NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Collaborating Centre for Cancer (UK). Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients. London: National Institute for Health and Clinical Excellence (NICE); 2012 Sep. (NICE Clinical Guidelines, No. 151.)

Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients.
Show details10. Timing of initial antibiotic therapy. (Topic E4)
Guideline subgroup members for this question
Anton Kruger (lead), Wendy King, Barbara Crosse, Bob Phillips and Rosemary Barnes.
Review question
Does the length of time before empiric antibiotics are given influence patient outcomes?
Rationale
Neutropenic sepsis is a serious complication of myelo-suppressive anticancer treatment or of bone marrow failure for other reasons. Very early observations established that this is a lethal condition with high mortality rates especially when the infective organism is a gram negative bacterium. Early studies of the active management of this condition showed that delaying treatment, for instance while waiting for culture results, was dangerous and carried a significant risk of death, again particularly when the infective organism was a gram negative bacterium. This led to the concept of empiric antibiotic treatment where a broad-spectrum antibiotic or combination of antibiotics is administered before the results of microbiological tests are available. A further extension of this concept implies that if time to treatment is critical, empiric treatment should be given to potentially neutropenic patients with clinical signs of sepsis even before the neutrophil count is known. This time between onset of symptoms and administration of antibiotics can be termed the “symptom-to-needle time”.
There are a large number of factors that will influence the symptom-to-needle time. It may be possible to influence these factors and it would therefore be useful to establish if there is a safe or optimum interval between the onset of symptoms and treatment. Although it would appear obvious that treatment delays are a bad thing, it is possible that over-hasty treatment may also confer disadvantages. For instance, patients who are not neutropenic or who do not even have an infection may be given unnecessary antibiotics with potential adverse side effects.
This question seeks to establish whether there is an evidence base for the relationship between symptom-to-needle time and outcome in patients with potential (blood count unknown) or established (blood count known) neutropenic sepsis.
Question in PICO format
| Patients/population | Factors | Outcomes |
|---|---|---|
| Patients with suspected neutropenic sepsis, (before neutrophil count is known) | Length of time before empiric antibiotics are given (symptom to needle time) |
|
METHODS
Information sources and eligibility criteria
The information specialist (SB) searched the following electronic databases: Medline, Premedline, Embase, Cochrane Library, Cinahl, BNI, Psychinfo, Web of Science (SCI & SSCI), ISI proceedings and Biomed Central. The search was done on 31st May 2011 and updated on 7th November 2011.
Study selection
The information specialist (SB) did the first screen of the literature search results. Two reviewers (NB and CL) subsequently selected potentially eligible studies by comparing titles and abstracts to the inclusion criteria presented in the PICO question. Full text articles were obtained for all studies identified as being potentially eligible. These articles were checked against the inclusion criteria. Data were extracted by one reviewer (CL) and checked by another (NB).
RESULTS
Results of literature searches
Two observational studies of the timing of initial antibiotic therapy were identified (Larche et al 2003 and Lin et al 2008). Neither directly met the criteria set out by the PICO. One was a study of cancer patients (some neutropenic) with septic shock (Larche et al 2003); the other was a study of patients with bacteremia, some of whom were neutropenic, but it was unclear whether or not they were cancer patients (Lin et al 2008). Both were retrospective cohort studies. Both studies evaluated early versus delayed administration of antibiotics. The study by Larche et al. defined a delay as > 2 hours from ICU admission. The study by Lin et al. defined a delay as > 24 hours from index blood culture.
Evidence statements
Short term mortality (febrile neutropenia studies)
A multivariate analysis by Larche, et al., (2003) found that 30 day mortality was higher when time to antibiotic therapy was more than two hours (odds ratio (OR) = 7.05 (95% CI, 1.17 to 42.21 (P = 0.03)). (Table 10.1).
Table 10.1
GRADE profile: Does the length of time before empiric antibiotics are given influence patient outcomes?
A multivariate analysis by Lin, et al., found that mortality was higher in patients with an ANC of <0.1 × 109/L when time to antibiotic therapy was > 24 hours in a non-ICU setting (OR = 18.0; 95% CI, 2.84 to 114.5; P < 0.01); and in an ICU setting (OR, 5.56; 95% CI, 0.85 to 36.3; P = 0.07). However, for patients who were non-neutropenic (ANC, >0.5 × 109/L) or had ANCs of 0.1 to 0.5 × 109/L, delay was not associated with increased mortality in ICU (OR (ANC 0.1 to 0.5 × 109/L) = 0.59; 95% CI, 0.06 to 6.22; P = 0.66; OR (ANC > 0.5 × 109/L ) = 0.55; 95% CI 0.29 to 1.02) or non-ICU (OR (ANC 0.1 to 0.5 × 109/L) = 1.92; 95% CI, 0.17 to 21.3; P = 0.60; OR (ANC > 500) = 1.78; 95% CI 0.89 to 3.44).
This evidence is of very low quality and is indirect on the basis that patients had bacteraemia or septic shock
Overtreatment, Severe sepsis, Length of stay, Duration of fever and Quality of life
These outcomes were not reported by the identified studies. The outcome of severe sepsis was not relevant to the included studies, which included only participants who had bacteraemia or severe sepsis at study entry.
EVIDENCE TABLES
Download PDF (457K)
10.1. Timing of initial antibiotic: a wider search of timing of antibiotic therapy: removing the requirement of neutropenia
Rationale
No studies meeting the criteria set out by the PICO were identified. Furthermore, only two studies containing indirect evidence were found. On this basis, a wider search was necessary to identify additional studies of time to antibiotic therapy. The requirement of participants having neutropenia was removed in the second search, in a bid to identify further indirect evidence. The search produced over 35,000 hits. It was not feasible to consider this number of studies. Consequently, ‘systematic review’ filter was applied.
Question in PICO format
| Patients/population | Factors | Outcomes |
|---|---|---|
| Patients with suspected bacterial infection | Length of time before empiric antibiotics are given (symptom to needle time) |
|
RESULTS
Study selection
The information specialist (SB) did the first screen of the literature search results. Two reviewers (NB and CL) subsequently selected potentially eligible studies by comparing titles and abstracts to the inclusion criteria presented in the PICO question. Full text articles were obtained for all studies identified as being potentially eligible. These articles were checked against the inclusion criteria. Data were extracted by one reviewer (CL) and checked by another (NB).
Study characteristics
Four systematic reviews considering the timing of antibiotic therapy were identified (Pines et al. 2009, Yu et al. 2008, Siddiqui et al 2010 and McGregor et al. 2007). Two were systematic reviews of studies evaluating the impact of time to antibiotic therapy in Community Acquired Pneumonia (Pines et al. 2009 and Yu et al. 2008); one was concerned with bacteremia (McGregor et al. 2007); and one was concerned with severe sepsis (Siddiqui et al. 2010). Three were systematic reviews of observational studies (Pines et al. 2009, Yu et al. 2008, and McGregor et al. 2007); one was a systematic review of Randomised Controlled Trials (RCTs). Two included only studies of adult participants (McGregor et al. 2007 and Siddiqui et al 2010); two included studies of adult and paediatric participants (Pines et al. 2009 and Yu et al. 2008). None of the identified reviews included meta-analyses. These were small systematic reviews. The number of papers identified related to the timing of antibiotic therapy ranged from 0 (Siddiqui et al 2010) to 13 (Yu et al. 2008).
Evidence statements
Overtreatment
Overtreatment was not reported by the identified systematic reviews.
Short term mortality
Two of the four systematic reviews reported data on short term mortality related to the timing of antibiotic therapy (Pines et al. 2009 and Yu et al. 2008).
Yu et al calculated individual odds ratios for each study for delayed versus non delayed administration; these ranged from 0.24 (95% CI, 0.08 to 0.71) to 1.99 (95% CI, 1.22 to 13.45) in studies with delay < 4 hours and 0.60 (95% CI, 0.37 to 1.35) to 0.96 (95% CI, 0.70 to 1.30) in studies defining a delay as < 8 hours.
Pines et al. took the approach of categorising studies in terms of whether or not they supported early administration of antibiotics: 2 supported early administration; 1 was neutral; and 5 opposed early administration. The criteria used for categorisation were unclear.
Severe sepsis
Severe sepsis was not reported by the identified systematic reviews.
Length of stay
Length of stay was not reported in relation to timing of antibiotic therapy by any of the identified systematic reviews.
Duration of fever was not reported by the identified systematic reviews
Duration of fever and quality of life were not reported by the identified systematic reviews.
EVIDENCE TABLES
Download PDF (221K)
REFERENCES
- Larche J, Azoulay E, Fieux F, Mesnard L, Moreau D, Thiery G, et al. Improved survival of critically ill cancer patients with septic shock. Intensive Care Medicine. 2003;29:1688–1695. [PubMed: 13680115]
- Lin MY, Weinstein RA, Hota B. Delay of active antimicrobial therapy and mortality among patients with bacteremia: impact of severe neutropenia. Antimicrobial Agents & Chemotherapy. 2008;52:3188–3194. [PMC free article: PMC2533498] [PubMed: 18625778]
- McGregor JC, Rich SE, Harris AD, Perencevich EN, Osih R, Lodise TP, et al. A Systematic Review of the Methods Used to Assess the Association between Appropriate Antibiotic Therapy and Mortality in Bacteremic Patients. Clinical Infectious Diseases. 2007;45:329–337. [PubMed: 17599310]
- Pines JM, Isserman JA, Hinfey PB. The measurement of time to first antibiotic dose for pneumonia in the emergency department: a white paper and position statement prepared for the American Academy of Emergency Medicine. Journal of Emergency Medicine. 2009;37:335–340. [Review] [17 refs] [PubMed: 19717266]
- Siddiqui S, Razzak J. Early versus late pre-intensive care unit admission broad spectrum antibiotics for severe sepsis in adults. Cochrane Database of Systematic Reviews. 2010;(10) [Review] CD007081, 2010., CD007081. [PMC free article: PMC6516895] [PubMed: 20927754]
- Yu KT, Wyer PC. Evidence-based emergency medicine/critically appraised topic. Evidence behind the 4-hour rule for initiation of antibiotic therapy in community-acquired pneumonia. Annals of Emergency Medicine. 51:651–662. (662) [Review] [34 refs] [PubMed: 18272253]
11. Empiric intravenous antibiotic monotherapy or empiric intravenous antibiotic dual therapy. (Topic E3)
Guideline subgroup members
Anton Kruger (lead), Wendy King, Barbara Crosse, Bob Phillips and Rosemary Barnes.
Review question
Is there a difference in the effectiveness of empiric intravenous antibiotic monotherapy and empiric dual therapy in the treatment of patients with neutropenic sepsis?
Rationale
Neutropenic sepsis is a potentially lethal condition especially when the infection is due to gram negative bacteria. Early studies focussed on empiric antibiotic treatment combinations using two, three and even five drug regimens. These early trials were small and produced inconsistent and clinically poor outcomes by today's standards. In 1973 the European Organisation for Research on Treatment of Cancer (EORTC) formed a cooperative group to research the problem. In parallel over the next three decades, a stream of new drugs based on the beta-lactam structure entered the market: some of these and the older drugs have now disappeared. Early treatments were assessed in the empiric setting, but emphasis was also placed on the effectiveness of agents in controlling infections subsequently shown to have been caused by known pathogens.
Combination therapy including a beta lactam antibiotic (penicillin or cephalosporin) combined with an aminoglycoside formed the backbone of the early studies due to theoretical and in-vitro synergism predicted for the combination and also because of known gaps in microbiological sensitivities for the earlier beta lactams. The effectiveness of these combinations was confirmed in the first EORTC study. From the early 1980's and for more than 20 years on, a number of randomised comparisons of monotherapy based on emerging new Beta-lactam antibiotics with a particularly broad spectrum of activity (and known effectiveness against dangerous organisms such as Pseudomas) versus combination therapy (beta-lactam plus aminoglycoside) have been undertaken. Many of these studies involved more than two drugs, with a “newer” Beta lactam in the trial arm being compared with an “older” cephalosporin or penicillin combined with an aminoglycoside in the control arm.
Monotherapy has potential advantages over combination therapy. These could include cost, resource and staff time and avoidance of the side effects and need for monitoring of drug levels associated with aminoglycosides. Aminoglycoside kidney toxicity is usually immediately apparent and can interfere with ongoing cancer treatment. On the other hand inner ear toxicity (deafness and balance problems) can be insidious and often presents many years after the exposure. This can result in an underestimation of this potentially crippling side effect.
A Cochrane review and meta analysis published in 2003 concluded that monotherapy (based on newer broad spectrum beta-lactams) was superior to combination regimens (with narrower spectrum beta-lactams) in terms of efficacy and associated with fewer side effects. Despite this, combination regimens are still widely employed and a further analysis of the question is warranted. There are additional reasons why aminoglycosides may still be used, including concerns about secondary infection with clostridium difficile and emerging forms of antibiotic resistance. In addition, particular subgroups of patients may fare better with combination therapy and local knowledge of microbiological flora may also affect treatment choices. An up to date evidence base is needed to guide modern treatment decisions. This will have to take into account the historical perspective and potential microbiological consequences.
Question in PICO format
| Patients/population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with neutropenic sepsis | Intravenous antibiotic monotherapy (Piperacillin/tazobactam Ceftazidime Meropenem Imipenem Aztreonam Ciprofloxacin) | Intravenous antibiotic dual therapy (Monotherapies plus aminoglycosides) |
|
METHODS
Information sources and eligibility criteria
The information specialist (SB) searched the following electronic databases: Medline, Premedline, Embase, Cochrane Library, Cinahl, BNI, Psychinfo, Web of Science (SCI & SSCI), ISI proceedings and Biomed Central. The full strategy will be available in the full guideline.
