The relatively recent discovery that HTLV and HIV induce fatal human diseases has reinforced the need to understand the epidemiology and transmission of all pathogenic retroviruses. The niche these agents occupy in nature and the ways in which they are maintained within the host population are not always emphasized by studies conducted in tissue culture and laboratory animal models. However, these features influence the frequency with which disease arises and they provide clues to methods that may control and eventually eliminate the virus. Although such goals may seem to be a new priority, stimulated by the devastating effects of these viruses on humans, retrovirus-induced diseases have posed significant agricultural problems for some time. EIAV was recognized as an important infection of horses in the mid nineteenth century (for review, see Montelaro et al. 1993), and VMV was discovered in association with a devasting epidemic that affected Icelandic sheep from the 1930s until the virus was eradicated from Iceland in 1985 (for review, see Petursson 1994). Problems caused by ALV infection were so severe that the Department of Agriculture established the Regional Poultry Laboratory to devise ways to control the infections.
Naturally Occurring Retroviral Infections of Animals
Many farm animal populations contain significant proportions of animals that are infected with pathogenic retroviruses (Table 9). ALV is still present in some chicken flocks (for review, see Payne 1992) and significant percentages of the sheep in some herds in the United States are infected with ovine lentiviruses (Crawford and Adams 1981; Cheevers and McGuire 1988; McGuire et al. 1990; Cutlip et al. 1992a,b). EIAV-infected horses can also be found in southern United States. The frequency of infected animals may be drastically underestimated because only a subset of premium horses are tested routinely (for review, see Montelaro et al. 1993). Infections with these and other retroviruses, including BLV, are common in other parts of the world as well. Large numbers of infected animals are not always required to maintain the infections. As few as 5–10% of the chickens in a flock can sustain an ALV infection (Solomon et al. 1966; L.N. Payne et al. (1979, 1982)). These statistics highlight the difficulties associated with eliminating the virus.
Table 9
Transmission of Pathogenic Retroviruses.
A second problem inherent in controlling naturally occurring retroviral infections is that many of them do not result in disease. Thus, identifying and culling infected animals are complicated. Asymptomatic infections may not pose a threat to resistant animals. However, such animals can transmit the infection to more susceptible animals when they are housed in close quarters or during veterinary procedures in which contaminated blood is inadvertently transferred from animal to animal. The drastic consequences that can occur when susceptible animals are exposed to resistant, infected animals are well illustrated by the Icelandic VMV epidemic. The factors that control such differential susceptibility remain elusive in most cases. However, the delicate balance that can be achieved between a virus and host population is well illustrated by the dynamics of the natural MLV infection in wild mice in Lake Casitas, California (for review, see Gardner et al. 1991). In this instance, a single host gene, Akvr1/ Fv4, corresponding to an endogenous provirus (see above Host Genes That Influence Pathogenesis), facilitates maintenance of a virus in a population by controlling susceptibility to infection.
Domestic cats are a second group of animals that are infected with retroviruses. Both FeLV-A and FIV are prevalent, and infections with either of these viruses pose difficulties for pet owners (for review, see Hardy 1993; Pedersen 1993). These infections are especially common in multi-cat households where close contact increases the risks of infection. FeLV transmission usually occurs via saliva during mutual grooming, although bites are an equally effective method of transfer (for review, see Hardy 1993). The virus is also present in urine and feces and can be transmitted from infected mothers to their young via milk (Pedersen et al. 1977). FIV infection appears to be most effectively transmitted by biting (Pedersen et al. 1987; Yamamoto et al. 1989), but it can also be transmitted from infected females to kittens in the milk (Sellon et al. 1994; O'Neill et al. 1995).
