NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Guideline Alliance (UK). Endometriosis: diagnosis and management. London: National Institute for Health and Care Excellence (NICE); 2017 Sep. (NICE Guideline, No. 73.)
11.1. Pharmacological management
11.1.1. Analgesics
Review question: What is the effectiveness of analgesics for reducing pain in women with endometriosis, including recurrent and asymptomatic endometriosis?
11.1.1.1. Introduction
Pain is the most debilitating and common symptom of endometriosis. Endometriosis may cause cyclical pelvic pain, typically during menstruation, and often starting a few days before a woman’s period. Referred pain to the back and legs is common. Apart from acute pain during menstruation, women may also experience non-cyclical pain, deep pain during sexual intercourse, and pain associated with bowel and bladder functions. For many women, pain becomes persistent or chronic.
Most women who experience menstrual pain and who would like pharmacological analgesia will buy over-the-counter medications or be prescribed simple analgesics such as paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs), for example, ibuprofen, naproxen or aspirin. Mefanamic acid, another NSAID, is also commonly chosen for menstrual pain. For moderate to severe pain, weak opioids such as codeine are often used but the side effects of these are often limiting; constipation in particular may aggravate endometriosis symptoms. Stronger medication such as morphine is also prescribed if the pain is severe and does not respond to other treatments.
Symptomatic management of pain using analgesics is thus very important for women with endometriosis. Because of disease recurrence and potential chronicity of pain, women need access to analgesics throughout a lifetime living with endometriosis.
11.1.1.2. Description of clinical evidence
The objective of this review is to determine the clinical and cost effectiveness of analgesics in reducing pain in women with endometriosis.
For full details, see review protocol in Appendix D.
One study was included (Kauppila 1985) that used a crossover design to evaluate the effect of non-steroidal anti-inflammatory drugs (NSAIDs) compared with placebo in 24 women with ‘moderate’ to ‘very severe’ painful menstrual periods secondary to endometriosis. Endometriosis was diagnosed by pelvic examination, or by visualisation (for example, laparoscopy or laparotomy). One group of women received naproxen tablets for 2 menstrual cycles and then crossed over to placebo for 2 further menstrual cycles. The second group received placebo for the first and second menstrual cycles, then crossed over to naproxen sodium for 2 further menstrual cycles. Both groups received 275 mg naproxen tablets (1 or 2 tablets 4 times a day).
Results are presented from the first treatment period for 20 women who used a questionnaire immediately after each menstrual cycle to self-record outcomes of pain severity, use of supplementary analgesia and unintended effects from treatment. For severity of pain a score (range 1–3) was used where ‘mild improvement’ was scored as 1, ‘moderate improvement’ was scored 2 and ‘excellent relief’ was scored 3. It is not clear how the questionnaire was developed or validated.
No evidence was identified for the critical outcome of quality of life or for the important outcomes of effect on daily activities, absence from work or school, number of women requiring more invasive treatment and participant satisfaction with treatment.
Evidence is summarised in the clinical GRADE evidence profile below Table 53. See also the study selection flow chart in Appendix F, study exclusion list in Appendix H, forest plots in Appendix I, full GRADE profiles in Appendix J and study evidence tables in Appendix G. Summary of included studies
A summary of the studies that were included in this review are presented in Table 52.
11.1.1.3. Clinical evidence profile
The clinical evidence profile for this review question (NSAIDs for treatment of endometriosis) is presented in Table 53.
11.1.1.4. Economic evidence
No economic evidence was found on the use of analgesics in women with endometriosis.
Consequently, data from NICE CG173 (neuropathic pain) was used to inform an economic model that is described in more detail in Appendix K.
The economic cost of analgesics is very difficult to quantify. Although the drugs and the dosing regimen are normally very well understood, compliance and indirect costs (such as additional GP visits) can create uncertainty over the ‘true’ cost of prescribing one drug over another. In addition, many patients will self-medicate with over-the-counter analgesics, meaning that the cost to the NHS of recommending over-the-counter medicines such as paracetamol is only a fraction of the cost of recommending prescription-only medicines such as codeine (moreover, over-the-counter medicines tend to be less expensive to begin with).
Table 54 gives the direct cost of the 3 analgesics considered in the economic model for endometriosis (selected because of the availability of evidence on their cost and effectiveness). Table 55 gives indicative costs of all other analgesics specified in the protocol. The true economic cost of prescribing one over the other depends on factors not included in this table, including side effects, compliance and indirect costs.
The cost of ‘Generic’ analgesia is given as the cost of aspirin. Aspirin has a slightly higher cost than some other NSAIDs according to the electronic drug tariff; for example, Ibuprofen costs £0.86 for 24 400g tabs giving an annual cost of £40.05 and Naproxen costs £0.93 for 28 250g tabs giving an annual cost of £36.37. Nevertheless, it was thought appropriate to use the cost of aspirin as it is probably the most commonly prescribed NSAID, and the slightly higher cost is expected to offset indirect costs from drug prescription, such as side-effects, which are not included in Electronic Drug Tariff prices.
The economic model suggests that no analgesic is likely to be better than hormonal treatment; hormonal treatment is likely to be both more effective and cheaper than the best analgesics. These results are demonstrated in Table 56. The table shows that Tramadol likely dominates no treatment – being both cheaper and more effective – but that the next most effective set of analgesics are outside the range which would normally be considered for the NICE cost-effectiveness threshold of around £20,000
NSAIDs were excluded from most runs of the model; the evidence for their effectiveness was weak and contradictory (and the evidence upon which this was based was not clear in specifying which exact analgesic was used; NSAIDs were inferred from a description of the side effects). If the results for NSAIDs are accepted at face value, they would be more effective than hormonal treatment at a slightly higher cost, which would nonetheless be cost-effective at £20,000/quality adjusted life year (QALY) threshold. The Committee discussed how this could well be important evidence highlighting the effectiveness of NSAIDs versus other analgesics.
11.1.1.5. Clinical evidence statements
Very low quality evidence from 1 crossover RCT (n=20) showed that there was no difference in overall pain relief, unintended effects or need for supplementary analgesia when women with endometriosis received naproxen sodium compared to placebo for 2 menstrual cycles, although there was uncertainty around the estimate.
11.1.1.6. Evidence to recommendations
11.1.1.6.1. Relative value placed on the outcomes considered
The Committee prioritised pain relief, health-related quality or life and adverse events from analgesics (particularly those leading to withdrawal from treatment) as critical outcomes.
The Committee also discussed the need to take further supplementary analgesia, which was another outcome that was reported. No evidence was identified that reported on health-related quality of life.
11.1.1.6.2. Consideration of clinical benefits and harms
Pain is a common symptom of endometriosis and, when severe and/or persistent, can be completely debilitating, affecting one’s ability to perform routine daily activities, greatly limiting lifestyle and quality of life.
The Committee acknowledged that analgesia would only provide symptomatic relief of pain, rather than addressing any underlying pathology, but that effective pain relief can provide an alternative to more invasive treatment. The Committee noted that hormonal therapies used to treat endometriosis may take at least 1 menstrual cycle to become effective. For this reason, pain relief medication may be used until the long-term treatment begins to work.
The Committee noted that some women might tolerate significant harms associated with side effects of analgesics in order to have respite from their pain and that this trade off was variable depending on the severity of the woman’s symptoms and her individual circumstances.
11.1.1.6.3. Consideration of economic benefits and harms
The Committee acknowledged that hormonal treatment was likely to be more cost-effective than the best analgesics but reflected that this did not exclude giving an analgesic with another kind of treatment as, in general, analgesics were not thought to interact with other forms of treatment. The Committee also noted that analgesics might be considered cost-effective in the absence of other treatments. However, as there was no direct evidence on the effectiveness of analgesics in combination with other treatments for endometriosis the Committee made it clear that clinical judgement would be required if considering analgesics in combination with other treatments (e.g. hormonal or surgical treatments).
Although there are no results for the impact of analgesics on fertility (as this was not modelled), the Committee considered that the presence or absence of analgesics would be unlikely to alter a woman’s fertility and have a relatively smaller impact on fertility than other treatment options considered in this guideline. The Committee acknowledged some limited evidence that NSAIDs might inhibit ovulation if taken continuously during the cycle, (making conception less likely), but noted that if taken during the period, would not have an effect on ovulation. Members further pointed out that severe pain might reduce the likelihood of intercourse and hence analgesics might improve the chance of conception. Overall the Committee concluded that the impact of analgesics on fertility (especially NSAIDs) was not sufficiently researched to underpin a recommendation.
Estimating the resource impact of analgesics is difficult as many women will chose to self-medicate if prescribed over-the-counter analgesia (as this can often work out cheaper for both the woman and the NHS). The Committee described how the general principle of their recommendations – trialling cheap medication and considering more expensive analgesia if this failed – was current NHS practice, and so the recommendations are unlikely to represent a significant resource impact.
11.1.1.6.4. Quality of evidence
The available evidence was drawn from a single small trial conducted in 1985 and was of very low quality. A self-reported questionnaire to assess pain was used, although the validity of the pain scoring system was unclear. While the study indicated that 24 women were randomised, the results for only 20 women were reported for unintended effects of treatment and 19 for overall pain relief and for supplementary analgesia needed. There were other methodological flaws such as unclear allocation concealment and unclear reporting of exclusion criteria. The direction of the effect for overall pain relief, unintended effects and need for supplementary analgesia outcomes was in favour of naproxen sodium but, due to the small sample size, the study was underpowered and outcome effects had wide confidence intervals (CIs). No evidence was available for the other outcomes prioritised and no other relevant evidence assessing the effectiveness of any other type of analgesic for endometriosis-related pain was available.
The Committee considered that the small number of women included in the study and its short duration made it difficult to draw any valid conclusions. The Committee agreed that although there is no good evidence for use of analgesics in management of acute pain specific to endometriosis, there is robust evidence of effectiveness of analgesics for pain management in other areas and hence gave little weight to the limited evidence.
11.1.1.6.5. Other considerations
Due to the poor quality and limited evidence available, the Committee based their decisions on consensus and the experience and expertise of its members.
The Committee discussed the Pain Ladder developed by the World Health Organization (WHO) for analgesia for cancer-related pain but which has since been adopted for acute and chronic non-malignant pain relief. This describes a 3-step progressive approach to use of pharmacologic agents proportional to the level of pain reported. The initial step uses oral administration of non-opioids such as paracetamol or NSAIDs. If pain is not controlled, then mild opioids such as codeine are tried and, as a last step, strong opioids such as morphine are used until the patient’s pain is alleviated. One benefit of the stepped approach is that adverse events can be discovered throughout the process.
The Committee discussed whether the addition of an opioid analgesic could be considered if pain was not adequately controlled after a trial period. However, the potential adverse effects of opioid analgesia, such as dependency, were recognised, given the chronic nature of endometriosis-related pain and, particularly, constipation. Therefore, the Committee concluded that a referral for diagnosis might be more appropriate and that there were other treatment options available.
The Committee also considered whether a research recommendation should be drafted for this topic. They agreed that research into analgesia in the management of pain related to endometriosis is not a priority for this guideline because there is sufficient indirect evidence from other conditions available to draw upon.
The Committee considered whether any different recommendations were necessary for adolescent women but concluded that none were required.
11.1.1.6.6. Key conclusions
The Committee concluded that a short trial of analgesics for first line management of pain in women with endometriosis-related pain is appropriate.
11.1.1.7. Recommendations
- 32.
For women with endometriosis-related pain, discuss the benefits and risks of analgesics, taking into account any comorbidities and the woman’s preferences.
- 33.
Consider a short trial (for example, 3 months) of paracetamol or a non-steroidal anti-inflammatory drug (NSAID) alone or in combination for first-line management of endometriosis-related pain.
- 34.
If a trial of paracetamol or an NSAID (alone or in combination) does not provide adequate pain relief, consider other forms of pain management and referral for further assessment.
11.1.2. Neuromodulators (neuropathic pain treatment)
Review question: What is the effectiveness of neuromodulators for treating endometriosis, including recurrent and asymptomatic endometriosis?
11.1.2.1. Introduction
Neuromodulators, otherwise known as neuropathic analgesics, are used mainly by pain specialists and general practitioners (GPs) in the management of chronic, also known as, persistent pain. Neuromodulators differ from conventional analgesics such as NSAIDs in that they primarily affect the central nervous system’s modulation of pain, rather than peripheral meditators of inflammation. An overactive and hypersensitive nervous system contributes to the development and maintenance of chronic pain. Neuromodulators exert their effects via their modulation of this overactive and hypersensitive nervous system.
Many neuromodulators were originally developed with different aims, for example, as antidepressants or anticonvulsants. The main classes of neuromodulators are: the tricyclic antidepressants, for example, amitriptyline and nortriptyline; the selective serotonin re-uptake inhibitors such as duloxetine; and the gabapentinoids (gabapentin and pregabalin). Under this heading we also considered capsaicin, ketamine, local anaesthetics (lidocaine) and nerve blocks. Certain opioid medications, such as tramadol and tapentadol, also have neuromodulating properties.
These medicines may also have important other effects, depending on their dose, on other related conditions that may be concurrently present, such as anxiety and/or depression. NICE already recommends a choice of amitriptyline, duloxetine, gabapentin or pregabalin as the initial treatment for neuropathic pain (CG 173).
Understanding the effectiveness of neuromodulators for women with endometriosis is important as, if useful, they might reduce the burden of pain and/or side effects from other medications, or offer an alternative to other types of treatment such as hormonal. If effective, they might reduce the need for surgery and prevent or reduce the chronicity of pain with its far-reaching consequences.
11.1.2.2. Description of clinical evidence
The objective of this review is to determine the clinical and cost effectiveness of neuromodulators to improve outcomes in women with endometriosis.
For full details, see review protocol in Appendix D.
We looked for systematic reviews, randomised and comparative observational studies assessing the effectiveness of neuromodulators in the management of endometriosis of any stage or severity. These may also include suspected diagnoses as described in detail in the protocol.
Two trials were identified that used local anaesthetics with a procedure called perturbation, which involves the insertion of a thin plastic catheter in the cervical canal. This catheter is then used to infuse the local anaesthetic through the uterine cavity and is then pertubated into the peritoneal cavity.
One trial was conducted in Sweden (Wickström 2013) with a number of associated published abstracts and 1 further full article are both associated with this particular trial (Edelstam 2012, Wickström 2012a, 2012b, 2012c). The local anaesthetic used in this trial was lidocaine. The second trial was conducted in Egypt, using the same procedure but with a different local anaesthetic bupivacaine (Shokeir 2015). In both trials the inclusion criteria included the requirement that endometriosis had been confirmed by laparoscopy.
Both trials reported pain as an outcome (as indicated on the visual analogue scale [VAS]). One of them also reported the rate of women who were overall satisfied with the procedure. The other trial also reported health-related quality of life as measured by the Endometriosis Health Profile-30 (EHP-30) as well as recurrence and need for other therapies. Fertility outcomes cannot be assessed because both studies excluded women who intended to become pregnant within the forthcoming year.
No further evidence was identified for any other type of neuromodulator or neuropathic analgesia.
Evidence for the outcomes from these trials is summarised in the clinical GRADE evidence profile below (Table 58). See also the study selection flow chart in Appendix F study exclusion list in Appendix H, forest plots in Appendix I, full GRADE profiles in Appendix J and study evidence tables in Appendix G. Summary of included studies
A brief summary of the studies that were included in this review is presented in Table 57.
11.1.2.3. Clinical evidence profile
The clinical evidence profile for this review question is presented in Table 58.
Other reported findings – EHP-30 (endometriosis-related quality of life)
Quality of life scores were reported as medians with interquartile ranges and therefore could not be graphically presented as forest plots. They are presented in Table 59 below.
11.1.2.4. Economic evidence
No economic evidence was found on the use of neuromodulators in women with endometriosis.
As no evidence was found on the use of neuromodulators in women with endometriosis, the effectiveness of these treatments was calculated from. Consequently, not all treatments listed in the protocol could be included in the economic model.
Table 61 demonstrates which neuromodulators might be selected as a cost-effective treatment on average. Both amitriptyline and gabapentin perform well relative to an incremental cost-utility ratio (ICER) of £20,000/QALY and are cheap enough that a diagnostic strategy of ‘empirical diagnosis’ – treating based on symptoms rather than a definitive diagnosis - can be pursued. However, this is only with reference to the class of neuromodulators; the main economic model indicates that neuromodulators are neither cheap enough to be considered in preference to hormonal treatment nor effective enough to be considered in preference to surgery. Given that there are some women who cannot tolerate hormonal therapy (usually because they are seeking a pregnancy, which is discussed below) these results might be important, as it is possible neuromodulators will be cost-effective in these women. This is relevant as, if a woman cannot have hormonal therapy but responds to neuromodulators, then it is unlikely surgery will be cost-effective for this woman.
It was thought that neuromodulators would not have a positive effect on women seeking to conceive and some neuromodulators would be harmful to a developing fetus. For these reasons, neuromodulators were not considered in an analysis of women where infertility was the main reason for their seeking treatment.
11.1.2.5. Clinical evidence statements
No evidence was identified that addressed the effectiveness of commonly used neuropathic analgesics.
11.1.2.5.1. Pertubation of lidocaine vs. placebo
Pain up to 12 months
Very low to low quality evidence from 1 randomised controlled trial (RCT) with 42 women with endometriosis suggested higher rates of women who reported a significant improvement in pain associated with pertubation of lidocaine compared to placebo at 3, 6, 9 and 12 months. However the uncertainty around this improvement was too large to draw clear conclusions about its clinical effectiveness.
EHP-30
Very low quality evidence from 1 RCT with 42 women with endometriosis reported no clear differences between women treated with lidocaine compared to placebo at 6 and 12 months for the subscales pain, control and powerlessness, emotional well-being, self-image and sexual intercourse. A small difference on the social support subscale was reported at 6 but not 12 months (Table 59).
Recurrence at 12 months
Very low quality evidence from 1 RCT (N=42) suggested a higher rate of recurrence in those receiving lidocaine compared to those in the placebo group. However, the uncertainty around this effect was too large to draw clear conclusions about this finding.
Escalating levels of pain with a need for other therapies at 12 months
Very low quality evidence from 1 RCT (N=42) suggested that there were fewer women needing other treatments in the lidocaine group compared to the control group. However, there was too much uncertainty around this effect to draw clear conclusions from these findings
11.1.2.5.2. Pertubation of bipuvacaine vs. Placebo
Pain up to 3 months
Moderate to high quality evidence from 1 randomised controlled trial (RCT) conducted with 60 women who have endometriosis reported improvements in pain at 1, 2 and 3 months associated with bipuvacaine pertubation. However, the uncertainty around this effect make it difficult to draw conclusions about the clinical significance of this finding.
Satisfaction with treatment at 3 months
High quality evidence from 1 RCT conducted with 60 women who have endometriosis showed a higher rate of satisfaction with bipuvacaine treatment compared to placebo.
11.1.2.6. Evidence to recommendations
11.1.2.6.1. Relative value placed on the outcomes considered
All reported outcomes (pain, endometriosis health profile, recurrence, satisfaction and need for further therapies) are critical for decision-making. However, the Committee did not place trust in the evidence for these outcomes since pertubation with local anaesthetic is not used in current practice.
11.1.2.6.2. Consideration of clinical benefits and harms
The Committee agreed that it was disappointing that there was no clinical evidence for the effectiveness of commonly used neuromodulators.
They recognised that there was much useful guidance in the NICE guidance Neuropathic pain in adults: pharmacological management in non-specialist settings (Clinical Guideline 96). The Committee discussed how this guidance could be useful for professionals looking to manage pain in certain settings as it was unlikely to interact with surgical or hormonal treatments, which would be the main alternative pharmacological management strategies. Therefore a neuromodulator for pain management in addition to first line treatment might help reduce pain further. The Committee was made aware that because of the well-established value of neuromodulators in pain management the evidence for these treatments for endometriosis specifically was almost entirely lacking and consequently an expert consensus was reached that there was no feature of endometriosis that would specifically indicate that neuromodulators would behave systematically differently in endometriosis than other long-term conditions, and therefore that the findings of CG173 would be appropriate to rely on. The Committee discussed the risks of extrapolating the CG79 guidance which focuses on neuropathic pain. Endometriosis could be considered to have similar pathophysiological processes via central sensitisation to neuropathic pain conditions but the CG79 guidance which may mean that it may be questionable whether it is directly translatable.
Even though the trials indicated that there might be benefits of the pertubation method for the administration of local anaesthesia, the Committee considered the invasive nature of this. They agreed that this is a procedure that is not currently used in the NHS and that the evidence is not convincing to warrant a change in practice. The Committee raised concerns that the discomfort and possible side effects from the intervention would outweigh the possible benefits.
The Committee was of the opinion that the nature of this treatment make it unlikely to be adopted because it would require repeated monthly administrations (to co-occur with the menstrual cycle).
11.1.2.6.3. Consideration of economic benefits and harms
Based on NICE guidance CG173, both amitriptyline and gabapentin perform well relative to an ICER of £20,000/QALY and are inexpensive enough that a diagnostic strategy of ‘empirical diagnosis’ – treating all those with symptoms of endometriosis without a confirmatory diagnostic test - can be pursued. However, this is only with reference to the class of neuromodulators. The Committee discussed comparative economic considerations indicating that neuromodulators are neither inexpensive enough to be considered in preference to hormonal treatment nor effective enough to be considered in preference to surgery. There are also some women who cannot tolerate or do not want to take hormonal therapy (usually because they are seeking to conceive, at which time neuromodulators would not be the appropriate option). In other cases where a woman cannot have hormonal therapy, does not consider pregnancy but responds to neuromodulators, then it is unlikely surgery will be cost-effective for this woman.
In the very specific case of a woman who cannot have hormonal therapy, is not considering pregnancy and yet responds to neuromodulators, then the economic model indicates that neuromodulators should be trialled as a first line treatment (before considering surgery). It is difficult to imagine the personal circumstances of such a woman, and so it may be that in most cases where neuromodulators are recommended by the economic model as a first line treatment that the economic model does not accurately capture these specific circumstances.
As the Committee is only recommending neuromodulators in line with the NICE Guideline on the topic, there will be no significant resource impact.
11.1.2.6.4. Quality of evidence
The evidence was of very low to moderate quality, according to GRADE criteria. Even though the methodology of the trials was well described, there were inconsistencies in the results reported (for instance, differences in results when pain was reported as a categorical or continuous measure). There were also a number of outcomes that were only reported as medians, for which it is difficult to estimate the confidence in the effect size.
The Committee therefore had little confidence in the findings of the trials.
11.1.2.6.5. Other considerations
The Committee noted that there is a substantial amount of evidence for nerve ablation, specifically in the form of Laparoscopic uterine nerve ablation (LUNA). However LUNA has been covered by a NICE Interventional Procedure Guideline (IPG234) and so was outside the scope of this Guideline. The IPG concluded that the evidence on laparoscopic uterine nerve ablation for chronic pelvic pain suggests that it is not efficacious and therefore should not be used.
11.1.2.6.6. Key conclusions
The Committee concluded that there was currently insufficient evidence for the effectiveness of neuromodulators in managing pain of women with endometriosis. Little confidence was placed in the evidence for a method of administering local anaesthetics, which is not currently used in the NHS and hence the Committee decided not to make any recommendation regarding this technique. They agreed that the recommendations set out in NICE guidance CG173 would be generalizable to women with endometriosis and therefore cross-referenced to this guidance.
11.1.2.7. Recommendations
- 35.
For recommendations on using neuromodulators to treat neuropathic pain, see the NICE guideline on neuropathic pain.
11.1.3. Hormonal medical treatments
Review question: What is the effectiveness of hormonal medical treatments for treating endometriosis compared to placebo, other hormonal medical treatments, usual care, surgery, or surgery in combination with hormonal treatment?
11.1.3.1. Introduction
Endometriosis is considered a predominantly oestrogen-dependent condition. Thus, ovarian suppression with hormones is currently offered as an alternative to surgical excision to treat the disease and its symptoms. However, clinical practice with regards to hormonal treatment varies widely, because of the implications of each option. None of the hormones used to manage endometriosis (or, in fact, any drug) are free of side effects, but the severity and tolerability of the side effects can vary quite significantly. Many of the hormones used to manage endometriosis-associated pain will also reduce menstrual bleeding and this may be advantageous. Similarly, the contraceptive properties of the hormones may be welcome if the woman does not wish to become pregnant at this moment in time, or unwanted if fertility is an issue. All these factors should be taken into consideration when prescribing hormones to women for the treatment of endometriosis. The effects of hormonal contraceptives, progestogens, anti-progestogens, gonadotrophin releasing hormone agonists (GnRH agonists) and aromatase inhibitors on endometriosis symptoms are discussed below.
The principal aim of this review is to determine the clinical and cost effectiveness of hormonal medical treatments in reducing pain in women with endometriosis.
For full details, see the review protocols in Appendix D.
11.1.3.2. Network Meta-analysis
11.1.3.2.1. Methods
The results of conventional pairwise comparison (and meta-analyses) of direct evidence alone do not help to fully inform which intervention is most effective in the treatment of endometriosis. The challenge of interpretation arises for 2 main reasons:
- In isolation, each pairwise comparison does not fully inform the choice between the different treatments and having a series of discrete pairwise comparisons can be disjointed and difficult to interpret.
- RCT evidence is not available that directly compares treatments of clinical interest are not fully available, for example, comparison between certain types of hormonal therapy. This makes choice difficult unless based on patient preference or cost.
To overcome these issues, a hierarchical Bayesian network meta-analysis (NMA) was performed in addition to a pairwise comparison of hormonal treatments. Advantages of performing this type of analysis are:
- It allows the synthesis of data from direct and indirect comparisons without breaking randomisation, to produce measures of treatment effect and ranking of different interventions. If treatment A has never been compared against treatment B head to head, but these 2 interventions have been compared to a common comparator directly, then an indirect treatment comparison can use the relative effects of the 2 treatments versus the common comparator. Indirect estimates can be calculated whenever there is a path linking 2 treatments through a set of common comparators. All the randomised evidence is considered simultaneously within the same model.
- For every intervention in a connected network, a relative effect estimate (with its 95% credible intervals) can be estimated versus any other intervention. These estimates provide a useful clinical summary of the results and facilitate the formation of recommendations based on all of the best available evidence, while appropriately accounting for uncertainty.
- Estimates from the NMA can be used to directly parameterise treatment effectiveness in cost-effectiveness modelling of multiple treatments.
The terms indirect treatment comparisons, mixed treatment comparisons and network meta-analysis are used interchangeably, though we use the term NMA throughout the guideline.
Study selection and data collection
For full details, see review and analysis protocols in Appendices K and L.
11.1.3.2.2. Outcome measures for NMA
For assessing the effectiveness of treatments, the Committee identified pain relief, health-related quality of life (QoL) and adverse events as critical outcomes for which NMA could be used to aid decision-making. NMAs were performed on these outcomes where evidence was available.
Pain relief
For pain relief, the visual analogue scale (VAS) was considered by the Committee to be the most widely used useful pain scale for which data would be available. A series of subscales first reported by Biberoglu and Behrman (1981) were also frequently used in studies of hormonal treatments and NMAs of these subscales were also performed to provide additional information on pain relief. There was sufficient evidence available for NMA for dysmenorrhoea, dyspareunia and non-menstrual pelvic pain subscales, though not for induration and pelvic tenderness subscales. Therefore induration and pelvic tenderness were analysed within a separate pairwise comparison analysis. Dysmenorrhoea and nonmenstrual pelvic pain were used in a multivariate analysis to inform the VAS scale, so their results are not presented separately here.
Health-related QoL
For health-related QoL, the Short Form 36 Health Survey (SF-36) was determined by the Committee to the most useful scale that was widely used in the literature. However, there were not a sufficient number of studies available from the systematic review to allow for NMA. Therefore these studies were analysed within the separate pairwise comparison analysis where appropriate.
Adverse events
As adverse events varied substantially depending on the treatment in question, the Committee felt that the number of women discontinuing treatments due to adverse events was a more generalizable and useful outcome, as this also accounted for how severe women felt an adverse event to be (i.e. it had to be sufficiently severe for them to discontinue treatment).
11.1.3.2.3. Statistical methodology
Due to difficulty in obtaining stable estimates from the model, NMAs were conducted separately for hormonal and non-pharmacological therapies, and for surgery and surgery plus hormonal treatment. The Committee felt that the difficulties in model estimation were likely to be because the populations may not have been sufficiently homogeneous, as patients receiving surgical treatment were likely to have failed on hormonal treatments, thus violating the assumption of transitivity.
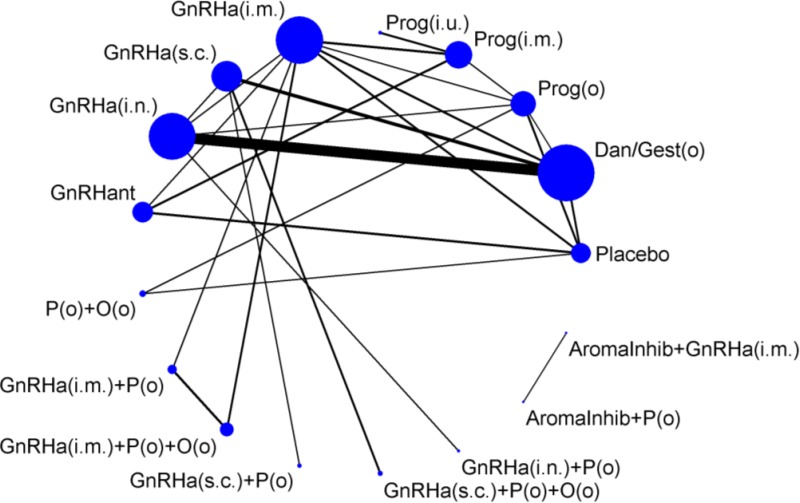
Data were available for a number of treatments and routes of administration. Due to the sparseness of the networks, it was necessary to group treatments within different classes and assume a common class effect (Table 62). The common class effects were assessed to identify if it was reasonable to assume similarity of treatment effects within classes. Though data were often too limited to be able to closely examine within-class variation there was no evidence to suggest that treatment effects differed substantially within classes. Multi-level NMA models with treatments nested within classes were also examined, though this added complexity did not improve model fit for any of the analyses. Therefore common class effects were assumed throughout the analyses.
There are 3 key assumptions behind an NMA: similarity, transitivity and consistency.
Similarity across trials is the critical rationale for the consistency assumption to be valid as, by ensuring the clinical characteristics of the trials are similar, we ensure consistency in the data analysis.
More specifically, randomisation holds only within individual trials, not across the trials. Therefore, if the trials differ in terms of patient characteristics, measurement and/or definition of outcome, length of follow-up across the direct comparisons, the similarity assumption is violated and this can bias the analysis. Potential sources of heterogeneity arising from trials of interventions for endometriosis and attempts made to identify and account for heterogeneity are:
- Different population: for example, mixed populations of women with and without endometrioma.
- Sensitivity analyses were performed to test the validity of the assumption of similarity of effect for treatments for women with and without endometrioma.
- Different duration of treatment or study follow-up:
- Although data were limited to reliably assess the effect of study duration, relative treatment effects appeared to be similar across studies of different duration that fitted the inclusion criteria specified in the analysis protocol.
- Sensitivity analyses were conducted to assess the impact of removing studies of short duration.
- Different dosages of pharmacological treatments:
- These typically showed little variation and were within the dose ranges specified by the British National Formulary (BNF).
Transitivity is the assumption that an intervention (A) will have the same efficacy in a study comparing A vs. B as it will in a study comparing A vs. C. Another way of looking at it, in terms of the study participants, is that we assume that it is equally likely that any patient in the network could have been given any of the treatments in the network and would have responded to the treatments in the same way (depending on how efficacious the treatments are).
This assumption is closely related to similarity in that if participants in a study comparing A vs. B are not the same as those in a study comparing A vs. C. For example, if those in a comparison of A vs. B were women seeking treatment to improve fertility and those in A vs. C were women whose primary concern was pain relief, then both the similarity and transitivity assumptions would be violated, hence the importance in our analysis of keeping these populations distinct.
The final assumption is consistency/coherence of the network. It is important that for a network that contains closed loops of treatments (e.g. with studies comparing A vs. B, B vs. C and A vs. C), the indirect comparisons are consistent with the direct comparisons. Discrepancies between direct and indirect estimates of effect may result from several possible causes. One possible cause is ‘chance’ and if this is the case then the NMA results are likely to be more precise as they pool together more data than conventional meta-analysis estimates alone. However, a second possible cause could be due to differences between the trials included in terms of their clinical or methodological characteristics, which would therefore raise concerns about the validity of the network.
There were no studies that fitted the NMA inclusion criteria for the following treatments in Table 62: anti-androgens, selective oestrogen receptor modulators, tibolone, nutritional supplements, Chinese herbal medicine, and dietary interventions. As no studies investigating non-pharmacological treatments fitted the inclusion criteria for the NMA, the analyses presented are only of hormonal treatments.
11.1.3.2.4. Summary of included studies
Studies included in the NMA
All studies included women with laparoscopic confirmation of endometriosis.
11.1.3.2.5. Studies excluded from the NMA
Table 64 lists the studies that were excluded from the NMA for statistical reasons.
11.1.3.2.6. Clinical evidence profile
Pain relief – VAS
Due to difficulty in achieving convergence during estimation, NMAs were conducted separately for hormonal and non-pharmacological therapies, and for surgery and surgery plus hormonal treatment. The Committee felt that this was likely to be because the populations may not have been sufficiently homogeneous, as patients receiving surgical treatment were likely to have failed on hormonal treatments, thus violating the assumption of transitivity.
Hormonal treatments
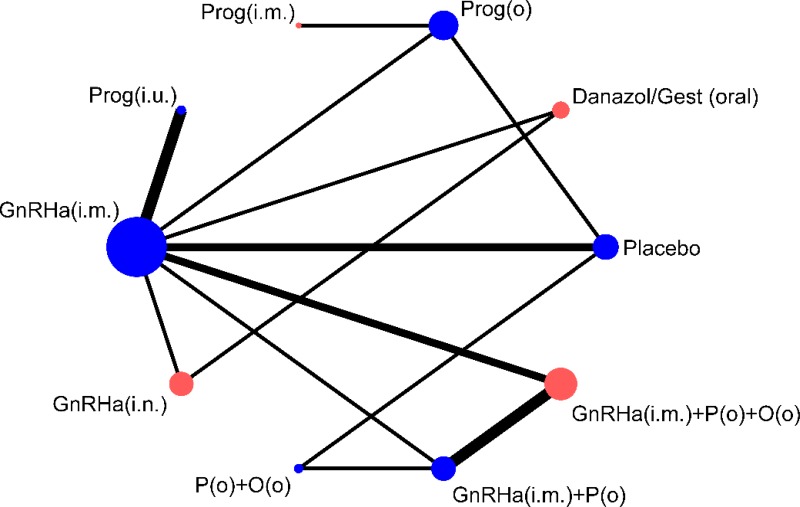
Fifteen trials of 10 hormonal treatment classes were included in the network for the outcome of pain relief on the VAS, with a total sample size of 1,680 women (Figure 13). No studies reported data on non-pharmacological treatment that could be used in the network. One study was at high risk of bias, 7 were at moderate risk of bias and 7 at low risk of bias.
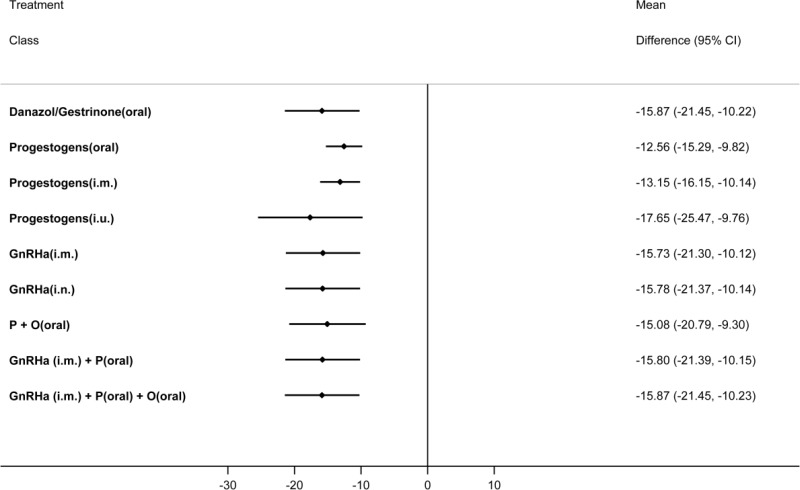
Table 65 presents the results of the pairwise meta-analyses of the VAS where they were available (direct comparisons; upper right section of table) together with the results from the multivariate NMA for every possible class comparison (lower left section of table), presented as mean differences. A multivariate NMA was performed as this allowed for the incorporation of additional information from dysmenorrhoea and non-menstrual pelvic pain Biberoglu and Behrman subscales, allowing estimation of the efficacy of treatments not investigated using the VAS (progestogens (i.m.), danazol/gestrinone, GnRHa (i.n.) and GnRHa (i.m.) plus the pill). The VAS is a 0–100 patient-reported scale, on which a difference of 10 points has been shown to be clinically significant to patients (Gerlinger 2012).
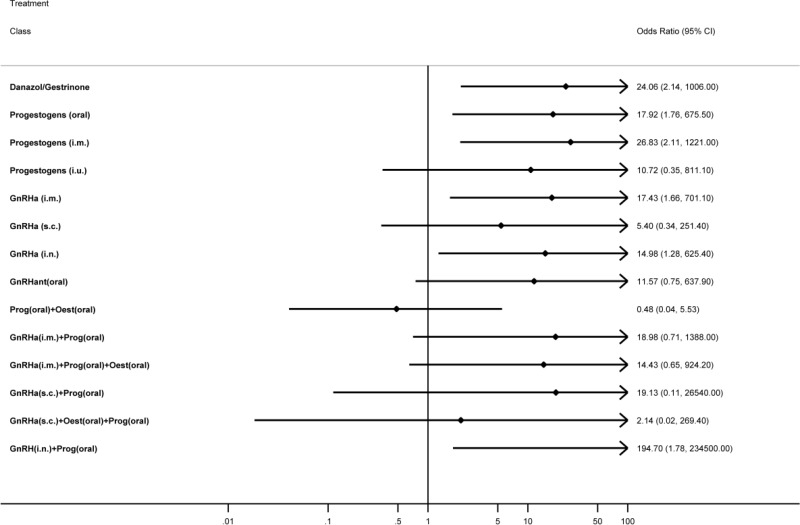
NMA results were derived from a fixed effects multivariate model. Figure 14 graphically presents the results computed by the NMA for each treatment versus placebo.
All treatments led to a clinically significant reduction in pain on the VAS when compared to placebo. The magnitude of this treatment effect was similar for all treatments, suggesting that there was little difference between them in their capacity to reduce pain. No other significant differences were found between the hormonal treatments.
The levornorgestrel implant (progestogens (i.u.)) had the highest probability of being among the best 3 treatments (74.2%), followed by danazol/gestrinone (52.6%) and GnRHa (i.m.) plus the pill (52.5%). The results of this are described in Table 66.
Results were broadly similar from the multivariate and univariate NMA where information was available for comparison. The largest differences were for the progestogens (i.u.) (considerably more effective in the multivariate than in the univariate NMA) and GnRHa (i.m) (less effective in the multivariate than in the univariate NMA) (Appendix L).
Sufficient data to calculate standard errors (SEs) was not available in 4 of the 15 trials. However, sensitivity analyses using the upper 95% CrI of the posterior for the imputed SEs showed that estimates and their 95% CrIs were very insensitive to the imputed SEs (Appendix L).
The multivariate nature of the network did not allow for simple assessment of incoherence, though it was assessed for each of the univariate outcomes and was not found to be present in any closed loops. However there were some differences between the direct estimates on the VAS scale and those from the NMA, particularly for progestogens (oral) versus GnRHa (i.m.). These differences are due to the multivariate analysis and the inclusion of evidence from the Biberoglu and Behrman scales and therefore reflect incoherence between the outcomes rather than between the treatment comparisons. Although this appears to change the direction of effect in some comparisons, the change is very small and not clinically meaningful.