We restricted the search to published randomised (or quasi randomised) trials and systematic reviews of such trials. A comprehensive and good quality systematic review of this question was published in 2007 (Paul et al, 2007). Our literature search was therefore limited to papers published after 2005, to identify new evidence not included in their review. The search was done on the 23rd of October 2010 and updated on 7th November 2011.
Drug names for the literature search
- Drug names for monotherapy
- Penicillins: Piperacillin with tazobactam [AK note: Piperacillin with Tazobactam is the only surviving Ureidopenicillin in the market. Other discontinued drugs (Azlocillin, Mezlocillon) would have been important agents in earlier randomised studies and their exclusion might inappropriately influence the review outcome if insufficient recent studies are found. Ticarcillin, a carboxypenicillin (and now combined with clavulanic acid) is still available and may appear in relevant papers. It is a less desirable drug on microbiological sensitivity criteria alone but should be included]
- Quinolones: Ciprofloxacin
- Cephalosporins: Ceftazidime
- Monobactams: Aztreonam
- Carbapenems: , Meropenem, Imipenem.
- Aminoglycosides: Gentamicin [AK note: some of the original aminoglycosides such as Netilmicin are no longer listed in the BNF and are presumably no longer marketed. Nevertheless, relevant studies may still exist and as there relatively few differences between the drugs in this group I would include all of the currently used parenteral drugs (Amikacin, Gentamicin, Tobramycin) and Netilmicin. I am not aware of any studies that included Streptomycin but there may be some. Also be aware that some papers have used “y” instead of “i” in the “mYcin”. An alternative strategy might be to search under the generic term aminoglycoside]
Selection of studies
The information specialist (SB) did the first screen of the literature search results. One reviewer (NB) then selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO question. The full articles were then obtained for possibly eligible studies and checked against the inclusion criteria.
Data synthesis
Where the searches identified new data we updated the meta-analyses reported by Paul et al (2007). For consistency between updated and original analyses we used the same statistical methods as the original review. Dichotomous outcomes were analysed by calculating the relative risk and its 95% confidence interval for each study. A Mantel-Haenzel fixed effect model was used for all meta-analyses in the Paul et al (2007) review, unless significant heterogeneity was observed (defined as P < 0.1 or I2 > 50%) in which case the random effect model was used.
Forest plots were generated whenever additional trials were added to the meta-analyses of Paul et al (2007) (see figures 1 to 4).
Paul et al (2007) considered several patient and treatment subgroups, we can update these subgroup analyses with any new trial data if the guideline group thinks it appropriate. Patient subgroups included: patients with severe neutropenia (absolute neutrophil count < 100/m3), those with microbiologically documented infections, those with documented Pseudomonas aeruginosa infections, those with bacteraemia, adults versus children and those with underlying haematological malignancy or bone marrow transplantation. Treatment subgroups included: monotherapy drug, same beta-lactam used in both combined and monotherapy and aminoglycoside dosing regimen.
RESULTS
Description of included studies
Initial screening identified 116 relevant papers, 15 of these were ordered and three included as evidence. The reasons for exclusion are noted in the list of excluded references below.
Seventy randomised or quasi randomised trials were included: 68 from the Paul et al (2007) systematic review and three later trials (Pereira et al., 2009; Yildirim et al., 2008 and Zengin et al., 2011).
Populations in the included trials
Most of the trials were in patients with haematological cancers: 34/70 trials included only patients with haematological cancers and in a further 32/70 trials a majority of the patients had haematological cancers.
43/70 trials were in adult cancer patients, 14/70 trials included only children and 13/70 included both adults and children.
Antibiotics used in the included trials
In 15 trials the same beta-lactam was used in both arms of the trial. In these trials the beta-lactam was: ceftazidime (seven trials), piperacillin-tazobactam (three trials), cefepime (three trials), imipenem (two trials) and in one trial cefoperazone (one trial assessed more than one beta-lactam monotherapy). The other 55 trials compared a beta-lactam (typically a new drug) to a narrower spectrum beta-lactam plus an aminoglycoside. See Table 11.1 for summary of antibiotics used in the trials.
Table 11.1
Beta-lactam classes used for montherapy and combined therapy.
The following aminoglycosides were used in combined therapy: amikacin (42 trials), tobramycin (14 trials), gentamicin (11 trials) and netilmicin (3 trials).
Overall risk of bias in the included trials
Allocation concealment was judged to be adequate in 27/70 trials. Blinding was reported in 10/70 trials (six single blinding and four double blinding). Intention to treat (ITT) analysis of treatment failure was reported in 23/70 trials; ITT analysis of mortality was reported in 25/48 trials.
The unit of randomisation was the patient in 24/70 studies and the episode of neutropenia / fever in the other trials. Studies reporting multiple episodes from the same patients did not adjust their analyses for the correlation between multiple data points from the same patient.
Fourteen trials used a pre-specified follow-up period, ranging from three days to one month following the end of treatment. Some trials described follow-up until the end of treatment, without reporting the actual duration. Two trials reported follow-up of greater than one month.
Evidence Statements
Evidence from trials directly comparing single agent with combined treatment
There was moderate quality evidence from 44 studies with over seven thousand episodes of neutropenia and fever which did not show a significant difference in the risk of all cause mortality between monotherapy and combined therapy. This evidence is summarised in table 11.2.
Table 11.2
GRADE evidence profile for empiric IV antibiotic monotherapy versus empiric IV antibiotic dual therapy.
Moderate quality evidence from 55 studies showed that treatment failure was less likely with monotherapy than combined therapy, when combined therapy used a narrower spectrum antibiotic than was used for monotherapy. Fifteen studies where the same beta-lactam was used for both monotherapy and combined therapy, however, found treatment failure more likely with monotherapy.
Moderate quality evidence showed that monotherapy was associated with fewer adverse events, including nephrotoxicity.
Moderate quality evidence showed that monotherapy and combined therapy had similar rates of bacterial secondary infection.
Low quality evidence showed fungal secondary infection was more likely with combined therapy.
Very low quality evidence from two studies with 152 patients suggested that colonisation of resistant Gram-negative bacteria was more likely with monotherapy, but such bacteria were only detected in six patients overall.
There was no evidence about quality of life and no useful evidence about the duration of hospital stay.
Death from any cause
All cause mortality (typically within one month of the start of treatment) was reported in 44 trials including 7171 episodes of neutropenia and fever. One additional trial (Pereira et al, 2009) was added to the Paul et al (2007) meta-analysis (see figure 11.2). The relative risk (RR) of mortality in the monotherapy group versus the combined therapy group was 0.88 (95% C.I. 0.75 to 1.03) suggesting a non-statistically significant 12% reduction in the risk of mortality with monotherapy.

Figure 11.2
Forest plot of all cause mortality.
The subgroup analyses of mortality of Paul et al (2007) were updated (Table 11.3). Data from Pereira et al (2009) were added to the different beta-lactam, haematological malignancy and children subgroup analyses. There were no significant differences in mortality in any subgroup.
Table 11.3
Subgroup analyses for all cause mortality.
Duration of fever / Treatment failure
Duration of fever was not reported in the Paul et al (2007) review. After discussion with the lead GDG member for this topic (AK) “treatment failure” was included as an outcome because it incorporates duration of fever in its definition. Treatment failure was defined as any of the following: death; persistence, recurrence or worsening of clinical signs or symptoms of the presenting infection; any modification of the assigned empirical antibiotic treatment. A problem with treatment failure as an outcome (as noted by Paul et al, 2007) is that treatment modification might have been biased. Most of the trials were open trials, where clinicians knew the empirical therapy the patient was receiving and this knowledge may have biased their decision to modify antibiotic treatment.
Treatment failure was reported in all 70 trials including 10429 episodes of neutropenia and fever. Two additional trials (Pereira et al, 2009; Yildirim et al, 2008) were added to the original Paul et al (2007) meta-analyses (see figure 11.3). Pooling all 70 trials gave a relative risk of 0.94 (95% C.I. 0.97 to 1.01) but there was significant there was significant heterogeneity (P=0.04, I2 = 24%).
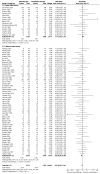
Figure 11.3
Forest plot of treatment failure.
The subgroup analyses of treatment failure in Paul et al (2007) were updated with data from Pereira et al (2009), Yildirim et al (2008) and Zengin et al (2011). These analyses suggested that using the same beta-lactam for both monotherapy and combined therapy was related to the risk of treatment failure. In the 15 trials where the same beta-lactam was used in both trial arms, the risk of treatment failure in the monotherapy group was greater than in the combined therapy group, RR = 1.11 (95% C.I. 1.02 to 1.21). In the 55 trials where a different beta-lactam was used in each trial arm, the risk of treatment failure in the monotherapy group was less than in the combined therapy group, RR = 0.92 (95% C.I. 0.87 to 0.96).
Table 11.4
Subgroup analyses for treatment failure.
Adverse events
Any adverse event was reported 48 trials including 7340 episodes of neutropenia and fever. An additional trial (Pereira et al, 2009) was added to the Paul et al (2007) meta-analysis (see Figure 11.4). Monotherapy was associated with a lower risk of adverse events than combined therapy, RR=0.86 (95 %C.I. 0.80 to 0.93), but there was significant heterogeneity in this meta-analysis (P<0.0001, I=51%).

Figure 11.4
Forest plot of any adverse event.
Subgroup analyses according to specific monotherapy drugs showed a statistically significant reduction in the risk of adverse events only with ceftazidime monotherapy (RR = 0.64; 95% C.I. 0.53 to 0.76) and moxalactam monotherapy (RR = 0.64; 95% C.I. 0.53 to 0.76), suggesting the drug used for monotherapy might account for some of the heterogeneity seen in the overall meta-analysis.
Nephrotoxicity was reported in 37 trials including 6411 episodes of neutropenia and fever. No new evidence about nephrotoxicity was identified in our literature search. The risk of any nephrotoxicity was significantly lower with monotherapy than with combined therapy, RR=0.45 (95% C.I. 0.35 to 0.57). With severe nephrotoxicity the effect in favour of monotherapy was more marked: RR=0.16 (95% C.I. 0.05 to 0.49; from 18 trials with 4002 episodes).
Paul et al (2007) did subgroup analyses of any nephrotoxicity and severe nephrotoxicity according to aminoglycoside dosing regimen (see Tables 11.5 and 11.6). No new nephrotoxicity data were identified in our literature searches. The risk of any nephrotoxicity was significantly lower with monotherapy than with combined therapy for both once daily and multiple daily aminoglycoside dosing regimens. A similar pattern was seen for severe nephrotoxicity although the difference was not statistically significant in the four studies using a once daily aminoglycoside dosing regimen.
Table 11.5
Subgroup analyses for any nephrotoxicity.
Table 11.6
Subgroup analyses for severe nephrotoxicity.
Secondary infection
Superinfection was defined as new, persistent or worsening symptoms and/or signs of infection associated with the isolation of a new pathogen or the development of a new site of infection.
Bacterial superinfection was reported 29 trials including 4961 episodes of neutropenia and fever. An additional trial (Pereira et al, 2009) was added to the Paul et al (2007) meta-analysis (Figure 11.5). There was no significant difference between treatment groups in the risk of bacterial superinfection: RR=1.02 (95% C.I. 0.87 to 1.19).

Figure 11.5
Forest plot of bacterial superinfection.
Fungal superinfection was reported 20 trials including 3437 episodes of neutropenia and fever. Our searches identified no new evidence for this outcome. The risk of fungal superinfection was lower in the monotherapy group than in the combined therapy group, RR=0.70 (95% C.I. 0.49 to 1.00). However the data for fungal superinfection were relatively sparse, with 114 events in total. Fungal superinfection occurred in around 3% of episodes and bacterial superinfection in around 11% of episodes.
Resistant colonisation
Resistant colonisation was defined as the isolation (during or following therapy) of Gram-negative bacteria resistant to the beta-lactam included in the empiric regimen, without symptoms or signs of infection. There was very little evidence about this outcome. Although seven trials supplied reported resistant colonisation only two trials reported the relative rates of resistant colonisation between the treatment groups (Cornelissen 1992; Norrby, 1987). In these trials resistant Gram-negative bacteria were detected in 5/152 patients in the monotherapy group compared with 1/152 patients in the combination therapy group.
Length of hospital stay
Four trials reported this outcome; in three of the trials the duration of hospital stay was shorter in the monotherapy group. In the other trial the duration of hospital stay was shorter in the combined therapy group. The difference was not statistically significant (at P<0.05) in any of these trials. Data were not pooled due to the different ways in which the trials reported hospital stay.
Quality of life
Quality of life was not an included as an outcome in Paul et al (2007). The abstracts of the trials included in the Paul et al (2007) review were checked for mention of quality of life outcomes, but none was found and neither of the new studies (Pereira et al 2009; Yildrim et al, 2008) reported quality of life as an outcome. It is debateable whether differences between the quality of life of the treatment groups would be measureable over the short period of follow-up used in these trials.
REFERENCES
- Paul M, Grozinsky S, Soares-Weiser K, Leibovici L. Cochrane Database of Systematic Reviews. Chichester (UK): John Wiley & Sons, Ltd; 2007. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database of Systematic Reviews: Reviews.
