Foxa2 promoter raises the possibility that it is a direct nodal target (Howell and Hill, 1997). Autoregulatory loops-positive or negative- have been demonstrated in different species. (Davidson et al., 2002; Di Gregorio et al., 2001).
FoxA targets have been studied comprehensively in C. elegans (Gaudet and Mango, 2002). Beyond compiling a list of targets, Mango and co-authors have shown that the late targets have lower affinity binding sites and thus are only induced once the levels of FoxA reach a critical threshold. Several studies have proposed that GATAs and FoxA together form a preinitiation complex that is required but not sufficient for endoderm gene transcription (Bossard and Zaret, 1998; Cirillo et al., 2002).
Sox and Mix, vertebrate players
Specific to vertebrates, other key components of the network downstream of Nodal comprise Sox17, Mix, and several related genes.
Sox17
Sox17 was first implicated in endoderm development in Xenopus and has also been extensively studied in Zebrafish where there are several members of the family (Alexander and Stainier, 1999; Hudson et al., 1997). In mouse, Sox17 is first expressed in visceral endoderm nearest to the ectoplacental cone at 6.0 dpc and progressively spreads to the entire extraembryonic VE. It is also expressed in definitive endoderm from 7.5–8.5 dpc (Kanai-Azuma et al., 2002). Mid- and hindgut expression persists until 8.5 dpc, whereas foregut expression decreases by 8 dpc. In Sox17 knockout mice, definitive endoderm is depleted and visceral endoderm-like tissue replaces it in the most posterior and lateral regions. Anterior endoderm is generated, but posterior and lateral endoderm down from the midgut level are reduced and later fail to expand (Kanai-Azuma et al., 2002). In contrast to Foxa2-knockout cells that can form hindgut but not fore- and midgut, Sox17 mutant cells can contribute to some extent to the foregut but not mid- and hindgut (Dufort et al., 1998). Elevated levels of apoptosis in the foregut later lead to foregut reduction suggesting that Sox17 is also a maintenance factor for endoderm. Promoter studies in Xenopus have demonstrated that Sox17 is directly regulated by TGFβs (Vg1 or Nodal) and through a different promoter element by Sox17 itself and VegT (Howard et al., 2007). Mixer also participates in Sox17 regulation by stimulating an autoregulatory loop which also involves GATAs (Sinner et al., 2006). Sox17 directly activates the endodermal genes HNF1β, FoxA1, FoxA2 and Endodermin in Xenopus, in part through synergistic interactions with β-catenin (Ahmed et al., 2004; Sinner et al., 2004). Other endodermal genes are exclusively under transcriptional control by Mixer or require synergy between Mixer and Sox17 (Sinner et al., 2006). In mice, Sox7 and Sox17 may be redundant in extraembryonic visceral endoderm.
Mix family
The Mix family encodes homeodomain proteins initially described in Xenopus. Mixer is predominantly expressed at the endoderm/mesoderm boundary, and is the only gene of the family that induces endoderm specifically (Henry and Melton, 1998; Kofron et al., 2004; Sinner et al., 2006). Mix1 and Bix1/Mix4 induce endoderm at high levels and can repress mesodermal genes like Xbra whereas at low level, they induce mesoderm (Henry and Melton, 1998; Latinkic and Smith, 1999; Latinkic et al., 1997; Lemaire et al., 1998; Tada et al., 1998).
Only one Mix gene has been found in Amniotes (Peale et al., 1998; Pearce and Evans, 1999; Robb et al., 2000; Stein et al., 1998). Mouse Mixl1 is first detected in the visceral endoderm and later in nascent primitive streak, but not in the node or definitive endoderm. Mice lacking Mixl1 have reduced definitive endoderm and mid- hindgut lies at the level of the foregut (Hart et al., 2002; Tam et al., 2007). However, visceral endoderm is displaced normally to the periphery. Mixl1 mutant cells in chimeras contribute to all organs but the hindgut. Conversely, Mixl1 overexpression in frog injection assays can induce excess endoderm formation (Hart et al., 2002). Nodal expression is expanded in Mixl1 mutants suggesting that a feedback loop regulates NODAL. SMAD2/4 dimers bind the activin-responsive element of the mix2 promoter (Howell et al., 1999). Mixer recruits SMAD2/4 to activin responsive elements of mesendodermal genes such as Gsc (Germain et al., 2000).
In addition to these genes, many genes have been described in endoderm and may be used as markers as compiled in Table 1.
Table 1
Selected endoderm-enriched genes Yellow: membrane proteins; green: membrane proteins used for cell sorting. (HSCs) Hematopoietic stem cells (OV) Otic vesicle (YS) Yolk Sac (NT) Neural tube.
Engineering endoderm
Generating endoderm from ES cells using Activin or Nodal
ES cells spontaneously form endoderm, including definitive endoderm, but in a small proportion (Itskovitz-Eldor et al., 2000). Early work aimed at generating clinically relevant endodermal derivatives from ES cells did not characterize intermediate steps in the differentiation and rather focused on the end products such as hepatocytes or pancreatic beta cells. Such attempts were characterized by either controversy in their reproducibility at generating functional differentiated cells (Hori et al., 2002; Lumelsky et al., 2001; Rajagopal et al., 2003) or their low efficiency (Hamazaki and Terada, 2003; Jones et al., 2002; Blyszczuk et al., 2003; Vincent et al., 2006; Yamada et al., 2002; Yamada et al., 2002). Based on the knowledge of endoderm development, strategies have more recently been devised to generate endoderm from mouse and human embryonic stem cells (ESCs). Developmental knowledge provided triggers and markers in this process. As in vivo, Nodal is necessary for endoderm induction from ES cells (Gadue et al., 2006; McLean et al., 2007). Due to the limited availability and price of biologically active Nodal protein (Tada et al., 2005), most efforts have made use of Activin as a surrogate for Nodal. Initial experiments by Kubo et al. (Kubo et al., 2004) and Yasunaga et al. (Yasunaga et al., 2005) on mouse ES cells (mESCs) and D’Amour et al. (D’Amour et al., 2005) on human ES cells (hESCs) have shown that endoderm is efficiently generated with both species in the presence of Activin and low serum. This protocol functions with mESCs aggregated into embryoid bodies (Kubo et al., 2004) as well as mESCs or hESCs cultured as a monolayer (D’Amour et al., 2005; Yasunaga et al., 2005). The efficiency of the protocol appears to vary largely depending on the cell lines used but has been successfully used in many laboratories (D’Amour et al., 2006). The low serum most likely limits phosphatidyl Inositol 3 kinase (PI3K) activity, a condition needed for definitive endoderm formation from ES cells (McLean et al., 2007). Nodal has also in some instances been provided by the use of MEF-conditioned medium (McLean et al., 2007).
As expected from the developmental response to Nodal morphogen the authors could demonstrate that 25–100 ng/ml activin lead to 50–60% endoderm whereas lower doses of activin (1–10 ng/ml) induced skeletal muscle markers (Gadue et al., 2006; Kubo et al., 2004). The duration of activin exposure was explored in less detail but Kubo et al. reported that hematopoietic progenitors emerged after 5 days of activin (3–100 ng/ml) treatment whereas 6 days of exposure were necessary for induction of the endodermal markers Sox17 and Hex. Shorter durations of high activin exposure (3 days) can nevertheless induce endoderm (Yasunaga et al., 2005), in particular when Wnts are added (D’Amour et al., 2006).
More recently the tetracycline-inducible system was used to drive expression of Nodal and was demonstrated to be more effective at inducing endoderm that adding exogenous Activin (Takenaga et al., 2007). This may be due to different intrinsic properties of Nodal and activin. Although they share the same receptors, their mechanism of action is somewhat different as for example their different requirement for Cripto. The differences observed may however be due to the method of delivery: endogenously produced Nodal may be differently post-translationnally processed, may traffic differently or may be expressed more evenly.
Molecular characterization of endoderm induced from ES cells
The expression of Foxa2 and Sox17 show that the endoderm induced is at least in majority definitive endoderm. This idea is also confirmed by the transient expression of primitive streak markers such as Brachyury, Goosecoid, Lhx1, MixL1, PDGFRα and Wnt3a (D’Amour et al., 2005; Gadue et al., 2006; Kubo et al., 2004; Yasunaga et al., 2005). ES cells have been used to decipher the hierarchy of protein activity downstream of activin, confirming that Eomesodermin is acting upstream of Mixl1 during endoderm differentiation (Izumi et al., 2007; Russ et al., 2000). Of particular interest, a recent study comparing two protocols of endoderm induction from ES cells reported a list of potential new endoderm markers using microarray RNA profiling of the genes enriched in both conditions (McLean et al., 2007). An earlier microarray study provided several additional validated markers including Cxcr4 that can be used to sort cells (Yasunaga et al., 2005). The eventual test for their endodermal nature will be transplantation assays and the proof that they can integrate to mouse or chick endoderm and further differentiate and contribute to endodermal organs.
Role of the Wnt pathway in endoderm induction from ES cells
Although the most ancient signaling pathway for endoderm induction has been marginally used for endoderm induction, Wnt pathway activity is necessary during endoderm induction from ES cells (D’Amour et al., 2006; Gadue et al., 2006; Lindsley et al., 2006). Wnt3 (10ng/ml or 100 ng/ml) on its own does not have the ability to induce endoderm but at least in some instances can potentiate Activin activity (D’Amour et al., 2006; Gadue et al., 2006). During development, many other genes described in the previous paragraphs are necessary to form endoderm but there is limited knowledge as to whether they may be sufficient to induce endoderm from ES cells. Forced expression of Sox17 was rtecently shown to promote endoderm differentiation from ES cells (Seguin et al., 2008).
ES cells as a tool to answer developmental and medical questions
Experiments with ES cells are helpful to answer questions that may be difficult to address in mice or human. The best example is the demonstration using ES cells of mouse mesendoderm progenitors. Clonal analysis showed that sorted Gsc+ single cells generated by activin treatment of ES cells could generate clones made of mesoderm only or mesoderm and endoderm (Tada et al., 2005). This experiment provides strong support in favor of mesendoderm progenitors and the lack of endoderm-only progenitors. ES cells may be very useful to study the antero-posterior commitment of endoderm cells. ES cells may also provide the number of cells needed to perform biochemistry or chromatin immunoprecipitations in endoderm-like cells. In the longer term, it would be interesting to have endodermal stem cells that can only give rise to endodermal lineages and be stably maintained as has been achieved for the ectoderm (Conti et al., 2005; Tada et al., 2005).
Lastly, human ES cells with mutated genes could also represent a wonderful tool to study endodermal organ disease.