Pain relief – dyspareunia (Biberoglu and Behrman)
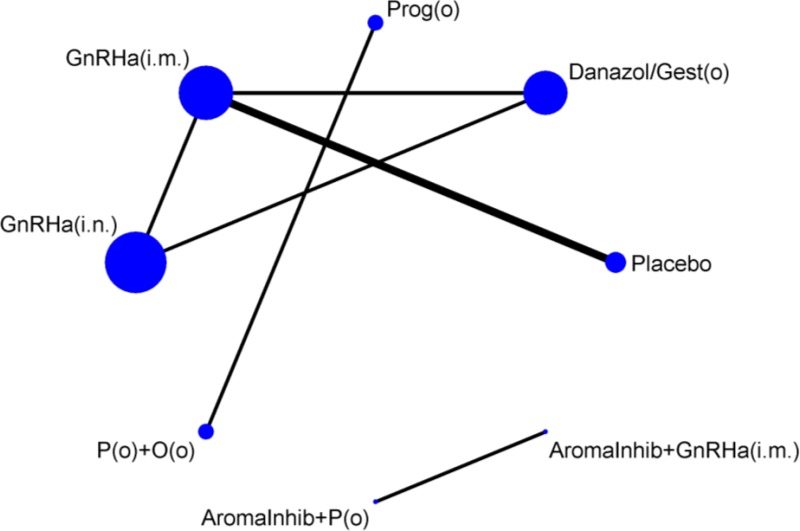
Five trials of 4 treatment classes were included in the network for the outcome of dyspareunia, with a total sample size of 572 women (Figure 15). One study was at high risk of bias, 2 were at moderate risk of bias and 2 were at low risk of bias.
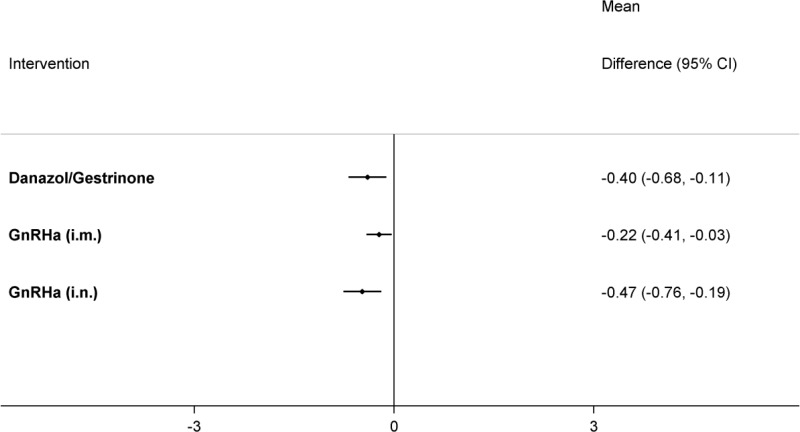
Table 67 presents the results of the conventional pairwise meta-analyses (direct comparisons; upper right section of table) together with the results from the NMA for every possible class comparison (lower left section of table), presented as mean differences. Dyspareunia was assessed using a 0–3 patient-reported scale developed by Biberoglu and Behrman (1981). NMA results were derived from a fixed effects model.
All treatments were significantly better at relieving dyspareunia than placebo/no treatment, although the improvement was quite small. GnRHa (i.n.) was also found to be significantly better at relieving dyspareunia than GnRHa (i.m.), which led to it having the highest probability of being the best treatment (85.1%), followed by danazol/gestrinone (14.3%) (see Table 68). Results from this NMA should be interpreted with caution, as sufficient data to calculate SEs was only available in 2 of the 5 trials. Sensitivity analyses using the upper 95% CrI of the posterior for the imputed SEs showed that the probability of being the best treatment results were sensitive to the imputed SEs. With larger SEs, there was more uncertainty regarding whether GnRHa (i.n.) or danazol/gestrinone were the better treatment (Appendix L).
There was no clear evidence of incoherence in the closed loop of GnRHa (i.m.), danazol/gestrinone and GnRHa (i.n.). However, there was very limited statistical power to test for this and, as the direction of effect differs between 2 of the direct and indirect estimates, results of this network should be treated with caution.
- GnRHa (i.m.) vs. danazol/gestrinone (p=0.123)
- direct MD=0.33 (95% CrI: 0.04 to 0.65)
- indirect MD=−0.01 (95% CrI: −0.33 to 0.31)
- GnRHa (i.n.) vs. danazol/gestrinone (p=0.115)
- direct MD=−0.12 (95% CrI: −0.27 to 0.03)
- indirect MD=0.22 (95% CrI: −0.17 to 0.62)
- GnRHa (i.n.) vs. GnRHa (i.m.) (p=0.115)
- direct MD=−0.11 (95% CrI: −0.38 to 0.17)
- indirect MD=−0.45 (95% CrI: −0.77 to −0.13)
Discontinuation of treatment due to adverse events
36 trials of 15 treatment classes were included in the network for the outcome of discontinuation of treatment due to adverse events, with a total sample size of 5,319 women (Figure 17). No studies that reported data on non-pharmacological treatments could be included in the network. Five studies were at high risk of bias, 21 studies were at moderate risk of bias and 10 studies were at low risk of bias.
Table 69 presents the results of the pairwise meta-analyses (direct comparisons; upper right section of table) together with the results from the NMA for every possible class comparison (lower left section of table), presented as odds ratios (ORs). These results were derived from a random effects model with very high heterogeneity (between-study SD: 0.94 (95% CrI: 0.45 to 1.69)). Accounting for severity of endometriosis (as measured by the rAFS) did not further explain the high heterogeneity.
Several treatment classes were found to result in significantly more discontinuations of treatment due to adverse events than placebo/no treatment (danazol/gestrinone, progestogens (oral), progestogens (i.m.), GnRHa (i.m.), GnRHa (i.n.) and GnRHa (i.n.) plus progestogen). The combined oral contraceptive pill (progestogen plus oestrogen (oral)), was found to lead to significantly less discontinuation than danazol/gestrinone, progestogen alone (oral), progestogen (i.m.), GnRHa (i.m.) and GnRHa (i.n.) plus progestogen. Figure 18 graphically presents the results computed by the NMA for each treatment versus placebo.
Though this outcome was taken where reported in studies as discontinuation due to adverse events, there may be some degree of reporting bias for this outcome - it is likely that women who are not finding the treatment effective or women who have difficulty with treatment compliance, may also be likely to discontinue treatment. For these women, even though they may cite adverse events as their reason for discontinuing treatment, treatment efficacy may play a part. Therefore this outcome is not independent of treatment efficacy. So because the combined oral contraceptive pill (progestogen plus oestrogen (oral)) was found to be effective, this may in part explain why it had the highest probability of being 1 of the best 3 treatments for discontinuation due to adverse events (87.8%). Placebo/no treatment had the next highest probability (82.13%) (Table 70).
The treatments with the highest probability of being 1 of the 3 worst for discontinuation were GnRHa (i.n.) plus progestogen (oral) (78.8%), progestogen (i.m.) (39.1%), GnRHa (s.c.) plus progestogen (38.8%).
There was strong evidence of serious incoherence in the closed loop of GnRHa (s.c.), danazol/gestrinone and GnRHa (i.n.). As the direction of effect differs between direct and indirect estimates, results of this network should be treated with caution. No significant incoherence was found in any other closed loops of treatments (Appendix L).
- GnRHa (s.c.) vs. danazol/gestrinone (p=0.005)
- direct OR = 0.10 (95% CrI: 0.03 to 0.25)
- indirect OR = 2.25 (95% CrI: 0.41 to 12.18)
- GnRHa (i.n.) vs. danazol/gestrinone (p=0.025)
- direct OR = 1.09 (95% CrI: 0.45 to 2.34)
- indirect OR = 0.15 (95% CrI: 0.05 to 0.59)
- GnRHa (i.n.) vs. GnRHa (s.c.) (p=0.04)
- direct OR = 0.42 (95% CrI: 0.09 to 1.88)
- indirect OR = 9.03 (95% CrI: 3.00 to 33.12).
11.1.3.3. Pairwise comparison
11.1.3.3.1. Description of clinical evidence
This pairwise comparison analysis accompanies the NMA that examined pain (VAS total scores and Biblioglu and Behrman criteria and) and withdrawal due to adverse events. The potential evidence for this analysis included RCTs identified from the searches performed on the basis of the protocol (see Appendix D) as well as RCTs that were considered for the NMA.
In total, 7 studies were included in this review. Three were Cochrane systematic reviews (Brown 2010, 2012 and Davis 2007) and 4 were RCTs (Harada 2008, Ling 1999, Parazzini 2000 and Schlaff 2006). 10 RCTs from the Brown 2010 (Agarwal 1997, Bergqvist 1998, Burry 1992, Cheng 2005, Fedele 1989, Fedele 1993, Fraser 1991, NEET 1992, Petta 2005, Wheeler 1992), 2 RCTs from the Brown 2012 (Bergqvist 2001, Vercellini 1996) and 1 RCT from the Davis 2007 (Vercellini 1993) Cochrane systematic reviews were relevant.
The population of interest was women with suspected or confirmed endometriosis of any stage or severity who did not receive surgery in conjunction with the hormonal medical treatments, although who may have had surgery prior to trial recruitment. Evidence was available for comparisons of hormonal treatments with placebo or no treatment (4 RCTs), for head to head hormonal treatment comparisons with placebo (6 RCTs) or without placebo (5 RCT) use in each treatment arm and for hormonal treatment combinations compared with a single hormonal treatment (2 RCTs).
The Committee specified critical outcomes of pain (for outcomes not included in the NMA), quality of life and unintended effects from treatment. However, many reports of unintended effects were identified (type, incidence and duration of side effects), preventing their meaningful inclusion in the pairwise analysis. Therefore these were addressed as ‘withdrawal from hormonal treatment due to adverse events’ in the NMA.
Hormonal treatments compared with placebo
Evidence was available from 3 studies that compared hormonal treatments with placebo or no treatment. One was a Cochrane systematic review (Brown 2010) and 2 were RCTs (Harada 2008 and Ling 1999). Two RCTs within the Cochrane systematic review were relevant (Bergqvist 1998, Fedele 1993).
All participants had a diagnosis or symptoms of endometriosis. One RCT examined a comparison of a GnRH agonist (buserelin intranasal (IN)) to expectant management in a population of women whose main symptom was infertility and who had undergone diagnostic laparoscopy combined with dilation and curettage (D&C) (Fedele 1993).
Two RCTs examined comparisons of GnRH agonists to placebo (triptorelin IM (intramuscular) depot and leuprolide IM depot) (Bergqvist 1998 and Ling 1999, respectively). One RCT compared a combined oral contraceptive pill to placebo (Harada 2008).
Evidence was only available for the critical outcome of pain (outcomes not included in the NMAs). There was no evidence available for any other critical or important outcomes.
Hormonal treatment compared with another hormonal treatment
Evidence was available from 2 studies comparing a hormonal treatment to another hormonal treatment. One was a Cochrane systematic review (Brown 2010) and one was a RCT (Schlaff 2006). Four RCTs within the Cochrane systematic review were relevant (Burry 1992, Cheng 2005, Fedele 1989, Petta 2005).
Three RCTs examined a comparison of a GnRH agonist (nafarelin IN or buserelin IN) to danazol (Burry 1992, Cheng 2005, Fedele 1989). One RCT compared leuprolide IM to a levonorgestrel-releasing intrauterine system (LNG-IUS) to (Petta 2010) and 1 RCT compared leuprolide to depot medroxyprogesterone acetate (DMPA) subcutaneous (SC) injections (Schlaff 2006). All participants had laparoscopically confirmed endometriosis. One trial (Fedele 1989) included infertile women only.
Evidence was available for the critical outcomes of pain (outcomes not included in the NMA) and quality of life, and for the important outcomes of patients requiring surgery because of reappearance of symptoms and the effect on daily activities. There was no evidence available for any other important outcomes.
Hormonal treatment with placebo compared with another hormonal treatment with placebo
Evidence was available from 2 Cochrane systematic reviews (Brown 2010; Brown 2012) comparing a GnRH agonist to another hormonal treatment with use of placebos in each trial arm to blind for route of administration. Five RCTs were relevant in total: 4 RCTs from the Brown 2010 Cochrane systematic review (Agarwal 1997, Fraser 1991, NEET 1992, Wheeler 1992); and 1 RCT from the Brown 2012 Cochrane systematic review (Bergqvist 2001).
Four trials examined intranasal nafarelin (Agarwal 1997, Bergqvist 2001, Fraser 1991, NEET 1992) and 1 trial examined the use of depot leuprolide (Wheeler 1992).
One RCT examined a comparison of nafarelin IN and placebo IM injections to leuprolide acetate depot intramuscular (IM) injections and placebo IN (Agarwal 1997). One RCT compared nafarelin IN plus placebo tablets twice daily to MPA tablets and placebo IN (Bergqvist 2001).
Three trials compared the use of a GnRH agonist to danazol (Fraser 1991, NEET 1992, Wheeler 1992). Two trials compared the use of nafarelin IN to danazol with placebo in both treatment arms (Fraser 1991, NEET 1992). The first RCT compared of nafarelin IN and oral placebo to oral danazol and placebo IN over 6 months (Fraser 1991). The second RCT compared nafarelin IN and oral placebo capsules to oral danazol capsules and IN placebo (NEET 1992).
The final RCT compared a form of leuprolide depot injections and oral placebo to danazol and placebo IM injections (Wheeler 1992).
Evidence was available for the critical outcomes of pain relief (those outcomes not included in the NMA) and quality of life and for the important outcome of effects on daily activities. There was no evidence available for any important outcomes.
Hormonal treatment compared with combined oral contraceptive pill
Three studies comparing hormonal treatment to combined oral contraceptive pill (cOCP) were included in this review. Evidence was available from 2 Cochrane systematic reviews (Davis 2007, Brown 2012) and 1 RCT (Parazzini 2000). One RCT within each Cochrane systematic review was relevant (Vercellini 1993 and 1996, respectively).
All participants had laparoscopically confirmed endometriosis. One RCT examined a comparison of a GnRH agonist (triptorelin slow release for 4 months) followed by treatment with gestodene and ethinylestradiol (E/P pill) to E/P pill alone (Parazzini 2000). One RCT compared a GnRH agonist (goserelin subcutaneous depot) to cOCP (ethinylestradiol and desogestrel) (Vercellini 1993) and 1 RCT compared depot medroxyprogesterone acetate to cOCP (ethinylestradiol and desogestrel) plus danazol (Vercellini 2012). In 1 study (Parazzini 2000) additional treatment for relief of pain with naproxen sodium as first-line treatment was allowed.
Evidence was available for the critical outcome of pain (outcomes not included in the NMA) and for an important outcome of patient satisfaction. There was no evidence available for any other critical or important outcomes.
Studies are summarised in the tables below Table 71 and the available evidence is presented by comparison in the clinical GRADE evidence profiles below (Table 72 to Table 85). See also the study selection flow chart in Appendix F, study exclusion list in Appendix H, forest plots in Appendix I, full GRADE profiles in Appendix J and study evidence tables in Appendix G. Summary of included studies
A summary of the studies that were included in this review are presented in Table 71.
11.1.3.3.2. Clinical evidence profile
The clinical evidence profiles for this review question are presented in Table 72 to Table 85.
11.1.3.3.3. Economic evidence
Cost effectiveness papers
Three studies were identified concerned with the cost-effectiveness of hormonal therapy in the treatment of endometriosis.
Lukac (2011a)
This paper refers to an analysis of the Slovakian AU19 trial on endometriosis-associated pelvic pain. It compares dienogest with Gonadotrophin Releasing Hormone agonists (GnRHa) over a period of 2 years. The source for costing data are “published price lists, clinical guidelines, product labels and expert opinion” and the source for QALY data is the SF-36 QoL instrument. The paper describes a Markov Chain model with a discount rate of 5% although it reports some data on the direct costs of these treatments with and without diagnostic laparoscopy.
The paper finds dienogest saves €506 and gains 0.002 QALYs relative to GnRHas. This indicates dienogest dominates GnRHa and would be considered cost-effective in any system. The authors include a cost-effectiveness acceptability curve (CEAC) implying that dienogest is cost-effective at a threshold of €18,000 / QALY (the Slovakian threshold, equivalent to around £15,000 / QALY) in 69% of cases
Lukac (2011b)
This paper appears to be a re-analysis of Lukac (2011a) as it refers to the same AU19 trial and finds similar results. The difference appears to be that this paper looks at a 5-year time horizon whereas the first paper looks at a 2-year time horizon. This paper finds a cost saving of €426 and a QALY gain of 0.069 QALYs, again indicating dienogest dominates GnRHas.
Bodner, Vale, Ratcliffe & Farrar (1996)
This paper refers to a subpopulation of 60 women with infertility taken from a full cohort of 273 enrolled in the Gynaecology Audit Project in Scotland (GAPS). It was intended principally to demonstrate a methodological point around using medical audit data to underpin economic evaluation, but was still considered relevant to include in this review as part of the audit data considered were costs and health outcomes. 35 women were treated with ‘expectant management’, 21 treated medically and 2 treated surgically (the remaining 2 women were on a surgical waiting list – it is not clear why these women were not included in the expectant management group).
The main outcome measure considered was fertility rates, but participants also completed an SF-36 QoL questionnaire. The source of cost data was NHS Reference Costs and estimates obtained by interviews with clinical managers. The time horizon was 6 months and the discount rate 6%.
The cost per patient alternative were £387.29 for expectant management, £645.02 for medical management and £1594.06 for surgical management. The SF-36 general health scores (and SDs) were an improvement of 61.0 (21.1) to 61.4 (29.9) in the medical group and a deterioration of 76.4 (18.2) to 75.3 (22.0) in the expectant management group. There were not enough women in the surgical group to report accurate scores. Neither of these changes would be considered statistically significant by any reasonable criteria, but – if they were significant – would represent an ICER of £17,200 indicating medical management is likely to be cost-effective compared to no treatment at the standard threshold of £20,000 / QALY – although it should be cautioned that the short follow up means that the effect of the (contraceptive) hormonal medical management on long-term QALYs may not have been properly accounted for.
Only 2 of the 60 women became pregnant by the end of the study, which is consistent with a view where endometriosis is highly damaging to fertility but does not give much analysable information about the cost-effectiveness of strategies to treat endometriosis-related infertility.
Cost only papers
Additionally, 5 studies were identified looking only at the costs of hormonal therapy. Since none of these papers were based on a UK perspective it was thought that conventional NHS costing sources were likely to be more relevant and so the Committee did not weight their evidence strongly in making a final recommendation, but Table 86 gives a high-level summary of the relevant information.
11.1.3.3.4. Economic model output
The cost of hormonal treatments can vary greatly depending on the dose required to achieve amenorrhea, the route of administration and any issues relating to unwanted side effects (perhaps the most important of which is infertility). Nevertheless it is known that there are a cluster of extremely cheap hormonal treatments (including the combined oral contraceptive pill) and a cluster of extremely high-cost treatments including dienogest and GnRHas.
Owing to a lack of evidence on a number of these treatments, only 4 were included for analysis in the final model as other treatments were not suitable for inclusion in the NMA.
Note that there is a significant issue with the costing of the 2 more routine contraceptives, which is that some women take these contraceptives purely to prevent pregnancy. This means that the opportunity cost of the NHS prescribing these drugs to these women is zero, which is a consideration the Committee made when discussing whether there was a case to recommend the more expensive hormonal treatments.
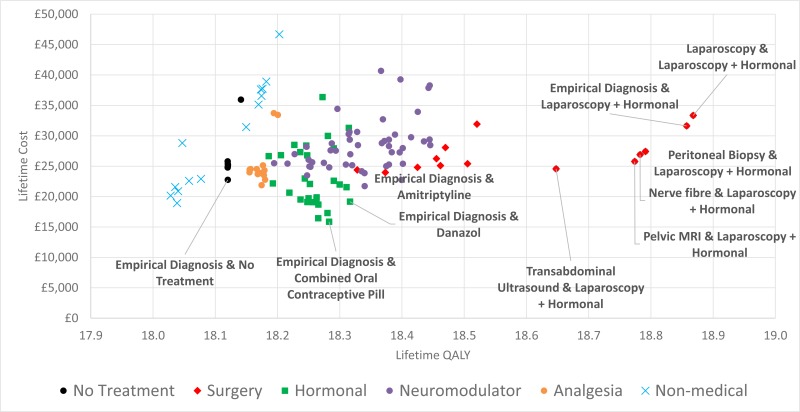
Hormonal treatments are both highly cost-effective on average and highly likely to be cost-effective vs. no treatment for any individual patient. This effect explains why Empirical Diagnosis & Danazol can have such a high ICER (£98,467) but also such a high probability of being cost-effective relative to no treatment. Another important point is how little difference there is between the combined oral contraceptive pill and Progestogen treatment – Progestogen treatment is fractionally cheaper based on the economic evidence and fractionally less effective based on the NMA, but patient-level analysis suggests that at £20,000 / QALY around 45% - 50% of patients offered the one treatment would actually have done better if offered the other. This indicates that the type of contraceptive might not be as important as the model implies as there is so little difference between them. This does not apply to GnRHas and Danazol, which are notably more expensive and only cost-effective at cost/QALY thresholds around one hundred thousand pounds (GnRHas are dominated by Danazol in this model, but if Danazol is removed the ICER for the most cost-effective GnRHa is £173,760).
The Committee discussed how this was entirely expected; hormonal treatments are known to be effective for endometriosis and known to be cheap and safe to prescribe, with few side-effects. The Committee also discussed how empirical diagnosis followed by hormonal treatment was extremely likely to be the most cost-effective strategy; the cheaper hormonal treatments are so cheap that even if the number of women presenting with endometriosis was small (and even if hormonal treatments had no effect on superficially similar conditions like dysmenorrhoea) that the cost of prescribing these drugs to otherwise healthy women was negligible.
It was expected that hormonal treatments are harmful for fertility. In actual fact the NMA suggested that progestogen treatment might improve fertility, but this is thought to be an inconsistency with the evidence underpinning the NMA and not reflective of the actual effects of progestogen treatment on fertility. As a result of this, no analysis has been conducted on the best hormonal treatment for preserving fertility.
However, in women who have both pain and infertility as a symptom of endometriosis, the effectiveness of hormonal treatment at controlling pain coupled with its low cost meant hormonal treatment was preferred at ICERs less than £13,027 / QALY, where it is replaced with surgical treatment with adjunct hormonal therapy.
11.1.3.3.5. Clinical evidence statements
Comparison 1: GnRH agonist versus no treatment
Pain
Very low quality evidence from 1 trial (n=35) found a clinically significant beneficial effect of GnRH agonist treatment (buserelin IN) compared with expectant management for dysmenorrhoea relief (measured using VAS) at 12 weeks after starting treatment.
Comparison 2: GnRH agonist versus placebo
Dysmenorrhoea
Moderate quality evidence from 1 trial (n=88) demonstrated a clinically significant beneficial effect of GnRH agonist treatment (leuprorelin IM depot) compared with placebo in the reduction of dysmenorrhoea (measured using VAS) at 12 weeks after starting treatment.
Pelvic pain
Moderate quality evidence from 1 trial (n=88) demonstrated a clinically significant beneficial effect of GnRH agonist treatment (leuprorelin IM depot) compared with placebo in the reduction of pelvic pain (measured using VAS) at 12 weeks after starting treatment.
Moderate quality evidence from 1 trial (n=46) found a clinically significant beneficial effect of GnRH agonist treatment (triptorelin IM depot) compared with placebo in the cessation of pelvic tenderness at 6 months after starting treatment.
Dyspareunia
Moderate quality evidence from 1 trial (n=88) demonstrated a clinically significant beneficial effect of GnRH agonist treatment (leuprorelin IM depot) compared with placebo in the reduction of deep dyspareunia (measured using VAS) at 12 weeks after starting treatment.
Very low quality evidence from 1 trial (n=46) found a clinically significant difference between GnRH agonist treatment (triptorelin IM depot) and placebo in the cessation of pelvic tenderness at 6 months after starting treatment.
Comparison 3: Combined oral contraceptive pill versus placebo
Pain
Low and moderate quality evidence from 1 trial (n=96) found a clinically significant beneficial effect of treatment with a combined oral contraceptive compared with placebo for dysmenorrhoea (measured using VAS), but no clinically significant difference between treatments for non-menstrual pelvic pain score (measured using VAS) or induration.
Comparison 4: GnRH agonist versus danazol
Pain
Moderate quality evidence from 1 RCT (n=59) found no clinically significant difference between GnRH agonist treatment (nafarelin IN) compared with danazol for pelvic tenderness and pelvic induration at 3 months (during treatment period) and at the end of the 6 month treatment period.
Patient requiring surgery because of reappearance of symptoms and positive findings at pelvic examination
Moderate quality evidence from 1 RCT (n=62) reported no clinically significant difference between GnRH agonist treatment (buserelin IN) and danazol in the number of patients requiring surgery because of reappearance of symptoms and positive findings at pelvic examination at follow-up at least 12 months after treatment ended.
Quality of life
Low quality evidence from 1 RCT (n=169) found no statistically significant difference in quality of life (PGWBI and modified Nottingham Health Profile) between GnRH agonist (nafarelin IN) and danazol at the end of the 6 month treatment period. Clinical significance was not calculable as the data reported in the paper were descriptive.
Comparison 5: GnRH agonist versus levonorgestrel-releasing intrauterine system
Quality of life
Moderate quality evidence from 1 RCT (n=83) reported no clinically significant difference between GnRH agonist treatment (leuprolide IM) and levonorgestrel-releasing intrauterine system in quality of life (PGWBI) at the end of the 6 month treatment period.
Comparison 6: GnRH agonist versus DMPA-SC
Effect on daily activities
High to moderate quality evidence from 1 RCT (n=274) found no clinically significant difference between GnRH agonist treatment (leuprolide IM) and depot MPA (given by SC injection) regarding the mean number of hours of productivity lost at employment and housework at the end of the 6 month treatment period and at 18 months (12 months post-treatment).
Comparison 7: GnRH agonist 1 + placebo versus GnRH agonist 2 + placebo
Pain
Low quality evidence from 1 RCT (n=192) found no clinical significant differences between GnRH agonist treatments (nafarelin 200mcg twice per day (BDS) IN and IM placebo compared with leuprolide depot 3.75mg IM plus IN placebo) for pelvic tenderness and pelvic induration at 6 months after the end of the treatment period.
Comparison 8: GnRH agonist + placebo versus progestin + placebo
Quality of life
Very low quality evidence from 1 RCT (n=48) reported no clinical significant differences between treatment with a GnRH agonist (nafarelin 200 µg IN BDS) and oral placebo compared with oral medroxyprogesterone (BDS 15 mg) and IN placebo in terms of overall quality of life (measured using Goldberg’s general health and Nottingham Health Profile Questionnaire) at 6 months after the end of the treatment period. Results were poorly reported.
Effect on daily activities
Very low quality evidence from 1 trial (n=48) reported no clinical significant differences between treatment with a GnRH agonist (nafarelin 200 µg IN BDS) and oral placebo compared with oral medroxyprogesterone (BDS 15 mg) and IN placebo in terms of the effects on daily activities (measured using the Coping wheel, Inventory of Social Support and Interaction – ISSI and demands, control and support questionnaires) including sleep disturbances, anxiety-depression, household work, vacation life and leisure, sexual life, motivation, emotional balance and work activities (including psychological work demands, intellectual discretion at work, authority over decisions at work and social support) at 6 months after the end of the treatment period. Results were poorly reported.
Comparison 9: GnRH agonist + placebo versus danazol + placebo
Pain
Very low quality evidence from 1 RCT (n=49) found no clinically significant difference between GnRH agonist treatment (nafarelin 200mcg BDS -400mcg/d- IN) and oral placebo compared with oral danazol (200mg 3 times per day (TDS)) plus IN placebo for pelvic tenderness and pelvic induration at 6 months after the end of the treatment period.
Very low quality evidence from 1 RCT (n=96) found no clinically significant differences between GnRH agonist treatment (nafarelin 200mcg BDS -400mcg/d- IN) and oral placebo compared with danazol (200mg TDS) plus IN placebo for pelvic tenderness and pelvic induration at 12 months after the end of the treatment period.
Low quality evidence from 1 RCT (n=253) found no clinically significant difference between GnRH agonist treatment (leuprolide 3.75mg monthly IM) and oral placebo compared with oral danazol (800mg once daily) plus IM placebo for pelvic tenderness at 6 months after the end of the treatment period.
Comparison 10: Depot medroxyprogesterone acetate versus cOCP + danazol
Pain
Moderate quality evidence from 1 RCT (n=80) found a clinically significant beneficial effect of depot medroxyprogesterone acetate treatment compared with cOCP plus danazol for dysmenorrhoea at 6 months after starting treatment and at the end of the treatment period (at 12 months). Very low- to low-quality evidence from the same study reported no clinically significant difference between the 2 intervention groups for dyspareunia and non-menstrual pelvic pain at 6 months after starting treatment and at the end of the treatment period (at 12 months).
Patient satisfaction
Low quality evidence from the same RCT (n=80) reported no clinically significant difference between depot medroxyprogesterone acetate treatment compared with cOCP plus danazol regarding patient satisfaction with treatment (very satisfied/satisfied) at the end of the treatment period (at 12 months).
Comparison 11: GnRH agonist (triptorelin) + E/P pill versus E/P pill
Pain
One RCT (n=102) reported a clinically significant beneficial effect of GnRH agonist (triptorelin) + E/P pill (gestodene 0.75 mg/ethinylestradiol 0.03 mg) treatment compared with E/P pill (gestodene 0.75 mg/ethinylestradiol 0.03 mg) alone for dysmenorrhoea and nonmenstrual pelvic pain at 8 months during the treatment period and for dysmenorrhoea at the end of the treatment period (at 12 months). Evidence was of low to moderate quality.
Low quality evidence from the same study found no clinically significant beneficial effect of E/P pill (gestodene 0.75 mg/ethinylestradiol 0.03 mg) compared with GnRH agonist (triptorelin) + E/P pill (gestodene 0.75 mg/ethinylestradiol 0.03 mg) treatment for nonmenstrual pelvic pain at the end of treatment period (at 12 months).
Comparison 12: GnRH agonist (goserelin) versus cOCP
Pain
Low quality evidence from 1 RCT (n=57) demonstrated a clinically significant beneficial effect of GnRH agonist (goserelin) treatment compared with cOCP (0.02 mg ethinylestradiol and 0.15 mg desogestrel) for dyspareunia at the end of the treatment period (at 6 months). The same study reported no clinically significant difference between the 2 study arms for nonmenstrual pelvic pain and dysmenorrhoea at the end of the treatment period (at 6 months) and for dyspareunia, non-menstrual pelvic pain and dysmenorrhoea at 6 months after the treatment period. Evidence was of very low to low quality.
11.1.3.4. Evidence to recommendations
11.1.3.4.1. Relative value placed on the outcomes considered
As pain relief is the primary reason for patients seeking treatment, this was the most critical outcome for the NMA, pairwise meta-analysis and pairwise comparison within this review. Health-related quality of life was also critical as this might be considered to give a more broad reflection of patient experience than pain relief alone, but data were only available for the pairwise comparison. Withdrawal due to adverse events and adherence to treatment were also critical outcomes as these reflected specific issues relating to the use of certain treatments and were addressed within the NMA and pairwise meta-analysis.
Rate of success, satisfaction with treatment, effect on daily activities and reduction in size and extent of endometriotic cysts were considered important outcomes as they were less clear indicators of effectiveness and were addressed within the pairwise comparison.
11.1.3.4.2. Consideration of clinical benefits and harms
The evidence from the NMA supported the use of hormonal treatments for pain relief in women with endometriosis and evidence from the pairwise comparison was broadly consistent with this, therefore the Committee used the NMA for most decision-making. The Committee agreed with the evidence and further highlighted that the benefit from hormonal treatments was due to their efficacy in stopping or reducing periods. There was a desire from the Committee to reduce the number of repeated operations for women with endometriosis, further supporting maintenance of pain relief using hormonal treatments wherever possible.
Although they chose not to be specific about recommending a particular hormonal treatment in the recommendations, they stated that the first-line hormonal treatment would generally be the oral combined contraceptive pill or progestogens as they have good efficacy and typically have side effects that women may find more tolerable. The evidence showed that cyclic use of the combined oral contraceptive pill is effective, but the Committee were also aware that continuous and tricycling (where three packets are taken in a row, followed by a pill free interval) use of the pill are used in clinical practice, and although evidence was not available on these regimens in the literature, the Committee have found in their experience that these were also effective with limited adverse events.
The Committee recommended that if first-line hormonal treatment was contraindicated or not tolerated, then women should be referred to a gynaecologist for possible further treatment which could include other hormonal treatments (for example, with a GnRH-a) or surgery. The Committee discussed the results of clinical effectiveness of other hormonal treatments such as GnRH-as and danazol. Even though highly effective, use of GnRH-as requires guidance from a specialist as the NMA showed that they had higher risk of withdrawal due to adverse events and the Committee identified them as having more serious adverse events (e.g. bone density changes). The Committee noted that GnRH-as are only licensed for a 6 month period and therefore require special considerations to ensure that women do not stay on this treatment indefinitely. They also discussed that to negate their adverse events add-back therapy using oestrogens, progestogens or both would usually be prescribed as well. The Committee’s view was that women found the androgenic adverse events related to other hormonal treatments such as danazol in particular to be very unpleasant (e.g. voice alteration, hair growth). The Committee therefore decided not to be prescriptive about which treatment path to follow when first line treatment is not effective, not tolerated or is contraindicated and that clinical judgement was required to weigh up the benefits and harms of options that could be used.
Throughout the care pathway, the Committee stressed the importance of a full discussion with women of their symptoms and priorities with respect to pain and fertility and of the importance of the woman’s choice. Such a discussion should also relieve any concerns over future fertility with regards to taking hormonal treatments, as their use was not considered to have any detrimental effect on subsequent fertility.
Adverse events were very varied across different types of hormonal treatments (androgenic, etc.) but were consistent within the classes of hormonal treatments. Overall the Committee highlighted that potential adverse events should be discussed with women alongside the potential benefit for pain relief.
There was no evidence to recommend whether to use or not use aromatase inhibitors, selective oestrogen receptor modulators (SERMs) or selective progestogen receptor modulators (SPRMs).
11.1.3.4.3. Consideration of economic benefits and harms
The Committee agreed with the output of the health economic model that hormonal treatment was likely to be the most cost-effective first-line treatment for endometriosis. Hormonal treatments are so effective that they can be prescribed without any confirmatory testing, although the Committee discussed how such testing might be useful anyway for reasons unrelated to symptom control (for example, to ensure that the lesions were not adhering to the bowel wall). There was some discussion about whether the more expensive classes of hormonal treatment (for example, GnRHas) were likely to give better results than the simple oral contraceptive, but health economic modelling demonstrates the gain would have to be far in excess of the uncertainty intervals of the NMA model in order for the treatments to be cost-effective.
The Committee discussed how the certainty of the finding of the model was not sufficient to recommend the combined pill over progestogen treatment, although the contraceptive pill generated slightly more QALYs on average; the Committee decided it was best to offer whichever cheap oral hormonal contraceptive the woman preferred, especially with reference to any treatment she might currently be taking.
The Committee believed that the result from the model indicating that progestogen treatment was likely to improve fertility was an artefact. In general, the Committee argued that as hormonal treatments have no plausible biological pathway to improving fertility they should not be recommended to women seeking to conceive on health economic grounds.
As many women will already be taking hormonal contraception for reasons unrelated to their endometriosis, it is difficult to estimate precisely the resource impact of these recommendations. Although the contraception itself carries a small cost, it is expected to displace unnecessary prescriptions of expensive treatments such as GnRHas and therefore the overall effect is of uncertain direction. Assuming the most expensive scenario for the NHS (all women with symptomatic endometriosis are prescribed hormonal treatment they would not otherwise have been taking) the total cost to the NHS is fractionally above the NHS threshold for high resource impact, so it is assumed with the fact that there is a preexisting base of women taking the treatment that the net resource impact is not high.
11.1.3.4.4. Quality of the evidence
The quality of the evidence used to make recommendations on hormonal treatments for pain relief was generally moderate and was drawn from the NMA. Although the majority of studies were appropriately blinded, they rarely reported appropriate allocation concealment or details of the randomisation procedure. Several did not report measures of variability or uncertainty in their estimates, which meant that statistical imputation of missing information was needed. However, a variety of sensitivity of analyses were performed to test assumptions made during modelling and the results seemed robust. Studies were relatively consistent in their inclusion and exclusion criteria, which led to low inconsistency within the evidence.
However, the quality of the evidence was poorer when making recommendations on potential adverse events. Withdrawal from studies due to adverse events was relatively rare, giving very low precision to the analyses, and for the NMA some of the direct and indirect evidence did not agree, raising concerns as to the validity of this network and its use in decision-making.
11.1.3.4.5. Other considerations
One of the key considerations throughout treatment for pain relief in endometriosis is women’s fertility. Fertility may be a strongly influencing factor in many women’s treatment choices and a timely discussion on how different treatments will impact this is essential. The Committee suggested that a particular point to highlight in such a discussion is that although there can be a delay in return to fertility after stopping treatment with hormones (which might be a particular consideration for perimenopausal women), spontaneous pregnancy rates are not affected..
The different treatment options recommended here are based on RCT evidence from a number of different studies, which was in agreement with the experience of the Committee. Recommendations on information provision and the pathway of care were developed primarily from Committee experience and opinion, supported in part by the literature.
11.1.3.4.6. Key conclusions
The Committee concluded that women should be offered the oral combined contraceptive pill or progestogens as the first-line treatment for pain relief. However, if these were contraindicated or if women did not tolerate them, or found the treatments to be ineffective, they should be referred to a gynaecologist to discuss the alternative management options of hormonal treatment or laparoscopy. Throughout the process, the Committee stressed the importance of the woman’s choice and of fully informing them about their options.
11.1.3.5. Recommendations
- 36.
Explain to women with suspected or confirmed endometriosis that hormonal treatment for endometriosis can reduce pain and has no permanent negative effect on subsequent fertility.
- 37.
Offer hormonal treatment (for example, the combined oral contraceptive pill or a progestogen)a to women with suspected, confirmed or recurrent endometriosis.
- 38.
If initial hormonal treatment for endometriosis is not effective, not tolerated or is contraindicated, refer the woman to gynaecology service, specialist endometriosis service (endometriosis centres) or paediatric and adolescent gynaecology service for investigation and treatment options.
11.2. Non-pharmacological management
Review question: What is the effectiveness of non-pharmacological therapies (for example, acupuncture) for managing pain associated with endometriosis?
11.2.1. Introduction
The symptoms associated with endometriosis differ with each woman; however, pain is almost always a factor, whether it be pelvic pain, painful periods, pain on intercourse, pain on urination or on defecation.
The level of pain experienced does not always relate to the extent of the disease and minor disease can be as or more painful than severe disease. It is often related to the location of the disease.
For many women treatment will involve a combination of therapies given over their lifetime depending on their circumstances at any given time. The aim of any management is primarily to reduce symptoms and maintain or improve quality of life.
There are many reasons why women may choose to use non-pharmacological therapies, for example, being offered counselling or acupuncture as alternatives or adjuncts to medical and surgical management.
In addition to reduction in pain, these therapies may be chosen to enable the woman to feel she is taking an active role in the treatment of her symptoms. Women who use self-management strategies may report regaining control over their lives and feel less dependent on healthcare professionals.
Some women have exhausted all possible hormonal and medical treatments and have discontinued these due to intolerable side effects or found them to be ineffective and are keen to seek further alternative or additional solutions for their pain. They may report reduction in medication use and potentially therefore in side effects.
Women who are trying to conceive may decide to postpone treatment for a certain time period with the hope of a resulting pregnancy. While trying to become pregnant she may still be experiencing painful symptoms but would be unable to use medical treatments during this time as most can be harmful to the developing fetus. Women often give up trying to conceive as their pain is intolerable therefore non-pharmacological therapies that have been shown to be safe for use in early pregnancy may be chosen to help them continue in their desire for a pregnancy.
The aim of this review is to determine the clinical and cost effectiveness of non-pharmacological therapies in reducing pain in women with endometriosis or suspected endometriosis
For full details, see the review protocol in Appendix D, the study selection flow chart in Appendix F, study exclusion list in Appendix H, forest plots in Appendix I, full GRADE profiles in Appendix J and study evidence tables in Appendix G.
11.2.2. Description of clinical evidence
Ten studies were included in the evidence review (Chen 2012, de Sousa 2016, Flower 2011, Mira 2012, Sesti 2009, Wayne 2008; Wu 2006 (Flower 2012); Xia 2006; Xiang 2002; Zhu 2014). Nine of these were RCTs and the tenth was a Cochrane systematic review that provided data on 1 further RCT (Flower 2012) (Table 89).
Five RCTs were conducted in China (Chen 2012, Wu 2006 (Flower 2012), Xia 2006, Xiang 2002, Zhu 2014). Two RCTs were from Europe – 1 from the UK (Flower 2011) and 1 from Italy (Sesti 2009). Two RCTs were conducted in Brazil (de Sousa 2016, Mira 2015) and 1 in the USA (Wayne 2008).