- Agaoglu L, Devecioglu O, Anak S, Karakas Z, Yalman N, Biner B, Eryilmaz E, Goksan B, Unuvar A, Agirbasli H, Can M, Bilgen H, Gedikoglu G. Cost-effectiveness of cefepime + netilmicin or ceftazidime + amikacin or meropenem monotherapy in febrile neutropenic children with malignancy in Turkey. Journal of Chemotherapy. 2001;13(3):281–7. [PubMed: 11450887]
- Ahmed N, El-Mahallawy HA, Ahmed IA, Nassif S, El-Beshlawy A, El-Haddad A. Early hospital discharge versus continued hospitalization in febrile pediatric cancer patients with prolonged neutropenia: A randomized, prospective study. Pediatric Blood Cancer. 2007;49:786–92. [PubMed: 17366527]
- Akova M, Akan H, Korten V, Biberoglu K, Hayran M, Unal S, Kars A, Kansu E. Comparison of meropenem with amikacin plus ceftazidime in the empirical treatment of febrile neutropenia: a prospective randomised multicentre trial in patients without previous prophylactic antibiotics. Meropenem Study Group of Turkey. International Journal of Antimicrobial Agents. 1999;13(1):15–9. [PubMed: 10563400]
- Alanis A, Rehm S, Weinstein AJ. Comparative efficacy and toxicity of moxalactam and the combination of nafcillin and tobramycin in febrile granulocytopenic patients. Cleve Clin Q. 1983;50(4):385–95. [PubMed: 6365358]
- Antmen B, Sasmaz I, Tanyeli A, Yaman A, Kocabas E, Bayram I, Kilinc Y. Initial empiric antibiotic treatments in childhood febrile neutropenia: meropenem versus ceftazidime plus amikacin combination; 11th European Congress of Clinical Microbiology and Infectious Diseases; 1-4 April 2001; Istanbul, Turkey. 2001. p. P1087. (Adana, TR) [MEDLINE: http://www
.akm.ch/eccmid2001] - Au E, Tow A, Allen DM, Ang PT. Randomised study comparing imipenem/cilastatin to ceftriaxone plus gentamicin in cancer chemotherapy-induced neutropenic fever. Annals of the Academy of Medicine, Singapore. 1994;23(6):819–22. [PubMed: 7741492]
- Behre G, Link H, Maschmeyer G, Meyer P, Paaz U, Wilhelm M, Hiddemann W. Meropenem monotherapy versus combination therapy with ceftazidime and amikacin for empirical treatment of febrile neutropenic patients. Annals of Hematology. 1998;76(2):73–80. [PubMed: 9540761]
- Bezwoda WR, Derman DP, Perkins S, Cassel R. Treatment of neutropenic infection: a randomized trial comparing latamoxef (moxalactam) with cephradine plus tobramycin. Journal of Antimicrobial Chemotherapy. 1985;15(2):239–45. [PubMed: 3884566]
- Borbolla JR, Lopez-Hernandez MA, Gonzalez-Avante M, DeDiego J, Trueba E, Alvarado ML, Jimenez RM. Comparison of cefepime versus ceftriaxone-amikacin as empirical regimens for the treatment of febrile neutropenia in acute leukemia patients. Chemotherapy. 2001;47(5):381–4. [PubMed: 11561142]
- Cometta A, Calandra T, Gaya H, Zinner SH, De Bock R, Del Favero A, Bucaneve G, Crokaert F, Kern WV, Klastersky J, Langenaeken J, Micozzi A, Padmos A, Paesmans M, Viscoli C, Glauser MP, Martino P, Caballero D, Kern WV, et al. Monotherapy with meropenem versus combination therapy with ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. Antimicrobial Agents and Chemotherapy. 1996;40(5):1108–15. [PMC free article: PMC163274] [PubMed: 8723449]
- Conte G, Flores C, Alfaro J, Araos D, Thompson L, Barahona O, et al. Single agent sulperazone vs. two agent ceftazidime-amikacin in high risk febrile neutropenic patients. Orlando, Florida: Blood. 1996;88(10):30b. Conte G, Flores C, Alfaro J, Araos D, Thompson L, Barahona O, et al.
- Corapcioglu F, Sarper N. Cefepime versus ceftazifdime + amikacin as empirical therapy for febrile neutropenia in children with cancer: a prospective randomized trial of the treatment efficacy and cost. Pediatr Hematol Oncol. 2005;22(1):59–70. [PubMed: 15770833]
- Cornelissen JJ, de Graeff A, Verdonck LF, Branger T, Rozenberg-Arska M, Verhoef J, Dekker AW. Imipenem versus gentamicin combined with either cefuroxime or cephalothin as initial therapy for febrile neutropenic patients. Antimicrobial Agents and Chemotherapy. 1992;36(4):801–7. [PMC free article: PMC189424] [PubMed: 1503442]
- Cornely OA, Reichert D, Buchheidt D, Maschmeyer G, Wilhelm M, Chiel X. For the Paul-Erlich-Gesellschaft. Three-armed multicenter randomized study on the empiric treatment of neutropenic fever in a high risk patient population (PEG study III); 41th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2001. p. 41. (Abstract L-775)
- De la Camara R, Figuera A, Sureda A, Hermida G, Verge G, Olalla I, Fernandez Ranada JM, Domingo Albos A. Meropenem versus ceftazidime plus amikacin in the treatment of febrile episodes in neutropenic patients: a randomized study. Haematologica. 1997;82(6):668–75. [PubMed: 9580087]
- de Pauw BE, Kauw F, Muytjens H, Williams KJ, Bothof T. Randomized study of ceftazidime versus gentamicin plus cefotaxime for infections in severe granulocytopenic patients. Journal of Antimicrobial Chemotherapy. 1983;12(suppl A):93–9. [PubMed: 6311790]
- De Pauw BE, Deresinski SC, Feld R, Lane Allman EF, Donnelly JP. Ceftazidime compared with piperacillin and tobramycin for the empiric treatment of fever in neutropenic patients with cancer. A multicenter randomized trial. The Intercontinental Antimicrobial Study Group. Annals of Internal Medicine. 1994;120(10):834–44. [PubMed: 8154643]
- Del Favero A, Menichetti F, Martino P, Bucaneve G, Micozzi A, Gentile G, Furno P, Russo D, D'Antonio D, Ricci P, Martino B, Mandelli F. A multicenter, double-blind, placebo-controlled trial comparing piperacillin-tazobactam with and without amikacin as empiric therapy for febrile neutropenia. Clinical Infectious Diseases. 2001;33(8):1295–301. [PubMed: 11565068]
- Dincol D, Arican A, Aydin F, Samur M, Willke A, Akbulut H, Pamin A, Cay F, Icli F. A comparison of imipenem monotherapy versus cefoperazone/sulbactam plus amikacin combination treatment in febrile neutropenic cancer patients. Cancer Journal. 1998;11(2):89–93.
- Doyen C, Tepantondele JM, Wauters G, Michaux JL. A randomized therapeutic trial for ceftazidime versus ceftazidime and amikacin in febrile granulopenic patients. In: Spitzy KH, editor. Proceedings of the 13th International congress of chemotherapy; Vienna. 1983. pp. 26–29.
- Duzova A, Kutluk T, Kanra G, Buyukpamukcu M, Akyuz C, Secmeer G, Ceyhan M. Monotherapy with meropenem versus combination therapy with piperacillin plus amikacin as empiric therapy for neutropenic fever in children with lymphoma and solid tumors. The Turkish Journal of Pediatrics. 2001;43(2):105–9. [PubMed: 11432485]
- El Haddad AMA. Comparison of cefoperazone-sulbactam versus piperacillin plus amikacin as empiric therapy in pediatric febrile neutropenic cancer patients. Current Therapeutic Research Clinical and Experimental. Current Ther Res Clin Exp. 1995;56(10):1094–9.
- Erjavec Z, de Vries Hospers HG, van Kamp H, van der Waaij D, Halie MR, Daenen SM. Comparison of imipenem versus cefuroxime plus tobramycin as empirical therapy for febrile granulocytopenic patients and efficacy of vancomycin and aztreonam in case of failure. Scandinavian Journal of Infectious Diseases. 1994;26(5):585–95. [PubMed: 7855556]
- Esteve J, Nomdedeu B, Mensa J, Guardia R, Marco F, Montserrat E. Piperacillin/tazobactam vs. piperacillin/tazobactam plus amikacin as empiric therapy for fever in neutropenic patients. Blood. 1997;90(10 Suppl 1 (Pt 2)):229b. Abstract 3767.
- Gaytan-Martinez JE, Mateos-Garcia E, Casanova LJ, Fuentes-Allen JL, Sanchez-Cortes E, Manjarrez-Tellez B, Vargas-Valerio A, Cedillo de la Cerda JL, Javier-Gonzalez L, Rodriguez J. Efficacy of Empirical Therapy with Cefepime Compared with Ceftazidime Plus Amikacin in Febrile Neutropenic Patients. Annual meeting of the American Society of Hematology. 2002. Abstract no. 3655.
- Gibson J, Date L, Joshua DE, Young GA, Wilson A, Benn R, Benson W, Iland H, Vincent PC, Kronenberg H. A randomised trial of empirical antibiotic therapy in febrile neutropenic patients with hematological disorders: ceftazidime versus azlocillin plus amikacin. Australian and New Zealand Journal of Medicine. 1989;19(5):417–25. [PubMed: 2686610]
- Gorschluter M, Hahn C, Fixson A, Mey U, Ziske C, Molitor E, et al. Piperacillin-tazobactam is more effective than ceftriaxone plus gentamicin in febrile neutropenic patients with hematological malignancies: a randomized comparison. Supportive care in cancer. 2003;11(6):362–370. [PubMed: 12690546]
- Gribble MJ, Chow AW, Naiman SC, Smith JA, Bowie WR, Sacks SL, Grossman L, Buskard N, Growe GH, Plenderleith LH. Prospective randomized trial of piperacillin monotherapy versus carboxypenicillin-aminoglycoside combination regimens in the empirical treatment of serious bacterial infections. Antimicrobial Agents and Chemotherapy. 1983;24(3):388–93. [PMC free article: PMC185330] [PubMed: 6357076]
- Hansen SW, Friis H, Ernst P, Vejlsgaard R, Hansen HH. Latamoxef versus carbenicillin plus gentamicin or carbenicillin plus mecillinam in leukopenic, febrile patients with solid tumors. Acta Med Scand. 1986;220(3):249–54. [PubMed: 3535399]
- Hense J, Bertz H, Leifert J, Meusers P, Mertelsmann R, Brittinger G. Final results of a prospective randomized trial of bolus meropenem versus infusion meropenem versus ceftazidime/amikacin as empiric initial therapy for infections and fever of unknown origin in neutropenic patients with hematologic malignancies. Supportive care in cancer. 2000;8 Suppl:160 (abstract 64)
- Hess U BC, Rey K, Senn Hj. Monotherapy with piperacillin/tazobactam versus combination therapy with ceftazidime plus amikacin as an empiric therapy for fever in neutropenic cancer patients. Support Care Cancer. 1998;6:402–409. [PubMed: 9695210]
- Hung KC, Chiu HH, Tseng YC, Wang JH, Lin HC, Tsai FJ, Peng CT. Monotherapy with meropenem versus combination therapy with ceftazidime plus amikacin as empirical therapy for neutropenic fever in children with malignancy. Journal of microbiology, immunology, and infection. 2003;36(4):254–9. [PubMed: 14723254]
- Jacobs RF, Vats TS, Pappa KA, Chaudhary S, Kletzel M, Becton DL. Ceftazidime versus ceftazidime plus tobramycin in febrile neutropenic children. Infection. 1993;21(4):223–8. [PubMed: 8225625]
- Jimeno A, Arcediano A, Bazares S, Amador ML, Gonzalez-Cortijo L, Ciruelos E, Robles L, Castellano D, Paz-Ares L, Lumbreras C, Hornedo J, Cortes-Funes H. Randomized Study of Cefepime versus Ceftazidime plus Amikacin in Patients with Solid Tumors Treated with High Dose Chemotherapy (HDC) and Peripheral Blood Stem Cell Support (PBSCS) with Febrile Neutropenia. Clinical transplantation and oncology. 2006;8(12):889–95. [PubMed: 17169762]
- Kiehl MG, Bischoff M, Basara N, Guenzelmann S, Fauser AA. A Prospective Randomized Trial Comparing the Efficacy and Safety of Piperacillin/Tazobactam Versus Piperacillin/Tazobactam Plus Netilmicin in the Treatment of Febrile Neutropenia in Allogeneic Stem Cell Recipients. Proceeding of the Interscience Conference on Antimicrobial Agents and Chemotherapy. 2001:267.