Endoderm regionalization and morphogenesis
Markers and fate maps reveal progressive patterning of endoderm into organs
In chick and mouse, the cells recruited early in the primitive streak will form more anterior endoderm derivatives. The position of endoderm progenitors along the primitive streak reflects their later antero-posterior (AP) and medio-lateral position (Franklin et al., 2008; Lawson et al., 1986; Tam et al., 2004). Subsequently, folding of the gut tube anteriorly brings the most anterior cells to the most ventral positions (Franklin et al., 2008). The endoderm at this stage appears to be roughly divided into anterior and posterior areas. Cerberus 1 (m-Cer1/Cerr)1 (Belo et al., 1997; Biben et al., 1998; Shawlot et al., 1998), Orthodenticle homologue 2 (Otx2) (Ang et al., 1994), Homeo box gene expressed in ES cells 1 (Hesx1) (Thomas and Beddington, 1996) and Hematopoietically expressed homeobox (Hex) (Thomas et al., 1998) are restricted to anterior endoderm. Antero-posterior asymmetry of the endoderm at the same stages is also demonstrated by the specific ability of the anterior endoderm to induce heart differentiation in the mesoderm (Schultheiss et al., 1995). However, at this early stage association of the anterior endoderm half with posterior mesoderm can still induce posterior genes in endoderm and vice versa (Wells and Melton, 2000), suggesting that AP patterning of the endoderm is not yet determined. At somitic stages, the identity of more regions exhibiting different gene expression profiles is progressively specified (Grapin-Botton, 2005; Grapin-Botton and Melton, 2000).
Molecular mechanisms of endoderm patterning
The molecular mechanisms responsible for early patterning along the AP and medio-lateral/dorso-ventral axes in endoderm are beginning to emerge. They are schematized in Figure 5. Thus far, they appear to be very similar to the mechanisms of AP patterning of the neurectoderm, involving wnts, Fgf4 and retinoic acid. Retinoic acid was recently shown to control AP patterning in endoderm at the time of gastrulation in Xenopus, Zebrafish and Amphioxus (Chen et al., 2004; Escriva et al., 2002; Schubert et al., 2005; Stafford et al., 2004; Stafford and Prince, 2002; Stafford et al., 2006). This work suggests that increasing levels of retinoic acid activity gradually induces posterior organs. In mouse retinoic acid is required to form the pancreas and pattern branchial arch endoderm (Huang et al., 2002; Huang et al., 1998; Martin et al., 2005; Matt et al., 2003; Molotkov et al., 2005). However, it is unclear whether graded activity orchestrates the relative position of organs along the entire AP axis in this layer. Retinoic acid is produced by the mesoderm, a tissue that sends patterning signals to endoderm (Kumar et al., 2003; Pan et al., 2007; Wells and Melton, 2000). Fgfs are also necessary for endoderm patterning from gastrulation to somitogenesis (Dessimoz et al., 2006; Serls et al., 2005; Wells and Melton, 2000). Exposing endoderm to FGF4, a node-derived factor, shifts posterior endoderm markers anteriorly and represses anterior markers at gastrulation. After gastrulation the patterning role of FGF4 becomes restricted to the mid- and hindgut, where increasing levels of signalling progressively induce more posterior fates in endodermal cells (Dessimoz et al., 2006; Wells and Melton, 2000). Although these pathways appear to pattern endoderm at least in part through direct signaling to this layer, more work is needed to elucidate how they cooperate (Dessimoz and Grapin-Botton, 2006; Huang et al., 2002; Pan et al., 2007; Stafford et al., 2006). Recent experiments in Xenopus show that endoderm is also patterned by the Wnt pathway: the foregut forms in the absence of Wnt activity whereas Wnt signalling is necessary to form the intestine (McLin et al., 2007). The lack of anterior Wnt activity is permitted by the expression of several Wnt inhibitors (Cerberus, dickkopf, Frzb) in anterior endoderm (Belo et al., 1997; Lewis et al., 2008; Mukhopadhyay et al., 2001; Pfeffer et al., 1997). The role of this pathway in endoderm development is likely to be conserved in rodents as mice deficient for Tcf1 and Tcf4 exhibit posterior endoderm defects (Gregorieff et al., 2004).
Local signals lead to organ formation
There is evidence that in addition to the signals that lead to the regionalization of endoderm along the AP axis, other signals are necessary locally for the induction of organ primordia. These signals are discussed in more details in the chapters pertaining to specific endoderm organs. One such signal is Fgf2 secreted by the cardiac primordium which is necessary to liver and lung development (Deutsch et al., 2001; Gualdi et al., 1996; Jung et al., 1999; Serls et al., 2005). Similarly, BMP4 from the septum transversum is necessary for liver induction whereas the pancreas appears to form in the absence of BMPs (Rossi et al., 2001; Spagnoli and Brivanlou, 2008). In contrast to most of the digestive tract epithelium, the pancreas can only develop in an area free of Shh (Apelqvist et al., 1997). Lastly, Fgf10 is necessary for the development of several organs budding off the digestive tract such as the pancreas, caecum, lungs and stomach glands (Bhushan et al., 2001; Burns et al., 2004; Nyeng et al., 2007; Sekine et al., 1999).
Patterning endodermalized ES cells
In spite of our limited knowledge of endoderm patterning, the exploitation of such information to generate organ-restricted progenitors is promising. Exposure to FGF and BMP after endoderm induction by activin enriched in liver progenitors (Gouon-Evans et al., 2006). The role of retinoic acid, Fgf10 and the absence of Shh in pancreas development were exploited to enrich endodermalized ES cells in pancreas progenitors (D’Amour et al., 2006; Kroon et al., 2008). Since Nodal and Wnts used to induce endoderm from ES cells also play a role in AP patterning, a careful investigation of the regional endoderm markers obtained after different amounts and duration of exposure to these morphogens would be interesting. Further, ES cells could be very valuable to investigate that are difficult to address in vivo such as the possible graded activities of Nodal, Wnt, RA and Fgf signaling pathways in endoderm patterning.
Differentiation of extraembryonic endoderm lineages
In mouse, primitive endoderm (PrE) segregates from the inner cell mass (ICM) at the blastocyst stage as a squamous epithelium and covers the outside of the embryo (Weber et al., 1999; see Figure 2). Whereas some PrE cells remain attached to the basement membrane of the ICM and differentiate into cuboidal visceral endoderm (VE), others undergo an epithelial-mesenchymal transition to become parietal endoderm (PE). PE cells migrate along the basement membrane of trophectoderm (TE) cells which gives rise to Reichert's membrane of the parietal yolk sac. VE forms the epithelial lining of the yolk sac and some cells contribute to the definitive gut tube at least until 9.0dpc (Kadokawa et al., 1987; Kwon et al., 2008). Until placentation, PE and VE lineages together are responsible for nutrient and waste exchange between maternal tissue and the foetus. VE can contribute in a minor way to embryonic gut in the fore- and hindgut (Tam and Beddington, 1992). What we know of the differentiation of these 3 lineages arises from a cross-talk between in vivo experiments and experiments with ES cells. ES cells are thus already an experimental model of embryo development. Markers of extraembryonic endoderm lineages, some of which are shared with definitive endoderm are indicated in Table 1.
Primitive endoderm (PrE)
Before the segregation of epiblast and PrE, the inner cell mass (ICM) is a mosaic of cells expressing markers of one or the other lineage (Gerbe et al., 2008; Plusa et al., 2008). In ES cell lines and in vivo, Pou5f1 (Oct3/4) is required to maintain pluripotency and to prevent differentiation into TE cells (Nichols et al., 1998; Niwa et al., 2000). On the other hand, using an inducible Pou5f1 transgene, less than twofold increase in the expression level of Pou5f1 in ES cells is sufficient to induce markers of PrE and mesoderm differentiation (Niwa et al., 2000). Peak levels of Pou5f1 expression thus are likely to also specify PrE in the blastocyst. In normal ES cell cultures, differentiation is inhibited owing to the presence of LIF. However, if ES cells are aggregated to form embryoid bodies (EB), LIF can no longer prevent PrE differentiation and the segregation of these cells at surface of these aggregates (Murray and Edgar, 2001; Shen and Leder, 1992). Zebrafish MZ-Spg/Pou2/0ct4 mutants lack endodermal markers such as Cas/Sox32 and Sox17 but a similar activity in Amniotes may be hidden by early lethality (Lunde et al., 2004; Reim et al., 2004).
Communication between ICM cells is needed to segregate PrE from epiblast. Mice lacking either Fgf4, Fgf receptor2 (Fgfr2) or their downstream effector Grb2 do not form PrE (Arman et al., 1998; Feldman et al., 1995; Wilder et al., 1997; Chazaud et al., 2006; Cheng et al., 1998). In embryoid bodies, forced expression of a dominant-negative form of the Fgf receptor prevents formation of PrE, and overexpression of Gata6 and Gata4 rescues this phenotype (Li et al., 2004; Li et al., 2001). Gata factors are thus likely to control PrE formation downstream of Fgf signaling, although single knockouts for these genes do not elicit such early phenotypes, most likely due to redundancy (Hamazaki et al., 2006; Koutsourakis et al., 1999; Morrisey et al., 1998). The Fgf signaling pathway represses Nanog expression and Nanog represses Gata6 (Hamazaki and Terada, 2003). It is however unclear how an apparently random subset of ICM cells activate the FGF pathway. Several pieces of evidence suggest that Gatas control the sorting of PrE and epiblast cells (reviewed in (Yamanaka et al., 2006). Apoptosis may lead to the elimination of inappropriately sorted cells (Plusa et al., 2008). Genes enriched in PrE have been identified by gene expression arrays (Gerbe et al., 2008). Extraembryonic stem cells (XEN) have been isolated from PrE and contribute to their lineage of origin in chimeras (Kunath et al., 2005). Overexpression of GATA factors in ES cells leads to cells molecularly very similar to XEN cells and both contribute preferentially to parietal rather than visceral endoderm in chimeras (Shimosato et al., 2007). Subsequent withdrawal of GATAs however leads to their differentiation into cells endowed with visceral endoderm characters (Shimosato et al., 2007). Although XEN cells require mouse embryonic fibroblasts (MEFs) for their growth, GATA-induced ES cells do not, suggesting that MEF-derived factor(s) serve to maintain Gata expression.
Parietal endoderm (PE)
How PrE cells choose between PE and VE fates is poorly understood. Clonal analysis of cells from genetically marked E3.5 and E6.5–7.5 donor embryos revealed that PrE and VE both give rise to PE when transplanted into host blastocysts (Gardner, 1982). This suggests that PrE descendants adopt PE fate because of environmental cues, rather than owing to a lack of competence to express the characteristics of VE.