Much of the evidence came from small RCTs and sample sizes ranged from 18 (Wayne 2008) to 259 (Sesti 2009).
The severity or stage of endometriosis was not described in many of the articles. However, 1 RCT specifically included women with deep endometriosis who were suffering from persisting pelvic pain and dyspareunia, despite hormonal therapy (Mira 2015). One RCT included women with subfertility and minimal/mild endometriosis, all of whom underwent operative laparoscopy at the start of the trial (Zhu 2014). A third RCT only recruited women with an endometrioma, who underwent cystectomy at the start of the trial (Sesti 2009).
The majority of RCTs focused on outcomes of pain relief and health-related quality of life. Two RCTs reported on reduction in the size or recurrence of endometriomas (Wu 2006 (Flower 2012); Sesti 2009). One reported on fertility outcomes (live birth and miscarriage rates) (Zhu 2014).
Five RCTs investigated the use of different forms of acupuncture for endometriosis. Two RCTs compared acupuncture to sham acupuncture (de Sousa 2016, Wayne 2008). One RCT compared the use of acupuncture with danazol (Chen 2012) and another compared acupuncture plus Chinese herbal medicine (CHM) to danazol (Xia 2006). One RCT compared acupuncture to CHM (Xiang 2002).
Three further RCTs considered the use of CHM. One compared the use of individualised CHM preparations to placebo (Flower 2011). The Cochrane review included 1 RCT including 3 treatment groups: Nei Yi tablets; Nei Yi tablets and enemas; and danazol (Wu 2006 (Flower 2012)). The third RCT assessed fertility rates in women given short-term CHM plus the combined oral contraceptive pill (cOCP) after surgery for endometriosis, compared to women given cOCP alone, or no treatment (Zhu 2014).
A single RCT compared acupuncture-like transcutaneous electrical nerve stimulation (TENS) to self-applied TENS (Mira 2015)
Finally, 1 RCT compared dietary therapy (a nutritional supplement of vitamins, minerals, fatty acids and probiotics) with placebo, GnRH analogues or cOCP in prevention of endometrioma recurrence after cystectomy (Sesti 2009).
Evidence for 2 critical outcomes was available (relief of endometriosis-related pain and health-related quality of life). Evidence for 2 important outcomes was also available (fertility and reduction in size of endometriotic cysts). Some evidence was available on activities of daily living. No evidence was available for the remaining outcomes (improvement of endometriosis symptoms other than pain, adverse events resulting from the intervention and adherence to the treatment programme).
11.2.3. Summary of included studies
A summary of the studies that were included in this review are presented in Table 89.
11.2.4. Clinical evidence profile
The clinical evidence profiles for this review question are presented in Table 90 to Table 103.
11.2.5. Economic evidence
No health economic evidence was found on the cost effectiveness of non-pharmacological interventions for the treatment of endometriosis. Consequently this issue was considered in a de novo economic model. Some of the relevant sections to this review are described in the guideline (for further information on the complete model see Appendix K).
11.2.5.1. Summary of relevant section of the health economic model
Owing to a lack of clinical evidence, only 2 non-pharmacological techniques were considered for economic analysis. These were acupuncture and a generic category of Chinese Herbal Medicine (CHM). Unlike pharmacological or surgical interventions, the cost of non-pharmacological interventions is not well fixed and can vary greatly depending on the technique and supplier. Consequently there is a considerable margin for error on these estimates. Table 104 below gives the estimated annual cost for these interventions, but their estimation is described in more detail in the subsequent paragraphs.
The cost of acupuncture was taken from NICE guidance NG23 (Menopause). This estimates £65 for an initial appointment and then £40 for 12 subsequent appointments and is based on estimates from the UK Acupuncture Clinic (retrieved 15/11/16). Committee opinion was that this likely underestimated the cost of acupuncture in the case of endometriosis, as most women would not consider a 3-month-on treatment 9-month-off treatment schedule to be acceptable to them.
TCM is not typically prescribed on the NHS and thus it is difficult to acquire costings from the BNF. Anecdotally, most users purchase their TCM from health food stores or online from sites such as Amazon.com. This difficulty is compounded by inconsistency in labelling the active ingredient; for example, Dan’e is a mixture of Radix Salviae miltiorrhizae and Rhizoma Zedoariae with no clear indication of the typical ratio between them. Estimating dosage is also difficult, as typically users are advised to vary the dose until the desired effect is achieved. A TCM advocacy group (retrieved 15/11/16) recommends a dose of between 5g and 10g of Dan’e daily, which, based on purchasing a bulk bag from Amazon.com (retrieved 15/11/16) would require between 3 to 7 such bags a year. At the recommended maximum dose of 30g it would require 22 bags per year. Based on an average of a 7.5g daily dose, the total annual cost for the drugs would be £120.77.
Table 105 identifies the cost and effectiveness of all non-dominated treatment strategies containing a non-pharmacological intervention (in the subset of all treatment strategies containing a non-pharmacological intervention; the table does not imply that these interventions are likely to be superior to hormonal or surgical treatment); if a test/treat dyad is not listed then it is because an alternative treatment is available at the same cost that gives more QALYs. The results demonstrate that herbal medicine is both unlikely to be cost-effective on average and unlikely to benefit any woman more than placebo. Acupuncture is likely to be cost-effective on average and moderately likely to be cost-effective for an individual patient at the upper limit of the conventional NICE threshold (£30,000/QALY). However, this is only true when looking at non-pharmacological interventions in isolation; Figure 19 demonstrates that acupuncture is dominated by pharmacological methods of pain relief including hormonal treatments and a willingness to pay for acupuncture implies a willingness to pay for surgery if these methods are inappropriate (it is extendedly dominated).
11.2.6. Clinical evidence statements
11.2.6.1. Comparison 1: Conventional oral contraceptive pill and Dan’e Chinese herbal medicine vs. no treatment
Fertility
Low and moderate quality evidence from 1 RCT (n=156) found no clinically significant difference in incidence in live birth or miscarriage at 12 months after treatment ended when use of cOCP and Dan’e CHM in combination was compared to no treatment.
11.2.6.2. Comparison 2: Conventional oral contraceptive pill and Dan’e Chinese herbal medicine vs. conventional oral contraceptive pill
Fertility
Low and moderate quality evidence from 1 RCT (n=156) found no clinically significant difference in incidence in live birth or miscarriage at 12 months after treatment ended when use of cOCP and Dan’e CHM in combination was compared to use of cOCP alone.
11.2.6.3. Comparison 3–5: Dietary supplements vs. placebo, dietary supplements vs. GnRH agonist and dietary supplements vs. conventional oral contraceptive pill
Recurrence rates
Low quality evidence from 1 RCT (n=240) found no clinically significant difference in endometrioma recurrence at 18 months after surgery when post-operative use of a 6 month course of dietary supplements (including vitamin, mineral and fatty acid supplementation) was compared to placebo, GnRH agonist (tryptorelin or leuprorelin) or a cOCP (continuous, low-dose).
11.2.6.4. Comparison 6: Acupuncture vs. sham acupuncture
Pain
Very low and low quality evidence from 1 RCT (n=18) found a clinically significant improvement in pain reduction at 4 weeks during treatment when Japanese-style acupuncture was compared to sham acupuncture. However, there was no clinically significant difference between the 2 interventions for pain assessed at the end of 8 weeks of treatment and at 6 month follow-up.
Moderate quality evidence from 1 RCT (n=42) found a clinically significant improvement in pain reduction for chronic pelvic pain and dyspareunia at 2 months after treatment when acupuncture was compared to sham acupuncture.
Quality of life
Low quality evidence from 1 RCT (n=18) found a clinically significant improvement in quality of life (EHP total score) at 4 weeks during treatment, at the end of 8 weeks of treatment and at 6 month follow-up when Japanese-style acupuncture was compared to sham acupuncture.
Low quality evidence from 1 RCT (n=18) found no clinically significant difference in quality of life at 4 weeks during treatment (Pediatric QoL Inventory total score) when Japanese-style acupuncture was compared to sham acupuncture. There may be a clinically significant benefit of Japanese-style acupuncture compared to sham acupuncture for improvement in quality of life at the end of 8 weeks of treatment, but there is uncertainty around the estimate. However, there was a clinically significant improvement in quality of life at 6 month follow up when Japanese-style acupuncture was compared to sham acupuncture.
Activities of daily living
Very low and low quality evidence from 1 RCT (n=18) found a clinically significant benefit in improvement in activities of daily living at 4 weeks during treatment when Japanese-style acupuncture was compared to sham acupuncture. However, there was no clinically significant difference between the 2 interventions for activities of daily living assessed at the end of 8 weeks of treatment and at 6 months follow up.
11.2.6.5. Acupuncture vs. danazol
Cure of symptoms
Very low quality evidence from 1 RCT (n=70) found no clinically significant difference in cure of endometriosis symptoms at 3 months post-treatment when use of abdominal acupuncture was compared to danazol over 3 menstrual cycles.
11.2.6.6. Comparison 8: Acupuncture vs. Chinese herbal medicine
Dysmenorrhoea
Very low quality evidence from 1 RCT (n=67) found a clinically significant improvement in dysmenorrhoea at the end of 3 months treatment when use of ear acupuncture therapy was compared to oral administration of CHM.
Cure of symptoms
Low quality evidence from 1 RCT (n=67) found that there may be a clinically significant benefit at the end of 3 months treatment with ear acupuncture therapy compared to oral administration of CHM for cure of endometriosis symptoms, but there is uncertainty around the estimate.
11.2.6.7. Comparison 9: Chinese herbal medicine (individualised decoction) vs. placebo
Pain and quality of life
Very low and low quality evidence from 1 RCT (n=33) found no clinically significant differences in pain symptoms (VAS) or quality of life (MYMOP and EHP 30) at the end of 16 weeks treatment with an individualised CHM decoction compared to a placebo decoction.
11.2.6.8. Comparison 10: Chinese herbal medicine (Nei Yi pills) vs. danazol
Pain
Low quality evidence from 1 RCT (n=58) found clinically significant improvement in symptomatic relief within 3 years of stopping treatment. However, there was no clinically significant difference dysmenorrhoea score, lumbosacral pain relief, rectal irritation relief, tenderness of vaginal nodules in the posterior fornix at the end of 3 months treatment with CHM (Nei Yi pills) compared to danazol (low quality evidence).
Reduction in the size and extent of endometriotic cysts
Very low quality evidence from 1 RCT (n=58) found no clinically significant difference in disappearance or shrinkage of adnexal masses at the end of 3 months treatment with CHM (Nei Yi pills) compared to danazol.
11.2.6.9. Comparison 11: Chinese herbal medicine (Nei Yi pills plus Nei Yi enema) vs. danazol
Pain
Low quality evidence from 1 RCT (n=58) found clinically significant benefit in symptomatic relief (within 3 years of stopping treatment) and reduction in dysmenorrhoea score at the end of 3 months treatment with CHM (Nei Yi pills plus Nei Yi enema) compared to danazol. There may be a clinically significant benefit of CHM (Nei Yi pills plus Nei Yi enema) compared to danazol for rectal irritation relief, but there is uncertainty around the estimate. No clinically significant differences in lumbosacral pain relief or in tenderness of vaginal nodules in the posterior fornix were identified when CHM (Nei Yi pills plus Nei Yi enema) and danazol were compared.
Reduction in the size and extent of endometriotic cysts
Low quality evidence from 1 RCT (n=58) found clinically significant benefit in disappearance or shrinkage of adnexal masses at the end of 3 months treatment with CHM (Nei Yi pills plus Nei Yi enema) compared to danazol.
11.2.6.10. Comparison 12: Chinese herbal medicine (Nei Yi pills plus Nei Yi enema) vs. Chinese herbal medicine (Nei Yi pills)
Pain
Very low and Low quality evidence from 1 RCT (n=58) found that there may be a clinically significant improvement in dysmenorrhoea at the end of 3 months treatment when CHM administered orally and rectally (Nei Yi pills plus Nei Yi enema) compared to oral administration of CHM alone (Nei Yi pills), but there is uncertainty around the estimate. No clinically significant differences in symptomatic relief, lumbosacral pain relief, rectal irritation relief or tenderness of vaginal nodules in posterior fornix were found when the 2 interventions were compared.
Reduction in the size and extent of endometriotic cysts
Low quality evidence from 1 RCT (n=58) found no clinically significant difference in disappearance or shrinkage of adnexal masses at the end of 3 months treatment when CHM administered orally and rectally (Nei Yi pills plus Nei Yi enema) and oral administration of CHM alone (Nei Yi pills) were compared.
11.2.6.11. Comparison 13: Chinese herbal medicine (Gui-Zhi-Fu-Ling-Wan) and acupuncture vs. danazol
Pain
Very low quality evidence from 1 RCT (n=78) found no clinically significant differences in dysmenorrhoea, lumbosacral pain or dyspareunia at the end of 3 months treatment when use of CHM (Gui-Zhi-Fu-Ling-Wan) and acupuncture in combination was compared to danazol.
11.2.6.12. Comparison 14: Acupuncture-like transcutaneous electrical nerve stimulation vs. self-applied transcutaneous electrical nerve stimulation
Quality of life
Very low quality evidence from 1 RCT (n=22) found no clinically significant difference in quality of life (EHP-30 total score) when use of acupuncture-like TENS was compared to self-applied TENS.
11.2.7. Evidence to recommendations
11.2.7.1. Relative value placed on the outcomes considered
The principal aim of this review is to determine the clinical and cost effectiveness of non-pharmacological therapies in reducing pain in women with endometriosis or suspected endometriosis. The Committee prioritised relief of endometriosis-related pain, health-related quality of life and adherence to the treatment programme as critical outcomes when considering recommendations. The remaining outcomes of improvement in fertility rates (live birth), reduction in the size and extent of endometriotic cysts, improvement of endometriosis-related symptoms apart from pain (e.g. fatigue), adverse effects resulting from the intervention, rates of reoccurrence and activities of daily living were considered to be important.
11.2.7.2. Consideration of clinical benefits and harms
The Committee agreed that the evidence on non-pharmacological treatments for endometriosis-related pain management was very uncertain and of limited value.
The Committee noted that some of the non-pharmacological medicines, particularly CHM, are not available within the NHS or are not applicable in the UK setting. The Committee discussed and agreed that there is some evidence that CHM may be effective but expressed their concern regarding standardisation, regulation, efficacy and safety of these medicines.
The Committee’s opinion regarding recommending non-pharmacological treatments was divided: some of the Committee members would not discourage women who would like to try alternative treatment options but would warn them to be cautious, for example, regarding CHM or a particular diet; other Committee members felt that they would not encourage women to try alternative treatments and noted their potentially negative impact on health and interactions with standard treatment.
Some of the Committee members expressed concern that, for example, physiotherapy pain-management interventions are not necessarily disease-specific (for example, a population of women with chronic pelvic pain may also include some with endometriosis-related pain), therefore search criteria applied in this guideline may have led to an impression that there is no evidence regarding physio-related pain management interventions. Further, pain management (such as psychological and behaviour interventions) would be more broadly applicable to people with other reasons for chronic pain. However, when finalising the protocol, the Committee specified a threshold of 66% of women have a diagnosis of endometriosis that for studies with mixed populations of women with chronic pelvic pain.
Some of the Committee members, based on their experience, suggested that physiotherapy and psychological pain management approaches are definitely effective. However, the Committee stressed that there is no evidence for these approaches.
11.2.7.3. Consideration of economic benefits and harms
The Committee discussed the results of the health economic model which demonstrated that Herbal Medicine is both unlikely to be cost-effective on average and unlikely to benefit any woman more than placebo. Acupuncture is likely to be cost-effective versus placebo on average and moderately likely to be cost-effective for an individual patient (especially at a threshold of £30,000 / QALY). However the Committee agreed that this is only true when looking at non-pharmacological interventions in isolation. The Committee noted that the economic evidence clearly indicates that a willingness to pay for acupuncture implies a willingness to pay for surgery if these methods are inappropriate and therefore agreed not to recommend acupuncture on the basis of cost implications as it would only be appropriate if a woman could not tolerate any other treatment considered in the guideline and such a woman would have so idiopathic a condition that these recommendations would probably not apply to her.
The Committee discussed how certain interventions on the protocol but for which no evidence were found had a high probability of being cost-effective. This was especially true for behavioural interventions such as a Pain Management Programme and Psychosexual Counselling. The reason for the Committee’s observation is that these programmes are offered once early in the treatment of a woman with endometriosis (or sometimes shortly following diagnosis) but are expected to ‘pay off’ with a steady improvement in QALYs over the rest of the woman’s life. In pain management in particular, there may also be a positive economic impact if women are switched away from expensive drugs or treatments with unpleasant side effects and onto alternative methods of managing their pain. Given an expected cost of £1500 for any of these programmes the QALY gain required per year for cost-effectiveness at £20,000 would only be around 0.0025, which the Committee noted was easily achievable. Because of the extremely high potential for high value-of-information in this area, the Committee decided a Research Recommendation was especially important in this instance.
The Committee also noted that the interaction profile of certain herbal medicines was not well understood and so they might have an effect on other treatments women might want to try. Other non-pharmacological treatments, including acupuncture, would be unlikely to interact with any other treatment attempted – however there was no evidence that the full benefit of the non-pharmacological treatment would be felt in this instance. Consequently the Committee decided that there was insufficient evidence to recommend non-pharmacological treatment even in combination with pharmacological therapies.
11.2.7.4. Quality of evidence
Evidence was not available for the majority of interventions stipulated in the protocol e.g. no evidence was available for behavioural medicine. Acupuncture and TENS were the only physical interventions examined. Diet was considered in 1 study but the intervention was insufficiently described to be used in clinical practice. The majority of the evidence was regarding Chinese herbal medicines and the Committee considered that their use was not without potential harm.
The Committee noted that several of the studies were small and that although the range in quality of the evidence was from moderate to very low, the majority of evidence was of low or very low quality.
The Committee discussed the paucity of available evidence and concluded that there was a broader evidence base regarding the effectiveness of behavioural medicine and other interventions used in pain management but that this would be drawn from studies of mixed populations of women, not uniquely those with endometriosis and hence would be excluded from the review.
The Committee concluded that there is lack of evidence on physical activity, psychological pain management and particularly dietary interventions and made recommendations for research in populations of women with endometriosis. They also stressed that it is not only important to encourage research but also to improve its quality.
The Committee agreed that they should not only focus on the evidence presented but also discuss other interventions listed in the protocol for which no evidence was found.
11.2.7.5. Other considerations
The Committee considered that no additional recommendations were necessary for equality reasons.
The Committee did not believe that their recommendations would constitute a change of practice requiring additional support for implementation. They acknowledged that pain management clinics may use interventions for women with endometriosis on the basis of practice in a broader population of people experiencing pain.
It was noted that many of the interventions specified in the protocol would be accessible to women outside the NHS. A recommendation was made to ensure that healthcare professionals advise women that the Committee considered there to be insufficient evidence to recommend their use. There were specific concerns regarding herbal medicine preparations and the Committee drew upon recommendations made in the Menopause guideline to echo these concerns for women with endometriosis.
The Committee were concerned that many of the currently used non-pharmacological treatments were not supported by evidence. The Committee intended to look for evidence on a wide range of psychological, physical and lifestyle treatments (see Appendix D). However, the Committee agreed that the lack of evidence specifically addressing a population of women with endometriosis made it difficult to draft recommendations for these management strategies and particularly for dietary interventions.
The Committee agreed that this would be an important topic for future research and made recommendations for research in populations of women with endometriosis which would hopefully inform an update of this guideline. They also stressed that it is not only important to encourage research but also to improve its quality.
They decided that there would be benefit in research investigating commonly used pain management programmes specifically in populations of women with endometriosis. Moreover, the Committee noted that women with endometriosis in support groups discuss lifestyle interventions that they perceive as helpful. Generally these are related to nutrition (such as the Endo diet) and exercise. The Committee further noted that these would be important interventions that could be promoted to self-manage symptoms if found to be effective. They therefore decided that lifestyle interventions should also be proposed as a research recommendation to inform future guidance.
11.2.7.6. Key conclusions
The Committee concluded that there are no non-pharmacological treatments that are clinically and cost-effective and with good evidence. They therefore decided not to recommend any particular non-pharmacological intervention but agreed that future research should be prioritised in this topic, particularly relating to pain management programmes and lifestyle changes.
11.2.8. Recommendations
- 39.
Advise women that the available evidence does not support the use of traditional Chinese medicine or other Chinese herbal medicines or supplements for treating endometriosis.
11.2.9. Research recommendations
- 2.
Are pain management programmes a clinically and cost-effective intervention for women with endometriosis?
Why this is important
Pain is one of the most debilitating symptoms of endometriosis. Endometriosis-related pain can be acute or chronic, and can adversely affect the woman’s quality of life, ability to work, and can affect partners and their families.
Pain management programmes have been found to be effective in managing chronic pelvic pain, and can improve quality of life. However, it is unclear how much of this small evidence base can be generalised to women with endometriosis for which evidence is lacking. Furthermore, pain management programmes have not been compared with other treatments available for endometriosis. Pain management programmes promote self-management and are often provided in the community.
If found to be effective for endometriosis, pain management programmes would provide an additional or alternative treatment option for women experiencing endometriosis-related pain. Groups of particular interest are women for whom hormonal and surgical options have been exhausted, women who would prefer an alternative to a pharmacological or surgical approach, and women who may be prioritising trying to conceive.
- 3.
Are specialist lifestyle interventions (diet and exercise) effective, compared with no specialist lifestyle interventions, for women with endometriosis?
Why this is important
Endometriosis is a long-term condition that can cause acute and chronic pain, and fatigue. It has a significant and sometimes severe impact on the woman’s quality of life and activities of daily living, including relationships and sexuality, ability to work, fertility, fitness and mental health.
Supporting self-management is critical to improving quality of life for women living with endometriosis. In order to successfully self-manage the condition, women need evidence-based, easily accessible information about the condition and ways of managing it that support surgical and medical treatment. However, no high-quality research was identified on the effectiveness of lifestyle interventions such as diet or exercise and other non-medical treatments in reducing pain, fatigue and other symptoms.
Studies should aim to provide evidence-based options to support self-management of endometriosis. This would improve the quality of life of women with endometriosis, enabling them to manage pain and fatigue, and reducing the negative impact on their career, relationships, sex lives, fertility, and physical and emotional wellbeing.
11.3. Surgical management and combinations of treatment
11.3.1. Surgery, including ablation and excision (and the surgical network meta-analysis)
11.3.1.1. Introduction
Surgical treatment is an important part of the management of endometriosis, aiming to remove or destroy endometriotic deposits and divide adhesions with restoration of normal anatomy. Surgical treatments can be performed by laparoscopy (traditional or robotic) or as an open procedure (laparotomy). Current practice is to use a laparoscopic approach, as it offers several advantages when compared to open procedures, including improved visualisation, microsurgical techniques, shorter hospital stay, quicker return to normal function and cost.
Endometriotic deposits can be treated by excision (cutting them out) or ablation (destruction or evaporation using a variety of energy modalities). These techniques are used to treat endometriosis of all degrees of severity. Surgical techniques such as the choice of energy modality may be influenced by the surgeons’ training and preferences. Severe endometriosis involving the bowel, bladder and ureter may require additional surgical expertise, including colorectal surgeons and urologists. Surgery has a role in the management of recurrent disease, although it is recognised that outcomes may reduce with increasing numbers of operations.
Even if all endometriosis tissue is removed by excision or ablation, the risk of recurrence is high. Relapse of symptoms occurs in 40–45% of women and up to 30% of women are readmitted for surgery within 5 years. Half of all women diagnosed with endometriosis require a second operation and just over a quarter will undergo 3 or more procedures.
Reduction of pain due to presumed recurrence currently involves the use of hormonal treatments pre- or post-surgery. The rationale for this is that hormonal treatments reduce circulating levels of oestrogen leading to lighter or no periods, theoretically causing shrinkage of existing endometriosis lesions and preventing new lesions developing. The Committee were interested in assessing the clinical and cost effectiveness of surgery as well as the effectiveness of pre- and post-surgical hormonal treatment.
The aim of the review question was to assess the evidence for excisional and ablative surgical techniques and combinations of hormonal treatments with surgery, and to compare their clinical and cost effectiveness in the management of endometriosis, including the management of ovarian endometriomas. A review on laparoscopic uterine nerve ablation (LUNA) for chronic pelvic pain was not prioritised, because there is a NICE interventional procedure guideline on this topic.
For full details, see the review protocol in Appendix D, the study selection flow chart in Appendix F, study exclusion list in Appendix H, forest plots in Appendix I and full GRADE profiles in Appendix J.
11.3.1.2. Network Meta-analysis
What is the effectiveness of the following treatments for endometriosis, including recurrent and asymptomatic endometriosis:
- surgery
- combined surgery and hormonal treatment?
11.3.1.3. Methods
Study selection and data collection
For full details see review and analysis protocols in Appendix D.
Outcome measures for NMA
For assessing the effectiveness of different surgical or combined surgery plus hormonal treatments, the Committee identified pain relief and health-related Quality of Life (QoL) as critical outcomes for which NMA could be used to aid decision-making. NMAs were performed on these outcomes where evidence was available. Pain relief
For pain relief, the visual analogue scale (VAS) was considered to be the most widely used useful pain scale for which data would be available.
Health-related QoL
For health-related QoL, the SF-36 was determined to the most useful scale that was widely used in the literature. However, there were not a sufficient number of studies available from the systematic review to allow for NMA. Therefore these studies were analysed using pairwise meta-analysis where appropriate.
Statistical methodology
Data were available for a number of treatments and routes of administration. Due to the sparseness of the networks, it was necessary to group treatments within different classes and assume a common class effect (Table 110). All non-surgical treatments in the table were only included in the NMA if they were administered in combination with surgery.
The common class effects were assessed to identify if it was reasonable to assume similarity of treatment effects within classes. Multi-level NMA models with treatments nested within classes were also examined, though this added complexity did not improve model fit for any of the analyses.
11.3.1.4. Summary of included studies
Studies included in the NMA
All studies included women with laparoscopic confirmation of endometriosis.
11.3.1.4.1. Clinical evidence profile
Pain Relief – VAS
Due to difficulty in achieving convergence during estimation, NMAs were conducted separately for hormonal therapies and for surgery and surgery plus hormonal treatment. The Committee felt that this was likely to be because the populations may not have been sufficiently homogeneous, as patients receiving surgical treatment were likely to have failed on hormonal treatments, thus violating the assumption of transitivity.
Surgery and combined surgery plus hormonal therapy
Four trials of 6 surgery or combined surgery plus hormonal treatment classes were included in the network for the outcome of pain relief on the VAS, with a total sample size of 267 women (Figure 20). All studies of combined surgery and hormonal treatment administered hormonal treatment within 4 weeks post-surgery. One study was at high risk of bias, 7 were at moderate risk of bias and 8 at low risk of bias. Three of the 4 trials included women with endometrioma.
Table 112 presents the results of the pairwise meta-analyses (direct comparisons; upper right section of table) together with the results from the NMA for every possible class comparison (lower left section of table), presented as mean differences. The VAS is a 0–100 patient-reported scale, on which a difference of 10 points has been shown to be clinically significant to patients (Gerlinger 2012). NMA results were derived from a fixed effects model. As no closed treatment loops existed that were not from the same study, incoherence could not be assessed.
All treatments led to a clinically significant improvement when compared to diagnostic laparoscopy/no treatment. Use of a hormonal treatment after laparoscopy surgery led to a clinically significant improvement when compared to laparoscopic surgery alone, though evidence for this came exclusively from studies including a majority of women with endometrioma. There were no clinically significant differences between any of the hormonal treatments combined with laparoscopic surgery. Figure 21 graphically presents the results computed by the NMA for each treatment versus placebo.
The combined oral contraceptive pill (P(o) + O(o)) after laparoscopic surgery had the highest probability of being among the best 3 treatments (95.87%), followed by progestogen (oral) after laparoscopic surgery (85.10%) and GnRHa (i.m.) after laparoscopic surgery (84.49%) (Table 113).
Sufficient data to calculate SEs was only available in 2 of the 4 trials. However, sensitivity analyses using the upper 95% credible interval of the posterior for the imputed SEs showed that the probability of being the best treatment results were not sensitive to the imputed SEs (Appendix L). Two results compared to laparoscopic surgery (surgery plus progestogens (oral) and surgery plus the combined oral contraceptive pill plus Chinese herbal medicine) had 95% CrI that included 0, though the numerical was small and the point estimates still suggested strong clinical benefit.
11.3.1.5. Pairwise comparison of surgical ablation and excision
Review question: What is the effectiveness of surgery (ablation or excision) for the treatment of endometriosis, including recurrent and asymptomatic endometriosis?
11.3.1.5.1. Description of clinical evidence
The objective of this review is to determine the clinical and cost-effectiveness of surgery in improving health related quality of life and reducing adverse events.
Eight studies were included that evaluated the clinical and cost-effectiveness of ablation or excision for the management of endometriosis; 3 systematic reviews (Hart 2008; Dan 2013; Duffy 2014), of which 2 were Cochrane systematic reviews (Hart 2008 and Duffy 2014) and 5 RCTs (Abbott 2004, Carmona 2011; Wright 2005; Healey 2010; Healey 2014).
Two trials were carried out in the United Kingdom (Abbott 2004; Wright 2005), 2 in Australia (Healey, 2010; Healey, 2014) and 1 in Spain (Carmona, 2011). The included studies in the 3 systematic reviews (Duffy 2014; Dan 2013; Hart 2008) were carried out in various countries including Australia, Canada, Egypt, Iran and the United Kingdom.
Of the 3 included systematic reviews, 1 consisted of 3 trials (Hart 2008), 1 included 10 trials (Duffy 2014) and the third included 7 trials (Dan 2013).
Two systematic reviews (Hart 2008; Dan 2013) and 1 trial (Carmona 2011) were carried out to determine whether laparoscopic surgical excision or ablation is the optimum surgical management of ovarian endometrioma with respect to pain and fertility outcomes and recurrence rate.
The effectiveness and safety of laparoscopic surgery in the treatment of painful symptoms and subfertility associated with endometriosis was assessed by 1 systematic review (Duffy 2014).
Reduction of pain following laparoscopy after ablation or excision of endometriosis was examined by 1 trial (Healey 2010). A follow-up study was performed 5 years after the operation to assess reduction in the pain score (Healey 2014).
One trial (Wright 2005) compared excisional and ablative treatment modalities for mild endometriosis in the management of chronic pelvic pain.
One trial (Abbott 2004) reported on quality of life in women with endometriosis who received excisional laparoscopy at different time periods. In this evidence report, data on quality of life outcomes were reported from the Aboott 2004 study and pain-related outcomes were reported from the Duffy 2014 systematic review.
The following comparisons were examined using the available evidence:
- Laparoscopic treatment (excision or ablation) versus diagnostic laparoscopy
- Excision versus diagnostic laparoscopy
- Ablation versus diagnostic laparoscopy
- Excisional surgery versus ablative surgery
No evidence was identified for the following outcomes:
- Effect on daily activities
- Participant satisfaction with treatment
Evidence from these studies is summarised in the clinical GRADE evidence profiles below (Table 115 to Table 117). Descriptive data from the Healey 2004 and Abbott 2004 trials are presented in Table 118 and Table 119, respectively. See also the study selection flow chart in Appendix F, study exclusion list in Appendix H, forest plots in Appendix I, full GRADE profiles in Appendix J and study evidence tables in Appendix Summary of included studies
A summary of the studies that were included in this review are presented in Table 114.
11.3.1.5.2. Clinical evidence profile
The clinical evidence profiles for this review question are presented in Table 115 to Table 119.
The data provided by Wright 2005 comparing ablation with excision for pelvic pain associated with mild endometriosis demonstrated good symptom relief at 6 months for the majority of participants irrespective of the treatment modality. However, their data could not be included in the meta-analysis because the data were obtained using a ranked ordinal scale.
11.3.2. Economic evidence
No health economic studies were found contrasting ablation to excisional surgery for endometriosis.
One RCT was found looking at the costs of ablation compared to hormonal treatment (Lalchandani, 2005). This found an expected saving for surgery over hormonal treatment of £595 per patient. However this trial did not consider the opportunity cost of the use of equipment or clinician time and so was not appropriate for inclusion in a NICE Guideline.
Four large database studies were found estimating the costs of laparoscopic surgery in different healthcare systems. These studies were Allaire (2014) in Canada, Chvatal (2010) in Germany and Fuldeore (2010, 2011) in the US. Together these studies incorporated 94,605 women with endometriosis. As these looked at cost rather than cost-effectiveness and were conducted in non-NHS settings, they were considered less appropriate as a source of costs than the NHS Reference Costs, but were included to serve as a source of variation for sensitivity analysis.
Finally 1 US study was found contrasting laparoscopic surgery to laparotomy (Luciano, 1992). This trial was excluded as laparotomy vs. laparoscopy was not a comparison of interest to the Committee and the data were very out of date, although it should be noted that the total cost for a laparoscopy was £3004, which is consistent with other estimates of the cost of the procedure.
Because of the importance of this question to the Committee, it was prioritised for de novo health economic modelling. The model found that – relative to no treatment – surgical interventions increased the average cost of treatment by £703.36 and average lifetime QALYs by 0.47. By typical cost-effectiveness standards, paying £1506.40 per QALY would be considered cost-effective, but the Committee considered evidence from the model suggesting that pairing a laparoscopic treatment with a more sensitive diagnostic test could reduce the cost/QALY relative to the same test with no treatment quite substantially. Table 121 demonstrates that in particular MRI and laparoscopic diagnosis reduce costs greatly compared to no treatment. The table also shows that – in general – laparoscopy and adjunct hormonal treatment costs slightly more and adds more QALYs than laparoscopy alone. In fact, laparoscopic diagnosis & laparoscopy with adjunct hormonal treatment adds the most possible lifetimes QALYs, since it pairs the most effective treatment with the most sensitive diagnostic strategy (empirical diagnosis and laparoscopic treatment adds nearly as many, because it has identical sensitivity but patients do not benefit from the therapeutic effects of a diagnostic laparoscopy described elsewhere). This indicates that there is always some cost-effectiveness threshold at which the NHS would consider this treatment, although the model describes how the NHS would only consider this treatment at cost-effectiveness thresholds of >£160,000.
11.3.3. Clinical evidence statements
11.3.3.1. Endometriosis
11.3.3.1.1. Laparoscopic treatment (excision or ablation) versus diagnostic laparoscopy for endometriosis
Overall pain at 6 months
Very low quality evidence from 1 study of 69 women with endometriosis showed a clinically significant improvement in overall pain at 6 months associated with laparoscopic treatment compared with diagnostic laparoscopy for endometriosis.
Overall pain at 12 months
Low quality evidence from 1 study of 69 women with endometriosis found a clinically significant improvement in overall pain at 12 months associated with laparoscopic treatment compared with diagnostic laparoscopy for endometriosis.
11.3.3.1.2. Live birth or ongoing pregnancy
Very low quality evidence from 2 studies of 382 women found no clinically significant difference in live birth or ongoing pregnancy between laparoscopic treatment and diagnostic laparoscopy for endometriosis.
11.3.3.1.3. Clinical pregnancy
Very low quality evidence from 3 studies including 528 women with endometriosis found no clinically significant difference between laparoscopic treatment and diagnostic laparoscopy for the outcome of clinical pregnancy.
11.3.3.1.4. Miscarriage per pregnancy
Very low quality evidence from 2 studies including 112 women with endometriosis found no clinically significant difference between laparoscopic treatment and diagnostic laparoscopy for miscarriages per pregnancy.
11.3.3.1.5. Excision versus diagnostic laparoscopy for endometriosis
Overall pain at 6 months
High quality evidence from 1 study including 39 women with endometriosis found a clinically significant improvement in overall pain at 6 months associated with excision compared with diagnostic laparoscopy.
Overall pain score at 6 months
Very low quality evidence from 1 study including 16 women with endometriosis found a clinically significant reduction in overall pain score at 6 months associated with diagnostic laparoscopy compared with excision.
Overall pelvic pain score at 12 months
Moderate quality evidence from 1 study including 16 women with endometriosis found a clinically significant reduction in overall pain score at 12 months’ follow-up associated with diagnostic laparoscopy compared with excision.
Pelvic pain score at 6 months
Moderate quality evidence from 1 study including 39 women with endometriosis found no clinically significant difference in pelvic pain scores at 6 months associated with excision compared with diagnostic laparoscopy.
Dysmenorrhoea pain score at 6 months
Moderate quality evidence from 1 study including 39 women with endometriosis found that there was no clinically significant difference in dysmenorrhoea pain score at 6 months associated with excision compared with diagnostic laparoscopy.
Dyspareunia pain score at 6 months
Moderate quality evidence from 1 study including 39 women with endometriosis found that there was no clinically significant difference in dyspareunia pain score at 6 months associated with excision compared with diagnostic laparoscopy.
Health-related quality of life
Low quality evidence from 1 study including 39 women with endometriosis reported that there was no clinically significant difference in the mean EQ-5D index summary score at 6-month follow-up in the excision groups compared with the diagnostic laparoscopy group. Moderate quality evidence from the same study reported a clinically significant increase in the mean EQ-5D VAS summary score at 6 months associated with excision compared with diagnostic laparoscopy, but no clinically significant difference in the mean SF-12 physical and mental component scores at 6-month follow-up associated with excision compared with diagnostic laparoscopy.
11.3.3.2. Excisional surgery versus ablative surgery for endometriosis
Pain scores (improvement from baseline in VAS scores at 12 months)
Low to very low quality evidence from 1 randomised controlled trial comprising 103 women with endometriosis showed similar improvement in pain score in the laparoscopic excision and laparoscopic ablation groups for global pain as well as pelvic pain and dyspareunia at 12 months follow-up. One study reported the reduction in VAS score at 5-year follow-up, however, the clinical significance of reported outcomes could not be calculated.
Unintended effects of treatment (improvement from baseline in VAS score at 12 months follow up)
Moderate to low quality evidence from 1 randomised controlled trial comprising 103 women with endometriosis showed no clinically significant differences between the 2 treatments in nausea, vomiting and bloating at 12 months follow-up.
11.3.3.3. Endometrioma
Excisional surgery versus ablative surgery for endometrioma
Recurrence of pelvic pain
Moderate to low quality evidence from 2 randomised controlled trials with a total of 104 women with endometriosis showed clinically significant lower rates of recurrence of dysmenorrhoea and non-menstrual pelvic pain associated with laparoscopic excision when compared to laparoscopic ablation of endometrioma.
Pregnancy rate after surgical treatment
Moderate quality evidence from 3 randomised controlled trials with a total of 138 women with endometriosis showed higher rates of pregnancy associated with laparoscopic excision compared to laparoscopic ablation after surgical treatment of endometrioma, but there is some uncertainty around this finding which makes judgment of clinical benefit unclear.
Recurrence of endometrioma (at 12 months and at 60 months)
High quality evidence from 4 randomised controlled trials with a total of 258 women with endometriosis showed lower rates of recurrence of endometrioma associated with laparoscopic excision when compared to laparoscopic ablation at 12 months follow up. However, this result did not reach clinical significance. Low quality evidence from 1 randomised controlled trial comprising 74 women with endometriosis showed similar rates of recurrence of endometrioma in the laparoscopic excision and laparoscopic ablation groups at 60 months follow-up.
Reoperation after surgical treatment (up to 60 months)
Very low quality evidence from 2 randomised controlled trials comprising together of 174 women with endometriosis showed higher rates of reoperations associated with laparoscopic excision when compared to laparoscopic ablation up to 60 months follow up. However, this result did not reach clinical significance.
11.3.4. Evidence to recommendations
11.3.4.1. Relative value placed on the outcomes considered
As pain relief is the primary reason for patients seeking treatment, this was the most critical outcome for this review. Health-related quality of life was also critical as this might be considered to give a more broad reflection of patient experience than pain relief alone.
Rate of success, surgical complications, satisfaction with treatment, effect on daily activities, absence from work, number of women requiring more surgery and reduction in size and extent of endometriotic cysts were considered important outcomes as they were less clear indicators of effectiveness.
11.3.4.2. Consideration of clinical benefits and harms
Throughout the care pathway, the Committee stressed the importance of a full discussion with women of their symptoms and priorities with respect to pain and fertility. This was particularly important in gynaecology services and specialist endometriosis services (endometriosis centres) when discussing the benefits and harms of laparoscopic surgery. Such a discussion should highlight the potential negative impact of laparoscopic treatment on ovarian reserve.
The Committee recognised that a woman might be referred from a GP for a consultation with a general gynaecologist, a gynaecologist with a specialist interest or at a specialist centre and noted that women with suspected rectovaginal endometriosis would require the expertise available at a specialist centre.