- Kinsey SE, Machin SJ, Goldstone AH. Ceftazidime monotherapy is as effective as ceftazidime combined with gentamicin in the treatment of febrile neutropenic patients. The Journal of Hospital Infection. 1990;15(suppl A):49–53. [PubMed: 1971645]
- Kliasova G, Savchenko V, Lubimova L, Mendeleeva L, Parovichnikova E, Tolkacheva T, Petrova N, Singerman B. Monotherapy with meropenem versus combination therapy with ceftazidime plus amikacin as empiric therapy for febrile neutropenic bone marrow transplant patients; 11th European Congress of Clinical Microbiology and Infectious Diseases; 2001. [MEDLINE: http://www
.akm.ch/eccmid2001/] - Koehler M, Bubala H, Sonta-Jakimczyk D, Wieczorek M, Janik-Moszant A, Kuder K. [Assessment of the efficacy of treating infections in hematopoietic proliferative diseases: Monotherapy with ceftazidime and tobramycin combined with amoxycillin/ampicillin]. Pol Tyg Lek. 1990;45(21-22):417–20. [PubMed: 2267192]
- Kojima A, Shinkai T, Soejima Y, Okamoto H, Eguchi K, Sasaki Y, Tamura T, Oshita F, Ohe Y, Saijo N. A randomized prospective study of imipenem-cilastatin with or without amikacin as an empirical antibiotic treatment for febrile neutropenic patients. American Journal of Clinicial Oncology. 1994;17(5):400–4. [PubMed: 8092111]
- Leyland MJ, Bayst K, Cohen J, Warren R, Newland AC, Bint AJ, Bint AJ, Cefai C, White D, Murray S, Bareford D, et al. A comparative study of imipenem versus piperacillin plus gentamicin in the initial management of febrile neutropenic patients with haematological malignancies. Journal of Antimicrobial Chemotherapy. 1992;30(6):843–54. [PubMed: 1289359]
- Lieschke Gj, Bell D, Rawlinson W, Green M, Sheridan W, Morstyn G, Stuart Harris R, Kefford RF, Levi J, Sorrell T, Fox RM. Imipenem/cilastatin versus tobramycin and piperacillin as initial empiric therapy for febrile episodes in neutropenic patients: Interim analysis of a prospective randomized comparison. Australian and New Zealand Journal of Medicine. 1990;20(3) suppl 1:424. [abstract]
- Liu CY, Wang FD. A comparative study of ceftriaxone plus amikacin, ceftazidime plus amikacin and imipenem/cilastatin in the empiric therapy of febrile granulocytopenic cancer patients. Chemotherapy. 1989;35(suppl 2):16–22. [PubMed: 2692982]
- Marie JP, Pico J, Lapierre V, Maulard C, Pappo M, Chiche D, et al. [Comparative trial of ceftazidime alone, ceftazidime + amikacin and ceftazidime + vancomycin as empiric therapy of febrile cancer patients with induced prolonged neutropenia]. Traitement empirique des episodes febriles survenant chez les patients cancereux presentant une neutropenie prolongee: essai comparatif ceftazidime seule, ceftazidime+amikacine et ceftazidime+vancomycine. Medicine et Maladies Infectieuses. 1991;21:386–8.
- Matsui K, Masuda N, Takada M, Kusunoki Y, Fukuoka M. A randomized trial comparing imipenem/cilastatine alone with latamoxef plus tobramycin in febrile neutropenic patients with lung cancer. Jpn J Clin Oncol. 1991;21(6):428–34. [PubMed: 1805048]
- Miller JA, Butler T, Beveridge RA, Kales AN, Binder RA, Smith LJ, Ueno WM, Milkovich G, Goldwater S, Marion A, et al. Efficacy and tolerability of imipenem-cilastatin versus ceftazidime plus tobramycin as empiric therapy of presumed bacterial infection in neutropenic cancer patients. Clinical Therapeutics. 1993;15(3):486–99. [PubMed: 8364941]
- Morgan G, Duerden BI, Lilleyman JS. Ceftazidime as a single agent in the management of children with fever and neutropenia. Journal of Antimicrobial Chemotherapy. 1983;12(suppl A):347–51. [PubMed: 6352641]
- Norrby SR, Vandercam B, Louie T, Runde V, Norberg B, Anniko M, Andrien F, Baudrihaye M, Bow E, Burman LA, et al. Imipenem/cilastatin versus amikacin plus piperacillin in the treatment of infections in neutropenic patients: a prospective, randomized multi- clinic study. Scandinavian Journal of Infectious Diseases Supplement. 1987;52:65–78. [PubMed: 3331044]
- Novakova I, Donnelly P, De Pauw B. Amikacin plus piperacillin versus ceftazidime as initial therapy in granulocytopenic patients with presumed bacteremia. Scandinavian Journal of Infectious Diseases. 1990;22(6):705–11. [PubMed: 2284577]
- Novakova IR, Donnelly JP, de Pauw BE. Ceftazidime with or without amikacin for the empiric treatment of localized infections in febrile, granulocytopenic patients. Annals of Hematology. 1991;63(4):195–200. [PubMed: 1932297]
- Ozyilkan O, Yalcintas U, Baskan S. Imipenem-cilastatin versus sulbactam-cefoperazone plus amikacin in the initial treatment of febrile neutropenic cancer patients. The Korean Journal of Internal Medicine. 1999;14(2):15–9. [PMC free article: PMC4531927] [PubMed: 10461420]
- Papachristodoulou A, Vaslamatzis M, Xynogalos S, Papacharalambous A, Alexopoulos CG. Ann-Oncol. Oxford: Oxford Univerisity Press, published on behalf of The European Society for Medical Oncology; 1996. Ceftazidime (CFZ) monotherapy as empirical initial treatment of febrile neutropenia cancer patients (Pts). p. 140. Abstract 676.
- Pegram Sp, Muss HB, McGall CE, Cooper RM, White DR, Richards Jackson DV, Stuart JJ, Spurr CL. A comparative study of Moxalactam vs. Ticarcillin and Tobramycin in febrile, neutropenic cancer patients. Proceedings of the Annual Meetings of the American Society of Clinical Oncology. 1984. Proc Annu Meet Am Soc Clin Oncol 1984;Abs. Abstract no. 391.
- Pellegrin JL, Marit G, Fourche J, Broustet A, Texier MJ, Leng B, Reiffers J. [Response to infection in patients with acute leukemia during remission induction treatment: Ceftazidime versus cefotaxime + tobramycin]. Etude prospective randomisee de la ceftazidime versus l'association cefotaxime-tobramycine, dans les leucemies aigues en aplasie therapeutique. Presse Med. 1988;17(37):1960–3. [PubMed: 2973596]
- Perez C, Sirham M, Labarca J, Grebe G, Lira P, Oliva J, Duhalde M, Ocqueteau M, Acuna G. [Imipenem/cilastatin versus ceftazidime-amikacin in the treatment of febrile neutropenic patients]. Imipenem/cilastatina versus ceftazidima-amikacina para el tratmiento de pacientes neutropenicos febriles. Revista Medica de Chile. 1995;123(3):312–20. [PubMed: 8525170]
- Petrilli AS, Cypriano M, Dantas LS, Lee LM, Vercillo Luisi MF, Torres B Silva KV, Pires Pereira CA. Evaluation of ticarcillin/clavulanic acid versus ceftriaxone plus amikacin for fever and neutropenia in pediatric patients with leukemia and lymphoma. Brazilian Journal of Infectious Diseases. 2003;7(2):111–120. [PubMed: 12959681]
- Piccart M, Klastersky J, Meunier F, Lagast H, Van Laethem Y, Weerts D. Single-drug versus combination empirical therapy for gram-negative bacillary infections in febrile cancer patients with and without granulocytopenia. Antimicrobial Agents and Chemotherapy. 1984;26(6):870–5. [PMC free article: PMC180041] [PubMed: 6524903]
- Pickard W, Durack D, Gallis H. A randomized trial of empiric therapy with moxalactam versus tobramycin plus ticarcillin in febrile, neutropenic patients. Current choices in antibiotic therapy for febrile episodes in neutropenic patients. 1983
- Piguet H, Pappo M. [First-line treatment of febrile episodes in leukemia in adults. Randomized, multicenter study of ceftazidime in single antibiotic therapy versus a cefotaxime-amikacin combination]. Traitment de premiere intention des episodes febriles des leucemies de l'adulte. Etude randomisee, multicentrique de la ceftazidime en monoantibiotherapie versus l'association cefotaxime-amikacine. Presse Med. 1988;17(37):1954–9. [PubMed: 2973595]
- Rodjer S, Alestig K, Bergmark J, Bergstrom T, Hultberg B, Jagenburg R, Olaisson L, Trollfors B, Westin J. Treatment of septicaemia in immunocompromised patients with ceftazidime or with tobramycin and cefuroxime, with special reference to renal effects. Journal of Antimicrobial Chemotherapy. 1987;20(1):109–16. [PubMed: 3305460]
- Rodriguez W, Gomez H, Silva ME, Vallejos C, Valdivia S, Casanova L, et al. Cefotaxime vs Cephalotin-Gentamicin in the first febrile episode of patients having solid tumors and short-term neutropenia. Acta cancerol. 1995;25:61–8.
- Rolston KV, Berkey P, Bodey GP, Anaissie EJ, Khardori NM, Joshi JH, Keating MJ, Holmes FA, Cabanillas FF, Elting L. A comparison of imipenem to ceftazidime with or without amikacin as empiric therapy in febrile neutropenic patients. Archives of Internal Medicine. 1992;152(2):283–91. [PubMed: 1739355]
- Schuchter L, Kaelin W, Petty B, Wingard J, Altomonte V, Dick J, Rizzo T. Ceftazidime vs ticalcillin and gentamicin in febrile neutropenic bone marrow transplant patients: a prospective, randomized, double-blind trial. Blood. 1988;72(5 Suppl 1):406a. abstract 1534.
- Smith L, Will AM, Williams RF, Stevens RF. Ceftriaxone vs. azlocillin and netilmicin in the treatment of febrile neutropenic children. The Journal of Infection. 1990;20(3):201–6. [PubMed: 2187928]
- Tamura K, Matsuoka H, Tsukada J, Masuda M, Ikeda S, Matsuishi E, et al. Cefepime or carbapenem treatment for febrile neutropenia as a single agent is as effective as a combination of 4th-generation cephalosporin + aminoglycosides: comparative study. Am J Hematol. 2002;71:248–255. [PubMed: 12447952]
- Tamura K, Imajo K, Akiyama N, Suzuki K, Urabe A, Ohyashiki K, Tanimoto M, Masaoka T., the Japan Febrile Neutropenia Study Group. Randomized Trial of Cefepime Monotherapy or Cefepime in Combination with Amikacin as Empirical Therapy for Febrile Neutropenia. Clinical Infectious Diseases. 2004;39:S15–S24. [PubMed: 15250016]
- Wade JCBC, Delin A, Finely R, Drusano G, Thompson B. Imipenem vs. piperacillin plus amikacin, empiric therapy for febrile neutropenic patients: a double blind trial; 27th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1987. Abstract no. 1251.
- Wrzesien-Kus A, Jamroziak K, Wierzbowska A, Robak T. Cefepime in monotherapy or in combination with amikacine as the empirical treatment of febrile neutropenic patients. Acta Haematologica Polonica. 2001;32(2):165–72.
- Yamamura D, Gucalp R, Carlisle P, Cimino M, Roberts J, Rotstein C. Open randomized study of cefepime versus piperacillin-gentamicin for treatment of febrile neutropenic cancer patients. Antimicrobial Agents and Chemotherapy. 1997;41(8):1704–8. [PMC free article: PMC163989] [PubMed: 9257745]
- Pereira CA, Petrilli AS, Carlesse FA, Luisi FA, da Silva KV, de Martino Lee ML. Cefepime monotherapy is as effective as ceftriaxone plus amikacin in pediatric patients with cancer and high-risk febrile neutropenia in a randomized comparison. Journal of Microbiology, Immunology & Infection. 2009 Apr;42(2):141–7. [PubMed: 19597646]
- Yildirim I, Aytac S, Ceyhan M, Cetin M, Tuncer M, Cengiz AB, et al. Piperacillin/tazobactam plus amikacin versus carbapenem monotherapy as empirical treatment of febrile neutropenia in childhood hematological malignancies. Pediatric Hematology & Oncology. 2008 Jun;25(4):291–9. [PubMed: 18484473]
- Zengin E, Sarper N, Kılıç SC. Piperacillin/tazobactam monotherapy versus piperacillin/tazobactam plus amikacin as initial empirical therapy for febrile neutropenia in children with acute leukemia. Pediatr Hematol Oncol. 2011;28(4):311–20. [PubMed: 21524156]
Trials included in Paul et al (2007)
Some trials had multiple publications but only the major publications are listed here.
New trials published since Paul et al (2007)
EVIDENCE TABLES
Download PDF (301K)
11.1. Mixed treatment comparison of empiric intravenous antibiotic monotherapy and empiric intravenous antibiotic dual therapy. (Topic E3)
Evidence Statements
A mixed treatment comparison was done using 108 trials identified in two Cochrane reviews by Paul, et al., (2007 and 2010). These trials were either comparing single agent beta-lactams with each other (Paul, et al., 2010) or comparing single agent beta-lactams with combined beta-lactam/aminoglycoside treatment (Paul, et al., 2007)
The treatment most likely to be best at reducing overall mortality was the use of a single agent ureidopenicillin. This was reflected in direct and indirect estimates (Tables 11.1.1 to 11.1.3). Carbapenems alone compared with ureidopenicillin had higher overall mortality, equivalent infectious mortality and marginally less risk of ‘treatment failure’.
Table 11.1.1
Results of Mixed Treatment Comparison Analysis.
Table 11.1.2
Comparison of pair wise and MTC analyses for mortality.
Table 11.1.3
Comparison of Results for Direct and Indirect Analysis of Mortality. Indirect comparisons in the upper triangle, direct comparisons in the lower triangle.