Although FIV was isolated only recently, both FeLV-A and FIV have probably been present in feline populations for some time (for review, see Pedersen 1993). Lentiviruses have been isolated from nondomestic cats, but these are distantly related to FIV, suggesting that interspecies transmission of feline lentiviruses is not common (Barr et al. 1989; Olmsted et al. 1992). Whether these viruses cause disease in their natural hosts is difficult to assess. However, many individuals in at least one endangered puma species, the Florida panther, are infected with a lentivirus that may affect the health of the population (Olmsted et al. 1992). Widespread retroviral infection in the absence of disease is probably quite common. For example, bovine immunodeficiency virus (BIV) infects large numbers of cattle but does not appear to cause disease (Cockerell et al. 1992; McNab et al. 1994; St. Cyr Coats et al. 1994). However, a related virus does induce a fatal disease in Bali cattle (Soeharsono et al. 1990; Soesanto et al. 1990; Kertayadnya et al. 1993; Wilcox et al. 1995).
HTLV Infections
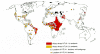
The association of HTLV-1 with a fatal malignancy and a devastating CNS disorder has stimulated large testing programs that provide a detailed picture of the prevalence of HTLV-1 worldwide (Fig. 21). The frequency of infection with this virus and with HTLV-2 is increasing, especially among sexually promiscuous populations and intravenous drug users. Even though the incidence of disease associated with HTLV-1 infection is low and no diseases are firmly associated with HTLV-2 (see above Oncogenesis, Tumor Induction by Viruses Containing the v-onc Gene), the dissemination of these viruses in the human population is a matter of considerable concern. The infection is spread primarily from mothers to their children, most commonly through milk (Kinoshita et al. 1984; Ando et al. 1987; Hino et al. 1987; Tsuji et al. 1990; Hirata et al. 1992). The virus can probably also be transmitted via intrauterine or transvaginal routes in rare instances (Saito et al. 1990; Saji et al. 1990; Tsuji et al. 1990). A second common route of transmission is from infected men to women via virus-infected cells present in semen (Tajima et al. 1982; Nakano et al. 1984); sexually mediated transmission from women to men does not appear to occur at a significant frequency. The third common route of transmission of HTLV-1 is via blood or blood cell products, a mode of transmission that accounts for the high frequency of intravenous drug users that have become infected (Gotoh et al. 1982; Maeda et al. 1984; Miyamoto et al. 1984; Jason et al. 1985; Sato and Okochi 1986; Minamoto et al. 1988). Unlike HIV (see Chapter 11, transmission of HTLV usually requires the transfer of lymphocytes.
Although increased travel has spread HTLV throughout the world, HTLV-1 is endemic in areas where ATL is most common, such as Southern Japan (Hinuma et al. 1982; Hinuma 1986; Tajima 1990) and the Caribbean basin (Blattner et al. 1982; Murphy et al. 1991). HTLV-2 strains are found in pockets of infection in parts of Central and South America, Africa and Melanesia, and in some Native American populations (Hunsmann et al. 1983; Merino et al. 1984; Saxinger et al. 1984; Asher et al. 1988; Reeves et al. 1988). Molecular analyses have shown that independent isolates from each endemic area are highly similar (Komurian et al. 1991; Ratner et al. 1991; Schulz et al. 1991; Gessain et al. 1992, 1993). Indeed, these viruses are more closely related to strains of simian T-cell leukemia virus (STLV) that are found in nonhuman primates indigenous to the same area than they are to strains of HTLV from other locations (Koralnik et al. 1994). These data suggest that ancestors of HTLV and STLV circulated between humans and nonhuman primates and that the different strains arose independently in at least two different parts of the world. The relationship of HTLV-1 strains found in developed countries, including Japan, to those found in West Africa supports the hypothesis that the slave trade had a role in disseminating this virus (Gallo et al. 1983; Gessain et al. 1992). Similar events may have generated HTLV-2. Long thought to be endemic in Native Amerindian tribes (Lairmore et al. 1990; Pardi et al. 1991; Dube et al. 1993; Hjelle et al. 1993), recent evidence suggests that a second subtype may have existed in Africa for many years (Gessain et al. 1995).
Publication Details
Copyright
Publisher
Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY)
NLM Citation
Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 1997. Transmission and Epidemiology.