The Committee discussed what a referral would provide for a woman and agreed that the gynaecologists would firstly discuss the woman’s symptoms and priorities with her and what her treatment options would be. For example, her primary symptom could be pain in which case offering alternative hormonal therapy to that offered by the GP might be appropriate - the type of hormone and its duration of effect being determined on an individual basis considering the woman’s preferences. However, this treatment might not be appropriate if the woman’s primary concern was fertility.
The Committee discussed whether a diagnostic laparoscopy should be offered prior to further management and concluded that the decision for a diagnostic laparoscopy would be on individual symptoms and priorities (and may require a further referral). The Committee agreed that diagnostic laparoscopy is a valuable tool which provides the most accurate diagnosis and also provides the opportunity to treat. The Committee noted that once diagnosed (either by laparoscopy or incidental other confirmatory findings from ultrasound, MRI or biomarkers), the most suitable long-term treatment options can then be discussed with the women with the aim to tailor these to her needs and priorities.
The Committee agreed that if a diagnostic laparoscopy was performed and minor endometriosis was found, it should be treated during the laparoscopy by a suitably trained surgeon. To describe the minor type endometriosis they agree that there were 2 types that could be treated at the same time. Peritoneal endometriosis not involving the bowel, bladder or ureter and uncomplicated endometriomas. They intentionally used ‘uncomplicated’ to allow for clinical judgement, based on the surgeon’s skill and experience, since it would be difficult to define all possible complex cases of endometriomas. Treatment of an uncomplicated endometrioma could be performed at the initial procedure by a suitably trained surgeon, but decision-making would be influenced by the findings at laparoscopy; more extensive surgery, for example, treatment of a large endometrioma or bilateral endometriomas would not be performed at the time of diagnostic laparoscopy. It was noted that for instance an endometrioma that is adherent to its surrounding structures may be a complex procedure and may require further surgery after referral to a specialist centre. That the diagnostic laparoscopy may include treatment should be agreed with women prior to the procedure. Therefore the discussion with the woman was key to guide surgical decision-making. The Committee further noted that surgical diagnosis with treatment might not be suitable for all women (e.g. young women with mild disease) and that therapeutic treatment at laparoscopy would only be performed with mild or moderate endometriosis and not if there was extensive disease.
Excisional treatment was recommended over ablative treatment as the evidence showed that there was lower risk of recurrence of endometrioma and the Committee suggested that ablative surgery had a greater negative impact on ovarian reserve.
In reviewing pre-operative pharmacological treatment, the Committee noted the limitations of the evidence and discussed whether there was a role for hormonal therapies in women with severe (deep infiltrating) endometriosis and felt that this should be considered, based on their surgical expertise and experience, and on discussion with the woman to ensure that she understands the possible benefits, risks and complications of the treatment (particularly highlighting the side effect profile of GnRH agonists). The Committee acknowledged that there was another school of thought that hypothesises that this adjunct treatment could lead to co-occuring superficial endometriosis being missed. However, consensus was reached that this prior treatment would facilitate surgery (by reducing bleeding and inflammation) and therefore reduce reoperation rate.
11.3.4.3. Consideration of economic benefits and harms
The difference between endometrial excision and ablation surgery is highly unlikely to carry a significant cost, as the most significant cost of the operations is not the technique itself but the cost of the support network required to employ the technique – the surgical time, operating theatre use and recovery time. The Committee believed that these would be similar for both techniques and that any difference between the 2 techniques would come down to individual patient / disease characteristics (such as location of the endometriosis), or possibly the familiarity of the surgeon with a particular piece of equipment. Consequently health economic evidence was not used to inform the discussion of ablation vs. excision.
The difference between diagnostic, therapeutic and no laparoscopy was considered sufficiently important to warrant de novo economic modelling. Details of this model are available in Appendix K.
The difference in cost between diagnostic and therapeutic laparoscopy is not strictly relevant to health economic analysis as they are not competing alternatives – the NHS could offer one, both or neither. Additionally, the Committee suggested it would be very common to offer minor therapeutic surgery during a ‘diagnostic’ laparoscopy and a therapeutic laparoscopy would – by definition – require a diagnosis of the pathology that was the target of the surgery. Consequently in health economic terms the distinction between the 2 forms of surgery is a little artificial.
Committee members suggested that there might be some value in a diagnostic laparoscopy that went beyond the placebo effect, for example, receiving a definitive diagnosis might have positive psychological consequences. Although the economic model tries to account for this in sensitivity analysis, it is likely the economic benefit of a diagnostic laparoscopy will vary depending on the other potential diagnoses a woman might be considering and the value she places on knowing her condition for certain. The Committee took this fact into account when making recommendations, arguing that although the diagnostic health economic model typically found laparoscopy fell outside the range that NICE would typically pay for, the fact that the laparoscopy had other benefits, could be used to rule out malignancy and was anyway required for a therapeutic laparoscopy justified its inclusion.
Laparoscopic treatment (with or without subsequent hormonal treatment) is the gold-standard for treating endometriosis. Consequently there is always some willingness-to-pay threshold at which laparoscopic treatment becomes cost-effective. In general, NICE considers treatments more than £20,000 / QALY to be poor candidates for being cost-effective, and so the Committee observed that whether therapeutic laparoscopy was cost-effective or not depended on whether the woman was able to take hormonal therapy (and whether the treatment was having any positive effect). If the woman was currently on hormonal treatment and this was improving her symptoms relative to no treatment, the cost per QALY of operating was just above £20,000 / QALY and might be regarded as borderline cost-effective. But if the woman could not tolerate hormonal and neuromodulator treatment or was not receiving any benefit from the therapy the cost per QALY of operating was around £14,000 / QALY, which would normally be considered cost-effective. The Committee noted that the situation where this was most likely to occur was where a woman was trying to conceive and therefore could not take contraceptives.
Surgery for endometriosis that is not well controlled with other treatments is extremely common and consequently it is not thought that the Committee’s recommendations will lead to a substantial change of resources.
11.3.4.4. Quality of the evidence
The quality of the evidence used to make recommendations on combined surgery plus hormonal treatments for pain relief was generally moderate. Although the majority of studies were appropriately blinded, they rarely reported appropriate allocation concealment or details of the randomisation procedure. Two of the 4 studies in the NMA did not report measures of variability or uncertainty in their estimates, which meant that statistical imputation of missing information was needed. However, a variety of sensitivity of analyses were performed to test assumptions made during modelling and the results seemed robust.
For comparison between different surgical techniques the quality of the evidence was very low. The Committee discussed the difficulty of conducting high quality randomised studies, particularly as randomising patients to either excisional or ablative laparoscopic treatment can be impractical especially where there is deep endometriosis affecting bowel, bladder and ureter.
Evidence of the effectiveness of hormonal treatment combined with surgery only came from studies that followed surgery with hormonal treatment.
11.3.4.5. Other considerations
One of the key considerations throughout treatment for pain relief in endometriosis is women’s fertility. Fertility may be a strongly influencing factor in many women’s treatment choices and a timely discussion on how different treatments will impact this is essential. The Committee suggested that a particular point to highlight in such a discussion is that laparoscopic treatment (ablation or excision) of ovarian endometrioma may negatively affect ovarian reserve.
The different treatment options recommended here are based on RCT evidence from a number of different studies, which was in agreement with the experience of the Committee. Recommendations on information provision and the pathway of care were developed primarily from Committee experience and opinion, supported in part by the literature.
The Committee was aware of an ongoing trial investigating the effectiveness of post-surgical hormonal treatments. They agreed that the results of the NMA were consistent with their experience that hormonal treatments after surgery delay recurrence of endometriosis. However, they also noted that there was still some uncertainty around the size of the effect and that results from the ongoing trial would be important by adding to the evidence base and thus informing future guidance.
The Committee considered whether any additional recommendations were necessary for adolescent women. It was concluded that none were required, but that it was important to highlight that treatment options may be different for these women and that there was an even greater need to minimise repeat surgery in this population.
Although there were no studies that looked specifically at pre-surgical hormonal treatment, the Committee felt that this may often be the case in practice, as women may have been receiving ovulation suppression for many months/years prior to surgery. The Committee concluded that there was insufficient evidence to recommend hormonal therapy as a standard treatment prior to surgery although they acknowledged that that there may be some benefit for some women with deep endometriosis involving the bowel, bladder or ureter, as based on their clinical experience and knowledge, pre-operative GnRH agonists can reduce surgical complications such as bleeding. However, the Committee agreed that the decision to use GnRH agonists pre-operatively should be made on an individual level based on surgeon and patient preference and informed consent.
The Committee also discussed whether this topic should be prioritised for a research recommendation. They decided that there was a gap in the evidence with regards to the effectiveness of ablation or excision related to peritoneal endometriosis. The research recommendations are provided below.
The Committee discussed whether to cross-refer to recommendations in the laparoscopic uterosacral nerve ablation (LUNA) for chronic pelvic pain NICE interventional procedure guideline (IPG). They agreed that there was considerable uncertainty about the conclusions of this IPG for women with endometriosis and therefore did not feel sufficiently confident to refer to this.
11.3.4.6. Key conclusions
The Committee concluded that clinicians should discuss with women whether they would like uncomplicated endometriomas or peritoneal endometriosis to be treated if found during diagnostic laparoscopy. The discussion should highlight the potential risks and benefits of the laparoscopy and allow women to make an informed choice regarding their treatment.
As there was evidence that post-surgical hormonal therapy gave additional benefit over surgery alone, the Committee recommended that this be offered after surgery. Although there was no evidence available regarding the use of GnRH agonists prior to surgery, the Committee agreed that a recommendation should be made to support this because based on their experience and knowledge, pre-operative GnRH agonists can reduce surgical complications such as bleeding. The decision to use GnRH agonists pre-operatively should be made on an individual patient basis and only in severe deep disease
11.3.5. Recommendations
- 40.
Ask women with suspected or confirmed endometriosis about their symptoms, preferences and priorities with respect to pain and fertility, to guide surgical decision-making.
- 41.
Discuss surgical management options with women with suspected or confirmed endometriosis. Discussions may include:
- what a laparoscopy involves
- that laparoscopy may include surgical treatment (with prior patient consent)
- how laparoscopic surgery could affect endometriosis symptoms
- the possible benefits and risks of laparoscopic surgery
- the possible need for further surgery (for example, for recurrent endometriosis or if complications arise)
- the possible need for further planned surgery for deep endometriosis involving the bowel, bladder or ureter.
- 42.
Perform surgery for endometriosis laparoscopically unless there are contraindications.
- 43.
During a laparoscopy to diagnose endometriosis, consider laparoscopic treatment of the following, if present:
- peritoneal endometriosis not involving the bowel, bladder or ureter.
- uncomplicated ovarian endometriomas.
- 44.
As an adjunct to surgery for deep endometriosis involving the bowel, bladder or ureter, consider 3 months of gonadotrophin-releasing hormone agonistsb before surgery.
- 45.
Consider excision rather than ablation to treat endometriomas, taking into account the woman’s desire for fertility and her ovarian reserve. Also see ovarian reserve testing in the NICE guideline on fertility problems.
11.3.6. Research recommendations
- 4.
Is laparoscopic treatment (excision or ablation) of peritoneal disease in isolation effective for managing endometriosis-related pain?
Why this is important
Isolated peritoneal endometriosis can be an incidental finding in women who may or may not experience pain or other symptoms.
Research is needed to determine whether laparoscopic treatment of isolated peritoneal endometriosis in women with endometriosis-related pain results in a clinical and cost-effective improvement in symptoms.
The current literature does not provide a clear answer because the stage of endometriosis is often not sufficiently clearly defined in research studies, and the treatment modalities used are multiple and varied. The resultant amalgamation of various stages of endometriosis and variable treatment modalities leads to loss of certainty of outcome in this specific group of women.
Establishing whether treating isolated peritoneal endometriosis is cost effective is important, because this forms a large part of the workload in general gynaecology, and uses considerable resources.
11.3.7. Pairwise comparison of combinations of treatments
Review question: What is the effectiveness of hormonal treatment before or after surgery for treatment of endometriosis?
11.3.7.1. Description of clinical evidence
The aim of this review is to determine the clinical and cost effectiveness of pharmacological therapy in combination with surgery in women with endometriosis. Pharmacological therapy specifically included hormonal suppression treatments available in the UK and 4 comparisons are examined:
- pharmacological therapy before surgery vs. placebo or no pharmacological therapy before surgery
- pharmacological therapy after surgery vs. placebo or no pharmacological therapy after surgery
- pharmacological therapy before surgery vs. pharmacological therapy after surgery
- pharmacological therapy before and after surgery vs. placebo or no pharmacological therapy before and after surgery
For full details see review protocol in Appendix D.
In total 12 studies were included, but the evidence of these studies only addressed 1 of the 4 possible comparisons.
No studies were identified for inclusion for the following 3 comparisons:
- pharmacological therapy before surgery vs. placebo or no pharmacological therapy before surgery
- pharmacological therapy before surgery vs. pharmacological therapy after surgery
- pharmacological therapy before and after surgery vs. placebo or no pharmacological therapy before and after surgery
For the comparison ‘pharmacological therapy after surgery vs. placebo or no pharmacological therapy after surgery’ 12 studies were included in total (Abou-Setta 2013, Alborzi 2011, Bianchi 1999, Busacca 2001, Furness 2011, Loverro 2008, Mettler 2014, Muzii 2000, Parazzini 1994, Serrachioli 2010, Sesti 2007, Sesti 2009).
Two were Cochrane systematic reviews (Abou-Setta 2013 and Furness 2011) and the remaining 10 studies were randomised controlled trials (Alborzi 2011, Bianchi 1999, Busacca 2001, Loverro 2008, Mettler 2014, Muzii 2000, Parazzini 1994, Serrachioli 2010, Sesti 2007, Sesti 2009). The full text of all trials included in the Cochrane reviews were considered for inclusion according to the protocol. Additional outcomes from 6 trials were also included in this review (Bianchi 1999, Busacca 2001, Loverro 2008, Muzii 2000, Parazzini 1994, Sesti 2007).
Of the remaining 4 trials, one reported outcomes relevant to this review (Sesti 2009) and 3 were published subsequently to the searches performed within the Furness 2011 Cochrane review (Alborzi 2011, Mettler 2014, Serrachioli 2010).
All studies included women who had confirmed endometriosis and who had undergone surgery prior to being randomised to hormonal suppression treatment compared to no treatment or placebo. Available details of the surgery performed are noted in Table 124.
The post-surgical hormonal suppression treatments in the intervention arms were gonadotropin-releasing hormone agonists (GnRHa) (including leuprorelin, triptorelin, goserelin, nafarelin, and decapeptyl), letrozole, combined oral contraceptives, medroxyprogesterone acetate and danazol and the 2 trials in the Abou-Setta 2013 Cochrane review used a levonorgestrel-releasing intrauterine device (LGN-IUS).
Evidence was available for the critical outcomes of pain relief and health related quality of life. Evidence was available for the important outcome of rate of success (subsequent reoperation rate and disease recurrence) and participant satisfaction with treatment. No evidence was available for effect on daily activities or number of live births. Evidence relating to fertility is covered in the Network Meta-Analysis (NMA).
Evidence from the included studies are summarised in the clinical GRADE evidence profile below (Table 124).
Stratified analysis according to the specifications in the protocol was not possible due to the presentation of the available data.
See also the study selection flow chart in Appendix F, study exclusion list in Appendix H, forest plots in Appendix I, full GRADE profiles in Appendix J and study evidence tables in Appendix G. Summary of included studies
A summary of the studies that were included in this review are presented in Table 124.
11.3.7.2. Clinical evidence profile
The clinical evidence profile for this review question (Post-surgical pharmacological therapy versus placebo or no treatment) is presented in Table 125.
11.3.8. Economic evidence
One paper was found contrasting the use of medical treatments following surgery. Additionally, 1 paper was found looking at the costs of medical treatments before surgery.
Sanghera (2016)
This paper refers to a de novo economic model intended to assess the use of levonorgestrel-releasing intrauterine system, depot-medroxyprogesterone acetate, combined oral contraceptive pill (cOCP) and ‘no treatment’ after conservative surgery to prevent recurrence of endometriosis.
The model was a Markov Chain design with a time horizon of 36 months and a cycle length of 1 month. A discount rate of 3.5% is used and no half-cycle correction is applied as the model is based on discrete transitions. Cost estimates were taken from a ‘recent primary parallel study’ and appear to be taken from standard sources such as the NHS Reference Costs and NICE evaluations, uprated to 2016 values. Costs for increase indirect health resource use were not estimated. Utility estimates were taken from clinical consensus using a modified visual analogue scale.
LNG-IUS cost £650.94 and generated 1.88 quality adjusted life years (QALYs), Depot medroxyprogesterone acetate (DMPA) cost £622.56 and generated 1.92 QALYs, cOCP cost £599.93 and also generated 1.92 QALYs and no treatment cost £371.34 and generated 2.27 QALYs. This indicates that no treatment dominates, as it is both the most effective and cheapest option following surgery. The paper is significantly limited by having to rely on estimates for the utility values of treatment states, as the results are heavily influenced by estimates of these values. As the result is highly counter-intuitive and contradicts estimates made by members of the Committee, less weight is put on this finding in the economic model and subsequent Committee discussion.
The paper also conducts a literature review and finds no other papers conducting an economic evaluation of hormonal treatment for endometriosis following conservative surgery, consistent with our findings.
Ferracini & Nakada (2013)
This paper is a cost minimisation study contrasting 3 possible pre-surgical hormonal treatment strategies. These strategies were: dienogest then surgery, leuprorelin acetate then surgery and finally one drug, then the other drug if no effect, then surgery in any case.
The source of cost data was ‘Brazilian official data’ and the time horizon was 6 months. The unit of cost measure was the Brazilian Real, BRL. As the time horizon was below 1 year, no discount rate has been applied. The Brazilian system is partially privately funded, so the authors disaggregated these costs.
For the comparison of dienogest vs. leuprorelin, the private cost of dienogest was 1020.42 Brazilian Real (BRL) (~£250) and the public cost was 1461.22 BRL (~£350) while the private cost of leuprorelin was 2328.94 (~£580) BRL and the public cost 2377.52 BRL (~£585)
For the comparison of both drugs together, the private cost of dienogest first was 882.74 BRL (~£200) and public cost 942.18 BRL (~£220), whereas the private cost of leuprorelin first was 768.13 BRL (~£170) and the public cost 856.77 BRL (~£210).
This does not provide good evidence on whether it is cost-effective to offer hormonal treatment before surgery, but does indicate there is a cost saving to providing robust hormonal treatment if hormonal treatment is offered before surgery.
Summary of findings from economic model
The cost of providing hormonal treatment after surgery is assumed to be simply the cost of the surgery itself plus the cost of a course of hormonal treatment to follow. The literature is inconsistent around which drug should be provided and for how long – a range of 3 months to 24 months has been identified in the clinical review. An estimate of 12 months of additional treatment with danazol is used for the purpose of economic modelling, making the total cost £1,546.42 for the initial surgery and a maximum of £597.56 for the subsequent hormonal treatment (this could be less if the woman relapses before the end of the full course of treatment) – the maximum cost of this technique is therefore £2,143.98.
An important health economic issue is whether the addition of hormonal treatment delays the recurrence of endometriosis. For example, doubling average recurrence time would halve the number of operations required to treat a woman over the course of her lifetime, with clear cost implications. The Committee thought it biologically plausible that such an effect might occur, but conceded that the evidence was not strong enough to recommend one way or the other.
Table 127 demonstrates that considering surgical treatments alone, combination treatment with an MRI or laparoscopic diagnosis extendedly dominates surgery alone. This is unsurprising as the NMA finds that combination treatment is extremely effective at controlling symptoms of pain.
Table 128 that the opposite effect is true if the primary concern of the woman is to preserve fertility. The NMA showed that hormonal treatment suppressed fertility, so the most effective method of accruing quality adjusted life year (QALYs) for a woman (which were highly conditional on a live birth) was to offer surgery alone, without subsequent hormones.
The final column (probability of live birth) demonstrates that the effect of surgery appears greater than the effect of subsequent hormonal treatment; by the end of their lives more women on a combination treatment plan had had a live birth than women on no treatment at all. However since treatment with no subsequent hormonal treatment is cheaper than hormonal treatment and hormonal treatment suppress fertility the overall effect is for laparoscopy + hormonal treatment to be dominated by laparoscopy alone.
11.3.9. Clinical evidence statements
11.3.9.1. Pain
Pain recurrence
Low quality evidence from 1 trial (n= 53) reported that there is no clinically significant difference between intranasal nafarelin and placebo after surgery for pain recurrence (measured using Andersch and Milsom scale).
Very low quality evidence from 4 trials (n= 476) found that there is no clinically significant difference between hormonal treatment (triptorelin, goserelin, decapeptyl, letrozole and danazol) and no treatment after surgery for pain recurrence at 12 months.
Very low quality evidence from 3 trials (n= 312) reported that there is no clinically significant difference between hormonal treatment (leuprolide, goserelin and cyclic combined oral contraceptives) and no treatment after surgery for pain recurrence at 13 to 24 months.
Very low quality evidence from 1 trial (n=54) reported that there is no clinically significant difference between triptorelin treatment and no treatment after surgery for pain recurrence at 5 years.
Pelvic pain
Moderate evidence from 1 trial (n=187) found a clinically significant beneficial effect of hormonal treatments (triptorelin, leuprorelin and oestroprogestin) compared with placebo for pelvic pain (measured using VAS) after surgery although there was low and very low quality evidence of no clinically significant difference between the 2 interventions for dysmenorrhoea and deep dyspareunia.
Dyspareunia
Low quality evidence from 1 trial (n=120) found a clinically significant beneficial effect of leuprorelin treatment compared with no treatment for dyspareunia (measured using a questionnaire) after surgery at 12 months although there was low and very quality evidence of no clinically significant difference between the 2 interventions for abdominal pain or dysmenorrhoea.
Dysmenorrhoea
Moderate quality evidence from 2 trials (n= 95) found a clinically significant beneficial effect of LGN-IUS treatment compared with no treatment after surgery for dysmenorrhoea at 12 months.
Recurrence of endometriosis
Very low quality evidence from 1 trial (n=285) reported that there is no clinically significant difference between leuprolide treatment and no treatment after surgery for recurrence of endometriosis at 5–6 months after starting treatment.
Very low quality evidence from 3 trials (n=310) reported that there is no clinically significant difference between hormonal treatment (triptorelin, letrozole, leuprolide and danazol) and no treatment after surgery for recurrence of endometriosis at 12 months
Very low quality evidence from 1 trial (n=45) reported that there is no clinically significant difference between hormonal treatment (danazol or an unspecified GnRH agonist) compared with no treatment after surgery for endometriosis recurrence at 24 months.
11.3.9.2. Recurrence of endometrioma
Low quality evidence from 3 trials (n= 463) reported a clinically significant beneficial effect of between hormonal treatment (triptorelin, leuprolide and combined oral contraceptives) and placebo or no treatment after surgery for endometrioma recurrence at 13–36 months.
Very low quality evidence from 1 trial (n=35) reported that there is no clinically significant difference between triptorelin treatment and no treatment after surgery for endometrioma recurrence at 5 years.
11.3.9.3. Health related quality of life
Very low quality evidence from 1 trial (n=187) reported that women receiving hormone treatment with GnRH agonist or oestroprogestin (oestradiol plus medroxyprogesterone) and women receiving placebo had improved quality of life (improved scores in all domains of the SF-36 general health survey) at 12 months.
Satisfaction
Low quality evidence from 2 trials (n=95) reported no clinically significant difference in patient satisfaction with treatment results when LGN-IUS treatment was compared with no treatment after surgery.
11.3.9.4. Reoperation rates
Very low quality evidence from 3 trials (n=327) reported that there is no clinically significant difference between hormonal treatment (triptorelin, leuprolide, danazol and oestroprogestin) and placebo or no treatment after surgery on reoperation rates.
11.3.10. Evidence to recommendations
11.3.10.1. Relative value placed on the outcomes considered
The Committee prioritised pain relief, health related quality of life and adverse events as critical outcomes for their decision making and number of women requiring more surgery, absence from work and other activities of daily living and fertility as important outcomes. However, when the outcomes for the NMA were discussed subsequently, it was decided that adverse events causing withdrawal from the study and fertility would be more appropriately considered as outcomes in the NMA.
11.3.10.2. Consideration of clinical benefits and harms
In view of the high rate of recurrence of endometriosis, affecting long-term quality of life for many women, improvement in long-term control of the condition was felt by the Committee to be clinically very important. The Committee were aware of the high rate of reoperation for endometriosis with associated risks of surgery and, as there was strong evidence to support this, considered that avoidance of repeat surgery by the use of long-term medical therapy would be beneficial. The Committee noted that the duration of follow-up in most studies was insufficient, but brought additional clinical experience to the discussion. Based on the evidence, the beneficial effect of all hormonal therapies was similar (probably because all work through similar mechanisms) and so the Committee considered the adverse effects of the various treatments in making their recommendation, as there are known side effects with hormonal treatments that some women may wish to avoid.
In general, the Committee considered that the combined oral contraceptive pill or long-acting reversible progestogen contraceptives were the most acceptable treatments. The Committee noted that these would not be appropriate for women who were trying to conceive, although they could be used by women who were planning pregnancy at some time in the future. They also felt it was important to note that GnRHa are only licensed for 6 months due to a loss of bone density.
11.3.10.3. Consideration of economic benefits and harms
The Committee discussed how the addition of hormonal treatments either before or after surgery was likely to carry a very low direct cost to the NHS and therefore could be recommended if the clinical evidence was thought strong enough to support such a recommendation. Many studies identified in the literature review used a more expensive hormonal treatment such as GnRHa in their pre / post-surgical dosage, and the economic evidence for this is more equivocal – although the model suggests that it would be cost-effective to offer such treatment at £20,000 / QALY, the Committee were told that cheaper hormonal treatments like the combined oral contraceptive pill were likely to be more cost-effective
The above holds true for fertility treatments too. If fertility outcomes are improved by adding a hormonal treatment then this could be considered as it is likely to be cost-effective, but the Committee thought in this instance that the clinical evidence did not support offering a hormonal treatment to women attempting to become pregnant.
The likely resource impact of these recommendations is somewhere between low and negative – most women who are able to tolerate hormonal treatments already receive these for endometriosis, so the Committee’s recommendations were not a significant departure from current practice. Even if they were, the cost of long-acting reversible contraception is not substantial. The recommendations may cause a small cost saving, since Committee opinion is that some clinicians prescribe more expensive hormonal treatments such as GnRHas before trialling combined oral contraceptive pill or long-acting reversible progestogen contraceptives. These recommendations should prevent that unwarranted clinical variation, saving NHS resources.
11.3.10.4. Quality of evidence
Evidence was available from 12 studies in total and the quality ranged from moderate to very low. Studies that reported pain as dichotomously or results from scoring systems not included in the NMA were included in the pairwise reviews. Pain outcomes using the Biberoglu and Behrman scale (B&B) were also reported in the pairwise analysis where these were presented as separate components (dysmenorrhoea, dyspareunia and pelvic pain).
The Committee commented that the descriptions of the surgery performed were poor and that the included studies had been published over a 30 year period. Although the techniques used over this time had not changed greatly, there had been significant improvement in laparoscopic technology resulting in a surgeon’s ability to remove more diseased tissue through improved visualisation. Thus it was difficult to draw overall conclusions from the included studies regarding the quality of the surgery performed. The Committee further noted that this might also affect assessment of the effectiveness of the additional hormonal suppression therapy as women might have a comparatively greater treatment effect where less diseased tissue had been removed by surgery.
The Committee noted that 3 trials had used excision techniques to remove endometrioma rather than ablative techniques and that excision had been demonstrated to be superior to ablation in a separate review.
Various hormone suppression therapies were examined in the included studies. The Committee questioned the relevance and accuracy of reporting of dysmenorrhoea and dyspareunia pain outcomes in trials using GnRH analogues. These therapies can suppress menstruation and decrease libido to such an extent that assessment of pain associated with menstruation or sexual intercourse might be irrelevant if neither were occurring and studies did not report any confirmation of questioning women as to whether either were.
Further, different types of GnRHa therapies have different routes of administration. For example, leuprorelin is administered as a depot injection which diminishes uncertainty regarding dose received and user compliance compared to intranasal administration of nafarelin where there can be variability in the dose retained and which needs to be administered every 12 hours or so,
The results of 1 trial conducted in 1994 were particularly unreliable as surgery had been performed using laparotomy combined with intranasal nafarelin.
11.3.10.5. Other considerations
The Committee gave special consideration to young women (aged 17 and under) and discussed whether any additional recommendations were necessary but concluded that none were required.
Based on consensus the Committee agreed that hormonal treatment prior to surgery would only be suitable for women with deep endometriosis involving the bowel, bladder or ureter. The Committee noted that this would usually lead to less bleeding and would therefore aid the surgical procedure.
11.3.10.6. Key conclusions
The Committee based their recommendations on the findings of the NMA, which demonstrated that adding hormonal treatment following surgery (laparoscopic excision or ablation) reduces the risk of recurrence and symptoms, so it should be offered to women post-surgery unless they want to conceive.
11.3.11. Recommendations
- 46.
After laparoscopic excision or ablation of endometriosis, consider hormonal treatment (with, for example, the combined oral contraceptive pill)c, to prolong the benefits of surgery and manage symptoms.
11.4. Hysterectomy (with or without oophorectomy) in combination with surgical management
Review question: What is the effectiveness of hysterectomy with or without oophorectomy, including recurrent and asymptomatic endometriosis, in managing endometriosis?
11.4.1. Introduction
Hysterectomy combined with surgical excision/ablation of endometriosis is currently offered for the treatment of endometriosis when medical and hysterectomy sparing surgical options have been offered, failed or are inappropriate. Hysterectomy is associated with potential morbidity and a very low risk of mortality. Due to the fact that endometriosis is thought to be a predominantly oestrogen-dependent disease, women can opt to have their ovaries removed at the time of hysterectomy, often depending on the severity and location of their endometriosis. However, it is unclear whether a hysterectomy without oophorectomy may be as clinically effective as with oophorectomy and there is currently variation in clinical practice.
In either case it is critical that women are appropriately counselled about the fact that they will no longer be able to have children after a hysterectomy, the risks of early oophorectomy (e.g. osteoporosis, cardiovascular disease), the effects of a surgical menopause, the need for hormone replacement until the age of natural menopause and the potential for recurrence of the disease. There are also different routes by which this could be carried out, i.e. laparoscopic or abdominal. However, individual assessment and the experience of the clinician are very important because patient characteristics and surgical expertise are determinants of the chosen approach. Hysterectomy is not currently offered for the treatment of asymptomatic endometriosis. The effectiveness of hysterectomy with and without oophorectomy is discussed below.
11.4.2. Description of clinical evidence
The objective of this review is to determine the clinical and cost-effectiveness of hysterectomy with or without oophorectomy in reducing pain, improving health-related quality of life and reducing adverse events.
For full details see the review protocol in Appendix D.
Two observational studies were included in this review (Shakiba 2008, Namnoum 1995). No other evidence was identified.
Both of the included studies were retrospective cohort studies that were carried out in the USA.
In both studies, a retrospective review of medical records was completed. In Shakiba 2008, records were searched for women who had surgery for chronic pelvic pain with histological confirmation of endometriosis, of any stage and severity between January 1995 and December 2003. Women who had surgery for infertility or menorrhagia as the primary indication were excluded from the study. Follow-up information was obtained in 2006 from medical records (operative reports, pathology reports, outpatient charts and a telephone survey consisting of a questionnaire about reoperation, pain clinic visit, medical treatment and level of satisfaction). Surgery was only performed if other therapies failed to control symptoms.
In Namnoum 1995 the inclusion criteria were women who underwent a hysterectomy with a diagnosis of endometriosis (unclear diagnostic method) between 1979 and 1991. The study excluded women who were older than 45 years at the time of hysterectomy in order to prevent confounding the results by including data from women with menopausal changes. Follow-up data were obtained primarily from outpatient charts and telephone questionnaires. However, written questionnaires were sent if the patient could not be reached by telephone
Shakiba 2008 evaluated the need for further surgery after laparoscopic excision of endometriosis or hysterectomy. Even though the focus of the study does not match our current protocol, women who had hysterectomy (n=97) were divided into 2 subgroups depending on whether they had bilateral oophorectomy. For this review we selected data for hysterectomy subgroups (hysterectomy with or without oophorectomy; n=47 and n=50 respectively). The only outcome reported was the effect of ovarian preservation on reoperation-free survival for each surgery group. In this review, the data for the outcome for 7 years follow-up in the 2 hysterectomy subgroups (hysterectomy with or without oophorectomy) was presented.
Namnoum 1995 compared the rates of reoperation and symptom recurrence (pain) between groups with some ovarian preservation (n=29) compared with women who had all ovarian tissue removed (n=109). The mean duration of follow-up was 58 months (4 years 10 months) post hysterectomy.
We did not identify any evidence for the following outcomes:
- Quality of life
- Effect on daily activities
- Unintended effects from treatment
- Participant satisfaction with treatment
Evidence is summarised in the clinical GRADE evidence profile below (Table 130). See also the study selection flow chart in Appendix F, the study selection flow chart in Appendix F, study exclusion list in Appendix H, forest plots in Appendix I, full GRADE profiles in Appendix J and study evidence tables in Appendix G. Summary of included studies
A summary of the studies that were included in this review are presented in Table 129.
11.4.3. Clinical evidence profile
The clinical evidence profile for this review question (hysterectomy with or without oophorectomy for the treatment of endometriosis) is presented in Table 130.
In Namnoum 1995, a Cox proportional hazards model was used to investigate the relative risk of pain recurrence and relative risk of reoperation when adjusted for age at time of hysterectomy (≤35 years vs. >35 years), stage of disease (revised AFS criteria), previous medical therapy and previous surgical therapy. The results for the risk of pain recurrence showed that the relative risk for pain 6.1 (95% confidence interval (CI) 2.5% to 14.6%) with ovarian conservation compared with bilateral oophorectomy. The results for reoperation showed that the relative risk of reoperation was 8.1 (95% CI 2.1% to 31.2%) with ovarian conservation compared with bilateral oophorectomy.
In Shakiba 2008, a Cox proportional hazards ratio investigating time to reoperation when adjusted for age and stage of disease was reported for hysterectomy plus bilateral oophorectomy compared with hysterectomy only and showed that preservation of both ovaries increased the risk of reoperation by 2.44 times compared with both ovaries removed, but there was a lot of uncertainty around this result (P=0.18) with a wide 95% CI (0.65% to 9.10%), due to the small sample size. The authors reported that confounding factors such as stage of disease did not have any effect on surgery free time in either group, but age at the time of surgery was important in determining the outcome.
A Kaplan-Meier graph showed reoperation free survival estimates at 2, 5 and 7 years in the hysterectomy subgroups. In the hysterectomy only group, the 2, 5 and 7 year percentages of women who avoided reoperation were 95.7%, 86.6% and 77.0% respectively. In the hysterectomy with bilateral oophorectomy group, the 2, 5 and 7 year percentages of women who avoided were 96.0%, 91.7% and 91.7% respectively. It would suggest that women who had oophorectomy at the time of hysterectomy had a lower reoperation rate compared with women who had hysterectomy alone.
11.4.4. Clinical evidence statements
Very low quality evidence from 1 retrospective cohort study with 97 participants showed that there was no clinically significant difference between the 2 interventions for reoperation free survival up to 7 years.
Very low quality evidence from 1 retrospective cohort study with 136 participants that after a mean follow-up of 4 years 10 months, there was a lower rate of reoperation after hysterectomy with oophorectomy compared to hysterectomy with ovarian conservation.
Very low quality evidence from 1 retrospective cohort study with 136 participant that after a mean follow-up of 4 years 10 months, there was a lower rate of pain recurrence after hysterectomy with oophorectomy compared to hysterectomy with ovarian conservation.
11.4.5. Economic evidence
No health economic evidence was found on the cost-effectiveness of hysterectomy for endometriosis.
The costs of hysterectomy to the NHS are largely driven by the cost of the operation itself. Additionally, there may be complications or long-term effects of the operation which should be taken into account. Table 131 presents various costs for the initial operation given in the NHS Reference Costs for 2013/14. There is no specific code for a hysterectomy (either with or without oophorectomy), so the table presents a variety of plausible codes.
Excluding pelvic exenteration (which is included as an upper bound figure only), it seems the cost of a hysterectomy to the NHS is somewhere between £3500 and £4000.
Hysterectomy is likely to be a highly cost-effective treatment for endometriosis if it is clinically effective, especially if given to young women as it requires a one-off payment. However the economic harm of such a strategy is that the woman will be infertile for the rest of her life. Another harm is that this treatment will induce a surgical menopause; if menopause has Quality of life (QoL) implications (for example, affecting a woman’s mental health more than if the menopause happens naturally over a number of years) so too will this strategy.
Table 132 indicates that a hysterectomy is cost-effective at a very low threshold of £574 / quality adjusted life year (QALY) and every technique is highly likely to be cost-effective vs. no treatment for a given patient. In comparison to the most cost-effective treatment in the model (empirical diagnosis and oral hormonal contraceptive pill), hysterectomy is cost-effective at a threshold of £4239 / QALY. Note that this model does not estimate the harm of giving a hysterectomy to a woman who does not have endometriosis (and so therefore is not cured by giving up her fertility) but this would likely be large.
11.4.6. Evidence to recommendations
11.4.6.1. Relative value placed on the outcomes considered
The Committee considered the following outcomes to be important for their decision-making:
- Pain relief
- Quality of life
- Unintended effects from treatment
Evidence was only available for pain relief in 2 old, small retrospective cohort studies. Evidence for the other critical outcomes was not identified.
11.4.6.2. Consideration of clinical benefits and harms
As only very low quality evidence was identified, the Committee based their recommendations on their experience and expertise.
The Committee was of the opinion that endometriosis by definition is endometriotic tissue outside the uterus, which means that it is not expected to be cured by hysterectomy. However, they agreed to highlight some indications for hysterectomy (for instance in presence of adenomyosis or heavy menstrual bleeding not responding to other treatments) and that it would then be important that the endometriotic lesions would be removed at the same time. Since this excision or ablation of the endometriotic tissue would be carried out laparoscopically the Committee agreed that the hysterectomy should also be carried out laparoscopically to enable both to happen in one procedure. The Committee noted that laparoscopic surgery may be contraindicated for a few women for example, those who cannot undergo procedures under anaesthetic, where there are large fibroids or where there are severe adhesions perhaps following major bowel resection, but that generally decisions regarding surgery would be based on relative harms and benefits. Also, the Committee noted that endometriosis is a hormone-dependent condition and it is therefore plausible that oophorectomy would be more effective than hysterectomy alone.
They concluded that making an informed choice was very important to women and that therefore all necessary details should be discussed. The Committee identified that women would need to know that hysterectomy was not a treatment for endometriosis but that excision of the endometriotic lesions at the same time is the actual treatment. Having the hysterectomy could then prevent or delay recurrence of endometriosis. Healthcare professionals should inform women about the procedure how it would affect their symptoms and the implications of oophorectomy (such as possible risks related to osteoporosis and cardiovascular conditions). The Committee recognised that there can be significant social / psychological effects of hysterectomy. The Committee considered cross-referencing to the NICE guideline on heavy menstrual bleeding, but because the population within that guideline would be different (they would not necessarily be suspected of having endometriosis), elected not to do so. The Committee noted that bilateral oophorectomy induces surgical menopause and therefore cross referenced the menopause guideline because the symptoms of menopause may be severe and the benefits and risks of hormone replacement therapy should be discussed.
11.4.6.3. Consideration of economic benefits and harms
The Committee understood that health economic costs did not indicate hysterectomy should be a first-line treatment, but that hysterectomy was a cost-effective option to consider in women who would prefer this option over and above their other options which would preserve fertility.
Hysterectomy for women with endometriosis is cost-saving for the NHS and so these recommendations are likely to have a small negative resource impact
11.4.6.4. Quality of evidence
Evidence from 2 retrospective cohort studies was identified for inclusion and was of very low quality due to risk of bias in both studies (study design, outcome selection and detection bias), imprecision of results in 1 study (width of the confidence interval) and indirectness in 1 study (age of study limiting applicability for modern surgical techniques). Therefore there is uncertainty around the evidence that these studies provides.
The data in both studies were limited due to their retrospective cohort design. In 1 study the main comparison was between laparoscopic excision and hysterectomy. The results for the current review rely on a subgroup analysis only and were therefore underpowered. The second study was old (hysterectomies were conducted between 1979 and 1991) and it is unclear whether the outcomes would have changed based on modern techniques.