METHODS
Statistical analysis
We considered antibiotics in seven groups, based around antibiotic class, and agreed a priori with clinical experts in the GDG. We considered combinations of antibiotics, for example cephalosporin plus aminoglycoside, as an intervention-group, rather than the sum of effects of cephalosporin plus aminoglycoside. This decision was made considering the additional antimicrobial coverage of a second agent could vary depending on the paired therapy, and so the simple ‘sum’ approach may not reflect the underlying treatment. The groups were:
- carbapenem
- ureidopenicillins
- 3rd Generation Cephalosporin
- 4th Generation Cephalosporin
- ureidopenicillins +aminoglycoside
- 3rd Generation Cephalosporin +aminolycoside
- 4th Generation Cephalosporin+aminoglycoside
We carried out a mixed treatment comparison using a Bayesian network model by Markov Chain Monte Carlo simulations using WinBugs software to obtain estimates of the effects of all interventions and estimates of the ranking of interventions (Caldwell, Ades & Higgins, 2005; Higgins & Whitehead, 1996; Lu & Ades, 2004 and Smith, Spiegelhalter & Thomas, 1989) . Log odds ratios of the effects of interventions were modeled using non-informative prior distributions: for normal priors, a mean of zero and variance of 10 000, for standard deviation of log-odds ratios, a uniform prior between 0 and 2. The effect size covariance was adjusted for multi-arm trials. The models are available on request. These priors were assessed in a sensitivity analysis. A burn-in sample of 10 000 iterations was followed by 100 000 iterations used to compute estimates, at which point the MCMC error was less than 1% of the standard deviation. Results are reported as median estimates of efficacy with 95% credibility intervals. We modeled the effects of specific covariates on these estimates in a series of planned sensitivity analyses. Model fit was evaluated by comparing the residual deviance with the number of data points, and selected the most parsimonious model to describe effects by comparing deviance information criterion (DIC) values.
For direct comparisons of treatments effect upon overall mortality we used the DerSimonian-Laird random effects models (DerSimonian & Laird, 1986) in STATA using the metan package. Results are presented as point estimates with 95% confidence intervals. Statistical heterogeneity was quantified using the I2 statistic. A value greater than 50% was considered to be substantial heterogeneity (Higgins & Thompson, 2002 and Higgins et al., 2003).
We compared indirect and direct comparisons for consistency for all pairs where direct evidence was available (Dias et al., 2010).
RESULTS
The summary estimates from the mixed treatment comparisons showed good model fit (residual deviance ∼ 126, compared with 148 data points). The DIC was minimised when covariates indicating year of publication, age of patients, and proportion of haematological malignancy were NOT entered into the model. Additionally, none of these covariates were significant (i.e. their 95% credible intervals all crossed log-zero; no effect).
The treatment most likely to be best at reducing overall mortality was the use of a single agent ureidopenicillin (see Figure 11.1.1). This was reflective in direct and indirect estimates (see Tables 11.1.1 to 11.1.3).
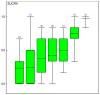
Figure 11.1.1
Cumulative chance of being best at reducing overall mortality. Treatment groups are carbapenem (1), ureidopenicillins (2), 3rd Generation Cephalosporin (3), 4th Generation Cephalosporin (4), ureidopenicillins +aminoglycoside (5), 3rd Generation Cephalosporin (more...)
REFERENCES
- Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900. [PMC free article: PMC1255806] [PubMed: 16223826]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. [PubMed: 3802833]
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison. Stat Med. 2010;29:932–44. [PubMed: 20213715]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [PubMed: 12111919]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [PMC free article: PMC192859] [PubMed: 12958120]
- Higgins JP, Whitehead A. Borrowing strength from external trials in a meta-analysis. Stat Med. 1996;15:2733–49. [PubMed: 8981683]
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–24. [PubMed: 15449338]
- Paul M, Yahav D, Bivas A, Fraser A, Leibovici L. Anti-pseudomonal beta-lactams for the initial, empirical, treatment of febrile neutropenia: comparison of beta-lactams (2010). Cochrane Database of Systematic Reviews. 2010;(11) Art. No.: CD005197. [PMC free article: PMC9022089] [PubMed: 21069685] [CrossRef]
- Paul M, Schlesinger A, Grozinsky-Glasberg S, Soares-Weiser K, Leibovici L. Beta-lactam versus beta-lactam-aminoglycoside combination therapy in cancer patients with neutropenia. Cochrane Database of Systematic Reviews. 2007 Art. No.: CD003038. [PMC free article: PMC6457814] [PubMed: 23813455] [CrossRef]
- Smith TC, Spiegelhalter DJ, Thomas A. Bayesian approaches to random-effects meta-analysis: a comparative study. Stat Med. 1995;14:2685–99. [PubMed: 8619108]
12. Role of empiric glycopeptide antibiotics (antibiotics chosen in the absence of an identified bacterium) in patients with central lines and neutropenia or neutropenic sepsis. (Topic G)
Guideline subgroup members for this question
Jeanette Hawkins (lead), Bob Phillips, Anne Higgins, Barbara Crosse and Rosemary Barnes.
Review question
In patients with a central venous access device with no external signs of line infection but with suspected neutropenia or neutropenic sepsis, what are the benefits and risks of adding vancomycin, teicoplanin or linezolid to first-line antibiotics?
Rationale
It is common for cancer unit antibiotics policies to include specific guidance on the management of cancer patients with fever and neutropenia (proven or suspected) who also have a central venous access device (CVAD) inserted, to minimise the risk of potentially life threatening bacteraemia originating from or colonizing the CVAD. There is usually an assessment of the likelihood of infection in or around the catheter as a potential cause of infection, and often a “step-up” to a different line of more targeted antibiotic therapy if CVAD infection is suspected (i.e. different from broad spectrum untargeted standard treatment). Targeted antibiotic therapy is usually aimed at aerobic and anaerobic Gram-positive bacteria, including multi-resistant Staphylococci based on research showing that these are the most common pathogens for CVAD infection.
The assessment of the CVAD as a potential source of infection will usually include;
- Inspection of the catheter exit site for central lines and skin over the hub for implanted devices, for redness, swelling, or exudate.
- Inspection of the areas around the CVAD for swelling, pain or tenderness especially along the tunnel or port pocket, local joint movement restrictions, e.g. pain on movement of neck or shoulders.
- Patient history for;
- Rigour after CVAD flush
- Mild and / or self resolving low grade fever on several occasions after CVAD flushing
- Pain, tenderness.
- History of previous CVAD infection
If there are obvious signs of infection (e.g. redness, swelling, exudate) the “step-up” to targeted antibiotics covering typical colonizing organisms is accepted practice and not included in this enquiry.
If there are no external signs of infection - is there evidence for the empirical use of “step-up” targeted antibiotics, such as vancomycin, which may be higher in cost and have increased toxicity compared with standard broad spectrum antibiotics?
In the situation of “no external signs of infection” the following factors are usually taken into consideration in clinical practice in assessing the possibility of CVAD infection.
Immunocompromised patients may not produce “pus” due to lack of competent neutrophils and macrophages and therefore external signs of infection may be absent. The principle of “treating blind” is often used for treating infections in cancer patients
Rigours or low grade fevers within the first few hours after a line flush commonly indicate CVAD infection. (Myth, Experience or Research?)
History of previous CVAD infection is a common indicator of recurrent infection. (Myth, Experience or Research?)
Clinical experience that patients who have no apparent sign of CVAD infection at presentation can go on to have proven bacteraemia on blood culture from CVAD or colonisation of catheter-tip on venogram. (Myth, Experience or Research?).
Question in PICO format
| Patients/population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients with central venous access devices and neutropenia or neutropenic sepsis, without an identified bacterium. | Empiric vancomycin, teicoplanin, linezolid in addition to first line antibiotics | First-line, broad spectrum antibiotics |
|
METHODS
Information sources and eligibility criteria
The information specialist (SB) searched the following electronic databases: Medline, Premedline, Embase, Cochrane Library, Cinahl, BNI, Psychinfo, Web of Science (SCI & SSCI), ISI proceedings and Biomed Central. The search was done on 19th July 2011 and updated on 7th November 2011. The search was restricted to published randomised (or quasi randomised) trials and systematic reviews of randomised trials.
Selection of studies
The information specialist (SB) conducted the first screen of the literature search results. Two reviewers (NB and CL) then selected potentially eligible studies by comparing their title and abstract with the inclusion criteria set out by the PICO question. Full text articles were obtained for studies identified as potentially relevant. These were read and checked against the inclusion criteria.
RESULTS
Results of searches
Eight Randomised Controlled Trials (RCTs) were identified that compared empiric vancomycin / teicoplanin / linezolid plus first-line antibiotics, to first-line broad spectrum antibiotics presented alone. Only one of these RCTs (Karp et al 1986) included only patients with a central venous access device. The proportion with a central line was unclear in 6 studies (de Pauw et al 1990; del Favero et al 1987; Novakova et al 1991; Marie et al 1991; Molina et al 1993; EORTC 1991). In the remaining study (Ramphal et al 1992) 61% had a central line. One systematic review that included these studies (in addition to other studies that did not meet the criteria set out by the PICO) was identified.
Types of participant
All participants were neutropenic cancer patients with a fever. At least a proportion of participants in each study had a central venous access device.
Evidence statements
The evidence for all outcomes is summarised in Table 12.1
Table 12.1
GRADE profile: Role of empiric glycopeptide antibiotics (antibiotics chosen in the absence of an identified bacterium) in patients with central lines and suspected neutropenia or neutropenic sepsis.
Short term mortality
Five studies reported short term mortality (de Pauw, et al., 1990; EORTC, 1991; Ramphal, et al., 1992; Molina, et al., 1993; Novakova, et al., 1991). There was very low quality evidence of uncertainty about the difference between antibiotics administered alone, and the same empiric antibiotics administered with the addition of glycopeptides, RR = 0.97 (95% CI 0.63 – 1.50) in four studies with1083 participants.
Critical care, length of stay and line preservation
These outcomes were not reported by any of the included studies.
Antibiotic resistance
Only one study reported antibiotic resistance (Novakova, et al., 1991). Rates of resistance were very low in both groups (2/51 (4%) in the group who received empiric antibiotics alone and 0/52 (0%) in the group who received empiric antibiotics plus glycopeptides).
Proven Bacteraemia
Two studies with 150 particpants reported proven bacteremia as an outcome (Del Favero, et al., 1987; Novakova, et al., 1991). There was very low quality evidence of uncertainty about whether antibiotics administered alone or empiric antibiotics administered with glycopeptides was more effective in terms of proven bacteraemia, RR = 0.80 (95% CI 0.42 – 1.53)
Nephrotoxicity
In five studies with 1160 participants, there was very low quality evidence of a significant difference between antibiotics administered alone, and the same empiric antibiotics administered with glycopeptides, with a greater number of individuals receiving the latter regimen experiencing nephrotoxicity, RR = 0.57 (95% CI 0.33 – 0.99).
Hepatic toxicity
Two studies with 856 participants reported hepatic toxicity as an outcome. There was very low quality evidence of a significant difference between empiric antibiotics administered alone, and antibiotics administered with the addition of glycopeptides. A greater number of individuals in the latter group experienced hepatic toxicity, RR = 0.53 (95% CI 0.33 – 0.99).
Evidence summary and figures
Short-term mortality
There was no significant difference between antibiotics administered alone, and the same empiric antibiotics administered with glycopeptides (RR = 0.97 (0.63 – 1.50) P = 0.88; 4 studies; 1083 participants).

Figure 12.2
Forest plot of all cause short-term mortality.
Bacteraemia
There was no significant difference between antibiotics administered alone, and the same empiric antibiotics administered with glycopeptides (RR = 0.80 (0.42 – 1.53) P = 0.10; 2 studies; 150 participants).
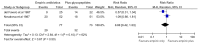
Figure 12.3
Forest plot of bacteraemia.
Toxicity
Nephrotoxicity
Five studies reported nephrotoxicity as an outcome. There was a significant difference between antibiotics administered alone, and the same empiric antibiotics administered with glycopeptides, with a greater number of individuals receiving the latter regimen experiencing nephrotoxicity (RR = 0.57 (0.33 – 0.99) P = 0.05; 5 studies; 1160 participants).

Figure 12.4
Forest plot of nephrotoxicity.
Hepatic toxicity
Two studies reported hepatic toxicity as an outcome. There was a significant difference between antibiotics administered alone, and the same empiric antibiotics administered with glycopeptides, with a greater number of individuals receiving the latter regimen experiencing hepatic toxicity (RR = 0.53 (0.33 – 0.99) P = 0.0008; 2 studies; 856 participants).

Figure 12.4
Forest plot of hepatic toxicity.