Terminally differentiated PE cells have been derived ex vivo from ES cells upon forced expression of GATA4 or GATA6 (Fujikura et al., 2002). The signals governing the differentiation of extraembryonic endoderm have been studied best in F9 cells. Upon treatment with retinoic acid, this embryonal carcinoma cell line differentiates to become PrE and VE (Strickland and Mahdavi, 1978; Strickland et al., 1980). The transcription factor Sox7 is needed downstream of RA for the induction of GATA4 and GATA6, and subsequent parietal endoderm differentiation (Futaki et al., 2004). Although Sox17 is not required for PE and VE formation from PrE in vivo recent work with ES cells shows a requirement in vitro (Shimoda et al., 2007). The effect of RA on F9 cells appears to be mediated by activated Ras (Verheijen et al., 1999). Parathyroid hormone-related peptide (PTHrP) produced by trophoblast cells and the deciduum immediately adjacent to the implantation site or induces an epithelial to mesenchymal transition (EMT) in PrE cells and their conversion into a PE-like cells (Behrendtsen et al., 1995; Chan et al., 1990; Karperien et al., 1996; Smyth et al., 1999; Strickland et al., 1980; Veltmaat et al., 2000). cAMP, the intracellular mediator of PTHrP triggers the same effect.
visceral endoderm (VE)
Differentiation and survival of VE depends on a number of transcription factors, including the orphan nuclear receptor HNF4 (Chen et al., 1994; Duncan et al., 1997). HNF4 is expressed in PrE as early as day E4.5, but after E5.25 becomes restricted to the visceral yolk sac endoderm (Duncan et al., 1994; Mesnard et al., 2006). Signals upstream of HNF4 include BMP2/4 (Coucouvanis and Martin, 1999), and the activin receptor Alk2 (Sirard et al., 1998) which is essential in extraembryonic lineages (Gu et al., 1999). Also the homeodomain protein HNF1ß and GATA6 are required to induce HNF4 expression in the VE (Barbacci et al., 1999; Coffinier et al., 1999; Morrisey et al., 1998), suggesting that both of these transcription factors may act within or in parallel to the BMP pathway. HNF1ß (Tcf2) also stimulates expression of HNF1α (Tcf1) and Foxa2 (Barbacci et al., 1999). GATA6 is responsible for activating expression of GATA4 (Morrisey et al., 1998), which in turn acts in the VE lineage to enable ventral closure of the primitive gut tube (Molkentin et al., 1997; Narita et al., 1997). Toghether, these observations indicate that differentiation of the VE lineage is brought about by the concerted action of a cascade of transcription factors which later also regulate gene expression in the definitive endoderm and its various derivatives.
The rules as to how specific combinations of these and other transciption factors might pattern the VE remain poorly understood. Initially, VE cells form a columnar epithelium which is subsequently patterned along the proximal distal axis of the conceptus by inductive interactions with adjacent ectodermal cells (Brennan et al., 2001; Dziadek, 1978; Gardner, 1982). Single-cell labelling at E5.5 shows that cells can contribute to embryonic and extraembryonic visceral endoderm (Perea-Gomez et al., 2007). The AVE is formed from a PrE population of Cerl-expressing cells and cells that acquire Cer1 expression later (Torres-Padilla et al., 2007). Thus, expression of HNF1ß and TTF (Transthyretin) is confined to the extraembryonic region, whereas VE cells overlying the egg cylinder express α-fetoprotein (Dziadek and Adamson, 1978) and Ihh (Becker et al., 1997; Belaoussoff et al., 1998) and adopt a squamous morphology. Recent experiments have shown that Nodal is expressed in the PrE. Nodal signaling is essential to downregulate a subset of PrE markers and thus induce embryonic visceral endoderm (Mesnard et al., 2006). In the embryonic region, patterning of the VE further becomes evident with the expression of Wnt3 in the proximal-posterior region. By contrast, cells differentiating at the distal tip express elevated levels of Otx2, and in response move to the prospective anterior pole to become anterior visceral endoderm (AVE; Kimura et al., 2000). Foxa2 binds Otx2 promoter and is essential for its expression in AVE (Kimura-Yoshida et al., 2007). The specific gene expression pattern of the distal tip is restricted by signalling from the extraembryonic ectoderm as well as nodal signalling (Mesnard et al., 2006; Rodriguez et al., 2005). The AVE also expresses a number of other specific markers, including Lefty-1 (Ebaf) and Cerberus-like. These secreted proteins function redundantly as negative feedback inhibitors in the Nodal pathway and thereby confine primitive streak formation to the posterior epiblast (Perea-Gomez et al., 2002).
Concluding remarks
Although our understanding of endoderm development is less extensive than that of ectoderm and mesoderm, it has been successfully exploited to generate endodermal cells from ES cells with high efficiency but not yet from induced Pluripotent Stem cells (iPS cells). This knowledge has also been helpful with regards to quality control by providing a set of markers that collectively define endoderm identity. This protocol is expensive and labour intensive. It would therefore be of great interest to develop endodermal stem cells endowed with the ability to self-renew and differentiate into all endodermal organs. Promising results in this direction have recently been published. Sorted Cxcr4/Hex double positive cells generated after endoderm induction from mES cells are likely to represent an anterior definitive endoderm population that can be stably propagated and further differentiated into liver and pancreatic cell types (Morrison et al., 2008). Forced expression of Sox17 in hEScells leads to the differentiation of mesendodermal cells which can also be propagated and further differentiated into liver and pancreatic cell types (Seguin et al., 2008). A major gap remains to be filled to understand how different organ primordia are induced from endoderm and use this strategy on endoderm cells in vitro. These recent advances in endoderm generation in vitro will most likely allow the development of new strategies to address questions that are difficult to address in vivo such as those requiring large numbers of cells or live monitoring of cells.
Acknowledgements
The author's work on endoderm development is funded by the Swiss National Science Foundation (3100AO-109606), the European 6th Framework Program BetaCellTherapy (LSHB-CT-2005-521145) and the NIDDK Beta Cell Biology Consortium (NIH 1-U19-DK072495-01).
References
- Afouda B.A, Ciau-Uitz A, Patient R. GATA4, 5 and 6 mediate TGFbeta maintenance of endodermal gene expression in Xenopus embryos. Development. 2005;132:763–774. [PubMed: 15659482]
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis E.M. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. [PMC free article: PMC2292107] [PubMed: 10683171]
- Ahmed N, Howard L, Woodland H.R. Early endodermal expression of the Xenopus Endodermin gene is driven by regulatory sequences containing essential Sox protein-binding elements. Differentiation. 2004;72:171–184. [PubMed: 15157240]
- Alexander J, Stainier D.Y. A molecular pathway leading to endoderm formation in zebrafish. Curr Biol. 1999;9:1147–1157. [PubMed: 10531029]
- Anderson W.J, Zhou Q, Alcalde V, Kaneko O.F, Blank L.J, Sherwood R.I, Guseh J.S, Rajagopal J, Melton D.A. Genetic targeting of the endoderm with claudin-6CreER. Dev Dyn. 2008;237:504–512. [PMC free article: PMC2665265] [PubMed: 18213590]
- Andersson O, Bertolino P, Ibanez C.F. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. Dev Biol. 2007;311:500–511. [PubMed: 17936261]
- Ang S.L, Conlon R.A, Jin O, Rossant J. Positive and negative signals from mesoderm regulate the expression of mouse Otx2 in ectoderm explants. Development. 1994;120:2979–2989. [PubMed: 7607086]
- Ang S.L, Wierda A, Wong D, Stevens K.A, Cascio S, Rossant J, Zaret K.S. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301–1315. [PubMed: 8306889]
- Apelqvist A, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. 1997;7:801–804. [PubMed: 9368764]
- Arman E, Haffner-Krausz R, Chen Y, Heath J.K, Lonai P. Targeted disruption of fibroblast growth factor (FGF) receptor 2 suggests a role for FGF signaling in pregastrulation mammalian development. Proc Natl Acad Sci USA. 1998;95:5082–5087. [PMC free article: PMC20217] [PubMed: 9560232]
- Arnold S.J, Hofmann U.K, Bikoff E.K, Robertson E.J. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Development. 2008;135:501–511. [PMC free article: PMC7116389] [PubMed: 18171685]
- Azzaria M, Goszczynski B, Chung M.A, Kalb J.M, McGhee J.D. A fork head/HNF-3 homolog expressed in the pharynx and intestine of the Caenorhabditis elegans embryo. Dev Biol. 1996;178:289–303. [PubMed: 8812130]
- Barbacci E, Reber M, Ott M.O, Breillat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126:4795–4805. [PubMed: 10518496]
- Beck S, Le Good J.A, Guzman M, Haim N.B, Roy K, Beermann F, Constam D.B. Extraembryonic proteases regulate Nodal signalling during gastrulation. Nat Cell Biol. 2002;25:25. [PubMed: 12447384]
- Becker S, Wang Z.J, Massey H, Arauz A, Labosky P, Hammerschmidt M, St-Jacques B, Bumcrot D, McMahon A, Grabel L. A role for Indian hedgehog in extraembryonic endoderm differentiation in F9 cells and the early mouse embryo. Dev Biol. 1997;187:298–310. [PubMed: 9242425]
- Behrendtsen O, Alexander C.M, Werb Z. Cooperative interactions between extracellular matrix, integrins and parathyroid hormone-related peptide regulate parietal endoderm differentiation in mouse embryos. Development. 1995;121:4137–4148. [PubMed: 8575314]