It is difficult to say whether the results are generalizable to all women who would have such surgery, because women who were included in the studies were from tertiary care referral centres. In both studies more than 50% of the women had advanced disease and more than 50% had at least 1 previous surgery, with the rates being as high as 77% in 1 study.
Although the result from these studies may show clinical benefit, it should be applied with caution as there are limitations in study design and the ability to be applied to the current population. In addition, the Shakiba 2008 result is not precise and also both studies had a small sample size and no other, better quality evidence has been identified.
11.4.6.5. Other considerations
The Committee noted that that the laparoscopic approach to hysterectomy is possibly safer and is a better use of resources than laparotomy which is no longer widely used.
11.4.6.6. Key conclusions
The Committee concluded that the 2 included studies provided too little and very low quality evidence to draw clear conclusions about the comparative effects between hysterectomy only and hysterectomy plus oophorectomy. The Committee therefore based the recommendations on expertise, experience and consensus highlighting examples of some possible indications, noting that the endometriotic tissue should be removed at the same time, and stating how this procedure should be carried out. The Committee also agreed that it was important to provide information to women who consider a hysterectomy and specified what a discussion about this should include.
11.4.7. Recommendations
- 47.
If hysterectomy is indicated (for example, if the woman has adenomyosis or heavy menstrual bleeding that has not responded to other treatments), excise all visible endometriotic lesions at the time of the hysterectomy.
- 48.
Perform hysterectomy (with or without oophorectomy) laparoscopically when combined with surgical treatment of endometriosis, unless there are contraindications.
- 49.
For women thinking about having a hysterectomy, discuss:
- what a hysterectomy involves and when it may be needed
- the possible benefits and risks of hysterectomy
- the possible benefits and risks of having oophorectomy at the same time
- how a hysterectomy (with or without oophorectomy) could affect endometriosis symptoms
- that hysterectomy should be combined with excision of all visible endometriotic lesions
- endometriosis recurrence and the possible need for further surgery
- the possible benefits and risks of hormone replacement therapy after hysterectomy with oophorectomy (also see the NICE guideline on menopause).
Footnotes
- a
At the time of publication (September 2017), not all combined oral contraceptive pills or progestogens have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council’s Prescribing guidance: prescribing unlicensed medicines for further information.
- b
At the time of publication (September 2017), not all gonadotrophin-releasing hormone agonists have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council’s Prescribing guidance: prescribing unlicensed medicines for further information.
- c
At the time of publication (September 2017), not all hormonal treatments (including not all combined oral contraceptive pills) have a UK marketing authorisation for this indication. The prescriber should follow relevant professional guidance, taking full responsibility for the decision. Informed consent should be obtained and documented. See the General Medical Council’s Prescribing guidance: prescribing unlicensed medicines for further information.
Figures
Figure 13Network for hormonal therapy for pain relief (VAS)
The size of nodes is proportional to the number of women in the network who were given a particular treatment class. The thickness of connecting lines is proportional to the number of studies directly comparing 2 treatment classes. Red nodes indicate treatment classes that are informed only from Biberoglu and Behrman scales. For treatment name abbreviations, see Table 62.
Figure 14Forest plot showing mean differences (95% CrI) of multivariate NMA estimates for each treatment versus placebo/no treatment for pain relief on the VAS
For treatment name abbreviations, see Table 61
Figure 15Network for treatments for relief of dyspareunia
The size of nodes is proportional to the number of women in the network who were given a particular treatment class. The thickness of connecting lines is proportional to the number of studies directly comparing 2 treatment classes. Four treatment classes were not connected and could not be compared in the NMA (progestogens (oral), progestogen + oestrogen (oral), aromatase inhibitor + progestogen (oral), aromatase inhibitor + GnRHa (i.m.)). For treatment name abbreviations, see Table 62.
Figure 16Forest plot showing mean differences (95% CrI) of NMA estimates for each treatment versus placebo for the relief of dyspareunia
For treatment name abbreviations, see Table 61
Figure 17Network for discontinuation of treatment due to adverse events
The size of nodes is proportional to the number of women in the network who were given a particular treatment class. The thickness of connecting lines is proportional to the number of studies directly comparing 2 treatment classes. Two treatment classes were not connected and could not be compared in the NMA (aromatase inhibitors + progestogens (oral) and aromatase inhibitors + GnRHa (i.m.)). For treatment name abbreviations, see Table 62.
Figure 18Forest plot showing odds ratios (95% CrI) of NMA estimates for each treatment versus placebo/no treatment for discontinuation due to adverse events
For treatment name abbreviations, see Table 62
Figure 20Network for surgery and combined surgery plus hormonal therapy for pain relief (VAS)
The size of nodes is proportional to the number of women in the network who were given a particular treatment class. The thickness of connecting lines is proportional to the number of studies directly comparing 2 treatment classes. 2 treatment classes were not connected and could not be compared in the NMA (laparoscopic surgery + GnRHa (s.c.) and laparoscopic surgery + GnRHa (s.c.) + progestogen + oestrogen (oral)). For treatment name abbreviations, see Table 110.
Figure 21Forest plot showing mean differences (95% CrI) of NMA estimates for each treatment versus diagnostic laparoscopy/no treatment for pain relief on the VAS
For treatment name abbreviations see Table 110
Tables
Table 52Summary of included studies
| Study | Intervention/comparison | Population | Outcomes | Comments |
|---|---|---|---|---|
| Kauppila 1985 Finland | NSAIDs (Naproxen Sodium)/placebo | 20 women with endometriosis classified using American Fertility Society (AFS) criteria |
| Crossover trial Study funded by pharmaceutical company |
NSAIDs: non-steroidal anti-inflammatory drugs; RCT: randomised controlled trial
Table 53Summary clinical evidence profile: analgesics versus placebo
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | Absolute effect | No. of participants (studies) | Evidence quality (GRADE) | Comments | |
|---|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||||
| Placebo | Intervention (analgesics) | ||||||
| Overall pain relief (self-measured by questionnaire) | 625 per 1,000 | 906 per 1000 (512–1,000) | RR 1.45 (0.82 to 2.57) | 281 more per 1,000 (from 113 fewer-981 more) | 19 (1 study) | ⊕⊝⊝⊝ Very low1,2,3,4 | Measured with 3 point scale questionnaire |
| Unintended effects from treatments (hypo-menorrhea, diarrhoea. increased diuresis, headache, epigastric pain nausea, tremor and dizziness) | 778 per 1,000 | 366 per 1,000 | RR 0.47 (0.2 to 1.1) | 412 fewer per 1,000 (from 622 fewer-78 more) | 20 (1 study) | ⊕⊝⊝⊝ Very low1,3,4 | |
| Supplement ary analgesia needed | 222 per 1,000 | 91 per 1,000 (9–849) | RR 0.36 (0.04 to 3.35) | 160 fewer per 1,000 (from 240 fewer587 more) | 19 (1 study) | ⊕⊝⊝⊝ Very low1,3,5 | Additional medication needed |
CI: confidence interval; RR: risk ratio
- 1
Unclear allocation concealment, sequence generation and selective reporting
- 2
Unvalidated tool used for pain assessment
- 3
n=24 randomised, n=20 analysed (19 for overall pain relief and supplementary analgesia needed), no clear exclusion criteria hence high risk of selection bias
- 4
Wide confidence interval
- 5
Very wide confidence interval
Table 54Estimated annual direct cost of analgesics included in economic model
| Treatment | Cost | Source |
|---|---|---|
| Codeine | £563.42 | NICE CG 173 |
| Tramadol | £542.13 | NICE CG 173 |
| ‘Generic’ analgesiaa | £93.15 | Electronic Drug Tariff, retrieved 14/12/16 |
- (a)
There is a lack of clarity in the evidence regarding exactly which analgesic was given to patients in a handful of trials – it appears to be simple NSAIDs, but to avoid confusion it is labelled in the model as ‘generic’ treatment
Table 55Estimated annual direct costs of analgesics specified in protocol
| Compound | Cost per annum (min)b | Source |
|---|---|---|
| Paracetamol | £87.60 | Electronic Drug Tariff, retrieved 14/12/16 |
| Diclofenac | £29.20 | Electronic Drug Tariff, retrieved 14/12/16 |
| Ibuprofen | £36.50 | Electronic Drug Tariff, retrieved 14/12/16 |
| Naproxen | £54.75 | Electronic Drug Tariff, retrieved 14/12/16 |
| Celecoxib | £394.20 | Electronic Drug Tariff, retrieved 14/12/16 |
| Mefenamic acid (tabs) | £219.00 | Electronic Drug Tariff, retrieved 14/12/16 |
| Mefenamic acid (caps) | £186.15 | Electronic Drug Tariff, retrieved 14/12/16 |
| Etoricoxib | £299.30 | Electronic Drug Tariff, retrieved 14/12/16 |
| Indomethacin | £52.93 | Electronic Drug Tariff, retrieved 14/12/16 |
| Tolfenamic acid (as Clotam Rapid) | £698.98 | Electronic Drug Tariff, retrieved 14/12/16 |
| High-dose aspirin | £536.55 | Electronic Drug Tariff, retrieved 14/12/16 |
| Co-codamol | £169.73 | Electronic Drug Tariff, retrieved 14/12/16 |
| Co-codaprin | £2,642.60 | Electronic Drug Tariff, retrieved 14/12/16 |
| Co-dydramol | £114.98 | Electronic Drug Tariff, retrieved 14/12/16 |
| Dyhydrocodeinea | £1,053.03 |
https://www |
| Buprenorphine (as Temgesic) | £202.58 | Electronic Drug Tariff, retrieved 14/12/16 |
- (a)
Whereas all other costings taken from Electronic Drug Tariff, dyhydrocodeine costs were not available and were estimated from online pharmacy costs.
- (b)
The cost is given by taking the average of the minimum and maximum daily dose multiplied by 365. For example, if the recommendation was to take 1–2 capsules of a drug 4–6 times a day, we assume the average daily dose is 1.5*5=7.5 capsules per day.
Table 56Cost and effectiveness of all treatment strategies containing an analgesic
| Treatment | Cost | QALY | ICER | Pr. cost-effective vs. no treatment (£20k/QALY) | Pr. cost-effective vs. no treatment (£30k/QALY) |
|---|---|---|---|---|---|
| Empirical Diagnosis & No Treatment | £22,752.60 | 18.120 | Base Case | 100.0% | 100.0% |
| Empirical Diagnosis & Tramadol | £21,875.58 | 18.174 | -£16,159.27 | 85.7% | 86.8% |
| Empirical Diagnosis & Codeine (as Morphine) | £22,776.51 | 18.180 | £161,978.83 | 86.8% | 87.9% |
| Laparoscopy & Codeine (as Morphine) | £33,431.95 | 18.200 | £518,261.74 | 75.8% | 80.2% |
QALY: quality adjusted life year; ICER: incremental cost-effectiveness ratio; Pr.: probability NICE CG 173 does not have QALY data on codeine, so it is assumed the opioid codeine behaves (as morphine), a different opioid, for the purpose of determining between-class performance
Table 57Summary of included studies
| Study | Intervention/Comparison | Population | Outcomes | Comments |
|---|---|---|---|---|
| Shokeir 2015 Egypt | Pertubal 10ml diluted bupivacaine infusion (0.25%) versus placebo infusion (sterile water) N=62 | Women with chronic pelvic pain for at least 6 months who had a pain score of at least 5 (on a VAS ranging from 0 to 10 cm) and had laparoscopically confirmed endometriosis of any stage |
|
|
| Wickström 2012, 2013 Sweden | Pertubation of 10 ml lidocaine/lignocaine versus placebo N=42 | Women with chronic pelvic pain for at least 6 months who had a pain score of at least 5 (on a VAS ranging from 0 to 10 cm) and had laparoscopically confirmed endometriosis of any stage |
|
|
N: number of participants in study; VAS: Visual analogue scale
Table 58Summary clinical evidence profile: Local anaesthetic (pertubation) versus placebo
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Placebo | Local anaesthetic | ||||
| Pain score – VAS >50% improved – at 3 months | 56 per 1,000 | 375 per 1,000 (52 to 1,000) | RR 6.75 (0.94 to 48.57) | 42 (1 study) | ⊕⊕⊝⊝ Low1,2 |
| Pain score – VAS >50% improved – at 6 months | 56 per 1,000 | 167 per 1,000 (21 to 1,000) | RR 3 (0.37 to 24.61) | 42 (1 study) | ⊕⊝⊝⊝ Very low1,3 |
| Pain score–- VAS >50% improved – at 9 months | 0 per 1,000 | 0 per 1,000 (0 to 0)6 | Peto OR 6.01 (0.35 to 102.4) | 42 (1 study) | ⊕⊝⊝⊝ Very low1,3 |
| Pain score – VAS >50% improved – at 12 months | 0 per 1,000 | 0 per 1,000 (0 to 0)6 | Peto OR 6.81 (0.84 to 51.68) | 42 (1 study) | ⊕⊝⊝⊝ Very low1,3 |
| Pain – VAS continuous – at 1 month | - | The mean pain - vas continuous - at 1 month in the intervention groups was 1.3 lower (2.18 to 0.42 lower) | MD −1.3 (−2.18 to −0.42) | 60 (1 study) | ⊕⊕⊝⊝ Low4,5 |
| Pain – VAS continuous – at 2 months | - | The mean pain - vas continuous - at 2 months in the intervention groups was 1.9 lower (2.92 to 0.88 lower) | MD −1.9 (−2.92 to −0.88) | 60 (1 study) | ⊕⊕⊕⊝ Moderate4 |
| Pain – VAS continuous – at 3 months | - | The mean pain - vas continuous - at 3 months in the intervention groups was 2.3 lower (3.46 to 1.14 lower) | MD −2.3 (−3.46 to −1.14) | 60 (1 study) | ⊕⊕⊕⊝ Moderate4 |
| Rate of satisfaction with treatment at 3 months | 67 per 1,000 | 733 per 1,000 (189 to 1,000) | RR 11 (2.83 to 42.7) | 60 (1 study) | ⊕⊕⊕⊝ Moderate4 |
| Rate of recurrence at 12 months | 0 per 1,000 | 0 per 1,000 (0 to 0)6 | Peto OR 6.01 (0.34 to 102.42) | 42 (1 study) | ⊕⊝⊝⊝ Very low3,6 |
| Escalating pain with a need for other therapies at 12 months | 167 per 1,000 | 42 per 1,000 (5 to 368) | RR 0.25 (0.03 to 2.21) | 42 (1 study) | ⊕⊝⊝⊝ Very low1,3 |
CI: confidence interval; RR: risk ratio; Pero OR: Peto odds ratio; MD: mean difference; VAS: visual analogue scale
- 1
The patient flow is a little unclear and there is a difference in results using 2 types of analyses. The categorisation of the pain scale favours the treatment group and there are conflicting results with another pain outcome used in the same trial.
- 2
The CI is large ranging from no effect to effect favouring the treatment.
- 3
The CI for this outcome ranges from an affect favouring placebo to an effect favouring the treatment. There is therefore too much uncertainty around this effect.
- 4
Some of the reported CIs seem to be incorrectly reported.
- 5
The CI ranged from a high effect to no appreciable benefit.
- 6
Due to zero events in the control group Peto OR were used rather than Risk Ratios because this method performs well when events are very rare (Bradburn 2007). This means that the risk difference is reported with confidence intervals.
Table 59Clinical evidence table: local anaesthetic (pertubation) versus placebo - endometriosis health related quality of life scores
| Change after 6 months | Change after 12 months | |||||
|---|---|---|---|---|---|---|
| EHP-30 dimension | Lidocaine median IQR | Placebo median IQR | Mann-Whitney U-test p-value | Lidocaine median IQR | Placebo median IQR | Mann-Whitney U-test p-value |
| Pain | −13.6 (−27.3 to 2.3) | −11.4 (−22.7 to 2.3) | 0.99 | −8 (−29.5 to 2.3) | −11.4 (−20.5 to 4.5) | 0.69 |
| Control and powerlessness | −8.3 (−33.3 to 2.1) | −6.3 (−35.4 to 2.1) | 0.84 | −12.5 (−37.5 to −8.3) | −20.8 (−41.7 to 0) | 0.74 |
| Emotional wellbeing | −4.2 (−37.5 to −4.17) | −12.5 (−20.8 to −6.25) | 0.99 | −20.8 (−37.5 to 0) | −12.5 (−25.to 4.17) | 0.63 |
| Social support | −18.8 (−31.25 to 0) | −6.3 (−12.5 to −6.25) | 0.034 | −12.5 (−37.5 to 0) | −6.3 (−31.25 to 12.5) | 0.50 |
| Self-image | −8.3 (−16.7 to −0) | 0 (−16.67 to 8.33) | 0.24 | −8.3 (−16.7 to 0) | 0 (−16.7 to −0) | 0.57 |
| Sexual intercourse | −10 (−25.to 10) | −5 (−10.to 5) | 0.24 | −7.5 (−15.to 5) | −7.5 (−20.to 7.5) | 0.97 |
EHP-30: Endometriosis Health Profile-30; IQR: interquartile range
Table 60Annual cost of neuromodulator treatments included in the model
| Treatment | Cost per year | Source |
|---|---|---|
| Amitriptyline | £227.25 | CG173 |
| Nortriptyline | £1,086.43 | CG173 |
| Duloxetine | £871.00 | CG173 |
| Venlafaxine | £383.44 | CG173 |
| Capsaicin patches | £1,210.80 | CG173 |
| Gabapentin | £365.62 | CG173 |
| Pregabalin | £1,000.77 | CG173 |
| Topiramate | £63.07 | CG173 |
CG96: Neuropathic pain in adults: pharmacological management in non-specialist settings
Table 61Cost and effectiveness of all non-dominated treatment strategies containing a neuromodulator treatment
| Treatment | Cost | QALY | ICER | Pr. cost-effective vs. no treatment (£20k/QALY) | Pr. cost-effective vs. no treatment (£30k/QALY) |
|---|---|---|---|---|---|
| Empirical Diagnosis & No Treatment | £22,752.60 | 18.120 | Base Case | N/A | N/A |
| Empirical Diagnosis & Amitriptyline | £21,702.24 | 18.340 | -£4,774.17 | 92.3% | 95.6% |
| Empirical Diagnosis & Gabapentin | £22,734.50 | 18.399 | £17,458.76 | 94.5% | 95.6% |
| Peritoneal biopsy & Gabapentin | £25,400.16 | 18.401 | Extendedly Dominated | 86.8% | 89.0% |
| Empirical Diagnosis & Pregabalin | £27,488.25 | 18.448 | £96,666.23 | 85.7% | 94.5% |
Note: ICER: incremental cost-effectiveness ratio; QALY: quality adjusted life year
Table 62Dose ranges of treatments in different classes of interventions, with abbreviations used in tables and figures within this chapter
| Class | Treatment | Abbreviation |
|---|---|---|
| Placebo/no treatment | Placebo No treatment/waiting list | - |
| Danazol/gestrinone | Danazol (100–800 mg/d) Gestrinone | Dan/gest |
| Oestrogens (oral) | Oestradiol (1–2 mg/d) Conjugated equine oestrogens (0.3–1.25 mg/d) | Oest(o) |
| Progestogens (oral) | Norethisterone (2.5 mg/d) Medroxyprogesterone (15–30 mg/d) Levonorgestrel (30 micrograms/d) Desogestrel (75 micrograms/d) Dienogest (2 mg/d) | Prog(o) |
| Progestogens (depot) | Medroxyprogesterone (150 mg/3 months) Gestodene (5–10 mg) | Prog(i.m.) |
| Progestogens (subcutaneous) | Medroxyprogesterone (104 mg/3 months) Promegestone | Prog(s.c.) |
| Progestogens (intrauterine) | Levonorgestrel (20 micrograms/day) | Prog(i.u.) |
| GnRH agonists (depot) | Leuprorelide (3.75 mg/m) Triptorelin (3 mg/m) | GnRHa(i.m.) |
| GnRH agonists (subcutaneous) | Goserelin (3.6 mg/m) | GnRHa(s.c.) |
| GnRH agonists (nasal spray) | Nafarelin (200 micrograms b.d.) Buserelin (300 micrograms t.d.) | GnRHa(i.n.) |
| GnRH antagonists | Elagolix | GnRHant |
| Aromatase inhibitors | Anastrozole (1 mg/d) Letrozole (2.5 mg/d) | AromaInhib |
| Anti-androgens | Cyproterone acetate (only in combination as combined oral contraceptive) | Anti-And |
| Selective oestrogen receptor modulators | Raloxifene (60 mg/d) | SERM |
| Tibolone | Tibolone (2.5 mg/d) | - |
| Nutritional supplements | Calcium Vitamin D | Supp |
| Chinese herbal medicine | Nei yi pills Dan’e mixture | CHM |
| Dietary interventions | Dietary intervention | Diet |
Table only includes treatments in full-text studies assessed for inclusion/exclusion. Treatments only in studies that were not included in the NMA could not be included in the network.
Table 63Characteristics of included studies
| First author | Pub date | rAFS | Surgery type | Endometriomas included | Risk of biasa | Outcomes reported in study (1=reported, 0=not reported) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disc | VAS | Dysmen | Dyspar | Pelv pain | ||||||
| Acs | 2015 | NR | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Agarwal | 1997 | I–II | None | NR | Low | 1 | 0 | 1 | 1 | 1 |
| Bergqvist | 1997 | I–II | None | NR | Low | 1 | 0 | 0 | 0 | 0 |
| Bergqvist | 1998 | I–II | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Bergqvist | 2000 | I-IV | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Burry | 1989 | I–IV | None | None | Mod | 1 | 0 | 0 | 0 | 0 |
| Burry | 1992 | NR | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Carr | 2014 | I–IV | None | NR | Low | 1 | 0 | 0 | 0 | 0 |
| Crosignani | 2006 | NR | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Diamond | 2014 | I–IV | None | NR | Low | 1 | 0 | 0 | 0 | 0 |
| Dlugi | 1990 | I–IV | None | Some | Mod | 0 | 0 | 1 | 1 | 1 |
| Dmowski | 1989 | I–IV | None | NR | High | 0 | 0 | 1 | 1 | 1 |
| Fedele | 1989 | I–IV | None | None | Mod | 1 | 0 | 0 | 0 | 0 |
| Fernandez | 2004 | III–IV | None | NR | Low | 0 | 0 | 0 | 0 | 1 |
| Ferreira | 2010 | NR | None | None | Mod | 0 | 1 | 0 | 0 | 0 |
| Franke | 2000 | III–IV | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| GISG (Vercellini) | 1996 | I–II | None | NR | Low | 0 | 0 | 1 | 1 | 1 |
| Gomes | 2007 | III–IV | None | NR | Mod | 0 | 1 | 0 | 0 | 0 |
| Granese | 2015 | III–IV | Excision/ablation | Some | High | 0 | 1 | 0 | 0 | 0 |
| Guzick | 2011 | NR | None | NR | Mod | 0 | 1 | 0 | 0 | 0 |
| Harada | 2008 | NR | None | All | Low | 1 | 1 | 1 | 0 | 1 |
| Harada | 2009 | NR | None | Some | Low | 1 | 0 | 0 | 0 | 0 |
| Henzl | 1989 | I–IV | None | Some | Mod | 1 | 0 | 0 | 0 | 0 |
| Hornstein | 1998 | I–II | None | NR | Low | 1 | 0 | 1 | 0 | 1 |
| Jelley | 1988 | I–IV | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Kennedy | 1990 | I–II | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Kiesel | 1996 | NR | None | NR | Low | 1 | 0 | 0 | 0 | 0 |
| Kiilholma | 1995 | III–IV | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Ling | 1999 | NR | None | NR | Mod | 0 | 0 | 1 | 1 | 1 |
| NEET | 1992 | I–IV | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Petta | 2005 | III–IV | None | NR | Low | 0 | 1 | 0 | 0 | 0 |
| Razzi | 2007 | NR | Excision | All | Mod | 0 | 1 | 0 | 0 | 0 |
| Rock | 1993 | I–IV | None | NR | High | 1 | 0 | 0 | 0 | 0 |
| Rolland | 1990 | I–IV | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Rotondi | 2002 | I–IV | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Schlaff | 2006 | NR | None | NR | Low | 1 | 0 | 0 | 0 | 0 |
| Seibel | 1982 | NR | None | None | High | 1 | 0 | 0 | 0 | 0 |
| Shaw | 1990 | NR | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Shaw | 1992 | I–IV | None | Some | Mod | 1 | 0 | 0 | 0 | 0 |
| Strowitzki | 2010 | III–IV | None | NR | Mod | 1 | 1 | 0 | 0 | 0 |
| Strowitzki | 2010b | I–IV | None | NR | Mod | 1 | 1 | 0 | 0 | 0 |
| Sutton | 1994 | I–II | Ablation | None | High | 0 | 1 | 0 | 0 | 0 |
| Telimaa | 1987 | I–II | None | Some | High | 1 | 0 | 0 | 0 | 0 |
| Vercellini | 1996 | I–IV | None | NR | Low | 1 | 0 | 1 | 0 | 0 |
| Walch | 2009 | I–IV | None | None | High | 1 | 0 | 0 | 0 | 0 |
| Wheeler | 1993 | I–IV | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
| Wong | 2010 | III–IV | None | NR | High | 1 | 0 | 0 | 0 | 0 |
| Zhu | 2014 | I–II | Excision/ablation | None | Mod | 0 | 1 | 0 | 0 | 0 |
| ZOLADEX | 1996 | I–II | None | NR | Mod | 1 | 0 | 0 | 0 | 0 |
- (a)
Cochrane risk of bias checklist
- (b)
Abbreviations - rAFS: revised American Fertility Scale; Mod: moderate; NR: not reported; Disc: discontinuation of treatment due to adverse events; Dysmen: dysmenorrhoea; Dyspar: dyspareunia; Pelv Pain: non-menstrual pelvic pain.
Table 64Table of studies excluded from the NMA for statistical reasons
| First author | Pub date | Reason for exclusion |
|---|---|---|
| Fernandez | 2004 | Study adds no information to any network |
| Ferrero | 2011 | Treatment not connected to any network |
| Howell | 1995 | Study adds no information to any network |
| Soysal | 2004 | Treatment not connected to any network |
Table 65Matrix of results for the multivariate NMA of hormonal therapy for pain relief on the VAS
| Placebo/no treatment | −12.3 (−12.7 to −11.9) | −18.6 (−20.4 to −16.8) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| −15.9 (−21.5 to −10.2) | Danazol/gestrinone (oral) | ||||||||
| −12.6 (−15.3 to −9.8) | 3.3 (−2.1 to - 8.7) | Progestogens (oral) | 1.5 (0.7 to 2.3) | ||||||
| −13.2 (−16.2 to −10.1) | 2.7 (−2.8 to 8.2) | −0.6 (−1.8 to 0.6) | Progestogens (i.m.) | ||||||
| −17.7 (−25.5 to −9.8) | −1.8 (−7.2 to 3.6) | −5.1 (−12.8 to 2.7) | −4.5 (−12.4 to 3.4) | Progestogens (i.u.) | −1.4 (−2.8 to 0.1) | ||||
| −15.7 (−21.3 to −10.1) | 0.1 (−0.5 to 0.8) | −3.2 (−8.5 to 2.2) | −2.6 (−8.1 to 2.9) | 1.9 (−3.4 to 7.3) | GnRHa (i.m.) | ||||
| −15.8 (−21.4 to −10.1) | 0.1 (−0.6 to 0.8) | −3.2 (−8.6 to 2.2) | −2.6 (−8.2 to 2.9) | 1.8 (−3.5 to 7.3) | 0.0 (−0.7 to 0.6) | GnRHa (i.n.) | |||
| −15.1 (−20.8 to −9.3) | 0.8 (−0.1 to 1.6) | −2.5 (−8.0 to 3.0) | −1.9 (−7.6 to 3.7) | 2.5 (−2.8 to 8.1) | 0.7 (−0.2 to 1.5) | 0.7 (−0.2 to 1.5) | Prog(oral)+ Oest(oral) | 3.5 (−1.7 to 8.7) | |
| −15.8 (−21.4 to −10.2) | 0.1 (−0.7 to 0.8) | −3.3 (−8.6 to 2.2) | −2.7 (−8.2 to 2.9) | 1.8 (−3.5 to 7.3) | −0.1 (−0.8 to 0.6) | 0 (−0.8 to 0.7) | −0.7 (−1.6 to 0.2) | GnRHa(i.m.) +Prog(oral) | |
| −15.9 (−21.5 to −10.2) | 0.0 (−0.7 to 0.7) | −3.3 (−8.7 to 2.1) | −2.7 (−8.3 to 2.8) | 1.8 (−3.6 to 7.2) | −0.1 (−0.8 to 0.5) | −0.1 (−0.8 to 0.6) | −0.8 (−1.7 to 0.1) | −0.1 (−0.8 to 0.6) | GnRHa(i.m.)+Prog(oral)+Oest(oral) |
Mean differences and 95% CrIs from the multivariate NMA (bottom left diagonal) and conventional pairwise VAS meta-analyses (top right diagonal) treatment effects between the column-defined and row-defined treatments. Mean differences less than 0 favour the row-defined treatment. Numbers in bold, grey-shaded cells denote results where the 95% CrI do not include 0. Treatment effects for danazol/gestrinone (oral), progestogens (i.m.), GnRHa (i.n.), progestogen plus oestrogen (oral) and GnRHa (i.m.) plus progestogen (oral) plus oestrogen (oral) are informed from Biberoglu subscales for dysmenorrhoea and non-menstrual pelvic pain. Pairwise results for these treatments are therefore not shown here as they would be reported on different scales. For treatment name abbreviations, see Table 62.
Table 66Mean differences versus placebo from multivariate and univariate NMAs for each treatment, with probabilities of being among the best 3 treatments and the worst 3 treatments, and the rank and 95% CrI from the multivariate NMA for each treatment
| Treatment class | Probability of being within the best 3 (%) | Probability of being within the worst 3 (%) | Rank (95% CrI) |
|---|---|---|---|
| Placebo/no treatment | 0.00% | 100.00% | 10 (10 to 0) |
| Danazol/gestrinone (o) | 57.33% | 0.47% | 3 (1 to 7) |
| Progestogens (o) | 0.05% | 95.70% | 9 (7 to 9) |
| Progestogens (i.m.) | 14.27% | 31.98% | 7 (1 to 9) |
| Progestogens (i.u.) | 74.46% | 16.01% | 1 (1 to 9) |
| GnRHa (i.m.) | 22.14% | 0.80% | 5 (2 to 7) |
| GnRHa (i.n.) | 34.64% | 0.87% | 4 (1 to 7) |
| Prog (o) + Oest (o) | 0.57% | 52.83% | 8 (6 to 9) |
| GnRHa (i.m.)+Prog (o) | 39.65% | 0.87% | 4 (1 to 7) |
| GnRHa (i.m.) + Prog (o) + Oest (o) | 56.88% | 0.47% | 3 (1 to 7) |
For treatment name abbreviations, see Table 62
Table 67Matrix of results for the NMA of dyspareunia
| Placebo/no treatment | −0.22 (−0.41 to −0.03) | ||
|---|---|---|---|
| −0.4 (−0.68 to −0.11) | Danazol/gestrinone | 0.32 (0.04 to 0.61) | −0.12 (−0.27 to 0.03) |
| −0.22 (−0.41 to −0.03) | 0.18 (−0.04 to 0.39) | GnRHa (i.m.) | −0.11 (−0.39 to 0.17) |
| −0.47 (−0.76 to −0.19) | −0.08 (−0.22 to 0.06) | −0.25 (−0.46 to −0.04) | GnRHa (i.n.) |
Mean differences and 95% CrIs from the NMA (bottom left diagonal) and conventional meta-analyses (top right diagonal) treatment effects between the column-defined and row-defined treatments. Mean differences less than 0 favour the row-defined treatment. Numbers in bold, grey-shaded cells denote results where the 95% CrI do not include 0. For treatment name abbreviations, see Table 62.