EVIDENCE TABLES
Download PDF (719K)
REFERENCES
- de Pauw BE, Novakova IR, Donnelly JP. Options and limitations of teicoplanin in febrile granulocytopenic patients. British Journal of Haematology. 1990;76 Suppl-5 [PubMed: 2149042]
- Del Favero A, Menichetti F, Guerciolini R, Bucaneve G, Baldelli F, Aversa F, et al. Prospective randomized clinical trial of teicoplanin for empiric combined antibiotic therapy in febrile, granulocytopenic acute leukemia patients. Antimicrobial Agents & Chemotherapy. 1987;31:1126–1129. [PMC free article: PMC174882] [PubMed: 2959198]
- EORTC. Vancomycin added to empirical combination antibiotic therapy for fever in granulocytopenic cancer patients. European Organization for Research and Treatment of Cancer (EORTC) International Antimicrobial Therapy Cooperative Group and the National Cancer Institute of Canada-Clinical Trials Group. Journal of Infectious Diseases. 1991;163:951–958. [Erratum appears in J Infect Dis 1991 Oct; 164(4):832] [PubMed: 2019772]
- Karp JE, Dick JD, Angelopulos C, Charache P, Green L, Burke PJ, et al. Empiric use of vancomycin during prolonged treatment-induced granulocytopenia. Randomized, double-blind, placebo-controlled clinical trial in patients with acute leukemia. American Journal of Medicine. 1986;81:237–242. [PubMed: 3526884]
- Marie JP, Pico J, Lapierre V, Maulard C, Pappo M, Chiche D, et al. Comparative trial of ceftazidime alone, ceftazidime + amikacin and ceftazidime + vancomycin as empiric therapy of febrile cancer patients with induced prolonged neutropenia. Medecine et Maladies Infectieuses. 1991;21:386–388. [French]
- Molina F, Pedro L, Rosell R, Barnadas A, Font A, Maurel J. Randomized open and prospective study of two antibiotic schedules (with and without teicoplanin) for post-chemotherapy episodes of neutropenic fever. Oncologia: IV Congresso Nacional de la SEOM. 1993;16:247.
- Novakova I, Donnelly JP, de PB. Ceftazidime as monotherapy or combined with teicoplanin for initial empiric treatment of presumed bacteremia in febrile granulocytopenic patients. Antimicrobial Agents and Chemotherapy. 1991;35:672–678. [PMC free article: PMC245077] [PubMed: 1829879]
- Paul M, Brook S, Fraser A, Vidal L, Leibovici L. Empirical antibiotics against Gram positive infections for febrile neutropenia: systematic review and meta-analysis of randomised controlled trials. Journal of Antimicrobial Chemotherapy. 2005;55:436–44. [PubMed: 15722392]
- Ramphal R, Bolger M, Oblon DJ, Sherertz RJ, Malone JD, Rand KH, et al. Vancomycin is not an essential component of the initial empiric treatment regimen for febrile neutropenic patients receiving ceftazidime: A randomized prospective study. Antimicrobial Agents and Chemotherapy. 1992;36:1062–1067. [PMC free article: PMC188836] [PubMed: 1510394]
13. Indications for removing central lines in patients with neutropenia or neutropenic sepsis. (Topic H)
Guideline subgroup members for this question
Jeanette Hawkins (lead), Bob Phillips, Anne Higgins, Barbara Crosse and Rosemary Barnes
Review question
Which patients with central venous access devices and neutropenic sepsis will benefit from removal of their central line?
Rationale
Tunnel, intra-luminal or pocket infection associated with central venous access devices (CVAD) is a potentially life threatening complication, with heightened risk in immunocompromised patients. Cancer patients with neutropenic sepsis and suspected or proven CVAD infection, require early detection of infection and prompt intervention to prevent morbidity, mortality and (where possible) to preserve long-term devices.
CVADs are frequently intended to be ‘long-term’ devices in cancer patients to support long-term therapy and improve quality of life for patients on treatment (and in palliative care) by reducing exposure to frequent needle sticks. CVADs reduce the risks of extravasation injury from vesicant & irritant cytotoxic infusions. CVADs also facilitate the infusion of multiple therapies more readily, e.g. concurrent chemotherapy, parenteral nutrition and antibiotics. Replacement devices are often considered when long term CVADs are removed due to infection, but device replacement is not without risk and inconvenience to the patient, and costly in terms of additional theatre and anaesthetic time for the NHS. For these reasons there has been a shift towards line preservation where possible, by attempting to treat CVAD infections. Clinicians need evidence based guidelines to weigh up the risk / benefit equation in attempting to preserve devices without increasing the risk of serious morbidity and mortality.
Assessing the need for line removal usually includes;
- Proven Catheter related sepsis (CRS) or Catheter related blood stream infection (CRBSI) due to isolated pathogens.
- Location of infection (proven or suspected) exit site, tunnel, intra-luminal, pocket.
- Prolonged unresponsive fever after commencing antibiotics.
- Severity of clinical illness
- Recurrent infection in same CVAD
- Failures of CVAD function with or without evidence of colonised fibrin sheath at catheter tip.
Question in PICO format
| Patients/population | Prognostic factors | Outcomes |
|---|---|---|
| Patients with central venous access device and neutropenic sepsis |
|
|
METHODS
Information sources and eligibility criteria
The information specialist (SB) searched the following electronic databases: Medline, Premedline, Embase, Psychinfo and BMI. The full strategy will be available in the full guideline. There were no publication date limits set. The date of the search was 10th of August 2011 and was updated on 7th November 2011.
Papers ordered for topic G and topic C, were also checked for eligibility for this topic.
Selection of studies
The information specialist (SB) did the first screen of the literature search results. Two reviewers (NB and CL) then independently selected possibly eligible studies by comparing their title and abstract to the inclusion criteria in the PICO question. The full articles were then obtained and checked against the inclusion criteria.
RESULTS
Results of the literature searches
Study characteristics
- All studies were observational, five studies were prospective.
- Six studies included only children or teenagers.
- Nine studies included a majority of patients with haematological cancers.
- Five studies reported results only for patients with presumed CVC (central venous catheter)-related infections.
- Three studies reported results only for patients with specific microbiologically documented infections. De Pauw et al (1990) included only episodes with Gram positive bacterial infections, Hanna et al (2004) Gram negative infections and Park et al (2010) patients with presumed catheter-related staphylococcus aureus bacteraemia.
- Three studies came from the 1980s, four from the 1990s and seven from 2000 onwards.
Study quality
The evidence was of very low quality because there was a lack of studies comparing criteria for central line removal. Instead studies reported outcomes according to the site of the infection or infecting micro-organism. All 14 included studies were observational of which five were prospective. Six studies included only children or teenagers, nine studies included a majority of patients with haematological cancers and five studies reported results only for patients with presumed central venous catheter related infections.
Evidence Statements
Mortality
No studies considered prognostic factors for overall survival, but some reported infectious mortality.
Two studies (Al Bahar, et al., 2000; Elishoov, et al., 1998) reported infectious mortality according to the site of infection (Table 13.1). All 16 cases of infectious mortality were associated with bacteraemia or fungaemia and there were no cases of infectious mortality attributed to tunnel or exit site infections.
Table 13.1
Outcome according to infection site.
Elishoov, et al., (1998) reported ten occurrences of infectious mortality according to the infecting microorganisms. Microorganisms associated with infectious mortality were coagulase negative Staphylococcus aureus (1 infectious mortality in 29 infections), Streptococcus virididans (1/3), Pseudomonas aeruginosa (4/13), Candida species (2/10). There were 2 polymicrobial infectious deaths involving Escherichia coli, Enterococcus faecalis, Klebsiella pneumoniae and Proteus vulgaris in one case and Pseudomonas aeruginosa, Escherichia coli and Klebsiella pneumoniae in another.
Park, et al., (2010) reported 2 infectious deaths in a series of 48 cases of catheter-related Staphylococcus aureus bacteraemia.
Length of hospital stay, duration of fever and duration of antibiotics
None of the included studies reported length of hospital stay.
Millar, et al., (2011) considered prognostic factors for length of the febrile episode in a prospective multicentre study of children with central venous catheters and fever. The febrile neutropeniaFN episode was longer in patients with fever, rigors and chills (FRC): HR 0.49 (95% C.I. 0.27 to - 0.88), than in those without FRC. Children infected with pathogens (organisms which would normally prompt central venous catheter removal such as Staphylococcus aureus or Pseudomonas aeruginosa) had longer febrile episodes than children without microbiologically documented infections: HR 0.48 (95% C.I. 0.19 to - 1.17). Similarly children infected with organisms typically treated with antibiotic lock or skin bacteria had longer febrile episodes than children without microbiologically documented infections: HR 0.57 (95% C.I. 0.38 to- 0.84).
The total duration of IV treatment was 3.61 times longer in patients with FRC (95% CI 0.55 to - 6.68) than without, 4.39 times longer in patients with pathogenic organisms (95% CI -0.39 to - 9.18) than those without microbiologically documented infections and 2.99 times longer in patients with other organisms or skin bacteria than in those without microbiologically documented infections (95% CI 0.91 to - 5.08).
Line preservation
Several studies (Viscoli, et al., 1988, Junqueria, et al., 2010, Holloway, et al., 1995, Al Bahar., et al., 2000, Hartman, et al., 1987, Elishoov, et al., 1998 and Hanna, et al.,, 2004) reported whether or not the central venous catheter was removed according to the site of infection (Table 13.1). Central venous catheters were often preserved in those with exit site infection or bacteraemia, but were removed in all but one case of tunnel infection.
In Millar et al., (2011) the presence of fever, rigors, chills and/or hypotension was associated with a greatly increased likelihood of central venous catheter removal, HR=16.39 (95% C.I. 4.73 to - 56.79).
Park, et al., (2010) reported the outcome of attempted Hickman catheter salvage in 33 patients with presumed catheter-related Staphylococcus aureus bacteraemia (Table 13.2). Several factors were associated with an increased chance of salvage failure: external signs of infection (tunnel or exit-site infection), positive follow up blood cultures (at 48 to 96 hours) and methicillin resistance (at a statistical significance level of P<0.05). Catheter salvage failed in both patients with septic shock in this study.
Table 13.2
Line preservation according to infecting microorganism.
Joo, et al., (2011) reported the outcome of attempted catheter salvage in 38 patients with a central venous catheter related infection. There was a greater proportion of Gram-negative bacteria in the salvage failure group (8/18) than in the successful salvage group (2/20), (pP=0.027). The majority of the successful central venous catheter salvage attempts (13/20) were in patients with coagulase negative Staphylococcus infections .
Millar, et al., (2011) found in children infected with pathogens traditionally leading to central venous catheter removal, the time to central venous catheter removal was much shorter than when there was no microbiologically documented infection (HR 25.71; 95% C.I. 4.27 to - 154.7). If the child was infected with a microorganism usually treated with antibiotic lock or a skin bacteria, the time to central venous catheter removal was also shorter than if there was no microbiologically documented infection ( HR 8.40; 95% C.I. 2.01 to - 35.14), ).
Infection-control complications
This outcome was not reported in the included studies.
EVIDENCE TABLES
Download PDF (426K)
REFERENCES
- Apostolopoulou E, Raftopoulos V, Terzis K, Elefsiniotis I. Infection Probability Score, APACHE II and KARNOFSKY scoring systems as predictors of bloodstream infection onset in hematology-oncology patients. BMC Infectious Diseases. 2010;10:135. [PMC free article: PMC2890000] [PubMed: 20504343]
- Averbuch D, Makhoul R, Rotshild V, Weintraub M, Engelhard D. Empiric treatment with once-daily cefonicid and gentamicin for febrile non-neutropenic pediatric cancer patients with indwelling central venous catheters. Journal of Pediatric Hematology/Oncology. 2008 July;30(7):527–532. 2008.Date of Publication: July 2008. [PubMed: 18797200]
- Bahar SA, Pandita R, Bavishi K, Savani B. Febrile neutropenia in patients with acute leukemia with long-term central venous access in Kuwait: Microbial spectrum, outcome and catheter management. Medical Principles and Practice. 2000 Jan;9(1):35–41. 2000.Date of Publication: Jan 2000.