- Belaoussoff M, Farrington S.M, Baron M.H. Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development. 1998;125:5009–5018. [PubMed: 9811585]
- Belo J.A, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis E.M. Cerberus-like is a secreted factor with neuralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. [PubMed: 9431803]
- Belo J.A, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis E.M. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. [PubMed: 9431803]
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson E.J, Constam D.B. The Nodal Precursor Acting via Activin Receptors Induces Mesoderm by Maintaining a Source of Its Convertases and BMP4. Dev Cell. 2006;11:313–323. [PubMed: 16950123]
- Bertocchini F, Skromne I, Wolpert L, Stern C.D. Determination of embryonic polarity in a regulative system: evidence for endogenous inhibitors acting sequentially during primitive streak formation in the chick embryo. Development. 2004;131:3381–3390. [PubMed: 15226255]
- Bhushan A, Itoh N, Kato S, Thiery J.P, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. [PubMed: 11748146]
- Biben C, Stanley E, Fabri L, Kotecha S, Rhinn M, Drinkwater C, Lah M, Wang C.C, Nash A, Hilton D. et al. Murine cerberus homologue mCer-1: a candidate anterior patterning molecule. Dev Biol. 1998;194:135–151. [PubMed: 9501024]
- Blanco M.J, Barrallo-Gimeno A, Acloque H, Reyes A.E, Tada M, Allende M.L, Mayor R, Nieto M.A. Snail1a and Snail1b cooperate in the anterior migration of the axial mesendoderm in the zebrafish embryo. Development. 2007;134:4073–4081. [PubMed: 17965052]
- Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus A.M. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci USA. 2003;100:998–1003. [PMC free article: PMC298715] [PubMed: 12525695]
- Bossard P, Zaret K.S. GATA transcription factors as potentiators of gut endoderm differentiation. Development. 1998;125:4909–4917. [PubMed: 9811575]
- Brennan J, Lu C.C, Norris D.P, Rodriguez T.A, Beddington R.S, Robertson E.J. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. [PubMed: 11418863]
- Brennan J, Norris D.P, Robertson E.J. Nodal activity in the node governs left-right asymmetry. Genes Dev. 2002;16:2339–2344. [PMC free article: PMC187443] [PubMed: 12231623]
- Brown J.L, Snir M, Noushmehr H, Kirby M, Hong S.K, Elkahloun A.G, Feldman B. Transcriptional profiling of endogenous germ layer precursor cells identifies dusp4 as an essential gene in zebrafish endoderm specification. Proc Natl Acad Sci USA. 2008;105:12337–12342. [PMC free article: PMC2527912] [PubMed: 18719100]
- Burns R.C, Fairbanks T.J, Sala F, De Langhe S, Mailleux A, Thiery J.P, Dickson C, Itoh N, Warburton D, Anderson K.D, Bellusci S. Requirement for fibroblast growth factor 10 or fibroblast growth factor receptor 2-IIIb signaling for cecal development in mouse. Dev Biol. 2004;265:61–74. [PubMed: 14697353]
- Carmany-Rampey A, Schier A.F. Single-cell internalization during zebrafish gastrulation. Curr Biol. 2001;11:1261–1265. [PubMed: 11525740]
- Casanova J. Pattern formation under the control of the terminal system in the Drosophila embryo. Development. 1990;110:621–628. [PubMed: 2133557]
- Chan S.D, Strewler G.J, King K.L, Nissenson R.A. Expression of a parathyroid hormone-like protein and its receptor during differentiation of embryonal carcinoma cells. Mol Endocrinol. 1990;4:638–646. [PubMed: 2177844]
- Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–624. [PubMed: 16678776]
- Chen W.S, Manova K, Weinstein D.C, Duncan S.A, Plump A.S, Prezioso V.R, Bachvarova R.F, Darnell J.E Jr. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 1994;8:2466–2477. [PubMed: 7958910]
- Chen Y, Pan F.C, Brandes N, Afelik S, Solter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Dev Biol. 2004;271:144–160. [PubMed: 15196957]
- Chen Y, Schier A.F. The zebrafish Nodal signal Squint functions as a morphogen. Nature. 2001;411:607–610. [PubMed: 11385578]
- Cheng A.M, Saxton T.M, Sakai R, Kulkarni S, Mbamalu G, Vogel W, Tortorice C.G, Cardiff R.D, Cross J.C, Muller W.J, Pawson T. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. [PubMed: 9865697]
- Cirillo L.A, Lin F.R, Cuesta I, Friedman D, Jarnik M, Zaret K.S. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9:279–289. [PubMed: 11864602]
- Clements D, Woodland H.R. Changes in embryonic cell fate produced by expression of an endodermal transcription factor, Xsox17. Mech Dev. 2000;99:65–70. [PubMed: 11091074]
- Coffinier C, Thepot D, Babinet C, Yaniv M, Barra J. Essential role for the homeoprotein vHNF1/HNF1beta in visceral endoderm differentiation. Development. 1999;126:4785–4794. [PubMed: 10518495]
- Conlon F.L, Lyons K.M, Takaesu N, Barth K.S, Kispert A, Herrmann B, Robertson E.J. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. [PubMed: 7924997]
- Constam D.B, Robertson E.J. Regulation of bone morphogenetic protein activities by pro domains and proprotein convertases. J Cell Biol. 1999;144:139–149. [PMC free article: PMC2148113] [PubMed: 9885250]
- Conti L, Pollard S.M, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S, Ying Q.L, Cattaneo E, Smith A. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. [PMC free article: PMC1184591] [PubMed: 16086633]
- Coucouvanis E, Martin G.R. BMP signaling plays a role in visceral endoderm differentiation and cavitation in the early mouse embryo. Development. 1999;126:535–546. [PubMed: 9876182]
- D’Amour K.A, Agulnick A.D, Eliazer S, Kelly O.G, Kroon E, Baetge E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. [PubMed: 16258519]
- D’Amour K.A, Bang A.G, Eliazer S, Kelly O.G, Agulnick A.D, Smart N.G, Moorman M.A, Kroon E, Carpenter M.K, Baetge E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. [PubMed: 17053790]
- Davidson E.H, Rast J.P, Oliveri P, Ransick A, Calestani C, Yuh C.H, Minokawa T, Amore G, Hinman V, Arenas-Mena C. et al. A genomic regulatory network for development. Science. 2002;295:1669–1678. [PubMed: 11872831]
- Davidson E.H, Rast J.P, Oliveri P, Ransick A, Calestani C, Yuh C.H, Minokawa T, Amore G, Hinman V, Arenas-Mena C. et al. A provisional regulatory gene network for specification of endomesoderm in the sea urchin embryo. Dev Biol. 2002;246:162–190. [PubMed: 12027441]
- Dessimoz J, Grapin-Botton A. Pancreas development and cancer: Wnt/beta-catenin at issue. Cell Cycle. 2006;5:7–10. [PubMed: 16322692]
- Dessimoz J, Opoka R, Kordich J.J, Grapin-Botton A, Wells J.M. FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev. 2006;123:42–55. [PubMed: 16326079]
- Deutsch G, Jung J, Zheng M, Lora J, Zaret K.S. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. [PubMed: 11222142]
- Di Gregorio A, Corbo J.C, Levine M. The regulation of forkhead/HNF-3beta expression in the Ciona embryo. Dev Biol. 2001;229:31–43. [PubMed: 11133152]
- Dohrmann C.E, Kessler D.S, Melton D.A. Induction of axial mesoderm by zDVR-1, the zebrafish orthologue of Xenopus Vg1. Dev Biol. 1996;175:108–117. [PubMed: 8608857]
- Dougan S.T, Warga R.M, Kane D.A, Schier A.F, Talbot W.S. The role of the zebrafish nodal-related genes squint and cyclops in patterning of mesendoderm. Development. 2003;130:1837–1851. [PubMed: 12642489]
- Duboc V, Rottinger E, Besnardeau L, Lepage T. Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev Cell. 2004;6:397–410. [PubMed: 15030762]
- Dufort D, Schwartz L, Harpal K, Rossant J. The transcription factor HNF3beta is required in visceral endoderm for normal primitive streak morphogenesis. Development. 1998;125:3015–3025. [PubMed: 9671576]
- Duncan S.A, Manova K, Chen W.S, Hoodless P, Weinstein D.C, Bachvarova R.F, Darnell J.E Jr. Expression of transcription factor HNF-4 in the extraembryonic endoderm, gut, and nephrogenic tissue of the developing mouse embryo: HNF-4 is a marker for primary endoderm in the implanting blastocyst. Proc Natl Acad Sci USA. 1994;91:7598–7602. [PMC free article: PMC44449] [PubMed: 8052626]
- Duncan S.A, Nagy A, Chan W. Murine gastrulation requires HNF-4 regulated gene expression in the visceral endoderm: tetraploid rescue of Hnf-4(-/-) embryos. Development. 1997;124:279–287. [PubMed: 9053305]
- Dunn N.R, Vincent S.D, Oxburgh L, Robertson E.J, Bikoff E.K. Combinatorial activities of Smad2 and Smad3 regulate mesoderm formation and patterning in the mouse embryo. Development. 2004;131:1717–1728. [PubMed: 15084457]
- Dziadek M. Modulation of alphafetoprotein synthesis in the early postimplantation mouse embryo. J Embryol Exp Morphol. 1978;46:135–146. [PubMed: 81255]
- Dziadek M, Adamson E. Localization and synthesis of alphafoetoprotein in post-implantation mouse embryos. J Embryol Exp Morphol. 1978;43:289–313. [PubMed: 75937]
- Escriva H, Holland N.D, Gronemeyer H, Laudet V, Holland L.Z. The retinoic acid signaling pathway regulates anterior/posterior patterning in the nerve cord and pharynx of amphioxus, a chordate lacking neural crest. Development. 2002;129:2905–2916. [PubMed: 12050138]
- Fan Q.W, Kadomatsu K, Uchimura K, Muramatsu T. Embigin/basigin subgroup of the immunoglobulin superfamily: different modes of expression during mouse embryogenesis and correlated expression with carbohydrate antigenic markers. Dev Growth Differ. 1998;40:277–286. [PubMed: 9639355]
- Feldman B, Poueymirou W, Papaioannou V.E, DeChiara T.M, Goldfarb M. Requirement of FGF-4 for postimplantation mouse development. Science. 1995;267:246–249. [PubMed: 7809630]
- Franklin V, Khoo P.L, Bildsoe H, Wong N, Lewis S, Tam P.P. Regionalisation of the endoderm progenitors and morphogenesis of the gut portals of the mouse embryo. Mech Dev. 2008;125:587–600. [PubMed: 18486455]
- Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, Nakao K, Miyazaki Ji J, Niwa H. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16:784–789. [PMC free article: PMC186328] [PubMed: 11937486]