Table 68Probabilities of being the best treatment and the rank (with 95% CrI) for each treatment
| Treatment class | Probability of being the best treatment (%) | Rank (95% CrI) |
|---|---|---|
| Placebo/no treatment | 0.03% | 4 (4 to 4) |
| Danazol/gestrinone | 14.26% | 2 (1 to 3) |
| GnRHa (i.m.) | 0.67% | 3 (2 to 3) |
| GnRHa (i.n.) | 85.05% | 1 (1 to 2) |
For treatment name abbreviations, see Table 62
Table 69Matrix of results for the NMA of discontinuation of treatment due to adverse events
| Placebo/no treatment | 5.28 (0.28 to 305) | 10.19 (0.96 to 371) | >999 (12.8 to >999) | >999 (12.2 to >999) | 0.49 (0.07 to 3.18) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24.1 (2.14 to >999) | Danazol/gestrinone | 0.47 (0.12 to 1.63) | 0.11 (0.02 to 0.31) | 1.12 (0.45 to 2.33) | ||||||||||
| 17.9 (1.76 to 676) | 0.75 (0.16 to 3.27) | Progestogens(oral) | 1.66 (0.15 to 21.3) | 0.79 (0.12 to 4.95) | 1.22 (0.21 to 7.26) | |||||||||
| 26.8 (2.11 to 999) | 1.1 (0.18 to 6.89) | 1.47 (0.27 to 9.1) | Progestogens(i.m.) | 0.4 (0.06 to 2.37) | 0.88 (0.22 to 3.26) | 0.34 (0.09 to 1.17) | ||||||||
| 10.7 (0.35 to 811) | 0.42 (0.02 to 7.11) | 0.57 (0.03 to 9.32) | 0.39 (0.04 to 3.23) | Progestogens(i.u.) | ||||||||||
| 17.4 (1.66 to 701) | 0.73 (0.21 to 2.52) | 0.98 (0.23 to 4.23) | 0.66 (0.15 to 2.72) | 1.71 (0.13 to 24.4) | GnRHa(i.m.) | 0.48 (0.09 to 2.54) | 1.04 (0.19 to 5.66) | 0.8 (0.21 to 3.09) | ||||||
| 5.4 (0.34 to 251) | 0.23 (0.05 to 0.73) | 0.3 (0.04 to 1.87) | 0.21 (0.02 to 1.59) | 0.53 (0.02 to 10.9) | 0.31 (0.05 to 1.57) | GnRHa(s.c.) | 0.42 (0.08 to 2.18) | 2.94 (0.07 to >999) | 0.43 (0.01 to 6.83) | |||||
| 14.98 (1.28 to 625) | 0.63 (0.25 to 1.42) | 0.84 (0.19 to 3.72) | 0.57 (0.08 to 3.36) | 1.48 (0.08 to 25.6) | 0.86 (0.23 to 3.06) | 2.76 (0.75 to 12.5) | GnRHa(i.n.) | 10.31 (0.36 to >999) | ||||||
| 11.57 (0.75 to 638) | 0.47 (0.05 to 4.97) | 0.63 (0.07 to 6.77) | 0.43 (0.08 to 2.56) | 1.11 (0.07 to 20.0) | 0.65 (0.09 to 5.24) | 2.08 (0.18 to 35.2) | 0.75 (0.08 to 8.5) | GnRHant(oral) | ||||||
| 0.48 (0.04 to 5.53) | 0.02 (<0.01 to 0.61) | 0.03 (<0.01 to 0.77) | 0.02 (<0.01 to 0.6) | 0.04 (<0.01 to 2.92) | 0.03 (<0.01 to 0.81) | 0.09 (<0.01 to 3.57) | 0.03 (<0.01 to 1.02) | 0.04 (<0.01 to 1.56) | Prog(oral)+Oest(oral) | |||||
| 18.98 (0.71 to 999) | 0.76 (0.06 to 10.2) | 1.02 (0.07 to 15.6) | 0.69 (0.04 to 10.0) | 1.8 (0.06 to 58.5) | 1.04 (0.11 to 10.31) | 3.35 (0.21 to 68.1) | 1.21 (0.09 to 17.4) | 1.62 (0.07 to 31.9) | 39.98 (0.67 to >999) | GnRHa(i.m.)+Prog(oral) | ||||
| 14.43 (0.65 to 924) | 0.58 (0.05 to 6.06) | 0.78 (0.07 to 9.35) | 0.53 (0.04 to 6.08) | 1.37 (0.05 to 36.9) | 0.8 (0.11 to 5.93) | 2.57 (0.2 to 41.8) | 0.93 (0.09 to 10.39) | 1.24 (0.07 to 19.6) | 30.71 (0.59 to >999) | 0.76 (0.1 to 5.67) | GnRHa(i.m.)+Prog(oral)+Oest(oral) | |||
| 19.13 (0.11 to >999) | 0.66 (0.01 to 438) | 0.92 (0.01 to 670) | 0.62 (0.01 to 493) | 1.69 (0.01 to >999) | 0.93 (0.01 to 668) | 2.97 (0.05 to >999) | 1.07 (0.01 to 726) | 1.47 (0.01 to >999) | 40.65 (0.14 to >999) | 0.93 (0.01 to 888) | 1.2 (0.01 to >999) | GnRHa(s.c.)+Prog(oral) | ||
| 2.14 (0.02 to 269) | 0.08 (<0.01 to 2.26) | 0.11 (<0.01 to 4.05) | 0.08 (<0.01 to 3.06) | 0.2 (<0.01 to 14.6) | 0.12 (<0.01 to 3.79) | 0.38 (0.01 to 8.22) | 0.14 (<0.01 to 3.8) | 0.18 (<0.01 to 9.04) | 4.42 (0.02 to 980) | 0.11 (<0.01 to 6.75) | 0.14 (<0.01 to 7.71) | 0.11 (<0.01 to 21.4) | GnRHa(s.c.)+Oest(oral)+Prog(oral) | |
| 196 (1.78 to >999) | 6.73 (0.14 to >999) | 9.18 (0.16 to >999) | 6.28 (0.09 to >999) | 17.12 (0.14 to >999) | 9.32 (0.17 to >999) | 30.95 (0.58 to >999) | 10.74 (0.26 to >999) | 14.87 (0.16 to >999) | 416 (2.05 to >999) | 9.37 (0.09 to >999) | 12.19 (0.13 to >999) | 10.51 (0.01 to >999) | 94.31 (0.52 to >999) | GnRH(i.n.)+Prog(oral) |
Odds ratios and 95% credible intervals (CrI) from the NMA (bottom left diagonal) and conventional meta-analyses (top right diagonal) treatment effects between the column-defined and row-defined treatments. Odds ratios less than 1 favour the row-defined treatment. Numbers in bold, grey-shaded cells denote results where the 95% CrI do not
Table 70Probabilities of being among the best 3 treatments and the worst 3 treatments, and the rank and 95% Credible Interval (95%CrI) for each treatment
| Treatment class | Probability of being within the best 3 (%) | Probability of being within the worst 3 (%) | Rank (95% CrI) |
|---|---|---|---|
| Placebo/no treatment | 82.13% | 0.05% | 2 (1 to 6) |
| Danazol/gestrinone | 0.01% | 31.10% | 11 (6 to 14) |
| Progestogens (o) | 0.68% | 17.46% | 10 (4 to 14) |
| Progestogens (i.m.) | 0.09% | 39.11% | 12 (6 to 15) |
| Progestogens (i.u.) | 14.69% | 15.91% | 7 (2 to15) |
| GnRHa (i.m.) | 0.14% | 7.64% | 10 (5 to 13) |
| GnRHa (s.c.) | 16.33% | 0.35% | 5 (2 to 10) |
| GnRHa (i.n.) | 0.42% | 6.07% | 9 (5 to 13) |
| GnRHant (o) | 7.43% | 11.83% | 7 (3 to 14) |
| Prog (o) + Oest (o) | 87.77% | 0.55% | 1 (1 to 8) |
| GnRHa (i.m.) + Prog (o) | 4.91% | 29.72% | 10 (3 to 15) |
| GnRHa (i.m.) + Prog (o) + Oest (o) | 5.87% | 17.80% | 8 (3 to 14) |
| GnRHa (s.c.) + Prog (o) | 19.13% | 38.84% | 9 (1 to 15) |
| GnRHa (s.c.) + Oest (o) + Prog (o) | 58.78% | 4.82% | 3 (1 to 14) |
| GnRH (i.n.) + Prog (o) | 1.64% | 78.75% | 15 (4 to 15) |
For treatment name abbreviations, see Table 57
Table 71Summary of included studies
| Study | Intervention/Comparison | Population | Outcomes | Comments |
|---|---|---|---|---|
| Agarwal 1997 (Brown 2010 CSR) USA | Nafarelin 200mcg BDS IN + placebo every 4 weeks IM for 6 months (n=105) versus LA Depot 3.75mg every 4 weeks IM + placebo BDS IN for 6 months (n=103) | n=208* Inclusion criteria: Laparoscopically diagnosed endometriosis within 18 months prior to study, 19–44 years old, patients demonstrating clinical symptoms and signs, bone mineral density within normal age range | Assessed at 6 months after the end of treatment period Pelvic tenderness Pelvic induration Measured using a 4-point numerical scale: 0=none; 1=mild; 2=moderate; 3=severe | *No information on stages of endometriosis provided |
| Bergqvist 1998 (Brown 2010 CSR) Sweden | Triptorelin 3.75mg IM depot every 4 weeks for 24 weeks (n=24) vs. Placebo IM every 4 weeks for 24 weeks (n=25) | Inclusion criteria: Menstruating regularly 3 months before study Clinical symptoms of endometriosis Not taken oral contraceptive or oral steroid therapy for 3 months Not taken long-acting depot gestagens or GnRH agonists within past 6 months Not pregnant in prior 3 months Not breastfeeding No history of osteoporosis or coagulation disorders | PAIN: VAS (0 to 10) and direct questions about pelvic pain, dysmenorrhoea and dyspareunia (none, mild, moderate, severe) | |
| Bergqvist 2001 (Brown 2012) Sweden | Nafarelin 200 µg BDS IN and placebo MPA tablets (n=23) for 6 months versus MPA 15mg PO BDS and placebo nafarelin IN (n=25) for 6 months | n=48* Inclusion criteria: Diagnosis of endometriosis by laparoscopy or laparotomy, within 3 months regular menstruating and complaining of dysmenorrhoea, dyspareunia and/or pelvic pain | Assessed at 6 months (at the end of treatment) and 12 months (6 months after the end of the treatment period) QoL (Goldberg’s general health and Nottingham Health Profile Questionnaire) Effect on daily activities (coping wheel, Inventory of Social Support and Interaction – (ISSI) and demands, control and support questionnaires) | *No information on stages of endometriosis provided |
| Burry 1992 (Brown 2010 CSR) USA | Nafarelin 400mcg daily IN for 6 months (n=111) versus Danazol 600mg daily PO for 6 months (n=58) | N=169 Inclusion criteria: Laparoscopically diagnosed endometriosis | Assessed at the end of the 6 month treatment period QoL (PGWBI plus a modification of Part II of the Nottingham Health Profile) | |
| Cheng 2005 (Brown 2010 CSR) Taiwan | Nafarelin acetate 200mcg BDS (400mcg/day) IN for 180 days (n=29) versus Danazol 200mg TDS (600mg/day) PO for 180 days (n=30) | N=59 Inclusion criteria: Laparoscopically diagnosed endometriosis within 3 months prior to study, age 18–48 years, barrier contraception | Assessed at 3 months (during treatment period) and at the end of the 6 month treatment period Pelvic tenderness Pelvic induration (TSSS, scale not defined) | |
| Fedele 1989 (Brown 2010 CSR) Italy | Buserelin 400mcg TDS IN for 6 months (n=30) versus Danazol 200mg TDS PO for 6 months (n=32) | N=62 Inclusion criteria: Laparoscopically diagnosed endometriosis within 3 months prior to study and no therapeutic intervention | Assessed at 12 months post-treatment Patients requiring surgery because of reappearance of symptoms and positive findings at pelvic examination | Infertile women included |
| Fedele 1993 (Brown 2010 CSR) Italy | Buserelin acetate 1200mcg daily IN for 6 months (n=19) versus Expectant management (n=16) Treatment group followed up for 18 months and expectant management group for 12 months | Inclusion criteria: Laparoscopically diagnosed endometriosis One or more of dysmenorrhoea, pelvic pain and deep dyspareunia | Pain: dysmenorrhoea and pelvic pain measured by VAS (0 to 10): 0 (no pain); 1 to 4 (mild;, 5 to 7 (moderate); 8 to 10 (severe); deep dyspareunia | Population of women whose main symptom is infertility and who may not have had pain as a symptom at baseline |
| Fraser 1991 (Brown 2010 CSR) Australia/New Zealand | Nafarelin 200mcg BDS (400mcg/d) IN + placebo PO for 6 months (n=33) versus Danazol 200mg TDS (600mg/d) PO + placebo IN for 6 months (n=16) | n=49* n=40 patients with stage I–II n=9 patients with stage III Inclusion criteria: Laparoscopically diagnosed endometriosis, symptomatic, regular menstrual cycle 24–36 days, not pregnant, negative pap smear, barrier contraception | Assessed at 6 months after the end of the treatment period Pelvic tenderness Pelvic induration Measured using a 4-point numerical scale: 0=none; 1=mild; 2=moderate; 3=severe | * American Fertility Society classification. 18 women dropped out of the study – no reasons were provided. However, 17 of these women responded to the psychosocial questions. Anxiety-depression was significantly more common among women who dropped out compared to the 30 women analysed (p=0.04). |
| Harada 2008 Japan | Monophasic oral contraceptive pill (ethinylestradiol 0.035mg plus norethisterone 1mg) for 21 days plus 7 days of placebo for 3 cycles (n=49) versus Placebo for 28 days for 3 cycles (n=47) | Inclusion criteria: Aged 18 or over Regular menstrual cycles (28+/− 2 days) Symptomatic endometriosis (diagnosed by laparoscopy or laparotomy) or ovarian | PAIN: dysmenorrhoea and non-menstrual pelvic pain scores Pelvic induration (hardening of soft tissues): physical examination endometrioma (diagnosed by ultrasound or MRI) Normal cervical and endometrial smear cytology Moderate or severe dysmenorrhoea (evaluated by a modified pain scale) No medical or surgical treatment for endometriosis within 8 weeks before entry into the study | VAS not defined |
| Ling 1999 USA | Leuprolide acetate 3.75mg IM depot every 4 weeks on day 0, week 4 and week 8 (n=49) versus Placebo IM every 4 weeks on day 0, week 4 and week 8 (n=46) | Inclusion criteria: 18–45 years of age Moderate to severe chronic pelvic pain for at least 6 months, unrelated to menstruation and incompletely resolved with NSAIDs Physician-assessed pain severity (B&B) Regular menstrual bleeding and menstrual cycles for 3 months prior to enrolment | PAIN: dysmenorrhoea, pelvic pain, dyspareunia, based on an 11-point VAS (0 to 10) | |
| NEET 1992 (Brown 2010 CSR) Europe | Nafarelin 200mcg BDS IN + placebo PO for 6 months (n=206) versus Danazol 200mg TDS PO + placebo IN for 6 months (n=101) | n=263*: n=160 patients with stage I–II n=103 patients with stage III–IV Inclusion criteria: Laparoscopically diagnosed endometriosis, 18–45 years old, not pregnant, pap smear negative for malignancy, normal menstrual cycle 21–36 days for previous 4 months, weight between 45 and 110 kg | Assessed at 12 months after the end of the treatment period** Pelvic tenderness Pelvic induration | * American Fertility Society classification ** after 12 months follow-up only 96 out of the 263 included women were analysed, main reason for dropping out the study were: 1) pregnancy 2) further medical therapy for endometriosis 3) hormonal therapy for other medical conditions 4) loss to follow-up |
| Parazzini 2000 Italy | GnRH agonist (triptorelin 3.75 mg) slow release every 28 days for 4 months followed by gestodene 0.75 mg/ethinylestradiol 0.03 mg (E/P pill) for 8 months (n=55) versus gestodene 0.75 mg/ethinylestradiol 0.03 mg (E/P pill) for 12 months (n=47) | N=102 Inclusion criteria: Laparoscopically diagnosed endometriosis and pelvic pain lasting 3–12 months after diagnosis. Additionally, only women who reported a score of >=3 for the multidimensional scale and/or >=5 for the analogue scale for dysmenorrhoea and/or non-menstrual pelvic pain were eligible 51.9% in the GnRH agonist+ E/P group and 57.8% in the E/P group had stage I–II endometriosis | Assessed at 8 months during treatment period and at the end of the treatment period (12 months) Dysmenorrhoea Non-menstrual pelvic pain (a 10-point VAS scale where 0 = the absence of pain, 10 = unbearable pain) | Additional treatment for relief of pain with naproxen sodium as first-line treatment was allowed |
| Petta 2005 (Brown 2010 CSR) Brazilian | Lupron 3.75mg every 28 days IM for 6 months (n=43) versus LNG-IUS (Mirena) 20mcg/day 5 years IU for 6 months (n=40) | N=83 Inclusion criteria: Laparoscopically and histologically confirmed endometriosis within 3 to 24 months prior to study enrolment, 18–40 years old, complaints of cyclic chronic pelvic pain with or without dysmenorrhoea, VAS pain score of greater or equal to 3 during the pre-treatment cycle, regular menstrual cycle of 25–35 days for at least 3 months prior to study, not used hormone treatment for at least 3 months prior to study, not taken any long-acting progestogens or GnRH agonist within 9 months prior to study, no osteoporosis, coagulation disorders or contra-indications Stage: I to IV | Assessed at the end of the 6 month treatment period QoL – psychological wellbeing (PGWBI) | |
| Schlaff 2006 USA | Leuprolide (11.25 mg given by IM injection) versus DMPA-SC 104 (104 mg/0.65 mL given by SC injection) | N=274 Inclusion criteria: Premenopausal women who ranged in age from 18 to 49 years, with persistent symptoms of pain caused by endometriosis (surgically diagnosed within the previous 42 months). A patient’s pain must have returned to its previous level within 30 days after a diagnostic laparoscopy or within 3 months after laparoscopy or laparotomy with surgical treatment, and it must have persisted for a minimum of 3 months. | Assessed at the end of the 6 month treatment period and 18 months (12 months post-treatment) Effect on daily activities: Total hours of productivity lost at employment Total hours of productivity lost at housework (Endometriosis-impact diary) | |
| Vercellini 1993 (Davis 2007 CSR) Italy | GnRH agonist (goserelin 3.6 mg) subcutaneous depot formulation monthly for 6 months (n=29) versus a low-dose cyclic monophasic contraceptive pill, containing 0.02 mg ethinylestradiol and 0.15 mg desogestrel (n=28) for 6 months In the goserelin group the follow-up period was considered to start 4 weeks after the last injection | N=57 Inclusion criteria: Laparoscopically diagnosed endometriosis and no attempts at endometriosis reduction other than biopsy within 3 months of study entry 76% and 24% in the GnRH agonist group has stage I–II endometriosis; 82% and 28% in the cOCP group had stage III–IV endometriosis | Assessed at the end of the treatment period (6 months) and 6 months after the treatment period Dysmenorrhoea Dyspareunia Non-menstrual pelvic pain (a 10-point VAS scale where 0 = the absence of pain, 1–5 = mild pain, 6–7 = moderate pain, 8–10 = unbearable pain) | In the cOCP group, if spotting or breakthrough bleeding occurred, women could switch to a contraceptive with EE2, 0.03 mg and desogestrel 0.15 mg per pill |
| Vercellini 1996 (Brown 2012 CSR) Italy | Depot medroxyprogesterone acetate 150 mg every 90 days (n=40) versus Ethinylestradiol 0.02 mg + desogestrel 0.15mg plus 50 mg danazol daily for 21 days out of 28-day cycle (n=40) for 12 months | N=80 Inclusion criteria: Laparoscopically diagnosed endometriosis with attempt at implant reduction other than biopsy in the previous 3 months, pelvic pain of greater than 6 months duration. Additionally, only women who had at least 1 moderate or severe symptom on a verbal rating scale modified from the one devised by Biberoglu and Behrman and on a 10 point visual analogue scale were eligible 55% and 45% women in both groups had stage I–II or III-IV endometriosis | Assessed at 6 months during the treatment period and at the end of the treatment period (12 months) Dysmenorrhoea Dyspareunia Non-menstrual pelvic pain (a 10-cm VAS where 0 = absence of pain, >0–5 = mild pain, >5–8 = moderate pain, >8–10 = unbearable pain) Patient satisfaction (no particular scale defined: very satisfied; satisfied; uncertain; dissatisfied; very dissatisfied) | |
| Wheeler 1992 (Brown 2010 CSR) USA | Leuprolide 3.75mg monthly IM + placebo OD PO for 24 weeks (n=134) versus Danazol 800mg OD PO + placebo monthly IM for 24 weeks (n=136) | n=270* Inclusion criteria: Laparoscopically diagnosed endometriosis within 4 months prior to study, over 18 years of age, no surgical treatment at time of laparoscopy, premenopausal, not pregnant or lactating, never previously taken GnRH agonist, any other treatment completed at least 3 months prior to study | Assessed at 6 months after the end of the treatment period Pelvic tenderness | *No information on stages of endometriosis provided |
BDS: twice per day; cOCP: combined oral contraceptive; CSR: Cochrane systematic review; DMPA-SC: depot medroxyprogesterone acetate; GnRH: gonadotrophin releasing hormone; IM: intramuscular; IN: intranasal; LA: leuprolide acetate; LNG-IUS: levonorgestrel-releasing intrauterine system; MPA: medroxyprogesterone acetate; NSAID: non-steroidal anti-inflammatory drug; OD: once per day; PGWBI: psychological well-being index questionnaire (scale 0–110); PO: by mouth; QoL: quality of life; SF-36: Short form questionnaire (36 items); TDS: 3 times per day; TSSS: total symptom severity score; 4-point scale: each symptom or sign was scored on a 4point system at each visit from the cards prospectively recorded by each patient or at the vaginal examination (0=none, 1=mild, 2=moderate, 3 severe); VAS: Visual Analogue Scale
Table 72Summary clinical evidence profile, comparison 1: GnRH agonist versus no treatment
| Outcomes | Illustrative comparative risks (95% CI) | Rela-tive effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| No treatment | GnRH agonist | ||||
| Dysmenorrhoea relief at 12 months scale: 0 (no pain); 1 to 4 (mild); 5 to 7 (moderate); 8 to 10 (severe) | 188 per 1,000 | 579 per 1,000 (195 to 1,000) | RR 3.09 (1.04 to 9.18) | 35 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
CI: confidence interval; GnRH: gonadotrophin releasing hormone; RR: risk ratio
- 1
The main symptom of the study population was not pain (infertility)
- 2
CI crosses 1 threshold
Table 73Summary clinical evidence profile, comparison 2: GnRH agonist versus placebo
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Placebo | GnRH agonist | ||||
| Mean dysmenorrhoea at week 12 (11-point VAS) | - | The mean dysmenorrhoea at week 12 in the intervention groups was 6.30 lower (9.93 to 2.67 lower) | MD −6.30 (−9.93 to −2.67) | 88 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Mean pelvic pain at week 12 (11-point VAS) | - | The mean pelvic pain at week 12 in the intervention groups was 4.4 lower (6.93 to 1.87 lower) | MD −4.4 (6.93 to −1.87) | 88 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Mean deep dyspareunia at week 12 (11-point VAS) | - | The mean deep dyspareunia at week 12 in the intervention groups was 3.1 lower (4.85 to 1.35 lower) | MD −3.1 (−4.85 to −1.35) | 61 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Pelvic tenderness cessation at 6 months | 174 per 1,000 | 696 per 1,000 (275 to 1,000) | RR 4 (1.58 to 10.15) | 46 (1 study) | ⊕⊕⊕⊝ Moderate2 |
| Dyspareunia cessation at 6 months | 391 per 1,000 | 434 per 1,000 (219 to 869) | RR 1.11 (0.56 to 2.22) | 46 (1 study) | ⊕⊝⊝⊝ Very low2,3 |
CI: confidence interval; CSR: Cochrane systematic review; GnRH: gonadotrophin releasing hormone; RR: risk ratio; MD: mean difference; VAS: visual analogue scale
- 1
Outcomes measured immediately after treatment period are of less clinical relevance than sustained posttreatment effects
- 2
No details provided regarding sequence generation and allocation concealment (unclear risk)
- 3
CIs for estimate are very wide crossing 2 thresholds
Table 74Summary clinical evidence profile, comparison 3: Combined oral contraceptive pill versus placebo
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Placebo | Oral contraceptive | ||||
| Dysmenorrhoea (VAS not defined, reported on a scale 0 to 100) | - | The mean dysmenorrhoea in the intervention groups was 21.5 lower (28.14 to 14.86 lower) | MD −21.5 (−28.14 to 14.86) | 96 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Non-menstrual pelvic pain score (VAS not defined, reported on a scale 0 to 100) | - | The mean non-menstrual pelvic pain score(VAS) in the intervention groups was 6.6 lower (14.27 lower to 1.07 higher) | - | 96 (1 study) | ⊕⊕⊝⊝ Low1,2 |
| Induration | 404 per 1,000 | 226 per 1,000 (121 to 420) | RR 0.56 (0.3 to 1.04) | 96 (1 study) | ⊕⊕⊝⊝ Low1,2 |
CI: confidence interval; RR: risk ratio; MD: mean difference; VAS: visual analogue scale
- 1
Short duration of treatment is of limited relevance to clinical practice
- 2
CI crosses 1 threshold
Table 75Summary clinical evidence profile, comparison 4: GnRH agonist versus danazol
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95 % CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Control | GnRH agonist versus danazol | ||||
| Pelvic tenderness at 3 months (TSSS, scale not defined) | - | The mean pelvic tenderness at 3 months in the intervention groups was 0.2 lower (0.78 lower to 0.38 higher) | MD −0.2 (−0.78 to −0.38) | 41 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Pelvic tenderness at 6 months (TSSS, scale not defined) | - | The mean pelvic tenderness at 6 months in the intervention groups was 0.2 lower (0.75 lower to 0.35 higher) | MD −0.2 (−0.75 to 0.35) | 41 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Pelvic induration at 3 months (TSSS, scale not defined) | - | The mean pelvic induration at 3 months in the intervention groups was 0.1 lower (0.59 lower to 0.39 higher) | MD −0.1 (−0.59 to 0.39) | 41 (1 study) | ⊕⊕⊝⊝ Low2 |
| Pelvic induration- at 6 months (TSSS, scale not defined) | - | The mean pelvic induration at 6 months in the intervention groups was 0.2 higher (0.29 lower to 0.69 higher) | MD 0.2 (−0.29 to 0.69) | 41 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Patients requiring surgery because of reappearance of symptoms and positive findings at pelvic examination at 6 months | 357 per 1,000 | 364 per 1,000 (129 to 1,000) | RR 1.02 (0.36 to 2.91) | 25 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| QoL at 6 months (PGWBI plus a modification of Part II of the Nottingham Health Profile | - | No statistically significant difference between the 2 intervention groups | Not estimable | 169 (1 study) | ⊕⊕⊝⊝ Low3 |
CI: confidence interval; GnRH: Gonadotrophin Releasing Hormone; RR: relative risk; MD: mean difference; PGWBI: Psychological General Well-Being Index; TSSS: Total Symptom Severity Score; 4-point scale: each symptom or sign was scored on a 4-point system at each visit from the cards prospectively recorded by each patient or at the vaginal examination (0=none, 1=mild, 2=moderate, 3 severe); QoL: quality of life
- 1
CI crosses 1 threshold
- 2
CI crosses 2 thresholds
- 3
Reporting bias, i.e. not possible to access imprecision as only descriptive data reported
Table 76Summary clinical evidence profile, comparison 5: GnRH agonist versus levonorgestrel-releasing intrauterine system
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Control | GnRH agonist versus levonorgestrel-releasing intrauterine system | ||||
| QoL at 6 months (PGWBI, 0–110 scale) | - | The mean QoL (PGWBI) at 6 months in the intervention groups was 1.2 lower (7.79 lower to 5.39 higher) | MD −1.2 (−7.79 to 5.39) | 72 (1 study) | ⊕⊕⊕⊝ Moderate1 |
CI: confidence interval; GnRH: gonadotrophin releasing hormone; QoL: quality of life; PGWBI: Psychological Well-Being Index questionnaire
- 1
CI crosses 1 threshold
Table 77Summary clinical evidence profile, comparison 6: GnRH agonist versus DMPA-SC
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Control | GnRH agonist versus DMPA-SC | ||||
| Effect on daily activities from baseline to 6 month follow-up (Endometriosisimpact diary) | - | The mean number of hours of productivity lost at employment at 6 months in the intervention groups was 6.15 higher(2.17 lower to 14.47 higher) | MD 6.15 (−2.17 to 14.47) | 190 (1 study) | ⊕⊕⊕⊕ High |
| Effect on daily activities from baseline to 18 month follow-up (Endometriosisimpact diary) | - | The mean number of hours of productivity lost at employment at 18 months in the intervention groups was 6.38 higher (1.94 lower to 14.7 higher) | MD 6.38 (−1.94 to 14.7) | 190 (1 study) | ⊕⊕⊕⊕ High1 |
| Effect on daily activities from baseline to 6 month follow-up (Endometriosisimpact diary) | - | The mean number of hours of productivity lost at housework at 6 months in the intervention groups was 7.35 lower (−16.63 lower to 1.93 higher) | MD −7.35 (−16.63 to 1.93) | 81 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Effect on daily activities from baseline to 18 month follow-up (Endometriosisimpact diary) | - | The mean number of hours of productivity lost at housework at 18 months in the intervention groups was 3.64 lower (12.92 lower to 5.64 higher) | MD −3.64 (−12.92 to 5.64) | 81 (1 study) | ⊕⊕⊕⊝ Moderate1 |
CI: confidence interval; GnRH: gonadotrophin releasing hormone; DMPA-SC: depot medroxyprogesterone acetate
- 1
CI crosses 1 threshold
Table 78Summary clinical evidence profile, comparison 7: GnRH agonist 1 + placebo versus GnRH agonist 2 + placebo
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95 % CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| GnRH agonist (LA depot IM) + placebo IN | GnRH agonist (nafarelin IN) + placebo IM | ||||
| Relief of painful symptoms – Pelvic tenderness Follow-up: 6 months1 | 624 per 1,000 | 536 per 1,000 (418 to 680) | RR 0.86 (0.67 to 1.09) | 192 (1 study) | ⊕⊕⊝⊝ Low2 |
| Relief of painful symptoms–Pelvic induration Follow-up: 6 months1 | 813 per 1,000 | 740 per 1,000 (634 to 862) | RR 0.91 (0.78 to 1.06) | 190 (1 study) | ⊕⊕⊝⊝ Low2 |
CI: confidence interval; GnRH: gonadotrophin releasing hormone; IM: intramuscular; IN: intranasal; RR: risk ratio
- 1
Assessed after the end of the treatment period
- 2
Quality of evidence was downgraded by 2 points owing to very serious imprecision: CI crosses 2 default thresholds
Table 79Summary clinical evidence profile, comparison 8: GnRH agonist + placebo versus progestin + placebo
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| MPA and placebo nasal spray | GnRH agonist (nafarelin) IN + placebo tablets | |||||
| Paid working life Nottingham Health Profile Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate an improvement in the nafarelin group, but not in the MPA group (p=0.06) |
| Household work Nottingham Health Profile Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in household work score (data not shown) |
| Vacation life Nottingham Health Profile Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in vacation life score (p=0.72) |
| Leisure Nottingham Health Profile Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in leisure score (p=0.93) |
| Sexual life Nottingham Health Profile Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in sexual life score (p=0.90) |
| Disturbed sleep Goldberg’s General Health Q Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in sleep disturbance (difficulties of falling asleep, early wakening and nightmares) score (p=0.19) |
| Anxiety-depression Goldberg’s General Health Q Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in anxietydepression score (p=0.20) |
| Motivation coping wheel, ISSI and demands, control and support Q Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in motivation score (p=0.41) |
| Emotional balance Coping wheel, ISSI and demands, control and support Q Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in emotional balance score (p=0.44) |
| Structure Coping wheel, ISSI and demands, control and support Q Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in structure score (p=0.41) |
| Coping Coping wheel, ISSI and demands, control and support Q Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in coping score (p=0.39) |
| Psychological work demands Coping wheel, ISSI and demands, control and support Q Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in ‘psychological work demands’ score (p=0.51) |
| Intellectual discretion at work Coping wheel, ISSI and demands, control and support Q Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in ‘intellectual discretion at work’ score (p=0.95) |
| Authority over decisions at work Coping wheel, ISSI and demands, control and support Q Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in ‘authority over decisions at work’ score (p=0.39) |
| Social support at work Coping wheel, ISSI and demands, control and support Q Follow-up3 | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊝⊝⊝ Very low1,2 | The results indicate no significant difference between groups in ‘social support at work’ score (p=0.68) |
CI: confidence interval; GnRH: gonadotrophin releasing hormone; ISSI: Inventory of Social Support and Interaction; Q: questionnaire
- 1
The quality of the evidence was downgraded of 2 points because of the high risk of reporting bias (i.e. not Possible to access imprecision as only descriptive data with p values reported) and the potential risk of detection Bias (no details were given about randomisation and allocation concealment methods).
- 2
Only descriptive data reported, insufficient details given to assess the minimally important difference threshold and the imprecision
- 3
Follow-up at 6 months (at the end of the treatment period) and 12 months (6 months after the end of the treatment period) using analysis of variance (ANOVA) for repeated measures (mixed model)
Table 80Summary clinical evidence profile Comparison 9: GnRH agonist + placebo versus danazol + placebo
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95 % CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Oral danazol + IN placebo | GnRH agonist (nafarelin) + oral placebo | ||||
| Relief of painful symptoms – Pelvic tenderness 4-point numerical scale Follow-up: 6 months1 | - | The mean relief of painful symptoms – - pelvic tenderness in the intervention groups was 0.1 lower (0.38 lower to 0.18 higher) | Not estimable | 49 (1 study) | ⊕⊝⊝⊝ Very low2,3 |
| Relief of painful symptoms - Pelvic induration 4-point numerical scale. Follow-up: 6 months1 | - | The mean relief of painful symptoms – pelvic induration in the intervention groups was 0 higher (0.28 lower to 0.28 higher) | Not estimable | 49 (1 study) | ⊕⊝⊝⊝ Very low2,3 |
CI: confidence interval; GnRH: gonadotrophin releasing hormone; IN: intranasal; 4-point scale: each symptom or sign was scored on a 4-point system at each visit from the cards prospectively recorded by each patient or at the vaginal examination (0=none, 1=mild, 2=moderate, 3 severe)
- 1
Assessed after the end of the treatment period
- 2
Quality of evidence was downgraded by 1 point owing to unclear risk of selection bias (no details given about allocation concealment methods)
- 3
Quality of evidence was further downgraded by 2 points owing to very serious imprecision: CI crosses 2 default thresholds
Table 81Summary clinical evidence profile Comparison 9: GnRH agonist + placebo versus danazol + placebo
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95 % CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Danazol + placebo nasal spray | GnRH agonist (nafarelin IN) + oral placebo TDS | ||||
| Relief of painful symptoms – Pelvic tenderness Follow-up: 12 months1 | 742 per 1,000 | 772 per 1,000 (601 to 987) | RR 1.04 (0.81 to 1.33) | 96 (1 study) | ⊕⊝⊝⊝ very Low2,3 |
| Relief of painful symptoms – Pelvic induration Follow-up: 12 months1 | 871 per 1,000 | 906 per 1,000 (775 to 1,000) | RR 1.04 (0.89 to 1.22) | 96 (1 study) | ⊕⊝⊝⊝ Very low2,3 |
CI: confidence interval; GnRH: gonadotrophin releasing hormone; IN: intranasal; RR: risk ratio; TDS: 3 times per day
- 1
Assessed after the end of the treatment period
- 2
Quality of evidence was downgraded by 1 point owing to unclear risk of selection bias (no details about allocation concealment method and unclear description of the allocation concealment procedure)
- 3
Quality of evidence was downgraded by 2 points owing to very serious imprecision: CI crosses 2 default thresholds
Table 82Summary clinical evidence profile, comparison 9: GnRH agonist + placebo versus danazol + placebo
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95 % CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Danazol OD PO + placebo IM | GnRH agonist (leuprolide IM) + placebo OD PO | ||||
| Relief of painful symptoms – Pelvic tenderness Follow-up: 6 months1 | 760 per 1,000 | 730 per 1,000 (631 to 844) | RR 0.96 (0.83 to 1.11) | 253 (1 study) | ⊕⊕⊝⊝ Low2,3 |
CI: confidence interval; GnRH: gonadotrophin releasing hormone; IM: intramuscular; OD: once per day; PO: by mouth; RR: risk ratio;
- 1
Assessed after the end of the treatment period
- 2
Quality of evidence was downgraded by 1 point owing to unclear risk of detection bias (no details were given about randomisation and allocation concealment methods)
- 3
Quality of evidence was further downgraded by 1 point owing to serious imprecision: CI crosses 1 default threshold and p is higher than 0.1
Table 83Summary clinical evidence profile, comparison 10: Depot medroxyprogesterone acetate versus cOCP + danazol
| Outcomes | Illustrative comparative risks (95% CI) | Rela-tive effect (95 % CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| cOCP + desogestrel | Depot medroxyprogesterone | ||||
| Pain at 6 months during treatment period – Dysmenorrhoea (Scale: 10 cm VAS)4 | - | The mean pain at 6 months during treatment period – dysmenorrhoea in the intervention groups was 1.84 lower (2.23 to 1.45 lower) | MD −1.84 (−2.23 to −1.45) | 68 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Pain at 6 months during treatment period – Dyspareunia (Scale: 10 cm VAS)4 | - | The mean pain at 6 months during treatment period - dyspareunia in the intervention groups was 0.3 lower (1.18 lower to 0.58 higher) | MD −0.3 (−1.18 to 0.58) | 59 (1 study) | ⊕⊕⊝⊝ Low1,2 |
| Pain at 6 months during treatment period – Non-menstrual pelvic pain (Scale: 10 cm VAS)4 | - | The mean pain at 6 months during treatment period – non-menstrual pelvic pain in the intervention groups was 0.6 higher (0.09 lower to 1.29 higher) | MD 0.6 (−0.09 to 1.29) | 68 (1 study) | ⊕⊝⊝⊝ Very low1,3 |
| Pain at the end of treatment period (12 months) – Dysmenorrhoea (Scale: 10 cm VAS)4 | - | The mean pain at the end of treatment period (12 months) – dysmenorrhoea in the intervention groups was 1.3 lower (1.79 to 0.81 lower) | MD −1.3 (−1.79 to −0.81) | 68 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Pain at the end of treatment period (12 months) – Dyspareunia (Scale: 10 cm VAS)4 | - | The mean pain at the end of treatment period (12 months) – dyspareunia in the intervention groups was 0.3 lower (1.41 lower to 0.81 higher) | MD −0.3 (−1.41 to 0.81) | 59 (1 study) | ⊕⊕⊝⊝ Low1,2 |
| Pain at the end of treatment period (12 months) – Non-menstrual pelvic pain (Scale: 10 cm VAS)4 | - | The mean pain at the end of treatment period (12 months) – non-menstrual pelvic pain in the intervention groups was 0.4 higher (0.35 lower to 1.15 higher) | MD 0.4 (−0.35 to 1.15) | 68 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Patient satisfaction (very satisfied/satisfied) with treatment at the end of treatment period (12 months) | 575 per 1,000 | 724 per 1,000 (523 to 1,000) | RR 1.26 (0.91 to 1.75) | 80 (1 study) | ⊕⊕⊝⊝ Low1,2 |
CI: confidence interval; cOCP: combined oral contraceptive pill; RR: relative risk; MD: mean difference; VAS: visual analogue scale;
- 1
‘Open label’ study, subjects not blinded
- 2
CI crosses 1 default threshold
- 3
CI crosses 2 default thresholds
- 4
VAS Scale: 10cm scale where 0 = absence of pain, >0–5 = mild pain, >5–8 = moderate pain, >8–10 = unbearable pain
Table 84Summary clinical evidence profile, comparison 11: GnRH agonist + E/P pill versus E/P pill
| Outcomes | Illustrative comparative risks* (95% CI) | Rela-tive effect (95 % CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| E/P pill | GnRH agonist + E/P pill | ||||
| Pain at 8 months during treatment period – Dysmenorrhoea (Scale: 10-point VAS)3 | - | The mean pain at 8 months during treatment period – dysmenorrhoea in the intervention groups was 1.9 lower (2.54 to 1.26 lower) | MD −1.9 (−2.54 to −1.26) | 101 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Pain at 8 months during treatment period – Non-menstrual pelvic pain (Scale: 10-point VAS)3 | - | The mean pain at 8 months during treatment period – non-menstrual pelvic pain in the intervention groups was 2.5 lower (3 to 2 lower) | MD −2.5 (−3.to −2) | 101 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Pain at the end of treatment period (12 months) – Dysmenorrhoea (Scale: 10-point VAS)3 | - | The mean pain at the end of treatment period (12 months) – dysmenorrhoea in the intervention groups was 2.7 lower (3.34 to 2.06 lower) | MD −2.7 (−3.34 to −2.06) | 95 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Pain at the end of treatment period (12 months) – Non-menstrual pelvic pain (Scale: 10-point VAS)3 | - | The mean pain at the end of treatment period (12 months) – non-menstrual pelvic pain in the intervention groups was 0.8 higher (0.33 to 1.27 higher) | MD 0.8 (0.33 to 1.27) | 95 (1 study) | ⊕⊕⊝⊝ Low1,2 |
CI: confidence interval; GnRH: gonadotrophin releasing hormone; E/P: ethinylestradiol pill; MD: mean difference; VAS: visual analogue scale
- 1
No blinding of study participants, investigators or assessors reported
- 2
CI crosses 1 default threshold
- 3
VAS scale: 0 = the absence of pain, 10 = unbearable pain
Table 85Summary clinical evidence profile, comparison 12: GnRH agonist versus cOCP
| Outcomes | Illustrative comparative risks (95% CI) | Rela-tive effect (95 % CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| cOCP | Goserelin | ||||
| Pain at the end of treatment period (6 months) – Dyspareunia (Scale: 10-point VAS)4 | - | The mean pain at the end of treatment period (6 months) – dyspareunia in the intervention groups was 1.8 lower (3.4 to 0.2 lower) | MD −1.8 (−3.4 to −0.2) | 44 (1 study) | ⊕⊕⊝⊝ Low1,2 |
| Pain at the end of treatment period (6 months) – Non-menstrual pelvic pain (Scale: 10-point VAS)4 | - | The mean pain at the end of treatment period (6 months)–- non-menstrual pelvic pain in the intervention groups was 0.2 higher (1.11 lower to 1.51 higher) | MD 0.2 (−1.11 to 1.51) | 50 (1 study) | ⊕⊕⊝⊝ Low1,2 |
| Pain at 6 months after treatment period – Dysmenorrhoea (Scale: 10-point VAS)4 | - | The mean pain at 6 months after treatment period – dysmenorrhoea in the intervention groups was 0.1 higher (1.08 lower to 1.28 higher) | MD 0.1 (−1.08 to 1.28) | 50 (1 study) | ⊕⊝⊝⊝ Very low1,3 |
| Pain at 6 months after treatment period – Dyspareunia (Scale: 10-point VAS)4 | - | The mean pain at 6 months after treatment period - dyspareunia in the intervention groups was 0.4 lower (2.1 lower to 1.3 higher) | MD −0.4 (−2.1 to 1.3) | 43 (1 study) | ⊕⊕⊝⊝ Low1,2 |
| Pain at 6 months after treatment period – Non-menstrual pelvic pain (Scale: 10-point VAS)4 | - | The mean pain at 6 months after treatment period – non-menstrual pelvic pain in the intervention groups was 0.3 higher (1.25 lower to 1.85 higher) | MD −0.3 (−1.25 to 1.85) | 50 (1 study) | ⊕⊕⊝⊝ Low1,2 |
CI: confidence interval; cOCP: combined oral contraceptive pill; MD: mean difference; VAS: visual analogue scale
- 1
No blinding of participants, investigators or assessors reported
- 2
CI crosses 1 default threshold
- 3
CI crosses 2 default thresholds
- 4
VAS scale: 0 = the absence of pain, 1–5 = mild pain, 6–7 = moderate pain, 8–10 = unbearable pain
Table 86Summary characteristics of cost-only studies excluded from review
| Lead Author | Date | Country | Comparison A | Comparison B | Outcomes |
|---|---|---|---|---|---|
| Araujo | 2011 | Brazil | Goserelin acetate for those with confirmed deep endometriosis | Goserelin acetate for all with pelvic pain | Treating all US$1662 cheaper |
| Avxentyeva | 2013 | Russia | Triptorelin, Leuprorelin, Buserelin, Dydrogesterone, Dienogest | N/A | Triprorelin = €1102 Leuprorelin = €1118 Buserelin = €340 Dydrogesterone = €369 Dienogest = €295 |
| Romero | 2012 | Columbia | 12 months Dienogest | 6 months GnRHa | Diogenest = US$986.16 vs. GnRH US$2855.57 |
| Zalis’ka | 2014 | Ukraine | Dydogesterone, Dienogest, Triptorelin | N/A | Dydogesterone = US$345 Dienogest = US$1347 Triptorelin = US$1347 |
| Zhao | 1998 | US | Nafarelin | Leuprolide | Nafarelin = US$2261 vs. Leuprolide US$3245 |
Table 87Annual cost of 4 hormonal treatments included in the model
| Treatment | Cost per 3 monthsa | Source |
|---|---|---|
| Combined oral contraceptive pill (as ethinylestradiol / gestodene tablet) | £19.31 | Electronic Drug Tariff, January 2017b |
| Progestogen treatment (as Desogestrel) | £14.35 | Electronic Drug Tariff, January 2017b |
| Danazol | £86.63 | Electronic Drug Tariff, January 2017b |
| GnRHa (as Leuprorelin) | £236 | Electronic Drug Tariff, January 2017b |
- (a)
The economic model uses 3-month cycles as the standard step in its Markov Chains. As hormonal treatments are typically given cyclically (for example, 21 days on followed by 7 days off) the 3-month cost reflects an average of the cost over this time.
- (b)
Including placebo-arm costs from NICE CG 173 Table F16 to account for, for example, increased GP visits not accounted for in Electronic Drug Tariff.