- Biagi E, Arrigo C, Dell'Orto MG, Balduzzi A, Pezzini C, Rovelli A, et al. Mechanical and infective central venous catheter-related complications: A prospective non-randomized study using Hickman and Groshong catheters in children with hematological malignancies. Supportive Care in Cancer. 1997 May;5(3):228–233. 1997.Date of Publication: May 1997. [PubMed: 9176970]
- Body JJ, Richard V, Pector JC, Lemaire A, Deshpande S, Verheye E, et al. Septicemias in cancer patients during parenteral nutrition: Contributing factors and detection by weekly blood cultures. Clinical Nutrition. 1989;8(4):191–195. 1989.Date of Publication: 1989., 1989. [PubMed: 16837288]
- Carr E, Jayabose S, Stringel G, Slim M, Ozkaynak MF, Tugal O, et al. The safety of central line placement prior to treatment of pediatric acute lymphoblastic leukemia. Pediatric Blood & Cancer. 2006;47:886–888. [PubMed: 16200633]
- Chaberny IF, Ruseva E, Sohr D, Buchholz S, Ganser A, Mattner F, et al. Surveillance with successful reduction of central line-associated bloodstream infections among neutropenic patients with hematologic or oncologic malignancies. Annals of Hematology. 2009;88:907–912. [PubMed: 19139891]
- Chaftari AM, Kassis C, El Issa H, Al Wohoush I, Jiang Y, Rangaraj G, et al. Novel Approach Using Antimicrobial Catheters to Improve the Management of Central Line-Associated Bloodstream Infections in Cancer Patients. Cancer. 2011;117:2551–2558. [PubMed: 24048803]
- De Pauw BE, Novakova IR, Donnelly JP. Options and limitations of teicoplanin in febrile granulocytopenic patients. British Journal of Haematology. 1990;76 Suppl-5 [PubMed: 2149042]
- Elihu A, Gollin G. Complications of implanted central venous catheters in neutropenic children. American Surgeon. 2007;73:1079–1082. [PubMed: 17983086]
- Elishoov H, Or R, Strauss N, Engelhard D. Nosocomial colonization, septicemia, and Hickman/Broviac catheter related infections in bone marrow transplant recipients: A 5-year prospective study. Medicine. 1998 Mar;77(2):83–101. 1998.Date of Publication: Mar 1998. [PubMed: 9556701]
- Fatkenheuer G, Buchheidt D, Cornely OA, Fuhr HG, Karthaus M, Kisro J, et al. Central venous catheter (CVC)-related infections in neutropenic patients--guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Annals of Hematology. 2003;82 Suppl-57 [PubMed: 13680168]
- Gorelick MH, Owen WC, Seibel NL, Reaman GH. Lack of association between neutropenia and the incidence of bacteremia associated with indwelling central venous catheters in febrile pediatric cancer patients. Pediatric Infectious Disease Journal. 1991;10:506–510. [PubMed: 1876466]
- Groeger JS, Lucas AB, Thaler HT, Friedlander-Klar H, Brown AE, Kiehn TE, et al. Infectious morbidity associated with long-term use of venous access devices in patients with cancer. Annals of Internal Medicine. 1993;119:1168–1174. [PubMed: 8239247]
- Gutierrez I, Gollin G. Exclusion of neutropenic children from implanted central venous catheter placement: impact on early catheter removal. Journal of Pediatric Surgery. 2010;45:1115–1119. [PubMed: 20620305]
- Hanna H, Afif C, Alakech B, Boktour M, Tarrand J, Hachem R, et al. Central venous catheter-related bacteremia due to gram-negative bacilli: Significance of catheter removal in preventing relapse. Infection Control and Hospital Epidemiology. 2004 Aug;25(8):646–649. 2004.Date of Publication: Aug 2004. [PubMed: 15357155]
- Hartman GE, Shochat SJ. Management of septic complications associated with Silastic catheters in childhood malignancy. Pediatric Infectious Disease Journal. 1987;6:1042–1047. [PubMed: 3696842]
- Holloway RW, Orr JW. An evaluation of Groshong central venous catheters on a gynecologic oncology service. Gynecologic Oncology. 1995;56(2):211–217. 1995.Date of Publication: 1995., 1995. [PubMed: 7896188]
- Howell PB, Walters PE, Donowitz GR, Farr BM. Risk factors for infection of adult patients with cancer who have tunnelled central venous catheters. Cancer. 1995;75:1367–1375. [PubMed: 7882288]
- Joo E-J, Kang C-I, Ha YE, Park SY, Kang SJ, Joung M-K, et al. Clinical outcome of catheter salvage in neutropenic cancer patients with catheter-related infection. Scandinavian Journal of Infectious Diseases. 2011 April;43(4):258–263. 2011.Date of Publication: April 2011. [PubMed: 21189047]
- Junqueira BLP, Connolly B, Abla O, Tomlinson GA, Amaral JGPV. Port-a-catheter infection rate and associated risk factors in children diagnosed with acute lymphoblastic Leukemia; Blood; Conference: 51st Annual Meeting of the American Society of Hematology; ASH New Orleans, LA United States. 2009. Conference Start: 20091205 Conference End: 20091208. Conference Publication: (var.pagings) 2009.Date of Publication: 20 Nov 2009., 20.
- Junqueira BL, Connolly B, Abla O, Tomlinson G, Amaral JG. Severe neutropenia at time of port insertion is not a risk factor for catheter-associated infections in children with acute lymphoblastic leukemia. Cancer. 2010;116:4368–4375. [PubMed: 20564151]
- Karthaus M, Doellmann T, Klimasch T, Krauter J, Heil G, Ganser A. Central venous catheter infections in patients with acute leukemia. Chemotherapy. 2002;48(3):154–157. 2002.Date of Publication: 2002., 2002. [PubMed: 12138333]
- Kassar R, Hachem R, Jiang Y, Chaftari AM, Raad I. Management of Bacillus bacteremia: the need for catheter removal. Medicine. 2009;88:279–283. [PubMed: 19745686]
- Katsibardi K, Papadakis V, Charisiadou A, Pangalis A, Polychronopoulou S. Blood stream infections throught the entire course of acute lymphoblastic leukemia treatment. Neoplasma. 2011;58:326–330. [PubMed: 21520989]
- Kelly M, Conway M, Wirth K, Billett A, Sandora T. Risk factors for central line-associated bloodstream infection in pediatric oncology patients; Pediatric Blood and Cancer; Conference: 23rd Annual Meeting of the American Society of Pediatric Hematology/Oncology, ASPHO 2010 Montreal; QC Canada. Conference Start: 20100407 Conference End: 20100410; Jun, 2010. p. 82. Conference Publication: (var.pagings)
- Kinsey SE. Experience with teicoplanin in non-inpatient therapy in children with central line infections. European Journal of Haematology.Supplementum. 1998;62:11–14. [PubMed: 9658687]
- Lordick F, Hentrich M, Decker T, Hennig M, Pohlmann H, Hartenstein R, et al. Ultrasound screening for internal jugular vein thrombosis aids the detection of central venous catheter-related infections in patients with haemato-oncological diseases: a prospective observational study. British Journal of Haematology. 2003;120:1073–1078. [PubMed: 12648081]
- Miceli MH, Dong L, Coria P, Vila A, Estrada S, Garcia-Damiano MC, et al. Leaving previously implanted central venous catheters (ports) in place does not increase morbidity in patients undergoing autologous peripheral stem cell transplantation. Bone Marrow Transplantation. 2005;36:131–134. [PubMed: 15908970]
- Millar MM, Zhou W, Skinner R, Pizer B, Hennessy E, Wilks M, et al. Accuracy of bacterial DNA testing for central venous catheter-associated bloodstream infection in children with cancer. Health Technology Assessment. 2011;15:XI. -+ [PubMed: 21294989]
- Morrison VA, Peterson BA, Bloomfield CD. Nosocomial septicemia in the cancer patient: The influence of central venous access devices, neutropenia, and type of malignancy. Medical and Pediatric Oncology. 1990;18(3):209–216. 1990.Date of Publication: 1990., 1990. [PubMed: 2329966]
- Mullan FJ, Hood JM, Barros D'Sa AAB. Use of the Hickman catheter for central venous access in patients with haematological disorders. British Journal of Clinical Practice. 1992;46(3):167–170. 1992.Date of Publication: 1992., 1992. [PubMed: 1286014]
- Nieboer P, de Vries EG, Mulder NH, Rodenhuis S, Bontenbal M, van der Wall E, et al. Factors influencing catheter-related infections in the Dutch multicenter study on high-dose chemotherapy followed by peripheral SCT in high-risk breast cancer patients. Bone Marrow Transplantation. 2008;42:475–481. [PubMed: 18622420]
- Nosari AM, Nador G, De GA, Ortisi G, Volonterio A, Cantoni S, et al. Prospective monocentric study of non-tunnelled central venous catheter-related complications in hematological patients. Leukemia & Lymphoma. 2008;49:2148–2155. [PubMed: 19021058]
- Park KH, Cho OH, Lee SO, Choi SH, Kim YS, Woo JH, et al. Outcome of attempted Hickman catheter salvage in febrile neutropenic cancer patients with Staphylococcus aureus bacteremia. Annals of Hematology. 2010;89:1163–1169. [PubMed: 20574733]
- Press OW, Ramsey PG, Larson EB, Fefer A, Hickman RO. Hickman catheter infections in patients with malignancies. Medicine. 1984;63:189–200. [PubMed: 6377003]
- Raad I, Hachem R, Hanna H, Bahna P, Chatzinikolaou I, Fang X, et al. Sources and outcome of bloodstream infections in cancer patients: the role of central venous catheters. European Journal of Clinical Microbiology & Infectious Diseases. 2007;26:549–556. [PubMed: 17582536]
- Ruggiero A, Barone G, Margani G, Nanni L, Pittiruti M, Riccardi R. Groshong catheter-related complications in children with cancer. Pediatric Blood & Cancer. 2010;54:947–951. [PubMed: 20162685]
- Sariosmanoglu N, Ugurlu B, Turgut NH, Demirkan F, Ozsan H, Ergor G, et al. Use of tunnelled catheters in haematological malignancy patients with neutropenia. Journal of International Medical Research. 2008;36:1103–1111. [PubMed: 18831907]
- Tacconelli E, Tumbarello M, Pittiruti M, Leone F, Lucia MB, Cauda R, et al. Central venous catheter-related sepsis in a cohort of 366 hospitalised patients. European Journal of Clinical Microbiology & Infectious Diseases. 1997;16:203–209. [PubMed: 9131322]
- van de Wetering MD, Poole J, Friedland I, Caron HN. Bacteraemia in a paediatric oncology unit in South Africa. Medical & Pediatric Oncology. 2001;37:525–531. [PubMed: 11745891]
- Viscoli C, Garaventa A, Boni L, Melodia A, Dini G, Cuneo R, et al. Role of Broviac catheters in infections in children with cancer. Pediatric Infectious Disease Journal. 1988;7:556–560. [PubMed: 3174299]
- Wilcox MH, Tack KJ, Bouza E, Herr DL, Ruf BR, Ijzerman MM, et al. Complicated skin and skin-structure infections and catheter-related bloodstream infections: Noninferiority of linezolid in a phase 3 study. Clinical Infectious Diseases. 2009;48(2):203–212. 2009.Date of Publication: 15 Jan 2009., 15. [PubMed: 19072714]
- Wolf HH, Leithauser M, Maschmeyer G, Salwender H, Klein U, Chaberny I, et al. Central venous catheter-related infections in hematology and oncology : guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Annals of Hematology. 2008;87:863–876. [Review] [116 refs] [PubMed: 18629501]
- Worth LJ, Seymour JF, Slavin MA. Infective and thrombotic complications of central venous catheters in patients with hematological malignancy: Prospective evaluation of nontunneled devices. Supportive Care in Cancer. 2009 July;17(7):811–818. 2009.Date of Publication: July 2009. [PubMed: 19096883]
14. Inpatient versus ambulatory (non-hospitalised) management strategies. (Topic E2)
Guideline subgroup members for this question
Anton Kruger (lead), Wendy King, Barbara Crosse, Bob Phillips and Rosemary Barnes.
Review question
Is there any difference between the outcome of patients with neutropenic sepsis managed in hospital and those managed as outpatients?
Rationale
Neutropenic sepsis is a potentially lethal condition with potentially high mortality rates especially when the infection is due to gram negative bacteria. Early studies focussed on empiric antibiotic treatment combinations specifically targeting this group of organisms and because of the historically poor outcomes and the fact that these regimens had to be given intravenously in multiple daily doses, hospital based care became the norm. In addition, many of the early studies were based on patient populations comprising a high proportion of patients with acute leukaemia. These patients represent the worst risk cases for depth and duration of neutropenia. A further driver to their inpatient management was the recognition that the physical environment may present an additional risk for such high risk patients to acquire mould infections, hence the introduction of hepa filtered rooms.
However, it is apparent that not all patients with neutropenia are at the same risk for an adverse outcome of a septic episode and that treatment and location of treatment may be tailored according to risk factors. These include patient specific factors, on the anti infective treatment received and the environment. Patient specific factors would include the underlying illness, chemotherapy regimen, presence of indwelling intravenous catheters or other devices and co- morbidities. The sensitivities and prevalence of local microbiological flora add an environmental background.
Having defined a group of “low risk” patients it has been possible to design ambulatory care treatment strategies as an alternative to inpatient intravenous care. Ambulatory care strategies include intravenous antibiotic regimens as well as oral. The advantages for ambulatory care are obvious. Most patients prefer to be treated at home, the risks of nosocomial infections is reduced and there are potential cost and resource savings. On the other hand, some ambulatory care programs might remain resource intensive, especially if based on intravenous drug regimens. There are also risks of failure of this strategy and risks particular to oral antibiotics, such as diarrhoea and infection with clostridium difficile. Some patients may also prefer the reassurance of inpatient care.
This review should establish if there is any difference between the outcome of patients with neutropenic sepsis managed in hospital and those managed as outpatients and in which groups ambulatory care may be safe?
Question in PICO format
| Patients/population | Intervention | Comparison | Outcomes |
|---|---|---|---|
| Patients receiving treatment for neutropenic sepsis | In patient care | Ambulatory care (all different forms Community Outpatient Home) |
|
METHODS
Information sources and eligibility criteria
The information specialist (SB) searched the following electronic databases: Medline, Premedline, Embase, Cochrane Library, Cinahl, BNI, Psychinfo, Web of Science (SCI & SSCI), ISI proceedings and Biomed Central. The search was restricted to published randomised (or quasi randomised) trials and systematic reviews of randomised trials.
Selection of studies
The information specialist (SB) conducted the first screen of the literature search results. Two reviewers (NB and CL) then selected potentially eligible studies by comparing their title and abstract to the inclusion criteria set out by the PICO question. Full text articles were obtained for studies identified as potentially relevant. These were read and checked against the inclusion criteria. The final literature search was done on 7th November 2011. The titles and abstracts of 9 papers were compared to the PICO. One was eligible for inclusion.
RESULTS
Description of included studies
One recent, comprehensive systematic review of the literature was identified (Teuffel et al, 2011). This review included 6 RCTs comparing inpatient antibiotic treatment to outpatient antibiotic treatment (Rapoport et al. 1999, Innes et al. 2003, Hidalgo et al. 1999, Malik et al. 1995, Ahmed et al. 2007, Santolaya et al. 2004), and 8 RCTs comparing outpatient oral antibiotic treatment to outpatient intravenous antibiotic treatment (Sebban et al. 2008, Monotti et al. 1999, Rubenstein et al 1993, Gupta et al. 2009, Petrilli et al. 2000, Paganini et al. 2003, Paganini et al 2000, Mullen et al. 1999). One additional RCT comparing inpatient antibiotic treatment to outpatient antibiotic treatment was identified by the update search (Talcott et al. 2011).