- Fukui A, Goto T, Kitamoto J, Homma M, Asashima M. SDF-1 alpha regulates mesendodermal cell migration during frog gastrulation. Biochem Biophys Res Commun. 2007;354:472–477. [PubMed: 17239342]
- Futaki S, Hayashi Y, Emoto T, Weber C.N, Sekiguchi K. Sox7 plays crucial roles in parietal endoderm differentiation in F9 embryonal carcinoma cells through regulating Gata-4 and Gata-6 expression. Mol Cell Biol. 2004;24:10492–10503. [PMC free article: PMC529033] [PubMed: 15542856]
- Gadue P, Huber T.L, Paddison P.J, Keller G.M. Wnt and TGF-beta signaling are required for the induction of an in vitro model of primitive streak formation using embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:16806–16811. [PMC free article: PMC1636536] [PubMed: 17077151]
- Gao X, Sedgwick T, Shi Y.B, Evans T. Distinct functions are implicated for the GATA-4, -5, and -6 transcription factors in the regulation of intestine epithelial cell differentiation. Mol Cell Biol. 1998;18:2901–2911. [PMC free article: PMC110669] [PubMed: 9566909]
- Gardner R.L. Investigation of cell lineage and differentiation in the extraembryonic endoderm of the mouse embryo. J Embryol Exp Morphol. 1982;68:175–198. [PubMed: 7108421]
- Gaudet J, Mango S.E. Regulation of organogenesis by the Caenorhabditis elegans FoxA protein PHA-4. Science. 2002;295:821–825. [PubMed: 11823633]
- Gerbe F, Cox B, Rossant J, Chazaud C. Dynamic expression of Lrp2 pathway members reveals progressive epithelial differentiation of primitive endoderm in mouse blastocyst. Dev Biol. 2008;313:594–602. [PubMed: 18083160]
- Germain S, Howell M, Esslemont G.M, Hill C.S. Homeodomain and winged-helix transcription factors recruit activated Smads to distinct promoter elements via a common Smad interaction motif. Genes Dev. 2000;14:435–451. [PMC free article: PMC316385] [PubMed: 10691736]
- Gouon-Evans V, Boussemart L, Gadue P, Nierhoff D, Koehler C.I, Kubo A, Shafritz D.A, Keller G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24:1402–1411. [PubMed: 17086172]
- Grapin-Botton A. Antero-posterior patterning of the vertebrate digestive tract: 40 years after Nicole Le Douarin's PhD thesis. Int J Dev Biol. 2005;49:335–347. [PubMed: 15906249]
- Grapin-Botton A, Constam D. Evolution of the mechanisms and molecular control of endoderm formation. Mech Dev. 2007;124:253–278. [PubMed: 17307341]
- Grapin-Botton A, Melton D.A. Endoderm development: from patterning to organogenesis. Trends Genet. 2000;16:124–130. [PubMed: 10689353]
- Gregorieff A, Grosschedl R, Clevers H. Hindgut defects and transformation of the gastro-intestinal tract in Tcf4(-/-)/Tcf1(-/-) embryos. Embo J. 2004;23:1825–1833. [PMC free article: PMC394245] [PubMed: 15057272]
- Gu Z, Reynolds E.M, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin D.T, van den Eijnden-van Raaij J, Donahoe P.K, Li E. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551–2561. [PubMed: 10226013]
- Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman J.R, Zaret K.S. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 1996;10:1670–1682. [PubMed: 8682297]
- Hagos E.G, Dougan S.T. Time-dependent patterning of the mesoderm and endoderm by Nodal signals in zebrafish. BMC Dev Biol. 2007;7:22. [PMC free article: PMC1851950] [PubMed: 17391517]
- Hamazaki T, Kehoe S.M, Nakano T, Terada N. The Grb2/Mek pathway represses Nanog in murine embryonic stem cells. Mol Cell Biol. 2006;26:7539–7549. [PMC free article: PMC1636849] [PubMed: 16908534]
- Hamazaki T, Terada N. In vitro differentiation of embryonic stem cells into hepatocytes. Methods Enzymol. 2003;365:277–287. [PubMed: 14696353]
- Harada Y, Akasaka K, Shimada H, Peterson K.J, Davidson E.H, Satoh N. Spatial expression of a forkhead homologue in the sea urchin embryo. Mech Dev. 1996;60:163–173. [PubMed: 9025069]
- Hart A.H, Hartley L, Sourris K, Stadler E.S, Li R, Stanley E.G, Tam P.P, Elefanty A.G, Robb L. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development. 2002;129:3597–3608. [PubMed: 12117810]
- Helde K.A, Grunwald D.J. The DVR-1 (Vg1) transcript of zebrafish is maternally supplied and distributed throughout the embryo. Dev Biol. 1993;159:418–426. [PubMed: 8405668]
- Henry G.L, Brivanlou I.H, Kessler D.S, Hemmati-Brivanlou A, Melton D.A. TGF-beta signals and a pattern in Xenopus laevis endodermal development. Development. 1996;122:1007–1015. [PubMed: 8631246]
- Henry G.L, Melton D.A. Mixer, a homeobox gene required for endoderm development. Science. 1998;281:91–96. [PubMed: 9651252]
- Hoodless P.A, Pye M, Chazaud C, Labbe E, Attisano L, Rossant J, Wrana J.L. FoxH1 (Fast) functions to specify the anterior primitive streak in the mouse. Genes Dev. 2001;15:1257–1271. [PMC free article: PMC313796] [PubMed: 11358869]
- Hori Y, Rulifson I.C, Tsai B.C, Heit J.J, Cahoy J.D, Kim S.K. Growth inhibitors promote differentiation of insulin-producing tissue from embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:16105–16110. [PMC free article: PMC138572] [PubMed: 12441403]
- Horner M.A, Quintin S, Domeier M.E, Kimble J, Labouesse M, Mango S.E. pha-4, an HNF-3 homolog, specifies pharyngeal organ identity in Caenorhabditis elegans. Genes Dev. 1998;12:1947–1952. [PMC free article: PMC316969] [PubMed: 9649499]
- Hou J, Charters A.M, Lee S.C, Zhao Y, Wu M.K, Jones S.J, Marra M.A, Hoodless P.A. A systematic screen for genes expressed in definitive endoderm by Serial Analysis of Gene Expression (SAGE). BMC Dev Biol. 2007;7:92. [PMC free article: PMC1950885] [PubMed: 17683524]
- Howard L, Rex M, Clements D, Woodland H.R. Regulation of the Xenopus Xsox17alpha(1) promoter by co-operating VegT and Sox17 sites. Dev Biol. 2007;310:402–415. [PMC free article: PMC2098691] [PubMed: 17719026]
- Howell M, Hill C.S. XSmad2 directly activates the activin-inducible, dorsal mesoderm gene XFKH1 in Xenopus embryos. Embo J. 1997;16:7411–7421. [PMC free article: PMC1170341] [PubMed: 9405370]
- Howell M, Itoh F, Pierreux C.E, Valgeirsdottir S, Itoh S, ten Dijke P, Hill C.S. Xenopus Smad4beta is the co-Smad component of developmentally regulated transcription factor complexes responsible for induction of early mesodermal genes. Dev Biol. 1999;214:354–369. [PubMed: 10525340]
- Huang D, Chen S.W, Gudas L.J. Analysis of two distinct retinoic acid response elements in the homeobox gene Hoxb1 in transgenic mice. Dev Dyn. 2002;223:353–370. [PubMed: 11891985]
- Huang D, Chen S.W, Langston A.W, Gudas L.J. A conserved retinoic acid responsive element in the murine Hoxb-1 gene is required for expression in the developing gut. Development. 1998;125:3235–3246. [PubMed: 9671595]
- Huang R.P, Ozawa M, Kadomatsu K, Muramatsu T. Developmentally regulated expression of embigin, a member of the immunoglobulin superfamily found in embryonal carcinoma cells. Differentiation. 1990;45:76–83. [PubMed: 1965893]
- Hudson C, Clements D, Friday R.V, Stott D, Woodland H.R. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. [PubMed: 9363948]
- Hudson C, Yasuo H. A signalling relay involving Nodal and Delta ligands acts during secondary notochord induction in Ciona embryos. Development. 2006;133:2855–2864. [PubMed: 16835438]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. [PMC free article: PMC2174807] [PubMed: 10662781]
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article: PMC1949933] [PubMed: 10859025]
- Izumi N, Era T, Akimaru H, Yasunaga M, Nishikawa S. Dissecting the molecular hierarchy for mesendoderm differentiation through a combination of embryonic stem cell culture and RNA interference. Stem Cells. 2007;25:1664–1674. [PubMed: 17446562]
- Jacobsen C.M, Narita N, Bielinska M, Syder A.J, Gordon J.I, Wilson D.B. Genetic mosaic analysis reveals that GATA-4 is required for proper differentiation of mouse gastric epithelium. Dev Biol. 2002;241:34–46. [PubMed: 11784093]
- Jiang Y, Evans T. The Xenopus GATA-4/5/6 genes are associated with cardiac specification and can regulate cardiac-specific transcription during embryogenesis. Dev Biol. 1996;174:258–270. [PubMed: 8631498]
- Jones E.A, Tosh D, Wilson D.I, Lindsay S, Forrester L.M. Hepatic differentiation of murine embryonic stem cells. Exp Cell Res. 2002;272:15–22. [PubMed: 11740861]
- Jung J, Zheng M, Goldfarb M, Zaret K.S. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. [PubMed: 10373120]
- Kadokawa Y, Kato Y, Eguchi G. Cell lineage analysis of the primitive and visceral endoderm of mouse embryos cultured in vitro. Cell Differ. 1987;21:69–76. [PubMed: 3607886]
- Kaestner K.H, Hiemisch H, Schutz G. Targeted disruption of the gene encoding hepatocyte nuclear factor 3gamma results in reduced transcription of hepatocyte-specific genes. Mol Cell Biol. 1998;18:4245–4251. [PMC free article: PMC109008] [PubMed: 9632808]
- Kaestner K.H, Knochel W, Martinez D.E. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed: 10702024]
- Kalantry S, Manning S, Haub O, Tomihara-Newberger C, Lee H.G, Fangman J, Disteche C.M, Manova K, Lacy E. The amnionless gene, essential for mouse gastrulation, encodes a visceral-endoderm-specific protein with an extracellular cysteine-rich domain. Nat Genet. 2001;27:412–416. [PubMed: 11279523]
- Kalb J.M, Lau K.K, Goszczynski B, Fukushige T, Moons D, Okkema P.G, McGhee J.D. pha-4 is Ce-fkh-1, a fork head/HNF-3alpha,beta,gamma homolog that functions in organogenesis of the C. elegans pharynx. Development. 1998;125:2171–2180. [PubMed: 9584117]
- Kanai-Azuma M, Kanai Y, Gad J.M, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam P.P, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. [PubMed: 11973269]