GnRHa: Gonadotropin-releasing hormone agonist
Table 88Cost and effectiveness of all non-dominated treatment strategies containing a hormonal treatment
| Treatment | Cost | QALY | ICER | Pr. cost-effective vs. no treatment (£20k / QALY) | Pr. cost-effective vs. no treatment (£30k / QALY) |
|---|---|---|---|---|---|
| Empirical Diagnosis & No Treatment | £22,752.60 | 18.120 | Base Case | N/A | N/A |
| Empirical Diagnosis & Combined Oral Contraceptive Pill | £15,845.16 | 18.283 | -£42,434.80 | 96.7% | 96.7% |
| Empirical Diagnosis & Danazol | £19,158.84 | 18.316 | £98,467.20 | 92.3% | 93.4% |
QALY: Quality Adjusted Life-Year; ICER: Incremental Cost-Effectiveness Ratio
Table 89Summary of included studies
| Study | Intervention/comparison | Population | Outcomes | Comments |
|---|---|---|---|---|
| Chen 2012 China | Intervention: Abdominal acupuncture, administered prior to and during menses for 3 consecutive menstrual cycles. Acupuncture was performed approximately 7 times during each treatment cycle Comparison: Danazol, 200mg twice daily was administered (starting on day 1 of a menstrual cycle), for 3 consecutive cycles | Severity of endometriosis symptoms:
N=70 | Assessed at 6 months (3 months of treatment, then 3 months without treatment). Cure of symptoms – defined as complete relief of pain and other symptoms after medication and no relapse in the next 3 menstrual cycles | |
| de Sousa 2016 Brazil | Intervention: experimental treatment of acupuncture, 5 sessions of acupuncture in 5 weeks Comparison: sham-acupuncture, 5 sessions of acupuncture in 5 weeks | Mean age of 30.81 years (±6.38). These women were suffering from endometriosis for an average of 4.79 years (±2.48). No other information given. N=42 | All outcomes measured at 2 months (follow up). Pain score (VAS of 0–10) for:
| |
| Flower 2011 UK | Intervention: an individualised CHM decoction was administered twice daily for 16 weeks Comparison: a placebo decoction comprising inactive ingredients was administered twice daily for 16 weeks | 15% of participants were using concomitant medical therapy (not described). N=33 | Assessed at the end of treatment (16 weeks) Pain scores, measured with VAS 0–10:
Endometriosis Health Profile-30 scores (range 0–100) | |
| Mira 2015 Brazil | Intervention: acupuncture-like TENS to S3–S4 region, 30 minute sessions were applied once a week for a period of 8 weeks Comparison: self-applied TENS to the S3–S4 region, 20 minute sessions were conducted twice daily for a period of 8 weeks | Women with deep endometriosis diagnosed in the cul-de-sac and intestinal loop who sustained pelvic pain and/or deep dyspareunia, despite continuous clinical medication. All women were undergoing hormone therapy with continuous progestin alone or combined oral contraceptives for at least 3 months. N=22 | Assessed at the end of treatment (8 weeks). Endometriosis Health Profile-30 scores (range 0–100) | Women were undergoing hormone therapy with continuous progestin alone or combined contraceptives for at least 3 months |
| Sesti 2009 Italy | Intervention: Group 1: GnRH analogue 3.75mg every 28 days post-operatively Group 2: continuous lowdose cOCP for 6 months post-operatively Group 3: dietary intervention (including probiotics, vitamin, mineral and fatty acid supplementation) postoperatively Comparison: placebo | All women underwent laparoscopic cystectomy for endometrioma at the start of the trial. N=259 | Assessed at 18 months follow up: recurrence of endometrioma >20mm diameter. Cysts suspected to be endometriomas with ultrasound were then confirmed laparoscopically | Women with an endometrioma, who underwent cystectomy at the start of the trial. |
| Wayne 2008 USA | Intervention: Japanese-style acupuncture, twice per week for 8 consecutive weeks Comparison: sham-acupuncture, twice per week for 8 consecutive weeks | All women had stage I endometriosis. Eligible participants were aged 13–22 years old. N=18 | All outcomes measured at 4 weeks, 8 weeks (during treatment) and 6 months (follow up). Pain score (numerical analogue scale of 0–10). Endometriosis Health Profile-30 (range 0–100). Pediatric Quality of Life Inventory score (range 0–100). Activity scale (to assess activities limited by endometriosis) (range 0–10) | |
| Wu 2006 (Flower 2012 CSR) China | Intervention: CHM Group 1: Nei Yi pills 10g twice daily Group 2: Nei Yi pills 10g twice daily plus Nei Yi enema 70ml once daily Comparison: danazol 400mg per day | Laparoscopically confirmed endometriosis. No other details given N=58 | Symptomatic relief was assessed within 3 years of stopping treatment, other outcomes – at the end of 3 months treatment. Five outcomes were assessed:
| |
| Xia 2006 China | Intervention: acupuncture (started 9 days before menses and discontinued during menses) and CHM (GuiZhi-Fu-Ling-Wan) Comparison: danazol 200mg twice daily. Treatment was continued for 3 consecutive cycles | N=78 | Assessed at the end of treatment (3 months of treatment). Dysmenorrhoea (pain scale not reported). Dichotomous outcome of ‘cessation of signs and symptoms’ of
| |
| Xiang 2002 China | Intervention: Ear acupuncture therapy, beginning 5 days before menses and given fo4ur times every other day, for 3 menstrual cycles Comparison: CHM. A decoction was given 5 days before menstruation, 1 dose for 7 days, for 3 menstrual cycles | Laparoscopically confirmed endometriosis. No other information given. N = 67 | Assessed at the end of treatment (3 menstrual cycles). Dysmenorrhoea score (5–15). Symptom cure (dichotomous outcome) | |
| Zhu 2014 China | Intervention: Group 1: cOCP (30μg ethinyloestradiol and 150μg desogestrel) administered once per day for 63 days after surgery Group 2: as group 1, but also received Dan’e CHM 30g per day for the latter 30 days of treatment. Comparison: no medical treatment after surgery | Women with minimal/mild endometriosis (wishing to conceive), who had failed to become pregnant after at least 12 months of unprotected intercourse. All women underwent surgery at the start of the trial, including ablation/excision of all visible lesions and division of adhesions to restore normal pelvic anatomy N=156 | Fertility outcomes assessed at 12 months after treatment:
| Women with subfertility and minimal/mild endometriosis, all of whom underwent operative laparoscopy at the start of the trial |
N: number of participants in study; CSR: Cochrane systematic review
Table 90Summary clinical evidence profile, Comparison 1: cOCP and Dan’e compared to no treatment for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| No treatment | cOCP and Dan’e | ||||
| Live birth (denominator pregnancy) at 12 months after treatment completion | 792 per 1,000 | 815 per 1,000 (594 to 1,000) | RR 1.03 (0.75 to 1.4) | 40 (1 study) | ⊕⊕⊝⊝ Low1 |
| Miscarriage (denominator pregnancy) at 12 months after treatment completion | 125 per 1,000 | 188 per 1,000 (43 to 815) | RR 1.5 (0.34 to 6.52) | 40 (1 study) | ⊕⊕⊝⊝ Low1 |
CI: confidence interval; RR: risk ratio; cOCP: combined oral contraceptive pill
- 1
CI for estimate is very wide, crossing 2 thresholds
Table 91Summary clinical evidence profile, Comparison 2: cOCP and Dan’e compared to cOCP for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| cOCP | cOCP and Dan’e | ||||
| Live birth (denominator pregnancy) at 12 months after treatment completion | 700 per 1,000 | 812 per 1,000 (560 to 1,000) | RR 1.16 (0.8 to 1.68) | 36 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Miscarriage (denominator pregnancy) at 12 months after treatment completion | 200 per 1,000 | 188 per 1,000 (48 to 720) | RR 0.94 (0.24 to 3.6) | 36 (1 study) | ⊕⊕⊝⊝ Low2 |
CI: confidence interval; RR: risk ratio; cOCP: combined oral contraceptive pill
- 1
CI for estimate is very wide, crossing 1 threshold
- 2
CI for estimate is very wide, crossing 2 thresholds
Table 92Summary clinical evidence profile, Comparison 3: Diet compared to placebo for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Placebo | Diet | ||||
| Endometrioma recurrence1 | 167 per 1,000 | 177 per 1,000 (82 to 387) | RR 1.06 (0.49 to 2.32) | 122 (1 study) | ⊕⊕⊝⊝ Low2 |
CI: confidence interval; RR: risk ratio
- 1
The recurrence of endometrioma was defined as the presence of a cyst, detected by transvaginal ultrasonography, with a pattern suggesting an endometrioma more than 20 mm in diameter.
- 2
CI for estimate is very wide, crossing 2 thresholds
Table 93Summary clinical evidence profile, Comparison 4: Diet compared to GnRHa for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| GnRHa | Diet | ||||
| Endometrioma recurrence1 | 103 per 1,000 | 178 per 1,000 (70 to 449) | RR 1.72 (0.68 to 4.34) | 120 (1 study) | ⊕⊕⊝⊝ Low2 |
CI: confidence interval; RR: risk ratio
- 1
The recurrence of endometrioma was defined as the presence of a cyst, detected by transvaginal ultrasonography, with a pattern suggesting an endometrioma more than 20 mm in diameter.
- 2
CI for estimate is very wide, crossing 2 thresholds
Table 94Summary clinical evidence profile, Comparison 5: Diet compared to cOCP for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| cOCP | Diet | ||||
| Endometrioma recurrence1 | 150 per 1,000 | 177 per 1,000 (79 to 398) | RR 1.18 (0.53 to 2.65) | 122 (1 study) | ⊕⊕⊝⊝ Low2 |
CI: confidence interval; RR: risk ratio; cOCP: combined oral contraceptive pill
- 1
The recurrence of endometrioma was defined as the presence of cyst, detected by transvaginal ultrasonography, with a pattern suggesting an endometrioma more than 20 mm in diameter.
- 2
Confidence interval for estimate is very wide crossing 2 thresholds
Table 95Summary clinical evidence profile, Comparison 6: Acupuncture compared to sham acupuncture for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Sham acupuncture | Acupuncture | ||||
| Change (from baseline) in pain in last 4 weeks – at 4 weeks (ESSS) | - | The mean change (from baseline) in pain in last 4 weeks – at 4 weeks (ESSS) in the intervention groups was 3.4 lower (5.82 to 0.98 lower) | MD −3.4 (−5.82 to −0.98) | 14 (1 study) | ⊕⊕⊝⊝ Low3,4 |
| Change (from baseline) in pain in last 4 weeks – at 8 weeks (ESSS) | - | The mean change (from baseline) in pain in last 4 weeks – at 8 weeks (ESSS) in the intervention groups was 0.5 lower (3.22 lower to 2.22 higher) | MD −0.5 (−3.22 to 2.22) | 15 (1 study) | ⊕⊝⊝⊝ Very low3,4 |
| Change (from baseline) in pain in last 2 months–chronic pelvic pain | - | The mean change (from baseline) in last 2 months – chronic pelvic pain in the intervention groups was 3.29 lower (3.97 to 2.61 lower) | MD −3.29 (−3.97 to −2.61) | 42 (1 study) | ⊕⊕⊕⊝ Moderate6 |
| Change (from baseline) in pain in last 2 months – dyspareunia | - | The mean change (from baseline) in last 2 months – dyspareunia in the intervention groups was 3.76 lower (4.55 to 2.97 lower) | MD −3.76 (−4.55 to −2.97) | 42 (1 study) | ⊕⊕⊕⊝ Moderate6 |
| Change (from baseline) in pain in last 4 weeks – at 6 months (ESSS) | - | The mean change (from baseline) in pain in last 4 weeks–- at 6 months (ESSS) in the intervention groups was 0.8 lower (4.66 lower to 3.06 higher) | MD −0.8 (−4.66 to 3.06) | 14 (1 study) | ⊕⊝⊝⊝ Very low3,5 |
| Change (from baseline) in QoL (EHP total score) - at 4 weeks (EHP) | - | The mean change (from baseline) in QoL (EHP total score)–- at 4 weeks (EHP in the intervention groups was 21.5 lower (39.27 to 3.73 lower) | MD −21.5 (−39.27 to −3.73) | 14 (1 study) | ⊕⊕⊝⊝ Low3,4 |
| Change (from baseline) in QoL (EHP total score) - at 8 weeks (EHP) | - | The mean change (from baseline) in QoL (EHP total score) – at 8 weeks (EHP) in the intervention groups was 19.7 lower (38.7 to 0.7 lower) | MD −19.7 (−38.7 to −0.7) | 15 (1 study) | ⊕⊕⊝⊝ Low3,4 |
| Change (from baseline) in QoL (EHP Total score) – at 6 months (EHP) | - | The mean change (from baseline) in QoL (EHP total score) - at 6 months (EHP) in the intervention groups was 20.9 lower (37.57 to 4.23 lower) | MD −20.9 (−37.57 to −4.23) | 14 (1 study) | ⊕⊕⊝⊝ Low3,4 |
| Change (from baseline) in QoL (Paediatric QoL Inventory total score)1 – at 4 weeks | - | The mean change (from baseline) in QoL (paediatric QoL inventory total score) – at 4 weeks in the intervention groups was 10.1 higher (3.26 lower to 23.46 higher) | MD 10.1 (−3.26 to 23.46) | 14 (1 study) | ⊕⊕⊝⊝ Low3,4 |
| Change (from baseline) in QoL (Paediatric QoL Inventory total score)1 – at 8 weeks | - | The mean change (from baseline) in QoL (paediatric QoL inventory total score) – at 8 weeks in the intervention groups was 14.2 higher (0.94 lower to 29.34 higher) | MD 14.2 (−0.94 to 29.34) | 15 (1 study) | ⊕⊕⊝⊝ Low3,4 |
| Change (from baseline) in QoL (Paediatric QoL Inventory total score)1 – at 6 months | - | The mean change (from baseline) in QoL (paediatric QoL inventory total score) – at 6 months in the intervention groups was 14.9 higher (1.18 to 28.62 higher) | MD 14.9 (1.18 to 28.62) | 14 (1 study) | ⊕⊕⊝⊝ Low3,4 |
| Change (from baseline) in activities of daily living (3 activity score)2 – at 4 weeks | - | The mean change (from baseline) in activities of daily living (3 activity score) – at 4 weeks in the intervention groups was 2.9 lower (4.85 to 0.95 lower) | MD −2.9 (−4.85 to −0.95) | 14 (1 study) | ⊕⊝⊝⊝ Very low3,5 |
| Change (from baseline) in activities of daily living (3 activity score)2 – at 8 weeks | - | The mean change (from baseline) in activities of daily living (3 activity score) – at 8 weeks in the intervention groups was 1.8 lower (4.48 lower to 0.88 higher) | MD −1.8 (−4.48 to 0.88) | 14 (1 study) | ⊕⊕⊝⊝ Low3,4 |
| Change (from baseline) in activities of daily living (3 activity score)2 – at 6 months | - | The mean change (from baseline) in activities of daily living (3 activity score) – at 6 months in the intervention groups was 1.7 lower (5.21 lower to 1.81 higher) | MD −1.7 (−5.21 to 1.81) | 14 (1 study) | ⊕⊝⊝⊝ Very low3,5 |
CI: confidence interval; MD: mean difference; ESSS: Endometriosis Symptom Severity Scale (0–10); EHP: Endometriosis Health Profile-30 (subscales range 0–100)
- 1
Paediatric QoL Inventory Total score (subscales range 0–100)
- 2
Activity scale scores range 0–10
- 3
Due to dropouts
- 4
CI for estimate is very wide, crossing 1 threshold
- 5
CI for estimate is very wide, crossing 2 thresholds
- 6
The quality of the evidence was downgraded because of the unclear risk of attrition bias (no details provided in the text), besides the unclear risk of detection bias
Table 96Summary clinical evidence profile, Comparison 7: Acupuncture compared to danazol for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Danazol | Acupuncture | ||||
| Cure of symptoms1 | 143 per 1,000 | 86 per 1,000 (23 to 331) | RR 0.6 (0.16 to 2.32) | 70 (1 study) | ⊕⊝⊝⊝ Very low2,3 |
CI: confidence interval; RR: risk ratio
- 1
Defined as complete relief of pain and other symptoms after medication and no relapse in the next 3 menstrual cycles
- 2
No blinding
- 3
CI for estimate is very wide, crossing 2 thresholds
Table 97Summary clinical evidence profile, Comparison 8: Acupuncture compared to CHM for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| CHM | Acupuncture | ||||
| Dysmenorrhoea | - | The mean dysmenorrhoea in the intervention groups was 4.81 lower (6.25 to 3.37 lower) | MD −4.81 (−6.25 to 3.37) | 67 (1 study) | ⊕⊝⊝⊝ Very low2,3 |
| Cure of symptoms1 | 100 per 1,000 | 297 per 1,000 (91 to 970) | RR 2.97 (0.91 to 9.7) | 67 (1 study) | ⊕⊕⊝⊝ Low2,4 |
CI: confidence interval; RR: risk ratio
- 1
Defined according Guideline for Clinical Research on New Chinese Drugs for Treatment of Pelvic Endometriosis
- 2
No blinding
- 3
CI for estimate is very wide, crossing 2 thresholds
- 4
CI for estimate is very wide crossing 1 threshold
Table 98Summary clinical evidence profile, Comparison 9: CHM compared to placebo for endometriosis
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Placebo | CHM | ||||
| Change (from baseline) in pain (VAS) at week 16 – period pain | - | The mean change (from baseline) in pain (VAS) at week 16 – period pain in the intervention groups was 1.22 lower (3.81 lower to 1.37 higher) | MD −1.22 (−3.81 to 1.37) | 12 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Change (from baseline) in pain (VAS) at week 16 – pain during sex | - | The mean change (from baseline) in pain (VAS) at week 16 – pain during sex in the intervention groups was 0.76 higher (1.53 lower to 3.05 higher) | MD 0.76 (−1.53 to 3.05) | 8 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Change (from baseline) in pain (VAS) at week 16 – pain on bowel movement | - | The mean change (from baseline) in pain (VAS) at week 16 – pain on bowel movement in the intervention groups was 0.08 higher (2.87 lower to 3.03 higher) | MD 0.08 (−2.87 to 3.03) | 12 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Change (from baseline) in pain (VAS) at week 16 – daily pain | - | The mean change (from baseline) in pain (vas) at week 16 – daily pain in the intervention groups was 0.74 higher (1.81 lower to 3.29 higher) | MD 0.74 (−1.81 to 3.29) | 13 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Change (from baseline) in patient assessed QoL (MYMOP) at week 16 – symptom 1 | - | The mean change (from baseline) in patient assessed QoL (MYMOP) at week 16 – symptom 1 in the intervention groups was 0.58 lower (2.41 lower to 1.25 higher) | MD −0.58 (−2.41 to 1.25) | 18 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Change (from baseline) in patient assessed QoL (MYMOP) at week 16 – symptom 2 | - | The mean change (from baseline) in patient assessed QoL (MYMOP) at week 16–- symptom 2 in the intervention groups was 0.9 lower (2.68 lower to 0.88 higher) | MD −0.9 (−2.68 to 0.88) | 18 (1 study) | ⊕⊕⊝⊝ Low1,3 |
| Change (from baseline) in patient assessed QoL (MYMOP) at week 16 – activity | - | The mean change (from baseline) in patient assessed QoL (MYMOP) at week 16 – activity in the intervention groups was 0.69 lower (2.31 lower to 0.93 higher) | MD −0.69 (−2.31 to 0.93) | 17 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Change (from baseline) in patient assessed QoL (MYMOP) at week 16 – wellbeing | - | The mean change (from baseline) in patient assessed QoL (MYMOP) at week 16 – wellbeing in the intervention groups was 1.06 lower (2.95 lower to 0.83 higher) | MD −1.06 (−2.95 to 0.83) | 17 (1 study) | ⊕⊕⊝⊝ low1,3 |
| Change (from baseline) in QoL (EHP 30) at week 16 – pain | - | The mean change (from baseline) in QoL (EHP-30) at week 16 – pain in the intervention groups was 0.32 lower (10.01 lower to 9.37 higher) | MD −0.32 (−10.01 to 9.37) | 18 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Change (from baseline) in QoL (EHP 30) at week 16 – control and powerlessness | - | The mean change (from baseline) in QoL (EHP-30) at week 16–- control and powerlessness in the intervention groups was 1.73 lower (7.35 lower to 3.89 higher) | MD −1.73 (−7.35 to 3.89) | 18 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Change (from baseline) in QoL (EHP 30) at week 16 – emotional wellbeing | - | The mean change (from baseline) in QoL (EHP-30) at week 16 – emotional wellbeing in the intervention groups was 0.37 lower (4.38 lower to 3.64 higher) | MD −0.37 (−4.38 to 3.64) | 18 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Change (from baseline) in QoL (EHP 30) at week 16 – social support | - | The mean change (from baseline) in QoL (EHP-30) at week 16 – social support in the intervention groups was 2.71 lower (7.09 lower to 1.67 higher) | MD −2.71 (−7.09 to 1.67) | 18 (1 study) | ⊕⊕⊝⊝ Low1,3 |
| Change (from baseline) in QoL (EHP 30) at week 16 – self-image | - | The mean change (from baseline) in QoL (EHP-30) at week 16 – self-image in the intervention groups was 0.46 higher (2.22 lower to 3.14 higher) | MD 0.46 (−2.22 to 3.14) | 18 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
CI: confidence interval; MD: mean difference; MYMOP: Measure Your own Medical Outcomes Profile (1–7-point Likert scale); QoL: quality of life
- 1
Due to drop outs
- 2
CI for estimate is very wide, crossing 2 thresholds
- 3
CI for estimate is very wide, crossing 1 threshold
Table 99Summary clinical evidence profile, Comparison 10: CHM (oral) compared to danazol for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Danazol | CHM (oral) | ||||
| Symptomatic relief1 | 111 per 1,000 | 562 per 1,000 (142 to 1,000) | RR 5.06 (1.28 to 20.05) | 34 (1 study) | ⊕⊕⊝⊝ low2,3 |
| Dysmenorrhoea score | - | The mean dysmenorrhoea score in the intervention groups was 1.01 lower (3.11 lower to 1.09 higher) | MD −1.01 (−3.11 to 1.09) | 34 (1 study) | ⊕⊕⊝⊝ Low2,4 |
| Lumbosacral pain relief | 722 per 1,000 | 874 per 1,000 (621 to 1,000) | RR 1.21 (0.86 to 1.7) | 34 (1 study) | ⊕⊕⊝⊝ Low2,4 |
| Rectal irritation relief | 500 per 1,000 | 835 per 1,000 (450 to 1,000) | RR 1.67 (0.9 to 3.1) | 24 (1 study) | ⊕⊕⊝⊝ Low2,4 |
| Tenderness of vaginal nodules in posterior fornix | 692 per 1,000 | 907 per 1,000 (602 to 1,000) | RR 1.31 (0.87 to 1.97) | 24 (1 study) | ⊕⊕⊝⊝ Low2,4 |
| Adnexal masses disappearance or shrinkage | 533 per 1,000 | 752 per 1,000 (421 to 1,000) | RR 1.41 (0.79 to 2.5) | 27 (1 study) | ⊕⊝⊝⊝ Very low2,5 |
CI: confidence interval; RR: risk ratio; MD: mean difference
- 1
Defined as a complete resolution of all symptoms and signs and included pregnancy, when desired, within 3 years of stopping treatment
- 2
Not clear if blinding was performed
- 3
Although the outcome is defined, it is wide, encompassing different symptoms and signs.
- 4
CI for estimate is very wide, crossing o1ne threshold
- 5
CI for estimate is very wide, crossing 2 thresholds
Table 100Summary clinical evidence profile, Comparison 11: CHM (oral + enema) compared to danazol for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Danazol | CHM (oral + enema) | ||||
| Symptomatic relief1 | 111 per 1,000 | 624 per 1,000 (163 to 1,000) | RR 5.63 (1.47 to 21.54) | 42 (1 study) | ⊕⊕⊝⊝ Low2,3 |
| Dysmenorrhoea score | - | The mean dysmenorrhoea score in the intervention groups was 2.9 lower (4.55 to 1.25 lower) | MD −2.9 (−4.5 to −1.25) | 42 (1 study) | ⊕⊕⊝⊝ Low2,4 |
| Lumbosacral pain relief | 722 per 1,000 | 831 per 1,000 (592 to 1,000) | RR 1.15 (0.82 to 1.62) | 42 (1 study) | ⊕⊕⊝⊝ Low2,4 |
| Rectal irritation relief | 500 per 1,000 | 890 per 1,000 (495 to 1,000) | RR 1.78 (0.99 to 3.2) | 30 (1 study) | ⊕⊕⊝⊝ Low2,4 |
| Tenderness of vaginal nodules in posterior fornix | 692 per 1,000 | 872 per 1,000 (582 to 1,000) | RR 1.26 (0.84 to 1.9) | 29 (1 study) | ⊕⊕⊝⊝ Low2,4 |
| Adnexal masses disappearance or shrinkage | 533 per 1,000 | 907 per 1,000 (555 to 1,000) | RR 1.7 (1.04 to 2.78) | 36 (1 study) | ⊕⊕⊝⊝ Low2,4 |
CI: confidence interval; RR: risk ratio; MD: mean difference; CSR: Cochrane systematic review
- 1
Defined as a complete resolution of all symptoms and signs and included pregnancy, when desired, within 3 years of stopping treatment
- 2
Not clear if blinding was performed
- 3
Although the outcome is defined, it is wide, encompassing different symptoms and signs.
- 4
CI for estimate is very wide, crossing 1 threshold
Table 101Summary clinical evidence profile, Comparison 12: CHM (oral+ enema) compared to CHM (oral) for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| CHM (oral) | CHM (oral+ enema) | ||||
| Symptomatic relief1 | 562 per 1,000 | 624 per 1,000 (366 to 1,000) | RR 1.11 (0.65 to 1.89) | 40 (1 study) | ⊕⊝⊝⊝ Very low2,3,4 |
| Dysmenorrhoea score | - | The mean dysmenorrhoea score in the intervention groups was 1.89 lower (3.89 lower to 0.11 higher) | MD −1.89 (−3.89 to 0.11) | 40 (1 study) | ⊕⊕⊝⊝ Low2,5 |
| Lumbosacral pain relief | 875 per 1,000 | 831 per 1,000 (648 to 1,000) | RR 0.95 (0.74 to 1.23) | 40 (1 study) | ⊕⊕⊝⊝ Low2,5 |
| Rectal irritation relief | 833 per 1,000 | 892 per 1,000 (658 to 1,000) | RR 1.07 (0.79 to 1.44) | 30 (1 study) | ⊕⊝⊝⊝ Low2,5 |
| Tenderness of vaginal nodules in posterior fornix | 909 per 1,000 | 873 per 1,000 (673 to 1,000) | RR 0.96 (0.74 to 1.25) | 27 (1 study) | ⊕⊕⊝⊝ Low2,5 |
| Adnexal masses disappearance or shrinkage | 750 per 1,000 | 908 per 1,000 (638 to 1,000) | RR 1.21 (0.85 to 1.72) | 33 (1 study) | ⊕⊕⊝⊝ Low2,5 |
CI: confidence interval; RR: risk ratio; MD: mean difference
- 1
Defined as a complete resolution of all symptoms and signs and included pregnancy, when desired, within 3 years of stopping treatment
- 2
Not clear if blinding was performed
- 3
Although the outcome is defined, it is wide, encompassing different symptoms and signs
- 4
CI for estimate is very wide, crossing 2 thresholds
- 5
CI for estimate is very wide, crossing 1 threshold
Table 102Summary clinical evidence profile, Comparison 13: CHM and acupuncture
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Danazol | Chinese herbal medicine and Acupuncture | ||||
| Dysmenorrhoea (cessation) | 342 per 1,000 | 400 per 1,000 (222 to 715) | RR 1.17 (0.65 to 2.09) | 78 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Lumbosacral pain (cessation) | 316 per 1,000 | 376 per 1,000 (202 to 695) | RR 1.19 (0.64 to 2.2) | 78 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Dyspareunia (cessation) | 53 per 1,000 | 125 per 1,000 (26 to 606) | RR 2.38 (0.49 to 11.51) | 78 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
CI: confidence interval; RR: risk ratio
- 1
No blinding
- 2
CI for estimate is very wide, crossing 2 thresholds
Table 103Summary clinical evidence profile, Comparison 14: Acupuncture TENS compared to Self-applied TENS for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Self-applied TENS | Acupuncture TENS | |||||
| Change (from baseline) in QoL (EHP-30 total score) | - | The mean change (from baseline) in QoL (EHP-30 total score) in the intervention groups was 1.39 lower (8.94 lower to 6.16 higher) | MD −1.39 (−8.94 to 6.16) | 22 (1 study) | ⊕⊝⊝⊝ Very low1,2 | All women were undergoing hormone therapy with continuous progestin or combined oral contraceptives for at least 3 months |
CI: confidence interval; MD: mean difference; EHP-30: Endometriosis Health Profile
- 1
No blinding
- 2
CI for estimate is very wide, crossing 2 thresholds
Table 104Estimated annual direct cost of non-pharmacological interventions included in economic model
| Technique | Cost | Source (see above for details) |
|---|---|---|
| Acupuncture | £545.00 | NG23 |
| TCM | £120.77 |
Amazon |
TCM: Traditional Chinese Medicine
Table 105Cost and effectiveness of all non-dominated treatment strategies containing a non-pharmacological intervention
| Treatment | Cost | QALY | ICER | Pr. cost-effective vs. no treatment (£20k/QALY) | Pr. cost-effective vs. no treatment (£30k/QALY) |
|---|---|---|---|---|---|
| Empirical diagnosis and no treatment | £22,899.35 | 18.739 | Base case | N/A | N/A |
| Empirical diagnosis and herbal medicine | £17,215.53 | 18.645 | Extendedly dominated | 14.29% | 19.05% |
| Pelvic MRI and herbal medicine | £19,355.67 | 18.653 | Extendedly dominated | 14.29% | 14.29% |
| Transabdominal ultrasound and herbal medicine | £21,234.45 | 18.665 | Extendedly dominated | 28.57% | 33.33% |
| Empirical diagnosis and acupuncture | £22,984.66 | 18.799 | £1,419.84 | 52.38% | 61.90% |
| Laparoscopy and acupuncture | £32,746.25 | 18.822 | £428,026.09 | 61.90% | 66.67% |
Table 106Research recommendation rationale
| Research question | Are Pain Management Programmes (PMPs) a clinically and cost-effective intervention for women with endometriosis? |
|---|---|
| Importance to ‘patients’ or the population | Minimising distress and disability associated with chronic pelvic pain is of prime importance for women with endometriosis to maximise their overall quality of life and emotional wellbeing. PMPs are well established as interventions for people with chronic pain conditions, to minimise the physical disability and psychological distress associated with chronic pain by developing effective self-management techniques. PMPs may also reduce longer term healthcare costs. However, few services and programmes exist to support the specific needs of women with endometriosis. While there is some published research evaluating the efficacy of pelvic pain specialist PMPs (which include women with endometriosis), there is little evidence specifically for women with endometriosis. This is a developing area within the field of pain management but many women experiencing endometriosis have valued PMPs and considered these to be an effective multidisciplinary intervention to support them. |
| Relevance to NICE guidance | This is relevant to NHS guidance because it could help to minimise the functional, psychological and social impact of endometriosis, thereby improving quality of life, emotional wellbeing and minimising associated financial costs. Since this is currently lacking evidence future guidelines would benefit from this information which could lead to recommendations in an update of this guideline. |
| Relevance to the NHS | This is highly relevant because appropriate self-management of chronic pain can reduce unnecessary repeated visits to GPs, A&Es, outpatients and repeated investigations. It can also minimise the impact on emotional wellbeing and mental health. This would reduce the requirement in health, social and educational settings and therefore also reduce costs. |
| National priorities | Since the Chief Medical Officer’s Report of 2008, which made chronic pain a focus, the management of chronic pain has been recognised as of huge national importance. Since then a national pain summit, pain audit and pain service specifications have been achieved: https://www The Royal College of GPs made treatment of chronic pain a priority (http://content Active support for self-management is now also seen as the first priority for commissioners (Kings Fund 2015) (https://www |
| Current evidence base | There is a small evidence base for women with chronic pelvic pain. No evidence was identified that addressed this topic in women with endometriosis. |
| Equality | Pain management programmes will have to take into consideration any protected equalities groups, such as age, sexuality, or people with learning difficulties. Possible communication difficulties or need for interpreters may also need to be taken into consideration when designing materials. Cultural differences may also impact on the way pelvic pain is described or interpreted. |
| Feasibility | PMPs are audited as standard clinical practice, therefore evaluating the efficacy and outcomes is feasible. It may be difficult to collect follow up data for several years after the intervention as most clinical services do not offer follow up for longer than 1 year. |
| Other comments | Pain management programmes are multi-faceted and can therefore be tailored to individual needs. Therefore they actively promote equalities. |
Table 107Research recommendation PICO table
| Criterion | Explanation |
|---|---|
| Population | An RCT of women with endometriosis who are suitable for and complete a specialist Pelvic Pain Management Programme. Multi-centre research may be feasible if services are matched for specialism and PMP intervention quality. Cross-over study against wait list control. |
| Intervention | Completion of a specialist PMP specifically designed for women with endometriosis (and/or other diagnoses which result in pelvic pain). It should be gender specific i.e. a programme for women only and delivered by a multidisciplinary team including clinical psychologists, pain management physiotherapists and consultants in pain medicine with experience managing pelvic pain. It should specifically address issues such as sexual, bowel and bladder function in the context of pain. It may also touch on important issues for individuals for example, fertility. Specialised PMPS are currently run by NHSE-recognised Specialised Pain Services. |
| Comparator | Outcomes for women with endometriosis related chronic pain treated by PMP could be compared with outcome data for other patient groups attending PMPs e.g. those with other pelvic pain, musculoskeletal and neuropathic pain to benchmark effectiveness of PMPS for endometriosis against other pain conditions. Women with endometriosis who have completed medical and surgical management wait list control. |
| Outcome | Validated pain (physical, functional and psychological outcome measures recognised in pain management specialism such as the Brief Pain Inventory. Healthcare and medicines utilisation health related quality of life and costs associated with the delivery of the PMP. |
| Study design | Study design: A multi-centre RCT evaluating the outcomes and long-term efficacy of specialised pelvic PMPs on women with a diagnosis of endometriosis. The study should also collect prospective community (GP) service and hospital data to evaluate medicines and healthcare utilisation economics. |
| Timeframe | Within 5 years |
Table 108Research recommendation rationale
| Research question | Are specialist lifestyle interventions (diet and exercise) effective, compared with no specialist lifestyle interventions, for women with endometriosis? |
|---|---|
| Importance to ‘patients’ or the population | Effective self-management is critical for the wellbeing of women with endometriosis. They receive care in a range of settings where information and support on self-management of endometriosis varies widely. They consistently report uncertainty on lifestyle interventions relating to self-management of endometriosis. Many patients seek self-management lifestyle intervention information online. However, this information is not based on high quality research and therefore is not evidence-based guidance. High quality, evidence-based research would enable clinical staff to provide accurate, safe and consistent guidance to complement surgical and medical treatment and advice, enabling endometriosis patients to plan effective self-management options. |
| Relevance to NICE guidance | This is highly relevant to NHS guidance as it could help to establish the safety and effectiveness of specialist lifestyle interventions in the management of endometriosis. Since there is lack of high quality evidence, future NICE guidance would greatly benefit from the identification of appropriate strategies to self-manage the condition through specialist lifestyle interventions. |
| Relevance to the NHS | This is highly relevant to the NHS as effective self-management of endometriosis can reduce unnecessary repeated visits to GPs, A&Es, outpatients, repeated investigations and other interventions. This could also minimise associated financial costs if more women are empowered to self-manage their condition outside of the NHS. |
| National priorities | Since the Chief Medical Officer’s Report of 2008, which made chronic pain a focus, the management of chronic pain has been recognised as of huge national importance. Since then a national pain summit, pain audit and pain service specifications have been achieved: https://www The Royal College of GPs made treatment of chronic pain a priority (http://content Active support for self-management is now also seen as the first priority for commissioners (Kings Fund 2015) (https://www |
| Current evidence base | There is currently no high quality research on the effectiveness of specialist lifestyle interventions. |
| Equality | Women have the right to accessible, safe and effective information and guidance on how to manage this long-term, life altering condition that can have a negative impact on many aspects of a woman’s life. |
| Feasibility | There are always ethical issues in conducting studies in vulnerable populations. These would require careful consideration, but could be overcome. |
| Other comments | Not applicable |
Table 109Research recommendation PICO table
| Criterion | Explanation |
|---|---|
| Population | An RCT of women and/or girls with diagnosed or suspected endometriosis, who are suitable for a specialist lifestyle intervention. |
| Intervention | Completion of a specialist diet and/or exercise intervention designed for women and/or girls with diagnosed or suspected endometriosis. |
| Comparator | Outcomes for women and/or girls participating in a specialist diet and/or exercise intervention could be compared with outcomes for those participating in a non-specialist diet and/or exercise intervention. |
| Outcome | Validated outcome measures or questionnaires recognised in lifestyle intervention specialism/field should be used to assess, for example, reduction in pain improvement in energy levels and fitness improvement of menorrhagia and dysmenorrhoea improvement in emotional wellbeing improvement in autonomy and ability to manage activities of daily living increased ability to self-manage the condition fewer medical appointments |
| Study design | A multicentre RCT evaluating the outcomes and the long-term effectiveness of specialised diet and/or exercise interventions. |
| Timeframe | Within 5 years |
Table 110Dose ranges of treatments in different classes of interventions, with abbreviations used in tables and figures within this chapter
| Class | Treatment | Abbreviation |
|---|---|---|
| Diagnostic laparoscopy / No treatment | Diagnostic laparoscopy No treatment/Waiting list | Diagnostic/no treat |
| Danazol/gestrinone | Danazol (100–800 mg/d) Gestrinone | Dan/gest |
| Oestrogens (oral) | Oestradiol (1–2 mg/d) Conjugated equine oestrogens (0.3–1.25 mg/d) | Oest(o) |
| Progestogens (oral) | Norethisterone (2.5 mg/d) Medroxyprogesterone (15–30 mg/d) Levonorgestrel (30 micrograms/d) Desogestrel (75 micrograms/d) Dienogest (2 mg/d) | Prog(o) |
| Progestogens (depot) | Medroxyprogesterone (150 mg/3m) Gestodene (5–10 mg) | Prog(i.m.) |
| Progestogens (subcutaneous) | Medroxyprogesterone (104 mg/3m) Promegestone | Prog(s.c.) |
| Progestogens (intrauterine) | Levonorgestrel (20 micrograms/d) | Prog(i.u.) |
| GnRH agonists (depot) | Leuprorelide (3.75 mg/m) Triptorelin (3 mg/m) | GnRHa(i.m.) |
| GnRH agonists (subcutaneous) | Goserelin (3.6 mg/m) | GnRHa(s.c.) |
| GnRH agonists (nasal spray) | Nafarelin (200 micrograms b.d.) Buserelin (300 micrograms t.d.) | GnRHa(i.n.) |
| GnRH antagonists | Elagolix | GnRHant |
| Aromatase inhibitors | Anastrozole (1 mg/d) Letrozole (2.5 mg/d) | AromaInhib |
| Anti-androgens | Cyproterone acetate (only in combination as combined oral contraceptive 2 mg) | Anti-And |
| Selective oestrogen receptor modulators | Raloxifene (60 mg/d) | SERM |
| Tibolone | Tibolone (2.5 mg/d) | Laparoscopy |
| Laparoscopic surgery | Ablation (laser, diathermy, etc.) Excision (laser, diathermy, etc.) | Laparoscopy |
| Nutritional supplements | Calcium Vitamin D | Supp |
| Chinese herbal medicine | Nei yi pills Dan’e mixture | CHM |
| Dietary interventions | Dietary intervention | Diet |
Table only includes treatments in full-text studies assessed for inclusion/exclusion. Treatments only in studies that were not included in the NMA could not be included in the network.