All of the RCTs included in the Teuffel et al. review had been identified by our literature search. The remaining 15 studies were excluded (6 studies treated all participants as inpatients, 8 were not RCTs, and 1 randomised participants to different antibiotics as opposed to randomising to inpatient/outpatient treatment). Excluded studies are listed at the end of the document.
Types of study
RCTs comparing any inpatient antibiotic treatment to outpatient antibiotic treatment for the management of FN in cancer patients were included. RCTs comparing any oral outpatient antibiotic treatment to any intravenous outpatient antibiotic treatment were also included and analysed separately. Characteristics of the included studies are presented in the table 14.2.
Table 14.2
Characteristics of included studies (from Teuffel et al 2011; updated with data from Talcott et al. 2011 ).
Evidence statements
Short term mortality
Low quality evidence from seven randomised trials (reviewed in Teuffel, et al., 2011), showed no statistically significant difference in the 30 day mortality of inpatients and outpatients, RR 1.11 (95% C.I. 0.41 to 3.05). Low quality evidence from eight randomised trials found no statistically significant difference in 30 day mortality according to route of drug administration in the outpatient setting (intravenous versus oral), but no patients died in these studies
Critical care
Critical care was not considered as an outcome by the Teuffel, et al., (2011), systematic review. However critical care events were probably included in the composite outcome of treatment failure. Which was defined as one or more of the following: death; persistence, recurrence or worsening of clinical signs or symptoms; any addition to, or modification of the assigned intervention, including readmission.
Low quality evidence from six randomised trials showed no significant difference between the rate of treatment failure of inpatients and outpatients RR = 0.81; (95% CI 0.55 - 1.19).
Low quality evidence from eight randomised trials showed no association between route of drug administration in the outpatient setting (intravenous versus oral) and treatment failure, RR 0.93 (95% CI 0.65 –1.32)).
Three of the six studies comparing inpatient to outpatient treatment reported critical care admission. No patients were admitted to ICU in these studies (350 episodes). Four of the eight studies of outpatient IV versus outpatient oral antibiotics reported critical care admission. No patients were admitted to ICU in these studies (520 episodes).
Length of stay
Only three studies comparing inpatient to outpatient management reported length of stay in the inpatient group. Means were reported as 4.41 days, range 2 – 8 (Innes, et al., 2003), 10.4 days, range 7-19 (Ahmed et al 2007) and 5.3 days, range 3-9 (Santolaya, et al., 2004). Length of stay was not a relevant outcome in studies considering only outpatients.
Hospital readmission (outpatients)
Low quality evidence suggested that hospital readmission was less likely in patients treated with outpatient intravenous therapy than in those who received outpatient oral therapy, RR 0.46 (95% CI 0.22 - 0.97).
Quality of life
Quality of life was not considered as an outcome by the Teuffel, et al., (2011), a systematic review, and none of the included studies reported quality of life. A later study (Talcott, et al., 2011) reported results from subscales of the EORTC QLQ C-30. Moderate quality evidence suggested that role function increased more for hospitalised patients than home care patients (mean change 0.78 versus 0.58 respectively, P = 0.05). Moderate quality evidence showed emotional function scores declined for hospitalised patients but increased for home care patients (mean change -6.94 versus 3.27; P = 0.04). No other QLQ-C30 subscale differences were evident but the data for these subscales were not reported.
Table 14.1
GRADE profile: Is inpatient management more effective than outpatient management for patients with neutropenic sepsis.
Table 14.3
Summary of outcomes.

Figure 14.2
Inpatient versus Outpatient treatment – Treatment Failure.

Figure 14.3
Outpatient Oral Antibiotics versus Outpatient Intravenous Antibiotics – Treatment Failure.
Additional data extracted from original papers
Critical care - Inpatient versus Outpatient treatment
| Study ID | Inpatient | Outpatient |
|---|---|---|
| Rapoport 1999 | Not reported | Not reported |
| Innes 2003 | 0/60 (0%) | 0/66 (0%) |
| Hidalgo 1999 | 0/48 (0%) | 0/47 (0%) |
| Malik 1995 | Not reported | Not reported |
| Ahmed 2007 | 0/66 (0%) | 0/63 (0%) |
| Santolaya 2004 | Not reported | Not reported |
0/174 (0%) episodes treated on an inpatient basis were admitted to ICU.
0/176 (0%) episodes treated on an outpatient basis were admitted to ICU.
Critical care - Outpatient Oral Antibiotics versus Outpatient Intravenous Antibiotics
| Study ID | Intravenous | Oral |
|---|---|---|
| Sebban 2008 | Not reported | Not reported |
| Minotti 1999 | Not reported | Not reported |
| Rubenstein 1993 | Not reported | Not reported |
| Gupta 2009 | 0/54 (0%) | 0/61 (0%) |
| Petrilli 2007 | Not reported by group | Not reported by group |
| Mullen 1999 | 0/33 (0%) | 0/40 (0%) |
| Paganini 2003 | 0/80 (0%) | 0/74 (0%) |
| Paganini 2000 | 0/89 (0%) | 0/89 (0%) |
| Talcott et al. 2011 | Not reported | Not reported |
0/256 (0%) episodes treated on an inpatient basis were admitted to ICU.
0/264 (0%) episodes treated on an outpatient basis were admitted to ICU.
Hospital readmission - Inpatient versus Outpatient treatment
| Study ID | Inpatient | Outpatient |
|---|---|---|
| Rapoport 1999 | Not reported | Not reported |
| Innes 2003 | Not applicable | 5/66 (8%) |
| Hidalgo 1999 | Not applicable | 8/47 (16%) |
| Malik 1995 | Not applicable | 18/48 (21%) |
| Ahmed 2007 | Not applicable | 2/63 (6%) |
| Santolaya 2004 | Not applicable | Not reported |
| Talcott et al. 2011 | Not applicable | 4/47 (9%) |
33/224 (15%) episodes treated on an outpatient basis required hospital readmission.
Hospital readmission - Outpatient Oral Antibiotics versus Outpatient Intravenous Antibiotics
| Intravenous | Oral | |
|---|---|---|
| Sebban 2008 | Not reported | Not reported |
| Minotti 1999 | Not reported | Not reported |
| Rubenstein 1993 | 0/43 (0%) | 6/40 (15%) |
| Gupta 2009 | 0/54 (0%) | 3/61 (5%) |
| Petrilli 2007 | Not reported by group | Not reported by group |
| Mullen 1999 | 2/33 (6%) | 8/44 (18%) |
| Paganini 2003 | 6/80 (7%) | 4/74 (5%) |
| Paganini 2000 | 2/89 (3%) | 1/89 (1%) |
10/299 (3%) episodes treated with intravenous antibiotics resulted in admission to hospital.
22/308 (7%) episodes treated with oral antibiotics resulted in admission to hospital.
EVIDENCE TABLES
Download PDF (712K)
REFERENCES
- Ahmed N, El-Mahallawy HA, Ahmed IA, Nassif S, El-Beshlawy A, El-Haddad A. Early hospital discharge versus continued hospitalization in febrile pediatric cancer patients with prolonged neutropenia: A randomized, prospective study. Pediatric Blood & Cancer. 2007;49:786–792. [PubMed: 17366527]
- Gupta A, Swaroop C, Agarwala S, Pandey RM, Bakhshi S. Randomized controlled trial comparing oral amoxicillin-clavulanate and ofloxacin with intravenous ceftriaxone and amikacin as outpatient therapy in pediatric low-risk febrile neutropenia. Journal of Pediatric Hematology/Oncology. 2009;31:635–641. [PubMed: 19684522]
- Hidalgo M, Hornedo J, Lumbreras C, Trigo JM, Colomer R, Perea S, et al. Outpatient therapy with oral ofloxacin for patients with low risk neutropenia and fever: a prospective, randomized clinical trial. Cancer. 1999;85:213–219. [PubMed: 9921995]
- Innes HE, Smith DB, O'Reilly SM, Clark PI, Kelly V, Marshall E. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: a prospective randomised controlled single centre study. British Journal of Cancer. 2003;89:43–49. [PMC free article: PMC2394220] [PubMed: 12838298]
- Malik IA, Khan WA, Karim M, Aziz Z, Khan MA. Feasibility of outpatient management of fever in cancer patients with low-risk neutropenia: results of a prospective randomized trial. The American journal of medicine. 1995;98:224–231. [PubMed: 7872337]
- Minotti V, Gentile G, Bucaneve G, Iori AP, Micozzi A, Cavicchi F, et al. Domiciliary treatment of febrile episodes in cancer patients: a prospective randomized trial comparing oral versus parenteral empirical antibiotic treatment. Supportive care in cancer: official journal of the Multinational Association of Supportive Care in Cancer. 1999;7:134–139. [PubMed: 10335931]
- Mullen CA, Petropoulos D, Roberts WM, Rytting M, Zipf T, Chan KW, et al. Economic and resource utilization analysis of outpatient management of fever and neutropenia in low-risk pediatric patients with cancer. Journal of pediatric hematology/oncology : official journal of the American Society of Pediatric Hematology/Oncology. 1999;21:212–218. [PubMed: 10363854]
- Mullen CA, Petropoulos D, Roberts WM, Rytting M, Zipf T, Chan KW, et al. Outpatient treatment of fever and neutropenia for low risk pediatric cancer patients. Cancer. 1999;86:126–134. [PubMed: 10391572]
- Paganini H, Gómez S, Ruvinsky S, Zubizarreta P, Latella A, Fraquelli L, et al. Outpatient, sequential, parenteral-oral antibiotic therapy for lower risk febrile neutropenia in children with malignant disease: a single-center, randomized, controlled trial in Argentina. Cancer. 2003;97:1775–1780. [PubMed: 12655535]
- Paganini HR, Sarkis CM, De Martino MG, Zubizarreta PA, Casimir L, Fernandez C, et al. Oral administration of cefixime to lower risk febrile neutropenic children with cancer. Cancer. 2000;88(12):2848–2852. Date of Publication: 15 Jun 2000., 15. [PubMed: 10870071]
- Petrilli A, Altruda CF, Alberto Pires PC. Oral gatifloxacin in the outpatient treatment of children with cancer fever and neutropenia. Pediatric Blood & Cancer. 2007;49:682–686. [PubMed: 17253640]
- Rapoport BL, Sussmann O, Herrera MV, Schlaeffer F, Otero JC, Pavlovsky S, et al. Ceftriaxone plus once daily aminoglycoside with filgrastim for treatment of febrile neutropenia: early hospital discharge vs. Standard In-patient care. Chemotherapy. 1999;45:466–476. [PubMed: 10567777]
- Rubenstein EB, Rolston K, Benjamin RS, Loewy J, Escalante C, Manzullo E, et al. Outpatient treatment of febrile episodes in low-risk neutropenic patients with cancer. Cancer. 1993;71:3640–3646. [PubMed: 8490912]
- Santolaya ME, Alvarez AM, Avilés CL, Becker A, Cofré J, Cumsille MA, et al. Early hospital discharge followed by outpatient management versus continued hospitalization of children with cancer, fever, and neutropenia at low risk for invasive bacterial infection. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:3784–3789. [PubMed: 15365075]
- Sebban C, Dussart S, Fuhrmann C, Ghesquieres H, Rodrigues I, Geoffrois L, et al. Oral moxifloxacin or intravenous ceftriaxone for the treatment of low-risk neutropenic fever in cancer patients suitable for early hospital discharge. Supportive Care in Cancer. 2008;16:1017–1023. [PubMed: 18197434]
- Talcott JA, et al. Safety of early discharge for low-risk patients with febrile neutropenia: A multicenter randomized controlled trial. Journal of Clinical Oncology. 2011;29(30):3977–3983. Date of Publication: 20 Oct 2011.30 (2011): 20. [PMC free article: PMC3675706] [PubMed: 21931024]
- Teuffel O, Ethier MC, Alibhai S, Beyene J, Sung L. Outpatient management of cancer patients with febrile neutropenia: a systematic review and meta-analysis. Annals of Oncology, Advance Access. 2011 [PubMed: 21363878]
- Timing of initial antibiotic therapy. (Topic E4)
- Timing of initial antibiotic: a wider search of timing of antibiotic therapy: removing the requirement of neutropenia
- Empiric intravenous antibiotic monotherapy or empiric intravenous antibiotic dual therapy. (Topic E3)
- Mixed treatment comparison of empiric intravenous antibiotic monotherapy and empiric intravenous antibiotic dual therapy. (Topic E3)
- Role of empiric glycopeptide antibiotics (antibiotics chosen in the absence of an identified bacterium) in patients with central lines and neutropenia or neutropenic sepsis. (Topic G)
- Indications for removing central lines in patients with neutropenia or neutropenic sepsis. (Topic H)
- Inpatient versus ambulatory (non-hospitalised) management strategies. (Topic E2)
- Initial Treatment: guideline chapter six - Neutropenic Sepsis: Prevention and Ma...Initial Treatment: guideline chapter six - Neutropenic Sepsis: Prevention and Management of Neutropenic Sepsis in Cancer Patients
Your browsing activity is empty.
Activity recording is turned off.
See more...