- Karperien M, Lanser P, de Laat S.W, Boonstra J, Defize L.H. Parathyroid hormone related peptide mRNA expression during murine postimplantation development: evidence for involvement in multiple differentiation processes. Int J Dev Biol. 1996;40:599–608. [PubMed: 8840192]
- Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005;233:1064–1075. [PubMed: 15880404]
- Kimura C, Yoshinaga K, Tian E, Suzuki M, Aizawa S, Matsuo I. Visceral endoderm mediates forebrain development by suppressing posteriorizing signals. Dev Biol. 2000;225:304–321. [PubMed: 10985852]
- Kimura W, Yasugi S, Stern C.D, Fukuda K. Fate and plasticity of the endoderm in the early chick embryo. Dev Biol. 2006;289:283–295. [PubMed: 16337933]
- Kimura-Yoshida C, Tian E, Nakano H, Amazaki S, Shimokawa K, Rossant J, Aizawa S, Matsuo I. Crucial roles of Foxa2 in mouse anterior-posterior axis polarization via regulation of anterior visceral endoderm-specific genes. Proc Natl Acad Sci USA. 2007;104:5919–5924. [PMC free article: PMC1851592] [PubMed: 17389379]
- Kinder S.J, Loebel D.A, Tam P.P. Allocation and early differentiation of cardiovascular progenitors in the mouse embryo. Trends Cardiovasc Med. 2001;11:177–184. [PubMed: 11597828]
- Kofron M, Wylie C, Heasman J. The role of Mixer in patterning the early Xenopus embryo. Development. 2004;131:2431–2441. [PubMed: 15128672]
- Koutsourakis M, Langeveld A, Patient R, Beddington R, Grosveld F. The transcription factor GATA6 is essential for early extraembryonic development. Development. 1999;126:723–732. [PubMed: 9895320]
- Kroon E, Martinson L.A, Kadoya K, Bang A.G, Kelly O.G, Eliazer S, Young H, Richardson M, Smart N.G, Cunningham J. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. [PubMed: 18288110]
- Kubo A, Shinozaki K, Shannon J.M, Kouskoff V, Kennedy M, Woo S, Fehling H.J, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. [PubMed: 14998924]
- Kumar M, Jordan N, Melton D, Grapin-Botton A. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Dev Biol. 2003;259:109–122. [PubMed: 12812792]
- Kunath T, Arnaud D, Uy G.D, Okamoto I, Chureau C, Yamanaka Y, Heard E, Gardner R.L, Avner P, Rossant J. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. [PubMed: 15753215]
- Kwon G.S, Viotti M, Hadjantonakis A.K. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev Cell. 2008;15:509–520. [PMC free article: PMC2677989] [PubMed: 18854136]
- Latinkic B.V, Smith J.C. Goosecoid and mix.1 repress Brachyury expression and are required for head formation in Xenopus. Development. 1999;126:1769–1779. [PubMed: 10079237]
- Latinkic B.V, Umbhauer M, Neal K.A, Lerchner W, Smith J.C, Cunliffe V. The Xenopus Brachyury promoter is activated by FGF and low concentrations of activin and suppressed by high concentrations of activin and by paired-type homeodomain proteins. Genes Dev. 1997;11:3265–3276. [PMC free article: PMC316753] [PubMed: 9389657]
- Laufer J.S, Bazzicalupo P, Wood W.B. Segregation of developmental potential in early embryos of Caenorhabditis elegans. Cell. 1980;19:569–577. [PubMed: 7363324]
- Lawson K.A, Meneses J.J, Pedersen R.A. Cell fate and cell lineage in the endoderm of the presomite mouse embryo, studied with an intracellular tracer. Dev Biol. 1986;115:325–339. [PubMed: 3709966]
- Leahy A, Xiong J.W, Kuhnert F, Stuhlmann H. Use of developmental marker genes to define temporal and spatial patterns of differentiation during embryoid body formation. J Exp Zool. 1999;284:67–81. [PubMed: 10368935]
- Lemaire P, Darras S, Caillol D, Kodjabachian L. A role for the vegetally expressed Xenopus gene Mix.1 in endoderm formation and in the restriction of mesoderm to the marginal zone. Development. 1998;125:2371–2380. [PubMed: 9609820]
- Leung B, Hermann G.J, Priess J.R. Organogenesis of the Caenorhabditis elegans intestine. Dev Biol. 1999;216:114–134. [PubMed: 10588867]
- Lewis S.L, Khoo P.L, De Young R.A, Steiner K, Wilcock C, Mukhopadhyay M, Westphal H, Jamieson R.V, Robb L, Tam P.P. Dkk1 and Wnt3 interact to control head morphogenesis in the mouse. Development. 2008;135:1791–1801. [PubMed: 18403408]
- Li L, Arman E, Ekblom P, Edgar D, Murray P, Lonai P. Distinct GATA6- and laminin-dependent mechanisms regulate endodermal and ectodermal embryonic stem cell fates. Development. 2004;131:5277–5286. [PubMed: 15456727]
- Li X, Chen Y, Scheele S, Arman E, Haffner-Krausz R, Ekblom P, Lonai P. Fibroblast growth factor signaling and basement membrane assembly are connected during epithelial morphogenesis of the embryoid body. J Cell Biol. 2001;153:811–822. [PMC free article: PMC2192393] [PubMed: 11352941]
- Lindsley R.C, Gill J.G, Kyba M, Murphy T.L, Murphy K.M. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. [PubMed: 16943279]
- Liu P, Wakamiya M, Shea M.J, Albrecht U, Behringer R.R, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. [PubMed: 10431240]
- Liu Y, Festing M, Thompson J.C, Hester M, Rankin S, El-Hodiri H.M, Zorn A.M, Weinstein M. Smad2 and Smad3 coordinately regulate craniofacial and endodermal development. Dev Biol. 2004;270:411–426. [PubMed: 15183723]
- Loose M, Patient R. A genetic regulatory network for Xenopus mesendoderm formation. Dev Biol. 2004;271:467–478. [PubMed: 15223347]
- Lowe L.A, Yamada S, Kuehn M.R. Genetic dissection of nodal function in patterning the mouse embryo. Development. 2001;128:1831–1843. [PubMed: 11311163]
- Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. [PubMed: 11326082]
- Lunde K, Belting H.G, Driever W. Zebrafish pou5f1/pou2, homolog of mammalian Oct4, functions in the endoderm specification cascade. Curr Biol. 2004;14:48–55. [PubMed: 14711414]
- Maeda M, Kubo K, Nishi T, Futai M. Roles of gastric GATA DNA-binding proteins. J Exp Biol. 1996;199(Pt 3):513–520. [PubMed: 8867276]
- Mango S.E, Lambie E.J, Kimble J. The pha-4 gene is required to generate the pharyngeal primordium of Caenorhabditis elegans. Development. 1994;120:3019–3031. [PubMed: 7607089]
- Martin M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dolle P, Gradwohl G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Dev Biol. 2005;284:399–411. [PubMed: 16026781]
- Matt N, Ghyselinck N.B, Wendling O, Chambon P, Mark M. Retinoic acid-induced developmental defects are mediated by RARbeta/RXR heterodimers in the pharyngeal endoderm. Development. 2003;130:2083–2093. [PubMed: 12668623]
- McGrath K.E, Koniski A.D, Maltby K.M, McGann J.K, Palis J. Embryonic expression and function of the chemokine SDF-1 and its receptor, CXCR4. Dev Biol. 1999;213:442–456. [PubMed: 10479460]
- McLean A.B, D’Amour K.A, Jones K.L, Krishnamoorthy M, Kulik M.J, Reynolds D.M, Sheppard A.M, Liu H, Xu Y, Baetge E.E, Dalton S. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells. 2007;25:29–38. [PubMed: 17204604]
- McLin V.A, Rankin S.A, Zorn A.M. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. [PubMed: 17507400]
- Meno C, Gritsman K, Ohishi S, Ohfuji Y, Heckscher E, Mochida K, Shimono A, Kondoh H, Talbot W.S, Robertson E.J. et al. Mouse Lefty-2 and zebrafish Antivin are feedback inhibitors of Nodal signaling during vertebrate gastrulation. Mol Cell. 1999;4:287–298. [PubMed: 10518210]
- Mesnard D, Guzman-Ayala M, Constam D.B. Nodal specifies embryonic visceral endoderm and sustains pluripotent cells in the epiblast before overt axial patterning. Development. 2006;133:2497–2505. [PubMed: 16728477]
- Mizoguchi T, Verkade H, Heath J.K, Kuroiwa A, Kikuchi Y. Sdf1/Cxcr4 signaling controls the dorsal migration of endodermal cells during zebrafish gastrulation. Development. 2008 [PubMed: 18579679]
- Molkentin J.D, Lin Q, Duncan S.A, Olson E.N. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. [PubMed: 9136933]
- Molkentin J.D, Tymitz K.M, Richardson J.A, Olson E.N. Abnormalities of the genitourinary tract in female mice lacking GATA5. Mol Cell Biol. 2000;20:5256–5260. [PMC free article: PMC85974] [PubMed: 10866681]
- Molotkov A, Molotkova N, Duester G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005;232:950–957. [PubMed: 15739227]
- Monaghan A.P, Kaestner K.H, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–578. [PubMed: 8187630]
- Moore-Scott B.A, Opoka R, Lin S.C, Kordich J.J, Wells J.M. Identification of molecular markers that are expressed in discrete anterior-posterior domains of the endoderm from the gastrula stage to mid-gestation. Dev Dyn. 2007;236:1997–2003. [PubMed: 17576135]
- Morrisey E.E, Tang Z, Sigrist K, Lu M.M, Jiang F, Ip H.S, Parmacek M.S. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590. [PMC free article: PMC317242] [PubMed: 9832509]
- Morrison G.M, Oikonomopoulou I, Migueles R.P, Soneji S, Livigni A, Enver T, Brickman J.M. Anterior definitive endoderm from ESCs reveals a role for FGF signaling. Cell Stem Cell. 2008;3:402–415. [PubMed: 18940732]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward D.W, Glinka A, Grinberg A, Huang S.P. et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–434. [PubMed: 11702953]
- Murray P, Edgar D. Regulation of the differentiation and behaviour of extra-embryonic endodermal cells by basement membranes. J Cell Sci. 2001;114:931–939. [PubMed: 11181176]
- Nair S, Schilling T.F. Chemokine signaling controls endodermal migration during zebrafish gastrulation. Science. 2008;322:89–92. [PMC free article: PMC2770598] [PubMed: 18719251]
- Nakaya Y, Sukowati E.W, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008 [PubMed: 18552836]
- Narita N, Bielinska M, Wilson D.B. Wild-type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev Biol. 1997;189:270–274. [PubMed: 9299119]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. [PubMed: 9814708]
- Niwa H, Miyazaki J, Smith A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376. [PubMed: 10742100]