Table 111Characteristics of included studies
| First Author | Pub Date | rAFS | Surgery type | Endometriomas included | Risk of Bias |
|---|---|---|---|---|---|
| Granese | 2015 | III-IV | Excision/ablation | Some | High |
| Razzi | 2007 | NR | Excision | All | Mod |
| Sutton | 1994 | I-II | Ablation | None | High |
| Zhu | 2014 | I-II | Excision/ablation | None | Mod |
Pub Date: Date of publication; rAFS: revised American Fertility Scale; Mod: Moderate; NR: Not reported
Table 112Matrix of results for the NMA of surgery and combined surgery plus hormonal therapy for pain relief on the VAS
| Diagnostic / no treatment | −26.8 (−40.9 to −12.7) | ||||
|---|---|---|---|---|---|
| −26.8 (−40.9 to −12.7) | Laparoscopic surgery | −23.9 (−35.0 to −12.9) | −16.6 (−27.7 to −5.53) | ||
| −54.0 (−80.5 to −27.4) | −27.2 (−49.8 to −4.44) | Laparosc and Prog (o) | 3.25 (−16.7 to 23.1) | ||
| −56.4 (−87.6 to −25.4) | −29.7 (−57.6 to −1.83) | −2.54 (−35.0 to 30.0) | Laparosc and GnRH (i.m.) | 5.75 (−19.9 to 31.4) | |
| −50.7 (−68.6 to −33.0) | −23.9 (−35.0 to −12.9) | 3.25 (−16.7 to 23.1) | 5.75 (−19.9 to 31.4) | Laparosc and Prog (o) and Oest (o) | 7.32 (−3.79 to 18.4) |
| −43.4 (−61.3 to −25.6) | −16.6 (−27.7 to −5.53) | 10.6 (−12.1 to 33.2) | 13.09 (−14.9 to 41.0) | 7.32 (−3.79 to 18.4) | Laparosc and P (o) and O (o) and CMH |
Mean differences and 95% credible intervals from the NMA (bottom left diagonal) and conventional meta-analyses (top right diagonal) treatment effects between the column-defined and row-defined treatments. Mean differences less than 0 favour the row-defined treatment. Numbers in bold, grey-shaded cells denote results where the 95% CrI credible intervals do not include 0. For treatment name abbreviations see Table 110
Table 113Probabilities of being among the best 3 treatments and the worst 3 treatments, and the rank and 95% CrI for each treatment
| Treatment Class | Probability of being within the best 3 (%) | Probability of being within the worst 3 (%) | Rank (95% CrI) |
|---|---|---|---|
| Diagnostic/no treatment | 0.00% | 100.00% | 6 (6, 6) |
| Laparoscopic surgery | 0.08% | 99.92% | 5 (4, 5) |
| Laparosc + P (o) | 85.10% | 14.90% | 2 (1, 4) |
| Laparosc + GnRH (i.m.) | 84.49% | 15.51% | 1 (1, 4) |
| Laparosc + P (o) + O (o) | 95.87% | 4.13% | 2 (1, 4) |
| Laparosc + P (o) + O (o) + CHM | 34.46% | 65.54% | (2, 4) |
For treatment name abbreviations see Table 110
Table 114Summary of included studies
| Study | Intervention/Comparison | Population | Outcomes |
|---|---|---|---|
| Abbott 2004 UK | Excision versus diagnostic laparoscopy in a crossover trial | Women with clinically proven endometriosis N=39 Median rAFS scores (range):
|
|
| Carmona 2011 Spain | Laparoscopic cystectomy versus laser vaporisation | Women undergoing laparoscopy for adnexal mass with the diagnosis of endometrioma N=90 Median rAFS scores (range):
|
|
| Dan 2013 (Systematic review) | Laparoscopic ovarian cystectomy versus fenestration/coagulation Laparoscopic ovarian cystectomy versus laser ablation | Women with endometrioma N=7 RCTs included Median rAFS scores (range) across studies ranged from 27 (16 - 136) or 27 (19 – 96) to 32 (16–133); mean (±SD) ranged from 38 ± 3.8 to 81.22 ± 11.88 |
|
| Duffy 2014 (Systematic review) | Laparoscopic surgery compared with diagnostic laparoscopy Laparoscopic ablation versus laparoscopic excision | Women with clinical symptoms and signs suggestive of endometriosis N=973 rAFS scores one to 4 |
|
| Hart 2008 (CSR) | Planned surgical excision (stripping) of endometrioma Planned ablation of the endometrioma capsule | Women with ovarian endometrioma N=304 rAFS score not reported |
|
| Healey 2010 (as reported in Duffy 2013 CSR) (*outcomes reported only in Healey 2010) | Ablation versus excision | Women with endometriosis N=103 Median rAFS scores (95% CI):
|
|
| Healey 2014 Australia | Ablation versus excision | Women of reproductive age with pelvic pain and visually proved endometriosis N=82 Median rAFS scores (range):
|
|
| Wright 2005 UK | Ablation versus excision | Women with mild endometriosis N=24 rAFS scores one to 2 |
|
rAFS: Revised American Fertility Society; EQ-5D: EuroQol 5 dimensions; SF-12: 12-Item Short Form Survey; CI: confidence Intervals; VAS: Visual Analogue Scale; CSR: Cochrane systematic review
Table 115Summary clinical evidence profile: Laparoscopic treatment (excision or ablation) versus diagnostic laparoscopy for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95 % CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Diagnostic laparoscopy | Excision/ablation | ||||
| Overall pain better or improved - At 6 months | 429 per 1,000 | 399 more per 1,000 (from 99 more-870 more) | RR 1.93 (1.23 to 3.03) | 69 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 |
| Overall pain better or improved - At 12 months | 214 per 1,000 | 516 more per 1,000 (from 137 more-1,000 more) | RR 3.41 (1.64 to 7.11) | 69 (1 study) | ⊕⊕⊝⊝ Low1,2 |
| Live birth or ongoing pregnancy | 205 per 1,000 | 135 more per 1,000 (from 29 more-291 more) | RR 1.66 (1.14 to 2.42) | 382 (2 studies) | ⊕⊝⊝⊝ Very low2,3 |
| Miscarriage per pregnancy | 108 per 1,000 | 5 fewer per 1,000 (from 60 fewer118 more) | RR 0.95 (0.44 to2.09) | 112 (2 studies) | ⊕⊝⊝⊝ Very low5,6 |
CI: confidence interval; RR: risk ratio
Table 116Summary clinical evidence profile: Excision versus diagnostic laparoscopy for endometriosis
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95 % CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Diagnostic laparoscopy | Excision/ablation | ||||
| Overall pain better or improved – At 6 months | 316 per 1,000 | 483 more per 1,000 (from 82 more to 1,000 more) | RR 2.53 (1.26 to 5.09) | 39 (1 study) | ⊕⊕⊕⊕ High1 |
| Overall pain score - At 6 months | - | The mean overall pain score - at 6 months in the intervention groups was 0.9 higher (0.31 to 1.49 higher) | MD 0.90 (0.31 to 1.49) | 16 (1 study) | ⊕⊝⊝⊝ Very low2,3 |
| Overall pain score - At 12 months | - | The mean overall pain score - at 12 months in the intervention groups was 1.65 higher (1.11 to 2.19 higher) | MD 1.65 (1.11 to 2.19) | 16 (1 study) | ⊕⊕⊕⊝ Moderate3 |
| Pelvic pain scores - At 6 months | - | The mean pelvic pain scores - at 6 months in the intervention groups was 5.1 lower (16.64 lower to 6.44 higher) | MD −5.10 (−16.64 to 6.44) | 39 (1 study) | ⊕⊕⊕⊝ Moderate1,3 |
| Dysmenorrhoea pain score - At 6 months | - | The mean dysmenorrhoea pain score - at 6 months in the intervention groups was 2.4 higher (6.18 lower to 10.98 higher) | MD 2.40 (−6.18 to 10.98) | 39 (1 study) | ⊕⊕⊕⊝ Moderate1,3 |
| Dyspareunia pain score - At 6 months | - | The mean dyspareunia pain score - at 6 months in the intervention groups was 6.3 higher (8.18 lower to 20.78 higher) | MD 6.30 (−8.18 to 20.78) | 39 (1 study) | ⊕⊕⊕⊝ Moderate1,3 |
| EQ-5D index summary score - At 6 months | - | The mean EQ-5D index summary at 6 months in the intervention groups was 0.03 higher (0.12 lower to 0.18 higher) | MD 0.03 (−0.12 to 0.18) | 39 (1 study) | ⊕⊕⊝⊝ Low1,5 |
| EQ-5D VAS – At 6 months | - | The mean EQ-5D VAS at 6 months in the intervention groups was 17.7 higher (7.02 to 28.38 higher) | MD 17.7 (7.02 to 28.38) | 39 (1 study) | ⊕⊕⊕⊝ Moderate1,3 |
| SF-12 Physical component score - At 6 months | - | The mean SF-12 physical component score at 6 months in the intervention groups was 2.7 higher (2.9 lower to 8.3 higher) | MD 2.7 (2.9 to 8.3) | 39 (1 study) | ⊕⊕⊕⊝ Moderate1,3 |
| SF-12 Mental component score – At 6 months | - | The mean FS12 mental component score at 6 months in the intervention groups was 2.3 higher (4.5 lower to 9.1 higher) | MD 2.3 (4.5 to 9.1) | 39 (1 study) | ⊕⊕⊕⊝ Moderate1,3 |
CI: confidence interval; RR: Risk ratio; MD: mean difference; EQ-5D: EuroQol five dimensions, SF-12: 12-Item Short Form Survey
- 1
Unclear if selective reporting
- 2
Evidence was downgraded by two due to performance bias (blinding of participants and personnel and attrition bias (incomplete outcome data)
- 3
Evidence was downgraded by one due to serious imprecision as 95%CI crossed one default MID
- 4
No blinding of participants and personnel and incomplete outcome data
- 5
Evidence was downgraded by two due to very serious imprecision as 95%CI crossed two default MIDs
Table 117Summary clinical evidence profile: Excisional surgery versus ablative surgery for endometriosis and endometrioma
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Diagnostic laparoscopy | Excisional/ablation | ||||
| Endometriosis | |||||
| Pain score (reduction in VAS at 12 months) - Overall | - | The mean pain score (reduction in VAS at 12 months) - overall in the intervention groups was 0 higher (1.22 lower to 1.22 higher) | MD 0 (−1.22 to 1.22) | 103 (1 study) | ⊕⊕⊝⊝ Low1 |
| Pain score (reduction in VAS at 12 months) - Pelvic | - | The mean pain score (reduction in VAS at 12 months) - pelvic in the intervention groups was 0.1 lower (1.3 lower to 1.1 higher) | MD −0.1 (−1.3 to 1.1) | 103 (1 study) | ⊕⊕⊝⊝ Low1 |
| Pain score (reduction in VAS at 12 months) - Dyspareunia | - | The mean pain score (reduction in VAS at 12 months) - dyspareunia in the intervention groups was 1.3 higher (0.29 lower to 2.89 higher) | MD 1.3 (−0.29 to 2.89) | 103 (1 study) | ⊕⊝⊝⊝ Very low1,2 |
| Unintended effects (reduction from VAS score by 12 months after operation (nausea, vomiting) - Nausea | - | The mean unintended effects (reduction from VAS score by 12 months after operation (nausea, vomiting) - nausea in the intervention groups was 1.1 higher (0.14 lower to 2.34 higher) | MD 1.1 (−0.14 to 2.34) | 103 (1 study) | ⊕⊕⊝⊝ Low2,3 |
| Unintended effects (reduction from VAS score by 12 months after operation (nausea, vomiting) - Vomiting | - | The mean unintended effects (reduction from VAS score by 12 months after operation (nausea, vomiting) - vomiting in the intervention groups was 0.2 higher (0.71 lower to 1.11 higher) | MD 0.2 (−0.71 to 1.11) | 103 (1 study) | ⊕⊕⊕⊝ Moderate3 |
| Unintended effects (reduction from VAS score by 12 months after operation (nausea, vomiting) - Bloating | - | The mean unintended effects (reduction from VAS score by 12 months after operation (nausea, vomiting) - bloating in the intervention groups was 0.9 higher (0.3 lower to 2.1 higher) | MD 0.9 (−0.3 to 2.1) | 103 (1 study) | ⊕⊕⊝⊝ Low2,3 |
| Endometrioma | |||||
| Recurrence of pelvic pain - Dysmenorrhoea | 548 per 1,000 | 389 fewer per 1,000 (from 247 fewer to 466 fewer) | RR 0.29 (0.15 to 0.55) | 104 (2 studies) | ⊕⊕⊕⊝ Moderate3 |
| Recurrence of pelvic pain - Non-menstrual pelvic pain | 529 per 1,000 | 429 fewer per 1,000 (from 127 fewer to 503 fewer) | RR 0.19 (0.05 to 0.76) | 37 (1 study) | ⊕⊕⊝⊝ Low2,3 |
| Pregnancy rate after surgical treatment | 233 per 1,000 | 242 more per 1,000 (from 56 more to 552 more) | RR 2.04 (1.24 to 3.37) | 138 (3 studies) | ⊕⊕⊕⊝ Moderate2,4 |
| Recurrence of endometrioma - At 12 months | 256 per 1,000 | 146 fewer per 1,000 (from 69 fewer to 192 fewer) | RR 0.43 (0.25 to 0.73) | 258 (4 studies) | ⊕⊕⊕⊕ High |
| Recurrence of endometrioma - At 60 months | 368 per 1,000 | 147 fewer per 1,000 (from 261 fewer to 96 more) | RR 0.6 (0.29 to 1.26) | 74 (1 study) | ⊕⊕⊝⊝ Low5 |
| Reoperation after surgical treatment up to 60 months follow-up | 94 per 1,000 | 59 fewer per 1,000 (from 85 fewer to 33 more) | RR 0.37 (0.1 to 1.35) | 174 (2 studies) | ⊕⊝⊝⊝ Very low4,5 |
CI: confidence interval; RR: Risk ratio; MD: mean difference
- 1
Evidence was downgraded by two due to performance bias (lack of blinding) and attrition bias.
- 2
Evidence was downgraded by one due to serious imprecision as 95%CI crossed one default MID
- 3
Evidence was downgraded by one due to lack of blinding.
- 4
Taking into account weighting in a meta-analysis and the likely contribution from each component, evidence was downgraded by one due to lack of blinding.
- 5
Evidence was downgraded by two due to very serious imprecision as 95%CI crossed two default MIDs.
Table 118Reduction in VAS score by 5 years after surgery (Healey 2014)
| Outcome | Excision group, median (range) | Ablation group, median (range) | P-Value (Mann-Whitney U test) |
|---|---|---|---|
| Overall pain | 5.8 (−3.4 to 10.0) | 5.5 (−0.2 to 10.0) | 0.46 |
| Pelvic pain | 6.2 (−2.6 to 9.3) | 5.9 (−3.9 to 10.0) | 0.81 |
| Dyspareunia | 6.0 (0 to 10.0) | 3.2 (−4.3 to 10.0) | 0.03 |
VAS: Visual Analogue Scale
Table 119Change in quality of life: excision versus diagnostic laparoscopy at 6month follow-up (Abbott 2004)
| Outcome | DSG (mean (SD)) | ISG (mean (SD)) | DSG vs. ISG p-value (t-test) |
|---|---|---|---|
| EQ-5D index summary | 0.74 (0.23) | 0.77 (0.25) | 0.07 |
| EQ-5D VAS summary score | 65.9 (21.3) | 83.6 (10.8) | 0.01 |
| SF-12 physical component score | 45.5 (10.0) | 48.2 (7.6) | 0.36 |
| SF-12 mental component score | 45.3 (11.8) | 47.6 (9.7) | 0.55 |
EQ-5D: EuroQol 5 dimensions questionnaire, DSG: Delayed Surgery Group, ISG: Immediate Surgery Group
Table 120Estimates of cost of laparoscopic surgery from different sources
| Study | Population | Cost estimate in local currency | Cost estimate in 2016 GBP |
|---|---|---|---|
| Allaire (2014) | 57,879 Canadian women recruited over 5 years receiving laparoscopic surgery | $1529.89 CAD | £949.09 |
| Chvatal (2010) | 20,835 German women receiving inpatient treatment of any kind for endometriosis | 3056.12 EUR | £3189.23 |
| Fuldeore (2010) | 15,891 US women receiving therapeutic laparoscopy and 63,564 controls | $5886 USD | £5506.23 |
| Fuldeore (2011) | As above – paper is re-analysis of Fuldeore (2010) | $6856 USD | £6096.35 |
EUR: Euro, CAD: Canadian Dollar, GBP: British Pound, USD: US Dollar
Table 121Estimates of cost of laparoscopic surgery from different sources
| Strategy | Lifetime Cost | Lifetime QALY | ICER vs same diagnostic strategy, no treatment |
|---|---|---|---|
| Empirical Diagnosis & No Treatment | £22,752.60 | 18.12 | N/A |
| Empirical Diagnosis & Laparoscopy + Hormonal | £31,626.43 | 18.86 | £12,034.14 |
| Empirical Diagnosis & Laparoscopic Treatment | £28,052.06 | 18.47 | £15,156.19 |
| Pelvic MRI & No Treatment | £24,929.53 | 18.12 | N/A |
| Pelvic MRI & Laparoscopy + Hormonal | £25,772.03 | 18.77 | £1,288.34 |
| Pelvic MRI & Laparoscopic Treatment | £24,783.78 | 18.42 | -£478.24 |
| Nerve fibre & No Treatment | £25,795.25 | 18.12 | N/A |
| Nerve fibre & Laparoscopy + Hormonal | £26,875.57 | 18.78 | £1,630.21 |
| Nerve fibre & Laparoscopic Treatment | £26,222.93 | 18.46 | £1,274.89 |
| Laparoscopy & No Treatment | £35,933.66 | 18.14 | N/A |
| Laparoscopy & Laparoscopy + Hormonal | £33,344.74 | 18.87 | -£3,562.28 |
| Laparoscopy & Laparoscopic Treatment | £31,899.07 | 18.52 | -£10,637.82 |
| Peritoneal biopsy & No Treatment | £25,362.71 | 18.12 | N/A |
| Peritoneal biopsy & Laparoscopy + Hormonal | £27,422.18 | 18.79 | £3,069.05 |
| Peritoneal biopsy & Laparoscopic Treatment | £25,079.29 | 18.46 | -£829.43 |
| Transabdominal Ultrasound & No Treatment | £24,775.14 | 18.12 | N/A |
| Transabdominal Ultrasound & Laparoscopy + Hormonal | £24,562.05 | 18.65 | -£403.77 |
| Transabdominal Ultrasound & Laparoscopic Treatment | £23,948.36 | 18.37 | -£3,262.92 |
| CA-125 & No Treatment | £25,201.29 | 18.12 | N/A |
| CA-125 & Laparoscopy + Hormonal | £25,381.47 | 18.51 | £467.87 |
| CA-125 & Laparoscopic Treatment | £24,377.37 | 18.33 | -£3,964.13 |
| Pelvic MRI & No Treatment | £24,929.53 | 18.12 | N/A |
| Pelvic MRI & Laparoscopy + Hormonal | £25,772.03 | 18.77 | £1,288.34 |
| Pelvic MRI & Laparoscopic Treatment | £24,783.78 | 18.42 | -£478.24 |
Table 122Research recommendation rationale
| Research question | Is laparoscopic treatment of peritoneal disease in minimal and mild endometriosis cost-effective for the management of suspected endometriosis-associated pain? |
|---|---|
| Importance to ‘patients’ or the population | This is important as all surgery carries with it a potential morbidity and mortality. Thus any surgical interventions needs to have a likelihood of relief of symptoms to be clinically justified. |
| Relevance to NICE guidance | The answer to this question has not been able to be determined from the current literature available. The impact of surgery on this subset of women with endometriosis needs to be known so that the cost-effectiveness of surgery can be determined. |
| Relevance to the NHS | Pain associated with endometriosis costs the NHS significant amounts of money. In addition surgical time and bed usage are limited resources so that cost-effective utilisation is essential. |
| National priorities | This is a large group of women who require evidence based care. |
| Current evidence base | Not available regards this group in relation to pain outcomes. |
| Equality | A study population should include the full age spectrum of women who suffer endometriosis associated pain. Adolescents as well as adults will need to be studied. |
| Feasibility | This has been done in relation to fertility outcomes and hence the same research model could be adopted. |
| Other comments | Not applicable |
Table 123Research recommendation PICO table
| Criterion | Explanation |
|---|---|
| Population | Women with proven isolated peritoneal disease which is classed as minimal or mild endometriosis. This should be determined at diagnostic laparoscopy and the position and extent of the endometriosis described as accurately and fully as possible. |
| Intervention | Complete laparoscopic excision of all peritoneal endometriosis, with histological confirmation. |
| Comparator | Laparoscopy without excision of any endometriosis |
| Outcome | Standardised patient symptom questionnaire and Quality of Life using a validated measurement system at 6 months and annually after surgery. Secondary outcomes would include additional surgical and/or medical treatment required by the patient in the follow up interval. |
| Study design | Randomised controlled trial, ideally with participant blinding to treatment allocation. |
| Timeframe | Two years of randomisation and 2 years of follow up, providing recruitment numbers are sufficient to achieve population numbers of sufficient size to answer the research question. |
Table 124Summary of included studies
| Study | Intervention/Comparison | Population | Outcomes |
|---|---|---|---|
| Abou-Setta 2013 (CSR) | Postoperative insertion of the LNG-IUS versus
| 2 Trials comparing insertion of the LNG-IUS versus no postoperative treatment, placebo (inert IUD), or any other active systemic treatment in women undergoing surgery for endometriosis Tanmahasamut 2012 trial – using ASRM staging.
|
|
| Alborzi 2011 Iran | Letrozole for 2 months and triptorelin for 2 months (2 arms) versus no treatment | Women who had been infertile for at least 12 months and some of whom had symptoms (dysmenorrhoea, dyspareunia and pelvic pain.
|
|
| Bianchi 1999 Italy | Surgery: Cook and Rock technique of laparoscopy (conservative surgery) was used Pharmacological comparison: Danazol, 600 mg/day versus no medical therapy for 3 months. | Inclusion criteria: < 40 yrs.
N randomised = 77 N analysed = 77 | Included in Furness 2011 and additionally reported
|
| Busacca 2001 Italy | Surgery: Cook and Rock technique of laparoscopy (conservative surgery) was used Pharmacological comparison: GnRHa (leuprolide) versus no medical therapy every 4 weeks for a period of 12 weeks | Inclusion criteria: < 40 years, laparoscopic diagnosis of endometriosis
N randomised = 89 N analysed = 89 | Included in Furness 2011 and additionally reported
|
| Furness 2011 (CSR) | All systemic medical treatments for the hormonal suppression of endometriosis including GnRHas, danazol, progestogens, gestrinone or the oral contraceptive pill (or combinations of these) administered after surgery to no medical treatment, or placebo were studied. The use of medical therapy was considered at any dosage and for a period of at least 3 months duration before or after surgery. Only agents used with the aim of hormonal suppression were included. | Trials (N=12) The study population included women of reproductive age who were undergoing surgery for endometriosis | Pain recurrence (VAS)
|
| Loverro 2008 Italy | Surgery: Laparoscopic diathermy, laser vaporisation or surgical excision of endometriomas Pharmacological comparison: Triptorelin depot versus placebo over a 3 month period | Inclusion criteria: women of reproductive age with stage III - IV endometriosis, associated with chronic pelvic pain, adnexial mass or infertility, who had undergone complete laparoscopic excision, had rAFS score > 15 and no previous hormonal treatment
N analysed = 54 | Included in Furness 2011 and additionally reported
|
| Mettler 2014 Germany | Surgery: laparoscopic excision of endometrial foci, removal of adhesions and restoration of normal reproductive anatomy. Ureter and superficial bowel lesions were removed Pharmacological comparison: Leuprorelin depot subcutaneously injected monthly or no treatment over a 3 month period | Inclusion criteria: Women with symptomatic endometriosis (18–44 years old) in whom 2 consecutive laparoscopic interventions were to be assessed.
|
|
| Muzii 2000 Italy | Surgery: Laparoscopic excision of ovarian endometriomas with drainage, adhesionolysis or bipolar coagulation if necessary Pharmacological comparison: Cyclic monophasic combined oral contraceptives versus no medical therapy for 6 months | Inclusion criteria: 2035 years, moderate to severe dysmenorrhoea and/or chronic pelvic pain, not desiring fertility. Mean AFS scores 43.4 SD 22.3 in treatment group and 46.1 SD 23.9 in control group. Exclusion criteria: treatment for endometriosis in previous 6 months. N randomised = 70 N analysed = 68 | Included in Furness 2011 and additionally reported
|
| Parazzini 1994 Italy | Surgery: Laparotomy as first surgical treatment for debulking or radical surgery of endometriotic lesions Pharmacological comparison: Intranasal nafarelin (400 µG/day) versus placebo over a period of 3 months | Inclusion criteria: age < 38 yrs, normal medical examination, unexplained infertility for at least 1 year, with/without chronic pelvic pain, endometriosis AFS stage III-IV, partners with normal sperm analysis and postcoital tests.
N randomised =75 N analysed (pain scores) =68 | Included in Furness 2011 and additionally reported
|
| Seracchioli 2010 Italy | Surgery: Laparoscopic excision of ovarian endometriomas using the classic stripping technique Pharmacological comparison: 2 groups using continuous low dose monophasic oral contraceptives and cyclic therapy (combined in this analysis) vs. no treatment for 24 months | Inclusion criteria: Nulliparous women (20–40 years old) not attempting to conceive at study entre of for at least 2 years postsurgery. No previous surgical or medical treatment of endometriosis and no receipt of oral contraceptives for at least 6 months prior to surgery.
|
|
| Sesti 2007 Italy | Surgery: Conservative pelvic surgery Pharmacological comparison: GnRHa (either triptorelin or leuprorelin) or continuous oestroprogestin (cOCP) versus placebo for 6 months | Inclusion criteria: women of reproductive age <40, with endometriosis related symptoms (dysmenorrhoea, pelvic pain, deep dyspareunia), laparoscopic diagnosis of St III-IV endometriosis, desiring pregnancy, nulliparous.
N randomised = 234 N analysed = 222 | Included in Furness 2011 and additionally reported
|
| Sesti 2009 Italy | Surgery: Laparoscopic removal of endometriomas with enucleation of the entire cyst and stripping from the normal ovarian tissue and with drainage, adhesionolysis and bipolar coagulation if necessary Pharmacological comparison: Tryptorelin or leuprorelin and continuous low dose monophasic oral contraceptives (2 arms) vs. placebo for 6 months | Women of reproductive age, up to 40 years at time of surgery, US evidence of endometrioma, moderate to severe endometriosis, laparoscopic diagnosis of endometrioma first laparoscopic surgery for endometriosis, conservative treatment, complete excision of all evident ovarian and peritoneal disease, UC and clinical follow up after surgery.
|
|
CSR: Cochrane systematic review; N: number of participants in study
- *
Pelvic pain was assessed using Andersch and Milsom’s multidimensional verbal rating scale, which defines pain according to limitation of ability to work (unaffected, 0; rarely affected, 1; moderately affected, 2; clearly inhibited, 3), presence of systemic symptoms (absent, 0; present, 1), and need for analgesics (no, 0; rarely, 1; regularly, 2). The score of each dimension is added to provide an overall summary score; AFS: American Fertility Society Score
Table 125Summary clinical evidence profile for Comparison: Pharmacological therapy after surgery vs. placebo or no pharmacological therapy after surgery
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95 % CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | ||||
| Control | Post-surgical pharmacological therapy | ||||
| Pain recurrence (VAS) cm - Pelvic pain Follow-up: 12 months | Control group mean 6.2 (SD 0.9) | The mean pain recurrence (VAS) - pelvic pain in the intervention groups was 1.2 lower (1.47 to 0.93 lower) | MD −1.2 (1.47 to −0.93) | 187 (1 study) | ⊕⊕⊕⊝ Moderate1 |
| Pain recurrence (VAS) cm-Dysmenorrhoea Follow-up: 12 months | Control group mean 6.4 (SD 1.3) | The mean pain recurrence (VAS) - dysmenorrhoea in the intervention groups was 0.7 lower (1.04 to 0.36 lower) | MD −0.7 (1.04 to −0.36) | 187 (1 study) | ⊕⊕⊝⊝ Low1,2 |
| Pain recurrence (VAS) cm - Deep dyspareunia Follow-up: 12 months | Control group mean 4.8 (SD 1.2) | The mean pain recurrence (VAS) - deep dyspareunia in the intervention groups was 0.4 lower (0.76 to 0.04 lower) | MD −0.4 (0.76 to −0.04) | 187 (1 study) | ⊕⊝⊝⊝ Very low1,3 |
| Pain recurrence (questionnaire based) - Abdominal pain at 12 months post treatment completion | 569 per 1,000 | 404 per 1,000 (279 to 586) | RR 0.71 (0.49 to 1.03) | 120 (1 study) | ⊕⊕⊝⊝ Low2,4 |
| Pain recurrence (questionnaire based) - Dysmenorrhoea at 12 months post treatment completion | 346 per 1,000 | 301 per 1,000 (190 to 471) | RR 0.87 (0.55 to 1.36) | 158 (1 study) | ⊕⊝⊝⊝ Very low3,4 |
| Pain recurrence (questionnaire based) - Dyspareunia at 12 months post treatment completion | 304 per 1,000 | 161 per 1,000 (85 to 301) | RR 0.53 (0.28 to 0.99) | 144 (1 study) | ⊕⊕⊝⊝ Low2,4 |
| Pain recurrence (Andersch and Milsom) - Pelvic pain Follow-up: 12 months | Control group mean 4 (SD 3.6) | The mean pain recurrence (Andersch and Milsom) - pelvic pain in the intervention groups was 0.4 lower (2.15 lower to 1.35 higher) | MD −0.4 (2.15 to 1.35) | 53 (1 study) | ⊕⊕⊝⊝ Low3 |
| Pain recurrence (dichotomous) Follow-up: 12 months | 216 per 1,000 | 168 per 1,000 (119 to 241) | RR 0.78 (0.55 to 1.12) | 476 (4 studies) | ⊕⊝⊝⊝ Very low2,5 |
| Pain recurrence (dichotomous) Follow-up: 13–24 months | 286 per 1,000 | 200 per 1,000 (134 to 294) | RR 0.7 (0.47 to 1.03) | 312 (3 studies) | ⊕⊝⊝⊝ Very low2,6 |
| Pain recurrence (dichotomous) Follow-up: 60 months | 480 per 1,000 | 446 per 1,000 (254 to 797) | RR 0.93 (0.53 to 1.66) | 54 (1 study) | ⊕⊝⊝⊝ Very low3,7 |
| Dysmenorrhoea Follow-up: 12 months | 383 per 1,000 | 84 per 1,000 (31 to 230) | RR 0.22 (0.08 to 0.6) | 95 (2 studies) | ⊕⊕⊕⊝ Moderate8 |
| Reoperation (women with endometriosis) | 30 per 1,000 | 35 per 1,000 (12 to 101) | RR 1.17 (0.4 to 3.4) | 327 (3 studies) | ⊕⊝⊝⊝ Very low3,9 |
| Endometriosis recurrence (dichotomous) - Disease recurrence at 5–6 months Follow-up: 5–6 months | 401 per 1,000 | 397 per 1,000 (301 to 530) | RR 0.99 (0.75 to 1.32) | 285 (1 study) | ⊕⊝⊝⊝ Very low3,4 |
| Endometriosis recurrence (dichotomous) Follow-up: 12 months | 70 per 1,000 | 101 per 1,000 (20 to 515) | RR 1.44 (0.28 to 7.36) | 310 (3 studies) | ⊕⊝⊝⊝ Very low3,10,11 |
| Endometriosis recurrence (dichotomous) Follow-up: 24 months | 133 per 1,000 | 29 per 1,000 (1 to 500) | RR 0.22 (0.01 to 3.75) | 45 (1 study) | ⊕⊝⊝⊝ Very low3,12 |
| Endometrioma recurrence (dichotomous) - Recurrence at 13–36 months | 189 per 1,000 | 104 per 1,000 (68 to 163) | RR 0.55 (0.36 to 0.86) | 463 (3 studies) | ⊕⊕⊝⊝ Low2,13,14 |
| Endometrioma recurrence (dichotomous) Follow-up: 60 months | 125 per 1,000 | 210 per 1,000 (44 to 1,000) | RR 1.68 (0.35 to 8.03) | 35 (1 study) | ⊕⊕⊝⊝ Low3,7 |
| Patient Satisfaction | Not estimable | Not estimable | RR 1.21 (0.80 to 1.82) | 95 (2 studies) | See comment |
- 1
Blinding: unclear risk. Placebo is not described and seems unlikely that blinding could be maintained when the interventions are depot and oral hormonal treatments
- 2
95% Confidence Interval crosses 1imprecision threshold
- 3
95% Confidence Interval crosses 2 imprecision thresholds
- 4
Randomisation, Allocation concealment: unclear risk. No information provided. Blinding: High risk. No placebo used
- 5
Allocation concealment: unclear risk. Not mentioned in Alborzi 2011, Loverro 2001 or Bianchi 1999. Blinding: high risk. No placebo used in Alborzi 2011, Bianchi 1999 or Vercellini 1999. Incomplete data reporting: unclear risk. 22% withdrawal overall in Vercellini 1999 due to reasons other than symptom recurrence or major protocol violations (similar in each group). 18% withdrawal overall in Alborzi 2011 after randomisation due to “poor patients follow up” with reasons not reported and unequal loss across groups (11/58 letrozole group, 18/58 dipherelin group and 1/59 no treatment group)
- 6
Allocation concealment: unclear risk. Not mentioned in Busacca 2001 or Muzii 2000. Blinding: high risk. No placebo use in Busacca 2001, Muzii 2000 or Vercellini1999. Incomplete data reporting: unclear risk. 22% withdrawal overall in Vercellini 1999 due to reasons other than symptom recurrence or major protocol violations (similar in each group). Other bias: unclear risk. No baseline characteristics reported in Muzii 2000
- 7
Allocation concealment: unclear risk. Not mentioned.
- 8
Blinding: unclear/high risk of performance bias. Unclear how patients were blinded to IUD presence in Tanmahasamut 2012 and Vercellini 2003 reported as an open label study with outcome assessors not blinded to treatment group (high risk of detection bias).
- 9
Allocation concealment: unclear risk. Not mentioned in Bianchi 1999, Busacca 2001 or Sesti 2009. Blinding: high risk. No placebo use in Bianchi 1999 or Busacca 2001.
- 10
Allocation concealment: unclear risk. Not mentioned in Alborzi 2011, Bianchi 1999 or Busacca 2001. Blinding: high risk. No placebo used in Alborzi 2011, Bianchi 1999 or Busacca 2001. Incomplete data reporting: unclear risk. 18% withdrawal overall in Alborzi 2011 after randomisation due to “poor patients follow up” with reasons not reported and unequal loss across groups (11/58 letrozole group, 18/58 dipherelin group and 1/59 no treatment group)
- 11
Using random effects model. Heterogeneity: Chi² = 5.72, df = 2 (P = 0.06); I² = 65%. Removal of Alborzi 2011 (RR = 16.48 95%CI 0.99 - 272.92) from the pooled analysis removes inconsistency (Heterogeneity: Chi² = 0.38, df = 1 (P = 0.54); I² = 0%) and the pooled fixed effects result for Bianchi 1999 and Busacca 2001 becomes RR = 0.76 (95%CI 0.30 - 1.90)
- 12
Blinding: high risk. No placebo used. Incomplete data reporting: high risk. 4/15 (27%) loss to follow up in treatment group in Tsai 2004.
- 13
Allocation concealment: unclear risk. Not mentioned in Muzii 2000 or Sesti 2009. Blinding: unclear risk - no placebo use in Muzii 2000 or in Seracchioli 2010 (although outcome assessors were blinded to treatment group. Incomplete data reporting: unclear risk. 8% withdrawal overall in relevant treatment arms in Sesti 2009. Other bias: unclear risk. No baseline characteristics reported in Muzii 2000
- 14
Using fixed effects model Heterogeneity: Chi² = 3.25, df = 2 (P = 0.20); I² = 39%
Table 126narratively reported results
| Study ID | Hormone treatment groups (GnRH agonist or estroprogestin n=77) | Control Group (Placebo n=110) |
|---|---|---|
| Sesti 2007 Italy | Short form 36 general health survey: Improvement of scores in all domains at 12 months | Short form 36 general health survey: Improvement of scores in all domains at 12 months |
Even though this outcome could not be assessed using GRADE, this would be rated as very low quality evidence because of outcome reporting bias and lack of detail provided.
Table 127Cost and effectiveness of all non-dominated treatment strategies containing a combination treatment for pain
| Treatment | Cost | QALY | ICER | Pr. cost-effective vs. no treatment (£20k / QALY) | Pr. cost-effective vs. no treatment (£30k / QALY) |
|---|---|---|---|---|---|
| Empirical Diagnosis and No Treatment | £22,899.35 | 18.739 | Base Case | N/A | N/A |
| CA-125 and Laparoscopic Treatment | £25,368.67 | 19.016 | Extendedly Dominated | 71.43% | 71.43% |
| Pelvic MRI and Laparoscopic Treatment | £26,686.53 | 19.085 | Extendedly Dominated | 85.71% | 85.71% |
| Transabdominal Ultrasound and Laparoscopy + Hormonal | £27,908.07 | 19.251 | Extendedly Dominated | 76.19% | 80.95% |
| Pelvic MRI and Laparoscopy + Hormonal | £28,125.90 | 19.404 | £7,864.31 | 80.95% | 85.71% |
| Laparoscopy and Laparoscopy + Hormonal | £34,123.57 | 19.493 | £67,337.99 | 90.48% | 95.24% |
Table 128Cost and effectiveness of all treatment strategies containing a combination treatment for fertility
| Treatment | Cost | QALY | ICER | Pr. cost-effective vs. no treatment (£20k / QALY) | Pr. cost-effective vs. no treatment (£30k / QALY) | Pr. Live Birth |
|---|---|---|---|---|---|---|
| Empirical Diagnosis and No Treatment | £16,028.47 | 19.184 | Base Case | N/A | N/A | 11.90% |
| CA-125 and Laparoscopy + Hormonal | £16,564.63 | 19.202 | Dominated | 61.90% | 61.90% | 15.48% |
| Transabdomin al Ultrasound and Laparoscopy + Hormonal | £18,216.92 | 19.208 | Dominated | 63.69% | 63.69% | 15.48% |
| CA-125 and Laparoscopic Treatment | £14,605.81 | 19.227 | -£33,216.05 | 64.29% | 64.29% | 15.48% |
| Empirical Diagnosis and Laparoscopy + Hormonal | £34,692.83 | 19.294 | Dominated | 66.07% | 66.67% | 15.48% |
| Laparoscopy and Laparoscopy + Hormonal | £29,321.88 | 19.320 | Dominated | 59.52% | 59.52% | 20.24% |
| Pelvic MRI and Laparoscopy + Hormonal | £22,248.26 | 19.350 | Dominated | 63.10% | 63.10% | 18.45% |
| Empirical Diagnosis and Laparoscopic Treatment | £27,712.68 | 19.355 | Dominated | 62.50% | 62.50% | 19.64% |
| Transabdomin al Ultrasound and Laparoscopic Treatment | £17,058.07 | 19.407 | £13,607.13 | 66.67% | 67.26% | 22.02% |
| Laparoscopy and Laparoscopic Treatment | £27,444.47 | 19.409 | Dominated | 63.69% | 63.69% | 22.02% |
| Pelvic MRI and Laparoscopic Treatment | £19,424.28 | 19.415 | £300,633.64 | 58.33% | 58.33% | 23.21% |
Table 129Summary of included studies
| Study | Intervention/Comparison | Population | Outcomes | Comments |
|---|---|---|---|---|
Shakiba 2008 USA |
| Women diagnosed with endometriosis who had undergone surgery for chronic pelvic pain with histological confirmation of endometriosis N=97 | Requirement of reoperation (Effect of ovarian preservation on reoperation free survival) | Endometriosis was staged according to the revised American Fertility Society Mean follow-up of 7 years. |
Namnoum 1995 USA |
| Women undergoing hysterectomy with a diagnosis of endometriosis. Women undergoing hysterectomy aged 45 years or older were excluded. N=138 | Requirement of reoperation. Recurrence of symptoms (pain) | Endometriosis was staged according to the revised American Fertility Society. Mean follow-up of 4 years 10 months. |
Table 130Summary clinical evidence profile
| Outcomes | Hazard ratio (95% CI) | Absolute effect | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
|---|---|---|---|---|---|
| Reoperation-free survival (effect of ovarian preservation (Hysterectomy only versus hysterectomy plus bilateral oophorectomy) | HR 2.44 (0.65 to 9.10) | An absolute effect could not be calculated3 | N=97 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | |
| Relative risk for reoperation (effect of ovarian preservation) | RR 8.1 (2.1 to 31.3) | An absolute effect could not be calculated5 | N=138 (1 study) | ⊕⊝⊝⊝ Very low4,5,6 | Mean follow up 4 years 10 months |
| Relative risk for symptom recurrence (pain) | RR 6.1 (95% CI 2.5 to 14.6) | An absolute effect could not be calculated5 | N=138 (1 study) | ⊕⊝⊝⊝ Very low4,5,6, | Mean follow up 4 years 10 months |
CI: confidence interval; HR: Hazard ratio; RR: risk ratio
- 1
Evidence was downgraded by 1 due to outcome selection bias
- 2
Evidence was downgraded by 2 due to very serious imprecision as 95% confidence interval crossed 2 default minimally important differences (MIDs).
- 3
Adjusted for age, stage of disease, or operative time predictive for reoperation. Age and time of surgery were considered important confounding factors, stage of disease did not have any effect on surgery-free time in any group, but stratification for multiple factors reduced the statistical power and even large differences may not reach statistical significance even though the size of the difference may be clinically important. The P value for the comparison was 0.18.
- 4
Evidence was downgraded by 2 due to risk of bias; study design was a retrospective cohort with outpatient chart review.
- 5
Cox proportional hazards model adjusting for adjusting for revised AFS classification of endometriosis stage, previous medical therapy, previous surgical therapy and age at time of hysterectomy (≤35 years vs. > 35 years)
- 6
Evidence was downgraded by 1 due to indirectness: The hysterectomies in the study took place between 1979 to 1991, which may limit the applicability of the study with regards to current surgical techniques and outcomes. In addition, women over 45 years were excluded.
Table 131Summary of Hysterectomy Costs
| Currency Code | Procedure Name | National Average Cost |
|---|---|---|
| LB71Z | Total Pelvic Exenteration | £16,361 |
| MA02C | Very Major Open, Upper or Lower Genital Tract Procedures, with CC Score 0–1 | £4,013 |
| MA07G | Major Open Upper Genital Tract Procedures with CC Score 0–2 | £3,586 |
| MA28Z | Complex, Laparoscopic or Endoscopic, Upper Genital Tract Procedures | £3,636 |
CC: Complications and comorbidities
Table 132Cost and effectiveness of all non-dominated treatment strategies containing a hysterectomy
| Treatment | Cost | QALY | ICER | Pr. cost-effective vs. no treatment (£20k / QALY) | Pr. cost-effective vs. no treatment (£30k / QALY) |
|---|---|---|---|---|---|
| Empirical Diagnosis & No Treatment | £23,150.21 | 18.424 | Base Case | 62.22% | 62.22% |
| CA-125 & Hysterectomy | £24,318.30 | 19.742 | Extendedly Dominated | 80.00% | 80.00% |
| Pelvic MRI & Hysterectomy | £24,407.45 | 20.616 | £573.50 | 91.11% | 91.11% |
| Laparoscopy & Hysterectomy | £28,913.62 | 20.702 | £52,403.82 | 88.89% | 91.11% |
| Empirical Diagnosis & Hysterectomy | £38,856.92 | 20.777 | £132,467.23 | 93.33% | 95.56% |
ICER: incremental cost-effectiveness ratio; MRI: Magnetic resonance imaging; Pr.: Probability; QALY: quality adjusted life years