- Norris D.P, Brennan J, Bikoff E.K, Robertson E.J. The Foxh1-dependent autoregulatory enhancer controls the level of Nodal signals in the mouse embryo. Development. 2002;129:3455–3468. [PubMed: 12091315]
- Nyeng P, Norgaard G.A, Kobberup S, Jensen J. FGF10 signaling controls stomach morphogenesis. Dev Biol. 2007;303:295–310. [PMC free article: PMC1864952] [PubMed: 17196193]
- Odenthal J, Nusslein-Volhard C. Fork head domain genes in zebrafish. Dev Genes Evol. 1998;208:245–258. [PubMed: 9683740]
- Pagan-Westphal S.M, Tabin C.J. The transfer of left-right positional information during chick embryogenesis. Cell. 1998;93:25–35. [PubMed: 9546389]
- Pan F.C, Chen Y, Bayha E, Pieler T. Retinoic acid-mediated patterning of the pre-pancreatic endoderm in Xenopus operates via direct and indirect mechanisms. Mech Dev. 2007;124:518–531. [PubMed: 17643968]
- Peale F.V Jr., Sugden L, Bothwell M. Characterization of CMIX, a chicken homeobox gene related to the Xenopus gene mix.1. Mech Dev. 1998;75:167–170. [PubMed: 9739137]
- Pearce J.J, Evans M.J. Mml, a mouse Mix-like gene expressed in the primitive streak. Mech Dev. 1999;87:189–192. [PubMed: 10495285]
- Perea-Gomez A, Meilhac S.M, Piotrowska-Nitsche K, Gray D, Collignon J, Zernicka-Goetz M. Regionalization of the mouse visceral endoderm as the blastocyst transforms into the egg cylinder. BMC Dev Biol. 2007;7:96. [PMC free article: PMC1978209] [PubMed: 17705827]
- Perea-Gomez A, Vella F.D, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, Pfister V, Chen L, Robertson E, Hamada H. et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell. 2002;3:745–756. [PubMed: 12431380]
- Pezeron G, Mourrain P, Courty S, Ghislain J, Becker T.S, Rosa F.M, David N.B. Live analysis of endodermal layer formation identifies random walk as a novel gastrulation movement. Curr Biol. 2008;18:276–281. [PubMed: 18291651]
- Pfeffer P.L, De Robertis E.M, Izpisua-Belmonte J.C. Crescent, a novel chick gene encoding a Frizzled-like cysteine-rich domain, is expressed in anterior regions during early embryogenesis. Int J Dev Biol. 1997;41:449–458. [PubMed: 9240561]
- Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis A.K. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development. 2008;135:3081–3091. [PMC free article: PMC2768606] [PubMed: 18725515]
- Poelmann R.E. The head-process and the formation of the definitive endoderm in the mouse embryo. Anat Embryol (Berl). 1981;162:41–49. [PubMed: 7283172]
- Priess J.R, Thomson J.N. Cellular interactions in early C. elegans embryos. Cell. 1987;48:241–250. [PubMed: 3802194]
- Rajagopal J, Anderson W.J, Kume S, Martinez O.I, Melton D.A. Insulin staining of ES cell progeny from insulin uptake. Science. 2003;299:363. [PubMed: 12532008]
- Rehorn K.P, Thelen H, Michelson A.M, Reuter R. A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development. 1996;122:4023–4031. [PubMed: 9012522]
- Reim G, Mizoguchi T, Stainier D.Y, Kikuchi Y, Brand M. The POU domain protein spg (pou2/Oct4) is essential for endoderm formation in cooperation with the HMG domain protein casanova. Dev Cell. 2004;6:91–101. [PubMed: 14723850]
- Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico M.G, Ibanez C.F, Brivanlou A.H. The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 2001;15:2010–2022. [PMC free article: PMC312747] [PubMed: 11485994]
- Reiter J.F, Alexander J, Rodaway A, Yelon D, Patient R, Holder N, Stainier D.Y. Gata5 is required for the development of the heart and endoderm in zebrafish. Genes Dev. 1999;13:2983–2995. [PMC free article: PMC317161] [PubMed: 10580005]
- Reuter R. The gene serpent has homeotic properties and specifies endoderm versus ectoderm within the Drosophila gut. Development. 1994;120:1123–1135. [PubMed: 7913013]
- Robb L, Hartley L, Begley C.G, Brodnicki T.C, Copeland N.G, Gilbert D.J, Jenkins N.A, Elefanty A.G. Cloning, expression analysis, and chromosomal localization of murine and human homologues of a Xenopus mix gene. Dev Dyn. 2000;219:497–504. [PubMed: 11084649]
- Rodaway A, Patient R. Mesendoderm: an ancient germ layer? Cell. 2001;105:169–172. [PubMed: 11336666]
- Rodaway A, Takeda H, Koshida S, Broadbent J, Price B, Smith J.C, Patient R, Holder N. Induction of the mesendoderm in the zebrafish germ ring by yolk cell- derived TGF-beta family signals and discrimination of mesoderm and endoderm by FGF. Development. 1999;126:3067–3078. [PubMed: 10375499]
- Rodriguez T.A, Sparrow D.B, Scott A.N, Withington S.L, Preis J.I, Michalicek J, Clements M, Tsang T.E, Shioda T, Beddington R.S, Dunwoodie S.L. Cited1 is required in trophoblasts for placental development and for embryo growth and survival. Mol Cell Biol. 2004;24:228–244. [PMC free article: PMC303371] [PubMed: 14673158]
- Rodriguez T.A, Srinivas S, Clements M.P, Smith J.C, Beddington R.S. Induction and migration of the anterior visceral endoderm is regulated by the extra-embryonic ectoderm. Development. 2005;132:2513–2520. [PubMed: 15857911]
- Rossi J.M, Dunn N.R, Hogan B.L, Zaret K.S. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes Dev. 2001;15:1998–2009. [PMC free article: PMC312750] [PubMed: 11485993]
- Russ A.P, Wattler S, Colledge W.H, Aparicio S.A, Carlton M.B, Pearce J.J, Barton S.C, Surani M.A, Ryan K, Nehls M.C. et al. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404:95–99. [PubMed: 10716450]
- Sasaki H, Hogan B.L. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47–59. [PubMed: 8375339]
- Schier A.F, Neuhauss S.C, Helde K.A, Talbot W.S, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. [PubMed: 9053309]
- Schroeder D.F, McGhee J.D. Anterior-posterior patterning within the Caenorhabditis elegans endoderm. Development. 1998;125:4877–4887. [PubMed: 9811572]
- Schubert M, Yu J.K, Holland N.D, Escriva H, Laudet V, Holland L.Z. Retinoic acid signaling acts via Hox1 to establish the posterior limit of the pharynx in the chordate amphioxus. Development. 2005;132:61–73. [PubMed: 15576409]
- Schultheiss T.M, Xydas S, Lassar A.B. Induction of avian cardiac myogenesis by anterior endoderm. Development. 1995;121:4203–4214. [PubMed: 8575320]
- Seguin C.A, Draper J.S, Nagy A, Rossant J. Establishment of endoderm progenitors by SOX transcription factor expression in human embryonic stem cells. Cell Stem Cell. 2008;3:182–195. [PubMed: 18682240]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. [PubMed: 9916808]
- Seleiro E.A, Connolly D.J, Cooke J. Early developmental expression and experimental axis determination by the chicken Vg1 gene. Curr Biol. 1996;6:1476–1486. [PubMed: 8939612]
- Serls A.E, Doherty S, Parvatiyar P, Wells J.M, Deutsch G.H. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. [PubMed: 15576401]
- Shah S.B, Skromne I, Hume C.R, Kessler D.S, Lee K.J, Stern C.D, Dodd J. Misexpression of chick Vg1 in the marginal zone induces primitive streak formation. Development. 1997;124:5127–5138. [PubMed: 9362470]
- Shawlot W, Deng J.M, Behringer R.R. Expression of the mouse cerberus-related gene, Cerr1, suggests a role in anterior neural induction and somitogenesis. Proc Natl Acad Sci USA. 1998;95:6198–6203. [PMC free article: PMC27625] [PubMed: 9600941]
- Shen M.M, Leder P. Leukemia inhibitory factor is expressed by the preimplantation uterus and selectively blocks primitive ectoderm formation in vitro. Proc Natl Acad Sci USA. 1992;89:8240–8244. [PMC free article: PMC49893] [PubMed: 1518852]
- Sherwood R.I, Jitianu C, Cleaver O, Shaywitz D.A, Lamenzo J.O, Chen A.E, Golub T.R, Melton D.A. Prospective isolation and global gene expression analysis of definitive and visceral endoderm. Dev Biol. 2007;304:541–555. [PubMed: 17328885]
- Shimoda M, Kanai-Azuma M, Hara K, Miyazaki S, Kanai Y, Monden M, Miyazaki J. Sox17 plays a substantial role in late-stage differentiation of the extraembryonic endoderm in vitro. J Cell Sci. 2007;120:3859–3869. [PubMed: 17940068]
- Shimono A, Behringer R.R. Angiomotin regulates visceral endoderm movements during mouse embryogenesis. Curr Biol. 2003;13:613–617. [PubMed: 12676095]
- Shimosato D, Shiki M, Niwa H. Extra-embryonic endoderm cells derived from ES cells induced by GATA factors acquire the character of XEN cells. BMC Dev Biol. 2007;7:80. [PMC free article: PMC1933422] [PubMed: 17605826]
- Sinner D, Kirilenko P, Rankin S, Wei E, Howard L, Kofron M, Heasman J, Woodland H.R, Zorn A.M. Global analysis of the transcriptional network controlling Xenopus endoderm formation. Development. 2006;133:1955–1966. [PubMed: 16651540]
- Sinner D, Rankin S, Lee M, Zorn A.M. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131:3069–3080. [PubMed: 15163629]
- Sirard C, de la Pompa J.L, Elia A, Itie A, Mirtsos C, Cheung A, Hahn S, Wakeham A, Schwartz L, Kern S.E. et al. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes & Dev. 1998;12:107–119. [PMC free article: PMC316400] [PubMed: 9420335]
- Skromne I, Stern C.D. Interactions between Wnt and Vg1 signalling pathways initiate primitive streak formation in the chick embryo. Development. 2001;128:2915–2927. [PubMed: 11532915]
- Smyth N, Vatansever H.S, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–160. [PMC free article: PMC2148127] [PubMed: 9885251]
- Soudais C, Bielinska M, Heikinheimo M, MacArthur C.A, Narita N, Saffitz J.E, Simon M.C, Leiden J.M, Wilson D.B. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development. 1995;121:3877–3888. [PubMed: 8582296]
- Sousa-Nunes R, Rana A.A, Kettleborough R, Brickman J.M, Clements M, Forrest A, Grimmond S, Avner P, Smith J.C, Dunwoodie S.L, Beddington R.S. Characterizing embryonic gene expression patterns in the mouse using nonredundant sequence-based selection. Genome Res. 2003;13:2609–2620. [PMC free article: PMC403803] [PubMed: 14613977]
- Spagnoli F.M, Brivanlou A.H. The Gata5 target, TGIF2, defines the pancreatic region by modulating BMP signals within the endoderm. Development. 2008;135:451–461. [PubMed: 18094028]
- Stafford D, Hornbruch A, Mueller P.R, Prince V.E. A conserved role for retinoid signaling in vertebrate pancreas development. Dev Genes Evol. 2004;214:432–441. [PubMed: 15322880]
- Stafford D, Prince V.E. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. [PubMed: 12176331]
- Stafford D, White R.J, Kinkel M.D, Linville A, Schilling T.F, Prince V.E. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133:949–956. [PubMed: 16452093]
- Stainier D.Y. A glimpse into the molecular entrails of endoderm formation. Genes Dev. 2002;16:893–907. [PubMed: 11959838]
- Stainier D.Y. A glimpse into the molecular entrails of endoderm formation. Genes Dev. 2002;16:893–907. [PubMed: 11959838]
- Stein S, Roeser T, Kessel M. CMIX, a paired-type homeobox gene expressed before and during formation of the avian primitive streak. Mech Dev. 1998;75:163–165. [PubMed: 9739135]
- Strahle U, Blader P, Henrique D, Ingham P.W. Axial, a zebrafish gene expressed along the developing body axis, shows altered expression in cyclops mutant embryos. Genes Dev. 1993;7:1436–1446. [PubMed: 7687227]
- Strickland S, Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978;15:393–403. [PubMed: 214238]
- Strickland S, Smith K.K, Marotti K.R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980;21:347–355. [PubMed: 6250719]
- Tada M, Casey E.S, Fairclough L, Smith J.C. Bix1, a direct target of Xenopus T-box genes, causes formation of ventral mesoderm and endoderm. Development. 1998;125:3997–4006. [PubMed: 9735361]
- Tada S, Era T, Furusawa C, Sakurai H, Nishikawa S, Kinoshita M, Nakao K, Chiba T. Characterization of mesendoderm: a diverging point of the definitive endoderm and mesoderm in embryonic stem cell differentiation culture. Development. 2005;132:4363–4374. [PubMed: 16141227]
- Tam P.P, Beddington R.S. Establishment and organization of germ layers in the gastrulating mouse embryo. Ciba Found Symp. 1992;165:27–41;. 42–29. discussion. [PubMed: 1516473]
- Tam P.P, Khoo P.L, Lewis S.L, Bildsoe H, Wong N, Tsang T.E, Gad J.M, Robb L. Sequential allocation and global pattern of movement of the definitive endoderm in the mouse embryo during gastrulation. Development. 2007;134:251–260. [PubMed: 17151016]
- Tam P.P, Khoo P.L, Wong N, Tsang T.E, Behringer R.R. Regionalization of cell fates and cell movement in the endoderm of the mouse gastrula and the impact of loss of Lhx1(Lim1) function. Dev Biol. 2004;274:171–187. [PubMed: 15355796]
- Tam P.P, Williams E.A, Chan W.Y. Gastrulation in the mouse embryo: ultrastructural and molecular aspects of germ layer morphogenesis. Microsc Res Tech. 1993;26:301–328. [PubMed: 8305722]
- Tanaka C, Sakuma R, Nakamura T, Hamada H, Saijoh Y. Long-range action of Nodal requires interaction with GDF1. Genes Dev. 2007;21:3272–3282. [PMC free article: PMC2113028] [PubMed: 18079174]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie C.C, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. [PubMed: 15797385]
- Thisse B, Wright C.V, Thisse C. Activin- and Nodal-related factors control antero-posterior patterning of the zebrafish embryo. Nature. 2000;403:425–428. [PubMed: 10667793]
- Thisse C, Thisse B, Postlethwait J.H. Expression of snail2, a second member of the zebrafish snail family, in cephalic mesendoderm and presumptive neural crest of wild-type and spadetail mutant embryos. Dev Biol. 1995;172:86–99. [PubMed: 7589816]
- Thomas P, Beddington R. Anterior primitive endoderm may be responsible for patterning the anterior neural plate in the mouse embryo. Curr Biol. 1996;6:1487–1496. [PubMed: 8939602]
- Thomas P.Q, Brown A, Beddington R.S. Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development. 1998;125:85–94. [PubMed: 9389666]
- Torres-Padilla M.E, Richardson L, Kolasinska P, Meilhac S.M, Luetke-Eversloh M.V, Zernicka-Goetz M. The anterior visceral endoderm of the mouse embryo is established from both preimplantation precursor cells and by de novo gene expression after implantation. Dev Biol. 2007;309:97–112. [PMC free article: PMC3353121] [PubMed: 17662710]
- Veltmaat J.M, Orelio C.C, Ward-Van Oostwaard D, Van Rooijen M.A, Mummery C.L, Defize L.H. Snail is an immediate early target gene of parathyroid hormone related peptide signaling in parietal endoderm formation. Int J Dev Biol. 2000;44:297–307. [PubMed: 10853826]
- Verheijen M.H, Wolthuis R.M, Bos J.L, Defize L.H. The Ras/Erk pathway induces primitive endoderm but prevents parietal endoderm differentiation of F9 embryonal carcinoma cells. J Biol Chem. 1999;274:1487–1494. [PubMed: 9880524]
- Vincent R, Treff N, Budde M, Kastenberg Z, Odorico J. Generation and characterization of novel tetracycline-inducible pancreatic transcription factor-expressing murine embryonic stem cell lines. Stem Cells Dev. 2006;15:953–962. [PubMed: 17253956]
- Vincent S.D, Robertson E.J. Targeted insertion of an IRES Cre into the Hnf4alpha locus: Cre-mediated recombination in the liver, kidney, and gut epithelium. Genesis. 2004;39:206–211. [PubMed: 15282747]
- Weber H, Symes C.E, Walmsley M.E, Rodaway A.R, Patient R.K. A role for GATA5 in Xenopus endoderm specification. Development. 2000;127:4345–4360. [PubMed: 11003835]
- Weber R.J, Pedersen R.A, Wianny F, Evans M.J, Zernicka-Goetz M. Polarity of the mouse embryo is anticipated before implantation. Development. 1999;126:5591–5598. [PubMed: 10572036]
- Weigel D, Jurgens G, Kuttner F, Seifert E, Jackle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57:645–658. [PubMed: 2566386]
- Weinstein D.C, Ruiz i Altaba A, Chen W.S, Hoodless P, Prezioso V.R, Jessell T.M, Darnell J.E Jr. The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575–588. [PubMed: 8069910]
- Wells J.M, Melton D.A. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. [PubMed: 10725233]
- Wilder P.J, Kelly D, Brigman K, Peterson C.L, Nowling T, Gao Q.S, McComb R.D, Capecchi M.R, Rizzino A. Inactivation of the FGF-4 gene in embryonic stem cells alters the growth and/or the survival of their early differentiated progeny. Dev Biol. 1997;192:614–629. [PubMed: 9441693]
- Yaguchi S, Yaguchi J, Angerer R.C, Angerer L.M. A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Dev Cell. 2008;14:97–107. [PubMed: 18194656]
- Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20:146–154. [PubMed: 11897871]
- Yamada T, Yoshikawa M, Takaki M, Torihashi S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y. In vitro functional gut-like organ formation from mouse embryonic stem cells. Stem Cells. 2002;20:41–49. [PubMed: 11796921]
- Yamamoto M, Meno C, Sakai Y, Shiratori H, Mochida K, Ikawa Y, Saijoh Y, Hamada H. The transcription factor FoxH1 (FAST) mediates Nodal signaling during anterior-posterior patterning and node formation in the mouse. Genes Dev. 2001;15:1242–1256. [PMC free article: PMC313795] [PubMed: 11358868]
- Yamanaka Y, Ralston A, Stephenson R.O, Rossant J. Cell and molecular regulation of the mouse blastocyst. Dev Dyn. 2006 [PubMed: 16773657]
- Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt L.M, Nishikawa S, Chiba T, Era T. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. [PubMed: 16311587]
- Yeo C.-Y, Whitman M. Nodal Signals to Smads through Cripto-Dependent and Cripto-Independent Mechanisms. Mol Cell. 2001;7:949–957. [PubMed: 11389842]
- Yu J.K, Holland L.Z, Holland N.D. An amphioxus nodal gene (AmphiNodal) with early symmetrical expression in the organizer and mesoderm and later asymmetrical expression associated with left-right axis formation. Evol Dev. 2002;4:418–425. [PubMed: 12492142]
- Yu J.K, Holland N.D, Holland L.Z. AmphiFoxQ2, a novel winged helix/forkhead gene, exclusively marks the anterior end of the amphioxus embryo. Dev Genes Evol. 2003;213:102–105. [PubMed: 12632180]
- Zhou X, Sasaki H, Lowe L, Hogan B.L.M, Kuehn M.R. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–547. [PubMed: 8429908]
- *
Edited by Alexander F. Schier. Last revised October 31, 2008. Published November 30, 2008. This chapter should be cited as: Grapin-Botton, A., Endoderm specification (November 30, 2008), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.30.1, http://www.stembook.org.
- Review Endoderm development in vertebrates: fate mapping, induction and regional specification.[Dev Growth Differ. 2005]Review Endoderm development in vertebrates: fate mapping, induction and regional specification.Fukuda K, Kikuchi Y. Dev Growth Differ. 2005 Aug; 47(6):343-55.
- Notch signaling can regulate endoderm formation in zebrafish.[Dev Dyn. 2004]Notch signaling can regulate endoderm formation in zebrafish.Kikuchi Y, Verkade H, Reiter JF, Kim CH, Chitnis AB, Kuroiwa A, Stainier DY. Dev Dyn. 2004 Apr; 229(4):756-62.
- Origin and development of the zebrafish endoderm.[Development. 1999]Origin and development of the zebrafish endoderm.Warga RM, Nüsslein-Volhard C. Development. 1999 Feb; 126(4):827-38.
- Review Extraembryonic endoderm cells as a model of endoderm development.[Dev Growth Differ. 2013]Review Extraembryonic endoderm cells as a model of endoderm development.Moerkamp AT, Paca A, Goumans MJ, Kunath T, Kruithof BP, Kruithof-de Julio M. Dev Growth Differ. 2013 Apr; 55(3):301-8. Epub 2013 Feb 18.
- Review The guts of endoderm formation.[Results Probl Cell Differ. 2002]Review The guts of endoderm formation.Warga RM, Stainier DY. Results Probl Cell Differ. 2002; 40:28-47.
- Endoderm specification - StemBookEndoderm specification - StemBook
Your browsing activity is empty.
Activity recording is turned off.
See more...

 .
.